Chapter 11
Cardiac Positron Emission Tomography
1. What is positron emission tomography (PET)?
2. Which are the two most common PET radiopharmaceuticals used for myocardial perfusion imaging (MPI)?
Rubidium-82 (Rb-82) and nitrogen-13 (N-13) ammonia.
3. What are the characteristics of Rb-82?
Rb-82 is a monovalent cation analog of potassium. It is commercially available as a strontium-82 generator. Its physical half-life is 75 seconds. It is extracted with high efficiency by myocardial cells through the Na+,K+-ATPase pump. The adult radiation dose from a Rb-82 MPI varies from 1.75 to 7.5 mSv (Fig. 11-1).
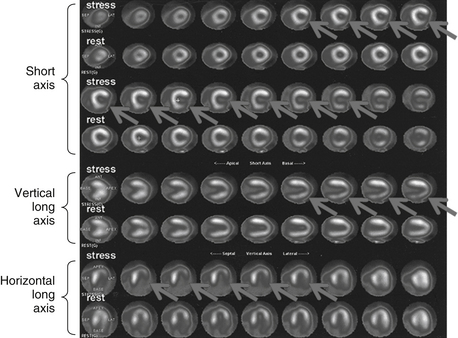
Figure 11-1 Abnormal Rb-82 myocardial perfusion imaging demonstrating inducible ischemia of left circumflex vascular territory. The figure shows stress and rest images displayed in three planes: short axis (apex to base), vertical long axis (septum to lateral wall), and horizontal long axis (from inferior to anterior). The rest images demonstrate good perfusion throughout the left ventricle. The stress images demonstrate decreased tracer in the lateral wall (arrows). (Modified with permission from Branch KR, Caldwell JH, Soine LS, et al: Vascular [humoral] cardiac allograft rejection manifesting as inducible myocardial ischemia on nuclear perfusion imaging, J Nucl Cardiol. 12(1):123-124, 2005.)
4. What are the characteristics of N-13 ammonia?
5. What are the advantages of PET MPI over single-photon emission computed tomography (SPECT)?
 It is more sensitive and specific (93% and 92%, respectively).
It is more sensitive and specific (93% and 92%, respectively).
 It is cost effective. This is primarily due to a reduction in unnecessary coronary angiographies.
It is cost effective. This is primarily due to a reduction in unnecessary coronary angiographies.
 It results in lower radiation dose to the patient.
It results in lower radiation dose to the patient.
 It allows for absolute quantification in myocardial blood flow, expressed as mL/min/g of tissue.
It allows for absolute quantification in myocardial blood flow, expressed as mL/min/g of tissue.
6. My patient’s PET myocardial perfusion study was reported as demonstrating severe ischemia, but the coronary angiogram showed only nonobstructive coronary artery disease (CAD). How can this be?
7. What is the radiopharmaceutical used in cardiac PET for the assessment of myocardial viability?
Fluorine-18 (F-18) fluorodeoxyglucose (FDG).
8. What is meant by “glucose loading” when a viability F-18 FDG cardiac PET is being performed?
9. What is meant by perfusion-metabolism (P-M) “match” or “mismatch” on cardiac PET viability imaging?
A P-M mismatch would be present if the area of decreased perfusion demonstrates normal or increased FDG uptake (Fig. 11-2). This finding would be consistent with an area of viable hibernating myocardium.
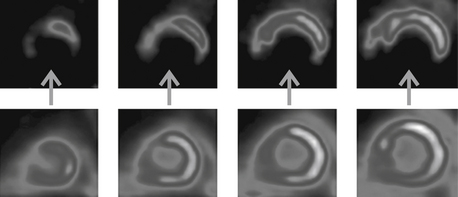
Figure 11-2 Viability study showing four short-axis slices of the left ventricle. The top row of images display perfusion with Rb-82, demonstrating markedly decreased perfusion of the inferior wall (arrows). The bottom row of images display F-18 fluorodeoxyglucose (FDG) uptake in the inferior wall, indicating that the poorly perfused inferior wall still consists of viable, hibernating myocardium. (Modified with permission from Taegtmeyer H, Dilsizian V: Imaging myocardial metabolism and ischemic memory. Nat Clin Pract Cardiovasc Med. Suppl 2:S42-8, 2008.)
10. Are there any other tracers that can be used for PET cardiac imaging?
Bibliography, Suggested Readings, and Websites
1. Bengel, F., Schwaiger, M. PET metabolism, innervations and receptors. In Dilsizian V., Pohost G.M., eds.: Cardiac CT, PET and MR, ed 1, Malden, MA: Blackwell Futura, 2006.
2. Di Carli. PET assessment of myocardial perfusion. In Dilsizian V., Pohost G.M., eds.: Cardiac CT, PET and MR, ed 1, Malden, MA: Blackwell Futura, 2006.
3. Machac, J. Cardiac positron emission tomography imaging. Semin Nucl Med. 2005;35(1):17–36.
4. Dilsizian, V., Bacharach, S.L., Beanlands, R.S., et al. ASNC Imaging guidelines for nuclear cardiology procedures: PET myocardial perfusion and metabolism clinical imaging. Available at http://asnc.org/imageuploads/ImagingGuidelinesPETJuly2009.pdf. Accessed March 27, 2013