Chapter 19 Cardiac pacing and implantable cardioverter/defibrillators
Cardiac pacing has rapidly evolved since its introduction by Zoll in 1952.1 The technological knowledge gained in pacing has assisted in the even more rapidly advancing field of implantable cardioverter/defibrillators (ICDs). Although implantation and follow-up of permanent pacemakers and ICDs are in the domain of appropriately trained cardiologists, intensive care physicians should be familiar with such devices as a significant number of critically ill patients will have them in situ. It is also essential, when urgent pacing is required, that the intensivist is skilled in all aspects of temporary pacing, including lead insertion and testing.
CARDIAC PACING IN BRADYARRHYTHMIAS2,3
ELECTRODES
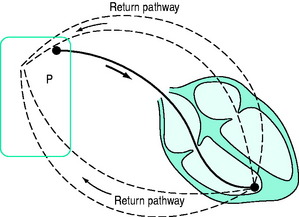
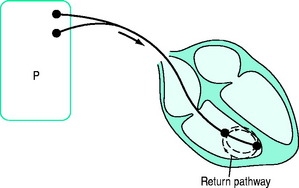
BIPOLAR (Figure 19.2)
A bipolar lead has two conducting wires surrounded by a layer of insulation. Electric current travels down one wire to an electrode (usually the distal), passes through cardiac tissue to cause depolarisation, and returns to the pacemaker via the second electrode. Inappropriate sensing of EMI or myopotentials is uncommon. Bipolar pacing is the method of choice for temporary pacing. If one limb fails, a bipolar system can be converted to a unipolar system (see Figure 19.1) by connecting the other limb to one pacemaker pole (usually positive). The other pole (usually negative) is connected to an electrocardiogram (ECG) skin electrode to complete a unipolar pacing circuit.
PACING SITES
PACEMAKER MODES
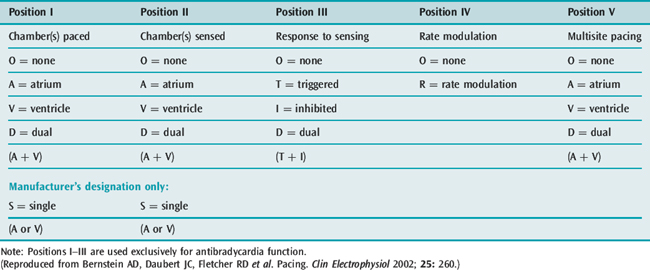
The North American Society of Pacing and Electrophysiology (NASPE) and the British Pacing and Electrophysiology Group (BPEG) developed the NBG code4 for pacing. It is a generic code used to identify modes of pacing (Table 19.1). The code was updated in 2002 to include multisite pacing therapy (position V).5
SPECIFIC PACING MODES
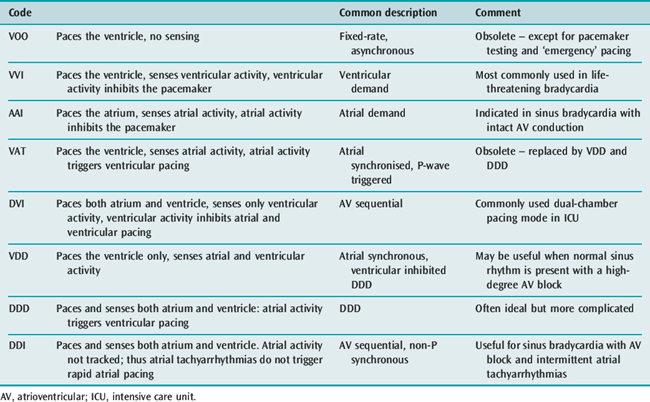
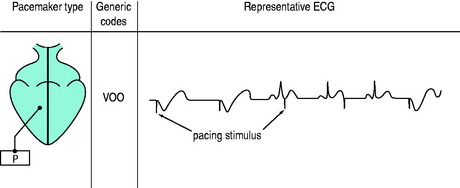
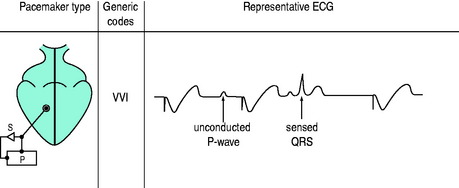
The three-position code (Figure 19.3) is adequate to describe emergency temporary pacing and most forms of permanent pacing in the intensive care unit (ICU) (Table 19.2).
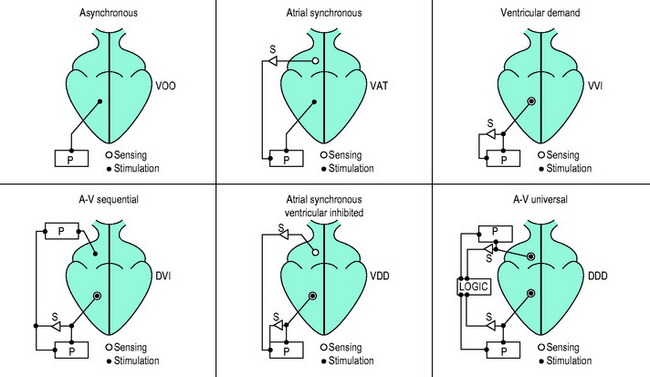
Figure 19.3 Examples of pacemaker modes and their three-position (letter) codes. P, pacemaker; S, sensing.
SINGLE-CHAMBER PACING
DUAL-CHAMBER PACING
Two sets of electrodes are required (atrial and ventricular).
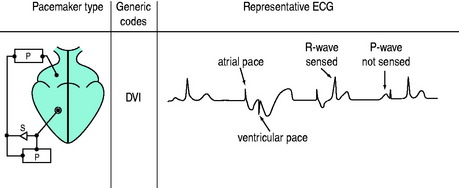
DVI (AV SEQUENTIAL) PACING
Atria and ventricles are paced in sequence (Figure 19.7). After a stimulus is delivered to the atrium, there is a delay and an impulse is then delivered after atrial stimulation, to the ventricles. If AV conduction is successful, the ventricular output of the pacemaker is inhibited; otherwise the ventricle is paced. The advantage of this mode is that the atria and ventricles usually contract in sequence. To maintain AV synchrony in the absence of atrial sensing, the pacemaker discharge rate must be greater than the spontaneous atrial rate; asynchronous atrial pacing can precipitate AF. Self-inhibition (‘cross-talk’) can occasionally occur, that is, inappropriate detection of the atrial pacing stimulus by the ventricular channel. If there is no escape rhythm, asystole may result. DVI pacing is indicated when there is impaired AV conduction with an atrial bradycardia. It is of no value when atrial tachyarrhythmias are present.
VDD (ATRIAL SYNCHRONOUS VENTRICULAR INHIBITED) PACING
This mode paces only the ventricle. Sensing takes place in both atrium and ventricle. A sensed P-wave triggers ventricular pacing. VDD pacing is used in some permanent pacemaker systems as a single-lead system capable of pacing the ventricle in response to atrial sensing (from an electrode situated in the intra-atrial part of the lead) with ventricular electrodes at the apex of the RV.
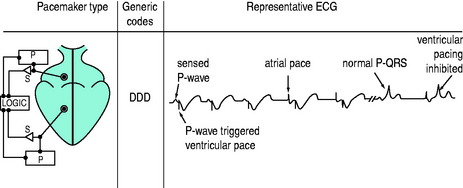
DDD PACING
There is pacing and sensing in both chambers (Figure 19.8). An atrial impulse will trigger a ventricular output and simultaneously inhibit an atrial output. If the impulse is conducted normally to the ventricle, the ventricular output is then inhibited, as with the DVI mode. Upper rate-limiters prevent the pacemaker from following excessive atrial activity with paced ventricular responses. DDD pacemaker function depends on the underlying cardiac rhythm:
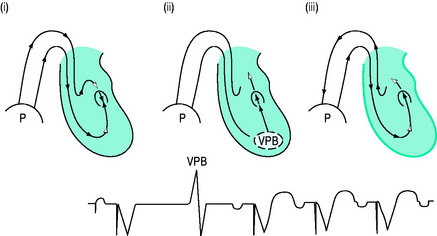
Self-inhibition can be prevented by introducing a ventricular blanking (refractory) period coinciding with the atrial pacing stimulus. With DDD and VDD pacing, re-entry pacemaker-mediated ‘endless-loop’ tachycardias are possible.7 These are commonly initiated by a ventricular premature beat (Figure 19.9) conducted retrogradely to the atria, where it is sensed and ventricular pacing is triggered with an endless loop: the circuit’s anterograde limb is the pacemaker, and the retrograde limb is via the AV node. Conversion to asynchronous (non-sensing) mode or DDI or increasing the postventricular atrial refractory period (PVARP) will prevent endless-loop tachycardia.
HAEMODYNAMICS OF CARDIAC PACING
In the normal heart, cardiac output increases three- to fourfold during exercise, due mainly to increased heart rate and increases in stroke volume. Atrioventricular synchrony – the normal activation sequence of the heart in which the atria contract first and then, after an appropriate delay, the ventricles contract – contributes only about 20% of cardiac output.8 Thus the ability of pacemakers to increase heart rate is paramount, although AV synchrony may on occasions be vital (e.g. in low-cardiac-output states). Many permanent pacemakers are rate-adaptive. When temporary pacing is used, the arbitrary back-up rate of 70–80 beats/min may need to be increased if oxygen delivery is inadequate. For life-threatening bradyarrhythmias, increasing heart rate with VVI mode pacing is the treatment of choice. Permanent VVIR pacing (most commonly using an activity or respiration sensor) will allow heart rate modulation. However, in the ICU, adaptive rate changes may not occur. For example, no activity will be sensed in a patient with septic shock, even when the cardiac output is low.
During VVI and VVIR pacing the atria and ventricles beat independently and AV synchrony is lost. Occasionally this can have deleterious effects and cause a ‘pacemaker syndrome’.9 The pacemaker syndrome is, however, a complex of clinical signs and symptoms associated with loss of AV synchrony. A fall in blood pressure coinciding with the onset of ventricular pacing is consistent with the pacemaker syndrome. It was initially recognised with VVI pacing but can occur with any pacing mode if there is AV dissociation, and can be compared to the effects in patients with complete heart block and AV dissociation. The atria contract against closed AV valves with significant regurgitation of blood into the pulmonary and systemic circulation. Blood pressure, stroke volume and cardiac output may fall. The pacemaker syndrome can be eliminated by restoring AV synchrony.
Emergency and long-term haemodynamic effects of DDD or AAI pacing are generally superior to VVI pacing. In studies of patients with permanent pacing, there is a consistent lower mortality with DDD or AAI pacing compared to VVI pacing, and a significantly lower incidence of AF.2 DDD and/or DVI pacing requires two pacing leads, one each in the RA and RV. Under appropriate conditions AAI and DVI pacing provide AV synchrony but not rate adaptation. DDD pacing will usually ensure AV synchrony and heart rate responsiveness, provided the SA node is normal.
Dual-chamber pacemakers require the AV interval to be set as close as possible to the normal P-R interval (140–200 ms). Traditionally, the pacing AV interval is arbitrarily set at about 150–200 ms. If interatrial conduction time (between the RA and LA) is significantly prolonged, the LV may contract before or at the same time as the LA, causing DDD pacemaker syndrome10 with decreased stroke volume and cardiac output (due to LA contraction against a closed mitral valve). Hence, if there is evidence of inadequate cardiac output or impaired oxygen delivery, the AV interval may need to be increased appropriately, or optimised using thermodilution cardiac output or echocardiographic techniques at various AV intervals.
INDICATIONS FOR CARDIAC PACING IN BRADYARRHYTHMIAS
TEMPORARY PACING
Temporary pacing is indicated for the treatment of heart block or sinus bradycardia after cardiac surgery. In high-risk patients, for example, aortic or mitral valve replacement, prophylactic epicardial electrodes are often attached during surgery. DDD (dual-chamber) epicardial pacing has been shown to increase cardiac output at any given heart rate compared to VVI (single-chamber) pacing.11 Temporary perioperative cardiac pacing strategies to increase stroke volume and cardiac output include optimisation of AV delay and occasionally multisite pacing.12 Temporary pacing may be required for bradycardia during cardiac catheterisation and percutaneous transluminal coronary angioplasty. Asymptomatic patients with bifascicular block do not require prophylactic pacing prior to general anaesthesia, although transcutaneous pacing should be readily available. Patients with second- or third-degree AV block should be paced prior to general anaesthesia and surgery.
Caution should be exercised in patients with acute myocardial infarction as many patients will have received thrombolytic agents; central venous cannulation should be avoided, although if necessary the femoral vein can be used. Prognosis is related to infarct size rather than the degree of AV block. Transcutaneous pacing obviates the need for prophylactic temporary transvenous pacing leads in high-risk patients. Pacing is sometimes required for patients with anterior or inferior myocardial infarction (Table 19.3).
Table 19.3 Complete atrioventricular (AV) block in acute myocardial infarction
| Feature | Inferior | Anterior |
|---|---|---|
| Onset | Slow (usually via Mobitz 1) | Sudden (usually via Mobitz II) |
| QRS complex | Narrow | Wide |
| Ventricular rate | > 45 beats/min | < 45 beats/min (often 20–30 beats/min) |
| Escape pacemaker | Stable | Unstable |
| Drug response (e.g. atropine) | Yes | No |
| Haemodynamic effects | No (usually) | Yes |
| Permanent pacing | No (usually) | Yes (if high-degree AV block persists) |
| Prognosis | Good | Very poor |
PERMANENT PACING
Guidelines for permanent pacing have been published.13
OTHER INDICATIONS
There are also non-bradycardia indications for pacing for haemodynamic improvement. Evolving indications include the following:2,3
ELECTROMAGNETIC INTERFERENCE
Any signal that can be sensed by the pacemaker, or ICD, constitutes EMI and can cause pacing problems such as failure to pace (inappropriate sensing), asynchronous pacing (‘safety pacing’ when excess ‘noise’ is detected), pacemaker reprogramming and occasionally damage to the pacemaker (DC shock, diathermy) or the heart itself. The most important source of potentially harmful electromagnetic radiation in hospitals is magnetic resonance imaging (MRI). MRI is generally considered to be contraindicated in patients with a pacemaker or ICD. Recently, several studies have demonstrated the potential for MRI to be performed in pacemaker or ICD patients.20 The risks presented by MRI must be outweighed by the diagnostic benefit of this powerful imaging modality on a case-by-case assessment.
TECHNIQUE OF TEMPORARY TRANSVENOUS PACING
VENTRICULAR PACING
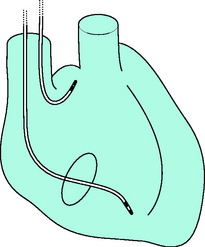
Percutaneous insertion via the right internal jugular vein probably offers least complications with ease of manipulation and stability of the lead. The infraclavicular (subclavian vein) approach allows more freedom for patient arm movement. Antecubital veins are associated with lead instability and thrombophlebitis. The femoral vein is best avoided except in patients who have received thrombolytic agents, because of problems with sterility and increased risk of deep-vein thrombosis. The pacing lead is introduced using the Seldinger technique and manipulated under fluoroscopic control to the apex of the RV (Figure 19.10). After the lead has been manipulated to a satisfactory position, a chest X-ray should be obtained to exclude pneumothorax and to confirm adequate lead positioning. The lead should be sutured to the skin at two different sites: one where the lead exits the skin, and a second to a loop formed with the lead. Occasionally, transvenous RV pacing may not be feasible, owing to the presence of a prosthetic tricuspid valve or a congenital anomaly.
ATRIAL PACING
Insertion of the atrial J-lead is similar to that of the ventricular lead. Correct positioning requires experience. The right atrial appendage is anterior and medial, and the tip is advanced anteriorly and to the patient’s left, after passing from the superior vena cava to the RA (see Figure 19.10). The correct position can be confirmed by a lateral chest X-ray or fluoroscopy.
DUAL-CHAMBER PACING
Modern external pacemakers are available (Figure 19.11) which will pace in all modes. These units are small, easy to use and can fit into a small pouch suitable for mobile patients.
TESTING THE PACING LEADS
Pacing threshold and sensing should be tested adequately using the external pacemaker.
COMPLICATIONS OF TEMPORARY PACING21
Temporary transvenous pacemaker insertions should have a low complication rate. There are, however, problems when inexperienced and/or inadequately trained operators insert the pacing lead. Several studies from the UK report complications in a third to a half of all cases.22,23 Temporary pacing involves two components: (1) obtaining central venous access; and (2) intracardiac placement of the pacing lead. Complications include:
FAILURE TO PACE
PACEMAKER PROGRAMMING
An external programmer emits signals capable of changing (‘programming’) various pacing parameters (Table 19.4). Virtually all modern permanent pacemakers are capable of programming rate, voltage, output, pulse width, sensitivity, mode and AV interval. Programmability is also helpful in assessing various pacemaker problems. The pacemaker format best suited to a particular patient’s needs may change with time, and programmability enables optimal pacemaker function to be achieved, i.e. ‘prescribed’. Pacing changes may be indicated under certain circumstances, for example, decreased pacing rate in a patient with angina or acute myocardial infarction to reduce myocardial oxygen consumption, or increased rate in a patient with haemorrhagic shock.
Table 19.4 Multiprogrammable permanent dual-chamber pacemakers
| Parameter | Adjustment | Comments |
|---|---|---|
| Rate | Increase | Increase cardiac output |
| Decrease | Reduce myocardial oxygen consumption To assess underlying cardiac rhythm | |
| Output | Increase | Increasing output may result in successful pacing when there is failure to capture |
| Decrease | Increases battery life | |
| Sensitivity | Increase | Reducing numerical value increases sensing ability May cause oversensing occasionally |
| Decrease | Increasing numerical value decreases sensing ability Useful in oversensing, e.g. T-wave sensing | |
| Mode | DDD to DDI | To prevent endless-loop tachycardias and to prevent tracking of episodic atrial tachyarrhythmias Mode switching can be automated and programmed either ‘on’ or ‘off’ |
| Atrioventricular interval (AVI) | Increase/decrease | To optimise stroke volume and cardiac output AVI can be rate-adaptive, i.e. AVI shortens as the heart rate increases |
| Refractory period (atrial/ventricular) | Increase | To minimise oversensing, e.g. to prevent ventricular sensing in AAI pacing |
| Decrease | To optimise sensing under certain circumstances | |
| Hysteresis | To preserve AV synchrony by delaying the onset of VVI pacing until the spontaneous heart rate is significantly less than the back-up ventricular pacing rate | |
| Polarity | Unipolar mode | To improve sensing. Occasionally to allow pacing to continue when fracture of the other conducting wire occurs |
| Bipolar mode | To prevent oversensing, e.g. muscle potentials |
CARDIAC PACING IN TACHYARRHYTHMIAS
Certain tachyarrhythmias may be treated safely and effectively by rapid pacing and/or premature electrical stimulation. These include:
AVNRT and AVRT rarely require rapid atrial pacing as treatment with drugs such as adenosine or verapamil is usually successful. Atrial flutter, however, is often resistant to drug therapy, and rapid atrial pacing will usually convert it to sinus rhythm. When unifocal atrial tachycardia is associated with an atrial re-entry circuit, rapid atrial pacing will often revert this arrhythmia; conversely, when due to automatic focus discharging at a rapid rate, the arrhythmia is usually incessant and will respond only intermittently, if at all, to atrial pacing. Occasionally, dual-chamber pacing with a short AV interval can be used to prevent drug-resistant AVNRT or AVRT. Rapid continuous atrial pacing can be used to slow ventricular rate during resistant SVTs associated with a rapid ventricular response, by inducing AF and a high degree of AV block. Sustained VT is responsive to rapid ventricular pacing, but this should not be used for very rapid ventricular rates (e.g. > 300/min), or when severe haemodynamic compromise is present, as immediate DC cardioversion is indicated. Rapid cardiac pacing may at times have advantages over DC cardioversion (Table 19.5) and drug therapy (Table 19.6), but is of no value in sinus tachycardia, AF and VF (Table 19.7).
Table 19.5 Pacing versus cardioversion for the treatment of tachyarrhythmias
Table 19.6 Pacing versus drug therapy for the treatment of tachyarrhythmias
Table 19.7 Indications for rapid cardiac pacing in suitable arrhythmias
SUPRAVENTRICULAR TACHYARRHYTHMIAS
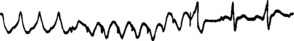
The pacing lead is manipulated to a suitable position in the RA. Atrial pacing is started at a slow rate (e.g. 60–80/min) and slowly increased to about 10–20% faster than the spontaneous atrial rate. Inadvertent ventricular pacing, especially at rapid rates, must be avoided. The atrium is paced for about 30 seconds and the pacemaker is then switched off. Normal sinus rhythm should ensue (Figure 19.12). If not, the pacing lead is manipulated to a different position and/or a faster pacing rate is tried. A more prolonged pacing period may be effective. If sinus rhythm still does not result, AF is deliberately precipitated by rapid atrial pacing at 400–800/min. This is an unstable rhythm which usually reverts spontaneously to normal sinus rhythm. AF persists occasionally, but the ventricular rate is usually slower and more responsive to drug therapy than with SVT.
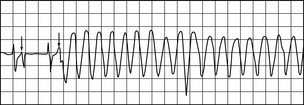
VENTRICULAR TACHYCARDIA
Useful techniques to pace VT include the following:
IMPLANTABLE CARDIOVERTER/DEFIBRILLATOR2
This is an implantable device which can recognise and automatically terminate VT and VF. Guidelines13 have been published for ICD implantation; nevertheless, this is a rapidly evolving field with many trials in progress. ICDs are of proven survival benefit (class I) in patients who have had a cardiac arrest due to VF or VT, not due to transient or reversible cause. In other clinical situations ICD implantation may be a reasonable approach, for example, syncope with inducible VT at electrophysiology study which is poorly tolerated haemodynamically, and antiarrhythmic drugs are ineffective and/or contraindicated. A trial24 in over 1200 patients with recent myocardial infarction (> 1 month) and low LV ejection fraction (30%) has demonstrated a decrease in mortality when prophylactic ICD implantation was compared to conventional treatment. ICDs are standard therapy to prevent sudden cardiac death in such high-risk patients.
Virtually all ICD systems are implanted transvenously and include antitachycardia pacing and back-up ventricular bradycardia pacing, dual-chamber pacing with rate-adaptive options. Single- or dual-chamber ICDs may be implanted. One trial25 suggests that single-chamber ICDs may be superior to dual-chamber ICDs in patients who do not have an associated bradycardia. Current devices continuously monitor the patient’s heart rate and deliver therapy when the heart rate exceeds a predetermined limit. There is a graded response which is programmable:
1 Zoll PM. Resuscitation of the heart in ventricular standstill by external electric stimulation. N Engl J Med. 1952;747:768-771.
2 Hayes DL, Zipes DP. Cardiac pacemakers and cardioverter-defibrillators. In: Zipes DP, Libby P, Bonow RO, et al, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th edn. Philadelphia: Elsevier Saunders; 2005:767-802.
3 Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet. 2004;364:1701-1719.
4 Bernstein AD, Camm AJ, Fletcher RD, et al. The NASPE/BPEG generic pacemaker code for antibradyarrhythmic and adaptive rate pacing and antitachyarrhythmic devices. Pace. 1987;10:794-799.
5 Bernstein AD, Daubert JC, Fletcher RD, et al. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group: the revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. Pacing Clin Electrophysiol. 2002;25:260-264.
6 Leung S-K, Lau C-P. Developments in sensor-driven pacing. Cardiol Clin. 2000;18:113-155.
7 Furman S, Fisher JD. Endless-loop tachycardia in an AV universal (DDD) pacemaker. Pace. 1982;5:486-489.
8 Donovan KD, Dobb GJ, Lee KY. The haemodynamic importance of maintaining atrioventricular synchrony during cardiac pacing in critically ill patients. Crit Care Med. 1991;19:320-326.
9 Johnson AD, Laiken SL, Engler RL. Hemodynamic compromise associated with ventriculoatrial conduction following transvenous pacemaker placement. Am J Med. 1978;65:75-81.
10 Pierantozzi A, Bocconcelli P, Sgarbi E. DDD pacemaker syndrome and atrial conduction time. Pace. 1994;17:374-376.
11 Baller D, Hoeft A, Korb H, et al. Basic physiological studies on cardiac pacing with special reference to the optimal mode and rate after cardiac surgery. Thorac Cardiovasc Surg. 1981;29:168-173.
12 Spotnitz HM. Optimizing temporary perioperative cardiac pacing. J Thorac Cardiovasc Surg. 2005;129:5-8.
13 Gregoratos G, Abrams J, Epstein ET, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices. Summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE commmittee to update the 1998 pacemaker guidelines). Circulation. 2002;106:2145-2161.
14 Guo H, Hahn D, Olshansky B. Temporary biventricular pacing in a patient with subacute myocardial infarction, cardiogenic shock, and third-degree atrioventricular block. Heart Rhythm. 2005;2:112.
15 Bryce M, Spielman SR, Greenspan AM, et al. Evolving indications for permanent pacemakers. Ann Intern Med. 2001;134:1130-1141.
16 Greenberg MD, Katz NM, Iuliano S, et al. Atrial pacing for the prevention of atrial fibrillation after cardiovascular surgery. J Am Coll Cardiol. 2000;35:1416-1422.
17 Blommaert D, Gonzalez M, Muccumbitsi J, et al. Effective prevention of atrial fibrillation by continuous atrial overdrive pacing after coronary artery bypass surgery. J Am Coll Cardiol. 2000;35:1411-1415.
18 Levy T, Fotopoulos G, Walker S, et al. Randomized controlled study investigating the effect of biatrial pacing in prevention of atrial fibrillation after coronary artery bypass grafting. Circulation. 2000;102:1382-1387.
19 Daubert JC, Mabo P. Atrial pacing for the prevention of postoperative atrial fibrillation: how and where to pace? J Am Coll Cardiol. 2000;35:1423-1427.
20 Faris OP, Mitchell S. Magnetic resonance imaging of pacemaker and implantable cardioverter-defibrillator patients. Circulation. 2006;114:1232-1233.
21 Donovan KD, Lee KY. Indications for and complications of temporary transvenous cardiac pacing. Anaesth Intens Care. 1985;13:63-70.
22 Betts TR. Regional survey of temporary transvenous pacing procedures and complications. Postgrad Med J. 2003;79:463-465.
23 Murphy JJ. Problems with temporary cardiac pacing. Br Med J. 2001;323:527.
24 Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883.
25 Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA. 2002;288:3115-3123.