Chapter 19 Cardiac Pacing and Defibrillation
PACEMAKERS
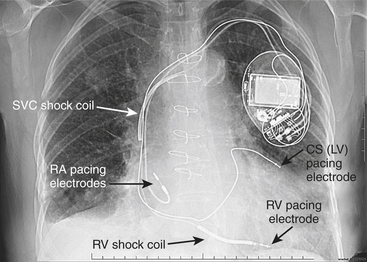
Battery-operated, implantable pacing devices were first introduced in 1958, just 4 years after the invention of the transistor. The complexity, calculation, and data storage abilities of these devices have grown in a manner similar to that seen within the computer industry. The natural progression of pacemaker developments led to the invention of the implanted cardioverter-defibrillator (ICD) around 1980. As this technology has advanced, the divisions between these devices have become less clear. For example, every ICD currently implanted has anti-bradycardia pacing capability, and patients, news media, and even physicians often misidentify an implanted defibrillator as a pacemaker. The consequence of mistaking an ICD for a conventional pacemaker can lead to patient harm, either due to electromagnetic interference (EMI) issues resulting in inappropriate ICD therapy or the unintentional disabling of ICD therapies in some ICDs that can be permanently disabled by magnet placement. Figure 19-1 shows a three-lead defibrillation system and identifies the right ventricular shock coil, which differentiates an ICD system from a conventional pacemaking system. The complexity of cardiac pulse generators, as well as the multitude of programmable parameters, limits the number of sweeping generalizations that can be made about the perioperative care of the patient with an implanted pulse generator. Population aging, continued enhancements in implantable technology, and new indications for implantation will lead to growing numbers of patients with these devices. Both the American College of Cardiology (ACC) and the North American Society for Pacing and Electrophysiology-The Heart Rhythm Society (HRS-NASPE)* have taken note of these issues, and guidelines have been published regarding the care of the perioperative patient with such a device.1 The American Society of Anesthesiology (ASA) has issued a practice advisory.2
Pacemaker Overview
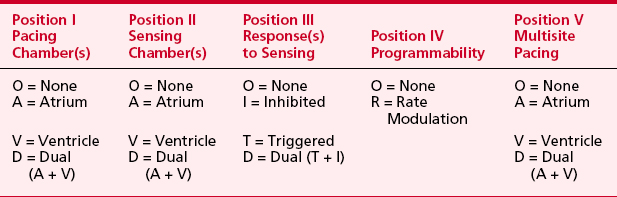
Pacemaker manufacturers (more than 26 companies) have produced over 2000 models to date. More than 220,000 adults and children in the United States undergo new pacemaker placement each year, and nearly 3 million patients have pacemakers today. Many factors can lead to confusion regarding the behavior of a device and the perioperative care of a patient with a device.3 An understanding of pulse generators and their likely idiosyncrasies in the operating or procedure room is needed. Whether the patient with a pacemaker is at increased perioperative risk remains unknown, but reports suggest that these patients deserve extra perioperative attention. No discussion of pacemakers can take place without an understanding of the generic pacemaker code, which has been published by the HRS-NASPE and The British Pacing and Electrophysiology Group (BPEG). This code, initially published in 1983, was revised in February 2002. Shown in Table 19-1, the code (NBG) describes the basic behavior of the pacing device.4
Pacemaker Indications
Indications for permanent pacing are shown in Box 19-1. Devices have also been approved by the U.S. Food and Drug Administration (FDA) for three-chamber pacing (right atrium, both ventricles) to treat dilated cardiomyopathy (DCM) (also called biventricular pacing [Bi-V] or cardiac resynchronization therapy [CRT]).5 Also, specially programmed devices are used to treat hypertrophic cardiomyopathy (HCM) in both adults and children. Bi-V and HCM indications require careful attention to pacemaker programming, because effective pacing in these patients often requires a pacing rate greater than native sinus or junctional escape rate (often accomplished with drugs) and an atrioventricular (AV) delay shorter than the native PR interval so that the ventricle is paced 100% of the time. Inhibition or loss of pacing (e.g., from native conduction, atrial irregularity, ventricular irregularity, development of junctional rhythm, or EMI) can lead to deteriorating hemodynamics in these patients. Bi-V pacing can lengthen the QT interval in some patients, producing torsades de pointes.
Pacemaker Magnets
Placement of a magnet over a generator might produce no change in pacing because NOT ALL PACEMAKERS SWITCH TO A CONTINUOUS ASYNCHRONOUS MODE WHEN A MAGNET IS PLACED. Also, not all models from a given company behave the same way. Although most pacemakers have “high-rate” (80 to 100 beats per minute) asynchronous pacing with a magnet some still switch to asynchronous pacing at program rate, and some will respond with a brief (10 to 64 beats) asynchronous pacing event before reverting to original programmed behavior. Possible effects of magnet placement are shown in Box 19-2. In some devices, magnet behavior can be altered via programming. Also, any pacemaker from CPI-Guidant ignores magnet placement after any electrical reset, which is a possibility in the presence of strong EMI. For all generators, calling the manufacturer remains the most reliable method for determining magnet response and using this response to predict remaining battery life. For generators with programmable magnet behavior (Biotronik, CPI-Guidant, Pacesetter, and St. Jude Medical), only an interrogation with a programmer can reveal current settings. Most manufacturers publish a reference guide, although not all of these guides list all magnet idiosyncrasies. A telephone call can also alert the clinician to any recalls or alerts, which are not uncommon with these devices.
BOX 19-2 Pacemaker Magnet Behavior
*See text and Pacemaker Programming for special precautions.
PREANESTHETIC EVALUATION AND PACEMAKER REPROGRAMMING
The prudent anesthesiologist reviews the patient’s pacemaker history and follow-up schedule. Under the name NASPE, the HRS has published a consensus statement suggesting that pacemakers should be routinely evaluated with telephone checks for battery condition at least every 3 months. NASPE also recommends a comprehensive evaluation (interrogation) at least once per year. There are additional checks for devices implanted less than 6 or more than 48 (dual-chamber) or 72 (single-chamber) months. Rozner and associates reported a 2-year retrospective review of follow-up intervals in patients who presented for an anesthetic, and they found that more than 32% of 172 patients presenting for an anesthetic at their hospital did not meet the HRS-NASPE guideline for comprehensive evaluation.6 They also reported that 5% of the patients presented for their anesthetic with a pacemaker in need of replacement for battery depletion and that nearly 10% of patients had less-than-optimal pacing settings. Note that a recent, preoperative interrogation is now part of the ASA Pacemaker Advisory and the ACC guidelines.1,2
Important features of the preanesthetic device evaluation are shown in Box 19-3. Determining dependency on the pacemaker function might require temporary reprogramming to a VVI mode with a low rate. In patients from countries where pacemakers might be reused, battery performance might not be related to length of implantation in the current patient. It should also be noted that in a registry of 345 pacemaker generator failures, 7% of failures were not related to battery depletion.7
Appropriate reprogramming (Box 19-4) might be the safest way to avoid intraoperative problems, especially if monopolar “Bovie” electrocautery will be used. For lithotripsy, consideration should be given to programming the pacing function out of an atrial-paced mode, because some lithotriptors are designed to fire on the R wave and the atrial pacing stimulus could be misinterpreted as the contraction of the ventricle. All of the manufacturers stand ready to assist with this task. Reprogramming a pacemaker to asynchronous pacing at a rate greater than the patient’s underlying rate usually ensures that no oversensing or undersensing during EMI will take place, thus protecting the patient. Reprogramming a device will not protect it from internal damage or reset caused by EMI.
BOX 19-4 Pacemaker Reprogramming Probably Needed
In general, rate responsiveness and other “enhancements” (hysteresis, sleep rate, AV search, etc.) should be disabled by programming. Note that for many CPI devices, the Guidant Corporation recommends increasing the pacing voltage to “5 volts or higher” in any case in which the monopolar electrosurgical unit (ESU) will be used. Rozner and associates reported increases in both atrial and ventricular thresholds in 6 of 141 consecutive operations involving pacemaker cases in which the monopolar ESU was used, large volume and blood shifts were observed, or both.6 Although many of the operations were thoracic explorations, no pacing threshold changes were noted for these cases. No cardiopulmonary bypass cases were included in this cohort. Special attention must be given to any device with a minute ventilation (bioimpedance) sensor, because inappropriate tachycardia has been observed secondary to mechanical ventilation, monopolar “Bovie” ESU, and connection to an ECG monitor with respiratory rate monitoring.
Intraoperative (or Procedure) Management
With respect to anesthetic technique, no studies have championed one over another. Nevertheless, a number of reports of prolongation of the QT interval with the use of isoflurane, desflurane, or sevoflurane have been published, whereas halothane appears to reduce this interval.8 No interactions have been reported for enflurane.
Monopolar “Bovie” electrocautery (ESU) use remains the principal intraoperative issue for the patient with a pacemaker. Between 1984 and 1997, the U.S. FDA was notified of 456 adverse events with pulse generators, 255 from electrocautery, and a “significant number” of device failures.9 Monopolar ESU is more likely to cause problems than is bipolar ESU, and patients with unipolar electrode configuration are more sensitive to EMI than are those with bipolar configurations. Coagulation ESU will likely cause more problems than nonblended “cutting” ESU. Magnet placement during electrocautery might allow reprogramming of an older (pre-1990) generator; however, newer generators are relatively immune to such effects. In fact, most devices from CPI-Guidant as well as St. Jude cannot be reprogrammed in the presence of a magnet. Note, however, that strong EMI can produce an electrical reset or improper detection of battery depletion, which might change the programming mode, rate, or both. If monopolar electrocautery is to be used, then the current return pad should be placed to ensure that the electrocautery current path does not cross the pacemaking system. For cases such as head and neck surgery, the pad might be best placed on the shoulder contralateral to the implanted device. For breast and axillary cases, the pad might need to be placed on the ipsilateral arm with the wire prepped into the field by sterile plastic cover. Procedures with special pacing ramifications are shown in Box 19-5.
BOX 19-5 Special Procedures in Patients with Implantable Generators
Magnetic resonance imaging (MRI) deserves special mention. In general, MRI has been contraindicated in pacemaker and ICD patients. However, reports suggests that MRI is probably safe for some patients with newer devices, as well as any patient who will be wide awake in the MRI tunnel, who is not dependent on his or her pacemaker for heart rhythm or survival, who will not need medication to undergo the MRI, and who can communicate regularly with the MRI care team. Nevertheless, not all MRI sequences and energy levels have been studied, and caution is advised.10
TEMPORARY PACEMAKERS
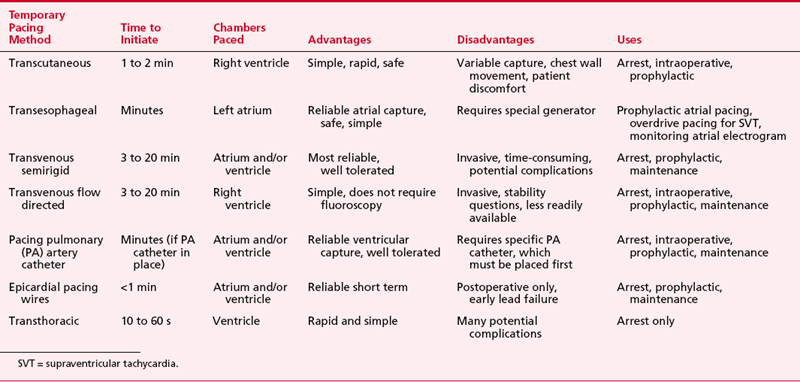
The various forms of temporary pacing include many transvenous catheter systems, transcutaneous pads, transthoracic wires, and esophageal pacing techniques. This section reviews the indications for temporary cardiac pacing and discusses the techniques available to the anesthesiologist. Table 19-2 summarizes these techniques.
Indications for Temporary Pacing
Temporary pacemakers are commonly used postoperatively after cardiac surgery, in the treatment of drug toxicity resulting in arrhythmias, with certain arrhythmias complicating myocardial infarction, and for intraoperative bradycardia due to β-blocker use. The placement of a temporary pacing system can assist in the hemodynamic management in the perioperative period. Abnormal electrolytes, preoperative β-blocker use, and many of the intraoperative drugs have the potential to aggravate bradycardia and bradycardia-dependent arrhythmias. Because drugs used to treat bradyarrhythmias have a number of important disadvantages compared with temporary pacing, hemodynamically unstable perioperative bradyarrhythmias should be considered an indication for temporary pacing (Table 19-3).If the patient already has epicardial wires or a pacing catheter or wires, or transesophageal pacing is feasible, pacing is preferred to pharmacologic therapy. However, transcutaneous and ventricular-only transvenous pacing, even if feasible, may exacerbate hemodynamic problems in patients with heart disease because these pacing modalities do not preserve AV synchrony (i.e., produces ventricular or global activation).
| Patient Condition | Event Requiring Temporary Pacing |
|---|---|
| AMI |
AF = atrial fibrillation; AMI = acute myocardial infarction; AV = atrioventricular; SVT = supraventricular tachycardia; VT = ventricular tachycardia.
Transvenous Temporary Pacing
Once catheters are positioned, pacing is initiated using the distal electrode as the cathode and the proximal electrode as the anode. Ideally, the capture thresholds should be less than 1 mA and generator output should be maintained at three times threshold as a safety margin. In dual-chamber pacing, AV delays of between 100 and 200 ms are used. Many patients are sensitive to this parameter. Cardiac output optimization with echocardiography and/or mixed venous oxygen saturation can be used to maximize hemodynamics by adjusting AV delay.11 AV sequential pacing is clearly beneficial in many patients, but it should be remembered that emergency pacing starts with ventricular capture alone. There is a potential risk of interference of external pacemaker generators by walkie-talkies and digital cellular phones. Clinicians should also be aware of all complications related to transvenous lead placement.
Pacing Pulmonary Artery Catheters
The pulmonary artery AV pacing thermodilution (TD) catheter allows for AV sequential pacing via electrodes attached to the outside of the catheter, as well as routine pulmonary artery (PA) catheter functions. Combination of the two functions into one catheter eliminates the need for separate insertion of temporary transvenous pacing electrodes. However, several potential disadvantages exist with this catheter, including (1) varying success in initiating and maintaining capture, (2) external electrode displacement from the catheter, and (3) relatively high cost compared to standard pacing PA catheters. The Paceport PA catheter provides ventricular pacing with a separate bipolar pacing lead (Chandler probe), which allows for more stable ventricular pacing as well as hemodynamic measurements. This catheter has been used for successful resuscitation after cardiac arrest during closed-chest cardiac massage when attempts to capture with transcutaneous and transvenous flow-directed bipolar pacing catheters had failed. However, this unit does not provide the potential advantages associated with atrial pacing capability. The newer pulmonary artery A-V Paceport adds a sixth lumen to the older Paceport to allow placement of an atrial J-wire, flexible-tip bipolar pacing lead. Both of these Paceport catheters are placed by transducing the right ventricular pressure port to ensure correct positioning of the port 1 to 2 cm distal to the tricuspid valve. This position usually guides the ventricular wire (Chandler probe) to the apex where adequate capture should occur with minimal current requirements. Although ventricular capture is easily obtained, atrial capture can be more difficult and less reliable.11 This catheter has been used successfully after cardiac surgery. The atrial wire can be used to diagnose supraventricular tachycardia (SVT) by atrial electrograms and to overdrive atrial flutter and reentrant SVT.
Transcutaneous Pacing
The large patches typically are placed anteriorly (negative electrode or cathode) over the palpable cardiac apex (or V3 lead location) and posteriorly (positive electrode or anode) at the inferior aspect of the scapula. The anode has also been placed on the anterior right chest with success in healthy volunteers. The skin should be cleansed with alcohol (but not abraded) to reduce capture threshold and improve patient comfort. Abraded skin can cause more discomfort. Typical thresholds are 20 to 120 mA, but pacing may require up to 200 mA at long pulse durations of 20 to 40 ms.12 Transcutaneous pacing appears to capture the right ventricle followed by near-simultaneous activation of the entire left ventricle. The hemodynamic response is similar to that of right ventricular endocardial pacing. Both methods can cause reductions in left ventricular systolic pressure, a decrease in stroke volume, and an increase in right-sided pressures due to AV dyssynchrony. Capture should be confirmed by palpation or display of a peripheral pulse. Maintenance current is set 5 to 10 mA above threshold as tolerated by the patient. Success rates appear to be highest when the system is used prophylactically or early after arrest—upward of 90%.
Esophageal Pacing
The newest technique available to anesthesiologists is esophageal pacing, and it has been shown to be quite reliable.13 Significant bradycardia, secondary to underlying pathology or pharmacologic effects, can occur during anesthesia. The response to pharmacologic therapy for significant bradycardia with vagolytic drugs can be unpredictable and difficult to sustain accurately. Chronotropic drugs may have little effect and can lead to tachyarrhythmias and/or myocardial ischemia. Esophageal pacing is relatively noninvasive and well tolerated even in the majority of awake patients, and it appears to be devoid of serious complications. This modality is useful for heart rate support of cardiac output, for overdrive suppression of reentrant SVT, and for diagnostic atrial electrograms. Ventricular capture must be excluded before attempts at rapid atrial pacing for overdrive suppression to prevent potential VT or VF.
IMPLANTED CARDIOVERTER-DEFIBRILLATORS
A significant number of technologic advances have occurred since the first ICD was placed, including considerable miniaturization (pectoral pocket placement with transvenous leads is the norm) and battery improvements that permit permanent pacing with these devices. Thus, a pectoral ICD could easily be confused with a pacemaker. Like pacemakers, ICDs have a generic code to indicate lead placement and function, which is shown in Table 19-4.
Table 19-4 North American Society for Pacing and Electrophysiology/The British Pacing and Electrophysiology Group Generic Defibrillator Code (NBD)
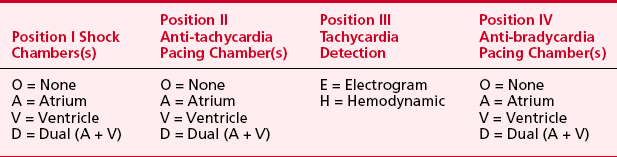
Twenty to 40 percent of shocks are for rhythms other than VT or VF despite reconfirmation. SVT remains the most common cause of inappropriate shock therapy. Advances in ICDs to include dual-chamber detection might be able to lower the inappropriate shock rate, although it is still reported as high as 17%.14 Whether inappropriate shocks injure patients remains a subject of considerable debate, but a significant number of patients who receive an inappropriate shock demonstrate elevated troponin levels in the absence of an ischemic event.
Indications
Initially, ICDs were placed for hemodynamically significant VT or VF. Newer indications associated with sudden death include long QT syndrome, Brugada syndrome (right bundle-branch block, ST-segment elevation in leads V1 to V3), and arrhythmogenic right ventricular dysplasia. Studies suggest that ICDs can be used for primary prevention of sudden death (i.e., before the first episode of VT or VF) in young patients with hypertrophic cardiomyopathy, and data from the second Multicenter Automatic Defibrillator Intervention Trial (MADIT II) suggest that any patient after myocardial infarction with an ejection fraction less than 30% should undergo prophylactic implantation of an ICD.15
Trials are under way for the cardiomyopathy patient with nonischemic cardiomyopathy as well. The Sudden Cardiac Death-Heart Failure Trial (SCD-HeFT) results and the previously published Defibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) study suggest that ICD placement will result in lower mortality in any patient with an ejection fraction less than 35% regardless of the cause of the cardiomyopathy. The DEFINITE results are important, because the patients in this study were randomized only after initiation of β-blockade and angiotensin-converting enzyme inhibitor therapy, which form the backbone of medical therapy for cardiomyopathy. The SCD-HeFT results showed ICDs reduced mortality by 23%. Based on these results, Medicare expanded its ICD coverage.16,17
Box 19-6 reviews ICD indications.
Dilated Cardiomyopathy
With the advent of Bi-V pacing for the patient with a DCM and prolonged QRS interval, and the approval of ICDs with Bi-V capability, the presence of a defibrillator with Bi-V pacing will become more common. About 550,000 new diagnoses of congestive heart failure are made in the United States every year, and the prevalence of this disease includes nearly 5 million patients. Significant risk factors for the development of congestive heart failure include ischemic heart disease and/or hypertension. These data, combined with the results from SCD-HeFT and MADIT II trials (ICD is indicated in any patient with cardiomyopathy and ejection fraction < 30% to 35%), suggest that the number of patients eligible to receive a defibrillator to include Bi-V pacing will increase dramatically. Whether any country’s economy can absorb this economic burden remains to be seen. At present, Bi-V pacing without defibrillation capability improves functional status and quality of life and was shown to reduce mortality.5,18
Preanesthetic Evaluation and Implanted Cardioverter-Defibrillator Reprogramming
All ICDs should have their anti-tachycardia therapy disabled before the induction of anesthesia and commencement of the procedure.1 Guidelines from HRS-NASPE and the ASA Advisory suggest that every patient with an ICD have an in-office comprehensive evaluation every 1 to 4 months. Devices with Bi-V pacing must have a sufficiently short AV delay for sensed events to ensure that all ventricular activity is paced. Failure of ventricular pacing (either right or left) owing to native AV conduction or threshold issues has been associated with inappropriate antitachycardia therapy (i.e., shock).
Anesthetic Considerations for Insertion of an Implanted Cardioverter-Defibrillator
Complications associated with ICD insertion include those related to insertion and those associated specifically with the device. Percutaneous insertion is typically via the subclavian vein, predisposing to pneumothorax. Cardiac injury including perforation is a remote possibility. Device-related complications include those associated with multiple shocks that may lead to myocardial injury or refractory hypotension.19
Postanesthesia Implanted Cardioverter-Defibrillator Evaluation
The ICD must be reinterrogated and reenabled. All events should be reviewed and counters should be cleared, because the next device evaluator might not receive information about the EMI experience of the patient and might make erroneous conclusions regarding the patient’s arrhythmia events.20
SUMMARY
Preoperative
Intraoperative
1. Fleisher L., Beckman J., Brown K., et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. Circulation. 2007;116:1971.
2. American Society of Anesthesiology Practice Advisory for the Perioperative Management of Patients with Rhythm Management Devices. Pacemakers and implantable cardioverter-defibrillators. Anesthesiology. 2005;103:186.
3. Rozner M. Pacemaker misinformation in the perioperative period: Programming around the problem. Anesth Analg. 2004;99:1582.
4. Bernstein A.D., Daubert J.C., Fletcher R.D., et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol. 2002;25:260.
5. Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140.
6. Rozner M.A., Roberson J.C., Nguyen A.D. Unexpected high incidence of serious pacemaker problems detected by pre- and postoperative interrogations: A two-year experience. J Am Coll Cardiol. 2004;43:113A.
7. Hauser R., Hayes D., Parsonnet V., et al. Feasibility and initial results of an Internet-based pacemaker and ICD pulse generator and lead registry. Pacing Clin Electrophysiol. 2001;24:82.
8. Yildirim H., Adanair T., Atay A., et al. The effects of sevoflurane, isoflurane, and desflurane on QT interval of the ECG. Eur J Anaesthesiol. 2004;21:566.
9 Pressly N: Review of MDR Reports reinforces concern about EMI. FDA User Facility Reporting No. 20.Published 1997. Available at: http://www.fda.gov/cdrh/fuse20.pdf. Accessed December 1, 2002
10. Rozner M.A. MRI in the patient with a pacemaker: Caution is indicated. J Am Coll Cardiol. 2004.
11. Trankina M.F., White R.D. Perioperative cardiac pacing using an atrioventricular pacing pulmonary artery catheter. J Cardiothorac Anesth. 1989;3:154.
12. Gauss A., Hubner C., Meierhenrich R., et al. Perioperative transcutaneous pacemaker in patients with chronic bifascicular block or left bundle branch block and additional first-degree atrioventricular block. Acta Anaesthesiol Scand. 1999;43:731.
13. Atlee J.L.III, Pattison C.Z., Mathews E.L., Hedman A.G. Transesophageal atrial pacing for intraoperative sinus bradycardia or AV junctional rhythm: Feasibility as prophylaxis in 200 anesthetized adults and hemodynamic effects of treatment. J Cardiothorac Vasc Anesth. 1993;7:436.
14. Niehaus M., de Sousa M., Klein G., et al. Chronic experiences with a single lead dual chamber implantable cardioverter defibrillator system. Pacing Clin Electrophysiol. 2003;26:1937.
15. Moss A.J., Zareba W., Hall W.J., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877.
16. Bardy G.H., Lee K.L., Mark D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225.
17. McClellan M., Tunis S. Medicare coverage of ICDs. N Engl J Med. 2005;352:222.
18. Gras D., Leclercq C., Tang A.S., et al. Cardiac resynchronization therapy in advanced heart failure: The multicenter InSync clinical study. Eur J Heart Fail. 2002;4:311.
19. DiMarco J.P. Implantable cardioverter-defibrillators. N Engl J Med. 2003;349:1836-1847.
20. Goldberger Z., Lampert R. Implantable cardioverter-defibrillators: expanding implications and technologies. JAMA. 2006;295:809.