Chapter 3 Cardiac Malpositions
Dextrocardia in situs inversus was known to the anatomist-surgeon Marco Aurelio Severino in 1643 and was one of the first recognized congenital malformations of the heart.1 Nearly a century and a half elapsed before Matthew Baillie’s2 account of “complete transposition in the human subject, of the thoracic and abdominal viscera, to the opposite side from what is natural.”
Cardiac malpositions, which have a prevalence rate of 0.10 per 1000 live births,3 refer to hearts that are located abnormally within the thoracic cavity or that are located outside the thoracic cavity—ectopia cordis.4 In 1901, Paltauf5 published remarkable illustrations that distinguished the various types of dextrocardia; and in 1928, the first useful classification of cardiac malpositions was proposed.6 Subsequent observations by Lichtman7 and by de la Cruz8 shed light on the embryologic bases of the malpositions; the landmark observations of van Praagh9 confirmed the validity of those assumptions. Campbell’s10–12 diagrams in the 1950s and 1960s, and Elliott’s13 radiologic classification in 1966, set the stage for the clinical recognition of cardiac malpositions.
The genetics of cardiac midline and lateral defects occur along three geometric axes: anteroposterior, dorsal-ventral, and left-right.14 Genes expressed in dorsal midline cells coordinate the development of the three embryonic axes, driving the cardiac tube to loop in the appropriate direction relative to body axes. The left-right axis is established at approximately the 18th day after fertilization.14 Both bilateral left-sidedness and bilateral right-sidedness have been reported in members of the same family, which implies that the two conditions are different manifestations of a primary defect in lateralization.15
The literature on cardiac malpositions is replete with an arcane vocabulary that often confounds rather than clarifies. Terms have been fully abbreviated, minimally abbreviated, or unabbreviated. In this chapter, unabbreviated terms are used because they are accessible to the widest audience.16
Definitions and terminology*
Situs Solitus
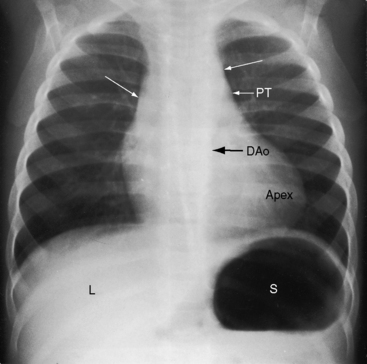
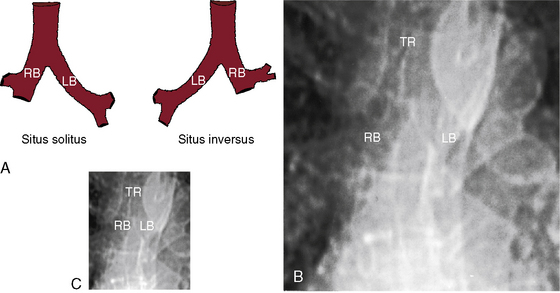
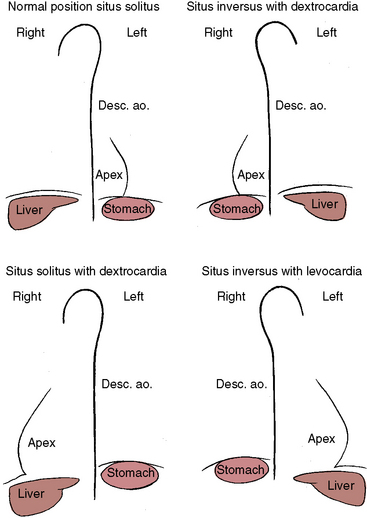
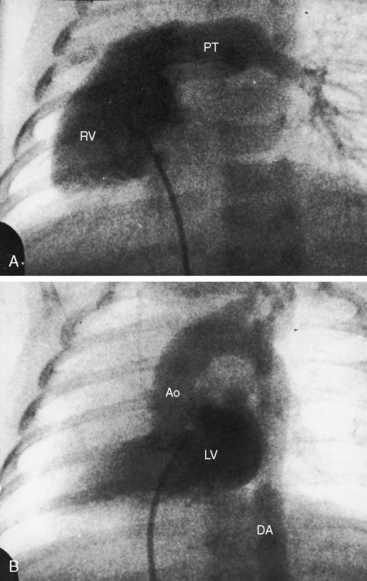
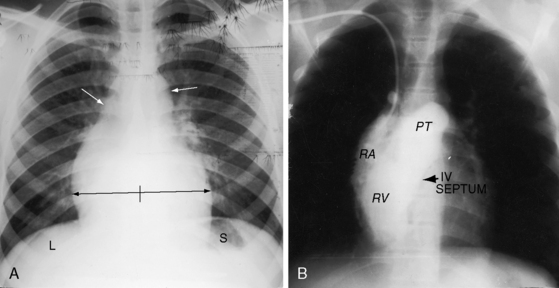
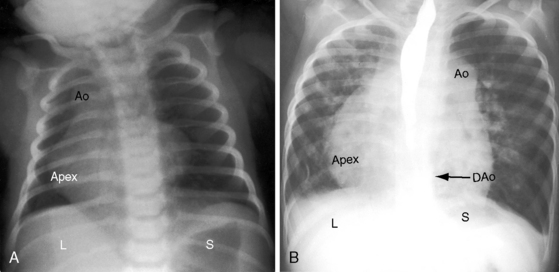
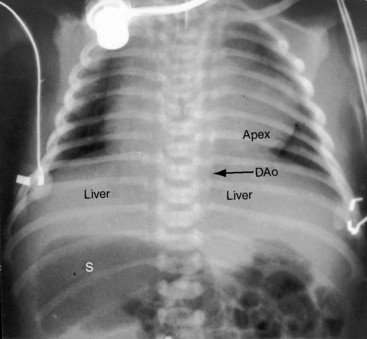
Because atrial situs and abdominal situs are usually concordant, atrial situs solitus can be inferred at the bedside with percussion of a left-sided stomach, a right-sided liver, and a left-sided heart. The chest x-ray confirms the positions of the stomach, liver, and heart (see Figure 3-1) and discloses bronchial morphology, which is a reliable predictor of atrial situs.19 A morphologic right bronchus is relatively short and straight, whereas a morphologic left bronchus is relatively long and curved (Figure 3-5). A morphologic right bronchus is concordant with a trilobed morphologic right lung, and a morphologic left bronchus is concordant with a bilobed morphologic left lung. The chest x-ray establishes the direction of the base to apex axis, which points to the left because the straight heart tube of the embryo initially bends to the right (d-loop) and then pivots to the left until the ventricular portion comes to occupy its normal left thoracic position (see Figure 3-1).9,20 The relative levels of the two hemidiaphragms are determined by the location of the cardiac apex, not by the location of the liver, so the left hemidiaphragm is normally lower than the right hemidiaphragm.21 Thoracoabdominal discordance is represented by thoracic situs solitus, a left thoracic heart, and abdominal situs inversus (Figure 3-6)22,23 or by thoracic situs inversus, a right thoracic heart (dextrocardia), and abdominal situs solitus.
The next step in the systematic analysis concerns the great arteries. The chest x-ray provides information on the spatial relationships of aorta and pulmonary trunk and on ventriculoarterial alignments. In situs solitus with atrioventricular and ventriculo/great arterial concordance, the ascending aorta forms a convex shadow at the right basal aspect of the cardiac silhouette, the aortic arch forms a left basal knuckle below which lies the slightly convex main pulmonary artery segment, and the descending thoracic aorta runs parallel to the left border of the vertebral column (see Figure 3-1).
Malpositions
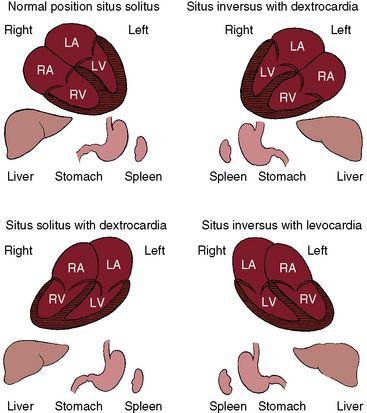
Three major cardiac malpositions occur in the presence of right/left asymmetry (Figures 3-7 and 3-8): (1) visceroatrial situs inversus with dextrocardia; (2) visceroatrial situs solitus with dextrocardia; and (3) visceroatrial situs inversus with levocardia. Mesocardia, a midline heart, is sometimes regarded as a fourth malposition. A midline heart in situs solitus with a d-bulboventricular loop is a variation of normal, but a midline heart with visceroatrial situs inversus and an l-bulboventricular loop occurs with major congenital malformations.18
Situs Inversus with Dextrocardia
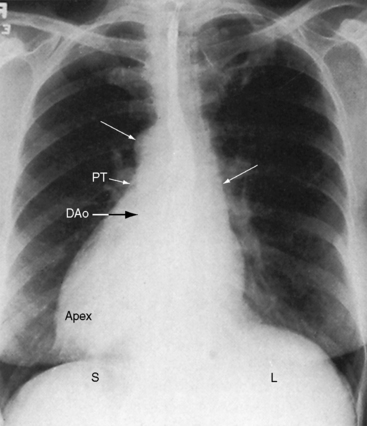
The incidence rate in the general population is estimated at 1/8000 to 1/25,000.24 The heart and the thoracic and abdominal viscera are mirror images of normal (see Figure 3-2).25 The bronchi are inverted (see Figure 3-5A), with the morphologic right bronchus concordant with the morphologic right atrium and the trilobed lung and with the morphologic left bronchus concordant with the morphologic left atrium and the bilobed lung (see Figure 3-5). The heart is right-sided, and the right hemidiaphragm is lower than the left hemidiaphragm (see Figure 3-2). The descending aorta is on the right; the ascending aorta, aortic knuckle, and pulmonary trunk are in their mirror image positions; and the anatomic right ventricle lies to the left of the anatomic left ventricle (l-bulboventricular loop), which is normal for situs inversus just as a d-bulboventricular loop is normal for situs solitus.
Situs Solitus with Dextrocardia
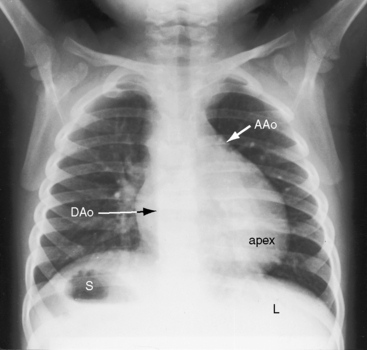
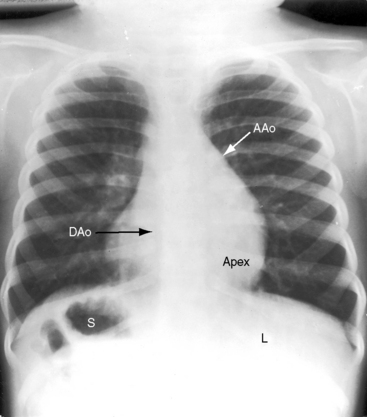
The lungs and abdominal viscera are situs solitus, but the heart is right thoracic (dextrocardia) (Figures 3-7 through 3-10).25 The ascending aorta and aortic knuckle occupy their normal positions and the descending aorta runs its normal course along the left vertebral border (see Figure 3-9), but the major cardiac shadow lies to the right of midline (dextrocardia), the base to apex axis points to the right, and the right hemidiaphragm is lower than the left hemidiaphragm (see Figure 3-9). In the type of situs solitus with dextrocardia shown in Figure 3-9, the anatomic right ventricle lies to the right of the anatomic left ventricle (d-loop) because the straight heart tube of the embryo initially bends in a rightward direction (d-loop) but then fails to pivot into the left chest. Varying degrees of incomplete pivoting determine the degree to which the ventricular portion of the heart lies to the right of midline (see Figure 3-10).
Situs Inversus with Levocardia
The defining characteristics of this malposition are situs inversus of thoracic and abdominal viscera in the presence of a left thoracic heart (levocardia; Figures 3-7 and 3-11). The left hemidiaphragm is lower than the right hemidiaphragm because the apex is on the left (see Figure 3-11). Inversion of the bronchi (see Figure 3-5A) coincides with inversion of the atria and lungs. The stomach is on the right, and the liver is on the left (abdominal situs inversus; see Figure 3-11). The major cardiac mass lies in the left chest for one of two morphogenetic reasons. First, an embryonic l-loop, which is concordant for situs inversus, fails to pivot into the right side of the chest. Second, an embryonic d-loop, which is discordant for situs inversus, fails to pivot into the left side of the chest. When a d-loop in situs inversus is associated with congenitally corrected transposition of the great arteries (ventricular inversion), the ascending aorta forms a smooth shadow at the left basal aspect of the heart (see Figure 3-11).
Midline Heart (Mesocardia)
The example shown in Figure 3-12 is a midline cardiac position in the presence of thoracic and abdominal situs solitus. The cardiac silhouette extends equally to the right and left of midline (Figure 3-12A). A d-bulboventricular loop stops in the midline as it pivots to the left (Figure 3-12B).18,26 Much less commonly, mesocardia is associated with situs inversus and an l-loop that stops in the midline as it incompletely pivots to the right.18,26
In brief, two varieties of right-thoracic hearts exist (see Figures 3-7 and 3-8): namely, situs inversus with dextrocardia and situs solitus with dextrocardia. And two varieties of left-thoracic hearts exist: namely, situs solitus with levocardia (normal) and situs inversus with levocardia. A midline heart (mesocardia) is exceptional but occurs either in situs solitus or rarely in situs inversus. Once the cardiac malposition has been defined, clinical assessment turns to the presence and type of associated congenital heart disease.
Situs inversus with dextrocardia (complete situs inversus, mirror image dextrocardia; see Figure 3-2) usually occurs without coexisting congenital heart disease. Isolated atrial inversion is rare.27 Situs solitus with dextrocardia is only occasionally associated with a structurally normal heart (see Figures 3-9 and 3-10); left-to-right shunts at atrial level or ventricular level usually coexist. When situs solitus with dextrocardia occurs with a bulboventricular loop that initially bends to the left and then pivots to the right (where an l-loop belongs),18 ventricular inversion, ventricular septal defect, and obstruction to venous ventricular outflow usually coexist.13,18,28
Situs inversus with levocardia is consistently associated with coexisting congenital heart disease (see Figure 3-11),18 whether the left thoracic heart results from a discordant d-loop that pivots into the left hemithorax or from a concordant l-loop that fails to pivot into the right hemithorax. A discordant d-loop in situs inversus results in ventricular inversion, as does a discordant l-loop in situs solitus.18 Coexisting congenital heart disease is invariable and complex but occurs without prevailing patterns.13,18
A midline cardiac position (mesocardia) occurs in situs solitus (see Figure 3-12) or in situs inversus.18,26 If the bulboventricular loop is discordant, ventricular inversion coexists.
History
Situs inversus with dextrocardia and a structurally normal heart is usually discovered by chance in a chest x-ray, which is often considered normal because the film is inadvertently reversed when first read. A tendency for left handedness in complete situs inversus is reported,1 but Matthew Baillie2 wrote: “The person seems to have used his right hand in preference to his left … which was readily discovered by the greater bulk and hardness of that hand as well as the greater fleshiness of the arm.” Baillie’s conclusion has been confirmed.29
Investigations of the human brain, which is asymmetric in both structure and function, are important.29 The developmental factors that determine functional asymmetry of the brain independently recognize laterality (asymmetry) in visceral situs.29 Developmental factors that determine anatomic asymmetry of the brain are distinct from those that determine visceral asymmetry and lateralization of language.29
Situs inversus with dextrocardia is the malposition most likely to occur with an otherwise structurally normal heart and with normal longevity. Symptoms caused by coexisting acquired cardiac or noncardiac disease may lead to the discovery of hitherto unsuspected situs inversus. The pain of ischemic heart disease is located in the right anterior chest with radiation to the right shoulder and right arm. The pain of appendicitis is referred to the left lower quadrant,30 and the pain of biliary colic presents in the left upper quadrant (Figure 3-13).
In 1933, Kartagener31 called attention to the association of sinusitis, bronchiectasis, and situs inversus, a combination subsequently called Kartagener’s syndrome or triad.32 In the first English-language publication of the syndrome (1937), as many as one fifth of patients with situs inversus had bronchiectasis, underscoring that the association was not fortuitous.33 In 1986, a blinded controlled study of cilia ultrastructure in Kartagener’s syndrome found a widespread inherited ciliary disorder34 that included the upper and lower respiratory tracts (bronchitis, bronchiectasis, sinusitis)32 and the testis (immobile sperm, male infertility).35,36 Situs inversus is common in infertile men, an observation that contributed to the identification of a generalized disorder of ciliary motility.36 Respiratory symptoms are a significant part of the history and may lead to the discovery of situs inversus. Familial situs inversus has been reported,37 and Kartagener’s syndrome is sometimes familial.34 One family of six siblings included two cases of Kartagener’s syndrome and two cases of isolated bronchiectasis.
Situs solitus with dextrocardia occasionally occurs without coexisting congenital heart disease and escapes recognition. A routine chest x-ray may provide the first evidence (see Figure 3-10). As a rule, accompanying congenital cardiac malformations bring the patient to medical attention. Situs inversus with levocardia (see Figure 3-11) invariably occurs with coexisting congenital heart disease that leads to the discovery of the cardiac malposition.
Physical Appearance, Arterial Pulse, and Jugular Venous Pulse
These features are determined by coexisting congenital heart disease rather than the cardiac malposition. The left testicle in the healthy upright male is lower than the right testicle, whereas the opposite is the case in situs inversus. Poland’s syndrome, which is characterized by the absence of a pectoralis major muscle (usually right-sided), ipsilateral syndactyly, brachydactyly, and hypoplasia of a hand, (see Chapter 15, Figure 15-16) has been reported with situs solitus and dextrocardia38; and Goldenhar’s syndrome (oculoauricular vertebral dysplasia, hemifacial microsomia) has been reported with complete situs inversus.39
Percussion and Palpation
A right anterior chest bulge with asymmetry arouses suspicion of dextrocardia. Percussion and palpation are useful in the clinical recognition of cardiac malpositions because these physical signs are influenced by the malposition per se and establish the right or left thoracic location of the heart and the abdominal location of hepatic dullness and gastric tympany. If the stomach is not sufficiently air-filled to generate a tympanitic percussion note, a carbonated beverage or deliberate aerophagia (an infant can suck an empty bottle) solves the problem. Percussion begins over the sternum and then is used to compare left and right parasternal sites. The side of major cardiac dullness is more accurately established with percussion with the patient turned moderately to the left and then moderately to the right. The heart tends to fall to the side toward which the base to apex axis points. Situs inversus with dextrocardia is characterized by gastric tympany on the right, hepatic dullness on the left, and cardiac dullness on the right (see Figures 3-2 and 3-7). Situs solitus with dextrocardia is characterized by normal locations of gastric tympany and hepatic dullness and by cardiac dullness on the right (see Figures 3-7 and 3-10). Situs inversus with levocardia is the converse of situs solitus with dextrocardia (see Figures 3-7 and 3-11).
Palpation is undertaken with the patient supine and then in both left and right lateral decubitus positions. The normal situs solitus heart (see Figure 3-1) is represented by a morphologic left ventricle that occupies the apex and a morphologic right ventricle that underlies the lower left sternal border.40 Situs inversus with dextrocardia (see Figure 3-2) is represented by a morphologic left ventricle that occupies the apex in the right hemithorax and a morphologic right ventricle that underlies the lower right sternal border. Situs solitus with dextrocardia is represented by a right thoracic apical low-pressure morphologic right ventricle that retracts and a high-pressure systemic morphologic left ventricle that generates outward systolic movement adjacent to the lower right sternal border (see Figures 3-9 and 3-10). Situs inversus with levocardia and l-bulboventricular loop is represented by a left thoracic apical low-pressure morphologic right ventricle that retracts and a high-pressure systemic morphologic left ventricle that generates outward systolic movement adjacent to the lower left sternal border (see Figures 3-11).
Auscultation
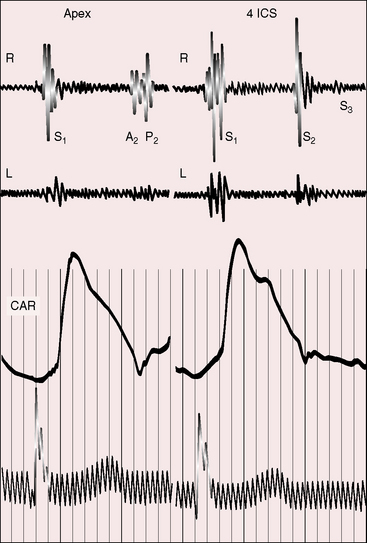
The relative prominence of auscultatory events should be compared in the left and right anterior hemithorax, more specifically along the left and right sternal borders and at the apices (Figure 3-14). The stethoscope should alternate from one side to the other for comparison of analogous right and left thoracic sites. With dextrocardia, the first and second heart sounds are louder in the right anterior chest (see Figure 3-14); splitting of the second sound in the second right intercostal space is a feature of dextrocardia just as splitting of the second heart sound in the second left interspace is a feature of a left thoracic heart. In situs solitus with dextrocardia and a d-bulboventricular loop (see Figures 3-9 and 3-10), the position of the pulmonary valve results in splitting of the second sound in the second right interspace and the anterior position of the aorta results in amplification of the aortic component (Figure 3-15). In situs inversus with levocardia, splitting of the second sound is more prominent in the second left interspace.
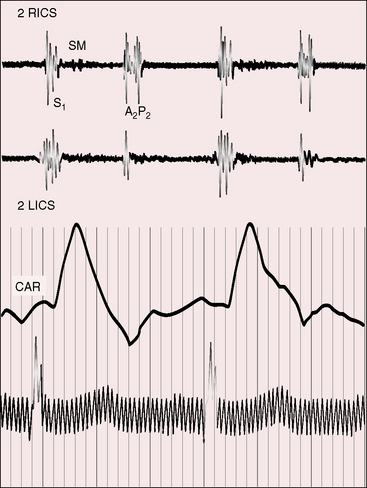
Figure 3-15 Phonocardiograms from the 7-year-old boy referred to in Figure 3-14. A short soft pulmonary midsystolic murmur was recorded in the second right interspace (2 RICS) together with persistent splitting of the second heart sound. (A2 = aortic component; P2 = pulmonary component; CAR = carotid pulse; 2 LICS = second left intercostal space.)
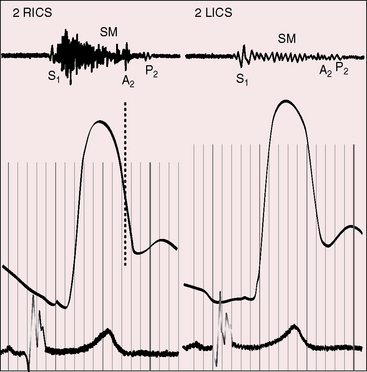
The location and radiation of murmurs are governed by the type of cardiac malposition. In situs inversus with dextrocardia, murmur sites are the mirror images of normal. In situs solitus with dextrocardia, a pulmonary stenotic murmur is louder to the right of the sternum (Figure 3-16) and radiates upward and to the left because of the direction taken by the pulmonary trunk (see Figure 3-9A).
Electrocardiogram
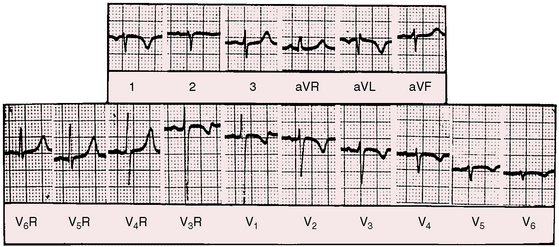
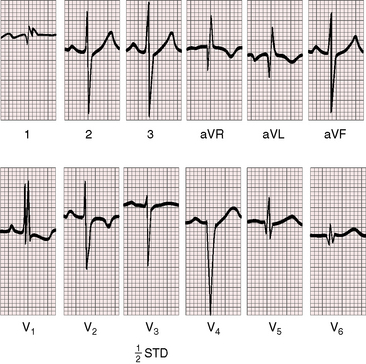
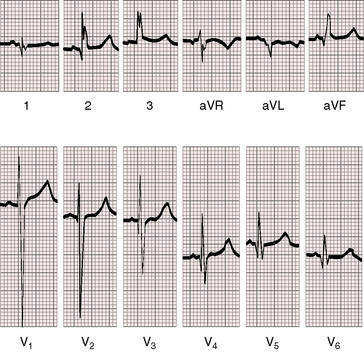
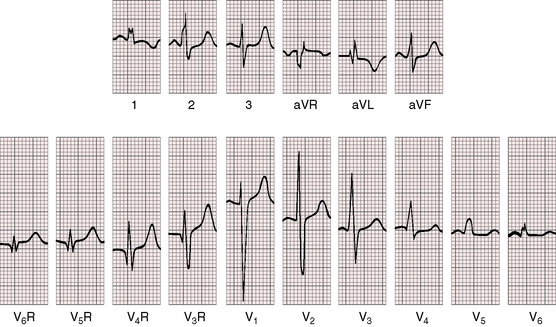
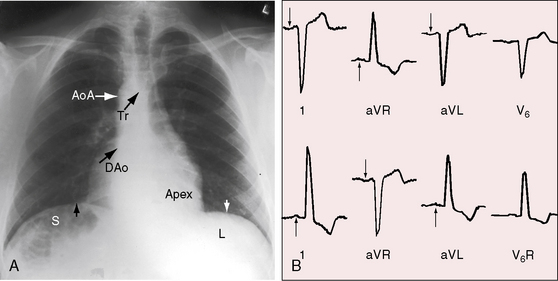
As early as 1889, well before the advent of electrocardiography, it was postulated that ventricular potentials in complete situs inversus should be diametrically opposite the ventricular potentials of the normal heart.41 In situs inversus with dextrocardia, a mirror image sinus node lies at the junction of a left superior vena cava and the mirror image left-sided morphologic right atrium. The right and left bundle branches supply their corresponding mirror image right and left ventricles.42 Certainty that the limb leads are properly attached is essential before proceeding with interpretation of the 12-lead electrocardiogram.43 Interpretation in mirror image dextrocardia is easier when the arm leads are intentionally reversed and when right precordial leads are intentionally recorded from locations that are the exact opposites of standard left precordial lead positions (Figure 3-17).11 In situs solitus with dextrocardia, the limb leads are best left unchanged, and the precordial leads are recorded from the right anterior chest (Figure 3-18). This recommendation is appropriate because atrial situs is normal (sinus node is at the junction of the right superior vena cava and morphologic right atrium on the right)42 and because the base to apex axis points to the right whether the bundle branches supply their corresponding ventricles as d-loop or l-loop.42 In situs inversus with levocardia, standard limb lead and precordial lead positions suffice (Figure 3-19).
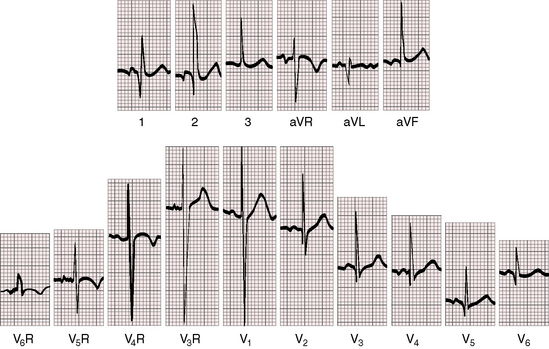
Figure 3-18 Electrocardiogram from the 7-year-old boy in situs solitus with dextrocardia referred to in Figure 3-14. The direction of the P wave is abnormal because of a left atrial ectopic focus. The frontal QRS axis is vertical. The deep Q wave in lead 1 is a sign of right ventricular hypertrophy. Septal depolarization proceeds from left to right as in normally positioned hearts. Septal Q waves in left precordial leads indicate that ventricular inversion does not coexist. The dominant R wave in V6R is evidence of right ventricular hypertrophy because the right-sided right ventricle occupies the apex.
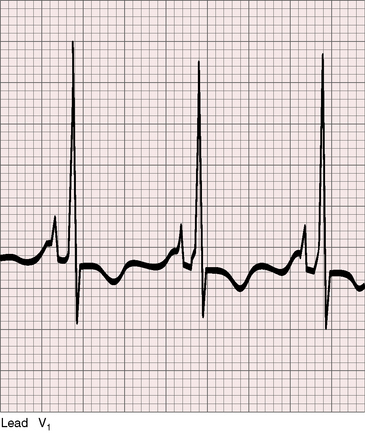
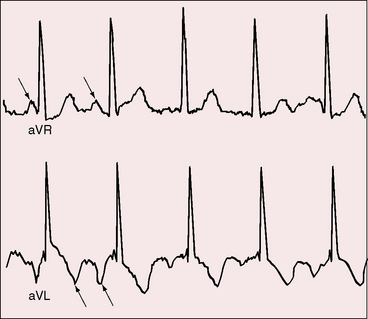
Analysis of the electrocardiogram commences with the P wave direction, which is determined by atrial situs unless the atrial pacemaker is ectopic.44–46 In situs solitus with either levocardia or dextrocardia, atrial depolarization proceeds from a normally positioned right sinus node, so upright P waves appear in leads 1 and aVL and an inverted P wave appears in lead aVR (Figure 3-20).11,12,45,47 Conversely, in situs inversus with either dextrocardia or levocardia, atrial depolarization proceeds from a left sinus node, so inverted P waves appear in leads 1 and aVL and an upright P wave appears in lead aVR (see Figures 3-17 and 3-19).12 In the presence of a right sinus node, the direction of the P wave can be altered by a left atrial ectopic focus.12,47–49 Valsalva’s maneuver, ocular pressure, or exercise may transiently shift the ectopic focus to the right sinus node.49 Left atrial ectopic rhythm is manifested by a negative P wave in lead 1 and isoelectric or negative P waves in left precordial leads (see Figure 3-18). A less common but more distinctive configuration is the dome and dart P wave in lead V1 (Figure 3-21).49 A negative P wave in lead 1 or lead V1 does not distinguish situs solitus with a left atrial ectopic rhythm from situs inversus, but a dome and dart P wave in lead V1 or V2 confirms a left atrial ectopic focus irrespective of atrial situs.48–50
In situs inversus with dextrocardia, ventricular activation and repolarization are the reverse of normal as predicted in 1889 (see Figure 3-17).41 In lead 1, the major QRS deflection is negative and the T wave is inverted; lead aVR resembles lead aVL, and vice versa; and right precordial leads resemble leads from corresponding left precordial sites (see Figure 3-17). Septal Q waves appear in right lateral precordial leads rather than in left lateral precordial leads because septal depolarization proceeds from right to left (see Figure 3-17). The electrocardiogram can be “corrected” when limb leads are reversed and chest leads are recorded from right precordial sites (see previous discussion).
In situs solitus with dextrocardia and a d-loop, the left ventricle is relatively anterior and the right ventricle lies to the right (see Figure 3-10); left ventricular electrical activity is directed anteriorly, and right ventricular activity is directed to the right. Depolarization in the frontal plane is counterclockwise, so Q waves appear in leads 1 and aVL (see Figures 3-18 and 3-20). Precordial leads display relatively prominent R waves in leads V1 and V2 (anterior left ventricular forces) and display prominent RS complexes in most of the remaining right precordial leads (see Figure 3-18). Normal left-to-right septal depolarization (d-loop) results in Q waves in standard left precordial locations (see Figures 3-18 and 3-20). The converse is the case for an l-loop with which Q waves are absent in left precordial leads and are present in right precordial leads, indicating right-to-left septal depolarization (Figure 3-22). Left ventricular hypertrophy is manifested by tall R waves in leads V1 and V2, and right ventricular hypertrophy is manifested by tall R waves in leads V5R and V6R and deep Q waves in lead 1 (see Figures 3-18 and 3-20). In situs inversus with levocardia and l-loop, septal depolarization is right to left, so precordial Q waves are present at right thoracic sites and are absent on the left.
X-Ray
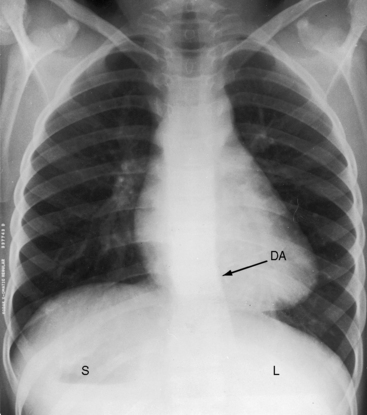
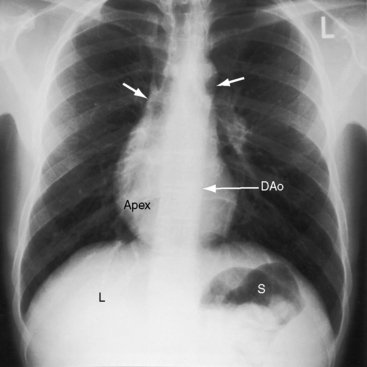
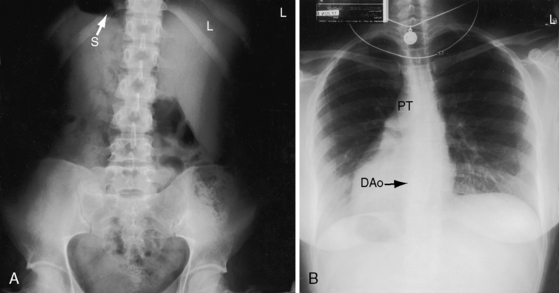
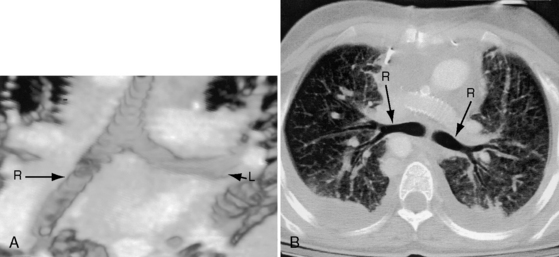
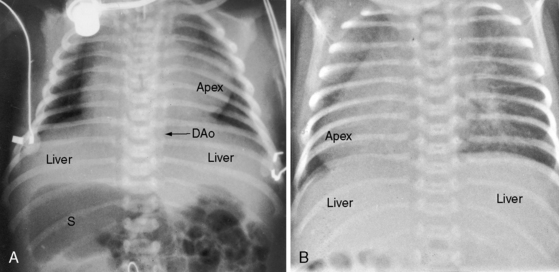
The x-ray permits confident recognition of cardiac malpositions.13,18,19,51 The first necessity is to identify the orienting letters L and R or analogous symbols that designate left and right. From the radiologic point of view, this is all that is required to diagnose situs inversus with dextrocardia (see Figure 3-2). The aorta is in its inverted position with the arch deviating the trachea toward the left, the descending thoracic aorta runs as a fine line along the right vertebral border, and the major cardiac shadow to the right of midline (Figures 3-2 and 3-23A). Situs inversus is missed if the film is inadvertently read in a reversed position because it then appears correct except for the L and R designations that are on the wrong side (Figure 3-23B). Complete situs inversus implies atrial situs inversus (visceroatrial concordance; see Figures 3-8 and 3-23), which is established by identifying the inverted morphologic right and left bronchi (see Figure 3-5).19
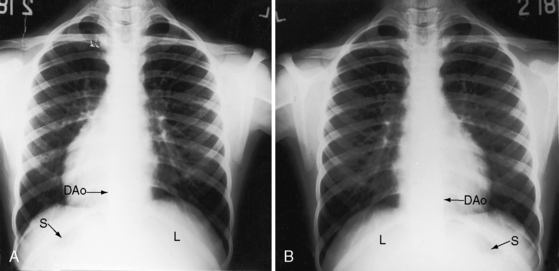
Figure 3-23 X-rays from an 11-year-old girl in situs inversus with dextrocardia and no associated congenital heart disease (see Figure 3-17). A, The L in the upper right corner of the film indicates that the x-ray is viewed properly. The stomach (S) and liver (L) are inverted. B, When the x-ray is reversed, it normalizes except for the L designation. (DAo = descending aorta.)
Situs solitus with dextrocardia (Figures 3-9, 3-10, and 3-24) is represented by normal positions of the stomach, liver, descending thoracic aorta, and right and left bronchi; by the major cardiac shadow to the right of midline; and by the right hemidiaphragm positioned lower than the left hemidiaphragm because the cardiac apex is on the right.21,52 The position of the ascending aorta permits identification of a d-bulboventricular loop (Figure 3-24A).
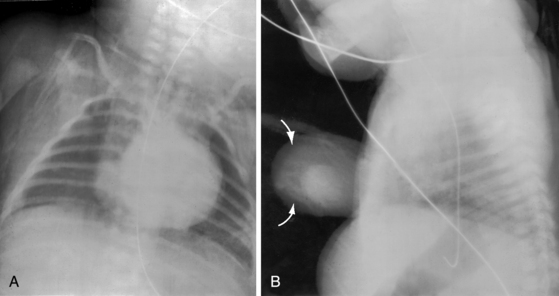
Situs inversus with levocardia is represented by inverted positions of the stomach, liver, descending aorta, and bronchi; the major cardiac shadow is to the left of midline; and the left hemidiaphragm is lower than the right hemidiaphragm because the cardiac apex is on the left (Figures 3-11, 3-25, and 3-26). When the bulboventricular loop is discordant for situs inversus (d-loop), the ascending aorta forms a smooth contour at the left basal aspect of the heart (see Figure 3-25).
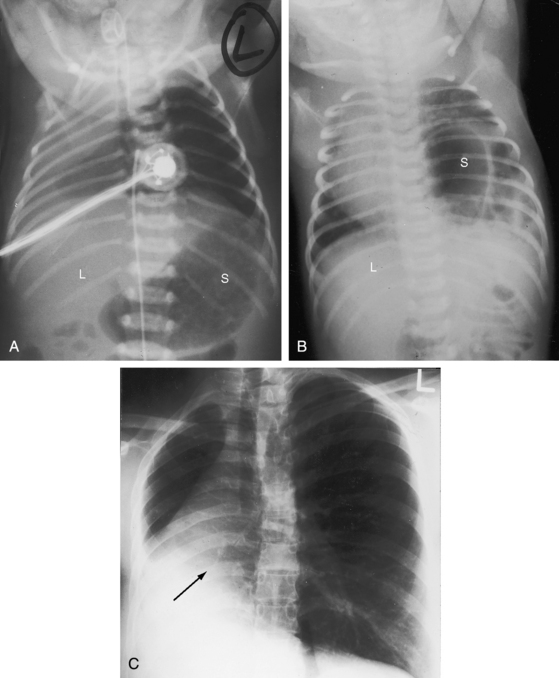
A midline cardiac position, mesocardia, is uncommon and is usually represented by situs solitus with a d-bulboventricular loop that stops at midline as it pivots from right to left (see Figure 3-12). A hump-shaped contour of the right cardiac border is to the result of superimposition of right ventricular and right atrial shadows (see Figure 3-12B).
Echocardiogram
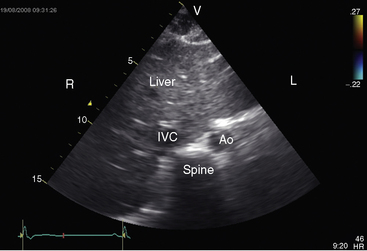
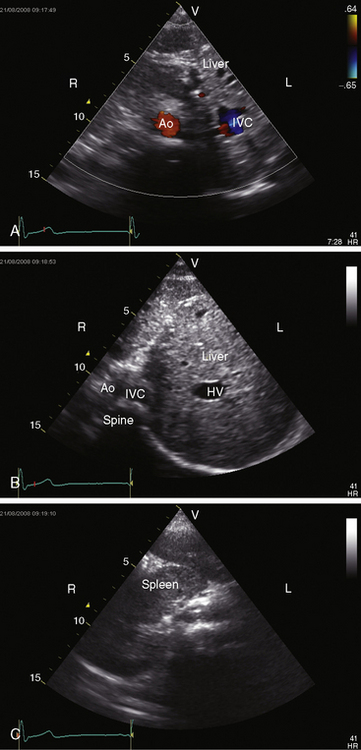
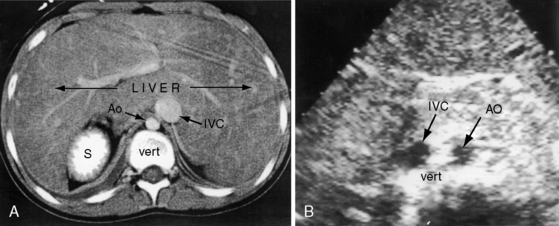
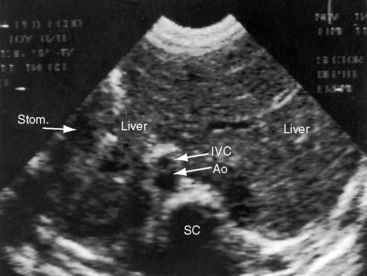
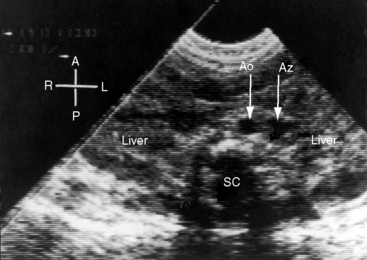
Echocardiography with color flow imaging lends itself to systematic segmental analysis of visceroatrial situs, atrioventricular connections, ventricular locations, and the spatial relationships and ventricular alignments of the great arteries.53,54 Atrial morphology and atrial situs are established with identification of the right atrial appendage with its broad junction and the left atrial appendage with its narrow junction. However, atrial morphology and situs are easier to infer from the abdominal echocardiogram. The normal situs solitus with left thoracic heart is represented by an aorta on the left side of the spinal column and an inferior cava on the right side of the spinal column (Figure 3-27). The morphologic right atrium resides on the same side as the inferior vena cava (atrial situs solitus). The inferior vena cava and aorta are distinguished from each other with color flow imaging because the aorta pulsates. The liver is on the right and the stomach on the left—normal positions (see Figure 3-27). Hepatic venous connections to the inferior vena cava can be identified as can the course of the inferior vena cava to the right-sided morphologic right atrium.
Situs inversus with dextrocardia is the reverse (mirror image) of the normal arrangements.53,54 The short-axis view recognizes the left atrial appendage with its narrow junction to the right of the aorta and recognizes the right atrial appendage with its broad junction to the left of the aorta. The abdominal echocardiogram identifies the aorta to the right of the spinal column and identifies the inferior vena cava to the left of the spinal column (Figure 3-28A and video 3-1). Color flow imaging refines identification of the aorta and inferior vena cava (Figures 3-28B and 3-28C). The echocardiogram is then used to determine hepatic venous connections to the inferior vena cava and determine the course of the left-sided inferior vena cava to the left-sided morphologic right atrium.54 Situs solitus with dextrocardia has echocardiographic features of normal atrial situs. Situs inversus with levocardia has the echocardiographic features of atrial situs inversus. Once atrial situs is established, echocardiography focuses on the atrioventricular junction, ventricular morphology, ventricular location, and ventricular/great arterial connections.
Visceral heterotaxy
Visceral heterotaxy18,55,56 occurs in 0.8% of cases of congenital heart disease.3 Isolated congenital asplenia in otherwise healthy individuals is rare. Right and left bronchi are normally asymmetric (Figures 3-29A and 3-30A); the right lung is trilobed, the left lung is bilobed, and the right and left atrial appendages are morphologically distinctive. The spleen is the only organ that is normally left-sided from its inception because it develops in the left side of the dorsal mesogastrium.57 Unpaired structures such as the liver, spleen, and stomach are normally confined to either the right or left upper quadrant of the abdomen.
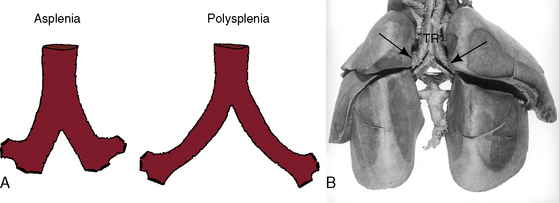
The two bronchi can be morphologically right or morphologically left (Figures 3-29 and 3-30B), the two lungs can be bilaterally trilobed or bilaterally bilobed (Figure 3-29B), and the two atrial appendages can exhibit right or left morphologic features. A relationship exists, albeit imperfect, between right isomerism and asplenia and left isomerism and polysplenia.58 In right isomerism (asplenia) and left isomerism (polysplenia), the liver is typically transverse (bilaterally symmetric; Figures 3-31 and 3-32) and the superior vena cavae are typically bilateral (Figure 3-33). There is strong but not invariable concordance between a morphologic right bronchus (see Figures 3-30A), a morphologic right atrial appendage, and a trilobed morphologic right lung (see Figure 3-29B).58 There is similar strong but not invariable concordance between a morphologic left bronchus (see Figure 3-30A), a morphologic left atrial appendage, and a bilobed morphologic left lung.58 Accordingly, and with few exceptions,58 bilaterally symmetric morphologic right bronchi (see Figures 3-29 and 3-30B) are coupled with bilateral morphologic right atrial appendages and with bilateral trilobed right lungs (see Figure 3-29B), and bilaterally symmetric morphologic left bronchi (see Figures 3-29A and 30A) are coupled with bilateral morphologic left atrial appendages and with bilateral bilobed left lungs. In about 15% of cases, splenic tissue does not coincide with the type of isomerism; and in about 5% of necropsy cases, a normal-sized spleen is located in the right upper quadrant. Rarely, bronchial morphology is not concordant with atrial morphology.58,59
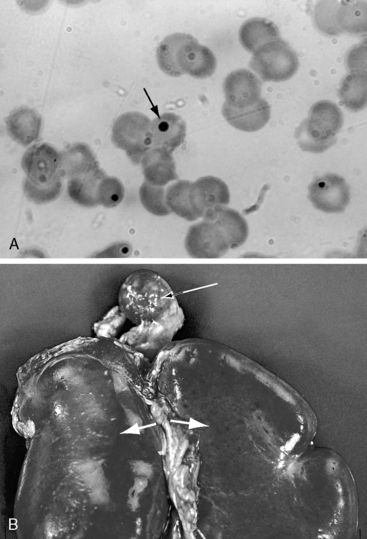
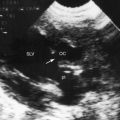
Asplenia can be diagnosed with ultrasound or computed tomographic scan and has been diagnosed with fetal ultrasound scan.60 A simple and readily accessible method for identification of asplenia is the presence of Howell-Jolly bodies in peripheral blood smears (Figure 3-34),61 although Howell-Jolly bodies are occasionally found in healthy infants during the first week of life.46,62 Pitted red cells are also evidence of asplenia, but visualization of the pits requires examination of wet preparations with a special optical system. A wandering spleen is highly mobile and may be located anywhere in the abdomen or pelvis. Oversight has been mistaken for absent spleen.59,63 What is important in a clinical setting is not the presence, absence, or multiplicity of the spleen, but the relatively consistent relationships that exist between the type of isomerism and the type of congenital heart disease.59 It is this relationship that forms the basis of the following sections.
Visceral Heterotaxy with Right Isomerism
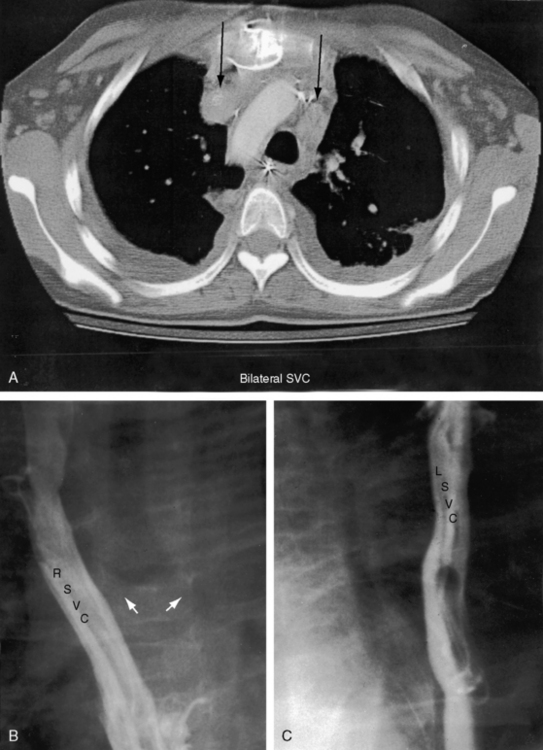
Right isomerism as characterized in Box 3-1 is accompanied by the congenital cardiovascular malformations listed in Box 3-2. The heart is likely to reside to the left of midline.64,65 The liver is transverse (Figures 3-31 and 3-35). The superior vena cavae are bilateral,66 hence the tendency for bilateral sinus nodes.42,67 Exceptionally, one vena cava is partially or completely atretic.66 Bilateral morphologic right bronchi (see Figures 3-29 and 3-30) are closely coupled with bilateral morphologic right atrial appendages and bilateral trilobed lungs (see Figure 3-29B).59,68 Ventricular/great arterial connections are usually discordant.64 Total anomalous pulmonary venous connection is a common arrangement.57,59,62,66,67 The ductus arteriosus may be bilateral.69 Intracardiac malformations approximate a bilocular heart (see Figure 3-35), namely, common atrium, common atrioventricular valve, functionally single ventricle (hypoplastic right or left ventricle) or anatomically single ventricle, and severe pulmonary stenosis or atresia.59,62,64,67
Extracardiac malformations have focused chiefly on the spleen, the largest lymphoid organ in the body. Because of the spleen’s manifold immunologic functions, asplenia is accompanied by recurrent, serious, and even life-threatening infections.70 Gastrointestinal abnormalities are the rule. Biliary atresia71 and midline defects such as tracheoesophageal fistula are prevalent,58 but the most common gastrointestinal disorder is intestinal malrotation that predisposes to volvulus.58,64 Midline defects also include central nervous system (meningomyelocele, cerebellar agenesis, encephalocele), craniofacial (cleft lip and palate), genitourinary (horseshoe kidney), and musculoskeletal (kyphoscoliosis, pectus deformity) defects.58,64
History
The gender distribution of visceral heterotaxy with right isomerism is approximately equal65 or with male predominance.57,58,62 Asplenia has been reported in siblings and in families.62,72 Cyanosis is often evident in the first 24 hours.64 Survival is determined chiefly by coexisting congenital heart disease (see Box 3-2), but extracardiac anomalies (see previous discussion) weigh heavily in determination of morbidity and mortality. Most deaths are within the first few months of life, with only sporadic survivals after the first year57,62,73 and a single remarkable survival to age 21 years.74 Because asplenia increases the risk of bacterial infection and septicemia,70 the clinical presentation may be characterized by high fever, vomiting, hypotension, coma, and death shortly after the onset of symptoms.
Physical Appearance
Infants with visceral heterotaxy and right isomerism are usually born at term, are normally formed, and have normal birth weights.62 Neonatal cyanosis is invariable (see previous discussion) and conspicuous.
Palpation and Percussion
Palpation of a transverse liver edge crossing the upper abdomen (see Figure 3-35) is presumptive evidence of visceral heterotaxy. Percussion is likely to reveal a left rather than a right thoracic heart.57,64,65 The location of the stomach is variable (see subsequent discussion).
Electrocardiogram
The sinus node is normally at the junction of a morphologic right atrium and a right superior vena cava. In right isomerism, paired sinus nodes are seen because each of the bilateral morphologic right atria is equipped with a junction to each of the bilateral superior vena cavae.67,73,75,76 The P wave axis is usually normal, however, which indicates that the right sinus node is the dominant atrial pacemaker.75 AP wave axis that is directed inferior and to the right implies that atrial depolarization is from the left sinus node. The conduction system is also equipped with two atrioventricular nodes that are connected by a sling of conducting tissue.77 Supraventricular tachycardia has been attributed to reentry between the paired atrioventricular nodes.76 Atrioventricular block is rare in contrast to left isomerism.65,73
X-Ray
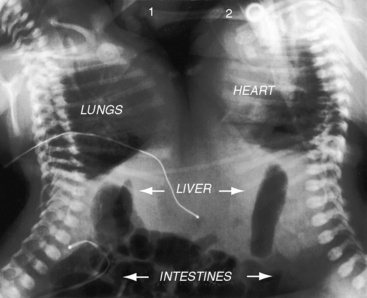
The x-ray is especially valuable when the upper abdomen is included (see Figure 3-31). A transverse liver (see Figure 3-32A) implies visceral heterotaxy but not its type. In right isomerism, the position of the stomach is variable (right, left, or occasionally central; see Figures 3-31 and 3-34),57 and the heart can be either to the right or left of midline (see Figure 3-31). Once visceral heterotaxy is suspected because of a transverse liver, bilateral symmetry (isomerism) is confirmed by bilaterally symmetric bronchi (see Figure 3-5A).19,65 The next step is determination of whether the symmetric bronchi are morphologic right or morphologic left. Overpenetrated films or tomographic scans serve to make this distinction (see Figures 3-5B,C and 3-30).
Echocardiogram
Echocardiography can be used to identify morphologic right and morphologic left atrial appendages with their respective broad and narrow junctions. Abdominal imaging of the liver, aorta, inferior vena cava, and hepatic veins provides a reliable basis for the diagnosis of visceral heterotaxy.53,54,65,78 The liver is transverse, and the inferior vena cava and aorta are anterior to or on the same side of the spinal column (Figures 3-32 and 3-36). Hepatic veins drain into the inferior vena cava, which joins the right-sided atrium. Imaging of the upper quadrants fails in identification of a spleen. Visceral heterotaxy has been diagnosed with fetal echocardiography.79 The largely predictable coexisting cardiac malformations are as in Box 3-2.
Visceral Heterotaxy with Left Isomerism
Left isomerism as characterized in Box 3-3 is associated with the cardiovascular anomalies representated in Box 3-4. Polysplenia is a common but not invariable feature of left isomerism.59,65,80,81 Matthew Baillie2 wrote, “There were three spleens, nearly of the size of a pullet’s egg, found adhering to the larger spleen by short adhesions, besides two other still smaller spleens which were involved in the epiploon at the great end of the stomach.” Baillie’s larger spleen was accompanied by additional spleens of different size and was an example of a normally formed spleen with accessory spleens. Accessory spleens or splenules are present in about 10% of the general population and are usually located in the vicinity of a normally formed spleen (see Figure 3-34B) but may reside elsewhere in the abdomen.82 Two well-formed spleens may be accompanied by one or more splenules.57 Because splenic tissue develops in the dorsal mesogastrium, accessory spleens tend to be located along the greater curvature of the stomach.57 Normal single spleens are occasionally bilobed, trilobed, or multilobed.73,81 Polysplenia is characterized by a cluster of multiple splenules that collectively approximates the mass of one normal spleen.73,81
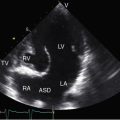
Left isomerism—bilateral left-sidedness—is characterized by bilateral morphologic left bronchi, bilateral morphologic left atria,83 and bilateral morphologic bilobed left lungs. The superior vena cavae are bilateral (see Figure 3-33) and attach to morphologic left atria.68,84 Paired morphologic left atria seemingly preclude the existence of an atrial septum, but attention has been called to a divided left-sided atrium, an intact atrial septum, and a sinus venosus atrial septal defect.85 The answer may lie in the nature of atrial isomerism in which the appendages are morphologically the same, but bilateral similarity (isomerism) may not include the rest of the atria.58
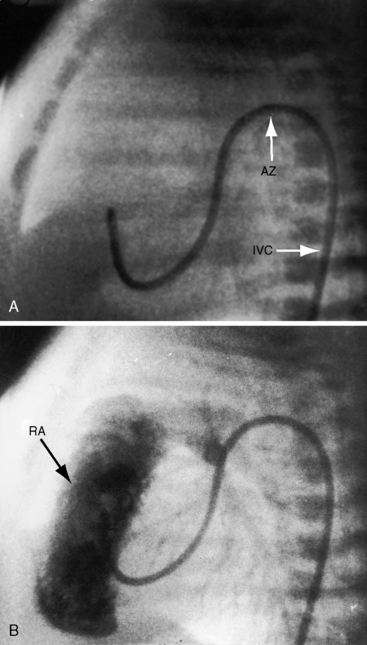
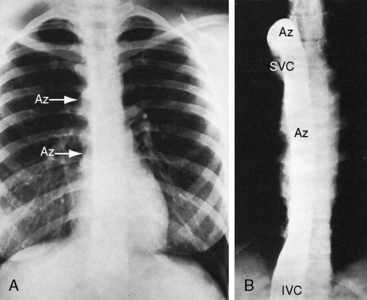
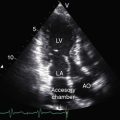
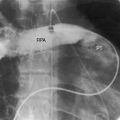
However, it is interruption of the inferior vena cava with azygous continuation that is distinctive and diagnostically important.57,59,62,81,86 The suprarenal segment of the inferior cava is absent, and the infrarenal segment continues as the azygos or hemiazygous vein (Figure 3-37).59 Rarely, isolated inferior caval interruption with azygos continuation occurs without visceral heterotaxy, without isomerism, with normal hearts, and with a normal single spleen (Figure 3-38).
Anomalous pulmonary venous connection in left isomerism is partial, ventriculo/great arterial alignments are concordant, and the pulmonary valve is occasionally stenotic but rarely atretic. The atrioventricular orifices are guarded by two valves or a common valve with an atrioventricular septal defect or a separate inlet ventricular septal defect.57,62,73,81 Two normally formed noninverted ventricles are present, as is a moderate increase in incidence of left ventricular outflow obstruction.87
The most prevalent extracardiac anomalies in left isomerism are gastrointestinal and, apart from the ubiquitous transverse liver, include intestinal malrotation,58,85 biliary atresia,58,71,85 esophageal atresia, and congenital short pancreas.88 Nongastrointestinal extracardiac anomalies are analogous to those with right isomerism, namely, musculoskeletal, neurologic, and craniofacial.58,85
History
Visceral heterotaxy with left isomerism tends to occur in females,85 and right isomerism tends to occur in males.65 The clinical course (morbidity and mortality) of left isomerism is determined by coexisting cardiovascular malformations (see Box 3-4) and by extracardiac anomalies but not by the septicemia of asplenia (see previous discussion). An investigation of fetal bradycardia from complete heart block serves to identify intrauterine left isomerism.89,90 Most patients present as neonates with congestive heart failure or cyanosis,73,81,85 but an important minority present with symptoms related to an extracardiac malformation, especially biliary atresia.85 Approximately 20% die as neonates, and 50% survive adolescence.85 Adult survival is uncommon but not unknown.62,81,91,92 Reports exist of familial polysplenia and familial asplenia,27,62,93 and attention has been called to sibling pairs94 in which one had polysplenia and the other had asplenia.95
Physical Appearance
Infants are normally formed and usually acyanotic. Abnormal physical appearance is likely to reflect the extracardiac anomalies, such as biliary atresia (jaundice), myelomeningocele, or cleft lip/palate.85
Percussion and Palpation
Palpation of a transverse liver in an acyanotic infant is presumptive evidence of left isomerism.
Electrocardiogram
The normal sinus node is a right-sided structure that arises at the junction of a right superior vena cava and a morphologic right atrium (see previous discussion). In left isomerism, a sinus node is absent or hypoplastic because vena caval connection is to a morphologic left atrium. The atrial pacemaker is therefore ectopic (Figure 3-39), located in the atrial wall or near the ostium of a coronary sinus,67,75,86,96 so the P wave axis is abnormal.57,62,73,75,97 The ectopic pacemaker may shift from one site to another85,96 or may fire slowly (ectopic atrial bradycardia).85 Atrial fibrillation or atrial flutter occasionally develops.85,98 Complete atrioventricular block occurs in approximately one in five cases of left isomerism and can be present in the fetus and neonate (see previous discussion)77 with a significant impact on morbidity and mortality.57,62,97 Conduction is interrupted at the level of the penetrating bundle, producing nodoventricular discontinuity and a narrow QRS complex.77
X-Ray
A transverse liver is an important radiologic sign of visceral heterotaxy (see previous discussion; Figure 3-40), and when accompanied by symmetric morphologic left bronchi (see Figure 3-29A), the diagnosis of heterotaxy with left isomerism is secure.57,62,81 The heart is usually left-sided,62,81 and the stomach tends to be on the side opposite the descending aorta (see Figure 3-40). A distinctive radiologic feature of left isomerism is inferior vena caval interruption with azygous continuation that is best seen in the frontal projection (see Figure 3-38).62,85 Absence of an inferior vena caval shadow in the lateral projection is not a reliable sign of interruption because azygos continuation may create the impression of a normal uninterrupted inferior cava.86,99 The lung fields usually show increased pulmonary blood flow because left-to-right shunts occur without obstruction to right ventricular outflow. A transverse liver is a feature of conjoined twins without heterotaxy (Figure 3-41).
Echocardiogram
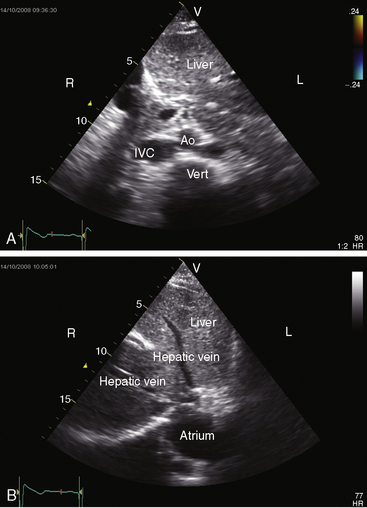
The echocardiogram confirms a transverse liver (Figure 3-42).53,54 The aorta and inferior vena cava lie anterior to or on the same side of the spinal column (Figures 3-42 and 3-43). When inferior vena caval interruption with azygous continuation is seen, the hepatic veins connect directly to the atria and do not join the inferior cava (Figure 3-43B). Echocardiographic diagnosis can be made in the fetus.79
1 Brown J.W. Congenital heart disease. London: Staples Press; 1950.
2 Baillie M. Of a remarkable transposition of the viscera. Philos Trans R Soc Lond B Biol Sci. 1785–1790;16:483.
3 Fyler D.C., Buckley L.P., Hellenbrand W.E. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375-461.
4 Khoury M.J., Cordero J.F., Rasmussen S. Ectopia cordis, midline defects and chromosome abnormalities: an epidemiologic perspective. Am J Med Genet. 1988;30:811-817.
5 Paltauf R. Dextrocardie und Dextroversio Cardis. Wien Klin Wochenschr. 1901;14:1032.
6 Mandelstam M.E., Reinberg S.A. Die Dextrokardie. Ergeb Med Kinderheilkd. 1928;34:154.
7 Lichtman S.S. Isolated congenital dextrocardia: report of two cases with unusual electrocardiographic findings; anatomic, clinical, roentgenologic and electrocardiographic studies of the cases reported in the literature. Arch Intern Med. 1931;48:866-903.
8 De La Cruz M.V., Da Rocha J.P. An ontogenetic theory for the explanation of congenital malformations involving the truncus and conus. Am Heart J. 1956;51:782-805.
9 Van Praagh R., Van Praagh S., Vlad P., Keith J.D. Anatomic types of congenital dextrocardia: diagnostic and embryologic implications. Am J Cardiol. 1964;13:510-531.
10 Campbell M., Deuchar D.C. Dextrocardia and isolated laevocardia. I. Isolated laevocardia. Br Heart J. 1965;27:69-82.
11 Campbell M., Deuchar D.C. Dextrocardia and isolated laevocardia. II. Situs inversus and isolated dextrocardia. Br Heart J. 1966;28:472-487.
12 Campbell M., Reynolds G. The significance of the direction of the P wave in dextrocardia and isolated laevocardia. Br Heart J. 1952;14:481-488.
13 Elliott L.P., Jue K.L., Amplatz K. A roentgen classification of cardiac malpositions. Invest Radiol. 1966;1:17-28.
14 Yost H.J. The genetics of midline and cardiac laterality defects. Curr Opin Cardiol. 1998;13:185-189.
15 Casey B., Devoto M., Jones K.L., Ballabio A. Mapping a gene for familial situs abnormalities to human chromosome Xq24-q27.1. Nat Genet. 1993;5:403-407.
16 Van Praagh R. Terminology of congenital heart disease. Glossary and commentary. Circulation. 1977;56:139-143.
17 Anderson R.H., Macartney F.J., Shinebourne E.A., Tynan M. Terminology. In: Paediatric Cardiology. Edinburgh: Churchill Livingstone; 1987:65.
18 Van Praagh R., Weinberg P.M., Smith S.D. Malpositions of the heart. In Moss A.J., Adams F.H., Emmanouilides G.C., Riemenschnider T.A., editors: Heart disease in infants, children and adolescents, 4th ed, Baltimore: Williams & Wilkins, 1989.
19 Van Mierop L.H., Eisen S., Schiebler G.L. The radiographic appearance of the tracheobronchial tree as an indicator of visceral situs. Am J Cardiol. 1970;26:432-435.
20 Stalsberg H. Development and ultrastructure of the embryonic heart. II. Mechanism of dextral looping of the embryonic heart. Am J Cardiol. 1970;25:265-271.
21 Reddy V., Sharma S., Cobanoglu A. What dictates the position of the diaphragm—the heart or the liver? A review of sixty-five cases. J Thorac Cardiovasc Surg. 1994;108:687-691.
22 Hastreiter A.R., Rodriguez-Coronel A. Discordant situs of thoracic and abdominal viscera. Am J Cardiol. 1968;22:111-118.
23 Sacks L.V., Rifkin I.R. Mirror image arrangement of the abdominal organs with a left-sided morphologically normal heart. Br Heart J. 1987;58:534-536.
24 Douard R., Feldman A., Bargy F., Loric S., Delmas V. Anomalies of lateralization in man: a case of total situs inversus. Surg Radiol Anat. 2000;22:293-297.
25 Garg N., Agarwal B.L., Modi N., Radhakrishnan S., Sinha N. Dextrocardia: an analysis of cardiac structures in 125 patients. Int J Cardiol. 2003;88:143-155. discussion 155–156
26 Lev M., Liberthson R.R., Golden J.G., Eckner F.A., Arcilla R.A. The pathologic anatomy of mesocardia. Am J Cardiol. 1971;28:428-435.
27 Santoro G., Masiello P., Farina R., Baldi C., Di Leo L., Di Benedetto G. Isolated atrial inversion in situs inversus: a rare anatomic arrangement. Ann Thorac Surg. 1995;59:1019-1021.
28 Schiebler G.L., Edwards J.E., Burchell H.B., Dushane J.W., Ongley P.A., Wood E.H. Congenital corrected transposition of the great vessels: a study of 33 cases. Pediatrics. 1961;27(suppl 5):849-888.
29 Kennedy D.N., O’craven K.M., Ticho B.S., Goldstein A.M., Makris N., Henson J.W. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53:1260-1265.
30 Nagaratnam N., Kotagama L.S. Dextrocardia, situs inversus totalis and appendicular abscess. Postgrad Med J. 1957;33:287-288.
31 Kartagener M. Zur Pathogenese der Bronchiektasien; Bronchiektasien bei Situs Viscerum Inversus. Beitr Klin Tuberk. 1933;83:489.
32 Katz M., Benzier E., Nangeroni L., Sussman B. Kartagener’s syndrome (situs inversus, bronchiectasis and chronic sinusitis). N Engl J Med. 1953;248:730-731.
33 Adams R., Churchill E. Situs inversus, sinusitis, bronchiectasis. J Thorac Surg. 1937;7:206.
34 Narayan D., Krishnan S.N., Upender M., et al. Unusual inheritance of primary ciliary dyskinesia (Kartagener’s syndrome). J Med Genet. 1994;31:493-496.
35 Abu-Musa A., Hannoun A., Khabbaz A., Devroey P. Failure of fertilization after intracytoplasmic sperm injection in a patient with Kartagener’s syndrome and totally immotile spermatozoa: case report. Hum Reprod. 1999;14:2517-2518.
36 Gershoni-Baruch R., Gottfried E., Pery M., Sahin A., Etzioni A. Immotile cilia syndrome including polysplenia, situs inversus, and extrahepatic biliary atresia. Am J Med Genet. 1989;33:390-393.
37 Campbell M. The mode of inheritance in isolated laevocardia and dextrocardia and situs inversus. Br Heart J. 1963;25:803-813.
38 Fraser F.C., Teebi A.S., Walsh S., Pinsky L. Poland sequence with dextrocardia: which comes first? Am J Med Genet. 1997;73:194-196.
39 Gorgu M., Aslan G., Erdooan B., Karaca C., Akoz T. Goldenhar syndrome with situs inversus totalis. Int J Oral Maxillofac Surg. 1998;27:404.
40 Perloff J.K. Physical examination of the heart and circulation, 4th ed. Shelton, Connecticut: People’s Medical Publishing House; 2009.
41 Waller A.D. On the electromotive charges connected with the beat of the mammalian heart and of the human heart in particular. Philos Trans R Soc Lond B Biol Sci. 1889;180:169-194.
42 Bharati S., Lev M. The course of the conduction system in dextrocardia. Circulation. 1978;57:163-171.
43 Edenbrandt L., Rittner R. Recognition of lead reversals in pediatric electrocardiograms. Am J Cardiol. 1998;82:1290-1292. A1210
44 Blieden L.C., Moller J.H. Analysis of the P wave in congenital cardiac malformations associated with splenic anomalies. Am Heart J. 1973;85:439-444.
45 Momma K., Linde L.M. Cardiac rhythms in dextrocardia. Am J Cardiol. 1970;25:420-427.
46 Rao P.S. Dextrocardia: systematic approach to differential diagnosis. Am Heart J. 1981;102:389-403.
47 Mirowski M., Neill C.A., Bahnson H.T., Taussig H.B. Negative P waves in lead I in dextroversion: differential diagnosis from mirror-image dextrocardia, with a report of a successful closure of a ventricular septal defect in a patient with dextroversion associated with agenesis of the right lung. Circulation. 1962;26:413-420.
48 Frankl W.S., Soloff L.A. Left atrial rhythm. Analysis by intra-atrial electrocardiogram and the vectorcardiogram. Am J Cardiol. 1968;22:645-656.
49 Mirowski M., Neill C.A., Taussig H.B. Left atrial ectopic rhythm in mirror-image dextrocardia and in normally placed malformed hearts: Report of twelve cases with“ dome and dart” P waves. Circulation. 1963;27:864.
50 Harris B.C., Shaver J.A., Gray S.3rd, Kroetz F.W., Leonard J.J. Left atrial rhythm. Experimental production in man. Circulation. 1968;37:1000-1014.
51 Maldjian P.D., Saric M. Approach to dextrocardia in adults: review. AJR Am J Roentgenol. 2007;188:S39-S49. quiz S35–38
52 Martinez E., Divekar A. Which diaphragm is lower and why? Pediatr Cardiol. 2007;28:243.
53 Huhta J.C., Hagler D.J., Seward J.B., Tajik A.J., Julsrud P.R., Ritter D.G. Two-dimensional echocardiographic assessment of dextrocardia: a segmental approach. Am J Cardiol. 1982;50:1351-1360.
54 Huhta J.C., Smallhorn J.F., Macartney F.J. Two dimensional echocardiographic diagnosis of situs. Br Heart J. 1982;48:97-108.
55 Jacobs J.P., Anderson R.H., Weinberg P.M., et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young. 2007;17(suppl 2):1-28.
56 Van Praagh R., Van Praagh S. Atrial isomerism in the heterotaxy syndromes with asplenia, or polysplenia, or normally formed spleen: an erroneous concept. Am J Cardiol. 1990;66:1504-1506.
57 Van Mierop L.H.S., Gessner I.H., Schiebler G.L. Asplenia and polysplenia syndromes. Birth Defects. 1972;8:36.
58 Ticho B.S., Goldstein A.M., Van Praagh R. Extracardiac anomalies in the heterotaxy syndromes with focus on anomalies of midline-associated structures. Am J Cardiol. 2000;85:729-734.
59 Anderson C., Devine W.A., Anderson R.H., Debich D.E., Zuberbuhler J.R. Abnormalities of the spleen in relation to congenital malformations of the heart: a survey of necropsy findings in children. Br Heart J. 1990;63:122-128.
60 Chitayat D., Lao A., Wilson R.D., Fagerstrom C., Hayden M. Prenatal diagnosis of asplenia/polysplenia syndrome. Am J Obstet Gynecol. 1988;158:1085-1087.
61 Corazza G.R., Ginaldi L., Zoli G., et al. Howell-Jolly body counting as a measure of splenic function. A reassessment. Clin Lab Haematol. 1990;12:269-275.
62 Rose V., Izukawa T., Moes C.A. Syndromes of asplenia and polysplenia. A review of cardiac and non-cardiac malformations in 60 cases withspecial reference to diagnosis and prognosis. Br Heart J. 1975;37:840-852.
63 Abell I. Wandering spleen with torsion of the pedicle. Ann Surg. 1933;98:722-735.
64 Hashmi A., Abu-Sulaiman R., Mccrindle B.W., Smallhorn J.F., Williams W.G., Freedom R.M. Management and outcomes of right atrial isomerism: a 26-year experience. J Am Coll Cardiol. 1998;31:1120-1126.
65 Sapire D.W., Ho S.Y., Anderson R.H., Rigby M.L. Diagnosis and significance of atrial isomerism. Am J Cardiol. 1986;58:342-346.
66 Rubino M., Van Praagh S., Kadoba K., Pessotto R., Van Praagh R. Systemic and pulmonary venous connections in visceral heterotaxy with asplenia. Diagnostic and surgical considerations based on seventy-two autopsied cases. J Thorac Cardiovasc Surg. 1995;110:641-650.
67 Macartney F.J., Zuberbuhler J.R., Anderson R.H. Morphological considerations pertaining to recognition of atrial isomerism. Consequences for sequential chamber localisation. Br Heart J. 1980;44:657-667.
68 Uemura H., Ho S.Y., Devine W.A., Anderson R.H. Analysis of visceral heterotaxy according to splenic status, appendage morphology, or both. Am J Cardiol. 1995;76:846-849.
69 Formigari R., Vairo U., De Zorzi A., Santoro G., Marino B. Prevalence of bilateral patent ductus arteriosus in patients with pulmonic valve atresia and asplenia syndrome. Am J Cardiol. 1992;70:1219-1220.
70 Wang J.K., Hsieh K.H. Immunologic study of the asplenia syndrome. Pediatr Infect Dis J. 1991;10:819-822.
71 Herman T.E. Special imaging casebook. Left-isomerism (polysplenia) with congenital atrioventricular block and biliary atresia. J Perinatol. 1999;19:155-157.
72 Eronen M., Kajantie E., Boldt T., Pitkanen O., Aittomaki K. Right atrial isomerism in four siblings. Pediatr Cardiol. 2004;25:141-144.
73 Stanger P., Rudolph A.M., Edwards J.E. Cardiac malpositions. An overview based on study of sixty-five necropsy specimens. Circulation. 1977;56:159-172.
74 Wolfe M.W., Vacek J.L., Kinard R.E., Bailey C.G. Prolonged and functional survival with the asplenia syndrome. Am J Med. 1986;81:1089-1091.
75 Wren C., Macartney F.J., Deanfield J.E. Cardiac rhythm in atrial isomerism. Am J Cardiol. 1987;59:1156-1158.
76 Wu M.H., Wang J.K., Lin J.L., et al. Supraventricular tachycardia in patients with right atrial isomerism. J Am Coll Cardiol. 1998;32:773-779.
77 Ho S.Y., Fagg N., Anderson R.H., Cook A., Allan L. Disposition of the atrioventricular conduction tissues in the heart with isomerism of the atrial appendages: its relation to congenital complete heart block. J Am Coll Cardiol. 1992;20:904-910.
78 Arisawa J., Morimoto S., Ikezoe J., et al. Cross sectional echocardiographic anatomy of common atrioventricular valve in atrial isomerism. Br Heart J. 1989;62:291-297.
79 Atkinson D.E., Drant S. Diagnosis of heterotaxy syndrome by fetal echocardiography. Am J Cardiol. 1998;82:1147-1149. A1110
80 Merklin R.J. Cardiac lesions associated with visceral inversion. A study of 185 cases. J Int Coll Surg. 1964;41:597-606.
81 Peoples W.M., Moller J.H., Edwards J.E. Polysplenia: a review of 146 cases. Pediatr Cardiol. 1983;4:129-137.
82 Dodds W.J., Taylor A.J., Erickson S.J., Stewart E.T., Lawson T.L. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol. 1990;155:805-810.
83 Pepes S., Zidere V., Allan L.D. Prenatal diagnosis of left atrial isomerism. Heart. 2009;95:1974-1977.
84 Roguin N., Aydinalp A. Isomeric arrangement of the left atrial appendages and visceral heterotaxy. Cardiol Young. 2000;10:668.
85 Gilljam T., Mccrindle B.W., Smallhorn J.F., Williams W.G., Freedom R.M. Outcomes of left atrial isomerism over a 28-year period at a single institution. J Am Coll Cardiol. 2000;36:908-916.
86 Roguin N., Hammerman H., Korman S., Riss E. Angiography of azygos continuation of inferior vena cava in situs ambiguus with left isomerism (polysplenia syndrome). Pediatr Radiol. 1984;14:109-112.
87 Van Praagh S., Geva T., Friedberg D.Z., et al. Aortic outflow obstruction in visceral heterotaxy: a study based on twenty postmortem cases. Am Heart J. 1997;133:558-569.
88 Sener R.N., Alper H. Polysplenia syndrome: a case associated with transhepatic portal vein, short pancreas, and left inferior vena cava with hemiazygous continuation. Abdom Imaging. 1994;19:64-66.
89 Phoon C.K., Villegas M.D., Ursell P.C., Silverman N.H. Left atrial isomerism detected in fetal life. Am J Cardiol. 1996;77:1083-1088.
90 Schmidt K.G., Ulmer H.E., Silverman N.H., Kleinman C.S., Copel J.A. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol. 1991;17:1360-1366.
91 Gayer G., Apter S., Jonas T., et al. Polysplenia syndrome detected in adulthood: report of eight cases and review of the literature. Abdom Imaging. 1999;24:178-184.
92 Hojo Y., Kuroda T., Yamasawa M., et al. Polysplenia accompanied by major cardiovascular anomalies with prolonged survival. Internal Medicine. 1994;33:357-359.
93 De La Monte S.M., Hutchins G.M. Sisters with polysplenia. Am J Med Genet. 1985;21:171-176.
94 Thacker D., Gruber P.J., Weinberg P.M., Cohen M.S. Heterotaxy syndrome with mirror image anomalies in identical twins. Congenit Heart Dis. 2009;4:50-53.
95 Toriello H.V., Kokx N., Higgins J.V., Hofman R., Waterman D.F. Sibs with the polyasplenia developmental field defect. Am J Med Genet Suppl. 1986;2:31-36.
96 Momma K., Takao A., Shibata T. Characteristics and natural history of abnormal atrial rhythms in left isomerism. Am J Cardiol. 1990;65:231-236.
97 Garcia O.L., Metha A.V., Pickoff A.S., et al. Left isomerism and complete atrioventricular block: a report of six cases. Am J Cardiol. 1981;48:1103-1107.
98 Wang T.D., Tseng C.D., Lee Y.T. Left isomerism in a middle-aged woman with early-onset atrial fibrillation. Int J Cardiol. 1997;58:269-272.
99 Bartram U., Fischer G., Kramer H.H. Congenitally interrupted inferior vena cava without other features of the heterotaxy syndrome: report of five cases and characterization of a rare entity. Pediatr Dev Pathol. 2008;11:266-273.