Chapter 10
Cardiac Magnetic Resonance Imaging
1. How does cardiac magnetic resonance imaging (CMR) produce images?
Similar to echocardiography, CMR allows the generation of images of the heart without exposure to ionizing radiation. Although the spatial resolution of CMR is comparable to echocardiography (approximately 1 mm), the contrast-to-noise and signal-to-noise ratios are far superior (Fig. 10-1). The latter allows easier delineation of borders between tissues and between blood pool and tissue. The contrast between blood pool and myocardium (see Fig. 10-1) is generated using differences in signal properties of the different tissues without the use of contrast agents. CMR is also not limited by “acoustic windows” that may hinder echocardiography, and images can be obtained in any tomographic plane. Finally, CMR can provide information about tissue characteristics using differences in T1 and T2 signals, and with addition of contrast agents.
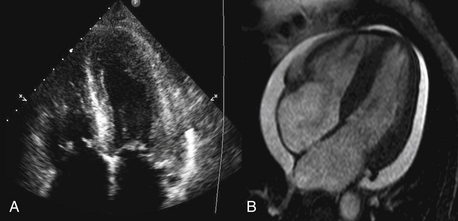
Figure 10-1 Comparison of (A) an echocardiographic and (B) cardiac MRI (CMR) cine image of a four-chamber view of the same patient. The superior contrast-to-noise and signal-to-noise ratios are clearly evident in the CMR image, with clear delineation of the endocardial and epicardial borders of both ventricles.
3. What are the limitations of CMR?
The major limitation of CMR is availability. Given the cost, the special construction necessary to host a CMR system, and the technical expertise and support necessary, CMR is not widely available at all centers. CMR is limited with respect to portability, unlike echocardiography where imaging can be performed at the patient’s bedside. There is also a set of contraindications (listed later) that limits the use of this technology in selected patient populations. Image acquisition can be challenging in patients with an irregular cardiac rhythm or difficulty holding their breath, though newer real-time techniques provide an opportunity to overcome these barriers (Fig. 10-2). Finally, because patients have to lie still in a long hollow tube for up to an hour during imaging, claustrophobia may become an important limiting factor.
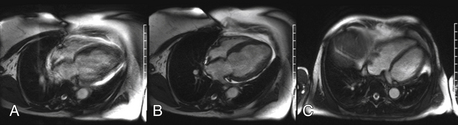
Figure 10-2 A, Four-chamber view in a patient with difficulty holding their breath (blurry). B, The same patient who was able to breath-hold after the acquisition period was shortened. C, Another patient with a real-time acquisition while breathing freely.
4. What are the common imaging pulse sequences used in CMR?
5. What are the appropriate uses of CMR?
6. What is delayed enhancement (DE) CMR imaging?
One of the unique aspects of CMR is the ability to identify myocardial scar and/or fibrosis. This is most commonly performed using DE imaging. Gadolinium-based contrast agents are first administered, then after waiting approximately 10 minutes and allowing time for the contrast to distribute into areas of scar and fibrosis, an inversion recovery sequence is performed. This sequence nulls (makes it black) normal myocardium and anything that is bright within the myocardium is most likely myocardial scar or fibrosis (Fig. 10-3).
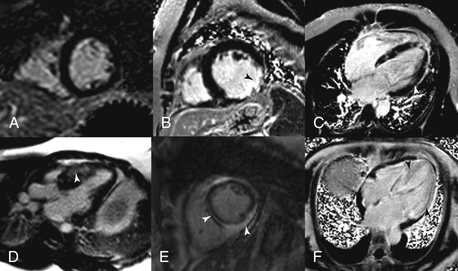
Figure 10-3 Delayed enhancement (DE) patterns. A, A normal short-axis DE image. B, A transmural scar in the circumflex territory. C, Cardiomyopathy secondary to sarcoidosis. D, Hypertrophic obstruction cardiomyopathy with basal anterior septal midmyocardial DE. E, Myocarditis with both midmyocardial and epicardial DE. F, Cardiac amyloidosis with diffuse enhancement of the entire left ventricle.
7. Does CMR have a role in the evaluation of chest pain?
In patients with chest pain syndrome, CMR stress testing can be performed to assess flow-limiting coronary stenosis. This is most appropriate in patients with intermediate pretest probability of CAD with uninterpretable ECG or who are unable to exercise. The two stress methods used are vasodilator perfusion CMR or dobutamine stress function CMR. Vasodilator stress testing is performed identically to nuclear stress testing with the use of adenosine. With peak coronary vasodilation, gadolinium contrast agent is administered and perfusion of the myocardium during the first pass of the contrast agent is captured. Areas of perfusion abnormality can be detected as dark areas (Fig. 10-4). Resting perfusion is also commonly performed, mainly to differentiate imaging artifacts from a true perfusion defect. This is then followed by DE imaging to assess for myocardial scar. Alternatively, graded doses of dobutamine can be administered to provide a sympathetic stress with cine imaging to identify regions of wall motion abnormality, in a manner similar to echocardiography.
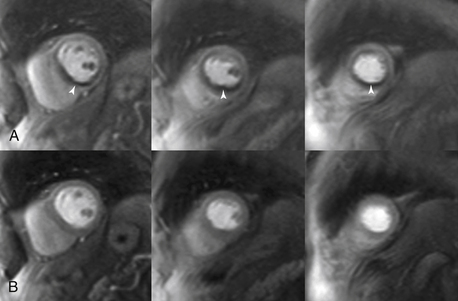
Figure 10-4 Adenosine perfusion imaging. A, Peak stress short-axis and four-chamber perfusion images showing ischemia (arrows) in the basal to apical inferior and inferior septal segments, consistent with ischemia in the right coronary artery territory. B, Rest images illustrate normal perfusion.
8. Can CMR coronary angiography be used to assess chest pain?
Coronary angiography for assessment of coronary stenosis is not commonly performed in clinical settings. However, it is appropriate to use coronary magnetic resonance angiography (MRA) to assess the origin, branching pattern, and proximal course of the coronary arteries to assess for coronary anomalies (Fig. 10-5). This can be performed without the use of contrast agents. However, the acquisition may require a substantial amount of time and is susceptible to artifacts. The use of this technique may be particularly important, however, in young patients, thereby avoiding radiation exposure from invasive coronary angiography or coronary CT angiography.
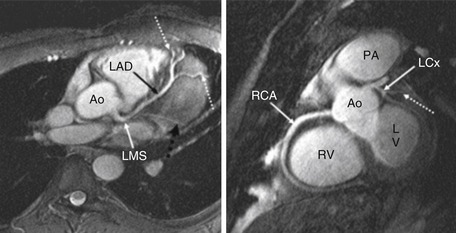
Figure 10-5 Cardiovascular magnetic resonance angiography at 3 Tesla. Left panel, Left coronary artery and branches (dotted arrows). Right panel, Right coronary artery (RCA). Ao, Aorta; LAD, left anterior descending artery; LCx, left circumflex artery; LMS, left main stem; LV, left ventricle; PA, pulmonary artery; RV, right ventricle. (From Stuber M, Botnar RM, Fischer SE, et al: Preliminary report on in vivo coronary MRA at 3 Tesla in humans, Magn Reson Med 48:425-429, 2002. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.)
9. What is the role of CMR in the assessment of ventricular function?
10. Does CMR have a role in the assessment of cardiomyopathies?
CMR has an important role in the evaluation of specific cardiomyopathies, particularly in the infiltrative diseases (e.g., amyloidosis and sarcoidosis), hypertrophic cardiomyopathy, and cardiomyopathies due to cardiotoxic therapies. Although CMR-based ventricular morphology and functional images can provide certain clues to the etiology of the underlying cardiomyopathy, the most important CMR technique in the assessment of cardiomyopathies is DE imaging. Certain DE patterns have been associated with particular cardiomyopathies and can be easily identified by experienced readers (see Fig. 10-3). Another specific cardiomyopathy that can be assessed by CMR is arrhythmogenic right ventricular cardiomyopathy (ARVC). CMR may be specifically used in patients presenting with syncope or ventricular arrhythmias to provide excellent assessment of right ventricular (RV) function, RV dilation, focal aneurysms, and also myocardial scar using DE imaging both in the LV and RV.
11. What is the role of CMR in myocardial infarction?
After an acute myocardial infarction, CMR can be used to assess the extent of myocardial necrosis (see Fig. 10-3, B) and areas of microvascular obstruction (“no reflow regions”) (Fig. 10-6) using DE imaging. Both the extent of necrosis and the presence of microvascular obstruction have been shown to have prognostic value. In addition, DE imaging can be used to determine the likelihood of recovery of function with revascularization (surgical or percutaneous) or medical therapy. Segments of myocardium with more than 50% transmural scar are less likely to recover with revascularization. Another important use of CMR is in patients who present with a positive troponin test but have normal coronary angiography. CMR can help identify myocarditis (see Fig. 10-3, E), Tako-tsubo syndrome, or even myocardial infarction with recanalized coronary artery.
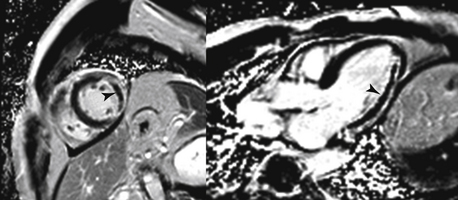
Figure 10-6 Microvascular obstruction post infarction. A short-axis (left) and long-axis (right) image of microvascular obstruction (arrows) seen on delayed enhancement imaging. Both images are from the same patient.
12. Can valvular disease by assessed by CMR?
Echocardiography remains the most commonly used method for assessment of stenotic and regurgitant lesions of both native and prosthetic valves. The role of CMR is mainly in patients with technically limited images from echocardiography. CMR can be used to assess the significance of the valve lesions using several techniques. First, an en face image of the valvular lesion can be obtained for visual assessment and planimetry of the stenotic or regurgitant orifices. However, the most useful CMR techniques are the quantitative assessment of valvular stenosis obtained by transvalvular gradients, or of regurgitant lesions obtained by volumes and flow measurements. The latter is often obtained using a combination of stroke volumes by planimetry of the ventricles and phase-contrast imaging (analogous to Doppler imaging in echocardiography) (Fig. 10-7).
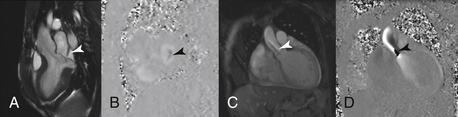
Figure 10-7 Valvular disease assessment. A, Three-chamber steady-state free precession (SSFP) image showing bileaflet mitral valve prolapse and a jet of mitral regurgitation (arrow). B, Short-axis phase-contrast image of the mitral valve during systole showing the mitral regurgitant orifice (arrow). C, D, Magnitude and phase contrast images, respectively, of the aortic valve showing flow acceleration at and beyond the aortic valve of aortic stenosis (arrows).
13. Can CMR be used in the assessment of congenital heart disease?
Among imaging tests, CMR is likely one of the best modalities for the assessment of congenital heart disease. The ability to assess the heart and its relationship to the arterial and venous circulation in three-dimensional space and obtain hemodynamic data in the same study is unique to CMR. Images can be obtained in any preferred plane, often essential in patients with complex congenital heart disease or with complex repairs. Also, important parameters, such as LV and RV volumes and function, that are used to make important clinical decisions, can be measured in an accurate and reproducible manner. Furthermore, as these patients are young and typically require multiple imaging studies throughout their lives, CMR provides excellent assessment without ionizing radiation exposure. The major limitation of CMR in this population is the susceptibility to artifacts from previous surgeries involving surgical clips, valves, and coils.
14. Can CMR be used to assess cardiac masses?
CMR is a good modality for the assessment of tumors (Fig. 10-8) as tissue characterization using various sequences may provide insights into the differential diagnosis of an identified mass. However, the initial hopes that CMR could definitively characterize tumor tissue (e.g., a noninvasive biopsy) have not quite come to fruition. Nevertheless, CMR can still help narrow the differential diagnosis and provide information about whether the tumor has characteristics suggestive of malignancy. However, small tumors and those with high frequency motion may not be appropriately assessed using CMR.
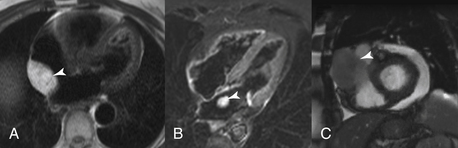
Figure 10-8 Cardiac masses. Three cardiac masses (arrows) are illustrated using three different cardiac magnetic resonance imaging sequences. A, Lipoma; B, left atrial myxoma; and C, metastatic melanoma.
15. What are some of the other clinical uses of CMR?
16. What are the contraindications to CMR?
All devices should be checked for magnetic resonance imaging (MRI) compatibility prior to taking the patient into the MRI room. A good reference website to check the compatibility of a device is www.mrisafety.com. Alternatively, the manufacturer of the device should be contacted to check for compatibility. Some of the most common contraindications to CMR are provided in the list that follows.
 Ocular foreign body (e.g., metal in eye)
Ocular foreign body (e.g., metal in eye)
 Central nervous system aneurysm clips
Central nervous system aneurysm clips
 Implanted cardiac pacemaker or defibrillator
Implanted cardiac pacemaker or defibrillator
 Other implanted medical devices (e.g., drug infusion ports, insulin pump)
Other implanted medical devices (e.g., drug infusion ports, insulin pump)
17. Can CMR be performed in patients with implanted cardiovascular devices?
Bibliography, Suggested Readings, and Websites
1. IMAIOS SAS. MRI step-by-step, interactive course on magnetic resonance imaging. Available at www.imaios.com/en/e-Courses/e-MRI. Accessed January 25, 2013
2. Shellock, F.G. Website for. MRIsafety.com Available at www.mrisafety.com. Accessed January 25, 2013
3. Society for Cardiovascular Magnetic Resonance. Website for SCMR. Available at www.scmr.org. Accessed January 25, 2013
4. U.S. Food and Drug Administration Information for Healthcare Professionals. Gadolinium-based contrast agents for magnetic resonance imaging (Marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142884.htm. Accessed January 25, 2013
5. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR. 2006 Appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Radiol. 2006;48:1476–1497.
6. Cummings, K.W., Bhalla, S., Javidan-Nejad, C., et al. A pattern-based approach to assessment of delayed enhancement in non-ischemic cardiomyopathy at MR imaging. Radiographics. 2009;29:89–103.
7. Kanal, E., Broome, D.R., Martin, D.R., et al. Response to the FDA’s May 23, 2007, nephrogenic systemic fibrosis update. Radiology. 2008;246:11–14.
8. Kim, R.J., Wu, E., Rafael, A., et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453.
9. Levine, G.N., Gomes, A.S., Arai, A.E., et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–2891.
10. Lin, D., Kramer, C. Late gadolinium-enhanced cardiac magnetic resonance. Curr Cardiol Rep. 2008;10:72–78.
11. Shinbane, J.S., Colletti, P.M., Shellock, F.G., et al. Magnetic resonance imaging in patients with cardiac pacemakers: era of “MR Conditional” designs. J Cardiovasc Magn Reson. 2011;2011(13):63.
12. Zou, Z., Zhang, H.L., Roditi, G.H., et al. Nephrogenic systemic fibrosis review of 370 biopsy-confirmed cases. JACC Cardiovasc Imaging. 2011;4:206–216.







