CHAPTER 40 CARDIAC INJURIES
PENETRATING CARDIAC INJURY
Historical Perspective
The earliest descriptions of a cardiac injury are found in the Iliad1 and in the Edwin Smith Papyrus,2,3 written in approximately 3000 BC. Hippocrates4 stated that all wounds of the heart were deadly. Ambrose Pare,5,6 the famous French trauma surgeon, described two cases of penetrating cardiac injuries, both detailed from autopsy studies. Wolf,7 in 1642, was the first to describe a healed wound of the heart, while Senac,8 in 1749, concluded that although all wounds of the heart were serious, some wounds might heal and not be fatal. Larrey9,10 was the first to describe the surgical approach to the pericardium to relieve a pericardial effusion, and is credited with pioneering the technique for pericardial window. Billroth, in 1875 and in 1883, proclaimed his strong resistance to any attempt at cardiac injury repair.11–13 Block,14 in 1882, created cardiac wounds in a rabbit model and was successful in achieving repair, thus demonstrating successful recovery and suggesting that the same techniques could be applicable to humans. Also, Del Vecchio15 demonstrated cardiac injury healing after suturing the heart in a canine model.
However, it took the courage of Cappelen16 from Norway to attempt cardiac injury repair in a human; in 1895 he repaired a 2-cm left ventricular laceration including ligation of a large branch of the distal left anterior descending coronary artery. This was followed by Farina17 in Italy in 1896, who also attempted to repair a left ventricular wound; however, both patients succumbed. Rehn18 in Germany in 1896 was successful in repairing a wound of the right ventricle, while in the United States, Hill,19 in 1902, was the first surgeon to successfully repair a left ventricular injury.
Duval20 described the median sternotomy incision, and Spangaro,21 in 1906, described the left anterolateral thoracotomy incision. Peck22 in 1909 was the first to describe successful repair of a stab wound of the right atrium, and he reported a total of 11 patients. Smith23 was the first to develop a comprehensive plan for cardiac injury management, and for the first time pointed out the dangers of dysrhythmias occurring during cardiac manipulation. He also described the use of an Allis clamp near the apex to stabilize and hold the heart during suture placement.
Beck24 in 1942 described the technique of placing mattress sutures under the bed of the coronary arteries. During the same year, Griswold25 refined the techniques in the management of cardiac injuries and recommended that every large general hospital should have available a sterile set of instruments plus an available operating room 24 hours a day. Elkin26–28 in 1944 recommended the administration of intravenous infusions before operation and pointed to the beneficial effects of increasing blood volume and thus cardiac output. Beall and colleagues29–32 were the first to describe the technique of emergency department (ED) thoracotomy. Meanwhile, Mattox et al.33–35 refined and protocolized ED thoracotomy and cardiorrhaphy, inclusive of the use of emergency cardiopulmonary bypass in the management of these injuries. These hallmark contributions have made it possible for patients sustaining penetrating cardiac injuries to survive today.
Incidence
Feliciano et al.36 in 1983 described a 1-year experience consisting of 48 cardiac injuries at Ben Taub Hospital in Houston. Mattox and associates37 in 1989 described a 30-year experience from the same institution reporting 539 cardiac injuries (18 cardiac injuries per year). Asensio and colleagues38,39 reported two prospective consecutive series reporting a total of 165 cardiac injuries in a 3-year period (55 cardiac injuries per year) at Los Angeles County/USC Medical Center in Los Angeles. A recent review by Asensio et al.,40 which focused on the National Trauma Databank (NTDB) of the American College of Surgeons (ACS), identified a total of 2016 patients sustaining penetrating cardiac injuries, and calculated the national incidence of 0.16% for these injuries. Thus, penetrating cardiac injuries are uncommon and are usually seen only in busy urban trauma centers.
Etiology
In the civilian arena, penetrating cardiac injuries are usually caused by gunshot wounds (GSWs), stab wounds (SWs), and rarely by shotgun wounds and ice picks.41 According to a recent review,40 63% of all reported cardiac injuries in America are caused by gunshot wounds and 36% are caused by stab wounds, while shotgun and impalement injuries accounted for approximately 1% of these injuries. In the military arena, Rich and Spencer42 reported 96 cardiac injuries from the Vietnam conflict. Most of these patients sustained injuries from grenade fragments or shrapnel, while a few of these patients were impaled by flechettes.
Clinical Presentation
Beck’s triad—muffled heart tones, jugular venous distention, and hypotension—describes the classical presentation of a patient with pericardial tamponade.3,41 Kussmaul’s sign, described as jugular venous distention upon inspiration, is another classic sign attributed to pericardial tamponade. In reality, the presence of Beck’s triad and Kussmaul’s signs represent the exception rather than the rule.3,41,43 It is estimated that Beck’s triad is only present in approximately 10% of patients.41
The clinical presentation of penetrating cardiac injuries may range from complete hemodynamic instability to cardiopulmonary arrest; in fact, some penetrating cardiac injuries can be very deceptive in their presentation.41,44 The clinical presentation of penetrating cardiac injuries may also be related to factors including the wounding mechanism; the length of time elapsed before arrival at a trauma center; and the extent of the injury, which if sufficiently large in terms of myocardial destruction will invariably lead to exsanguinating hemorrhage into the left hemithoracic cavity. The presentation of these injuries is also related to blood loss, as patients who lose between 40% and 50% of intravascular blood volume develop cardiopulmonary arrest. The muscular nature of the left ventricle, and to a lesser extent that of the right ventricle, may seal penetrating injuries and prevent exsanguinating hemorrhage, allowing these patients to arrive with some signs of life at a trauma center.41,44
The most unique presentation of a penetrating cardiac injury is pericardial tamponade. The tough fibrous nature, lack of elasticity, and noncompliance of this structure translate to acute rises in intrapericardial pressure leading to compression of the thin wall of the right ventricle, impairing its ability to accept the returning blood volume, resulting in a concomitant decrease in left ventricular filling and ejection fraction. This results in a drastic decrease in cardiac output (CO) and stroke volume (SV). The impaired ability to generate both right and left ventricular ejection fractions increases cardiac work and myocardial wall tension. This results in an increase in myocardial volume of oxygen consumption (MVO2) which cannot be met, leading to myocardial hypoxemia and lactic acidosis.41,44
It is well known that the pericardium is able to accommodate gradual quantities of blood, provided that the rate of hemorrhage is slow and does not cause acute rises in intrapericardial pressures exceeding the right ventricle and subsequently the left ventricle’s ability to fill. Pericardial tamponade can have both deleterious and protective effects. Its deleterious effects can lead to a rapid rise in pericardial pressure and cardiopulmonary arrest, whereas its protective effect will limit extrapericardial hemorrhage into the left hemithoracic cavity, preventing exsanguinating hemorrhage. Moreno et al.,45 in a retrospective study consisting of 100 patients presenting with penetrating cardiac injuries, reported 77 patients who presented with pericardial tamponade. The authors reported that for patients presenting with pericardial tamponade, the survival rate was much higher—73% versus 11%—thereby ascribing tamponade a protective effect. These findings were statistically significant, leading the authors to conclude that pericardial tamponade is a critical independent factor in patient survival.
Asensio et al.,39 in a prospective 2-year study reporting 105 patients failed to find any statistical significance to the presence of pericardial tamponade in terms of survival, and were not able to identify it as a critical independent factor for survival. What remains undefined is the actual period of time after which the protective effect of pericardial tamponade is lost, and when exactly this transition occurs causing its adverse effect on cardiac function.
Diagnosis
Physical Examination
The clinical presentation of patients with penetrating cardiac injury may range from presenting hemodynamically stable to cardiopulmonary arrest. Frequently, these patients present with associated pneumohemothoraces and decreased breath sounds in the ipsilateral hemithoracic cavity. Occasionally, patients presenting with precordial injuries are restless and refuse to lie down; this may be a subtle indicator denoting the presence of hemopericardium and/or incipient pericardial tamponade. The most dramatic presentation for a patient sustaining a penetrating cardiac injury is, of course, cardiopulmonary arrest, which will require ED thoracotomy as a life-saving intervention.41,42 Pericardiocentesis is only mentioned to note that it currently has no role in establishing the diagnosis of cardiac injuries.
Subxiphoid Pericardial Window
The original technique of pericardial window was described by Larrey9,10,41 in the 1800s, and only small variations in the original technique have been added to this procedure. This technique has seen a marked diminution in its role during recent times because of the advent of two-dimensional echocardiography a part of the focused assessment and sonographic examination of the trauma patient (FAST).41 Nevertheless, the technique is still widely employed in many countries where medical personnel do not have access to ultrasound equipment.
Two-Dimensional Echocardiography
Echocardiography as part of FAST has become the gold standard in the evaluation of patients with penetrating thoracic injury. Major benefits of echocardiography include being noninvasive, rapid, and accurate; its ability to be repeated at any time; and most importantly, its painlessness. Data from two multicenter studies46,47 conclusively support the role of FAST as the initial investigative tool for the evaluation of patients with penetrating cardiac injuries, given its accuracy and ease of performance. Other techniques such as transesophageal echocardiography (TEE) have no role in the immediate evaluation sustaining penetrating precordial injuries.41
Minimally Invasive Methods
Thoracoscopy
Morales et al.48 reported a 31% incidence of positive windows describing a technique that was both accurate and well tolerated without any complications, and the authors recommend this technique to be used in patients also requiring evacuation of a retained hemothorax. In our opinion, thoracoscopic pericardial window has no role in the acute evaluation of penetrating cardiac injuries.
Management
Prehospital
Emergency medical systems in large urban areas providing rapid transport to trauma centers have allowed patients with penetrating cardiac injuries an opportunity to undergo life-saving surgical procedures. Field stabilization of patients with penetrating cardiac injuries should consist of intubation and closed cardiopulmonary resuscitation for patients found in cardiopulmonary arrest. Several studies49–52 strongly support and advocate for the need of immediate transport of patients with penetrating thoracic injuries to a trauma center, with the only predictors of outcome being the achievement of an airway via endotracheal intubation. Endotracheal intubation has been proven to increase both duration and tolerance of cardiopulmonary resuscitation administered for a period of less than 5 minutes. The return of organized cardiac electrical activity will provide the best opportunity at survival for these patients.
Emergency Department
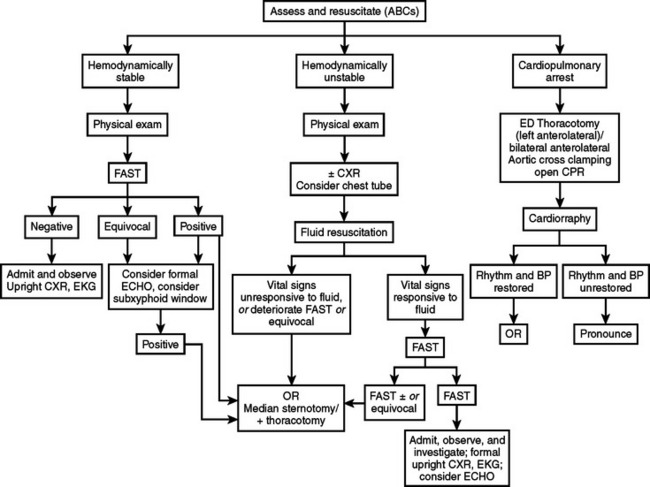
All patients with penetrating cardiac injuries should undergo rapid initial assessment and resuscitation following Advanced Trauma Life Support (ATLS) protocols.53 Patients will usually self-stratify into those who are hemodynamically stable and may undergo diagnostic studies, those who are hemodynamically unstable but will respond to fluid resuscitation and allow for rapid transport to the operating room (OR), and those who present in cardiopulmonary arrest and will necessitate life-saving surgical interventions such as ED thoracotomy. Patients can be initially and rapidly evaluated with FAST, chest x-ray, and optionally an electrocardiogram (EKG). Volume resuscitation with lactated Ringer and O- or type-specific blood should be initiated. An arterial blood gas to determine initial pH and base deficit and lactic acid level should also be obtained. However, a significant majority of these patients will arrive “in extremis” requiring life-saving interventions.38,39,41,44
Emergency department thoracotomy
Emergency department thoracotomy is a surgical procedure of great value if undertaken after strict indications for its performance. This procedure is routinely performed in urban trauma centers that receive patients “in extremis.” When performed in an expedient fashion, ED thoracotomy, aortic cross-clamping, and cardiorrhaphy are successful in salvaging approximately 10% of all penetrating cardiac injuries. Open cardiopulmonary massage after definitive repair of penetrating cardiac injuries is more effective in producing a greater ejection fraction. Similarly, lacerations of major thoracic blood vessels can also be controlled by means of vascular clamps.41,44
Prehospital factors predictive of poor outcome include absence of vital signs, fixed and dilated pupils, absence of cardiac rhythm, absence of motion in the extremities, absence of a palpable pulse, and the presence of cardiopulmonary arrest are predictors of poor outcome.41,44
Generally accepted indications for this procedure include cardiopulmonary arrest secondary to penetrating thoracic injuries and profound shock with systolic blood pressures of less than 60 mm Hg because of exsanguinating hemorrhage or pericardial tamponade. Cardiopulmonary arrest secondary to blunt injury is generally a contraindication to the performance of this procedure.41,42,44
Objectives to be achieved with this procedure include resuscitation of agonal patients arriving with penetrating cardiothoracic injuries, evacuation of pericardial tamponade, control of massive intrathoracic hemorrhage secondary to cardiovascular injuries, prevention of air emboli, and restoration of cardiac function using open cardiopulmonary massage. Other objectives accomplished include definitive repairs of penetrating cardiac injuries and control of exsanguinating thoracic vascular injuries. Similarly, cross-clamping of the descending thoracic aorta, redistributing the remaining blood volume to perfuse the carotid and coronary arteries, is achieved with this technique.41,42,44
Emergency department thoracotomy should be performed simultaneously with the initial assessment evaluation and resuscitation, using the ATLS53 protocols by trained trauma surgeons. Similarly, immediate venous access with simultaneous use of rapid infusion techniques complements the resuscitative process. A left anterolateral thoracotomy commencing at the lateral border of the left sternocostal junction and inferior to the nipple is carried out and extended laterally to the latissimus dorsi. In females, the breast is retracted cephalad. This incision is rapidly carried through skin until the intercostal muscles have been reached and sharply transected. A Finochietto retractor is then placed to separate the ribs. The lung is then elevated medially, and the thoracic aorta is located immediately as it enters the abdomen via the aortic hiatus. The aorta should then be palpated to assess the status of the remaining blood volume. It can also be temporarily occluded digitally against the bodies of the lower thoracic vertebrae. To fully cross-clamp the aorta, a combination of sharp and blunt dissection commencing at both the superior and inferior borders of the aorta is performed, so that the aorta may be encircled between the thumb and index fingers; this facilitates the aortic cross-clamp to be placed safely. Trauma surgeons should then observe the pericardium and search for the presence of an injury. The pericardium is usually tense and discolored in the presence of tamponade. A longitudinal opening in the pericardial sac is then made anterior to the phrenic nerve and extended both inferiorly and superiorly. Usually it is necessary to grasp the pericardium and then make a small incision sharply, followed by opening the pericardium with Metzenbaum scissors.
Digital control of penetrating ventricular injuries as they are simultaneously sutured prevents further hemorrhage. We generally recommend the use of monofilament suture, such as 2-0 polypropylene. If the injury or injuries are quite large, balloon tamponade using a Foley catheter can temporarily arrest the hemorrhage either to allow the performance of cardiorrhaphy or to gain time so that the patient may be transferred expeditiously to an OR for a more definitive surgical procedure. We do not recommend the use of bioprosthetic materials such as Teflon patches in the ED. This is a time-consuming technique that, if needed, should be performed in the OR.41,44
In our experience, staples do not effectively control hemorrhage, tend to enlarge the cardiac injury, and prove to be rather difficult to remove, although they have worked in the hands of others.54
Outcomes of emergency department thoracotomy for penetrating cardiac injuries
Wide disparity in the reporting of outcomes exists in the literature, ranging from 0% to 72%. Most of these series are retrospective, and the patients reported have been injured because of stab wounds. Asensio, Wall, and others in the Working Group of the Committee on Trauma of the American College of Surgeons,55 after an extensive analysis of the literature, generated practice management guidelines for ED thoracotomy (Table 1).
Techniques for Cardiac Injury Repair
Incisions
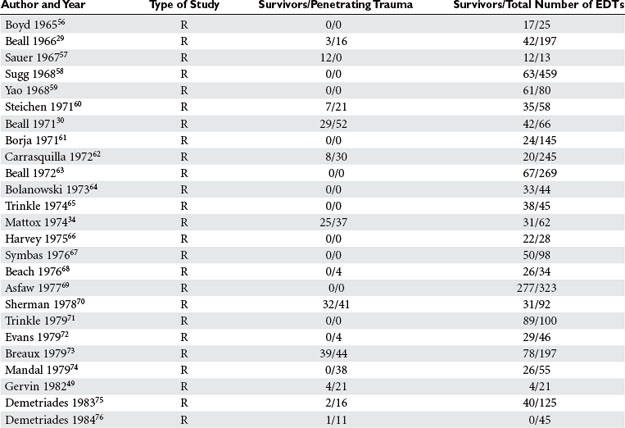
Two main incisions are used in the management of penetrating cardiac injuries. Median sternotomy described by Duval20 is the incision of choice for patients admitted with penetrating precordial injuries that arrive with some degree of hemodynamic instability and may either undergo preoperative investigation with FAST and/or chest x-ray. The left anterolateral thoracotomy described by Spangaro21 is the incision of choice in the management of patients who arrive “in extremis.” This incision is used in the ED for resuscitative purposes, and it can also be extended across the sternum as bilateral anterolateral thoracotomies. Extension into bilateral anterolateral thoracotomies is also the incision of choice for patients who are hemodynamically unstable after incurring mediastinal-traversing injuries (Figure 1). It is important to note that upon transection of the sternum, both internal mammary arteries are also transected and must be ligated after restoration of perfusion pressure. For patients who sustain thoracoabdominal injuries, the left anterolateral thoracotomy is also the incision of choice if patients deteriorate in the OR while undergoing a laparotomy.44,92
Adjunct Maneuvers
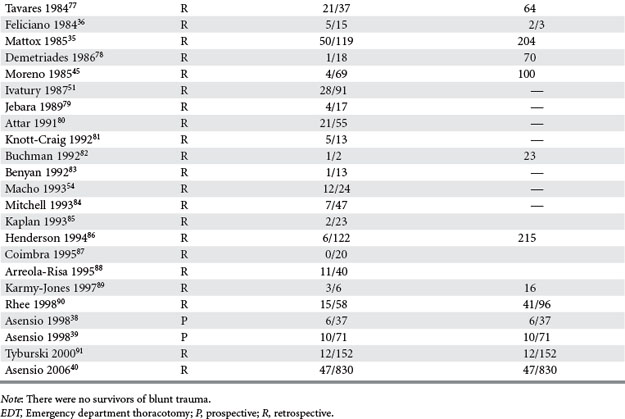
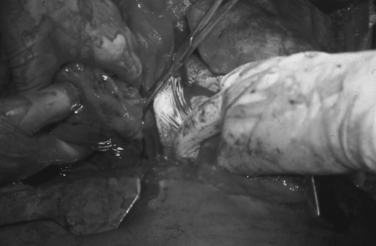
Trauma surgeons must possess several maneuvers in their armamentarium to deal with penetrating cardiothoracic injuries. Total inflow occlusion to the heart is a complex maneuver which entails cross-clamping both the superior (SVC) and inferior vena cava (IVC) in their intrapericardial location to arrest total blood flow to the heart (Figure 2). It is indicated for the management of injuries in the lateral-most aspect of the right atrium and/or the superior or inferior atriocaval junction. The safe period for this maneuver is unknown, although a 1–3-minute range is often quoted in the literature as the period of time after which clamps must be released.
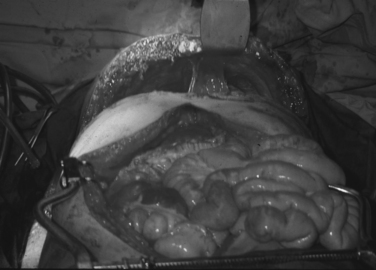
Cross-clamping of the pulmonary hilum is another valuable maneuver indicated for the management of associated pulmonary injuries, particularly those that present with hilar central hematomas and/or active bleeding (Figure 3). This maneuver arrests bleeding from the lung and prevents air emboli from reaching the systemic circulation. However, in the presence of acidosis, hypothermia, and ischemia, the right ventricle may not be able to tolerate this maneuver, leading to fibrillation and arrest.
Repair of Ventricular Injuries
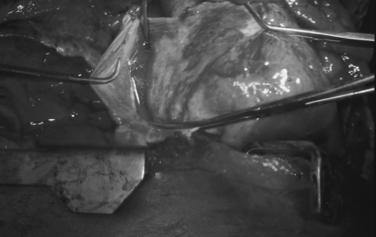
Ventricular injuries usually cause significant hemorrhage. They should be occluded digitally while simultaneously repaired by either simple interrupted or horizontal mattress sutures of Halsted. Performing cardiorrhaphy for ventricular stab wounds is usually less challenging than for gunshot wounds. Missile injuries often produce some degree of blast effect that causes myocardial fibers to retract, and frequently require multiple sutures to control significant hemorrhage. In the presence of this scenario, bioprosthetic materials such as Teflon strips and/or pledgets are often needed to buttress the suture line (Figure 4). We recommend 2-0 monofilament sutures of polypropylene on an MH needle.
Complex and Combined Injuries
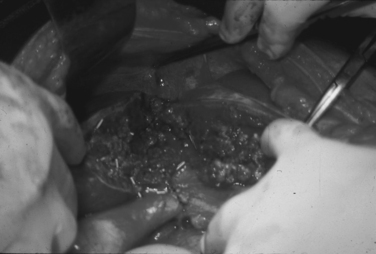
A significant number of patients arrive harboring multiple associated injuries in addition to their penetrating cardiac injuries. Complex and combined cardiac injuries can be defined as a penetrating cardiac injury plus associated neck, thoracic, thoracic-vascular, abdominal, abdominal vascular, or peripheral vascular injuries (Figures 5 and 6). These injuries are quite challenging to manage. Priority should be given early to the injury causing the greatest blood loss or threatening the patient’s life.
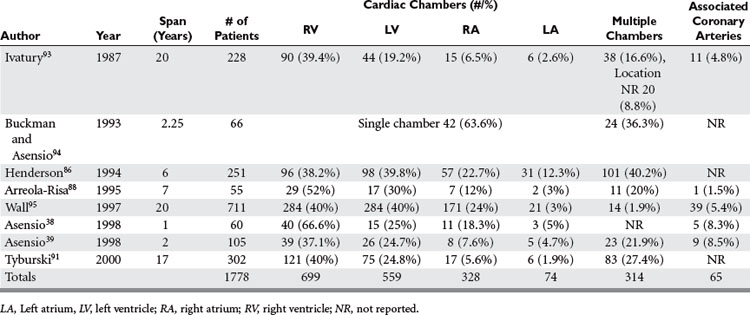
Anatomic Location of Injury
A great deal of variability exists in the literature when it comes to reporting the breakdown of cardiac injuries by chambers (Table 2). Ventricular injuries occur with an incidence ranging from 37% to 67% of all cardiac injuries, whereas left ventricular injuries occur with an incidence ranging from 19% to 40%. Right atrial injuries appear to occur with greater frequency ranging from 5% to 20%, whereas the left atrium, the most recessed chamber of the heart, is injured between 2% and 12% of the times.38,39,86,88,91,93–95
Prognostic Factors
The American Association for the Surgery of Trauma and its Organ Injury Scaling (AAST-OIS)96 committee have developed a scale to uniformly describe cardiac injuries (Table 3). This scale comprehensively describes these injuries and, although the scale has been available since 1994, few studies have correlated cardiac injury grade with mortality.
Table 3 American Association for the Surgery of Trauma, Organ Injury Scale: Cardiac Injury
| Gradea | Injury Description |
|---|---|
| I | Blunt cardiac injury with minor electrocardiographic abnormality (nonspecific T or T-wave changes, premature atrial or ventricular contraction, or persistent sinus tachycardia). |
| Blunt or penetrating pericardial wound without cardiac injury, cardiac tamponade, or cardiac herniation. | |
| II | Blunt cardiac injury with heart block (right or left bundle branch, left anterior fascicular, or atrioventricular) or ischemic changes (ST depression or T-wave inversion) without cardiac failure. |
| Penetrating tangential myocardial wound up to, but not extending through, endocardium, without tamponade. | |
| III | Blunt cardiac injury with sustained (>5 beats per minute) or multifocal ventricular contractions. |
| Blunt or penetrating cardiac injury with septal rupture, pulmonary or tricuspid valvular incompetence, papillary muscle dysfunction, or distal coronary arterial occlusion without cardiac failure. | |
| Blunt pericardial laceration with cardiac herniation. | |
| Blunt cardiac injury with cardiac failure. | |
| Penetrating tangential myocardial wound up to, but not extending through, endocardium, with tamponade. | |
| IV | Blunt or penetrating cardiac injury with septal rupture, pulmonary or tricuspid valvular incompetence, papillary muscle dysfunction, or distal coronary arterial occlusion producing cardiac failure. |
| Blunt or penetrating cardiac injury with aortic or mitral valve incompetence. | |
| Blunt or penetrating cardiac injury of the right ventricle, right atrium, or left atrium. | |
| V | Blunt or penetrating cardiac injury with proximal coronary arterial occlusion. |
| Blunt or penetrating left ventricular perforation. | |
| Stellate wound with <50% tissue loss of the right ventricle, right atrium, or left atrium. | |
| VI | Blunt avulsion of the heart; penetrating wound producing >50% tissue loss of a chamber. |
a Advance one grade for multiple penetrating wounds to a single chamber or multiple chamber involvement.
Modified from Moore EE, Malangoni MA, Cogbill TH, et al: Organ injury scaling IV: thoracic, vascular, lung, cardiac, and diaphragm. J Trauma 36:229–300, 1994, with permission.
Asensio and colleagues,39 in a 2-year prospective study of 105 patients, correlated AAST-OIS for cardiac injuries with mortality, in which 99 (94%) of the 105 patients incurred grade IV–VI injuries. Mortality progressively increased with injury grade. Grade IV injuries incurred mortality of 56%, grade V 76%, and grade VI 91%. Although findings of both these studies would appear to validate the correlation between mortality and organ injury grade, the authors feel that further work is necessary to confirm their findings. Furthermore, the authors strongly believe that all cardiac injuries should be graded according to this scale.
Prognostic factors such as mechanism of injury, physiologic parameters at the scene of the traumatic incident, during transport and upon arrival such as pupillary response, spontaneous ventilation, presence of a carotid pulse, presence of a measurable blood pressure, sinus rhythm, any extremity movement, need for intubation and cardiopulmonary resuscitation, as well as scene times greater than 10 minutes have been prospectively validated in many studies.38,39,49,94 The presence of cardiopulmonary arrest upon arrival as a poor predictor of outcome has been confirmed.36,37,51,86,91 Similarly, physiologic parameters upon arrival such as these measured by the cardiovascular respiratory score (CVRS) component of the trauma score (TS) have been validated by Buckman et al.94
BLUNT CARDIAC INJURY
Historical Perspective
The first unquestionable case of myocardial contusion was reported in 1764 by Akenside,97 and the first recorded case of blunt cardiac chamber rupture was reported in 1679 by Borch.98,99 According to Warburg,100 for almost 200 years, from 1676 to 1868, only 27 cases of blunt traumatic cardiac injuries were reported in the literature.101
In 1958, Parmley et al.102 reviewed 207,548 autopsy cases from the Armed Forces Institute of Pathology (AFIP), and described 546 patients with nonpenetrating traumatic injury to the heart reporting an incidence of 0.1%. In this hallmark study, the authors reported 353 cases of cardiac chamber rupture, of which 273 were isolated and 80 associated with combined aortic ruptures. The breakdown per chamber included 66 ruptured right ventricles, 59 ruptured left ventricles, 41 ruptured right atria, and 26 ruptured left atria. A total of 106 patients sustained multiple-chamber injuries.
Mechanism, Pathophysiology, and Incidence
Blunt cardiac injury can range from a mild myocardial contusion to frank cardiac chamber rupture including the rare entity of “comotio cordis” described as sudden cardiac arrest from a sternal blow, leading to cardiogenic shock. Blunt cardiac injuries may occur secondary to compression, deceleration, blast, and direct forces applied to the chest, or transmitted increases in intravascular abdominal pressures associated with compression of the abdominal contents. High-speed motor vehicular collisions, causing crushing injuries to the thoracic cage or objects of great weight falling directly onto the sternum or thoracic cage will directly compress the heart against the vertebral column causing BCI. Similarly, accidental falls from great heights as well as blast injuries can also cause BCIs. The true incidence of BCIs remains difficult to estimate from the literature.103
Clinical Presentation
Blunt cardiac injury encompasses an entire spectrum of different processes; therefore, clinical presentation for patients sustaining BCI may range from complete hemodynamic instability to cardiopulmonary arrest. These patients may also present with the classical syndrome of pericardial tamponade. Chest pain experienced by some of these patients and its distribution may be indistinguishable from the classical pain of myocardial infarction. Physical findings may include pain and tenderness over the anterior chest wall, contusion, ecchymosis, anterior rib fractures, and even a central flail chest.103
Diagnosis
A number of different modalities have been employed to establish a diagnosis of BCI including chest x-ray, EKG, Holter monitoring, measurement of cardiac enzymes, transthoracic (TTE) and TEE echocardiography, and nuclear medicine scans including radionuclide angiography (RNA), thallium 201, single-photon emission computed tomography (SPECT), and multiple-gated image acquisition scans (MUGAs).103 Chest x-rays are routinely obtained in all trauma patients; they may detect the presence of fractured ribs and flail chest and rarely may reveal a globular-shaped cardiac silhouette. EKG is used to screen patients and detect conduction disturbances. However, there is no pathonogmonic finding that can reliably establish the diagnosis of BCI.
The measurement of creatine phosphokinase (CPK) or creatine kinase (CK) with measurement of the myocardial band (MB) was for a time used to establish the diagnosis of BCI. However, more specific measurements of contractile proteins of different muscle types including troponin (cTn) have emerged as tools to diagnose BCI as both troponin T and I belong to a group of proteins of the contractile apparatus that are unique to cardiac muscle. Fulda et al.104 prospectively evaluated 71 patients with thoracic wall injuries utilizing signal-averaged EKG, serum troponin T levels, standard EKG, and CPK-MB measurements. Patients were monitored electrocardiographically and by serial measurements of troponin-T and CPK-MB fractions. The authors reported that the sensitivity and specificity of troponin-T in predicting clinically significant abnormalities were 27% and 91%, respectively. From the findings of this study, the authors concluded that the best predictors for the development of significant electrocardiographic changes are an abnormality detected in the initial EKG as well as an elevated serum troponin T level, recommending that both of these tests be obtained to diagnose BCI.
Salim and colleagues105 investigated the role of serum cardiac troponin I and EKG to identify patients at risk for the development of cardiac complications after blunt cardiac trauma. In this prospective 115-patient study, the authors identified patients at risk for significant BCI defined as cardiogenic shock, arrhythmias requiring treatment, or structural cardiac abnormalities directly related to blunt cardiac trauma. All patients were evaluated with EKG upon admission, which was repeated at an 8-hour interval. Cardiac troponin I was obtained at admission and also at 4 and 8 hours. Two-dimensional EKGs were obtained when clinically indicated. Nineteen patients (16.5%) had significant BCIs. In 18 of the 19, symptoms were present within 24 hours. Of the 115 patients, 58 (50%) had abnormal EKGs and 27 (23.5%) had increased cTnI. From these data, the authors concluded that the combination of EKG and troponin I reliably identified the presence or absence of significant BCIs.
The use of two-dimensional echocardiography (TTE) has been extensively used as diagnostic modality for the evaluation of patients with suspected BCI. Patients are selected for evaluation by this modality after abnormal EKG and abnormal cardiac enzymes and troponin level measurements are detected. TTE evaluates segmental wall abnormalities or valvular dysfunction.103 However, although useful, it has not been shown to correlate with complications or eventual outcome in BCI, and whereas it may detect and identify structural defects and abnormalities with wall motion, it is limited by chest wall edema, and traumatic structural abnormalities such as fractured ribs and flail chest, but most importantly it cannot detect the electrical instability that is a hallmark for BCI.
Transesophageal echocardiography has been shown to be a useful adjunct to evaluate patients with BCI, as it is more versatile in its ability to detect BCI. However, it is an invasive procedure that is operator dependent and not always available around the clock.106–108
A number of different radionuclide scans have been used in the past for the diagnosis of BCI, but none were sufficiently sensitive or specific to reliably establish the diagnosis of BCI and have been abandoned.103
Spectrum of Blunt Cardiac Injury
Clinically, BCI can be divided into two types: acute and subacute. The acute type is usually the catastrophic injury that causes death immediately or rapidly if surgical intervention is not instituted, including cardiac chamber rupture with acute pericardial tamponade, combined chamber and pericardial rupture with hemorrhage into the pleural cavity, and acute myocardial injury with cardiogenic shock. Subacute cardiac injury may not lead to immediate death, but does impact cardiac hemodynamics, while placing the patient at risk for the development of significant arrhythmias and hemodynamic complications, and includes myocardial contusion, subacute pericardial tamponade, myocardial infarction, valvular injury, intracardiac shunts, mural thrombi, and of course, arrhythmias.
Valvular, Papillary Muscle/Chordae Tendineae, and Septal Injury
Septal ruptures are also uncommon. Hewett109 in 1847 first described the rupture of the intraventricular septum caused by blunt trauma. Bright and Beck110 in 1935 described 11 patients with septal rupture in a series of 152 patients who sustained fatal cardiac injury.
Blunt Coronary Artery Injury
Blunt coronary artery injuries are extremely rare. Direct impacts may cause acute coronary thrombosis, and may result in intimal disruption caused by significant application of blunt energy to the chest. Blunt coronary artery injuries are usually associated with severe myocardial contusions, generally along the distribution of the left anterior descending coronary artery (LAD). The clinical presentation of these patients cannot be distinguished from acute myocardial infarction. Long-term sequelae of these injuries may lead to the development of a ventricular aneurysm with its potential complications such as rupture, ventricular failure, and production of emboli or malignant arrhythmias.102
Cardiac Chamber Rupture
Türk and Tsokos111 reported that blunt atrial injuries are more common than ventricular injuries. The most frequently injured cardiac chambers are the right atrium followed by the right ventricle. Left-sided chamber injuries occur with a smaller frequency. Several patients have been reported with multiple chamber injuries; however, none survived.
Myocardial Contusion
Out of all the BCIs, the least important and more difficult to define is myocardial contusion/concussion. The definition of myocardial contusion has evolved over several decades of discussion among trauma surgeons. This diagnosis is more often established out of proportion to its incidence, severity, and clinical relevance. Mattox et al.112 suggested that the terms of myocardial contusion and concussion be eliminated in favor of a more reasonable definition for this entity, and proposed that they be defined as BCI either with cardiac failure, with the presence of complex arrhythmia, and those with minor EKG or enzyme abnormality. On this basis, it is recommended that asymptomatic patients with anterior chest wall injuries should not be admitted to a surgical intensive care unit for continuous EKG monitoring, serial determination of CPK-MB enzyme levels, or further cardiac imaging.
Civetta and colleagues113 concluded that significant cardiac events are uncommon in young patients with chest trauma, and pointed out that initial EKG abnormalities are better indicators of cardiac complications in critically injured patients.
After a thorough review of the literature, Pasquale et al.114 identified well-conducted primary studies or reviews involving the identification of BCI. On the basis of this literature review, the Eastern Association for the Surgery of Trauma (EAST) generated the following three recommendations:
An admission EKG should be performed for all patients in whom there is suspected BCI.
CONCLUSIONS
Cardiac injuries continue to be both challenging and fascinating entities. Only with serious scientific inquiry based on prospective collection and analysis of data can we extend the frontiers in the management of these critical injuries (Figure 7).
1 Homer, Lang, Leaf, Myers. The Iliad. XIII. New York: McMillan & Co; 1922:259. line 442
2 Breasted JH. The Edwin Smith Papyrus. Chicago: University of Chicago Press; 1930;1.
3 Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227.
4 Hippocrates, Adams Francis, editor. The Genuine Works of Hippocrates. 2. New York: William Wood and Co; 1886:252. Sec. 6, aphorism 18
5 Pare A, Johnson T, editor. The works of that Famous Chirurgion Ambroise Pare. London: Gates & Co, 1634. Beck CS. Wounds of the heart: the technique of suture. Arch Surg. 1926;13:205-227. (As cited by
6 Pare A, Keynes G, editor. The Apologia and Treatise of Ambroise Pare (Concerning the Voyages Made into Divers Places with Many of His Writings on Surgery). Chicago: University of Chicago Press, 1952.
7 Wolf I. Cited by Fischer G. Die Wunden des Herzens und des Herzbentels. Arch Klin Chir. 1868;9:571.
8 Senac JB, Traite de la structure du Coeur, de son action, et de ses maladies. Paris: Breasson; 1749;2. Beck CS. Wounds of the heart: the technique of suture. Arch Surg. 1926;13:205-227. (As cited by
9 Larrey DJ. Bull Sci Med. 1810;6:284.
10 Larrey DJ. Chirurgie. 1829;2:303.
11 Billroth T, Jeger E, editor. Die Chirurgie der Blutgefasse und des Herzens. Berlin: A Hirschwald; 1913:295. Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
12 Billroth T, Richardson RG, editor. The Scalpel and the Heart. New York: Scribner; 1970:27. Cited by
13 Billroth T. Offenes Schreibner and Herr der Wittelshofer uver die erste mil gustingen susgange ausgefuhrte pylorectomie. Wiener Med Wochensch. 1881;31:161.
14 Block MH Verhandlunge der Deutchsen Gesselhoff fur Chirurgie. Elfren Congress, Berlin, 1882, part I, p. 108.Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
15 Del Vecchio S, Sutura del cuore. Napoli: Reforma Med; 1985:xi-xii. 38 Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
16 Cappelen A Vinia cordis, suture of hjertet. Norsk, Mag. F. Laegy; Kristiania, 4, R; xi, 285, 1896.Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
17 Farina G, Discussion. Centralbl Chir. 1896;23:1224. Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
18 Rehn L. Ueber penetrerende herzwunden und herznaht. Arch Klin Chir. 1897;55:315.
19 Hill IX, A report of case of successful suturing of the heart, and table of 37 other cases of suturing by different operators with various terminations and conclusions drawn. Med Record. 1902;62:846. Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
20 Duval P, Le incision median thoraco-laparotomie. Bull Mem Soc Chir Paris. 1907;33:15. Ballana C. Bradshaw Lecture. The surgery of the heart. Lancet. 1920;CXCVIII:73-79. (As cited by
21 Spangaro S, Sulla tecnica da seguire negli interventi chirurgici per ferrite del cuore e su di un nuovo processo di toracotomia. Clin Chir. 1906;14:227. Beck CS. Wounds of the heart. The technique of suture. Arch Surg. 1926;13:205-227. (As cited by
22 Peck CH. The operative treatment of heart wounds. Ann Surg. 1909;50:100-134.
23 Smith WR. Cardiorraphy in acute injuries. Ann Surg. 1923;78:696-710.
24 Beck C. Further observations on stab wounds of the heart. Ann Surg. 1942;115:698-704.
25 Griswold A, Maguire CH. Penetrating wounds of the heart and pericardium. Surg Gynecol Obstet. 1942;74:406-418.
26 Elkin DC. The diagnosis and treatment of cardiac trauma. Ann Surg. 1941;114:169.
27 Elkin DC. Wounds of the heart. Ann Surg. 1941;120:817-821.
28 Elkin DC, Campbell RE. Cardiac tamponade: treatment by aspiration. Ann Surg. 1941:623-630.
29 Beall AC, Dietrich EB, Crawford HW. Surgical management of penetrating cardiac injuries. Am J Surg. 1966;112:686-692.
30 Beall AC, Gasior RM, Bricker DL. Gunshot wounds of the heart. Changing patterns of surgical management. Ann Thorac Surg. 1971;11:523-531.
31 Beall AC, Morris GC, Cooley DA. Temporary cardiopulmonary bypass in the management of penetrating wounds of the heart. Surgery. 1962;52:330-337.
32 Beall AC, Oschner JL, Morris GC, et al. Penetrating wounds of the heart. J Trauma. 1961;1:195-207.
33 Mattox KL, Espada R, Beall AC, et al. Performing thoracotomy in the emergency center. J Am Coll Emerg Phys. 1974;3:13-17.
34 Mattox KL, Beall AC, Jordan GL, et al. Cardiorraphy in the emergency center. J Am Coll Emerg Phys. 1974;68:886-895.
35 Mattox KL, Limacher MC, Feliciano DR, et al. Cardiac evaluation following heart injury. J Trauma. 1985;25:758-765.
36 Feliciano DV, Bitondo CG, Mattox KL, et al. Civilian trauma in the 1980’s. A 1-year experience with 456 vascular and cardiac injuries. Ann Surg. 1984;199:717-724.
37 Mattox KL, Feliciano DV, Burch J, Beall ACJr, et al. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg. 1989;210:698-707.
38 Asensio JA, Murray J, Demetriades D, et al. Penetrating cardiac injuries: Prospective one year preliminary report; an analysis of variables predicting outcome. J Am Coll Surg. 1998;186:24-33.
39 Asensio JA, Berne JD, Demetriades D, et al. One hundred and five penetrating cardiac injuries. A two year prospective evaluation. J Trauma. 1998;44:1073-1083.
40 Asensio JA, Garcia-Núñez LM, Healy M, Petrone P, Lavery R: Penetrating cardiac injuries in America—Predictors of outcome in 2016 patients from the National Trauma Data Bank. Abstract submitted to the American Association for the Surgery of Trauma Annual Meeting, 2006.
41 Asensio JA, Stewart BM, Murray JA, Fox AH, et al. Penetrating cardiac injuries. Surg Clin North Am. 1996;76:685-724.
42 Rich NM, Spencer FC. Wounds of the heart. In: Rich NM, Spencer FC, editors. Vascular Trauma. Philadelphia: WB Saunders; 1978:384-424.
43 Borchdart E, Sammlung Klin. Leipzig: Vortrage; 1906. Peck CH. The operative treatment of heart wounds. Ann Surg. 1909;50:100-134. (As cited by
44 Asensio JA, Petrone P, Costa D, et al. An evidence-based critical appraisal of emergency department thoracotomy. Evidence-Based Surg. 2003;1:11-21.
45 Moreno C, Moore EE, Majure JA, et al. Pericardial tamponade. A critical determinant for survival following penetrating cardiac wounds. J Trauma. 1986;26:821-825.
46 Rozycki GS, Feliciano DV, Schmidt JA, et al. The role of surgeon-performed ultrasound in patients with possible penetrating cardiac wounds. Ann Surg. 1996;223:737-746.
47 Rozycki GS, Feliciano DV, Oschner MG, et al. The role of ultrasound in patients with possible penetrating cardiac wounds: a prospective multi-center study. J Trauma. 1999;46:543-552.
48 Morales CH, Salinas CM, Henao CA, et al. Thoracoscopic pericardial window and penetrating cardiac trauma. J Trauma. 1997;42:273-275.
49 Gervin AS, Fischer RP. The importance of prompt transport in salvage of patients with penetrating heart wounds. J Trauma. 1982;22:443-448.
50 Durham LA3rd, Richardson RJ, Wall MJJr, et al. Emergency center thoracotomy: impact of prehospital resuscitation. J Trauma. 1992;32:775-779.
51 Ivatury RR, Nallathambi MN, Roberge RJ, et al. Penetrating thoracic injuries: in-field stabilization vs. prompt transport. J Trauma. 1987;27:1066-1073.
52 Millham V, Grindlinger G. Survival determinants in patients undergoing emergency room thoracotomy for penetrating chest injury. J Trauma. 1993;34:332-336.
53 American College of Surgeons, Committee on Trauma (ACS-COT). Advanced Trauma Life Support Manual, 7th ed. Chicago: American College of Surgeons, 2005.
54 Macho JR, Markinson RE, Schecter WP. Cardiac stapling in the management of penetrating injuries of the heart: rapid control of hemorrhage and decreased risk of personal contamination. J Trauma. 1993;34:711-716.
55 Working Group, Ad Hoc Subcommittee on Outcomes, American College of Surgeons, Committee on Trauma. practice management guidelines for emergency department thoracotomy. J Am Coll Surg. 2001;193:303-309.
56 Boyd TF, Strieder JW. Immediate surgery for traumatic heart disease. J Thorac Cardiovasc Surg. 1965;50:305-315.
57 Sauer PE, Murdock CE. Immediate surgery for cardiac and great vessel wounds. Arch Surg. 1967;95:7-11.
58 Sugg WL, Rea WJ, Ecker RR, et al. Penetrating wounds of the heart. J Thorac Cardiovasc Surg. 1968;56:530-545.
59 Yao ST, Vanecko RM, Printen K, Shoemaker WC. Penetrating wounds of the heart: a review of 80 cases. Ann Surg. 1968;168:67-78.
60 Steichen FM, Dargan EL, Efron G, et al. A graded approach to the management of penetrating wounds of the heart. Arch Surg. 1971;103:574-580.
61 Borja AR, Randsell HT. Treatment of penetrating gunshot wounds of the chest. Am J Surg. 1971;122:81-84.
62 Carrasquilla C, Wilson RF, Walt AJ, et al. Gunshot wounds of the heart. Ann Thorac Surg. 1972;13:208-213.
63 Beall AC, Patrick TD, Ikles JE, et al. Penetrating wounds of the heart: changing patterns of surgical management. J Trauma. 1972;12:468-473.
64 Bolanowski PS, Swaminathan AP, Neville WE. Aggressive surgical management of penetrating cardiac injuries. J Thorac Cardiovasc Surg. 1973;66:52-57.
65 Trinkle JK, Marcos J, Grover FL, et al. Management of the wounded heart. Ann Thorac Surg. 1974;17:231-236.
66 Harvey JC, Pacifico AD. Primary operative management method of choice for stab wound to the heart. South Med J. 1975;68:149-152.
67 Symbas PN, Harlaftis N, Waldo WJ. Penetrating cardiac wounds: a comparison of different therapeutic methods. Ann Surg. 1976;183:377-381.
68 Beach PM, Bognolo D, Hutchinson JE. Penetrating cardiac trauma. Experience with thirty four patients in a hospital without cardiopulmonary bypass capability. Am J Surg. 1976;131:411-415.
69 Asfaw I, Arbulu A. Penetrating wounds of the pericardium and heart. Surg Clin North Am. 1977;57:37-49.
70 Sherman MM, Saini UK, Yardoz MD, et al. Management of penetrating cardiac wounds. Am J Surg. 1978;135:553-558.
71 Trinkle JK, Toon R, Franz JL, et al. Affairs of the wounded heart: penetrating cardiac wounds. J Trauma. 1978;19:467-472.
72 Evans J, Gray LA, Payner A, et al. Principles for the management of penetrating cardiac wounds. Ann Surg. 1979;189:777-784.
73 Breaux EP, Dupont JB, Albert HM, et al. Cardiac tamponade following penetrating mediastinal injuries: improved survival with early pericardiocentesis. J Trauma. 1979;19:461-466.
74 Mandal AK, Awariefe SO, Oparah SS. Experience in the management of 50 consecutive penetrating wounds of the heart. Br J Surg. 1979;66:565-568.
75 Demetriades D, Vander Veen BW. Penetrating injuries of the heart: experience over two years in South Africa. J Trauma. 1983;23:1034-1041.
76 Demetriades D. Cardiac penetrating injuries: personal experience of 45 cases. Br J Surg. 1984;71:95-97.
77 Tavares S, Hankins JR, Moulton AL, et al. Management of penetrating cardiac injuries: the role of emergency thoracotomy. Ann Thorac Surg. 1984;38:183-187.
78 Demetriades D. Cardiac wounds. Experience with 70 patients. Ann Surg. 1986;203:315-317.
79 Jebara VA, Saade B. Penetrating wounds to the heart: a wartime experience. Ann Thorac Surg. 1989;47:250-253.
80 Attar S, Suter CM, Hankins JR, et al. Penetrating cardiac injuries. Ann Thorac Surg. 1991;51:711-716.
81 Knott-Craig CJ, Dalton RP, Rossouw GJ, et al. Penetrating cardiac trauma: management strategy based on 129 surgical emergencies over 2 years. Ann Thorac Surg. 1992;53:1006-1009.
82 Buchman TG, Phillips J, Menker JB. Recognition, resuscitation and management of patients with penetrating cardiac injuries. Surg Gynecol Obstet. 1992;174:205-210.
83 Benyan AK Z, Al-A’Ragy HH. The pattern of penetrating cardiac trauma on Basrah province: personal experience with seventy-two cases in a hospital without cardiopulmonary bypass facility. Int Surg. 1992;77:111-113.
84 Mitchell ME, Muakkassa EE, Poole GV, et al. Surgical approach of choice for penetrating cardiac wounds. J Trauma. 1993;34:17-20.
85 Kaplan AJ, Norcross ED, Crawford FA. Predictors of mortality in penetrating cardiac injury. Am Surg. 1993;59:338-342.
86 Henderson VJ, Smith SR, Fry WR, et al. Cardiac injuries: analysis of an unselected series of 251 cases. J Trauma. 1994;36:341-348.
87 Coimbra R, Pinto MC C, Razuk A, et al. Penetrating cardiac wounds: predictive value of trauma indices and the necessity of terminology standardization. Am Surg. 1995;61:448-452.
88 Arreola-Risa C, Rhee P, Boyle EM, et al. Factors influencing outcome in stab wounds of the heart. Am J Surg. 1995;169:553-556.
89 Karmy-Jones R, Van Wijngaarden MH, Talwar MK, et al. Penetrating cardiac injuries. Injury. 1997;28:57-61.
90 Rhee PM, Foy H, Kaufman C, et al. Penetrating cardiac injuries: a population based study. J Trauma. 1998;45:366-370.
91 Tyburski JG, Astra L, Wilson RF, et al. Factors affecting prognosis with penetrating wounds of the heart. J Trauma. 2000;48:587-591.
92 Asensio JA, Hanpeter D, Demetriades D, et al: The futility of the liberal utilization of emergency department thoracotomy. A prospective study. In Proceedings of the American Association for the Surgery of Trauma 58th Annual Meeting, Baltimore, MD, September 1998, p. 210.
93 Ivatury RR, Rohman M, Steichen FM, et al. Penetrating cardiac injuries: twenty-year experience. Am Surg. 1987;53:310-317.
94 Buckman RF, Badellino MM, Mauro LH, et al. Penetrating cardiac wounds: prospective study of factors influencing initial resuscitation. J Trauma. 1993;34:717-727.
95 Wall MJJr, Mattox KL, Chen C, Baldwin JC. Acute management of complex cardiac injuries. J Trauma. 1997;42:905-912.
96 Moore EE, Malangoni MA, Cogbill TH, et al. Organ injury scaling IV: thoracic, vascular, lung, cardiac and diaphragm. J Trauma. 1994;36:299-300.
97 Akenside M. An account of a blow upon the heart and its effects. Philosophical Transactions. 1764:353.
98 Osborn LR. Findings in 262 fatal accidents. Lancet. 1943;2:277.
99 Urbach J. Die Verletzungen des Herzens durch stumple Gewalt. Beiir Ger Med. 1940;4:653.
100 Warburg E. Traumatic Heart Lesions. London: Humphrey Milford, Oxford University Press, 1938.
101 Kissane RW. Traumatic heart disease: non-penetrating injuries. Circulation. 1952;6:421-425.
102 Parmley LF, Manion WC, Mattingly TW. Non penetrating traumatic injury of the heart. Circulation. 1958;18:371-396.
103 Newman PG, Feliciano DV. Blunt cardiac injury. New Horiz. 1999;7:26-34.
104 Fulda GJ, Giberson F, Hailstone D, et al. An evaluation of serum troponin T and signal averaged electrocardiography in predicting electrocardiographic abnormalities after blunt chest trauma. J Trauma. 1997;43:304-312.
105 Salim A, Velmahos GC, Jindal A, et al. Clinically significant blunt cardiac trauma. Role of serum troponin levels combined with electrocardiographic findings. J Trauma. 2001;50:237-243.
106 Biffl WL, Moore FA, Moore EE, et al. Cardiac enzymes are irrelevant in the patient with suspected myocardial contusion. Am J Surg. 1994;169:523-528.
107 Hiatt JR, Yeatman LAJr, Child JS. The value of echocardiography in blunt chest trauma. J Trauma. 1988;28:914-922.
108 King MR, Mucha PJr, Seward JB, et al. Cardiac contusion: a new diagnostic approach utilizing two-dimensional echocardiography. J Trauma. 1983;23:610-614.
109 Hewett P. Rupture of the heart and large vessels: the result of injuries. Lond Med Gaz. 1847;1:870.
110 Bright EF, Beck CS. Non-penetrating wounds of the heart: a clinical and experimental study. Am Heart J. 1935;10:293.
111 Türk EE, Tsokos M. Blunt cardiac trauma caused by fatal falls from height: an autopsy-based assessment of the injury pattern. J Trauma. 2004;57:301-304.
112 Mattox KL, Flint LM, Carrico CJ, et al. Blunt cardiac injury (editorial). J Trauma. 1992;33:649-650.
113 Civetta J. The clinical significance of myocardial contusion (discussion of Cachecho R, Grindlinger GA, Lee VW). J Trauma. 1992;38:68-73.
114 Pasquale M, Fabian TC. Practice management guidelines for trauma from the Eastern Association for the Surgery of Trauma. J Trauma. 1998;44:941-957.