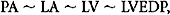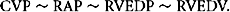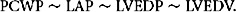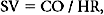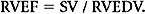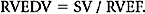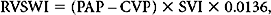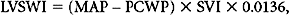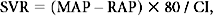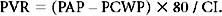CHAPTER 82 CARDIAC HEMODYNAMICS: THE PULMONARY ARTERY CATHETER AND THE MEANING OF ITS READINGS
The pulmonary artery catheter (PAC) is a physical object creating a conundrum. Since its introduction in the 1970s, the PAC has been simultaneously hailed for its ability to provide physiological data not easily obtainable by other means, and condemned as a useless and potentially harmful invasive monitor. Very little hard data support continued use of the PAC, and some data support avoiding it altogether. Despite considerable controversy over the clinical utility and safety of the PAC, which has intermittently led to confusion and conjecture regarding its use and future, the PAC remains a mainstay of invasive intensive care unit (ICU) monitoring. In this chapter, we aim to provide a concise history of right-heart catheterization. We will then examine the basis of insertion, data collection, interpretation, and troubleshooting. Finally, we will reexamine the clinical data for and against the use of PA catheterization and determination of resuscitation in critically ill surgical patients.
HISTORY OF CONTROVERSY
The PAC was introduced in the late 1960s, approved for clinical use in 1970 and quickly became the de facto tool of the critical care physician. By 1999, 1.5 million catheters were sold, and presumably used, each year in the United States. Due to its introduction prior to a 1976 policy change, which mandated the testing of medical devices, the PAC was grandfathered by the Food and Drug Administration (FDA), and has to date never been required to undergo safety testing. Even as considerable controversy and political debate over its utility continues, the catheter has never been considered a life-saving device and, as a result, is exempt from licensing and required study.1
Since its invention, the PAC has enjoyed growing use and acceptance as a monitoring tool. Indeed, its popularity has followed closely the advent of critical care as a specialty, and thus is considered by many a primary tool of the critical care physician. After years of use with little data supporting benefits, concerns about the overall utility and safety of the PAC first appeared in several papers written in the late 1980s.1 Gore et al. examined 3000 patients with acute myocardial infarction (MI) and the relationship of outcome to PA catheterization.2 This study of over 3000 patients with acute MI reported higher mortality in patients with hypotension who received a PAC (42% vs. 32%). Higher mortality was also reported in the subgroup or patients with CHF who received a PAC (44% vs. 25%). In addition, patients who received a PAC had longer hospital stay. Several observational and retrospective studies quickly followed with similar concerning results.3 Many in the critical care community discounted these trials, believing that PAC placement was more common in patients with greater illness, which would explain the higher mortality rates. A 1990 Canadian trial was the first to attempt to prospectively study the use of PAC in critically ill patients. In what was to become a recurring theme, however, the study failed due to a 35% exclusion rate as many clinicians refused to randomize their patients, arguing instead that it was unethical not to use a PAC (which had become the de facto tool of the ICU physician). A lack of clinical agreement regarding PAC use or perhaps just a lack of interest in the problem followed, and the PAC enjoyed continued widespread use until the debate was reignited by Connors et al., who studied PAC use in 5735 critically ill ICU patients. The Connors group was careful to attempt to match illness severity and other confounding variables between the PAC and control groups. Ultimately, his group found that patients treated with PAC had increased 30-day mortality, mean cost, and ICU stay. Subgroup analysis identified no groups of patients who benefited from PAC.4
As a result of these and similar data, in the same issue of the Journal of the American Medical Association, Bone and Drhen called for a National Heart, Lung, and Blood Institute (NHLBI) randomized prospective clinical trail to test the efficacy and safety of the PAC. They went on to spark considerable controversy by suggesting that the FDA issue a moratorium on the use of the pulmonary catheter until such time that the safety and use be measured in an appropriate clinical trial. In response to this call for a moratorium, both a National Heart, Lung, and Blood Institute (NHLBI) and a Society of Critical Care Medicine (SCCM) consensus conference were convened in 1997. The Pulmonary Artery Catheter Consensus Conference Consensus Statement was published that same year. According to the statement, there was no need of a moratorium on PACs, given adequate level-IV evidence to support the possibility of benefit of PAC in patient groups including MI and trauma, but conceded that appropriate clinical trials should be undertaken to measure its use and safety.5,6 The NHLBI conference came to similar findings.7 Many trials followed, all with limited numbers of patients or low randomization rates.
In 2003, a Canadian group published the first prospective randomized study with sufficient patient enrollment to have statistical power and authority.8 In this study, 1994 patients were randomized to surgery without a pulmonary catheter versus with a PAC. The authors found that there were no differences in hospital survival, and in 6- and 12-month survival. There was however, an increase in the number of pulmonary embolism (PE) events with eight reported in the catheter group versus 0 in the observation group. The authors concluded that no benefit from PA catheterization could be found in elderly high-risk surgical patients. While this study was important in that it was the first to randomize a significant cohort of patients, the randomization rate remained a low 52%. Furthermore, it represented a subset of older critically ill patients, while excluding younger trauma or septic patients. Other trials have followed and shown mixed results.9–12 More recently Shah et al. performed a meta-analysis of 13 randomized clinical trials between 1985 and 2005.13 This study totaling 5051 patients was performed using a random effects model to estimate the odds ratio for death, hospital days, and pressors and found no difference between patients with and without a PAC.
No randomized prospective trial to date, however, has shown a definite benefit to PA catheterization in critically injured patients. In data limited to trauma patients, there is some recent evidence to show a benefit for PAC use in the injured. A database study of over 53,000 patients drawn from the National Trauma Data Bank showed a reduction in mortality in older patients and patients with higher injury severity scores. Overall, PAC use was shown to be beneficial in patients with severe shock with a base deficit of 11 or more, injury severity scores higher than 25, and age over 61. This large cohort database study is the first and only study to show a clear benefit from PAC use in the severely injured patient.14
One bias confounding PAC use and study are disparate factors that affect which patients are treated with the PAC. In 2000, Rapoport et al. reported a comprehensive look at the characteristics of PAC use.15 This group retrospectively examined 10,217 patients in 34 ICUs in the United States and showed that full-time ICU staffing was associated with a decreased likelihood of PAC use. Catheter use was associated with white race/ethnicity and private insurance. Patients admitted to a surgical ICU were two times more likely to have a PAC. This study is revealing in that it is indicative of the lack of an established protocol and the presence of an established bias in PAC placement.
PULMONARY ARTERY CATHETER USE AND INSERTION: WHAT IT IS AND HOW IT WORKS
Insertion Tips and Guidelines
Insertion of the catheter is done through the gasketed introducer port of a Cordis™ catheter. Full sterile technique is paramount in order to reduce infection rates. We commonly place the introducer catheter and the PAC sequentially with one sterile prep and setup, which prevents contamination at the introducer gasket as well as the necessity of reprepping of a previously placed introducer. At times, however, this cannot be avoided, or a PAC will be placed through a previously located introducer line. In this instance, the introducer catheter and surrounding skin should be prepped widely with chlorohexidine preparation. In all cases, wide sterile prep with chlorohexidine, full sterile precautions including gown, hat, mask, and sterile gloves are essential. Any break in sterile technique has been shown in many studies to significantly increase the infection rate and implementation of the above precautions has been reported to reduce the infection rate to near zero.16–21 Another tip is to prep widely. We cannot stress this seemingly trivial point too strongly. Opening, preparing, and inserting a PAC results in an often-unwieldy octopus of catheter, tubing, and transducers that have to cross through a sterile to unsterile transition zone. Wide prep of all or most of the bed with a sterile half-sheet facilitates easy handling and reduced risk of contamination. Once an introducer catheter is in place, the PAC is removed from the packaging and the proximal end is passed to an assistant who will connect and flush the catheter ports. At this time, the transducer is connected, zeroed, and tested. The balloon is also tested. Placement of the catheter sheath allows future adjustments without additional sterile prep.
Often several attempts to ensure proper wedging are required. When a RV waveform is not evident after 35–45 cm or no wedge tracing occurs after the catheter has advanced 10–15 cm past the RV tracing, the balloon should be deflated, and the catheter pulled back to the RA or RV. Once a distinct tracing identifies the catheter location, another careful attempt at reinsertion and wedging can begin. When the catheter is inserted and wedged, the pulmonary capillary wedge pressure (PCWP) is noted and the balloon deflated. The catheter is locked into place and the sterile covering advanced to cover the full length of the catheter. Placement of the catheter is confirmed by a chest x-ray.
INTERPRETATION: WHAT DOES IT MEASURE AND WHAT DOES IT MEAN?
Initial Warnings and Potential Measurement Problems
While the PAC provides a lot of otherwise unobtainable data, only diligent attention and educated interpretation make PA catheterization worth the risk, time, and cost. Without proper placement, all obtained data are erroneous and will lead to false interpretation and incorrect (and possibly harmful) intervention. Careful attention to the PAC waveform helps confirm catheter placement. Entry into the pulmonary artery is evidenced by a decrease in mean pressure and characteristic wave form. Upon entering the PA, a triphasic waveform is seen, which reflects atrial and ventricular contraction. From the PA careful advancement of the catheter will “wedge” the catheter.22
where LVEDP is left ventricle end-diastolic pressure. It is crucial to note that this relationship holds true only when there is a contiguous fluid column between the PA and LV. A common reason for improper interpretation of PAOP occurs when this contiguous fluid column is interrupted or affected. Examples of interrupted fluid column include mitral valve disease (stenosis or insufficiency) and pulmonary venous obstruction. Also affecting the relationship between PAOP and left ventricular end-diastolic volume (LVEDV) is the ability to extrapolate volume from pressure measurements. Since PCWP is ultimately used as a measure of ventricular filling (volume), this necessitates the ability to predictably extrapolate volume from pressure, which is only possible with normal compliance. Indeed, LVEDP is analogous to left ventricular end-diastolic volume (LVEDV) only in a normally compliant heart and thorax. Many factors influence the ability to use PCWP as an accurate measure of left heart–filling pressures. As described previously, the analogous relationship between LVEDP and LVEDV is accurate only insofar as the ventricle is normally compliant. In cases where left heart compliance is reduced such as ventricular hypertrophy or ischemic myocardial damage, the PCWP will be elevated for a corresponding left ventricular pressure/volume and cannot be considered an accurate measure. While trend analysis can be used to monitor fluid resuscitation in these patients, injured or septic patients often manifest rapidly changing cardiac compliance making interpretation difficult and suspect. Indeed multiple animal models have shown a rapid flux of cardiac edema resulting from injury, resuscitation, and inflammation, all of which can rapidly change ventricular compliance and render the PAC an unreliable measure of cardiac filling.22
Positive end-expiratory pressure (PEEP) also affects the PAC tracing, and interpretation of the PCWP in a patient affected by intrinsic or intrinsic PEEP is the subject of much conjecture and misinformation. PEEP (both intrinsic and extrinsic) causes transmural thoracic pressure, which positively effects PAWP measurements. To correctly determine the PAWP, the PEEP can be temporally disconnected (for a few seconds or breath cycles). Alternatively, the effect of intrinsic or extrinsic PEEP can be estimated by adding 2–3 cm H2O to each 5 cm of PEEP >10.22
Pressure, Volume, and Work Measures
Once CO is known, stroke volume (SV) can be calculated:
where HR is heart rate.
Right-sided ejection fraction (RVEF) is then calculated as follows:
where PAP is pulmonary artery pressure, and SVI is stroke volume index.
where LVSWI is left ventricle stroke work index, and MAP is mean arterial pressure.
where RAP is renal arterial pressure.
Goal-Directed Therapy Using Pulmonary Artery Catheter
There is little disagreement regarding the large amount of data provided by a properly placed and interpreted PA catheter. Whether the data provided result in better patient outcome is the source of considerable debate. Much clinical research has been done in an attempt to determine which PAC-derived physiologic parameters should be measured and which are predictive of outcome. Shoemaker and his group provided the initial work toward defining and grouping patient physiologic response to trauma.23 As early as 1973 Shoemaker and colleagues showed in several studies that PAWP measured reduced cardiac output. They further showed that decreased oxygen delivery along with increased peripheral vascular resistance characterized non-survivors after trauma.24 Based on these and other similar study results, many groups targeted resuscitation of severely injured trauma patients to achieve physiologic values retrospectively associated with survival. Ten years after his initial studies, Shoemaker and colleagues showed an overall survival benefit in patients who achieved normal physiologic values.25,26 Finally, this group showed that there was less oxygen debt as measured by VO2 in surviving patients.27 Interestingly, there was no difference in oxygen consumption between the groups. In similar research, Bishop et al. examined physiologic parameters in 90 trauma patients and found that patients who achieved higher levels of CI, oxygen delivery, and oxygen consumption within the first 24 hours after admission had a lower incidence of ARDS and reduced overall mortality.28 Based on these and similar findings, several groups then randomized patients to supernormal resuscitation versus traditional resuscitation with the intent of proving that invasive monitoring of aggressive resuscitation would allow achievement of supranormal physiology and better outcome. These studies showed mixed results. Bishop’s group randomized patients to be supranormally resuscitated to a cardiac index of more than 4.5, DO2I greater than 670, and a VO2I greater than 166, versus traditional resuscitation as defined by achievement of normal urine output and CVP.29 The supranormally resuscitated group showed significantly lower mortality and decreased organ failure. Interestingly, optimal parameters were reached in 70% of the study group and 29% of the control group. Similarly, Flemming et al. randomized trauma patients with blood loss of more than 2000 cc to supernormal CI, DO2, and VO2, and found that attainment of these goals resulted in statistically significant decreases in organ failure, ICU stay, and ventilator days, and a trend toward decreased mortality.30
Other studies, however, pointed out that attainment of resuscitation was more important than the means by which it was achieved and many more recent studies have been unable to show similar benefit to monitoring or goal-directed resuscitation. Velmahos et al. randomized severely injured patients to supranormal resuscitation versus traditional hemodynamic monitoring. While there was no difference in mortality, organ failure, sepsis, ICU days, or hospital stay between the two groups, they found that 70% of the supranormal group and 40% of the traditional group achieved supranormal physiologic parameters, and that attainment of these optimal values was associated with better outcome independent of treatment protocol. There were no outcome differences between the two groups. What was perhaps most interesting in this study, however, was the finding that nonresponders (those who failed to reach the set goals despite volume and ionotropic manipulation) fared significantly worse than the control group. This suggests the likelihood that aggressive resuscitation toward supranormal physiology will be harmful in those whose physiology cannot attain it, and is in fact exhausted such attempts. Other groups have reported similar results, which indicate that that the ability to achieve normal parameters is associated with the mortality benefit, and is ultimately more important than whatever treatment was designed to achieve them.31 Taken together, these results cast doubt regarding the usefulness of interventional monitoring and resuscitation. Indeed, many believe that aggressive resuscitation should be instituted in all patients without any need for invasive monitoring. Others argue for limited supraphysiologic resuscitation. Some data points to poorer outcome from PAC use. Indeed, deleterious consequences of aggressive resuscitation and the PAC have been reported. Hayes et al. reported that increasing oxygen delivery with ionotropic augmentation with dobutamine was associated with increased mortality.32 Others have reported similar increased mortality in supranormally resuscitated patients. At least one group has purported to show that the PAC is itself a predictor of morbidity independent of protocol-driven resuscitation. Rhodes et al. performed a prospective randomized trial of 200 patients where no protocol-driven resuscitation was instituted.33 In this study, patients were randomized to PAC placement versus no PAC where patients were resuscitated based up the clinical judgment of the ICU staff. Data were obtained prospectively and examined retrospectively, and revealed that the PAC group received significantly more fluid in the first 24 hours and exhibited increased morbidity. Evidence for the deleterious effects of PAC protocol–driven resuscitation were seen in a study by Hayes et al. that showed an increased mortality in PAC-treated patients (54% vs. 34%).32 In this study there was a rigorous goal-directed attempt to achieve supranormal physiologic parameters. To achieve these goals, aggressive fluid resuscitation and ionotropic support were used.
In an attempt to make sense of the huge amount of data regarding PACs and endpoints of resuscitation, Kern and Shoemaker performed a meta-analysis on 21 randomized clinical trials and found that early hemodynamic optimization achieved significant mortality reduction only if optimized parameters were achieved prior to the onset of sepsis or organ dysfunction.34 Heyland and associates analyzed seven studies and found no significant reductions in mortality achieved with optomization.35 Lastly, an analysis by Poeze et al. showed that physiologic optimization resulted in decreased mortality. In this analysis the entire benefit was from patients who hemodynamically optimized perioperatively. Patients with established sepsis or organ failure prior to treatment were unlikely to benefit from “optimization.”36
Others have questioned the accuracy of the actual PAC measurements. Epstein et al. studied the accuracy of VO2 measurements by the calculated reverse Fick method versus direct measurement by indirect calorimetry in an attempt to determine the accuracy of PAC determined oxygen consumption measures. Their study showed a bias of 41 ml/min/m2, indicative of an undermeasurement of VO2 by PAC measures.37 Finally, Luchette and colleagues studied the accuracy of continuous cardiac output measurements and found that there was higher signal-to-noise ratio and degraded accuracy when patient temperature was above 38.5° C, resulting in further questioning of the accuracy and usefulness of PAC measurements in severely injured or septic patients.38
Right Ventricle End-Diastolic Pressure as Measure of Cardiac Index and Cardiac Function
As predicted by the Starling curve, end-diastolic volume is the best predictor of preload. Unfortunately, PAC pressure measurements as discussed previously are confounded in the setting of changing intrinsic ventricular compliance and extrinsic PEEP. RVEDV monitoring provides the first actual measurement of preload. Rather than being a pressure surrogate for volume, RVEDV utilizes a PAC with a fast thermistor to give a directly measured volume measure. In an attempt to find an alternative to PAOP as a measure of cardiac function, Chetham et al. showed that CI correlated better with RVEDVI than PAOP.39 The same group then studied 64 patients with respiratory failure and high PEEP requirements, and suggested that RVEDVI was a better measure of CI than PAOP at all levels of PEEP. CI was inversely correlated with PCWP at PEEP levels over 15. Taken together, these findings caused this group to conclude that RVEDVI should be used in lieu of PAOP as a measure of cardiac function.
Despite initial reservations that mathematical coupling was responsible for the close correlation between RVEDV and CI, several groups have shown that the directly measured volume measures are superior to extrapolations from pressure (CVP and PAWP) measures. Chang and colleagues showed that splanchnic malperfusion was better predicted by RVEDV than PCWP, which was in turn associated with MODS and mortality.40 Other correlations among RVEDV, tissue perfusion, and outcome have been similarly reported by several groups.42 Lastly Kincaid et al.41 presented data to show that optimal RVEDV could be calculated for each individual patient. Taken together, these data support the use of RVEDV as a superior measure of cardiac function and goal-directed resuscitation.
1 Williams G, Grounds M, Rhodes A. Pulmonary artery catheter. Curr Opin Crit Care. 2002;8:251-256.
2 Gore JM, Goldberg RJ, Spodick DH, Alpert JS, Dalen JE. A community-wide assessment of the use of pulmonary artery catheters in patients with acute myocardial infarction. Chest. 1987;92:721-727.
3 Zion MM, et al. Use of pulmonary artery catheters in patients with acute myocardial infarction. Analysis of experience in 5,841 patients in the SPRINT Registry. SPRINT Study Group. Chest. 1990;98:1331-1335.
4 Connors AFJr, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276:889-897.
5 Pulmonary Artery Catheter Consensus Conference. Consensus statement. N Horiz. 1997;5:175-194.
6 Sibbald WJ, Keenan SP. Show me the evidence: a critical appraisal of the Pulmonary Artery Catheter Consensus Conference and other musings on how critical care practitioners need to improve the way we conduct business. Crit Care Med. 1997;25:2060-2063.
7 Bernard GR, et al. Pulmonary artery catheterization and clinical outcomes: National Heart, Lung, and Blood Institute and Food and Drug Administration Workshop Report. Consensus statement. JAMA. 2000;283:2568-2572.
8 Sandham JD, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5-14.
9 Polanczyk CA, et al. Right heart catheterization and cardiac complications in patients undergoing noncardiac surgery: an observational study. JAMA. 2001;286:309-314.
10 Guyatt G. A randomized control trial of right-heart catheterization in critically ill patients. Ontario Intensive Care Study Group. Intensive Care Med J Intensive Care Med. 1991;6:91-95.
11 Wilson J, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ. 1999;318:1099-1103.
12 Afessa B, et al. Association of pulmonary artery catheter use with in-hospital mortality. Crit Care Med. 2001;29:1145-1148.
13 Shah MR, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294:1664-1670.
14 Friese RS, Shafi S, Gentilello LM. Pulmonary artery catheter use is associated with reduced mortality in severely injured patients: a National Trauma Data Bank analysis of 53,312 patients. Crit Care Med. 2006;34:1597-1601.
15 Rapoport J, et al. Patient characteristics and ICU organizational factors that influence frequency of pulmonary artery catheterization. JAMA. 2000;283:2559-2567.
16 Chen YY, et al. Comparison between replacement at 4 days and 7 days of the infection rate for pulmonary artery catheters in an intensive care unit. Crit Care Med. 2003;31:1353-1358.
17 Blot F, et al. Mechanisms and risk factors for infection of pulmonary artery catheters and introducer sheaths in cancer patients admitted to an intensive care unit. J Hosp Infect. 2001;48:289-297.
18 Kac G, et al. Colonization and infection of pulmonary artery catheter in cardiac surgery patients: epidemiology and multivariate analysis of risk factors. Crit Care Med. 2001;29:971-975.
19 Rello J, Coll P, Net A, Prats G. Infection of pulmonary artery catheters. Epidemiologic characteristics and multivariate analysis of risk factors. Chest. 1993;103:132-136.
20 Horowitz HW, Dworkin BM, Savino JA, Byrne DW, Pecora NA. Central catheter-related infections: comparison of pulmonary artery catheters and triple lumen catheters for the delivery of hyperalimentation in a critical care setting. J Parenter Enteral Nutr. 1990;14:588-592.
21 Eyer S, Brummitt C, Crossley K, Siegel R, Cerra F. Catheter-related sepsis: prospective, randomized study of three methods of long-term catheter maintenance. Crit Care Med. 1990;18:1073-1079.
22 Marino PL. The ICU Book. Baltimore: Williams and Wilkins, 1998.
23 Shoemaker WC, Reinhard JM. Tissue perfusion defects in shock and trauma states. Surg Gynecol Obstet. 1973;137:980-986.
24 Shoemaker WC, Montgomery ES, Kaplan E, Elwyn DH. Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch Surg. 1973;106:630-636.
25 Shoemaker WC, Hopkins JA. Clinical aspects of resuscitation with and without an algorithm: relative importance of various decisions. Crit Care Med. 1983;11:630-639.
26 Shoemaker WC, Appel P, Bland R. Use of physiologic monitoring to predict outcome and to assist in clinical decisions in critically ill postoperative patients. Am J Surg. 1983;146:43-50.
27 Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176-1186.
28 Bishop MH, et al. Relationship between supranormal circulatory values, time delays, and outcome in severely traumatized patients. Crit Care Med. 1993;21:56-63.
29 Bishop MH, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma. 1995;38:780-787.
30 Fleming A, et al. Prospective trial of supranormal values as goals of resuscitation in severe trauma. Arch Surg. 1992;127:1175-1179. discussion 1179–1181
31 McKinley BA, et al. Normal versus supranormal oxygen delivery goals in shock resuscitation: the response is the same. J Trauma. 2002;53:825-832.
32 Hayes MA, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717-1722.
33 Rhodes A, Cusack RJ, Newman PJ, Grounds RM, Bennett ED. A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med. 2002;28:256-264.
34 Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686-1692.
35 Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24:517-524.
36 Poeze M, Solberg BC, Greve JW, Ramsay G. Monitoring global volume-related hemodynamic or regional variables after initial resuscitation: What is a better predictor of outcome in critically ill septic patients? Crit Care Med. 2005;33:2494-2500.
37 Epstein CD, Peerless JR, Martin JE, Malangoni MA. Comparison of methods of measurements of oxygen consumption in mechanically ventilated patients with multiple trauma: the Fick method versus indirect calorimetry. Crit Care Med. 2000;28:1363-1369.
38 Luchette FA, et al. Effects of body temperature on accuracy of continuous cardiac output measurements. J Invest Surg. 2000;13:147-152.
39 Cheatham ML, Nelson LD, Chang MC, Safcsak K. Right ventricular end-diastolic volume index as a predictor of preload status in patients on positive end-expiratory pressure. Crit Care Med. 1998;26:1801-1806.
40 Chang MC, Meredith JW. Cardiac preload, splanchnic perfusion, and their relationship during resuscitation in trauma patients. J Trauma. 1997;42:577-582. discussion 582–584
41 Kincaid EH, Meredith JW, Chang MC. Determining optimal cardiac preload during resuscitation using measurements of ventricular compliance. J Trauma. 2001;50:665-669.
42 Miller PR, Meredith JW, Chang MC. Randomized, prospective comparison of increased preload versus inotropes in the resuscitation of trauma patients: effects on cardiopulmonary function and visceral perfusion. J Trauma. 1998;44:107-113.