4 Cardiac Electrophysiology
Diagnosis and Treatment
Cardiac rhythm disturbances are common and an important source of morbidity and mortality.1,2 Supraventricular tachycardias (SVTs) have an estimated incidence from 35 per 100,000 person-years for paroxysmal SVT to 5 to 587 per 100,000 person-years for atrial flutter for individuals age 50 years versus those older than 80 years, respectively.3,4 But it is atrial fibrillation that has proved to be the most common sustained cardiac arrhythmia in the general population, affecting more than 2.3 million Americans.5 The prevalence of atrial fibrillation is strongly related to age, occurring in fewer than 1% of individuals younger than 55 years but in nearly 10% of those older than 80 years.5 The occurrence of atrial fibrillation increases health resource utilization, heightens the risk for stroke, and is associated with long-term mortality.6
There has been a major shift in the treatment of cardiac arrhythmias since the 1980s; this is due, in part, to advances made in catheter- and surgical-based ablations, as well as widely held views that pharmacologic treatments have limited efficacy and, in some instances, may actually increase risk for mortality. These latter observations are mostly due to the negative inotropic and proarrhythmic effects of these drugs.7,8 Data from prospective randomized trials showing improved survival for patients with implantable cardioverter-defibrillators (ICDs) compared with those given antiarrhythmic drugs have further contributed to a shift to nonpharmacologic treatments.9
Basic electrophysiologic principles
Anatomy and Physiology of the Cardiac Pacemaker and Conduction Systems
Sinus Node
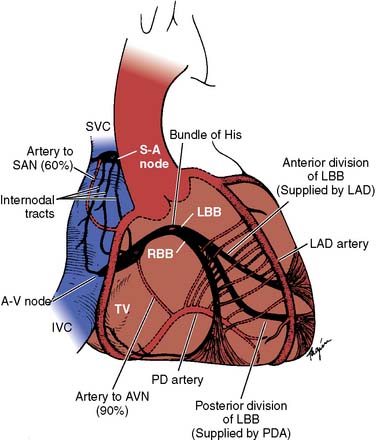
The sinoatrial node (SAN; Figure 4-1) is a spindle-shaped structure composed of highly specialized cells located in the right atrial sulcus terminalis, lateral to the junction of the superior vena cava (SVC) and the right atrium10,11 (see Box 4-1 for a summary of the anatomy of the cardiac pacemaker and conduction system). Three cell types have been identified in the SAN (nodal, transitional, and atrial muscle cells), but no single cell appears to be solely responsible for initiating the pacemaker impulse. Rather, multiple cells in the SAN discharge synchronously through complex interactions.12–14 Rather than a discrete and isolated structure, studies suggest that the SAN consists of three distinct regions, each responsive to a separate group of neural and circulatory stimuli.15 The interrelationship of these three regions appears to determine the ultimate rate of output of the SAN. Although the SAN is the site of primary impulse formation, subsidiary atrial pacemakers located throughout the right and left atria also can initiate cardiac impulses.16–18 In a series of studies both in dogs and humans, it was confirmed that there is an extensive system of atrial pacemakers widely distributed in the right and left atria, as well as in the atrial septum.15,19–21 Because the atrial pacemaker system occupies a much larger area than the SAN, it can be severed during arrhythmia surgery, resulting in impaired rate responsiveness.10 However, it is extremely difficult to completely abolish SAN activity through catheter-based ablation techniques.
The arterial supply to the SAN (SAN artery) is provided from either the right coronary artery (RCA; in 60% of the population) or the left circumflex coronary artery (see Figure 4-1). The SAN is richly innervated with postganglionic adrenergic and cholinergic nerve terminals. Vagal stimulation, by releasing acetylcholine, slows SA nodal automaticity and prolongs intranodal conduction time, whereas adrenergic stimulation increases the discharge rate of the SAN.10
Internodal Conduction
For many years, there has been much controversy concerning the existence of specialized conduction pathways connecting the SAN to the atrioventricular (AV) node. Most electrophysiologists now agree that preferential conduction is unequivocally present, and that spread of activation from the SAN to the AV node follows distinct routes by necessity because of the peculiar geometry of the right atrium.10 The orifices of the superior and inferior cavae, the fossa ovalis, and the ostium of the coronary sinus divide the right atrium into muscle bands, thus limiting the number of routes available for internodal conduction (see Figure 4-1). These routes, however, do not represent discrete bundles of histologically specialized internodal tracts comparable with the ventricular bundle branches.22 It has been suggested that a parallel arrangement of myocardial cells in bundles, such as the crista terminalis and the limbus of the fossa ovalis, may account for preferential internodal conduction. Although electrical impulses travel more rapidly through these thick atrial muscle bundles, surgical transection will not block internodal conduction because alternate pathways of conduction through atrial muscle are available.23
Atrioventricular Junction and Intraventricular Conduction System
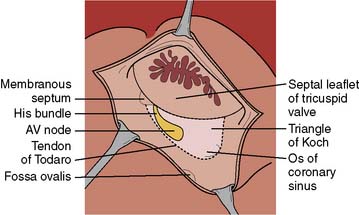
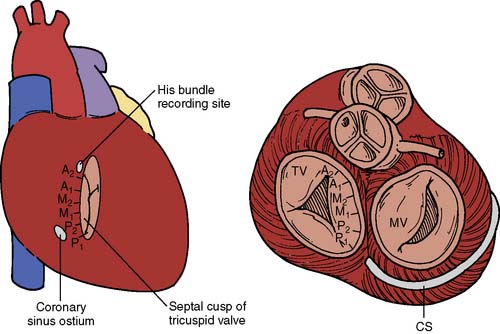
The AV junction (Figure 4-2) corresponds anatomically to a group of discrete specialized cells, morphologically distinct from working myocardium and divided into a transitional cell zone, compact portion, and penetrating AV bundle (bundle of His).24 Based on animal experiments, the transitional zone appears to connect atrial myocardium with the compact AV node.25 The compact portion of the AV node is located superficially, anterior to the ostium of the coronary sinus above the insertion of the septal leaflet of the tricuspid valve. The longitudinal segment of the compact AV node penetrates the central fibrous body and becomes the bundle of His. As the nodal-bundle axis descends into the ventricular musculature, it gradually becomes completely isolated by collagen and is no longer in contact with atrial fibers. The AV junction is contained within the triangle of Koch, an anatomically discrete region bounded by the tendon of Todaro, the tricuspid valve annulus, and the ostium of the coronary sinus (Figure 4-3). This triangle is avoided in all cardiac operative procedures to prevent surgical damage to AV conduction. Individual variability in the specific anatomy of the AV nodal area is dependent on the degree of central fibrous body development.10
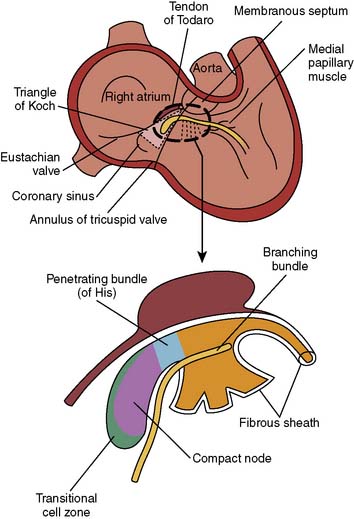
Figure 4-2 Anatomic relation of the atrioventricular junction in relation to other cardiac structures.
(From Harrison DC [ed]: Cardiac Arrhythmias: A Decade of Progress. Boston: GK Hall Medical Publishers, 1981.)
The branching of the nodal-bundle axis begins at the superior margin of the muscular interventricular septum. At this level, the bundle of His emits a broad band of fasciculi, forming the left bundle branch that extends downward as a continuous sheet into the left side of the septum beneath the noncoronary aortic cusp (see Figure 4-1). The left bundle divides into smaller anterior and broader posterior fascicles, although this is not a consistent anatomic delineation. The right bundle branch usually originates as the final continuation of the bundle of His, traveling subendocardially on the right side of the interventricular septum toward the apex of the right ventricle. The distal branches of the conduction system connect with an interweaving network of Purkinje fibers, expanding broadly on the endocardial surface of both ventricles. Blood supply to the AV node is mostly from the RCA (in 85% of the population) or from the left circumflex artery. The bundle of His is supplied by branches from the anterior and posterior descending coronary arteries. Innervation to the SA and AV nodes is complex because of substantial overlapping of vagal and sympathetic nerve branches. Stimulation of the right cervical vagus nerve causes sinus bradycardia, whereas stimulation of the left vagus produces prolongation of AV nodal conduction. Stimulation of the right stellate ganglion speeds SA nodal discharge rate, whereas stimulation of the left ganglion produces a shift in the pacemaker from the SAN to an ectopic site.26
Basic Arrhythmia Mechanisms
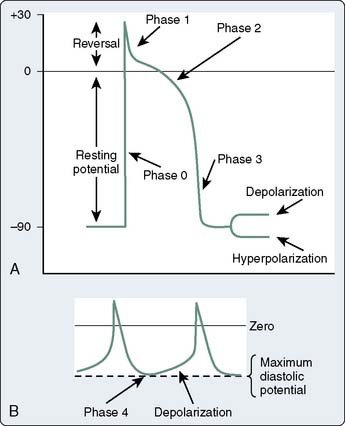
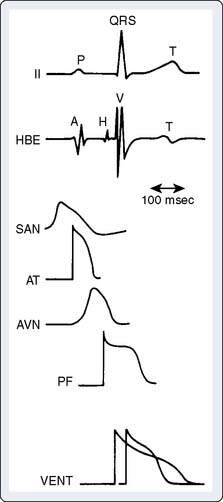
The mechanisms of cardiac arrhythmias are broadly classified as: (1) focal mechanisms that include automatic or triggered arrhythmias, or (2) reentrant arrhythmias (Box 4-2). Cells that display automaticity lack a true resting membrane potential and, instead, undergo slow depolarization during diastole (Figures 4-4 and 4-5). Diastolic depolarization results in the transmembrane potential becoming more positive between successive action potentials until the threshold potential is reached, leading to cellular excitation. Cells possessing normal automaticity can be found in the SAN, subsidiary atrial foci, AV node, and His-Purkinje system.10,13,27–29 The property of slow diastolic depolarization is termed spontaneous diastolic, or phase 4 depolarization. Factors that may modify spontaneous diastolic depolarization are shown in Figure 4-5 and include alterations in the maximum diastolic potential, threshold potential, and rate or slope of diastolic depolarization. The net effect of these factors is to influence the rate (increased or decreased) at which the threshold potential is achieved, resulting in either an increase or a decrease in automaticity. The ionic mechanism of diastolic depolarization involves the “funny” current, which, in turn, may involve a decrease in net outward K+ movement and/or an increase in net inward Na+ movement.26,30–33 Pacemaker cells with the fastest rate of phase 4 depolarization become dominant in initiating the cardiac impulse, with other automatic foci subject to overdrive suppression.
When cells that normally display automaticity (e.g., SAN, AV node, Purkinje fibers) change the rate of pacemaker firing, altered normal automaticity is said to occur. Although the ionic mechanisms resulting in altered normal automaticity are unchanged, other factors such as those seen in Figure 4-5 can contribute to an increase in automaticity. In contrast, automaticity resulting from abnormal ionic mechanisms, even if occurring in cells that are usually considered automatic (e.g., Purkinje fibers), is referred to as “abnormal automaticity.” Abnormal automaticity also may occur in cells in which automaticity is not normally observed (e.g., ventricular myocardium).
Arrhythmias arising from a “triggered” mechanism are initiated from cells that experience repetitive afterdepolarizations. Afterdepolarizations are oscillations in the transmembrane potential that occur either before (early afterdepolarizations) or after (delayed afterdepolarizations) membrane repolarization. Different ionic mechanisms are responsible for each form of afterdepolarization, and if the oscillations in membrane potential reach the threshold potential, a triggered cardiac impulse can be generated.13 Triggered activity is often considered an abnormal form of automaticity. However, because triggered activity requires a prior cardiac impulse (in contrast with automaticity), this abnormal electrophysiologic event cannot purely be considered a form of automaticity.
Reentry is a condition in which a cardiac impulse persists to re-excite myocardium that is no longer refractory.10 Unidirectional block of impulse conduction is a necessary condition for reentry. This unidirectional block may be in the form of differences in membrane refractoriness (dispersion of refractoriness) such that some areas of myocardium are unexcitable, whereas other areas allow impulse propagation. On repolarization, previously refractory membranes will be available for depolarization if the initial impulse has found an alternate route of propagation and returns to the prior site of conduction block. For reentry to occur, slowed conduction in the alternate pathway must exceed the refractory period of cells at the site of unidirectional block. Partial depolarization of fast-response fibers (depressed fast response) results in reduced Na+ channel availability with consequent reduced rate of phase 0 of the action potential. This reduced rate of action potential upstroke of phase 0 can result in slowed conduction and contribute to the above conditions conducive to reentry. Arrhythmias produced by reentrant or triggered mechanisms, but not those secondary to increased automaticity, can be induced with programmed stimulation in the setting of a diagnostic electrophysiology study (EPS). Pacemaker-induced overdrive suppression is a characteristic of arrhythmias produced by automaticity (see Chapter 25).
Diagnostic Evaluation
The history of symptoms often can provide clues in determining the cause of a patient’s palpitations. Abrupt onset and abrupt termination of regular palpitations, for example, are consistent with a paroxysmal SVT most often caused by atrioventricular nodal reentrant tachycardia (AVNRT), atrioventricular reentrant tachycardia (AVRT) associated with an accessory AV bypass tract, or atrial tachycardia. Although a history of syncope does not definitively point toward a ventricular or supraventricular cause, its presence is helpful in determining how urgently this condition should be evaluated. Whether palpitations are regular or irregular is useful in differentiating atrial fibrillation as a cause of the symptoms. Precipitating events, number and duration of episodes, presence of dyspnea, fatigue, or other constitutional symptoms should be sought from the history (Box 4-3).
A 12-lead electrocardiogram (ECG) should be obtained during tachycardia whenever possible and compared with baseline sinus rhythm ECGs. It also is helpful to run a rhythm strip during periods of intervention such as carotid sinus massage or adenosine administration. Patients with a history of pre-excitation presenting with an arrhythmia should be evaluated immediately because atrial fibrillation in the presence of an accessory pathway can lead to sudden death. For all patients undergoing evaluation of an arrhythmia, an echocardiogram is essential to evaluate for cardiac structural abnormalities and ventricular function. The latter is particularly germane for patients with persistent tachycardia because this can lead to tachycardia-associated cardiomyopathy.34 Twenty-four-hour Holter monitoring of patient-triggered events also may be useful in some patients with frequent but transient symptoms. Other evaluations such as exercise or pharmacologic stress testing also have been used to elicit episodes of tachycardia or determine how robust pre-excitation is present with increasing heart rates.
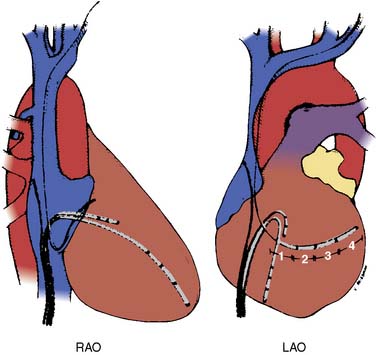
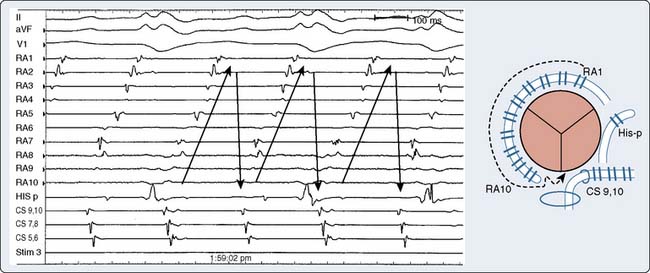
The ultimate diagnosis of the underlying mechanisms of the arrhythmia may require invasive electrophysiologic testing. These studies involve percutaneous introduction of catheters capable of electrical stimulation and recording of electrograms from various intra-cardiac sites. Initial recording sites often include the high right atrium, bundle of His, coronary sinus, and the right ventricle10,35 (Figures 4-6 and 4-7). The sequence of cardiac activation can be discerned from these recordings together with the surface ECG. This is illustrated in Figure 4-8 from a patient undergoing evaluation for an accessory AV conduction pathway. The sequence of the activation is observed by noting the timing of depolarization recorded by the respective electrodes positioned fluoroscopically at various anatomic sites. An example of a recording obtained during diagnostic evaluation of a patient with a ventricular arrhythmia is shown in Figure 4-9.
The catheters are most often introduced via the femoral vessels under local anesthesia. Systemic heparinization is required, particularly when catheters are introduced into the left atrium or left ventricle. The most common complications from electrophysiologic testing are those associated with vascular catheterization.10,36 Other complications include hypotension (in 1% of patients), hemorrhage, deep venous thrombosis (in 0.4% of patients), embolic phenomena (0.4%), infection (0.2%), and cardiac perforation (0.1%).10,37 Proper application of adhesive cardioversion electrodes before the procedure facilitates rapid cardioversion/defibrillation in the event of persistent or hemodynamically unstable tachyarrhythmia resulting from stimulation protocols.
Principles of Electrophysiologic Treatment
The paradigm for ablative treatment of cardiac arrhythmias evolved from the surgical treatment of Wolff–Parkinson–White (WPW) syndrome and then ventricular tachycardia (VT) developed by Sealy, Boineau, and colleagues.38–40 The fundamental paradigm for this approach is precise localization of the electrophysiologic substrate for the arrhythmia and then ablating the pathway. In the case of WPW syndrome, the accessory pathway is identified with intraoperative electrophysiologic mapping that initially used handheld electrodes.10,41 Development of multichannel computer-based mapping systems allowed for the identification of both the mechanisms for many arrhythmias, including VT, and their termination by interruption of the underlying substrate. Experience and insights into arrhythmia mechanisms led to the development of catheter-based methods now routinely used for a variety of supraventricular and ventricular arrhythmias. General indications for ablative treatments include drug-resistant arrhythmias, drug intolerance, severe symptoms, and desire to avoid lifelong drug treatments (Box 4-4).
Manipulation of catheter electrodes in the heart for precise mapping and treatment of arrhythmias can be laborious and time consuming. Newer catheters, as well as robotically and magnetically driven navigational systems, have been developed to facilitate this process and improve both catheter positioning and stability. With these navigational systems, the catheter tip is localized with three-dimensional fluoroscopy and/or advanced three-dimensional mapping applications and precisely moved to the myocardial area of interest using either a robotic arm or a magnetic field42 (Figure 4-10).
Specific Arrhythmias
Supraventricular Tachyarrhythmias
Supraventricular arrhythmias are defined as cardiac rhythms with a heart rate greater than 100 beats/min originating above the division of the common bundle of His. These arrhythmias are often seen as a narrow-complex tachycardia and, in some cases, can be hemodynamically unstable in the presence of structural heart disease. Further, persistent tachycardias for weeks to months may lead to tachycardia-associated cardiomyopathy and to disabling symptoms.34 The differential diagnosis of SVTs includes atrioventricular reciprocating tachycardia (AVRT), AVNRT, atrial tachycardia, inappropriate sinus tachycardia or sinus node reentry, atrial flutter, and atrial fibrillation. Antiarrhythmic medications traditionally have been used with mixed success. Hence surgical and catheter-based procedures have been developed for the management of these arrhythmias.
Atrioventricular Reciprocating Tachycardia
Accessory pathways are abnormal strands of myocardium connecting the atria and ventricles across the AV groove, providing alternate routes for conduction that bypass the AV node and bundle of His (Box 4-5). Various classifications are used to describe accessory pathways and are based on their location (e.g., tricuspid, mitral), whether they are manifest or concealed on a surface ECG, and the conduction properties exhibited by the pathway (e.g., antegrade, retrograde, decremental, nondecremental).42 Decremental conduction along any myocardial tissue refers to the concept that conduction through that tissue is slower as the frequency of impulses reaching it increases. Accessory pathways are more often nondecremental, meaning that regardless of how quickly impulses reach the pathway, the conduction velocity across the pathway remains the same. Concealed pathways refer to the situation in which the accessory pathway only exhibits retrograde conduction; thus, there is no conduction from the atrium to the ventricles through the pathway, thereby showing no evidence of ventricular pre-excitation. This is in contrast with manifest pathways displaying antegrade conduction from the atrium to the ventricles. Because electrical signals can enter the ventricles both from the AV node and the accessory pathway, ventricular pre-excitation will be “manifest” on the surface ECG as delta waves. Manifest pathways typically conduct in both antegrade and retrograde directions. The presence of a manifest pathway allows for the ventricle to be depolarized or “pre-excited” before that occurring via the normal route of conduction through the AV node (Figures 4-11 and 4-12). During pre-excitation, an activation wavefront propagates simultaneously to the ventricles across the bundle of His and the accessory pathway. Because anterograde conduction is delayed at the AV node but not the accessory pathway, the impulse passing through the accessory pathway initiates ventricular depolarization before the impulse traveling via the normal AV conduction system. The ventricle is thus pre-excited, resulting in a delta wave preceding the QRS complex (see Figure 4-11). These ECG findings (short PR interval and delta wave) were noted by Wolff, Parkinson, and White in the 1930s in association with SVT.44 WPW syndrome describes the condition of pre-excitation when accompanied by tachyarrhythmias caused by reentry via the accessory pathway. Not all individuals with the classic WPW ECG findings experience tachyarrhythmias. In fact, it is estimated that about 30% of individuals with WPW ECG findings exhibit tachyarrhythmias. Individuals with WPW ECG findings but without tachyarrhythmias are said to have the WPW signature. AVRT occurs in the absence of the WPW syndrome when the pathway is concealed, and not all tachyarrhythmias in patients with WPW result from the AVRT mechanism.
BOX 4-5. Atrioventricular Reciprocating Tachycardia Accessory Pathway Characteristics
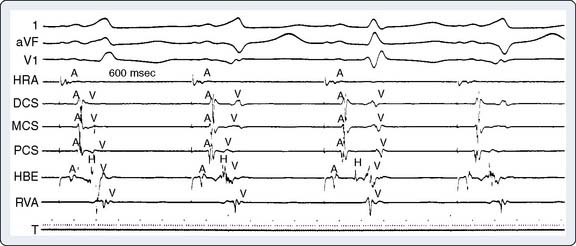
Figure 4-11 The presence of two accessory pathways is shown during pacing. The site of earliest ventricular activation is noted with the distal coronary sinus (DCS) electrode, indicating left free-wall accessory pathway. The second paced beat shows the site of earliest ventricular activation from the proximal coronary sinus (PCS) electrode, indicating posterior septal accessory pathway. After the third paced beat, neither site is activated due to anterograde conduction block. In this instance, conduction follows the normal AV-His bundle and bundle-branch pathways. Surface electrocardiogram leads and intracardiac electrograms are organized as in Figure 4-8. HBE, His-bundle region; HRA, high right atrium; MCA, mid-coronary sinus; RVA, right ventricular apex.
(From Cain ME, Cox JL: Surgical treatment of supraventricular tachyarrhythmias. In Platia EV [ed]: Management of Cardiac Arrhythmias. Philadelphia: JB Lippincott, 1987, p 312.)
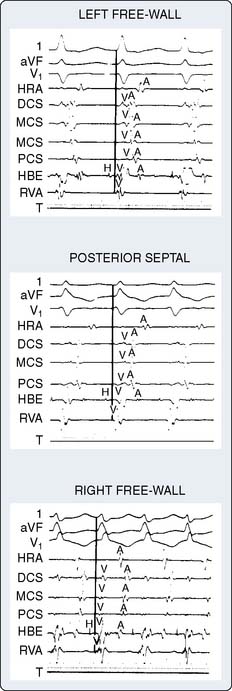
Figure 4-12 Atrial activation recordings from three different patients during orthodromic tachycardia via accessory pathways at distinct locations. Using the solid vertical line as a reference for the QRS complex from the surface electrocardiogram (ECG), the first example demonstrates the earliest atrial activation at the distal coronary sinus (DCS) site, indicating a left free-wall accessory pathway. The posterior septal accessory pathway is indicated by earliest activation of the electrode located in the proximal coronary sinus (PCS). In the last example, atrial activation at the high right atrium (HRA) and bundle of His area (HBE) occurs before all the coronary sinus recording sites, indicative of a right free-wall accessory pathway. Surface ECG leads and intracardiac electrograms are organized as in Figure 4-8. MCA, mid-coronary sinus; RVA, right ventricular apex.
(From Cain ME, Cox JL: Surgical treatment of supraventricular tachyarrhythmias. In Platia EV [ed]: Management of Cardiac Arrhythmias. Philadelphia: JB Lippincott, 1987, p 313.)
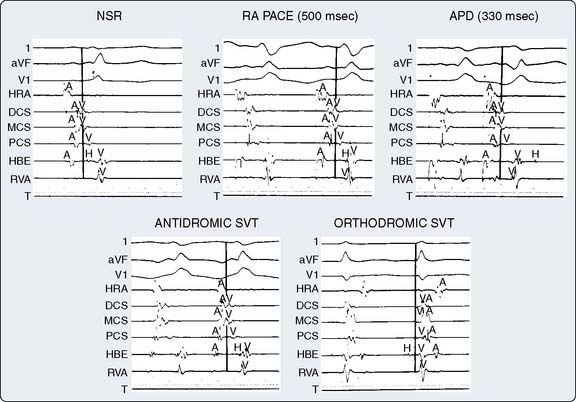
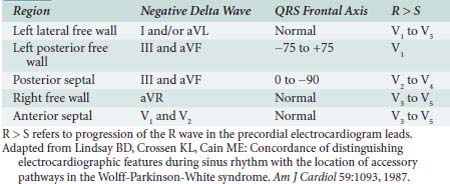
By noting polarity of the delta wave (QRS axis) and precordial R-wave progression, the resting 12-lead ECG can provide clues about the location of the accessory pathway in either the left lateral, left posterior, posterior septal, right free wall, or anterior septal regions45 (Table 4-1). Precise localization, though, is dependent on EPS. Additional information provided by such investigation includes documentation of the mechanism for the arrhythmia (AV vs. other mechanism) and the conduction properties of the accessory pathways. The atrial and ventricular insertion sites of the accessory pathway are identified by observing ventricular activation patterns during sinus rhythm and during atrial pacing (see Figure 4-11). In the presence of an accessory pathway, the interval between the deflection denoting activation of the bundle of His and the earliest ventricular activation (delta wave) is less than the H-V interval. The area with the shortest delta-to-V interval localizes the accessory pathway’s ventricular insertion. More than one accessory pathway may be present, which is suggested by observing different delta-wave morphology with increasing atrial pacing rates or with introduced atrial premature beats (see Figure 4-12). Observing atrial activation patterns during ventricular pacing, after a ventricular premature beat or during induced orthodromic SVT, can identify the location of the atrial insertion sites.
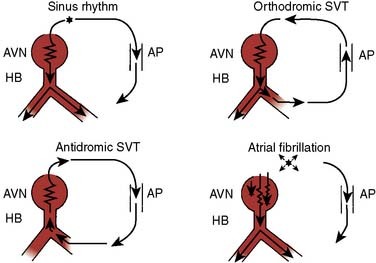
AVRT can occur in one of two fashions: orthodromic reciprocating tachycardia (ORT) and antidromic reciprocating tachycardia (ART)46–48 (Figure 4-13). ORT is by far the most common type and involves antegrade conduction via the normal AV nodal conduction system and retrograde conduction via the accessory pathway. ART, in contrast, involves antegrade conduction down the accessory pathway and retrograde conduction via the AV node. As suggested by these mechanisms, ORT appears as a narrow-complex tachycardia, whereas ART appears as a wide-complex tachycardia that at times can be difficult to distinguish from VT. Importantly, atrial fibrillation occurring in patients with a pathway capable of conducting in an antegrade fashion run the risk for rapid conduction to the ventricles and development of ventricular fibrillation and sudden death. The potential for sudden death caused by atrial fibrillation in patients with WPW provides an argument for aggressive ablative treatment when the procedure can be performed in centers with low periprocedural morbidity.
Catheter-Based Therapy for Accessory Pathways
Percutaneous catheter ablation of accessory pathways has largely supplanted the surgical approach to treatment. RF ablation is typically performed during EPS once the accessory pathway has been localized. Transseptal or retrograde aortic catheter approaches are used to ablate left-sided accessory pathways, and right-heart catheterization via a venous approach is used to ablate right-sided pathways. Success rates of 95% have been reported using these methods.43,49–51 Recurrence rates after successful catheter ablation of an accessory pathway are generally less than 5% and are a function of pathway location, as well as stability of the catheter during energy delivery. Overall, reported complications are low and include those related to vascular access such as hematoma and AV fistula. Other complications are related to catheter manipulations of the left- and right-sided circulation such as valvular or cardiac damage from the catheter, systemic and cerebral embolization caused by catheter manipulation in the aorta, coronary sinus damage, coronary thrombosis and dissection, cardiac perforation, and cardiac tamponade. Complete AV block, cardiac perforation, and coronary spasm caused by RF also may occur. A 1995 survey involving 5427 patients reported serious complications from catheter ablation of accessory pathways in 1.8% of patients and procedure-related mortality in 0.08%.43,49 Complete AV block is more common with ablation of accessory pathways close to the bundle of His. Procedural success with catheter ablation methods is reported to be 87% to 99%.43,50,51 In a randomized study comparing ablation with drug treatment, quality of life, symptom scores, and exercise performance were improved with successful RF ablation.52
Atrioventricular Nodal Reentrant Tachycardia
AVNRT is due to altered electrophysiologic properties of the anterior fast pathway and posterior slow pathway fibers providing input to the AV node.10,43,51 In the past, the only treatment for recurrent SVT caused by AVNRT was total ablation of the His bundle and permanent pacemaker insertion. Surgical techniques developed in the 1980s provided an alternate treatment that was associated with high procedural success, acceptable morbidity, and preservation of AV conduction.53–56 Fundamentals developed with this surgical approach and increased understanding of the physiologic basis of AVNRT led to the development of percutaneous catheter-based treatments. Interruption of either the slow or fast pathway with RF ablation can eliminate AVNRT, with greater success rates reported for ablation of the slow pathway (slow-pathway ablation [68% to 100%] vs. fast-pathway ablation [46% to 94%]).43,57–60 Complication rates are lower with slow-pathway RF ablation and include AV block requiring pacemaker insertion (1%)43 (Box 4-6).
BOX 4-6. Atrioventricular Nodal Reentrant Tachycardia
Catheter-Based Therapy for Atrioventricular Nodal Reentrant Tachycardia
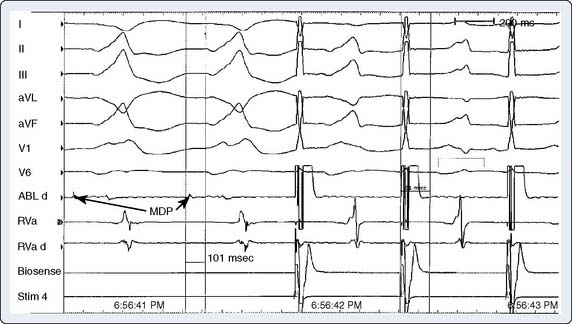
Historically, fast-pathway ablation is performed by positioning the catheter adjacent to the AV node–His bundle anterosuperior to the tricuspid valve annulus. The catheter is withdrawn until the atrial electrogram is larger than the ventricular electrogram and the His recording small or absent. The ECG is closely monitored as RF energy is applied for PR prolongation/heart block. The energy is delivered until there is PR prolongation or the retrograde fast-pathway conduction is eliminated. Noninducibility of AVNRT then is confirmed. Given the increased incidence of complete heart block with fast-pathway ablation, most electrophysiologists have adopted ablation of the slow pathway as a safer alternative. Slow-pathway ablation is performed by identifying the pathway along the posteromedial tricuspid annulus near the coronary sinus. One approach using fluoroscopy is to divide the level of the coronary sinus os and His bundle recordings into six anatomic regions61 (Figure 4-14). Lesions then are placed beginning with the most posterior region moving anteriorly. Rather than the anatomic approach, the slow pathway can be mapped and then ablated by performing ventricular pacing. The end point of slow-pathway ablation is elimination of induced AVNRT.43,57–60 The development of junctional ectopy during RF ablation of the slow pathway is associated with successful slow-pathway ablation.10
Focal Atrial Tachycardia
Focal atrial tachycardia accounts for less than 15% of patients undergoing evaluation for SVT.62 The arrhythmia is due to atrial activation from a discrete atrial area, resulting in heart rates between 100 and 250 beats/min.63 Although the 12-lead ECG might provide clues to the origin of the tachycardia based on P-wave axis, localization of the site of atrial tachycardia is made by electrophysiologic investigations and tends to “cluster” in certain anatomic zones.43 Right-sided tachycardias typically originate along the crista terminalis from the SAN to the AV node and left-sided ones from the pulmonary veins, atrial septum, or mitral valve annulus.63,64 The mechanisms for atrial tachycardia include abnormal automaticity, triggered activity, or micro-reentry. Characteristics of the arrhythmia might provide clues to the underlying mechanisms. Abrupt onset and offset suggest a reentrant mechanism, whereas a gradual onset (“warm-up”) and offset (“cool-down”) pattern suggests automaticity (Box 4-7).
Catheter-Based Therapy for Focal Atrial Tachycardia
Because of the discrete localized area involved in generating atrial tachycardia, the approach to catheter ablation is the same regardless of the mechanisms for the arrhythmia. The site of tachycardia onset is identified with electrophysiologic mapping and then isolated from the remaining atrium by application of RF current. Success of this approach is reported to be 86%, and recurrence rates, 8%.43,64–68 Complications reported in these series occur in 1% to 2% of cases and include rare myocardial perforation, phrenic nerve injury, and sinus node dysfunction.69
Inappropriate Sinus Tachycardia
Sinus tachycardia is deemed inappropriate when it occurs in the absence of physiologic stressors (e.g., increased body temperature, hypovolemia, anemia, hyperthyroidism, anxiety, postural changes, drugs), indicating failure of normal mechanisms controlling sinus rate. Proposed mechanisms are enhanced sinus node automaticity or abnormal autonomic regulation, or both. Clinically, this entity is seen most often in female health-care providers. The diagnosis is made based on nonparoxysmal, persistent resting sinus tachycardia and excessive increases in response to normal physiologic stressors and nocturnal normalization of the rate based on Holter monitoring.51 The P-wave morphology and endocardial activation are consistent with a sinus origin and secondary causes have been excluded. Catheter-based or surgical treatments are considered for a minority of patients not responding to β-blockers and when symptoms are truly disabling. The aim of this treatment is RF ablative modification of the sinus node to promote dominance of slower depolarizing sinus nodal tissues. An esophageal electrode is placed and connected to the operating room ECG monitor to guide treatment. The end point of application of RF energy is change in the P-wave morphology. Reported complications include need for permanent pacemaker, SVC syndrome, phrenic nerve injury, and pericarditis.43,70 Acute and long-term reported success rates are 76% and 66%, respectively.43,70
Sinus Node Reentrant Tachycardia
Reentrant pathways involving the sinus node may lead to paroxysmal tachycardia, in contrast with the nonparoxysmal inappropriate sinus tachycardia.71 The P-wave morphology is similar to that occurring during sinus rhythm. Similar to other reentrant tachycardias, the arrhythmia is usually triggered by a premature atrial beat. Endocardial activation sequence during EPS is in the high right atrium and is similar to sinus rhythm. The arrhythmia can be initiated with a premature pace beat and is terminated by vagal maneuvers or adenosine.43 Clinically, the arrhythmia also is responsive to β-blockers, nonhydropyridine calcium channel antagonists, and amiodarone. RF ablation of the identified reentrant pathway can be used for frequently occurring tachycardia episodes not responsive to other treatments.72
Atrial Flutter
Atrial flutter usually presents with acute onset of symptoms (e.g., palpitations, shortness of breath, fatigue) accompanied by tachycardia and typical “flutter” waves on the ECG (Box 4-8). Fixed 2:1 conduction is usually present with flutter rate of 300 beats/min and ventricular rate of 150 beats/min. When AV conduction is fixed, the heart rate is regular, but varying AV conduction results in an irregular rhythm. Rapid AV conduction can occur with exercise, in patients with accessory pathways, and, paradoxically, after administration of class 1C antiarrhythmic drugs.43 This results from the antiarrhythmic drugs slowing the atrial flutter rate, thus allowing the AV node to support more rapid conduction to the ventricles. This maneuver requires coadministration of drugs with AV conduction-slowing properties (e.g., β-blockers).
Atrial flutter is due to reentry that is referred to as “macroreentry” because the anatomic circuit is large. “Typical” atrial flutter occupies a circuit that circles the tricuspid valve, crossing the myocardial isthmus between the inferior vena cava (IVC) and the tricuspid valve43,62 (Figure 4-15). Counterclockwise rotation through the cava-tricuspid region is usually observed, although other patterns such as clockwise rotation, double waves, and “lower-loop” reentry (i.e., reentry around the IVC) might be observed.43,73,74 Polarity of the flutter waves on the 12-lead ECG provides insight into the pattern of atrial flutter. Counterclockwise rotation is associated with negative flutter waves in the inferior leads and positive flutter waves in V1, whereas the opposite is observed with clockwise rotation.43
The anatomic location of this macroreentrant pathway is amenable to catheter ablation and cure of atrial flutter by creating a linear conduction block across the tricuspid-IVC isthmus. Testing for bidirectional conduction block through the cavo-tricuspid region after application of RF energy enhances success.75,76
Atrial flutter and atrial fibrillation may coexist, complicating success with catheter ablation methods. Procedural success with pure atrial flutter is reported in 80% to 100% of cases, with recurrence occurring in 16% of patients.77–81 In a prospective, randomized trial, catheter ablation resulted in sinus rhythm in 80% of patients, compared with 36% of patients treated with antiarrhythmic drugs (mean follow-up, 21 months).81 Fewer hospitalizations and higher scores on quality-of-life surveys are reported after catheter ablation compared with drug treatment. In the absence of atrial fibrillation, subsequent RF ablation procedures may result in successful elimination of atrial flutter. Even when not present during initial treatment, atrial fibrillation may develop after successful catheter ablation for atrial flutter in 8% to 12% of patients.43,79
Atrial scar tissue from prior cardiac surgery (e.g., congenital heart surgery, mitral valve surgery, Maze procedure) may provide an area for reentry leading to atrial flutter.64,82–85 Reentrant circuits involving the cavo-tricuspid area may coexist, leading to complicated, multiple reentry pathways.43,85 Characterization of the reentry circuit with electrophysiologic mapping studies may allow for successful RF ablation in these circumstances.
Anesthetic Considerations for Supraventricular Arrhythmia Surgery/Ablation Procedures
The approach to the care of patients undergoing percutaneous therapies for supraventricular arrhythmias involves similar basic principles (Box 4-9). Patients with WPW are usually young and free of other cardiac disease, although the syndrome can be accompanied by Ebstein’s anomaly in up to 10% of cases.41,86 Anesthesiologists must be familiar with preoperative EPS results and the characteristics of associated supraventricular arrhythmias (rate, associated hemodynamic disturbances, syncope, etc.), including treatments. Tachyarrhythmias might recur at any time during surgical and percutaneous treatments. Transcutaneous cardioversion/defibrillation adhesive pads are placed before anesthesia induction and connected to a defibrillator/cardioverter. The development of periprocedural tachyarrhythmias is unrelated to any single anesthetic or adjuvant drug.
BOX 4-9. Anesthetic Considerations for Supraventricular Arrhythmia Surgery and Ablation Procedures
Accessory pathway ablation is typically performed under conscious sedation, with general anesthesia reserved for selected patients such as those unable to tolerate the supine position. There is considerable experience with anesthetizing patients with WPW for surgical ablation when this treatment approach was prevalent. The effects of anesthetics on accessory pathway conduction have been investigated mostly to evaluate whether these agents might interfere with electrophysiologic mapping. Droperidol has been demonstrated to depress accessory pathway conduction, but the clinical significance of small antiemetic doses is likely minimal.87,88 Opioids and barbiturates have no proven electrophysiologic effect on accessory pathways and have been shown to be safe in patients with WPW syndrome.89–92 Normal AV conduction is depressed by halothane, isoflurane, and enflurane, and preliminary evidence suggests that these volatile anesthetics also may depress accessory pathway conduction.92,93 Although muscle relaxants with anticholinergic effects (e.g., pancuronium) have been used safely in patients with WPW, drugs lacking autonomic side effects are most often chosen.94
The major goal of the management of patients undergoing supraventricular ablative procedures is to avoid sympathetic stimulation and the development of tachyarrhythmias. Clinical studies have evaluated the efficacy of various anesthetic techniques in maintaining intraoperative hemodynamic stability and in preventing arrhythmias in patients with WPW syndrome.10,95,96 An opioid-based anesthetic technique with supplemental volatile anesthetics is typically used.
Atrial Fibrillation
Atrial fibrillation, the most common sustained cardiac arrhythmia in the general population, can lead to palpitations, shortness of breath, chest discomfort, or anxiety because of the irregular-irregular heart rate pattern5 (Box 4-10). The treatment aims for atrial fibrillation include anticoagulation to decrease the risk for stroke, and heart rate control to limit symptoms and reduce the risk for tachycardia-associated cardiomyopathy. Restoration of sinus rhythm with cardioversion, antiarrhythmic drugs, or both are considered in some instances, but data suggest this strategy is no more effective than a strategy of anticoagulation/heart rate control for improving mortality in certain populations.97 Because antiarrhythmic drugs are associated with life-threatening proarrhythmic side effects, speculation exists that any benefits of restoring sinus rhythm might be outweighed by mortality caused by drug-induced ventricular arrhythmias.7,8 Regardless, the increasing prevalence of atrial fibrillation and the limitations of pharmacologic treatments have led to much interest in nonpharmacologic treatments.
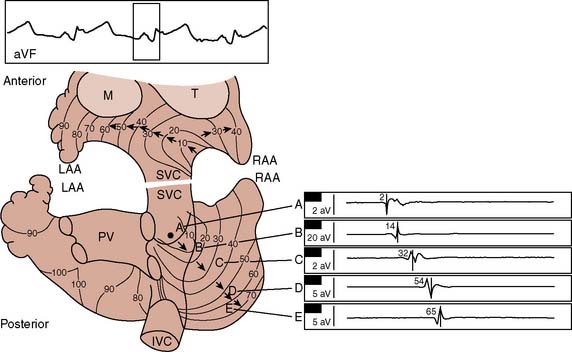
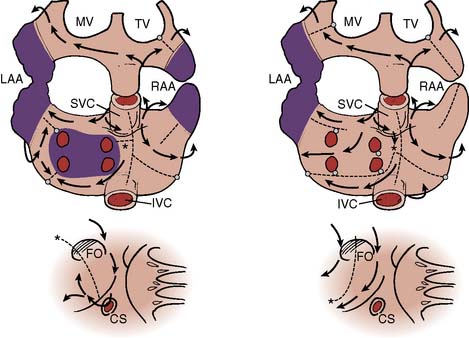
A growing understanding of the mechanisms of atrial fibrillation has led to the introduction of surgical and catheter-based procedures to restore sinus rhythm. Experimental and clinical investigations demonstrate that atrial fibrillation is associated with multiple reentrant circuits in the atrium (“multiple wavelets”) that rapidly and unpredictably change their anatomic location.98–101 Intraoperative electrophysiologic mapping of a patient in sinus rhythm (Figure 4-16), and then after atrial fibrillation was induced by introducing atrial ectopic beats (Figure 4-17), demonstrates the random and fleeting nature of the reentrant circuits.10, 101 The rapidly changing nature of the reentrant circuits precludes a map-directed surgical or ablative strategy for atrial fibrillation. Nonetheless, the realization that certain cardiac structures (e.g., pulmonary veins, valve annulus, vena cava) were necessary substrates for the fibrillatory reentrant circuits led to the development of an anatomically based surgical procedure for atrial fibrillation (the Cox–Maze procedure), whereby macroreentrant circuits are interrupted by a series of atrial incisions and cryoablation lesions.10,101,102
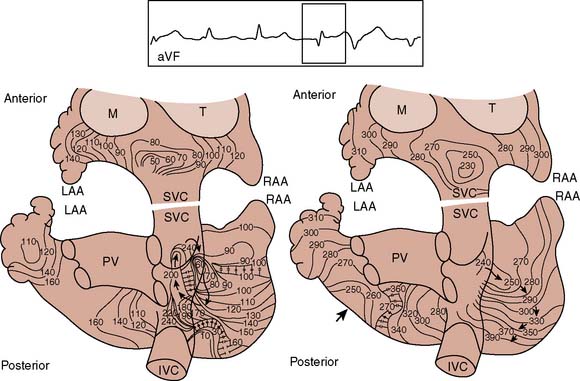
Figure 4-17 Atrial activation mapping from a human during atrial fibrillation indicating a single reentrant circuit. Recordings and isochronous mapping are the same as in Figure 4-20. The map on the left shows the first 240 milliseconds, with 230 to 400 milliseconds in the right map. The beat spreads along the anterior and posterior atria (left). Posteriorly, the beat encounters several areas of conduction block, but as it spreads, it encounters myocardium now repolarized and capable of sustaining conduction. The clockwise, rotating reentrant circuit circulates around natural obstacles such as the orifices of the vena cava. IVC, inferior vena cava; LAA, left atrial appendage; M, mitral valve; PV, pulmonary veins; RAA, right atrial appendage; SVC, superior vena cava; T, tricuspid valve.
(From Cox JL, Canavan TE, Schuessler RB, et al: The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 101:406, 1991.)
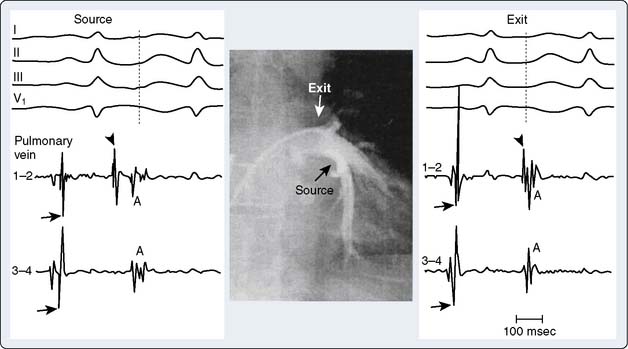
Investigators have demonstrated that atrial fibrillation in some instances originates from automatic foci in the pulmonary veins or vena cava and that isolating these sites may restore sinus rhythm103 (Figure 4-18). Other data have demonstrated focal sources of atrial fibrillation in patients with mitral valve disease.104,106 These findings are supported by laboratory investigations showing that atrial fibrillation can be maintained by a single atrial source of fibrillatory waves moving away from the originating circuit.106,107 These and other findings, together with advances in computer-based electrophysiologic mapping systems, open up the possibility of map-guided strategies to eliminate the substrate for atrial fibrillation in some patients.108–118 The latter strategy would have the benefit of perhaps greater success rates and lower complications than what occur with current procedures.
Catheter-Based Therapy for Atrial Fibrillation
Catheter ablation approaches for atrial fibrillation include AV node ablation with permanent pacemaker placement to control ventricular rate and catheter ablation procedures that aim to restore sinus rhythm. AV node ablation is used for medically refractory tachycardia caused by atrial fibrillation or to eliminate intolerable symptoms caused by an irregular heart rate. The procedure requires pacemaker implantation, does not aim to restore sinus rhythm, and does not eliminate the need for anticoagulation. In this latter class of procedures, many different strategies are employed, but all tend to involve electrical isolation of the pulmonary veins. It is thought that myocardial sleeves involving the os of the pulmonary veins can initiate atrial fibrillation because of their inherently different electrophysiologic properties. By electrically isolating them, the goal is to prevent atrial fibrillation from developing. Pulmonary vein isolation can be achieved in one of two ways. In the first, complete electrical isolation is achieved by sequential, segmental application of RF ablation at each pulmonary vein ostium.103,119 An alternate strategy is to regionally isolate the posterior left atrium by encircling not only the pulmonary vein ostia but the surrounding posterior left atrial wall by a circular pattern of adjacent RF ablation lesions.105 A randomized comparison of these two strategies has demonstrated a significantly greater success rate with the regional isolation strategy; 88% of patients were free of atrial fibrillation at 6 months compared with 67% free of atrial fibrillation at 6 months with the segmental isolation strategy.112 The regional isolation procedure also reduces the risk for creating pulmonary venous stenosis that can be associated with the segmental isolation procedure.
Research into methods emulating the surgical Maze procedure continues to evolve but remains investigative (see later). Linear ablation techniques involve RF energy application along critical sites for the maintenance of atrial fibrillation.113–116,118,120 Success has been limited with this approach (28% to 57%), and the procedures are associated with long procedure duration and associated radiation exposure. Further, complication rates remain high (4% to 50%).
Surgical Therapy for Atrial Fibrillation
The growing understanding of the underlying mechanisms for atrial fibrillation led to the development of a surgical procedure developed by Cox et al termed the Maze procedure.101,102,121–125 This moniker stems from the basic design of the operation to surgically create a “Maze” of functional myocardium, allowing propagation of atrial depolarization throughout the atrium to the AV node while interposed scar tissue interrupts possible routes of reentry (Figure 4-19). The principal goals of the Maze procedure are (a) to interrupt the electrophysiologic substrate for atrial fibrillation (reentrant circuits) restoring sinus rhythm; (b) to maintain sinus nodal–to–AV nodal conduction, thus preserving AV synchrony; and (c) to preserve atrial mechanical function (“atrial kick”) to improve hemodynamic function.
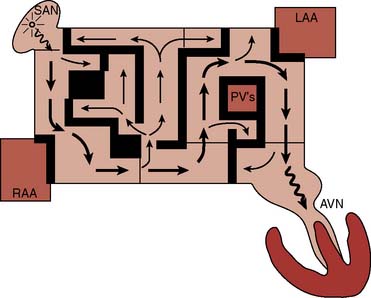
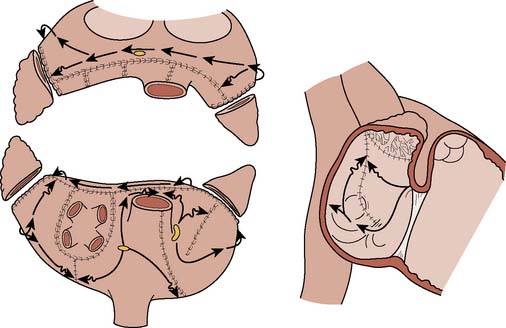
The Maze procedure has evolved from the original procedure (Maze I) introduced in the early 1990s. The Maze I procedure consisted of multiple atrial incisions around the SAN including an incision anterior to the atrial-SVC junction102–104,124 (Figure 4-20). The latter incision is through the sinus tachycardia region of the SAN, resulting in the unintended consequence of blunted heart rate response to exercise and obtunded atrial mechanical function.125–127 Subsequently, the procedure was modified (Maze II procedure) to include an incision on the anterior right atrium while allowing the sinus impulse to travel anteriorly across the left atrium but preventing it from reentering the right atrial–SVC junction (Figure 4-21). Although successfully addressing the limitations with the original procedure, the Maze II procedure was technically challenging, particularly the approach to the left atrium that necessitated division and then reapproximation of the SVC. This was addressed by moving the left atrial incision to a more posterior location (Figure 4-22). These and other modifications led to the introduction of the Maze III procedure, which reduced the frequency of chronotropic incompetence, improved atrial transport function, and shortened the procedure.124
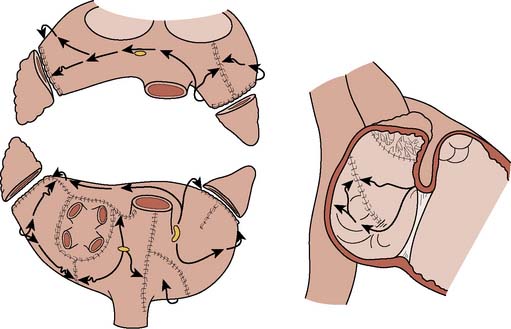
The combination of conduction blocks imparted surgically for treating atrial fibrillation is called the lesion set, which consists of three basic components: pulmonary vein isolation alone, pulmonary vein isolation with connecting lesions to the mitral valve, and lesions involving the right atrium. The Cox–Maze III represents the gold standard of lesion sets in atrial fibrillation surgery. As shown in Figure 4-22, the pulmonary veins are isolated with connection lesions to the mitral annulus and left atrial appendage (LAA). This constitutes the “left-sided” lesion set. On the right side, the SVC and IVC line is combined with connecting lesions to the tricuspid annulus and right atrial appendage. The coronary sinus is ablated in one spot using cryothermy and both atrial appendages are removed. The complexity of this procedure combined with alternative energy sources has motivated surgeons to use other combinations of lesion sets. These other combinations are collectively called “modified Maze” procedures.
Based on Haissaguerre’s103 seminal article in 1998, electrical isolation of the pulmonary veins has been used extensively. With modern devices, pulmonary vein isolation is straightforward and may be performed epicardially and without CPB. In patients with paroxysmal atrial fibrillation, most centers report that up to 80% of patients remain free of atrial fibrillation 6 months after surgery. For persistent atrial fibrillation, sinus rhythm is reported to be successfully restored in 30% to 40% of patients. The addition of connecting lesions increases the efficacy of the modified Maze. This is particularly true in patients with persistent or permanent atrial fibrillation.
To simplify the Cox-Maze III operation, Cox et al157 proposed cryothermy. Tissue is exposed to −60°C temperature using a handheld probe, which leads to a consistent transmural scar despite being applied only to the heart surface. A variety of flexible and colder probes is available that allows the creation of all lesion sets.
Another energy form for surgical arrhythmia ablation is RF energy in which alternating electrical current is used to generate thermal injury and, thus, localized atrial scar. Unipolar RF, however, can be associated with collateral atrial injury and tissue charring. This has led to the development of bipolar probes that minimize this risk. Although bipolar RF energy may be used epicardially for pulmonary vein isolation, any other ablative lesions require opening the heart. The latter can be accomplished via a small incision using a purse-string technique. Because of the generated heat, contiguous structures like the esophagus have been injured with pulmonary vein isolation (PVI) or posterior atrial lesion generation.128 Thus, when RF energy is used, it is advised to retract the transesophageal echocardiography probe to hopefully decrease the risk for this complication. Nonetheless, esophageal injury from RF energy may occur regardless of whether TEE is used during surgery. Monitoring esophageal temperature using a probe fluoroscopically placed behind the left atrium may provide guidance to the operator.
Operative results from multiple centers show that greater than 90% of patients remain free of atrial fibrillation after the classic Maze procedure.127 Episodes of atrial flutter during the immediate perioperative period do not alter the long-term success of restoration of sinus rhythm.127 Procedure-specific complications have included an attenuated heart rate response to exercise resulting in the need for permanent pacemaker implantation.127 The frequency of these complications is less with newer versions of the procedure. Fluid retention is a common problem after the Maze procedure, which is attributed to reduced secretion of atrial natriuretic peptide and increase of antidiuretic hormone, as well as aldosterone.129,130 Furosemide, spironolactone, or both perioperatively can limit the consequences of this complication.131
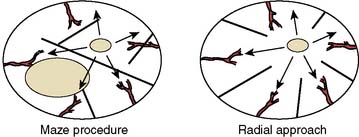
An intended goal of the Maze procedure is preservation of atrial transport function. Follow-up of patients early in the experience at Washington University School of Medicine demonstrated that this was achieved in 98% of patients for the right atrium but only in 86% of patients for the left atrium.132 More detailed analyses further demonstrated that even when left atrial contraction was present, quantitative mechanical function was lower compared with control patients.133,134 The latter consequence was believed to be related to the incisions used to isolate the pulmonary veins that resulted in isolating nearly 30% of the left atrium myocardium from excitation.135 A new approach to the Maze procedure was developed whereby incisions radiate from the SAN (Figure 4-23) along the path of coronary arteries supplying the atrium (rather than across as in the Maze III procedure), to better preserve left atrial transport function.136,137 This modification is termed the radial procedure and is further designed to preserve the right atrial appendage, which is an important source of atrial natriuretic peptide.138 Compared with the standard Maze III procedure, the radial approach results in a more synchronous activation sequence of the left atrium, preserving atrial transport function, although it is equally effective in eliminating the reentrant circuits of atrial fibrillation.
Anesthetic Considerations
Patient monitoring modalities for the surgical procedures are similar to those used for other cardiac surgical procedures including TEE to evaluate for ventricular and valvular function, monitor for new wall motion abnormalities, and assist in evacuation of air from the cardiac chambers at the conclusion of surgery. Ventricular dysfunction (right more often than left ventricle), at least transiently, as well as echocardiographic and ECG ischemic changes (inferiorly more often), is common.10 The proposed cause includes coronary artery air embolization or inadequate myocardial protection, or both. Because the Maze procedure entails placement of multiple atrial incisions, initial atrial compliance and performance of the atria appear altered. TEE evaluation of atrial activity is performed after separation from the extracorporeal circulation and decannulation.10
Ventricular arrhythmias
As with supraventricular arrhythmias, the treatment of ventricular fibrillation and VT is aimed at addressing underlying mechanisms (e.g., myocardial ischemia, drug induced, electrolyte, or metabolic abnormalities). In most patients with life-threatening ventricular arrhythmias and structural heart disease, ICD placement is the standard of care with or without concomitant antiarrhythmic drug therapy.139 In patients with significant structural heart disease, catheter ablation is considered as an adjuvant therapy for medically refractory monomorphic VT. Rarely, VT occurs in the setting of a structurally normal heart. This syndrome of a primary electrical disorder is typically due to a focal, triggered mechanism that occurs mostly in younger patients and originates from the right ventricular outflow tract or apical septum140–142 (Box 4-11). ICDs are typically not indicated in these individuals.
BOX 4-11. Ventricular Arrhythmias
Catheter Ablation Therapy for Ventricular Tachycardia
The mechanism for VT can be identified in the electrophysiology laboratory using programmed stimulation.143,144 Single or multiple extrastimuli are introduced during the vulnerable period of cardiac repolarization (near T wave) until sustained VT develops that is similar in morphology to that of the spontaneous arrhythmia. The diagnostic hallmark of VT caused by a reentrant circuit is the ability to entrain the tachycardia by pacing slightly faster than the tachycardia cycle length.145 Traditional catheter mapping techniques for guiding catheter ablation of VT serve to position the ablation catheter within a protected isthmus of the reentrant circuit. The pathologic characteristics of this site are thought to be viable myocardium surrounded by scar tissue that is electrically isolated from the bulk of the ventricular myocardium except at the entrance and exit sites. Important shortcomings of these techniques are that most VTs are not hemodynamically stable enough for mapping, and that multiple morphologies of inducible VT are commonly present in a single patient. As a result, newer strategies that rely on three-dimensional computerized mapping techniques attempt to identify important areas of myocardial scar, of which the perimeter may participate in reentrant circuits. By strategic placement of areas of conduction block guided by these maps, significant cure rates have been obtained without the necessity of mapping individual reentrant circuits.146 In rare instances, the VT circuits might involve the conduction system as in bundle-branch reentry or fascicular VT that is easily ablated with RF energy.147
There are no data from prospective randomized trials of VT ablation, but results from case series report success rates ranging from 37% to 86%.129,147–153 The latter represent mostly patients with drug-resistant VT or multiple VT morphologies and the treatment performed as a “last-ditch effort” to control the arrhythmia. Reported success rates are greater after RF ablation for primary VT. Major complications from catheter ablation procedures for VT in the setting of structural heart disease include stroke, myocardial infarction, heart failure exacerbation, vascular injury, and death. The incidence of these complications appears to be low despite the lengthy procedure times that are commonly required.146
Anesthetic Considerations
Prior or current treatment with amiodarone is a particular concern. The long elimination half-life (about 60 days) of amiodarone requires that potential side effects such as hypothyroidism be considered perioperatively.154 The α- and β-adrenergic properties of amiodarone might lead to hypotension during anesthesia, but most anesthesiologists in contemporary practice are familiar with the management of these complications. Much attention has been given to bradycardia associated with amiodarone during anesthesia that might be resistant to atropine.155–159 Methods for temporary cardiac pacing should be readily available to care for patients receiving long-term amiodarone. Retrospective reports further suggest a greater need for inotropic support for patients receiving preoperative amiodarone therapy because a low systemic vascular resistance has been observed in these patients.156,157 Pulmonary complications speculated to be related to pulmonary toxicity from amiodarone have also been reported.106,159 In a series of 67 patients receiving preoperative amiodarone, 50% experienced development of acute respiratory distress syndrome that could not be attributed to other factors including intraoperative Fio2 (see Chapter 10).159
Monitoring includes direct arterial pressure monitoring, and central venous access is necessary for administration of vasoactive drugs, if needed. Means for rapid cardioversion/defibrillation should be readily available when inserting any central venous catheter. Self-adhesive electrode pads are most often used and connected to a cardioverter/defibrillator before anesthesia induction. Premature ventricular beats induced during these procedures can easily precipitate the patient’s underlying ventricular arrhythmia that might be difficult to convert to sinus rhythm.157,160 Selection of anesthetics for arrhythmia ablation is dictated mostly by the patient’s underlying physical state. General anesthesia with endotracheal intubation is typically chosen because of the duration of the procedures. Because anesthetics can influence cardiac conduction and arrhythmogenesis, there is a concern about the potential of anesthetics to alter the electrophysiologic mapping procedures.161,162 The effects of the various volatile anesthetics on ventricular arrhythmias vary among the experimental models and, importantly, because of the mechanism of the arrhythmia. Data showing proarrhythmic, antiarrhythmic, and no effects of volatile anesthetics on experimental arrhythmias have been reported.13,161–168 Nonetheless, the small doses administered during ablative procedures may have minimal effects on electrophysiologic mapping. Opioids have been shown to have no effects on inducibility of VT.165,168,169
Implantable cardioverter-defibrillator
Considerable progress has occurred with the ICD, including decreased device size, improved battery life, and improved treatment algorithms, all contributing to enhanced reliability (Box 4-12). Current ICDs are capable of providing tiered therapy consisting of antitachycardia pacing and shocks to terminate potentially life-threatening ventricular arrhythmias. All ICDs also have the ability to pace the heart to treat bradycardia either as a single-chamber, dual-chamber, or biventricular system. Advances in lead technology, as well as the implementation of a biphasic waveform, have considerably reduced defibrillation energy requirements.170,171 These improvements have led to simplification of lead implantation for the use of transvenous insertion methods rather than epicardial patch electrodes used in prior generations. As a result, insertion of modern devices is nearly exclusively via percutaneous techniques rather than more invasive median sternotomy, except in cases in which the body habitus would preclude this approach (e.g., pediatric population).
BOX 4-12. Implantable Cardioverter-Defibrillator
The ICD consists of a pulse generator and transvenous leads that continuously monitor the heart rate. When the heart rate exceeds a programmable limit, therapy is initiated that might include a brief burst of rapid pacing (i.e., antitachycardia pacing) followed by a biphasic shock if the arrhythmia persists. Electrogram storage capabilities allow for review of appropriateness of delivered treatments, as well as changes in ventricular arrhythmia characteristics. The style of ICD, either one, two, or three leads, is chosen based on a patient’s requirement for antibradycardia pacing (single- or dual-lead devices) or cardiac resynchronization therapy, also known as biventricular pacing, when medically refractory heart failure and interventricular conduction delay are present (see Chapter 25).
Technologic aspects of ICDs have been reviewed and are discussed in more detail in Chapter 25.170 Defibrillation voltage is much greater than can be delivered with existing batteries, necessitating the use of storage capacitors and transformers. Once the ICD has detected an arrhythmic event, the device begins to charge its capacitor. During charging and immediately after the capacitor has been fully charged, continued presence of the arrhythmia is confirmed and, if present, the device delivers therapy. If during the charge or immediately after charging is complete the arrhythmia spontaneously terminates, the energy is then dumped to avoid unnecessary energy delivery. If energy is delivered, the device enters into a redetection algorithm to assess whether the arrhythmia was successfully terminated. If the arrhythmia persists, then the device recharges its capacitor and repeats the process. If the arrhythmia has terminated, then the episode is declared complete. Although much of the ICD’s ability to determine whether an arrhythmia needs therapy is based on the rate, all ICDs have the ability to apply various algorithms to discriminate whether the arrhythmia is ventricular or supraventricular. These include criteria for abruptness of onset, intracardiac signal morphology, and rate stability (stable with VT but irregular with atrial fibrillation).170 Presence of an atrial lead can sometimes enhance the discrimination of atrial fibrillation with rapid ventricular response from VT.170
Guidelines for implantation of ICDs have been issued by the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society172 (Table 4-2). In general, ICDs are indicated for the primary or secondary prevention of sudden cardiac death. These recommendations are based on data from large, multicenter investigations that have compared ICD therapy with standard care including antiarrhythmic drugs. For patients with prior cardiac arrest caused by VT or ventricular fibrillation (secondary prevention), the data show that ICDs reduce the risk for subsequent mortality by 20% to 30% compared mostly with amiodarone or β-adrenergic receptor blockers.172–175 Similarly, relative mortality is reduced by 49% to 54% with ICD treatment for patients with nonsustained VT or inducible ventricular arrhythmias with programmed stimulation compared with standard care or serial drug testing in patients with ischemic left ventricular dysfunction.176,177
TABLE 4-2 American College of Cardiology/American Heart Association/Heart Rhythm Society Guidelines for Insertion of ICD216
ICD, implantable cardioverter-defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; RV, right ventricular; VF, ventricular fibrillation; VT, ventricular tachycardia.
The most convincing data regarding primary prevention of sudden death with ICD treatment for patients with ischemic and nonischemic cardiomyopathy come from the MADIT II (Multicenter Automatic Defibrillator Implantation Trial II)176 and SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial)178 trials. In contrast with other studies, these two randomized trials did not require a history of inducible or spontaneous ventricular arrhythmias. Rather, enrollment criteria were based on the ejection fraction alone (≤ 30%) in the presence of ischemic cardiomyopathy (MADIT II) or the ejection fraction (≤ 35%) with New York Heart Association Class II/III heart failure symptoms in the presence of any type of end-stage cardiomyopathy (SCD-HeFT).178 Patients were continued on conventional treatments including β-blockers, angiotensin-converting enzyme inhibitors, and 3-hydroxy-3-methylglutaryl-coenzyme A (hMG-CoA) reductase inhibitors (“statins”). After more than 4 years of follow-up, ICD treatment was associated with a significant reduction in all-cause mortality compared with those randomized to only conventional treatment. Other conditions such as inherited long QT syndrome, hypertrophic cardiomyopathy, Brugada syndrome, arrhythmogenic right ventricular dysplasia, and infiltrative disorders including cardiac sarcoidosis may warrant ICD insertion for prevention of sudden cardiac death, although data from large randomized studies are lacking because of the relative rarity of the conditions. In the future, genetic screening might provide valuable information about the risk for sudden death for patients with these less common entities.179,180
Anesthetic Considerations
In addition to standard patient monitoring, continuous arterial blood pressure monitoring might be considered even during monitored anesthesia care to rapidly assess for return of blood pressure after defibrillation testing. Defibrillation testing was demonstrated to be associated with ischemic electroencephalographic (EEG) changes 7.5 ± 1.8 seconds (mean ± SD) after arrest.181 These changes were transient and not associated with persistent ischemic EEG changes or exacerbation of an existing neurologic deficit, nor was significant deterioration in neuropsychometric performance detected. Repeated defibrillation testing is usually well tolerated without deterioration of cardiac function even in patients with left ventricular ejection fractions less than 35%. Nonetheless, means of pacing must be available should bradycardia develop after cardioversion/defibrillation. Often, however, restoration of circulatory function after defibrillation testing is accompanied by tachycardia and hypertension, necessitating treatment with a short-acting β-blocker or vasoactive drugs, or both.
Complications associated with ICD insertion include those related to insertion and those associated specifically with the device. Percutaneous insertion is typically via the subclavian vein, predisposing to pneumothorax. Cardiac injury including perforation is a remote possibility. Cerebrovascular accident and myocardial infarction have been reported, but mostly with older device insertion methods.10 Device-related complications include those associated with multiple shocks that may lead to myocardial injury or refractory hypotension.182,183 Device infections are particularly difficult to manage, often requiring device and lead explantation.
1 National Center for Health Statistics. Report of final mortality statistics, 1995. Monthly Vital Stat Rep. 45(Suppl 2), 1997.
2 Gillum R.E. Sudden coronary death in the United States: 1980-1985. Circulation. 1989;79:756.
3 Orejarena L.A., Vidailles H.Jr, DeStefano F., et al. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150.
4 Granada J., Uribe W., Chyou P.H., et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242.
5 Sra J., Dhala A., Blanck Z., et al. Atrial fibrillation: Epidemiology, mechanisms, and management. Curr Probl Cardiol. 2000;25:405.
6 Benjamin E.J., Wolf P.A., D’Agostino R.B., et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946.
7 The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406.
8 Ben-David J., Zipes D.P. Torsades de pointes and proarrhythmia. Lancet. 1993;341:1578.
9 Zipes D.P. Implantable cardioverter-defibrillator: A Volkswagen or Rolls Royce? Circulation. 2001;103:1372.
10 Andritsos M., Faddis M., Hogue C.W.Jr. Cardiac electrophysiology: Diagnosis and treatment. In: Kaplan’s Cardiac Anesthesia. WB Saunders; 2006:355-382.
11 Anderson K.R., Ho S.Y., Anderson R.H. The location and vascular supply of the sinus node in the human heart. Br Heart J. 1979;41:28.
12 Cranefield P.F. Action potentials, afterpotentials and arrhythmias. Circ Res. 1977;41:415.
13 Atlee J.L., Bosnjak Z.J. Mechanism for cardiac arrhythmias during anesthesia. Anesthesiology. 1990;72:347.
14 Fozzard H.A., Gunn R.B. Membrane transport. In: Fozzard H.A., Haber E., Jennings R.B., et al, editors. The Heart and Cardiovascular System. New York: Raven Press; 1986:1-30.
15 Boineau J.P., Schuessler R.B., Mooney C.R., et al. Multicentric origin of the atrial depolarization wave: The pacemaker complex. Circulation. 1978;58:1036.
16 Goldberg J.M. Intra-SA-nodal pacemaker shifts induced by autonomic nerve stimulation in the dog. Am J Physiol. 1975;229:1116.
17 Randall W.C., Talano J., Kaye M.P., et al. Cardiac pacemakers in absence of the SA node: Responses to exercises and autonomic blockade. Am J Physiol. 1978;234:H465.
18 Sealy W.C., Bache R.J., Seaber A.V., Bhattacharga S.K. The atrial pacemaking site after surgical exclusion of the sinoatrial node. J Thorac Cardiovasc Surg. 1973;65:841.
19 Sealy W.C., Seaber A.V. Surgical isolation of the atrial septum from the atria: Identification of an atrial septal pacemaker. J Thorac Cardiovasc Surg. 1980;80:742.
20 Boineau J.P., Schuessler R.B., Hackel D.B., et al. Widespread distribution and rate differentiation of the atrial pacemaker complex. Am J Physiol. 1980;239:H406.
21 Boineau J.P., Miller C.B., Schuessler R.B., et al. Activation sequence and potential distribution maps demonstrating multicentre atrial impulse origin in dogs. Circ Res. 1984;54:332.
22 Hoffman B.F. Fine structure of internodal pathways. Am J Cardiol. 1979;44:385.
23 Cox J.L. The surgical treatment of cardiac arrhythmias. In: Sabiston D.C.Jr, editor. Textbook of Surgery. Philadelphia: WB Saunders; 1991:2058.
24 Anderson R.H., Davies M.J., Becker A.E. Atrioventricular ring specialized tissue in the normal heart. Eur J Cardiol. 1974;2:219.
25 Anderson R.H., Becker A.E. Anatomy of conducting tissues revisited. Br Heart J. 1979;40:2.
26 Zipes D.P. Genesis of cardiac arrhythmias: Electrophysiologic considerations. In: Braunwald E., editor. Heart Disease. ed 3. Philadelphia: WB Saunders; 1990:581-620.
27 Kreitner D. Electrophysiological study of the two main pacemaker mechanisms in the rabbit sinus node. Cardiovasc Res. 1985;19:304.
28 Rozanski G.J., Lipsius S.L. Electrophysiology of functional subsidiary pacemakers in canine right atrium. Am J Physiol. 1985;249:H504.
29 Watanabe Y., Dreifus L.S. Sites of impulse formation within the atrioventricular junction of the rabbit. Circ Res. 1968;22:717.
30 DiFrancesco D. A new interpretation of the pacemaker current in calf Purkinje fibers. J Physiol (Lond). 1981;314:359.
31 Noble D., Tsien R.W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibers. J Physiol (Lond). 1968;195:185.
32 Vassalle M. Cardiac pacemaker potentials at different extracellular and intracellular K+ concentrations. Am J Physiol. 1965;208:770.
33 Vassalle M. Analysis of cardiac pacemaker potential using “voltage clamp” technique. Am J Physiol. 1966;210:1335.
34 Wu E.B., Chia H.M., Gill J.S. Reversible cardiomyopathy after radiofrequency ablation of lateral free-wall pathway-mediated incessant supraventricular tachycardia. PACE Pacing Clin Electrophysiol. 2000;23:1308.
35 Cain M.E., Lindsay B.D. The preoperative electrophysiologic study. Cardiac Surg. 1990;4:53.
36 DiMarco J.P., Garan H., Ruskin J.N. Complications in patients undergoing cardiac electrophysiologic procedures. Ann Intern Med. 1982;97:490.
37 Horowitz L.N., Kay H.R., Kutalek S.P., et al. Risks and complications of clinical cardiac electrophysiologic studies: A prospective analysis of 1,000 consecutive patients. J Am Coll Cardiol. 1987;9:1261.
38 Cobb F.R., Blumenschein S.D., Sealy W.C., et al. Successful surgical interruption of the bundle of Kent in a patient with Wolff-Parkinson-White syndrome. Circulation. 1968;38:1018.
39 Sealy W.C., Boineau J.P., Wallace A.G. The identification and division of the bundle of Kent for premature ventricular excitation and supraventricular tachycardia. Surgery. 1970;68:1009.
40 Boineau J.P., Moore E.N., Sealy W.C., Kasell J.H. Epicardial mapping in Wolff-Parkinson-White syndrome. Arch Intern Med. 1975;135:422.
41 Cox J.L., Gallagher J.J., Cain M.E. Experience with 118 consecutive patients undergoing surgery for the Wolff-Parkinson-White syndrome. J Thorac Cardiovasc Surg. 1985;90:490.
42 Faddis M.N., Chen J., Osborn J., et al. Magnetic guidance system for cardiac electrophysiology: A prospective trial of safety and efficacy in humans. J Am Coll Cardiol. 2003;42:1952.
43 Blomström-Lundqvist C., Scheinman M.M., Aliot E.M., et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. J Am Coll Cardiol. 2003;108:1871.
44 Wolff L., Parkinson J., White P.D. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachycardia. Am Heart J. 1930;5:685.
45 Lindsay B.D., Crossen K.J., Cain M.E. Concordance of distinguishing electrocardiographic features during sinus rhythm with the location of accessory pathways in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1987;59:1093.
46 Gallagher J.J., Pritchett E.L.C, Sealy W.C., et al. The preexcitation syndromes. Prog Cardiovasc Dis. 1978;20:285.
47 Bardy G.H., Packer D.L., German L.D., et al. Pre-excited reciprocating tachycardia in patients with Wolff-Parkinson-White syndrome: Incidence and mechanisms. Circulation. 1984;70:377.
48 Kuck K., Brugada P., Wellens H.J. Observations on the antidromic type of circus movement tachycardia in the Wolff-Parkinson-White syndrome. J Am Coll Cardiol. 1983;2:1003.
49 Scheinman M.M., Huang S. The 1998 NASPE prospective catheter ablation registry. PACE Pacing Clin Electrophysiol. 2000;23:1020.
50 Calkins H., Yong P., Miller J.M., et al. For the Atakr Multicenter Investigator Group: Catheter ablation of accessory pathways, atrioventricular nodal re-entrant tachycardia, and the atrioventricular junction: Final results of a prospective, multicenter clinical trial. Circulation. 1999;99:262.
51 Yee R., Connolly S., Noorani H. Clinical review of radiofrequency catheter ablation for cardiac arrhythmias. Can J Cardiol. 2003;19:1273.
52 Lau C.P., Tai Y.T., Lee P.W. The effects of radiofrequency ablation versus medical therapy on the quality-of-life and exercise capacity in patients with accessory pathway-mediate supraventricular tachycardia: A treatment comparison study. PACE Pacing Clin Electrophysiol. 1995;18:424.
53 Holman W., Ikeshita M., Lease J., et al. Elective prolongation of atrioventricular conduction by multiple discrete cryolesions: A new technique for the treatment of paroxysmal supraventricular tachycardia. J Thorac Cardiovasc Surg. 1982;84:554.
54 Holman W.L., Ikeshita M., Lease J.G., et al. Alteration of antegrade atrioventricular conduction by cryoablation of periatrioventricular nodal tissue. J Thorac Cardiovasc Surg. 1984;88:67.
55 Holman W.L., Ikeshita M., Lease J.G., et al. Cryosurgical modification of retrograde atrioventricular conduction: Implications for the surgical treatment of atrioventricular node reentry tachycardia. J Thorac Cardiovasc Surg. 1986;91:826.
56 Cox J.L., Holman W.L., Cain M.E. Cryosurgical treatment of atrioventricular node reentry tachycardia. Circulation. 1987;76:1329.
57 Stein K.M., Lerman B.B. Evidence for functionally distinct dual atrial inputs to the human AV node. Am J Physiol. 1994;267:H2333.
58 Langberg J.J., Leon A., Borganelli M., et al. A randomized, prospective comparison of anterior and posterior approaches to radiofrequency catheter ablation of atrioventricular nodal reentry tachycardia. Circulation. 1993;87:1551.
59 Jazayeri M.R., Akhtar M. Electrophysiological behavior of atrioventricular node after selective fast or slow pathway ablation in patients with atrioventricular nodal reentrant tachycardia. PACE Pacing Clin Electrophysiol. 1993;16:623.
60 Chen S.A., Chiang C.E., Tsang W.P., et al. Selective radiofrequency catheter ablation of fast and slow pathways in 100 patients with atrioventricular nodal reentrant tachycardia. Am Heart J. 1993;125:1.
61 Akhtar M., Jazayeri M.R., Sra J.S., et al. Atrioventricular nodal reentry: Clinical, electrophysiologic and therapeutic considerations. Circulation. 1993;88:282.
62 Steinbeck G., Hoffmann E. ‘True’ atrial tachycardia. Eur Heart J. 1998;19:E-10.
63 Saoudi N., Cosio F., Waldo A., et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanism and anatomical bases: A statement from a joint expert group from The Working Groups of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1162.
64 Hoffmann E., Reithmann C., Nimmermann P., et al. Clinical experience with electroanatomic mapping of ectopic atrial tachycardia. PACE Pacing Clin Electrophysiol. 2002;25:49.
65 Lai L.P., Lin J.L., Chen T.F., et al. Clinical, electrophysiological characteristics, and radiofrequency catheter ablation of atrial tachycardia near the apex of Koch’s triangle. PACE Pacing Clin Electrophysiol. 1998;21:367.
66 Chen S.A., Tai C.T., Chiang C.E., et al. Focal atrial tachycardia: Reanalysis of the clinical and electrophysiologic characteristics and prediction of successful radiofrequency ablation. J Cardiovasc Electrophysiol. 1998;9:355.
67 Schmitt C., Zrenner B., Schneider M., et al. Clinical experience with a novel multielectrode basket catheter in right atrial tachycardia. Circulation. 1999;99:2414.
68 Natale A., Breeding L., Tomassoni G., et al. Ablation of right and left ectopic atrial tachycardia using a three-dimensional nonfluoroscopic mapping system. Am J Cardiol. 1998;82:989.
69 Anguera I., Brugada J., Roba M., et al. Outcomes after radiofrequency catheter ablation of atrial tachycardia. Am J Cardiol. 2001;87:886.
70 Man K.C., Knight B., Tse H.F., et al. Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol. 2000;35:451.
71 Cossu S.F., Steinberg J.S. Supraventricular tachyarrhythmias involving the sinus node: Clinical and electrophysiologic characteristics. Prog Cardiovasc Dis. 1998;41:51.
72 Goya M., Iesaka Y., Takahashi A., et al. Radiofrequency catheter ablation for sinoatrial node reentrant tachycardia: Electrophysiologic features of ablation sites. Jpn Circ J. 1999;63:177.
73 Cheng J., Scheinman M.M. Acceleration of typical atrial flutter due to double-wave reentry induced by programmed electrical stimulation. Circulation. 1998;97:1589.
74 Cheng J., Cabeen W.R.Jr, Scheinman M.M. Right atrial flutter due to lower loop reentry: Mechanism and anatomic substrates. Circulation. 1999;99:1700.
75 Willems S., Weiss C., Ventura R., et al. Catheter ablation of atrial flutter guided by electroanatomic mapping (CARTO): A randomized comparison to the conventional approach. J Cardiovasc Electrophysiol. 2000;11:1223.
76 Kottkamp H., Hugl B., Krauss B., et al. Electromagnetic versus fluoroscopic mapping of the inferior isthmus for ablation of typical atrial flutter: A prospective randomized study. Circulation. 2000;102:2082.
77 Ward D.E., Xie B., Rowland E. Ablation of atrial flutter using the anatomical method: Results and long-term follow-up. J Interv Cardiol. 1995;8:697.
78 Tai C.T., Chen S.A., Chiang C.E., et al. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: Risk prediction of recurrent arrhythmia. J Cardiovasc Electrophysiol. 1998;9:115.
79 Lee S.H., Tai C.T., Yu W.C., et al. Effects of radiofrequency catheter ablation on quality of life in patients with atrial flutter. Am J Cardiol. 1999;84:278.
80 Anselmen F., Saoudi N., Poty H., et al. Radiofrequency catheter ablation of common atrial flutter: Significance of palpitations and quality-of-life evaluation in a patient with proven isthmus block. Circulation. 1999;99:534.
81 Natale A., Newby K.H., Pisano E., et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol. 2000;35:1898.
82 Nakagawa H., Shah N., Matsudaira K., et al. Characterization of re-entrant circuit in macro-re-entrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal” ablation. Circulation. 2001;103:699.
83 Shah D., Jais P., Takahashi A., et al. Dual-loop intra-atrial re-entry in humans. Circulation. 2000;101:631.
84 Duru F., Hindricks G., Kottkamp H. Atypical left atrial flutter after intraoperative radiofrequency ablation of chronic atrial fibrillation: Successful ablation using three-dimensional electroanatomic mapping. J Cardiovasc Electrophysil. 2001;12:602.
85 Akar J.G., Kok L.C., Haines D.E., et al. Coexistence of type I atrial flutter and intra-atrial reentrant tachycardia in patients with surgically corrected congenital heart disease. J Am Coll Cardiol. 2001;38:377.
86 Lev M., Gibson S., Miller D.A. Ebstein’s disease with Wolff-Parkinson-White syndrome. Am Heart J. 1955;49:724.
87 Henzi I., Sonderegger J., Tramer M.R. Efficacy, dose-response, and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anesth. 2000;47:537-551.
88 Bertolo L., Novakovic B.S., Penna M. Antiarrhythmic effects of droperidol. Anesthesiology. 1972;29:529.
89 Gomez-Arnau J., Mones J.M., Auello F. Fentanyl and droperidol effects on the refractoriness of the accessory pathway in the Wolff-Parkinson-White syndrome. Anesthesiology. 1983;58:307.
90 Sadowski A.R., Moyers J.R. Anesthetic management of the Wolff-Parkinson-White syndrome. Anesthesiology. 1979;51:553.
91 Suppan P. Althesin in the Wolff-Parkinson-White syndrome. Br J Anaesth. 1979;51:69.
92 Sharpe M.D., Dobkowski W.B., Murkin J.M. The electrophysiologic effects of volatile anesthetics on the normal AV conduction system and accessory pathways in WPW. Anesthesiology. 1994;80:63-70.
93 Dobkowski W.B., Murkin J.M., Sharpe M.D., et al. The effect of enflurane (IMAC) on the normal AV conduction system and accessory pathways. Anesth Analg. 1991;72:550.
94 Geha G.D., Rozelle B.C., Raessler K.L., et al. Pancuronium bromide enhances atrioventricular conduction in halothane-anesthetized dogs. Anesthesiology. 1977;46:342.
95 Kumazawa T. Wolff-Parkinson-White syndrome and anesthesia. Jpn J Anesthesiol. 1970;19:68.
96 Van der Starr P.J.A. Wolff-Parkinson-White syndrome during anesthesia. Anesthesiology. 1978;48:369.
97 Wyse D.G., Waldo A.L., DiMarco J.P., et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825.
98 Moe G.K. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn. 1962;140:183.
99 Boineau J.P., Schuessler R.B., Mooney C.R., et al. Natural and evoked atrial flutter due to circus movement in dogs: Role of abnormal atrial pathways, slow conduction, nonuniform refractory period distribution and premature beats. Am J Cardiol. 1980;45:1167.
100 Allessie M.A., Bonke F.I.M., Schopman F.G. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block as studied with multiple microelectrodes. Circ Res. 1976;39:168.
101 Cox J.L., Boineau J.P., Schuessler R.B., et al. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991;266:1976.
102 Cox J.L., Boineau J.P., Schuessler R.B., et al. Operations for atrial fibrillation. Clin Cardiol. 1991;14:827.
103 Haissaguerre M., Jais P., Shah D.C., et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659.
104 Yamauchi S., Tanaka S., Asano T., et al. Efficacy of combining mapping with surgery for atrial fibrillation. Rinsho Kyobu Geka. 1994;14:344-345.
105 Harada A., Sasaki K., Fukushima T., et al. Atrial activation during chronic atrial fibrillation in patients with isolated mitral valve disease. Ann Thorac Surg. 1996;61:104.
106 Schuessler R.B., Grayson T.M., Bromberg B.I., et al. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992;71:1254.
107 Yamauchi S., Boineau J.P., Schuessler R.B., Cox J.L. Varying types of circus movement reentry with both normal and dissociated contralateral conduction causing different right and left atrial rhythms in canine atrial flutter. Jpn Circ J. 1998;62:201.
108 Nitta T., Ishii Y., Miyagi Y., et al. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or reentrant activation as the mechanisms in atrial fibrillation. J Thorac Cardiovasc Surg. 2004;127:770.
109 Schuessler R.B. Do we need a map to get through the Maze? J Thorac Cardiovasc Surg. 2004;127:627.
110 Haissaguerre M., Shah D.C., Jais P., et al. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000;102:2463.
111 Pappone C., Rosario S., Oreto G., et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619.
112 Oral H., Scharf C., Chugh A., et al. Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355.
113 Haissaguerre M., Jais P., Shah D.C., et al. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1996;7:1132.
114 Jais P., Shah D.C., Haissaguerre M., et al. Efficacy and safety of septal and left-atrial linear ablation for atrial fibrillation. Am J Cardiol. 1999;84:R139.
115 Natale A., Leonelli F., Bheiry S., et al. Catheter ablation approach on the right side only for paroxysmal atrial fibrillation therapy: Long-term results. PACE Pacing Clin Electrophysiol. 2000;23:224.
116 Shah D.C., Haissaguerre M., Jais P., et al. Electrophysiological endpoint for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409.
117 Hsieh M.H., Chen S.A., Tai C.T., et al. Double multi-electrode mapping catheters facilitate radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. J Cardiovasc Electrophysiol. 1999;10:136.
118 Chen S.A., Hsieh M.H., Tai C.T., et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological response, and effect of radiofrequency ablation. Circulation. 1999;100:1879.
119 Haissaguerre M., Jais P., Shah D.C., et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409-1417.
120 Hsieh M.H., Chem S.A., Tai C.T., et al. Double multielectrode mapping catheters facilitate radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. J Cardiovasc Electrophysiol. 1999;10:136-144.
121 Cox J.L., Canavan T.E., Schuessler R.B., et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406.
122 Cox J.L., Schuessler R.B., D’Agostino H.J., et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569.
123 Cox J.L. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg. 1991;101:584.
124 Cox J.L., Boineau J.P., Schuessler R.B., et al. Modification of the Maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg. 1995;110:473.
125 Cox J.L., Jaquiss R.D.B., Schuessler R.B., et al. Modification of the Maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the Maze III procedure. J Thorac Cardiovasc Surg. 1995;110:485.
126 Pasic M., Musci M., Siniawski H.K., et al. Transient sinus node dysfunction after the Cox-Maze III procedure in patients with organic heart disease and chronic fixed atrial fibrillation. J Am Coll Cardiol. 1998;32:1040.
127 Gillinov A.M., Blackstone E.H., McCarthy P.M. Atrial fibrillation: Current surgical options and their assessment. Ann Thorac Surg. 2002;74:2210.
128 Doll N., Borger M.A., Fabricius A., et al. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high? J Thorac Cardiovasc Surg. 2003;125:836.
129 Kim Y.H., Sosa-Suarez G., Trouton T.G., et al. Treatment of ventricular tachycardia by transcatheter radiofrequency ablation in patients with ischemic heart disease. Circulation. 1994;89:1094.
130 Albåge A., van der Linden J., Bengtsson L., et al. Elevations in antidiuretic hormone and aldosterone as possible causes of fluid retention in the Maze procedure. Ann Thorac Surg. 2001;72:58.
131 Ad N., Suyderhoud J.P., Kim Y.D., et al. Benefits of prophylactic continuous infusion of furosemide after the Maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2002;123:232.
132 Cox J.L., Schuessler R.B., Lappas D.G., Boineau J.P. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg. 1996;224:267.
133 Isobe F., Kawashima Y. The outcome and indications of the Cox Maze III procedure for chronic atrial fibrillation with mitral valve disease. J Thorac Cardiovasc Surg. 1998;116:220.
134 Feinberg M.S., Waggoner A.D., Kater K.M., et al. Restoration of atrial function after the Maze procedure for patients with atrial fibrillation: Assessment by Doppler echocardiography. Circulation. 1994;90(Suppl II):II-285.
135 Tsui S.S., Grace A.A., Ludman P.F., et al. Maze 3 for atrial fibrillation: Two cuts too few? PACE Pacing Clin Electrophysiol. 1994;17:2163.
136 Nitta T., Lee R., Schuessler R.B., et al. Radial approach: A new concept in surgical treatment for atrial fibrillation. I. Concept, anatomic and physiologic bases and development of the procedure. Ann Thorac Surg. 1999;67:27.
137 Nitta T., Lee R., Watanabe H., et al. Radial procedure: A new concept in surgical treatment for atrial fibrillation. II. Electrophysiologic effects and atrial contribution to ventricular filling. Ann Thorac Surg. 1999;67:36.
138 Omari B.O., Nelson R.J., Robertson J.M. Effect of right atrial appendectomy on the release of atrial natriuretic hormone. J Thorac Cardiovasc Surg. 1991;102:272.
139 The Antiarrhythmics Versus Implantable Defibrillator (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576.
140 Calkins H., Kalbfleisch S.J., el-Atassi R., et al. Relation between efficacy of radiofrequency catheter ablation and site of origin of idiopathic ventricular tachycardia. Am J Cardiol. 1993;71:827.
141 Coggins D.L., Lee R.J., Sweeney J., et al. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994;23:1333.
142 Thakur R.K., Klein G.J., Sivaram C.A., et al. Anatomic substrate for idiopathic left ventricular tachycardia. Circulation. 1996;93:497.
143 Prystowsky E.N., Miles W.M., Evans J.J., et al. Induction of ventricular tachycardia during programmed electrical stimulation: Analysis of pacing methods. Circulation. 1986;73(Suppl II):II-132.
144 Bigger J.T., Reiffel J.A., Livelli F.D.Jr, et al. Sensitivity, specificity, and reproducibility of programmed ventricular stimulation. Circulation. 1986;73(Suppl II):II.
145 Stevenson W.G., Sager P.T., Friedman P.L. Entrainment techniques for mapping atrial and ventricular tachycardias. J Cardiovasc Eletrophysiol. 1995;6:201.
146 Marchlinski F.E., Callans D.J., Gottlieb C.D., et al. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288.
147 Mehdirad A.A., Keim S., Rist K., Tchou P. Long-term clinical outcome of right bundle branch radiofrequency catheter ablation for treatment of bundle branch reentrant ventricular tachycardia. Pacing Clin Electrophysiol. 1995;18:2135.
148 Rothman S.A., Hsia H.H., Cossu S.F., et al. Radiofrequency catheter ablation of postinfarction ventricular tachycardia: Long-term success and the significance of inducible nonclinical arrhythmias. Circulation. 1997;96:3499.
149 Callans D.J., Zado E., Sarter B.Y., et al. Efficacy of radiofrequency catheter ablation for ventricular tachycardia in healed myocardial infarction. Am J Cardiol. 1998;82:42.
150 Stevenson W.G., Friedman P.L., Kocovic D., et al. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998;98:308.
151 Stevenson W.G., Khan H., Sage P., et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647.
152 Gonska B.D., Cao K., Schaumann A., et al. Catheter ablation of ventricular tachycardia in 136 patients with coronary artery disease: Result and long-term follow-up. J Am Coll Cardiol. 1994;24:1506.
153 Jadonath R.L., Snow J.S., Goldner B.G., Cohen T.J. Radiofrequency catheter ablation as primary therapy for symptomatic ventricular tachycardia. J Invasive Cardiol. 1994;6:289.
154 Haffajee C., Love J., Canada A., et al. Clinical pharmacokinetics and efficacy of amiodarone for refractory tachyarrhythmias. Circulation. 1981;67:1347-1355.
155 Gallagher J.D., Lieberman R.W., Meranze J., et al. Amiodarone-induced complications during coronary artery surgery. Anesthesiology. 1981;55:186.
156 Liberman B.A., Teasdale S.J. Anesthesia and amiodarone. Can Anaesth Soc J. 1985;32:629.
157 Feinberg B.I., LaMantia K.R. Ventricular tachyarrhythmias during placement of pulmonary artery catheters in patients with recurrent ventricular tachycardia. Mt Sinai J Med. 1986;53:545.
158 Schmid J.P., Rosengant T.K., McIntosh C.L., et al. Amiodarone-induced complications after cardiac operation for obstructive hypertrophic cardiomyopathy. Ann Thorac Surg. 1989;48:359.
159 Greenspon A.J., Kidwell G.A., Hurley W., Mannion J. Amiodarone-related postoperative adult respiratory distress syndrome. Circulation. 1991;84(Suppl III):III-1407.
160 Eliot G.C., Zimmerman G.A., Clemmen T.P. Complications of pulmonary artery catheterization in the case of critically ill patients. Chest. 1979;76:647.
161 Kroll D.A., Knight P.R. Antifibrillatory effects of volatile anesthetics in acute occlusion/reperfusion arrhythmias. Anesthesiology. 1984;61:657.
162 Turner L.A., Bosnjak Z.J., Kampine J.P. Electrophysiological effects of halothane on Purkinje fibers from normal and infarcted canine hearts. Anesthesiology. 1987;67:619.
163 Hunt G.B., Ross D.L. Comparison of effects of three anesthetic agents on induction of ventricular tachycardia in a canine model of myocardial infarction. Circulation. 1988;78:221.
164 Deutsch N., Huntler C.B., Tait A.R., et al. Suppression of ventricular arrhythmias by volatile anesthetics in a canine model of chronic myocardial infarction. Anesthesiology. 1990;72:1012.
165 MacLeod B.A., Augerean P., Walker M.J.A. Effects of halothane anesthesia compared with fentanyl anesthesia and no anesthesia during coronary ligation in rats. Anesthesiology. 1983;58:44.
166 Denniss A.R., Richards D.A., Taylor A.T., Uther J.B. Halothane anesthesia reduces inducibility of ventricular tachyarrhythmias in chronic canine myocardial infarction. Basic Res Cardiol. 1989;84:5.
167 Atlee J.L. Halothane: Cause or cure for arrhythmias. Anesthesiology. 1987;67:617.
168 Saini V., Carr D.B., Hagestod E.L., et al. Antifibrillatory action of the narcotic agonist fentanyl. Am Heart J. 1988;115:598.
169 DeSilva R.A., Verrier R.L., Lown B. Protective effect of the vagotonic action of morphine sulphate or ventricular vulnerability. Cardiovasc Res. 1978;12:167.
170 Atlee J.L., Bernstein A.D. Cardiac rhythm management devices. Part I. Anesthesiology. 2001;95:1265.
171 Saksena S., An H., Mehra R., et al. Prospective comparison of biphasic and monophasic shocks for implantable cardioverter-defibrillators using endocardial leads. Am J Cardiol. 1992;70:304.
172 Epstein A.E. An update on implantable cardioverter-defibrillator guidelines. Curr Opin Cardiol. 2004;19:23-25.
173 The Antiarrhythmics Versus Implantable Defibrillator (AVID) Investigators. A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated for near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576.
174 Connolly S.J., Gent M., Roberts R.S., et al. Canadian Implantable Defibrillator Study (CIDS): A randomized trial of the implantable cardioverter-defibrillator against amiodarone. Circulation. 2000;101:129.
175 Kuck K.H., Capitol R., Seibel J., et al. Randomized comparisons of anti-arrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748.
176 Moss A.J., Hall W.J., Cannon D.S., et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia: Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933.
177 Buxton A.E., Lee K.L., Fisher J.D., et al. A randomized study of the prevention of sudden death in patients with coronary artery disease: Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882.
178 Bardy G.H., Lee K.L., Mark D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
179 Zareba W., Moss A.J., Schwartz P.J., et al. Influence of genotype on the clinical course of the long-QT syndrome: International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960.
180 Watkins H., McKenna W.J., Thierfelder L., et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058.
181 Adams D.C., Heyer E.J., Emerson R.G., et al. Implantable cardioverter-defibrillator. Evaluation of clinical neurologic outcome and electroencephalographic changes during implantation. J Thorac Cardiovasc Surg. 1995;109:565.
182 Meyer J., Mollhoff T., Seifert T., et al. Cardiac output is not affected during testing of the AICD. J Cardiovasc Electrophysiol. 1996;7:211.
183 Hurst T.M., Hinrichs M., Breidenbach C., et al. Detection of myocardial injury during transvenous implantation of automatic cardioverter-defibrillators. J Am Coll Cardiol. 1999;34:402.