Chapter 16 Cardiac Disease and Pregnancy
Cardiac disease complicates approximately 1% to 2% of all pregnancies and is a leading cause of maternal death.1,2 Historically, rheumatic valvular heart disease, particularly mitral stenosis, has been the predominant cause of heart disease in pregnancy, and in developing countries this is still the case. However, in the United States and other developed nations, corrected or palliated congenital heart disease is becoming more common. In this chapter the management of pregnant patients with cardiac disease is reviewed briefly.
PHYSIOLOGIC CHANGES DURING PREGNANCY
Pregnancy and labor impose a huge metabolic burden on the mother; the result is profound alterations in maternal physiology, particularly of the cardiovascular system.2–4 These physiologic changes begin about week 6, become maximal early in the third trimester (about week 30), and thereafter remain relatively stable until labor.
Labor
Cardiac output rises throughout labor, particularly during uterine contractions. Contractions cause the expulsion of blood from the uteroplacental capacitance vessels, resulting in an autotransfusion of 300 to 500 ml of blood. Cardiac output remains elevated by 10% to 20% above predelivery levels for 24 to 48 hours after delivery, then slowly declines to prepregnancy levels over the next 10 to 14 days. Uterine contractions are associated with acute increases in heart rate and blood pressure. These hemodynamic effects are exacerbated by maternal pain and anxiety and minimized by epidural analgesia. Maternal blood loss averages 300 to 400 ml in a normal vaginal delivery and 500 to 800 ml in a cesarean section.
CARDIAC DISEASE DURING PREGNANCY
A number of kinds of cardiac lesions are associated with increased risk for adverse outcome during pregnancy (Table 16-1).5–8 In general, regurgitant valvular lesions are better tolerated than stenotic lesions. With a stenotic lesion, in which cardiac output is relatively fixed, the vasodilatation and increased preload associated with pregnancy can result in hypotension and pulmonary edema. In contrast, vasodilatation and increased preload in the setting of a regurgitant lesion tend to encourage forward flow. However, any valve lesion causing New York Heart Association (NYHA) class III or IV symptoms prior to pregnancy is unlikely to be compatible with progress to term. Some conditions, notably Eisenmenger syndrome and severe cyanotic heart disease, are associated with such a high maternal and fetal mortality rate that pregnancy is inadvisable.
Table 16-1 Cardiac Lesions Associated With High Maternal and Fetal Risk During Pregnancy
| Cyanotic heart disease |
| Severe aortic stenosis with or without symptoms |
| Mitral stenosis with NYHA functional class II to class IV symptoms |
| Mitral or aortic regurgitation with NYHA functional class III to class IV symptoms |
| Valvular heart disease resulting in severe pulmonary hypertension (pulmonary artery pressure > 75% systemic pressure) |
| Left ventricular dysfunction (ejection fraction < 40%) |
| Mechanical prosthetic valve requiring anticoagulation |
| Aortic regurgitation in Marfan syndrome |
From Siu SC, Sermer M, Harrison DA, et al: Risk and predictors for pregnancy-related complications in women with heart disease. Circulation 96:2789-2794, 1997; and from Bonow RO, Carabello B, de Leon AC, et al: ACC/AHA guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee on Management of Patients With Valvular Heart Disease]. J Am Coll Cardiol 32:1486-1588, 1998. NYHA, New York Heart Association.
Mitral Stenosis
Rheumatic mitral stenosis (see Chapter 10) is the valve lesion that most commonly complicates pregnancy. The increased circulating volume and heart rate that occur during pregnancy can cause pulmonary congestion or frank pulmonary edema. The development of rapid atrial fibrillation can precipitate acute cardiac decompensation.
Severe Mitral Stenosis
Patients with severe mitral stenosis should be considered for percutaneous valvuloplasty or, if the mitral anatomy is unsuitable, for mitral valve surgery prior to conception. Patients who develop persistent heart failure during pregnancy should be considered for percutaneous valvuloplasty during the second trimester,9 or if the mitral anatomy is unsuitable (see Chapter 10), for open mitral valve surgery. Excellent results can usually be obtained with percutaneous valvuloplasty, and complications are rare.
Aortic Stenosis
Aortic stenosis (see Chapter 10) is an uncommon problem in pregnancy. Patients with known severe aortic stenosis should undergo valve replacement prior to conception—preferably involving a homograft or tissue valve, thus eliminating the need for warfarin.
Severe aortic stenosis presents a very high risk for maternal and fetal mortality. If symptoms related to aortic stenosis are present in the first trimester, termination of the pregnancy should be considered. If the pregnancy proceeds, intervention in the form of percutaneous valvuloplasty or valve replacement will be required during the second trimester. In contrast to percutaneous mitral valvuloplasty, aortic valvuloplasty is associated with a high rate of procedural complications (particularly aortic regurgitation) and provides only limited benefit. Patients with bicuspid aortic valve are at increased risk for aortic dissection during pregnancy.
Mitral and Aortic Regurgitation
Mitral regurgitation (see Chapter 10) as a complication of pregnancy is most commonly caused by mitral valve prolapse. Chronic aortic regurgitation in young women is usually associated with aortic root dilatation (e.g., Marfan syndrome) or with a bicuspid or rheumatic valve. In patients who are in NYHA functional class I or II prior to conception, mitral and aortic regurgitation are usually well tolerated during pregnancy. Treatment involves diuretics and, if vasodilation is required, hydralazine or a calcium channel blocker. Angiotensin-converting enzyme inhibitors are associated with fetal toxicity secondary to fetal hypotension and decreased renal blood flow, resulting in anuria-associated oligohydramnios. Thus, angiotensin-converting enzyme inhibitors are contraindicated in pregnancy. Surgery for mitral or aortic regurgitation during pregnancy should be considered only for patients with NYHA class III or IV symptoms.
Marfan Syndrome
Patients with Marfan syndrome5 (see Chapter 11) are at increased risk for aortic dissection and rupture during pregnancy, even in the absence of aortic aneurysm. An ascending aortic dimension greater than 5 cm is an indication for elective repair prior to conception. Patients with Marfan syndrome who are considering pregnancy should undergo an echocardiogram prior to becoming pregnant and should receive prophylactic β blockers during pregnancy. In the absence of any detectable cardiac abnormalities, it is reasonable to plan on vaginal delivery. A carefully titrated epidural and an assisted delivery reduce the need for pushing during labor. Thus, acute increases in blood pressure, which put the patient at risk for aortic dissection, are avoided. If an aortic root diameter greater than 4 cm is discovered during pregnancy, some authorities recommend termination of the pregnancy and surgical repair. If the pregnancy proceeds, blood pressure should be tightly controlled. It may be prudent to avoid labor by performing an elective cesarean section.
Cardiopulmonary Bypass During Pregnancy
Cardiac surgery is only rarely required during pregnancy,10,11 most commonly for a decompensated valvular lesion. Although cardiac surgery and cardiopulmonary bypass (CPB) can be carried out with a relatively low maternal mortality rates (around 3%), fetal mortality rates are high (around 20%). If possible, surgery should be delayed until the fetus is viable (≥24 weeks’ gestation) and for 48 hours after corticosteroids have been administered to promote fetal lung maturity, at which time cesarean section and cardiac surgery can be carried out as a combined procedure, with the cesarean section being performed first.
Anticoagulation
Anticoagulation5,12,13 during pregnancy is required for patients with mechanical heart valves, atrial fibrillation, or both. However, anticoagulation in pregnancy is problematic for a number of reasons. Pregnancy represents a hypercoagulable state. Maternal thromboembolism occurs in 4% to 14% of patients with mechanical heart valves, despite adequate anticoagulation, and it has high maternal mortality rates. Warfarin can cause fetal hemorrhage and is teratogenic if used during weeks 6 through 12 of pregnancy. Heparins do not cross the placenta and are not teratogenic, but there are problems with their use in pregnancy. Unfractionated heparin has been used extensively for anticoagulation during pregnancy. However, it is difficult to maintain satisfactory anticoagulation with this drug, and its use has been associated with catastrophic valve thrombosis resulting in maternal death. Furthermore, prolonged use of unfractionated heparin is associated with thrombocytopenia and osteoporosis. Low molecular weight heparins (LMWHs) are an attractive option because of their prolonged duration of action, their more predictable antithrombotic effects, and their minimal bone and platelet effects. Because the pharmacokinetics of LMWHs change during pregnancy, the monitoring of antifactor Xa levels and dose adjustment to ensure therapeutic anticoagulation are recommended. However, there are only limited data to support their use and, at least for enoxaparin, use in pregnancy is not supported by the manufacturer.14
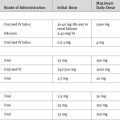
Thus, anticoagulation during pregnancy is controversial, and a number of regimens are used. Warfarin may be used throughout the pregnancy (aiming for INR 2.5 to 3.5 for mechanical valves) except during weeks 6 through 12 and beyond 36 weeks, when heparin can be substituted. Unfractionated heparin may be administered either subcutaneously (17,500 to 20,000 IU twice daily, adjusted to prolong the aPTT to 2 to 3 times normal) or as a continuous intravenous infusion (dose-adjusted to prolong the aPTT to 2 to 3 times normal). LMWH is also used subcutaneously twice daily, adjusted to maintain therapeutic antifactor Xa levels. Adjunctive aspirin is also recommended with all regimens. Delivery should be planned and the patient converted to intravenous heparin at least 36 hours prior to induction of labor or cesarean section. Heparin should then be stopped at least 4 hours prior to labor or surgery. If labor begins while the mother is receiving warfarin, anticoagulation should be reversed and cesarean delivery should be performed.8 Heparin is restarted 4 to 6 hours after delivery and warfarin is resumed the night after delivery if there are no bleeding complications.
Endocarditis Prophylaxis
The American College of Cardiology/American Heart Association guidelines5 do not recommend that antibiotic prophylaxis be given to patients with valvular heart disease who are undergoing uncomplicated vaginal or cesarean delivery unless infection is suspected. However, antibiotics may be considered in patients at high risk for endocarditis (Table 10-8).
MANAGEMENT IN THE INTENSIVE CARE UNIT
Maternal Cardiac and Respiratory Decompensation
Data concerning the effects of inotropic agents on placental blood flow are conflicting and are derived mainly from animal studies.3 Catecholamines with α1-receptor activity cause uterine vasoconstriction. Drugs with β2-receptor activity cause uterine vasodilatation but may exacerbate hypotension and cause uterine relaxation, which may be good in the presence of premature labor but bad during the postpartum period. In one study, milrinone did not adversely affect uterine blood flow.15 It is interesting to note that, in contrast to common opinion, the use of the α1-agonists metaraminol and phenylephrine to treat hypotension due to spinal anesthesia has been associated with improved fetal pH measurements as compared to ephedrine, a mixed α and β agonist.16,17 For the treatment of low cardiac output, an inodilating drug (e.g., dobutamine, milrinone, low-dose epinephrine) may be used. Inoconstricting drugs such as norepinephrine should be avoided unless there is documented inappropriate vasodilatation.
The indications for mechanical ventilation are the same as those for the nonpregnant patient. Intubation should be performed as a rapid-sequence induction (see Chapter 40), and the clinician should be prepared for brisk desaturation (due to the low functional residual capacity and high metabolic rate), difficult intubation (due to body habitus, the lateral tilt position, and airway edema), and the reflux of gastric contents. A smaller than usual endotracheal tube should be available (i.e., 7.0 to 7.5 mm internal diameter). Minute ventilation must be 20% to 40% higher than normal. Assuming high airway pressures are not required, it is reasonable to ventilate to an arterial carbon dioxide tension of 4 kPa (30 mmHg), the normal value for pregnancy.
Cardiac Arrest
Several factors make cardiac resuscitation difficult in the pregnant patient.18,19 Aortocaval compression contributes to hemodynamic instability when the patient is in the supine position, but external cardiac massage is less effective when in the lateral tilt position. Hypoxemia develops rapidly, and there is a high risk for aspiration of gastric contents. Endotracheal intubation may be difficult. External cardiac compression and bag-mask ventilation are unable to meet the high metabolic requirements of the mother and the fetus. Epinephrine causes marked uterine vasoconstriction. Despite these problems, standard resuscitation protocols should be employed. In addition to placing a wedge under the right hip, manual sideways traction may be applied directly to the abdomen to reduce the effect of aortocaval compression.
1 Cooper GM, Lewis G, Neilson J. Confidential enquiries into maternal deaths, 1997-1999. B J Anaesth. 2002;89:369-372.
2 Gei AF, Hankins GD. Cardiac disease and pregnancy. Obstet Gynecol Clin North Am. 2001;28:465-512.
3 Lapinsky SE, Kruczynski K, Slutsky AS. Critical care in the pregnant patient. Am J Respir Crit Care Med. 1995;152:427-455.
4 Naylor DFJr, Olson MM. Critical care obstetrics and gynecology. Crit Care Clin. 2003;19:127-149.
5 Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease). J Am Coll Cardiol. 1998;32:1486-1588.
6 Siu SC, Sermer M, Harrison DA, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. 1997;96:2789-2794.
7 Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515-521.
8 Reimold SC, Rutherford JD. Clinical practice: valvular heart disease in pregnancy. N Engl J Med. 2003;349:52-59.
9 Martinez-Reding J, Cordero A, Kuri J, et al. Treatment of severe mitral stenosis with percutaneous balloon valvotomy in pregnant patients. Clin Cardiol. 1998;21:659-663.
10 Parry AJ, Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 1996;61:1865-1869.
11 Pomini F, Mercogliano D, Cavalletti C, et al. Cardiopulmonary bypass in pregnancy. Ann Thorac Surg. 1996;61:259-268.
12 Born D, Martinez EE, Almeida PA, et al. Pregnancy in patients with prosthetic heart valves: the effects of anticoagulation on mother, fetus, and neonate. Am Heart J. 1992;124:413-417.
13 Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000;160:191-196.
14 Ginsberg JS, Chan WS, Bates SM, et al. Anticoagulation of pregnant women with mechanical heart valves. Arch Intern Med. 2003;163:694-698.
15 Santos AC, Baumann AL, Wlody D, et al. The maternal and fetal effects of milrinone and dopamine in normotensive pregnant ewes. Am J Obstet Gynecol. 1992;166:257-262.
16 Ngan Kee WD, Lau TK, Khaw KS, et al. Comparison of metaraminol and ephedrine infusions for maintaining arterial pressure during spinal anesthesia for elective cesarean section. Anesthesiology. 2001;95:307-313.
17 Thomas DG, Robson SC, Redfern N, et al. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:61-65.
18 Whitty JE. Maternal cardiac arrest in pregnancy. Clin Obstet Gynecol. 2002;45:377-392.
19 Rees GA, Willis BA. Resuscitation in late pregnancy. Anaesthesia. 1988;43:347-349.