5.9 Cardiac Arrhythmias
Normal conduction system
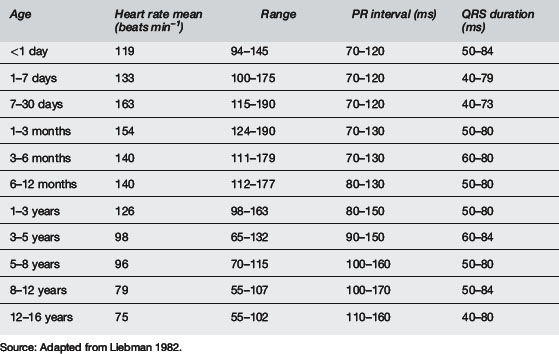
The SA node is normally the dominant (fastest) cardiac pacemaker but this can change if sinus node dysfunction occurs or if other parts of the conduction system develop increased automaticity. As stated above, the SA node is located near the junction of the superior vena cava (SVC) and the right atrium. The SA node is innervated by both sympathetic and parasympathetic nerve endings. Parasympathetic tone predominates during rest. Children are known to have relatively greater parasympathetic tone than adults and are also known to have developmental and age-dependent differences in action potential amplitude and conduction speed. Accordingly, there are age-dependent normal values for resting heart rate as well as PR interval and QRS duration, in addition to many other electrophysiology parameters (see Table 5.9.1).
The cardiac action potential
The cardiac action potential is divided into five phases: 0, 1, 2, 3, 4.
Pathogenesis of arrhythmias
General principles for arrhythmia management
 If patient has an acute arrhythmia with haemodynamic compromise (e.g. shock or loss of consciousness), assess whether the rhythm is fast and/or disorganised or slow and/or irregular. If the rhythm is fast or disorganised, cardioversion or defibrillation should take priority. If slow and/or irregular, cardiopulmonary resuscitation should commence.
If patient has an acute arrhythmia with haemodynamic compromise (e.g. shock or loss of consciousness), assess whether the rhythm is fast and/or disorganised or slow and/or irregular. If the rhythm is fast or disorganised, cardioversion or defibrillation should take priority. If slow and/or irregular, cardiopulmonary resuscitation should commence. Obtain venous access and draw blood for biochemistry, specifically Na, K, Ca, Mg and blood sugar level.
Obtain venous access and draw blood for biochemistry, specifically Na, K, Ca, Mg and blood sugar level.Sinus bradycardia
Sinus node dysfunction
Conduction disturbances: atrioventricular block
Wide complex tachyarrhythmia
Ventricular fibrillation
 There is debate about the applicability and safety of automated external defibrillators for use in young children.
There is debate about the applicability and safety of automated external defibrillators for use in young children. Waveform analysis has the potential to reliably predict success of defibrillation and if so allow treatment alteration to improve outcome.
Waveform analysis has the potential to reliably predict success of defibrillation and if so allow treatment alteration to improve outcome.Alexander M.E., Berul C.I. Ventricular arrhythmias: When to worry. Pediatr Cardiol. 2000;21(6):532-541.
Anonymous. The International Liason Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for paediatric and neonatal patients: paediatric basic and advanced life support. Pediatrics. 2006;117(5):e955-e977.
Bink-Boelkens M.T.E. Pharmacologic management of arrhythmias. Pediatr Cardiol. 2000;21(6):508-515.
Celiker A., Ayabakan C., Ozer S., et al. Sotalol in the treatment of pediatric cardiac arrhythmias. Pediatr Int. 2001;43:624-630.
Chun T.U., Dehart G.F. Advances in the approach to treatment of supraventricular tachycardia in the pediatric population. Curr Cardiol Rep. 2004;6:322-326.
Kudenchuk P.J., Cobb L.A., Copass M.K., et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341(12):871-878.
Liebman J. Tables of normal standards. In: Liebman J., Plonsey R., Gillette P.C., editors. Pediatric electrocardiography. 1st ed. Baltimore: Williams & Wilkins; 1982:82-133.
Luedtke S.A., Kuhn R.J., McCaffrey F.M. Pharmacologic management of supraventricular tachycardias in children. Part 1, WPW and AV nodal re-entry. Ann Pharmacother. 1997;31:1227-1243.
Luedtke S.A., Kuhn R.J., McCaffrey F.M. Pharmacologic management of supraventricular tachycardias in children. Part 2, Atrial flutter, atrial fibrillation and junctional and atrial ectopic tachycardia. Ann Pharmacother. 1997;31:1347-1359.
McKee M.R. Amiodarone – an ‘old’ drug with new recommendations. Curr Opin Pediatr. 2004;15:193-199.
Saul J.P., Scott W.A., Brown S., et al. Intravenous amiodarone for incessant tachyarrhythmias in children: a randomized, double-blind, antiarrhythmia drug trial. Circulation. 2005;112:3470-3477.