Carcinoid syndrome
1. What are carcinoid tumors? How are they classified?
Carcinoid tumors are neoplasms that arise from enterochromaffin cells. They are classified according to their site of origin as foregut (bronchus, stomach, duodenum, bile ducts, pancreas), midgut (jejunum, ileum, appendix, ascending colon), or hindgut (transverse and descending colon, rectum) carcinoids. They also develop in the ovaries, testes, prostate, kidney, breast, thymus, or skin. Approximately 55% of carcinoid tumors occur in the gastrointestinal (GI) tract, and 30% are found in the bronchopulmonary system. Of those tumors in the GI tract, the location frequencies are as follows: small intestine (45%, most commonly the ileum), rectum (20%), appendix (16%), colon (11%), and stomach (7%).
Carcinoid syndrome is a humorally mediated disorder consisting of cutaneous flushing (90%), diarrhea (75%), bronchospasm (20%), endocardial fibrosis (33%), right-sided heart valvular lesions, and occasionally pleural, peritoneal, or retroperitoneal fibrosis.
3. What are the biochemical mediators of carcinoid syndrome?
Carcinoid tumors produce a variety of humoral mediators, including serotonin, chromogranin A, neuron-specific enolase (NSE), histamine, prostaglandins, bradykinin, tachykinins, neurotensin, motilin, and substance P. Diarrhea and fibrous tissue formation may be caused by serotonin, whereas flushing and wheezing are likely the result of histamine, prostaglandins, or kinins (Fig. 54-1). The formation and metabolism of serotonin are shown in Figure 54-2.
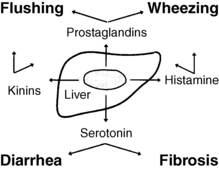
Figure 54-1. Carcinoid syndrome.
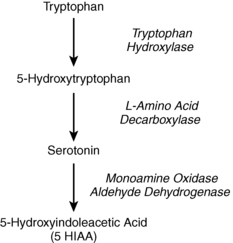
Figure 54-2. Serotonin metabolism.
4. Why does pellagra often accompany carcinoid syndrome?
Pellagra is caused by niacin deficiency that results when a carcinoid tumor diverts large amounts of tryptophan from niacin synthesis to produce serotonin (see Fig. 54-2).
5. Why do intestinal carcinoid tumors so infrequently cause carcinoid syndrome?
Carcinoid syndrome occurs when humoral mediators enter the systemic circulation in large quantities. Solitary intestinal carcinoids secrete mediators into the portal circulation, where they are almost totally metabolized by the liver and never reach the systemic circulation. Carcinoid syndrome does not usually occur with these tumors unless the patient has hepatic metastases that impair mediator metabolism or that secrete mediators directly into the hepatic vein. Extraintestinal carcinoids, however, may cause carcinoid syndrome in the absence of metastases because they secrete mediators into venous systems that do not first pass through the liver.
6. Do carcinoid tumors cause any other humoral syndromes?
Carcinoids may also secrete corticotropin-releasing factor (CRF) or corticotropin (adrenocorticotropin [ACTH]), thus causing Cushing’s syndrome, or growth hormone–releasing factor (GRF), thus causing acromegaly. These syndromes have been reported mainly with bronchial and pancreatic carcinoid tumors.
7. How is the diagnosis of carcinoid syndrome usually made?
The diagnosis is made by finding markedly elevated urinary excretion of 5-hydroxyindoleacetic acid (5-HIAA), a breakdown product of serotonin (see Fig. 54-2). Normal urinary 5-HIAA excretion is less than 8 mg/24 hours. Malabsorption syndromes, ingestion of tryptophan-rich foods, and use of certain medications can elevate urinary 5-HIAA, but the value usually remains lower than 30 mg/24 hours (Table 54-1). Carcinoid syndrome is most often associated with urinary 5-HIAA excretion greater than 100 mg/24 hours, although mild or no elevation may be seen in some patients.
TABLE 54-1.
NONCARCINOID CAUSES OF ABNORMAL 5-HYDROXYINDOLEACETIC ACID EXCRETION
Diseases: malabsorption disorders
Food (tryptophan rich) : bananas, pineapples, kiwi, plums, avocados, eggplant, pecans, walnuts, hickory nuts
Medications that increase 5-HIAA : acetaminophen, ephedrine, guaifenesin, mephenesin, methocarbamol, phenacetin, caffeine, nicotine, methamphetamine, phenobarbital, acetanilid, reserpine, phentolamine, phenmetrazine, coumaric acid, melphalan, fluorouracil
Medications that decrease 5-HIAA : aspirin, ethanol, heparin, imipramine, levodopa, methyldopa, monoamine oxidase inhibitors, phenothiazines, isoniazid, corticotropin, gentisic acid, methenamine, streptozotocin
8. How is carcinoid syndrome diagnosed if urinary 5-HIAA is normal?
Foregut carcinoid tumors often lack the enzyme L-amino acid decarboxylase and therefore do not make serotonin or 5-HIAA (see Fig. 54-2). Chromogranin A, a sensitive but nonspecific marker of neuroendocrine tumors, is a good alternative test. Mild elevations may occur in many conditions (Table 54-2), but values greater than 31 U/L have 75% sensitivity and 84% specificity for diagnosing carcinoid syndrome. Whole blood serotonin and NSE, when available, may also be helpful in equivocal cases.
TABLE 54-2.
CAUSES OF ELEVATED SERUM CHROMOGRANIN A
Neuroendocrine disorders: carcinoid tumors, pheochromocytoma, islet cell tumors, medullary carcinoma of the thyroid, neurofibromatosis
Other conditions: prostate cancer, hyperthyroidism, renal failure, heart failure, hypertension, atrophic gastritis
Medications: proton pump inhibitors
9. What procedures are best to localize the source of carcinoid syndrome?
Abdominal computed tomography (CT) scan and octreotide scintigraphy (OctreoScan) are currently the most accurate options. When these scans are negative or not feasible, positron emission tomography (PET) with fluorine-18 [18F]-fluorodeoxyglucose (FDG-PET) is often helpful. Other specific functional PET imaging agents, such as 6-[18-F]-fluorodopa and carbon-11 [11C]-5- hydroxytryptophan, are in development but are not yet available.
10. What is the treatment for carcinoid syndrome?
Surgery can be curative when carcinoid syndrome results from a carcinoid tumor that has not metastasized. However, approximately 90% of patients with carcinoid syndrome have extensive metastases at the time of diagnosis. The usual goals of therapy are therefore is to provide palliation and to prolong survival.
11. How does one control carcinoid syndrome symptoms?
The most troublesome symptoms patients with carcinoid syndrome experience are intense flushing and frequent diarrhea. Somatostatin analogs (octreotide, lanreotide) are often highly effective in controlling carcinoid symptoms. Other antiflushing and antidiarrheal strategies can be added if symptom control is inadequate. Table 54-3 lists various medication options for relief of refractory symptoms. Hepatic resection can be very effective in patients with focal liver metastases. Other options include hepatic artery embolization, chemoembolization, 90-Y microsphere radioembolization, radiolabeled somatostatin analog therapy, and alpha-interferon.
12. What chemotherapy regimens are most effective in carcinoid tumors?
Cytotoxic chemotherapy has produced disappointing results in patients with carcinoid syndrome. Etoposide and cisplatin combination therapy is the most commonly used regimen for poorly differentiated carcinoid tumors. Everolimus, an antagonist of mammalian target of rapamycin (mTOR), and vascular endothelial growth factor receptor (VEGF-R) antagonists, such as Vatalanib, Sunitinib, Sorafenib, and bevacizumab, are also under investigation. Other agents that have been used with limited success include streptozotocin, 5-fluorouracil, lomustine, doxorubicin, and dacarbazine.
13. What is a carcinoid crisis?
Carcinoid crisis is a life-threatening episode of hypotension, flushing, and bronchospasm that is triggered most often by tumor manipulation or anesthesia and less commonly by chemotherapy, hepatic artery embolization, or radionuclide therapy. It can also be provoked by the administration of adrenergic agents, such as epinephrine and sympathomimetic amines, or monoamine oxidase (MAO) inhibitors in patients with underlying carcinoid tumors.
14. How can a carcinoid crisis be prevented?
Epinephrine, sympathomimetic amines, and MAO inhibitors should be avoided in patients with carcinoid syndrome. When these patients undergo surgery or hepatic artery embolization for their tumor or metastases, they should be pretreated with octreotide (Sandostatin), 300 to 500 μg subcutaneously or intravenously, 30 to 60 minutes before the procedure. Anesthesiologists should be specifically notified that the patient has carcinoid syndrome.
15. Can a carcinoid crisis be predicted?
Patients with carcinoid tumors who have not developed carcinoid syndrome can be tested for their potential to have a carcinoid crisis. This is most commonly accomplished with an epinephrine provocation test; patients are given progressive intravenous (IV) boluses of epinephrine every 5 minutes, starting with a dose of 1 μg and increasing, if necessary, to 10 μg, while heart rate and blood pressure are monitored every 60 seconds. A positive response consists of flushing or a blood pressure drop of 20 mm Hg systolic or 10 mm Hg diastolic 45 to 120 minutes after an injection. All patients undergoing this test must have venous catheters and be monitored carefully throughout the test; IV phentolamine (Regitine) 5-mg and methoxamine (Vasoxyl) 3-mg preparations must also be available to reverse a crisis should it occur.
16. Describe the management of a carcinoid crisis.
Effective treatment for carcinoid crisis consists of IV administration of octreotide and glucocorticoids. If this does not abort the episode, additional options include methotrimeprazine (an antiserotonin agent), methoxamine (a direct vasoconstrictor), phentolamine (an alpha-adrenergic blocker), ondansetron (a serotonin receptor antagonist), and glucagon. It is critical to avoid the use of adrenergic and sympathomimetic agents in patients with suspected carcinoid crisis because these drugs can significantly worsen the condition. Effective medication dose regimens for this condition are listed in Table 54-4.
TABLE 54-4.
MANAGEMENT OF CARCINOID CRISIS
| MEDICATION | DOSE REGIMEN |
| Octreotide (Sandostatin) | 50 μg IV over 1 min, then 50 mg IV over 15 min |
| Hydrocortisone (Solu-Cortef) | 100 mg IV over 15 min |
| Methotrimeprazine (Levoprome) | 2.5-5.0 mg slow IV push |
| Methoxamine (Vasoxyl) | 3-5 mg slow IV push, followed by an infusion |
| Phentolamine (Regitine) | 5 mg slow IV push |
| Ondansetron (Zofran) | 20 mg IV over 15 min |
| Glucagon | 0.5-1.5 mg slow IV push |
From Warner RRP: Gut neuroendocrine tumors. In Bardin CW, editor: Current therapy in endocrinology and metabolism, ed 6, St. Louis, 1997, Mosby, pp 606–614.
Baudin, E, Gastroenteropancreatic endocrine tumors. clinical characterization before therapy. Nat Clin Pract Endocrinol Metab 2007;3:228–238.
Campana, D, Nori, F, Piscitelli, L, et al, Chromogranin A. is it a useful marker of neuroendocrine tumors. J Clin Oncol 2007;25:1963–1967.
Eriksson, B, Kloppel, G, Krenning, E, et al, Consensus guidelines for the management of patients with digestive neuroendocrine tumors. well differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology 2008;87:8–19.
Feldman, JM. The carcinoid syndrome. Endocrinologist. 1993;3:129–135.
Galanis, E, Kvols, LK, Rubin, J. Carcinoid syndrome. J Clin Oncol. 1998;16:796–798.
Koopmans, KP, Neels, OC, Kema, IP, et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26:1489–1495.
Maggard, MA, O’Connell, JB, Ko, CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240:117–122.
Modlin, IM, Lye, KD, Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959.
Moldin, IM, Oberg, K, Chung, CD, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol. 2008;9:61–72.
O’Toole, D, Ducreaux, M, Bommelaer, G, et al, Treatment of carcinoid syndrome. a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 2000;88:770–776.
Plockinger, U, Wiedenmann, B. Management of metastatic endocrine tumors. Best Pract Res Clin Gastroentero. 2005;19:553–576.
Soga, J, Yakuwa, Y, Osaka, M, Carcinoid syndrome. a statistical evaluation of 748 reported cases. J Exp Clin Cancer Res 1999;18:133–141.
Stuart, K, Levy, DE, Anderson, T, et al, Phase II study of interferon gamma in malignant carcinoid tumors (E9292). a trial of the Eastern Oncology Group. Invest New Drugs 2004;22:75–81.


