70 Carbon Ion Radiotherapy
The use of charged particles in medical radiotherapy (RT) was first suggested in 1946 by physicist Robert Wilson1, a former student of Ernest Lawrence, Nobel Prize winner for developing the cyclotron at the University of California, Berkeley. Following his participation in the Manhattan project and having been disappointed at the use of atomic weapons, Wilson was anxious to propose something useful for the welfare of mankind in the medical literature. At about the same time, Cornelius Tobias, also a student of Ernest Lawrence, was encouraged by Lawrence to work in the field of medical physics. Tobias was instrumental in preparing for clinical use of charged-particle beams with a long and productive series of biophysical studies beginning in the early 1950s2,3 at the University of California Lawrence Berkeley National Laboratory (LBNL). The rationale for using charged-particle beams of carbon ions for RT is based on dose-distribution advantages with less multiple straggling and enhanced biologic effects at depth resulting from the nonhomogeneous distribution of increased energy deposition around stopping particle tracks in the Bragg ionization peak, allowing more dose to the tumor and sparing of the surrounding normal tissues.2
When Robert Wilson proposed the use of protons in 1946, he also noted that carbon ions might be a useful beam and perhaps superior to protons. During the three decades between Wilson’s proposal until 1977, when the first carbon ion patient was treated in phase I trials by Castro and colleagues4,5 at the LBNL, a critical expansion of knowledge in RT occurred. Significant therapeutic gains were made with the use of high-energy x-ray beams with resultant increase in tumor control and fewer undesirable side effects. Much was learned from the pioneers of RT about the clinical application of these megavoltage beams, providing the basis for using charged particles in cancer therapy.
John Lawrence, brother of Ernest Lawrence, and John Lawrence’s colleagues in the late 1950s and 1960s used plateau proton beams for treatment of pituitary tumors at LBNL, employing a precise patient positioner with side-to-side head rotation.6,7 However, spread Bragg peak charged-particle therapy of cancer was only made practical with the advent of computed tomography (CT) in the 1970s. CT allowed rapid, accurate determination of the beam path through varying tissue densities in a patient, and powerful computers also became available for rapid treatment planning calculations.
Relative Biologic Effectiveness
The concept of relative biologic effectiveness (RBE) arose from observations that ionizing particulate radiations can be several times more effective per unit dose in producing biologic effects than x-rays. RBE is defined as the ratio of absorbed doses of two radiations required to produce the same biologic effect. One extension of this concept has been the clinical use of the terminology gray-equivalent (GyE) dose. This is the dose of a particle modality that yields an equivalent biologic response to a dose of megavoltage x-ray or cobalt-60 gamma-rays. For 160- to 230-meV proton beams, the RBE is generally considered to be approximately 1.1 to 1.2, and for heavier particles (helium, carbon, neon, silicon), the RBE ranges from 1.2 to 4.5. RBE depends on dose fraction size as well as the type of tissue irradiated and the position on the depth-dose curve at which it is measured. No single RBE value is adequate to characterize the variable range of RBE values in the stopping carbon ion beam, whether delivered by passive or active scanning techniques. When different clinical situations are considered, the biologic advantages of carbon ions over photons and protons are expected to be most pronounced for tumors that have a low radiosensitivity when treated with photons and for situations in which the tumor is surrounded by radiosensitive normal tissues. Values for RBE can be as high as 4 for carbon ion RT and depend on many factors, which have to be addressed during treatment planning. Experiments conducted with fast neutrons and carbon ions have demonstrated that increasing the dose per fraction results in a decrease of the RBE of both the tumor and normal tissues. The tumor RBE did not decrease as rapidly as the normal tissue RBE.8,9 As a consequence, short-course hypofractionation schemes are likely to enhance the therapeutic ratio in carbon ion RT. At the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, hypofractionated carbon ion RT has been systematically investigated and a significant reduction of overall treatment time has been accomplished for many tumors without increasing toxicity.10–12
Decreased Radioresistance of Tumor Cells to High-Let Radiation
Although factors other than hypoxia are important in tumor control, most tumors have significant numbers of hypoxic cells that are preferentially killed with high-LET irradiations. Whether high-LET RT is effective in treating a given disease depends on the relative values of the tumor and normal-tissue RBE, as well as on reduction in the OER. Recent work with radioresistant tumor cells under even aerobic conditions in vitro indicate that stopping carbon ions can overcome tumor resistance as a result of the over-expression of Bcl-2, an anti-apoptotic protein that is frequently over-expressed in 30% to 50% of tumors and is associated with radioresistance.13 Carbon ions have also been shown to increase the gene expression of another protein, SPHK1 (a kinase related to cell growth and control of proliferation), in human oral squamous cell carcinoma in a manner not seen with x-ray doses.14 Qualitative differences between low-LET and high-LET molecular mechanisms of cell killing such as these need further study. Gene expression profiles are being used to identify novel candidate genes as biomarkers in human normal tissues and tumor cells associated with carbon ion radiosensitivity.15,16
Accelerated Tumor Reoxygenation
Carbon ion irradiation has been shown experimentally to accelerate the reoxygenation of murine fibrosarcoma compared with x-ray irradiation.17 This suggests that high-LET radiations would be effective with shortened treatment durations compared with low-LET RT.
Possible Increased Risk of Carcinogenesis and Other Late Radiation Effects
In high-LET RT the cost-benefit analysis must include an awareness of enhanced late sequelae, including undesirable tissue fibrosis, vascular damage, central nervous system (CNS) toxicities, genomic instability, and secondary cancers. In the clinical trial at LNBL, relatively short-term follow-up (24-173 months, mean of 67 months) of neon-ion treated patients suggested a radiation-induced tumor rate of approximately 2%.18 There was no increase over conventional x-ray rates for the helium patients. Carbon ion late effects should be similar to or slightly increased over helium ion effects. In the laboratory, there is clear genetic variability in the susceptibility to carbon ion–induced mammary carcinoma among rat strains studied.19 Sprague-Dawley rats were more susceptible to carbon ion–induced mammary carcinomas in a mechanism without the common H-ras and Tp-53 mutations seen with low-LET radiation-induced tumors. Further basic investigations should explore these molecular differences further with powerful new genomic and proteomic tools.
Preliminary Clinical Studies at Lawrence Berkeley National Laboratory with Heavy Ions
Clinical studies with fractionated Bragg peak charged-particle therapy, including carbon ions, were carried out from 1975 to 1992 at LBNL20,21 in collaboration with the Medical Center at the University of California, San Francisco. Helium ions were used instead of protons at LBNL, as they were readily produced in the LBNL accelerators at about 232 meV/U, giving a range in tissue of approximately 26 cm. They deposit a small amount of high LET, which must be accounted for in treatment planning. Their clinical effects are similar to protons, with normal-tissue RBE values of 1.2 to 1.4 compared with megavoltage x-rays (except in CNS, in which the RBE is approximately 1.6). Approximately 700 patients were treated with helium ions, and demonstrated excellent tumor control in critical sites in the skull base, eye, head and neck, and paraspinal-sacral regions, similar to results with protons.22–26
Carbon ions were used for the first time in the treatment of human tumors at LBNL in 1977. These studies were preliminary in nature, aimed primarily at assessing toxicity and tumor effect. Interestingly, the first patient treated with carbon ions in a skin RBE comparison with 6-meV electron-beam RT lived for more than 10 years. The late skin irradiation RBE values obtained for carbon ions of approximately 2.5 to 2.7 in the middle of a 4-cm spread Bragg peak was in line with estimates made later in Japan and Germany. A small number of patients with advanced tumors were treated with carbon ions in phase I studies at LBNL in 1977 and 1978. Subsequently, neon ions were picked over carbon ions for extended study at LBNL largely because of their greater potential for high-LET advantage. From 1978 through 1992, 433 patients at LBNL were treated with neon ion RT, of whom 299 received at least 10 Gy (approximately 30 GyE) of neon ions. A small number of patients (approximately 20) received treatment with silicon ions, and two patients were treated with argon ions. The LBNL clinical studies showed that neon and carbon ions could be delivered safely, providing careful treatment planning and delivery occurred, with evidence of beneficial effect especially in slow-growing tumors.21,27–30,30a Late reactions were increased in CNS tissues and GI tissues with neon ion RT. Concerns over significant late effects in normal tissues31–33 halted further study of silicon and argon ions.
Concomitant with the development of accelerator-based methods to provide carbon ion beams has been the advancement in tumor and normal-tissue imaging and beam-delivery techniques, including beam scanning and three-dimensional (3-D) and four-dimensional (4-D) breathing-gated delivery to provide individually-tailored image-guided dose plans.26,34–37 New technologies are also being developed commercially to accelerate carbon ions in facilities having a much smaller footprint and energy requirement for operation.38
Clinical Results of Carbon Ion Radiation Therapy in Japan and Germany
Carbon ion RT became available for the first time in a medically dedicated facility in 1994 when the NIRS in Japan started the first extensive clinical trials with carbon ions. The second carbon ion therapy facility, which began clinical operation in 1997, was developed by Kraft and associates39,40 at the GSI in Germany. This work will be continued at a new clinical heavy ion medical facility at the University of Heidelberg. A third carbon ion therapy facility began patient treatments at the Hyogo Ion Beam Medical Center facility in Harima, Japan, in 2004.41 Additional medical proton-carbon ion therapy facilities are planned or under construction in Italy, France, Germany, Austria, the United States, and Japan.
Beam Delivery Techniques at NIRS and GSI
Beam delivery is performed with passive methods using modulators, collimators, and compensators at the NIRS. The advantage of passive beam delivery systems is that the treatment planning for this system is simple. This approach has already been applied in many clinical situations, including in the treatment of moving targets such as lung cancer and liver cancer. The major disadvantage is that a significant amount of the dose is also delivered along the entrance path that often includes nontarget normal tissue. As an alternative to passive beam delivery, active beam delivery using the raster scan method has been developed. At GSI, focused pencil beams produced in a Synchroton are deflected laterally by two magnetic dipoles while the energy of the incoming beam is varied during the treatment. Thus 3-D intensity-modulated carbon ion RT can be accomplished, and the dose distribution can be tailored optimally to any irregular tumor shape without adding passive absorbers, compensators, or collimators.42 The dose distribution can also be conformed to the proximal edge of the target volume, and normal tissue that resides along the entrance channel of the beam can thus be spared. For both passive and active beam delivery techniques, patient immobilization and its day-to-day reproducibility need to be consistent with the high spatial accuracy achievable with particle beams. Precision head and body immobilization systems, stereotactic target localization, and image guidance with pretreatment correction of even small patient set-up deviations are commonly used at modern particle therapy centers. Using passive beam delivery, interfractional and intrafractional movements are addressed by adding a safety margin to ensure that the clinical target volume (CTV) is fully covered during treatment. For active beam delivery, adding a safety margin around the CTV is not sufficient, because movement of the target during the scanning process can cause regions already irradiated to again move into the path of the beam, whereas regions that have not been irradiated can escape the path of the beam. Different strategies of tumor tracking are currently being explored.43–45 The use of an active beam delivery system also has implications in terms of the treatment planning procedure, which becomes more complex and requires biologic plan optimization. New computational modeling strategies to optimize the LET distribution over the tumor volume are under development for each type of beam delivery mode.46,47 The physical advantages of particle-beam therapy can only be properly exploited when it is possible to use multiple fields at the same level of complexity commonly used for modern photon therapy. Although gantries for proton therapy have been installed at several facilities, carbon ion RT is delivered with fixed-beam lines. The charged-particle therapy facility at Heidelberg University is the first to be equipped with a rotating gantry for proton and carbon ion RT. The optimal design of a modern particle therapy facility is still actively being discussed.
Uveal Melanoma
RT of uveal melanoma aims at local tumor control and eye retention. Although brachytherapy and proton RT are accepted function-preserving alternatives to surgery with 5-year local control rates in the range of 96% and eye retention rates between 75% and 92%,22,48–54 carbon ion RT has been investigated recently at the NIRS in a dose-escalation trial in large-size uveal melanoma. Data is preliminary because of the limited patient number and follow-up time. Between January 2001 and February 2006, 59 patients with locally advanced or unfavorably located choroidal melanoma were enrolled in a phase I and II trial investigating an optimal dose of carbon ion RT. The primary endpoint of the study was morbidity, and local control and overall survival were secondary endpoints. Local control, overall survival, and disease-free survival rates at 3 years were 97.4%, 88.2%, and 84.8%, respectively. Twenty-three patients (40%) developed neovascular glaucoma and three patients underwent enucleation.55 It was found that the volume of the ciliary body irradiated to more than 50 GyE and irradiation of the optic disc were independent risk factors for development of neovascular glaucoma after carbon ion RT in choroidal melanoma.56
Skull-Base Chordoma and Low-Grade Chondrosarcoma
Chordoma is a rare tumour with a slow growth pattern. Thirty-five percent of chordomas occur in the skull-base region, displaying a challenge for neurosurgeons as well as radiation oncologists. Complete resection is exceptional and local recurrences are common. The results after treatment of chordoma and low-grade chondrosarcoma with conventional photon RT and stereotactic photon RT have been poor, with prognosis ranging between 17% and 50% at 5 years.57–59 At the Massachusetts General Hospital, 375 patients with skull-base chordoma and chondrosarcoma have been treated with proton RT between 1975 and 1998. Local control rates at 5 and 10 years were reported to be 73% and 54% for chordoma and 98% for chondrosarcoma, respectively.60 Other proton centers report similar results.61–63 Currently, proton RT is considered the treatment of choice for patients with chordoma and low-grade chondrosarcoma of the skull base.
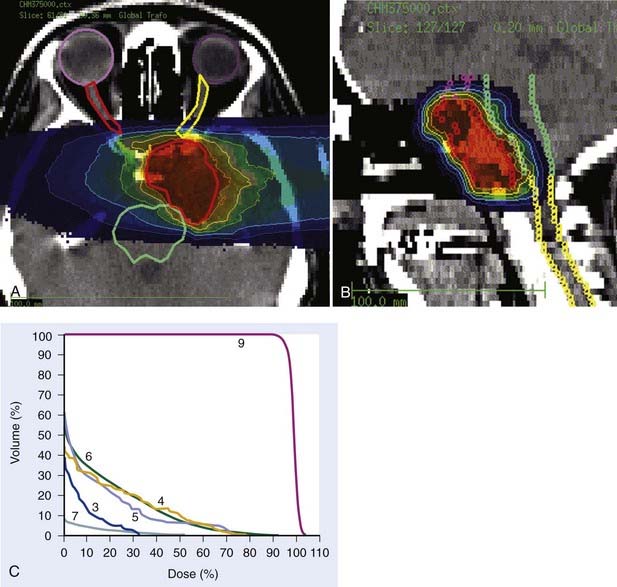
Carbon ion RT also offers biologic advantages in treatment of these tumors, particularly chordoma. Between 1997 and 2001, a clinical phase I and II study was carried out at GSI to investigate the feasibility and effectiveness in chordoma and chondrosarcoma of the skull base. Sixty-seven patients with chordoma (n = 44) and low-grade chondrosarcoma (n = 23) were enrolled in the study and received a full course of carbon ion RT with a median total tumor dose of 60 cobalt gray equivalent (CGE) (range 57 to 70 CGE/20 fractions/20 days). A typical carbon ion treatment plan of a patient with skull-base chordoma is shown in Fig. 70-1. Median follow-up was 32 months (range 3 to 66 months). Local control rates for chordoma and low-grade chondrosarcoma of the skull base were 74% and 87% at 4 years, respectively. Overall survival at 4 years was 86% and 100% for chordoma and chondrosarcoma, respectively. Acute toxicity was very mild. No patient developed acute skin reaction greater than the National Cancer Institute common toxicity criteria (NCI-CTC) grade 2. Only three patients developed grade 3 mucositis.64 A follow-up analysis in 2005 of this study included 96 patients with chordoma and 54 patients with chondrosarcoma. Cumulative local control and overall survival rates at 4 years were 89.8% and 98.2% for chondrosarcoma.65 Local control and overall survival rates at 5 years were 70% and 88.5% for chordoma of the skull base.66 Severe late toxicity was observed in less than 5% of all patients, and overall treatment time was significantly reduced to 3 weeks.
Carbon ion RT–induced toxicity to the temporal lobes was analyzed in 59 patients with chordoma and chondrosarcoma treated between 2002 and 2003 at GSI with carbon ion RT. Of these 59 patients, 10 developed circumscribed white-matter changes in the temporal lobes during their regular follow-up with magnetic resonance imaging. In most of these patients, the white-matter changes were of a temporary nature and diminished without treatment. Only two patients developed neurologic deficits. Analysis of the dose-volume histograms revealed that age and maximum dose, after subtracting the maximum dose volume of 1 ml, significantly influenced the risk for white-matter changes after carbon ion RT. The 5% and 50% probabilities for temporal-lobe injury after carbon ion RT were determined to be 68.8 and 87.3 GyE,67 consistent with highly conformal photon and proton irradiations.
When the local control rates in chordoma were analyzed together with the data available for proton RT, a dose-response relationship could be derived for chordoma of the skull base. The optimal dose is estimated to be between 75 and 85 GyE.66
The results of carbon ion RT at NIRS in 29 patients with chordoma and seven patients with chondrosarcoma were recently reported.12 The total dose was escalated from 48.0 to 60.8 GyE given in 16 fractions for 4 weeks. Overall local control was achieved in 74% of chordoma patients and in 100% of those with chondrosarcoma. With higher total doses, an improvement in tumor control was observed with no severe adverse reactions. For chordoma, the fractionation regimen of 60.8 GyE given in 16 fractions for 4 weeks yielded the best local control of 91% at 5 years, whereas local control was only 60% after 48.0 to 57.6 GyE. The overall 5-year survival rates were 88% in chordoma and 54% in chondrosarcoma. There were three deaths with chondrosarcoma, all with intercurrent disease.
Glioblastoma Multiforme
The current treatment in glioblastoma multiforme is surgical removal with adjuvant radiochemotherapy using photons and temozolomide.68 A tumor dose of 60 Gy given in conventional fractionation is recommended. The planning target volume (PTV) includes a visible tumor volume plus a generous margin of 3 to 5 cm for subclinical disease. The overall prognosis of these patients remains poor, with a median survival time of 14.6 months and further improvements are strongly needed. The addition of new agents with antitumor activity is under investigation. Another strategy to improve effectiveness of therapy in these tumors is to use beams with a higher biologic effectiveness such as carbon ions.
The first clinical phase I and II trial investigating optimal dose in carbon ion RT in patients with anaplastic glioma and glioblastoma was performed at the NIRS between 1994 and 2002. A total of 48 patients were treated with combined conventional photon RT with 50 Gy in 25 fractions followed by eight fractions of carbon ion RT given within 2 weeks. Carbon ion dose was increased from 16.8 to 24.8 GyE in 10% incremental steps. Nimustine hydrochloride (ACNU) was administered twice at a dose of 100 mg/m2 concurrently in week 1 and in week 4 or 5 of photon RT. The median survival time was 35 months in patients with anaplastic glioma and 17 months in patients with glioblastoma. Increased progression-free survival with dose was found in glioblastoma patients.69 The results of this trial are promising and may be further improved by replacing ACNU by temozolomide. A clinical phase I and II trial combining carbon ion RT with temozolomide is currently being prepared in NIRS and Heidelberg.
Prostate Cancer
Dose escalation has been found to be favorable, especially for locally advanced prostate cancer in a number of photon intensity-modulated radiation therapy (IMRT) and proton trials. Disease-free survival rates at 5 years in patients with intermediate-risk prostate cancer are reported to be 51% after conventional RT with doses less than 76 Gy versus 82% for doses larger than 76 Gy.70 However, the delivery of such high tumor doses is limited by the close vicinity of the prostate to the anterior rectal wall, which might result in a higher rate of severe radiation reactions of the rectum. Modern RT techniques such as intensity-modulated photon RT might help spare organs at risk and at the same time escalate the dose to the prostate. The resulting clinical benefit is being evaluated in clinical photon trials.71
Prostate cancer cells are considered to show a low radioresponsiveness against photons represented by relatively low α/β values in the range of 1.5 Gy, whereas a higher radiosensitivity is assumed for dose-limiting, late-reacting tissues such as the rectal wall.72 Hypofractionated carbon ion RT has been applied to more than 760 patients at NIRS.73 After the early dose escalation study was conducted,74 patients were successively treated with three different fractionation regimens using 66 GyE in 20 fractions for 5 weeks, 63 GyE in 20 fractions, and 57.6 GyE in 16 fractions.12,75 The fractionation of 66 GyE in 20 fractions for 5 weeks was used until January 2005, when the total dose was reduced to 63 GyE given in the same fractions and treatment period. Patients were immobilized prior to carbon ion RT in a low-temperature thermoplastic material. When the anterior beams were applied for 4 out of 16 fractions, the bladder was filled with 100 ml of sterilized water for both the treatment-planning CT and prior to each treatment. The rectum was emptied as much as possible by the patient, or by the use of a laxative or enema. The CTV included the prostate and seminal vesicles, irrespective of T stage or other risk factors. The PTV1 included the CTV and anterior and lateral safety margins of 10 mm and a posterior margin of 5 mm. To reduce the dose to the rectum, a rectum-sparing target volume (PTV2) was defined. Irrespective of the size of the spread-out Bragg peak (SOBP), the RBE value for carbon ions was estimated to be 3.0 at the distal part of the SOBP. Carbon ion RT was given once a day, 4 days per week.
Of the 466 patients treated with 63 or 66 GyE in 20 fractions for 5 weeks and observed for 6 months or longer, the 5-year local control rate was 99.2%, the 5-year survival rate was 94.8%, the 5-year cause-specific survival rate was 98.6%, and the 5-year biochemical nonrecurrence rate was 89.9%.12 Analysis of prognostic factors using the biochemical nonrecurrence rate as an endpoint revealed that the clinical T stage and the Gleason score determined by the same pathologist were significant prognostic factors.
Lung Cancer
Surgical resection is the treatment of choice for localized non–small cell lung cancer (NSCLC). Primary photon RT is considered in medically inoperable patients. With fractionated proton RT, 5-year in-field control rates of 89% and 39% have been observed in stage IA and stage IB NSCLC patients, respectively. Overall survival rates were 70% and 16% for stage IA and stage IB patients, respectively.76 Similar results have been found in other proton trials.77,78
At the NIRS, the first phase I and II study investigating carbon ion RT in localized NSCLC was conducted from 1994 to 1999 to determine the optimal dose in stage I tumors. Irradiation was applied in 18 fractions over 6 weeks in 47 patients (protocol #9303) and in nine fractions over 3 weeks in 34 patients (protocol #9701). According to protocol #9303, dose was escalated from 59.4 to 95.4 GyE in 10% increments and in protocol #9701 from 68.4 to 79.2 GyE in 5% increments, respectively. Carbon ion irradiation was applied 3 to 4 days per week. Before treatment planning two or three iridium seeds were placed into the lung parenchyma in close vicinity to the tumor using a video bronchoscope. Patients were treated with two to four portals, with one portal applied in each session using a respiratory gating system.79 Digital orthogonal x-ray images were taken prior to radiation, set-up deviations were determined with the help of the iridium seeds, and misalignments greater than 1 mm were corrected. The optimal dose fractionation scheme of carbon ion RT in stage I NSCLC was determined to be 68.4 to 79.2 GyE given in nine fractions. Local control probability at 5 years was 84%; 3 out of 81 developed grade 3 pneumonitis.80
As a next step, a phase II study employing 72.0 GyE in nine fractions over 3 weeks was carried out in 50 patients at the NIRS from April 1999 to December 2000. The local control rate was 94.7%. The 5-year overall and cause-specific survival rates were 50% (stage IA: 55.2%, IB: 42.9%) and 75.7% (stage IA: 89.4%, IB: 55.1%), respectively.81
A further hypofractionated carbon ion RT plan was investigated in a phase I and II study for stage I NSCLC in NIRS.82 Seventy-nine patients were included in the study and were treated with a fixed dose of 52.8 GyE for stage IA tumors and with 60.0 GyE for stage IB tumors in four fractions during 1 week. The local control rate was 90% for all patients (T1: 98%, T2: 80%) and the overall survival rate was 68%. No radiation-induced lung toxicity greater than grade 3 was detected.
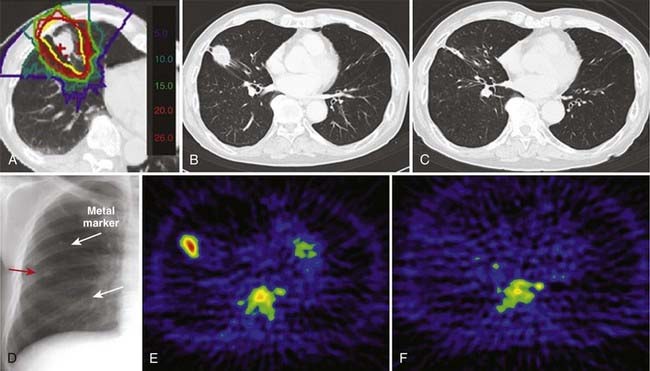
At present, single-fraction carbon ion RT is being investigated for stage I NSCLC at the NIRS.12 A single total dose has been escalated from 28.0 GyE to 44.0 GyE at increments of 5%. The starting dose of 28 GyE was estimated on the basis of an NIRS modification of the linear quadratic model for the end point of local tumor control.83–85 The biologically effective dose (BED) was calculated based on the result of the first dose escalation study using 18 fractions. The dose response of the BED was fitted by a sigmoid function and the curve for a single fraction treatment was derived, from which a single dose of 28.0 GyE was calculated to yield 90% of tumor control probability.
So far, almost 200 patients have been treated, in whom no serious adverse events have been observed and in whom the local control appears to be improving with increases in total dose. A typical patient treated with a single fraction of carbon ion RT given from four directions is shown in Fig. 70-2.
Hepatocellular Carcinoma
Common therapy modalities for hepatocellular carcinoma (HCC) are hepatectomy, transcatheter arterial embolization, percutaneous ethanol injection (PEI) therapy, and radio-frequency ablation (RFA) therapy. Treatment outcomes are, however, still disappointing, especially in large tumors. RT of HCC is therefore a treatment option, especially for inoperable cases. Toxicity to the liver parenchyma is considered to be the dose-limiting endpoint. Local control and overall survival rates at 5 years reported after proton RT are in the range of 86.9% to 87.8% and 23.5% to 55.6%, respectively.86–88 Toxicity has been shown to be low, but coexisting liver cirrhosis has been shown to adversely affect overall survival.86,87 Any therapeutic method applied to HCC must be used with caution so as to retain sufficient liver function.
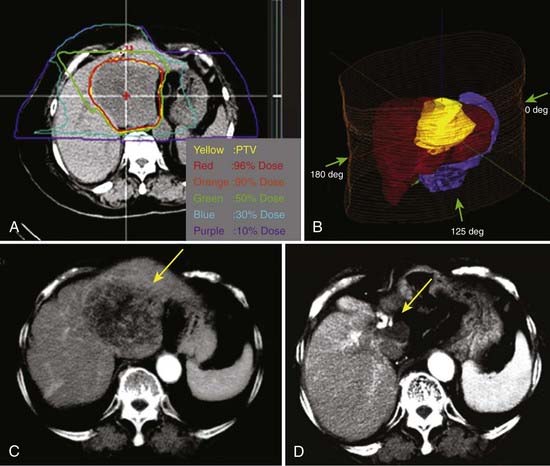
The first clinical phase I and II trial was conducted at NIRS between 1995 and 1997 to evaluate the safety and effectiveness of carbon ion RT in patients with HCC.89 Twenty-four patients were treated in 15 fractions delivered in 5 weeks. The total dose ranged from 49.5 to 79.5 GyE. The dose per fraction was increased in the absence of early and late reactions of the normal tissue. The PTVs included the tumor plus a 1.0-cm margin (Fig. 70-3). Respiratory gating was employed at the acquisition of the planning CT scan and during irradiation. The local control and overall survival rates were 92% and 92% at 1 year, 81% and 50% at 3 years, and 81% and 25% at 5 years.
Since 2003, a dose escalation study has been performed in 36 HCC patients, delivering a total dose of 32 to 38.8 GyE in two fractions over 2 days.89 No patients developed severe side effects, and the 3-year local control and survival rates were 84% and 77%, respectively. It was decided for the two-fraction RT to adopt a total dose of 38.8 GyE or larger. Carbon ion RT can be given to patients with small restrictions because of liver function (Child-Pugh grade A or B). In small lesions of 3 cm or less, carbon ion RT might be offered to patients not eligible for PEI or RFA for any reason. For patients with lesions larger than 3 cm, effective tumor control is rarely achieved with PEI or RFA alone. These patients are ideally suited for carbon ion RT. The results with carbon ion RT are particularly encouraging for large lesions exceeding 5 cm.
Head and Neck Tumors
The clinical phase I and II dose-escalation study was carried out at the NIRS between 1994 and 1997, in which a total of 36 patients with locally advanced head and neck tumors were treated with carbon ion RT. Patients received 18 fractions in 6 weeks (group A) and 16 fractions in 4 weeks (group B). Total doses were escalated in increments of 10% after careful observation of at least three patients treated at the same dose level. Local control was 75% at 5 years for all patients. Subanalysis showed the local control rates of 100% in malignant melanoma, 50% in adenoid cystic carcinoma, and 34% in squamous cell carcinoma. Toxicity was acceptable.90
Although the outcome in patients with squamous cell carcinoma does not seem to be superior to outcome reported after photon or proton RT, local control rates in malignant melanoma and adenoid cystic carcinoma appear very promising as compared with results obtained with low-LET irradiation.91 Between April 1997 and August 2007, a total of 295 patients were treated in a third study at NIRS using 57.6 or 64.0 GyE given in 16 fractions over 4 weeks.12 Local control was achieved in 75% to 81% of the patients with adenocarcinoma, adenoid cystic carcinoma, and malignant melanoma. The most common histologic type has been malignant melanoma, and a total of 95 patients with this tumor were treated. Local control was 75%, but survival was only 36% because of distant metastases. Based on this result, a new protocol was designed for treatment of malignant melanoma, using carbon ion RT and concomitant chemotherapy. To date, a total of 64 patients have been treated and the 5-year local control and survival rates are 78.8% and 48%, respectively. It is possible that better tumor control will reduce distant metastases and result in improved survival in malignant melanoma.
In locally advanced adenoid cystic carcinoma treatment results after primary or postoperative conventional low-LET RT are poor. Neutron RT has been found to be superior to photon RT in a clinical phase III trial. Local-regional control rates were 56% versus 17% after neutron RT versus photon RT at 10 years.92 The drawback of neutron RT is, however, the high rate of late toxicity. In a meta-analysis of 570 patients with adenoid cystic carcinoma treated with neutron RT at different European neutron facilities, a rate of 10.6% has been noted for severe late effects.93
At GSI and Heidelberg University a clinical phase I and II trial has been carried out investigating combined photon RT plus carbon ion boost in patients with locally advanced adenoid cystic carcinoma. The results have been compared with results obtained with photon IMRT alone in similar patients treated at Heidelberg University. Twenty-nine patients were treated with combined photon IMRT and a carbon ion RT boost within this clinical phase I and II study. The CTV covered the course of the involved cranial nerves to the point where they enter the base of the skull. The gross tumor volume included the macroscopic tumor with a safety margin of 1 to 2 mm. The CTV was treated with combined stereotactic photon RT (fractionated stereotactic RT or intensity-modulated stereotactic RT) to a total dose of 54 Gy in a weekly fractionation of 5 × 1.8 Gy and a carbon ion boost of 18 GyE in a weekly fractionation of 6 × 3.0 GyE to the macroscopic tumor residual. At a median follow-up of 16 months (range 2 to 60 months) 4-year locoregional control rates were 77%. The 4-year overall survival rate was 75.8%. Toxicity was acceptable, and severe late toxicity grade 4 occurred in one patient only. When the local control rates were compared with the control rates obtained for 23 patients with locally advanced adenoid cystic carcinoma treated with photon IMRT alone, outcome appeared to be improved after combined treatment, and toxicity was comparable.94
Bone and Soft-Tissue Sarcoma
Bone and soft-tissue sarcomas consist of a variety of histologic subtypes. Complete resection is the treatment of choice, followed by adjuvant therapy in large and high-grade tumors. Although tumors of the extremities are accessible to complete surgery in most patients, a great proportion of bone and soft-tissue sarcomas of the trunk and paraspinal tumors are considered unresectable, or microscopic and macroscopic tumor residuals remain after surgery. Results with photons are poor because of the inability to deliver the needed target doses while respecting the tolerance doses of the neighboring organs at risk such as the spinal cord, liver, kidneys, and small bowel.95 In the past, proton RT has been investigated especially for chordoma and chondrosarcoma of the spine. In studying treatment of axial osteo and chondrogenic tumors, Hug and colleagues report 5-year actuarial local recurrence–free survival rates of 53% for chordoma and 100% for chondrosarcoma after proton RT.96 In a small group of patients with bone sarcoma, mainly in the spine, treated at LBNL with helium or neon particles, 5-year actuarial local control and survival were 59% and 45% respectively.97
At NIRS, a dose-escalation trial has been carried out in patients with different bone and soft-tissue tumors such as osteosarcoma, chondrosarcoma, chordoma, and retroperitoneal sarcoma. Customized cradles and a low-temperature thermoplastic sheets were used for immobilization. Respiratory gating for both CT acquisition and therapy was performed whenever indicated. Irrespective of the size of the SOBP, the RBE value of carbon ions was estimated to be 3.0 at the distal part of the SOBP. Compensators were fabricated for each patient. Carbon ion RT was applied 4 days a week (16 fractions within 4 weeks). The total dose ranged from 52.8 to 73.6 GyE in 16 fractions. To determine the maximum tolerated dose (MTD), patients were divided into the following three groups: spinal tumors, pelvic tumors, and extremity tumors. A total dose of 73.6 GyE was determined to be the MTD. The local control rate increased as the total dose increased from 52.8 to 73.6 GyE, reaching more than 80% in patients treated with 70.4 GyE or more.98 In the up-date analysis for the same patients, the 3-year local control rate was 63%, and 3-year and 5-year survival rates were 47% and 36%, respectively. To confirm these findings, a phase II clinical trial was initiated at NIRS. A total dose of 73.6 GyE was prescribed in cases with no subcutaneous tumor, and 70.4 GyE in patients presenting with subcutaneous tumor invasion. A total of 323 lesions in 307 patients were treated between 2000 and August 2007, and the 5-year local control and survival rates were 80% and 56%, respectively. Severe side effects in the form of skin and soft-tissue toxicities developed in approximately 3% in the previous study, which has since been reduced to zero following changed techniques to decrease the skin doses. In 58 patients with osteosarcoma in the pelvis or spine, the 5-year local control and survival rates were 65% and 29%, respectively, as compared with 5-year survival rates of 10% or less reported in the literature for conventional RT.99–102
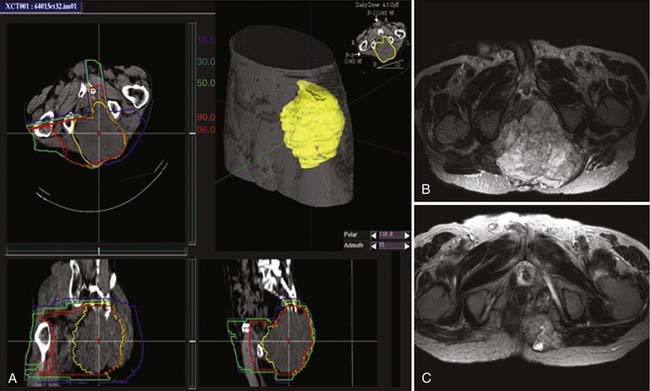
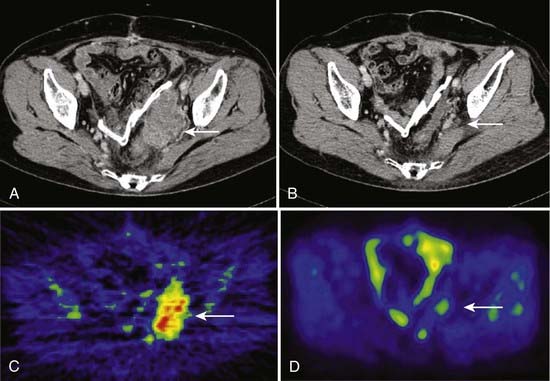
Imai and colleagues retrospectively analyzed the subgroup of patients with sacral chordoma treated with carbon ion RT. A high local control rate of 96% was found.102,103 The result is favorably compared to other sacral and spinal chordoma series treated with high-dose proton RT and other charged particles.29,104 The results obtained with carbon ions alone provide evidence that carbon ion doses between 70.4 and 74 CGE are highly effective in controlling chordoma within the target volume. An example of carbon ion treatment of chordoma is shown in Fig. 70-4.
Carbon ion RT is an effective local treatment for patients with unresectable bone and soft-tissue sarcoma. In contrast to neutron RT, the morbidity has been quite acceptable in carbon ion RT.105
Recurrent Rectal Cancers
For postoperative pelvic recurrence of rectal cancer, surgical resection has been the first choice of treatment, but often these tumors prove unresectable. If the pelvic recurrent lesion is successfully resected, however, the 5-year survival rate after resection is relatively favorable at around 30%.106 This means that, if the local recurrent lesions can be securely controlled, a favorable prognosis may be expected, but the prognosis with conventional RT alone is not satisfactory, with many reports describing a 50% survival period of 12 months and a 3-year survival rate of approximately 10%.107 After carbon ion RT with a total dose of 73.6 GyE, the 5-year local control rate was 92.7% and the 5-year survival rate was 57.4%, both favorably comparable to the results of surgical resection and superior to conventional RT.
At the NIRS, a total of 95 lesions in 90 patients with locally recurrent rectal cancers were treated with carbon ion RT. Total doses of 67.2 GyE, 70.4 GyE, and 73.6 GyE were delivered in 16 fractions for 4 weeks.108 The treatment results were favorable; the overall local control rate was 88.6% at 3 years and 80.5% at 5 years, and the overall survival rate was 60.0% at 3 years and 42.8% at 5 years. With respect to side effects, none of the patients developed severe reactions of NCI-CTC grade 3 or worse, either acute or late, in the gastrointestinal tract, urinary tract, or subcutaneous tissues. With increases in total dose, improvements of both local control and survival rates were observed in 63 lesions in 61 patients treated with 73.6 GyE in 16 fractions for 4weeks, and their 5-year local control and survival rates were 92.7% and 57.4%, respectively (Fig. 70-5), both favorably comparable to the results of surgical resection and superior to conventional RT.
Carcinoma of the Uterine Cervix
The treatment results for uterine cervix cancer have been favorable when a combination of intracavitary brachytherapy and external-beam RT with or without chemotherapy is used. In the case of advanced lesions, however, the results are not satisfactory. Carbon ion RT has been used mainly for locally advanced tumors in an attempt to make a new breakthrough in treating tumor stages in which there have been poor treatment results. In dose escalation trials at the NIRS, carbon ion RT was found to be effective in 44 patients with locally advanced carcinoma of the uterine cervix, including squamous cell carcinoma,107a adenocarcinoma, and adenosquamous carcinoma. Local control rates were encouraging even in patients with stage IVA tumors. With the total doses of more than 62.4 GyE delivered in 16 fractions to the pelvis and eight fractions to the primary tumor site, 5-year local control rates of 69% and 64% were reached in stage IVA tumors and in tumors with a dimension of at least 6.0 cm. No patient developed severe acute toxicity, but eight patients developed major late gastrointestinal complications. These were patients treated with doses higher than 60 Gy E to the intestine.109
Future Perspectives
More than 4500 patients have been treated worldwide using carbon ion RT. Most of the patients treated with carbon ion RT have been included in prospective clinical phase I and II and phase II trials. Carbon ion RT has been found to be effective in a number of prospective nonrandomized trials for non–squamous cell head and neck tumors, locally advanced adenoid cystic carcinoma, chordoma and chondrosarcoma, prostate cancer, lung cancer, HCC, and locally advanced bone and soft-tissue tumors.110 The physical and biologic benefits of carbon ion RT have permitted significantly shorter courses of RT, which means that a carbon therapy facility can be operated more efficiently, treating a larger number of patients than is possible with other modalities during the same time. Further investigation of carbon ion RT with controlled clinical trials comparing particle therapy with modern photon and proton RT with respect to tumor control, toxicity, and treatment cost is clearly warranted.110 Current and future developments in molecular biology, targeted therapy, imaging, and radiation technology need to be fully integrated into the charged-particle therapy process.111 The future must also contain further attempts to reduce morbidity, especially in children and young adults.112 Protons and light ions offer the best chance to reduce dose to normal tissue, with concomitant reduction in long-term morbidity and incidence of secondary tumors. It will be particularly important to study combined therapy in which chemotherapy and ion beam therapy is used. Carbon ion RT and the associated disciplines of physics, biology, and engineering represent a rapidly growing field with an encouraging prospect for better control of human cancers.
1 Wilson RR. Radiological use of fast protons. Radiology. 1946;47:491-498.
2 Tobias CA, Anger HO, Lawrence JH. Radiological use of high energy deuterons and alpha particles. Am J Roentgen Rad Ther Nucl Med. 1952;67:1.
3 Tobias CA, Alpen E, Blakely E, et al. Radiobiological basis for heavy ion therapy. In: treatment of radioresistant cancers. Elsevier/North Holland Biomedical Press; 1979.
4 Castro JR, Tobias CA, Quivey JM, et al, Results of tumor treatments with alpha particles and heavy ions at Lawrence Berkeley Laboratory, (Suppl); Barendsen G, Broerse J, Breur K, editors; High LET Radiations in Clinical Radiotherapy, Eur J Cancer; 1979.
5 Castro JR, Quivey JM, Lyman T, et al. Current status of clinical particle radiotherapy at Lawrence Berkeley National Laboratory. Cancer. 1980;46(4):633.
6 Lawrence JH. Proton irradiation of the pituitary. Cancer. 1957;10:795.
7 Tobias CA, Lawrence JH, Born JL, et al. Pituitary irradiation with high energy proton beams: a preliminary report. Cancer Res. 1958;18:121.
8 Denekamp J, Waites T, Fowler JF. Predicting realistic RBE values for clinically relevant radiotherapy schedules. Int J Radiat Biol. 1997;71:681-694.
9 Ando K, Koike S, Uzawa A, et al. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res. 2005;46:51-57.
10 Tsujii H, Mizoe J, Kamada T, et al. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother Oncol. 2004;73(S2):41-49.
11 Tsujii H, Mizoe J, Kamada T, et al. Clinical results of carbon ion radiotherapy at NIRS. J Radiat Res. 2007;48:A1-13.
12 Tsujii H, Kamada T, Baba M, et al: Clinical advantages of carbon-ion radiotherapy, New J Phys (in press).
13 Hamada N, Hara T, Omura-Minamisawa M, et al. Energetic heavy ions overcome tumor radioresistance caused by overexpression of Bcl-2. Radiother Oncol. 2008;89(2):231-236.
14 Higo M, Uzawa K, Kawata T, et al. Enhancement of SPHKl in vitro by carbon ion irradiation in oral squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;65(3):867-875.
15 Suga T, Iwakawa M, Tsuii H, et al. Influence of multiple genetic polymorphisms on genitourinary morbidity after carbon ion radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):808-813.
16 Yamamoto N, Ikeda C, Yakushiji T, et al. Genetic effects of x-ray and carbon ion irradiation in head and neck carcinoma cell lines. Bull Tokyo Dent Coll. 2007;48(4):177-185.
17 Ando K, Koike S, Ohira C, et al. Accelerated reoxygenation of a murine fibrosarcoma after carbon ion irradiation. Int J Radiat Biol Phys. 1999;75(4):505-512.
18 Castro JR: Unpublished data.
19 Imaoka T, Nishimura M, Kakinuma S, et al. High relative biologic effectiveness of carbon ion radiation on induction of rat mammary carcinoma and its lack of H-ras and TP53 mutations. Int J Radiat Oncol Biol Phys. 2007;69(1):194-203.
20 Castro JR, Chen GTY, Blakely EA. Current considerations in heavy charged particle radiotherapy. Radiat Res. 1985;104(2):s263.
21 Linstadt D, Castro J, Phillips T. Neon ion radiotherapy. Results of the phase I-II clinical trial. Int J Radiat Oncol Biol. 1991;20:761.
22 Castro JR, Char DH, Petti PL, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 1997;39:989-996.
23 Castro JR, Linstadt DE, Bahary JP, et al. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int J Radiat Oncol Biol Phys. 1994;29(4):647.
24 Castro JR, Collier JM, Petti PL, et al. Charged particle radiotherapy for lesions encircling the brain stem or spinal cord. Int J Radiat Oncol Biol Phys. 1989;17(3):477.
25 Castro JR, Petti PL, Daftari IK, et al. Clinical gain from improved beam delivery systems. Radiat Envir Phys. 1992;31:233.
26 Evans PM. Topical review: anatomical imaging for radiotherapy. Phys Med Biol. 2008;53:R151-R191.
27 Schoenthaler R, Castro J, Halberg F, et al. Definitive postoperative irradiation of bile duct carcinoma with charged particles and/or photons. Int J Radiat Oncol Biol Phys. 1993;27:75.
28 Lillis-Hearne PK, Castro JR. Indications for heavy ions: lessons from Berkeley. In: Linz U, editor. Ion beams in tumor therapy. New York: Chapman & Hall; 1995:133.
29 Schoenthaler R, Castro JR, Petti PL, et al. Charged particle irradiation of sacral chordomas. Int J Radiat Oncol Biol Phys. 1993;26(2):291-298.
30 Castro JR, Saunders WM, Tobias CA, et al. Treatment of cancer with heavy charged particles. Int J Radiat Oncol Biol Phys. 1982;8(12):2191-2198.
30a Kaplan, et al. IJROBP. 1994;28:257-261.
31 Castro JR. Future research strategy for heavy ion radiotherapy. Progress in radio-oncology V. In: Kogelnik HD, editor. Proceedings of the 5th International Meeting on Progress in Radio-Oncology, ICRO/OGRO 5. Salzburg, Austria; Bologna, Italy: Monduzzi Editore; May 10–14, 1995:643.
32 Castro JR, Petti PL, Blakely EA, et al. Particle radiation therapy. In Leibel SA, Phillips TL, editors: Textbook of radiation oncology, 2nd ed, Philadelphia: W.B. Saunders, 2004.
33 Castro JR. Results of heavy ion radiotherapy. Radiat Environ Biophys. 1995;34:45-48.
34 Kramer M, Jakel O, Haberer T, et al. Treatment planning for heavy-ion radiotherapy: physical beam model and dose optimization, Phys. Med Biol. 2000;45:3299-3317.
35 Kanematsu N, Yonai S, Ishizaki A, et al. Computational modeling of beam-customization devices for heavy-charged particle radiotherapy. Phys Med Biol. 2008;53:3113-3127.
36 Jake O, Reiss P. The influence of metal artifacts on the range of ion beams. Phys Med Biol. 2007;52:635-644.
37 Inaniwa T, Kohno T, Tomitani T, et al. Monitoring the irradiation field of 12C and 16O SOBP beams using positron emitters produced through projectile fragmentation reactions. Phys Med Biol. 2008;53:529-542.
38 Peggs S, Satogata T: A survey of hadron therapy accelerator technologies, IEEE Proceedings of PAC07, Albuquerque, New Mexico, 2007, pp 115–119.
Kaplan ID, Castro JR, Phillips TL. Helium charged particle radiotherapy for meningioma: Experience at UCLBL. Int J Radiat Onc Biol Phys. 1994;28:257-261.
39 Kraft G. Tumor therapy with heavy charged particles. Progress in particle and nuclear physics. 2000;45:S473-S544.
40 Kraft G. Tumor therapy with heavy ions. Darmstadt, Germany: Verein zum Forderung der tumortherapie mit Schwerenionen e.V.; 2007.
41 Abe M. Charged particle radiotherapy at the Hyogo ion beam medical center: characteristics, technology and clinical results. Proc Japan Acad Ser B. 2007;83:6.
42 Haberer T, Becher W, Schardt D, et al. Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods Phys Res A. 1993;330:296-305.
43 Grözinger SO, Rietzel E, Li Q, et al. Simulations to design an online motion compensation system for scanned particle beams. Phys Med Biol. 2006;51(14):3517-3531.
44 Minohara S, Kanai T, Endo M, et al. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(4):1097-1103.
45 Shirato H, Shimizu S, Kunieda T, et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(4):1187-1195.
46 Elsasser T, Kramer M, Scholz M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2007;71(3):866-872.
47 Kanematsu N, Yonai S, Ishizaki A, et al. Computational modeling of beam-customization devices for heavy-charged-particle radiotherapy. Phys Med Biol. 2008;53:3113-3127.
48 Dendale R, Lumbroso-Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma: results of Institut Curie-Orsay Proton Therapy Center (ICPO). Int J Radiat Oncol Biol Phys. 2006;65(3):780-787.
49 Damato B, Kacperek A, Chopra M, et al. Proton beam therapy of iris melanoma. IJROBP. 2005;63(1):109-115.
50 Hocht S, Bechrakis NE, Nausner M, et al. Proton therapy of uveal melanomas in Berlin. 5 years of experience at the Hahn-Meitner Institute. Strahlenther Onkol. 2004;180(7):419-424.
51 Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55(4):867-880.
52 Fuss M, Loredo LN, Blacharski PA, et al. Proton radiation therapy for medium and large choroidal melanoma: preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys. 2001;49(4):1053-1059.
53 Courdi A, Caujolle JP, Grange JD, et al. Results of proton therapy of uveal melanomas treated in Nice. Int J Radiat Oncol Biol Phys. 1999;45(1):5-11.
54 Desjardins L, Lumbroso L, Levy C, et al. Treatment of uveal melanoma with iodine 125 plaques or proton beam therapy: indications and comparison of local recurrence rates. J Fr Ophtalmol. 2003;26(3):269-276.
55 Tsujii H, Ishikawa H, Yanagi T, et al. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a phase I/II dose escalation study. Int J Radiat Oncol Biol Phys. 2007;67(3):857-862.
56 Hirasawa N, Tsuji H, Ishikawa H, et al. Risk factors for neovascular glaucoma after carbon ion radiotherapy of choroidal melanoma using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2007;67(2):538-543.
57 Catton C, O’Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67-72.
58 Romero J, Cardenes H, la Torre A, et al. Chordoma: results of radiation therapy in eighteen patients. Radiother Oncol. 1993;29:27-32.
59 Debus J, Schulz-Ertner D, Schad L, et al. Stereotactic fractionated radiation therapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys. 2000;47:591-596.
60 Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(Suppl 2):57-63.
61 Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91(3):432-439.
62 Noel G, Feuvret L, Calugaru V, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44(7):700-708.
63 Weber DC, Rutz HP, Pedroni ES, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institute experience. Int J Radiat Oncol Biol Phys. 2005;63(2):401-409.
64 Schulz-Ertner D, Nikoghosyan A, Thilmann C, et al. Carbon ion radiotherapy for chordomas and low-grade chondrosarcomas of the skull base. Results in 67 patients. Strahlenther Onkol. 2003;179(9):598-605.
65 Schulz-Ertner D, Nikoghosyan A, Hof H, et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys. 2007;67(1):171-177.
66 Schulz-Ertner D, Karger CP, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68(2):449-457.
67 Schlampp, et al: Personal communication, Presented at ASTRO 2008.
68 Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
69 Mizoe JE, Tsujii H, Hasegawa A, et al. Phase I/II clinical trial of carbon ion radiotherapy for malignant gliomas: combined x-ray radiotherapy, chemotherapy, and carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(2):390-396.
70 Hanks GE, Hanlon AL, Pinover WH, et al. Dose escalation for prostate cancer patients based on dose comparison and dose response studies. Int J Radiat Oncol Biol Phys. 2000;46(4):823-832.
71 Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53(5):1111-1116.
72 Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44(3):265-276.
73 Tsuji H, Yanagi T, Ishikawa H, et al. Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1153-1160.
74 Akakura K, Tsujii H, Morita S, et al. Phase I/II clinical trials of carbon ion therapy for prostate cancer. Prostate. 2004;58(3):252-258.
75 Ishikawa H, Tsuji H, Kamada T, et al. Carbon ion radiation therapy for prostate cancer. Results of a prospective phase II study. Radiother Oncol. 2006;81:57-64.
76 Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56(1):7-13.
77 Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest. 1999;116(5):1313-1319.
78 Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(1):107-111.
79 Minohara S, Kanai T, Endo M, et al. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(4):1097-1103.
80 Miyamoto T, Yamamoto N, Nishimura H, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2003;66(2):127-140.
81 Miyamoto T, Baba M, Yamamoto N, et al. Curative treatment of stage I non-small cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67(3):750-758.
82 Miyamoto T, Baba M, Sugane T, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2(10):916-926.
83 Webb S, Nahum AE. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol. 1993;38:653-666.
84 Dasu A, Toma-Dasu I, Fowler JF. Should single or distributed parameters be used to explain the steepness of tumor control probability curves? Phys Med Biol. 2003;48:387-397.
85 Kanai T, Matsufuji N, Miyamoto T, et al. Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys. 2006;64:650-656.
86 Hashimoto T, Tokuuye K, Fukumitsu N, et al. Repeated proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;65(1):196-202.
87 Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11(10):3799-3805.
88 Bush DA, Hillebrand DJ, Slater JM, et al. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127(5 Suppl 1):S189-193.
89 Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468-1476.
90 Mizoe J, Tsujii H, Kamada T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2004;55:358-364.
91 Tsujii H, Mizoe J, Kamada T, et al. Overview of clinical experience on carbon ion radiotherapy at NIRS. Radiother Oncol. 2002;73(S2):41-49.
92 Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Int J Radiat Oncol Biol phys. 1993;27:235-240.
93 Krüll A, Schwarz R, Brackrock S, et al. Neutron therapy in malignant salivary gland tumors: results at European centers. Recent results. Cancer Res. 1998;150:88-99.
94 Schulz-Ertner D, Nikoghosyan A, Didinger B, et al. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer. 2005;104(2):338-344.
95 Zagars GK, Ballo MT. Significance of dose in postoperative radiotherapy for soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2003;56(2):473-481.
96 Hug EB, Fitzek MM, Liebsch NJ, et al. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys. 1995;31(3):467-476.
97 Uhl V, Castro J, Knopf K, et al. Preliminary results of heavy charged particle irradiation of bone sarcoma. Int J Radiat Oncol Biol Phys. 1992;24:775.
98 Kamada T, Tsujii H, Yanagi T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466-4477.
99 Grimer RJ, Carter SR, Tillman RM, et al. Osteosarcoma of the pelvis. J Bone Joint Surg [Br]. 1999;81-B:796-802.
100 Bielack S, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776-790.
101 Kawai A, Huvos AG, Meyers PA, et al. Osteosarcoma of the pelvis. Oncologic results of 40 patients. Clin Orthop. 1998;348:196-207.
102 Imai R, Kamada T, Tsujii H, et al. Carbon ion radiotherapy for unresectable sacral chordomas. Clin Cancer Res. 2004;10:5741-5746.
103 Leoehrer P, Arececi R, Glatstein E, et al. The year book of oncology. Philadelphia: Elsevier Mosby; 2006. pp 368–370
104 Park L, Delaney TF, Liebsch NJ, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65(5):1514-1521.
105 Laramore GE. The use of neutrons in cancer therapy: a historical perspective through the modern era. Sem Oncol. 1997;24:672-685.
106 McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis. 1995;10:126-132.
107 Ciatti S. Radiation therapy of recurrences of carcinoma of the rectum and sigmoid after surgery. Acta Radiol Oncol. 1982;21:105-109.
107a Kato S, Ohno T, Tsujii H, et al. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2006;65(2):388-397.
108 Yamada S, Yasuda S, Kato H, et al. Carbon-ion therapy for patients with locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:S272-S273.
109 Kato H, Yamada S, Yasuda S, et al. Two-fraction carbon ion radiotherapy for hepatocellular carcinoma. Preliminary results of a phase I/II clinical trial. J Clin Oncol. 2005;23:338s.
110 Schulz-Ertner D, Tsujii H. Particle radiation therapy using protons and heavier ion beams. J Clin Oncol. 2007;25:953-964.
111 Karger CP, Jakel O. Current status and new developments in ion therapy. Strahlenther Onkol. 2007;183:295-300.
112 Greco C, Wolden S. Current status of radiotherapy with proton and light ion beams. Cancer. 2007;109:1227-1238.