51 Cancer of the Vagina
Epidemiology
Primary carcinoma of the vagina, a rare malignancy found primarily in older women, constitutes 1% to 2% of all gynecologic malignancies. The American Cancer Society estimates that there were 2210 new cases of vaginal cancer in 2008 with 760 deaths resulting from vaginal cancer in the same period.1 The majority of women diagnosed with vaginal cancer range in age from 50 to 70 years.2,3 Most malignant lesions detected in the vagina are metastases from other primary gynecologic malignancies. In one study of 141 cases of vaginal carcinoma, 37 were primary lesions and 104 were classified as secondary.4 For this reason, strict criteria are necessary to classify a vaginal neoplasm as primary. Tumors that involve both the vagina and cervix or the vagina and vulva are classified as cervical and vulvar lesions, respectively. In addition, vaginal tumors detected less than 5 years after the diagnosis of a previous gynecologic malignancy are considered metastatic. Although various intervals of time have been used to separate primary from secondary carcinomas, Murad and colleagues4 found that subsequent vaginal lesions detected after treatment for cervical cancer occurred either within the first year or after 5 years. Vaginal lesions detected 5 years or more after treatment for cervical cancer more closely resembled, histologically, primary carcinomas than did vaginal lesions detected within 1 year after treatment for cervical cancer. They concluded that lesions detected more than 5 years after treatment for cervical cancer were primary vaginal carcinomas.
Because of its rare occurrence, risk factors relating to primary vaginal carcinoma have been elusive. In general, in situ and invasive vaginal neoplasia are associated with the same risk factors as in cervical neoplasia. A population-based case-control study of 156 women with in situ or invasive vaginal cancer found that women with vaginal cancer were more likely to have five or more lifetime sexual partners, to have an early age at first intercourse, and to be current smokers at diagnosis than control women.5 Approximately 30% of all cases had been treated for a prior anogenital cancer. As with other squamous cell cancers of the gynecologic tract, human papillomavirus (HPV) has been implicated in the development of both in situ and invasive squamous cell carcinomas of the vagina. Daling and colleagues identified HPV 16/18 deoxyribonucleic acid in 60% of invasive cancer biopsy specimens and in more than 80% of patients with in situ vaginal disease.5 HPV-positive patients were noted to have improved survival rates as compared with HPV-negative patients.6 An HPV association of the same order of magnitude has been found in cervical cancer.7 Human immunodeficiency virus (HIV)-infected women also appear to be at increased risk for development of vaginal carcinoma, which may demonstrate more aggressive behavior and less response to therapy as compared with HIV-negative patients.8
Chronic irritation of the vaginal mucosa can lead to hyperkeratosis, thickening, acanthosis, and inflammation.9 These changes may progress to metaplastic and dysplastic changes. Earlier studies noting a predominance of posterior wall lesions gave rise to the theory that pooling of irritating substances in the posterior fornix led to chronic irritation and the development of vaginal cancers on the posterior wall. Recent series weaken this theory by finding approximately equal distribution of lesions on the anterior and posterior vaginal walls.10–14 The use of a vaginal pessary for uterine prolapse can cause chronic vaginitis, which has also been implicated in the development of vaginal cancer.9
Hysterectomy has also been linked to the development of vaginal cancer. Patients with vaginal cancer have a history of prior hysterectomy, for both benign and malignant disease, that ranges from 47% to 60%.10,11,13,15,16 The interval of time from hysterectomy to the diagnosis of vaginal cancer can be many years, with a median time of 10 years.11 The case-control study of Brinton and colleagues found that hysterectomy was associated with a 6.7-fold excess risk for the subsequent development of vaginal cancer.17 Herman and associates18 challenged this association. They compared 49 patients with vaginal cancer with 49 matched control subjects and found that the incidence of prior hysterectomy was 49% in both groups.18
In utero exposure to the synthetic estrogen diethylstilbestrol (DES) has been linked to the development of clear cell adenocarcinoma of the vagina and cervix. DES was prescribed in the mid-1940s to 1950s to manage high-risk pregnancies. Clear cell adenocarcinoma of the vagina was virtually unknown until 1970, when Herbst and Scully19 reported six cases of primary vaginal clear cell adenocarcinoma. These patients were 15 to 22 years of age; five of the six had been exposed to DES in utero in the first trimester. In 1986, Melnick and colleagues20 reported on 519 cases of clear cell adenocarcinoma of the vagina and cervix. In 60% of all cases, the patient’s mother received DES during pregnancy. In an additional 12% of patients, the mothers were treated with another hormone or an unidentified medication. These patients ranged in age from 15 to 27, with a median age of 19 years in 91% of patients exposed to DES. The risk of developing clear cell adenocarcinoma in exposed females from birth to age 34 was estimated to be 1 case per 1000. This low risk suggested that DES is not a complete carcinogen and that other factors probably contribute to the pathogenesis of these cancers. Other factors thought to increase risk were DES exposure before the 12th week of pregnancy, a maternal history of prior miscarriage, birth in autumn, and prematurity.21 It is recommended that women exposed to DES in utero undergo their first gynecologic examination at menarche with close attention paid to the vagina and cervix, including vaginal and cervical cytologic examination.
Anatomy
The vagina is a fibromuscular tube lined with a mucous membrane, extending from the uterus superiorly down to the vestibule, or the cleft between the labia minora (Fig. 51-1). It lies between the rectum posteriorly and the urethra and bladder anteriorly. Superiorly, the vagina joins the uterus at an angle of more than 90 degrees. Because of the angle of the junction, the posterior wall, which measures about 9 cm, is longer than the anterior wall, which measures approximately 7 cm. The cervix projects into the lumen of the vagina, resulting in a circular invagination between the walls of the vagina and the cervix. These invaginations are called the anterior, posterior, and lateral fornices. The walls of the vagina are usually in contact and form a lumen with the shape of the letter H on transverse section. Running in a longitudinal direction along the midline of the anterior and posterior walls are ridges called the vaginal columns. From these columns originate transverse rugae, which extend laterally on each side.
Pathologic Conditions
Carcinoma In Situ
Carcinoma in situ (CIS), or vaginal intraepithelial neoplasia (VAIN), is a preinvasive lesion seen with much less frequency than invasive vaginal cancer. The relationship between VAIN and invasive vaginal cancer is similar to the relationship of cervical CIS and invasive cervical cancer, although VAIN is a less frequent finding than CIS of the cervix. Histopathologically, most of the lesions are epidermoid and demonstrate full-thickness alterations in maturation with atypical mitoses, individual cell maturation, and hyperchromatism.22 The majority of lesions are multifocal and can involve all the vaginal surfaces, although the most common site of involvement is in the superior one third of the vaginal canal.23,24
Squamous Cell Carcinoma
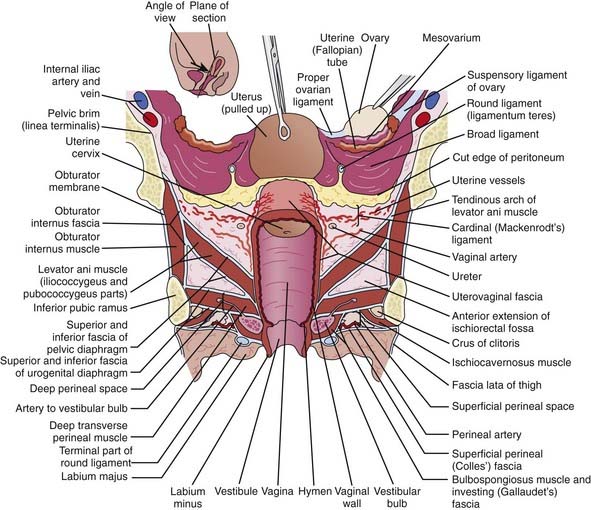
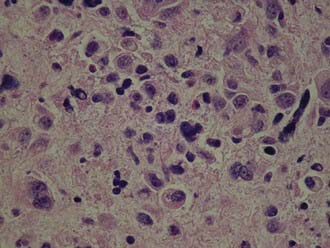
Invasive squamous cell carcinoma is found in 75% to 95% of primary vaginal carcinomas (Fig. 51-2). The majority of these lesions tend to be nonkeratinizing and moderately differentiated.25 The well-differentiated lesions may demonstrate keratinization, manifested by squamous pearls and intracellular bridges.26 Grossly, these tumors may manifest as nodular, ulcerated, indurated, exophytic, or endophytic lesions. Verrucous carcinoma is an uncommon variant of vaginal squamous cell carcinoma. It is well differentiated and has low malignant potential. These lesions usually present with a large fungating mass, which may demonstrate locally aggressive behavior, but rarely metastasizes.
Vaginal Adenosis and Adenocarcinoma
Adenocarcinoma is found in 5% to 10% of all vaginal cancers.3,11,13,27–29 The non–clear cell adenocarcinoma frequently arises in the submucosa. When a biopsy of a vaginal lesion reveals adenocarcinoma, it is important to look for a primary lesion, such as endometrial cancer, elsewhere.
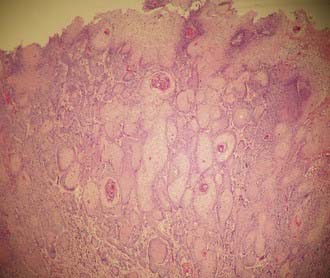
Vaginal adenosis defines the abnormal presence of glandular epithelium in the vagina, which is normally devoid of glandular elements (Fig. 51-3). The glandular epithelial cells may line glands in the submucosa or cover or replace surface squamous cells and are usually located near the surface epithelium.30 Three types of epithelial cells have been observed: mucinous, which resemble endocervical glandular elements; tubo-endometrial; and embryonic. Histologic changes in the female genital tract in women exposed to DES in utero include vaginal adenosis, vaginal and cervical ridges, and cervical erosion.31
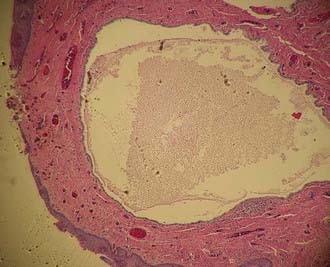
Vaginal adenosis has been found in one third of patients exposed to DES.31 The microscopic appearance of the adenosis in this subset of patients is identical to the adenosis seen in patients born before the DES era.32 Vaginal adenosis is intimately associated with the presence of clear cell adenocarcinoma of the vagina (Fig. 51-4). Robboy and colleagues33 found that 95% of cases of vaginal clear cell adenocarcinoma were associated with vaginal adenosis. Of the three types of epithelium seen in vaginal adenosis, the tubo-endometrial type was most commonly associated with clear cell adenocarcinoma, suggesting that this type of epithelium provides the bed from which clear cell adenocarcinoma develops.34 Clear cell adenocarcinoma is found in 0% to 12% of all vaginal cancers, depending on the study, but has been recognized less often during the past 15 years.2,10,35,36 These lesions tend to arise in the upper one third of the vaginal canal and involve the anterior wall.33,35,37 The most common histologic pattern is tubulocystic,37,38 followed by a solid pattern. The most common cells noted are the clear cell, hobnail cell, and endometrioid cell.38
Melanoma
Malignant melanoma of the vagina represents approximately 5% of all vaginal neoplasms and approximately 0.7% of all melanomas (Fig. 51-5).36,39–41 Almost all of the reported cases have been in white women. Most of these mucosal lesions project into the vaginal lumen, and there is greater involvement along the mucosal surface rather than penetration into the vaginal wall. Clinically, these tumors present as pigmented masses, plaques or ulcerative lesions, most frequently on the distal one-third of the anterior vaginal wall. However, they may present in a nonpigmented manner. Melanomas may display aggressive biological behavior with early and rapid local and systemic failure.
Location
Vaginal carcinomas involve the anterior and posterior walls with equal frequencies.10–14 The lateral walls are less frequently involved. Vaginal carcinoma more frequently involves the superior one third of the vaginal canal compared with the middle or inferior thirds.3,10,12,13,16,28,44,45 The middle and inferior thirds are involved with approximately equal frequencies.3,10,12,46 A factor contributing to this distribution may be the high percentage of patients with a history of prior hysterectomy and the increased incidence of upper one-third lesions in this subset of patients.11
Clinical Presentation
The majority of patients with vaginal cancer are symptomatic, and vaginal bleeding, either postmenopausal or postcoital, is the most common presenting symptom, occurring in 50% to 65% of patients.13,27,44,47 The next most common presenting symptom is vaginal discharge, which occurs in 10% to 15% of patients.13,16,19,27,47 Other signs and symptoms that occur less frequently are pain, urinary symptoms, gastrointestinal complaints, or the presence of a mass. Urinary symptoms are more common in vaginal cancer than in cervical cancer because of the proximity of anterior vaginal wall lesions to the urethra and bladder. Up to 20% of women may be asymptomatic at the time of diagnosis, with these lesions often discovered by abnormal cytological findings on a Papanicolaou smear or by the presence of a mass on speculum examination.
Routes of Spread
Lymphatics
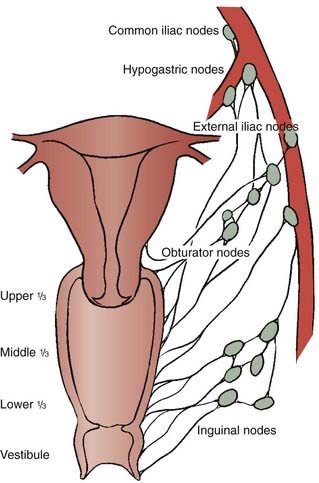
Lymphatic channels in the mucosa run parallel to a network of lymphatic channels in the submucosa and muscular layer, converging to form trunks at the periphery of the vaginal wall. These trunks drain to the major pelvic nodal groups. The upper vagina’s lymphatic drainage pattern is similar to that of the cervix, draining to the obturator, hypogastric, external iliac, and common iliac nodal groups. The lower vagina has trunks that drain to the inguinal, femoral, and external iliac nodes.48 Vaginal cancers, particularly those that involve the posterior wall, can drain to the inferior gluteal, presacral, or perirectal nodes.47 Because there is considerable crossover drainage as well as atypical drainage patterns, any pelvic lymph node group can serve as a drainage site (Fig. 51-6).
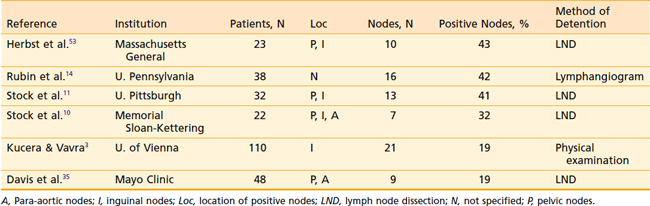
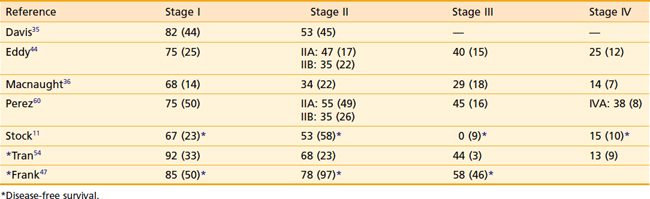
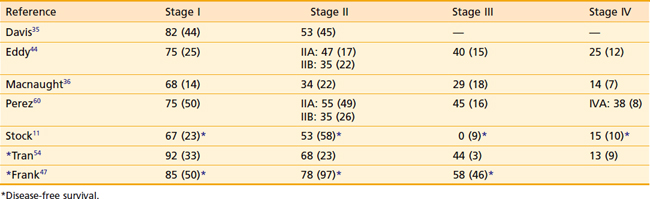
The true incidence of positive lymph nodes is difficult to determine, because the majority of patients are treated with radiation therapy and do not undergo surgical exploration. Most of the information concerning nodal metastases derives from series in which exploratory laparotomies and lymph node dissections were performed. Table 51-1 lists the occurrence of positive nodes in series that recorded this finding. Lymph node involvement in patients with stage I and II disease has been reported in 0% to 6%, and 21% to 26% of cases, respectively.14,37 The incidence of nodal involvement increases with stage and has been reported at 78% and 83% for stages III and IV, respectively.14
Prognostic Factors
The most important factor influencing survival is the stage of disease at the time of intervention.3,11,35,44,45 Increasing stage is associated with a decrease in complete response rates to radiation therapy and an increase in local, pelvic, and distant failure. In addition to the International Federation of Gynecology and Obstetrics (FIGO) stage, several authors have shown that the FIGO modification by Perez,49 which divides stage II disease into stage IIA (paravaginal extension) and IIB (parametrial extension), is also of prognostic significance. Patients with stage IIB disease have a poorer outcome than patients with stage IIA disease.47,50 The presence of symptoms carries an unfavorable prognosis, because larger tumors are more likely to cause symptoms than smaller lesions. Tumor histology has also been identified as an important prognostic factor, with (non–clear cell) adenocarcinoma having more than twice the rates of local and metastatic relapse of squamous cell carcinoma.51 Patients with non–DES-associated clear cell carcinoma have significantly poorer outcomes as compared with women with DES-associated clear cell vaginal cancers.
The extent of vaginal canal involvement also affects prognosis. Lesions limited to one third of the vaginal length carry a better prognosis than lesions extending farther.11,52 In the series by Stock and colleagues,11 of 100 cases, 64 patients with involvement of only one third of the vaginal canal had a significantly improved 5-year disease-free survival (DFS) rate of 61% compared with 36 patients with a more extensive involvement (25% 5-year DFS). In addition, patients with lesions of the upper one third of the vaginal canal have a better prognosis than patients with involvement of other areas of the vaginal canal. In an analysis of 110 patients, Kucera and Vavra3 found 5-year survival rates of 60%, 37.5%, and 37% for patients with lesions involving the upper, middle, and lower one third, respectively. Chyle and colleagues also found the tumor circumferential location to be of independent prognostic significance, with lesions involving the posterior wall faring worse than lesions located elsewhere in the vagina.51 Lymph node involvement carries an unfavorable prognosis.53 In their retrospective review of 78 patients with primary squamous cell carcinoma of the vagina treated with radiation, Tran and colleagues found the level of hemoglobin prior to definitive treatment and during treatment to be significant prognostic variable, with hemoglobin levels of 12.5 mg/dl or less, denoting poorer pelvic control and DFS outcomes.54
Diagnosis and Staging
Diagnosis
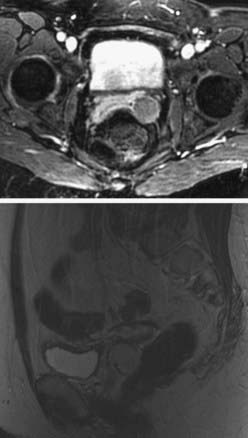
Ultimately, an examination under anesthesia is required to delineate the extent of the tumor. Adequate biopsies of the tumor should include biopsies of the cervix as well, to rule out a cervical primary. A Schiller test using Lugol solution, which stains normal mucosal cells but not malignant cells, and colposcopy can be useful in identifying lesions that are difficult to visualize on speculum examination. The workup should include a chest x-ray film, intravenous pyelography (IVP), barium enema, cystoscopy, and proctosigmoidoscopy. Although not officially sanctioned by FIGO as a staging tool, computed tomographic (CT) imaging is commonly used instead of IVP to evaluate kidney function. Pelvic CT and magnetic resonance imaging (MRI) studies are not part of the FIGO clinical staging system, but can be very important in defining the extent of disease. MRI technology produces excellent images of the pelvis and, unlike CT scans, often can differentiate malignant tissue from normal structures or fibrosis (Fig. 51-7).55
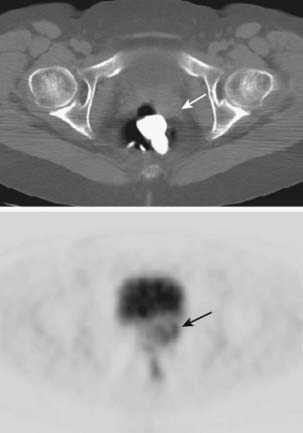
Positron emission tomography (PET) with the glucose analogue [18F]-fluoro-2-deoxy-d-glucose (FDG) has been demonstrated to be the most sensitive and specific imaging test for detecting lymph node tumor involvement in patients with carcinoma of the cervix.56 Because of the many similarities of carcinomas of the cervix and vagina, particularly regarding patterns of spread, whole-body FDG-PET should strongly be considered as a standard imaging evaluation for patients with carcinoma of the vagina (Fig. 51-8). Lamoreaux and colleagues57 reported on 23 patients with primary vaginal carcinoma who were evaluated by FDG-PET and CT during the primary staging work-up. The CT scan visualized the primary tumor in nine patients (43%), three with positive groin lymph nodes and one with positive pelvic nodes. PET identified all 21 (100%) primary vaginal cancers; four with positive groin lymph nodes and two with positive pelvic nodes.
Staging
Vaginal carcinomas are staged by either the American Joint Committee on Cancer system or the FIGO system (Table 51-2 and Table 51-3).58,59
Table 51-3 International Federation of Gynecology and Obstetrics Staging System
| I | Tumor is limited to the vaginal wall |
| II | Tumor infiltration into subvaginal tissue not extending to pelvic wall |
| III | Tumor extending to pelvic wall |
| IVA | Tumor extension to bladder and/or rectal mucosa and/or direct extension beyond true pelvis |
| IVB | Distant metastasis |
Treatment
Radiation Therapy
Radiation therapy is the most common treatment for this disease. Both external-beam radiation therapy (EBRT) and brachytherapy are used as part of the treatment plan. External-beam therapy is usually delivered via a whole-pelvic field, to treat the vaginal tumor and for prophylaxis of the entire vaginal mucosa. In addition, the pelvic lymph nodes are treated in most cases, because of the relatively high incidence of positive pelvic nodes found on pelvic lymph node dissections.10,11,35,53 Inguinal lymph nodes are usually included in a modified whole-pelvic field for lesions involving the distal vaginal canal. External-beam therapy alone is inferior to combined external-beam therapy and brachytherapy or brachytherapy alone.10,12,60,61
Many forms of brachytherapy have been used in the treatment of vaginal cancer and include a tandem and colpostat system (as employed to treat cervical cancer), vaginal cylinders, vaginal plaques, and interstitial needle implants. There are no accepted guidelines for the treatment of vaginal cancer with those various techniques. Treatment with brachytherapy is individualized based on the clinical stage, tumor location, and extent. A summary of external-beam therapy and brachytherapy by stages, showing the total doses used in the radiotherapeutic management of vaginal cancer, is provided in Table 51-4.
Table 51-4 External Beam, Brachytherapy, and Total Doses (cGy) in the Treatment of Vaginal Carcinoma by Stage Low-Dose-Rate Brachytherapy
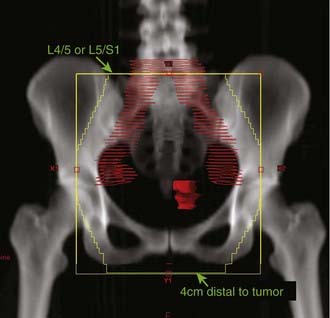
A standard whole-pelvic field should extend superiorly to the L5-S1 interspace. If positive pelvic lymph nodes have been identified, the superior border is raised to the L4-L5 interspace to include the proximal common iliac nodes. The inferior border is usually placed 4 cm below the most caudad extent of disease, which should be localized with the insertion of a small radiopaque marker seed, inserted at the distal edge of the tumor at the time of initial physical examination (Fig. 51-9).
When the distal third of the vagina is involved by tumor, the medial inguinofemoral lymph nodes (i.e., medial to the femoral vessels) are included in the treatment field. In patients with very distal lesions, it is often necessary to treat the vulva to achieve adequate tumor coverage. Treating these patients in an open-leg position may reduce the vulvar skin reaction (Fig. 51-10).
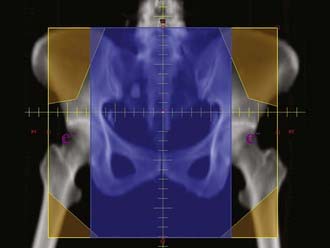
FIGURE 51-10 • Typical anterior-to-posterior fields for lesions in the distal vagina with positive inguinal nodes.
After the patient has received 4000 to 4500 cGy, she is re-evaluated with a pelvic examination to determine whether additional treatment to the primary tumor would be given most effectively in the form of interstitial or intracavitary brachytherapy. The group at M.D. Anderson Cancer Center (MDACC) has proposed a treatment algorithm after completion of the initial EBRT, which may involve additional EBRT.47 The factors that are considered include the extent, thickness, morphology, and location of initial and residual disease; the presence or absence of the uterus; the proximity of critical structures; and the patient’s general medical condition. The MDACC approach favors brachytherapy if the target is well defined, involves less than 50% to 60% of the circumference of the vagina, and does not extensively involve the rectovaginal septum.47 In some cases, an additional 500 to 1000 cGy EBRT boost is delivered to a small volume encompassing the central tumor (usually using lateral fields) before brachytherapy.
Certain advanced cases, particularly when tumors are extensive, very deeply infiltrating with indistinct margins, or extensively involve the retrovaginal septum or bladder, tend to be poor candidates for intracavitary or interstitial brachytherapy. In these cases, the MDACC group suggests that treatment with EBRT may yield improved outcomes versus suboptimal brachytherapy.47 These tumors may be boosted using conformal techniques. To minimize daily radiation in position of the target, for most proximal and some distal lesions, simulation and daily treatment should be performed with a fixed volume of saline that is instilled in the bladder using a Foley catheter. Depending on the size, location, and response of the tumor, a total dose of 6400 to 7000 cGy may be delivered to the gross tumor volume when conformal boost therapy is used.
The role of intensity-modulated radiation therapy (IMRT) in patients with gynecologic malignancies is evolving. Pelvic IMRT, especially in the postoperative setting, treats less small bowel than the standard four-field technique, which will likely translate into lower rates of acute and chronic gastrointestinal side effects. IMRT may also treat less bone marrow, which may allow for improved tolerance of concurrent chemotherapy. In addition, IMRT may allow for dose escalation, and potentially improving local control without increasing the risk of normal tissue injury particularly to the bowel and rectum.62,63
Based on limited preclinical data and anecdotal clinical reports, IMRT appears to have some advantages over conventional radiation therapy in some difficult situations in patients with vaginal cancer. IMRT may be useful in treating patients with locally advanced vaginal cancer in whom an implant would not be possible, or in situations in which there are grossly positive nodes. However, as the grossly positive disease will shrink during the course of radiation therapy, more than one IMRT plan may be needed during the course of treatment. The effects of internal organ motion and bladder and rectal filling need to be addressed.47
Chemotherapy
Concurrent cisplatin-based chemotherapy with irradiation is considered standard therapy for patients with advanced carcinoma of the cervix. Considering that the causes, histologic features, and natural history of vaginal carcinoma are similar to that of invasive carcinoma of the uterine cervix, it may be reasonable to advocate the use of concurrent cisplatin-based chemotherapy and irradiation as standard therapy for patients with high-risk vaginal cancers and good performance status. Patients who have stage III or IVA disease and those with tumors larger than 4 cm are most likely to benefit from the combined treatment.47 Patients with more advanced lesions have pelvic disease recurrence rates of at least 25% to 30%, similar to the pelvic recurrence rates of cervical cancer patients that have been found to benefit from chemotherapy.47,64–67 Because vaginal cancers affect a more elderly population than cervical cancer, the potential side effects of chemotherapy should be carefully considered.
Carcinoma In Situ
Many forms of therapy have been tried in the treatment of CIS, including surgical excision, carbon dioxide laser therapy, radiation therapy, and topical 5-fluorouracil. None has proved superior to the others. Surgical excision is usually accomplished with a wide local excision or partial vaginectomy, which provides a pathologic specimen to rule out invasive disease.26 Limited surgical procedures fail to treat the entire vaginal surface. Because this disease is frequently multifocal, areas of CIS may be overlooked. A Schiller mucosa test is mandatory to define abnormal mucosa. If there are multiple areas of abnormal mucosa, other treatment options must be considered, because a total vaginectomy is often necessary.
Carbon dioxide laser ablation has been used to treat multifocal disease. It has the advantage of precise microscopic control of tissue destruction with minimal destruction of normal tissue.68 The procedure may also be repeated for persistent or recurrent disease, and carries a failure rate ranging from 10% to 50%.68–70
Radiation therapy is effective but less often employed. Treatment involves the use of an intravaginal cylinder to deliver radiation to the entire vaginal mucosa. Low-dose-rate brachytherapy doses of 6000 to 8000 cGy can be delivered to the mucosal surface. In addition, in cases with discrete lesions, small portions of the mucosa can be boosted with a shielded cylinder to deliver an additional 1000 to 2000 cGy. Perez and colleagues60,67 reported that disease recurred in only 1 of 20 patients after intravaginal therapy, and Prempree and Amornmarn71 reported that disease was controlled in all their eight patients with CIS using intravaginal brachytherapy.
Stage I
Both radiation therapy and surgical excision are treatment options for stage I disease. Surgical excision can yield results equivalent to those obtained with radiation therapy.11,14,35 Limited surgical procedures can often provide adequate margins.11 Although these lesions are limited to the vaginal wall, involvement of the entire length of the vagina or extensive involvement of the anterior wall often requires a more radical surgical approach such as a total vaginectomy or anterior exenteration, respectively. Treatment must be individualized and surgical treatment selected only if the expected morbidity is limited. Younger patients with clear cell adenocarcinoma and smaller lesions are often candidates for limited surgical resection, which has the added advantage of preserving ovarian function in these patients.
Superficial lesions can also be treated with high-dose-rate brachytherapy, avoiding a 2- to 4-day hospital stay and confinement to bed rest. High-dose-rate brachytherapy can be particularly useful in obese patients or individuals with other medical problems who are at risk for the development of a pulmonary embolism. Unfortunately, there are few published data describing this form of treatment for vaginal cancer. Stock and colleagues10 reported 15 patients treated with a fractionated high-dose-rate system. Of the 15 patients, 14 were treated with high-dose-rate vaginal brachytherapy combined with whole-pelvic external-beam irradiation. The median whole-pelvic dose was 4000 cGy, and the median brachytherapy dose was 2100 cGy. This was delivered using a median dose per fraction of 700 cGy given 1 to 2 weeks apart. Nanavati and colleagues72 also reported on the combined use of whole-pelvic irradiation and high-dose-rate vaginal cylinder therapy. External doses of 4500 cGy were used in combination with high-dose-rate doses of 2000 to 2800 cGy given in three to four weekly doses.
When treatment consists of high-dose-rate brachytherapy alone, doses of 2100 to 2500 cGy prescribed to a depth of 0.5 cm are given to the entire vaginal mucosa by means of weekly fractions of 500 to 700 cGy. This prophylactic dose is designed to control microscopic disease. This fractionation regimen has been successfully and safely used to prophylactically treat the vaginal cuff after hysterectomy for endometrial cancer.73 An additional 2100 to 2500 cGy prescribed to a depth of 0.5 cm is delivered to the tumor via a shielded vaginal cylinder; weekly fractions of 500 to 700 cGy are used. This boost brings the total dose to 4200 to 5000 cGy.
Kucera and colleagues reported on 80 patients with invasive carcinoma of the vagina who were treated with high-dose-rate brachytherapy (HDRB) with or without external-beam therapy.74 These patients were compared with a historical group of 110 patients treated with intracavitary low-dose-rate brachytherapy (LDRB) with radium-226 or cesium-137, with or without external-beam irradiation. There were no significant differences in local and distant recurrences between the treatment modalities. The comparison of treatment with or without external-beam radiation and of complications showed no significant differences between the HDRB and LDRB series. HDRB would appear to be the preferred treatment modality because of the decreased radiation exposure, less variability in patient positioning, and better dose distribution as compared with LDRB.75
There is concern that the extent of submucosal disease and the role of microscopic paravaginal or nodal disease may be underestimated at initial presentation.47 This can result in unexpected recurrences when these early tumors are treated with intracavitary or interstitial therapy alone, and the group at MDACChave suggested including EBRT in the treatment plan in stage I patients, except in those with very small and very superficial lesions.47
Stage II
Radiation therapy is the primary treatment for stage II disease. Surgery is infrequently indicated because radical procedures are usually required to excise these lesions adequately and are often associated with significant morbidity and functional impairment. Stock and colleagues11 noted that 23 of 33 (70%) stage II patients treated with surgery required either a total vaginectomy or an exenterative procedure. Radical surgical procedures such as these are often associated with significant morbidity and functional impairment. Herbst and colleagues53 reported that 27% of patients undergoing this type of radical surgery developed serious complications that were often fatal. For this reason, surgical treatment is usually reserved for radiation therapy failures.
Treatment with radiation therapy involves a combination of external-beam therapy and brachytherapy. External-beam therapy covers a whole or a modified pelvic field that includes the inguinal region. Doses of 4500 to 5040 cGy are given to the pelvic field by means of 180 cGy fractions. If there is metastatic disease to the pelvic or groin lymph nodes, these regions should receive an external-beam boost to 6000 to 6600 cGy. The next stage of treatment should be delivered with brachytherapy. Unlike stage I lesions, stage II lesions should be treated with an interstitial implant. Because these are deeper lesions, an intracavitary system is inadequate because it fails to provide the needed dose to the deepest part of the tumor volume without exceeding the tolerance of the mucosal surface. Interstitial brachytherapy has been shown to improve local control when compared with intracavitary therapy.10,29 Perez26 recommends total tumor doses of 7500 to 8000 cGy given to the tumor volume by combined external-beam irradiation and interstitial implant, but Perez and colleagues60 acknowledge relatively high failure rates with these doses, with local and parametrial failure rates of 39% for stage IIA and 46.1% for stage IIB. Fleming and colleagues76 and Puthawala and colleagues77 recommend higher total doses of 8000 to 10,000 cGy of combined external-beam irradiation and brachytherapy with 4500 to 5000 cGy of the dose delivered with two interstitial implants. Puthawala and associates77 report improved local control with seven of seven stage IIA and seven of nine stage IIB patients locally controlled with total doses of 8000 to 10,000 cGy. These data suggest improved local control with higher doses of interstitial therapy. Based on these data, the recommended dose to be delivered to the tumor volume with interstitial therapy is 3500 to 4500 cGy, given in one or two applications. Two applications are usually scheduled, with a 1- to 2-week break between applications when higher interstitial doses are delivered. The use of interstitial HDRB appears to be reasonable alternative, although the number of patients treated with this technique is small, with limited follow-up available.78,79
Stage IVA
A similar radiotherapeutic approach can be taken with stage IV disease. One must keep in mind that the use of HDRB in combination with external-beam therapy to areas of tumor volume that invade bladder or rectum will be associated with a high incidence of fistula formation if local control is achieved. If a curative approach is taken, then treatment may involve an exenterative surgical procedure either for persistent disease or for complications. The MDACC has advocated the use of tailored EBRT fields to boost the primary site in selected cases, particularly in patients with extensive, very deeply infiltrating tumors that have indistinct margins or that extensively involve the rectovaginal septum or bladder. These patients tend to be poor candidates for intracavitary or interstitial brachytherapy.47
Vaginal Melanoma
There are relatively few reports on the management of vaginal melanoma, which is a rare lesion. Treatment has varied and has included wide local excision, radical surgery, radiation therapy, or combinations of these treatments. The limited data available preclude definitive treatment recommendations. The overall prognosis for patients with vaginal melanoma is extremely poor, with reported 5-year survival rates of 5% to 29%.80–82 Distant metastases, with rates ranging from 66% to 100%, contribute to the poor outcome.39,41,80,81,83 Patients with primary tumor size of less than 3 cm appeared to have improved survival rates.84,85
Morrow and Disaia,82 in a review of the literature, recommend radical surgery for these patients. Chung and colleagues81 reported local vaginal control in six of eight patients treated with radical surgery. Radical surgical procedures can still be associated with a high local failure rate, especially when pelvic failure including inguinal and pelvic nodal failure is considered. Bonner and colleagues80 treated five patients with radical surgery; four developed pelvic lesions as their first site of recurrence.
Radiation therapy has also been employed, particularly when more advanced lesions or lesions to be treated only for palliative intent are selected for radiotherapeutic management.36 This may explain the poor results reported with radiation therapy.81 In earlier stage disease, radiation therapy may provide adequate local control. Harrison and colleagues39 reported on three cases of vaginal melanoma, two stage I cases, and one isolated vaginal recurrence after local excision. Disease in all three patients was locally controlled with radiation therapy.
Given the high rate of distant metastases and the limited overall survival rate, radical surgical procedures are difficult to justify when there is no clear evidence that this approach provides increased local control or increased survival. Bonner and associates80 suggest adding pelvic radiation therapy to radical surgical procedures to increase local control. A less morbid treatment schema combines a wide local excision followed by postoperative whole-pelvic irradiation and intravaginal brachytherapy.
Radiation therapy alone can also be used. Some evidence suggests that high doses per fraction may be more beneficial in treating melanomas because of the large shoulder demonstrated in dose-response curves of cells in vitro. These data suggest a greater ability of these cells to undergo sublethal damage repair.86 The clinical relevance of these findings is unclear. Radiation Therapy Oncology Group 83-05, a prospective randomized trial, compared a standard fractionation scheme for the treatment of melanomas (5000 cGy in 20 daily fractions) to 3200 cGy by means of 800 cGy fractions given once a week, and found no difference in response rates between the two arms of the study.87 Because of these data, it is reasonable to treat vaginal melanomas using a dose and fractionation scheme similar to that employed in the treatment of squamous cell vaginal carcinomas of comparable stages.
External-Beam Radiation Therapy
If external-beam therapy is employed in the treatment plan, a whole-pelvic field or pelvic field modified to include the inguinal nodes is used to deliver a dose of 4500 cGy in 25 fractions with 15-mV or 18-mV photons. Techniques include a four-field plan with an anterior and posterior field, and two lateral fields. Alternatively, a parallel opposed, anterior and posterior field plan can also be applied and is often required because of the extensive anterior and posterior nodal drainage of these cancers (see Fig. 51-9). When lateral treatment fields are used, they must be carefully designed to avoid shielding potential nodal areas of involvement (particularly presacral, perirectal, or anterior external iliac nodes).
The treatment technique and the selection of the appropriate energy level must be individualized. In the presence of significant loops of small bowel in the field, a four-field plan may be preferable, because the small bowel can be blocked on the lateral fields. Energies from 6- to 18-mV photons can be selected, based on the patient’s size and isodose treatment plan. If the inguinal nodes are to be included in an anterior-posterior plan or a four-field technique, then the dose to the inguinal nodes (which usually lie 3 to 5 cm below the skin surface) should be calculated by means of a treatment plan. An inadequate dose to the inguinal nodes can be compensated by concurrent electron boosts to the inguinal regions (see Fig. 51-10). If there is no small bowel in the pelvic field, then an anterior-posterior plan (using 15- to 18-mV photons) may be preferable, especially if the inguinal nodes are to be treated. A standard whole-pelvic field should extend superiorly to the L5-S1 interspace and inferiorly to cover the inferior extent of the vagina, or to extend at least 4 cm distal to the most caudal aspect of the vaginal tumor. The lateral borders should be 1.5 to 2.0 cm lateral to the pelvic brim. If lateral fields are used, the posterior border should lie at the S2-S3 interspace and the anterior border 1 cm anterior to the symphysis pubis. These fields are designed to treat the vagina and the common iliac, external iliac, hypogastric, and obturator lymph nodes. If positive pelvic lymph nodes have been identified, the superior border is raised to the L4-L5 interspace to include the proximal common iliac nodes. If the inguinal nodes are to be treated, the lateral border should be extended to include these nodes. The inguinal nodes lie beneath and inferior to the inguinal ligament, which runs from the pubic tubercle to the anterior superior iliac spine.
Brachytherapy
Intracavitary Brachytherapy
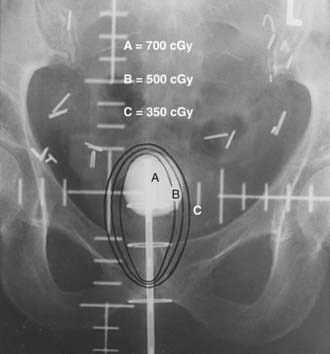
Intracavitary therapy is indicated primarily for superficial lesions. The most commonly employed intracavitary system is the vaginal cylinder. Many vaginal applicators are available, including the Bloedorn, Burnett, and Delclos cylinders. In addition, vaginal cylinders are available for use with high-dose-rate units (Fig. 51-11 and Fig. 51-12). Most of these applicators are available in different diameters. The largest-diameter cylinder that fits comfortably into the vaginal canal should be used. A 3-cm-diameter cylinder is the size most commonly used. The center of these cylinders contains a hollow tandem, through which the radioactive sources are inserted. The most common isotope employed for a low-dose-rate system is cesium-137 and iridium-192 for a high-dose-rate system. Usually two to three cesium sources are placed in the central tandem of the cylinder, determined by the extent of the vaginal canal to be treated. In a high-dose-rate system, one iridium source is used. It travels along the length of the central tandem, occupying specific dwell locations for prescribed times, determined by the extent of vaginal canal to be treated and the dose to be delivered. The dose delivered declines rapidly as the distance from the source increases. The differential between the dose delivered to the vaginal mucosa that lies adjacent to the vaginal cylinder and the prescription depth varies with the length of vaginal canal treated and the diameter of the cylinder. The larger the length of the canal treated and the diameter of the cylinder, the smaller the differential between mucosal and prescription dose. The prescription depth should be based on the estimated depth of tumor involvement. Doses are usually prescribed up to a depth of 1 cm into the mucosa. A depth of 0.5 cm is the most commonly prescribed depth. In patients with an intact cervix and superficial lesions in the fornices, vaginal ovoids can be employed.
Interstitial
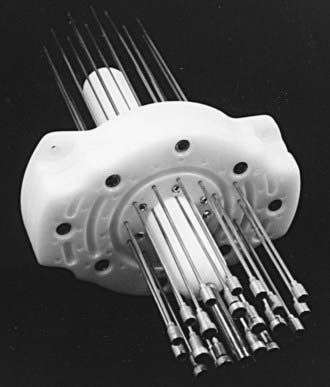
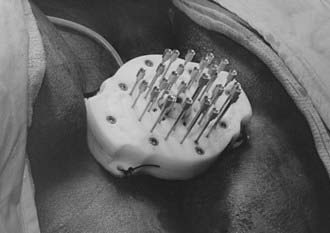
The two most commonly used applicators for transperineal interstitial brachytherapy are the Syed-Neblett template and the multiple-site perineal applicator (Fig. 51-13 and Fig. 51-14).88,89 These applicators contain a template that is placed adjacent to the perineum and vulva. This template has an opening in the center through which a cylinder inserts into the vaginal canal. The template contains holes through which needles can be inserted and guided along the vaginal mucosa and into the paravaginal space, parametria, cervix, and uterus. In the operating room the patient is placed in the dorsal-lithotomy position, anesthetized with either general or spinal anesthesia, and then prepared and draped. A careful speculum and bimanual examination is performed to assess the tumor’s dimensions and location. Small hemostat clips or titanium seeds are used to mark the vaginal mucosal component of the tumor. A diagram of the area to be implanted should be drawn. In patients with an intact uterus, the cervical os should be identified and dilated. By means of the Syed-Neblett applicator, the central tandem is inserted into the uterus through the dilated cervix. The vaginal cylinder is placed over the tandem by guiding the tandem through the central hole in the cylinder. A needle is then placed into one of the grooves on the cylinder and inserted into the cervix. This secures the central cylinder. If the patient has had a hysterectomy, the central tandem is not used. Rather, the template is inserted over the cylinder and secured in place by tightening the screws around the cylinder. The tumor volume is implanted by inserting needles through the holes in the template, which are arranged in concentric circles 1 cm apart. Planning an interstitial implant requires a three-dimensional concept. The goal of the implant is to cover the tumor volume with a 1- to 2-cm margin by placing needles into a three-dimensional volume. The needles are placed 1 to 2 cm apart, superior to the original tumor volume. The needles in the grooves of the cylinder will lie just outside the mucosal component of the tumor. Needles placed in the outer rows should implant an area at least 1 cm deep to the original volume. Once the needles are in place, they are secured by tightening the screws in the periphery of the template (Fig. 51-15). These implants can be optimally preplanned using CT scans and a template and cylinder placed in the vagina for the scan.
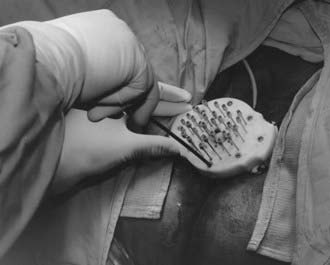
FIGURE 51-15 • Securing the needle template system by tightening the screws in the periphery of the template.
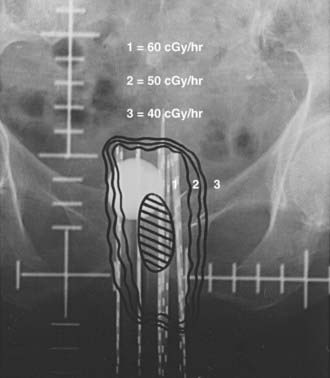
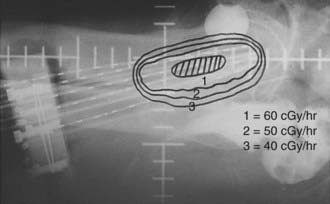
After the implant is complete, localization films are taken of the implanted area (Fig. 51-16 and Fig. 51-17). The information from the original simulation film, pelvic examination, pretreatment CT and MRI scans, and postimplant CT simulation is used to map out the original tumor volume. By providing a 1- to 2-cm margin to this volume, one can determine the number of needles to be implanted as well as the active length of each needle. Iridium-192 is the most common isotope used with this type of implant. Iridium-192 ribbons can be ordered with radioactive seeds placed 1 cm apart. Activities of 0.25 to 0.35 milligram-radium-equivalent (mgRaEq) per seed are used. The lower-activity ribbons are usually placed in the center of the implant to decrease central hot spots and create a uniform dose distribution. These lower-strength ribbons can also be placed in needles close to the urethra or rectum to decrease the dose delivered to these adjacent structures. The central tandem can be loaded with cesium-137 if needed. If the central tandem is loaded with cesium-137, the six central needles that lie in the obturator grooves should not be loaded to avoid hot spots in the bladder and rectum.88 The goal is a uniform dose distribution that covers the treatment volume with a dose rate of 50 to 70 cGy per hour.
The inability to visualize the tumor and surrounding normal structures during interstitial implantation has hampered the accuracy and safety of such implants.90 Consideration should be given to performing these implants with imaging guidance or under direct visualization, particularly in patients with a prior hysterectomy. When the implant is performed in conjunction with an open laparotomy, the needles can be visualized entering the tumor volume as well as the adjacent normal structures. This procedure can help avoid small-bowel or bladder perforation. The surgeon can assist in guiding the needles into the tumor volume and determining the depth of needle placement. The major problem with laparotomy-guided implants is that structures can only be visualized from an intraperitoneal view. Extraperitoneal structures, such as parts of the bladder, uterus, and cervix, as well as the vagina and paravaginal tissues, cannot be visualized. At the time of laparotomy, placement of an intraperitoneal sling can prevent small-bowel loops from contacting the top of the implant. Disaia and Creasman91 describe creating an “omental carpet” at the time of laparotomy. A section of omentum is placed along the descending colon into the pelvis; it separates the bladder and rectum from the implant and prevents small bowel from adhering to the implant. Laparotomy is usually not performed for the second implant in a two-application treatment.92
Other methods have been attempted to improve the accuracy of needle placement. Both CT and MRI preplanning have been used.93 These systems rely on accurate imaging of the target volume with the template and obturator in place. The limited accuracy of CT in visualizing pelvic structures has rendered this method to be of marginal value. MRI has been shown to be more accurate than CT in assessing tumor size and locoregional disease extent in cervical carcinoma.94 However, there are significant limitations in using MRI for preplanning, primarily because patients cannot be scanned through the MRI in the dorsal lithotomy position, which is the position of template insertion. For this reason, exact duplication of the plan is difficult. In addition, the position of the pelvic structures changes continually with filling and emptying of the bladder as well as changes in rectal fullness. Changes in body position can also alter the position of these organs.
To overcome the inaccuracies and limitations mentioned earlier, Stock and colleagues developed a system that relies on real-time transrectal ultrasound for guidance.90 Transrectal ultrasound, as opposed to transabdominal imaging, was used because it brings the ultrasound probe in closer proximity to the structures of interest (cervix, parametria, vagina) than abdominal ultrasound. The ultrasound probe can visualize all of the pelvic structures during the implant and allows for placement of needles into the target volume under direct visualization. Transverse ultrasound imaging is used to ensure that needles cover the target area and do not enter the bladder, rectum, or small bowel. The longitudinal mode of the ultrasound probe is extremely useful and accurate in determining the optimum depth of needle insertion. Needles can be inserted under direct guidance and placed at the appropriate depth. In addition, these images can be used to assess the overall length of the target volume, which will help to determine the active length of the implant. Using this technique, complicated preplanning or invasive laparotomy or laparoscopy can be avoided. CT scans with the needles in place can be used to optimize the dwell times and the plan when HDRB is used.
Critical Normal Tissues—Radiation Injury
Most late complications develop within the first 2 years after treatment, with a median time of 1 year.11,52 Evaluating complication rates from the literature is problematic; most studies report crude complication rates. Such figures fail to provide a true account of the risk of developing complications, because a large percentage of patients develop recurrences and die of their disease and thus would no longer be at risk for developing treatment complications. Thus, most reports tend to underestimate the actual risk, which is more accurately calculated by using an actuarial complication rate. Chyle and colleagues reported an actuarial incidence of serious complications of 19% at 20 years.51 This illustrates the importance of prolonged follow-up in determining the true incidence of late complications. Although most complications were evident by 5 years, a significant fraction appeared beyond this time, even up to nearly 16 years after treatment.
One of the most common treatment sequelae is vaginal stenosis. The incidence of vaginal stenosis ranges from 10% to 50%.2,12,13,95 Among 16 disease-free patients, Hintz and colleagues95 found 50% with some degree of vaginal stenosis. To avoid or minimize this problem, patients are encouraged to use vaginal dilators post-treatment because many women in this age group are sexually inactive. Vaginal stenosis interferes with follow-up examinations and may hinder discovery of local recurrences. Injury to the vulva and introitus can occur if the vaginal cylinder is loaded below the introitus. Care should be taken not to deliver high doses to this area unless it is involved with tumor.
Major complications requiring hospitalization or surgical intervention occur in 5% to 19% of patients.* The most common serious complications from radiation therapy are cystitis, proctitis, rectovaginal and vesicovaginal fistula formation, and vaginal necrosis. Kucera and Vavra3 reported a 40% incidence of proctitis and cystitis, although they did not comment on the extent of these complications. The incidence of fistula formation ranges from 1% to 7%.2,3,52,60,96 This serious complication may not resolve with conservative management. Exenterative surgical procedures are often necessary to prevent septicemia.
In their retrospective review of 36 patients treated with EBRT and interstitial brachytherapy for advanced gynecologic cancers, Kasibhatia and colleagues96 reported a 3-year risk of rectovaginal fistula of 18%. The risk was significantly higher in patients who received cumulative rectal doses of 7600 cGy or less.96 Vaginal necrosis has been reported in 4% to 15% of cases.12,77,97 The distal vagina has a lower radiation tolerance than the upper vagina, with both HDRB and LDRB.98 Hintz and colleagues95 found that none of their 16 patients developed vaginal necrosis in the upper vagina with summated doses up to 14,000 cGy. The lower vagina was more susceptible to vaginal necrosis when summated doses exceeded 9800 cGy. Cases of vaginal necrosis refractory to conservative management have been managed with hyperbaric oxygen treatments.99 In a series from the MDACC, two factors, namely FIGO stage and smoking history, were significantly correlated with subsequent complications in univariate analysis.47 The 5-year incidence of complications by stage were as follows: stage I, 4%; stage II, 9%; and stage III or IVA, 21%. At 5 years, the risk of major complications was 25% in current smokers compared with 5% who had no smoking history.
Patterns of Recurrence
Local recurrence is the most common pattern of treatment failure. In addition, persistent disease after radiation therapy is often a problem in more advanced lesions. In the series of Dixit and colleagues,52 68% of local failures in stage III patients were due to persistent disease. Although complete response rates are higher in earlier-stage disease, vaginal failure continues to be the main pattern of failure. Overall crude local failure rates range from 15% to 45%.* Extravaginal, intrapelvic failure, including nodal failure, is less common, and is seen in 7% to 18% of cases.1,11,35 A list of pelvic and local control rates by stage can be found in Table 51-5. Most locoregional recurrences and distant failures are noted in the first 5 years following treatment.47 Distant failures can also represent a significant problem, particularly in advanced disease.
Distant failure tends to occur later in the course of the disease than does local or pelvic recurrence. Reported incidences of distant failures vary widely. This is due to the difficulty, in retrospective reviews, of assessing the true incidence of distant failure, because thorough metastatic workups are seldom done in the setting of an incurable local failure. Distant failure rates range from 7% to 33%.2,11,28,29,35,52 At least half of all distant failures demonstrate concurrent local failure.29,53,60
Quality-of-Life Issues
Minimal data have been reported regarding quality-of-life issues for women with carcinoma of the vagina.57 Preservation of the anatomy and function of the vagina have been the reported advantage of radiation therapy versus surgical management of vaginal carcinomas. In addition, it has been presumed that preservation of the vagina confers a psychological benefit. However, it is unclear as to the percentage of women who actually maintain a functional vagina following definitive therapy for an invasive vaginal cancer.
Quality-of-life assessments in women with cervical carcinoma have demonstrated equivalent outcomes whether they are treated with primary irradiation or surgery. These treatments, however, are primarily directed to the cervix and upper vagina. Patients with vaginal cancer may present with tumor involvement of the upper, middle, and distal vagina, and tumor may spread to involve the urinary bladder and urethra. Therefore, depending on the tumor location and extent, vaginal function may be differentially affected.57
In an attempt to maintain vaginal functioning following definitive irradiation, the use of a vaginal dilator (two to three times per week) is strongly recommended. Topical intravaginal estrogen therapy and lubrication during intercourse is also advised. Some authors advocate the use of a vaginal douche during radiation therapy. A mixture of hydrogen peroxide and water (1 : 10 mixture) on a twice-daily basis is prescribed.57 After completion of irradiation, patients are instructed to continue to douche with this mixture.
Ultimately, functional outcome depends on the initial tumor size and extent, type of therapy, personal hygiene, the addition of hormone replacement therapy, and compliance with the prescribed post-treatment regimen.57
Results
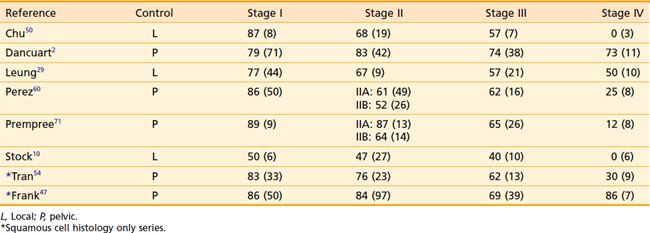
Overall survival rates by stage are listed in Table 51-6. Figure 51-18 shows the actuarial survival of patients by stage from an analysis by Stock and colleagues11 from the University of Pittsburgh.
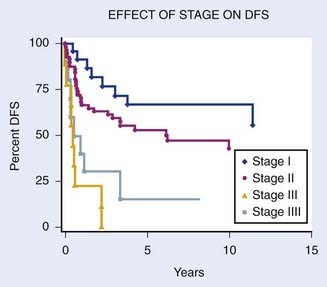
FIGURE 51-18 • Overall disease-free survival by stage.
(Data from Magee-Women’s Hospital, University of Pittsburgh School of Medicine.11)
Stage I
Actuarial survival rates for stage I disease range from 60% to 85%.* The results of treatment for stage I patients generally support the use of local therapy directed to the vagina and tumor volume and reveal local failure to be the primary site of recurrence. Among the 71 stage I patients of Dancourt and colleagues,2 of whom 36 were treated with local therapy alone (brachytherapy [intracavitary or interstitial] or transvaginal cone irradiation), the local failure rate was 18%. In only 2 of their 71 patients did the recurrence occur outside the central vaginal area. Perez and associates60 reported a pelvic failure rate of 14% for 50 stage I patients. In more recent years, there has been a greater tendency to treat stage I patients with an initial course of pelvic external-beam irradiation, prior to brachytherapy. Frank and colleagues reported a 5-year pelvic control rate of 86% for stage I patients.47 At 10 years, the pelvic relapse rate was 18% for stage I lesions, but all these pelvic failures occurred in patients who were treated with brachytherapy alone.47 Tran and colleagues reported a 5-year pelvic control rate of 83% for stage I patients, the majority of whom were treated with external-beam irradiation and brachytherapy.54 Distant metastases in this stage of disease are uncommon, occurring in about 5% of patients.2,35
Stage II
The actuarial survival rates for stage II disease range from 48% to 78%.*
Among stage II patients, disease can vary in extent. There can be minimal extension beyond the wall of the vagina or there can be extensive spread with involvement of the rectovaginal septum, parametria, bladder wall, or rectal wall (excluding mucosal involvement). These varied presentations are all considered stage II disease. Perez and colleagues49 suggested a modification of the FIGO staging system, dividing stage II into IIA (subvaginal involvement) and stage IIB (parametrial involvement). They found an improved 10-year DFS of 55% in 49 stage IIA patients compared with 43% in 26 stage IIB patients.60 Other data to support this modification reveal survival rates of 51% to 66% for stage IIA patients compared with rates of 24% to 31% for stage IIB.47,52 Local recurrence continues to be the main site of failure. Stock and associates,10 in a series from Memorial Sloan-Kettering Cancer Center, recorded an actuarial local failure rate of 53% for 27 patients with stage II disease. In a series from Magee-Women’s Hospital, the actuarial local failure rate in 58 stage II patients was 38%.11 More recent series suggest improved pelvic control rates of 76% to 84% at 5 years for patients with squamous cell carcinoma of the vagina.47,54 Frank and colleagues noted 91% 5-year vaginal disease control for patients with stage I or II disease.47 Compared with stage I, patients with stage II disease, in addition to demonstrating increased local failure, have a higher likelihood of developing distant metastases, with reported rates ranging from 22% to 46.1%.35,60 The distant metastasis rate has been shown to be higher in stage IIB lesions when compared with stage IIA.60,71
Stages III and IV
The actuarial survival rates for stage III disease range from 29% to 58%.* The local failure rates for stage III lesions range from 30% to 75%, reflecting the extent of disease as well as the treatment intent.10,47,54,60,71 Treatment with external-beam therapy alone has been associated with a poorer outcome than treatment with external-beam therapy combined with brachytherapy.10,47,53 Many stage III lesions are often managed with palliative intent and treated with external-beam irradiation alone. This partially accounts for the high reported local failure rates. In addition to an increasing local failure rate, distant failure is also increased. Reported distant failure rates have ranged from 23% to 50%.29,60,67,71
The actuarial survival rates for stage IV disease range from 0% to 25%.† This high incidence of distant metastases and decreased survival rates emphasizes the need for earlier diagnosis and effective systemic cytotoxic agents. These uniformly poor results reflect the inability of radiation therapy to control these lesions, with doses limited by the tolerance of the adjacent pelvic organs. Occasionally, lesions that have spread to the bowel or bladder but are not yet fixed to the pelvic sidewall can be treated or salvaged with a partial or total exenteration.11,44
Salvage Treatment
Theoretically, stage I and stage II lesions that fail radiation therapy might be salvaged with radical surgical procedures such as total vaginectomy, anterior or posterior exenteration, or total pelvic exenteration. Likewise, early lesions that recur after limited surgical procedures could theoretically be salvaged with more radical surgical procedures or radiation therapy. Unfortunately, salvage results have been uniformly poor. Stock and colleagues11 found that salvage was possible in only 5 of 50 patients with local failures (10%). These five patients had stage I or stage II disease at presentation. Chu and Beechinor50 reported a 15% salvage rate. These poor results reflect the fact that local recurrences are often more advanced and aggressive than the disease at initial presentation.
1 Jamal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA cancer J Clin. 2008;58:71.
2 Dancuart F, Delclos L, Wharton JT, et al. Primary squamous cell carcinoma of the vagina treated by radiotherapy: a failures analysis—the M.D. Anderson Hospital experience 1955–1982. Int J Radiat Oncol Biol Phys. 1988;14:745.
3 Kucera H, Vavra N. Radiation management of primary carcinoma of the vagina: clinical and histopathological variables associated with survival. Gynecol Oncol. 1991;40:12.
4 Murad TM, Durant JR, Maddox WA, et al. The pathologic behavior of primary vaginal carcinoma and its relationship to cervical cancer. Cancer. 1975;35:787.
5 Daling JR, Madeleine MM, Schwartz SM, et al. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol. 2002;84:263.
6 Ikenberg H, Runge M, Goppinger A, et al. Human papillomavirus DNA in invasive carcinoma of the vagina. Obstet Gynecol. 1990;76:432.
7 Ostrow RS, Manias DA, Clark BA, et al. Detection of human papillomavirus DNA in invasive carcinomas of the cervix by in situ hybridization. Cancer Res. 1987;47:47.
8 Lee YC, Holcomb K, Buhl A, et al. Rapid progression of primary vaginal squamous cell carcinoma in a young HIV-infected woman. Gynecol Oncol. 2000;78:380.
9 Merino MJ. Vaginal cancer: The role of infectious and environmental factors. Am J Obstet Gynecol. 1991;165:1255.
10 Stock RG, Mychalczak B, Armstrong JG, et al. The importance of brachytherapy technique in the management of primary carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 1992;24:747.
11 Stock RG, Chen ASJ, Seski J. A thirty-year experience in the management of primary carcinoma of the vagina: Analysis of prognostic factors and treatment modalities. Gynecol Oncol. 1995;56:45.
12 Benedet JL, Murphy KJ, Fairey RN, et al. Primary invasive carcinoma of the vagina. Obstet Gynecol. 1983;62:715.
13 Gallup DG, Talledo OE, Shah KJ, et al. Invasive squamous carcinoma of the vagina: a 14-year study. Obstet Gynecol. 1987;69:782.
14 Rubin SC, Young J, Mikuta JJ. Squamous cell carcinoma of the vagina: treatment, complications, and long-term follow-up. Gynecol Oncol. 1985;20:346.
15 Bell J, Sevin BU, Averette H, et al. Vaginal cancer after hysterectomy for benign disease: value of cytologic screening. Obstet Gynecol. 1984;64:699.
16 Manetta A, Gutrecht EL, Berman ML, et al. Primary invasive carcinoma of the vagina. Obstet Gynecol. 1990;76:639.
17 Brinton LA, Nasca PC, Malein K, et al. Case-control study of in situ and invasive carcinoma of the vagina. Gynecol Oncol. 1990;38:49.
18 Herman JM, Homesly HD, Dignan MB. Is hysterectomy a risk factor for vaginal cancer? JAMA. 1986;256:601.
19 Herbst AL, Scully RE. Adenocarcinoma of the vagina and cervix. A report of 7 cases including 6 clear cell carcinomas. Cancer. 1970;25:745.
20 Melnick S, Cole P, Anderson D, et al. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. An update. N Engl J Med. 1987;316:514.
21 Herbst AL, Anderson S, Hubby MM, et al. Risk factors for the development of diethylstilbestrol-associated clear cell adeno carcinoma: A case-controlled study. Am J Obstet Gynecol. 1986;154:814.
22 Woodruff JD. Carcinoma in situ of the vagina. Clin Obstet Gynecol. 1981;24:485.
23 Aho M, Vesterinen E, Meyer B, et al. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195.
24 Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:33.
25 Perez CA, Arneson AN, Dehner LP, et al. Radiation therapy in carcinoma of the vagina. Obstet Gynecol. 1974;44:862.
26 Perez CA. Vagina. In: Hoskins WJ, Perez CA, Young RC, editors. Principles and practice of gynecologic oncology. Philadelphia: JB Lippincott; 1992:567.
27 Frick HC, Jacox HW, Taylor HC. Primary carcinoma of the vagina. Am J Obstet Gynecol. 1968;101:695.
28 Houghton CRS, Iversen T. Squamous cell carcinoma of the vagina: A clinical study of the location of the tumor. Gynecol Oncol. 1982;13:365.
29 Leung S, Sexton M. Radical radiation therapy for carcinoma of the vagina—impact of treatment modalities on outcome: Peter MacCallum Cancer Institute experience 1970–1990. Int J Radiat Oncol Biol Phys. 1993;25:413.
30 Kurman RJ, Scully RE. The incidence and histogenesis of vaginal adenosis. Hum Pathol. 1974;5:265.
31 Robboy SJ, Scully RE, Herbst AL. Pathology of vaginal and cervical abnormalities associated with prenatal exposure to diethylstilbestrol (DES). J Reprod Med. 1975;15:13.
32 Robboy SJ, Hill EC, Sandberg EC. Vaginal adenosis in women born prior to the diethylstilbestrol era. Hum Pathol. 1986;17:488.
33 Robboy SJ, Young RH, Welch WR, et al. Atypical vaginal adenosis and cervical ectropion. Cancer. 1984;54:869.
34 Robboy SJ, Welch WR, Young RH, et al. Topographic relation of cervical ectropion and vaginal adenosis to clear cell adenocarcinoma. Obstet Gynecol. 1982;60:546.
35 Davis KP, Stanhope R, Garton GR, et al. Invasive vaginal carcinoma: Analysis of early-stage disease. Gynecol Oncol. 1991;42:131.
36 Macnaught R, Symonds RP, Hole D, et al. Improved control of primary vaginal tumors by combined external-beam and interstitial radiotherapy. Clin Radiol. 1986;37:29.
37 Senekjian EK, Frey BA, Stone C, et al. An evaluation of stage II vaginal clear cell adenocarcinoma according to substages. Gynecol Oncol. 1988;31:56.
38 Jones WB, Koulos JP, Saigo PE, et al. Clear-cell adenocarcinoma of the lower genital tract: Memorial Hospital 1974–1984. Obstet Gynecol. 1987;70:573.
39 Harrison LB, Fogel TD, Peschel RE. Primary vaginal cancer and vaginal melanoma: a review of therapy with external beam radiation and a simple intracavitary brachytherapy system. Endocurie Hypertherm. 1987;3:67.
40 Iversen K, Robins RE. Mucosal malignant melanomas. Am J Surg. 1980;139:660.
41 Norris HJ, Taylor HB. Melanomas of the vagina. Am J Clin Pathol. 1966;46:420.
42 Branton PA, Tavassoli FA. Spindle cell epithelioma, the so-called mixed tumor of the vagina. A clinicopathologic immunohistochemical, and ultrastructural analysis of 28 cases. Am J Surg Pathol. 1993;17:509.
43 Tavassoli FA, Norris HJ. Smooth muscle tumors of the vagina. Obstet Gynecol. 1979;53:689.
44 Eddy GL, Marks RD, Miller C, et al. Primary invasive vaginal carcinoma. Am J Obstet Gynecol. 1991;165:292.
45 Peters WA, Kumar NB, Morley GW. Carcinoma of the vagina: factors influencing treatment outcome. Cancer. 1985;55:892.
46 Rutledge F. Cancer of the vagina. Am J Obstet Gynecol. 1967;97:635.
47 Frank SJ, Jhingran A, Levenback C, et al. Definitive radiation therapy for squamous cell carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 2005;62:138.
48 Plentl AA, Friedman EA. Lymphatic system of the female genitalia: the morphologic basis of oncologic diagnosis and therapy. Philadelphia: WB Saunders; 1971. p. 51
49 Perez CA, Arneson AN, Galakatos A, et al. Malignant tumors of the vagina. Cancer. 1973;31:36.
50 Chu AM, Beechinor R. Survival and recurrence patterns in the radiation treatment of carcinoma of the vagina. Gynecol Oncol. 1984;19:298.
51 Chyle V, Zagars GK, Wheeler JA, et al. Definitive radiotherapy for carcinoma of the vagina: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 1996;35:891.
52 Dixit S, Singhal S, Baboo HA. Squamous cell carcinoma of the vagina: a review of 70 cases. Gynecol Oncol. 1993;48:80.
53 Herbst AL, Thomas GH, Ulfelder H. Primary carcincma of the vagina. Am J Obstet Gynecol. 1970;106:210.
54 Tran PT, Zheng S, Lee P, et al. Prognostic factors for outcomes and complications for primary squamous cell carcinoma of the vagina treated with radiation. Gynecol Oncol. 2007;105:641.
55 Chang YCF, Hricak H, Thurnher S, et al. Vagina: evaluation with MR imaging. Radiology. 1988;169:175.
56 Grigsby PW, Siegal BA, Dehdashti F. Lymph-node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745.
57 Lamoreaux WT, Grigsby PW, Dehd Ashti F, et al. FDG-PET evaluation of vaginal carcinoma. Int J Radiat Onvol Biol Phys. 2005;62:733.
58 Greene FL, Page DL, Fleming ID, et al, editors. AJCC cancer staging manual, ed 6, New York: Springer-Verlag, 2002.
59 Beller U, Benedet JL, Creasman WT, et al. Carcinoma of the vagina. FIGO 6th annual report on the results of treatment in gynecological cancer. Int J Gynecol Obstet. 2006;95(Suppl 1):S29-S42.
60 Perez CA, Camel M, Galakatos AE, et al. Definitive irradiation in carcinoma of the vagina: Long-term evaluation of results. Int J Radiat Oncol Biol Phys. 1988;15:1283.
61 Spiritos NM, Doshi BP, Kapp DS, et al. Radiation therapy for primary squamous cell carcinoma of the vagina: Stanford University experience. Gynecol Oncol. 1989;35:20.
62 Mundt AJ, Lujam AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330.
63 Jhingran A. Potential advantages of intensity-modulated radiation therapy in gynecologic malignancies. Semin Radiat Oncol. 2006;16:144.
64 Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1990;340:1154-1161.
65 Morris M, Eifel PF, Jiandong L, et al. Pelvic radiation and concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137-1143.
66 Rose PG, Bundy BN, Watkins J, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144-1153.
67 Perez CA, Grigsby PW, Garipagaoglu M, et al. Factors affecting long-term outcome of irradiation in carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 1999;44:37.
68 Jobson VW, Homesley HD. Treatment of vaginal intraepithelial neoplasia with the carbon dioxide laser. Obstet Gynecol. 1983;62:90.
69 Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: Biologic aspects and management. Obstet Gynecol. 1986;68:33.
70 Petrilli ES, Townsend DE, Morrow CP, et al. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321.
71 Prempree T, Amornmarn. Radiation treatment of primary carcinoma of the vagina. Acta Radiol Oncol. 1985;24:51.
72 Nanavati PJ, Fanning J, Hilgers RD, et al. High-dose rate brachytherapy in primary stage I and II vaginal cancer. Gynecol Oncol. 1993;51:67.
73 Mandell L, Nori D, Anderson L, Hilaris B. Postoperative vaginal radiation in endometrial cancer using a remote afterloading technique. Int J Radiat Oncol Biol Phys. 1985;11:473.
74 Kucera H, Mock U, Knocke TH, et al. Radiotherapy for invasive vaginal cancer: Outcome with intracavitary high dose rate brachytherapy versus conventional low dose rate brachytherapy. Acta Obstet Gynecol Scand. 2001;80:355.
75 Gore E, Gillin MT, Albano K, et al. Comparison of high dose rate and low dose rate dose distribution for vaginal cylinders. Int J Radiat Oncol Biol Phys. 1995;31:165.
76 Fleming P, Syed N, Neblett D, et al. Description of an afterloading Ir-192 interstitial-intracavitary technique in the treatment of carcinoma of the vagina. Obstet Gynecol. 1980;55:525.
77 Puthawala A, Syed N, Nalick R, et al. Integrated external and interstitial radiation therapy for primary carcinoma of the vagina. Obstet Gynecol. 1983;62:367.
78 Beriwal S, Heron DE, Mogus R, et al. High dose rate brachytherapy (HDRB) for primary or recurrent cancer in the vagina. Radiation Oncology. 2008;3:7.
79 Mock U, Kucera H, Fellmer C, et al. High dose rage (HDR) brachytherapy with or without external beam radiotherapy in the treatment of primary vaginal carcinoma: long-term results and side effects. Int J Radiat Oncol Biol Phys. 2003;56:950.
80 Bonner JA, Perez-Tamayo C, Reid GC, et al. The management of vaginal melanoma. Cancer. 1988;62:2066.
81 Chung AF, Casey MJ, Flannery JT, et al. Malignant melanoma of the vagina—report of 19 cases. Obstet Gynecol. 1980;55:720.
82 Morrow CP, Disaia PJ. Malignant melanoma of the female genitalia: A clinical analysis. Obstet Gynecol Surv. 1976;31:233.
83 Jentys W, Sikorowa L, Morkrzanowsk A. Primary melanoma of the vagina. Oncology. 1975;31:83.
84 Petru E, Nagele F, Czerwerka K, et al. Primary malignant melanoma of the vagina: Long-term remission following radiation therapy. Gynecol Oncol. 1998;70:23.
85 Buchanan DJ, Schlaerth J, Kurasaki T. Primary vaginal melanoma: Thirteen-year disease-free survival after wide local excision and review of recent literature. Am J Obstet Gynecol. 1998;178:1177.
86 Barranco SC, Romsdahl MM, Humphrey RM. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971;31:830.
87 Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20:429.
88 Syed AMS, Puthawala AA, Neblett D, et al. Transperineal interstitial-intracavitary “Syed-Neblett” applicator in the treatment of carcinoma of the uterine cervix. Endocurie Hypertherm Oncol. 1986;2:1.
89 Martinez A, Cox RS, Edmunson GK. A multiple-site perineal applicator (MUPIT) for treatment of prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1984;10:297.
90 Stock RG, Chan K, Terk M, et al. A new technique for performing Syed-Neblett template interstitial implants for gynecologic malignancies using transrectal-ultrasound guidance. Int J Radiat Oncol Biol Phys. 1997;37:819.
91 Disaia PJ, Creasman WT. Clinical Gynecologic Oncology, ed 4. St Louis: Mosby-Year Book; 1993. p 273
92 Monk BJ, Walker JL, Tewari K, et al. Open interstitial brachytherapy for the treatment of local-regional recurrences of the uterine corpus and cervix cancer after primary surgery. Gynecol Oncol. 1994;52:222.
93 Corn BW, Lanciano RM, Rosenblum N, et al. Improved treatment planning for the Syed-Neblett template using endorectal-coil magnetic resonance and intraoperative (laparotomy/laparoscopy) guidance: a new integrated technique for hysterectomized women with vaginal tumors. Gynecol Oncol. 1995;56:255.
94 Subak LL, Hricak H, Powell CB, et al. Cervical carcinoma: Computed tomography and magnetic resonance imaging for preoperative staging. Obstet Gynecol. 1995;86:43.
95 Hintz BL, Kagan AR, Chan P, et al. Radiation tolerance of the vaginal mucosa. Int J Radiat Oncol Biol Phys. 1980;6:711.
96 Kasibhatia M, Clough RW, Montana GS, et al. Predictors of severe gastrointestinal toxicity after external beam radiotherapy and interstitial brachytherapy for advanced or recurrent gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2006;65:398.
97 Reddy S, Lee MS, Graham JE, et al. Radiation therapy in primary carcinoma of the vagina. Gynecol Oncol. 1987;26:19.
98 Tyree WE, Cardenes H, Randall M, et al. High dose rate brachytherapy for vaginal cancer: Learning from treatment complications. Int J Gynecol Cancer. 2002;12:27.
99 Williams JA, Clarke D, Dennis WA, et al. The treatment of pelvic soft tissue radiation necrosis with hyperbaric oxygen. Am J Obstet Gynecol. 1992;167:412.