46 Cancer of the Testis
From a radiation oncologist’s point of view, when evaluating tumors of the testis one must first distinguish between seminomas, in which radiotherapy plays a major role in the care of the patient,1 and nonseminoma germ cell tumors (NSGCTs), in which the role of radiotherapy is primarily palliation and local control. This chapter focuses mainly on seminomas.
Incidence and Epidemiology
Testicular cancer accounted for some 8090 new cases and 380 estimated deaths in 2008.2 Although it represents only 1% of cancers in men it is the most common malignancy among men aged 20 to 30 years. Bilateral involvement is seen in 2% to 4% of patients. These tumors are highly curable even in advanced stage. The peak age incidence for seminoma is slightly older compared to NSGCT, with the histologic variant spermatocytic seminoma occurring in men older than age 60.3 Interestingly the worldwide incidence of testicular cancers has doubled in the past 40 years.2 Although germ cell tumors can rarely occur in extragonadal sites, their management follows that of testicular germ cell tumors.
Cryptorchidism, or an undescended testis, is the most studied risk factor for developing testis cancer. The relative risk of developing testicular cancer is 3.7 to 7.5.4,5 Orchiopexy before puberty decreases this risk.6 HIV as a risk factor is controversial. Although many reports show an increase in incidence,7 population-based studies have failed to confirm this.8,9 Chromosomal abnormalities most commonly involve chromosome 1 and 12. Deletions are seen in both arms of chromosome 1. The most frequent, and sometimes used diagnostically, is the isochrome of the short arm of chromosome 12, denoted as i(12p), and has been reported in up to 90% of germ cell tumors.10 Testicular cancers are observed 5 times more frequently among White men than among Black men. An association with the dysplastic nevus syndrome and Klinefelter’s syndrome has also been noted.
Anatomy
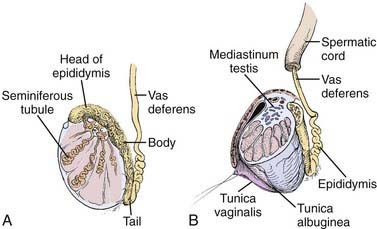
The adult testicle typically measures 4 to 5 cm in length and 2.5 cm in diameter. The testicle is covered by a parietal and visceral layer of the tunica vaginalis (Fig. 46-1). When this potential space accumulates fluid, a hydrocele forms. The testicle is encased by a hard outer capsule, the tunica albuginea. On the posterior-lateral aspect of the testicle, the tunica albuginea projects inwards, creating the mediastinum testis, the point of entry of blood vessels and ducts transporting sperm. Within the testicle, approximately 300 lobules exist, containing one or more seminiferous tubules: the site of spermatogenesis as well as site of origin for germ cell tumors. Each lobule of seminiferous tubules has short straight tubes that enter the mediastinum testis which then coalesce, forming the “rete testis” which, in turn, become fewer in number and wider in caliber, forming “efferent ductules.” These exit the testis and enter the head of the epididymis and subsequently the body and tail, where they become the vas deferens. Leydig cells reside in the interstitial tissue between seminiferous tubules and are responsible for testosterone production.
Pathology
The majority of testicular tumors are of germ cell origin. From a therapeutic perspective, these histologies are divided into two types: seminoma and NSGCTs. The histologies are listed in Table 46-1. The frequency of germ cell tumor histologies encountered is given in parentheses.
| Germ Cell Tumors (95%) |
| Pure seminoma (40%) |
Seminoma (35%)
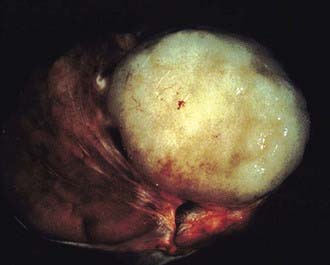
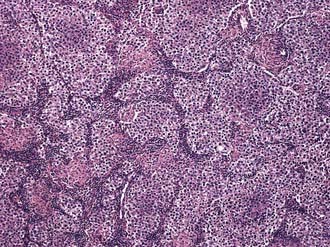
Three histologic subtypes of pure seminoma have been described. However, stage for stage, there is no prognostic significance to any of these subtypes. Classic seminoma accounts for 85% of all seminomas and is most common in the fourth decade of life. Grossly, coalescing gray nodules are observed (Fig. 46-2). Microscopically, monotonous sheets of large cells with clear cytoplasm and densely staining nuclei are seen (Fig. 46-3). It is noteworthy that syncytiotrophoblastic elements are seen in approximately 10% to 15% of cases, an incidence that corresponds approximately to the incidence of human chorionic gonadotropin (hCG) production in seminomas.
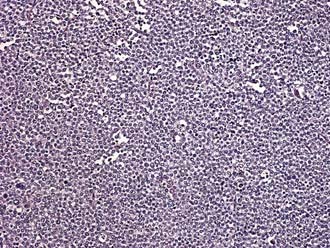
Spermatocytic seminoma accounts for 5% to 10% of all seminomas. Microscopically, cells vary in size and are characterized by densely staining cytoplasm and round nuclei that contain condensed chromatin (Fig. 46-4). More than half the patients with spermatocytic seminoma are over the age of 50. The important clinical aspect of spermatocytic seminoma is that they do not metastasize, thus, orchiectomy is curative and no additional therapy is needed.
Carcinoma in situ
A series of 250 patients with unilateral testicular cancer11 demonstrated the presence of carcinoma in situ (CIS) in 13 (5.2%) of the contralateral testes. This is approximately twice the overall incidence of bilateral testicular cancer. The presence of contralateral atrophy or ultrasonographic microlithiasis in patients with testicular tumors warrants contralateral biopsy. If diagnosed, CIS (also called testicular intraepithelial neoplasia, TIN) is usually treated by external beam radiotherapy to the involved testis with doses in the range of 18 to 20 Gy.12,13
Clinical Presentation
The classic presentation is that of a painless mass in the testes. Among the differential diagnoses are epididymitis, orchitis, and hydrocele. Trauma may lead to the discovery of an existing tumor but is not thought to be causative. Pain, though not common can be associated with presentation 40% of the time. Delay in diagnosis of greater than 3 to 6 months have been associated with worse prognosis.14 Evaluation of infertility occasionally leads to the diagnosis.15,16 Analysis of 38,000 men from the Surveillance, Epidemiology and End Results (SEER) database revealed that infertile men with abnormal semen analyses have a 20-fold greater incidence of testicular cancer compared to the general population.15 Gynecomastia and breast tenderness may be seen in 5% of patients17 owing to the production of estradiol in response to elevated levels of hCG. Patients with metastases to lymph nodes in the retroperitoneum most commonly present with low back pain and may or may not have hydronephrosis with secondary ureteral obstruction.
Serum Tumor Markers
Germ cell tumors frequently produce proteins that can serve as tumor markers for disease burden as well as diagnosis (Table 46-2). The two most important of these are AFP and the β subunit of human chorionic gonadotropin (β-hCG). Lactate dehydrogenase (LDH) level is also used as a marker for disease burden. It should be kept in mind, however, that these proteins are not specific to germ cell tumors. The serum half-life of these tumor markers is of clinical relevance and is 4 to 6 days for AFP; for β-hCG, 1 to 2 days. After 5 half-lives a previously elevated serum marker should return to within normal levels if all tumor cells have been eradicated. In pure seminoma elevated β-hCG is present about 10% of the time, yet if present tends to be modest in magnitude (usually <100). AFP, by contrast, is never present with pure seminoma.
Table 46-2 Frequency of Serum Marker Elevation in Testicular Tumors
| Histology | AFP, % | β-hCG, % |
|---|---|---|
| Seminoma | 0 | 9 |
| Teratoma | 38 | 25 |
| Embryonal | 70 | 60 |
| Choriocarcinoma | 0 | 100 |
| Yolk sac tumor | 75 | 25 |
AFP, Alpha-feto protein; hCG, human chorionic gonadotropin.
Routes of Spread
With the exception of choriocarcinoma, which demonstrates early hematogenous spread, germ cell tumors of the testis typically spread in a stepwise fashion via lymphatic channels. Lymph nodes of the testis extend from T11 to L4 but are concentrated at the level of the renal hila due to their common embryological origin with the kidney. The right and left testes possess somewhat different lymphatic pathways of clinical relevance in designing radiation fields. The primary landing site for the right testis is the interaortocaval area at the level of the right renal hilum. Stepwise spread, in order, is to the precaval, preaortic, paracaval, right common iliac, and right external iliac lymph nodes. The primary landing site for the left testis is the periaortic area at the level of the left renal hilum. Stepwise spread, in order, is to the preaortic, left common iliac, and left external iliac lymph nodes. In the absence of disease on the left side, no crossover metastases to the right side have ever been identified. However, right-to-left crossover metastases are common.18,19 These observations have resulted in modified surgical dissections to avoid injury to the sympathetic nervous system in select patients.
The retroperitoneum is the site most commonly involved in metastatic disease; however, visceral metastases may be seen in advanced disease. The sites involved in decreasing frequency include lung, liver, brain, bone, kidney, adrenal glands, gastrointestinal tract, and spleen.20 As mentioned, choriocarcinoma is the exception to the rule and is characterized by early hematogenous spread, especially to the lung. Choriocarcinoma also has a predilection for unusual sites of metastasis, such as the spleen.
Diagnostic Work-Up and Staging Studies
A testicular mass should be considered a tumor until proven otherwise. Work-up of a patient with a testicular mass should include an ultrasound of the both testes and tests for tumor markers, including AFP, β-hCG, and LDH. Once the diagnosis of testicular cancer has been pathologically established, one should then obtain a computed tomography (CT) scan of the abdomen and pelvis and a chest x-ray. Post-op serum markers should also be obtained, after an appropriate time interval, if they were elevated before surgery. A CT scan of the chest may be helpful to establish a baseline. Lymphangiography, although having the advantage of being able to detect nodal architecture, is now rarely used. The role of a positron-emission tomography (PET) scan in staging is controversial at this time. Its current utility lies in evaluating masses postchemotherapy to detect viable cancer cells. However, the decision to perform surgical resection of the residual mass should not be based exclusively on a positive PET image, since false-positive results appear to be common.21 The specificity of PET is significantly higher than that of CT when using a ≥3 cm cutoff. A negative PET scan excludes viable disease.22 Routine blood tests evaluating renal function and liver function would be required in patients with advanced disease. Counseling regarding sperm banking should be offered to all patients.
Prognostic Factors
Several prognostic factors for relapse have been identified. They include age (>30 years old), tumor size (>4 cm), presence of lymph/vascular invasion, histologic subtype (nonclassical), rete testes invasion, pT2, and preoperative levels of detectable hCG.23,24 Warde et al.23 demonstrated that primary tumor >4 cm was associated with a two-fold risk of relapse; the presence of rete testes invasion was associated with a 1.7-fold risk of relapse; and having both factors resulted in a 3.4-fold risk of relapse. Absence of rete testes invasion and tumor size <4 cm had a relapse rate of 12%, compared to tumor size >4 cm and absence of rete testes invasion at 17%. Patients with both rete testes invasion and tumors >4 cm had a relapse rate of 32%.25 Low-risk patients such as those with small tumors without rete testes invasion would be ideal candidates for surveillance.
For patients with advanced disease, the International Germ Cell Consensus Classification (IGCCC) risk stratification26 is commonly used (Table 46-3). It stratifies patients into good, intermediate, and poor risk based on the gonadal or extragonadal nature of the primary, the level of tumor markers, and sites of visceral metastases. Nonpulmonary sites of metastases have a poorer outcome. No patient with seminoma has poor risk.
| Classification | Seminoma | Nonseminoma |
|---|---|---|
| Good risk | Any HCG | AFP <1000 ng/mL |
| Any LDH | HCG <5000 miu/mL | |
| Nonpulmonary visceral metastases absent | LDH <1.5 × upper limit normalNonpulmonary visceral metastases | |
| Any primary site | Gonadal or retroperitoneal | |
| Intermediate risk | Any HCG | AFP 1000-10,000 ng/mL |
| Any LDH | HCG 5000-50,000 mIU/mL | |
| Nonpulmonary visceral metastases | LDH 1.5-10.0 × upper limit normalNonpulmonary visceral metastases | |
| Any primary site | Gonadal or retroperitoneal | |
| Poor risk | AFP >10,0000 ng/mLHCG >50,000 mIU/mLLDH >10.0 X upper limit normalNonpulmonary visceral metastasesMediastinal primary |
hCG, Human chorionic gonadotropin; AFP, α-feto protein; LDH, lactate dehydrogenase.
Staging Systems
The American Joint Committee of Cancer (AJCC) TNM staging system (Table 46-4) is currently standard.27 Only stage and disease burden are of clinical prognostic significance for seminomas. Neither the histologic subtype nor elevation of β-hCG has been shown to be of significant prognostic value in seminoma.
Standard Therapeutic Approaches
General Management Principles
Stage I (IA, IB, and IS)
The standard management for patients with stage I seminoma includes a radical inguinal orchiectomy followed by adjuvant radiation therapy to the para-aortic and ipsilateral pelvic lymph nodes (sometimes called “dog-leg” field) (Fig. 46-5). In instances where a scrotal approach to orchiectomy is performed, such patients are generally not considered for alternative management such as surveillance or reduced radiation fields. Although thought to be at higher risk for scrotal recurrence, several studies report no increase in pelvic, abdominal, or scrotal recurrence in patients with previous scrotal surgery.28
Surveillance for Stage I Seminoma
Surveillance, although commonly used in NSGCT, has only recently been considered for seminomas. In light of the high success rate of salvage chemotherapy (consisting of platinum-based regimens), the necessity of adjuvant radiation therapy after orchiectomy has been called into question. The advantage is that unnecessary treatment can be avoided in approximately 80% to 85% of patients and therefore spare these patients the toxicities of treatment. However, long surveillance follow-up of 7 to 10 years is needed and optimal patient selection is crucial to the success of these programs. However, any advantage should be weighed against the potential harm of whole-body CT scans over a 10-year surveillance period (20 scans) and the toxicities of salvage chemotherapy regimens in relapsed patients. Although it may offer some advantages in well-selected and compliant patients, the long-term benefit has yet to be proven. It is interesting to note that in recent patterns of care surveys the option of surveillance is often not discussed.29
To date, several investigators have published24,30–33 their experience with surveillance of patients with stage I seminoma (Table 46-5). With good follow-up data, the overall relapse rate varies from 15% to 20%. At relapse, these patients are offered systemic chemotherapy with excellent results. The protocol for following these patients on surveillance requires periodic exams, tumor markers, CT scans, and chest x-rays for a minimum of 7 years. The National Comprehensive Cancer Network (NCCN) guidelines recommend physical exams, markers every 3 to 4 months for years 1 to 3; every 6 months for years 4 to 7, and then annually. Abdominal and pelvic scans are recommended at each visit, and chest x-rays at every alternate visit up to 10 years.
Chemotherapy for Stage I Seminoma
Multiple nonrandomized trials have been published24,34–38 using either a single or 2 cycles of carboplatin. These trials have demonstrated the safety of this approach and have yielded low rates of relapses (Table 46-6).
The only randomized trial was done by the UK Medical Research Council/European Organization for Research and Treatment of Cancer (MRC/EORTC); 1447 patients were randomized to receive a single dose of carboplatin with an AUC of 7 versus radiotherapy (para-aortic or dog-leg field, 30 Gy). The primary endpoint was relapse-free survival (RFS). A noninferiority design was used and median follow-up was 4 years. RFS at 3-years was similar, 95.5% and 94.8% in the radiotherapy and chemotherapy arms, respectively. New primary second testicular tumors were seen in 10 patients in the RT arm and in two patients in the chemo arm. One seminoma-related death occurred in the RT arm. Recurrences in the RT arm were above the diaphragm and in the chemotherapy group in the retroperitoneum.39 In an updated follow-up presented in 2008 with a median follow-up of 6.5 years, the RFS at 5 years was 96% and 95%, respectively.
Risk-adapted approach involves selective treatment of those patients with increase risk. Such an approach was used in the Spanish Germ Cell Co-Op Group.40 Of the 314 patients, 100 had no risk factor (i.e., size >4 cm or rete testes invasion), were given no chemotherapy, and placed on a surveillance protocol. Two hundred fourteen patients had increased risk factors and were treated with two cycles of single-agent carboplatin with an AUC of 7. Overall, a 6% relapse was seen in the surveillance arm and 3.3% in the chemotherapy arm. An overall disease-free survival rate (DFS) was 93% on surveillance and 96% on chemotherapy. This approach spared chemotherapy for 30% of patients. A similar risk-adapted approach has been reported using two cycles of cisplatin and etoposide in patients with either a tumor >4 cm or age <34 years. With a median follow-up of 60 months, no relapses were noted.41
The long-term effects of carboplatin have been evaluated and no excess mortality was noted compared to an age- and sex-matched general UK population. Neither an increase in circulatory disease nor an increase in secondary cancers was noted.42
Chemotherapy for advanced disease has been well described.43 For patients with good-risk disease, three cycles of cisplatin, etoposide, and bleomycin (BEP) or two cycles of cisplatin and etoposide (EP) achieve a high cure rate.44 For patients with intermediate risk, four cycles of BEP is considered the standard of care.
Radiation Therapy, Simulation, and Treatment Planning
Radiotherapy Technique and Dose
Patients are simulated supine and immobilization devices are usually not necessary. With the availability of CT simulation, an intravenoces pyelogram (IVP) is now rarely performed to delineate the kidneys. The superior border of the field is at least at the T10 to 11 interspace, as T11 is the highest level of lymphatic drainage. The inferior border is at the top of the obturator foramen (although bottom of foramen is acceptable). Inclusion of the inguinal orchiectomy scar within the field is not indicated. The field is usually 10 to 12 cm wide, although care must be taken to include the left renal hilum in left-sided tumors that may require wider fields. Standard radiotherapy field is still the so-called “dog-leg” field that includes the para-aortic, ipsilateral iliac, and pelvic nodes (see Fig. 46-4).
Megavoltage photons are used in parallel opposed fields as described above, treated 5 days per week. Typical doses prescribed are 25 to 30 Gy in 1.25 to 2.0 Gy fractions. There are no data to support the use of higher doses. A lower dose-per-fraction may reduce the acute sequelae without compromising effectiveness. At our institution, we prescribe 25 Gy in 20 fractions. The optimal dose was tested in an EORTC randomized trial45 in which 625 patients with stage I testicular seminoma were randomized to receive either 20 Gy in 2 weeks or 30 Gy in 3 weeks with either dog-leg or para-aortic fields. Significant lethargy was more pronounced in the 30 Gy arm at 1 month, but by 3 months both arms were similar. With a median follow-up of 5 years, there were 10 and 11 relapses in the 30 and 20 Gy arm, respectively. One patient died from seminoma in the 20 Gy arm. This study, unfortunately, did not give any details of result comparisons between patients treated with dog-leg or para-aortic fields, thereby diminishing the strength of its conclusions. Longer follow-up is needed to confirm the duration of efficacy of 20 Gy and any potential reduction of late toxicities including secondary cancers.
Scattered radiation to the contralateral testis is minimized by use of a scrotal shield (“clamshell”) that can reduce the dose to about 1% to 2% of the prescribed dose, or 0.25-0.50 Gy, mostly from internal scatter. Further shielding can be achieved by extending the custom block by 5 cm below the edge of the field and adding a 10-cm lead shield supported over the scrotum.46 Diode measurements near the contralateral testicle can be obtained to monitor the scattered radiation during treatment.
Because fewer than 10% of patients with Stage I seminoma have disease in the pelvis there is increasing interest in omitting the pelvic nodes and treating the para-aortic nodes only. A randomized trial of 478 men with stage I testicular seminoma compared standard dog-leg to a para-aortic field. Dose was 30 Gy in 15 fractions.47 Patients with disturbed lymphatic drainage were excluded. With a median follow-up of 4.5 years, nine relapses occurred in each arm. In the para-aortic arm, four of those relapses were in the pelvis. The 3-year relapse-free survival rates were similar for both arms, about 96%. One seminoma-related death occurred in the para-aortic arm. Acute toxicity (nausea, vomiting, leucopenia) was less and sperm count higher in the para-aortic arm. Whether this strategy provides equal cure rates or reduces second malignancies must await longer follow-up.
For Stage IIA the radiation fields are the same as described above for Stage I. For Stage IIB modification of the fields, include a margin around the bulky nodal disease as seen on CT scan. Bilateral pelvic fields are sometimes recommended as well. Doses are 25 Gy with an additional boost to the bulky nodal disease for an additional 10 Gy. There does not appear to be any benefit to prophylactic mediastinal radiation therapy48 and it is not recommended.
Late Toxicities and Critical Normal Tissues
Sequelae of Radiotherapy
Acute side effects of radiation therapy are usually mild and predictable. They include nausea, vomiting and diarrhea, and a transient suppression of blood counts. The late sequelae of radiation therapy, although infrequent, are important considerations in the context of young patients and high cure rates. Among these are infertility, cardiotoxicity, gastrointestinal toxicity, second neoplasms, and immunosuppression. Secondary testicular tumors however are considered to stem from an underlying predisposition rather than to be radiation induced. Elective mediastinal radiation, which has been shown to be associated with an excessive number of cardiac deaths,49 has no role in stage I or II seminoma. Less than 2% will relapse within the mediastinum with standard fields.50 Late gastrointestinal toxicity, including gastric ulceration and dyspepsia, have been observed in patients receiving higher doses (40 Gy) than are currently prescribed.
Fertility
Infertility can be an important issue, since patients are typically aged 20 to 30 years. The contribution to infertility from surgery, RT, or a coexisting testicular abnormality is unknown. A lower testicular dose was associated with a more rapid recovery of sperm counts 1 year later.51 Inquiry should be made regarding the patient’s desire to retain fertility, as many such patients already have diminished fertility and may wish to consider sperm banking before radiotherapy. It would seem prudent to avoid insemination for 6 to 12 months after radiation therapy because of the potential presence of damaged genetic material.
Secondary Cancers After Radiotherapy for Seminomas
The incidence of secondary tumors attributable to radiation therapy is difficult to determine. However, available data suggest that patients with testicular cancers have higher rates of secondary tumors compared to an age-matched control. These included tumors of the stomach, colon, pancreas, kidney, and bladder as well as skin cancer and leukemia. In a large study by Travis et al.52 site-specific risk of second malignant neoplasm among 29,000 survivors of testicular cancer were reported from cancer registry databases. Second cancers were diagnosed among 5% of these patients. For patients with seminoma who are treated with adjuvant radiotherapy, the observed/expected risk was 1.5, and increased with time up to 20 years later. Significantly increased risk of tumors of the stomach, pancreas, bladder, colon, kidney, and leukemias was observed. Risk was similar for seminoma as well as nonseminoma testicular tumors. Although the cause of secondary cancers is often attributed to carcinogenic therapies, excess secondary cancers are likely multifactorial, including natural history, heightened diagnostic surveillance, environmental, and genetic determinants.
Outcomes
Testicular cancers are highly curable and very gratifying to treat. With modern approaches to therapy, the RFS rate for stage I seminoma is greater than 95%, and the cure rate approaches 99%.53 For stage II seminoma the cure rates are close to 98%.54 For more advanced stages the long-term survival approaches 85%. For stage I NSGCT, the survival rates are close to 100% and for more advanced disease is in the range of 70% to 80%.
1 National Comprehensive Cancer Network (NCCN), Clinical practice guidelines in oncology: testicular cancer available online at http://www.nccn.org
2 Jemal A, Siegel R, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
3 Carriere P, Baade P, et al. Population based incidence and age distribution of spermatocytic seminoma. J Urol. 2007;178:125-128.
4 Taran I, Elder JS. Results of orchiopexy for the undescended testis. World J Urol. 2006;24:231-239.
5 Swerdlow AJ, Higgins CD, et al. Risk of testicular cancer in cohort of boys with cryptorchidism. BMJ. 1997;314:1507-1511.
6 Pettersson A, Richiardi L, et al. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med. 2007;356:1835-1841.
7 Wilkinson M, Carroll PR. Testicular carcinoma in patients positive and at risk for human immunodeficiency virus. J Urol. 1990;144:1157-1159.
8 Roehrborn CG, Worrell JT, et al. Bilateral synchronous testis tumors of different histology in a patient with the acquired immunodeficiency syndrome related complex. J Urol. 1990;144:353-355.
9 Wilson WT, Frenkel E, et al. Testicular tumors in men with human immunodeficiency virus. J Urol. 1992;147:1038-1040.
10 Looijenga LH, de Munnik H, et al. A molecular model for the development of germ cell cancer. Int J Cancer. 1999;83:809-814.
11 Berthelsen JG, et al. Screening for carcinoma in situ of the contralateral testis in patients with germinal testicular cancer. Br Med J. 1982;285:1683.
12 Petersen PM, Giwercman A, Daugaard G, et al. Effect of graded testicular doses of radiotherapy in patients treated for carcinoma in-situ in the testis. J Clin Oncol. 2002;20:1537-1543.
13 Classen J, Dieckmann K, Bamberg M, et al. Radiotherapy with 16 Gy may fail to eradicate testicular intraepithelial neoplasia: preliminary communication of a dose-reduction trial of the German testicular Study Group. Br J Cancer. 2003;88:828-831.
14 Richie JP. Impact of diagnostic delay in testis cancer: Results of a large population-based study. Urol Oncol. 2008;26:220-221.
15 Raman JD, Nobert CF, et al. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol. 2005;174:1819-1822.
16 Phillips N, Jequier AM. Early testicular cancer: a problem in an infertility clinic. Reprod Biomed Online. 2007;15:520-525.
17 Javadpour N, McIntire KR, et al. Human chorionic gonadotropin (HCG) and alpha-fetoprotein (AFP) in sera and tumor cells of patients with testicular seminoma: a prospective study. Cancer. 1978;42:2768-2772.
18 Donahue JP, Zachary JM, Magnard BR. Distribution of nodal metastases in nonseminomatous testis cancer. J Urol. 1982;128:315-320.
19 Biswamay R, Hajdu SI, Whitmore WFJr. Distribution of retroperitoneal lymph node metastases in testicular germinal tumors. Cancer. 1974;33:340-348.
20 Bredael JJ, Vugrin D, Whitmore WFJr. Autopsy findings in 154 patients with germ cell tumors of the testis. Cancer. 1982;50:548-551.
21 Hinz S, Schrader M, et al. The role of positron emission tomography in the evaluation of residual masses after chemotherapy for advanced stage seminoma. J Urol. 2008;179:936-940.
22 Becherer A, De Santis M, et al. FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol. 2005;54:284-288.
23 Warde P, Specht L, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20:4448-4452.
24 Aparicio J, Garcia del Muro X, et al. Multicenter study evaluating a dual policy of postorchiectomy surveillance and selective adjuvant single-agent carboplatin for patients with clinical stage I seminoma. Ann Oncol. 2003;14:867-872.
25 Consoli F, Sava T, et al. Clinical stage I seminoma. Tumori. 2008;94:1-6.
26 International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594-603.
27 AJCC Cancer Staging Handbook. ed 6. 2002; Springer-Verlag, New York.
28 Kennedy CL, Hendry F, Peckman MJ. The significance of scrotal interference in stage I testicular cancer managed by orchiectomy and surveillance. Br J Urol. 1986;58:705.
29 Choo R, Sandler H, Warde P, et al. Survey of radiation oncologists: Practice patterns of the management of stage I seminoma of the testis in Canada and a selected group in the United States. Can J Urol. 2002;9:1479-1485.
30 Francis R, Bower M, et al. Surveillance for stage I testicular germ cell tumours: results and cost benefit analysis of management options. Eur J Cancer. 2000;36:1925-1932.
31 Chung P, Parker C, et al. Surveillance in stage I testicular seminoma—risk of late relapse. Can J Urol. 2002;9:1637-1640.
32 Daugaard G, Petersen PM, et al. Surveillance in stage I testicular cancer. APMIS. 2003;111:76-83.
33 Choo R, Thomas G, et al. Long-term outcome of postorchiectomy surveillance for stage I testicular seminoma. Int J Radiat Oncol Biol Phys. 2005;61:736-740.
34 Reiter WJ, Brodowicz T, et al. Twelve-year experience with two courses of adjuvant single-agent carboplatin therapy for clinical stage I seminoma. J Clin Oncol. 2001;19:101-104.
35 Steiner H, Holtl L, et al. Long-term experience with carboplatin monotherapy for clinical stage I seminoma: a retrospective single-center study. Urology. 2002;60:324-328.
36 Krege S, Kalund G, et al. Phase II study: adjuvant single-agent carboplatin therapy for clinical stage I seminoma. Eur Urol. 1997;31:405-407.
37 Argirovic D. Germ cell testicular tumors in clinical stage A and normal values of serum tumor markers post-orchiectomy: the experience in the management of 300 consecutive patients. J BUON. 2005;10:195-200.
38 Dieckmann KP, Bruggeboes B, et al. Adjuvant treatment of clinical stage I seminoma: is a single course of carboplatin sufficient? Urology. 2000;55:102-106.
39 Oliver RT, Mason MD, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
40 Aparicio J, Germa JR, et al. Risk-adapted management for patients with clinical stage I seminoma: the Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol. 2005;23:8717-8723.
41 Bamias A, Aravantinos G, et al. Two cycles of etoposide/cisplatin cured all patients with stage I testicular seminoma: risk-adapted protocol of the Hellenic Cooperative Oncology Group. Urology. 2007;70:1179-1183.
42 Powles T, Robinson D, et al. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol. 2008;19:443-447.
43 Williams SD, Birch R, et al. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435-1440.
44 Einhorn LH, Williams SD, et al. Evaluation of optimal duration of chemotherapy in favorable-prognosis disseminated germ cell tumors: a Southeastern Cancer Study Group protocol. J Clin Oncol. 1989;7:387-391.
45 Jones WG, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: a report on Medical Research Council Trial TE18, EORTC trial 30942. J Clin Oncol. 2005;23:1200-1208.
46 Kubo H, Shipley WU. Reduction of the scatter dose to the testicle outside the radiation treatment field. Int J Radiat Oncol Biol Phys. 1982;8:1741.
47 Fossa SD, et al. Optimal planning target volume for stage I testicular seminoma: a Medical Research Council randomized trial. J Clin Oncol. 1999;17:1146-1154.
48 Gospodarowicz M, Sturgeon JFG, Jewitt MAS. Early stage and advanced seminoma: Role of radiation therapy, surgery, and chemotherapy. Semin Oncol. 1998;25:160-173.
49 Hanks GE, Peters T, Owen J. Seminoma of the testis: long-term beneficial and deleterious results of radiation. Int J Radiat Oncol Biol Phys. 1992;24:913-919.
50 Makfoor BM, Shipley WU, Zietman AL. Early stage testicular seminoma: the role of radiation therapy following orchiectomy. Front Radiat Ther Oncol. 1994;28:183-195.
51 Gordon WJr, Siegmund K, Stanisic K, et al. A study of reproductive function in patients with seminoma treated with radiotherapy and orchidectomy (SWOG-8711): Southwest Oncology Group. Int J Radiat Oncol Biol Phys. 1997;38:83-94.
52 Travis LB, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89:1429-1439.
53 Fossa SD, Aass N, Kaalhus O. Radiotherapy for testicular seminoma stage I: treatment results and long-term post-irradiation morbidity in 365 patients. Int J Radiat Oncol Biol Phys. 1989;16:383-388.
54 Whipple GL, Sagerman RH, van Rooy EM. Long-term evaluation of postorchiectomy radiotherapy for stage II seminoma. Am J Clin Oncol. 1997;20:196-201.