37 Cancer of the Stomach
Epidemiology, Etiology, Genetics, and Cytogenetic Abnormalities
Epidemiology
Gastric and gastroesophageal junction (GEJ) adenocarcinoma constitutes a major health problem worldwide. Gastric cancer is the fourth most prevalent malignancy and the second leading cause of cancer death worldwide.1 In 2008, cancer of the stomach has an expected incidence in the United States of 21,500 cases (13,190 men and 8,310 women) and 10,880 deaths.2
Although there has been an overall reduction in gastric cancer incidence in both high and low-incidence countries, the incidence of adenocarcinoma of the gastroesophageal junction and gastric cardia has risen faster than any other malignancy in the last 25 years in the United States and other Western countries.3 The intestinal subtype is seen more commonly in older patients whereas the diffuse type affects younger patients and has a more aggressive clinical course. In a number of studies, the decrease in incidence has been attributed to the decline in the intestinal subtype. However, epidemiologic studies indicate that the diffuse subtype, especially signet ring cell type, is increasing.4
Etiology
Environmental Risk Factors
No single dietary agent has been determined to be the causative factor of gastric cancer. However, in case-controlled studies, gastric cancer appears to be positively correlated with the ingestion of pickled vegetables, salted fish, excessive dietary salt, and smoked meats.5–7 Fruits and vegetables may have a protective effect.8,9
Infectious Risk Factors
Helicobacter pylori is associated with inflammatory conditions in the stomach, in particular chronic atrophic gastritis. Although H. pylori by itself cannot cause gastric cancer, a growing body of evidence, including meta-analysis, suggests that it plays an important role in the development of adenocarcinoma of the stomach.10 Additionally, an association between Epstein Barr virus (EBV) and gastric cancer has been observed in 10% of cases, leading to speculation that this virus may also play a role in the development of gastric cancer, although further studies are needed for validation.11
Genetic Risk Factors
The genetic basis of gastric cancer and its precursors is being actively investigated. Emerging data suggest that the development of gastric cancer, similar to many other epithelial-derived carcinomas, is a multistep process that involves a successive activation or deletion of genes and their protein products that can occur through both genetic and epigenetic mechanisms. This is mediated by regulatory genes. Advances in molecular biology techniques have allowed characterization of the genetic changes thought to be responsible for this multistep process.12
One gene that has been shown to be critical in the development of gastric cancer is CDH1, that encodes E-cadherin, an epithelial cell adhesion molecule. Over half of gastric cancers have inactivated CDH1, through either genetic or epigenetic mechanisms. Germline CDH1 mutations have been identified in families with hereditary diffuse gastric cancer (HDGC), an example of a rare cancer susceptibility syndrome that has been very informative regarding cancer genetics and clinical approaches to a high risk for cancer. HDGC is an autosomal dominant inherited cancer syndrome clinically characterized by (1) two or more documented cases of diffuse gastric cancer (DGC) in first/second degree relatives, with at least one diagnosed before the age of 50, or (2) three or more cases of documented DGC in first/second degree relatives, independent of age of onset. The average age of onset of gastric cancer in affected families is 38, however individuals as young as 14 have died of the disease. HDGC is caused by the inheritance of a germline CDH1 mutation, typically a truncating mutation. Within the gastric mucosa a somatic event, usually CDH1 promoter methylation or an inactivating point mutation, provides the “second hit” leading to complete loss of E-cadherin function. Overall penetrance of diffuse gastric cancer in patients carrying a CDH1 mutation is estimated at 83% for women and 67% for men. Women with CDH1 mutations carry an additional 20-40% risk of lobular breast cancer.13
One challenge in managing a patient with a known CDH1 mutation is the inadequacy of current screening modalities. Diffuse gastric cancers found in HDGC patients are characterized by multiple infiltrates of malignant signet-ring cells which may underlie the normal mucosa. The wide distribution and small size of the malignant foci make them difficult to identify with random endoscopic biopsy. Because of high cancer penetrance, poor outcomes and inadequacy of clinical screening in HDGC, gene-directed prophylactic total gastrectomy is now offered to carriers of germline CDH1 mutations. In one published series of CDH1 mutation carriers who have undergone prophylactic gastrectomy, all specimens were found to contain multiple foci of diffuse, signet-ring cell cancer. DGC found in asymptomatic CDH1 carriers is typically early-stage and completely resected by prophylactic gastrectomy, so surgery can be considered curative.14
Other Risk Factors
Limited data suggest that radiation delivered for benign disease may be a risk factor for gastric cancer. In the earlier part of the 20th century, gastric irradiation was used to suppress gastric acid secretion. Orthovoltage radiation doses ranging from 1500 R to 2000 R in 10 days were used before the development of effective pharmacologic means to treat duodenal ulcers.15 In a small cohort of patients described by Peters and colleagues, patients who underwent partial gastrectomy and external-beam radiation therapy had an increased incidence of gastric cancer.16 Griem and associates reported on 1831 patients with peptic ulcer disease who were treated with radiation and compared them with a similar group of medically managed patients for an average of 22 years. Radiation therapy was linked to an increased relative risk for cancers of the stomach (RR = 2.77, 95% confidence interval), as was partial gastrectomy (RR = 2.60, 95% confidence interval). When surgery was combined with radiation therapy, the risk increased 10-fold.17 These results were only apparent after extended follow-up.18
A variety of other causal agents have been identified such as previous gastric surgery, adenomatous polyps, and chronic atrophic gastritis.12
Pathologic Conditions
The most common histologic condition (90%) is adenocarcinoma. Subtypes include papillary, tubular, mucinous, signet ring cell, adenosquamous, and squamous cell. Other histologic conditions include sarcoma, carcinoid, and small cell and undifferentiated carcinomas. Lymphomas (mucosa-associated lymphoid tissue [MALT]) and leiomyosarcomas can occur, albeit much more infrequently. MALT lymphomas are discussed in Chapter 54.
Jarvi and Lauren, based on epidemiologic studies, divided stomach cancers into two main groups: intestinal (expansile) or diffuse (infiltrative). In 1977, Ming proposed a modification of the Jarvi-Lauren classification.19 He emphasized the growth patterns rather than the tumor’s architectural subtypes. The classification divides tumors into two main patterns, expanding and infiltrative, which roughly correspond to the intestinal and diffuse types, respectively. The expanding pattern is more common in his series (67%) and describes tumors that tend to be fungating, whereas the infiltrating tumors tend to be diffuse.
Borrmann classified stomach cancer into four categories based on the gross morphology. These include type I: polypoid, type II: ulcerative, type III: ulcerating and infiltrating, and type IV: infiltrating. Type IV corresponds to the appearance of linitis plastica, in which infiltration by tumor causes rigidity of the gastric wall. Borrmann types I and II have a more favorable prognosis than Borrmann types III and IV, independent of lymph node involvement. Similarly, the Japanese Research Society for Gastric Cancer has a classification system based on gross appearance and divides lesions into protruded (I); superficial (II), with elevated (IIa), flat (IIb), and depressed (IIc) subtypes; and excavated (III) types.24
Diagnostic and Staging Studies
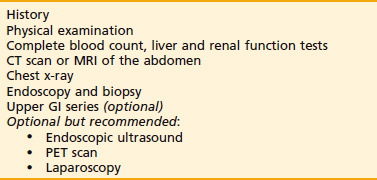
The workup for gastric cancer is described in Table 37-1. Numerous tumor markers have been described in association with gastric cancer, including CEA carcinoembryonic antigen) and Ca 19-9. However, the clinical utility of these tumor markers for screening, diagnostic staging, monitoring response to therapy, or as predictors of recurrence is controversial. Carcinoembryonic antigen (CEA) is positive in approximately one third of gastric carcinomas and reflects the extent of tumor burden. Postoperative elevated values of CEA are not helpful in predicting recurrence.
Endoscopy
Flexible fiberoptic endoscopy with biopsy is more than 90% accurate in diagnosing gastric cancer.20 The positive yield per biopsy is higher in exophytic tumors than in nonexophytic tumors. In the advanced infiltrating lesion (Borrmann type IV), the diagnosis by biopsy is less accurate. The location of the tumor can affect the diagnostic accuracy. It is particularly difficult to obtain a tissue diagnosis from lesions in the gastric cardia and antrum located behind the incisure angularis. Numerous studies have examined the correlation between radiologic diagnosis, endoscopy, and biopsy. No method alone is usually 100% reliable. The proper combination of imaging and endoscopy results in the highest number of positive diagnoses. Such information helps the endoscopist direct the biopsy to suspicious areas in which there may not be mucosal abnormalities.
The application of endoscopic ultrasonography (EUS) allows the identification of the five gastric wall layers.21 In staging gastric tumors, EUS diagnoses the depth of penetration of the primary gastric carcinoma with approximately 90% accuracy.22 EUS can also be used to stage lymph node metastases. However, the EUS is is less accurate compared with the T stage. In one series, EUS had an accuracy of 83%, sensitivity of 54%, and a specificity of 97%. The positive predictive value was 88% and the negative predictive value was 82%.
Imaging
The use of positron emission tomography with fluorodeoxyglucose (FDG-PET) is currently considered an optional component in the workup of gastric cancer. Recent studies have shown some added benefit in preoperative staging, predicting response to preoperative chemotherapy, and for the evaluation of recurrent gastric cancer.23 The role of FDG-PET in the evaluation of metastatic gastric cancer is unclear. Combination PET-CT may provide advantages over PET alone. Further studies are needed to validate this imaging modality in the evaluation of gastric cancer.24
Staging System
In 1988 the American Joint Commission on Cancer (AJCC) and the Union Internationale Contre le Cancer proposed a joint TNM staging system. This was based on the fifth edition of the AJCC TNM staging system. The sixth edition of the AJCC TNM staging system is seen in Table 37-2.25 The major change from the fifth edition is that T2 lesions have been divided into T2a (tumor invades the muscularis propria) and T2b (tumor invades the subserosa). There are also additional descriptors that, although they do not affect the stage grouping, do indicate cases needing separate analysis.
As with most other gastrointestinal malignancies, T stage is based on the extension through the layers of the stomach. Lymph node staging is based on the number of positive regional lymph nodes. Whether a lymph node is staged as nodal (N) or metastatic (M) disease depends on the relationship of the nodes to the primary tumor. In general, regional nodes include those located in the greater and lesser curvatures, as well as those in the left gastric, common hepatic, splenic, and celiac arteries. Involvement of other intra-abdominal nodes, including hepatoduodenal, retropancreatic, mesenteric, and paraaortic, are staged as metastatic disease. A list of specific regional and distant nodal disease is seen in Table 37-2. Patients with Epstein-Barr virus–involved gastric cancers have a lower incidence of positive nodes.26
Standard Therapeutic Approaches
Surgery
The primary treatment for local and regional gastric cancer is surgery. An analysis from the U.S. National Cancer Database of 50,169 patients treated from 1985 through 1996 who underwent a gastrectomy as a component of their therapy revealed a decrease in overall 5-year survival rate with increasing stage: IA, 78%; IB, 58%; II, 34%; IIIA, 20%; IIIB, 8%; and IV, 7%.27 In most cases, the determination of resectability for cure or palliation can only be assessed at the time of surgical exploration. The specific surgical procedure is based on the location and extent of the primary tumor and the respective lymph node drainage areas. Laparoscopy can detect disease in the peritoneal cavity and is helpful prior to a laparotomy.28 The most common site of failure is the abdomen.29
Subtotal versus Total Gastrectomy
The first randomized prospective trial comparing subtotal with total gastrectomy for distal gastric cancers was reported by Gouzi and colleagues.30 Both groups had similar morbidity (33%), mortality (1.3% versus 3.2% respectively), and 5-year survival (48%). Therefore, although elective total gastrectomy can be performed safely, subtotal and total gastrectomy offer equal long-term results in patients with carcinomas of the distal stomach. Most surgeons in the United States recommend a subtotal gastrectomy, providing that negative margins and an adequate lymphadenectomy can be achieved. Proper lymph node staging requires examination of 10 to 15 nodes.31 Negative margins usually require transection distally in the duodenum and at least 6 cm proximal to the palpable tumor. Margins must be confirmed with histopathology.
Conventional versus Extended Lymph Node Dissection
Lymphatic flow in the stomach is complex. Regardless of the location of the primary tumor, numerous nodal groups can be involved from either lymphatic spread occurring in a sequential manner or with metastases to distant lymph nodes without evidence of involvement of perigastric nodes (skipping). In rules established by the Japanese Research Society for Gastric Cancer, the lymph node groups are numbered 1 through 16, and are grouped into four lymph node levels or groups, N1 through N4. The determination of which nodal stations are placed in which nodal groups depends on the location of the primary tumor. By convention, N1 and N2 are considered regional nodes, whereas metastasis to N3 and N4 node groups is considered distant metastasis. Most Japanese surgeons recommend an extended dissection. The rationale is that improved local regional control results in improved patient survival. However, the data that support this hypothesis are controversial. In Japan, there is widespread endoscopic screening, and public health campaigns resulted in the diagnosis of a larger proportion of early stage tumors. The cure rates reported by Japanese investigators may be more related to the reduced stage of disease than the more radical surgical approach.32 Such “stage migration” may be responsible for the improved survival in each stage of the disease.
Three randomized trials have addressed the question of the extent of surgery.33–35 The three trials confirm that not only was there not an improvement in survival with more radical surgery (D2 versus D1 versus D0), but there was a corresponding increase in the incidence of complications. For example, in the Dutch trial, compared with D1 resection, patients who underwent a D2 resection had a significantly higher incidence of complications (43% versus 25%, p < 0.001), mortality (10% versus 4%, p = 0.004), and no difference in 11-year survival (35% versus 30%, p = 0.53).33 Almost all agree that a D0 resection is not adequate, and, in general, the recommended operation is at least a D1 for lesser curvature lesions and a full D2 for greater curvature lesions. The effect of a D2 resection on the results of the intergroup trial (INT) 0116 of postoperative adjuvant combined modality therapy36 is discussed in the “Outcomes” section of this chapter.
Sasako and colleagues randomized 523 patients with stages T2b through T4 gastric cancer to D2 resection with or without a prophylactic para-aortic lymph node dissection.37 There was no significant difference in 5-year survival (69% versus 70%, respectively).
Chemotherapy
The use of chemotherapy in the treatment of gastric cancer has been evaluated in a number of settings: neoadjuvant, adjuvant after complete resection, and for locally advanced or metastatic disease. Unfortunately, there are no accepted international standards for the optimal timing of chemotherapy or the combination of chemotherapeutic agents selected, leaving the decision to the provider. Two recent randomized trials reveal a significant improvement in survival with perioperative chemotherapy without radiation. Cunningham and colleagues reported the results of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial in which 503 patients with clinical stage II and III gastric cancer (11% GE junction) were randomized to three cycles of perioperative etoposide/cisplatin/5-Fluorocil (ECF) followed by surgery and three cycles of postoperative ECF, versus surgery alone.38 Approximately 40% of patients underwent a D2 resection. There was a significant improvement in 5-year survival in those who received chemotherapy versus observation (36% versus 23%, p = 0.009) The efficacy of the oral fluoropyrimidine S-1 has been reported in a randomized trial by Sakuramoto and colleagues. All patients underwent a resection (including D2 lymph node resection), and if they had stage II or III disease (89% were lymph node–positive) were randomized to surgery alone versus adjuvant S-1.39 Compared with surgery alone, those who received S-1 had a significant improvement in 3-year survival (80% versus 70%, HR 0.68, 95% CI 0.52–0.87, p = 0.003). Although local failure was 3% and nodal failure 9%, there was no significant improvement in distant control in patients receiving S-1.
Radiation therapy has been used as both an adjuvant treatment as well as a primary treatment modality. In the adjuvant setting it is limited to patients with local-regional disease who have undergone a complete resection with negative margins. Radiation as a primary treatment has been used after an incomplete or noncurative resection. The approximate 10% survival benefit seen in the two trials of chemotherapy discussed previously (ECF and S-1) is similar to that with postoperative chemoradiation in the INT 0116 trial.39 This has led to significant controversy as to the ideal adjuvant therapy for gastric cancer: neoadjuvant and postoperative ECF chemotherapy, postoperative S-1 chemotherapy alone, or postoperative combined-modality therapy. The results of INT 0116 are discussed in the “Outcome” section of this chapter.
Techniques of Radiation Therapy
Techniques
The design and delivery of radiation therapy for gastric cancer requires a knowledge of the natural history of the disease, patterns of failure, anatomy, and radiobiologic principles. Critical normal tissues that need to be considered include the liver, kidneys, small intestine, spinal cord, skin, bone marrow, and the remaining stomach. Furthermore, the use of proper equipment, implementation of methods to decrease treatment-related toxicity, and a close collaboration with the physics and technology staff is essential. General guidelines are seen in Table 37-3.
| General Considerations | |
| Target volume: includes the tumor bed, primary lymph nodes, plus an adequate (1.5-2.0 cm) margin.* | |
| The preoperative upper GI series, CT scan, operative findings, and clip placement define the tumor bed and lymph node areas. | |
| The tumor bed includes the maximum preoperative stomach volume. In patients with T3-T4 tumors, it should include the areas of local tumor extension as well as the medial two thirds to three fourths of the left hemidiaphragm. | |
| In the ideal setting, one half to two thirds of the left kidney can be blocked. Likewise, the porta hepatis and retroduodenal lymph nodes can be treated while including only a small portion of the right kidney. | |
| The nodes at risk include the celiac, porta hepatis, subpyloric, gastroduodenal, splenic-suprapancreatic, and retropancreaticoduodenal. | |
| The gastroepiploic nodes are usually removed with the operative specimen. | |
| The porta hepatic nodes are usually covered by a field that extends 2 cm to the right of T11-L1; however, their exact location is best determined from a CT scan. | |
| The celiac axis is located at approximately T12-L1. | |
| In proximal cardia and GE junction primaries, the paraesophageal nodes are at risk. The superior margin of the field should be extended to include 5 cm of the distal esophagus. | |
| Anteroposterior–Posteroanterior (AP–PA) Field | |
| Superior border: | The bottom of T8 or T9 to cover the celiac axis, GE junction, fundus, and the dome of the left hemidiaphragm. |
| Inferior border: | The bottom of L3 to cover the gastroduodenal nodes and the antrum. |
| Left border: | Include two thirds to three fourths of the left hemidiaphragm to cover fundus, suprapancreatic nodes, and splenic nodes. |
| Right border: | Field is 3 to 4 cm lateral to the vertebral bodies to cover the antrum, porta hepatis, and gastroduodenal nodes. |
| Lateral Field (If Appropriate) | |
| Superior border: | Same as AP–PA field. |
| Inferior border: | Same as AP–PA field. |
| Anterior border: | Anterior abdominal wall. |
| Posterior border: | Include one half to two thirds of the vertebral bodies. |
| Given the posterior location of fundus, suprapancreatic nodes, and the splenic hilar nodes, lateral fields should be used only if the location of these structures can be clearly identified and included in the lateral fields. | |
| Blocks | |
| Kidneys: | Blocks are used to spare the kidneys as much as possible and to decrease the amount of small bowel treated both superiorly and anteriorly. |
| Heart: | Since 5 cm of the distal esophagus are treated with proximal cardia and GE junction primaries, field blocks are needed to limit the dose to the heart. No more than 30% of the heart should receive >40 Gy. |
| Liver: | No more than 60% of the liver should received >30 Gy. |
CT, Computed tomography; GE, gastroesophageal; GI, gastrointestinal.
* It must be emphasized that these borders are idealized and they may require modification based on the preoperative imaging studies, operative findings, clip placement, lymph node involvement, and individual anatomy.
Radiation techniques for gastric cancer have evolved during the past decade. Initial recommendations included an anterior-to-posterior parallel pair (AP/PA) technique based on the University of Minnesota re-operation series from Gunderson and Sosin,40 followed by CT-based planning and a four-field technique41 to the more recent incorporation of intensity-modulated radiation therapy (IMRT).42–44
Radiation technique has an effect on acute and long-term toxicity. For example, in a series by Henning and colleagues, the incidence of grade 4 toxicity with postoperative radiation with or without chemotherapy was 14% acute and 5% chronic.45 The overall incidence of grade 4+ toxicity was lower in the 46 patients treated with four or more radiation fields (4% crude, and 5% 3-year actuarial) compared with the 18 treated with two radiation fields (22% crude, and 30% 3-year actuarial). These data are consistent with most other abdominal and pelvic malignancies in which a multiple-field technique allows a larger volume of small bowel to be excluded from the radiation field when compared with an AP/PA technique. Nonrandomized data comparing treatment plans with conformal versus conventional techniques suggest improved dose-volume histograms for dose-limiting organs.42–44
However, because of the location of the target volume as well as the proximity of adjacent organs (especially the kidneys and liver), sometimes a multiple-field technique may not always be advantageous. Furthermore, Ringash and colleagues examined organ motion during maximum inhalation and exhalation in 17 patients planned with three-dimensional (3-D) conformal techniques.46 The absolute displacement and range in cubic centimeters for all organs combined was 1.75 cc for cranial caudal, 0.59 cc for anterior-posterior, and 0.27 cc for right lateral locations.
CT-based treatment planning is helpful to determine if an AP/PA as opposed to a multiple-field technique should be used.41 Dose-volume histograms of the liver and kidneys are obtained. In general, if the dose to a given volume of kidney or liver exceeds its functional tolerance, then a higher dose to that volume is recommended to limit the dose to the remaining portion of the organ. For example, if 20% of the kidney receives 30 Gy, that 20% will not function. Therefore, delivering 45 Gy to that 20% will not further decrease function, whereas it may allow the delivery of a tolerable dose (<20 Gy) to the remaining 80% of the kidney. With this approach, the largest volume of the organ receives the smallest dose, thereby maintaining function. An analysis of late renal toxicity following conventional radiation for gastric cancer was presented by Verheij and colleagues.47 A total of 58 patients received postoperative chemoradiation (5-FU, capecitabine, or capecitabine/cisplatin) plus 45 Gy (1.8 Gy/fraction) with an AP/PA technique. Renal function was assessed by a 99mTc-mercaptoacetyltriglycine3 renal scan before and every 6 months after treatment. At 6-month follow-up, there was a 20% decrease in left renal function. By comparison, dose distribution was recalculated in a subset of patients using IMRT, which revealed that the dose to the left kidney was at least 50%, whereas the dose to the right kidney remained within the same range.
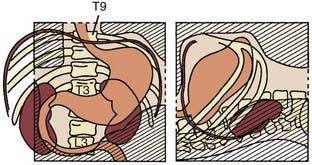
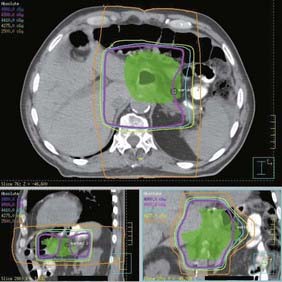
An example of a four-field technique is seen in Fig. 37-1. It must be emphasized that these are idealized treatment fields. The borders need to be modified based on the location of the primary tumor and the primary nodal drainage. Therefore, the radiation fields will usually be substantially smaller, reflecting this anatomic difference. An example of a 3-D treatment plan is seen in Fig. 37-2.
Critical Normal Tissues
The biologic mechanisms of acute and delayed toxicity as well as dietary interventions in patients receiving radiation for a variety of malignancies in which the small bowel is in the radiation field have been previously published.48–50
A number of simple radiotherapeutic techniques are available to decrease radiation-related toxicity (Table 37-4). For example, the treatment of all fields each day results in a lower integral dose and more homogeneous dose distribution. The treatment should be designed with the use of computerized radiation dosimetry and be delivered by high-energy linear accelerators, which, by nature of their depth-dose characteristics, deliver a higher dose to the tumor volume while sparing the surrounding normal structures. The fields need to be carefully tailored to the primary tumor location and nodal drainage.
| High-energy (≥10-15 MV) linear accelerators. |
| Treatment 5 days per week and all fields each day. |
| Port films once per week or more often if clinically indicated. |
| Computed dosimetry to minimize the hot spots and increase the homogeneity within the target volume. |
| Three-dimensional treatment planning to generate dose volume histograms for the liver, kidneys, and small intestine. |
| The ideal field arrangement (AP–PA vs. multiple fields) is the one that (a) delivers the most homogeneous distribution within the target volume while (b) limiting the dose volume to the surrounding organs so as not to exceed the functional tolerance of a given organ. Since the fundus of the stomach usually extends posteriorly, the use of lateral fields commonly increases the dose to the kidneys. Therefore, an AP–PA field arrangement is usually preferred. Occasionally a preoperative upper GI series or CT scan reveals that the stomach is sufficiently anterior to allow the addition of lateral fields. |
| The spinal cord should be limited to as close to 45 Gy as possible. |
| If the blood urea nitrogen, creatinine, or both are above normal range, then a renal scan is necessary to determine the relative function of each kidney. |
| Shaped blocks and, if needed, wedges. |
| Small bowel contrast for CT scan. |
| Dose considerations; 45 Gy at 1.8 Gy/d. |
AP–PA, Anteroposterior–posteroanterior; CT, computed tomography; GI, gastrointestinal.
Outcomes
Rationale for Adjuvant Radiation Therapy
The rationale for adjuvant radiation therapy is based on the patterns of failure following potentially curative surgery (Table 37-5). These failure patterns have been examined using clinical methods in a series from the Massachusetts General Hospital51 as well as re-operation methods in a series from the University of Minnesota.40 The series by Yoo and associates used both clinical and reoperation methods.52
Table 37-5 Patterns of Failure Following Potentially Curative Surgery in Gastric Cancer Cumulative Incidence of Failure (%)*
| Local Failure by TNM Stage | Clinical46 | Reoperation47 |
|---|---|---|
| T1N0 | 0 | — |
| T1-2N0 | 19 | — |
| T3N0 | 50 | — |
| T4N0 | 40 | — |
| T1-2 N1-2 | 24 | — |
| T3N1-2 | 36 | — |
| T4N1-2 | 56 | — |
| Failure Site | ||
| Total Local/Regional | 38 | 67 |
| -Gastric Bed | 21 | 54 |
| -Abdominal Scar | 5 | |
| -Anastomosis | 25 | 26 |
| -Nodes | 8 | 42 |
| Peritoneal Seeding | 23 | 41 |
| -Local | 19 | |
| -Diffuse | 22 | |
| Distant | 52 | 22 |
* Local failure as a component of failure.
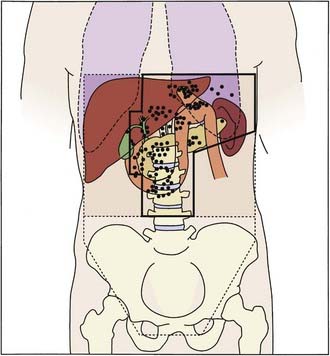
The clinical method relies on physical examination, radiologic studies, and, in some patients, operative findings. In contrast, the re-operation method used a planned second-look operation at least every 6 months. Although this method offers the most accurate appraisal of the anatomic distribution of failure, it is no longer used. The failure sites documented in the re-operation series from the University of Minnesota as well as idealized radiation fields covering these failure sites are seen in Fig. 37-3.
As seen with other gastrointestinal malignancies, the incidence of local failure increases with increasing penetration of the tumor through the wall of the organ and the presence of lymph node metastasis. Depending on the method used for analysis, the incidence of local failure following potentially curative surgery varies from 16% to 23% as the only site of failure and 33% to 67% as a component of failure.40,51,52 In the series of 508 patients reported by Yoo and colleagues, the mean time to local failure was 27 months and, by multivariate analysis, independent factors that predicted for a higher incidence of local failure included age older than 50, tumor size larger than 4 cm, proximal location, infiltrative or diffuse type, undifferentiated histology, serosal invasion, and lymph node metastasis.52 Even in patients in whom a complete (R0) resection is performed, local failure remains a significant clinical problem.
Results of Adjuvant Therapy
Postoperative Radiation Therapy With or Without Chemotherapy
For patients treated in the adjuvant setting (with negative margins) nonrandomized trials from Baeza and colleagues have reported encouraging results.53 There are three randomized trials of postoperative radiation therapy with or without chemotherapy following a complete resection with negative margins.54–57 The limited reports by Moertel and colleagues58 and Dent and associates59 did not reveal a clear survival advantage.
The landmark trial is INT 0116 (Fig. 37-4).36 Eligibility included patients with stages IB, II, IIIA, IIIB, and IV nonmetastatic adenocarcinoma of the stomach or GE junction. Following a resection with negative margins, patients were randomized to either observation alone or postoperative combined-modality therapy consisting of five monthly cycles of bolus chemotherapy with 45 Gy concurrent with cycles 2 and 3. During cycles 1, 2, 4, and 5, patients received 5-FU/leucovorin daily for 5 days, whereas during cycle 2 they received 3 days of 5-FU alone concurrent with the last 3 days of radiation.
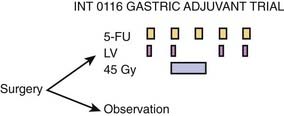
Figure 37-4 • Intergroup trial 0116 of adjuvant postoperative combined modality therapy versus observation for patients with T3 or N1-2 disease or both who undergo a complete resection with negative margins.36
(From Macdonald JS, Smalley SR, Benedetti J, et al: Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction, N Engl J Med 345:725, 2001.)
To minimize radiation-related toxicity, careful pretreatment review of the simulation films was performed. This resulted in the recommendation to the treating radiation oncologist to modify the design or volume of the radiation fields in 35% of cases. This illustrates the difficulty in designing gastric adjuvant radiation fields. Smalley and associates have reviewed the gastric anatomy and patterns of failure following surgery, and offer detailed radiation treatment-planning recommendations.41
Some physicians contend that adjuvant therapy is only a substitute for inferior surgery, and it is not necessary if patients undergo a D2 resection. This theory is based on the fact that in the INT 0116 trial, only 10% of patients underwent a D2 resection, whereas 54% underwent a D0 and 36% underwent a D1 resection. However, data do not support this conclusion. Although the INT 0116 trial was not stratified by the type of resection, there was no significant difference in survival based on the extent of surgery. Furthermore, as previously discussed, the three randomized trials addressing the question of the extent of surgery confirm that there is no improvement in survival with more radical surgery (D2 versus D1 versus D0).33–35 Careful surgical techniques are vital to the successful management of gastric cancer. However, adjuvant therapy is still necessary for patients with stages II through IV nonmetastatic disease, even following a D2 resection. This is further supported in a retrospective analysis of more than 500 patients who benefited from postoperative combined-modality therapy after a D2 resection.60 Although stage IB patients were included in INT 0116, most oncologists no longer offer them adjuvant therapy.
Most of the trials of combined-modality therapy seen in Table 37-6 have not used adequate doses of chemotherapy. In general, patients received 3 to 5 days of bolus 5-FU (350–500 mg/m2) during the first and sometimes the last 3 days of the radiation therapy. With this schedule, 5-FU is designed as a radiation sensitizer rather than as a systemic therapy. The European Organisation for Research and Treatment of Cancer randomized patients receiving combined-modality therapy to short-term 5-FU versus long-term 5-FU, and found that those randomized to receive short- plus long-term 5-FU had a median survival of 18 months compared with 10 months for those receiving short-term 5-FU.
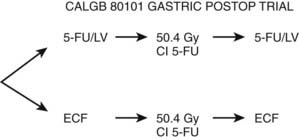
Based on the initial promising results of ECF chemotherapy (which were later confirmed in the MAGIC trial),38 this regimen was used before and after conventional postoperative combined-modality therapy. In a pilot trial, 21 patients received two cycles of postoperative ECF followed by 45 Gy plus continuous infusion 5-FU, followed by two additional cycles of ECF.61 Grade 3 toxicity was acceptable, with 29% white blood cell toxicity; 29% gastrointestinal toxicity; and 5% mucositis, fatigue, and hand-foot syndrome. Based on these pilot data, the Cancer and Leukemia Group B (CALGB) 80101 phase-III trial was developed (Fig. 37-5). The results are pending.
Preoperative Radiation Therapy
A randomized trial by Zhang and associates from Beijing revealed a significant improvement in survival with preoperative radiation.62 A total of 370 patients with adenocarcinoma of the gastric cardia who were younger than 65 and who, based on endoscopy and CT criteria, had clinically resectable disease, were randomized to preoperative radiation (40 Gy in 20 fractions) followed by surgery 2 to 4 weeks later versus surgery alone. With a median follow-up of 123 to 128 months, there was a significant improvement in survival with preoperative radiation (30% versus 20%, p = 0.0094). Preoperative radiation increased the rate of complete resection with negative margins (80% versus 62%, p < 0.001) without increasing the postoperative morbidity or mortality. The incidence of both local failure (33% versus 47%) and regional lymph node failure (31% versus 55%) was also decreased. There was no difference in distant failure (24% versus 25%). These data suggest that preoperative radiation improves local control and survival. However, randomized trials are needed to confirm the results in Western patients.
Preoperative Combined-Modality Therapy
Preoperative combined-modality therapy achieves a 25% pathologic complete response (pCR) rate and a 80% to 90% chance of a complete resection and negative margins in patients with adenocarcinoma of the GE junction. Based on these encouraging results, this approach is being used for the treatment of gastric cancer.63–65
In the Radiation Therapy Oncology Group (RTOG) 9904 trial, 43 patients with clinical-stage T2 or T3 or N1 or N2 (laparoscopy-negative) received two cycles of 5-FU/dl-leucovorin /cisplatin, then 45 Gy/5-FU/paclitaxel (Taxol).65 Overall, 36 had surgery (7 developed progression of disease ) and 50% had a D2 resection. The pCR rate was 26%, grade 4 acute toxicity was 21%, and the median survival was 23 months.
Although not standard of care, it is reasonable to treat T2 or lymph node–positive adenocarcinomas involving both the GE junction and stomach with preoperative combined-modality therapy. However, if this approach is used, a pretreatment laparoscopy is highly recommended to exclude patients with radiographically undetected peritoneal or liver metastasis.28
Treatment of Residual or Recurrent Disease
External-Beam Radiation With or Without Chemotherapy
The seminal trial examining the role of postoperative combined-modality therapy in gastric cancer was reported by Moertel and colleagues in 1969.66 Following laparotomy, patients with advanced gastric cancers were randomized to 40 Gy or 40 Gy plus 5-FU delivered as a radiation sensitizer. As seen in Table 37-6, there was a significant improvement in survival with the combination of radiation plus 5-FU.
The remaining randomized trials seen in Table 37-6 also include patients with unresectable or residual disease. It must be emphasized that none of the trials delivered adequate doses of radiation to control residual disease. Following a compete resection with negative margins, 45 Gy is recommended. The dose to adequately treat residual disease is least 55 to 65 Gy, which, when delivered by external beam, exceeds the tolerance of the stomach and small intestines. The use of intraoperative radiation therapy is an alternative method by which to achieve higher radiation doses, and this approach is discussed later.
Obtaining negative surgical margins is important because, as one would predict, patients who have microscopic or residual disease following surgery have a less favorable outcome than those with negative margins. In a report by Henning and colleagues of 63 patients treated with postoperative radiation with or without 5-FU-based chemotherapy, the median survival for the 25 patients with no residual disease was 19 months, versus 17 months for the 28 patients with microscopic residual disease, versus 9 months for the 10 patients with gross residual disease (P = 0.01).45 Likewise, there was a corresponding significant decrease in local failure (20% versus 36% versus 40%, p = 0.038, respectively). The actuarial 4-year survival for the 25 patients treated in the adjuvant setting (no residual disease) was 31%.
Chemotherapeutic agents other than 5-FU are being combined with radiation therapy. Taxanes have both systemic activity as well as radiation-sensitizing properties.67 Two paclitaxel-based postoperative combined-modality therapy regimens (paclitaxel plus radiation with or without 5-FU) were compared in the RTOG G0114 phase-II randomized trial.68 The trial was closed early because of the higher incidence of acute grade 3+ toxicity in the 5-FU–containing arm. Given the promising results of novel regimens such as irinotecan/cisplatin/bevacizumab in metastatic disease69 and S-1 for adjuvant disease,39 there are opportunities to explore new combined-modality therapy regimens in both the preoperative and postoperative settings.
Intraoperative Radiation Therapy
An alternative method of delivering radiation therapy is intraoperative radiation therapy (IORT).70–76 The theoretical advantage of this approach is the ability to deliver a more intensive dose of radiation to the tumor bed while excluding the surrounding normal tissues from the high-dose field.
The limited randomized trial performed by Sindelar and associates reported that the mean time to local failure was significantly improved in patients who received IORT (21 versus 8 months).70 Takahashi and Abe randomized patients based on the day of hospital admission to surgery plus IORT (28-35 Gy) versus surgery alone.71,72 There was an improvement in survival with IORT; however, it was limited to patients with stage III and IV disease.
There are a number of phase I and II trials of IORT with or without external-beam radiation or combined-modality therapy.73,74,77–80 Most include patients with residual disease. Calvo and colleagues reported a 39% 2-year survival.73 In the series from Coquard and associates, local failure was 25% and the 5-year survival was 47%.78
In the phase II RTOG 8504 trial, 27 patients with local-regional–only disease had a 19-month median survival and a 47% 2-year survival. Local failure within the IORT field was 37%.74 Ogata and colleagues reported a survival of 100% for stage II, 55% for stage III, and 12% for stage IV disease in 58 patients treated with 28 to 30 Gy of a wide field of IORT following a radical gastrectomy.79
In the IORT series by Henning and colleagues, the crude incidence of total grade 4 acute toxicity in the 60 patients who received a median of 46.8 Gy with or without IORT (91% received 5-FU–based chemotherapy) was 22% and the long-term toxicity was 18%.80
Palliative Radiation Therapy
In the palliative setting, radiation therapy provides relief of symptoms such as bleeding, obstruction, and pain in the majority of patients.81 Patients with favorable prognostic factors such as a favorable performance status, microscopic as compared with gross residual disease, and who received 5-FU–based chemotherapy tend to have a higher response rate. Overall, the median duration of palliation is 4 to 18 months.81–83 Rhomberg and colleagues treated 28 patients with gross unresectable (23) or with microscopic residual disease (5) with a median of 50 Gy plus the radiosensitizer dexrazoxane.84 The partial response rate was 89%, local control was 64%, and 96% had rapid pain relief.
1 Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150.
2 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
3 Parfitt JR, Miladinovic Z, Driman DK. Increasing incidence of adenocarcinoma of the gastroesophageal junction and distal stomach in Canada—an epidemiological study from 1964–2002. Can J Gastroenterol. 2006;20:271-276.
4 Henson DE, Diffus C, Younes M, et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770.
5 Chyou PH, Nomura AM, Hankin JH, et al. A case-cohort study of diet and stomach cancer. Cancer Res. 1990;50:7501-7511.
6 Demirer T, Icli F, Uzunalimoglu O, et al. Diet and stomach cancer incidence. A case-control study in Turkey. Cancer. 1990;65:2344-2355.
7 Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of selenium levels and incident esophageal and gastric cancer. J Natl Cancer Inst. 2000;92:1753-1763.
8 Zhang ZF, Kurtz RC, Yu GP, et al. Adenocarcinomas of the esophagus and gastric cardia: The role of diet. Nutrit Cancer. 1997;27:298-309.
9 Terry P, Lagergren J, Ye W, et al. Inverse association between intake of cereal fiber and risk of gastric cardia cancer. Gastroenterology. 2001;120:387-391.
10 Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131.
11 Koshiol J, Qiao YL, Mark SD, et al. Epstein-Barr virus serology and gastric cancer incidence and survival. Br J Cancer. 2007;97:1567-1569.
12 Schrump DS, Altorki N, Forastiere A, et al. Cancer of the esophagus. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins; 2001:1051-1091.
13 Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer. 2008;113:1850-1856.
14 Rogers WM, Dobo E, Norton JA, et al. Risk-reducing total gastrectomy for germline mutations in E-cadherin (CDH-1): pathologic findings with clinical implications. Am J Surg Pathol. 2008;32:799-809.
15 Carpender JW, Levin E, Clayman LB, et al. Radiation in the therapy of peptic ulcer. Am J Roentgen. 1956;75:274-279.
16 Peters M, Mackay IR, Buckley D. Occurrence of tumors and effects on longevity after limited x-irradiation in man. Am J Path. 1980;101:647-652.
17 Griem ML, Kleinerman RA, Boice JD, et al. Cancer following radiotherapy for peptic ulcer. J Natl Cancer Inst. 1994;86:842-851.
18 Griem ML, Justman J, Weiss L. The neoplastic potential of gastric irradiation. IV. Risk estimates. Am J Clin Oncol (CCT). 1984;7:675-678.
19 Munoz N, Connelly R. Time trends of intestinal and diffuse types of gastric cancer in the United States. Int J Cancer. 1971;8:158-170.
20 Nagao F, Takahashi N. Diagnosis of advanced gastric cancer. World J Surg. 1979;3:693-699.
21 Lambert R, Caletti G, Cho E, et al. International workshop on the clinical impact of endoscopic ultrasound in gastroenterology. Endoscopy. 2000;32:549-584.
22 Lightdale CJ. Diagnosis of esophago-gastric tumors. Endoscopy. 1992;24:18-29.
23 Jadvar H, Tatidil R, Garcia AA, et al. Evaluation of recurrent gastric malignancy with (F-18)-FDG positron emission tomography. Clin Radiol. 2003;58:215-221.
24 Rosenbaum SJ, Stergar H, Antoch G, et al. Staging and follow-up of gastrointestinal tumors with PET/CT. Abdom Imaging. 2006;31:25-35.
25 Stomach. In: Greene FL, Page DL, Fleming ID, et al, editors. AJCC Cancer Staging Manual. New York: Springer; 2002:99-106.
26 Beek J, Hausen A, Kranenbarg EK, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664-670.
27 Hundahl SA, Phillips JL, Menck HR. The national cancer data base report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921-932.
28 Nord HJ, Brady PG, Lightdale CJ, et al. Diagnostic laparoscopy guidelines for clinical application. Gastrointest Endosc. 2001;54:818-820.
29 D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816.
30 Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162-175.
31 Smith DS, Schwartz RR, Schwartz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124.
32 Otsuji E, Toma A, Kobayashi S, et al. Long-term benefit of extended lymphadenectomy with gastrectomy in distally located early gastric carcinoma. Am J Surg. 2000;180:127-132.
33 Hartgrink HH, van de Velde CJH, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch Gastric Cancer Group Trial. J Clin Oncol. 2004;22:2069-2077.
34 Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: Long term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530.
35 Roukos DH, Paraschou P, Lorenz M. Distal gastric cancer and extensive surgery: a new evaluation method based on the study of the status of residual lymph nodes after limited surgery. Ann Surg Oncol. 2000;7:719-726.
36 Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730.
37 Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462.
38 Cunningham D, Allum WH, Stenning SP, et al. Preoperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20.
39 Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, and oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820.
40 Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a reoperation series (second or symptomatic looks). Clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1-11.
41 Smalley SR, Gunderson L, Tepper JE, et al. Gastric surgical adjuvant radiotherapy consensus report—rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:283-293.
42 Kassam Z, Lockwood G, O’Brien C, et al. Conformal radiotherapy in the adjuvant treatment of gastric cancer: review of 82 cases. Int J Radiat Oncol Biol Phys. 2006;65:713-719.
43 Ringash JG, Wysocka B, Kassam Z, et al. Abdominal organ motion during conformal radiotherapy for gastric cancer. Int J Radiat Oncol Biol Phys. 2006;66:s271.
44 Leong T, Willis D, Joon DL, et al. 3D conformal radiotherapy for gastric cancer—results of a comparative planning study. Radiother Oncol. 2005;74:301-306.
45 Henning GT, Schild SE, Stafford SL, et al. Results of irradiation or chemoirradiation following resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:589-598.
46 Ringash J, Perkins G, Lockwood G, et al. IMRT for adjuvant radiation in gastric cancer: a preferred plan? Int J Radiat Oncol Biol Phys. 2006;65:311.
47 Verheij M, Oppedijk V, Boot H, et al. Late renal toxicity following post-operative chemoradiotherapy in gastric cancer. Proc ASCO/AGA/ASTRO/SSO GI Symp. 2005;2:83.
48 Kinsella TJ, Bloomer WD. Tolerance of the intestine to radiation therapy. Surg Gynecol Obstet. 1980;151:273-284.
49 Klimberg VS, Souba WW, Dolson DJ, et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62-68.
50 Coia L, Myerson R, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213-1236.
51 Landry J, Tepper JE, Wood WC, et al. Patterns of failure following curative resection of gastric cancer. Int J Radiat Oncol Biol Phys. 1990;19:1357-1362.
52 Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric adenocarcinoma. Br J Surg. 2000;87:236-242.
53 Baeza MR, Giannini O, Rivera R, et al. Adjuvant radiochemotherapy in the treatment of completely resected, locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2001;50:645-650.
54 Allum W, Hallissey MT, Ward LC, et al. A controlled, prospective randomized trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer: interim report. Br J Cancer. 1989;60:739-744.
55 Hallissey MT, Dunn JA, Ward LC, et al. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343:1309-1312.
56 Schein PS, Smith FP, Woolley PV, et al. Current management of advanced and locally unresectable gastric carcinoma. Cancer. 1982;50:2590-2596.
57 Gastrointestinal Tumor Study Group a. The concept of locally advanced gastric cancer. Effect of treatment on outcome. Cancer. 1990;66:2324-2330.
58 Moertel CG, Childs DS, O’Fallon JR, et al. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249-1254.
59 Dent DM, Werner ID, Novis B, et al. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer. 1979;44:385-392.
60 Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285.
61 Fuchs C, Fitzgerald T, Mamon H, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (RT): a multicenter study. Proc ASCO. 2003;22:257.
62 Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of the gastric cardia (AGC)—report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929-934.
63 Anne PR, Axelrod R, Rosato E, et al. A phase II trial of preoperative paclitaxel, carboplatin, 5-FU and radiation in patients with resectable esophageal or gastric cancer. Proc ASCO. 2004;22:320.
64 Allal AS, Zwahlen D, Brundler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: Long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2005;63:1286-1289.
65 Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): Quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958.
66 Moertel CG, Childs DS, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal carcinoma. Lancet. 1969;1:865-870.
67 Safran HS, Akerman P, Cioffi W, et al. Paclitaxel and concurrent radiation therapy for locally advanced adenocarcinomas of the pancreas, stomach, and gastroesophageal junction. Sem Radiat Oncol. 1999;53:57.
68 Schwartz GK, Winter K, Minsky B, et al. A randomized phase II trial comparing two paclitaxel (P)-cisplatin (C) containing chemoradiation (CRT) regimens as adjuvant therapy in resected gastric cancer (RTOG 0114). Int J Radiat Oncol Biol Phys. 2006;66:s169.
69 Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or esophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206.
70 Sindelar WF, Kinsella TJ. Randomized trial of resection and intraoperative radiotherapy in locally advanced gastric cancer. Proc ASCO. 1987;6:91.
71 Abe M. Intraoperative radiation therapy for gastric cancer. In: Dobelbower RR, Abe M, editors. Intraoperative Radiation Therapy. Boca Raton: CRC Press; 1991:166-179.
72 Takahashi T, Abe M. Intra-operative radiotherapy for carcinoma of the stomach. Eur J Surg Oncol. 1986;12:247-252.
73 Calvo FA, Aristu JJ, Azinovic I, et al. Intraoperative and external radiotherapy in resected gastric cancer: Updated report of a phase II trial. Int J Radiat Oncol Biol Phys. 1992;24:729-736.
74 Avizonis VN, Buzydolski J, Lanciano R, et al. Treatment of adenocarcinoma of the stomach with resection, intraoperative radiotherapy, and adjuvant external beam radiation: a phase II study from Radiation Therapy Oncology Group 85-04. Ann Surg Oncol. 1995;2:295-302.
75 Chen G, Song S. Evaluation of intraoperative radiotherapy for gastric carcinoma—analysis of 247 patients. In: Abe M, Takahashi M, editors. Intraoperative radiotherapy. New York: Pergamon Press; 1991:190-191.
76 Kramling HT, Willich N, Denecke H, et al. Prospective randomized study on IORT for resectable gastric carcinoma. In: Abe M, Takahashi M, editors. Intraoperative radiotherapy. New York: Pergamon Press; 1991:192-193.
77 Glehen O, Braujard AC, Romestaing P, et al. Intraoperative radiotherapy and external beam radiation therapy in gastric adenocarcinoma with R0-R1 surgical resection. Eur J Surg Oncol. 2000;26:s10-s12.
78 Coia LR, Engstrom PF, Paul AR, et al. Long-term results of infusional 5-FU, mitomycin-C, and radiation as primary management of esophageal cancer. Int J Radiat Oncol Biol Phys. 1991;20:29-36.
79 Ogata T, Araki K, Matsuura K, et al. A 10-year experience of intraoperative radiotherapy for gastric carcinoma and a new surgical method of creating a wider irradiation field for cases of total gastrectomy patients. Int J Radiat Oncol Biol Phys. 1995;32:341-347.
80 Henning GT, Schild SE, Stafford SL, et al. Results of irradiation or chemoirradiation for primary unresectable, locally recurrent, or grossly incomplete resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:109-118.
81 Falkson G, Van Eden EB, Sandison AG. A controlled clinical trial of fluorouracil plus imidazole carboxamide dimethyl triazeno plus vincristine plus bis-chloroethyl plus radiotherapy in stomach cancer. Med Pediatr Oncol. 1976;2:111-120.
82 Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373-381.
83 Mantell BS. Radiotherapy for dysphagia due to gastric carcinoma. Br J Surg. 1982;69:69-75.
84 Rhomberg W, Bohler F, Eiter H, et al. Radiotherapy and razoxane in the palliative treatment of gastric cancer. Radiat Oncol Invest. 1996;4:27-32.