41 Cancer of the Rectum
The sections in this chapter on epidemiology and genetics, pathologic conditions, routes of spread, and diagnostic and staging studies apply to both colon cancer and rectal cancer and are discussed in Chapter 40 on colon cancer. This chapter focuses exclusively on rectal cancer.
Epidemiology, Etiology, Genetics, and Cytogenetic Abnormalities
Information on epidemiology and genetics is covered in Chapter 40. In 2007 there were an estimated 41,420 new cases of rectal cancer in the United States with 23,840 occurring in men and 17,580 occurring in women.1
Anatomy
The rectum is approximately 15 cm in length. As seen in Fig. 41-1, it is divided into three 5-cm segments in relation to the anal verge (upper third, middle third, and lower third). However, the actual rectal length and division into surgical segments reflect a variety of features such as height, body habitus, pelvic width, and curve of the sacral hollow. For treatment purposes, large-bowel cancers that are located at or below the peritoneal reflection (rectosigmoid plus rectum) are collectively defined as rectal cancer.
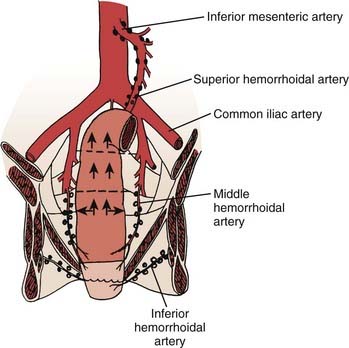
FIGURE 41-1 • Anatomy and lymphatic drainage of the rectum.
(From Pemberton JH: Anatomy and physiology of the anus and rectum. In Zuidema GD, ed. Shackelford’s Surgery of the Alimentary Tract. ed 2, Philadelphia, 1991, WB, Saunders, p 253.)
The major portion of the lymphatic drainage of the rectum passes along the superior hemorrhoidal arterial trunk toward the inferior mesenteric artery.2 Only a few lymphatics follow the inferior mesenteric vein. The pararectal nodes above the level of the middle rectal-valve drain exclusively along the superior hemorrhoidal lymphatic chain. Below this level (approximately 7-8 cm above the anal verge), some lymphatics pass to the lateral rectal pedicle. These lymphatics are associated with nodes along the middle hemorrhoidal artery, obturator fossa, and the hypogastric and common iliac arteries. Extensive lymphatics are also present in women contiguous with the rectovaginal septum, and in men along Denonvilliers fascia. The entire extraperitoneal soft tissue (mesorectum) is permeated with lymphatics.
Pathology
The majority of this information is covered in Chapter 40. However, the issue of lateral margins, which is most relevant to rectal cancer, is discussed.
In general, when pathologists describe negative margins of resection, they are referring to the proximal and distal margins. Because the staging of rectal cancer depends on the penetration of tumor through the bowel wall, the lateral (or circumferential or radial) margin is of equal importance. Birbeck and associates reviewed surgical specimens of 586 patients and found that 28% had a positive circumferential margin.3 There was a significant increase in local recurrence (38% versus 10%) and decrease in 5-year survival (40% versus 79%) in patients with positive versus negative circumferential margins. In a retrospective analysis of 504 patients reported by Bail and colleagues, even following preoperative combined-modality therapy (CMT), those with positive radial margins still had a higher local recurrence rate (35% versus 11%) and lower 5-year survival (27% versus 73%) compared with those with negative radial margins.4
Diagnostic and Staging Studies
The standard work-up for rectal cancer is seen in Table 41-1. The primary imaging modalities to assess the extent of the primary tumor are computed tomography (CT), magnetic resonance imaging (MRI), and transrectal ultrasound. The overall accuracy in predicting T stage is approximately 50% to 90% with transrectal ultrasound5–7 and 50% to 70% with CT or MRI.8–10 Positron emission tomography (PET) may offer a higher accuracy compared with CT for both primary and metastatic disease.11,12 PET is also helpful in predicting patients who will achieve negative margins at surgery.13 Obtaining a preoperative carcinoembryonic antigen (CEA) level is recommended; however, there is insufficient evidence at this time for the routine use of molecular markers.14
The identification of positive lymph nodes is more difficult. The overall accuracy in detecting positive lymph nodes with these techniques is approximately 50%. The accuracy of MRI is similar to CT; however, MRI may be further enhanced with the use of external or endorectal coils.10 Both CT and MRI can identify lymph nodes that measure 1 cm or larger, although enlarged lymph nodes are not pathognomonic of tumor involvement. Furthermore, using a nodal clearing technique, Herrera and colleagues found that 78% of positive lymph nodes in rectal cancer occur most frequently in lymph nodes measuring less than 5 mm.15 MRI may be further enhanced with the use of superparamagnetic iron oxide particles.16 Likewise, the accuracy of endorectal ultrasound for the detection of involved perirectal lymph nodes may be augmented if combined with fine-needle aspiration.7 The ability to accurately predict pathologic stage following preoperative CMT with MRI,17,18 ultrasound,19,20 PET,18,21 or physical examination22 is suboptimal.
Staging System
The sixth edition of the American Joint Commission on Cancer (AJCC) tumor-node-metastasis (TNM) staging system, as well as details regarding the evolution of pathologic staging of rectal cancer, are discussed in Chapter 40. Because many patients with rectal cancer are treated with preoperative CMT, most investigators recommend the TNM staging system with the provision that it is based on clinical or radiographic findings (e.g., ultrasound T3 [uT3] or clinical T4 [cT4]). The sixth edition of the AJCC TNM staging system recommends the y prefix (ycTNM or ypathologic [yp]TNM) when the tumor is staged during or after radiation or chemotherapy.23
One change is particularly relevant to rectal cancer. As with colon cancer, stage group II is subdivided into IIA (T3 disease) and IIB (T4 disease). Stage group III is subdivided into IIIA (T1-2 N1 M0 disease), IIIB (T3-4 N1 M0 disease), and IIIB (Tany N2 M0 disease). The prognostic validity of this change was supported by both the pooled analysis of the Intergroup and the National Surgical Breast and Bowel Project (NSABP) postoperative trials24 and the retrospective analysis of the American College of Surgeons National Cancer Database (NCDB).25 The 5-year survival by stages IIIA, IIIB, and IIIC in the pooled analysis was 81%, 57%, and 49%; and in the NCDB database was 55%, 35%, and 25%, respectively.
Standard Therapeutic Approaches
There are two conventional treatments for clinically resectable rectal cancer. The first is surgery and, if the tumor is pT3 or N1-2, this is followed by postoperative CMT.26 The second is used if the tumor is uT3 or cT4, and consists of preoperative CMT followed by surgery and postoperative chemotherapy.27 Based on the improved local control, toxicity profile, and incidence of sphincter preservation in patients who received preoperative CMT compared with postoperative CMT in the German Chirugische Arbeitsgemeinschaft Onkologie/Arbeitsgemeinschaft Radioonkologie/Arbeitsgemeinschaft Internistische Onkologie (CAO/ARO/AIO) 94 trial, preoperative CMT for patients with cT3 or N+ disease is now the standard of care.28
In patients with recurrent or clinically unresectable disease, intraoperative radiation therapy (IORT) is added at the time of surgery.29–32 In selected patients preoperative CMT followed by local excision33,34 or a local excision with or without postoperative CMT has been used.35,36 Radiation therapy alone has been used for patients who refuse surgery or are medically inoperable.37,38 The selection and results of these approaches are discussed in the Outcomes section. The National Comprehensive Cancer Network has published a clinical practice guideline for rectal cancer based on multidisciplinary panels, which use both evidence from randomized trials as well as lower-level sources such as clinical experience.39
Techniques of Radiation Therapy
The biologic mechanisms of acute and delayed toxicity as well as dietary interventions have been well described.40 Acute complications such as diarrhea and increased bowel frequency (small bowel), acute proctitis (large bowel), thrombocytopenia, leukopenia, and dysuria are common during treatment. These conditions are usually transient and resolve within a few weeks following the completion of radiation. The symptoms are related more to the dose rate and fraction size than the total dose. The mechanism is primarily the depletion of actively dividing cells in what is otherwise a stable cell-renewal system. In the small bowel, loss of the mucosal cells results in malabsorption of various substances, including fat, carbohydrate, protein, and bile salts. The bowel mucosa usually recovers completely in 1 to 3 months following radiation. Management usually involves the use of antispasmodic and anticholinergic medications.
The most common delayed severe complications are due to small-bowel damage and include small-bowel enteritis, adhesions, and small-bowel obstruction requiring surgical intervention. In the Massachusetts General Hospital (MGH) series, the incidence of small-bowel obestruction (SBO) with postoperative radiation therapy was 6% as compared with 5% with surgery alone.41
Preoperative radiation causes less acute and chronic toxicity compared with the postoperative treatment.28,42 This is likely the result of the fact that small bowel in an unviolated abdomen will be mobile and less likely to be adherent within a pelvic radiation portal. In the German CAO/ARO/AIO-94 trial, grade 3+ gastrointestinal toxicity was significantly reduced with the preoperative approach (acute 12% versus 18%, p = 0.04; and long-term 9% versus 15%, p = 0.07).28 Strictures at the anastomotic site were reduced 4% versus 12% (p = 0.003). The incidence of SBO requiring surgery in the preoperative arm of the German trial was 2%.
Toxicity of Pelvic Radiation Therapy
Complications of pelvic radiation therapy are a function of the volume of the radiation field, overall treatment time, fraction size, radiation energy, total dose, and technique. A number of simple radiotherapeutic techniques are available to decrease radiation-related small-bowel toxicity when using conventional techniques (Table 41-2).43,44 For example, the use of multiple-field techniques (preferably a three-field technique) allows a larger amount of small bowel to be blocked from the pelvis. The treatment of all fields each day results in a lower integral dose and more homogeneous dose distribution. The use of lateral fields for the boost, as well as positioning the patient in the prone position, further decreases the volume of small bowel in the lateral radiation fields. The treatment should be delivered by high-energy linear accelerators (>6 mV), which, by nature of their depth-dose characteristics, deliver a higher dose to the tumor volume while sparing the surrounding normal structures. When the perineal scar needs to be treated, it should be included in the pelvic radiation fields. The use of a separate perineal field is associated with an increased risk of overlap of the radiation fields and should be avoided. Split-course pelvic radiation is associated with increased chronic bowel complications.45
Table 41-2 Techniques to Decrease Radiation Toxicity in Small Bowel
Active inflammatory bowel disease is a contraindication to pelvic radiation, although there are some reports of patients who have tolerated it.46,47 Pelvic fractures following pelvic radiation are rare.48,49 Even with appropriate doses and techniques of radiation, approximately 1% of patients have significant long-term toxicity to pelvic organs. Testosterone levels are decreased when the testicles are near or in the radiation field.50,51 Radiation, alone or in combination with surgery, can have a negative effect on sexual function.52–54
Small-Bowel Contrast
The small bowel is a dose-limiting organ,55 and complications are proportional to the volume of small bowel in the radiation field.56 Small-bowel contrast is essential to determine the position of the small bowel during simulation. It should be used routinely during the simulation in patients receiving curative pelvic radiation therapy. Nonfixed small bowel normally moves, and a CT scan may be more accurate than traditional small-bowel series in detecting the position and volume of small bowel.57
Physical Maneuvers
Various physical maneuvers to exclude the small bowel from the pelvis have been examined. Gallagher and colleagues reported a significant decrease in the average small-bowel volume when the patients were treated in the prone position with the combination of abdominal-wall compression and bladder distension compared with the supine position.58 Use of a four-field technique further decreased the volume of small bowel. Treatment in the prone position without abdominal wall compression was not consistently effective in displacing small bowel and, in some patients, most commonly obese, the volume of small bowel increased.
Using a three-dimensional (3-D) planning system, Koelbl and associates found that in patients who received postoperative radiation, the use of the prone position plus a belly board decreased the small-bowel volume treated versus the use of the supine position.59 Intensity-modulated radiation therapy (IMRT) treatment-planning techniques can further decrease the volume of small bowel in the field.60
Immobilization Molds
The effectiveness of custom bowel immobilization molds (belly board) have been documented in a number of series.61,62 Shanahan and colleagues reported that the combination of the prone position and immobilization molds decreased the mean small-bowel volume in the radiation field by 66% compared with patients treated in the supine position without the immobilization mold.61
Any physical maneuver beyond the use of the prone position may be associated with patient discomfort, thereby leading to increased movement and daily set-up errors. Brierley and colleagues analyzed the variation of small-bowel volume in the pelvis before and during adjuvant pelvic radiation therapy for rectal cancer and reported that the displacement of small bowel from the posterior pelvis by bladder distension was not reliably maintained throughout the treatment course.63 Therefore, physical maneuvers beyond the prone position may not be beneficial in all patients. The use of such techniques should be tailored to the individual patient.
Radioprotectors and Radiosensitizers
Randomized trials have investigated the use of sucralfate enemas to decrease acute radiation proctitis, olsalazine and mesalazine to decrease acute enteritis, and butyric acid to decrease chronic radiation proctitis.64–68 All of these trials have been negative. The radioprotector WR-2721 did not reduce toxicity in early trials, but there is a suggestion of a benefit in a more recent study.69 Rectal-administered amifostine is well tolerated; however, its efficacy remains to be determined.70,71
Altered Radiation Fractionation Schemes
Hyperfractionated radiation has been examined in phase I and II trials.72,73 In general, the pathologic complete response (pCR) rates may be improved, but at the expense of increased acute toxicity. In the series by Janjan and associates, the use of a concomitant boost during standard CMT increased the incidence of perioperative morbidity.74 Movsas and colleagues recommend limiting the twice-daily component to the boost,75,76 whereas Myerson and colleagues deliver a concomitant 3-D boost one or twice a week.77 The Radiation Therapy Oncology Group R-0012 phase II randomized trial compared preoperative CMT with twice-daily radiation up to 60 Gy (1.2 Gy to 45.6 Gy, with a boost of 9.6-14.4 Gy) with conventional fractionation (1.8 Gy to 45 Gy, with a boost of 5.4-9.0 Gy) plus 5-fluorouracil (5-FU)/irinotecan.78 Both regimens resulted in a 28% pCR rate, but were also associated with a more than 40% rate of grade 3 or 4 acute toxicity. Hyperfractionated and accelerated fractionated radiation, especially in combination with chemotherapy, remains investigational.
Three-Dimensional Treatment Planning and Intensity-Modulated Radiation Therapy
Innovative techniques using 3-D treatment planning are being investigated.79 The most important contributions of 3-D treatment planning are the ability to plan and localize the target and normal tissues at all levels of the treatment volume, and to obtain dose-volume histogram data. A randomized trial of conformal versus conventional radiation therapy in 266 evaluable patients with pelvic malignancies has been reported by Tait and colleagues.80 Although there was a decrease in the volume of normal tissue volumes in the radiation field with conformal versus conventional treatment (689 cm3 versus 792 cm3), there was no difference in the level of symptoms or in medication prescribed. Meyerson and associates used a 3-D planned boost radiotherapy (0.9 Gy once or twice weekly to a total boost dose of 4.5 to 9 Gy) concurrently with pelvic irradiation (45 Gy/25 fractions).77 Dose-volume histogram information correlated with grade 3 and 4 toxicity, particularly with respect to small-bowel complications. The authors concluded that every effort should be made to limit the volume of small bowel receiving more then 40 Gy to less than 120 cc. Using a 3-D planning system, Koelbl and associates found that in patients receiving postoperative radiation, the use of the prone position plus a belly board decreased the small-bowel volume treated versus the supine position.81 An analysis of 3-D treatment planning techniques at the MGH suggests that the volume of small bowel in the radiation field is decreased with protons as compared with photons.82
To limit dose to previously irradiated critical structures, 3-D planning techniques are desirable for patients who undergo reirradiation. Although not well studied to date, the use of IMRT may further lower the dose to the critical structures while maintaining adequate doses in the planning target volume.83,84
Other Investigational Approaches
Investigational approaches such as neutrons,85 carbon ions,86 and hyperthermia87–89 have been examined. None have shown a clear advantage compared with conventional pelvic radiation therapy.
Surgical Techniques
Surgical techniques to minimize small-bowel injury in the postoperative setting include placing clips in the high-risk areas to better define the tumor volume and the use of absorbable mesh, which temporarily removes the small bowel from the pelvis.43 Because the radiation component of postoperative CMT does not begin until 4 months after surgery, the mesh may have already resorbed. Other techniques such as an inflatable pelvic small-bowel displacement prosthesis,90 reconstruction of the pelvic floor, construction of an omental pedicle flap,91 and retroversion of the uterus have had variable success.
Design of Radiation Therapy Fields for Rectal Cancer
The majority of local failures are in the posterior one half to two thirds of the true pelvis.43 Because the internal iliac and presacral nodes are posterior in reference to the external iliac nodes, much of the normal structures in the anterior pelvis can be spared with the use of lateral fields.
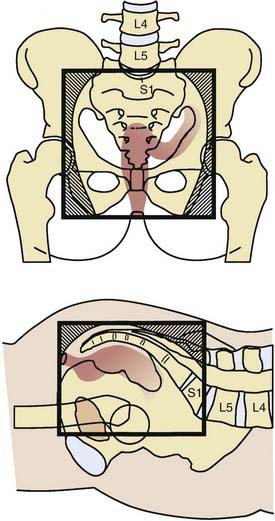
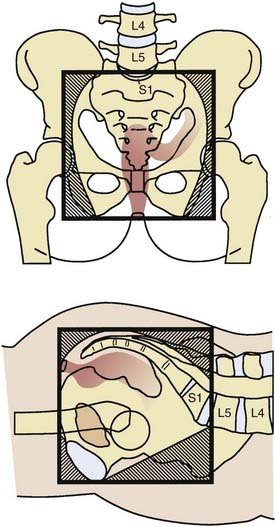
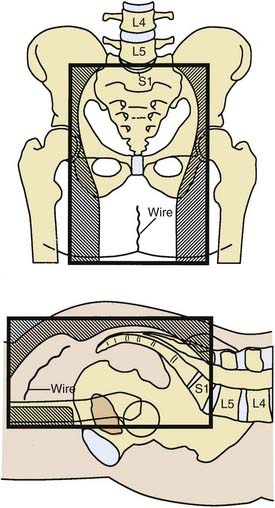
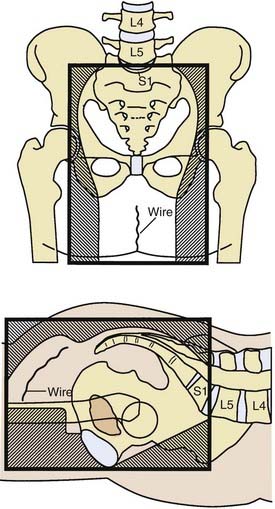
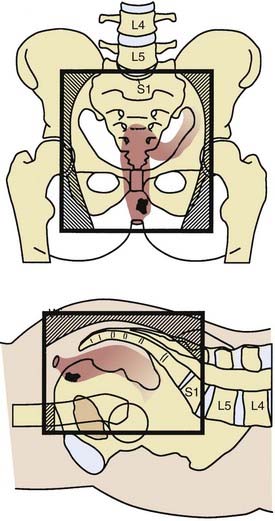
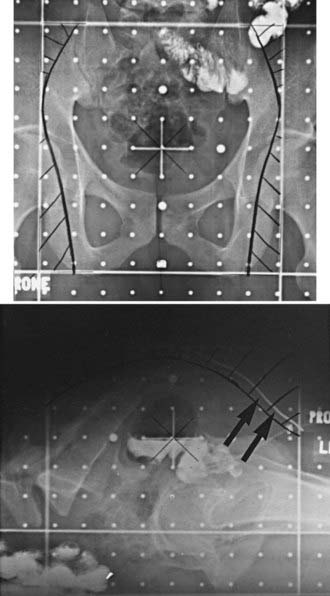
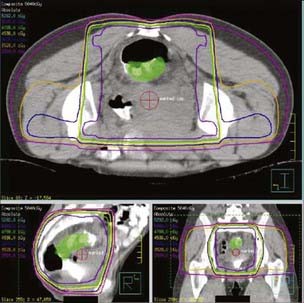
General guidelines for the design of conventional pelvic radiation therapy fields are listed in Table 41-3. The whole pelvic radiation field should adequately cover the primary bed as well as the primary nodes at risk. The intent of the boost is to treat the primary tumor and not to include the nodes. Therefore, the exact size is determined by the size and location of the primary tumor. Specific examples of field arrangements for a variety of clinical presentations are seen in Figs. 41-2 through Fig. 41-7. An example of a 3-D treatment plan is seen in Fig. 41-8.
Table 41-3 General Guidelines for Pelvic Radiation Therapy for Rectal Cancer
Outcome
Patterns of Failure
Local recurrence as a site of failure occurs frequently in patients with rectal cancer. The incidence is directly related to the extent of transmural penetration (microscopic versus gross) and the number and location of positive lymph nodes.92 The most common metastatic site is liver followed by lung. Brain93 and inguinal node94 metastases are uncommon. As more effective systemic chemotherapy is used, resulting in median survivals of up to 24 months in patients with metastatec disease,95,96 there is an increase in metastases seen in both these as well as other uncommon sites.
Clinically Resectable Rectal Cancer
Preoperative Radiation
A meta-analysis concluded that biologically effective preoperative doses of more than 30 Gy resulted in a statistically significant reduction in local-regional recurrences.97 With conventional fractionation (1.8-2.0 Gy fractions, 5 days per week), the doses most commonly used for the whole-pelvis fields, either preoperative or postoperative, range from 45 to 50.4 Gy in 5 to 6 weeks. These doses are necessary to control microscopic disease.98 An additional boost of 5.4 Gy to the primary tumor or tumor bed may be delivered if the small bowel is excluded from the high-dose field. However, it is not clear that higher doses improve local control. Doses up to 60 Gy are associated with increased pCR rates; however, they may also significantly increase acute and long-term morbidity.
In the Lyon R96-02 trial, 88 patients with cT2-3 rectal cancer received 39 Gy in 13 fractions preoperatively to the pelvis and were randomized to observation or a boost with contact radiation to a total dose of 85 Gy.99 Patients who received the boost had a decrease in local failure (2% versus 7%), but no difference in 2-year disease-free survival (92% versus 88%).
A unique clinical situation in which preoperative radiation therapy alone is recommended is when a patient has a distal uT2-N0 tumor and refuses an abdominoperineal resection (APR). Although APR is the standard therapy, an alternative is treatment with preoperative radiation followed by low anterior resection (LAR) and, if the pelvic nodes are positive, postoperative chemotherapy. In a series of 27 patients with cT2 N0 distal rectal cancer who refused APR, 78% of patients underwent a sphincter-sparing operation.100 The incidence of 5-year actuarial local recurrence of those undergoing sphincter preservation was 13%, colostomy-free survival was 100%, and overall survival was 85%. Overall, 77% of those undergoing a sphincter-sparing procedure had good or excellent bowel function at 24 to 36 months after surgery.
Short-Course Preoperative Radiation
There are 12 modern randomized trials of preoperative radiation therapy (without chemotherapy).27 All use low to moderate doses of radiation. Most of the trials showed a decrease in local recurrence, and in five of the trials, this difference reached statistical significance. Although in some trials a subset analysis revealed a significant improvement in survival, the Swedish Rectal Cancer Trial is the only one that reported a survival advantage for the total treatment group. Two meta-analyses report conflicting results. Although both revealed a decrease in local recurrence, the analysis by Camma and colleagues101 reported a survival advantage, whereas the analysis by the Colorectal Cancer Collaborative Group97 did not.
In the Swedish Rectal Cancer Trial, patients with cT1-3 rectal cancer were randomized to receive 25 Gy in 1 week followed by surgery 1 week later, versus surgery alone.102 Those who received preoperative radiation had a significant decrease in local recurrence (12% versus 27%) and a corresponding significant improvement in 5-year survival (58% versus 48%), with 13-year follow-up survival still significantly improved (38% versus 30%, p = 0.008).103 Of interest, the local recurrence rate in lymph node–positive (LN+) patients who underwent surgery alone was 46%, illustrating the inferior results of surgery prior to the adoption of total mesorectal excision (TME).
The Dutch CKVO 95-04 trial randomized 1805 patients with cT1-3 disease to TME or short-course preoperative radiation followed by TME.104 Although radiation significantly decreased local recurrence (8% versus 2%), there was no difference in 2-year survival (82%). With longer follow-up, 5-year local failure was higher with TME (11%); however, it was still significantly decreased to 6% with preoperative radiation.105 The acute toxicity in the Dutch CKVO 95-04 trial included 10% neurotoxicity, 29% perineal wound complications, and 12% postoperative leaks.106 In the patients who developed postoperative leaks, 80% required surgery resulting in 11% mortality.
The presence of a positive circumferential margin is an important negative prognostic sign. In the Dutch CKVO trial, 17% had positive circumferential margins. Although they received 50 Gy postoperatively, this did not compensate for positive margins.107 Few centers perform the necessary pathologic examination to detect positive circumferential margins.4,108 MRI can help identify patients who will have positive margins with surgery alone, as well as select those who may benefit from preoperative therapy.109–111
Combined Modality Therapy
There are two conventional treatments for clinically resectable rectal cancer. The first is surgery and, if the tumor is pT3 or N1-2, this is followed by postoperative CMT.26 The second is preoperative CMT followed by surgery and postoperative CMT if the tumor is uT3-4 or N+.27
Postoperative Therapy
The National Cancer Institute (NCI) Consensus Conference concluded in 1990 that CMT was the standard postoperative adjuvant treatment for patients with pT3 or N1-2 disease.26 Pelvic radiation therapy decreased local recurrence by approximately 50%, but did not improve survival. The standard design is to deliver six cycles of chemotherapy, two of which are given with concurrent radiation during the third and fourth cycles.
For patients treated with postoperative CMT receiving 5-FU as a single agent, there was a 10% survival advantage with continuous infusion (CI) 5-FU versus bolus 5-FU.112 The intergroup trial (INT) 0144 postoperative adjuvant rectal trial did not confirm this survival benefit; however, it did report a lower incidence of grade 3+ hematologic toxicity.113 Given these results, when 5-FU is used with either preoperative or postoperative CMT, CI is the standard. Although it is just now being studied in rectal cancer, the combination of CI–5-FU and oxaliplatin (FOLFOX) has replaced CI–5-FU as a standard postoperative chemotherapy treatment based on the efficacy demonstrated in stage III colon cancer patients.114
The INT 0114 4-arm trial randomized patients with pT3 or N+ rectal cancer to postoperative radiation and bolus 5-FU with or without leucovorin, levamisole, or leucovorin plus levamisole. There was no significant difference in local control or survival among the four arms.115 With longer follow-up, the study also revealed that local control and survival results continue to decrease after 5 years. At 7 years, local failure rate was 17% and the survival was 56% compared with 14% and 64%, respectively, at 5 years. Patients with high-risk (pT3 N+ or T4) disease had a lower survival compared with lower risk (pT1-2 N+ or T3 N0) disease (45% versus 70%). Further analysis of the INT 0114 trial has revealed that body mass is related to outcome and treatment-related toxicity,116 and both surgeons and hospitals with higher volumes of rectal cancer surgery have improved outcomes compared with those with lower volumes.117
Almost every randomized trial for the past 2 decades has confirmed a 10% to 15% survival benefit following 6 months of adjuvant 5-FU based chemotherapy for patients with LN+ colon or rectal cancer. One retrospective118 and two randomized trials119,120 question whether chemotherapy improves the results of preoperative radiation in patients with cT3 rectal cancer. The European Organisation for Research and Treatment of Cancer (EORTC) 22921 trial was a four-arm randomized trial of 1011 patients who received preoperative 45 Gy with or without concurrent bolus 5-FU/leucovorin followed by surgery with or without four cycles of postoperative 5-FU/leucovorin.121 Only 37% had a TME. The Fédération Francophone de Cancérologie Digestive (FFCD) 9203 trial was a two-arm randomized trial of 742 patients randomized to preoperative 45 Gy with or without bolus 5-FU/leucovorin.122 However, all patients were scheduled to receive postoperative chemotherapy, and 73% did.
The EORTC trial revealed a significant decrease in the local failure rate in those patients who receive CMT compared with radiation (8%-10% versus 17%, p < 0.001), but no difference in 5-year survival (65%). However, only 43% received 95% or more of the planned postoperative chemotherapy, which may explain the negative results. Furthermore, a subset analysis of the EORTC trial revealed that patients who responded to preoperative CMT had a survival benefit from postoperative chemotherapy.123
Are There Patients Who Do Not Require Postoperative Adjuvant Therapy?
The sixth edition of the AJCC staging system subdivides stage III into IIIA (T1-2 N1), IIIB (T3-4 N1), and IIIC (Tany N2).23 The prognostic validity of this change was supported by both the pooled analysis of Intergroup and NSABP postoperative trials24 and the retrospective analysis of the American College of Surgeons NCDB.25 The 5-year survival by stages IIIA, B, and C in the pooled analysis was 81%, 57%, and 49%, and in the NCDB was 55%, 35%, and 25%, respectively. The data suggest that patients with upper rectal cancers who undergo a TME, have at least 12 nodes examined and have stages pT3 N0 disease likely do not need the radiation component of CMT. The 4% to 5% benefit in local control with addition of radiation may not be worth the risks, especially in women of reproductive age.
These findings prompted further examination of this question. The 1990 NCI Consensus Conference recommendation was based on trials in which TME and examination of 12 or more nodes was not required.26 Retrospective data suggest that there may be a subset of patients with pT3 N0 disease who do not require adjuvant therapy if they meet those requirements. Nissan and associates reported results of 100 patients with uT2/3 N0 disease who underwent TME alone and had 12 or more nodes examined.124 In the subset of 49 patients with uT3 N0 disease, the overall local recurrence rate was 4%. For the total group, local recurrence was significantly higher in those with lymphovascular positive tumors (32% versus 6%, p = 0.006) and an elevated (>5.0) preoperative CEA (21% versus 0%). Therefore, the subset of patients with T3 N0 tumors with either of those clinicopathologic features should still receive postoperative CMT, even if they undergo TME and have 12 or more nodes examined.
Preoperative Therapy
Sphincter Preservation With Preoperative Radiation
An analysis of 1316 patients who received short-course radiation revealed that downstaging was most pronounced when the interval between the completion of radiation and surgery was at least 10 days.125 In the Dutch CKVO 95-04 trial, in which the interval was 1 week, there was no downstaging.126
When the goal of preoperative therapy is sphincter preservation, conventional radiation doses and techniques are recommended. These include multiple-field techniques to a total dose of 45 Gy to 50.4 Gy at 1.8 Gy/fraction. Surgery should be performed 4 to 8 weeks following the completion of radiation. Unlike the short course of radiation regimen, this conventional design allows for two important events to occur. First is the recovery from the acute side effects of radiation, and second is adequate time for tumor downstaging. Data from the Lyon R90-01 trial of preoperative radiation suggest that an interval of more than 2 weeks following the completion of radiation increases the chance of downstaging.127 Most series recommend a 4- to 8-week interval.128–130 Whether increasing the interval between the end of short-course radiation and surgery to more than 4 weeks will increase downstaging is not known. This question is being addressed in the ongoing Stockholm III trial.
Clinical Experience With Sphincter Preservation
A valid concern of surgeons is that to perform sphincter preservation in those patients who would otherwise require an APR, the distal resection margin may be suboptimal (<1 cm). Can preoperative therapy compensate for this? Retrospective data from the Memorial Sloan-Kettering Cancer Center (MSKCC) reveal that with preoperative CMT the 3-year local control rates were similar regardless of the margins being larger than 2 cm, smaller than 2 cm, larger than 1 cm, or less than 1 cm, providing they were negative.108,131 Similar data have been reported from MD Anderson.132
A positive circumferential margin following preoperative CMT is less favorable. Compared with 460 patients with negative circumferential margins, Bail and colleagues reported that the 44 patients with positive margins had a higher local recurrence (35% versus 11%) and decreased survival rates (27% versus 73%).4
Sphincter preservation without good function is of questionable benefit. In a series of 73 patients who underwent surgery, Grumann and associates reported that the 23 patients who underwent an APR had a more favorable quality of life compared with the 50 who underwent an LAR.133
Although preoperative CMT may adversely affect sphincter function,134 the effect is most likely less than postoperative CMT.135 In the four of eight preoperative series discussed previously, which report functional outcome, the majority (approximately 75%) have good to excellent sphincter outcome. Functional results continue to improve up to 1 year after surgery. Functional data from the German trial are pending.
In one series, the value of radical surgery in patients who had a biopsy-proven complete response was questioned.136 However, it included patients with cT1-3 disease and local failures <1 year were excluded. In series limited to patients with cT3 disease who received preoperative CMT, radical surgery is still necessary to fully evaluate whether a pathologic response has been achieved. Neither post-treatment ultrasound19,20 or physical examination (which is only 25% accurate)137 are sufficient. The use of PET scan21,138,139 and diffusion MRI140 as noninvasive measures of response are being investigated and have reported mixed results. Glynne-Jones and associates reviewed 218 phase II and 28 phase III trials of preoperative radiation or CMT and confirmed that clinical or radiologic response does not sufficiently correlate with pathologic response; the authors do not recommend a “wait-and-see” approach to surgery following preoperative therapy.141
Randomized Trials of Preoperative versus Postoperative Combined-Modality Therapy
Two randomized trials of preoperative versus postoperative CMT for clinically resectable rectal cancer have been performed: NSABP R0-3142 and the German CAO/ARO/AIO 94.28 The NSABP R-03 accrued only 267 of a planned 900 patients. They received induction chemotherapy followed by conventional CMT and were randomized to receive it either preoperatively or postoperatively. TME was not required and some patients underwent a local excision.
The German trial completed the planned accrual of more than 800 patients, and randomized patients with uT3/4 or LN+ rectal cancers smaller than 16 cm from the anal verge to preoperative CMT (with CI–5-FU) versus postoperative CMT.28 Patients were stratified by surgeon. Compared with postoperative CMT, patients who received preoperative therapy had a significant decrease in local failure (6% versus 15%, P = 0.006), acute toxicity (27% versus 40%, P = 0.001), chronic toxicity (14% versus 24%, P = 0.012), and in those 194 patients judged by the surgeon to require pretreatment and APR, a significant increase in sphincter preservation.(39% versus 20%, P = 0.004). With a median follow-up of 40 months, there was no difference in 5-year survival (74% versus 76%). A subsequent analysis revealed that the treatment center, schedule, and gender were independent prognostic factors for local control.143
Given the improved local control, acute and long-term toxicity profile, and sphincter preservation reported in the German trial, patients with cT3 rectal cancer who require CMT should receive it preoperatively. In the German trial, 18% of patients who were clinically staged as cT3 N0 preoperatively and underwent surgery without preoperative therapy had pT1-2 N0 disease. Therefore, those patients would have been over-treated if they had received preoperative therapy. Although not ideal, this is still preferred to performing surgery first because 20% to 40% of patients will have LN+ disease at the time of surgery and require postoperative CMT, which has inferior local control, higher acute and chronic toxicity, and, if a low anastomosis is performed, inferior functional results.144
Although pretreatment MRI can help predict patients who may have positive circumferential margins,145 neither MRI or any other imaging modality or clinicopathologic factor can reproducibly identify patients with LN+ disease with a >60%-70% occurocy.146 Tumor regression grade147–149 may help predict LN+. Clearly, the development of more accurate methods to identify LN+ disease including imaging techniques and molecular markers are essential as more patients are being treated with preoperative CMT.
Bujko and colleagues performed a randomized trial of two preoperative approaches.150,151 A total of 316 patients with cT3 rectal cancer were randomized to short course radiation followed by surgery (median 8 days) versus conventional preoperative CMT (50.4 Gy plus bolus 5-FU/leucovorin daily ×5 during weeks 1 and 5) followed by surgery (median 78 days). All tumors were above the anorectal ring, TME was performed for distal tumors, and there was no radiation quality-control review.
Preoperative Combined-Modality Therapy Programs With Novel Chemotherapeutic Agents
Chemotherapeutic agents such as capecitabine,114 oxaliplatin,152 and irinotecan,153 as well as targeted therapies such as bevacizumab154 and cetuximab,155 which have improved results of patients treated in the adjuvant and/or metastatic settings are currently incorporated into phase I and II combined-modality programs. Selected agents include uracil-tegafur,156 tomudex,157 oxaliplatin,158–160 irinotecan,78,161–163 getfitinib (Iressa),164 bevacizumab,165 cetuximab,160 and capecitabine158,166 with pelvic radiation therapy. Most suggest higher pCR rates compared with 5-FU alone. However, for some agents there is an associated increase in acute toxicity with this increased pCR rate. Phase III trials are needed to determine if these regimens offer a higher pCR, local control or survival rates compared with 5-FU–based CMT regimens.
Predicting the Response of the Primary Tumor
Although some series show no correlation,130,167 most series suggest that there is improved outcome with increasing pathologic response to preoperative CMT.137,168–173 Analysis of biopsies examining selected molecular markers174–176 have had varying success in helping to select patients who may best respond to preoperative therapy. Because the studies are limited retrospective trials and most do not examine multiple markers, the need for adjuvant therapy should still be based solely on T and N stage. Fortunately, INT rectal trials now prospectively collect tissues for molecular markers.
Alternative Methods for Sphincter Preservation
Endocavitary Radiation
Endocavitary radiation alone177–179 has been used for early, noninvasive tumors. For more advanced tumors (cT2-3 and LN+), it is combined with a temporary Iridium-192 implant and/or pelvic radiation.99,180–182 This technique is also known as the Papillon technique. Prior to delivery, the anus is dilated and a 4-cm proctoscope is introduced. A low-energy x-ray unit is placed through the scope almost against the tumor. Generally, 50-kV x-rays are delivered at 30 Gy per fraction in three or four fractions over 1 month. Winslow and colleagues report that patients who develop local failure can successfully undergo surgical salvage.183
Maingon and colleagues treated 151 patients and the incidence of initial local control and ultimate local control by stage was T1, 78% and 87%; T2, 58% and 79%; and T3, 54% and 69%, respectively.184 The Mayo Clinic treated 29 patients with curative intent with a total dose of up to 155 Gy in one to five fractions and local control was 76% at 10 years; survival was 65% at 5 years and 42% at 10 years.177 At Washington University, patients received pelvic radiation (20 Gy in five fractions for those with cT1, and the remainder received 45 Gy in 25 fractions) followed 6 to 7 weeks later by two endocavitary treatments of 30 Gy each.182 Results by stage were uT1,100% disease free survival; uT2, 85% local control; uT3 (who were not optimal candidates for surgery) or tethered uT2, 56% local control (67% after salvage surgery). Aumock and associates added external-beam radiation for T2-3 tumors and reported local control rates of T1,100%; mobile T2, 85%; and T3 or tethered T2, 56%.181 Because the 50 kVp radiation machine is not available, there are limited centers that continue to treat patients with contact radiation.
Local Excision and Radiation Therapy
Local excision has been performed both before and after radiation therapy. The advantage of performing a local excision prior to radiation is that pathologic details can be well characterized. Selected patients with pT1 tumors without adverse pathologic factors have local failure rates of 5% to 10%. However, once adverse pathologic factors are present (high grade, vascular invasion, or signet-ring cells) or the tumor invades into or through the muscularis propria, the local failure rate is at least 17% and the incidence of positive mesorectal and pelvic nodes is at least 10% to 15%.185
There are a variety of surgical approaches, including transanal local excision, posterior proctotomy, and transsphincteric excision. Transanal endoscopy microsurgery has emerged as another option for local treatment of rectal cancer.186 Regardless of the technique, the excision should be full thickness, nonfragmented, and have negative margins.187
Local Excision Followed by Postoperative Therapy
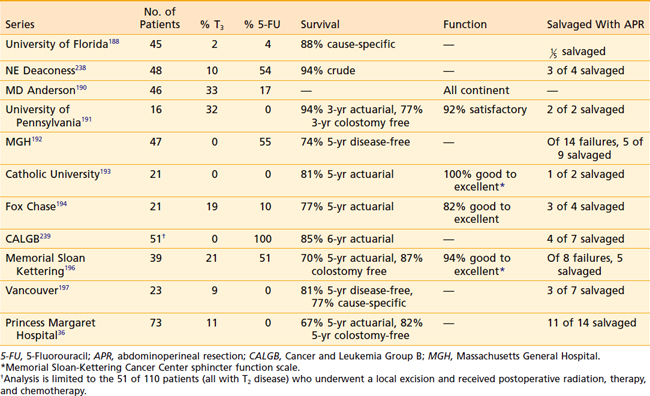
When the series are combined, the average crude local failure rate increases with T stage: pT1, 5%; pT2, 14%; and pT3, 22%.36,188–197 However, in the series with more than 4-year follow-up,36,192,195,198 the incidence of local failure for pT2 disease is 14% to 24% (Table 41-4). Therefore, patients who are treated with local excision and postoperative adjuvant therapy require close follow-up beyond 5 years.
Salvage of local failures is possible, with most series reporting that approximately half of the patients who undergo a salvage APR can be cured.199
Preoperative Therapy Followed by Local Excision
Experience with preoperative CMT followed by local excision is more limited.33,200–205 Most series select patients with cT3 disease who are either medically inoperable or refuse radical surgery. Local failure rates range from 0% to 20%, and 5-year survival ranges from 78% to 90%. Borschitz and colleagues reported local recurrence rates by pathologic stage: ypT1, 2%; ypT2, 6% to 20%; and ypT3 tumors that did not respond as high as 43%.205 This approach is being prospectively tested in the American College of Surgeons Oncology Group (ACSOG) 06031 trial.
Prospective assessment of functional results are limited. The groups from MSKCC196 and Gemelli Hospital, Rome,193 report 94% and 100% good to excellent function, respectively. Using a different scale, investigators from Fox Chase Cancer Center194 reported 82% good to excellent function, the University of Pennsylvania191 reported 92% satisfactory function, and MD Anderson190 reported that all patients were continent. In preoperative setting, sphincter function was reported good to excellent in 88% to 91%.200,204
Locally Advanced Rectal Cancer
The choice of one or more imaging studies for the work-up such as CT scan,10 MRI,145 and PET139,206 depend on the patient’s presenting symptoms. Sciatic pain suggests a situation unlikely to be helped by surgery because tumor invades in the sciatic notch. Patients with gross invasion of tumor into vital pelvic structures may be approached in a palliative rather than a curative fashion.
Approximately 10% of rectal cancers require pelvic exenteration to obtain negative margins.207 The 5-year survival rates range from 33% to 50% with significant morbidity (35%-50%) and mortality (1%-6%).208 The use of tissue flaps to facilitate the healing of pelvic and perineal wounds have improved the results.209 Extended surgery is still recommended even if there is a favorable response after preoperative therapy because there is still a risk of leaving microscopic residual tumor.210
Tethered cancers have the most favorable outcome of all T4 cancers. In a separate report from the MGH, the results of 28 patients with tethered rectal cancers treated with preoperative radiation were presented.211 A complete resection with negative margins was possible in 93%, and the local failure rate was 24%. Tobin and colleagues report a local failure rate of 14% and 5-year survival of 68% in 49 patients with tethered cancers treated with preoperative radiation.212
Intraoperative Radiation Therapy
IORT is delivered by two techniques: electron beam and brachytherapy. Brachytherapy is most commonly delivered by the high-dose rate technique and the dose rate is similar to that used for electron-beam IORT.213–215 The results and recommended dose of IORT depend on whether the patient has primary unresectable or recurrent disease, and on whether the margins of resection are negative or there is microscopic or gross residual disease. In general, series have used 10 Gy to 20 Gy. Some series report IORT following preoperative CMT for patients with clinically resectable disease.30
It is difficult to clearly separate treatment-related complications from disease-related complications in patients with unresectable primary or recurrent rectal cancers. The total incidence ranges from 15% to 50% in most series and is highest in patients with the most advanced disease.216,217 These consequences must be weighed against the chance of cure if the patient is treated and the disability eventually caused by uncontrolled tumor progression if the patient is not treated.
Primary Unresectable Disease
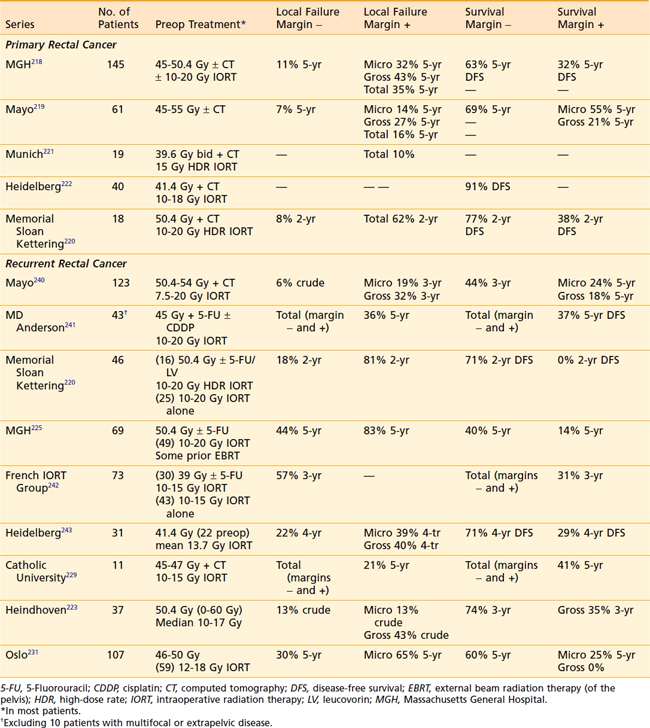
Results of selected series are seen in Table 41-5. The MGH reports local failure in patients with negative margins decreased from 18% without IORT to 11% with IORT.218 In patients with positive margins, local failure decreased from 83% without IORT to 43% with IORT if there was gross residual disease, and to 32% with IORT if there was microscopic residual disease. For all patients in the series (with or without IORT), the 5-year disease-free survival was 63% for patients with negative margins and 32% for patients with positive margins. These results underscore the importance of delivering preoperative therapy to help achieve negative margins. If negative margins cannot be obtained, then microscopic residual disease is still preferable to gross residual disease. Reports from the Mayo Clinic219 and MSKCC220 revealed similar local failure rates in patients with negative margins (7% and 8%, respectively). Similar series from Munich,221 Heidelberg,222 and Eindhoven223 have been reported. At the MGH, of the 95 patients with T4 disease who received preoperative irradiation and underwent complete resection, 40 patients had an IORT boost and 55 did not because it was not indicated secondary to a favorable response or it was not technically feasible.224,225 Regardless of the response to preoperative therapy, higher local failure rates were seen in patients not receiving IORT (responders: 16% versus 0%; and nonresponders: 12% versus 27%). These data suggest that IORT should be delivered independent of the extent of tumor downstaging.
Recurrent Disease
In general, the median survival ranges between 1 and 2 years.226 At the University of Wurzburg, sites of failure were analyzed in 155 patients.227 The incidence of failure sites were similar for APR versus LAR: local and nodal at 61% versus 66%, isolated lymph node at 4% versus 5%, internal iliac and presacral nodes at 47% versus 59%, and external iliac at 7% versus 2%. Local recurrence was most commonly seen in the presacral pelvis and in patients who underwent an LAR; the anastomosis was involved in 93%.
Recurrences can be heterogeneous and the pattern of extension is more infiltrative within the operative bed compared with primary rectal cancer. Localized pelvic recurrences may be classified according to the tumor location within the pelvis. At the Mayo Clinic, 106 patients with local recurrence treated by IORT and postoperative therapy were stratified during the surgical procedure according to the infiltration of the tumor to none (F0), one (F1), two (F2), or more than two pelvic sites (F3).228 This classification system significantly correlated with survival. At the Catholic University of the Sacred Heart, Rome, 47 patients with locally recurrent, nonmetastatic rectal carcinoma were treated by preoperative CMT and IORT, and were classified by CT scan according to Mayo Clinic system.229 A further (F4) class was added when tumor infiltrated small bowel or bone structures. The classification system significantly predicted R0 resectability (p = 0.01) and survival (P = 0.008).
As with primary unresectable disease, patients should receive preoperative CMT. However, some patients in these series had prior external-beam radiation and received either a limited dose or no external-beam radiation. Therefore, preoperative radiation may not be possible. Selected series are seen in Table 41-5. In the MGH series of 40 patients, the overall 5-year local control was 35% and was higher with negative margins (56%) versus positive margins (13%).225 The overall 5-year survival was 27% and was higher in those with negative margins (40%) versus positive margins (12%). Similar results were reported in 74 patients treated at MSKCC.214 The overall 5-year local control was 39% and was higher in patients with negative margins (43%) versus positive margins (26%). The overall 5-year survival was 23% and was higher with patients with negative margins (36%) versus positive margins (11%). In contrast, the Mayo Clinic reported no difference by margin status, but reported a higher 5-year local control (63% versus 34%) and survival (20% versus 12%) in patients who received external-beam radiation versus those who did not receive additional external-beam radiation. Investigators at MD Anderson also report no benefit of IORT for patients with positive margins.230 In a report from Olso, 107 patients with isolated pelvic recurrence received 46 Gy to 50 Gy preoperatively.231 Regardless of the volume of residual disease, there was no significant difference in local recurrence or survival, whether or not they received IORT. In summary, in contrast to patients who have negative or microscopically positive margins, it is unlikely that patients with gross positive margins benefit from aggressive therapy.
In the postoperative setting, if there is incomplete resection (R1 or R2 resection), radiation doses of more than 60 Gy are required. External-beam radiotherapy is limited in this situation by normal tissue tolerance, and results for patients with residual disease who received postoperative external radiation therapy are disappointing.107,232 IORT may help to overcome this problem by direct visualization and irradiation of the persistent tumor. Ferenschild and colleagues treated 27 patients with IORT who, following 50 Gy, had R1-2 margins.29 The 5-year local control rate was 58%.
Reirradiation Followed by Surgery
There is a subset of patients who present with local-only recurrence who have received previous pelvic radiation. Mohiuddin and colleagues recommend reirradiation with doses of 30 Gy, and if the small bowel can be excluded from the irradiation field, 40 Gy was used for limited volumes.233 A total of 103 patients with recurrent disease underwent reirradiation with concurrent 5-FU–based chemotherapy. The initial radiation dose to the pelvis ranged from 30 to 74 Gy with a median dose of 50.4 Gy. Irradiation techniques consisted of two lateral fields with or without a posterior pelvic field to include recurrent tumor with a margin of 2 to 4 cm. Doses ranged from 15 to 49.2 Gy (median 34.8 Gy). After reirradiation, 34 underwent surgical resection for residual disease. For the total group, the survival was 26 months median and 19% 5-year actuarial. Patients who underwent resection had significantly higher median (44 versus 14 months) and 5-year survival rates (22% versus 15%) (p = 0.001). Late complications were seen in 22 patients and were unrelated to radiation dose. These included 18 with persistent severe diarrhea, of which 10 required long-term parenteral support, 15 with small-bowel obstruction, four with fistula formation, and two with coloanal stricture.
A multicenter Italian trial of 59 patients with recurrent disease who had received less than 55 Gy were re-treated preoperatively with concurrent 5-FU plus 30 Gy (1.2 Gy bid) to the gross tumor volume (GTV) plus a 4-cm margin.234 A boost was delivered, with the same fractionation schedule, to the GTV plus a 2-cm margin (10.8 Gy). Acute grade 3+ toxicity was 5% and the clinical complete response (cCR) rate was 9%. With a median follow-up of 36 months, the local failure was 48%, median survival 42 months, and 5-year actuarial survival 39% (R0: 67% versus R1-2: 22%). Multivariate analysis confirmed the effect of longer disease-free interval on local control (p = 0.016) and disease-free survival (p = 0.002). Patients who underwent an R0 resection had improved local control and disease-free survival (p = 0.016). Of the 20 patients who presented with pelvic pain, 83% had a symptomatic response.
Radiation Therapy Alone
Patients selected for radiation therapy alone are usually medically inoperable or have advanced local disease such that resection would compromise a vital pelvic structure.37,180 In most series, patients received pelvic radiation therapy followed by a boost with either external-beam radiation or brachytherapy, or both.
Wang and colleagues from the Princess Margaret Hospital reported the results of external-beam therapy alone (40-60 Gy) in patients who refused surgery (90), or who had unresectable (55) or medically inoperable (99) disease.37 The results were related to the degree of tumor fixation at presentation. The cCR, local relapse, and actuarial 5-year survival rates by mobility were 103 mobile (49%, 72%, 48%), 51 partially fixed (22%, 73%, 26%), and 92 fixed (9%, 100%, 6%), respectively. These data suggest that patients with mobile or partially fixed rectal cancers who are medically inoperable should be treated aggressively with pelvic radiation therapy as a component of their therapy.
Gerard and associates reported the combination of external beam, intracavitary, and brachytherapy in 63 patients with uT2-3 tumors.38 For patients with uT3 disease, the 5-year local failure and survival rates were 20% and 35%, respectively.
Pelvic radiation offers effective palliation. In a subset of 80 patients with metastatic disease who received pelvic radiation, Crane and colleagues reported that 94% had complete resolution of pelvic symptoms and the 2-year pelvic symptom-free control was 82%.235 The Princess Margaret Hospital series reported similar palliative benefits.236 In the subset of 84 patients who received more than 45 Gy, the following presenting symptoms were palliated by 6 to 8 weeks following the completion of radiation: pain 89%, bleeding 79%, neurologic 52%, mass effect 71%, discharge 50%, urologic 22%, and other 42%. In the Thomas Jefferson University series, complete plus partial symptomatic relief was achieved in the following categories: pain (65% and 28%), bleeding (100%), and mass effect (24% and 64%), respectively.233 The duration of palliation was 8 to 10 months. In patients who are unable to receive radiation, laser or stents237 offer some palliative benefit.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66.
2 Cohen AM, Minsky BD, Friedman MJ. Cancer of the rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: J.B. Lippincott; 1993:978-1005.
3 Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449-457.
4 Bail SH, Kim NK, Lee YC, et al. Prognostic significance of circumferential resection margin following total mesorectal excision and adjuvant chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol. 2007;14:462-469.
5 Barbaro B, Valentini V, Coco C, et al. Tumor vascularity evaluated by transrectal color doppler US in predicting therapy outcome for low-lying rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1304-1308.
6 Schaffzin DM, Wong WD. Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorec Cancer. 2004;4:124-132.
7 Shami VM, Parmer KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59-65.
8 Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17-28.
9 Kim NK, Kim MJ, Park JK, et al. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000;7:732-737.
10 Kim NK, Kim MJ, Yun SH, et al. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42:770-775.
11 Chessin DB, Kiran RP, Akhurst T, et al. The emerging role of 18F-fluorodeoxyglucose positron emission tomography in the management of primary and recurrent rectal cancer. J Am Coll Surg. 2005;201:948-956.
12 Nahas CSR, Akhurst T, Yeung H, et al. Positron emission tomography detection of distant metastatic or synchronous disease in patients with locally advanced rectal cancer receiving preoperative chemoradiation. Ann Surg Oncol. 2008;15:704-711.
13 Beets-Tan RGH, Beets GL, Vliegen RFA, et al. Accuracy of magnetic resonance imaging in prediction of tumor-free resection margin in rectal cancer surgery. Lancet. 2001;357:497-504.
14 Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor makers in gastrointestinal cancer. J Clin Oncol. 2006;33:5313-5327.
15 Herrera L, Villarreal JR, Cert RT. Incidence of metastasis from rectal adenocarcinoma in small lymph nodes detected by a clearing technique. Dis Colon Rectum. 1992;35:783-788.
16 Harisinghani MG, Barentsz J, Hahn PF. Noninvasive detection of clinically occult lymph-node metastasis in prostate cancer. N Engl J Med. 2003;348:2491-2499.
17 Kim YH, Kim DY, Kim TH, et al. Usefulness of magnetic resonance volumetric evaluation in predicting response to preoperative concurrent chemoradiotherapy in patients with resectable rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:761-768.
18 Kuo LJ, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48:23-28.
19 Gavioli M, Bagni A, Piccagli I, et al. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer. Dis Colon Rectum. 2000;43:1075-1083.
20 Barbaro B, Schulsinger A, Valentini V, et al. The accuracy of transrectal ultrasound in predicting the pathological stage of low-lying rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 1999;43:1043-1047.
21 Kristiansen C, Loft A, Berthelsen AK, et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51:21-25.
22 Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate endpoint. J Clin Oncol. 2005;23:3475-3479.
23 Colon and rectum. In: Green FL, Page DL, Fleming ID, et al, editors. AJCC cancer staging manual. New York: Springer; 2002:113-124.
24 Gunderson LL, Sargent D, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785-1796.
25 Green FL, Stewart AK, Norton HJ. New tumor-node-metastasis staging system for node-positive (stage III) rectal cancer: An analysis. J Clin Oncol. 2004;22:1778-1784.
26 National Institutes of Health Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. J Amer Med Assoc. 1990;264:1444-1450.
27 Skibber JM, Hoff PM, Minsky BD. Cancer of the rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins; 2001:1271-1318.
28 Sauer R, Becker H, Hohenberger P, et al. Preoperative chemoradiotherapy as compared with postoperative chemoradiotherapy for locally advanced rectal cancer. N Engl J Med. 2004;351:11-20.
29 Ferenschild FT, Vermaas M, Nuyttens JJ, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum. 2006;49:1257-1265.
30 Diaz-Gonzalez JA, Calvo FA, Cortes J, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:1122-1128.
31 Haddock MG, Nelson H, Donohue JH, et al. Intraoperative electron radiotherapy as a component of salvage therapy for patients with colorectal cancer and advanced nodal metastasis. Int J Radiat Oncol Biol Phys. 2003;56:966-973.
32 Shoup M, Guillem JG, Alekitar KM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum. 2002;45:585-592.
33 Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004;60:1098-1105.
34 Tulchinsky H, Rabau M, Shacham-Shemueli E, et al. Can rectal cancers with pathologic T0 after neoadjuvant chemoradiation (ypT0) be treated by transanal excision alone? Ann Surg Oncol. 2006;13:347-352.
35 Paty PB, Nash GM, Baron P, et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522-530.
36 Benson R, Wong CS, Cummings BJ, et al. Local excision and postoperative radiotherapy for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2001;50:1309-1316.
37 Wang Y, Cummings B, Catton P, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005;77:126-132.
38 Gerard JP, Chapet O, Ramaioli A, et al. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2002;54:142-149.
39 Engstrom PF, Benson ABIII, Chen YJ, et al. Rectal cancer: clinical practice guidelines in oncology. J Natl Comp Cancer Network. 2005;3:492-508.
40 Guckenberger M, Flentje M. Late small bowel toxicity after adjuvant treatment for rectal cancer. Int J Colorec Dis. 2006;21:209-220.
41 Willett CG, Tepper JE, Kaufman DS, et al. Adjuvant postoperative radiation therapy for rectal adenocarcinoma. Am J Clin Oncol. 1992;15:371-375.
42 Minsky BD, Conti JA, Huang Y, et al. The relationship of acute gastrointestinal toxicity and the volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol. 1995;13:1409-1416.
43 Gunderson LL, Russell AH, Llewellyn HJ, et al. Treatment planning for colorectal cancer: Radiation and surgical techniques and value of small-bowel films. Int J Radiat Oncol Biol Phys. 1985;11:1379-1393.
44 Minsky BD, Cohen AM. Minimizing the toxicity of pelvic radiation therapy. Oncology. 1988;2:21-25.
45 Sigmon WR, Randall ME, Olds WE, et al. Increased chronic bowel complications with split-course pelvic irradiation. Int J Radiat Oncol Biol Phys. 1993;28:349-353.
46 Willett CG, Ooi CJ, Zeitman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995-998.
47 Lawrie WT, Song DS, Abrams RA, et al. Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Int J Radiat Oncol Biol Phys. 2000;48:226.
48 Tai P, Hammond A, Dyk JV, et al. Pelvic fractures following irradiation of endometrial and vaginal cancers—a case series and review of literature. Radiother Oncol. 2000;56:23-28.
49 Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. J Amer Med Assoc. 2005;294:2587-2593.
50 Dueland S, Guren MG, Olsen DR, et al. Radiation therapy induced changes in male sex hormone levels in rectal cancer patients. Radiother Oncol. 2003;68:249-253.
51 Hermann RM, Henkel K, Christiansen H, et al. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother Oncol. 2005;75:83-88.
52 Heriot AG, Tekkis PP, Fazio VW, et al. Adjuvant radiotherapy is associated with increased sexual dysfunction in male patients undergoing resection for rectal cancer: a predictive model. Ann Surg. 2005;242:502-511.
53 Marijnen CAM, van de Velde CJH, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847-1858.
54 Pietrzak L, Bujko K, Nowacki MP, et al. Quality of life, anorectal, and sexual functions after preoperative radiotherapy for rectal cancer: Report of a randomised trial. Radiother Oncol. 2007;84:217-225.
55 Miller AR, Martenson JAJr, Nelson H, et al. The incidence and clinical consequences of treatment-related bowel injury. Int J Radiat Oncol Biol Phys. 1999;43:817-825.
56 Robertson JM, Lockman D, Yan D, et al. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:413-418.
57 Nuyttens JJ, Robertson JM, Yan D, et al. The position and volume of the small bowel during adjuvant radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2001;51:1271-1280.
58 Gallagher MJ, Brereton HD, Rostock RA, et al. A prospective study of treatment techniques to minimize the volume of pelvic small bowel with reduction of acute and late effects associated with pelvic irradiation. Int J Radiat Oncol Biol Phys. 1986;12:1565-1573.
59 Koelbl O, Richter S, Flentje M. Influence of patient positioning on dose-volume histogram and normal tissue complication probability for small bowel and bladder in patients receiving pelvic irradiation: a prospective study using a 3D planning system and a radiobiological model. Int J Radiat Oncol Biol Phys. 1999;45:1193-1198.
60 Callister MD, Ezzell GA, Gunderson LL. IMRT reduces the dose to small bowel and other pelvic organs in the preoperative treatment of rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:s290.
61 Shanahan TG, Mehta MP, Bertelrud KL, et al. Minimization of small bowel volume within treatment fields utilizing customized “belly boards.”. Int J Radiat Oncol Biol Phys. 1990;19:469-476.
62 Kim TH, Chie EK, Kim DY, et al. Comparison of the belly board device method and the distended bladder method for reducing irradiated small bowel volumes in preoperative radiotherapy of rectal cancer patients. Int J Radiat Oncol Biol Phys. 2005;62:769-775.
63 Brierley JD, Cummings BJ, Wong CS, et al. The variation of small bowel volume within the pelvis before and during adjuvant radiation for rectal cancer. Radiother Oncol. 1994;31:110-116.
64 Talley NA, Chen F, King D, et al. Short-chain fatty acids in the treatment of radiation proctitis. A randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis Colon Rectum. 1997;40:1046-1050.
65 O’Brien PC, Franklin CI, Poulsen M, et al. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: Longer term follow-up of a phase III trial—Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:442-449.
66 Resbeut M, Marteau P, Cowen D, et al. A randomized double blind placebo controlled multicenter study of mesalazine for the prevention of acute radiation enteritis. Radiother Oncol. 1997;44:59-63.
67 Jahraus CD, Bettenhausen D, Sellitti M, et al. Randomized double-blind placebo controlled trial of balsalazide in the prevention of acute radiation enteritis as a consequence of pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:s253-s254.
68 Hadddock MG, Sloan JA, Bollinger JW, et al. Patient assessment of bowel function during and after pelvic radiotherapy: results of a prospective phase III North Central Cancer Treatment Group Clinical Trial. J Clin Oncol. 2007;25:1255-1259.
69 Antonadou D, Athanassiou H, Sarris N, et al. Final results of a randomized phase III trial of chemoradiation treatment + amifostine in patients with colorectal cancer: Clinical Radiation Oncology Hellenic Group. Int J Radiat Oncol Biol Phys. 2004;60:s140-s141.
70 Ben-Joseph E, Han S, Tobi M, et al. A pilot study of topical intrarectal application of amifostine for prevention of late radiation rectal injury. Int J Radiat Oncol Biol Phys. 2002;53:1160-1164.
71 Kouloulias VE, Kouvaris JR, Pissakas G, et al. Phase II multicenter randomized study of amifostine for prevention of acute rectal toxicity: Topical intrarectal versus subcutaneous application. Int J Radiat Oncol Biol Phys. 2005;62:486-493.
72 Ceelen W, Boterberg T, Pattyn P, et al. Neoadjuvant chemoradiation versus hyperfractionated accelerated radiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2007;14:424-431.
73 Coucke PA, Notter M, Stamm B, et al. Preoperative hyperfractionated accelerated radiotherapy (HART) in locally advanced rectal cancer (LARC) immediately followed by surgery. A prospective phase II trial. Radiother Oncol. 2006;79:52-58.
74 Janjan NA, Crane C, Feig BW, et al. Prospective trial of preoperative concomitant boost radiotherapy with continuous infusion 5-fluorouracil for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:713-718.
75 Movsas B, Hanlon A, Lanciano R, et al. Phase I dose escalating trial of hyperfractionated pre-operative chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:43-50.
76 Diratzouian H, Movsas B, Hanlon AL, et al. Phase II trial of preoperative chemoradiation with a hyperfractionated RT boost in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2001;51:10.
77 Myerson RJ, Valentini V, Birnbaum EH, et al. A phase I/II trial of three-dimensionally planned concurrent boost radiotherapy and protracted venous infusion of 5-FU chemotherapy for locally advanced rectal carcinoma. Int J Radiat Oncol Biol Phys. 2001;50:1299-1308.
78 Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group trial 0012. J Clin Oncol. 2006;24:650-655.
79 Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: Intensity-modulated photon therapy and proton therapy. Clin Colorec Cancer. 2007;6:348-356.
80 Tait DM, Nahum AE, Meyer LC, et al. Acute toxicity in pelvic radiotherapy: a randomised trial of conformal versus conventional treatment. Radiother Oncol. 1997;42:121-136.
81 Koelbl O, Vordermark D, Flentje M. The relationship between belly board position and patient anatomy and its influence on dose-volume histogram of small bowel for postoperative radiotherapy of rectal cancer. Radiother Oncol. 2003;67:345-349.
82 Tatsuzaki H, Urie MM, Willett CG. 3-D comparative study of proton vs. x-ray radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 1991;22:369-374.
83 Duthoy W, De Gersem W, Vergote K, et al. Clinical implementation of intensity-modulated arc therapy (IMAT) for rectal cancer. Int J Radiat Oncol Biol Phys. 2004;60:794-806.
84 Patel S, Vuong T, Ballivy O, et al. Phase II trial of pelvic intensity-modulated radiotherapy (IMRT) with concurrent chemotherapy for patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2004;60:s424-s425.
85 Duncan W, Arnott SJ, Jack WJL, et al. Results of two randomized trials of neutron therapy in rectal adenocarcinoma. Radiother Oncol. 1987;8:191-198.
86 Yamada S, Kamada T, Yasuda S, et al. Phase I/II trial of carbon-ion therapy for patients with locally recurrent rectal cancer. Proc ASCO. 2005;22:280s.
87 Anscher MS, Lee C, Hurwitz HI, et al. A pilot study of preoperative continuous infusion 5-fluorouracil, external microwave hyperthermia, and external beam radiotherapy for treatment of locally advanced, unresectable, or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:719-724.
88 Rau B, Wust P, Tilly W, et al. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys. 2000;48:381-391.
89 Van der Zee J, Gonzalez DG, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: a prospective, randomised, multicenter trial. Lancet. 2000;355:1119-1125.
90 Tuech JJ, Chaudron V, Thoma V, et al. Prevention of radiation enteritis by intrapelvic breast prosthesis. Eur J Surg Oncol. 2004;30:900-904.
91 Chen JS, Chang Chien CR, Wang JY, et al. Pelvic peritoneal reconstruction to prevent radiation enteritis in rectal carcinoma. Dis Colon Rectum. 1992;35:897-903.
92 Kim TH, Jeong SY, Choi DH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15:729-737.
93 Michels KB, Giovannucci E, Joshipura KJ, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740-1752.
94 Tocchi A, Lepre L, Costa G, et al. Rectal cancer and inguinal metastasis. Prognostic role and therapeutic indications. Dis Colon Rectum. 1999;42:1464-1466.
95 Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil, leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-1214.
96 Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544.
97 Colorectal Cancer Collaborative Group. adjuvant radiotherapy for rectal cancer: A systematic overview of 22 randomised trials involving 8507 patients. Lancet. 2001;358:1291-1304.
98 Withers HR, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys. 1995;31:353-359.
99 Gerard JP, Chapet O, Nemoz C, et al. Improved sphincter preservation in low rectal cancer with high dose preoperative radiotherapy: the Lyon R96–02 randomized trial. J Clin Oncol. 2004;22:2404-2409.
100 Rengan R, Paty P, Wong WD, et al. Distal cT2N0 rectal cancer: Is there an alternative to abdominoperineal resection? J Clin Oncol. 2005;23:4905-4912.
101 Camma C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer. a meta-analysis. J Amer Med Assoc. 2000;284:1008-1015.
102 Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987.
103 Birgisson H, Pahlman L, Glimelius B. Adverse effects of preoperative radiation therapy for rectal cancer: Long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23:8697-8705.
104 Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646.
105 Peeters KCMJ, Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701.
106 Marijnen CAM, Kapiteijn E, van de Velde CJH, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20:817-825.
107 Marijnen CAM, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1311-1320.
108 Guillem JG, Chessin DB, Shia J, et al. A prospective pathologic analysis using whole mount sections of rectal cancer following preoperative combined modality therapy. Implications for sphincter preservation. Ann Surg. 2007;245:88-93.
109 Branagan G, Chave H, Fuller C, et al. Can magnetic resonance imaging predict circumferential margins and TNM stage in rectal cancer? Dis Colon Rectum. 2004;47:1317-1322.
110 Burton S, Brown G, Daniels I, et al. MRI identified prognostic features of tumors in distal sigmoid, rectosigmoid, and upper rectum: Treatment with radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:445-451.
111 Rutten H, Sebag-Montefiore D, Glynne-Jones R, et al. Capecitabine, oxaliplatin, radiotherapy, and excision (CORE) in patients with MRI-defined locally advanced rectal adenocarcinoma: Results of an international multicenter phase II study. Proc ASCO. 2006;24:153s.
112 Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-715.
113 Smalley SR, Benedetti J, Williamson S, et al. Intergroup 0144 A phase III rectal surgical adjuvant study of pelvic radiation (XRT) plus 5-FU based chemotherapy (bolus 5-FU before and after PVI + XRT vs. PVI before, during, and after XRT vs. biochemically modulated bolus 5-FU and XRT): mature outcome results and pelvic railure analysis. Int J Radiat Oncol Biol Phys. 2004;60:s137-s138.
114 Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351.
115 Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control—final report of Intergroup 0114. J Clin Oncol. 2002;20:1744-1750.
116 Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup trial 0114. J Clin Oncol. 2004;22:648-657.
117 Meyerhardt JA, Tepper JE, Neidzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high risk curatively resected rectal cancer: findings from the Intergroup 0114 study. J Clin Oncol. 2004;22:166-174.
118 Chan AK, Wong A, Jenken DA, et al. Is postoperative adjuvant chemotherapy necessary in locally advanced rectal cancers after preoperative chemoradiation? Int J Radiat Oncol Biol Phys. 2004;60:s297.
119 Bosset JF, Calais G, Mineur L, et al. Does the addition of chemotherapy (CT) to preoperative radiotherapy (preop RT) increase the pathological response in patients with resected rectal cancer: report of the 22921 EORTC phase III trial. Proc ASCO. 2004;22:247.
120 Conroy T, Bonnetain F, Chapet O, et al. Preoperative (preop) radiotherapy (RT) + 5FU/folinic acid (FA) in T3,4 rectal cancers: preliminary results of the FFCD 9203 randomized trial. Proc ASCO. 2004;22:227.
121 Bosset JF, Collette L, Bardet E, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123.
122 Gerard JP, Conroy T, Bonnetain F. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;28:4620-4625.
123 Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386.
124 Nissan A, Stojadinovic A, Shia J, et al. Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated with surgery alone. J Clin Oncol. 2006;24:4078-4084.
125 Graf W, Dahlberg M, Osman MM, et al. Short-term preoperative radiotherapy results in down-staging of rectal cancer: a study of 1316 patients. Radiother Oncol. 1997;43:133-137.
126 Marijnen CAM, Nagtegaal ID, Kranenbarg EK, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976-1984.
127 Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90–01 randomized trial. J Clin Oncol. 1999;17:2396-2402.
128 Dolinsky CM, Mahmoud NN, Mick R, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96:207-212.
129 Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279-286.
130 Stein DE, Mahmoud NN, Anne PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum. 2003;46:448-453.
131 Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined modality therapy. Ann Surg Oncol. 2003;10:80-85.
132 Kuvshinoff B, Maghfoor I, Miedema B, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are <1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163-169.
133 Grumann MM, Noack EM, Hoffman IA, et al. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149-156.
134 Temple LKF, Wong WD, Minsky B. The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation. Sem Radiat Oncol. 2003;13:469-477.
135 Kollmorgen CF, Meagher AP, Pemberton JH, et al. The long term effect of adjuvant postoperative chemoradiotherapy for rectal cancer on bowel function. Ann Surg. 1994;220:676-682.
136 Habr-Gama A, de Souza PM, Ribeiro U, et al. Low rectal cancer. Impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087-1096.
137 Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-136.
138 Guillem JG, Moore HG, Akhurst T, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining long term outcomes of rectal cancer. J Am Coll Surg. 2004;199:1-7.
139 Heriot AG, Hicks RJ, Drummond EGP, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum. 2004;47:451-458.
140 Dzik-Jurask A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307-308.
141 Glynne-Jones R, Wallace M, Livingstone JI, et al. Complete clinical response after preoperative chemoradiation in rectal cancer: is a “wait and see” policy justified? Dis Colon Rectum. 2008;51:10-20.
142 Roh MS, Colangelo LH, OConnell MJ, et al. Pre-operative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum (NSABP-R-03). J Clin Oncol. 2009. in press
143 Fietkau R, Rodel C, Hohenberger W, et al. Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys. 2007;67:1008-1019.
144 Guillem JG, Diaz-Gonzalez J, Minsky BD, et al. cT3N0 rectal cancer: Potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26:368-373.
145 Beets-Tan RGH, Beets GL. Rectal cancer: how accurate can imaging predict the T stage and the circumferential resection margin? Int J Colorec Dis. 2003;18:385-391.
146 Kim JH, Beets GL, Kim MJ, et al. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78-83.
147 Roedel C, Fietkau R, Raab R, et al. Tumor regression grading as a prognostic factor in patients with locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Radiat Oncol Biol Phys. 2004;60:s140.
148 Read TE, Andujar JE, Caushaj FP, et al. Neoadjuvant therapy for rectal cancer: histologic response of the primary tumor predicts nodal status. Dis Colon Rectum. 2004;47:825-831.
149 Vecchio FM, Valentini V, Minsky B, et al. The relationship of pathologic tumor regression grade (trg) and outcome after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760.
150 Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomized trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15-24.
151 Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223.
152 Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704.
153 Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905-914.
154 Hurwitz HI, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
155 Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345.
156 Fernandez-Martos C, Aparicio J, Bosch C, et al. Preoperative uracil, tegafur, and concomitant radiotherapy in operable rectal cancer: a phase II multicenter study with 3 years follow-up. J Clin Oncol. 2004;22:3016-3022.
157 Valentini V, Coco C, Minsky BD, et al. Randomized multicenter phase IIB study of preoperative chemoradiotherapy in T3 mid-distal recta cancer: raltitrexed + oxaliplatin + radiotherapy versus cisplatin + 5-fluorouracil + radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:403-412.
158 Arnold D, Hipp M, Reese T, et al. Phase I/II study of cetuximab, capecitabine, and oxaliplatin (CAPOX) combined with standard radiotherapy (RTX) as neoadjuvant treatment of advanced rectal cancer (RC). Proc ASCO. 2006;24:164s.
159 Ryan DP, Niedzwiecki D, Hollis D, et al. Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901. J Clin Oncol. 2006;24:2557-2562.
160 Rodel C, Arnold D, Hipp M, et al. Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1081-1086.
161 Glynne-Jones R, Falk S, Maughan TS, et al. A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study. Br J Cancer. 2007;96:551-558.
162 Hofheinz RD, Horisberger K, Woernle C, et al. Phase I trial of cetuximab in combination with capecitabine, weekly irinotecan, and radiotherapy as neoadjuvant therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:1384-1390.
163 Navarro M, Dotor E, Rivera F, et al. A phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201-205.
164 Valentini V, DePaoli A, Gambacorta MA, et al. Chemoradiation with infusional 5-FU and ZD1839 (Gefitinib—Iressa) in patients with locally advanced rectal cancer: a phase II trial. Int J Radiat Oncol Biol Phys. 2006;66:s168.
165 Czito BG, Bendell JC, Willett CG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: phase I trial results. Int J Radiat Oncol Biol Phys. 2007;68:472-478.
166 Krishnan S, Janjan NA, Hoff PM, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:762-771.
167 Onaitis MW, Noone RB, Fields R, et al. Complete response to neoadjuvant chemoradiation for rectal cancer does not influence survival. Ann Surg Oncol. 2001;8:801-806.
168 Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107-112.
169 Mohiuddin M, Hayne M, Regine WF, et al. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000;48:1075-1080.
170 Read TE, Ogunbiyi OA, Fleshman JW, et al. Neoadjuvant external beam radiation and proctectomy for adenocarcinoma of the rectum. Dis Colon Rectum. 2001;44:1778-1790.
171 Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, et al. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298-304.
172 Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002;236:75-81.
173 Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664-674.
174 Bertolini F, Bengala C, Losi L, et al. Prognostic and predictive value of baseline and post treatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1455-1461.
175 Unsal D, Under A, Akyuerk N, et al. Matrix Metalloproteinase-9 expression correlated with tumor response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:196-203.
176 Johnston PG. Prognostic markers of local relapse in rectal cancer: are we any further forward? J Clin Oncol. 2006;24:4049-4050.
177 Lavertu S, Schild SE, Gunderson LL, et al. Endocavitary radiation therapy for rectal adenocarcinoma. 10 year results. Am J Clin Oncol. 2003;26:508-512.
178 Rauch P, Bey P, Peiffert D, et al. Factors affecting local control and survival after treatment of carcinoma of the rectum by endocavitary radiation: a retrospective study of 97 cases. Int J Radiat Oncol Biol Phys. 2001;49:117-124.
179 Gerard JP, Romestang P, Ardiet JM, et al. Endocavitary radiation therapy. Sem Radiat Oncol. 1998;8:13-23.
180 Coatmeur O, Truc G, Barillot I, et al. Treatment of T1-T2 rectal tumors by contact therapy and interstitial brachytherapy. Radiother Oncol. 2004;70:177-182.
181 Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys. 2001;51:363-370.
182 Myerson RJ. Conservative alternatives to radical surgery for favorable rectal cancers. Ann Ital Chir. 2001;72:605-609.
183 Winslow ER, Kodner IJ, Mutch MG, et al. Outcome of salvage abdominoperineal resection after failed endocavitary radiation in patients with rectal cancer. Dis Colon Rectum. 2005;47:2039-2046.
184 Maingon P, Guerif S, Darsouni R, et al. Conservative management of rectal adenocarcinoma by radiotherapy. Int J Radiat Oncol Biol Phys. 1998;40:1077-1085.
185 Minsky BD, Mies C, Rich TA, et al. Lymphatic vessel invasion is an independent prognostic factor for survival in colorectal cancer. Int J Radiat Oncol Biol Phys. 1989;17:311-318.
186 Neary PC, Makin GB, White TJ, et al. Transanal endoscopic microsurgery: a viable operative alternative in selected patients with rectal lesions. Ann Surg Oncol. 2003;10:1106-1111.
187 Willett CG. Local excision followed by postoperative radiation therapy. Sem Radiat Oncol. 1998;8:24-29.
188 Mendenhall WM, Rout WR, Vauthey JN, et al. Conservative treatment of rectal adenocarcinoma with endocavitary irradiation or wide local excision and post-operative irradiation. J Clin Oncol. 1997;15:3241-3248.
189 Bleday R, Breen E, Jessup JM, et al. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum. 1997;40:388-392.
190 Ota DM, Skibber J, Rich TA. MD Anderson Cancer Center experience with local excision and multimodality therapy for rectal cancer. Surg Oncol Clin N Am. 1992;1:147-152.
191 Rosenthal SA, Yeung RS, Weese JL, et al. Conservative management of extensive low-lying rectal carcinomas with transanal local excision and combined preoperative and postoperative radiation therapy: report of a phase I/II trial. Cancer. 1992;69:335-341.
192 Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49-54.
193 Valentini V, Morganti AG, De Santis M, et al. Local excision and external beam radiotherapy in early rectal cancer. Int J Radiat Oncol Biol Phys. 1996;35:759-764.
194 Fortunato L, Ahmad NR, Yeung RS, et al. Long-term follow-up of local excision and radiation therapy for invasive rectal cancer. Dis Colon Rectum. 1995;38:1193-1199.
195 Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of Radiation Therapy Oncology Group protocol 89–02. Int J Radiat Oncol Biol Phys. 2000;46:313-322.
196 Wagman RT, Minsky BD. Conservative management of rectal cancer with local excision and adjuvant therapy. Oncology. 2001;15:513-524.
197 Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175:360-363.
198 Butch RJ, Stark DD, Wittenberg J, et al. Staging rectal cancer by MR and CT. Am J Radiol. 1986;146:1155-1160.
199 Weiser MR, Landmann RG, Wong WD, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005;48:1169-1175.
200 Schell SR, Zlotecki RA, Mendenhall WM, et al. Transanal excision of locally advanced rectal cancers downstaged using neoadjuvant chemoradiotherapy. J Am Coll Surg. 2002;194:584-591.
201 Kim CJ, Yeatman TJ, Coppola D, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg. 2001;234:352-358.
202 Lezoche E, Guerrieri M, Paganini AM, et al. Long-term results of patients with pT2 rectal cancer treated with radiotherapy and transanal endoscopic microsurgical excision. World J Surg. 2002;26:1170-1174.
203 Ruo L, Guillem JG, Minsky BD, et al. Preoperative radiation with or without chemotherapy and full-thickness transanal excision for selected T2 and T3 distal rectal cancers. Int J Colorec Dis. 2002;17:54-58.
204 Ahmad NR, Nagle DA. Preoperative radiation therapy followed by local excision. Sem Radiat Oncol. 1998;8:36-38.
205 Borschitz T, Wachtlin D, Mohler M, et al. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720.
206 Larson SM, Schoder H, Yeung H. Positron emission tomography/computerized tomography functional imaging of esophageal and colorectal cancer. Cancer J. 2004;10:243-250.
207 Jimenez A, Shoup M, Cohen AM, et al. Contemporary outcomes of total pelvic exenteration in the treatment of colorectal carcinoma. Dis Colon Rectum. 2004;46:1619-1625.
208 Law WL, Chu KW, Choi HK. Total pelvic exenteration for locally advanced rectal cancer. J Am Coll Surg. 2004;190:78-83.
209 Goldberg JM, Piver MS, Hempling RE, et al. Improvements in pelvic exenteration: factors responsible for reducing morbidity and mortality. Ann Surg Oncol. 1998;5:399-406.
210 Reerink O, Verschueren RCJ, Szabo BG, et al. A favourable pathological stage after neoadjuvant radiochemotherapy in patients with initially irresectable rectal cancer correlates with a favorable prognosis. Eur J Cancer. 2003;39:192-195.
211 Willett CG, Shellito PC, Rodkey GV, et al. Preoperative irradiation for tethered rectal carcinoma. Radiother Oncol. 1991;21:141-142.
212 Tobin RL, Mohiuddin M, Marks G. Preoperative irradiation for cancer of the rectum with extrarectal fixation. Int J Radiat Oncol Biol Phys. 1991;21:1127-1132.
213 Strassmann G, Walter S, Kolotas C, et al. Reconstruction and navigation system for intraoperative brachytherapy using the flab technique for colorectal tumor bed irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1323-1329.
214 Alekitar KM, Zelefsky MJ, Paty PB, et al. High dose rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2000;48:219-226.
215 Nuyttens JJ, Kolkman-Deurloo IKK, Vermess M, et al. High dose intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:106-112.
216 Mannaerts GHH, Rutten HJT, Martijn H, et al. Effects on functional outcome after IORT-containing multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:1082-1088.
217 Azinovic I, Calvo FA, Puebla F, et al. Long-term normal tissue effects of intraoperative electron radiation therapy (IOERT): late sequelae, tumor recurrence, and second malignancies. Int J Radiat Oncol Biol Phys. 2001;49:597-604.
218 Nakfoor BM, Willett CG, Shellito PC, et al. The impact of 5-fluorouracil and intraoperative electron beam radiation therapy on the outcome of patients with locally advanced primary rectal and rectosigmoid cancer. Ann Surg. 1998;228:194-200.
219 Gunderson LL, Nelson H, Martenson JA, et al. Locally advanced primary colorectal cancer: intraoperative electron and external beam irradiation + 5-FU. Int J Radiat Oncol Biol Phys. 1997;37:601-614.
220 Harrison LB, Minsky BD, Enker WE, et al. High dose rate intraoperative radiation therapy (HDR-IORT) as part of the management strategy for locally advanced primary and recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:325-330.
221 Huber FT, Stepan R, Zimmermann F, et al. Locally advanced rectal cancer: resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy. Dis Colon Rectum. 1996;39:774-779.
222 Kallinowski F, Eble MJ, Buhr HJ, et al. Intraoperative radiotherapy for primary and recurrent rectal cancers. Eur J Surg Oncol. 1995;21:191-194.
223 Mannaerts GHH, Rutten HJT, Martijn H, et al. Comparison of intraoperative radiation therapy-containing multimodality treatment with historical treatment modalities for locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:1749-1758.
224 Willett CG, Nakfoor BM, Daley W, et al. Pathologic downstaging does not guide the need for IORT in primary locally advanced rectal cancer. Front Radiat Ther Oncol. 1997;31:245-256.
225 Lindel K, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol. 2001;58:83-87.
226 Gagliardi G, Hawley PR, Hershman MJ, et al. Prognostic factors in surgery for local recurrence of rectal cancer. Br J Surg. 1995;82:1401-1405.
227 Bagatzounis A, Kolbl O, Muller G, et al. The locoregional recurrence of rectal carcinoma. A computed tomographic analysis and a target volume concept for adjuvant radiotherapy. Strahlenther Onkol. 1997;173:68-75.
228 Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939-952.
229 Valentini V, Morganti AG, De Franco A, et al. Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma. Prognostic factors and long term outcome. Cancer. 1999;86:2612-2624.
230 Sanfilippo NJ, Crane CH, Skibber J, et al. T4 rectal cancer treated with preoperative chemoradiation to the posterior pelvis followed by multivisceral resection: Patterns of failure and limitations of treatment. Int J Radiat Oncol Biol Phys. 2001;51:176-183.
231 Wiig JN, Tveit KM, Poulsen JP, et al. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? Radiother Oncol. 2002;62:207-213.
232 De Neve W, Martijn H, Lybeert MM, et al. Incompletely resected rectum, rectosigmoid, or sigmoid carcinoma: Results of postoperative radiotherapy and prognostic factors. Int J Radiat Oncol Biol Phys. 1991;21:1297-1302.
233 Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144-1150.
234 Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129-1139.
235 Crane CH, Janjan NA, Abbruzzese JL, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys. 2001;49:107-116.
236 Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:255-259.
237 Liberman H, Adams DR, Blatchford GJ, et al. Clinical use of the self-expanding metallic stent in the management of colorectal cancer. Am J Surg. 2001;180:407-412.
238 Ohno S, Tomoda M, Tomisaki S, et al. Improved surgical results after combining preoperative hyperthermia with chemotherapy and radiotherapy for patients with carcinoma of the rectum. Dis Colon Rectum. 1997;40:401-406.
239 Steele GD, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol. 1999;6:433-441.
240 Gunderson LL, Martin JK, Beart RW. Intraoperative and external beam irradiation for locally advanced colorectal cancer. Ann Surg. 1988;207:52-60.
241 Lowy AM, Rich TA, Skibber JM, et al. Preoperative infusional chemoradiation, selective intraoperative radiation, and resection for locally advanced pelvic recurrence of colorectal adenocarcinoma. Ann Surg. 1996;223:177-185.
242 Bussieres E, Gilly FN, Rouanet P, et al. Recurrences of rectal cancers: results of a multimodal approach with intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1995;34:49-56.
243 Eble MJ, Lehnert T, Treiber M, et al. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol. 1998;49:169-174.