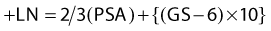45 Cancer of the Prostate
Epidemiology
Incidence, Etiology, and Genetics
It was estimated that 186,320 men would be diagnosed as having prostate cancer in 2008.1 It is also estimated that approximately 28,660 men would die of prostate cancer, making prostate cancer the number two cause of cancer death in men.1 The annual age-adjusted cancer incidence lifetime risk of a man developing prostate cancer appeared to peak between 1991 and 1993, reflecting the initial “harvesting effect” from widespread screening in the early to mid 1990s (Fig. 45-1A,B). The role of serum testosterone levels in the development of prostate cancer remains controversial; however, one recent study suggests that higher levels of serum-free testosterone are associated with an increased risk of aggressive prostate cancer among older men.2 On the other hand, another recent study suggests that baseline serum testosterone levels have no prognostic significance in men treated with ADT and external-beam radiotherapy (EBRT).3
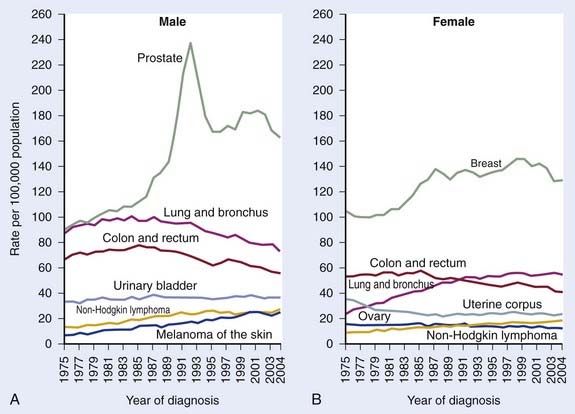
FIGURE 45-1 • A and B, Annual age-adjusted cancer incidence and death rates among males, US, prostate cancer by year.1
Early trials conducted in Canada and Europe supported PSA screening and early treatment, but two recently completed large prospective trials reached conflicting conclusions.4–10 The American Prostate, Lung, Colorectal, Ovarian Cancer screening trial included nearly 77,000 men, but at least 52% of the men on the control arm (“not screened”) got screened such that the relative risk of being treated was only 17% higher in the screened arm. Not surprisingly with short follow-up this was a negative study. In contrast, the European Randomized Study of Screening for Prostate Cancer included 182,000 men with a likelihood of being treated for prostate cancer 70% higher in the screened group. Not surprisingly, with far less screening in the control group they noted a 20% reduction in death from prostate cancer (P = 0.04).
Prostate cancer is generally considered a disease of “older men,” with an average age of 70 years at diagnosis. With routine screening, the number of men diagnosed at younger than 60 years of age will increase. There are wide variations in the incidence of prostate cancer internationally, with men from Scandinavian countries experiencing the highest rates and men from Asia the lowest. The highest incidence of prostate cancer in the United States is in black men (the ratio of blacks/ whites is 1.5-2.0 : 1). Diet, obesity, or environmental exposures related to urban living may account for a portion of the predisposition noted among black men. Black men also tend to present at an earlier age and with more advanced disease.11 Biological differences have been proposed by some investigators; however, socioeconomic and lifestyle factors are a more likely basis for this phenomenon. It appears to be reasonably well-established that the incidence of cytosine adenine glutamine (CAG) repeats is higher in black men.12 However, variations in polymorphisms in different populations are expected and alone are not proof of a cause-and-effect relationship.13 Most studies based on data from prospective randomized trials fail to identify race as an independent prognostic factor and therapy should not be modified based on race alone.11,13
A genetic predisposition may profoundly affect the risk of developing prostate cancer but a detailed description of this topic is beyond the scope of this chapter and can be found elsewhere.14 Recent studies have identified a number of genes that seem to correlate with susceptibility for prostate cancer in different populations. For example, single-nucleotide polymorphism–based, genome-wide linkage scans performed on 131 white families with a history of prostate cancer found the strongest evidence for linkage at 16q23 (LOD = 2.70). Prostate cancer linkage to the same region of 16q23 has been observed by others, but was not detected in other prior linkage studies.15 Genome-wide linkage analysis performed on another large prostate cancer pedigree from a population of cases and first-degree relatives identified a 14 Mb haplotype on chromosome 5 (5p13-q12). Two polymorphisms were associated with prostate cancer risk: rs3212649 (odds ratio [OR] = 1.67 [1.07-2.6], P = 0.0009) and rs1126643 (OR = 1.52 [1.01-2.28], P = 0.0088) with an association in both familial and sporadic prostate cancer.16 Screening first-degree relatives of prostate cancer patients may improve the cost-effectiveness of this practice. Thus the precise cause of prostate cancer is unknown, but it is likely to be multifactorial.
Prostate cancer does not appear to be related to benign prostatic hypertrophy (BPH); however, BPH increases the risk of a high PSA, which may lead incidentally to a diagnosis of disease.17 Vasectomy does not appear to be a risk factor.18–20 The number of sexual partners, venereal disease, dietary fat, alcohol, cadmium, and exercise have inconsistently been supported by studies as risk factors.21–26 Consumption of high doses of multivitamins and supplements is associated with an increased risk of advanced and fatal prostate cancer.27 Early epidemiologic and in vitro studies suggest a possible role of vitamin D and calcium, but larger and more recent studies do not.28–31 Early studies also suggested that a high intake of vitamin E was associated with a lower incidence of prostate cancer in one prospective randomized trial, but more recent large prospective studies have shown that vitamin E does not protect against prostate cancer, but increased consumption of gamma-tocopherol is associated with a reduced risk of clinically relevant disease.32–37 Another small study suggests that pomegranate juice may have a modest effect on PSA in patients failing therapy.38 Several studies suggest that smoking may be associated with more extensive and aggressive disease cancer.39,40 In general, smoking appears to be at most weakly related to the risk factor of developing prostate cancer.41–45
Alcohol consumption has not been studied as extensively as might be thought, with early reports finding no association.22 Velicer and co-workers examined the association between alcohol use and prostate cancer among 34,565 men, in the Vitamins and Lifestyle cohort in Washington State.46 White wine consumption was associated with increased risk (hazard ratio [HR] = 1.27; confidence interval [CI] = 1.08-1.49), but red wine, liquor, and beer were not associated with prostate cancer. In the Health Professionals Follow-up Study, 3348 cases of prostate cancer were diagnosed among 45,433 eligible participants.47 No linear trend was observed between red wine consumption and prostate cancer in the full analytic cohort. Among men younger than 65 years of age with unchanged alcohol consumption in the prior 10 years, slightly lower risks were observed for men who consumed four or fewer glasses of red wine per week, whereas null or slight increased risks were observed for men who consumed more than four glasses per week, with a trend noted for an increase in the risk with moderate white wine consumption. Most recently a meta-analysis was performed to address this question. Despite many caveats in a recently completed meta-analysis, investigators concluded, “the alcohol-prostate cancer association remained significant despite controlling for the degree to which studies endeavored to eliminate false negatives from their control groups.”48 Clearly, more work is needed in this area.
Natural History
Many men may not benefit from treatment because the risk of death from early-stage, low-volume, low-grade disease has been estimated to be approximately 10% at 10 years, with conservative management and delayed hormonal therapy (HT) considered reasonable initial options for such men if their life expectancy is less than 10 years.49,50 However, local progression to bulky (T3) disease approaches 50% at 10 years, and beyond 10 years the risk of death seems to climb substantially.51 Historically, many men have been treated with HT alone because only recently has it become clear that HT alone is not as effective as HT combined with radiotherapy.52
Patients at a reduced risk for death from prostate cancer were those with T1a stage disease (10%), low grade (43%), and age of 70 or older.3 For patients with higher stage and grade disease, the need for treatment appears to be less controversial. Such patients have a higher risk of death and the benefits of therapy have now been well-established.6,53–58
Anatomy
External Anatomy
The normal prostate in a young man is walnut-sized (20 cc) and similar in consistency to the tip of the nose. The average gland in a patient of 65 years is approximately 40 cc. The prostate is penetrated by the urethra as the urethral canal, which then descends to the urogenital diaphragm and then to the bulb of the penis. The distal (apical) portion of the prostate rests against the urogenital diaphragm and the proximal (base) portion of the prostate rests against the bladder. The surface anatomy of the prostate is shown diagrammatically in Fig. 45-2.59 The prostate is surrounded by a complex array of fibroconnective tissue that is penetrated by bundles of nerves and vessels that run superior-laterally and inferior-laterally called the superior and inferior pedicles, which are attached to the pubic symphysis by puboprostatic ligaments.
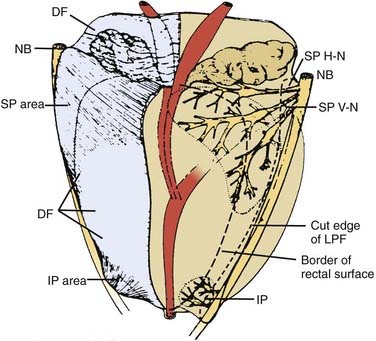
FIGURE 45-2 • Distribution of nerve branches to prostate—right posterolateral view. Nerves within neurovascular bundle (NB) branch to supply prostate in large superior pedicle (SP) at prostate base and small inferior pedicle (IP) at prostate apex. Branches leave lateral pelvic fascia (LPF) to travel in Denonvilliers fascia (DF), which has been cut away from right half of prostate. Nerve branches from superior pedicle fan out over large pedicle area (thickened area of DF). Small horizontal subdivision (H-N) crosses base to midline; large vertical subdivision (V-N) fans out extensively over prostate surface as far distally as midprostate. Branches continue their course within prostate after penetrating capsule within large nerve penetration area (dotted line). Small inferior pedicle has limited ramification and nerve penetration area (dotted line).59
As the prostate narrows distally, the inferior pedicles course medially and it becomes more difficult to spare these nerves during a prostatectomy without increasing the risk of positive apical margins. Efforts to obtain wide margins in this region may result in a shortened external sphincter and a higher risk of incontinence. Up to 80% of patients have apical involvement, so it is not surprising that positive margins in this region following radical prostatectomy are not uncommon.60 Suboptimal surgical technique is the cause of positive apical margins in 50%, with the remaining cases attributed to extracapsular extension (ECE).61 True ECE is most common at the base in the rectolateral and superior pedicle, in association with regions that are penetrated by nerves (considered “routes of least resistance”).
Investigators from Johns Hopkins have argued that focal penetration with low-grade disease is of little clinical significance but “established extracapsular extension” (defined as a penetration of approximately 1 cm into surrounding fat) is a major unfavorable prognostic factor if the tumor Gleason score (GS) is grade 7 or higher.62 It is unclear whether their generalizations hold true for patients with unfavorable pretreatment characteristics for whom less rigorous histopathologic evaluations are conducted. Data from the Mayo Clinic suggest patients with GSs of 7 or greater experience an increased risk of recurrences regardless of margin status.63,64
Internal Anatomy
The distribution of cancer does not appear to be random, with adenocarcinoma involving the first 5 to 8 mm of the apical portion of the gland in up to 80% and multifocal in up to 85% of patients. Even among the earliest cases, with mean volumes of only 0.11 ml, 40% are multifocal65 Historically, perineural invasion (PNI) was a common finding seen in up to 84% of patients. Because of stage migration and a higher incidence of pathologically confined disease, this incidence of PNI may be decreasing.66 In a study of men with T2 disease, ECE within perineural spaces was noted in 50% and in the remaining cases it occurred with PNI.59 This fact is in part responsible for discouraging the use of nerve sparing in patients with adverse pretreatment features.
Pathologic Considerations
Approximately 95% of all prostate cancers are adenocarcinomas. Small cell anaplastic carcinomas with neuroendocrine features and lymphomas are best managed with chemotherapy with or without EBRT, whereas sarcomas and various other types of carcinomas are frequently managed by surgery with or without EBRT. Patients with these unusual histologic types tend to do poorly. Tumors displaying a signet-ring pattern, for example, represent an uncommon subtype with a 3-year survival of less than 20%.67
The most widely used histologic grading system was described by Gleason and colleagues.68 The Gleason pattern score, a sum of the two most common histologic patterns (“primary” and “secondary”), correlates with survival. The GS may range from 2 to 10, with contemporary series dividing patients into GS 6 or lower, GS of 7, and GS of 8 to 10.58,66,69 Patients with an unusually low GS (e.g., 4 or less) should have their slides re-reviewed by an expert pathologist. When this is done, some will have no cancer, but most will have higher-grade cancers.70 The presence of a tertiary pattern 5 significantly affects prognosis.71–74 Patients with a GS of 7 on biopsy and a tertiary pattern 5 are by convention upgraded to a GS of 8.
Fine-needle aspiration of the prostate is very specific (false-positive rate, 0%-2%), but not nearly as sensitive as once thought when six or fewer biopsies are taken.75,76 There has been a dramatic increase in the number of biopsies taken during the preceding 10 years. In the mid 1980s a single biopsy was usually taken based on a palpable abnormality. By the early to mid 1990s, random or “blind” biopsies were in response to an elevated PSA in addition to those areas with a palpable abnormality. Sextant biopsy became standard in the 1990s. By the late 1990s, it became clear that sextant biopsies were no longer adequate for patients with an elevated PSA.77,78 Obtaining at least two additional lateral cores substantially reduces that risk of missing foci of disease, whereas midline biopsies increase morbidity although rarely increasing the yield. In addition to reducing the number of false negative biopsies, obtaining six or more cores may provide prognostic information (see following discussion).79–81
The clinical significance of PNI remains controversial in part because pathologists do not uniformly comment on its presence or absence, intraprostatic versus extraprostatic PNI, nor the extent of involvement.82–88 The significance may also be different for patients treated by surgery and radiotherapy and may depend on the dose of radiation delivered.84,87,89,90 It appears that PNI correlates with more advanced pathologic stage, and worse outcomes with EBRT in the postoperative and definitive setting, but less so after brachytherapy.91–97 In a “borderline patient” (i.e., features straddling between low- and intermediate-risk disease) it may be prudent to consider PNI a “tie-breaker.”
Routes of Spread
Tumor volume, grade, and serum PSA are independent risk factors for ECE. The initial enthusiasm for PSA as a marker resulted from the belief that the serum PSA correlated with the tumor volume. Because of BPH, the correlation between PSA and tumor volume is poor for patients with PSAs less than 9 ng/ml, with the correlation driven by large cancers with serum PSAs greater than 22 ng/ml.17
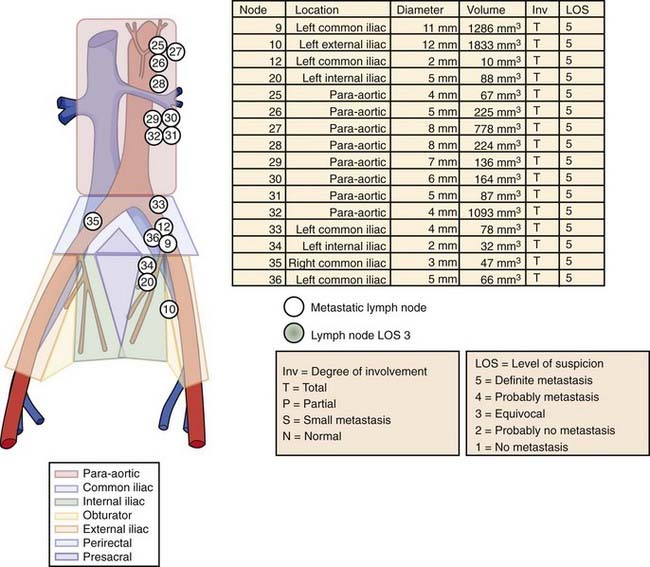
After local extension beyond the capsule of the prostate and seminal vesicles, tumor spreads to the pelvic lymph nodes.98 In addition to the classic involvement of the obturator nodes, high-risk patients frequently harbor metastasis to the external iliac, presacral, and presciatic nodes. Fig. 45-3 demonstrates by lymphangiography the location of the major nodal drainage areas. A more detailed discussion of the incidence of lymph node involvement by prostate cancer can be found in the subsection of “Clinical Presentation” later in this chapter.
Prognostic Factors
Prostate-Specific Antigen, Gleason Score, Stage, and Risk-Stratification Schemes
The major predictors of the extent of disease for men with localized prostate cancer are clinical stage, GS, pretreatment PSA, PNI, and the percentage of positive biopsies.91,92 Pretreatment PSA is the most important predictor of PSA failure, whereas GS, T stage, and age are the more important predictors of survival. Table 45-1 and Table 45-2 summarize the seventh edition of 2009 American Joint Committee on Cancer (AJCC) staging system.99 This major revision includes PSA, finally acknowledging the effect of pretreatment PSA on survival (Fig. 45-4A,B) making it more useful in the clinic,100,101
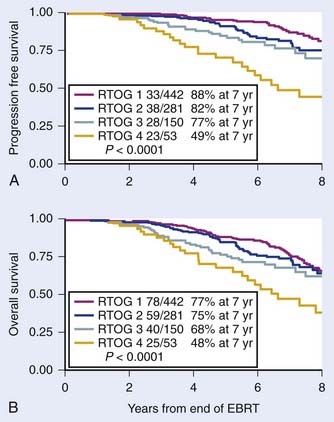
FIGURE 45-4 • First study to demonstrate progression free survival (PFS) defined as death after PSA failure (A) and overall survival for patients treated with radiotherapy alone by pretreatment PSA (B), respectively.100
A large number of risk-stratification schemes and predictive algorithms have developed, and the number keeps growing.102 These predictive algorithms fall into four major categories: (1) simple additive models initially designed to predict PSA failure, (2) computational approaches initially designed to predict PSA failure, (3) computational approaches to predict pathologic endpoints, and (4) computational approaches to predict disease-specific and overall survival. There is overlap between these models because factors that predict PSA failure or pathologic stage tend to correlate with survival, with long-term follow-up.
The most commonly used risk stratification schemes defined “low risk” as a GS of 2 to 6, PSA less than 10 ng/ml, and stage T1c to T2a.103,104 So-called intermediate risk included patients with T2b, a GS of 7 or PSA of 10 to 20; and “high risk” as GS to 8 to 10, T2c or T3, or PSA greater than 20 ng/ml. The clinical significance of a GS of 7 is assumed to be equal to having a PSA between 10 and 20 ng/ml and that a GS of 8 is similar in importance to having a PSA greater than 20 ng/ml. Patients with two or more intermediate factors behaved more like a high-risk patient.105 Including the percentage of positive biopsies improves the predictive outcome for intermediate risk patients, but may depend on treatment type.79,106,107
Models using multivariate statistical approaches have been developed and some incorporated into the design of clinical trials.108–111 Nomograms developed using well-established statistical methods, based on relatively large number of patients, and validated by several institutions are available on the Internet. These nomograms are useful tools for providing estimates of expected outcomes for the average patient. However these nomograms should be used cautiously because the follow-up is limited, the data are not based on the results of prospective trials, and most of these nomograms don’t address survival. The Kattan nomogram for predicting the risk of metastasis has been shown to be useful for predicting cause-specific and overall survival in patients treated with EBRT.112
Nomograms have been described that predict the probability of pathologic endpoints, including the risk of ECE, seminal vesicle involvement (+SV) and lymph node involvement (+LN) based on pretreatment stage, PSA, and biopsy GS.66,113,114 Based on Partin’s original report, Roach described three relatively simple equations that allowed estimates to be made of the risk of ECE, +SV, and +LN without a calculator.115 The simplicity of these equations in part comes from the fact that each uses a very similar format, as shown in the following equations.
The equation used for estimating the risk of ECE is:
The equation used for estimating the risk of +SV is:
The equation used for estimating the risk of lymph node involvement is:
These equations are simple enough to memorize, they have been validated, and they have been shown to predict biochemical failure in patients undergoing EBRT and have been used for stratification in a large phase I and II three-dimensional (3-D) dose escalation trial (9406) and defined eligibility for a large phase III trial (9413) conducted by the Radiation Therapy Oncology Group (RTOG).116–120
Recently investigators have questioned the merits of the so-called Roach lymph node equation.121 They concluded that the Roach scores overestimated the actual rate of positive lymph nodes in contemporary patients. However, their series included men who underwent a node sampling or conventional node dissection, which may result in missing 40% or more of involved nodes when compared with an extended lymph node dissection.98,122,123 Their series also suffered from the fact that the average number of nodes examined was only five (two to nine), resulting in an observed incidence of lymph node involvement of only 3.3%. In contrast, in a cohort of patients with similar clinical features, Briganti and colleagues have shown that the likelihood of finding lymph node involvement is critically dependent on the number of lymph nodes taken.124 They showed that in a cohort of patients with the mean number of nodes examined of 15, the observed incidence of lymph node involvement noted was 10.2%. They also noted that the rate of lymph node involvement increased with the number of nodes removed 2 to 10 (5.6%), 10 to 14 (8.6%), 15 to 19 (10.2%), and 20 to 40 (17.6%) and on multivariate analysis the number of lymph nodes removed was a major predictor of finding lymph node involvement (P < 0.001). Briganti et al.124 concluded 28 nodes were required to yield a 90% ability to detect lymph node involvement and conversely that examining 10 nodes or fewer resulted in nearly a zero probably of finding lymph node involvement. It is also now clear that with more sensitive processing of nodal tissue or the application of molecular assays (such as reverse transcriptase polymerase chain reaction [RT-PCR], or determining PSA n–ribonucleic acid [n-RNA] copy number), lymph node involvement that would otherwise be missed can be identified.125–127
The first empirical model created to predict overall and disease-specific survival was described by investigators from the RTOG (Fig. 45-5); since then a number of others have been described (Table 45-3A,B).69,112 The Kattan nomogram for predicting the risk of metastasis appears to be a more robust model for predicting survival (Fig. 45-6).112
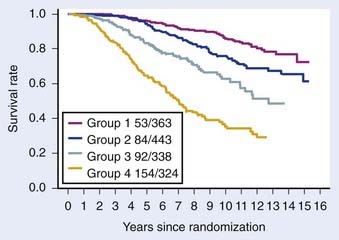
FIGURE 45-5 • Disease-specific survival, respectively, by four risk groups. From top to bottom: (1) group 1 patients had a GS = 2-6, and clinical stage T1-2Nx; (2) group 2 patients had a GS = 2-6, and T3Nx or N+ or GS = 7, T1-2Nx; (3) group 3 patients were clinically staged as T3Nx, with a GS = 7, or T1-2Nx, GS = 8-10; and (4) group 4 patients were clinically staged a T3Nx, S = 8-10, or N+, GS = 8-10.69
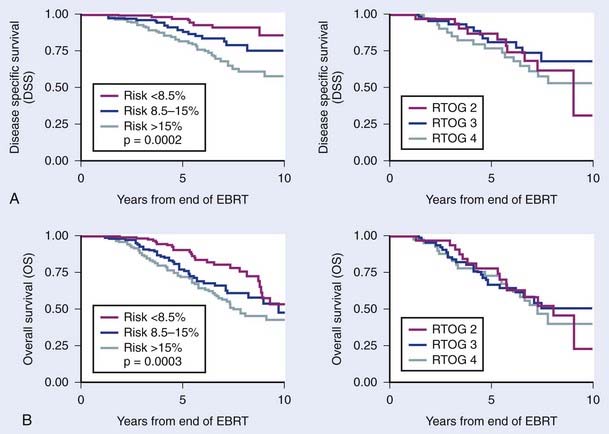
FIGURE 45-6 • Difference in freedom from biochemical failure, disease-specific survival, and overall survival among the three tertiles created by the nomogram using the cutoff points less than 8.5%, 8.5% to 15%, and greater than 15% (P < 0.001, 0.0002, and 0.0003, respectively). The risk of metastases using the categorized nomogram score (less than 8.5% and 8.5% to 15% versus greater than 15%), note preradiotherapy prostate specific antigen or Radiation Therapy Oncology Group risk (2 versus 3), was a significant predictor of disease-specific and overall survival for intermediate- and high-risk patients and intermediate- and high-risk patients with 15% or less risk for metastases.112
Predictive Markers for Prostate Cancer
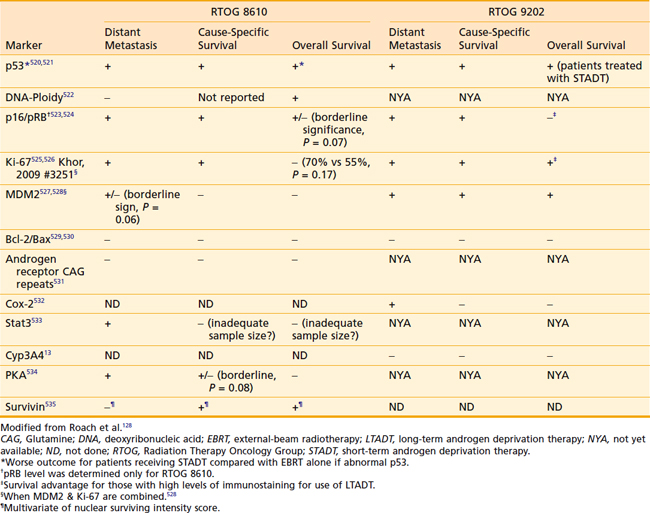
Increasingly, biomarkers are being added to predictive models in an effort to strengthen them but a detailed discussion of predictive markers is beyond the scope of this chapter. The RTOG has completed the largest number of studies to date including a wide range of markers using tissue from two phase III trials (RTOG 8610 and 9202). Preliminary assessments of p53, deoxyribonucleic acid ploidy, p16/pRB, Ki-67, MDM2, Bcl-2/Bax, CAG repeats, Cox-2, Stat3, Cyp3A4, and PKA have been completed and the findings are summarized in Table 45-4.128 Combining these markers with traditional pretreatment- and treatment-related variables may improve our ability to predict outcome and select the optimal treatment, but currently they do not appear to be ready for routine use.
Prostate-Specific Antigen, Lymphadenectomy, and Laparoscopic Nodal Sampling
There is no agreement on the indications for a lymphadenectomy or laparoscopic nodal sampling and there are widely disparate opinions as to the true incidence of lymph node involvement.121,129 Low-risk patients can be spared this procedure because they are very low-risk for lymph node involvement. High-risk patients who are found to have positive lymph nodes may benefit because of the potential benefits associated with early androgen deprivation.130,131 If a high-risk patient is going to undergo long-term androgen suppression and pelvic nodal irradiation, it may or may not be worth the cost and morbidity to undergo node sampling, particularly because of the false negative rate (see discussion later in this chapter).
The standard lymph node dissection is generally described as extending from the bifurcation of the common iliac vessels medially to the pelvic floor and to the inferior border of the prostate, and then superiorly along the hypogastric vessels back to the bifurcation of the common iliac vessels. If pelvic lymph nodes are negative, periaortic nodes are generally assumed to be negative, although skip metastases have been reported.132 There is a clear correlation between the side of palpable tumor and the side on which metastatic nodes are likely to be found.133 Frozen sections are highly specific (100%), but the sensitivity is modest at 65% or less.134 Although a standard lymph node dissection is usually considered a sensitive method of establishing lymph node status, it is far from 100% sensitive. In the series reported by Freeman and colleagues, 16% of patients who by standard approaches were defined as being pathologic stage T3N0 were found by a monoclonal epithelial PSA assay to have occult metastases.135 A more recent update of these data confirmed the highly important relevance of identifying occult positive nodes in approximately 13% of men with T3 disease.125 This estimate is likely to be an underestimation of the true incidence because not all of the nodes were sampled and occult nodal involvement may be more common than previously recognized.136 Some studies question the value of the extended node dissections but most confirm the problem of sampling errors and false negatives when limited sampling is done.137–139 Of note, however, studies including men undergoing extended dissections may underestimate the true incidence of metastatic disease, when assays thought to be more sensitive than a histopathologic evaluation are used.
The issue of occult nodal involvement was noted by Shariat et al.126 They compared the detection of human glandular kallikrein 2 (hK2) messenger RNA expression in archival lymph nodes and the risk of progression of disease and survival in patients who were found to have pathologically unfavorable but node-negative disease following prostatectomy.126 They evaluated total RNA extracted from fixed, paraffin-embedded, histopathologically normal pelvic lymph nodes from 199 pT3N0 prostate cancer patients for hK2-expressing cells using an RT-PCR/hK2 assay. RT-PCR/hK2 result was associated with disease progression (P = 0.001), distant metastases (P = 0.001), and cancer-specific survival (P = 0.005). These data suggest that the reported incidences of lymph node involvement in multiple surgical series must be taken with “a grain of salt” and provide a rationale for prophylactic pelvic nodal radiotherapy.66,140
The Significance of Lymph Node Involvement and Staging Studies
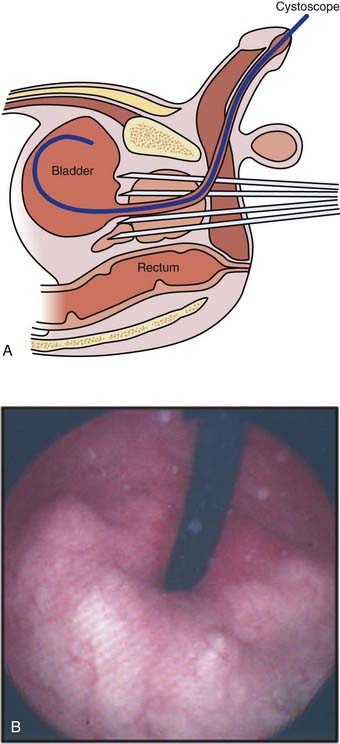
Ten-year survivors have been noted among patients with pathologically proven positive lymph nodes despite initial treatment with radiotherapy with and without HT, with the former generally doing substantially better than the latter group.58,69,141,142 Thus, there may be a subpopulation of patients with nodal disease that is controllable.140,143–145 Routine chest x-ray studies and cystoscopic examinations are unwarranted unless accompanied by the presence of a smoking history or urinary symptoms. Bone scans are not justified if the PSA is less than or equal to 10 ng/ml with a relatively poor yield for this test until the PSA exceeds 50 ng/ml when the serum alkaline phosphatase is normal or if the GS 7 or less. Routine lymphangiography has no role in the staging of prostate cancer. Neither abdominal-pelvic computed tomography (CT) nor conventional magnetic resonance imaging (MRI) appears to be warranted if the PSA is less than 20 ng/ml.146,147 These scans may be of limited value in this setting because of the poor correlation between the size of lymph nodes and the presence of cancer.148
Pelvic nodal disease can also be evaluated by using an MRI-compatible, nanoparticle-based imaging agent such as dextran-coated ferrous material (see discussion on whole-pelvic radiotherapy [WPRT] later in this chapter).149 Although the findings from this study were quite impressive, the United States Food and Drug Administration declined to give clearance to the use of this agent and as a result it was available only in Europe.150 The use of sentinel-node imaging techniques is also a promising strategy for identifying areas at risk to undergo lymph node radiotherapy.151,152 In a study of 25 patients, Ganswindt et al.151 concluded that using a standard CT-based planning target volume, relevant sentinel lymph nodes would have been missed in 19 of 25 patients (76%), mostly involving the presacral and perirectal nodes.
Endorectal MRI combined with magnetic resonance spectroscopic imaging (MRSI) may prove to be useful in staging and treatment planning for selected patients, but its use is not routinely supported by American College of Radiology Guidelines.153–156 In a study of 62 patients undergoing radical prostatectomy followed by an evaluation of histopathologic step-sections, they reported that the addition of MRSI (high specificity) to MRI (high sensitivity) resulted in improved localization of prostate cancer within the prostate. When both MRI and MRSI were positive, they reported a specificity of up to 91% (P < 0.05 versus MRI alone). MRSI may also improve the accuracy of cancer detection by MRI in patients with postbiopsy hemorrhage. An improvement in sensitivity and specificity of cancer localization can be attained when MRI and MRSI data are combined with biopsy data.157 MRSI might provide new insights into tumor aggressiveness, leading to an improvement in risk assessment in patients with clinically localized prostate cancer.158
Definitions of Failure Following Definitive Local Therapies
Definitions of Failure Following Radiation Therapy
Post-treatment biopsies have been used to assess local tumor eradication with a much higher incidence of residual tumor noted than suggested by palpation alone. The identification of histologically viable tumor after more than 3 years is usually reflective of persistent disease. A wide range of results in the incidence of positive biopsies following radiotherapy can be found in the literature. These differences reflect patient selection bias, differences in the effectiveness of treatment, and the PSA level at the time of biopsy. If patients with locally advanced disease or with palpable regrowth are biopsied, the positive biopsy rate approaches 90%. If patients with low PSAs, clinically controlled disease, and nonrising PSAs are biopsied, the positive biopsy rate is much lower.159–161 Routine post-treatment biopsies are hampered by the reluctance of patients to undergo this invasive procedure for which a certain course of action is lacking. Cost and ambiguity in interpretation of biopsies are also problematic. Pathologic interpretations may include “persistent disease with treatment effect” versus “persistent disease without treatment effect,” with the latter being considered “positive” and the former “equivocal” or “negative.”
Studies involving cohorts of consecutive patients undergoing post-treatment biopsies following conventional EBRT are instructive but post-treatment biopsies are not considered the gold standard after radiotherapy.160 Some studies support a relationship between the dose of radiation delivered and the risk of a positive biopsy, although others do not, leaving this issue unresolved. These conflicting results may reflect problems with sample size, duration of follow-up, differences in patient selection, the number of biopsies obtained, and the use of ADT (ADT reduces the risk of positive biopsies).
The use of the serum PSA has improved our ability to identify treatment failure much earlier. Eradication of local tumor results in a substantial decline (>90% reduction) in the serum PSA. An empirical “rule of thumb” is that the PSA half-life following EBRT is approximately 3 months. The lowest PSA value achieved following EBRT is typically reached at 24 to 36 months but continued declines may be seen at 4 to 5 years and beyond. The rate of decline following brachytherapy tends to be more variable, but the median nadir tends to be lower and to occur later.162
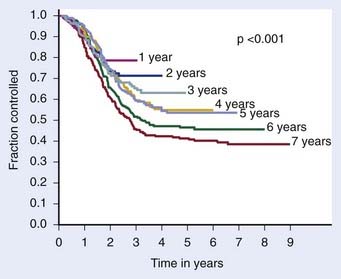
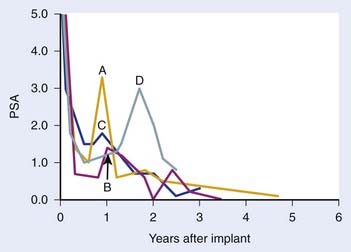
The definition of biochemical (PSA) failure following radiotherapy has evolved during the preceding 10 years, with the definition having a substantial effect on the percentage of patients considered free of disease. The first Consensus Conference sponsored by the American Society of Therapeutic Radiology and Oncology (ASTRO) defined failure based on three consecutive rises in the PSA.163 However, the ASTRO Consensus definition did not specify how much of a rise was significant.164 Because of random variations, occasionally three consecutive rises may occur by chance alone. It is well-known that the PSA tends to increase normally with age, so very small increases separated over a long period might not represent progression. The ASTRO definition also fails to specify whether the lowest value ever achieved should be used to define the nadir or whether the lowest value prior to rising should be used. The ASTRO definition also did not address how tied values (e.g., 0.1, 0.2, 0.3, 0.3, 0.7, and 0.9 ng/ml) should be dealt with or the slope of the PSA curve. Using the ASTRO definition after brachytherapy was particularly problematic because “PSA blips” or benign bounces are known to occur in 20% of patients. There is also a bias against PSA control in patients treated with short-term ADT because the PSA may rise in response to the recovery of a patient serum testosterone in the absence of true biochemical failure.164 One of the most important shortcomings associated with the ASTRO definition is the importance of the duration of follow-up and effect of backdating on creating an artificial flattening of the PSA control curve.164 Several studies have shown that to obtain an accurate outcome using the ASTRO consensus definition requires long-term follow-up.165,166 Fig. 45-7 demonstrates how duration of follow-up affects the estimated control rate and suggests the median follow-up should exceed the estimated date of biochemical control at 2 years before estimates of control stablize.165 For example, if the median follow-up is 5 years, only estimates at 3 years or less are likely to be accurate.
The “Phoenix Definition” for Prostate-Specific Antigen Failure after Radiotherapy
As a result of the shortcomings of the first ASTRO definition described previously, a second Consensus Conference jointly sponsored by ASTRO and the RTOG was held in 2005 in Phoenix, Arizona.164 The expert panel recommended that biochemical failure be declared when the PSA rose by 2 ng/ml or more above the nadir PSA after the completion of EBRT with or without HT and that the date of failure be determined “at call” (not backdated). This definition was shown to have a higher sensitivity and specificity than the previous ASTRO definition and to correlate more closely to clinical failure. The authors of the consensus statement acknowledge that it may not be the most accurate definitive for “cure.” They also recommended that investigators continue to be allowed to use the 1996 ASTRO consensus definition after EBRT alone (no HT) with strict adherence to guidelines as to “adequate follow-up.” To avoid the artifacts resulting from short-term follow-up, they recommended that the reported date of control should be listed as 2 years short of the median follow-up. They argued that by retaining a (stricter) version of the ASTRO definition, investigators would retain the opportunity to compare their outcomes with those found in a vast existing body of literature. This definition should not be used after cryosurgery or HIFU or other modalities without validation with long-term follow-up and clinical data.
Definitions of Disease-Free Survival Following Surgery
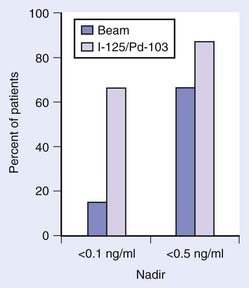
The most appropriate definition for biochemical (PSA) failure following a radical prostatectomy is controversial. Some have defined a PSA failure as a PSA greater than 0.5 ng/ml, greater than 0.4 ng/ml, and greater than 0.3 ng/ml, although 0.2 ng/ml is the most commonly used threshold in the literature.167–169 Some argue that a PSA greater than 0.4 ng/ml should become the new standard170 (Fig. 45-8). Because many normal men have PSAs of 0.4 ng/ml with an intact prostate, it is not logical that such a high value can be considered to be consistent with the notion of a “radical prostatectomy with curative intent.” Furthermore, Zincke et al.171 at the same institution previously demonstrated that the relative risk of failure was 1.3 times higher at 5 years if failure was defined as greater than 0.2 ng/ml compared with greater than 0.4 ng/ml. The importance of a detectable PSA was recently explored in detail, with a detectable PSA defined as failure to achieve PSA less than 0.03 ng/ml.172 A detectable PSA was associated with increased PSA recurrence and death (P < 0.001 and 0.041, respectively). In such patients the 1- and 5-year biochemical recurrence-free survival was 68% and 36%, significantly lower than 95% and 72%, respectively, in men without persistently detectable PSA. Overall survival at 10 years in patients with persistently detectable PSA was 63% versus 80% in patients without a persistently detectable PSA. Thus a persistently detectable PSA should not be ignored.172
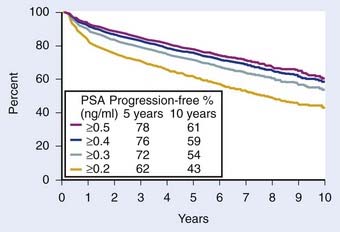
FIGURE 45-8 • Biochemical progression-free percent using different prostate-specific antigen cut points to define progression after radical prostatectomy. Number of patients at risk for progression at 5, 7, and 10 years ranged 1354 to 1728, 497 to 703, and 53 to 83, respectively. Amling curves.170
Of note, however, there does exist a small subset of patients with a detectable PSA after radical prostatectomy but with little or no subsequent rise in PSA and no clinical progression with long-term follow-up.173 In one series they identified 14 patients (8.8% of PSA failures) with a detectable serum PSA level and a mean PSA velocity after recurrence of 0.028 ng/ml per year after radical prostatectomy but without clinical or continued PSA progression at 10 years. They concluded that there is a subset of patients with PSA failure after prostatectomy who will not progress and cautioned against the use of early salvage therapy without first observing the kinetics of PSA progression. The presence of benign glands in the resection bed may account for this uncommon phenomenon.174–176
Some investigators have chosen to use the ASTRO consensus definition for patients treated surgically.177 However, if such an approach was used following prostatectomy, it would seem most reasonable to require the use of an ultrasensitive definition, so that the date of first rise is not missed because of an insensitive assay.178,179
Standard Therapeutic Approaches
Radical Prostatectomy: Surgical Technique and Complications
The radical perineal prostatectomy and the radical retropubic prostatectomy (RRP) for prostate cancer were first described by Young and Millin in 1905 and 1947, respectively.180,181 It was not until 1949 that Memmelaar first reported the successful use of the retropubic approach (RRP) for treatment of prostate cancer.182 However, in the 1950s and 1960s it was believed that radiotherapy provided comparable results with less morbidity. In late 1970s and early 1980s, Walsh and others demonstrated the value of performing a more sophisticated “anatomic” RRP to reduce blood loss, minimize the risk of incontinence, and preserve potency.183,184 With this advancement, the RRP became the surgical approach of choice in the eyes of most urologists. Thus the modern anatomic radical prostatectomy is only a few years older than 3-D conformal radiotherapy (3D-CRT). Brachytherapy has been around longer than both.
Although there was a surge in the use of brachytherapy in the late 1990s and early 2000s, in recent years there has been a shift toward an increased number of radical prostatectomies driven by robotic approaches.185–187 It appears that the major outcomes (cancer control, potency, positive margin rates) with the robot-assisted laparoscopic prostatectomy (RALP) are comparable to open prostatectomies when performed by expert hands.188 Despite this fact, in the next 5 years, RALPs are projected to account for as many as 80% of the radical prostatectomy procedures performed in the United States. Most experts believe that RALP is associated with shorter recovery, less blood loss, lower use of pain medications, earlier catheter removal, and an improved ability to perform a nerve-sparing operation because of the magnified images. Up to 98% of patients can be expected to achieve continence (no pad use at 12 months) following either RALP or an open prostatectomy.189,190 However there is no good evidence that outcomes are better and in some series they are worse following a RALP (see discussion below).
Recent series have demonstrated potency rates following open prostatectomy of 68% to 90% within 6 to 18 months, with potency rates of 68% to 84% within 6 to 24 months following RALP.191–194 In a trial of 2702 men treated by the so-called minimally invasive prostatectomy or open prostatectomy, the risk of salvage radiation or HT up to 6 months after surgery was substantially higher in the former compared with the latter group (28% versus 9%).195 Also of note, Schroeck and colleagues evaluated patient satisfaction and regret following RALP or open RRP, and found that patients were three times more likely to be dissatisfied and regret their treatment decision following RALP than following open RRP. Presumably this reflected unmet patient expectations as they related to urinary morbidity, sexual function, and cancer-control rates.196 To date, studies have shown that RALP results in lower transfusion rates, lower use of pain medications, and shorter convalescence, but without an advantage in continence or potency; RALP is associated with higher cost.197 Hu investigated the merits of the so-called minimally invasive radical prostatectomy (MIRP) and compared it with the open RRP to determine the comparative effectiveness.198 They performed a population-based observational cohort study using Surveillance, Epidemiology, and End Results Medicare-linked data based on men who underwent MIRP (N = 1938) versus RRP (N = 6899) between 2003 and 2007. They noted that the use of MIRP increased from 9% in 2003 to 43% in 2006 and 2007. They also noted that use of the MIRP was associated with shorter length of stay (median, 2 versus 3 days; P < 0.001) and lower rates of blood transfusions (2.7% versus 20.8%; P < 0.001), postoperative respiratory complications (4.3% versus 6.6%; P = 0.004), miscellaneous surgical complications (4.3% versus 5.6%; P = 0.03), and anastomotic stricture (5.8% versus 14.0%; P < 0.001). However, they also noted that the use of the MIRP was associated with an increased risk of genitourinary complications (4.7% versus 2.1%; P = .001), including incontinence (15.9 versus 12.2 per 100 person-years; P = 0.02) and erectile dysfunction (26.8 versus 19.2 per 100 person-years; P = 0.009). Failure rates did not appear to differ by surgical approach.198
Although the evidence that RALP is associated with clinically meaningful outcomes is lacking, it is clear that surgical experience can have a significant effect on outcomes.199 Based on an analysis of surgery performed on more than 7000 patients by 72 surgeons, there appears to be a statistically significant association between biochemical recurrence and surgeon experience.199
Complications of Radical Prostatectomy
One of the major criticisms of radical prostatectomy is that, although it is a widely performed procedure, the results that are usually quoted by urologists are those of a relatively small handful of experts. The rates of postoperative and late urinary complications are significantly reduced if the procedure is performed in a high-volume hospital and by a surgeon who performs a large number of prostatectomies. Unfortunately, less than 10% of urologists qualified as “high-volume surgeons”; thus the results reported by a relatively small handful of surgeons are not likely to be representative of the results of the other 90% of urologists.200 Given this limitation, it is clear that numbers quoted in the following text are based on the published literature and once again have to be taken with “a grain of salt.” The randomized trial of watchful waiting versus prostatectomy provides an important assessment of the effect of surgery on the overall quality of life (QOL).201 Urinary leakage occurred in 49% versus 21% after radical prostatectomy compared with “watchful waiting”; however, bowel dysfunction, anxiety, depression, well-being, and subjective QOL were similar in the two groups.
Pelvic pain, impotence, transient incontinence, and slight shortening of the penile length are among the immediate complications associated with radical prostatectomy.202 The reported intraoperative blood loss is typically in the order of 500 ml to 1 L (range, 300-4320 ml). Techniques that minimize injury to the anterior periprostatic veins, the dorsal vein complex, the numerous small branches from the neurovascular bundles (NVBs), the seminal vesicles, and the base of the bladder reduce the risk of blood loss. Among most urologists, the retropubic radical prostatectomy appears to be favored because of the reduced risk of rectal injury and the ease of completing a pelvic lymphadenectomy.
Operative mortality nationally has been reported to be nearly 2%, but in the hands of an experienced surgeon the risk is reported to be substantially lower.203 Similarly, rectal injury may occur infrequently in less than 1% of patients when reported by experienced surgeons, but is substantially more common based on national statistics. Thromboembolic events have been reported to occur in approximately 1% to 3% of patients, whereas myocardial infarctions and wound infections are reported to occur in 1% or less in selected series. However, based on a national sample of 20% of Medicare beneficiaries aged 65 and older, nearly 8% suffered major myocardial infarctions within 30 days of surgery.203 Urinary stress incontinence is reported by experienced surgeons to occur in 5% to 14% of patients, but is much more common based on national statistics or surveyed patients.
Potency After Radical Prostatectomy
The most widely quoted result of potency preservation after nerve-sparing radical prostatectomy has been reported in series from Johns Hopkins Medical Center and Washington University. Potency was reportedly maintained in approximately 60% of patients, with markedly better results in younger patients. At Washington University, patients younger than 60 years of age were reported to have a 68% likelihood of maintaining potency, versus 55% in patients 60 years or older. This patient selection phenomenon is highlighted by the prostatectomy series from Johns Hopkins, in which 86% of treated patients were potent preoperatively compared with only 50% of patients in the radiation series. It follows, as with other types of surgically induced morbidity, that the maintenance of potency depends on the skill and experience of the surgeon.200
In contrast, Robinson et al.204 recently updated a 1997 meta-analysis of rates of erectile function after treatment of localized prostate cancer. They conducted a comprehensive literature review and meta-analysis of the rates of erectile dysfunction associated with the use of nerve-sparing and non–nerve sparing prostatectomies, brachytherapy (permanent seed implants) with and without EBRT and cryosurgery for localized prostate cancer. The probability of maintaining erectile function after brachytherapy, brachytherapy plus EBRT, EBRT alone, nerve-sparing and non–nerve sparing prostatectomy and cryosurgery were 0.76, 0.60, 0.55, 0.34, 0.25, and 0.13, respectively. The major shortcoming of this analysis, however, is the fact that these are physician-reported rates of erectile dysfunction.
A more contemporary assessment of the effect of radical prostatectomy (with and without nerve sparing), EBRT, and brachytherapy on the QOL of men undergoing treatment for localized prostate cancer can be found in the report by Sanda et al.205 They prospectively measured outcomes reported by 1201 patients and 625 spouses or partners at multiple centers before and after radical prostatectomy, brachytherapy, or EBRT using the Expanded Prostate Cancer Index Composite (EPIC-26) and the Service Satisfaction Scale for Cancer Care.205–207 In their abstract they concluded, “Adjuvant hormone therapy was associated with worse outcomes across multiple quality-of-life domains among patients receiving brachytherapy or radiotherapy … and … [a]dverse effects of prostatectomy on sexual function were mitigated by nerve-sparing procedures.”
Unfortunately, the abstract failed to mention the most significant findings, and highlighted two of the least-relevant observations.208 Because adjuvant hormonal therapy (AHT) is supported by level I evidence for intermediate to high-risk patients treated with EBRT, highlighting the modest effect on QOL is misleading. In contrast, the single most remarkable QOL finding of this study was not even mentioned in the abstract. Despite starting with a higher mean sexual score at baseline and being 5 to 10 years younger, the mean sexual score of patients receiving prostatectomy had a much larger decline in sexual functioning at 2 years compared with those of patients receiving radiotherapy or brachytherapy. This finding should have been emphasized in the abstract and provided to patients who are attempting to make informed decisions about treatment.205
The randomized trial of watchful waiting versus prostatectomy provides an important multicenter international assessment of the effect of surgery on potency.201 These investigators surveyed 376 men treated on this trial and 87% of the men responded to the survey. All patients included were younger than 75 years of age, with the mean age being approximately 64.5 and 68.5 years at time of surgery and at the time of the survey, respectively. All were followed in excess of 1 year with the median follow-up being approximately 49 months, ample time for full recovery of function. In this study, erectile dysfunction occurred in 80% of men after radical prostatectomy compared with 45% on the watchful-waiting arm. To complicate matters further, potency is not an all-or-none phenomenon after prostatectomy. For example, in one report the authors noted that “no patient could get a hundred percent rigid penis any more” or “experience the exquisite sensation of inevitability, the so-called point ‘of no return,’ which is caused by contractions of seminal vesicles and prostate capsule.”209 These authors noted that it was not uncommon for men to abandon sexual intercourse because of orgasm-induced acute urinary incontinence.
Postoperative Radiotherapy: Adjuvant and Salvage Radiotherapy
Evidence for Adjuvant Radiotherapy
Support for postoperative EBRT following prostatectomy has grown with the completion of three phase III randomized trials demonstrating an improvement in biochemical failure, progression-free survival (PFS), time to metastasis, and overall survival.210–212 The most mature of these three trials conducted by the Southwest Oncology Group (SWOG) was recently updated.211 SWOG set out to determine whether adjuvant radiotherapy (ART) improves metastasis-free survival in patients with stage pT3N0M0 prostate cancer. In this study, 425 men were randomly assigned to receive 60 to 64 Gy of EBRT delivered to the prostatic fossa or observation (although, ultimately, 70 of these men received radiotherapy as well). ART resulted in an improvement in metastasis-free and overall survival compared with deferred therapy (HR 0.71; P = 0.016 and HR 0.72; P = 0.023, respectively). Among men randomized, there were 88 deaths of 214 on the radiotherapy arm versus 110 deaths of 211 on the observation arm (Fig. 45-9). Although adverse effects were more common with radiotherapy versus observation, by 5 years there were no differences in health-related QOL, and a subset analysis suggests that earlier treatment is better than delayed treatment.213,214
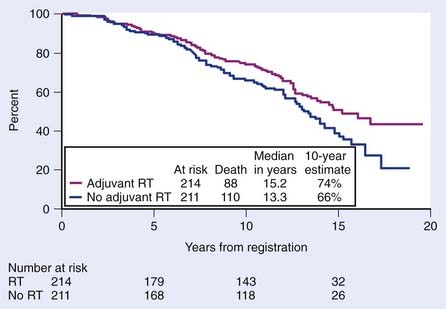
FIGURE 45-9 • Metastasis-free survival was significantly greater with radiotherapy (93 of 214 events on the radiotherapy arm versus 114 of 211 events on observation; hazard ratio [HR] 0.71; 95% confidence interval [CI] 0.54, 0.94; P = 0.016). Survival improved significantly with adjuvant radiation (88 deaths of 214 on the radiotherapy arm versus 110 deaths of 211 on observation; HR 0.72; 95% CI 0.55, 0.96; P = 0.023).211
The European Organisation for Research and Treatment of Cancer (EORTC) confirmed the value of ART, which reduces the risk of biochemical failure and prolongs the time to clinical progression.210 Patients eligible for this study had pN0M0 tumors and one or more pathologic risk factors (capsule perforation, positive margins, seminal vesicles invasion). After a median follow-up of 5 years, biochemical and clinical PFSs were significantly improved in the radiotherapy group (P < 0.0001 and P = 0.0009, respectively). The rate of local regional failure was also lower in the radiotherapy group. Severe toxic toxicity (grade 3 or higher) was similar, being 2.6% versus 4.2% at 5 years in the postoperative radiotherapy group (P = 0.07). Most recently, a German series confirmed the benefits of ART with a low incidence of late complications from radiotherapy.212 With modern techniques it is expected that the relatively small risk of late complications could be reduced further (see the following discussion).
Salvage Radiotherapy
Salvage radiotherapy is the only curative option for patients experiencing biochemical failure after radical prostatectomy. There is a growing body of literature suggesting that early intervention with radiotherapy is better than delayed intervention for patients with biochemical failure.215,216 Support can be found in a matched-pair analysis from a multi-institutional database of 2299 patients undergoing postoperative radiotherapy. This analysis included patients with pT3-4N0 who received either salvage radiation therapy (SRT) or early ART.215,216 In total, 211 patients receiving ART and 238 patients receiving SRT were matched in a 1 : 1 ratio according to preoperative PSA GS, seminal vesicle invasion, surgical margin status, and follow-up from date of surgery. Early ART for pT3-4N0 prostate cancer significantly reduces the risk of long-term biochemical progression after radical prostatectomy compared with SRT. Recently investigators from Johns Hopkins suggested that there was an increase in prostate cancer–specific and overall survival associated with SRT in men with a PSA doubling time of less than 6 months after adjustment for pathologic stage and other established prognostic factors.216 SRT initiated more than 2 years after recurrence or in men whose PSA never became undetectable provided no significant improvement in prostate cancer–specific survival.216 Stephenson et al.217 reported on 1540 patients who underwent SRT. They developed a nomogram that included PSA level before SRT (P < 0.001), prostatectomy Gleason grade (P < 0.001), PSA doubling time (P < 0.001), surgical margins (P < 0.001), ADT before or during SRT (P < 0.001), and lymph node metastasis (P = 0.019). This analysis provided evidence that even patients with seemingly very unfavorable prognostic factors may do relatively well if offered SRT.
Technical Considrerations
Set up error and organ (target) movement challenge the accuracy of radiotherapy (just as it does in definitive cases). Investigators from the University of California–San Francisco (UCSF) have shown that up to 30% to 40% of treatments would be off by more than 5 mm if no corrective measures were made. As a result, we have adopted the use of online imaging using gold markers to mark the anastomosis to guide the salvage approach.218 The role of dose escalation ( 70 Gy) in the salvage setting remains to be determined.219
Neoadjuvant Androgen Deprivation Therapy and Pelvic Radiotherapy in Postoperative Patients
Neoadjuvant ADT (NADT) may improve the outcomes of SRT in an analogous fashion to patients with locally advanced disease.217,220–222 Retrospective data demonstrated that patients with multiple adverse features have a better biochemical control rate when NADT was added to EBRT. Similar data have been reported from MD Anderson Cancer Center and in the large series described previously reported by Stephenson et al.217,222 Investigators from the Medical Research Council and Nation Cancer Institute of Canada have devised a trial attempting to define the role of adjuvant versus salvage radiotherapy and long- versus short-term ADT.223
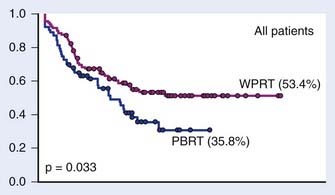
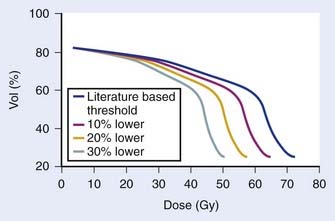
Retrospective data suggest that patients with adverse features have an improved biochemical control rate with the addition of WPRT compared with prostate bed–only radiation (Fig. 45-10).145 The RTOG is conducting a three-armed phase III trial, RTOG 0534, comparing radiation alone, radiation with HT, and radiation with HT and WPRT to answer this question.
Cryosurgery
Cryosurgery is an ablative treatment in which freezing is used to destroy prostatic tissue and its efficacy is well-established based on histopathologic studies. To avoid unacceptable toxicity, the urethra is spared from the full cryosurgical effect by using a warming device. Sparing the urethra from a full cryosurgical dose may spare portions of the apex as a matter of course, which may result in persistent microscopic disease. The failure to achieve a uniformly ablating dose is borne out by the fact that post-treatment biopsies show a high incidence of persistent viable-appearing prostatic tissue, and few patients have undetectable PSAs following a single session of cryosurgery. Despite these theoretical shortcomings, long-term results associated with cryosurgery are now available and are fairly encouraging.224 The use of multiple cryosurgery sessions appears to provide more complete ablation of the gland, complicated by a higher incidence of impotence than any other major treatment option for localized disease.204 More recent reports using more contemporary technology have demonstrated somewhat better potency.225 For example, in the report by Asterling et al.,225 prospective data were collected at 6 weeks, 3 months, then every 3 months up to 1 year, and then every 6 months up to 54 months. Following cryosurgery, 96.3% of patients had erectile dysfunction at 6 weeks and 3.7% had partial erections. Many of these men used phosphodiesterase type-5 inhibitors and by 24 months nearly 25% were described as fully potent and 9% partially potent.
Recent series suggests that in the hands of some surgeons, cryosurgery may compete favorably with radiotherapy.83,226,227 To date, two phase III trials (both Canadian) have been completed with somewhat conflicting results.228,229 Chin et al.228 recruited and randomized 64 of a planned 150 patients with clinically staged as T2C, T3A, or T3B disease. All patients received 6 months of ADT and were randomized between EBRT or cryosurgery. The 4-year biochemical disease-free survival was 47% and 13% respectively for EBRT and cryosurgery (P < 0.05). The authors concluded that “taking into account the relative deficiency in numbers and the original trial design, this prospective randomized trial indicated that the results of cryosurgery were less favorable compared to those of EBRT, and was suboptimal primary therapy in locally advanced prostate cancer.” The somewhat larger report (N = 244) by Donnelly and colleagues229 concluded that there were similar PSA control rates but fewer positive biopsies at 36 months among patients treated with cryosurgery compared with EBRT. Unfortunately this study appears to suffer from several methodologic issues. First, biochemical failure was defined using the so-called Phoenix definition” (nadir + 2), which may be inappropriate in patients undergoing ablative therapy.164 Second, although PSA control rates were similar, 94% of patients were treated to inadequate radiation doses of 70 Gy or less. Furthermore, retreatment in the first 6 months with cryosurgery was not considered failure. Also of note, 67% of patients treated with EBRT retained spontaneous erections compared with only 14% treated with cryosurgery.
The American Urologic Association generated a “Best Practice Statement on Cryosurgery.”230 The panel concluded that there was level II evidence for treating men with clinically confined disease, but that the role for patients with clinical T3 disease was still “currently undetermined.” They noted that there was no universally accepted definition of PSA failure, but that the 5-year biochemical disease-free survival rates since the year 2000 ranged from 65% to 92% for low-risk patients, 69% to 89% for intermediate-risk patients, and 48% to 91% for high-risk patients.230 Defining PSA failure after cryosurgery remains controversial.231 Levy et al.231 reported an analysis of 2427 patients treated with cryoablation in whom outcomes were studied for biochemical disease-free survival based initially on a “nadir + 2” definition. For PSA nadirs of 0.6 to 1.0 ng/ml, the 24-month biochemical disease-free survival was 70.5% for low-risk disease, 56.1% for intermediate-risk disease, and 46.7% for high-risk disease. They concluded that nadir PSA after prostate cryoablation is prognostic for biochemical disease-free survival, but cautioned against using it as a definition of disease-free survival because it has not been correlated with disease-specific or metastasis-free survival.
Complications After Primary and Salvage Cryosurgery
Short-term complications include urinary retention, which usually persist for 1 to 2 weeks and is treated with a suprapubic or Foley catheter.230 Penile and scrotal swelling and penile anesthesia may also occur. Physician-reported long-term complications include fistula formation (0%-0.5%), incontinence (<1% to 8%), erectile dysfunction in 49% to 93% of patients at 1 year, and urethral sloughing rates of 0% to 15%. Prospective studies assessing health-related quality of life (HRQL) are limited in number for cryosurgery.232 Urinary function was noted to be equivalent at 6 months with better American Urological Association (AUA) symptom scores noted after 3 months. Unlike radiation-related toxicities, delayed complications following cryosurgery appear to be rare.207,233 Performing cryosurgery in the postradiotherapy failure setting is technically more challenging and appears to be associated with greater morbidity. Urinary incontinence in early series approach 70% but more recently are 10% or less.230 Fistula and other complications are also probably more common in the salvage setting, with studies demonstrating a greater effect on HRQL.234
Cryosurgical salvage of radiation therapy failures has been tried with mixed results. The high incidence of urinary incontinence and rectal injury has discouraged many investigators from routinely recommending cryosurgery for salvage of radiation therapy failures. Several investigators have claimed more encouraging results.226,227,235 Robinson and colleagues prospectively evaluated the QOL after salvage cryosurgery in 46 men with recurrences after EBRT.236 They noted that at 24 months 29% and 56% of men, respectively, reported urinary bother and sexual bother as “moderate-to-big.” They concluded that long-term impairments in QOL were limited and overall QOL was high.236
Radiation Therapy
Conventional External-Beam Radiation Therapy
A number of retrospective and prospective studies support the long-term efficacy of radiation therapy in the management of clinically localized prostate cancer.69,237 Based on physical examination alone, these studies suggested that the local control rate following EBRT was between 70% and 90%. These endpoints are now known to underestimate the incidence of local failure.160 Older techniques were associated with inadequate coverage of the target volume in at least 20% to 41% of the patients treated and characterized by the use of relatively small boost fields, routinely using bony landmarks to define treatment borders.238 Early conventional series used total doses in the range of 60 to 70 Gy, because it was believed that this dose was sufficient and close to the maximum dose allowed by the surrounding normal tissues. These problems were addressed by the use of 3-D treatment planning technology (3D-CRT and intensity-modulated radiotherapy [IMRT]), but the adoption of image guidance has probably been equally important.239–243
“Conventional” versus Conformal Blocking and Three-Dimensional Conformal Radiation Therapy
3D-CRT allows delivery of higher doses of radiation to the target volume of interest while sparing more of the surrounding normal tissues than is possible using conventional techniques. There were many different approaches that were used in the early days of 3D-CRT. Two examples developed at UCSF are provided here. Early work demonstrated that the use of oblique fields placed at 35 degrees (from the lateral field), and delivering 50% of the dose by laterals, 25% by anterior obliques, 15% to 20% from posterior obliques, and 5% to 10% from an anterior field created an excellent dose distribution.244 Alternatively, using conformally blocked arcs to deliver radiation therapy to the prostate only for patients at very low risk for seminal vesicle involvement can simplify and shorten treatment time.245
Compared with treating a patient by standard technique, 3D-CRT is associated with a nearly 30% reduction in the dose received by 50% of the rectum. Based on this kind of analysis, it has been estimated that an increase in minimum tumor dose of greater than or equal to 10% should be possible without an increase in acute or chronic toxicity.246 An even greater increase is likely to be safely achieved if dose inhomogeneity constraints are relaxed and image guidance is used, as is common with IMRT.
Intensity-Modulated Radiation Therapy and the NCI-IMRT Working Group
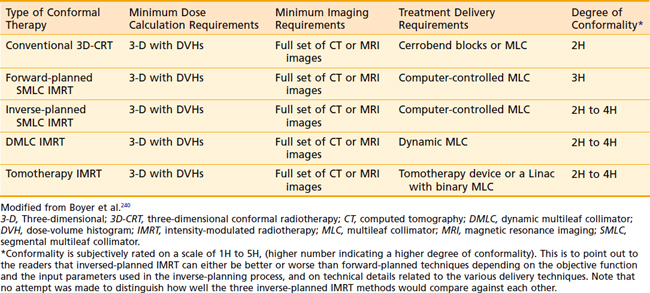
3D-CRT became the standard of care in nearly every major academic center in this country in the mid 1990s, with many centers moving to IMRT for the treatment of prostate cancer by the early to mid 2000s. In an effort to clarify the relationship between 3D-CRT and IMRT, the National Cancer Institute (NCI) formed an IMRT working group composed of a selected group of radiation physicists and oncologists with experience using this technology. The NCI-IMRT Collaborative Working Group (NCI-IMRT-CWG) set out to define an agreed-on set of jargon, standards for quality assurance, and to provide a clinical context through which this technology might be viewed.240 At the time the NCI-IMRT-CWG consensus paper was written, there were three major types of IMRT in common use (Table 45-5). Dynamic multileaf collimator (DMLC) IMRT is used to describe a complex delivery approach in which the gantry and leaves move simultaneously and inverse planning is incorporated.247–249 Tomotherapy (not to be confused with the commercial product), a second type of inverse planning–based IMRT, involved the use of a rotating multisegmented delivery system. Tomotherapy involves radiation delivered by using either sequential or continuous arcs much like a CT scanner.250–252 What had previously been called static-field IMRT by the conventions proposed by the NCI IMRT working group, became segmental multileaf collimated IMRT (SMLC-IMRT).239,244,253,254 When forward-planned, it was abbreviated F-SMLC. There are advantages and disadvantages to each form of IMRT, but all can deliver very complex dose distributions.240 The advantages associated with F-SMLC include the ability to generate port films that resemble conventional films from a 3-D plan, planning is more intuitive, and manual editing of blocks can be carried out relatively easily. Using automated sequence software SMLC-IMRT treatment can be delivered within a similar timeline as DMLC or tomotherapy-based IMRT.
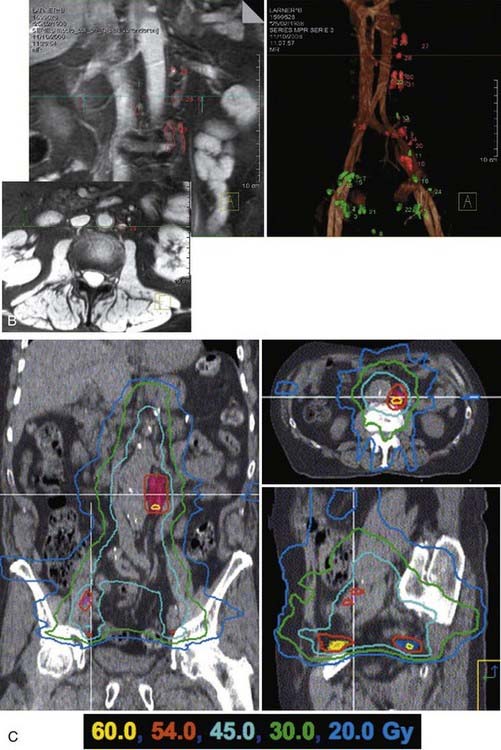
Fig. 45-11A-C shows axial, sagittal, and coronal distributions associated with tomotherapy, forward-planned SMLC, and inverse-planned SMLC all targeting a dominant intraprostatic lesion (DIL) to 90 Gy while treating the entire prostate to 75.6 Gy.242 Although long-term follow-up data demonstrating the benefits in outcome are not yet available, it was possible to selectively intensify the dose to selected regions of the prostate using IMRT by targeting DILs or using “dose-painting” techniques.239,242,253,255 This approach begged the question: “If the disease is not uniformly distributed throughout the gland, why uniformly distribute the dose?” Uniformly delivering a high dose to the entire prostate uniformly increases the risk of complications to surrounding normal tissues. Early studies conducted at UCSF had shown that MRI and MRSI can be used to define regions within the prostate that are at higher risk for involvement by tumor.153,256 Selective targeting is best accomplished by combining endorectal MRI and MRSI with biopsy information. Traditional sextant biopsies may have relatively low sensitivity.77,257,258 A number of studies suggest that MRI combined with MRSI can potentially enhance the sensitivity of biopsies.156,157,259
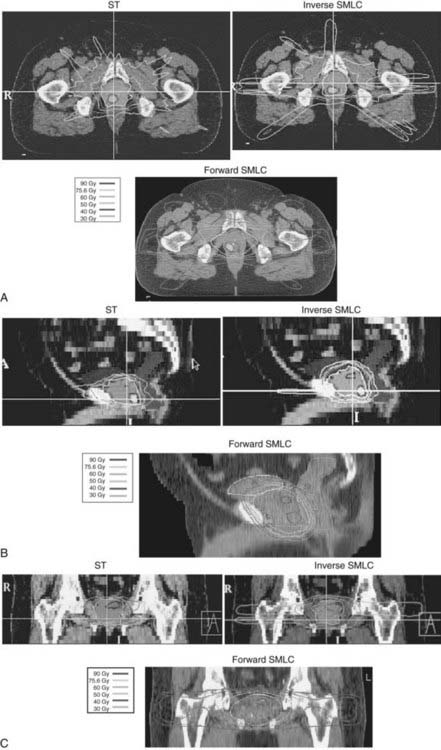
FIGURE 45-11 • A-C, Shows axial, sagittal, and coronal distributions associated with tomotherapy, forward-planned segmental multileaf collimation (SMLC) and inverse-planned SMLC all targeting a dominant intraprostatic lesion to 90 Gy while treating the entire prostate to 75.6 Gy.242
The Challenges of Day-to-Day Set-Up Variation and Organ Movement and IMRT: The Role of Image Guidance
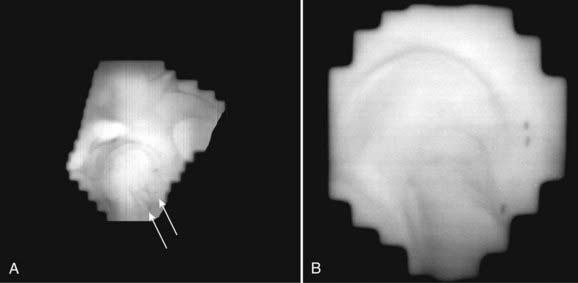
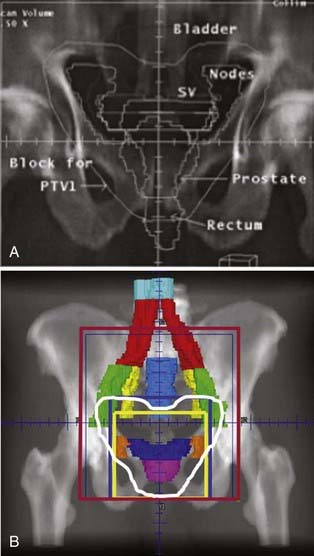
The ability to take full advantage of IMRT depends on the accuracy of reproducing the desired dose distribution from a computerized plan in the patient. A beautiful dose distribution if delivered even 5mm posterior to the intended location can have a substantial effect on the dose delivered to surrounding normal tissues.242,260 It is now abundantly clear that the use of weekly port films is inadequate for the accurate delivery of highly conformal dose distributions. Numerous studies have now demonstrated that organ movement and day-to-day set-up variations can result in significant treatment errors.261,262 The use of a rectal balloon, an ultrasound localization system, or an electronic portal imaging device (EPID) in conjunction with implanted markers are all options used to improve the accuracy of IMRT.251,263–265 The preferred approach at UCSF has involved the use of implanted gold marker seeds and the use of an EPID.266,267 We have compared these options and found the EPID-based process to be reproducible, fast, and accurate.268–270 Neither intrafraction movement or seed migration appear to be limiting factors in the application of the EPID-based approach.243,271 Fig. 45-12A and B demonstrate examples of lateral EPID images for WPRT and prostate-only radiotherapy (PORT), respectively. The use of gold marker seeds has also been routinely implemented at UCSF to address daily set-up error and target motion in management of postoperative patients.218 The routine use of one or more of these methods have replaced weekly port films as the standard of care (see the American College of Radiology Appropriateness Criteria 2008, available from the American College of Radiology website: www.acr.org/ac).
Rationale and Indications for Pelvic and Seminal Vesicle Irradiation
Nonrandomized Studies Addressing Whole-Pelvic Radiotherapy
Despite the uncertainty about the cure rates of patients with lymph node involvement, survival may be prolonged by prophylactic lymph node irradiation. That WPRT might be associated with a prolongation of survival would not be completely surprising. The survival of women treated to para-aortic lymph nodes for cervical cancer and with groin and pelvic radiotherapy in cancer of the vulva are associated with prolonged survival.272,273 Furthermore, prophylactic nodal radiotherapy in some subsets of women with breast cancer (a disease with a biology more closely resembling prostate cancer) has been shown to prolong survival.274,275
Several groups have published retrospective studies demonstrating an improvement in outcomes in subsets of patients treated with WPRT when compared with PORT.143–145,276–280 The reports by Seaward et al. (UCSF),278,279 Pan et al. (University of Michigan),280 Spiotto et al. (Stanford),145 Aizer (Yale),144 and Da Pozzo (Milan, Italy)143 (shown in Table 45-6) were based on patients treated in the PSA era. The UCSF group concluded that patients with an estimated risk of lymph node involvement of between 15% to 35% (based on the risk equation: +LN = 2/3 (PSA) + {(GS − 6) × 10} benefited the most from prophylactic pelvic radiotherapy. In contrast, the analysis by Pan and colleagues estimated each patient’s percentage of risk of lymph node (%rLN) involvement based on the updated Partin tables. They divided their patients into three different categories based on %rLN involvement: low, 0% to 5%; intermediate, more than 5% to 15%; and high, more than 15%. Multivariate analysis demonstrated a statistically significant benefit for the entire population treated with WPRT, with a relative risk reduction of 0.72 (95% CI, 0.54-0.97). Again, the greatest benefit to WPRT was seen in the intermediate risk (5%-15%) group. They concluded that WPRT appears to improve biochemical no evidence of disease rates in prostate cancer patients and that additional studies are needed to define the optimal group for WPRT. As shown in Table 45-6, there is a significant body of clinical literature supporting WPRT.
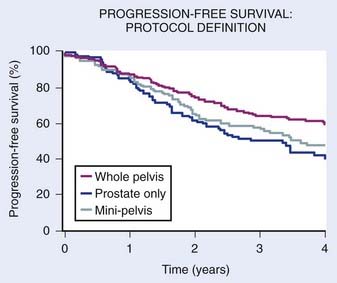
Several studies fail to demonstrate a benefit to prophylactic WPRT.281 Of particular note is the report by Jacob et al.281 from Fox Chase. Like the UCSF group, they evaluated patients with an estimated risk of lymph node involvement of greater than or equal to 15%. The negative findings from their report could have been due to the fact that patients treated with WPRT tended to have more advanced tumors with a poorer prognosis, or other factors (sample size, follow-up, method of estimating risk, inconsistent use of ADT). The major shortcoming of their analysis, however, is supported by the findings from a subset analysis of RTOG 9413. In this analysis it was shown that the PFS was directly related to field size. Using the criteria for defining WPRT from RTOG 9413, none of their patients received WPRT; all would have been treated on the prostate only arm. The importance of field size cannot be underestimated because in a subset analysis there was a clear relationship between field size and the benefits of WPRT.282 A comparison of field sizes used in the report by Jacob et al.281 with those used in RTOG 9413 is shown in Fig. 45-13A and B (see following discussion).281
Randomized Trials Addressing Whole-Pelvic Radiation Therapy
We are aware of four prospective randomized trials published to date that have compared WPRT with PORT. The oldest study conducted at Stanford was very small (N = 57), included only patients who were pathologically proven to be node-negative, and completed in the pre-PSA era. A small but statistically insignificant difference (perhaps because of sample size) in disease-free survival of 70% versus 53% favoring patients who received WPRT compared with PORT is noteworthy. RTOG 7706 also compared WPRT with prostate irradiation alone in patients at low risk for lymph node involvement (stages A2, B1, and B2; node-negative status confirmed by imaging or pathologic staging).283 No differences in outcome were noted between the two arms. The major problems with these studies are that even if pelvic irradiation were beneficial, the very low incidence of lymph node involvement in these patients and the small number of patients probably would not allow such a benefit to be recognized. It should not be surprising that WPRT is of little or no benefit in patients at low risk for lymph node involvement.
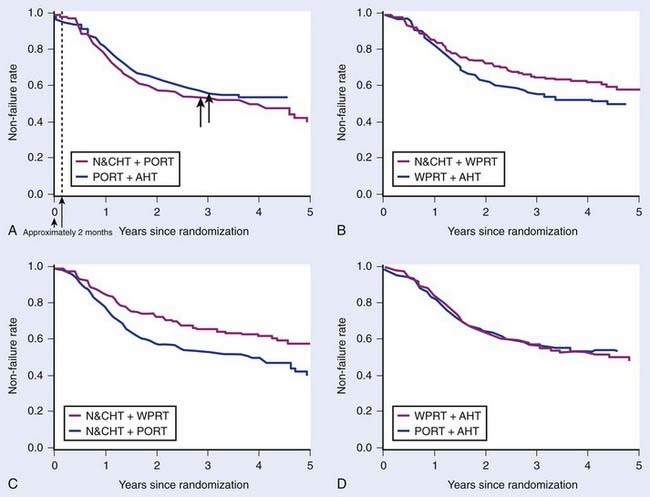
The most important phase III prospective randomized trial completed to date addressing the issue of prophylactic pelvic nodal irradiation is RTOG 9413.140 This large, four-arm trial assessed the value of WPRT as well as the effect of the timing of combined androgen suppression therapy in patients with an estimated risk of lymph node involvement exceeding 15%. More than 1300 men were accrued to this study in just over 4 years. This was the first major trial to use PSA to define PFS as the primary endpoint. At the time of the initial analysis, the follow-up for this study was too short to expect a difference in the secondary endpoints such as overall and cause-specific survivals. However, because having earlier progression is never more desirable and all patients received the same total dose of radiation and duration of HT, a primary endpoint of PFS was considered an appropriate intermediate endpoint.
With a median follow-up of nearly 5 years, patients treated with WPRT experienced a 4-year PFS of 54% compared with 47% in patients treated with PORT (P = 0.022). When simultaneously comparing all four arms, there was a PFS difference among WPRT and N&CHT, PORT and N&CHT, WPRT and AHT, and PORT and AHT (60% versus 44%, 49% and 50% respectively, P = 0.008). The results of 9413 are more impressive when viewed as pairs of curves, as shown in Fig. 45-14A-D. Thus this phase III trial demonstrated that WPRT and N&CHT is associated with an improvement in freedom from progression compared with PORT and N&CHT or AHT, and WPRT and AHT in patients with a risk of lymph node involvement greater than 15%. Because of the long follow-up required to determine the effect of treatment on survival in prostate cancer patients, as expected no survival difference was yet seen.
RTOG 9413 also suggests that the greatest benefit to WPRT and N&CHT was seen in patients with intermediate- to high-risk disease (GS = 7 and PSA < 20 ng/ml or GS = 6 and PSA > 30 ng/ml), compared with those with the lower-risk or higher-risk disease. This observation is consistent with retrospective data from the UCSF.278 This finding has major implications for the large number of patients receiving radiotherapy and CAB for clinically localized prostate cancer. However, the failure to identify an apparent benefit of pelvic nodal treatment in the lower-risk patients could have been due to sample size because relatively few patients in this category were included in this study. A larger phase III trial with survival as the primary endpoint is planned by the RTOG (0924) and is scheduled to be launched in the latter half of 2010. For this trial all patients will receive either short- or long-term ADT and high-dose radiation to the prostate by IMRT or brachytherapy boost.
Another phase III trial that is believed by many to address the issue of WPRT is the French study by the Groupe d’Etude des Tumeurs Uro-Génitales (GETUG)-01.284 The investigators of this study set out to assess the benefit and toxicity and QOL outcomes of pelvic node irradiation in nonmetastatic prostate carcinoma patients. They enrolled and randomized 444 patients with T1b-T3, N0 pNx, M0 prostate cancer to either pelvic and prostate radiotherapy or prostate radiotherapy only. The pelvic dose was 46 Gy with the superior border at S1/S2, thus by RTOG 9413 criteria none of the patients received WPRT. Again, the importance of field size cannot be underestimated because in a subset analysis there was a clear relationship between field size and the benefits of WPRT, as is shown in Fig. 45-15.282 This study allowed but did not mandate 4 to 8 months N&CHT allowed for “high-risk” patients. The patients were generally more favorable than those treated on RTOG 9413 with a median PSA 12 ng/ml (versus 22 ng/ml) and approximately 25% were T3, and approximately 10% had a GS of 8 to 10. Approximately 55% of patients had lymph node involvement risk of less than 15% using the “Roach formula”; thus more than half of the patients would not have been eligible for RTOG 9413. The total dose recommended to the prostate was changed during the course of the study from 66 Gy to 70 Gy. With a 42.1-month median follow-up time, the 5-year PFS and overall survival were similar in the two treatment arms for the whole series and for each stratified group. They concluded that there were no significant differences in acute and late digestive toxicities and in QOL outcomes, but pelvic radiotherapy did not improve PFS. Thus the patients treated on GETUG-01 were more favorable than on RTOG 9413, did not systematically receive ADT, and none received WPRT. Because of these features, this study does not address WPRT and does not contradict RTOG 9413.
Clinical Guidelines for WPRT
The equation used for estimating the risk of lymph node involvement is:
Based on the results of RTOG 9413 in general, WPRT is recommended in combination with neoadjuvant HT and neoadjuvant HT is recommended when WPRT is planned. Although it is recognized that using traditional treatment fields to deliver WPRT means that some lymph node groups may not be adequately encompassed, most experts believe that the first echelon of nodes are probably the most important to cover.285 As previously noted, treatment to the L5-S1 level is supported by the subset analysis from RTOG 9413.282 Using IMRT, investigators from UCSF have shown that WPRT can be executed and provide better coverage of lymph node chains while simultaneously resulting in lower doses to surrounding normal structures (Fig. 45-16).286–288 We have shown that using IMRT we can deliver doses 10% to 30% lower than the threshold doses expected to result in rectal toxicity (Table 45-7, Fig. 45-17). In addition, promising results may be further enhanced by the incorporation of dextran-coating nanoparticles to guide selective nodal coverage (Fig. 45-18A-C).
FIGURE 45-16 • New intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy for prostate cancer.286
Table 45-7 Advantages of IMRT versus 3D-CRT for Target Coverage and Normal Tissue Avoidance
| Target & Normal Tissue Doses | IMRT | 3D-CRT |
|---|---|---|
| Pelvic lymph nodes (Mean and D95) | 49.6 Gy/46 Gy* | 43.7 Gy/27.4 Gy* |
| Small bowel (V45)† | 51.5 cm3* | 283.6 cm3* |
| Penile bulb (mean) | 15.1 Gy* | 30.1 Gy* |
3D-CRT, Three-dimensional conformal radiotherapy; D95, dose to 95% of nodal targets; IMRT, intensity-modulated radiotherapy.
Modified from Wang-Chesebro et al.286
Seminal Vesicle Irradiation
At UCSF, our philosophy toward seminal vesicle irradiation is similar to that for lymph node involvement. We recommend treating them if positive by biopsy or as defined by transrectal ultrasound or endorectal MRI. In the absence of these findings, at UCSF patients are considered high-risk if the calculated risk of seminal vesicle invasion is greater than or equal to 15%. Other investigators seem to share a similar philosophy toward treatment of the seminal vesicles.289 For example, Kestin et al.289 performed a complete pathologic review of prostatectomy specimens to determine when the seminal vesicles should be irradiated and the appropriate length of seminal vesicles that should be included within the clinical target volume (CTV). They concluded that only 1% of low-risk patients (PSA < 10 ng/ml, Gleason ≤ 6, and clinical stage ≤ T2a) were found to have seminal vesicle involvement compared with 27% of high-risk patients. Patients with only one high-risk feature reached the 15% risk threshold for seminal vesicle involvement, whereas 58% of patients with all three high-risk features had seminal vesicle involvement. They reported that the median length of seminal vesicle involvement was 1 cm (90th percentile: 2 cm, range: 0.2-3.8 cm), and the risk of involvement beyond 2 cm was approximately 1%. They concluded that a portion of the seminal vesicles should be included in the CTV for higher-risk patients, but only the proximal −2 to 2.5 cm corresponding to approximately 60% of the seminal vesicle should be included within the CTV (high-dose area).
Even if the calculated risk is low, if the primary tumor is located at the base of the prostate (immediately adjacent to the seminal vesicles), seminal vesicle irradiation may be appropriate. At UCSF, a prophylactic dose for patients at risk for seminal vesicle involvement is 54 to 55.8 Gy. Patients with documented involvement received full dose to all or the proximal portion of the seminal vesicle. A single seminal vesicle is treated if there is a well-lateralized, low-volume lesion. If the calculated risk exceeds the threshold for high risk for seminal vesicle involvement but the tumor is apical in origin and imaging studies and seminal vesicle biopsies are negative, omitting elective seminal vesicle irradiation would also seem to be reasonable in some cases. Omitting seminal vesicle radiation therapy, when possible, should theoretically allow us to treat the prostate to a higher dose because of the rectal sparing resulting from this policy.290
Guidelines for Treatment Planning and Set-Up
The International Commission for Radiation Units 50 recommends standard terms for defining target volumes during treatment planning.291 Correlative pathologic studies have demonstrated that MRI is slightly more accurate in assessing volume than transrectal ultrasonography (TRUS), and both are superior to CT.292,293 When assessed by non-contrast CT, the prostate appears somewhat larger posteriorly and inferiorly than when assessed by MRI or TRUS. This region of discrepancy appears to correspond to the anterior wall of the rectum and the region of the levator ani muscles. Inclusion of peri-prostatic fat or blood vessels anteriorly may also contribute to overestimates of the prostatic volume when defined by CT. Lack of image resolution and not understanding these anatomic landmarks probably explain the tendencies for the discrepancies noted. Discrepancies in prostate volumes may also result in part from inaccurate assumptions about the length of the external sphincter when a retrograde urethrogram is used to assist in defining the apical portion of the prostate. Interobserver variability in defining the prostate size on CT probably also contributes to this problem.294
Patient Immobilization
Large day-to-day set-up errors (>0.5 mm) can be significantly reduced with the use of patient immobilization devices. Reducing the frequency of set-up errors reduces the likelihood of underdosing the tumor, allows the use of “tighter margins,” and reduces the dose unintentionally received by surrounding normal tissues. Regardless of the degree of patient immobilization, the potential for organ movement limits the advisability of using “very tight” margins unless online imaging is being used. The commonly used immobilization devices are constructed of a melted plastic mold material, a solidified polystyrene foam mold, or reusable inflatable mold devices.295,296 Patients may be simulated and treated supine or prone.297 The prone position in combination with a thermoplastic shell tends to be associated with less set-up error, but may be more prone to organ movement error caused by respirations.295,297,298 Regardless of the type of immobilization device used or the treatment position chosen, there is no replacement for a careful three-point set-up and clear instructions to patients on how to assume the same position every day.
Set-Up and Simulation
Before simulation, an immobilization device is usually made. Implementation of daily monitoring via a transabdominal ultrasound–based system or intraprostatic markers combined with EPIDs may eliminate the need to use immobilization devices.265 For patients beginning WPRT, the following directions are an example of the steps followed at UCSF. A week or more prior to simulation, two gold marker seeds are placed in the base and one in the apex. The patient is placed in the supine position with a full bladder and the rectum empty (following an enema).
Treatment Planning the Cone-Down Boost and Doses
Treatment planning CT scans should be obtained with the patient on a flat table with images obtained from the lower border of the sacroiliac joints, using 2- to 5-mm slices inferiorly to 1 cm below the ischial tuberosities. At UCSF we prefer to obtain the treatment planning CT with the rectum empty and the bladder full as stated previously, based on the assumption that this results in the prostate being in its most posterior inferior position; recent studies support this policy.299,300 Nonuniform field-edge margins varying from 0.75 to 2 cm are used to take into account the dose distribution associated with the technique used, day-to-day set-up variation, beam penumbra, and movement error. Nonuniform margins should allow the greatest opportunity for escalation of dose to the prostate, while maximizing tumor control probability and minimizing the normal tissue complication probability. Theoretically when anterior movement occurs, the post margins become slightly larger but in the absence of movement they are still adequate. When intraprostatic markers and an EPID-based system are used, all of these field edge margins may be decreased because errors resulting from day-to-day set-up variation and organ movement will also be reduced.
Treatment Verification
Monitoring treatment alignment by weekly port films has been a customary practice for many years. Significant gradual isocenter displacement may occur during the course of treatment in approximately 25% of patients. The magnitude of set-up error described is sufficient to reduce the tumor control probability and increase the normal tissue complication probability. Daily monitoring has been shown to reduce the magnitude of these errors and should allow the use of “tighter” margins. Endorectal balloons, ultrasound localization systems, or electronic portal imaging in conjunction with implanted markers are currently the major options for addressing the problem of organ movement.218,251,263–265,267 We have used intraprostatic markers at UCSF for more than 12 years and believe they have substantially improved the accuracy of our treatments.
Brachytherapy
Introduction to Permanent Implants
Prostate brachytherapy, in various forms, has fluctuated in popularity for more than 100 years. Interstitial prostate brachytherapy was first reported by Barringer in 1917, at what is now Memorial Sloan-Kettering Cancer Center (MSKCC).301,302 Its first widespread adoption occurred in the 1970s, when the retropubic method was popularized.303 A laparotomy was done for lymph node dissection and exposure of the prostate. Iodine-125 (125I) sources were implanted under direct visualization. The procedure was technically difficult to perform, in part because of limited working space in the pelvis. Retropubic implantation lost popularity in the 1980s because of the requirement for laparotomy, competition from emergence of modern prostatectomy and CT-based EBRT, poor seed localization, and inferior local control rates reported in some series.304 The last 15 years witnessed a tremendous surge in prostate brachytherapy, caused primarily by the introduction of TRUS technology in the early 1980s.
In 1983, Holm and coworkers reported the use of TRUS for transperitoneal transperineal implantation at the University of Copenhagen in Denmark.305 TRUS allowed visualization of the needle location within the prostate, facilitating real-time readjustments of needle position as necessary. Implants could be computer preplanned using transverse ultrasound images. Transperineal implants also could be done percutaneously on an outpatient basis, without laparotomy. Combined with modern, computer-based treatment planning, technological advances allowed for higher quality outpatient prostate brachytherapy.306
Brachytherapy offers substantial biologic advantages over EBRT in terms of dose localization and higher biologic doses. A modification of the time, dose, fractionation tables has been made to allow interconvertability between beam radiation and low-dose-rate (LDR) brachytherapy.307 There are also substantial practical advantages of brachytherapy, including vastly shorter treatment times and lower costs. These practical advantages have helped maintain widespread interest in brachytherapy, despite continuous improvements in beam radiation. Although enthusiasm remains high in some quarters, there are still vexing discrepancies in reported cure rates and morbidities. It is becoming clearer that such discrepancies result partly from different technical expertise and patient management policies.308 Brachytherapy, like surgery, is operator-dependent and outcomes vary with skill and experience.
Patient Selection
There are two principle criteria by which patients’ suitability for brachytherapy is judged—cancer status and morbidity risk. Contraindications to brachytherapy include metastatic disease (including lymph node involvement), gross seminal vesicle involvement, or large T3 disease that cannot be adequately implanted because of geometrical impediments to adequate tumor mass implantation (an unusual presentation). The rationale for excluding patients with substantial seminal vesicle invasion is that radioactive seeds are unlikely to be capable of sterilizing more than the most proximal 1 cm of seminal vesicle tissue.309
There is general agreement that cancer control in patients with low pretreatment PSA and GSs are similar between modalities, and that brachytherapy without supplemental beam radiation is sufficient. In contrast, there was a common perception that patients with a higher PSA or GS should receive beam radiation or surgery, based on their poorer outcomes in some brachytherapy series. In fact, patients with higher indices are at greater risk of cancer recurrence regardless of therapy, primarily because of their higher likelihood of subclinical metastatic disease at presentation. With good-quality brachytherapy, implant-based therapy is an acceptable component of treatment for higher-risk patients.310 Isolated series with inferior results in higher-risk patients have been widely promulgated, but inferior results were likely caused by inadequate technique.311
Big Prostates
Large prostate size is probably the most common and least understood alleged contraindication to brachytherapy. Patients with large prostates are at higher risk of acute postimplant retention, but such retention is typically short-lived, resolving after several days of an indwelling catheter or self-catheterization.312–314 In the past, patients with prostate volumes greater than 50 to 60 cc were commonly advised against brachytherapy or were placed on androgen therapy prior to implant to shrink their gland prior to implantation. Some series suggest, however, that hormonal ablation does not decrease the risk of retention, and may increase the risk of rectal complications.315
The primary technical concern regarding implantation of large prostates is that the anterior and lateral portion of the gland may be inadequately covered because of pubic arch interference (PAI) of needle placement. In fact, PAI is largely a function of arch anatomy, and less dependent on gland size. In addition, PAI is readily circumvented with technical maneuvers. With good technique, adequate coverage of large prostate glands is readily achievable. With increasing experience, prostate size is less likely to be perceived by brachytherapists as a technical contraindication.316 On the other extreme, small prostate volume should not be considered a contraindication to brachytherapy.317
Preimplant Retentive Symptoms
After prostate size, preimplant urinary retentive symptoms (high AUA score) is probably the most commonly cited contraindication to brachytherapy.314 Preimplant retentive symptoms may predispose to postimplant urinary retention or excessive obstructive symptoms. In most cases, however, retention is short-lived, with little long-term consequence. Although it is logical that patients with substantial pretreatment obstructive symptoms would be more prone to brachytherapy-related problems, AUA scores are only a weak predictor of the risk of postimplant urinary retention.316 Urodynamic studies, postvoid residual urine volume maximum flow rate, or cystoscopy are poor predictors for postimplant retention.318 Oddly enough, patients with higher preimplant scores experience lesser changes in their scores compared with those with low preimplant scores, one simplistic explanation being that patients with urinary obstructive symptoms are already having trouble, and won’t get much worse.319
Prior TURP
In an early report from Blasko and colleagues, approximately 50% of TURP patients developed postimplant incontinence.320 In contrast, the risk of incontinence in TURP patients has been low in more recent experience, probably because of the adoption of peripheral source loading patterns that avoid excessive urethral doses.321,322 If brachytherapy is performed for a patient with prior TURP, there should probably be enough tissue left around the defect to hold the seeds. CT, MRI, or TRUS are generally adequate to assess remaining tissue (Fig. 45-19).
Age
There has been a reluctance among oncologists to recommend brachytherapy for younger patients, primarily because of the lack of long-term tumor control data. However, with longer follow-up showing high tumor control rates in all ages, reluctance to implant young patients is waning.323 Although postimplant morbidity is not clearly higher in older patients, it is possible that more elderly patients would have more trouble coping with radiation prostatitis. The possibility of a more difficult postimplant course should be considered when deciding between brachytherapy and EBRT for older patients.
Other Considerations
Prostatitis is a poorly understood condition often treated empirically with antibiotics. Hughes and colleagues saw no relationship between preimplant clinical or pathologic findings of prostatitis and postimplant urinary morbidity.324
The presence of a penile prosthesis is a potential contraindication to brachytherapy, because of the possibility of periprosthetic infection or physical damage to the prosthesis. However, concerns over prosthesis-related complications may be exaggerated. No unusual complications were seen in four patients with pre-existing penile prosthesis, and such patients are routinely implanted by investigators from Seattle.325
Median lobe hyperplasia (MLH) refers to protrusion of transitional zone tissue into the bladder (Fig. 45-20). It has been considered by some to be a contraindication to brachytherapy, because of an increased risk of postimplant urinary morbidity or because of technical difficulties implanting intravesicular tissue. On the contrary, there appears to be little association between MLH and postimplant morbidity.326 Intraoperatively, MLH is readily viewed by TRUS, and there is little difficulty placing sources in the MLH tissue. There is no documented justification for prophylactic resection of hypertrophic tissue. The authors avoid patients with extreme MLH.
There is increasing evidence that obese patients have inferior tumor control rates after EBRT, in part because of daily positioning problems. In contrast, heavy patients pose a relatively minor problem for brachytherapy.327 Operative setup can take longer, and there is less room to maneuver between the patient’s legs, but this has not presented a huge problem. Standard 20-cm brachytherapy needles are almost always sufficient to reach the prostate. Clinical outcomes appear to be unaffected.328
IBD, including ulcerative colitis and regional enteritis (Crohn disease), has long been believed to be a contraindication to radiation, despite scant documentation of IBD-related complications. IBD patients treated with brachytherapy do not appear to be at increased risk of gastrointestinal morbidity.329 The use of supplemental beam radiation in the setting of IBD has not specifically been addressed, but data from patients treated with beam radiation alone suggest that it is not strongly contraindicated.
Patients with prior therapeutic pelvic radiation are presumably at higher risk for radiation-related complications. Reirradiation in other sites has generally been better tolerated than predicted radiobiologically, and it is likely reasonably safe in the setting of prostate cancer. Data regarding brachytherapy in previously irradiated patients is fragmentary, but suggestive that it is generally safe.330–332
Treatment Planning
Treatment planning refers to the determination of seed type, how many will be used, and where they will be placed. Planning and implementation has a significant effect on the likelihood of cure. Implants are typically planned from TRUS images of the prostate, taken at 5-mm intervals from base through the apex (Fig. 45-21). The patient should be in the lithotomy position, similar to that for the implant procedure itself (Fig. 45-22). It is important that the prostate is not deformed by excessive probe pressure (Fig. 45-23).
The most proximal image is considered the 0.0 plane or zero plane. It is located by visualizing the most proximal image or base, usually including a portion of the seminal vesicles. Images and source positions are typically specified by their caudal distance from the 0.0 plane. The most caudal images, through the prostatic apex, are frequently indistinct on transverse imaging but can be verified on sagittal imaging (Fig. 45-24).
Target Definitions
The gross target volume (GTV) is the prostate itself, as visualized on the TRUS images. The treatment margin (TM), is the perpendicular distance between the GTV and the CTV (Fig. 45-25). The treated volume is that enclosed by the prescription isodose. To start the treatment planning process, the prostatic margin (GTV) is identified on the TRUS images and transferred into a planning program. The margins appear fuzzy, especially at the apex, where the prostatic tissue blends into the nearly isodense pelvic floor musculature. When in doubt, it is probably best to err on the side of being too generous rather than too skimpy in identifying the prostatic borders. The goal of treatment planning is to deliver a cancercidal dose to the prostate, with a sufficient CTV to encompass the likely extent of extracapsular disease extension (ECE), keeping in mind the high incidence of early ECE even in patients with low PSA and GSs. ECE is nearly always limited to within 3 mm of the prostatic edge, so that a 3- to 5-mm TM should suffice (Fig. 45-26).
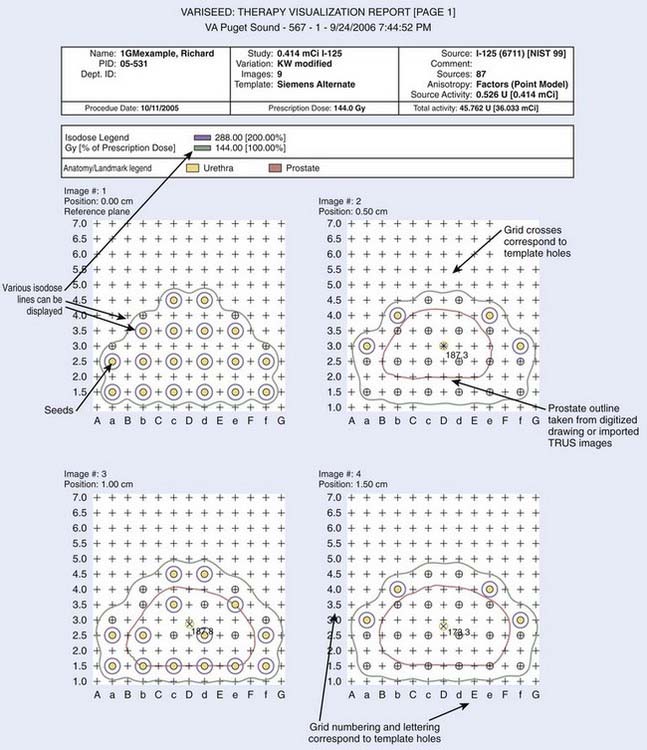
FIGURE 45-26 • Plan showing seed placement in the 0- to 1.5-cm transrectal ultrasonography planes for an iodine-125 implant by G. Merrick.
Unfortunately, achieving a sufficient postimplant TM is complicated by the substantial and unpredictable degree of implant-related prostate swelling. The prostate volume increases by approximately 20% by the completion of the implant procedure, but with a large degree of variability between patients.333 Predicting swelling in the individual patient has proven impossible, being unrelated to the prostate size or number of sources. To achieve adequate postimplant TMs, some allowance must be made for source placement error and implant-related swelling that continues over much of the active life of the isotopes. Adequate postimplant TMs are readily achievable using a heavily peripheral source placement, large planning TMs, and a central dose of 150% to 200% of the prescription dose.334 Achieving sufficient postimplant TMs is facilitated by placement of extraprostatic sources (Fig. 45-27).335 Before the availability of PSA screening, cancers were more frequently in the posterior-lateral regions. With the detection of PSA-screened cancers, all regions of the prostate are at risk of malignancies, so that adequate coverage of posterior, lateral, and anterior prostatic margins is recommended.336
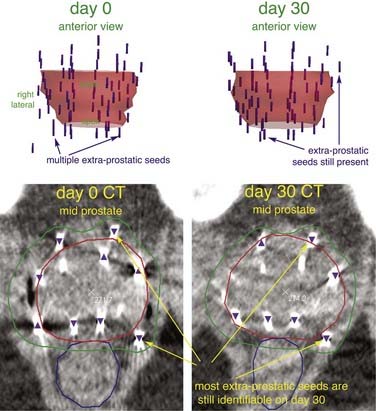
FIGURE 45-27 • Representative case from Nedea and colleagues335 showing three-dimensional views (top), and midprostatic computed tomography images on day 0 and day 30. This patient had 33 extraprostatic seeds (48% of 69 total seeds) and a V100 of 100% on day 0. He lost one seed between day 0 and day 30.
Early reports of correlations between central doses and urinary morbidity have not been well-corroborated in more recent series, probably because of adoption of peripheral loading patterns without the central dose extremes of early use of homogeneous placement patterns.314 A simple rule of thumb is to keep the urethral dose between 100% and 250% of the prescription dose, a goal that can be routinely met using modified peripheral source placement pattern, with the volume receiving 150% of the prescription dose (V150) typically 50% ± 20%.337
Much has been said regarding the importance of dose homogeneity, and detailed recommendations have been made regarding allegedly optimal V150, V200, and so on. In fact, the relationship between morbidity and dose homogeneity per se is unclear, at best. Homogeneity indices appear to be of little use, as long as guidelines for prostate coverage and urethral and rectal dose limitations are considered.338
Doses
Prescription doses for I-125 or palladium-103 (103Pd) monotherapy are typically 140 to 160 Gy or 110 to 130 Gy, respectively. It should be noted that monotherapy doses have been arrived at empirically, and can vary somewhat between practitioners. Also, the same nominal dose can be delivered with very different seed numbers and arrangement, depending on the design philosophy of the practitioners.308
When combining EBRT and brachytherapy, a wide variety of implant and beam radiation dose combinations are used. Used as a boost, implant doses are generally dropped to approximately 50% to 80% of monotherapy doses, ranging from 90 to 120 Gy with 125I and 60 to 113 Gy with 103Pd. Caution should be used when extrapolating combined brachytherapy and beam doses between institutions, because of differences in high-dose volumes and TMs. External-beam doses of 20 to 60 Gy have all been used extensively, with no clear evidence of superiority of one dose scheme over another.339
A wide variety of source strengths have been used for prostate brachytherapy, with no clinical evidence of any effect on outcome. 125I sources typically vary in strength from 0.2 to 0.9 mCi and 103Pd sources vary from 1.1 to 3.5 U (National Institute of Standards and Technology-99). Using higher activity sources reduces the number required. The only prospective clinical comparison showed better postimplant dosimetry with higher activity sources.340 However, when fewer seeds are used, each makes up a higher percentage of the total dose so that seed loss into the bladder or into the vasculature will have more effect on the dose. There is no evidence that end-to-end seed placement has any detrimental effect.
Preplanning versus Intraoperative Preplanning
In early versions of intraoperative treatment planning, the prostate size was estimated from preimplant TRUS imaging and the approximate number of sources needed was determined from a nomogram. At the time of the procedure, two thirds of the activity was placed at the prostatic periphery, and the remainder in the interior of the gland. More recently, faster treatment-planning systems allow intraoperative planning based on intraoperative image capture.341 The most touted advantage of intraoperative preplanning is better matching of the planning and implementation images, circumventing the potential problem of a discrepancy between the planning versus the intraoperative images some weeks later. However, in experienced hands such discrepancies are minimal and likely inconsequential. Reports of improved results with one technique over the other likely reflect evolving brachytherapy skill levels rather than a true advantage to one over the other. From a purely practical standpoint, intraoperative preplanning has some substantial disadvantages. It can increase operating time by 20 to 30 minutes or more, and requires a greater degree of physics support intraoperatively. In addition, it requires extra sources on hand, in case the intraoperative plan calls for more seeds than were anticipated.
Supplemental External-Beam Radiation
The use of supplemental beam radiation to cover the periprostatic prostate tissue has been widely practiced since the use of LDR iridium-192 (192Ir). In the TRUS era, the generally accepted policy has been to add EBRT for patients with a pretreatment PSA of more than 10 or a GS of more than 6. Although favorable tumor control rates have resulted from this policy, results with implant alone, even in patients with higher PSA and GSs, have been remarkably good in most series. Detailed pathologic studies have revealed that the radial extent of extraprostatic cancer extension, even in patients with higher PSA and GSs, is surprisingly limited and within reach of TMs achievable with a peripherally loaded implant. As such, the need for supplemental beam radiation is coming under increasing question. It is likely that for many patients supplemental beam radiation adds little other than increased morbidity and cost to a well-executed implant.342,343
Beyond the basic question of whether or not to use supplemental beam radiation, there are a dizzying menu of ways to combine brachytherapy and supplemental radiation, with little data to support one policy versus another. Basic issues, including sequencing, time gaps, isotope choice, and beam-field sizes, have been arrived at empirically.344 Optimal ways to combine radiation modalities should be a subject of future clinical investigation.
Anesthesia
Spinal (epidural) or general anesthesia are standardly used. Local anesthesia is a realistic option. It is accomplished with parenteral lidocaine is given in a 0.5% solution. Immediately following injection into the subcutaneous tissues, the pelvic floor and prostate are anesthetized by injecting lidocaine solution with small-gauge needles.345
Perioperative Corticosteroids
Prophylactically corticosteroids have been proposed as a way to decrease the likelihood of postoperative urinary retention. In prospective trials, corticosteroids did not appreciably alter the risk of retention.346
Patient Positioning
After induction of anesthesia, place the patient in the lithotomy position (see Fig. 45-22). Try to reproduce the positioning of the planning scan, realizing that there will usually be minor differences. The legs should be symmetrically rotated and extended at the hips and at the knees to avoid rotating the pelvis on the head-to-toe axis. In patients with PAI, the extended lithotomy position moves the pubic bones away from the anterior portion of the prostate.
Urethral Visualization
Most brachytherapists use a catheter or aerated gel to visualize the urethra during the procedure—a catheter is the simplest, most reliable method. Alternatively, aerated gel can be instilled through the urethra, using a penile clamp to keep it from leaking out (Fig. 45-28).
Transrectal Ultrasound Probe
TRUS frequencies of 5, 6, and 7 MHz are standard. Higher frequencies give better tissue visualization closer to the probe. The optimal MHz varies from patient to patient (Fig. 45-29). The probe should be covered generously with lubricant. A probe cover (condom) is used to facilitate postprocedure cleaning; water-filled covers are unnecessary. Transverse imaging alone is sufficient in most cases, but sagittal imaging helps visualize the prostatic base and apex and verify proper needle location and depth (Fig. 45-30). Biplane probes, capable of transverse and sagittal imaging, are standard in newer machines.
Stepping Unit and Stabilizer
The TRUS probe is supported on a stepping unit, allowing it to be moved caudal or cephalad at 0.5-cm intervals. The unit should hold the probe steady, without obstructing access to the perineum (Fig. 45-31). An easily adjustable stepping unit (and stabilizer) facilitates the procedure, and is well worth the initial investment. The stepping unit is supported by a stabilizing table-mounted or floor-mounted stand. Table-mounted stands are typically lighter, and move with the procedure table if the table position is changed during the case. They also tend to allow more foot space in front of the table, which can be a major advantage at teaching institutions.
Needle Insertion
Determining needle depth is as important to proper source placement as is correct positioning in the transverse images. Proper depth is typically determined by inserting the needle until it is viewed on the desired plane. When the tip of the needle is exactly at the same plane of the probe, rotating the needle generally allows two distinct lines to be seen, corresponding to the point at the bevel of the needle (Fig. 45-32). If in doubt, sagittal TRUS imaging or intraoperative fluoroscopy can be helpful (Fig. 45-33).
Isodose Overlays
The simplest way to evaluate an implant is visual inspection of the isodose overlays on the prostate (Fig. 45-34). Look at the location and size of underdosed regions. Scattered, small cool areas in the setting of a V100 of 90% or higher are fine. However, an implant with a V100 below 80% to 90%, or with a single large cool area should be considered for “touch-up.”
Quantifying Prostate Coverage
The simplest index to quantify prostate coverage is the V100, or the percent of the postimplant prostate volume covered by the prescription dose. Roy and colleagues first used postimplant CTs to calculate V100s, reporting typical values of only approximately 80%.347 It later became apparent that lesser values were due primarily to variable degrees of implant-related prostate swelling. In the past, V100s of 80% to 95% were typical among even experienced implant teams.348
Prostate Swelling
Postimplant dosimetry is complicated by prostate volume increases by 20% or more during the implant procedure, with substantial variability between patients.333,349 Implant-related swelling increases the prostate volume on which postimplant evaluation is based, making target coverage inferior to what would be determined if it was based on the smaller, preimplant prostate size. Target coverage decreases by 5% to 20%, depending on what indices are used. One way to account for the degree of swelling is to base the postimplant dosimetry on the preimplant volume, registered with the postimplant source locations. Doing so may be the most realistic way to assess how well the preplan was executed. But such a solution would likely be of little clinical utility because much of the dose will be delivered while the prostate is still larger than the pretreatment volume. Some have argued in favor of waiting 1 month to obtain postimplant dosimetry films, to allow implant-related swelling to resolve. The practical limitation of such a policy is that if target coverage is determined at that time to be inadequate, adding sources so long after the initial procedure would probably be radiobiologically suboptimal. Elegant formulas have been devised to account for the degree of swelling resolution over time. However, correction factors are impractical in clinical practice because of inconsistent edema resolution rates among patients.
Monitoring Treatment Response
PSA has dramatically decreased the time to detect cancer persistence, rendering routine follow-up digital rectal examination nearly obsolete. In the absence of a rising PSA, repeat prostatic biopsies rarely show residual cancers, and palpable tumor recurrence in the absence of a rising PSA is virtually unseen.350 Although still a subject of controversy, postimplant nadir PSA correlates with the likelihood of long-term biochemical control. Although there is no single nadir PSA value that guarantees against late biochemical failure, the lower the better. Because the great variability in the rate and consistency of PSA declines after brachytherapy, the optimal time to allow for nadiring to occur is unknown—some patients nadir at 6 months, whereas others take 6 years. The rate of decline is highly variable and has not, in itself, been correlated with prognosis. In the postbrachytherapy setting, any relation between the rate of decline and prognosis is made especially murky by the occurrence of temporary PSA rises.351
Temporary PSA rises, also referred to as PSA bumps, blips, or spikes, occur typically between 6 and 36 months after implantation352 (Fig. 45-35). The magnitude of most temporary rises is less than 2 ng/ml, but rises as high as 10 ng/ml or more are seen.353 If PSAs are taken at the typical 4- to 6-month intervals and any increase is considered, about one third of patients will experience a spike or bump. Their likelihood is independent of pretreatment PSA, GS, isotope, or use of supplemental EBRT. Some relationship to intraprostatic higher-dose regions and age may exist.354 The long-term prognosis for PSA-spike patients appears similar to patients without a spike.
Post-Treatment Biopsies
Postradiation biopsy has been used sporadically to assess intraprostatic tumor eradication. Patients with histologically viable tumor have a greater likelihood of developing local recurrence and distant metastases. Postimplant biopsies are conceptually appealing, but their routine use is limited by ambiguous interpretation and the fact that biopsies are almost always negative in patients with a low postradiation PSA.355 The clinical dilemma of a rising PSA in the first 3 postimplant years is made doubly confusing when a postimplant biopsy in the same period is positive, because biopsies may take 2 years or more to revert to negative.356
Suture-Mounted Sources
Suture-mounted 125I sources (RAPID Strand) are mounted in polyglactin 910, which dissolves in 1 to 3 months. Suture-mounted sources are more likely to maintain their position, and should decrease source migration. Theoretically, they could allow for wider periprostatic coverage, but their true clinical benefit is unclear. There is not a clear consensus of superiority of loose versus stranded sources.357 Newer versions of stranded or linked seeds are being introduced, but their clinical benefit is unclear.
Morbidity after Permanent Implants
Despite the assumption by many physicians and prospective patients that highly localized brachytherapy will result in less treatment-related morbidity, brachytherapy typically causes more marked urinary symptoms, at least in the first 6 to 12 postimplant months. Fortunately, nearly all implant-related symptoms resolve spontaneously, with patients returning to their baseline AUA scores within 1 year.314 Prophylactic institution of alpha-blockers substantially decreases the intensity and time-to-resolution of radiation-related symptoms.358
Although spontaneous resolution of radiation prostatitis is the norm, there is some chance of more serious complications, including superficial urethral necrosis (SUN) and incontinence. SUN, which is associated with severe dysuria and urinary incontinence, is a rare complication and likely results from extreme urethral doses.320
Reports regarding the incidence of postimplant urinary incontinence are mixed, with rates dependent on the measurement instrument, follow-up instrument, and implant technique. Since the widespread adoption of modified peripheral loading, frank urinary incontinence is unusual.314
Urethral stricture is an uncommon complication of modern brachytherapy, probably related to excessive apical radiation doses.337 When stricture does occur, it typically is successfully managed with one or more urethral dilations.
Proctitis
Some degree of subclinical postimplant radiation proctitis is almost inevitable, given the proximity of the prostate to the rectum. Symptoms, if they occur, usually resolve within a few months. In earlier series, rectal bleeding occurred in 2% to 10% of patients, and nearly always manifests between 6 and 18 months of implantation.359 It is partly related to rectal wall doses, and is more common in patients with lesser amounts of perirectal fat. Endoscopy typically reveals a circumscribed area of intense erythema, telangiectasias, and friability on the anterior rectal wall, overlying the prostate. Rectal ulceration, occurring in less than 1% of patients, is a more ominous complication that may lead to temporary or permanent colostomy. Rectal ulceration or fistula are exceedingly uncommon, providing that the volume of rectal wall receiving the prescription dose is kept below 0.5 cc on day 0 or 1 cc on day 30 dosimetry.360 The role of supplemental-beam radiation in rectal ulceration is probably minor, providing the implant-related rectal doses are limited.
Most patients who develop bleeding after EBRT or brachytherapy do not progress to rectal ulceration or fistula. Given enough time, most will heal spontaneously. However, healing is typically slow, sometimes requiring years. In contrast to medical therapies, more invasive therapies with electrocoagulation, laser, argon plasma coagulation, or topical formalin have been highly effective therapy for bleeding from radiation proctitis, with what appears to be a low likelihood of complications.361 Invasive therapies, however, might exacerbate radiation damage, so they should be undertaken with caution. Patients who undergo rectal wall biopsy in the course of evaluation for implant-related proctitis may be at higher risk of fistula formation. It appears that spontaneous healing of fistulas is unlikely, even with a diverting colostomy. Data regarding their surgical repair is limited, and an optimal management policy has not been determined.
Quality-of-Life Methodology
Partly in reaction to past inconsistencies in morbidity reports, there is a growing effort to quantify morbidities with far more detailed survey instruments, attempting to make lifestyle effects more uniformly reported. Although admirable in their intent, such efforts have culminated in scores like urinary bother, functional well-being, or EPIC scores, indices so nebulous that they are almost meaningless to patients and physicians. Although more standardized approaches to QOL measures are warranted, some degree of common sense seems to be in order. Making matters worse, brachytherapy-related sexual symptoms, especially, are different in nature to those of prostatectomy or even beam radiation, and are not accurately portrayed in generic QOL scales.362
Sexual Dysfunction after Brachytherapy
Despite some early claims, brachytherapy, like surgery and beam radiation, carries a high risk of short- and long-term sexual dysfunction. Although most discussion of treatment-related sexual dysfunction has focused on impotence, there are a variety of sexual dysfunctions following brachytherapy, not even mentioned in popular QOL scales.363 Patients commonly experience burning with orgasm (orgalmasia) in their first 6 to 12 postimplant months. Bloody ejaculate is very common in the first several weeks after implantation, and patients commonly report a diminished amount of ejaculate fluid. Intermittent hematospermia may occur for years after implantation. Approximately 10% of patients develop impotence immediately after their implant procedure, presumably caused by needle trauma to the NVBs or venous bodies. Patients with acute postimplant impotence may spontaneously improve with time.
As with EBRT, there is some evidence that potency loss may be ameliorated by limiting dose to the proximal penis. Merrick and colleagues found no relationship between NVB doses and impotence among patients followed up to 4 years posimplant.364 In contrast, radiation-induced impotence may be related to venous insufficiency from damage to the proximal corpus spongiosum, or bulb, of the penis. Separated from the prostate by the striated urethral sphincter, the bulb typically lies 0.5 to 1 cm below the prostatic apex and is fairly well visualized on CT, TRUS, or MRI.365 The bulb typically receives a maximal radiation dose of approximately 50% of the brachytherapy prescription dose. Because of the proximity of the bulb to the prostatic apex, excessive radiation dosage has been fairly common. Increased effort to limit proximal penile doses in the planning and implementation processes may result in a lower likelihood of implant-related impotence.
Potters et al.366 reported their experience with 482 men who were potent prior to undergoing brachytherapy for clinically localized prostate cancer. With a median follow-up of 34 months and a median age of 68 years, the 5-year actuarial potency rate was 76%, 56%, and 52% for brachytherapy alone, in combination EBRT and brachytherapy, with neoadjuvant androgen deprivation, respectively. However, only 29% of patients treated with brachytherapy, EBRT, and neoadjuvant androgen deprivation maintained potency. They reported that 62% (52/84) of patients treated with sildenafil responded successfully, with a higher percentage responding if not treated with neoadjuvant androgen deprivation.
Low-Risk Patients (Prostate-Specific Antigen < 10, Gleason Score ≤ 6)
Multiple series of patients treated with 125I or 103Pd alone, with median follow-up passing 10 years, show actuarial biochemical control rates are in the 70% to 90% range, comparing favorably with those reported from surgical series. To date, there is little evidence of excessive late failures beyond 10 years.367 Retrospective studies generally show that low-dose-rate brachytherapy compares favorably to competing modalities in terms of cancer eradication. That brachytherapy drives post-treatment PSA values to lower levels is a hint that longer-term studies will show superiority over beam radiation (Fig. 45-36).368
Higher-Risk Patients
Like low-risk patient groups, there are a rapidly increasing number of reports showing high biochemical cure rates using brachytherapy-based treatment for patients with a PSA of 10 to 20 ng/ml or a GS of 7 or more.369 The most remarkable aggregate finding is a relatively high percentage of patients who appear to be cured, evidenced by flattening of the freedom-from-failure curves past the 2-year follow-up period.
Outliers
The majority of brachytherapy reports have been surprisingly favorable. But like any modality, there are scattered reports to the contrary. The most publicized outliers for prostate brachytherapy are reported by D’Amico and colleagues.311 Poor results, far inferior to the norm, likely reflect poor technique or poor patient selection rather than inherent flaws in the modality. Unfortunately, aggressive publication of inferior outcomes may lead the casual observer to conclude that poor results are representative of other practitioners. Caution should be exercised in concluding that other therapies (hormonal or supplemental-beam radiation) are needed, when proper implant technique alone is likely to improve cancer eradication rates.
Implants as Salvage Therapy
There is substantial interest in the use of brachytherapy as salvage for patients with local tumor persistence after EBRT. A phase I and II trial is underway within the RTOG to test the safety of this option. Optimistic reports regarding salvage brachytherapy for failed external-beam patients have appeared. Grado reported on 49 patients salvaged with either 125I or 103Pd between 1990 and 1996. Biochemical freedom from failure in salvaged patients was 33% at 5 years, similar to that reported in surgical salvage series.331 In a similarly optimistic publication, Beyer reported 17 patients treated with reduced dose 125I or 103Pd salvage implants with an overall freedom from second relapse of 53%.330 Similarly, investigators from UCSF have also reported promising results with so-called salvage brachytherapy.332 In contrast to salvage prostatectomy, the reported risk of incontinence has been relatively low after salvage brachytherapy in these series. However, not all series have been so optimistic.370 In the Harvard MRI-guided series serious complications occurred in up to 30% of patients. Thus, although some published results with salvage brachytherapy are mildly encouraging, caution is in order, primarily because dosimetric details are missing from both reports. Simply stating the prescription dose of a salvage implant does not tell enough about the implant technique to safely duplicate it at other centers. The TMs used in the planning process, for instance, might have a substantial effect on the true dose to adjacent normal tissues. More meticulous information regarding salvage planning parameters is needed. Use of brachytherapy in the postprostatectomy patient would be considered unconventional.
Temporary Implants (High-Dose-Rate Brachytherapy)
Prostate brachytherapy has been the focus of brachytherapy development and has enjoyed many significant clinical and technical advancements during the last decade. The TRUS-guided implant technique is the backbone of modern prostate brachytherapy. Whether it is permanent or temporary, high-dose-rate (HDR) or LDR, each type of modern prostate brachytherapy uses a similar image-guided technique to transperineally insert seed-bearing needles or after-loading catheters. This approach is based on the original technique described by Holm and colleagues in 1983.305 Using ultrasound guidance, a real-time image of the implant volume can be seen and the position of each implant needle can be adjusted to ensure accurate placement. This method leads to improvements in the quality and reproducibility of prostate brachytherapy and has resulted in a resurgence of interest in this form of treatment.
Techniques of High-Dose-Rate Prostate Brachytherapy
Treatment of prostate cancer using HDR brachytherapy has been reported in the literature by various authors.371–373 Different techniques have been described. The treatment can be divided into three steps: (1) Insertion of the implant catheters, (2) treatment planning, (3) delivery of the treatment. As new equipment and software became available, improved techniques were increasing possible.
Insertion of Implant Catheters
Since the original description of the ultrasound guided technique by Holm and colleagues in 1983,305 many customdeveloped implant systems are now available on the market from various commercial vendors. TRUS allows the brachytherapist to see the prostate in real time during the implant procedure. This improved the accuracy, reproducibility, and ease of prostate implant.
Flexible cystoscopy is used to evaluate the depth of insertion of the implant catheter and to ensure the catheter is not erroneously inserted into the urethra or the bladder.372 During the cystoscopy, the scope is advanced so the tip of the scope is curved and deflected by the dome of the bladder, so the scope is retroflexed to visualize the bladder neck (Fig. 45-37). Catheters around the bladder neck can then be advanced under direct visual guidance until tenting of the bladder mucosa is produced by the tip of the catheter. Newer cystoscopes can be completely retroflexed on itself, without having to be deflecting from the dome bladder. Flexible cystoscopy is also useful for identification of accidental urethra puncture by the catheter. This often occurs below the level of urogenital diaphragm and frequently is not seen on the ultrasound image. It is not clear if puncturing the bladder neck can lead to more morbidity. Some brachytherapists advocate routine insertion of the implant catheter into the bladder to avoid underdosing the base of the prostate.374
Catheters are typically inserted using a prefabricated template under ultrasound guidance. Many types of prefabricated implant templates are available for temporary prostate implant. These templates are usually made out of semiflexible plastic or metal. All of these templates come with predetermined patterns of catheter insertion holes. These holes were designed to facilitate the insertion of the catheters or needles in a parallel and evenly distributed fashion throughout the implant volume. The template also serves as a fixation device that allows catheters to be secured to the patient and to maintain the geometric relationship between the catheters, and the target once the implant is inserted. Interfractional cephalad-caudal needle movement has been reported.375–377 Martinez et al.375,376 reported a mean displacement of 20 mm between the first and the second fraction measured using fluoroscopy. They reported the needle movement decreased between the subsequent fractions to an average of 4 mm. Damore et al.,377 using measurements obtained from plain films prior to treatment, reported a mean displacement of 7.6 mm and a maximum displacement of 28.5 mm. Interfractional needle movement poses an important challenge to prostate HDR brachytherapy.
The mean number of catheters per prostate implant reported range from 6 to 22.371,378,379 For Dinges et al.,380 the aim of not having more than 135% of the nominal dose to a volume of 10 cc can only be achieved with 20 or more catheters. On the other hand, a very low number of catheters (5 to 7) can result in up to 200% of the nominal dose being delivered to a 10-cc volume.381 For Mate et al.,382 18 to 22 needles enable 90% of the target volume to be encompassed by 100% isodose. Kini et al.379 implanted 14 to 18 needles and noticed that they got better apical coverage if a larger number of needles were implanted, but they didn’t apply this to their patients for fear of increasing the complication rate. Several authors implanted approximately 15 needles.371,383,384 Charra-Brunaud et al.385 analyzed the effect of increasing number of catheter on dosimetry. Results of the study suggest there should be a minimum of 15 catheters for an average-size prostate. Minimizing the number of catheters used may decrease patient discomfort and the risk of complication.
At UCSF we have developed a free-hand, ultrasound-guided technique that uses a custom mold made using dental putty for immobilization of the catheters. Instead of keeping all the catheters parallel to each other, our free-hand technique emphasizes the positioning of the catheter based on the target volume seen on the ultrasound. We typically place 12 catheters just inside of the TRUS-defined prostate capsule and four catheters around the urethra (Fig. 45-38). We routinely advance the posterior row of the catheter into the inferior portion of the seminal vesicles to improve coverage at the base of the prostate and add margin for needle migration (Fig. 45-39). Before starting the procedure, we place a friction collar on each catheter. After the catheters are inserted, we advance the friction collar to within approximately 1 cm from the skin surface. We use a grid to temporarily hold the catheters while applying the dental putty (Reprosil Type I, very high viscosity). We separate the catheters into two groups by dividing them at the median raphe. This allows each group to move independently and minimizes the risk that the template will be displaced because of its bulk. Once the putty is hardened, the grid is removed. The putty is then tightly secured against the perineum using sutures. Fig. 45-40 is an illustration of the procedure. We found the customized fit provided by this technique improves patient comfort and minimize catheter displacement between treatments.386
Treatment Planning
Treatment planning techniques can be accomplished using ultrasound images, x-ray films, CT scans, or MRI scans. Ultrasound-based systems have the capability of delivering the treatment in real time.371,387 Based on this approach, only one treatment is commonly given per implant. Currently the software for an ultrasound-based planning system is not commercially available. Martinez et al.375,388,389 have reported their clinical experience using this method. For an x-ray based system, localization of the implant catheters and volume of interest are based on orthogonal x-ray films, fluoroscopy, or CT images.372,383,390,391 HDR brachytherapy treatment planning software has significantly improved in recent years; 3-D planning can be done very quickly, minimizing the delay between localization and treatment.
Similar to conformal EBRT, CT- or MRI-based HDR 3-D brachytherapy planning systems are now commercially available. Because of the intrinsic dose heterogeneity and proximity of high dose to normal structures, accurate control and documentation of dose distribution is especially important for brachytherapy. Using CT- and MRI-compatible catheters, the position of catheters can be identified by following the air column or the CT-compatible dummy markers in the catheter (Fig. 45-41). As a result, during the localization process, extra attention should be given to identify the end of the catheter. The thickness of the image slices should be kept to a minimum to decrease the reconstruction error. Structures of interest can be contoured directly on the treatment planning system.392 Marker seeds placed at the time of implant, a Foley catheter, and contrast material placed in the bladder can be used to assist the planning process.
Once all the structures of interest are digitized into the treatment planning system, the appropriate dwell time at the selected dwell position must be determined. Various approaches have been described. In general, the process involves using an optimization algorithm with or without additional manual tweaking. Regardless of the approach, the basic goal of treatment planning is to ensure the target volume is covered by prescription dose. The simplest approach is to place dose points along the target contours and prescribe to the dose points. However, this approach can produce a large volume of high-dose regions within the treatment volume. To minimize the dose heterogeneity, dwell position–based optimization programs such as geometric optimization (GO) algorithms have been introduced. GO selects the dwell time of a particular dwell based on the sum of the square distances to other dwells.393,394 GO allows a more homogenous dose within the implant volume and thus better coverage of the target volume.395
An anatomy-based inverse planning simulated annealing (IPSA) optimization software has been developed at UCSF.396,397 IPSA allows the optimization process to account for multiple target structures and organs at risk based on preassigned priorities and multiple objectives simultaneously.398,399 This system is highly flexible and further improves coverage while minimizing the doses to normal structures.400,401 Clinical application of these treatment planning systems has significantly improved our work flow and may decrease the risk of treatment-related toxicities.391
Treatment Delivery
Various treatment fractionation schemes and normal tissue dose constraints for treatment planning have been reported in the literature (Table 45-8). The treatment protocols are a reflection of the available resources and philosophical approaches to a new treatment modality. Many programs started with higher number of fractions (>2) and aggressively hypofractionated treatments with a low number of fractions as they gained more experience and as the safety of such a treatment scheme was established. There are two general approaches to the number of fractions delivered per implant. If the procedure is done in the operating room, the number of implants performed per patient is often limited because of available operating room time. When the implants are done in the department, the number of implant procedures tends to be larger. Having the capability to deliver the treatment during the implant phase eliminates the problem of interfraction catheter displacement. However, this problem may be minimized if a new treatment plan is prepared prior to each fraction. Excellent results have been reported regardless of the treatment fractionation scheme and the number of implants used. It is unclear if there is a significant clinical difference when biological equivalent doses are used.
Table 45-8 Dose, Fractionation, and Constraints of HDR Brachytherapy Boost in Combination with External-Beam Radiotherapy
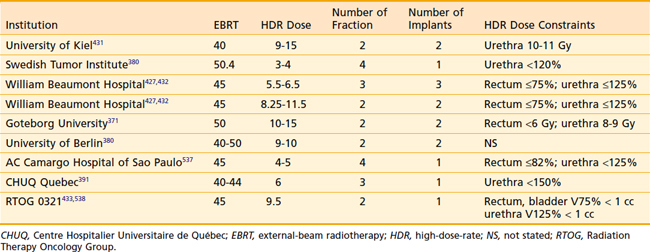
Different normal structure dose constraints and recommendations have been reported in the literature (see Table 45-8). Because the planning and delivery systems are quite different at each institute, these constraints may serve only as guidelines. These guidelines may not be appropriate if a different treatment protocol or treatment planning and delivery system is used.
Unique Aspects of High-Dose-Rate Prostate Brachytherapy
There are many reasons why HDR brachytherapy may be useful in the delivery of extremely conformal radiotherapy to the prostate. Hsu et al.402 compared the dosimetry data generated from 3-D conformal EBRT with HDR brachytherapy and showed significantly lower doses to rectal and bladder volumes with the latter. Besides the dosimetric advantages, there are other practical advantages of HDR brachytherapy compared with traditional LDR brachytherapy. Because the HDR treatment is delivered within a few minutes, similar to EBRT, and is always carried out in the shielded environment of the HDR treatment room, the patient does not need to be isolated between treatments and can stay in an unshielded room. There is no radiation exposure to hospital personnel or to the patient’s family members. In addition, it is well documented in the literature that the prostate can undergo significant change in its size after the permanent seed implant.333,349,403–409 As a result, there is some uncontrollable anatomic and biologic factors that may significantly affect dosimetry of permanent seed implants. Similarly, in EBRT the daily movement of prostate provides a challenging problem in dose escalation. Because an HDR implant is a closed system, there is no risk of seed migration and postimplant edema can be accounted for by performing dose calculation and treatment soon after the implant.
HDR prostate implants under TRUS guidance allows precise placement of the afterloading catheters and because of remote afterloading dwell time capability, the catheters have flexible spacing requirements. The ability to cover target volume using nonparallel catheters gives HDR brachytherapy the capability to treat prostate of almost any size without the problem of PAI.410 Having the source inside the catheter allows treatment of small cavities without the problem of seed migration. This gives HDR prostate brachytherapy the flexibility to treat ECE or involvement of the seminal vesicles.411,412 These capabilities make HDR ideal for dose escalation in treatment of locally advanced cancer (see Figure 45-41).
Radiobiology of High-Dose-Rate Prostate Brachytherapy
Clinical gains have been predicted and reported using alternative fractionation schemes in radiotherapy.413,414 The linear-quadratic model is the most commonly used method for estimating the clinical effects of alternative fractionation schemes. In this model, the response of tissue to altered fractionation is determined by the tissue’s alpha/beta ratio. In clinical practice, most tumors have a high alpha/beta ratio, with these tumors traditionally treated with standard fractionated or hyperfractionated radiotherapy. Conversely, tumors with a lower alpha/beta ratio can be treated effectively with higher dose per fraction or hypofractionated radiotherapy.
The alpha/beta value commonly used for acute responding tissue and most solid tumors is 10 to 12 Gy. Brenner et al.415 estimated the alpha/beta ratio for prostate carcinoma based on clinical data and their work has shown that prostate cancer has an exceptionally low alpha/beta ratio of 1.5 Gy. Recent studies have supported this finding.416–418 In a retrospective comparison of effect of fractionation using data from the HDR prostate dose escalation trial, Brenner et al.417 showed direct clinical evidence that suggest the alpha/beta ratio for prostate cancer is approximately 1.2. If this is indeed the case, then prostate cancer’s alpha/beta ratio is significantly lower than the alpha/beta ratio of dose-limiting structures around the prostate (3 to 5 Gy). This suggests there is a potential gain for treating prostate cancer using hypofractionated radiotherapy.419–421 Hsu et al.402 calculated this potential biologic dose gain based on the linear quadratic model and comparison of HDR dosimetry with conformal EBRT. They showed a potential increase of 7% to 64% in the biologic dose delivered to the prostate without an increase in the biologic dose delivered to the rectum using HDR boost. The effect of alpha/beta ratio on the biologic equivalent dose is illustrated in Table 45-9. It is clear from this study that if the radiobiologic hypothesis is correct, hypofractionation radiotherapy may indeed be a more effective form of radiotherapy for prostate cancer and when optimally used may result in fewer side effects.
Clinical Results of High-Dose-Rate Prostate Brachytherapy Boost
HDR prostate implant has been used as a boost in conjunction with EBRT. Typically this involves 4 to 5 weeks of EBRT treatment with one or multiple implants at the beginning, sandwiched between, or at the end of EBRT. Patients selected for the combination treatment are generally those with intermediate- or high-risk disease who are most likely to benefit from the higher doses that are possible when delivered using an implant. The technique of HDR prostate brachytherapy has been in clinical practice since the 1980s.371,375,380,383,422–430 Kovács et al.425,430 and Galalae et al.431 reported one of the earliest experiences using HDR brachytherapy boost at University of Kiel. Patients treated were mostly T2b-T3, G3, receiving a combination of split-course EBRT to 40 Gy to the prostate and two 15-Gy HDR treatments to the peripheral zone of the prostate. An 18% positive biopsy rate 18 months after treatment was reported. The results were updated with a median follow-up of 8 years.431 The disease-free survival rate was 82.6% and biochemical disease-free survival was 72.9% for 144 consecutively treated patients. An incidence of 2.3% RTOG-EORTC late G3 cystitis, and 4.1% G3 proctitis was reported. A history of a TURP was associated with a high risk of genitourinary toxicity.
Mate et al.382 at Swedish Medical Center reported their experience with HDR brachytherapy. They used a more moderate hypofractionated schema with four treatments of 3 to 4 Gy fractions of HDR treatments combined with 45 to 50 Gy of EBRT. They recommend routine cystoscopy at the end of the implant procedure to ensure that the catheters are placed at the proper depth and to avoid injuring the urethra. Pretreatment patient characteristics were stage T1b to T3c, mean initial PSA was 12.9, and Gleason grade ranges were 3 to 9. They reported 84% 5-year biochemical disease-free survival, 6% urethral stricture, and 1% urethral necrosis. There were no significant gastrointestinal complications.
Stromberg et al.,424 Edmundson et al.,426 Kestin et al.,427 and Martinez et al.432 at the William Beaumont Hospital reported the only on-going prospective dose escalation trial using HDR brachytherapy as a boost. There have been multiple updates of their results.424,426,427,432 They continued to dose-escalate using increasingly large fractions of HDR treatment ranging from 5.5 to 6.5 Gy for three treatments to 8.25 to 11.5 Gy for two treatments combined with 46 Gy of EBRT. As of their most recent update, they showed acceptable toxicity levels using 11.5 Gy for two treatments. Patients with PSA of 10 or more, T stage of T2b or more, and GS of 7 or more were selected for the trial. Despite a high frequency of poor prognostic factors, the actuarial biochemical control rate was 74% at 5 years using the ASTRO definition. The 5-year actuarial rates of local failure and distant metastasis were 8% and 6%, respectively.
Borghede et al.371,422 at Goteborg University in Sweden reported their experience using 50 Gy of EBRT combined with two fractions of 10-Gy HDR boost. They used ultrasound to target tumor nodules within the prostate and gave an additional 5-Gy boost during each HDR treatment. This means that a total of 30 Gy in two fractions was delivered to the tumor nodule from the HDR brachytherapy. They reported a 4% positive biopsy rate at 18 months after treatment. Patients included in the study were T1-3 and grade 1-3. No HT was used in the study. After a median follow-up of 45 months, post-treatment PSA level of 1 or less was obtained in 84% of the patients. Borghede et al.422 reported RTOG-EORTC G3 late effects of 4% for dysuria, 6% with urinary frequency, 8% with diarrhea, 2% with proctitis, and 12% with impotence, but no G4 late effects were reported.
Dinges et al.380,428 at University of Berlin reported their experience combining 40 to 45 Gy of EBRT with a 9 to 10-Gy HDR boost. Out of 82 patients with a median follow-up of 24 months, local control rate was 79.5% at 2 years. Dinges et al. reported that acute effects were similar to EBRT. Out of the 82 patients treated, they reported six with RTOG-EORTC G3 acute side effects. There were 13 (16%) with G3 late side effects. This included seven patients with urethral strictures and six patients with incontinence. Three patients (4%) developed G4 rectourethral fistula requiring colostomy after biopsies from the anterior rectal wall.
Table 45-10 summarizes the results of HDR brachytherapy boost in combination with EBRT. The results from these single-institutional clinical trials have shown the technique of HDR brachytherapy for prostate cancer is feasible with minimal morbidity. RTOG recently completed the first multi-institutional prospective trial using HDR boost. The preliminary results from RTOG 0321 suggest the HDR boost is well-tolerated with low toxicity.433
HDR boost also compared favorably against EBRT alone. From the only reported prospective randomized HDR trial, Hoskin et al.434 reported on 220 patients randomized to the external radiotherapy of 35.75 Gy for 13 fractions and HDR boost of 17 Gy for 2 fractions. At a median follow-up of 30 months, there was significant improvement in biochemical relapse-free survival favoring the HDR boost group. There was also lower incidence of acute rectal symptoms favoring the HDR boost group. The HDR boost group also had a significant better Functional Assessment of Cancer Therapy–Prostate score at 12 weeks. This trial represents the first evidence of clinical benefit by dose escalation using HDR brachytherapy compared with external-beam boost. Unlike the results of dose escalation studies using EBRT, the high-dose arm (HDR boost) actually reported less toxicity.434
High-Dose-Rate Prostate Brachytherapy Alone (Monotherapy)
There are interests in the development of HDR monotherapy for patients with early-stage prostate cancer. Although this approach is theoretically feasible, there is very limited published data on this approach at this time and one should use this approach with caution. There are two groups that have published studies using this approach. Yoshioka et al.378,435 have reported their results on patients treated with HDR monotherapy. Their patient population included those with T1 through T4 tumors. Higher-stage tumors were treated with AHT and higher implant dose. A total of 43 patients were treated with eight to nine twice-daily fractions of 6 Gy for 5 days. With a median follow-up of 24 months, the 3-year clinical local control rate was 100% and the biochemical relapse-free rate was 55%. Acute grade 4 toxicity was reported in one patient (2%). Five patients had G1-2 late toxicity.
Martinez et al.375 reported on the initial results from their on-going prospective phase II monotherapy trial. The selection criteria included GSs less than or equal to 7, PSA less than or equal to 10, and T stage less than or equal to T2a. All patients were treated with four twice-daily fractions of 9.5 Gy for 2 days. This protocol was used with 41 patients, and all patients tolerated treatment well. The clinical results and late toxicity are still too early to be reported.
Another prospective phase II monotherapy trial was done at Mount Vernon Cancer Center. Three dose levels were tested: 8.5 Gy for four treatments, 9 Gy for four treatments, and 10.5 Gy for three treatments. Grade 3 bladder toxicity was seen in two patients at 6 months, one from each of the last two dose regimens. The early results suggest an excellent biochemical response and no difference in the acute and late toxicity between the three regimens.436 Ongoing HDR monotherapy trials suggest hypofractionated HDR brachytherapy is both safe and effective. Future studies are likely to continue to push toward fewer numbers of highly hypofractionated treatments. This will no doubt make HDR monotherapy a potentially accurate and convenient option for patients.
Neutrons, Protons, and Heavy Charged Particles
Heavy particles (e.g., neutrons), charged particles (e.g., protons) and heavy charged particles (e.g., carbon or neon) are alternative forms of EBRT. The theoretical advantage of neutron irradiation relates to its relative lack of dependence on the presence of oxygen, and its relative resistance to the repair of sublethal radiation-induced damage. An early prospective randomized trial showed that fast neutrons were associated with a lower clinical local failure rate and PSA failures compared with x-ray therapy and an improvement in survival.437 The results were questioned, however, because the patients on the photon-only arm had larger tumors and a worse survival than expected based on other contemporary series of the time.
The major theoretical advantage to the use of protons and other charged particles is the dose distribution associated with this type of radiation. The first randomized trial demonstrated that there was an increase in local control only in the subset of patients with high-grade tumors.438 In the second trial Zietman et al.439 compared 70 with 79 Gy in patients with a PSA lower than 15 ng/ml, most of whom were low-risk and confirmed the dose-response data reported in other trials.440–443 The unique features of this trial include evidence of a dose response for low-risk patients and the failure to see increased toxicity in the high-dose arm of this study. Despite this trial, however, there is no reason to believe that proton beam–based treatments should render superior outcomes to x-ray–based therapy when similar doses are delivered. This assertion is based on the fact that there appear to be no obvious dose distribution advantages versus IMRT using this approach.444 There are in fact reasons to believe that well-delivered brachytherapy renders comparable or better PSA control rates and lower PSA nadirs, an endpoint associated with long-term control.368
Heavy charged particles are thought to have the advantages of both neutrons and protons. Tsuji et al.445 reported their experience with hypofractionated conformal carbon ion radiotherapy for localized prostate cancer including 201 patients treated with the dose-fractionation regimen established during three clinical trials. No grade 3 or higher toxicities were observed in either the rectum or genitourinary system, and the incidences of grade 2 rectum or genitourinary morbidity were only 1% and 6%, respectively. They noted an overall 5-year biochemical relapse-free survival rate of 83.2% without any local recurrence and 97.1% for patients with GSs of 7 or lower, with an initial PSA lower than 20 ng/ml.445 Longer follow-up will be required to assess the effect of these alternative types of irradiation on survival.