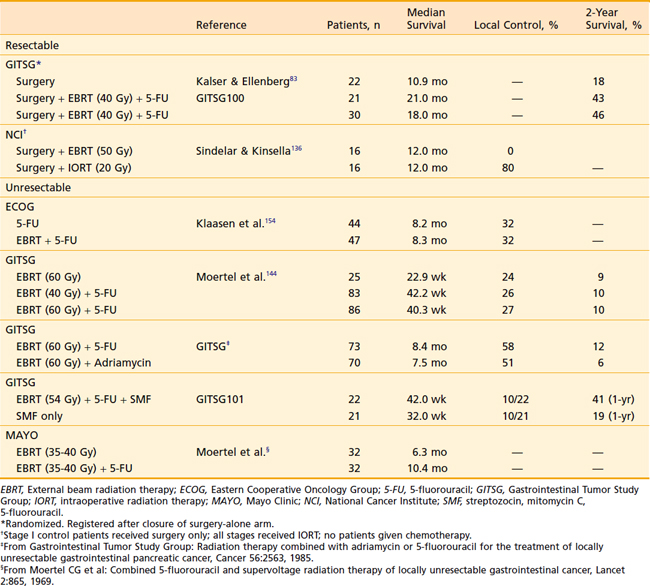
38 Cancer of the Pancreas
Epidemiology, Causal Factors, Genetics, and Cytogenetic Abnormalities
Pancreatic cancer is the fifth leading cause of cancer mortality among men and women of all ages; it strikes an estimated 37,680 Americans annually and caused 34,290 deaths in 2008.1 A National Cancer Institute (NCI) survey covering 1973 to 1988 showed less than 20% of affected patients survived 1 year and only 3% were alive 5 years after diagnosis.2,3 Even with the addition of adjuvant radiation and chemotherapy as well as recent decreases in surgical morbidity, only very modest improvements in survival have been realized. Worldwide, the reported incidence varies considerably. It is unclear whether this represents a true phenomenon or whether it is a reflection of more meticulous reporting patterns in more industrialized countries. In the United States, the incidence of pancreatic cancer is 9 in 100,000 in whites and 15.2 in 100,000 in blacks with a male-to-female ratio of 1.3 : 1. Diagnosis is rare before age 45 but rises sharply thereafter.2 Although the cause of pancreatic cancer is unknown, several environmental factors have been implicated. Smoking, in particular, raises the relative risk 1.5 times.4 Diets high in meat or fat also have been linked to increased risk, and a diet of fresh fruits and vegetables has been found to be protective.5 An increased incidence also is found with a prior history of surgery for peptic ulcer disease.6
Much controversy was generated in the early 1980s with a report linking pancreatic cancer with coffee consumption,7 but subsequent prospective studies have failed to confirm this.8 Both diabetes mellitus7,9 and chronic pancreatitis10 are associated with pancreatic cancer, but it is unclear whether this is a cause-and-effect relationship. Workers employed in manufacturing 2-naphthylamine, benzidine,11 and gasoline12 are reported to have a fivefold increased risk. More recently, the study of deoxyribonucleic acid content, oncogenes, and molecular biology has yielded intriguing data.13 Molecular histology data have been nicely summarized by Hruban.14 More and more evidence paints a picture of pancreatic cancer as a disease of acquired and inherited mutations. It was not until very recently that an international system of nomenclature was adopted that reflected the development of pancreatic ductal carcinoma as a continuum analogous to changes seen in the development of cervical carcinoma.15 Pancreatic intrepithelial neoplasia 1-3 is used to grade noninvasive early changes of pancreatic ductal neoplasia. Tumor suppressor genes regulate cell proliferation and their inactivation can play a profound role in the development of malignancies. There are four specific genes identified as crucial in the development of pancreatic cancer: p16, p53, DPC4, and BRCA2. An increased incidence of pancreatic cancer has been seen in families with the breast cancer-associated BRCA2 gene.16 Oncogenes become active by mutation or amputation. An example is the k-ras oncogene, which is often activated in human pancreatic duct carcinomas.17–21 Data from Almoguera and colleagues21 demonstrated that 95% of pancreatic adenocarcinomas contain k-ras oncogenes activated by a mutation at codon 12. This mutation is critical to oncogenesis and may mediate resistance to epidermal growth factor receptor inhibitors. Shibata and associates18 noted similar findings in 72% of patients undergoing fine needle aspiration. Kondo and colleagues22 also found specific mutations of the k-ras oncogene at codon 12 in 67% of pure pancreatic juice collected endoscopically in patients with proven pancreatic carcinoma. K-ras abnormalities were not detected in healthy controls, in patients with chronic pancreatitis, or in three patients with islet cell tumors.13
A summary of genetic abnormalities is provided in Table 38-1. The identification of consistent genetic abnormalities and the observation of higher cancer rates in some families were the genesis of the Johns Hopkins University Hospitals’ National Familial Pancreas Tumor Registry. Familial cases are defined as those in which two or more first-degree relatives are affected. In these individuals the risk extends to second-degree relatives who have a 3.7% chance of developing pancreatic cancer as opposed to 0.6% in second-degree relatives of sporadic cases.23
| Inactivated/Suppressor Genes | Frequency Seen, % |
|---|---|
| K-ras | >90 |
| p53 | 50-70 |
| p16 | 95 |
| MKK4 | <10 |
| DPC4 | 50 |
| BRCA2 | 70 |
Anatomy
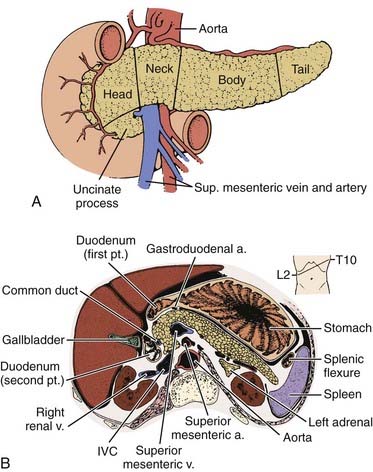
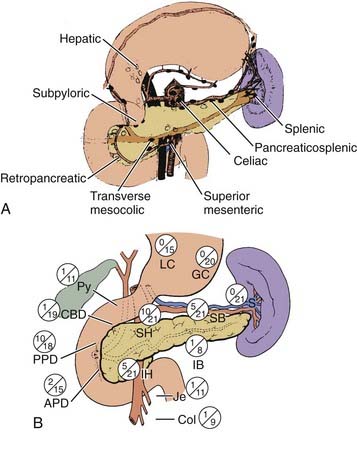
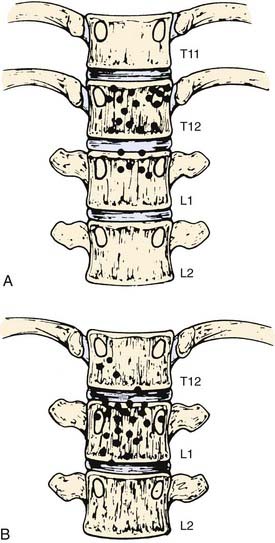
The pancreas is an elongated, coarsely lobulated gland lying transversely and retroperitoneally in the posterior abdomen at approximately the L1 to L2 level (Fig. 38-1A). The head lies in the duodenal flexure on the right and the tail extends to the spleen. The pancreas is divided into head, uncinate process (considered to be part of the head), neck, body, and tail. Tumors arising to the right of the superior mesenteric vein (SMV) are considered to be in the head, those arising to the left of that vein and left border of the aorta are part of the body, and those arising from the tail are located between the left border of the aorta and the hilum of the spleen. The pancreas is in close contact with surrounding organs including the spleen, stomach, duodenum, jejunum, and kidneys, all of which may be involved with tumor early on (see Fig. 38-1B). A rich lymphatic network surrounds the pancreas, including the celiac, superior and inferior pancreaticoduodenal, superior mesenteric, porta hepatis, and pancreaticosplenic nodes (Fig. 38-2A). With posterior extension of tumor, the lateral aortic nodes are also at risk. The main venous drainage of the pancreas is via the portal system to the liver. Tumors of the head of the pancreas often cause jaundice caused by invasion or compression of the common bile duct.
Pathologic Conditions
The most common type of pancreatic cancer is of ductal origin, comprising from 75% to 90% of patients.24 It is twice as common in the head as in the body or tail. Perineural invasion occurs in approximately 90% of patients, and in more than 85% of patients tumor has extended beyond the organ at the time of diagnosis. The most common extralymphatic sites of involvement are the liver and peritoneum; the lung is the most commonly involved extra-abdominal organ.
Less frequently occurring exocrine tumors, such as cystadenocarcinoma or intraductal carcinoma, are more common in women and may run a much more indolent course,25,26 remaining localized for many years and having up to a 50% five-year survival rate.27 Solid and cystic papillary neoplasms, also known as Hamoudi tumors, occur in women in their third decade of life, only rarely metastasize, and have a good prognosis. Rare acinar cell cancers are associated with fat necrosis and high lipase production and have a poor prognosis; they may be associated with a clinical picture that includes rash, eosinophilia, and polyarthralgia. Giant cell tumors, which account for only a small percentage of pancreatic cancers, are very large and aggressive and have a very poor survival rate.28 Clinical presentation of these less aggressive histologies, however, is indistinguishable from the more aggressive pathologic subtypes.25 Cancer metastatic to the pancreas is a relatively uncommon finding. In a series of 2587 consecutive autopsies at the Memorial Sloan-Kettering Cancer Center (MSKCC), 10% of patients were found to have metastases in the pancreas with the most frequent primary sites being breast, lung, or melanoma.24 The remaining 5% of pancreatic cancers are of endocrine origin and are discussed separately.
Clinical Presentation
More than 80% of patients present with pain, jaundice, or both29,30; these symptoms, along with weight loss, constitute a classic triad. Tumors in the body and tail may not cause jaundice or pain until far advanced; thus their prognosis is particularly poor,31 whereas periampullary cancers have a significantly better prognosis32 because of earlier clinical presentation. Tumors of the endocrine pancreas may present in a similar fashion but are often recognized because of various clinical syndromes produced by circulating polypeptides elaborated by the tumor.33 Rarely, presenting symptoms may be diabetes mellitus9 or acute pancreatitis.10 Infrequently, patients may present with migratory thrombophlebitis (Trousseau sign) or with a palpable gallbladder (Courvoisier sign). Abnormal laboratory tests, such as alteration in glucose, amylase, lipase, bilirubin, alkaline phosphatase, lactate dehydrogenase, or aspartate aminotransferase, may all be present but also are all nonspecific. The search for screening tests to allow for earlier diagnosis and, potentially, to effect cure has generally been elusive with the exception of the Cancer of the Pancreas 2 Screening Project. High-risk but asymptomatic individuals were assessed using endoscopic ultrasound (EUS), and the detection of premalignant and preinvasive lesions suggests that EUS may play a role in screening.34
Routes of Spread
Most pancreatic cancers are unresectable at presentation and thus there is sparse literature systematically analyzing patterns of failure. Cubilla and associates35 reported on the incidence of nodal involvement in completely resected early cancers of the head of the pancreas (see Fig. 38-2B) and showed that carcinomas in the head tended to spread to multiple nodal groups. In addition to initial spread to regional lymph nodes, early peritoneal seeding may be exceedingly common. A series from Massachusetts General Hospital (MGH) reports a 40% incidence of peritoneal studding in patients clinically considered to be resectable and who first underwent laparoscopy before definitive resection.36 In a patient who has undergone curative therapy, once the therapy fails, local recurrence again dominates the clinical picture.37,38 In a series of Griffen and associates,38 only 17% of patients developed disease purely outside the abdominal cavity (Fig. 38-3).
Diagnostic and Staging Studies
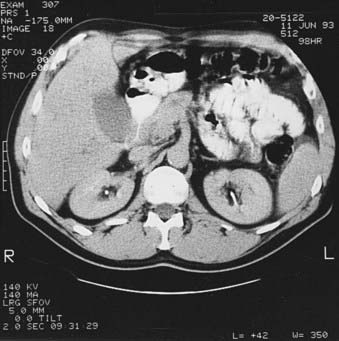
Preoperative staging of pancreatic cancer to detect resectability is extremely important to avoid unnecessary surgery. Table 38-2 reflects the staging system as revised in 2010, with the T classification distinguishing between potentially resectable (T3) and unresectable (T4) primary tumors. The stage grouping allows stage III to signify unresectable, locally advanced pancreatic cancer; stage IV is reserved for patients with metastatic disease. Pancreatic cancer is considered resectable if there is no portal vein or celiac axis involvement and no metastasis evident. Borderline resectability may include unilateral or bilateral SMV/portal vein impingement, less than 180° tumor abuttment, or encasement of the hepatic artery or SMV if the surgeon determines it is reconstructible.39 Magnetic resonance imaging (MRI), ultrasound, and computed tomography (CT) (Fig. 38-4) can detect pancreatic cancers as small as 1 cm. All three modalities easily detect dilatation of the pancreatic and bile ducts as well as liver metastases.40 Ultrasound can help distinguish between obstructive and nonobstructive jaundice, but CT is more helpful in providing definition of the tumor and surrounding structures. A clinical suspicion of pancreatic cancer or evidence of a dilated pancreatic duct warrants a pancreatic CT scan obtained via a defined pancreas protocol such as a helical or spiral triphasic crosectional CT with thin slices. The quality of modern imaging is so good as to predict resectabilty in 80% to 85% of cases when appropriate criteria are met.41,42 If cancer is fairly certain, the scan should include the chest to rule out metastatic lung disease. Positron emission tomography (PET) is considered a complementary study, as is EUS, which is of particular usefulness in the assessment of vascular invasion and thus resectability.43,44 Specifically, PET scan is helpful in characterizing equivocal lesions on CT and MRI, but is not helpful as a staging tool.45 Advances in technology have improved both the sensitivity and predictive value of cross-sectional imaging to such an extent that abdominal ultrasound, cholangiography, and angiography are no longer required for accurate staging. Percutaneous ultrasound or CT-guided biopsy can safely and easily provide pathologic diagnosis in approximately 90% of cases,46 but in the case of adenocarcinoma it may cause increased risk of local failure secondary to rapid seeding of tumor.47 A review from MGH48 showed a fourfold increased risk of positive peritoneal cytology in patients who had prior percutaneous fine needle aspirates. Some institutions thus advocate deferring preoperative biopsy in apparently resectable cases because histologic diagnosis is not required before surgery, although it is clearly required before embarking on neoadjuvant therapy or declaring a patient unresectable or having metastatic disease. Older literature suggested a benefit from treating patients already biopsied with low-dose (5 to 10 Gy) preoperative radiation in an attempt to decrease dissemination of tumor.49 This is now very rarely done and would not be considered standard. Endoscopic retrograde cholangiopancreatography (ERCP) is extremely useful in the differential diagnosis of tumors of the pancreaticobiliary junction (Fig. 38-5).50 Ampullary and duodenal cancers can be visualized and biopsied during ERCP; pancreatic duct stricture longer than 10 mm, especially if irregular, is more consistent with carcinoma than pancreatitis.51 Supportive serologic evidence of pancreatic cancer includes elevated levels of cancer antigen (CA) 19-9,52 carcinoembryonic antigen, pancreatic oncofetal antigen, tissue polypeptide antigen, and CA-125. A decrease in serial CA 19-9 levels has been found to correllate with survival; however, caution is warranted because of false elevations in the presence of benign biliary disease or obstruction or false negativity in individuals who are Lewis-a antigen negative. Unfortunately, no currently available marker is diagnostic or sufficiently sensitive or specific for screening purposes.53 Following serial levels of these markers, however, is very useful, and baseline and post-treatment levels of CA 19-9 do correlate with tumor response.54
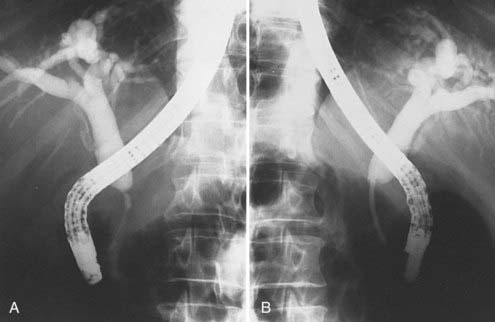
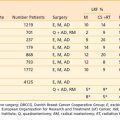
FIGURE 38-5 • Endoscopic retrograde cholangiopancreatography of the same patient shown in Fig. 38-4. A, Note thin ragged contour of the pancreatic duct—stenosis of the length (see text) is usually indicative of neoplasm. B, A stent has successfully been placed in the pancreatic duct to relieve obstructive jaundice.
Following this, in patients without prior abdominal surgery, laparoscopy may be used to rule out small hepatic or peritoneal metastasis. Laparoscopic ultrasound in conjunction with peritoneal cytologic findings significantly decreases the nontherapeutic laparotomy rate as positive peritoneal cytologic findings indicate advanced disease. Investigators at MGH prospectively evaluated 88 patients referred for curative resection using MRI and CT, angiography, and laparoscopy for staging. They preoperatively identified 89% of patients who could not have curative resection. These patients are best spared an operation unless duodenal obstruction is present.48 Excellent palliation for jaundice can be achieved with endoscopic placement of large stents.55 Diagnostic workup and tumor-node-metastasis (TNM) classification are outlined in Table 38-2 and Table 38-3. It should be noted that the TNM staging criteria include data obtained both preoperatively and postoperatively and that the American Joint Committee on Cancer also recommends that the surgeon assess the completeness of resection as R0 for complete resection with negative margins, R1 for incomplete resection with microscopically positive margins, and R2 for incomplete tumor resction with gross residual tumor.56 For practical purposes and to better understand the historical literature on the treatment of pancreatic cancer, much of it predating high-resolution imaging modalities, many of the clinical series have reported the conceptual classifications for pancreatic cancer patients as resectable, borderline resectable, locally advanced, and metastatic. This leads to confusion and difficulty in comparing results across institutions and trials.
CA, Cancer antigen; ERCP, endoscopic retrograde cholangiopancreatography; GI, gastrointestinal; MRI, magnetic resonance imaging; PET, positron emission tomography.
Therapeutic Approaches
Options for treating any malignancy include surgery, radiation, and chemotherapy, either singly, sequentially, or in combination. The overall disappointing outcomes in the treatment of pancreatic cancer have involved all modalities in every order and combination possible. The standard therapeutic approach to pancreatic carcinoma has been surgical, and the standard operation for cancers in the pancreatic head has been the pancreaticoduodenectomy or Whipple procedure. Complete surgical resection offers the only possibility for cure, yet more than 80% of patients presenting with pancreatic cancer cannot be cured with resection.57 Interestingly, there are no universally accepted criteria defining surgical resectability, although the driving consideration for the multidisciplinary team evaluating a potential surgical candidate is the likelihood of obtaining a complete resection with negative microscopic margins.58,59 Tumors of the body and tail of the pancreas generally present late and thus usually are not resectable. Although the Whipple is the standard procedure, some controversy still surrounds the debate of the optimal surgerical technique. One controversy is the role of preoperative biliary drainage, with a 1999 MSKCC series60 showing increased morbidity, including mortality, and a similar MD Anderson Cancer Center series61 showing no increase in sepsis or death. Thus most institutions selectively decompress the biliary tract in symptomatic patients or in those for whom surgery will be delayed. In most instances, this can be done by ERCP. CA-19-9 levels should be drawn after biliary decompression. Other surgical questions under investigation include the analysis of the optimal pancreatic anastamosis to minimize leaks and whether pylorus preservation offers a better quality of life and nutritional status. Total pancreatectomy has been advocated by some as a better operation because it removes more potentially involved tissue and because it avoids a pancreaticojejunal anastomosis, which is the source of significant surgical morbidity and mortality. However, it does result in exocrine insufficiency and brittle diabetes that is difficult to manage,62 and it confers no survival advantage. Fortner63 advocated a regional pancreatectomy, which is an en bloc resection of the pancreas and surrounding tissue along with a segment of the mesenteric-portal vein and occasionally major arterial segments. This operation is less frequently performed in the United States because of high operative mortality (8% in Fortner’s series) and no clear evidence of survival benefit. Some groups in Japan combine the Whipple procedure with extensive lymph node dissection, claiming better results with no apparent increase in operative mortality.64 The opposite approach has been taken by several groups in the United States65,66 who believe there is no compromise of survival when preserving the pylorus in an attempt to avoid postgastrectomy symptoms. In the 1960s and 1970s, the pancreaticoduodenectomy was associated with a 20% to 25% in-hospital mortality rate.67 By the 1980s and 1990s, surgical techniques and supportive care improved to the point at which this operation now carries only a 1% to 3% mortality rate in major centers.68 With certainty, the pylorus-preserving pancreaticoduodenectomy improves gastrointestinal function and does not appear to compromise further management. The dramatic improvements in surgical techniques have made it possible to conduct an ongoing prospective randomized trial with intraoperative randomization between a standard operation and one with extended retroperitoneal lymph node dissection. What is clear from the surgical literature is that, if tumor is knowingly left behind, survival after resection is not much better than after bypass alone.53 This has led to the question of appropriate surgical management of patients with positive lymph nodes at frozen section. Yeo and colleagues68 have shown that long-term survival out to 5 years is possible in both lymph node–positive and lymph node–negative tumors as long as the patient is otherwise technically suitable for resection. An additional important question answered recently involves determination of the best management of the elderly patient. Sohn and colleagues,69 Magistrelli and colleagues,70 and Balcom and colleagues71 clearly showed that it is appropriate to perform aggressive surgery in appropriately selected patients into their seventh and eighth decades of life. Several other important points from the surgical literature are worth noting. These involve the importance of both a multidisciplinary approach as well as the importance of an experienced team. The importance of an experienced team is further underscored by series from Robinson et al.72 and from Sohn et al.73 at two different institutions, showing the benefit of reoperation at an experienced center: 67% of patients, many deemed unresectable elsewhere, were in fact successfully resected without compromise of their overall outcome. Other series have clearly shown the benefit of decreased in-hospital morbidity and mortality rates as well as survival in those centers with a high volume of cases.74,75
Multiple large series have shown that only 5% to 30% of patients have resectable tumors.64,76–78 Remaining patients require some form of palliation for pain, duodenal obstruction, or jaundice. Obstructive jaundice can be relieved percutaneously, albeit with complications in up to 35% in some series,53 or with stents that have more than an 85% success rate and procedure-related mortality of only 1% to 2%.79 This represents a significant improvement over surgical morbidity. Two randomized trials80,81 have shown equal survival with both surgical bypass and endoscopic stent placement. In addition to analgesics, percutaneous neurolysis of the celiac ganglion and radiation therapy offer good palliation of pain.
Another strategy to treat in patients with resectable pancreatic adenocarcinomas is preoperative treatment with gemcitabine and radiotherapy. The rationale for this approach is as follows: (1) early systemic treatment with gemcitabine addresses risk of micrometastatic disease when tumor burden is lowest, (2) gemcitabine is a potent radiosensitizer, and (3) restaging studies performed after neoadjuvant therapy selects out those patients who are not good surgical candidates because of rapid disease progression. In a phase II study of 86 patients, this approach resulted in a resection rate of 74% having a median survival rate of 34 months. The median survival rate in the entire group of 86 was 22.7 months. The 5-year survival rate in those patients able to undergo successful surgical resection was 36% versus 0% in those who did not undergo surgery.82
For patients with resectable disease, the role of adjuvant chemoradiotherapy was established by the Gastrointestinal Tumor Study Group (GITSG) in 1985, when it showed that patients receiving adjuvant chemoradiation experienced a doubling of survival time.83 Small series using neoadjuvant treatment were reported in the 1980s, but that approach did not attract general interest until improved outcomes in surgical series were reported.84,85 Historically 5-fluorouracil (5-FU) was the chemotherapeutic agent of choice (Table 38-4) until Burris et al.86 showed an improvement in survival with gemcitabine over 5-FU as first-line therapy for patients with advanced pancreatic cancer. Subsequently, gemcitabine and 5-FU in varying doses and dose rates have been combined with and without radiation with varying results.87–89
Gemcitabine, now the most widely used chemotherapy in pancreatic cancer, has shown significant promise; but combination studies with other chemotherapies and targeted agents have been largely dissappointing. Gemcitabine has been combined with radiation therapy with modest success. However, because of the significant radiosensitizing properties of gemcitabine, reduced radiation dose and field size is necessary if full-dose gemcitabine is administered. Investigators from the University of Michigan90 reported a median overall survival (OS) of 11.2 months in patients (n = 74) with locally advanced pancreatic cancer treated with full-dose gemcitabine (1000 mg/m2, weekly) and concurrent radiotherapy (36 Gy in 2.4-Gy fractions). Using three-dimensional (3-D) conformal techniques, they treated the primary tumor with a 1-cm margin. Despite not treating the regional lymph nodes, only 5% of patients failed regionally, suggesting that treatment of the regional lymph nodes may be omitted. Similarly, Chang et al.91 reported that 0 of 77 patients developed an isolated nodal failure after stereotactic radiotherapy targeting only the primary tumor. Overall, there were nine nodal recurrences, but all were in the context of progressive disease at other sites.
Other strategies for improving the effectiveness of radiotherapy for pancreatic cancer include intensity-modulated radiotherapy (IMRT),92 image-guided radiotherapy (IGRT),93 and stereotactic radiotherapy.94,95
The major difficulty in treatment of pancreatic cancer is not the radioresistance of the tumor, but radiation dose limitations imposed by surrounding normal organs (see Fig. 38-1B) and the frequent presence of occult distant metastases. Attempts to overcome this problem have included the use of intraoperative electrons or implantation of the tumor either directly or percutaneously. Obviously, the advantage offered by IORT is precise field localization and the abilities to shield sensitive normal structures and to deliver a relatively high dose quickly. High-dose-rate brachytherapy has the advantage of reduced radiation exposure to medical personnel and the potential to overcome limitations of attempting to control tumors with a relatively fast doubling time with a low-dose-rate source.
Discouraging survival statistics, a 50% to 76%37,38 locoregional failure even after “curative” resection, and the proven survival benefit of adjuvant treatment83,96 make postoperative radiation with concurrent chemotherapy justifiable in virtually all patients who have had a potentially curative resection of pancreatic cancer. Patients with localized but unresectable cancer can expect doubling of survival with EBRT and concurrent 5-FU.97–99 They should be offered treatment unless poor clinical status precludes treatment.
Simulation
With the use of multiple fields and high-energy photons, doses of 60 to 65 Gy can be delivered safely.100 The addition of concurrent chemotherapy typically requires dose reduction to avoid excessive toxicity. With the widespread development and availability of IMRT techniques, increased dose intensity to the tumor is possible while minimizing radiation dose to adjacent normal tissues. Furthermore, reduced tumor margins also are possible with the application of IGRT techniques.
Anterior-Posterior–Posterior-Anterior Field: Lateral Borders
For lesions of the head of the pancreas, treatment of nodal groups, if indicated, should include the pancreaticoduodenal, suprapancreatic, celiac, and porta hepatis lymph nodes. Lesions in the head of the pancreas frequently invade the medial wall of the duodenum, and therefore the entire involved duodenal wall should be covered for lesions. In the direction of the pancreatic body, the treatment volume should extend to include a 2- to 3-cm margin beyond gross disease. Care must be taken to keep doses within renal tolerance. Often more than 50% of the right kidney is within the field; thus for lesions of the pancreatic head, at least two-thirds of the left kidney should be excluded from the anterior-posterior–posterior-anterior (AP–PA) portion of the treatment field. When the primary tumor is located in the pancreatic head, the risk of nodal involvement in this area is quite low (see Fig. 38-2B).35 The risk of renal injury is very low if half of the renal mass receives no more than 18 Gy.49
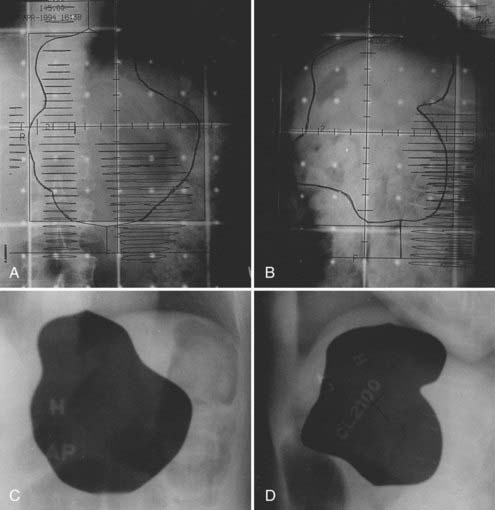
For lesions in the pancreatic body or tail, the target volume includes the tumor with a 2- to 3-cm margin, pancreaticoduodenal and porta hepatis nodes, lateral suprapancreatic nodes, and nodes of the splenic hilum. It is not necessary to include the whole duodenal loop in the treatment field, and thus most of the right kidney can be spared. Often 50% of the left kidney is in the field when the more lateral nodes are covered (Fig. 38-6).
FIGURE 38-6 • A and B, Simulation films of the patient shown in Fig. 38-4. See text for discussion. Care is taken to include all relevant at-risk regional nodes and to spare as much of one kidney as possible. On the lateral fields, a block should be placed to shield the spinal cord but not block at-risk para-aortic lymph nodes.
Anterior-Posterior–Posterior-Anterior Field: Superior and Inferior Borders
Kao and colleagues101 demonstrated significant variability in the location of the celiac axis and superior mesenteric nodes (Fig. 38-7), which emphasizes the need for individualized treatment planning when attempting to cover nodal groups associated with these vessels. For pancreatic head lesions, to ensure adequate coverage on the celiac nodes, the superior border should be at the T10/T11 level and the lower border at the L3/L4 level, depending on the preoperative staging studies. Guidelines are identical for lesions of the body and tail, although the superior margin occasionally is higher because of the location of the primary tumor.
Lateral Field Borders
Significantly larger fields encompassing the liver have been studied by some,102 but are not considered standard.
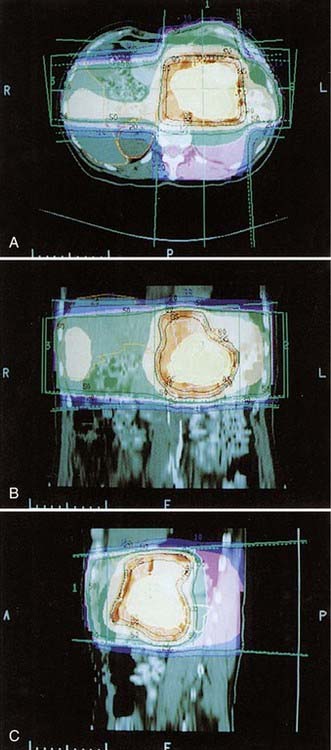
CT-based radiation treatment planning is widely available and should be considered the standard of care (Fig. 38-8). It is possible to determine complete anatomic and dose information for the entire tumor volume and surrounding normal tissues (Fig. 38-9A, B). An approach using tight conformal fields tailored to each patient is highly desired when treating aggressively with the newer chemotherapeutic agents that have higher toxicity than 5-FU. Construction of dose-volume histograms shows the volume of normal tissue in the treatment field (see Fig. 38-9C). Use of this information to assess the probability of normal tissue complications is under active investigation. The initial simulation is accomplished as described earlier with the patient placed in an immobilization device such as an alpha-cradle. The patient is then CT scanned in the immobilized treatment position on a flat CT tabletop with the CT alignment lasers positioned on the patient’s skin marks so that the simulation position can be exactly duplicated. Alternately, a dedicated CT simulator may be used. CT slices typically are obtained at 3- to 5-mm intervals, although even higher resolution imaging using biphasic high-speed helical CT scanning at 1.25-mm intervals is desirable for optimal definition of the tumor and regional anatomy. The clinician then outlines the target volume on each CT slice, which includes tumor, areas at risk for microscopic involvement, and an additional 10-mm margin to allow for setup error and patient motion. If daily IGRT techniques are used, the margin expansion can be reduced. Other critical organs, such as the kidneys and spinal cord, also are contoured, and these as well as the target volume and bony landmarks are reconstructed in three dimensions and can be displayed using a beam’s-eye view (BEV) technique. Beam shaping is then accomplished in this mode, ensuring adequate coverage of the target volume with minimal inclusion of normal tissue. Once the optimal beam arrangement has been determined, the CT image set is used to generate a BEV template at the same magnification as the initial simulation film for each treatment field. Often the isocenter may have been moved during the treatment planning process. At verification, the patient is set up again with the initial parameters, and changes are then made as determined by the 3-D plan. The new BEV template established by the 3-D plan is overlaid onto the verification simulation films (obtained for each field). The template can then be used to fabricate custom blocks (Fig. 38-10).
Intensity-Modulated Radiotherapy
Ben-Josef and colleagues92 described using an IMRT approach to treat locally advanced and resected pancreatic cancer with concurrent capecitabine. Using this technique, the primary tumor (or tumor bed if treating postoperatively) was treated with 54 Gy and the lymph nodes with 45 Gy. Acceptable toxicity occurred if the following dose limitations were observed: 50% of each kidney below 20 Gy, 67% of liver below 35 Gy, 90% of small bowel below 45 Gy, 90% of stomach below 45 Gy, and 90% of spinal cord below 45 Gy (max 50 Gy). Milano et al.103 also reported acceptable toxicity using IMRT for pancreatic and bile duct cancer. The majority of these patients also received 5-FU. These investigators recommended the following dose guidelines: 10% to 20% of the left kidney more than 12 Gy (max 30 Gy), 25% to 30% of the right kidney more than 20 Gy, 50% to 60% of the liver more than 8 Gy (max 44 Gy), and spinal cord less than 45 Gy. When treating pancreatic tail lesions, the dose constraints for the right kidney were tightened and the dose constraints for the left kidney were relaxed. Small bowel constraints were not considered in planning. In this series, unresectable tumors typically were treated to 59.4 Gy and regional lymphatics to 45 Gy.
Image-Guided Radiotherapy
Pancreatic tumors can move significantly in the course of normal respiration. The motion is greatest in the inferior-superior direction, but significant motion also exists in the AP and left-right directions. In one observational study of liver and pancreatic tumors with implanted fiducial clips, the average range of motion as determined by quantitative fluoroscopic analysis in the craniocaudal direction was 7.4 mm.104 The investigators concluded that tumor motion of this magnitude could significantly affect IMRT dose distributions. More recently, Minn and colleagues105 reported that, in a group of patients with pancreatic cancer, the maximum tumor motion that occurred during treatment was greatest in the superior-inferior direction (mean: 6.5 mm, range: 0.5-12.7 mm) but also occurred in the AP (mean: 2.9 mm, range: 0.6-5.5 mm) and left-right (mean: 2.4 mm, range: 0.4-9.4 mm) directions. Dynamic MRI and ultrasound studies suggest that pancreatic tumor motion occurs as much as 20 to 35 mm throughout the respiratory cycle.106,107
Chemotherapy Alone
A multitude of published series have shown improved disease-free survival (DFS) and OS with the addition of 5-FU to radiation. Various doses and schedules have been used; however, no prospective randomized study has clearly shown superiority of one schedule over another. Chemotherapy alone generally is reserved for patients with metastatic disease or poor-performance status patients with locally advanced disease. The only drugs currently approved by the U.S. Food and Drug Administration (FDA) for the management of patients with pancreatic ductal adenocarcinoma are gemcitabine and erlotinib. Gemcitabine initially was studied in a randomized trial and compared with weekly bolus 5-FU.86 The primary endpoint was clinical benefit as defined by performance status, narcotic use, and weight loss. In this pivotal trial, gemcitabine was found to be superior to 5-FU in clinical benefit. In addition, this trial showed a small but significant survival advantage. Subsequently, there have been many studies of gemcitabine combinations with both traditional chemotherapy drugs such as cisplatin, oxaliplatin, capecitabine, and irinotecan and new targeted therapeutics such as a farnesyl transferase inhibitor, bevacizumab, cetuximab, and erlotinib. Only the combination of gemcitabine and erlotinib108 has shown statistically significant improvement in OS compared with gemcitabine alone. However, because the OS benefit in this trial was small (hazard ratio 0.82), this combination has not been adopted as a standard of care, but is certainly an option for patients. Several other drugs have shown improvement in objective response rate or progression-free survival in randomized clinical trials.109–112 These combinations have included gemcitabine plus cisplatin,109 oxaliplatin,110 capecitabine,111 and bevacizumab.112 Improvement in progression-free survival with bevacizumab was seen only in combination with gemcitabine and erlotinib,113 not with gemcitabine alone.113 Current National Comprehensive Cancer Network guidelines suggest that chemotherapy combinations that include erlotinib, cisplatin, oxaliplatin, and capecitabine are acceptable options in patients with advanced disease who have a good performance status.114
Critical Normal Tissues
Critical normal tissues in a typical field treating pancreatic carcinoma include the liver, small bowel, stomach, kidney, and pancreas. The fact that the pancreas is more radioresistant than surrounding organs, coupled with overall poor long-term survival, has made reports of radiation injury to the pancreas exceedingly scarce in the literature. Fajardo and Berthrong115 describe radiation effects to be primarily stromal fibrosis, myointimal proliferation of the arteries, ductal stenosis, and parenchymal atrophy that is similar to the appearance of chronic pancreatitis without the active inflammatory component. Islet cells appear to be more resistant than the exocrine tissue. Autopsy studies in patients treated with helium ions116 also have shown prominent marked interstitial fibrosis and vascular sclerosis.
Outcome
Preoperative Radiation With and Without Chemotherapy
Preoperative radiation has been described in several small series in the United States. Pilepich and Miller117 treated 17 patients preoperatively. Of these, 16 patients had been explored and judged to be unresectable based on extension of tumor through the pancreatic capsule. They received 40 to 50 Gy with standard fractionation; of 11 re-explored patients, 6 were able to undergo radical resection. Two patients remained disease free for 5 years. A second series involved only seven patients,118 five of whom were given 45 Gy preoperatively. There were two 5-year survivors and no clinically detected local recurrence. In Jessup and colleagues’ series119 of 16 patients, 2 were able to undergo resection after neoadjuvant 5-FU and EBRT and had no evidence of disease at 20 and 22.5 months. In Japan, Ishikawa and colleagues documented excellent pathologic responses to preoperative radiation120 and also noted improved resectability in his series of 22 patients.121 An added benefit he noted was that no patient developed pancreatic fistulae as compared with a 17% rate of fistulae formation in similar nonirradiated patients who were treated concurrently.
The rationale for preoperative gemcitabine and radiation is as follows: (1) early systemic treatment with gemcitabine addresses the risk of micrometastatic disease when tumor burden is lowest, (2) gemcitabine is a potent radiosensitizer, (3) restaging studies performed after neoadjuvant therapy select out those patients who are not good surgical candidates because of rapid disease progression, and (4) prolonged recovery after a Whipple prevents treatment in up to 25% of patients.122 In a phase II study of 86 patients, this approach resulted in a 74% resection rate with a median survival rate of 34 months in the resected patients. The median survival in the entire group was 22.7 months. The 5-year survival rate in those patients able to undergo successful surgical resection was 36% versus 0% in those who did not undergo surgery.82
Neoadjuvant therapy is particularly attractive for borderline resectable patients,123 although definitive proof of its clinical benefit is still lacking.124
Postoperative Radiation With or Without Chemotherapy
Adjuvant Therapy After Curative Resection
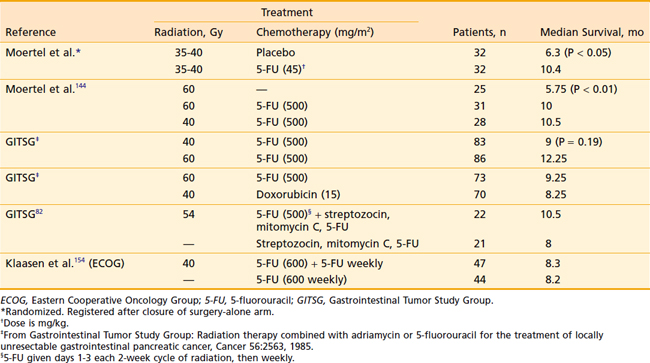
In 1974 the GITSG undertook a prospective randomized trial giving 40 Gy EBRT and weekly bolus 5-FU versus no adjuvant therapy in potentially curatively resected patients (see Table 38-4).83 There was a statistically significant improvement in median survival (21 months versus 11 months) in favor of the irradiated group. Two-year actuarial survival was 43% for the treated group versus 18% for the control group. Subsequently, the GITSG closed the observation arm and added an additional 30 patients to the treatment group, again showing the same improved survival.96 A variety of phase I studies have been carried out with the intent of defining an effective and tolerable regimen combining radiation therapy with gemcitabine.125–129
Although considerable debate is ongoing within the United States regarding the optimal approach to the treatment of pancreatic cancer, a tremendous gulf exists in the approach of U.S. clinicians versus those in Europe. The European Organization for Research and Treatment of Cancer conducted a phase III trial assessing adjuvant radiotherapy and 5-FU versus observation after surgery and found no significant benefit to treatment.130 Perhaps even more provocative are the results of the European Study Group for Pancreatic Cancer (ESPAC)–1131 study that not only showed the superiority of 5-FU over observation but also suggested that chemoradiation was in fact unneccessary and even harmful. Criticized for flaws in its conduct as well as lack of quality control for radiation therapy,132,133 there is general consensus that 5-FU–based chemoradiation remains within the standard of care in the adjuvant setting. In addition, the phase III Charité Onkologie (CONKO)-001 trial,134 in which patients were assigned to postoperative observation versus gemcitabine, showed significant improvement in DFS with adjuvant gemcitabine. The RTOG 97-04 phase III trial assessed pre- and post-chemoradiation continuous infusion 5-FU versus pre- and post-chemoradiation gemcitabine for resected pancreatic cancer.135 There was prospective quality assurance of radiation therapy to include central review of preoperative CT scans and simulation felds. For tumors of the pancreatic head, OS was significantly better for gemcitabine over continuous infusion 5-FU. Although the results of the RTOG 97-04, ESPAC-1, and CONKO trials cannot be directly compared because of differing patient characteristics, it is noteworthy that the treatment arms in each study have a similar OS of between 20.1 and 22.1 months. It is anticipated that the ongoing ESPAC-3 trial will provide more definitive answers as to the optimal postoperative treatment.
Intraoperative Radiation Therapy
Investigators at the NCI conducted a randomized trial of 32 patients with resected tumors,136 comparing 50 Gy EBRT to 50 Gy EBRT plus a 20 Gy IORT boost. OS was the same in both groups, but the IORT group had increased local control and DFS.
The use of intraoperative electrons to boost the dose to the tumor bed allows high-dose delivery to an area at risk, but minimizes damage to surrounding radiosensitive tissue (Table 38-5). In the United States, IORT has been used primarily for unresectable carcinomas, but in Japan it has been incorporated as part of the treatment for potentially curatively resected lesions. Shibamoto et al.137 reported a mean survival of 14 months in patients treated with both EBRT and IORT after total resection. Because of small numbers, this trend for improved survival was not statistically significant, but Kawamura et al.138 subsequently did show increased survival in a similar group of 32 patients. This study is difficult to assess because exact details of staging and selection criteria for treatment are not given. Hiraoka et al.139 treated 14 patients with extended surgery and extended-field IORT with a 5-year survival of 33%. Ozaki et al.140 reported on 16 patients treated with extended surgery, IORT, and mitomycin. Of these, 11 were International Union Against Cancer Criteria stage III, and the group had a 53% survival at 3 years.
Treatment for Localized but Unresectable Tumors
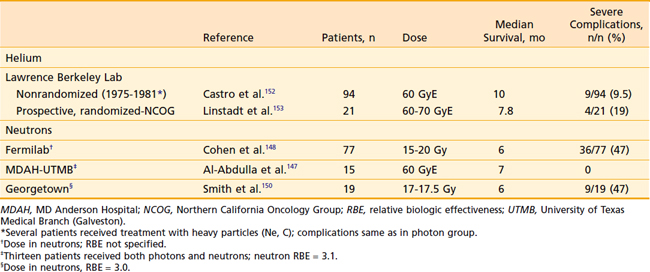
Optimal therapy for patients with locally advanced pancreatic carcinoma remains controversial largely because of the great clinical heterogeneity within that population. Median survival for localized unresectable cancer of the pancreas treated with bypass alone is 4 to 6 months.29,30,141–143 EBRT alone does not offer significant improvement.98,99,144 Hyperfractionated radiation, 1.2 Gy twice daily, was also disappointing, although the total dose of 50.4 Gy may have been too low to expect significant benefit.145 Particle therapy to include pions,146 neutrons,147–150 and helium ions151,152 has been disappointing (Table 38-6). Neutron therapy hoped to take advantage of a higher relative biologic effectiveness (2.6 to 3.0), and helium ion therapy was used to take advantage of improved dose localization properties. Even though initial data with helium appeared to be very promising151 and autopsy studies did confirm tumor kill with minimum effect on adjacent local tissue,116 a randomized prospective trial comparing helium ions with conventional radiation failed to show a survival benefit in spite of improved local control in the helium arm.153 The photon arm was split-course and the helium continuous; failures were due largely to distant metastases. EBRT combined with chemotherapy, however, has been shown to confer significant survival advantage over either EBRT or chemotherapy alone (Table 38-7). Although Klaasen et al.154 found no difference with combined modality treatment, the GITSG in a prospective study of 194 patients144 did find that the addition of 5-FU to EBRT almost doubled the mean survival time (from 5.5 to 10 months) and that at 1 year 40% of the combined modality group was alive versus only 10% of those treated with radiation alone. Other investigators have since duplicated these findings.98,99 The cooperative groups also have explored the newer chemotherapeutic agents. An Eastern Cooperative Oncology Group trial using protracted infusion 5-FU, gemcitabine, and EBRT showed unexpected early toxicity,155 necessitating early study closure. This underscored earlier studies that suggested that relatively high doses of radiation, large fields including lymph nodes at risk, and gemcitabine were too toxic. Similar experience of high toxicity suggesting a very narrow therapeutic index with gemcitabine chemoradiotherapy was demonstrated by researchers at MD Anderson Cancer Center.156
An alternate approach initially was investigated at the University of Michigan, consisting of a standard dose of gemcitabine (1000 mg/m2) weekly along with escalating doses of radiation.126 Radiation fields were tightly 3-D planned, covering only gross tumor volume. Doses ranged from 24 to 42 Gy delivered in 3 weeks with 1.6- to 2.8-Gy fractions. This study included 37 unresectable or incompletely resected patients, 14 of whom had confirmed or suspected metastatic disease. Median survival of 11.6 months and patterns of failure suggested that field-size reductions were not harmful and led to this approach being widely adopted. Subsequently, another approach—systemic chemotherapy prior to chemoradiation in the setting of locally advanced disease—was used. Both retrospective analyses157 and prospective studies158,159 suggest that treating patients with locally advanced disease with systemic chemotherapy first helps to establish overall disease control and selects for patients with locally advanced disease who are more likely to benefit from subsequent chemoradiation therapy. A significant number of patients who appear to have locally advanced but nonmetastatic cancer quickly fail and do not benefit from the locoregional treatment approach. The therapeutic strategy used by Huguet et al.157 in the GERCOR (French Oncology Research Group, Paris, Fance) II and III studies (n = 181 patients) was to administer initial chemotherapy for 3 months and thus select out those patients who would then benefit from the addition of further chemotherapy and radiation. In this study, tumor progressed in 29% of patients during the first 3 months and their median OS was only 4.5 months. Patients without tumor progression during the first 3 months had a median survival of 13.1 months. As a result, the patients who then received the course of chemotherapy followed by planned chemoradiotherapy had a significantly better OS of 15 months versus 11 months if treated with chemotherapy alone. Although registered on the trial in a prospective manner, Huguet’s study is a retrospective analysis of the 128 patients (from the original 181) who were without disease progression at 3 months, which leads to some criticism of the introduction of bias, mandating a future prospective trial to consider whether patients with locally advanced, nonmetastatic disease should all be offered up-front chemotherapy prior to radiation therapy. The GERCOR and Arbeitsgemeinschaft Internistische Onkologie groups are now conducting this phase III trial.
Brachytherapy
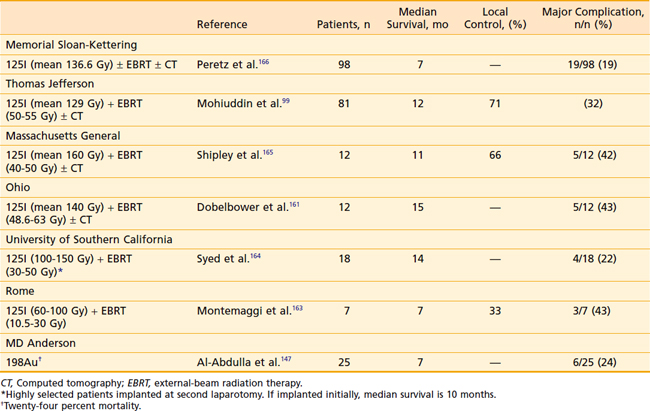
A more aggressive local approach to deliver high doses has been the use of intraoperative brachytherapy (Table 38-8). Direct or percutaneous160 implantation with I-125, Au-198, or Pd-103 has been reported. Numerous reports using this technique have appeared in the United States and abroad,49,98,99,161–164 yielding approximately equal results. Initial reports from MGH in 12 patients treated with bypass surgery, I-125 implantation, and postoperative 40 to 45 Gy showed an 11-month mean survival.165 Syed et al.164 reported on 18 patients treated in a similar fashion with a mean survival of 14 months. Their patients, however, were highly selected because they were implanted at second laparotomy; those implanted at initial laparotomy had a mean survival of only 10 months. The best survival (mean 15 months) was reported by Dobelbower et al.161 in their initial series of 12 patients. Long-term follow-up on 81 patients from Thomas Jefferson Medical School162 showed an excellent local control of 71% but unfortunately no increase in mean survival, which was 12 months for the entire group. Perioperative mortality was 5% and late complications were seen in 32% of patients. The largest series, reported for MSKCC by Peretz et al.,166 had a total of 98 patients. Mean peripheral dose to the tumor was 136.6 Gy; postoperative complications were high (19/98) and median survival for the whole group was only 7 months.
There are very sparse data on the use of high-dose-rate brachytherapy. Warszawski et al.167 developed a technique using CT treatment planning to ensure accurate delivery of high doses to the tumor and tolerable doses to surrounding organs. Nine patients were treated with 30-Gy high-activity Ir-192 and subsequently 52 Gy EBRT with concurrent 5-FU/leukovorin. Results are very preliminary, but all patients had significant pain relief, none had procedure-associated complications, and autopsy in one showed no residual cancer in the treatment field and minimal radiation effects on the adjacent small bowel. An alternate means of overcoming the potential drawback of low dose rate I-125 is with the use of Pd-103. With a short half-life of only 17 days, higher total activities can be implanted. The more rapid dose falloff for Pd-103 enhances the sparing effect of brachytherapy on the surrounding normal tissues, and the higher dose rate has a theoretical advantage in treating rapidly dividing tumors. Raben et al.168 reported a small series of patients from MSKCC treated with Pd-103. Median survival was 6.9 months, no different from a similar series of patients treated with I-125. The addition of EBRT or chemotherapy did not improve local control or survival. As in other brachytherapy series, palliation of pain was very good with 83% of symptomatic patients reporting durable pain control until the time of death. Complications in this series were felt to be unacceptably high.
Intraoperative Radiation Therapy
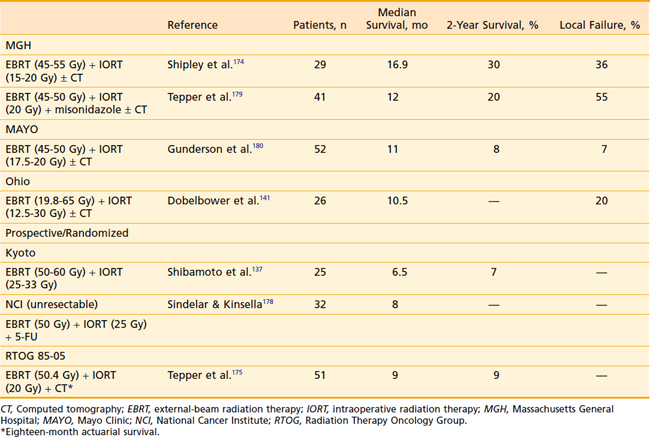
Since the report of Abe et al.169 dealing with the feasibility and safety of intraoperative electrons, many investigators have reported nonrandomized data.137–140,170–176 Single doses of 15 to 30 Gy are generally given with 15- to 29-meV electrons. Use of higher doses (up to 50 Gy) have up to a 75% incidence of treatment-related bleeding.172 IORT alone is no better than bypass surgery,141 with a mean survival of approximately 3.5 months; therefore, treatment is usually given in conjunction with 45 to 50 Gy EBRT and 5-FU. Mean survivals in that setting have ranged from 10.5 to 16.5 months.141,174,177 To ascertain whether this improvement in survival was real or due to selection bias, the NCI and RTOG both undertook randomized trials of IORT. In the NCI trial,178 patients received 25 Gy IORT with 18- to 22-meV electrons and postoperative 50 Gy at 1.5 to 1.75 Gy per day compared with controls who received split-course 60 Gy EBRT. Concurrently, both groups were given 5-FU. Treatment-related toxicity was similar in both groups and median survival in each arm was 8 months. Time to disease progression, however, was longer in the IORT group and for the subset of patients with stage III disease (locally infiltrative and involved nodes), time to progression and survival were both prolonged. In RTOG 85-05, patients were given 50.4 Gy EBRT in combination with 5-FU with or without 20 Gy IORT. For the 51 fully analyzable patients, the mean survival was only 9 months. This study did confirm the feasibility of using IORT in a multi-institution setting with acceptable morbidity, but it failed to show increased survival benefit of IORT over standard EBRT and 5-FU. The addition of misonidazole, a hypoxic cell sensitizer to IORT,179 also failed to show any advantage. Although OS rates were disappointing, one valuable effect of IORT is the ability to afford significant pain relief; Shipley et al.174 reported that 50% of their patients had complete pain relief for the duration of their survival. Others have also noted this in up to 90% of their patients.170,172 IORT unquestionably improves local control: in a series of 159 patients from Mayo,173 local control at 1 year was 82% for the IORT group versus 48% for standard radiation with 5-FU. Gunderson et al.180 documented local progression of only 7% in his series of 42 evaluable patients. Peritoneal failure occurred in 26% and distant metastasis in 57%. The majority of distant metastasis occurred in the liver. The vexing problem of local failure in the liver has been confirmed by Foo et al.181 and Griffen et al.38 in two series of curatively resected patients, thus underscoring the need for improved systemic treatment.
Endocrine Tumors
Endocrine tumors compose approximately 5% of all pancreatic tumors; the enteropancreatic cells are believed to be of neuroectodermal origin and tumors arising from these cells are termed APUDomas (from their ability for amine-precursor uptake and decarboxylation). These tumors are frequently recognized by the clinical syndromes caused by the circulating polypeptides that are elaborated by the malignant cells. These tumors are frequently slow-growing with a long natural history.33 Even with metastasis, survival may be quite long. The primary approach to these tumors is surgical, with chemotherapy reserved for unresectable or metastatic disease. There is very sparse anecdotal information on the effects of radiation. The rarity of these tumors has precluded prospective studies of any size. Phan et al.182 reported on a series of 50 patients, 29 of whom had malignant tumors. Even without adjuvant therapy, 5-year survival was 61%. Status of the surgical margins was of paramount importance. Median survival with negative margins was 71 months versus 13 months with positive margins.183–185
The medical management of pancreatic endocrine tumors is quite different from approaches taken with pancreatic ductal adenocarcinoma. Historic data support antitumor activity with classic chemotherapy drugs such as doxorubicin, streptozotocin, 5-FU and dacarbazine. More recently, temozolomide has been added to the list as an FDA-approved drug for neuroendocrine tumors.186 Current investigations are focusing on drugs that target the angiogenic pathways. Recently, sunitinib was shown to demonstrate improved OS in a randomized trial of patients with neuroendocrine tumors from pancreas and other sites.187 Radiolabeled somatostatin analog is also under investigation.188
Many patients with neuroendocrine tumors have bony metastases, and bisphosphonate therapy can be considered as a useful therapeutic adjunct. Because some neuroendocrine tumors are functioning neuroendocrine tumors, specific management of the hormone-related symptom complex is indicated. The reader is referred to several reviews detailing these syndromes and their specific management.189,190
1 Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71.
2 National Cancer Institute. Annual Cancer Statistics Review 1973–1988. Bethesda, Md: Dept of Health and Human Services; 1991. (NIH Publication no. 91-2789)
3 Gudjonsson B. Cancer of the pancreas: 50 years of surgery. Cancer. 1987;60:2284.
4 Farrow DC, Davis S. Risk of pancreatic cancer in relation to medical history and the use of tobacco, alcohol and coffee. Int J Cancer. 1990;45:816.
5 Howe GR, Jain M, Miller AB. Dietary factors and the risk of pancreatic cancer: results of a Canadian population based study. Int J Cancer. 1990;45:604.
6 Taylor PR, et al. Induction of pancreatic tumors by long-term duodenogastric reflux. Gut. 1989;30:1596.
7 Cuzick J, Babiker AG. Pancreatic cancer, alcohol, diabetes mellitus and gall-bladder disease. Int J Cancer. 1989;43:415.
8 Gordis L. Consumption of methylxanthine-containing beverages and pancreatic cancer. Cancer Letter. 1990;52:1.
9 Rosa JA, Van Linda BM, Abourizk NN. New onset diabetes mellitus as a harbinger of pancreatic carcinoma. J Clin Gastroenterol. 1989;11:211.
10 Lin A, Feller ER. Pancreatic carcinoma as a cause of unexplained pancreatitis: report of ten cases. Ann Intern Med. 1990;113:166.
11 Mancuso TF, el-Attar AA. Cohort study of workers exposed to betanaphthylamine and benzidine. J Occup Environ Med. 1967;9:277.
12 Lin RS, Kessler II. A multifactorial model for pancreatic cancer in man. J Am Med Assoc. 1981;245:147.
13 Abrams R, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: survival and observations regarding patterns of failure, radiotherapy dose and CA 19-19 levels. Int J Radiat Biol Phys. 1999;44:1039.
14 Hruban R. Pancreatic Cancer, 1999 Update Symposium. J Am Coll Surg. 1998;187:429.
15 Hruban RH, et al. Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am J Surg Path. 2001;25:579.
16 Hahn SA, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214.
17 Smit VHTB, et al. K-ras codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988;16:7773.
18 Shibata D, et al. Detection of c-K-ras mutations in fine needle aspirates from human pancreatic adenocarcinoma. Cancer Res. 1990;130:345.
19 Bos JL. Ras oncogene in human cancer: a review. Cancer Res. 1989;49:4682.
20 Gruenewald K, et al. High frequency of Ki-ras codon 12 mutations in pancreatic adenocarcinomas. Int J Cancer. 1989;43:1037.
21 Almoguera C, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549.
22 Kondo H, et al. Detection of point mutations in the K-ras oncogene at Codon 12 in pure pancreatic juice for diagnosis of pancreatic carcinoma. Cancer. 1994;73:1589.
23 Hruban RH, et al. Genetics of pancreatic cancer: from genes to families. Surg Oncol Clin North Am. 1998;7:1.
24 Cubilla AL, Fitzgerald PJ. Cancer of the exocrine pancreas: the pathologic aspects. CA Cancer J Clin. 1985;35:2.
25 Talamini MA, et al. Spectrum of cystic tumors of the pancreas. Am J Surg. 1992;163:117.
26 Milchgrub S, et al. Intraductal carcinoma of the pancreas. Cancer. 1992;69:651.
27 Abrams R, King D, Wilson J. Objective response of malignant carcinoid to radiation therapy. Int J Radiat Biol Phys. 1987;13:869.
28 Wilentz R, Hruban R. Pathology of cancer of the pancreas. Surg Oncol Clin N Am. 1998;7:43.
29 Kalser MH, Barkin J, MacIntyre MJ. Pancreatic cancer: assessment of prognosis by clinical presentation. Cancer. 1985;56:397.
30 COTP Taskforce. Staging of cancer of the pancreas. Cancer. 1981;47:1631.
31 Nordback IH, et al. Carcinoma of the body and tail of the pancreas. Am J Surg. 1992;164:26.
32 Winek T, et al. Prognostic factors for survival after pancreaticoduodenectomy for malignant disease. Am J Surg. 1990;159:454.
33 Friesen SR. Tumors of the endocrine pancreas. N Engl J Med. 1982;306:580.
34 Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606.
35 Cubilla A, Fortner J, Fitzgerald P. Lymph node involvement in carcinoma of the head of the pancreas area. Cancer. 1978;41:880.
36 Warshaw AL, Tepper JE, Shipley WU. Laparoscopy in the staging and planning of therapy for pancreatic cancer. Am J Surg. 1986;151:76.
37 Tepper JE, Nardi GL, Suit HD. Carcinoma of the pancreas: review of the MGH experience from 1963–1973: analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519.
38 Griffen JF, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56.
39 National Comprehensive Cancer Network, Clinical Practice Guidelines in Oncology V.1.2009 (online) Available at http://www.nccn.org Accessed 31 May 2009
40 Freeney PC, et al. Pancreatic ductal adenocarcinoma: diagnosis and staging with dynamic CT. Radiology. 1988;166:125.
41 Agarwal B, et al. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844.
42 Fuhrman GM, et al. Thin-section contrast-enhanced computed tomography accurately predicts the resectability of malignant pancreatic neoplasms. Am J Surg. 1994;167:104.
43 Santo E. Pancreatic cancer imaging: which method? JOP. 2004;5:253.
44 Varadarajulu S, Wallace MB. Applications of endoscopic ultrasonography in pancreatic cancer. Cancer Control. 2004;11:15.
45 Lytras D, et al. Positron emission tomography does not add to computed tomography for the diagnosis and staging of pancreatic cancer. Dig Surg. 2005;22:55.
46 Ferruci JT, et al. Diagnosis of abdominal malignancy by radiologic fine-needle aspiration biopsy. Am J Roentgenol. 1980;134:323.
47 Weiss SM, et al. Rapid intra-abdominal spread of pancreatic cancer. Arch Surg. 1985;120:415.
48 Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26.
49 Whittington R, et al. Radiotherapy of unresectable pancreatic carcinoma: a six-year experience with 104 patients. Int J Radiat Oncol Biol Phys. 1981;7:1639.
50 NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19:1.
51 Shemesh E, et al. Role of endoscopic retrograde cholioangiopancreatography in differentiating pancreatic cancer coexisting with chronic pancreatitis. Cancer. 1990;65:893.
52 Sakahara H, et al. Serum CA 19-19 concentrations and computed tomography findings in patients with pancreatic carcinoma. Cancer. 1986;57:1324.
53 Warshaw AL, Swanson RS. Pancreatic cancer in 1988: Possibilities and probabilities. Ann Surg. 1988;208:541.
54 Schifeling D, et al. Radiation therapy and 5-fluorouracil modulated by leucovorin for adenocarcinoma of the pancreas. Int J Pancreatol. 1992;12:239.
55 Speer AG, et al. Randomized trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987(11 July):57.
56 Edge SB, et al, editors; AJCC Cancer Staging Manual. ed 7. 2010; Springer, New York:241-246.
57 Li D, et al. Pancreatic cancer. Lancet. 2004;363:1049.
58 Varadhachary GR, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035.
59 Talamonti M. Borderline resectable pancreatic cancer: a new classification for an old challenge. Ann Surg Oncol. 2006;13:1019.
60 Povoski SP, et al. Preoperative biliary drainage: Impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg. Sep 1999;3:496.
61 Pisters PW, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47.
62 Van Heerden J, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas. Am J Surg. 1981;142:308.
63 Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Ann Surg. 1984;199:418.
64 Funovics JM, et al. Current trends in the management of carcinoma of the pancreatic head. Hepatogastroenterology. 1989;36:450.
65 Braasch JW, et al. Pyloric and gastric preserving pancreatic resection. Ann Surg. 1986;204:411.
66 Cameron JL, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120.
67 Cameron J, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430.
68 Yeo C, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. Ann Surg. 1995;221:721.
69 Sohn T, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998;2:207.
70 Magisterelli P, et al. Pancreatic resection for periampullary cancer in elderly patients. Hepatogastroenterology. 1998;45:242.
71 Balcom JHT, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391.
72 Robinson E, et al. Reoperative pancreaticoduodenectomy for periampullary carcinoma. Am J Surg. 1996;172:432.
73 Sohn TA, et al. Reexploration for periampullary carcinoma. Resectability, perioperative results, pathology, and long-term outcome. Ann Surg. 1999;229:393.
74 Sosa J, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429.
75 Birkmeyer JD, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128.
76 Moosa AR. Surgical treatment of pancreatic cancer. Am J Surg. 1986;152:503.
77 Connolly MM, et al. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366.
78 Tsuchiya R, et al. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77.
79 Soehendra N, et al. Malignant jaundice: results of diagnostic and therapeutic endoscopy. World J Surg. 1989;13:171.
80 Smith AC, Dowsett JF, Hatfield ARW. Prospective randomized trial of bypass surgery versus endoscopic stenting in patients with malignant obstructive jaundice. Gut. 1989;30:A1513.
81 Anderson JR, et al. Randomized trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132.
82 Evans DB, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496.
83 Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899.
84 Gnant M, et al. Neoadjuvant chemotherapy with gemcitabine and docetaxel for locally advanced pancreatic cancer. Proceed Am Coll Clin Oncol. 2002;21:149a.
85 Hoffman J, et al. A phase I study of preoperative gemcitabine with radiation therapy followed by postoperative gemcitabine in patients with localized resected pancreatic adenocarcinoma. Proceed Am Coll Clin Oncol. 1998;17:283a.
86 Burris HA3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403.
87 Haddock MG, et al. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: results of the North Central Cancer Treatment Group Phase II Study N9942. J Clin Oncol. 2007;25:2567.
88 Berlin JD, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270.
89 Tempero M, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402.
90 Murphy JD, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801.
91 Chang, et al: Cancer (in press).
92 Ben-Josef E, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454.
93 Fuss M, et al. Daily ultrasound-based image-guided targeting for radiotherapy of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 2004;59:1245.
94 Koong AC, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017.
95 Schellenberg D, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678.
96 Group TGTS. Further evidence of effective combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006.
97 Group TGS. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751.
98 Whittington R, et al. Multimodality therapy of localized unresectable pancreatic cancer. Cancer. 1984;54:1991.
99 Mohiuddin M, et al. Combined modality treatment of localized unresectable adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;14:79.
100 Dobelbower RRJr, et al. Precision radiotherapy for cancer of the pancreas: technique and results. Int J Radiat Oncol Biol Phys. 1980;6:1127.
101 Kao GD, Whittington R, Coia L. The anatomy of the celiac axis and superior mesenteric artery and its significance in radiation therapy. Int J Radiat Oncol Biol Phys. 1993;25:131.
102 Komaki R, et al. Phase I-II study of prophylactic hepatic irradiation with local irradiation and systemic chemotherapy for adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;15:1447.
103 Milano MT, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445.
104 Gierga DP, et al. Quantification of respiration-induced abdominal tumor motion and its impact on IMRT dose distributions. Int J Radiat Oncol Biol Phys. 2004;58:1584.
105 Minn AY, et al. Pancreatic tumor motion on a single planning 4D-CT does not correlate with intrafraction tumor motion during treatment. Am J Clin Oncol. 2009;32:364-368.
106 Bussels B, et al. Respiration-induced movement of the upper abdominal organs: a pitfall for the three-dimensional conformal radiation treatment of pancreatic cancer. Radiother Oncol. 2003;68:69.
107 Bryan PJ, et al. Respiratory movement of the pancreas: an ultrasonic study. J Ultrasound Med. 1984;3:317.
108 Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960.
109 Heinemann V, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946.
110 Louvet C, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509.
111 Cunningham D, et al. Phase III randomized comparison of gemcitabine (GEM) versus gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. European Cancer Conference (ECCO 13), presentation/abstract PS11, Paris, Franco, November 2, 2005. Eur J Cancer Suppl. 2005;3:4. Abstract PS11
112 Fogelman DR, et al, Final results of a bi-institution phase II study of gemcitabine, oxaliplatin, and bevacizumb for advanced pancreatic cancer ASCO 2009 Gastrointestinal Cancers Symposium. Abstract No. 182. Available at http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=63&abstractID=10801 Accessed 9/2/2009
113 Kindler HL, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033.
114 National Comprehensive Cancer Network, NCCN clinical practice guidelines in oncology: Pancreatic adenocarcinoma Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed 9/2/2009
115 Fajardo L, Berthrong M. Radiation injury in surgical pathology. Part III. Salivary glands, pancreas and skin. Int J Radiat Oncol Biol Phys. 1981;5:279.
116 Woodruff KH, et al. Postmortem examination of 22 pancreatic carcinoma patients treated with helium ion irradiation. Cancer. 1984;53:420.
117 Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer. 1980;46:1945.
118 Kopelson G. Curative surgery for adenocarcinoma of the pancreas/ampulla of Vater: The role of adjuvant pre or postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:911.
119 Jessup JM, et al. Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg. 1993;128:559.
120 Ishikawa O, et al. Clinical and histological appraisal of preoperative irradiation for adenocarcinoma of the pancreaticoduodenal region. J Surg Oncol. 1989;40:143.
121 Ishikawa O, et al. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreatoduodenectomy. Arch Surg. 1991;126:885.
122 Spitz FR, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928.
123 Pingpank JF, et al. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2001;5:121.
124 Brunner TB, et al. Primary resection versus neoadjuvant chemoradiation followed by resection for locally resectable or potentially resectable pancreatic carcinoma without distant metastasis. A multi-centre prospectively randomised phase II study of the Interdisciplinary Working Group Gastrointestinal Tumours (AIO, ARO, and CAO). BMC Cancer. 2007;7:41.
125 Blackstock A, et al. Phase I trial of twice-weekly gemcitabine and concurrrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208.
126 McGinn C, et al. Phase I trial of radiation dose escalation with concurrent weekly full dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202.
127 Wolff R, et al. Treatment-related toxicities with rapid fractionation external beam radiation and concomitant gemcitabine for locally advanced nonmetastatic adenocarcinoma of the pancreas. Int J Radiat Biol Phys. 1998;42:201.
128 Mason K, et al. Maximizing therapeutic gain with gemcitabine and fractionated radiation. Int J Radiat Biol Phys. 1999;44:1125.
129 Hoffman J, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas. J Clin Oncol. 1998;16:317.
130 Klinkenbijl JH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776.
131 Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic anncer. N Engl J Med. 2004;350:1200.
132 Crane CH, Ben-Josef E, Small WJr. Chemotherapy for pancreatic cancer. N Engl J Med. 2004;350:2713.
133 Koshy MC, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Radiat Oncol Biol Phys. 2005;61:965.
134 Oettle H, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. J Am Med Assoc. 2007;297:267.
135 Regine WF, et al. RTOG 9704 a phase III study of adjuvant pre and post chemoradiation (CRT) 5-FU vs gemcitabine (G) for resected pancreatic adenocarcinoma. J Clin Oncol. 24(18S), 2006. Abstract 4007
136 Sindelar WF, Kinsella TJ. Randomized trial of intraoperative radiotherapy in resected carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1986;12:148.
137 Shibamoto Y, et al. High dose, external beam and intraoperative radiotherapy in the treatment of resectable and unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 1990;19:605.
138 Kawamura M, et al. Intraoperative radiation for carcinoma of the pancreas. Nippon Igaku Hoshasen Gakkai Zasshi. 1991;51:1442.
139 Hiraoka T, et al. Combination of intraoperative radiation with resection of cancer of the pancreas. Int J Pancreatol. 1990;7:201.
140 Ozaki H, et al. Effectiveness of multimodality treatment for resectable pancreatic cancer. Int J Pancreatol. 1990;7:195.
141 Dobelbower RR, et al. Intraoperative electron beam radiation therapy (IOEBRT) for carcinoma of the exocrine pancreas. Int J Radiat Oncol Biol Phys. 1991;20:113.
142 Morrow M, Hilaris B, Brennan M. Comparison of conventional surgical resection, radioactive implantation, and bypass procedures for exocrine carcinoma of the pancreas 1975–1980. Ann Surg. 1984;199:1.
143 Sarr MG, Cameron JL. Surgical management of unresectable carcinoma of the pancreas. Surgery. 1982;91:123.
144 Moertel CG, et al. Therapy of locally unresectable pancreatic carcinoma: Randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. Cancer. 1981;48:1705.
145 Seydel HG, et al. Hyperfractionated radiation and chemotherapy for unresectable localized adenocarcinoma of the pancreas. Cancer. 1990;65:1478.
146 Hogstrom KR, et al. Static pion beam treatment planning of deep-seated tumors using computerized tomographic scans. Int J Radiat Oncol Biol Phys. 1979;5:875.
147 Al-Abdulla ASM, et al. Experience with fast neutron therapy for unresectable carcinoma of the pancreas. Int J Radiat Oncol Biol Physics. 1981;7:165.
148 Cohen L, et al. Response of pancreatic cancer to local irradiation with high energy neutrons. Cancer. 1985;56:1235.
149 Kaul R, et al. Pancreatic carcinoma: results with fast neutron therapy. Int J Radiat Oncol Biol Phys. 1981;7:173.
150 Smith FP, et al. Fast neutron irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Physics. 1981;7:1527.
151 Castro JR, et al. Current status of clinical particle radiotherapy at Lawrence Berkeley Laboratory. Cancer. 1980;46:633.
152 Castro JR, et al. Clinical problems in radiotherapy of carcinoma of the pancreas. Am J Clin Oncol. 1982;5:579.
153 Linstadt D, et al. Comparison of helium-ion radiation therapy and split-course megavoltage irradiation for unresectable adenocarcinoma of the pancreas. Final report of a Northern California Oncology Group Randomized Prospective Clinical Trial. Radiology. 1988;168:261.
154 Klaassen DJ, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-Flourouracil alone with radiation plus concurrent and maintenance 5-flourouracil—An Eastern Cooperative Oncology Group Study. J Clin Oncol. 1985;3:373.
155 Talamonti M, et al. Eastern Cooperative Oncology Group phase I trial of protracted venous infusion fluorouracil plus weekly gemcitabine with concurrrent radiation therapy in patients with locally advanced pancreas cancer. J Clin Oncol. 2000;18:3384.
156 Crane C, et al. Is the therapeutic index better with gemcitabine based chemoradiation than chemoradiation in locally advanced pancreatic cancer? Int J Radiat Biol Phys. 2002;52:1293.
157 Huguet F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326.
158 Krishnan S, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47.
159 Ko A, Tempero MA. Personalized medicine for pancreatic cancer: a step in the right direction. Gastroenterology. 2009;136:43. Epub 2008, Nov. 28
160 Joyce F, et al. Ultrasonically guided percutaneous implantation of Iodine-I-125 seeds in pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1049.
161 Dobelbower RR, et al. I-125 interstitial implant, precision high dose-rate external beam therapy, and 5-FU for unresectable adenocarcinoma of the pancreas and extrahepatic biliary tree. Cancer. 1986;58:2185.
162 Mohiuddin M, et al. Long-term results of combined modality treatment with I-125 implantation for carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1992;23:305.
163 Montemaggi P, et al. Interstitial brachytherapy for pancreatic cancer: report of seven cases and review of the literature. Int J Radiat Oncol Biol Phys. 1991;21:451.
164 Syed AMN, Puthawala AA, Neblett DL. Interstitial Iodine-125 implant in the management of unresectable pancreatic carcinoma. Cancer. 1983;52:808.
165 Shipley WU, et al. Iodine-125 implant and external beam irradiation in patients with localized pancreatic carcinoma. A comparative study to surgical resection. Cancer. 1980;45:709.
166 Peretz T, et al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys. 1989;17:931.
167 Warszawski N, et al. Combined isodose curves of high-dose rate interstitial brachytherapy with external beam radiation therapy in pancreatic carcinoma. Strahlentherapie und Onkologie. 1992;168:552.
168 Raben A, et al. Feasibility study of the treatment of primary unresectable carcinoma of the pancreas with 103-Palladium brachytherapy. Int J Radiat Oncol Biol Phys. 1996;35:351.
169 Abe M, et al. Clinical experience with intraoperative radiotherapy of locally advanced cancers. Cancer. 1980;45:40.
170 Dobelbower RR, et al. Treatment of cancer of the pancreas by precision high dose (PHD) external photon beam and intraoperative electron beam therapy (IOEBT). Int J Radiat Oncol Biol Phys. 1989;16:205.
171 Gunderson L, Martin JK, Earle JD. Intraoperative and external beam irradiation with or without resection: Mayo pilot experience. Mayo Clin Proc. 1984;59:691.
172 Latz D, Schraube P, Eble MJ. Primare strahlentherapie des inoperablen oder rezidivierten pankreaskarzinoms-heidelberger krankengut von 1982–1992. Strahlentherapie und Onkologie. 1993;169:387.
173 Roldan GE, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110.
174 Shipley WU, et al. Intraoperative electron beam irradiation for patients with unresectable pancreatic carcinoma. Ann Surg. 1984;200:289.
175 Tepper J, et al. Intraoperative radiation therapy of pancreatic carcinoma: A report of RTOG 85-05. Int J Radiat Oncol Biol Phys. 1991;21:1145.
176 Wood WC, et al. Intraoperative irradiation for unresectable pancreatic carcinoma. Cancer. 1982;49:1272.
177 Goldson AL. Intraoperative radiotherapy for pancreatic cancer—requiem or revival. Int J Radiat Oncol Biol Phys. 1991;21:1389.
178 Sindelar WF, Kinsella T. Randomized trial of intraoperative radiotherapy in unresectable carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1986;12:148.
179 Tepper JE, et al. The role of misonidazole combined with intraoperative radiation therapy in the treatment of pancreatic carcinoma. J Clin Oncol. 1987;5:579.
180 Gunderson LL, et al. Intraoperative and external beam irradiation ± 5-FU for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1987;13:319.
181 Foo ML, Gunderson LL, Nagorney DM. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation ± 5-fluorouracil. Int J Radiat Oncol Biol Phys. 1993;26:483.
182 Phan GQ, et al. Pancreaticoduodenectomy for selected periampullary neuroendocrine tumors: fifty patients. Surgery. 1997;122:989.
183 Samloski W, Eyre HJ, Sause W. Evaluation of the response of unresectable carcinoid tumor to radiotherapy. Int J Radiat Biol Phys. 1986;12:301.
184 Schupak K, Wallner K. The role of radiation therapy in the treatment of locally unresectable or metastatic carcinoid tumors. Int J Radiat Biol Phys. 1991;20:489.
185 Chakravarthy A, Abrams R. Radiation therapy in the management of patients with malignant carcinoid tumor. Cancer. 1995;75:1386.
186 Kulke MH, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401.
187 Van Cutsem E, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231. Epub March 23, 2009
188 Kwekkeboom DJ, et al. Radiolabeled somatostatin analog (177Lu-DOTA0, Tyr3) octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754.
189 Ong SL, et al. A fuller understanding of pancreatic neuroendocrine tumours combined with aggressive management improves outcome. Pancreatology. 2009 Aug 4;9:583. (Epub ahead of print)
190 National Comprehensive Cancer Network, NCCN clinical practice guidelines in oncology: neuroendocrine tumors. V.2.2009 Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed 9/2/2009