50 Cancer of the Ovary
Epidemiology and Diagnosis
In 2009, ovarian cancer will be diagnosed in approximately 21,550 American women, and an estimated 14,600 will die of the disease, making it the fifth most common cancer in women and the most common cause of gynecologic cancer mortality.1 Approximately 1 in 70 women will develop ovarian cancer in their lifetime. Ovarian cancer is more common in northern European and North American countries than in Asia, developing countries, or southern continents.
The etiology of ovarian cancer is unknown, but risk factors have been identified (Table 50-1). Recognized risk factors for the disease include advancing age,2 with an annual incidence of 1 in 11,000 at age 40, 1 in 4500 at age 50, 1 in 2600 at age 60, and 1 in 2000 at age 70. Other factors associated with an increased risk include infertility, endometriosis, the use of assisted reproductive technologies, and application of perineal talc.3 The incremental risk for developing ovarian cancer associated with any of these age-independent risk factors is relatively small, with relative risks of no more than 2 to 3 compared to the general population. Smoking, alcohol use, coffee consumption, and viral infections (such as mumps) have not been associated with increased risk.
Approximately 8% to 13% of ovarian cancer is due to inherited mutations4,5 in the cancer susceptibility genes BRCA1 and BRCA. Women with mutations in the BRCA1 gene have a 35% to 60% risk for developing ovarian cancer by age 70, corresponding to a relative risk of approximately 30 to 45 compared to the general population.6 BRCA2 mutations are associated with a 10% to 27% risk of ovarian cancer by age 70, which corresponds to a relative risk of 6 to 20. Approximately 1% to 2% of ovarian cancers are associated with inherited defects in the DNA mismatch repair genes MLH1, MSH2, and MSH6, associated with the hereditary nonpolyposis colon cancer (HNPCC) syndrome.7 Women with mutations in these genes have a 9% to 12% risk of developing ovarian cancer by age 70, corresponding to a relative risk of 6 to 9.
In the absence of genetic testing information, a family history of ovarian cancer or early-onset breast cancer has been associated with an increased risk of ovarian cancer, with a relative risk of approximately 3 to 5 compared to the general population.8 It is not clear, however, how much of this increased risk is accounted for by mutations in the known ovarian cancer susceptibility genes.
In regard to protective factors, multiple studies have demonstrated that oral contraceptive use and parity are associated with a decreased risk of ovarian cancer.9 Use of oral contraceptives is protective for ovarian cancer, with an average relative risk of about 0.7 for women who have used oral contraceptives for 2 years and a 0.5 relative risk for women who have used oral contraceptives for 5 years or longer. A woman’s risk for ovarian cancer also may be reduced if she has had tubal sterilization, a hysterectomy, or at least one child whom she has breastfed.10
Screening
One of the most significant ways to improve survival for patients with ovarian cancer would be to find a way to screen women for ovarian cancer and detect the disease before it spreads beyond the ovary. Recommendations for ovarian cancer screening have traditionally been stratified according to level of risk, with different recommendations put forth for women at average risk compared to women at increased risk. With the identification of autosomal dominant ovarian cancer susceptibility syndromes, the increased risk group has been further stratified to a group with a clear inherited risk and a group with increased risk by virtue of family history but in the absence of a known inherited cancer predisposition (Table 50-2).
| Women At or Near the General Population Risk (Relative Risk Less Than 3) |
HNPCC, Hereditary nonpolyposis colon cancer.
* A close relative is defined as a first-, second- or third-degree relative.
There have been a number of tests evaluated as potential methods for screening for ovarian cancer. Screening tests with the greatest evidence base include serum CA125 and transvaginal ultrasound. CA125, a high molecular-weight protein excreted by greater than 90% of advanced epithelial ovarian cancers, has been the most evaluated serum marker for ovarian cancer screening. In the largest study to date, 22,000 postmenopausal women at average risk of ovarian cancer were randomized to either annual serum CA125 or usual gynecologic care.11 Ultrasound was performed in women in the screening arm if the CA125 was greater than 30 U/mL. In this study, women with screen-detected ovarian cancer had improved survival compared to women diagnosed with ovarian cancer who were randomized to usual care. While these results were promising, there was no difference between the two groups in the number of deaths due to ovarian cancer. To improve the utility of CA125 measurements for ovarian cancer screening, a method has been proposed which focuses on the change in CA125 concentration over time as opposed to the absolute value.
In the largest study to date evaluating ultrasound as a screening method for ovarian cancer, 14,469 women predominantly at average risk for ovarian cancer were followed with annual transvaginal ultrasounds.13 In this study, ultrasound was associated with an 81% sensitivity and a 98.9% specificity, resulting in a positive predictive value of 9.4%. Several studies have evaluated the simultaneous use of transvaginal ultrasound and CA125. These studies have suggested that the combination of these tests result in a higher sensitivity for ovarian cancer detection, but at the cost of an increased rate of false-positive results. In the ongoing PLCO trial, 28,816 women were randomized to receive annual transvaginal ultrasound and CA125. At baseline, 1338 (4.7%) ultrasounds and 402 (1.4%) CA125s were abnormal. Workup of these abnormalities led to the diagnoses of 20 invasive ovarian cancers. The positive predictive values for an abnormal test were 1% for transvaginal ultrasound and 3.7% for CA125. This did, however, increase to 23.5% when both were abnormal.14
In 2002, a report from researchers at the National Cancer Institute suggested that by evaluating the low molecular protein spectrum in peripheral serum, one could identify a characteristic “proteomic” pattern that was both highly sensitive (100%) and specific (95%) for early ovarian cancer.15 The initial report was promising, but this approach has not been validated in a prospective screening study.
Numerous national organizations such as the American Cancer Society (ACS), the American Congress of Obstetricians and Gynecologists (ACOG), the Society of Gynecologic Oncologists (SGO), and the National Comprehensive Cancer Network (NCCN) have made ovarian cancer screening recommendations. Based on these recommendations, ovarian cancer screening guidelines stratified by ovarian cancer risk type are shown in Table 50-3.
Table 50-3 Ovarian Cancer Screening Guidelines
Histology
Ovarian tumors are usually categorized16 by their tissue of origin: epithelial (from the coelomic epithelial cells that line the ovary), germ cell (from the germinal epithelium), and sex cord–stromal (from the mesenchymal tissue of the ovary). Epithelial tumors account for approximately 85% of ovarian cancers, germ cell cancers account for approximately 10%, and the remaining 5% of tumors are sex cord–stromal (Table 50-4).
Table 50-4 Histologic Classification of Common Epithelial Tumors of the Ovary
| Serous Tumors |
Modified from Ozols RF, Rubin SC, Thomas GM, et al: Epithelial ovarian cancer. In Hoskins WJ, Perez CA, Young RC, et al, editors: Principles and practice of gynecologic oncology, ed 4, Philadelphia, 2005, Lippincott Williams & Wilkins, pp 895–987.
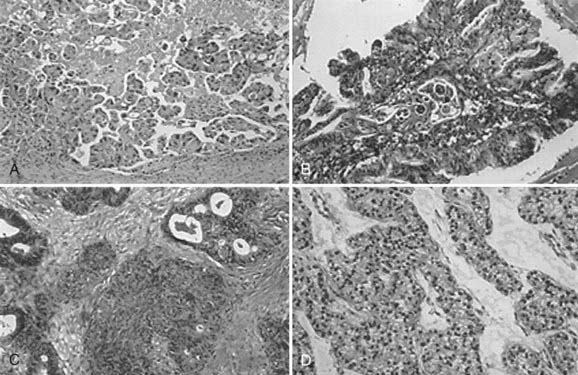
Of the malignant epithelial ovarian cancers, 40% to 50% are serous tumors, 15% to 25% are endometrioid tumors, 6% to 16% are mucinous tumors, and 5% to 11% are clear cell tumors (Fig. 50-1). Transitional cell (Brenner), mixed epithelial, and undifferentiated carcinomas are encountered less frequently. Epithelial tumors may be benign, have low malignant potential, or be frankly malignant. Tumors of low malignant potential (borderline tumors) have a much better prognosis than the frankly malignant cancers, but they are nevertheless malignant and can result in death. Malignant tumors are further subdivided by histologic grade into either three grades based on architecture (International Federation of Gynecology and Obstetrics [FIGO] classification) or four grades based on nuclear atypia (Broders’ index).
Molecular Biology
In addition to abnormalities in BRCA1 or BRCA2 and DNA mismatch repair genes, advances in the molecular biology of ovarian cancer have shed some light on its pathogenesis, classification, and prognosis.17,18 The TP53 gene located on chromosome 17p belongs to the family of tumor-suppressor genes, and mutations in these genes have been implicated in malignant transformation and tumor progression. Mutation and loss of TP53 function is the most common genetic abnormality in ovarian cancer, especially high-grade serous cancer, and is seen in 60% to 80% of both sporadic and familial cases. There seems to be a correlation between the frequency of mutation in p53 and the stage of ovarian cancer (4% of preinvasive borderline, 10% to 20% of early cancers, and 40% to 60% of advanced cancers) and spread.19–21 HER2-neu belongs to the epidermal growth factor (EGF) receptor gene family and is a potent proto-oncogene. HER2-neu is overexpressed in approximately 15% to 20% of ovarian cancers, especially mucinous. Berchuck et al.22 reported that patients with increased HER2-neu expression had a survival median of 15.7 months, compared with 32.8 months in patients with normal neu expression. Mutations in K-ras, also a proto-oncogene, is seen in about 25% to 50% of mucinous ovarian cancer and most low-grade serous tumors.18
Diagnosis
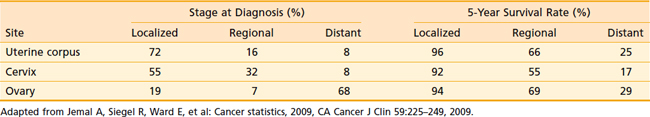
Table 50-5 illustrates the relative stage at diagnosis and the survival rate by stage for ovarian cancer in relation to the two other major gynecologic cancers. The 5-year survival rate for ovarian cancer by stage is not significantly different from the 5-year survival rate for other gynecologic cancers. However, at the time of diagnosis, two-thirds of patients with ovarian cancer have advanced-stage disease, unlike uterine corpus and cervical cancer, which are generally diagnosed in earlier stages. It is clear from these data that the single most important factor in the large number of deaths from ovarian cancer is failure to diagnose the disease at an early stage. Reasons for this failure correspond to the growth and spread patterns of this cancer. Because the ovary floats freely in the pelvic cavity, a tumor may grow for some time while producing only vague symptoms associated with involvement of, or pressure on, other organs. Thus ovarian cancer is diagnosed in most patients after the disease has spread beyond the ovary. In these patients, symptoms may be abdominal pain or a bloated feeling, gastrointestinal or urinary tract disturbances, or in many cases, the onset of clinically detectable ascites. Some patients with advanced disease have menstrual irregularity or postmenopausal bleeding. Occasionally a patient may have a palpable inguinal lymph node, tumor in a hernia sac, or a pleural effusion. For patients with advanced disease, diagnosis is established by tissue obtained at exploratory laparotomy. In rare instances when a patient cannot undergo surgery because of medical problems, the histologic or cytologic diagnosis is established by needle biopsy.
Table 50-5 Stage at Diagnosis and 5-Year Survival Rate of Gynecologic Cancers, United States, 1995-2001
Studies have shown that women with ovarian cancers, even women with early-stage disease, often experience symptoms several months before the diagnosis. In a survey of 1725 women with ovarian cancer, 70% recalled having had symptoms for 3 months or longer before the diagnosis, and 35% recalled having had symptoms for at least 6 months.23 About three-fourths of these women had abdominal symptoms, and half had pain or constitutional symptoms. Persistent symptoms such as an increase in abdominal size, abdominal bloating, fatigue, abdominal pain, indigestion, inability to eat normally, urinary frequency, pelvic pain, constipation, back pain, urinary incontinence of recent onset, or unexplained weight loss should be evaluated, with ovarian cancer being included in the differential diagnosis.
The use of tumor markers to assist in evaluation of a patient with an adnexal mass is appropriate, but misinformation may result. Serum CA125 is the only serum marker available with the potential accuracy to be beneficial, but even this marker is less than optimal. Approximately half of patients with early-stage ovarian and other cancers do not have elevated serum CA125 levels. Also, a variety of nonmalignant and nonovarian malignant conditions can result in elevated serum CA125 levels. These conditions are listed in Table 50-6. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful diagnostic and staging information, especially in delineating abdominal and pelvic masses and diagnosing retroperitoneal nodal involvement. The role of positron emission tomography (PET) is under investigation. In one study, the addition of PET to CT improved the staging accuracy from 85% to 93% outside the pelvis and from 79% to 81% in the pelvis.24 However, none of the procedures are sufficient enough for accurate staging, and laparotomy remains the single most important method for assessing the stage of disease and the design of treatment.
Table 50-6 Conditions That May Cause Elevated Serum CA125 Levels
| Malignant Conditions |
Staging
The FIGO staging classification scheme for ovarian cancer25 is outlined in Table 50-7. The staging of advanced disease (disease spread throughout the abdomen) may be obvious to most physicians, but a surgeon must be meticulous in the staging of early ovarian cancer. One study found that a third of patients referred with stage I or stage II disease were found to actually have stage III disease when the appropriate staging procedure was performed. Other researchers have reported similar results.
Table 50-7 FIGO Staging Classification for Ovarion Cancer
Rights were not granted to include this table in electronic media. Please refer to the printed book.

Table 50-8 outlines the recommended surgical staging procedures used for ovarian carcinoma that appears to be confined to the pelvis. The appropriate performance of a staging operation requires an understanding of the spread patterns of the cancer. Ovarian cancer can spread by direct infiltration of pelvic structures such as the pelvic peritoneum, bladder surface, rectal surface, fallopian tube, broad ligament, or uterus. Lymphatic spread occurs early, with nodal metastases present in 10% to 12% of patients with stage I cancer and 20% to 25% of patients with stage II disease. In stage III and stage IV, the incidence of positive lymph nodes is 50% to 70%. By far the most significant spread of ovarian cancer, however, is due to exfoliation of clonogenic cells into the peritoneal cavity. These cells are swept up the right abdominal gutter to the diaphragm and omentum by the clockwise flow of peritoneal fluid in the abdomen. The cells implant, form tumor nodules, and in turn exfoliate more cells. The normal daily activities of the patient and normal peristalsis of the intestine can result in spread of the disease throughout the abdominal cavity. Proper staging requires a generous lower and upper midline incision and meticulous exploration with multiple peritoneal and nodal biopsies.
Table 50-8 Diagnostic Procedures in the Diagnosis and Staging of Patients With Ovarian Carcinoma
Treatment of Primary Epithelial Ovarian Cancer
The treatment of epithelial ovarian cancer usually involves several types of therapy. Surgical therapy is the initial form of intervention, but it is curative in only a small percentage of cases. Usually, adjunctive chemotherapy is necessary. In a large percentage of patients, some type of salvage therapy is important. Table 50-9 outlines current therapeutic recommendations for epithelial ovarian cancer. The role of radiation in the management of ovarian carcinoma continues to represent a topic of considerable controversy, and the indications for its use are not fully established.
Table 50-9 Recommended Therapy for Epithelial Ovarian Cancer
| Category of Ovarian Cancer | Recommended (Standard) Therapy |
|---|---|
| Early Ovarian Cancer | |
| Low risk (stages IA and IB, grade 1*) | TAH, BSO†, full surgical staging |
| High risk (stages IA and IB, grades 2 and 3; stages IC, IIA, IIB, and IIC, no residual) |
BSO, Bilateral salpingo-oophorectomy; TAH, total abdominal hysterectomy.
* Some investigators include grade 2 in the low-risk category.
† Unilateral salpingo-oophorectomy is permissible in patients who desire further childbearing.
‡ Optimal (<1 cm residual tumor).
§ Suboptimal (stage III or IV, >1 cm residual tumor).
Surgery Alone for Early-Stage Disease
Although surgery alone is inadequate treatment for most ovarian cancers, a subset of patients exists in whom surgery alone is considered sufficient. In a Gynecologic Oncology Group (GOG) randomized trial, 81 patients with well-differentiated or moderately differentiated cancers confined to the ovaries (surgical stage IA and IB) were assigned to receive 12 cycles of melphalan or no further therapy.26 With a median follow-up of more than 6 years, there were no significant differences between the patients given no chemotherapy and those treated with melphalan with respect to either 5-year disease-free survival (91% versus 98%; P = 0.41) or overall survival (94% versus 98%; P = 0.43).
Primary Cytoreductive Surgery for Advanced-Stage Disease
In a meta-analysis of studies of the effects of cytoreductive surgery for patients with stage III or stage IV ovarian carcinoma, investigators found that survival was improved among patients referred to expert centers for primary surgery.27 Expert centers were described as those that attained optimal cytoreduction in 75% or more of advanced-stage cases. To achieve this goal, upper abdominal surgical procedures such as liver mobilization, diaphragm peritonectomy, and splenectomy are frequently required.28 In expert centers, the performance of these procedures if necessary to attain optimal cytoreduction can lead to significantly improved survival.29 Although different authors have used various cutoffs to define “optimal” cytoreduction, the cutoff used by GOG and the one most commonly reported is 1 cm or less. This amount of residual disease is associated with a survival advantage, but the best survival rates are achieved in cases where all grossly visible tumor is removed.30
Interval Cytoreductive Surgery for Advanced-Stage Disease
Even in expert centers, a significant percentage of patients with advanced ovarian cancer will not be able to have optimal cytoreduction. Thus, a significant number of patients begin initial adjunctive therapy with large-volume disease. To address this issue, a European cooperative group trial analyzed the benefit of a second cytoreductive procedure during the primary chemotherapy. This second surgery was referred to as interval cytoreduction. In this trial, patients with suboptimal residual disease were randomized to receive six courses of cisplatin and cyclophosphamide versus three courses of the same regimen, interval cytoreduction, and then another three courses of the same chemotherapy. These investigators reported a statistically significant improvement in both progression-free survival and overall survival for those undergoing interval cytoreduction.31
Subsequently, GOG performed another randomized trial of interval debulking. It was similar to the European trial except that in the American trial, the chemotherapeutic agents were cisplatin and paclitaxel.32 The researchers concluded that interval debulking did not improve progression-free survival or overall survival. The most accepted reason why these two trials still yielded conflicting results is that in the American trial, the initial surgeries were generally performed by gynecologic oncologists, so an aggressive attempt at cytoreduction was attempted, whereas in the European trial this was not the case.
Chemotherapy for Early-Stage Disease
With the exception of patients with well-differentiated to moderately differentiated surgical stage IA to IB, most patients with early-stage ovarian cancer receive adjuvant chemotherapy. The European Organization for Research and Treatment of Cancer (EORTC) reported the results of a randomized trial33 in 448 patients with stage IA to IB, grade II-III, and all stages IC to IIA. Patients were assigned to either observation or platinum-based chemotherapy. With a median follow-up of 5.5 years, the 5-year disease-free survival rate was 68% in the observation arm compared to 76% in the chemotherapy arm (P = 0.02), but this difference did not translate into overall survival advantage (78% for observation compared to 85% for chemotherapy; P = 0.1). The International Collaborative Ovarian Neoplasm (ICON) 1. There were 477 patients with early-stage disease, including grade 1, who were randomized to no further therapy or 6 cycles of chemotherapy. The 5-year overall survival was 70% in the observation arm compared to 79% in the chemotherapy arm (P = 0.03). Some of the shortcomings of this trial involved the inclusion of patients with low-risk disease (stage IA to IB, FIGO grade 1 or 2), where adjuvant chemotherapy was not needed, and the type of chemotherapy was to some extent left to the discretion of the treating physician.34 In the United States, most experts recommend adjuvant chemotherapy for patients with high-risk early-stage disease, and the emphasis has been on how much chemotherapy is needed in this group. In the GOG-157 trial, patients with stage IA to IB FIGO grade 3 and all stage IC to II were randomized to either 3 or 6 cycles of carboplatin/paclitaxel. The recurrence rate for 6 cycles was 24% lower (hazard ratio [HR]: 0.761; 95% confidence interval [CI]: 0.51-1.13, P = 0.18), and the estimated probability of recurrence within 5 years was 20.1% (6 cycles) versus 25.4% (3 cycles). The overall death rate was similar for these regimens (HR: 1.02; 95% CI: 0.662-1.57). The conclusion from this trial was that compared to 3 cycles, 6 cycles of carboplatin/paclitaxel do not significantly alter the recurrence rate in high-risk early-stage ovarian cancer but are associated with more toxicity.35
Chemotherapy for Advanced-Stage Disease
Currently in the United States, the standard treatment approach for advanced-stage ovarian cancer consists of total abdominal hysterectomy/bilateral salpingo-oophorectomy (TAH/BSO), staging, and an attempt at maximal surgical debulking followed by 6 cycles of carboplatin/paclitaxel. Two landmark trials (GOG-111 and Intergroup/EORTC) demonstrated that paclitaxel plus cisplatin improved response rates, progression-free survival, and overall survival compared to cyclophosphamide and cisplatin.36,37 GOG-158 compared cisplatin/paclitaxel to carboplatin/paclitaxel and found no difference in outcome but less toxicity with carboplatin/paclitaxel.38 Two other randomized trials comparing the same regimens demonstrated identical results.39,40 These results make paclitaxel plus carboplatin, with both drugs delivered intravenously (IV) every 3 weeks for a total of 6 cycles, a reasonable standard approach for most patients with advanced ovarian cancer following cytoreductive surgery. Prior to the availability of paclitaxel, a randomized GOG trial compared IV cisplatin and cyclophosphamide to intraperitoneal (IP) cisplatin plus IV cyclophosphamide in patients with optimal debulked stage III ovarian cancer. The risk of death was lower in the IP group (hazard ratio, 0.76; 95% CI: 0.61-0.96; P = 0.02), and the toxicity was less compared to the IV arm.41 An intergroup trial compared IV cisplatin plus paclitaxel to IV carboplatin followed by IV paclitaxel plus IP cisplatin. This study also demonstrated a trend toward improvement in survival.42 The most recent GOG-172 trial comparing IV paclitaxel and cisplatin to IV paclitaxel plus IP cisplatin and IP paclitaxel also showed significant improvement in disease-free and overall survival in favor of IP chemotherapy.43 This trial along with two others that have demonstrated improved survival for the IV/IP approach led the National Cancer Institute to issue an NCI Clinical Announcement in January 2006 that recommended that women with optimally debulked stage III ovarian cancer be counseled about the clinical benefit associated with combined IV and IP administration of chemotherapy.
Radiation
Because the entire peritoneal cavity is at risk for metastatic dissemination, the radiation treatment technique must encompass the whole peritoneum. One approach that has been extensively used to treat the peritoneal cavity is via IP radioisotopes. The radioisotope most commonly used for treating ovarian cancer is phosphorus-32 (32P) in the form of chromic phosphate colloid (32P-CP). The 32P isotope is a pure beta emitter and has a half-life of 14.3 days. Its beta particles have a mean energy of 0.69 Mev and tissue penetration of 3 to 5 mm. The fact that there is no gamma emission provides significant safety to the treating staff. The standard dose of 32P is 15 mCi given in a single IP application. The colloidal phase increases the isotope uptake by macrophages and mesothelial peritoneal cells, and decreases systemic elution. Several prospective randomized trials have looked at 32P versus chemotherapy, and all have shown equivalent survival rates. The GOG randomized 141 patients with poorly differentiated stage I and stage II tumors to melphalan or 15 mCi of intraperitoneal 32P. With a median follow-up of more than 6 years, outcomes for the two treatment groups were similar with respect to 5-year disease-free survival (80% in both groups) and overall survival (81% with melphalan versus 78% with 32P, P = 0.4). In the 32P arm, 6% of patients required laparotomy for bowel obstruction compared to 3% leukemia rate in the melphalan arm.26 The critics of this study point out that 32P was not compared to cisplatin-based chemotherapy, which represents the standard chemotherapy approach. A second reported GOG trial randomized 205 patients with stage I to IIA (high risk) to 15 mCi intraperitoneal 32P versus 3 cycles of cyclophosphamide and cisplatin. With a median follow-up of 6 years, there was no statistically significant difference in overall 5-year survival rates (84% chemotherapy arm versus 76% 32P arm). The authors concluded that chemotherapy is better because of the better progression-free interval.44
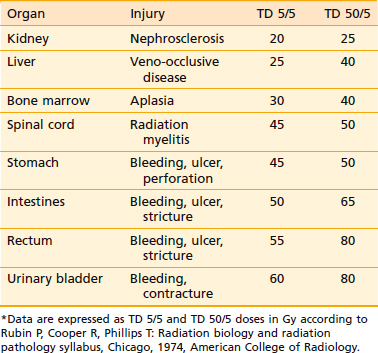
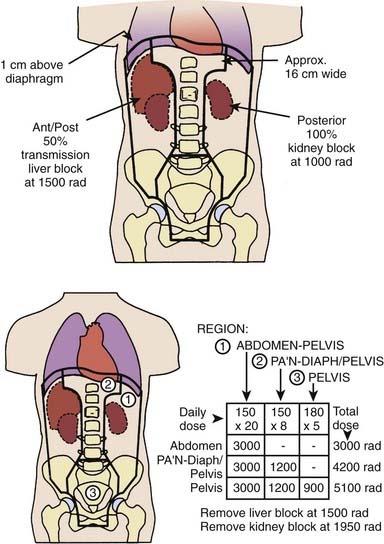
The other approach is whole abdominal irradiation (WAR), which is given by the open-field technique via anterior and posterior ports shaped to encompass the entire peritoneal cavity as well as pelvic and paraaortic lymph nodes.45 The probability of tumor control with radiation is dependent not only on the delivery of tumoricidal doses, but also on the feasibility of encompassing all sites of disease with sufficient radiation doses without significantly injuring normal tissues. Table 50-10 shows the tolerance of the critical normal organs encompassed by WAR, expressed in terms of the TD 5/5 and TD 5/50 according to Rubin et al.46 The data indicate that when whole abdominal fields are used, the limited tolerance of the liver, kidneys, and spinal cord would practically rule out eradication of visible tumor masses in the upper abdomen, regardless of tumor size. For pelvic fields, the tolerance of the intestines, rectum, and bladder would restrict the delivery of tumoricidal doses to tumors of less than 2 cm in size. This analysis suggests that only patients with minimal or microscopic residua would be candidates for cure by radiotherapy. The treatment fields extend from above the domes of the diaphragm to below the obturator foramina, and laterally beyond the peritoneal reflection (Fig. 50-2). The use of CT-based planning to accurately visualize the peritoneal reflections is very helpful.47 Radiation is delivered at a rate of 1.2 to 1.5 Gy/day. The total dose to this field is 30 Gy in 20 fractions over 33 days, although the kidneys are shielded at 20 Gy and the liver at 25 Gy to protect these organs from radiation damage. The paraaortic region is usually carried to an additional 15 Gy and the lower abdomen to an additional 20 to 25 Gy. There have been several modifications to the open-field technique, mostly designed to increase the dose to areas of high risk, such as the paraaortic region and the diaphragms. The technique described by Martinez et al.48 responds to these criteria and also implements a fractionation scheme that improves the tolerance of treatment. Treatment according to the Martinez technique (Fig. 50-3) is initiated with 9 Gy to the true pelvis via anterior and posterior open fields at daily fractions of 1.5 to 1.8 Gy. Subsequently, 30 Gy are given at this fractionation scheme to a classic whole abdominal field, and then 12 Gy through a “T-shaped” field, which includes the paraaortic and the medial diaphragmatic regions. Reports on the use of this technique have demonstrated it to be well tolerated.
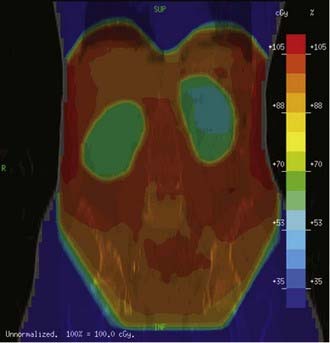
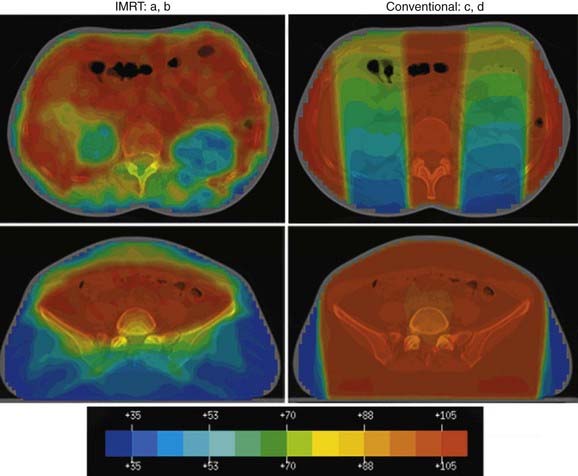
Because these fields are usually very large (40 cm × 30 cm or larger), most patients experience acute side effects, including myelosuppression, which can deplete bone marrow reserve and interfere with current or subsequent chemotherapy. Intensity-modulated radiation therapy (IMRT) has been shown to provide adequate coverage of the whole abdomen while minimizing the dose to the bone marrow (Fig. 50-4). An added benefit of IMRT was the ability to keep the dose to the liver and kidneys at tolerance levels, without having to use blocks that often lead to cold spots in the treated target.49,50
Several randomized trials have compared WAR to chemotherapy, including two trials with cisplatin-based chemotherapy. Most of these trials showed no statistically significant difference in survival but a trend toward improvement in disease-free survival in some. The Princess Margaret Hospital (PMH) group in Toronto reported on a group of 190 patients with stage IB, II, and asymptomatic stage III disease who were randomized to receive adjuvant pelvic irradiation, pelvic irradiation plus oral chlorambucil for 2 years, or WAR.51,52 Survival differences were observed only in those patients who had a complete resection of disease. In this group, WAR achieved a 78% 5-year and 64% 10-year survival, compared with 51% and 40%, respectively, in patients receiving pelvic irradiation with or without chlorambucil. The improved survival of patients treated with WAR appeared to be due to a 30% decrease of relapses in the upper abdomen. Two other randomized trials compared WAR (moving-strip technique) to melphalan. One trial from M.D. Anderson Cancer center53 compared WAR to 12 cycles of melphalan, reporting no difference in 5-year survival (71% versus 72%). The other was from the National Cancer Institute of Canada,54 comparing 3 arms: WAR, pelvic radiation, and melphalan, or pelvic radiation and intraperitoneal 32P. The corresponding 5-year overall survival rates were 62%, 61%, and 66%, respectively (P = 0.34). Sell et al. compared WAR (open field) to pelvic radiation and cyclophosphamide.55 There was no significant difference in disease-free survival or overall survival between the two arms. Two other randomized trials compared cisplatin to WAR. Redman et al.56 reported on 40 patients randomized to 5 cycles of cisplatin or WAR (moving strip) with 5-year survival rates of 62% and 58%, respectively (P = 0.66). Chiara et al. reported on 70 patients randomized to WAR or 6 cycles of cisplatin plus cyclophosphamide.57 The 5-year survival rate was 71% and 53% (P = 016), while disease-free survival was 74% and 50% (P = 0.07). Interestingly both of those trials were closed prematurely owing to lack of accrual, perhaps reflecting bias on the part of the treating physicians. The opponents of WAR point out that some of these trials showed a superior therapeutic ratio, since the complication rate with chemotherapy was lower. It is important to note, however, that the overlap of abutting fields with the moving-strip technique may have contributed significantly to the high incidence of gastrointestinal damage. When meticulous attention is paid to treatment techniques, the rate of serious toxicity should be low. In a randomized trial from Canada, 125 patients with optimally debulked stage I, II, and III disease were randomized to two different doses of WAR.58 The rate of any grade 3 complication in that trial was 4%. Most authors currently use the open-field technique with appropriate kidney shielding. The total dose is usually 25 to 30 Gy given at 10 to 15 Gy per fraction.
Consolidation Therapy
Approximately half of optimally cytoreduced patients who exhibit a complete pathologic response to chemotherapy eventually fail and succumb to ovarian cancer.59–61 Several approaches to treatment intensification have been tested in an attempt to improve the outcome.
Chemotherapy
A GOG/SWOG trial comparing 3 versus 12 months of paclitaxel in patients with advanced ovarian cancer who attained a clinically defined complete response following platinum/paclitaxel-based chemotherapy had to be closed prematurely because of significant improvement in disease-free survival (28 months for 12 monthly cycles compared to 21 months for 3 monthly cycles, P = 0.023). The data on overall survival, however, were not significantly different.62 In another trial from Italy, 200 patients with stages IIB to IV disease in clinical or pathologic complete response after 6 courses of paclitaxel/platinum-based chemotherapy were randomly allocated to either observation or 6 courses of paclitaxel 175 mg/m2 every 3 weeks. The conclusion from that study was that consolidation treatment with 6 cycles of paclitaxel did not prolong progression-free survival or overall survival in patients in complete response after first-line paclitaxel/platinum-based regimens.63
Another approach to dose intensification is intraperitoneal chemotherapy (IP). Barakat et al. reported on a phase II trial of IP cisplatin and etoposide as consolidation therapy in patients with stage II to IV ovarian cancer following negative surgical assessment.64 With a median follow-up of 36 months, 61% (22/36) of the treated patients were without evidence of recurrent disease. This group of patients was compared to a cohort of 46 contemporaneous patients who underwent observation only. The recurrence rate in this group was 54% (25/46), and when the protocol patients and the cohort were combined, the only predictor of improved disease-free survival was protocol treatment (P < 0.01). One important aspect of IP chemotherapy as consolidation is the size of disease at the time of initiation of IP chemotherapy. In a review of 433 patients who received IP chemotherapy65 at Memorial Sloan-Kettering Cancer Center (MSKCC), the median survival was 8.7 years for patients with negative second look, 4.8 years microscopic, 3.3 years for less than 1 cm, and 1.2 years for greater than 1 cm (237). Currently no phase III data exist on IP chemotherapy for consolidation, and since many of the patients who get this type of consolidation tend to have more favorable prognosis to begin with, it becomes difficult to determine its proper role.
Radiation
Several studies have attempted to consolidate complete responses to chemotherapy with radiation therapy. To evaluate intraperitoneal 32P, GOG completed a prospective randomized trial in 202 patients with negative second-look laparotomy.66 All patients were treated with postoperative platinum-based chemotherapy then were randomized to observation or 15 millicurie of 32P. There was no significant difference in disease-free survival (42% in the 32P arm versus 36% in observation; P = 0.27) or in overall survival (67% versus 63%, respectively; P = 0.19). Most protocols have used WAR, delivered after several courses of combination chemotherapy and a second-look cytoreductive effort. Lambert et al. reported on a randomized trial of 254 patients with advanced ovarian cancer who were treated with 5 cycles of carboplatin. Of the 254 patients, 117 patients with residual disease less than or equal to 2 cm were then randomized to WAR or another 5 cycles of carboplatin. There was no significant difference in disease-free or overall survival between the two arms.67 Sorbe reported on 742 patients with stage III ovarian cancer who received 4 cycles of cisplatin-based chemotherapy.68 Patients with complete pathologic response (n = 172) were randomized to no further therapy, 6 cycles of cisplatin-based chemotherapy, or WAR. In patients with microscopic residual disease (n = 74), the randomization was between WAR and chemotherapy. Disease-free survival in the group of patients with complete pathologic response was better with WAR or chemotherapy compared to observation (P = 0.04), but there was no difference between the chemotherapy and the WAR arms (P = 0.17). This difference in disease-free survival did not translate into survival improvement; the 3-year overall survival rate was 83% after WAR, 76% after chemotherapy, and 73% after observation (P = 0.11). In the group of patients with residual microscopic disease, there was no difference in overall survival; the 3-year survival rate was 46% after WAR and 56% after chemotherapy (P = 0.57).
In summary, consolidation with WAR seems to be at least equivalent to further chemotherapy. But if such investigations are to continue, attention to dosage and techniques in this subset of patients is very important, since most of them have had multiple laparotomies and several cycles of chemotherapy. Whelan et al. reported on 105 patients treated with WAR following chemotherapy for advanced ovarian cancer.69 The 5-year actuarial rate of severe bowel complication (requiring surgery and/or obstruction) was 13% ± 3.5%. The presence of both a dose of WAR greater than 2250 cGy and a second-look laparotomy prior to radiation was associated with an increased risk of serous bowel complications (P = 0.006).
Treatment of Recurrent/Persistent Disease
Surgery
Primary cytoreductive surgery is well accepted as the cornerstone of the initial management of ovarian cancer, but the use of cytoreductive surgery in the setting of recurrent disease is less clearly defined. Several studies have demonstrated a benefit of secondary cytoreduction, but a few have not. Due to the non-randomized nature of all the series on this topic, patient selection undoubtedly played a significant role in the findings and conclusions of these studies.70
A recent study suggested that until prospective, randomized data become available, the selection of patients who undergo secondary cytoreduction should be based on (1) the disease-free interval from the completion of primary therapy (6 to 12, 12 to 30, and over 30 months), (2) the number of sites of recurrence (single versus multiple versus carcinomatosis), and (3) the probability that cytoreduction to minimal residual disease can be achieved.71 For example, a patient who developed carcinomatosis 6 to 12 months from chemotherapy should not be considered for secondary cytoreduction. Conversely, a patient with single site of recurrence and a greater than 30-month interval represents an ideal scenario for surgery.
Chemotherapy
For patients with small-volume persistent or recurrent disease confined to the peritoneal cavity and who are felt to have platinum-sensitive disease, IP chemotherapy may be a reasonable regimen if they have not already received it.65 Systemic chemotherapy should be considered for patients with large tumors (>1 cm) or with disease located outside the peritoneal cavity. Patients who respond to platinum often can be re-treated with one of the platinum compounds alone or in combination with other agents. The longer the disease-free interval from primary chemotherapy, the better the chances of response; patients who have a disease-free interval of 2 years or more respond at a rate similar to that of patients with newly diagnosed cancer.72
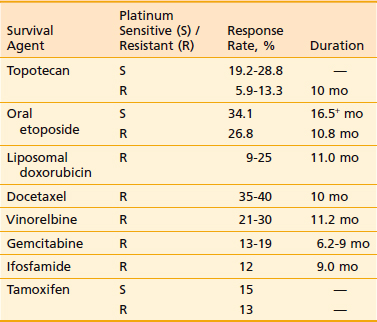
Patients who are platinum and paclitaxel resistant are candidates for experimental trials. In the absence of an experimental trial, modest response rates have been reported with topotecan, gemcitabine, doxorubicin, ifosfamide, hexamethylmelamine, and low-dose etoposide (VP-16) administered orally73–88 (Table 50-11). Low-dose etoposide administered orally, however, has been associated with an increased risk of leukemia, so its use has not been recommended for patients who have a long life expectancy (rarely a consideration for patients with platinum-resistant disease).
Radiation
The role of radiation in the adjuvant setting has been on the decline, but its use for patients with recurrent or persistent disease has been on the rise.89 Unfortunately, recurrent or persistent disease after curative-intent chemotherapy is rather common. The median survival in these patients is relatively short. Palliation of symptoms, especially in patients with pelvic disease, could have significant impact on the quality of life of patients in their final months of life. A review was conducted on patients treated with palliative radiotherapy (RT) for symptomatic ovarian cancer at The Ottawa Hospital Regional Cancer Centre between 1990 and 2003. There were 62 courses of RT delivered to 53 pts. The symptoms treated were: bleeding (40%), pain (37%), and “others” (23%). The most common dose fractionation scheme was 30 Gy in 10 fractions. The overall response rate was 100%, with 68% achieving a complete response. The complete-response rates were 88%, 65%, and 36% for the symptoms of bleeding, pain, and “others,” respectively (P = 0.003). The median duration of response was 4.8 months (range: 1 to 71 months). In multivariate analysis, the only factors found to be significant positive predictors of symptom control were: the symptom of bleeding (P = 0.015) and stage III to IV disease at presentation (P = 0.01). The most commonly reported toxicities were grades 1 and 2 nausea/vomiting and diarrhea. There were no grade 3/4 toxicities reported.90
Another important aspect of radiation in the setting of persistent/recurrent disease is limiting the field of radiation to the involved area. These patients are at high risk of radiation toxicity because they have been heavily treated with prior surgeries and multiple chemotherapeutic agents. Albuquerque et al.91 reported on 20 patients with a diagnosis of epithelial ovarian cancer who received tumor volume-directed RT for localized extraperitoneal recurrences (either as consolidation following debulking surgery or as attempted salvage if unresectable). All patients were heavily pretreated with multiple chemotherapy regimens. Eleven patients had optimal debulking of their recurrences prior to radiation. The median RT dose was 50.4 Gy. Of 20 patients, 17 had a complete response after RT. The actuarial LRFS, OS, and DFS at 5 years from date of radiation were 66%, 34%, and 34%, respectively. The LRFS at 3 years was 89% for those with optimal resection compared to 42% for those with gross residual/unresectable tumor, which was significantly better (P = 0.04). The corresponding 3-year DFS was 72% compared to 22% and 5-year OS was 50% compared to 19%, respectively. Acute complication of RT was mild; half had grade 1 to 2 gastrointestinal (GI) toxicity, and three patients had grade 3 to 4 late GI effects. The conclusion from this study was that involved-field RT is effective in controlling localized recurrences of ovarian cancer, especially after they are optimally debulked (89% local control and 50% 5-year overall survival in this subgroup), and it is relatively well tolerated in these heavily pretreated patients. At MSKCC, involved-field RT is generally used, and the dose is usually 45 to 50.4 Gy given at 1.8 Gy for patients with good performance status. When the aim of RT is purely palliative, especially in patients with poor performance status and short life expectancy, then 30 to 35 Gy given at 2.5 to 3 Gy per fraction could be used. With the increased use of chemotherapy in patients with ovarian cancer, the brain is becoming a sanctuary site for late site of relapse. There have been several reports in the literature on how to manage these patients.92–94
Ovarian Germ Cell Tumors and Stromal Tumors
The most common presentations are vague abdominal pain and a palpable pelvic mass. Elevated serum human chorionic gonadotropin (hCG) is seen in some dysgerminomas and most nongestational choriocarcinoma. High levels of α-fetoprotein (AFP) indicate the presence of yolk sac elements and are mostly elevated in endodermal sinus tumors. At the time of diagnosis, most women with germ cell tumors present with stage I disease (70%) and 25% to 30% with stage III. The main treatment is surgery, which includes unilateral salpingo-oophorectomy, to preserve fertility,95,96 and full surgical staging. For patients with surgical stage I disease, the decision on whether adjuvant chemotherapy is needed depends on the histology and grade of the tumors. Observation is a valid option for stage I dysgerminoma or low-grade immature teratoma. But for those with high-grade teratoma, endodermal sinus tumors, nongestational choriocarcinoma, or embryonal carcinoma, chemotherapy is recommended. Typically 3 to 4 cycles of bleomycin, etoposide, and cisplatin (BEP) are given every 21 days. For several decades, radiation therapy has been the traditional postoperative treatment for patients with dysgerminoma.97,98 Although dysgerminoma is exquisitely radiosensitive, and survival rates are excellent, fertility is almost always destroyed. To achieve equal efficacy with acceptable toxicity and also preserve fertility, chemotherapy has become the standard of care in these patients. For advanced-stage disease, the surgery should include maximal cytoreduction followed by 4 to 6 cycles of BEP. With the use of combination chemotherapy, cure rates of 90% and 60% to 80% are achievable in early-stage and advanced-stage disease, respectively.99–101
The surgical approach in patients with sex cord–stromal tumors is similar to that of epithelial tumors, except in young patients where fertility-sparing procedures should be considered. There is no role for adjuvant therapy in completely resected stage I disease. The role of chemotherapy in more advanced stages is not well defined. A GOG study looked at the influence of BEP on outcome in 57 patients with incompletely resected sex cord–stromal tumors, but the results were not as encouraging as those with germ-cell tumors using the same regimen.102 The data suggest that radiation therapy can induce a clinical response, with occasional long-term remission in patients with persistent or recurrent granulosa-cell tumors.103
Future Directions
With the large body of data on chemotherapy in ovarian cancer, it seems that the role of radiation has been completely abandoned. This is an unfortunate development, especially since radiation has been shown to be very effective. While it is unlikely for radiation to regain the upper hand, the focus should be on how best to integrate radiation and chemotherapy. Especially since the benefit of “conventional” chemotherapy seems to have plateaued. In a recent randomized trial, Bookman et al. reported no improvement in progression-free or overall survival in 4312 women with stages III to IV disease who were then randomly assigned among five arms that incorporated gemcitabine, methoxypolyethylene glycosylated liposomal doxorubicin, or topotecan compared with carboplatin and paclitaxel.104 As we have shown in this chapter, it is clear that about 50% of patients with locally advanced disease will still relapse even after achieving a complete response to paclitaxel/platinum-based chemotherapy. Data from the Gray Laboratory suggest that the initial shoulder of the cell survival curve is made of a hypersensitive initial slope to 0.6 to 0.8 Gy followed by a plateau to 1 to 1.2 Gy followed by a straight-line portion.105 This potential hyperkilling of tumor cells at low doses of radiation could be used in addition to standard chemotherapy without potentially increasing the risk of complications from radiation. With intensity-modulated radiation therapy, WAR could be delivered more accurately, since no kidney blocks are required. But more importantly, the dose to the bone marrow, a dose-limiting structure for chemotherapy, is much lower with intensity-modulated radiation therapy than conventional radiation. Such innovative approaches may allow radiation to gain back some of its preeminence in the multimodality management of this disease. Ultimately, better understanding of ovarian cancer at the molecular level will open new venues on how best to manage this disease. One such interesting area is antiangiogenesis targeted therapy. Burger et al. recently reported an impressive 17.7% objective response rate using bevacizumab.105 This led the GOG to conduct a randomized trial testing whether the addition of bevacizumab to paclitaxel and carboplatin will be beneficial.
1 Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59(4):225-249. 2009 Jul-Aug
2 Ries LAG, Harkins D, Krapcho M, et al, editors; SEER cancer statistics review, 1975–2003; 2006; National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2003/. Last accessed: August 4
3 Ness RB, Cramer DW, Goodman MT, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155:217-224.
4 Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700-710.
5 Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
6 Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-1130.
7 Lynch HT, Casey MJ, Snyder CL, et al. Hereditary ovarian carcinoma: heterogeneity, molecular genetics, pathology, and management. Mol Oncol. 2009 Apr;3(2):97-137.
8 Bergfeldt K, Rydh B, Granath F, et al. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: a population-based cohort study. Lancet. 2002;360:891-894.
9 Edmondson RJ, Monaghan JM. The epidemiology of ovarian cancer. Int J Gynecol Cancer. 2001;11:423-429.
10 Kjaer SK, Mellemkjaer L, Brinton LA, Johansen C, Gridley G, Olsen JH. Tubal sterilization and risk of ovarian, endometrial and cervical cancer. A Danish population-based follow-up study of more than 65,000 sterilized women. Int J Epidemiol. 2004;33:596-602.
11 Kauff ND, Mitra M, Robson ME, et al. Risk of ovarian cancer in BRCA1 and BRCA2 mutation negative hereditary breast cancer families. J Natl Cancer Inst. 2005;97:1382-1384.
12 Jacobs IJ, Skates SJ, MacDonald N, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207.
13 van Nagell JR, DePriest PD, Reedy MB, et al. The efficacy of transvaginal sonographic screening in asymptomatic women at risk for ovarian cancer. Gynecol Oncol. 2000;77:350-356.
14 Buys SS, Partridge E, Greene MH, et al. Ovarian cancer screening in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630-1639.
15 Petricoin EF, Ardekani AM, Hitt BA, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572-577.
16 Ozols RF, Rubin SC, Thomas GM, Robboy SJ. Epithelial ovarian cancer. In: Hoskins WJ, Perez CA, Young RC, Barakat R, Markman M, Randall M, editors. Principles and practice of gynecologic oncology. ed 4. Philadelphia (PA): Lippincott Williams & Wilkins; 2005:895-987.
17 Bast RCJr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009 Jun;9(6):415-428.
18 Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009 Sep;40(9):1213-1223.
19 Kohler MF, Marks JR, Wiseman RW, et al. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst. 1993;85:1513.
20 Berchuck A, et al. The p53 tumor suppressor gene frequently is altered in gynecologic cancers. Am J Obstet Gynecol. 1994;170:246-252.
21 Havrilesky L, et al. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3814-3825.
22 Berchuck A, Rodriguez GC, Kamel A, et al. Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am J Obstet Gynecol. 1991;164:669.
23 Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89:2068-2075.
24 Yoshida Y, Kurokawa T, Kawahara K, et al. Incremental benefits of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. AJR Am J Roentgenol. 2004 Jan;182(1):227-233.
25 Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006 Nov;95(Suppl 1):S161-S192.
26 Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials [see comments]. N Engl J Med. 1990;322:1021.
27 Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:248-259.
28 Chi DS, Franklin CC, Levine DA, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94(3):650-654.
29 Eisenhauer EL, Abu-Rustum NR, Sonoda Y, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stage IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083-1090.
30 Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma? Gynecol Oncol. 2006;103:559-564.
31 van der Burg ME, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1995;332:629-634.
32 Rose PG, Nerenstone S, Brady M, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351(24):2489-2497.
33 Trimbos JB, Vergote I, Bolis G, et al. EORTC-ACTION collaborators. European Organization for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organization for Research and Treatment of Cancer—Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113.
34 International Collaborative Ovarian Neoplasm (ICON 1) Collaborators. International Collaborative Ovarian Neoplasm Trial 1: A randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J Natl Cancer Inst. 2003;95:125.
35 Bell J, Brady M, Young RC, et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage ovarian epithelial carcinoma: a gynecologic Oncology Group Study. Gynecol Oncol. 2006;102:432-439.
36 McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and Stage IV ovarian cancer. N Engl J Med. 1996 Jan 4;334(1):1-6.
37 Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699.
38 Ozols R, Bundy B, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3194-3200.
39 du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17):1320-1329.
40 Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084.
41 Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950.
42 Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001.
43 Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
44 Young RC, Brady MF, Nieberg RK, et al. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal P32 or intravenous cyclophosphamide and cisplatin—a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:4350-4355.
45 Fuks Z. External radiotherapy of ovarian cancer: standard approaches and new frontiers. Semin Oncol. 1975;2:253.
46 Rubin P, Cooper R, Phillips T. Radiation biology and radiation pathology syllabus. Chicago: American College of Radiology; 1974.
47 LaRouere J, Perez-Tamayo C, Fraass B, et al. Optimal coverage of peritoneal surface in whole abdominal radiation for ovarian neoplasms. Int J Radiat Oncol Biol Phys. 1989;17:607.
48 Martinez A, Schray MF, Howes AE, Bagshaw MA. Postoperative radiation therapy for epithelial ovarian cancer: the curative role based on a 24-year experience. J Clin Oncol. 1985;3:901.
49 Hong L, Alektiar K, Chui C, et al. IMRT of large fields: whole-abdomen irradiation. Int J Radiat Oncol Biol Phys. 2002;54:278.
50 Rochet N, Sterzing F, Jensen AD, et al. Intensity-Modulated Whole Abdominal Radiotherapy After Surgery and Carboplatin/Taxane Chemotherapy for Advanced Ovarian Cancer: Phase I Study. Int J Radiat Oncol Biol Phys. 2009 Jul 21.
51 Dembo AJ. Abdominopelvic radiotherapy in ovarian cancer. A 10-year experience. Cancer. 1985;55:2285.
52 Dembo AJ, Bush RS, Beale FA, et al. The Princess Margaret Hospital study of ovarian cancer: stages I, II, and asymptomatic III presentations. Cancer Treat Rep. 1979;63:249.
53 Smith JP, Rutledge FN, Delclos L. Postoperative treatment of early cancer of the ovary: a random trial between postoperative irradiation and chemotherapy. Natl Cancer Inst Monogr. 1975;42:149.
54 Dent SF, Klaassen D, Pater JL, et al. Second primary malignancies following the treatment of early stage ovarian cancer: update of a study by the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann Oncol. 2000;11:65.
55 Sell A, Bertelsen K, Andersen JE, et al. Randomized study of whole-abdomen irradiation versus pelvic irradiation plus cyclophosphamide in treatment of early ovarian cancer. Gynecol Oncol. 1990;37:367.
56 Redman CW, Mould J, Warwick J, et al. The West Midlands epithelial ovarian cancer adjuvant therapy trial. Clin Oncol (R Coll Radiol). 1993;5:1.
57 Chiara S, Bruzzone M, Merlini L, et al. Randomized study comparing chemotherapy plus radiotherapy versus radiotherapy alone in FIGO stage IIB-III cervical carcinoma. GONO (North-West Oncologic Cooperative Group). Am J Clin Oncol. 1994;17:294.
58 Fyles AW, Thomas GM, Pintilie M, et al. A randomized study of two doses of abdominopelvic radiation therapy for patients with optimally debulked Stage I, II, and III ovarian cancer. Int J Radiat Oncol Biol Phys. 1998;41:543.
59 Louie KG, Ozols RF, Myers CE, et al. Long-term results of a cisplatin-containing combination chemotherapy regimen for the treatment of advanced ovarian carcinoma. J Clin Oncol. 1986;4:1579.
60 Neijt JP, Ten Bokkel Huinink WW, van der Burg ME, et al. Randomized trial comparing two combination chemotherapy regimens (CHAP-5 v CP) in advanced ovarian carcinoma. J Clin Oncol. 1987;5:1157.
61 Fuks Z, Rizel S, Biran S. Chemotherapeutic and surgical induction of pathological complete remission and whole abdominal irradiation for consolidation does not enhance the cure of stage III ovarian carcinoma. J Clin Oncol. 1988;6:509.
62 Markman M, Liu PY, Wilczynski S, et al, Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum/paclitaxel-based chemotherapy. A Southwest Oncology Group and Gynecologic Oncology Group Trial, J Clin Oncol; 13; 2003:2460-2465.
63 Pecorelli S, Favalli G, Gadducci A, et al. After 6 Italian Cooperative Group. Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol 1. J Clin Oncol. 2009 Oct 1;27(28):4642-4648.
64 Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with Stage II–IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol. 1998;69:17.
65 Barakat RR, Sabbatini P, Bhaskaran D, et al. Intraperitoneal chemotherapy for ovarian carcinoma: results of long-term follow-up. J Clin Oncol. 2002;20:694.
66 Varia MA, Stehman FB, Bundy BN, et al, Intraperitoneal radioactive phosphate (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: a Gynecologic Oncology Group Study, J Clin Oncol; 21; 2003:2849.
67 Lambert HE, Rustin GJ, Gregory WM, Nelstrop AE. A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer: a North Thames Ovary Group study. J Clin Oncol. 1993;11:440.
68 Sorbe B, On behalf of the Swedish-Norwegian cancer study group. Consolidation treatment of advanced (FIGO stage III) ovarian cancer in complete surgical remission, after induction chemo therapy: a randomized, controlled trial comparing whole abdominal radiotherapy, chemotherapy, and no further therapy, Int J Gynecol Cancer; 13; 2003:278.
69 Whelan TJ, Dembo AJ, Bush RS, et al. Complications of whole abdominal and pelvic radiotherapy following chemotherapy for advanced ovarian cancer. Int J Radiat Oncol Biol Phys. 1992;22:853.
70 Leitao MMJr, Chi DS. Surgical management of recurrent ovarian cancer. Semin Oncol. 2009 Apr;36(2):106-111.
71 Chi DS, McCaughty K, Schwabenbauer S, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent platinum sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933-1939.
72 Dizon DS, Hensley ML, Poynor EA, et al. Retrospective analysis of carboplatin and paclitaxel as initial second-line therapy for recurrent epithelial ovarian carcinoma: application toward a dynamic disease state model of ovarian cancer. J Clin Oncol. 2002;20:1238.
73 Kudelka AP, Tresukosol D, Edwards CL, et al. Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol. 1996;14:1552.
74 Bokkel Ten, Huinink W, Gore M, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer [see comments]. J Clin Oncol. 1997;15:2183.
75 Creemers GJ, Bolis G, Gore M, et al. Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer: results of a large European phase II study. J Clin Oncol. 1996;14:3056.
76 Bookman MA, Malmstrom H, Bolis G, et al. Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol. 1998;16:3345.
77 Rose PG, Blessing JA, Mayer AR, Homesley HD. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1998;16:405.
78 Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997;15:987.
79 Markman M, Kennedy A, Webster K, et al. Phase 2 trial of liposomal doxorubicin (40 mg/m2) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000;78:369.
80 Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093.
81 Kavanagh JJ, Kudelka AP, de Leon CG, et al. Phase II study of docetaxel in patients with epithelial ovarian carcinoma refractory to platinum. Clin Cancer Res. 1996;2:837.
82 Francis P, Schneider J, Hann L, et al. Phase II trial of docetaxel in patients with platinum-refractory advanced ovarian cancer. J Clin Oncol. 1994;12:2301.
83 Bajetta E, Di Leo A, Biganzoli L, et al. Phase II study of vinorelbine in patients with pretreated advanced ovarian cancer: activity in platinum-resistant disease. J Clin Oncol. 1996;14:2546.
84 Gershenson DM, Burke TW, Morris M, et al. A phase I study of a daily x3 schedule of intravenous vinorelbine for refractory epithelial ovarian cancer. Gynecol Oncol. 1996;70:404.
85 Shapiro JD, Millward MJ, Rischin D, et al. Activity of gemcitabine in patients with advanced ovarian cancer: responses seen following platinum and paclitaxel. Gynecol Oncol. 1996;63:89.
86 Lund B, Neijt JP. Gemcitabine in cisplatin-resistant ovarian cancer. Semin Oncol. 1996;23(suppl 10):72.
87 Markman M, Kennedy A, Sutton G, et al. Phase 2 trial of single agent ifosfamide/mesna in patients with platinum/paclitaxel refractory ovarian cancer who have not previously been treated with an alkylating agent. Gynecol Oncol. 1998;70:272.
88 Markman M, Iseminger KA, Hatch KD, et al. Tamoxifen in platinum-refractory ovarian cancer: a Gynecologic Oncology Group Ancillary Report. Gynecol Oncol. 1996;62:4.
89 Gelblum D, Mychalczak B, Almadrones L, et al. Palliative benefit of external-beam radiation in the management of platinum refractory epithelial ovarian carcinoma. Gynecol Oncol. 1998;69:36.
90 E C, Quon M, Gallant V, Samant R. Effective palliative radiotherapy for symptomatic recurrent or residual ovarian cancer. Gynecol Oncol. 2006 Aug;102(2):204-209.
91 Albuquerque KV, Singla R, Potkul RK, et al. Impact of tumor volume-directed involved field radiation therapy integrated in the management of recurrent ovarian cancer. Gynecol Oncol. 2005 Mar;96(3):701-704.
92 Ratner ES, Toy E, O’Malley DM, et al. Brain metastases in epithelial ovarian and primary peritoneal carcinoma. Int J Gynecol Cancer. 2009 Jul;19(5):856-859.
93 Lee YK, Park NH, Kim JW, Song YS, Kang SB, Lee HP. Gamma-knife radiosurgery as an optimal treatment modality for brain metastases from epithelial ovarian cancer. Gynecol Oncol. 2008 Mar;108(3):505-509.
94 Chen PG, Lee SY, Barnett GH, et al. Use of the Radiation Therapy Oncology Group recursive partitioning analysis classification system and predictors of survival in 19 women with brain metastases from ovarian carcinoma. Cancer. 2005 Nov 15;104(10):2174-2180.
95 Low JJ, Perrin LC, Crandon AJ, Hacker NF. Conservative surgery to preserve ovarian function in patients with malignant ovarian germ cell tumors. A review of 74 cases. Cancer. 2000;89:391.
96 Zanetta G, Bonazzi C, Cantu M, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19:1015.
97 Bjorkholm E, Lundell M, Gyftodimos A, Silfversward C. Dysgerminoma: the Radiumhemmet series 1927–1984. Cancer. 1990;65:38.
98 Zaghloul MS, Khattab TY. Dysgerminoma of the ovary: good prognosis even in advanced stages. Int J Radiat Oncol Biol Phys. 1992;24:161.
99 Williams S, Blessing JA, Liao SY, et al. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. 1994;12:701.
100 Williams SD, Blessing JA, Hatch KD, Homesley HD. Chemotherapy of advanced dysgerminoma: trials of the Gynecologic Oncology Group. J Clin Oncol. 1991;9:1950.
101 Gershenson DM, Morris M, Cangir A, et al. Treatment of malignant germ cell tumors of the ovary with bleomycin, etoposide, and cisplatin. J Clin Oncol. 1990;8:715.
102 Homesley HD, Bundy BN, Hurteau JA, Roth LM. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;72:131.
103 Wolf JK, Mullen J, Eifel PJ, et al. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecol Oncol. 1999;73:35.
104 Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009 Mar 20;27(9):1419-1425.
105 Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007 Nov 20;25(33):5165-5171.
106 Short SC, Joiner MC. Cellular response to low-dose irradiation (Review). Clin Oncol (R Coll Radiol). 1998;10:73.