28 Cancer of the Oropharynx
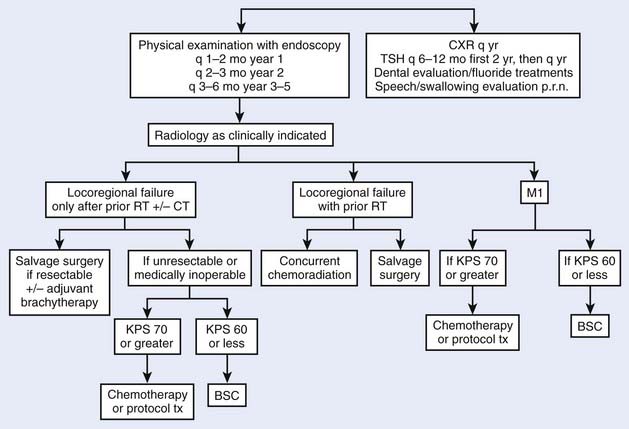
Tumors of the oropharynx comprise approximately 5000 new cases each year in the United States.1 The estimated incidence of pharyngeal cancers in the United States for 2007 was 11,800 new cases and 2180 deaths, with no breakdown by subsite of the pharynx.2 Patients with a history of tobacco or alcohol use are at increased risk for these tumors. Such a history can also be associated with other metachronous or synchronous tumors of the aerodigestive tract. The prognosis for oropharyngeal carcinoma depends on the location of the primary tumor and the stage at presentation. The most important cause of death is locoregional recurrence, which, if it occurs, usually manifests itself within 2 years. However, advances in radiation therapy as well as combined chemotherapy and radiotherapy have made local control much more successful, even for advanced primary site disease. Patients who survive are at risk for developing a second or third primary cancer in the upper aerodigestive tract or in the lower respiratory tract. The treatment strategies are numerous for these patients, and advances in organ preservation with attention to quality of life remain the major focus of research investigations.
Anatomy of the Oropharynx
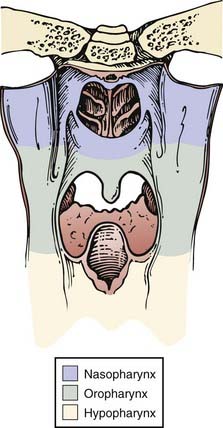
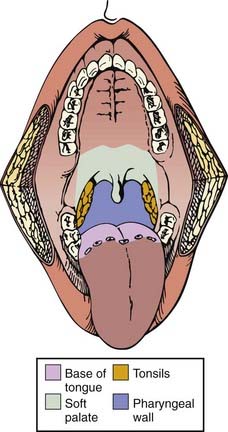
The pharynx is divided into the nasopharynx, oropharynx, and hypopharynx (Fig. 28-1). The oropharynx is located between the soft palate superiorly and the hyoid bone inferiorly. It is continuous with the oral cavity anteriorly and communicates with the nasopharynx above and the supraglottic larynx and hypopharynx below. Within the oropharynx are four different sites: soft palate, tonsillar region (fossa and pillars), base of tongue, and posterior and lateral oropharyngeal wall between the nasopharynx and the pharyngoepiglottic fold (Fig. 28-2).
Lymphatics of the Oropharynx
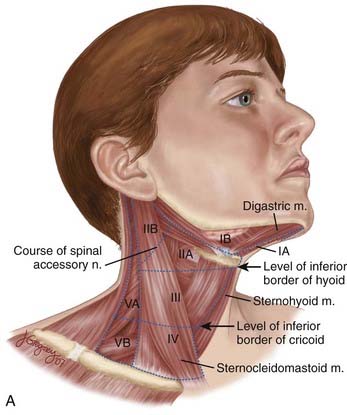
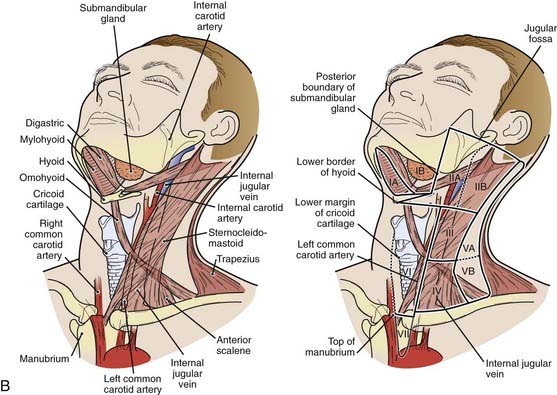
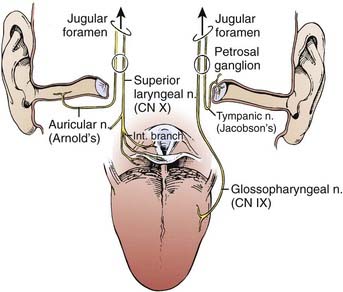
The lymphatic drainage of the neck was described in a classic paper by Rouviere3 in 1938 and has been refined by others.4,5 The lymph node groups are described by clinical levels I to V as depicted in Fig. 28-3.
The primary drainage of the oropharynx is to the jugulodigastric (level II) node(s) located in the upper deep jugular chain. The tonsillar region, pharyngeal portion of the soft palate, lateral and posterior oropharyngeal walls, and base of tongue also are drained by the retropharyngeal and parapharyngeal nodes. These nodes are located in the retropharyngeal and parapharyngeal space that is closely related to cranial nerves IX through XII, the internal jugular vein, and the internal carotid artery at the base of skull. The retropharyngeal lymph nodes are subdivided into lateral (Rouviere) and medial nodal chains.6–8 The lateral nodes lie posterolateral to the nasopharynx and oropharynx, just medial to the internal carotid artery. The parapharyngeal lymph nodes are known also as the junctional nodes, owing to the junction of the spinal accessory (level V) and upper internal jugular lymphatic chains.
The probability of lymphatic metastasis is related to size and location of the primary tumor within the oropharynx. The order of progression of lymph node metastases usually proceeds superiorly, from the high cervical first-echelon nodes (level II) inferiorly to the midcervical and lower cervical nodes (levels III and IV). Skip metastasis can occur in which a particular lymph node level is bypassed, but this is very unusual. Candela et al.,9 from 1965 to 1986, evaluated 333 previously untreated patients with primary squamous cell carcinoma (SCC) of the oropharynx or hypopharynx to ascertain the prevalence of neck node metastases by neck level. The patients underwent classic radical neck dissections. Isolated skip metastases outside of level II, III, or IV occurred in only one patient (0.3%). Otherwise, level I or V involvement always was associated with nodal metastases at other levels.9 Metastases to the retropharyngeal nodes are most commonly associated with cancers of the nasopharynx and pharyngeal wall,10–12 but can also occur from other subsites, particularly with advanced disease.13,14 Notably, these metastases occur primarily along the lateral retropharyngeal nodal chains. Involvement of the medial chain is extremely rare.3–10
Tumors located in the midline (base of tongue, soft palate, and posterior pharyngeal wall) exhibit a higher propensity for bilateral lymphadenopathy. The probability of cervical node metastases, as demonstrated by clinical examination of the soft palate, tonsillar fossa, base of tongue, and oropharyngeal wall, is shown in Table 28-1.13
Pathology
More than 90% of tumors of the oropharynx are SCC, the remainder being malignant melanomas, minor salivary gland tumors, sarcomas, plasmacytomas, lymphomas, and other rare tumors.15 Benign and malignant tumors that can be found in the oropharynx are listed in Table 28-2. Metastases to the oropharynx do occur.16–19 Lymphoepithelioma is more common in the tonsillar region and base of tongue. The distinction between lymphoepithelioma and SCC is important, with the former likely to be particularly radiosensitive.20 Non-Hodgkin lymphoma is seen in approximately 5% of tonsillar malignancies and rarely is encountered in the base of tongue.
| Malignant |
Soft Palate
Tumors of the soft palate are almost exclusively found on the anterior surface (the oropharynx portion as opposed to the nasopharynx portion) (Fig. 28-4). In a study of 359 male US military veterans diagnosed with 424 cancers of the oral cavity and oropharynx, tobacco smoking was found to be more strongly associated with soft palate lesions than with lesions in more anterior sites.21 Tumors can extend to involve the tonsillar pillars and the base of tongue. Occasionally, these lesions may extend laterally and superiorly as far as the nasopharynx. Involvement of the palatine nerve can result in tumor tracking along this pathway, with extension into the cranium. Lymphatic involvement is primarily to level II. Lesions of the midline and uvula can result in bilateral nodal metastases more frequently than do lateralized lesions.
Tonsil
The most common location for a primary tumor of the oropharynx is the anterior tonsillar pillar and tonsil. Common presenting symptoms are ipsilateral referred otalgia, discomfort, poorly fitting dentures, or a sensation of a lump or foreign body in the throat. Lesions involving the anterior tonsillar pillar can appear as areas of dysplasia, inflammation, or a superficial spreading or exophytic lesion. Frequently, these lesions become endophytic, ulcerate, and can spread laterally to the buccal mucosa and directly to the retromolar trigone Fig. 28-5. Inferior extension to the base of tongue is common. Perez et al.22 reported that of 218 patients presenting with SCC of the tonsillar region, the soft palate was involved in 131 (60%) and extension to the base of tongue occurred in 120 (55%). Superior extension can involve the soft and hard palate. Medial extension can involve the oral tongue. The close proximity of the anterior tonsillar pillar to the mandible places the periosteum and bone at risk in advanced cases. Posterior extension with destruction of the tonsillar pillars can involve the pterygoid muscles, with subsequent trismus and pain. The lymphatic drainage is primarily to level II but can involve level I and the retropharyngeal and parapharyngeal nodes.
The probability of clinical lymph node involvement is greater with tumors of the tonsillar fossa, especially contralateral involvement, in contrast to that of the tonsillar pillar. The lymphatic drainage depends on the location of the primary tumor. Lindberg13 describes nodal metastases in 76% of patients with tonsillar fossa tumors. The most common nodal group was level II. Contralateral lymph nodes were detected in 11% of patients. By contrast, tumors of the anterior tonsillar pillar or retromolar trigone region have an incidence of ipsilateral lymph node metastases of 45%, level II being the most common node-bearing region. Contralateral adenopathy was present in only 5% of patients.
Base of Tongue
SCC of the base of tongue is highly insidious. The base of tongue is almost devoid of pain fibers, and frequently these tumors are asymptomatic until they have progressed significantly. Many who present with a neck node are found, on examination, to have a base-of-tongue lesion. Visualization of this area on physical examination is difficult, and so a lesion often is missed.23 Patients may experience the sensation of a mass or discomfort in the throat, with bleeding and pain at later stages. Patients also might experience difficulty in swallowing or with speech. Occasionally, referred otalgia is the first symptom. If the size of the primary tumor increases such that it involves the pterygoid muscles, trismus can result.
Extension anteriorly can involve the oral tongue, superior and lateral extension can involve the tonsil, and inferior extension can involve the vallecula, epiglottis, and pre-epiglottic space (Fig. 28-6). Locally advanced base-of-tongue tumors can infiltrate the deep muscle and cause fixation.
Diagnostic Evaluation
History
The history should be part of a comprehensive evaluation of any patient with head and neck cancer. Patients usually present with pain and dysphagia and, occasionally, referred otalgia. If the history strongly reveals tobacco and alcohol use, efforts should be made to determine whether the patient is an alcoholic and continues to smoke. Alcoholic smokers are at risk for other chronic diseases of the heart, lung, peripheral vascular system, and liver, and may present with signs of malnutrition. Before the institution of any therapeutic modality, patients should cease alcohol and tobacco use. It may be necessary to guide the patient toward cessation programs. Clearly, those who discontinue smoking will better tolerate treatment and obtain a better result.24
Physical Examination
Specific aspects of the physical examination should include evaluation of the lesion (exophytic or infiltrative), tongue mobility, and palatal motion. Fixation of the tongue will result in incomplete protrusion or deviation of the tongue to the side of tumor involvement. In patients with a smoking and drinking history, three sites have a greater propensity for developing carcinoma than do others in the oral cavity and oropharynx: floor of mouth, ventrolateral tongue, and lingual aspect of the retromolar trigone and the anterior tonsillar pillar.25,26
For tumors of the tonsil or lateral pharyngeal wall, the examiner should test for anesthesia in the distribution of the ipsilateral mental nerve (V3). Any abnormality might suggest involvement of the inferior alveolar nerve in its pathway as it courses through the mandible or the base of skull and may direct the appropriate imaging study.20 The tonsil is adjacent to the ascending ramus of the mandible, and posterolateral to the tonsil is the parapharyngeal space. Tumors may extend into this area and be palpable in the neck on bimanual palpation. Owing to the relationship of the anterior tonsil (palatoglossus muscle) and the base of tongue, tonsillar cancers frequently extend into this region.
Initial Work-Up and Radiographic Evaluation
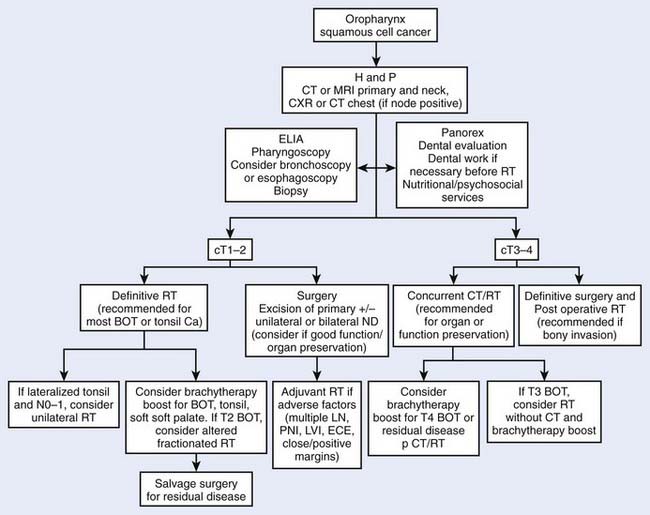
In addition to a history and physical examination, a complete blood cell count, screening profile, and chest roentgenogram are recommended (Fig. 28-8). Biopsy of a suspicious lesion is necessary to confirm the diagnosis.27 A more detailed metastatic work-up is indicated only when strong clinical or laboratory suspicion of metastatic disease exists.
Staging
The current staging criteria for tumors of the oropharynx, as defined by the American Joint Committee on Cancer (AJCC),28 are listed in Table 28-3. This is a clinical staging system and not a pathologic staging system. If radiographic information reveals a discrepancy from clinical staging, this should be noted. Current staging allows the radiographic findings to factor into the clinical staging designation.
Table 28-3 2002 AJCC Classification of Oropharyngeal Cancer

Early-Stage Disease: Site-Specific Management Strategies, Results, and Outcomes
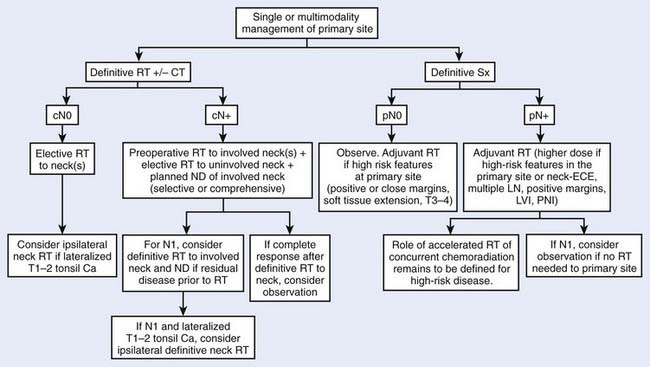
Treatment decisions depend both on the ability of a particular modality to control the primary tumor and on the state of the neck and its associated morbidity. In general, early stage disease can be treated by either radiation therapy (RT) or surgery, whereas more advanced lesions often are treated by combinations of these methods. RT is chosen more often than surgery for most early lesions because the cure rates are high and the functional outcome is better. Chemotherapy generally is reserved for patients with advanced disease or for certain organ-sparing protocols. In the following sections, the management guidelines, outcomes, and results of oropharyngeal tumors will be reported by subsite of the oropharynx (Figs. 28-9 and 28-10). Because the management of advanced disease is, in many cases, similar for the subsites of the oropharynx, they will be discussed together in the next section.
Soft Palate
Selection of Therapeutic Modality
For early stage lesions of the soft palate, either surgical resection or RT has provided excellent local control. Most patients will be treated with RT because the results are excellent and the probability of a good functional result is better. Also, because many lesions are near the midline, radiation treatment of the primary site and both sides of the neck is easy (see Radiation Therapy Techniques). In general, more morbidity ensues with surgical therapy to these same areas, especially if postoperative radiation becomes necessary.
Outcomes and Results
Definitive External Beam Radiotherapy
Two major series examine the use of EBRT alone or in conjunction with planned neck dissection. Erkal et al.29 analyzed 107 patients with SCC of the soft palate treated at the University of Florida between 1965 and 1996. Ninety-nine patients received external beam irradiation alone, while 8 patients (7%) received external beam RT followed by an interstitial brachytherapy boost. Patients received a median dose of 62.4 Gy via once-daily fractionation or 76.8 Gy via hyperfractionated techniques. The 5-year local control rates for T1 through T4 tumors were 86%, 91%, 67% and 36%, respectively. Ultimate 5-year local-regional control (LRC) after surgical salvage for Stages I through IV was 90%, 92%, 84%, and 60%, respectively. On multivariate analysis, overall treatment time significantly affected local control (P = .0002), regional control (P = .01) and overall survival (P = .04). Overall, 3% of patients treated with RT and 11% (3/27) patients treated with RT followed by neck dissection sustained severe late complications. Weber et al.30 reviewed the experience at the University of Texas M.D. Anderson Cancer Center. Treatment to the primary site consisted of RT for 150 patients, surgery alone for 28 patients, and combined therapy for 10 patients. Local control rates for T1 through T4 lesions were 91%, 77%, 77%, and 35%, respectively. Local control was obtained in 88% of patients with N0 necks and in 77% of patients with nodal involvement. In addition, these investigators found that patients with tumor extension to the tongue base, midline tumors, or tumors that extended across the palatine arch had inferior survival compared with those who did not exhibit those features (P < .05).30 Similar results have been reported in other single-institution retrospective series.31
Combined External Beam Radiation Therapy and Brachytherapy Implant
The use of a brachytherapy implant for small soft palate lesions has been reported extensively by French investigators. Esche et al.32 describe 43 patients with carcinoma of the soft palate and uvula who were treated by interstitial implant and EBRT. Patients received 5000 cGy external irradiation to the oropharynx and neck, followed by 2000 to 3500 cGy delivered by a low-dose rate (40 to 100 cGy/hour) interstitial iridium-192 (192Ir) implant. This therapy yielded a local control of 92%, with no local failures in 34 T1 primary tumors. One serious complication was seen. Overall actuarial survival was 60% at 3 years and 37% at 5 years, but cause-specific survivals were 81% and 64%, respectively. The leading cause of death was other aerodigestive cancers, with an actuarial rate of occurrence of 10% per year after treatment of a soft palate cancer.
Mazeron et al.33 reported on a subset of patients with early stage tumors who received EBRT to the primary tumor and neck nodes to a dose of 4500 cGy, followed by 3000 cGy delivered by a 192Ir implant to the primary tumor. Local control was 85% for soft palate tumors. Regional control was 97% for patients with N0 disease and 88% for patients with N1 through N3 disease. Soft tissue ulceration occurred in 17 patients, all of whom healed spontaneously.
Pernot et al.34 reported on 277 patients with velotonsillar cancer (oropharyngeal cancer excluding base of tongue and valleculae) who were treated by brachytherapy either alone (14 patients) or combined with external beam irradiation (263 patients) using an after-loading 192Ir technique. Thirty-five percent of the patients had soft palate lesions. Of the patients treated for early lesions in the soft palate, the local control rates for T1 to T2N0 and T1 to T2N1 to N3 were 90% and 86%, respectively. No local recurrence was detected after 3 years.
External Beam Radiation Therapy versus External Beam Radiation Therapy Plus Brachytherapy
The need for an implant was analyzed retrospectively by Mazeron et al.35 who reported T1 and T2 carcinomas of the soft palate and uvula treated definitively by EBRT alone (16 patients), 192Ir implant alone (14 patients), or a combination of the two methods (29 patients). Two techniques of implantation were used: the guide-gutter technique (33 patients) and the plastic-tube technique (10 patients). Local failure was 25% after EBRT alone, 0% after 192Ir implant alone, and 18% after combined therapy. No local failures were seen with the plastic tube technique compared with 15% for guide gutters. Only two nodal failures were observed (3%). Crude 5-year disease-free survival (DFS) was 33%. Severe complications were limited to one incident of osteonecrosis, one soft-tissue necrosis, and one case of partial palatal incompetence. Xerostomia was reduced when implantation was used for part or all of the treatment. This is not conclusive proof that an implant is required, and these results may be difficult to duplicate without the requisite expertise in brachytherapy. Clearly, patient selection factors are important in these results.
Fractionated High Dose Rate or Pulsed Dose Rate Brachytherapy
In an effort to minimize occupational radiation exposure and decrease the patient isolation period while minimizing normal tissue complications, Levendag et al. reported an initial experience combining EBRT with high dose rate (HDR—100 cGy/minute) or pulsed dose rate (PDR) brachytherapy in 38 patients—19 soft palate tumors (14 T1 to 2) and 19 tonsillar cancers.36 Twice-a-day fractions of 3.0 to 5.4 Gy HDR to a dose of 15 to 27 Gy or 4 × 2 Gy to 8 × 1 Gy per day of PDR to a dose of 20 to 28 Gy was delivered within 1 to 2 weeks after completion of EBRT (46 to 50 Gy, median cumulative dose of 66 Gy). At a mean follow-up of 2.6 years, 87% of soft palate tumors were locally controlled. The incidence of grade 3 mucositis or ulceration was no different between patients treated with PDR versus HDR and similar to those reported after low dose rate brachytherapy.
Tonsillar Region
Selection of Therapeutic Modality
Radiation Therapy
The use of RT as the definitive treatment for tumors of the tonsillar fossa is appropriate for T1, T2, and T3 (exophytic) tumors. For infiltrative or endophytic T3 or T4 lesions, either an organ-preserving approach involving concurrent chemotherapy or, in selected instances, surgery combined with postoperative RT (or concurrent chemoradiation) should be used. The target volume of the ipsilateral treatment should include the primary lesion with at least 2-cm margins, the ipsilateral jugular vein, and lateral retropharyngeal lymph nodes. If disease extends to the base of tongue, then EBRT alone is not as effective as EBRT plus implant to the tongue.37 In this case, full-dose EBRT is delivered, followed by an interstitial 192Ir implant as a boost to the tongue portion of the target.
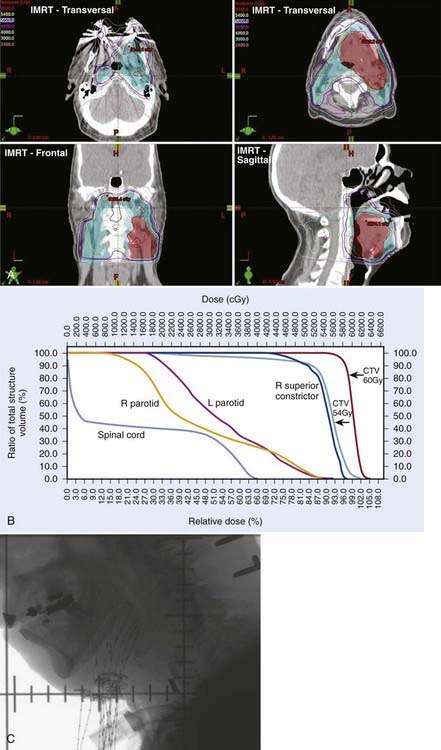
For early-stage, ipsilateral lesions with N0 or N1 nodal involvement, the primary site and ipsilateral neck can be treated while sparing the contralateral side, thus, optimizing the patient’s oncologic treatment and treatment-related morbidity. In the modern era, this is achieved with either three-dimensional conformal radiation therapy (3DCRT) or an intensity-modulated radiation therapy (IMRT) plan (see Radiation Therapy Techniques). In general, most T1 to T2 lesions in patients with an N0 or N1 neck can be treated with ipsilateral fields. Lesions that cross the midline, involve the tongue base, or involve N2 or more advanced neck disease should be treated to both the ipsilateral and contralateral neck. Treatment planning must ensure protection of critical normal structures including the spinal cord and larynx. A computed tomographic (CT) scan is performed for treatment planning.
Outcomes and Results
Surgery Alone
The use of surgery as the sole treatment for early tonsillar disease is not reported frequently. However, excellent local control rates ranging from 80% to 90% have been reported.34,38,39 When there is extension to the lateral pharyngeal wall or base of tongue, local recurrence approaches 33% and 47%, respectively.40–42 The degree to which local control can be obtained depends on the extension of disease outside the tonsillar fossa.
External Beam Radiation Therapy Alone
No definitive randomized studies comparing surgery versus EBRT have been reported. However, no obvious differences between these modalities in LRC or absolute survival can be determined based on the reported literature.38,43–54 EBRT alone has become the treatment of choice for most early stage lesions. If local control is maintained in patients by EBRT alone, the greatest risk to these patients is the development of future aerodigestive malignancies.55 In general, the results using conventionally fractionated EBRT alone (1.8 to 2.0 Gy/dose during 6.5 to 7 weeks to doses of 65 to 70 Gy) are excellent for early stage tumors.39,47–49,56
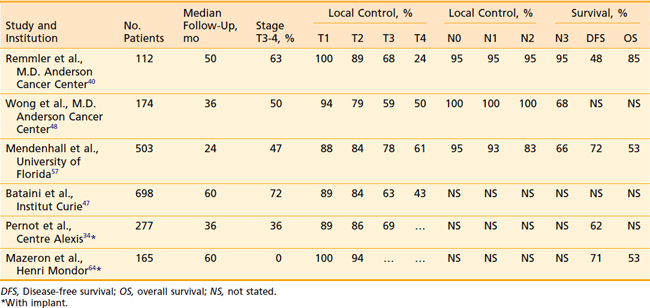
Mendenhall updated the University of Florida experience using definitive radiation to the primary site for treatment of 503 tonsillar cancers during a 38-year period, with a minimum 2-year follow-up.57 Patients were treated with continuous course radiation (n = 177, median total dose 66 Gy) or using hyperfractionation (n = 326, median total dose 76.8 Gy). Eighteen patients received induction chemotherapy, 39 received concomitant chemotherapy, 198 underwent planned neck dissection, and 106 underwent an interstitial brachytherapy boost. Five-year rates of local control were as follows: T1 (n = 75) 88%; T2 (n = 191) 84%; T3 (n = 151) 78%; and T4 (n = 86) 61%. Of the 95 local recurrences, 26 patients (27%) were successfully salvaged. The ultimate local control rates at 5 years were T1 94%, T2 91%, T3 82%, and T4 68%. Five-year cause-specific survivals by 1998 AJCC stage (5th ed.) were as follows: I 100%, II 86%, III 84%, IVA 73%, and IVB 46%. Absolute overall survival (OS) at 5 years was as follows: I 54%, II 61%, III 62%, IVA 57%, and IVB 33%. Only three patients required hospitalization during or shortly after RT; however, 9% (46/503) developed severe late complications including osteoradionecrosis requiring mandibulectomy (n = 15), dysphagia requiring gastrostomy (n = 18), bone exposure necessitating debridement and hyperbaric treatment (n = 10), orocutaneous fistula (n = 2), fatal radiation-induced sarcoma (n = 1) and fatal aspiration pneumonia (n = l).
Remmler et al.40 reported the results in 160 patients in whom EBRT was the sole therapy for the majority of patients. Primary tumor control rates were 100% for T1 lesions, 89% for T2, 68% for T3, and 24% for T4. In addition, control of cervical metastases by RT was excellent (95%). When a planned neck dissection was performed 5 weeks after RT, the control of cancer in the neck was 100%. The incidence of distant metastases was 10% and was not affected by the selection of therapy. The 2- and 5-year determinate survival figures for 112 patients treated with RT alone were 67% and 48%, respectively, whereas 31 patients treated with RT followed by neck dissection achieved a 5-year survival rate of 48% (Table 28-4).
Table 28-4 Tonsillar Carcinoma: Local Control and Survival with EBRT, With or Without Brachytherapy Implant
Lower doses or split-course radiation results in poorer LRC.58 Using RT alone, Bataini et al.47 reported that in tumors arising from the glossopalatine sulcus, characterized by involvement of the tongue, inferior local control is achieved compared with similar therapy for tumors arising from other sites within the tonsillar region.
Ipsilateral External Beam Radiation Therapy
For patients with T1 and T2 lesions, treatment of the ipsilateral neck alone usually is possible without having to irradiate the contralateral neck, which minimizes irradiation to the contralateral salivary gland and reduces the incidence of xerostomia. Eisbruch has demonstrated that patients treated unilaterally report less xerostomia and better quality of life compared with those treated with intensity-modulated RT delivery of bilateral radiation with contralateral parotid sparing.59 However, careful patient selection is required to minimize the risk for contralateral neck failure. Two recent major series document excellent outcomes using ipsilateral neck EBRT.
O’Sullivan et al. reported the results of 228 tonsillar carcinomas treated with ipsilateral RT during a 20-year period at Princess Margaret Hospital.60 Eligible patients typically had T1 or T2 tumors (191 T1-2, 30 T3, 7 T4) with N0 (133 N0, 35 N1, 27 N2 to 3) disease. During this period, only 16 patients were treated surgically. Radiation was typically delivered with wedged-pair cobalt beams and ipsilateral low anterior neck field delivering 50 Gy in 4 weeks (90% isodose line) to the primary volume. At a median follow-up of 5.7 years, the 3-year local control rate was 77%, regional control was 80%, and cause-specific survival was 76%. Contralateral neck failure occurred in 3% (8/228). All patients with T1 lesions or N0 neck status had 100% contralateral neck control. Patients with a 10% or greater risk for contralateral neck failure included those with T3 lesions, lesions involving the medial one third of the soft hemipalate, tumors invading the middle third of the ipsilateral base of tongue, and patients with N1 disease.
Jackson et al. reported an 18-year experience of 178 patients receiving ipsilateral treatment using limited fields for tonsillar cancers.61 Patients presented primarily with T1 to T2 (117/178 [66%]) and N0 (101/178 [57%]) disease, but 29% (52/178) had T3 tumors and 30% had N1 disease, with 63% presenting with stage III to IV disease. Sixty Gy per 25 fractions (50 to 66 Gy) was delivered to gross tumor volume with a 1-cm margin and first echelon lymph nodes using two or three wedged fields. The length of follow-up was not stated. The rates of LRC and contralateral neck recurrence by stage were as follows after ipsilateral RT: I (n = 23), 91%, 0%; II (n = 43), 74%, 2%; III (n = 82), 51%, 4%; IV (n = 30), 53%, 0%. Patients with N0 (n = 101) or N1 (n = 54) disease had contralateral failure rates of 0% and 4%, respectively. None of the 23 patients with N2 to N3 disease had contralateral failure; however, the determinate risk for contralateral failure may have been obscured by the 52% incidence of ipsilateral failure. The authors were unable to relate the risk for contralateral neck failure according to the degree of tumor extension along the glossopalatine fold due to the retrospective nature of the study. The overall rate of local control was 75% (T1-2, 84% and T3-4, 58%) and OS was 56%.
External Beam Radiation Therapy and Brachytherapy
Pernot et al.34 reported on 277 patients, 101 of whom had advanced disease (T3). The 5-year local control, disease-specific survival, and OS rates by T stage (T1, T2, T3) were as follows: local control, 89%, 86%, and 69%, respectively; disease-specific survival, 78%, 62%, and 46%, respectively; and OS, 62%, 53%, and 43%, respectively. No local recurrence was detected after 3 years. In a later update of 361 cases, the 5-year outcomes of patients with tonsil, posterior pillar, or soft palate cancers (group A) were compared with those involving the anterior pillar and glossopharyngeal sulcus (group B).46 Local control was better in group A patients compared with those in group B as follows: T1 94% versus 75%; T2 93% versus 67%; and T3 71% versus 51%, respectively. Disease-specific survival and OS was also better in group A as follows: T1: 88%, 65% versus 55%, 48%; T2 78%, 63% versus 43%, 38%; and T3 53%, 49% versus 27%, 27%, respectively. Multivariate analysis revealed that a treatment interval of less than 20 days between EBRT and brachytherapy and an overall treatment time of less than 55 days yielded better outcomes. Complications in this patient population appeared to be related to a total dose greater than 80 Gy, dose rate greater than 70 cGy per hour, treated surface area greater than 12 cm2, treatment volume greater than 30 cc, and absence of leaded protection.62
Levendag et al. reported on 248 patients with Stage T1-T3, N0-N+ SCC of the tonsillar fossa and/or soft palate treated at Erasmus Medical Center between 1986 and 2001.63 One hundred four patients were treated with external RT (median dose 46 Gy) followed by a brachytherapy implant, primarily using high-dose-rate (15 to 30 Gy at two daily fractions of 3 to 4 Gy to 15 to 30 Gy, n = 31) or pulsed-dose-rate (eight daily fractions of 1 to 2 Gy to 18 to 30 Gy, n = 62) treatment schedules. Patients who were node-positive underwent unilateral or bilateral neck dissection at the time of brachytherapy implant. Eighty-six patients who were felt to be unsuitable for brachytherapy at presentation (e.g., parapharyngeal extension) underwent surgery of the primary tumor and neck dissection, followed by postoperative RT to 50 to 70 Gy, depending on pathologic risk factors. Local control rates at 5 years for the definitive RT and surgical cohorts were 88% and 88%, respectively. Regional control, DFS, and OS rates were 93% versus 85%, 57% versus 52%, and 67% versus 57%, respectively. Significant late side effects in the brachytherapy group versus the surgery group included and trismus (1% versus 21%, P = .005) ulceration (39% versus 7%, P = .001). Ulceration healed spontaneously in 88% after a mean period of 6 months.
External Beam Radiation Therapy versus External Beam Radiation Therapy Plus Brachytherapy
Mazeron et al.64 reported on 165 T1 to T2 SCCs of the faucial arch. Because of institutional policy changes, these authors were able to compare patients who received EBRT alone with those with EBRT and implantation. Those who received an implant were first treated by EBRT to the tumor site and neck areas (4500 cGy in 25 fractions during 5 weeks) and then received a 3000-cGy low-dose-rate 192Ir implant. For patients with clinically positive nodes, either additional 2500- to 3000-cGy electron beam irradiation to the nodes or neck dissection was added. Both local control (77% versus 94% at 5 years; P < .01) and DFS (56% versus 71%; P = .03) were improved for the implant group. No randomized study has shown whether this combined approach is superior to EBRT alone. Nonetheless, even advocates of the use of EBRT alone agree that the addition of an implant can improve local control when disease extends into the tongue.37
High Dose Rate Brachytherapy
High dose rate brachytherapy as boost treatment with external beam radiation has been reported in two small series to offer excellent local control for early stage tonsil, base of tongue, and soft palate tumors with local control of 83% to 87% but lower with T3 to T4 tumors, 42% to 47%.36,65 Fractionated high dose rate brachytherapy using b.i.d. treatments of 1.2 to 3.0 Gy fractions was combined with EBRT to total cumulative doses of 66 to 72 Gy. Successful surgical salvage was 50% to 60% among those who failed locally with no obvious increase in surgical complications such as fistula or flap necrosis. Rates of serious complications (primarily soft tissue ulcer and mandibular osteoradionecrosis) were reported in 10% to 16% of patients and were similar to those reported by low dose rate brachytherapy reports.
Base of Tongue
Selection of Therapeutic Modality
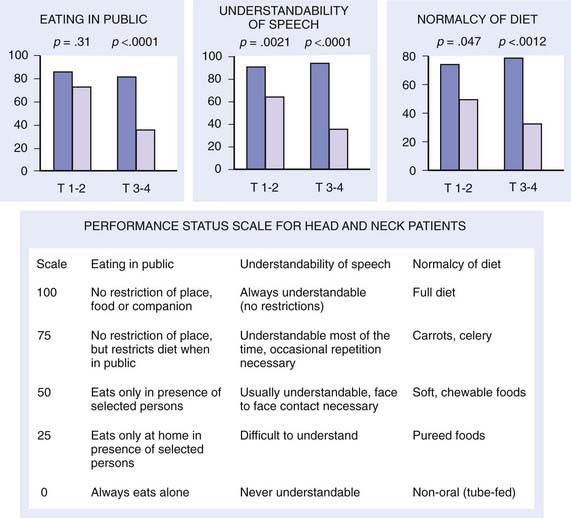
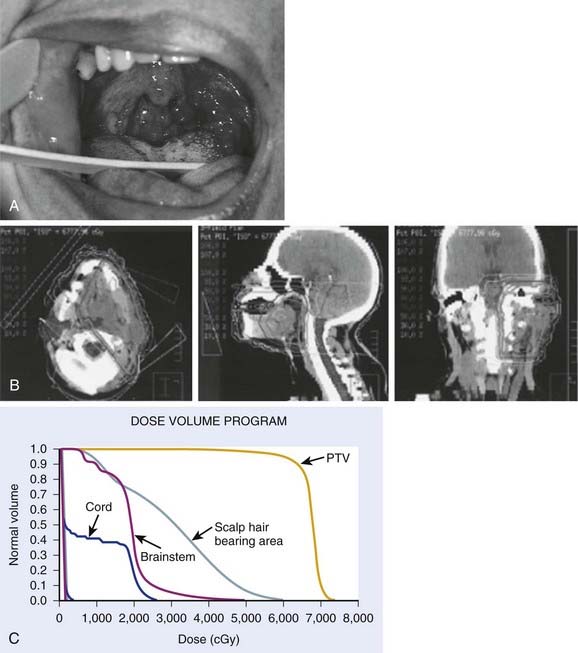
Early stage base-of-tongue disease is successfully treated with either definitive RT or surgery. The results are equivalent for local control and survival. The morbidity of a surgical procedure must be weighed against the morbidity of RT. In the overwhelming majority of cases, RT is selected because it provides a better functional result and quality-of-life (QOL) outcome (Fig. 28-11).
Radiation Therapy
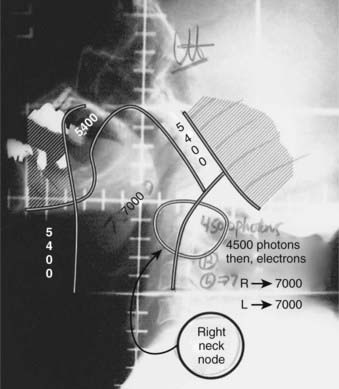
Our preferred strategy entails EBRT followed by an interstitial 192Ir implant as a boost to the base of tongue. We believe that this offers the most consistent excellent local control and best function results compared with either EBRT alone or to primary surgery. External beam radiation is delivered to a dose of 5400 cGy in 30 fractions (180 cGy/fraction). A brachytherapy boost to the tongue base (2000 to 3000 cGy) is performed approximately 3 weeks after EBRT (5000 to 5400 cGy). The implant consists of 192Ir after-loading catheters placed with a looping technique,66,67 which involves the percutaneous introduction of curved trocars by means of a submental approach through the base of tongue. The catheter is threaded through the trocar and looped back through an adjacent trocar, creating a loop in the tongue. For patients with disease extension toward the pharyngoepiglottic fold, lateral loops are added to encompass this region.66 Both ends exit the skin of the neck. An array of loops is fashioned to encompass the target volume plus a 1.0- to 1.5-cm margin. The spacing between each end of the loop is 1 cm and between each plane is 1.0 to 1.5 cm (see Radiation Therapy Techniques). As a safety precaution, a temporary tracheostomy is performed in patients immediately before the implantation. A temporary nasogastric feeding tube is placed at the completion of the procedure. The neck is managed by RT alone in the patient with N0 or N1 disease. For patients with more advanced neck disease, chemotherapy is given concomitantly with RT. When a neck dissection is planned, the implantation and dissection are performed during the same anesthetic period. Patients are loaded several days after the procedure, after the majority of swelling has subsided and patients are able to suction themselves and perform tracheostomy care. Efforts are made to protect the palate by use of a customized shield that is under patient control.
Surgical Therapy
By contrast, patients with nodal metastases routinely will undergo a surgical procedure in the neck if the primary tumor is being treated by radiation alone. The neck dissection preferably is performed at the completion of RT. If the primary tumor is being managed surgically, surgery followed by RT is preferred. In our practice, patients generally undergo a combination of EBRT and a brachytherapy implant (discussed later in this chapter). In this setting, the implant and the neck dissection are carried out during the same anesthetic period, approximately 3 weeks after the EBRT is completed. Andersen et al.68 have shown that preservation of the spinal accessory nerve in a modified neck dissection in patients with clinically evident nodal metastases was not associated with increased risk for treatment failure in the dissected neck.
Base-of-tongue tumors may be resected transorally or through a mandibulotomy and transhyoid pharyngotomy. The transoral approach is indicated only for small, well-circumscribed lesions that are located superficially. The mandibulotomy technique allows superior access and frequently is combined with a graft.69 When a patient has evidence of bone invasion or close encroachment of tumor to bone, frequently a mandibulectomy is performed. The use of a flap or plate with this procedure depends on the age of the patient, the amount of tissue resected, and the anticipated functional or cosmetic outcome.
After surgery, patients will spend a great deal of time learning to swallow and avoiding aspiration. If the dysfunction is significant, the patient may require placement of a percutaneous endoscopic gastrostomy (PEG), which can be performed at the time of surgery.70 A prosthesis may have to be constructed to aid speech and swallowing. Speech rehabilitation is started as soon as possible while the patient is still in the hospital.
Postoperative Adjuvant Radiation Therapy
After careful examination of the resected specimen by the pathologist, attention should be paid to the size of the primary tumor, histology, surgical margins, evidence of perineural extension, and lymph nodes. Patients with small tumors (stage T2), clear negative margins, no evidence of perineural extension, and histologically negative lymph nodes do not require postoperative RT. RT generally is used when the surgical wound is seeded, when close or positive margins are present, and in the presence of extranodal tumor spread (extracapsular extension), multiple positive nodes, or extensive vascular or perineural invasion.71 Because larger primary tumors are poorly controlled locally with surgery alone, postoperative RT is suggested in this group of patients. The retropharyngeal lymph nodes are included in the treatment of the primary tumor.72 Owing to the high probability for bilateral neck disease, RT is used electively to control the area of unoperated contralateral neck.
The interval between surgery and RT was examined by Vikram,73 who reports that a delay of 7 weeks or more was associated with increased locoregional failure and decreased survival. The neck disease recurrence rate was 2% if the postoperative RT was started within 6 weeks compared with 22% at later than 6 weeks. A reanalysis by Schiff et al.74 suggests that a prolonged delay in postoperative RT does not have a negative impact on LRC as long as appropriate tumoricidal doses of more than 6000 cGy are employed. Seventy-three percent in the delayed treatment group received doses less than 56 Gy, accounting for their increased risk for locoregional failure. Bastit et al. reported no difference in LRC or survival in a retrospective study of 420 oropharyngeal and hypopharyngeal carcinomas who were treated with similar doses (59 Gy/6 weeks) either within (n = 219) or after 30 days (n = 201) from surgery.75 However, Ang et al. reported that among high-risk patients (positive margins, extracapsular nodal extension, or multiple lymph nodes), a duration of less than 11 weeks from the end of surgery to the end of radiation resulted in better LRC and survival compared with those treated during a longer period.76 We recommend starting radiation as soon as possible.74
Outcomes and Results
Surgery Alone versus Radiotherapy
Surgical results for early base-of-tongue tumors reflect relatively high local control rates—from 75% to 85%.75,76 Primary EBRT, alone or followed by a planned neck dissection, also has a high local control rate of approximately 80% to 90% for T1 to T2 lesions and 70% to 85% for T3 tumors (Table 28-5). Survival for RT is similar to that after surgery, but with a lower risk for severe complications than surgery.77
Data from numerous authors have shown that the local control for early stage tumors is in the range of 80% to 100% when treated with combined EBRT and brachytherapy implant (Table 28-6). Pernot et al. reported a 5-year respective local control of 93% and 72% for T1 (n = 14) and T2 (n = 27) base-of-tongue tumors treated with a combined EBRT and brachytherapy approach. Corresponding 5-year disease-specific survivals were 76% and 62%, respectively.46
Table 28-6 Base-of-Tongue Carcinoma: Local Control and Survival With EBRT and Brachytherapy Implant Boosts
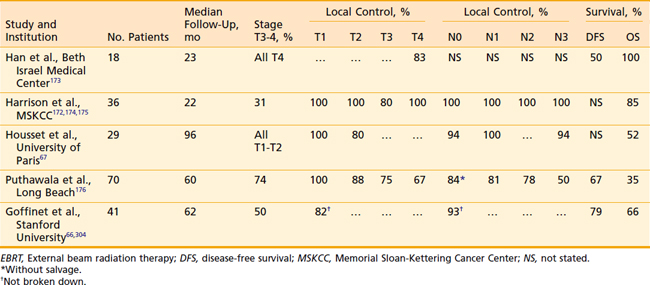
Housset et al.67 have evaluated a comparison among surgery plus postoperative RT, EBRT plus implantation, and EBRT alone for a series of patients with T1 to T2 base-of-tongue lesions. This series is unique in that it attempts to address the issue of which management strategy is optimal. Demographic and oncologic characteristics of the patients were well balanced except that, among the EBRT-alone group, there were significantly more patients with exophytic (more favorable) lesions. Despite this imbalance, the patients who received EBRT alone had approximately twice the local failure rate as those in the other two groups (40% versus 20%). This study suggests that EBRT plus brachytherapy is the preferred technique.
Pharyngeal Wall
Outcome and Results
Surgery Alone
The use of surgical resection alone for pharyngeal wall tumors has been reported by Guillamondegui et al.78 Twenty-eight percent of patients had recurrent tumors above the clavicles. Salvage in the form of RT or surgery was successful in less than one third of those patients. Patients with positive retropharyngeal nodes had an increased rate of distant metastases (22%) compared with those who did not have positive nodes (15%).
Definitive Radiation Therapy Alone
Hull et al.79 from the University of Florida retrospectively reviewed their experience with definitive radiation for pharyngeal wall tumors treated between 1964 and 2000. Thirty-seven percent of patients had oropharyngeal wall tumors, while the remainder was of hypopharyngeal origin. Sixty-four percent of patients were treated with a hyperfractionated technique, and 11 patients also received chemotherapy. Five-year local control rates for T1, T2, T3, and T4 tumors were 93%, 82%, 59%, and 50%, respectively. On multivariate analysis, factors associated with improved LRC included twice-daily fractionation (P = .0009), Stage I to II disease (P = .0051), and oropharyngeal primary site (P = .0193). The same group previously reported the effect of the use of once-daily to twice-daily fractionation on local control in 99 patients with carcinomas of the hypopharyngeal or oropharyngeal wall or both. The local control rates for patients treated with once-daily versus twice-daily fractionation were, for T1 lesions, 100% versus 100%; for T2 lesions, 67% versus 92%; for T3 lesions, 43% versus 80%; and for T4 lesions, 17% versus 50%. These investigators also examined their former technique (posterior border placed at middle of the vertebral body when the portals were reduced off the spinal cord) compared with their current, modified technique (posterior border placed at posterior edge of the vertebral body). The local control rates were 100% each for T1 lesions; 57% versus 100% for T2 lesions; 46% versus 73% for T3 lesions; and 29% versus 75% for T4 lesions.80
Meoz-Mendez et al.81 reported on 164 patients treated at the M.D. Anderson Cancer Center for carcinomas of the hypopharynx and pharyngeal walls. Local control rates for T1 through T4 lesions were 71%, 73%, 61%, and 37%, respectively. For patients with T3 and T4 tumors, the combination of surgery and RT was superior to RT alone (75% local control versus 51%).
Surgery and Radiation Therapy
Marks et al.82 retrospectively compared the results of treatment in 51 patients after low-dose (2500 to 3000 cGy) preoperative RT and surgery to the results in those who had definitive RT. No difference was found in local control or survival (17%); however, the surgery plus RT group experienced a significant number of complications (fistula, 31%; carotid rupture, 14%; operative mortality, 14%). The same authors updated their experience with a group consisting of 89 patients.83,84 These patients were treated with high doses of radiation (5000 to 7200 cGy) either for preoperative intent or for definitive therapy. Treatment outcome, survival, and tumor and nodal control were better for the RT-plus-surgery group than for the RT-alone group. The patterns of relapse differed for the two groups, with low-dose preoperative irradiation and surgery offering greater control of the primary tumor and high-dose irradiation achieving better control of nodal disease. To draw any conclusions from this article is difficult, as the reported study spanned almost 20 years, during which time many technologic advances were made in radiation oncology, anesthesia, and surgery.
Spiro et al.85 reviewed a 12-year experience with 295 patients treated for SCC of the pharynx at the Memorial Sloan-Kettering Cancer Center. Of these patients, 78 patients had lesions in the posterior wall. Surgery was the definitive therapy for the primary tumor in 73%. A second group of 21 patients with more extensive tumors required a laryngectomy and complex reconstruction, often with postoperative RT, and five had lesions that were implanted after access was provided by a mandibulotomy. The cumulative 5-year survival for the entire group was 32% and ranged from 44% in those with favorable lesions to 15% in those with extensive tumors. The overall complication rate was 50%. For the group that received an implant, local control was excellent.
Julieron et al.86 from the Institut Gustav-Roussy reported their results among 77 patients treated surgically between 1984 and 1995. Twenty-three of these patients had been previously irradiated, and all patients who were previously untreated underwent postoperative radiation. Of the 54 patients who were previously untreated, 89% were locally controlled and 78% were locoregionally controlled, with a 5-year survival rate of 35%.
The use of a brachytherapy implant (either 192Ir or iodine-125 [125I]) and EBRT was reported by Son and Kacinski87 for a small group of patients. The local control rate was 86% and actuarial survival was 82% at 5 years. These results are impressive but must be confirmed. In general, results in patients with extensive lesions still leave much to be desired, despite radical surgery and aggressive RT, and creative brachytherapy techniques warrant further investigation.
Advanced Disease: Management Strategies, Results, and Outcomes
Altered Fractionated Radiation
Withers et al. analyzed outcomes from nine centers using widely different dose-fractionation radiation regimens treating a total of 676 tonsil carcinomas (range of total dose 50 to 72 Gy, dose per fraction 1.8 to 3.3 Gy, and overall treatment time 3 to 8 weeks).88 Total dose and treatment duration were significant treatment parameters that impacted on local control, but dose per fraction was not important. The data were most consistent with the model of delayed accelerated tumor repopulation occurring 30 days after the beginning of treatment with a compensatory dose of 0.73 Gy per day of treatment prolongation. Thus, shortening treatment duration to minimize accelerated repopulation or increasing total dose without adding to late complications have become important areas of investigation. Attempts to improve LRC with altered fractionation have seemed promising in single-institution studies.43,50,89–92
On the basis of such evidence, the RTOG conducted a landmark trial (RTOG-90-03) comparing the leading US altered fractionated regimens for multiple head and neck cancer sites, including oropharynx cancers (60%).93 One thousand and seventy-three patients with primarily advanced SCCs of the head and neck were randomized to four arms:
 Split-course accelerated fractionation (S-AF) with 1.6 Gy b.i.d. to 67.2 Gy during 6 weeks with an intentional 2-week break after 38.4 Gy and an interfraction interval of 6 hours
Split-course accelerated fractionation (S-AF) with 1.6 Gy b.i.d. to 67.2 Gy during 6 weeks with an intentional 2-week break after 38.4 Gy and an interfraction interval of 6 hours Delayed concomitant boost (DCB) with daily morning 1.8 Gy treatments and a 1.5 Gy afternoon concomitant boost for the last 12 days of treatment with a 6-hour interfraction interval and a total dose of 72 Gy during 6 weeks
Delayed concomitant boost (DCB) with daily morning 1.8 Gy treatments and a 1.5 Gy afternoon concomitant boost for the last 12 days of treatment with a 6-hour interfraction interval and a total dose of 72 Gy during 6 weeks Pure hyperfractionation (HF) with 1.2 Gy twice daily with an interfraction interval of 6 hours to a dose of 81.6 Gy per 7 weeks.
Pure hyperfractionation (HF) with 1.2 Gy twice daily with an interfraction interval of 6 hours to a dose of 81.6 Gy per 7 weeks.Horiot et al. reported results of the EORTC 22791 randomized trial comparing an HF regimen of 1.15 cGy b.i.d. (4 to 6 hours between fractions) to 80.5 Gy during 7 weeks versus CF of 1.8 to 2.0 Gy to 70 Gy during 7 to 8 weeks in the treatment of 356 patients with T2 to T3N0 to 1 oropharyngeal cancers excluding the base of tongue.94 With a mean follow-up of about 4 years, HF improved 5-year actuarial LRC compared with CF (59% versus 40%, respectively P = .02) with a trend toward improved survival (38% versus 29%, respectively, P = .08). T3 tumors benefited from HF but T2 lesions did not. More severe acute mucositis occurred in the HF, but did not increase the incidence of treatment interruption. The incidence of grade 2 or 3 late effects was no different between the two arms.
Overgaard et al. reported results of the DAHANCA 6 and 7 trials, which randomized 1476 patients with stage I to IV head and neck cancer to either an accelerated radiotherapy regimen employing 6 weekly fractions of radiation with the standard regimen of 5 weekly fractions in the treatment of patients.95 Both arms were treated to a total dose of 66 to 68 Gy at 2 Gy per fraction (except for T1 glottic tumors, which received a total dose of 62 Gy). An oral dose of the hypoxic radiosensitizer nimorazole (1200 mg/m2) was administered with the first 30 fractions of radiation. The accelerated regimen proved to be superior in terms of 5-year local control (LC; 76% versus 64%, P = .0001), LRC (70% versus 60%, P = .0005) and disease-specific survival (DSS; 73% versus 66%, P = .01). OS was not improved with the shortened course of radiation. Confluent mucositis was significantly higher in the accelerated group (53% versus 33%, P < .0001), but 98% of both arms received the scheduled radiation dose, and late toxicity did not differ between the fractionation groups. Several other randomized trials examining altered fractionation schemes have been reported to date, most of which also demonstrate an improvement in LC and LRC at the expense of increased acute toxicity, but without an impact on OS.96–102
Intensity-Modulated Radiation Therapy as a Surrogate for Altered Fractionation
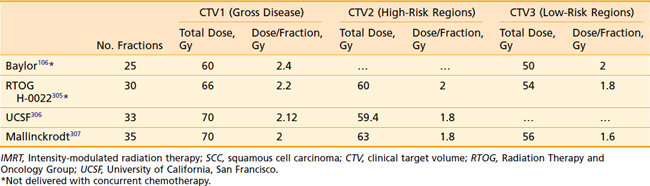
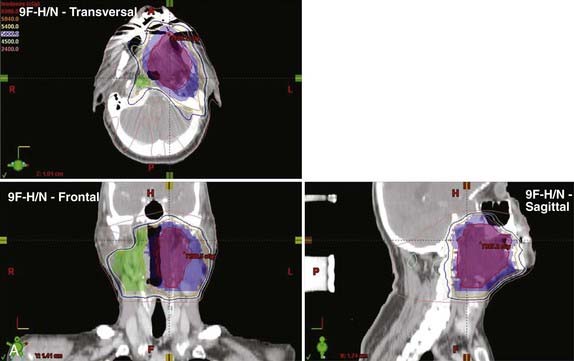
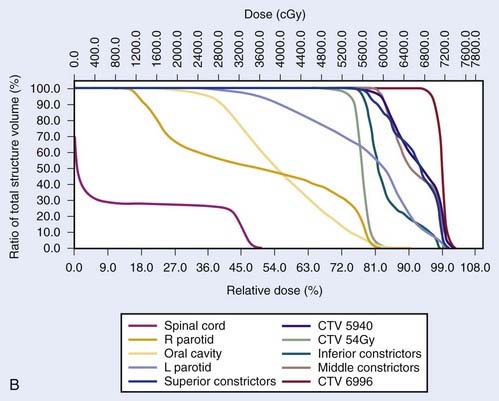
Another issue that arises in the modern era is the role of altered fractionation schemes in the context of IMRT. Although the use of hyperfractionated or accelerated treatment schedules with IMRT have been reported,103–105 because of the expense of time and resources required with IMRT, most radiation oncologists have reverted to treating patients once a day. An alternative to twice-daily radiation is the delivery of a simultaneous integrated boost (SIB), wherein multiple targets are treated simultaneously with different prescribed doses. For example, the primary tumor and gross nodal disease may be treated to 66 Gy in 30 fractions (2.2 Gy/fraction), while at the same time, high-risk areas receive 60 Gy (2 Gy/fraction) and low-risk areas receive 54 Gy (1.8 Gy/fraction). Because of the higher dose per fraction, however, this scheme may result in a greater risk of late morbidity. Alternatively, the gross disease may be treated with standard doses of 1.8 to 2.12 Gy per fraction, while subclinical disease is treated with a lower-than-standard daily dose (see Radiation Therapy Techniques). When this approach is employed, particularly in patients with advanced disease, concurrent chemotherapy should be administered. Common IMRT dose prescriptions are listed in Table 28-7. The optimal dose prescription has not been determined; however, it should be noted that those with the highest doses per fraction, such as that described by Butler et al.,106 should not be delivered with concurrent chemotherapy, due to severe acute mucositis. The use of SIB has not been compared with traditional altered-fractionated radiation in a randomized study, and therefore whether the employment of IMRT with a SIB offers the same benefit as the aforementioned altered fractionation schemes is, at present, unknown. However, the reported experience to date shows excellent LRC using IMRT with SIB with or without concurrent chemotherapy (Table 28-8). The most recent outcomes with IMRT indicate LRC rates of more than 90% in stage III/IV patients treated with primary concurrent chemoradiation.105,107,108 Longer follow-up is required for confirmation.
The Role of Chemotherapy in Advanced Oropharyngeal Cancer
Concurrent Chemoradiotherapy
Patients with advanced tumors of the oropharynx who require extensive surgery with or without total laryngectomy typically have experienced suboptimal results. Owing to the size and location of these tumors, primary surgery can have a significant impact on the functional, psychological, and cosmetic consequences for these patients. The previous standard for organ preservation was established by the Veterans Affairs (VA) Laryngeal Cancer Study Trial109,110 in which induction chemotherapy plus conventionally fractionated EBRT (which allowed preservation of the larynx in 66%) produced survival rates equivalent to those undergoing total laryngectomy with postoperative EBRT. However, recent studies and meta-analyses have demonstrated that a concurrent chemoradiation appears superior to the VA regimen or to EBRT alone and have brought into question the benefit of induction chemotherapy.111–121 Forastiere et al. reported the 5-year results of RTOG 91-11, demonstrating that in patients with larynx cancer requiring total laryngectomy and randomized to (A) conventional fractionated EBRT (70 Gy/7 weeks) alone, (B) the VA regimen, or (C) concurrent cisplatin (100 mg/m2 weeks 1, 4, and 7) with conventional fractionated EBRT (70 Gy/7 weeks), those in arms B and C had superior laryngectomy-free survival compared with conventional RT alone (46.6% and 44.6% versus 33.9%, P = .011), and arm C had a statistically superior 5-year larynx preservation rate (83.6%) compared with both arm A (65.7%, P = .00017) and arm B (70.5%, P = .0029). Moreover, arm C demonstrated a superior 5-year LRC compared with the other two arms. No significant difference in OS was seen.115–117 A number of trials restricted to oropharynx cancers have demonstrated similar findings.
A landmark French intergroup (GORTEC 94-01) phase III randomized trial tested whether the addition of concurrent chemotherapy to conventionally fractionated RT improves outcome compared with conventionally fractionated RT alone for patients with advanced stage oropharynx carcinoma.111–113122 Two hundred and twenty-six patients with stage III (32%) and IV (68%) tumors were randomized to 70 Gy per 7 weeks or 70 Gy with 3 cycles of concurrent carboplatin (70 mg/m2/day × 4) and fluorouracil (5-FU) (600 mg/m2/day × 4) (weeks 1, 4, and 7). The arms were balanced with regard to stage, sex, tumor site, performance status age, and histology. Acute toxicities were increased in the concurrent arm including grade 3 or 4 mucositis (71% versus 39%, P = .005), dermatitis (67% versus 59%, P = .02), and need for feeding tube (36% versus 15%, P = .02) compared with the control arm. However, the incidence of treatment-related mortality (1% [1/109] versus 0%), overall treatment duration (52 versus 50 days, respectively), and treatment interruption ≥3 days (19% versus 16%, respectively) were similar. Severe cervical fibrosis was increased in the combined modality treatment group (12% versus 3%, P = .08, respectively) but the overall rate grade 3 or 4 late toxicity was not statistically significant (56% versus 30%, P = .12). At a median follow-up of 5.5 years, patients in the combined modality arm had a higher 5-year LRC (47.6% versus 24.7%, P = .002), DFS (26.6% versus 14.6%, P = .01) and OS (22.4% versus 15.8%, P = .05) but no difference in the rate of distant metastases (18% versus 17%, respectively). On multivariate analysis, pretreatment hemoglobin levels <125 g/L, stage IV disease, and RT alone were independent prognostic factors of short survival and locoregional failure. The approximately 20% improvement in survival and LRC argues for the addition of concurrent chemotherapy in patients with advanced oropharynx cancer treated with definitive radiation. However, the optimal chemoradiotherapy regimen with regard to fractionation scheme and chemotherapy agents remains to be defined.
In 2006, Bonner et al.123 reported the results of a multicenter, international phase III study investigating the addition of concurrent cetuximab, a monoclonal antibody against the epidermal growth factor receptor (EGFR), to patients receiving definitive radiotherapy for stage III or IV head and neck carcinoma. Patients were treated via standard fractionation, hyperfractionation, or accelerated fractionation with concomitant boost schedules, with or without cetuximab. Sixty percent of the 424 patients enrolled in the study had oropharyngeal primaries. With a median follow-up of 54 months, the addition of cetuximab improved the 3-year LRC rate (47% versus 34%, P < .01), PFS (42% versus 31%, P = .04) and OS (55% versus 45%, P = .05). Although the trial was not sufficiently powered to detect differences among subgroups, the greatest survival benefit was among patients with oropharyngeal cancers, with a median survival of >66 versus 30.3 months. Importantly, although there was an increased incidence of grade 3 to 5 acneiform rash (17% versus 1%, P < .001) and infusion reaction (3% versus 0%, P = .01), the risk of other acute radiation-related side effects such as mucositis (56% versus 52%, P = .44) did not differ significantly between the two groups. Likewise, severe late effects were reported in about 20% of patients in each group. Currently, RTOG 0522 is randomizing patients with advanced head and neck SCC to receive concurrent accelerated radiation and cisplatin, with or without concurrent cetuximab124
Combining Chemotherapy With Altered Fractionated Radiation
As both the addition of concurrent chemotherapy and altered fractionated radiation have been have been shown independently to improve outcome for head and neck cancer patients, the present challenge is to find the optimal chemoradiation regimen(s) that offer high rates of LRC, are not unduly morbid, offer maximal preservation of organ function, and may be integrated with new biologic agents.93,111–113,115–117,123 Multiple phase II and randomized studies have reported possible regimens. The benefit of induction chemotherapy remains to be defined.
Phase II Studies
Harrison reported the results of a phase II trial that treated 82 patients with unresectable head and neck cancer using the DCB technique (70 Gy/6 weeks, b.i.d. RT last 2 weeks) with concurrent cisplatin (100 mg/m2) given every 3 weeks for 2 cycles.125 Adjuvant chemotherapy was given in 68%. Although 40% had initial skull base invasion, the response rate was 94% with 60% complete responses. Oropharynx cancers comprised 23% of all tumors treated. For all patients at a minimal follow-up of 3 years, the 3-year LC, OS, and distant-metastasis-free survival was 58%, 36%, and 58%, respectively. Seventy percent of patients with base of skull invasion were locally controlled. The treatment was reasonably well tolerated. The 3-year LC for oropharynx cancers was 64%. All patients experienced grade 3 acute mucositis usually during the concomitant boost phase. Twenty-four percent required a treatment break, the majority requiring less than one week. Two deaths due to sepsis occurred during treatment. Severe chronic toxicity occurred in three patients with one osteoradionecrosis, one frontal lobe necrosis, and one case of lung toxicity secondary to adjuvant chemotherapy. Given the especially poor prognosis of this group, the LC was good and survival better than expected with such an aggressive chemoradiation program. Our experience in treating advanced oropharyngeal disease was recently updated.126 Forty patients were treated with the above regimen, with either two cycles of cisplatin (100 mg/m2 on weeks 1 and 4) or daily carboplatin (10 to 17.5 mg/m2). Seventy-five percent of patients had T3 or T4 disease, and 57.5% of patients were stage N2 or N3. The 2-year LC, regional control, LRC, freedom from distant metastasis, DFS and OS rates were 81%, 97%, 81%, 74%, 76% and 80%, respectively. Grade 3 toxicity rates were as follows: mucositis, 67.5%; dysphagia, 62.5%, odynophagia, 62.5%. Patients required a median 2.24 days of treatment break. Late toxicities were mainly due to grade 3 to 4 xerostomia (36%, 2.2%, respectively) and grade 3 dysphagia (11.1%). One patient developed osteonecrosis and one patient developed soft tissue necrosis. Of the 80% of patients who had a gastrostomy tube placed before or during treatment, two patients were still dependent on parenteral feeds 5 and 17 months after treatment completion.
A modified version of DCB radiation was reported by Bieri et al. in which a planned total dose of 69.6 Gy was shortened to total of 5.5 weeks and one third of the patients received primarily concurrent cisplatin-based chemotherapy.127 Among the 55 patients with oropharynx carcinoma (76% stage III/IV), LRC at 3 years was 69.5% at a median follow-up of 32 months. Eighty-two percent experienced grade 3 or 4 mucositis. Patients receiving chemotherapy had a greater rate of grade 3 dysphagia (68% versus 25%, P = .003), hospitalization (37% versus 14%, P = .08), and need for nasogastric tube (68% versus 22%, P = .001). Late RTOG grade 3/4 complications occurred in 12% of patients and consisted of laryngeal edema, mucosal necrosis, and mandibular osteoradionecrosis.
Induction chemotherapy followed by RT was used in the treatment of patients with T4 (AJCC, 2nd ed.) oropharyngeal carcinomas at the University of Florida.128 Chemotherapy consisted of cisplatinum (100 mg/m2) and 5-FU (1000 mg/m2/day × 5 days) every 3 to 4 weeks for several cycles (63% received 3 cycles) followed by definitive RT (83% received hyperfractionated RT to 74.4 to 81.75 Gy). Twenty-six patients were able to complete the chemotherapy and high-dose radiation. The major response rate after induction chemotherapy was 97% (35% complete response and 62% partial response). These rates were compared with a similar group of 34 patients with T4 oropharyngeal tumors treated with a comparable radiation regimen without chemotherapy during the same time period and also to 83 patients with T4 tumors treated with RT alone during a three-decade time span. All patients were followed for a minimum of 1 year and none were lost to follow-up until death from 1964 to 1996. On multivariate analysis, no difference in local failure (37% versus 62% versus 52%, respectively) or regional or distant failure (13% versus 27% versus 24%, respectively) was detected in those receiving induction chemotherapy compared with patients treated with RT alone. However, disease-specific survival and OS were improved in those who received induction chemotherapy (58% versus 27% versus 37% and 42% versus 17% versus 23%, respectively). Because of the nonrandomized nature of the study and the lack of statistically significant improvement in parameters of tumor control, the authors cautioned against any conclusions regarding the benefit of induction chemotherapy.
A phase II study using induction chemotherapy followed by concurrent chemoradiation was reported by Vokes et al.129 Sixty-one patients with advanced oropharyngeal carcinomas (97% IV, 82% T3-4, and 85% N2 to N3) were treated with three cycles of cisplatinum (100 mg/m2), 5-FU (640 mg/m2/days × 5 days), leucovorin (300-600 mg/m2/day × 6 days), and interferon-α (2 million U/m2/day × 6 day) for three cycles then proceeded to concurrent chemoradiotherapy if at least a near complete response was obtained. This consisted of split courses of weekly concurrent hydroxyurea (2000 mg/day × 5 days) and 5-FU (800 mg/m2 × 5 days) with 900 to 1000 cGy per 5 days given every other week to a total dose of 68 to 75 Gy for gross disease. Neck dissections (n = 35) were performed for N2 to N3 disease. After induction chemotherapy, 65% obtained a complete response and 34% a partial response. Sixteen patients underwent resection of the primary tumor (11 organ-sparing and 13 after induction chemotherapy). At a median follow-up of 39 months (68 months among survivors), LRC was 70%, distant-metastasis-free survival 89%, DFS 64%, and OS 51%. LRC was 100% in patients with T1 to T2 (n = 11) or N0 (n = 1), 81% for T3 (n = 16), 53% for T4 (n = 34), and 67% to 69% N1 to N3 (n = 60). No difference was noted in LC among those undergoing surgery of the primary site versus those treated with chemoradiation alone (5-year LRC 66% versus 72%). Regional control was obtained in 98%. An update with 93 patients showed similar results.130 Acute toxicity was substantial with severe or life-threatening mucositis in 57% and leukopenia in 65% during the induction phase, whereas 81% had grade 3 or 4 mucositis during the concurrent chemoradiation. The authors concluded that the treatment sequence of induction chemotherapy followed by concurrent chemoradiation and optional organ-preservation surgery is promising but that less toxic regimens need to be identified.130
Bensadoun et al. reported on 54 patients with unresectable oropharynx and hypopharynx carcinoma treated with concomitant hyperfractionated radiation (75.6 to 80.4 Gy) and three cycles of 5-FU/cisplatin (CDDP) (750 mg/m2/day or 750 mg/day × 5 days and 100 mg/m2, respectively on weeks 1, 4, and 7).131 The regimen was acutely toxic (4% mortality from treatment-related septicemia, 86% grade 3/4 mucositis by cycle 2 of chemotherapy, grade 3 or 4 neutropenia in 43% by cycle 2) but no patient required a treatment break greater than 4 days due to mucositis. No grade ≥3 late toxicities were reported; grade 2 xerostomia was reported in 70% and grade 2 cervical fibrosis in 45%. At a median follow-up of 16 months, LRC was 67% at 6 months and disease-specific survival 72% at 2 years. Other chemoradiation regimens for oropharyngeal carcinomas with encouraging LRC rates but short-term follow-up or small patient numbers include those using concurrent weekly paclitaxel (30-50 mg/m2/week)132 and the use of induction chemotherapy with preoperative concurrent chemoradiation followed by organ-preserving surgery.133
Randomized Trials
Concurrent chemotherapy with hyperfractionated radiation was explored by Brizel et al. in a phase III randomized trial.134 One hundred and sixteen patients with advanced head and neck cancer were randomized to hyperfractionated radiation alone treated with 1.25 Gy b.i.d. (6-hour interfraction interval) 5 days per week to 75 Gy during a 6-week period versus a concurrent chemoradiation arm consisting of 5-FU/CDDP given on weeks 1 and 6 of split-course hyperfractionated radiation. In contrast to the control arm, an intentional 1-week treatment break was required in the experimental arm and the total dose was slightly lower (70 Gy). Both groups received two adjuvant courses of 5-FU/CDDP after completion of radiation. At a median follow-up of 41 months, the chemoradiation showed improved LRC (70% versus 44%, P = .01) and a trend toward improved 3-year OS (55% versus 34%, P = .07) and relapse-free survival (61% versus 41%, P = .08). However, patients in the chemoradiation arm developed more acute toxicity including the requirement for more feeding tubes (44% versus 29%) and worse hematologic suppression. Three fourths of patients in both arms experienced confluent mucositis. Chronic toxicity was no different, with about a 10% incidence of necrosis of the skin or bone in both arms. The trial has been criticized, not only for the added toxicity, but also because of the imbalance in the proportion of advanced neck disease (44% versus 63%) treated in the concurrent chemoradiation arm, which may have accounted for the difference in LRC.
Jeremic et al. reported a phase III randomized study testing whether daily low-dose cisplatin improved outcome for patients undergoing hyperfractionation radiation compared with those treated with the same hyperfractionated radiation alone in locally advanced head and neck SCCs (37% were oropharynx).135 One hundred and thirty patients with stage III or IV disease were randomized to 1.1 Gy b.i.d. to 77 Gy per 7 weeks alone or with cisplatin (6 mg/m2/day). At a median follow-up of 79 months, the investigational arm showed improved LRC (50% versus 36% at 5 years, P = .041), progression-free survival (46% versus 25% at 5 years, P = .0068 and OS (46% versus 25% at 5 years, P = .0075), and fewer distant metastases (14% versus 43% at 5 years, P = .0013). The latter result was unexpected and raised the possibility that concurrent daily cisplatin may impact on incidence of distant metastases by a direct systemic effect or secondarily through improved LRC. Daily concurrent chemotherapy was well tolerated with no increase in acute grade 3 mucositis and esophagitis. There were no increases in late skin or severe effects to bone or salivary gland.
A German multicenter randomized trial tested whether the combination of hyperfractionated accelerated radiation (69.6 Gy/5 5.5 weeks) with carboplatin (70 mg/m2) and 5-FU (600 mg/m2/day × 5 days) on weeks 1 and 5 of RT improved outcome compared with the same radiation regimen alone.136 Two hundred and forty patients with stage III (4%) to IV (96%) oropharyngeal (n = 178) and hypopharynx (n = 62) tumors were entered in a 2 × 2 study randomizing patients to receive radiation with or without chemotherapy and then again to receive granulocyte colony-stimulating factor (G-CSF) or not. G-CSF was administered to determine its effect on mucositis. Treatment was tolerable in both study arms with higher mucosal and hematologic toxicity in the chemotherapy/RT arm but no difference in duration of treatment (41 versus 42 days) or total radiation dose delivered (68.5 Gy versus 69.9 Gy) in the chemotherapy/RT versus RT arms, respectively. Response rates at 6 weeks after treatment were similar 92% (complete response = 40%) after chemotherapy/RT and 88% (complete response = 34%) after RT alone. At a median follow-up of 22 months, the 1- and 2-year respective rates of LRC were 69% and 51% after chemotherapy/RT compared with 58% and 45% after RT (P = .14). On subset analysis, patients with oropharyngeal carcinomas had l-year improved survival with LC after chemoradiation (60% versus 40%, P = .01) and trend toward improved LRC (70% versus 58%, l-year and 51% versus 42% 2-year, P = .07) compared with RT alone. Patients receiving chemotherapy/RT had increased grade 3 and 4 toxicities including mucositis (68% versus 52%, P = .01), vomiting (8% versus 2%, P = .02), and hematologic toxicity (neutropenia, 18% versus 0%) with similar rates of dermatitis (30% versus 28%). Patients receiving chemotherapy/RT had more swallowing problems and continuous need for feeding tube (51% versus 25%, P = .02). Interestingly, patients receiving G-CSF had reduced LRC (55% versus 38%, P = .0072) and decreased mucositis (P = .06). Unfortunately, this finding was not supported by RTOG 99-01, a large, multicenter randomized placebo-controlled study of GM-CSF that failed to show any improvement in radiation-induced mucositis rates.137
The GORTEC group conducted a study (GORTEC 96-01), randomizing 109 patients to receive either very accelerated RT (62 to 64 Gy/3 weeks) or moderately accelerated RT (62 to 64 Gy/5 weeks) with concurrent cisplatin (100 mg/m2 days 1, 16, and 32) and 5-FU (1 g/m2 days 1 to 5 and 31 to 35),138 followed by 2 additional cycles of chemotherapy 28 days and 56 days after RT. This trial was stopped early because of an increased incidence of treatment-related deaths in the chemoradiation arm. At preliminary report, no statistical difference on LRC, event-free, or OS could be detected between the two arms.
One question that remains to be answered is whether the employment of altered fractionation schedules further enhances the efficacy of concurrent chemotherapy and radiotherapy using a standard fractionation scheme. In order to address this issue, the RTOG139 has randomized patients with stage III and IV head and neck SCC to receive either standard RT (70 Gy / 7 weeks or accelerated RT (72 Gy / 6 weeks via DCB), with both groups receiving concomitant cisplatin. The accelerated RT group will receive 100 mg/m2 on days 1 and 22, while the standard RT group will receive an additional cycle on day 43. All patients with N2 to 3 disease, and N1 with residual disease at the completion of therapy, will undergo a planned neck dissection. This trial completed accrual in 2005, but preliminary results have not yet been reported.
Induction Chemotherapy
The concept of induction chemotherapy before definitive treatment has been studied for decades, and yet remains a controversial issue. The approach of induction chemotherapy can be considered for various endpoints, including organ preservation, reduction of distant metastatic disease, and improvement of OS rates. Induction chemotherapy has had success in organ preservation strategies in the context of larynx cancer and hypopharyngeal cancer, as described in the VA larynx preservation trial140 and the EORTC 24891 hypopharynx trial.141 The University of Michigan has further investigated the role of induction chemotherapy for larynx preservation. In an earlier study examining three cycles of induction CF followed by accelerated radiation for larynx cancer, it was found that the tumor response after the first cycle of chemotherapy significantly correlated with the response after three cycles, with a positive predictive value of a partial response after one cycle qualifying for organ preservation after three cycles of 90%.142 On the basis of this finding, a phase II trial examined the role of a single cycle of induction CF in advanced laryngeal cancer. Patients who achieved less than a 50% response underwent immediate laryngectomy, while those who responded underwent chemoradiotherapy.143 In this study, 75% of patients were able to undergo chemoradiation. Overall, the larynx preservation rate was 70% and the 3-year OS was 85%. In order to increase the organ preservation rates with induction chemotherapy, GORTEC 2000-01 compared docetaxel, cisplatin, and 5-FU (Taxotere, cisplatin 5-flurouracil [TPF]) to cisplatin and 5-FU (CPF). With TPF, there was an improvement in overall response (82.8% versus 60.8%, P = .0013), translating into an initial larynx preservation rate of 80% versus 57.6% and a 3-year larynx preservation rate of 73% versus 63% (P = .036). Although these results are promising, however, concomitant chemoradiation remains more effective in this context as shown in the RTOG 91-11 trial, which described a larynx preservation rate of 83.6% for concurrent chemoradiation, which was significantly better than both induction chemotherapy (68.8%) and radiation alone (51%), with no difference in OS.115,116
In most studies, the rationale for induction chemotherapy is twofold. First, systemic therapy may be used to suppress the development of distant metastatic disease. Second, induction chemotherapy may render subsequent definitive therapies more effective by reducing both the overall tumor volume and resistant areas of hypoxia. Wayne State University developed what has been until recently the most effective induction chemotherapeutic regimen, consisting of cisplatin and continuous-infusion 5-FU during 5 days for three cycles.140 With this regimen, induction chemotherapy achieves complete response rates of 30% to 50%.140,144,145 However, the response is transient, and definitive therapy is necessary to offer patients long-term control of their disease. Of the innumerable phase III trials that have tested the role of induction chemotherapy for head and neck SCC, only two have shown a survival benefit with this approach.
A French randomized multicenter study of strictly oropharyngeal cancers tested the benefit of adding induction chemotherapy to definitive locoregional treatment.114 Three hundred eighteen patients were randomized to three cycles of cisplatinum/5-FU (100 mg/m2 and 1000 mg/m2/day × 5 days, respectively on weeks 1, 4, and 7 followed by locoregional treatment (n = 157) within 2 to 3 weeks versus locoregional treatment alone (n = 161). About 75% were stage III or IV (49% stage III) and the remainder stage II. Patients underwent either definitive RT (70 Gy/7 weeks, n = 174) or radical resection followed by postoperative radiation (50 to 65 Gy/5-6.5 weeks, n = 142). The target enrollment was 760 patients, but the study was closed because of poor accrual near the end of the 6-year period. Among those receiving chemotherapy, the response rate was 56% (20% complete) with grade 3 or 4 toxicity in 16%, primarily of hematologic, gastrointestinal, and mucositis origin. At a median follow-up of 5 years, OS was higher (estimated 5-year actuarial OS 52% versus 42%, P = .03, respectively) but no statistically significant difference was found in locoregional failure (25% versus 30%), distant metastasis rate (8% versus 12%), or DFS in those receiving chemotherapy. The survival benefit appeared independent of the type of locoregional treatment received. However, the authors concluded that no definitive statement could be made about the role of neoadjuvant chemotherapy in oropharyngeal cancer, as the data were not “convincing enough” and the target accrual incomplete.
Paccagnella et al. reported on a multi-institutional trial that randomized 237 patients to receive either induction CF followed by locoregional treatment (i.e., surgery and/or radiation), or locoregional treatment alone. In a subset analysis of inoperable patients, there was an OS benefit with induction chemotherapy 24% versus 10% at 3 years, P = .04).146 This study was recently updated, and at 5 (23% versus 16%) and 10 (19% versus 9%) years, there was still no OS benefit among all patients. However, the survival benefit for inoperable patients remained significant (21% versus 8% at 5 years, 16% versus 6% at 10 years, P = .04).147
The overall experience with induction chemotherapy is summarized in the Meta Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC).119 This analysis of more than 30 trials comprising more than 5000 patients failed to reveal a survival advantage with induction chemotherapy, with only a 2% benefit at 5 years. There was, however, a significant benefit in trials utilizing a platin plus 5-FU (HR 0.88; 95% confidence interval = 0.69, 0.97). By contrast, this same meta-analysis reported an 8% OS benefit (P < .0001) with concurrent chemoradiation.
Nonetheless, as locoregional therapies have become highly effective in treating primary and nodal disease, patterns of failure have shifted, with distant metastases accounting for a significant proportion of treatment failures.148–152 This has led to heightened interest in finding more effective agents for use in induction regimens. The most promising combinations have added a taxane to platinum and 5-FU. Haddad et al.153 has reported a series of 72 patients with newly diagnosed head and neck SCC, 95% of whom had stage IV disease who were treated with three cycles of TPF and subsequently underwent biopsy of the primary tumor site. Patients subsequently underwent definitive chemoradiation with concomitant carboplatin plus paclitaxel or docetaxel, with neck dissections for patients with N3 or residual disease. With this regimen, an 89% pathologic complete response rate after induction TPF was reported. 5-year OS was 90% and PFS was 85%. Similarly high complete response rates and overall response rates have been reported in phase II studies.154,155
To date, three phase III studies have studied the addition of a taxane to platinum and 5-FU for induction therapy. Hitt et al.156 reported on a Spanish trial that randomized 382 patients with stage III or IV head and neck SCC to receive either induction TPF or CF, with the primary endpoint of complete response. Patients with greater than 80% response underwent definitive chemoradiation, while those with less than 80% response either underwent a neck dissection followed by chemoradiation or were treated off protocol. The treatment design resulted in nearly 40% of patients undergoing definitive treatment off protocol due to a less than 80% response, making the interpretation of secondary endpoints difficult. Nevertheless, there was a significant advantage with TPF in terms of complete response rates (33% versus 14%, P < .001) and overall response rates (80% versus 68%, P < .001).
A second randomized trial, EORTC 24971/TAX 323, has recently been published.157,158 This study randomized 358 patients to receive four cycles of induction TPF (docetaxel 75 mg/m2 day 1, cisplatin 75 mg/m2 day 1, 5-FU 750 mg/m2 continuous infusion days 1 to 5) or CF (cisplatin 100 mg/m2 day 1, 5-FU 1000 mg/m2 continuous infusion days 1 to 5), every 3 weeks. Patients without progression of disease then underwent radiotherapy (without concurrent chemotherapy), with or without planned neck dissection. The TPF regimen provided superior overall response rates to chemotherapy (68% versus 54%, P = .006) and to chemotherapy and radiotherapy (72% versus 59%, P = .006). Despite a similar complete-response rate after the induction chemotherapy phase of treatment, (8.5% versus 6.6%), the complete-response rate after completing radiation was significantly higher in the TPF group (33.3% versus 19.9%, P = .004). With a median follow-up of 32.5 months, this translated into an improvement in median survival (18.8 months versus 14.5 months) and 3-year OS (37% versus 26%, P = .02). These findings are supported by results of the TAX 324 study.159,160 This study randomized 501 patients with stage III or IV head and neck SCC to receive either three cycles of TPF (docetaxel 75 mg/m2 day 1, cisplatin 100 mg/m2 day 1, 5-FU 1000 mg/m2 continuous infusion days 1 to 4) every 3 weeks, or CF (cisplatin 100 mg/m2, 5-FU 1000 mg/m2 days 1 to 5), both followed by concomitant chemoradiation with weekly carboplatin (AUC 1.5). After the induction chemotherapy phase of treatment, there was a similar overall response rate (72% versus 64%, P = .07) and complete response rate (17% versus 15%, P = .66) between the two groups. With a median follow-up of 42 months, the estimated median survival (71 months versus 30 months, P = .006) and 3-year OS (62% versus 48%, P = .002) significantly favored the TPF arm. This translated into a 30% reduction in the risk of death for patients treated with TPF over PF (P = .006). The locoregional failure rate was significantly reduced in the TPF group (30% versus 38%, P = .04); however, the incidence of distant metastatic disease did not differ (5% versus 9%, P = .14). These studies have certainly changed the standard for induction chemotherapy, favoring the addition of a taxane in advanced disease. Despite these promising data, it is not clear yet, however, whether induction chemotherapy should be favored over concomitant chemoradiation without induction therapy. No trial to date has shown a survival benefit with induction chemotherapy compared with chemoradiation alone, and the incremental benefit achieved with induction therapy may be outweighed by added toxicity and the prolongation of overall treatment time. Currently, several trials are comparing chemoradiation with or without the addition of induction chemotherapy. The Southwest Oncology Group study S0427 randomized patients with stage III to IV oropharyngeal cancer to receive cisplatin-chemoradiotherapy with or without induction TPF. Patients in the induction arm with <50% response to TPF were to undergo surgical resection. Unfortunately, this study closed prematurely because of poor patient accrual. The University of Chicago is currently running a phase III trial comparing chemoradiation using docetaxel, hydroxyurea, and 5-FU and a split-course hyperfractionated radiotherapy regimen with the same treatment after 2 cycles of induction TPF. The Paradigm trial, a multi-institutional study headed by the Dana-Farber Cancer Institute, is randomizing patients to received chemoradiation with carboplatin and a DCB fractionation scheme versus induction chemotherapy followed by DCB chemoradiotherapy with either carboplatin for complete- or partial responders or docetaxel for nonresponders.
Special Considerations in the Management of Advanced Oropharyngeal Carcinoma
Management of Advanced Tonsillar Carcinoma
Patients with stage III and IV tonsillar disease can be managed in several ways. Definitive radiation with the addition of a neck dissection for node-positive patients often is used. However, for locally advanced lesions, recent studies have shown definitive concurrent chemoradiation to the primary, with or without planned neck dissection, to be the standard treatment in patients with advanced neck disease.111–113 Combined-modality treatment consisting of surgery followed by RT (or combined chemotherapy and radiotherapy) is sometimes employed, but this is becoming far less frequently advocated. The functional outcome of major oropharyngeal surgery can be poor, and there is no apparent oncologic advantage.
The management of patients with advanced tonsillar lesions is more complex and controversial. As is the case with other subsites of the oropharynx, definitive chemoradiation represents the current standard treatment option to be considered in eligible patients, particularly those with locally advanced and deeply infiltrating primary tumors. Other treatment options have also been used, including RT alone, reserving surgery for salvage,161,162 or surgery and postoperative RT.72,163
Surgery and Postoperative Radiation
In the past, definitive resection was recommended in patients with advanced disease. Hicks reported on the Roswell Park Cancer Institute experience of 76 patients with tonsillar cancer treated with single modality therapy—surgery (56 patients) and radiation (20 patients).44 Among stage III to IV patients (n = 52), surgery resulted in better 5-year DFS (47% versus 27%, P < .05) compared with radiation alone, although a greater percentage of patients were stage IV in the RT cohort (75% versus 44%, P < .05) and split-course treatment was delivered to about half those receiving radiation.
Surgery frequently results in close or positive margins and multiple positive neck nodes. Two reports highlight the importance of postoperative adjuvant RT in advanced cases. The first, by Zelefsky et al.,72 reported the long-term treatment results for advanced oropharyngeal carcinomas treated with surgery and postoperative RT at the Memorial Sloan-Kettering Cancer Center. Twenty patients with SCC of the tonsillar fossa were treated with surgery plus RT. The 7-year actuarial LC rate for tonsillar fossa lesions was 83%. LC was achieved in 94% of patients with T3 lesions and in 75% of patients with T4 lesions. Among patients with positive or close margins who received postoperative doses of 6000 cGy or more, the long-term control rate was 93%. At 7 years, the OS for all patients was 52%, and the DFS was 64%. The actuarial incidence of neck failure was 18%. For all patients, the likelihood of having distant metastasis at 7 years was 30%.
The second study, by Foote et al., evaluated 72 patients who had surgery either with or without postoperative adjuvant RT for advanced disease.38 These investigators note that the main pattern of treatment failure was above the clavicles. This occurred in 39% of patients treated with surgery alone compared with 31% of patients undergoing surgery and postoperative adjuvant RT (despite the more advanced neck disease of the surgery and RT group) and was significantly related (P = .002) to the overall clinical tumor, node, metastasis (TNM) stage. Five-year OS rates for patients with clinical stage III and IV disease who were treated with surgery and postoperative adjuvant RT were 100% and 78%, compared with 56% and 43%, respectively.
Perez et al.164 analyzed 296 patients with histologically proved epidermoid carcinoma of the tonsillar fossa: 127 were treated with irradiation alone (5500 to 7000 cGy), 133 received preoperative RT (2000 to 3000 cGy) or were planned initially for preoperative irradiation but were treated with RT alone, and 36 received postoperative irradiation (5000 to 6000 cGy). The primary tumor recurrence rate in the T1 to T2 groups was approximately 20% for patients treated with irradiation and surgery and 30% for those treated with irradiation alone (difference not statistically significant), 30% in patients with stage T3 lesions in all treatment groups, and 33% in patients with T4 disease treated with surgery and postoperative irradiation, compared with 52% for patients treated with irradiation alone (P = .03). Dasmahapatra et al.163 likewise reported for stage III disease that the 5-year survival after RT and surgery was 31%, compared with 11% for radiation alone; and for stage IV disease, the respective 3- and 5-year survival rates for RT and surgery were 24% and 15%, compared with 6% and 0%, respectively, for RT alone.
By contrast, Spiro and Spiro165 reviewed 162 patients with carcinoma of the tonsillar fossa treated between 1969 and 1983. Combined surgery and RT were used in 29% of patients with stage II disease, 40% of patients with stage III disease, and 67% of patients with stage IV disease. The 3-year determinate LC rates were 89%, 83%, 58%, and 49% for stages I through IV, respectively. The overall 2-year crude survival is 58%. A 60% 2-year survival for T3 lesions compares favorably with other series for treatment of T3 lesions. No survival benefit was seen in advanced stage disease, but this might reflect a selection bias against combined modality treatment compared with those receiving RT alone.
A number of retrospective reports have examined preoperative RT (4500 to 5000 cGy during 5 weeks) versus definitive RT or neck dissection.52,53,166 No advantage to preoperative RT was seen. Thus, preoperative RT for resectable lesions for which surgery is planned is no longer used as a strategy for the primary site. Patients with early stage primary lesions but advanced neck disease are often treated with definitive RT to the primary with preoperative radiation to the neck followed by planned neck dissection.
External Beam Radiation Therapy and Brachytherapy
The use of a combination of external megavoltage irradiation and interstitial 192Ir implants for T3 lesions has been reported by Puthawala et al.167 LC of disease in patients with T3 and T4 lesions was 79%, compared with 95% for T1 and T2 lesions. Treatment-related complications such as soft tissue necrosis or osteoradionecrosis occurred in 6% of patients in the primary group and in 23% in the recurrent group. No significant functional or aesthetic impairments were reported.
A number of investigators have reported that patients with tumor that extends to the tongue have an inferior outcome compared with patients with no tongue extension. However, when an implant is used, this difference is negated. In the retrospective report of Leborgne et al.,168 local relapse was 64% and the 3-year DFS rate was 23% in 39 patients with tongue extension who were treated with EBRT alone, compared with 33% and 43%, respectively, for 90 patients with no tongue extension. However, in those treated with EBRT plus brachytherapy, the local relapse rate was 40% and the 3-year survival rate 60%.
Recurrent Disease and Salvage
Surgical salvage of recurrent disease after RT has a greater chance of success for tumors of the anterior tonsillar pillar than for those in the tonsillar fossa. Gehanno et al.42 reported on salvage surgery for 50 patients with tumors of the tonsillar region. The actuarial survival rates at 3 and 5 years after salvage surgery were 38% and 24%, respectively. Compared with primary surgery, a higher postoperative mortality (8% versus 1.4%) also was seen. Tumor extension that required resection into the tongue base was found to be a negative prognostic factor; survival declined dramatically in such cases.
Peiffert et al.169 reported on using brachytherapy salvage (6000 cGy) in 73 patients who presented with velotonsillar SCC in a previously irradiated area. The 5-year actuarial LC rates for T1N0 and T2N0 disease were 80% and 67%, respectively. The regional relapse rate was 10% in both groups. Grade 2 complications occurred in 13% of patients, and these were not related to the volume treated or to the dose rate. No grade 3 or 4 complications occurred. The 5-year specific survival rate is 64%. Of note, 42% of the patients in this series died from another carcinoma.
Puthawala et al.167 using implantation alone, obtained a 75% LC rate in patients with recurrent disease, with a 2-year absolute DFS rate of 42%. Treatment-related complications such as soft tissue necrosis or osteoradionecrosis occurred in 23% of these patients who had received previous RT.
Management of Advanced Base-of-Tongue Carcinoma
Major resection had traditionally been recommended in patients with advanced base-of-tongue disease. This often entails a total laryngectomy as well as a bone or tongue resection followed by postoperative RT. However, in the modern era, advances in chemotherapy and RT have really removed primary surgery from initial therapeutic considerations. Although significant advances have been made for the laryngectomies, a significant rehabilitation process ensues for all patients, and some never regain the ability to communicate orally. In an effort to improve QOL, and even improve oncologic outcomes other approaches such as the combined use of chemotherapy and RT for organ preservation are now considered the standard of care. RT alone (with the addition of neck dissection for patients with palpable nodes at presentation) also is used for certain moderately advanced lesions. Most patients, even those with advanced disease, can be offered primary chemotherapy and RT options that provide LC equivalent to (or better than) that achieved surgically, but with better QOL.170 Primary surgery is not commonly employed in most centers, including our own.
Outcomes and Results
Advanced base-of-tongue tumors are poorly controlled with one treatment modality. The natural history of advanced tumors of the base of tongue can be gleaned from Dupont et al.,171 who reported on 34 patients with advanced SCC of the base of tongue (20 with T3 and 14 with T4 lesions) treated by surgical resection. These patients underwent an operative procedure as the sole definitive form of treatment. Twenty-eight patients (82%) presented with clinically positive cervical nodal metastases. The LC at 2 years was 27% (n = 9) and the OS was 20% (n = 7). Of note, 44% of patients (n = 15) required laryngectomy as part of the primary surgical treatment and 15% (n = 5) required laryngectomy owing to chronic aspiration, resulting in a total of 59% of patients who required total laryngectomy. Of the 19 patients who had a unilateral neck dissection, failure in the neck was experienced by 53% (70% contralateral, 30% ipsilateral).
Information regarding definitive EBRT for carcinomas of the base of tongue spans at least five decades. Data are typically from single institutions and do not take into account developments in diagnostic radiology and radiation oncology. For advanced tumors, definitive RT produced a LC rate of approximately 50%, compared with 75% to 90% for surgery and postoperative RT.75
The series reported by Harrison et al.172,173 involves mainly patients with stage III and IV disease who were treated with EBRT plus implantation and neck dissection. Patients who would have required laryngectomy had they undergone primary surgery received neoadjuvant chemotherapy followed by EBRT and implantation as part of a larynx preservation study. Sixty-eight patients were managed by this approach between 1981 and 1995. The range of follow-up was 1 to 151 months, with a median follow-up of 36 months. In this series, the actuarial LC rate at both 5 and 10 years was 88%, regional control was 96% at both 5 and 10 years, distant metastasis-free survival rates were 91% and 76%, respectively, DFS rates were 80% and 67%, respectively, and OS rates were 86% and 52%, respectively. After EBRT, 78% of dissected necks were pathologically negative. With surgical salvage, the LC rate was 94%.80,172,174,175 A recent update of the 18 patients with T4 tumors showed that at a median follow up of 23 months, the LC remained excellent at 83%, with a 100% LRC rate and a 94% OS rate. Of note, the one patient who did not receive concomitant chemotherapy developed a local failure. Twenty-eight percent of patients developed distant metastases, resulting in a 61% DFS rate.173 Almost identical results have been reported by Goffinet et al.66 and Puthawala et al.176 A dose–response effect appears to occur, with higher LC being associated with doses of at least 7500 cGy.177
In the opinion of the authors, the treatment of choice for patients with SCC of the base of the tongue is definitive RT, including a brachytherapy implant. The overwhelming majority of patients present with stage III or IV disease. In general, treatment consists of 5000 to 5400 cGy with EBRT and a 2000- to 3000-cGy boost to the base of tongue through a 192Ir implant using after-loading catheters. Necks are managed with elective radiation alone in the N0 group or with radiation plus neck dissection in the group with palpable neck node metastases. The data make clear that most patients’ disease will be controlled with this strategy. In addition because the reduced dose of EBRT decreases the dose to the constrictor muscles, swallowing function may be better preserved in patients treated with a combined approach (see Radiation Therapy Techniques).178 We believe that this approach should be considered the treatment of choice whenever feasible. For those few advanced stage patients who cannot be managed with an organ-preserving approach, surgery followed by RT generally is used.
Surgery and Postoperative Adjuvant Radiotherapy
Recognizing that advanced carcinomas treated surgically will need postoperative RT, Zelefsky et al.,72 reported the long-term treatment results for base-of-tongue and tonsillar fossa carcinomas treated at the Memorial Sloan-Kettering Cancer Center. Between 1973 and 1986, 51 patients were treated with surgery plus RT. Indications included advanced disease (stage T3 or T4, 66%), close or positive margins (64%), and multiple positive neck nodes (84%). The 7-year actuarial LC rates are impressive: 81% for carcinomas of the base of tongue. LC was achieved in 94% and 75% of patients with T3 and T4 lesions, respectively. For patients with positive or close margins who received postoperative doses of 6000 cGy or more, the long-term control rate was 93%. The authors also examined the influence of treatment interruptions. The actuarial control rate among patients who required a treatment break was 64%, in contrast to those who did not require interruption of their treatment, in whom the actuarial control rate was 93% (P = .05). At 7 years, the OS for all patients was 52%, and the DFS was 64%. For all patients, the likelihood of having distant metastasis at 7 years was 30%.
Two randomized trials have examined preoperative versus postoperative RT, and the results appear to be better with postoperative therapy. Although no trials address tumors of the oropharynx specifically, it is reasonable to infer from data on other head and neck sites. The Radiation Therapy Oncology Group (RTOG) trial 73-03 randomized patients with advanced operable tumors of the supraglottic larynx or hypopharynx to 5000 cGy before surgery or 6000 cGy after surgery, and patients with tumors of the oral cavity or oropharynx were assigned to preoperative, postoperative, or definitive RT.84 The LRC rate at 4 years was superior in the postoperative groups (58% versus 48%, respectively; P = .04). The Gustav-Roussy trial179 randomized patients with primary tumors of the hypopharynx to preoperative or postoperative RT. A statistically significant difference (P < .01) in survival rates, complications, and QOL existed in favor of postoperative RT. The postoperative RT group had a superior 5-year survival rate of 56% compared with 20% in the preoperative group.179 In the modern era, preoperative radiation no longer is used for lesions that are resectable and for which resection will be part of the planned management strategy.
In an attempt to reduce the probability of tumor repopulation during a long course of treatment, investigators have used the concomitant radiation boost schedule in a postoperative setting. The regimen is characterized by delivering the boost (10 fractions) as second daily treatments at a time when accelerated repopulation is theorized to occur.76,88 Ang et al. reported a multi-institutional randomized trial prospectively randomizing 213 patients who underwent surgical treatment to adjuvant radiation based on previously defined pathologic risk factors.76 Low-risk patients (n = 31) were observed, intermediate-risk patients (n = 31) received 57.6 Gy in 6.5 weeks, and high-risk patients were randomized to 63 Gy in 7 weeks (n = 75) or in 5 weeks (n = 76) using the DCB schedule. LRC was similar between low-risk patients who received no adjuvant radiation and intermediate-risk patients who received conventionally fractionated radiation to 57.6 Gy (5-year LRC 90% versus 94%, respectively). High-risk patients treated with DCB showed a trend toward improved LRC and survival compared with those treated with CF (P = .11, P = .08, respectively). This benefit was postulated to be due to the shortening of treatment duration between surgery and the end of radiation, thereby overcoming tumor repopulation. Among high-risk patients, shorter treatment duration significantly improved LRC (5-year LRC was as follows: 76% for <11 weeks, 62% for 11 to 13 weeks, and 38% for >13 weeks). Moreover, among high-risk patients treated with conventionally fractionated radiation, those treated below the median treatment duration had a higher 5-year LRC (71% versus 50%, P = .03, estimated from graph) and survival (45% versus 12%, P = .01, estimated from graph) compared with those whose treatment duration exceeded the median time. Acute toxicity was increased in the DCB arm compared with conventionally fractionated (confluent mucositis 62% versus 36%) but late grade 3 to 4 chronic toxicity was no different (38% versus 42%, respectively, at 5 years).
Bernier et al., reported results of the European Organization for the Research and Treatment of Cancer 22931 phase III multicenter randomized trial demonstrating the benefit of concurrent chemoradiation compared with conventional radiation as postoperative adjuvant treatment.180,181 Three hundred thirty-four patients with resected advanced head and neck cancers were randomized to conventional fractionated radiation (2 Gy up to 66 Gy) or to cisplatinum (100 mg/m2/cycle on weeks 1, 4, and 7) concurrent with the same radiation regimen. At a median follow-up of 60 months, patients receiving postoperative chemoradiation had better 5-year PFS (47% versus 36%, P = .04), 5-year OS (53% versus 40%, P = .02), and 5-year local-regional relapse (18% versus 31%, P = .007). Five-year distant metastatic (21% versus 25%) and secondary malignancy rates (12% versus 13%) were not significantly different between the two groups. Patients receiving concurrent chemoradiation had increased grade 3 or higher acute toxicity, the most common being mucositis (41% versus 21%, P = .001). Other severe toxicities in the group receiving chemoradiation included leukopenia (16%), granulocytopenia (13%), nausea (12%) and vomiting (11%). However, the cumulative incidence of late complications was not significantly different between the two groups.
Cooper et al182 reported findings from a similar trial from the RTOG. RTOG 95-01 randomized 459 patients with high-risk squamous-cell carcinoma of the head and neck after total resection to receive conventional radiotherapy alone (60 to 66 Gy in 30 to 33 fractions) or concurrent chemoradiation consisting of the same radiation treatment with three cycles of cisplatin (100 mg/m2 on days 1, 22, and 43). After a median follow-up of 45.9 months, patients in the chemoradiation arm had an improvement in 2-year LRC (82% versus 72%, P = .01) and 2-year DFS (70% versus 60%, P = .04). Two-year OS did not differ significantly between the two groups. The incidence of acute grade 3 toxicity was 77% in the combined-therapy group, compared with 34% in the radiotherapy alone group (P < .001).182 With longer follow-up, however, the improvements in LRC (and DFS rates were no longer significant. At five years, the LRF rate was 20.4% and 28.7% (P = .083) and the DFS rate was 37.4% versus 29.1% (P = .098).183 Nevertheless, based on the earlier results of the trial in conjunction with the findings from EORTC 22931, concomitant chemoradiation has become standard for postoperative patients with high-risk features.
Recurrent Disease and Salvage
The approach to the patient with recurrent base-of-tongue disease depends on the initial therapy the patient received. The use of surgery alone as the salvage procedure in cases of base-of-tongue cancer that were treated previously with RT was reported by Pradhan et al.184 In approximately one third of the patients LC was achieved for at least 1 year. Thirty-five patients required a total glossectomy, of whom 26 did not undergo removal of the larynx. Only 13 patients were alive more than 3 years after salvage.
Others have used 192Ir after-loading techniques in patients who received full-tolerance radiation (with or without previous surgery). Langlois et al.,185 reported on 123 patients treated for recurrence or new cancer of the tongue or oropharynx arising in previously irradiated tissues. The actuarial LC rate was 67% at 2 years and 59% at 5 years. LC of the tumor was achieved in most of these patients, though the actuarial survival was only 48% at 2 years and 24% at 5 years. The complication rate was slightly higher; 28 patients developed mucosal necrosis.
Mazeron et al.,186 had similar results: actuarial LC was 72% at 2 years and 69% at 5 years. Although LC of the tumor was achieved in the majority of these patients, only 14% remained alive at 5 years. The best results were achieved in patients with lesions of the faucial arch and posterior pharyngeal wall; LC was achieved in 100% of these patients. Patients with lesions of the base of tongue and of the glossotonsillar sulcus had suboptimal results; LC was achieved in only 61%.
Owing to the high complication rates, Housset et al.,187 compared two techniques of 192Ir implantation for salvage. Patients received either single-course implants, delivering 6000 cGy, or split-course implants with a source shift, the goal being to decrease treatment complications. The first and second course of the split-course implants delivered 3500 and 3000 cGy, respectively, at a 1-month interval. The active lines of the second implant were placed parallel to and between the lines of the first implant. This shift in the source position resulted in a more uniform dose within the treated volume, with a 60% reduction in the high-dose sleeves. The overall local failure rate was 45.5% (25 of 55). The difference between the local failure rates after single-course implants (52%) and after split-course implants (37.5%) was not statistically significant. The only complication noted in the 40 patients in whom immediate LC was achieved after either implantation technique was mucosal necrosis. Of note, the split-course implants were associated with a 2.5-fold decrease in the incidence of necrosis: 43% (9 of 21) in the single-course group and 16% (3 of 19) in the split-course group (P = .05).
Management of the Neck: The Role of Planned Neck Dissection
Management of the neck is an important and complex issue in head and neck SCC, and is discussed in greater detail elsewhere in this book. In the N0 neck, elective treatment of the neck is recommended in patients with a ≥20% risk of occult nodal disease.188 The selection of treatment modality for the neck should be based on the approach toward the primary tumor, in order to avoid use of multimodality treatment if unnecessary. This approach is recommended in node-positive disease as well; however, multimodality treatment plays a critical role in these patients. In cases where surgery is the primary treatment modality, neck dissection should be performed at the time of surgery, with the need for postoperative radiation or chemoradiation determined by pathologic findings. In oropharyngeal cancers, however, which are often treated with definitive chemoradiotherapy, the surgical management of the neck remains a debated issue.
For N1 patients, the chance of neck control after definitive radiation is very high. Therefore, patients do not require a neck dissection unless there is evidence of persistent disease after treatment.189 However, with increasing nodal disease, regional recurrence rates increase.190–193 It has long been recognized that patients treated with combined radiation and surgery for N2 to N3 disease have lower neck recurrences than after either modality alone194–197; planned neck dissections have therefore been standard for patients with N2 or N3 disease.198 However, in the setting of modern treatment with concurrent chemoradiation, the need for a planned neck dissection has been questioned, particularly in the setting of a complete clinical response after definitive doses of radiation.189,199–201 Several investigators advocate the incorporation of a planned neck dissection, regardless of the response after chemoirradiation,148,191,198,202–211 whereas others prefer to reserve neck dissection for those patients with clinical or radiographic evidence of residual nodal disease.189,199,212–217 Recently, the use of CT and positron-emission tomography (PET) scans has aided in further refining the management of the neck. Liauw et al., analyzed the records of 550 patients with node-positive disease treated with radiotherapy or chemoradiotherapy, with or without planned neck dissection.218 Patients typically underwent CT scans 30 days after completion of radiation; these were correlated with the pathologic findings from their planned neck dissection(s). Of 211 patients (266 heminecks) with CT data available for review, 28% had a radiologic complete response (rCR), with all nodes ≤1.5 cm and without focal abnormalities (calcifications or necrosis). When correlated with the pathologic specimens, a rCR demonstrated a sensitivity of 96%, specificity of 24%, positive predictive value of 35% and a negative predictive value of 94%. Amongst the 32 patients who did not undergo a neck dissection after a rCR, the 5-year neck control rate was 97% (with salvage, 100%); this was equivalent to those patients with a pathologically negative neck dissection (5-year neck control 98%). There was no difference in cause-specific or OS between these groups. Corry et al. reported similar results in a study of 43 patients, in which all patients who achieved a clinical and radiographic (CT scan) complete response remained without regional recurrence with a median follow-up of 3 years.201
FDG-PET scans have also been utilized to determine need for a planned neck dissection. Yao et al. reported on a series of 41 patients treated with definitive radiotherapy or chemoradiotherapy who underwent PET scans after treatment to assess response.107 Patients’ scans were correlated with biopsy or neck dissection specimens. In this study, a negative PET scan (maximum standardized uptake value [SUV] <3.0) was highly correlated with a negative pathologic examination, with a negative predictive value of 100%. Several studies support these very encouraging findings, with a negative predictive value of PET scans after radiation of 83% to 97%.219–222 Some studies, however, report significantly lower negative predictive values associated with postradiation PET scans if done earlier than 3 months after RT.223,224 In our estimation, this is reflective of the lack of standardization in interpretation and timing of PET, rather than a shortcoming of PET scans themselves. The majority of available evidence indicates that there is a significant proportion of patients in this category in whom a planned neck dissection is unnecessary. It has therefore become the policy at our institution to observe patients with N2 disease who attain a complete response after definitive chemoradiation. In patients with lymph nodes >6 cm, however, a planned neck dissection remains our standard approach (see Fig. 28-19). There are, however, disadvantages to this treatment approach. Patients who require neck dissections after receiving definitive doses of radiation (i.e., ≥70 Gy) have a higher likelihood of postoperative wound complications than would be the case after the lower doses that are used when a neck dissection is planned at the outset (60 Gy). It is also imperative that patients are followed rigorously with monthly examinations and surveillance imaging every 3 to 4 months for the first 1 to 2 years after therapy, as salvage after recurrence remains difficult.198,207,225
Prognostic Factors
Commonly reported prognostic factors for LRC, DFS, or OS include T stage, N status, and gender and performance status.39,58 Multiple studies have investigated various radiographic parameters and molecular markers as potential predictive factors in patients treated definitely for oropharynx cancers. In contrast to cancers involving the larynx,226 hypopharynx,227 and nasopharynx,228 pretreatment chemotherapy-determined primary tumor volume is not predictive for LC after definitive RT alone using conventional229 or altered fractionated radiation.230 Encouraging results from single institution studies have reported that a high intratumoral microvessel density predicts for poorer LC and worse OS after definitive radiation of oropharynx carcinoma231 and that a high Ki-67 labeling index is associated with local relapse232 and shorter mean time to relapse233 after definitive resection and postoperative radiation. Overexpression of proliferating cell nuclear antigen (PCNA; an estimate of growth fraction),233,234 vascular endothelial growth factor (VEGF), or thrombospondin-1 (both markers of angiogenesis) have shown no predictive value in patients with oropharynx cancer treated with either definitive RT or combined surgery and radiation.231,233,234 Studies that have reviewed p53 overexpression have yielded conflicting results regarding its prognostic value for this tumor site.231–233 Hypoxia as detected by Eppendorf measurement of cervical lymph nodes involved with head and neck cancer has been associated with worse radiation235–237 or chemoradiation response238 of the involved lymph node. Anemia has also been associated with poor outcome after radiation58,239 and its correction may improve outcome.240 Overexpression of a hypoxia-induced transcription factor, hif-l-α (hypoxia-inducible factor-1α), was shown in a study of 98 patients with oropharyngeal cancers treated with definitive radiation correlated with the chance of complete response at the primary and nodal sites after treatment as well as local failure-free, disease-free, and OS on multivariate analysis.241 These markers remain to be validated in larger, multi-institutional trials.
Human Papilloma Virus in Oropharynx Cancer: Prognostic Implications
In contrast to the incidence of other head and neck SCC sites, the incidence of oropharyngeal carcinoma has been steadily increasing during the past 2 decades.242 Human papilloma virus (HPV) is implicated for the increased incidence and established as an important prognostic and predictive determinant for tumor control. Mork suggests that the exposure to HPV precedes development of oropharyngeal cancer by 10 years.243 HPV is estimated to occur in at least 50% of oropharyngeal carcinomas although ranges from (49% to 72%),244,245 depending on the time period and country. The incidence of HPV associated oropharyngeal carcinomas has increased over time as shown in Sweden as the prevalence of HPV-associated cancer increased from 23% in the 1970s to 57% in the 1990’s and 68% from 2000 to 2002.245 HPV infection is related to sexual behavior as case-control studies have shown that a high life-time number of oral sex partners, early age at first intercourse, engagement in casual sex and infrequent use of condoms are strongly correlated with HPV-positive cancers.244 In contrast to HPV-negative oropharyngeal carcinomas which are associated with tobacco and alcohol use, HPV positive tumors are strongly associated with marijuana use.246 No evidence of synergy is found between combined HPV and tobacco or alcohol use. Virtually all HPV-positive cases are associated with the HPV-16 subtype in 85% to 90% of cases244,245 and the remainder are associated with HPV.246 Moreover, HPV-positive tumors are associated with younger age by 5 to 10 years with equal male and female risk, and unknown primary status.245,247 Morphologically, they tend to be poorly differentiated and of basaloid histology.247 There are geographic differences in the incidence of HPV positive tumors as an international literature study demonstrated the proportion to be 47% of oropharynx cases in North America compared to 28% in Europe.247
HPV-positive patients appear to have a better prognosis compared to tobacco-related tumors with up to a 60% to 80% risk reduction from disease related mortality.248–249 The risk reduction has been shown for oropharynx cancer patients treated with radiation,249,250 induction chemotherapy251 and primary surgery.252 In a phase II ECOG study of 96 oropharynx or larynx cancers treated with induction chemotherapy followed by concurrent chemoradiation, those patients who were HPV positive (40% of all patients, 63% of all oropharynx patients, 0% of all larynx patients) had higher response rates after induction chemotherapy (82% versus 55%, P = .01) and chemoradiation (84% versus 56%, P = .007) as well as improved 2-year survival (95% versus 62%, P = .005) compared to those who were HPV negative.251
The favorable prognostic outcome among HPV positive patients appears to be restricted to those that are also p16 positive.249 In a study of 77 patients with oropharynx cancers treated with primary radiation or postoperative radiation, 60% were HPV positive. Among the HPV positive patients (n = 18), those who also were p16 positive had improved LC (5-year 86% versus 26%, P = .027), DFS (75% versus 13%, P = .0025) and OS (79% versus 18%, P = .0095) compared to those who were p16 negative (n = 29).249 These study remains to be validated with larger numbers and prospective studies.
The biologic basis for improved survival in HPV-positive patients is unclear.252 HPV-positive tumors have a distinct molecular phenotype characterized by increased expression of p16, presence of wild-type p53,252 low EGFR and pRb expression253,254 and infrequent amplification of cyclin D252,255,256 HPV is thought to mediate oncogenesis by continued expression of two viral oncoproteins E6 and E7 which inactivate tumor suppressor proteins, retinoblastoma and p53, respectively.257–259 p16 binds to the cyclin D1/CDK4/CDK6 complex to prevent phosphorylation of the Rb protein.260 In tobacco-related head and neck cancer, p16 loss is a common and early event.261–263 p16 is an inhibitor of a cyclin-dependent kinase whose expression is negatively regulated by pRb. E7 is thought to bind pRb and release pRb mediated suppression of DNA synthesis as well as inhibition of p16 expression, thereby promoting cellular proliferation and increasing p16 expression.253 The correlation of p16 with HPV positivity is great enough such that p16 is felt to be a good surrogate for HPV infection.249,250
Treatment Sequelae and Complications
Surgical Resection
Sequelae related to surgery can be grouped into intraoperative, immediate, and delayed postoperative complications. Operative mortality for either primary tumor resection or neck dissections should be less than 5%. Among the intraoperative complications are damage to nerves (including cranial nerves V, VII, and XI), and vascular, lymphatic, and pulmonary complications.264,265 Delayed complications include fistula, dysarthria, mandibular necrosis, trismus, exposure of the carotid artery,266 lymphedema, and decreased function of muscle groups after a neck dissection. Even after preservation of the spinal accessory nerve, signs of muscle dysfunction can be seen.267 Extensive resections can affect speech and swallowing. Often, to prevent aspiration, a laryngectomy is performed,268 causing additional functional morbidity.
The base of tongue propels food past the larynx. Any reconstruction of the anterior floor of mouth that inhibits tongue-tip and lateral movement will reduce a patient’s ability to chew, to control foods in the mouth, and to initiate the swallow. The more tongue that is tethered or restricted in its motion after resection, the more severe will be the swallowing disorder. Surgical reconstruction that creates the greatest range of back and base-of-tongue movement will result in the best tongue control for swallowing.268
Salvage surgical procedures performed after induction chemotherapy and definitive RT have been reported to be associated with a higher rate of major wound complications.269 In a review of 96 patients, surgical salvage within 1 year of initial treatment resulted in a 77% incidence of major wound complications, compared with a 20% incidence if surgical salvage was performed 1 year after initial treatment.269 The mean time to resolution of fistulae and flap necrosis was 7.7 months. Two deaths were attributed to major wound complications: one patient had a carotid blowout, and another died of postoperative pneumonia.
Radiation Therapy
The late effects of RT after approximately 3500 to 4000 cGy can include xerostomia and altered sense of taste. The importance of preserving salivary production on a patient’s quality of life cannot be overstated. An average individual produces up to 1 to 1.5 liters of saliva per day, and loss of saliva predisposes to mucosal fissures and ulceration. Moreover, saliva aids in speech, taste, mastication and deglutition of food, and its loss may aggravate the nutritional deficiencies that are so prevalent among patients with head and neck SCC. Saliva also plays a role in preventing caries and oral cavity infections via secretion of salivary lysozyme, IgA and other antibacterial substances, and the production of salivary bicarbonate aids in preventing esophageal injury. All of these issues are magnified in this patient population. Amifostine has been shown to be effective in preventing xerostomia in clinical trials. Brizel et al.270 randomized 315 patients to receive radiation alone or with intravenous amifostine before each RT dose. Twenty-one percent of patients discontinued amifostine before completing radiation, most often due to nausea, vomiting or hypotension. Nonetheless, the use of amifostine was shown to reduce the incidence of grade 2 or greater acute xerostomia (51% versus 78%, P < .0001), as well as increasing the dose to onset of acute xerostomia (60 Gy versus 42 Gy, P = .0001). At 2 years, the incidence of grade 2 or greater late xerostomia was also improved (19% versus 36%, P = .038).271 There was no reduction in progression-free or OS associated with administration of amifostine. These results were not confirmed, however, by Buentzel et al.,272 who enrolled 132 patients in a multicenter randomized placebo-controlled study in which patient received either IV amifostine or placebo before receiving carboplatin-based chemoradiation. In this study there was no difference in either grade 2 or higher acute xerostomia (39% versus 34%, P = .715) or late xerostomia (37% versus 24%, P = .235) with or without the use of amifostine. Administration of subcutaneous (SC) amifostine is generally felt to be both more convenient and better tolerated than the intravenous route. In order to compare the two methods of administration, the GORTEC group ran a prospective randomized study comparing IV versus SC administration. Three hundred eleven patients receiving at least 40 Gy to the parotid glands were randomized to receive either IV amifostine (200 mg/m2/d, 15 to 30 minutes before each RT fraction) or SC amifostine (500 mg/d, 20 to 60 minutes before each RT fraction). Preliminary results273 suggested that the SC route was better tolerated than IV administration, with a compliance rate of 80% versus 70%, respectively. This was felt to be particularly due to the reduced incidence of hypotension (6% versus 0%). In an update of the results of this study,274 there was no difference in either compliance (70% versus 71%), and the incidence of hypotension was low in both groups (3% versus 1%). Moreover, the SC group had an increased incidence of rash (11% versus 22%, P = .02) and local pain at the injection site (0% versus 9%, P = .0008). Although the toxicity associated with amifostine was quite low, it should be noted that this was in the setting of a rigorously controlled clinical trial; this may not be the case in a less closely monitored setting.
Another approach to prevent xerostomia involves reduction in the dose of radiation received by the parotid glands, a major goal of head and neck IMRT (see Figs. 28-16 to 28-19). During the past several years, many clinical studies have reported on the role of IMRT in preventing xerostomia via parotid sparing. Eisbruch et al.275 reported that in a study of 88 patients and 152 parotid glands, limiting the mean parotid dose to <26 Gy resulted in retaining a substantial fraction of the baseline salivary output, while parotid glands receiving higher doses did not produce measurable saliva. Chao et al.276 studied 41 patients treated with either IMRT or 3DCRT and measured stimulated and unstimulated salivary flow, both before treatment and 6 months after completion of RT, and observed a 4% reduction in salivary flow per Gray of mean parotid dose, and calculated that a mean dose of ≤32 Gy would achieve <25% reduction in salivary flow. Several other investigators have reported similar findings.278–279
Recently, there has been emerging interest in sparing the submandibular glands to further aid in the reduction of xerostomia. Although the parotid glands contribute more than 50% of stimulated salivary flow, the submandibular glands are primarily responsible for salivary production in the resting state,280 which is important in patients’ subjective complaints of dry mouth. Attempts to spare the submandibular gland in the IMRT plan have been described281; however, as this would result in undertreating the level II lymph node station in at-risk patients, extreme caution should be employed when considering this approach. Submandibular sparing should certainly be possible in patients receiving ipsilateral radiation (see Fig. 28-16). An alternative approach to submandibular gland sparing involves surgical transfer of the gland into the submental space before radiotherapy. This was described by Seikaly and Jha et al.,282 who reported their series of patients with head and neck SCC and N0 to N2B disease who were undergoing surgery as part of their treatment plan. Patients with oral cavity or nasopharynx primaries were ineligible, as were patients with bilateral or N3 nodal disease, or involvement of level I nodes on either side of the neck. The reader is directed to their 2001 paper for further details regarding the technique of transfer. With a minimum of 2 years’ follow-up,283 83% of the 96 patients enrolled in this study reported normal salivary flow, based on the validated University of Washington QOL questionnaire.284 Measurement of stimulated salivary flow rate revealed preservation of saliva; this compared favorably with a matched cohort of 38 patients who did not undergo submandibular sparing, none of whom had normal salivary function. There were no disease recurrences on the side of the transferred gland or in the submental space, and no surgical complications attributed to the transfer procedure were reported. A subsequent study by the same group showed that submandibular transfer was also associated with improved swallowing outcomes.285 This method of salivary sparing has been reproduced with similar success by other groups,286,287 and a multicenter phase II trial is currently being run by the RTOG.288
Radiation-induced dysphagia is also an area of recent interest. Swallowing is a complex mechanism, involving the actions of 26 muscles and five cranial nerves; a normal adult swallows unconsciously approximately 600 times per day. Swallowing is separated into three phases. In the oral phase, the bolus is ingested, masticated and lubricated, and transported to the oropharynx. In the pharyngeal phase, the soft palate rises, preventing the bolus from entering the nasopharynx, while the larynx rises, the epiglottis retroflexes, and the focal folds close. The middle and inferior constrictors contract in a synchronized manner as the cricopharyngeal muscle relaxes to allow the bolus to enter into the esophagus. Finally, in the esophageal phase, peristaltic muscle contractions and relaxations carry the bolus through the esophagus, while the cricopharyngeal muscle again constricts, closing the upper esophageal sphincter. Perturbations in swallowing have great impact on quality of life after radiation, contributing to both nutritional issues and patient discomfort. Eisbruch et al.289 recently published a profound study examining the potential to reduce dysphagia via IMRT planning. In this study, the anatomic structures that are associated with dysphagia and aspiration were first identified in 26 patients undergoing chemoradiation. Patients were evaluated before and after treatment with videofluoroscopy and esophagography functional abnormalities in these studies were identified. Pre- and posttreatment CT scans and endoscopic examinations were then reviewed, and anatomic abnormalities were correlated to those functional abnormalities. On the basis of these studies, it was found that only one patient had abnormalities in the oral phase of swallowing, whereas multiple abnormalities were found in the pharyngeal phase, affecting every patient in this study. The dysphagia and aspiration related structures (DARS) were determined to be the circular pharyngeal constrictors and the glottic and supraglottic larynx. In the second phase of this study, the CT data sets of 20 consecutive patients who had been treated with one of two intensive chemoradiation regimens using standard IMRT planning techniques were obtained. In addition to a standard IMRT plan based on RTOG guidelines, two more treatment plans were generated, including a 3DCRT plan and a dysphagia/aspiration-optimized IMRT (doIMRT) plan. This plan minimized the portions of the DARS that lay outside the PTV receiving ≥50 Gy without compromising the dose to the PTVs. This study showed that it is possible to reduce the mean V50 Gy using a doIMRT plan compared with 3DCRT and standard IMRT planning to both the pharyngeal constrictors (69% versus 90% versus 80%, respectively P < .001) and the larynx (61% versus 79% versus 72%, respectively, P = .002 for 3DCRT, P = .001 for standard IMRT). This approach may hold great promise in reducing treatment-induced dysphagia.
Several centers have reported retrospective evidence of a dose-response relationship for potential DARS including the constrictor muscles (particularly the superior constrictors), larynx and hypopharynx.178,290–292 Threshold doses in the range of 45 to 54 Gy above which severe dysphagia and stricture is reported based on videoscopic swallowing evaluation and validated quality of life questionnaires such as EORTC Q-30 and M.D. Anderson dysphagia inventory. Levendag reports that patients with oropharynx cancers who are treated with a combination of external beam radiation and brachytherapy are the least likely to experience severe dysphagia compared to patients treated by IMRT or stereotactic radiosurgery boost.178,293
Potential long-term complications at doses exceeding 6000 cGy include soft-tissue and bone ulceration and necrosis. Radiation is believed to exert an avascular effect on tissues and epithelia that are thinner and more susceptible to injury. The process usually starts with ulceration of soft tissues, which can progress to bone exposure. If bone is then injured, bone necrosis or osteoradionecrosis can result. Treatment of this latter process is frustrating and difficult. For minor bone exposures, conservative measures are used, debridement being performed when indicated. For refractory cases, hyperbaric oxygen treatment has been advocated. Factors that can influence osteoradionecrosis include elective dental extraction after RT and treatment of tumors near bone.56,294 In the modern era, osteonecrosis should be an uncommon event (<5%).193
The use of brachytherapy implants also can contribute to osteoradionecrosis. Harrison et al.175 point out that the patients who develop complications usually received the brachytherapy implant as the initial mode of therapy and the entire tumor bed was implanted. When EBRT was used for initial treatment, the boost was administered to the smaller volume of residual disease and the incidence of soft-tissue ulceration and osteoradionecrosis was reduced greatly. Technique also plays a role. The nonlooping technique is associated with a higher reported injury rate than the looping technique.172,175
Quality of Life
A great deal of interest has been expressed in patient QOL since the turn of the millennium. Oncologists are recognizing that a cured patient who is disabled as a result of treatment is not the same as a patient who requires significant intervention by the health care system or dependence on society for social services. Measures of QOL, although still evolving, are now important parts of research protocols. We currently use a number of tools to study this process. One such tool is the Memorial Symptom Assessment Scale (MSAS), a comprehensive symptom measure that records (1) prevalence of 32 physical and psychological symptoms commonly experienced by cancer patients, (2) symptom characteristics, and (3) measures of symptom distress.295 Another such tool is the Functional Assessment of Cancer Therapy (FACT), a multidimensional 33-item QOL instrument. A study in a mixed cancer population (n = 545) demonstrated the internal consistency and validity of the total and subscale scores for various domains of well-being.296 Each score is calculated as the sum of the responses given to specific groups of questions relating to physical, emotional, social, and functional well-being.
A third QOL evaluation tool is the head and neck performance status scale (PSS). The PSS is an interviewer-rated head and neck cancer performance status scale that yields separate scores reflecting a patient’s ability to eat in public, comprehensibility of a patient’s speech, and normalcy of diet. The scale has been validated and used in previous surveys of patients with base-of-tongue cancer. Each score corresponds to a description of the functional capability that attends it. The higher the score, the better is the function. A score of 100 indicates normal function and is the best possible score.297
Harrison et al.170 retrospectively examined patients with SCC of the base of tongue who were treated with primary RT or primary surgery, comparing the QOL and functional outcome. Patients had been treated primarily either by RT or surgery depending on the philosophy of their primary physician. Primary RT consisted of 4500- to 5400-cGy EBRT followed by a 192Ir implant that delivered an additional 2000 to 3000 cGy during 2 to 3 days. In those with involved lymph nodes, a neck dissection was performed at the same time as the implantation. Primary surgery consisted of resection of the base-of-tongue lesion, neck dissection, and postoperative RT. Both groups had similar LC (80% to 90%). A subjective performance status scale for head and neck cancer patients was used to assess the QOL in these patients (0 to 100, where 0 = worst function and 100 = normal function), measuring the patient’s ability to eat in public, comprehensibility of the patient’s speech, and normalcy of diet (see Fig. 28-11).296 Patients treated with RT had consistently better performance status and QOL scores. This was true for those with early (T1 to T2) as well as more advanced (T3 to 4) disease. In addition, comparison of scores for early and advanced disease treated by primary RT revealed no difference in all three functional categories for T1 to T2 compared with T3 to T4 disease (P = .84), showing that QOL scores remain high for all stages. For surgery, functional status deteriorated significantly when comparing T1 to T2 and T3 to T4 lesions (P = .0014), consistent with the fact that larger tumors require more extensive operations. The investigators’ results show that RT provides a better performance status than surgery for base-of-tongue cancer, whether the patient exhibits early or advanced disease. Functional scores remained high for all T stages treated with irradiation but deteriorated with more advanced T stages for patients treated surgically.170
In addition to QOL issues, Harrison et al.170,298 have examined the long-term socioeconomic outcomes for patients with cancer of the base of tongue who were treated with an organ preservation approach. At the time of follow-up (median, 5 years), patients’ annual incomes were similar to those at the time of presentation (89% exceeding $20,000; 52% exceeding $60,000). Of those working full-time, 72% were still in full-time work, and 83% of those working part-time were in part-time work.
Radiation Therapy Techniques
Soft Palate
The two-dimensional (2D) fields begin with opposed lateral fields to the primary site and upper necks bilaterally (Fig. 28-12). The patient is simulated with head extension and a bite block. The field design include the retropharyngeal nodes, which are at risk for all soft palate cancers. Care is taken to block the roots of the teeth and the external auditory canal. The low neck is treated with a low anterior neck field matched at the thyroid notch below the hyoid bone with a block in the upper midline to protect the spinal cord and the glottic larynx. After 4500 cGy, the opposed lateral fields are split into an anterior photon and posterior electron field to protect the spinal cord. Cone down photon fields to areas of gross disease at the primary site and neck nodes are designed while the posterior necks are treated with electron fields of appropriate energy.
Tonsil
The chapter has extensively reviewed the relative indication for unilateral versus bilateral neck treatment for patients with tonsil carcinoma. The technique for each of these situations will be reviewed using 2D, 3D, and IMRT techniques. Fig. 28-13 shows a traditional 2D bilateral field arrangement, whereas Fig. 28-14 shows a patient treated with a unilateral 3D plan.
IMRT has been increasing utilized for tonsil cancers to preserve salivary gland function. It can be helpful in clinical situation in a case where the contralateral nodes may be spared to allow sparing not only of the contralateral parotid gland but also the contralateral submandibular gland (Fig. 28-15). If there is a clinical indication to treat the contralateral neck electively (e.g., a primary lesion that crosses midline or advanced ipsilateral neck disease but the contralateral neck is clinically negative), it is especially advantageous to spare the contralateral parotid gland (Fig. 28-16). In this situation, the contralateral jugular nodes may be treated up to the digastric muscle (bottom of the transverse process of C1) which significantly limits the volume of contralateral parotid gland exposed to radiation. In addition the bilateral retropharyngeal chain can still be covered.
Base of Tongue
Patients with cancer of the base of tongue are treated to the primary site and both sides of the neck. As with soft palate cancers, there are no situations where ipsilateral neck treatment is offered. The 2D treatment technique involves bilateral opposed fields for the primary site and upper neck, matched to a low neck field (Fig. 28-17). The patient is simulated with neck extension and a bite block. The initial fields include the primary site and upper neck nodes. The field is junctioned above the larynx so as to protect the glottic larynx from radiotherapy. The low neck field has a block in the upper midline for protection of the spinal cord and the larynx.
The authors’ practice is to use an implant for the boost treatment (see Fig. 28-17). In centers that use a concomitant boost technique, a field reduction will be designed to incorporate the base of tongue with adequate margin.
IMRT has become increasing utilized for base-of-tongue cancers. The most frequent indication is a patient in whom the contralateral neck is clinically negative. When this is the case, there is an opportunity to spare the contralateral parotid gland using an IMRT approach. Fig. 28-18 shows the contours, dose distributions and dose-volume histogram for a T2N2bM0 SCC of the base of tongue. In this case, the primary site as well as the bilateral neck nodes received 54 Gy in 30 fractions of 1.8 Gy. The contralateral parotid is relatively spared as shown in the dose-volume histogram. Completion of treatment to the primary site with a brachytherapy boost also allows decreases the dose to the constrictor muscles to maximize preservation of swallowing. The low neck would be treated with a separate, anterior field, which also block the larynx on the upper midline.
In a situation in which the low neck nodes are clinically involved, IMRT should be used to treat the upper and lower neck nodes to comprehensively treat the necks bilaterally without overdosing the brachial plexus (Fig. 28-19).
1 Parker SL, Tong T, Bolden S, et al. Cancer statistics. CA Cancer J Clin. 1996;46(1):5-27.
2 Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2007;57(1):43-66.
3 Rouviere H. Anatomy of the human lymphatic system. Ann Arbor, MI: Edwards Brothers; 1938.
4 Fisch UP. Cervical lymphography in cases of laryngo-pharyngeal carcinoma. J Laryngol Otol. 1964;78:715-726.
5 Haagensen CD, Feind CR. The lymphatics in cancer. Philadelphia: Saunders; 1972. xvi, 583
6 Wong YK, Novotny GM. Retropharyngeal space—a review of anatomy, pathology, and clinical presentation. J Otolaryngol. 1978;7(6):528-536.
7 Som PM. Lymph nodes of the neck. Radiology. 1987;165(3):593-600.
8 Mancuso AA, Harnsberger HR, Muraki AS, et al. Computed tomography of cervical and retropharyngeal lymph nodes: normal anatomy, variants of normal, and applications in staging head and neck cancer. Part I: normal anatomy. Radiology. 1983;148(3):709-714.
9 Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck. 1990;12(3):197-203.
10 McLaughlin MP, Mendenhall WM, Mancuso AA, et al. Retropharyngeal adenopathy as a predictor of outcome in squamous cell carcinoma of the head and neck. Head Neck. 1995;17(3):190-198.
11 Ballantyne AJ. Significance of retropharyngeal nodes in cancer of the head and neck. Am J Surg. 1964;108:500-504.
12 Chua DT, Sham JS, Kwong DL, et al. Retropharyngeal lymphadenopathy in patients with nasopharyngeal carcinoma: a computed tomography-based study. Cancer. 1997;79(5):869-877.
13 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446-1449.
14 Fletcher GH. Textbook of radiotherapy, 3d ed. Philadelphia: Lea & Febiger; 1980. xiv, 959
15 Crawford BE, Callihan MD, Corio RL, et al. Oral pathology. Otolaryngol Clin North Am. 1979;12(1):29-43.
16 Low WK, Sng I, Balakrishnan A. Palatine tonsillar metastasis from carcinoma of the colon. J Laryngol Otol. 1994;108(5):449-451.
17 Mochimatsu I, Tsukuda M, Furukawa S, et al. Tumours metastasizing to the head and neck–a report of seven cases. J Laryngol Otol. 1993;107(12):1171-1173.
18 Tesei F, Farneti G, Cavicchi O, et al. A case of Merkel-cell carcinoma metastatic to the tonsil. J Laryngol Otol. 1992;106(12):1100-1102.
19 Asami K, Yokoi H, Hattori T, et al. Metastatic gall bladder carcinoma of the palatine tonsil. J Laryngol Otol. 1989;103(2):211-213.
20 Gluckman JL, Gullane PJ, Johnson JT. Practical approach to head and neck tumors. New York: Raven Press; 1994. x, 212
21 Boffetta P, Mashberg A, Winkelmann R, et al. Carcinogenic effect of tobacco smoking and alcohol drinking on anatomic sites of the oral cavity and oropharynx. Int J Cancer. 1992;52(4):530-533.
22 Perez CA, Purdy JA, Breaux SR, et al. Carcinoma of the tonsillar fossa: a nonrandomized comparison of preoperative radiation and surgery or irradiation alone: long-term results. Cancer. 1982;50(11):2314-2322.
23 Shugar MA, Nosal P, Gavron JP. Technique for routine screening for carcinoma of the base of tongue. J Am Dent Assoc. 1982;104(5):646-647.
24 Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328(3):159-163.
25 Mashberg A, Samit A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers. CA Cancer J Clin. 1995;45(6):328-351.
26 Mashberg A, Meyers H. Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas: a continuing prospective study of oral cancer. II. Cancer. 1976;37(5):2149-2157.
27 Shaha AR, Shah JP. Biopsy techniques in head and neck. Surg Oncol Clin N Am. 1995;4(1):15-28.
28 Greene FL. American Joint Committee on Cancer, and American Cancer Society, AJCC cancer staging manual, 6th ed. New York: Springer-Verlag; 2002. xiv, 421
29 Erkal HS, Serin M, Amdur RJ, et al. Squamous cell carcinomas of the soft palate treated with radiation therapy alone or followed by planned neck dissection. Int J Radiat Oncol Biol Phys. 2001;50(2):359-366.
30 Weber RS, Peters LJ, Wolf P, et al. Squamous cell carcinoma of the soft palate, uvula, and anterior faucial pillar. Otolaryngol Head Neck Surg. 1988;99(1):16-23.
31 Keus RB, Pontvert D, Brunin F, et al. Results of irradiation in squamous cell carcinoma of the soft palate and uvula. Radiother Oncol. 1988;11(4):311-317.
32 Esche BA, Haie CM, Gerbaulet AP, et al. Interstitial and external radiotherapy in carcinoma of the soft palate and uvula. Int J Radiat Oncol Biol Phys. 1988;15(3):619-625.
33 Mazeron JJ, Crook J, Martin M, et al. Iridium 192 implantation of squamous cell carcinomas of the oropharynx. Am J Otolaryngol. 1989;10(5):317-321.
34 Pernot M, Malissard L, Taghian A, et al. Velotonsillar squamous cell carcinoma: 277 cases treated by combined external irradiation and brachytherapy–results according to extension, localization, and dose rate. Int J Radiat Oncol Biol Phys. 1992;23(4):715-723.
35 Mazeron JJ, Marinello G, Crook J, et al. Definitive radiation treatment for early stage carcinoma of the soft palate and uvula: the indications for iridium 192 implantation. Int J Radiat Oncol Biol Phys. 1987;13(12):1829-1837.
36 Levendag PC, Schmitz PI, Jansen PP, et al. Fractionated high-dose-rate and pulsed-dose-rate brachytherapy: first clinical experience in squamous cell carcinoma of the tonsillar fossa and soft palate. Int J Radiat Oncol Biol Phys. 1997;38(3):497-506.
37 Million RR. Squamous cell carcinoma of the head and neck: combined therapy: surgery and postoperative irradiation. Int J Radiat Oncol Biol Phys. 1979;5(11–12):2161-2162.
38 Foote RL, Schild SE, Thompson WM, et al. Tonsil cancer. Patterns of failure after surgery alone and surgery combined with postoperative radiation therapy. Cancer. 1994;73(10):2638-2647.
39 Perez CA, Patel MM, Chao KS, et al. Carcinoma of the tonsillar fossa: prognostic factors and long-term therapy outcome. Int J Radiat Oncol Biol Phys. 1998;42(5):1077-1084.
40 Remmler D, Medina JE, Byers RM, et al. Treatment of choice for squamous carcinoma of the tonsillar fossa. Head Neck Surg. 1985;7(3):206-211.
41 Weichert KA, Aron B, Maltz R, et al. Carcinoma of the tonsil: treatment by a planned combination of radiation and surgery. Int J Radiat Oncol Biol Phys. 1976;1(5–6):505-508.
42 Gehanno P, Depondt J, Guedon C, et al. Primary and salvage surgery for cancer of the tonsillar region: a retrospective study of 120 patients. Head Neck. 1993;15(3):185-189.
43 Mendenhall WM, Amdur RJ, Stringer SP, et al. Radiation therapy for squamous cell carcinoma of the tonsillar region: a preferred alternative to surgery? J Clin Oncol. 2000;18(11):2219-2225.
44 Hicks WLJr, Kuriakose MA, Loree TR, et al. Surgery versus radiation therapy as single-modality treatment of tonsillar fossa carcinoma: the Roswell Park Cancer Institute experience 1971–1991. Laryngoscope. 1998;108(7):1014-1019.
45 Mizono GS, Diaz RF, Fu KK, et al. Carcinoma of the tonsillar region. Laryngoscope. 1986;96(3):240-244.
46 Pernot M, Hoffstetter S, Peiffert D, et al. Role of interstitial brachytherapy in oral and oropharyngeal carcinoma: reflection of a series of 1344 patients treated at the time of initial presentation. Otolaryngol Head Neck Surg. 1996;115(6):519-526.
47 Bataini JP, Asselain B, Jaulerry C, et al. A multivariate primary tumour control analysis in 465 patients treated by radical radiotherapy for cancer of the tonsillar region: clinical and treatment parameters as prognostic factors. Radiother Oncol. 1989;14(4):265-277.
48 Wong CS, Ang KK, Fletcher GH, et al. Definitive radiotherapy for squamous cell carcinoma of the tonsillar fossa. Int J Radiat Oncol Biol Phys. 1989;16(3):657-662.
49 Amornmarn R, Prempree T, Jaiwatana J, et al. Radiation management of carcinoma of the tonsillar region. Cancer. 1984;54(7):1293-1299.
50 Gwozdz JT, Morrison WH, Garden AS, et al. Concomitant boost radiotherapy for squamous carcinoma of the tonsillar fossa. Int J Radiat Oncol Biol Phys. 1997;39(1):127-135.
51 Gluckman JL, Black RJ, Crissman JD. Cancer of the oropharynx. Otolaryngol Clin North Am. 1985;18(3):451-459.
52 Givens CDJr, Johns ME, Cantrell RW. Carcinoma of the tonsil. Analysis of 162 cases. Arch Otolaryngol. 1981;107(12):730-734.
53 Rabuzzi DD, Mickler AS, Clutter DJ, et al. Treatment results of combined high-dose preoperative radiotherapy and surgery for oropharyngeal cancer. Laryngoscope. 1982;92(9 Pt 1):989-992.
54 Garrett PG, Beale FA, Cummings BJ, et al. Carcinoma of the tonsil: the effect of dose-time-volume factors on local control. Int J Radiat Oncol Biol Phys. 1985;11(4):703-706.
55 Garrett PG, Beale FA, Cummings BJ, et al. Cancer of the tonsil: results of radical radiation therapy with surgery in reserve. Am J Surg. 1983;146(4):432-435.
56 Shukovsky LJ, Fletcher GH. Time-dose and tumor volume relationships in the irradiation of squamous cell carcinoma of the tonsillar fossa. Radiology. 1973;107(3):621-626.
57 Mendenhall WM, Morris CG, Amdur RJ, et al. Definitive radiotherapy for tonsillar squamous cell carcinoma. Am J Clin Oncol. 2006;29(3):290-297.
58 Johansen LV, Grau C, Overgaard J. Squamous cell carcinoma of the oropharynx–an analysis of treatment results in 289 consecutive patients. Acta Oncol. 2000;39(8):985-994.
59 Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):695-704.
60 O’Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 2001;51(2):332-343.
61 Jackson SM, Hay JH, Flores AD, et al. Cancer of the tonsil: the results of ipsilateral radiation treatment. Radiother Oncol. 1999;51(2):123-128.
62 Pernot M, Luporsi E, Hoffstetter S, et al. Complications following definitive irradiation for cancers of the oral cavity and the oropharynx (in a series of 1134 patients). Int J Radiat Oncol Biol Phys. 1997;37(3):577-585.
63 Levendag P, Nijdam W, Noever I, et al. Brachytherapy versus surgery in carcinoma of tonsillar fossa and/or soft palate: late adverse sequelae and performance status: can we be more selective and obtain better tissue sparing? Int J Radiat Oncol Biol Phys. 2004;59(3):713-724.
64 Mazeron JJ, Belkacemi Y, Simon JM, et al. Place of Iridium 192 implantation in definitive irradiation of faucial arch squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 1993;27(2):251-257.
65 Rudoltz MS, Perkins RS, Luthmann RW, et al. High-dose-rate brachytherapy for primary carcinomas of the oral cavity and oropharynx. Laryngoscope. 1999;109(12):1967-1973.
66 Goffinet DR, Fee WEJr, Wells J, et al. 192Ir pharyngoepiglottic fold interstitial implants. The key to successful treatment of base tongue carcinoma by radiation therapy. Cancer. 1985;55(5):941-948.
67 Housset M, Baillet F, Dessard-Diana B, et al. A retrospective study of three treatment techniques for T1-T2 base of tongue lesions: surgery plus postoperative radiation, external radiation plus interstitial implantation and external radiation alone. Int J Radiat Oncol Biol Phys. 1987;13(4):511-516.
68 Andersen PE, Shah JP, Cambronero E, et al. The role of comprehensive neck dissection with preservation of the spinal accessory nerve in the clinically positive neck. Am J Surg. 1994;168(5):499-502.
69 Spiro RH, Gerold FP, Strong EW. Mandibular “swing” approach for oral and oropharyngeal tumors. Head Neck Surg. 1981;3(5):371-378.
70 Shike M, Berner YN, Gerdes H, et al. Percutaneous endoscopic gastrostomy and jejunostomy for long-term feeding in patients with cancer of the head and neck. Otolaryngol Head Neck Surg. 1989;101(5):549-554.
71 Byrd SE, Richardson M, Gil G, et al. The spread of carcinoma of the base of the tongue as detected by computer tomography. Rev Laryngol Otol Rhinol (Bord). 1983;104(3):247-250.
72 Zelefsky MJ, Harrison LB, Armstrong JG. Long-term treatment results of postoperative radiation therapy for advanced stage oropharyngeal carcinoma. Cancer. 1992;70(10):2388-2395.
73 Vikram B. Importance of the time interval between surgery and postoperative radiation therapy in the combined management of head & neck cancer. Int J Radiat Oncol Biol Phys. 1979;5(10):1837-1840.
74 Schiff PB, Harrison LB, Strong EW, et al. Impact of the time interval between surgery and postoperative radiation therapy on locoregional control in advanced head and neck cancer. J Surg Oncol. 1990;43(4):203-208.
75 Bastit L, Blot E, Debourdeau P, et al. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2001;49(1):139-146.
76 Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571-578.
77 Hinerman RW, Parsons JT, Mendenhall WM, et al. External beam irradiation alone or combined with neck dissection for base of tongue carcinoma: an alternative to primary surgery. Laryngoscope. 1994;104(12):1466-1470.
78 Guillamondegui OM, Meoz R, Jesse RH. Surgical treatment of squamous cell carcinoma of the pharyngeal walls. Am J Surg. 1978;136(4):474-476.
79 Hull MC, Morris CG, Tannehill SP, et al. Definitive radiotherapy alone or combined with a planned neck dissection for squamous cell carcinoma of the pharyngeal wall. Cancer. 2003;98(10):2224-2231.
80 Harrison LB, Kraus DH, Zelefsky MJ. Long term results of primary radiation therapy for squamous cell cancer of the base of tongue. Proc Int Mtg Head Neck Cancer. 71, 1996. (abst)
81 Meoz-Mendez RT, Fletcher GH, Guillamondegui OM, et al. Analysis of the results of irradiation in the treatment of squamous cell carcinomas of the pharyngeal walls. Int J Radiat Oncol Biol Phys. 1978;4(7–8):579-585.
82 Marks JE, Freeman RB, Lee F, et al. Pharyngeal wall cancer: an analysis of treatment results complications and patterns of failure. Int J Radiat Oncol Biol Phys. 1978;4(7–8):587-593.
83 Marks JE, Smith PG, Sessions DG. Pharyngeal wall cancer. A reappraisal after comparison of treatment methods. Arch Otolaryngol. 1985;111(2):79-85.
84 Kramer S, Gelber RD, Snow JB, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73-03 of the Radiation Therapy Oncology Group. Head Neck Surg. 1987;10(1):19-30.
85 Spiro RH, Kelly J, Vega AL, et al. Squamous carcinoma of the posterior pharyngeal wall. Am J Surg. 1990;160(4):420-423.
86 Julieron M, Kolb F, Schwaab G, et al. Surgical management of posterior pharyngeal wall carcinomas: functional and oncologic results. Head Neck. 2001;23(2):80-86.
87 Son YH, Kacinski BM. Therapeutic concepts of brachytherapy/megavoltage in sequence for pharyngeal wall cancers. Results of integrated dose therapy. Cancer. 1987;59(7):1268-1273.
88 Withers HR, Peters LJ, Taylor JM, et al. Local control of carcinoma of the tonsil by radiation therapy: an analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys. 1995;33(3):549-562.
89 Ang KK. Altered fractionation trials in head and neck cancer. Semin Radiat Oncol. 1998;8(4):230-236.
90 Ang KK, Peters LJ, Weber RS, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19(6):1339-1345.
91 Wang CC. Local control of oropharyngeal carcinoma after two accelerated hyperfractionation radiation therapy schemes. Int J Radiat Oncol Biol Phys. 1988;14(6):1143-1146.
92 Million RR, Parsons JT, Cassisi NJ. Twice-a-day irradiation technique for squamous cell carcinomas of the head and neck. Cancer. 1985;55(9 Suppl):2096-2099.
93 Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48(1):7-16.
94 Horiot JC, Le Fur R, N’Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25(4):231-241.
95 Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362(9388):933-940.
96 Pinto LH, Canary PC, Araujo CM, et al. Prospective randomized trial comparing hyperfractionated versus conventional radiotherapy in stages III and IV oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1991;21(3):557-562.
97 Jackson SM, Weir LM, Hay JH, et al. A randomised trial of accelerated versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;43(1):39-46.
98 Dische S, Saunders M, Barrett A, et al. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;44(2):123-136.
99 Skladowski K, Maciejewski B, Golen M, et al. Randomized clinical trial on 7-day-continuous accelerated irradiation (CAIR) of head and neck cancer—report on 3-year tumour control and normal tissue toxicity. Radiother Oncol. 2000;55(2):101-110.
100 Hliniak A, Gwiazdowska B, Szutkowski Z, et al. A multicentre randomized/controlled trial of a conventional versus modestly accelerated radiotherapy in the laryngeal cancer: influence of a 1 week shortening overall time. Radiother Oncol. 2002;62(1):1-10.
101 Poulsen MG, Denham JW, Peters LJ, et al. A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: a Trans-Tasman Radiation Oncology Group Study. Radiother Oncol. 2001;60(2):113-122.
102 Bourhis J, Lapeyre M, Tortochaux J, et al. Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. J Clin Oncol. 2006;24(18):2873-2878.
103 Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006;24(17):2618-2623.
104 Sanguineti G, Pou AM, Newlands SD. Accelerated hyperfractionated intensity modulated radiotherapy (AHI) for T2-3 oropharyngeal carcinoma: preliminary results from a phase I-II study. Int J Radiat Oncol Biol Phys. 2005;63(2S):S380.
105 de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64(2):363-373.
106 Butler EB, Teh BS, Grant WH3rd, et al. Smart (simultaneous modulated accelerated radiation therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45(1):21-32.
107 Yao M, Graham MM, Hoffman HT, et al. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59(4):1001-1010.
108 Lawson JD, Otto K, Chen A, et al. Concurrent platinum-based chemotherapy and simultaneous modulated accelerated radiation therapy for locally advanced squamous cell carcinoma of the tongue base. Head Neck. 2008;30(3):327-335.
109 The Department of Veterans Affairs Laryngeal Cancer Study Group. induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685-1690.
110 Spaulding MB, Fischer SG, Wolf GT. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. The Department of Veterans Affairs Cooperative Laryngeal Cancer Study Group. J Clin Oncol. 1994;12(8):1592-1599.
111 Calais G. Radiation (RT) alone versus RT with concomitant chemotherapy (CT) in stages III and IV oropharynx carcinoma. Final results of the 94-01 GORTEC randomized study. Int J Radiat Oncol Biol Phys. 2001;51(3 Suppl 1):1.
112 Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91(24):2081-2086.
113 Calais G, Alfonsi M, Bardet E, et al. Stage III and IV cancers of the oropharynx: results of a randomized study of Gortec comparing radiotherapy alone with concomitant chemotherapy. Bull Cancer. 2000;87:48-53. Spec No
114 Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d’Etude des Tumeurs de la Tete et du Cou (GETTEC). Br J Cancer. 2000;83(12):1594-1598.
115 Forastiere AA, Berkey B, Maor M, et al. Phase III Trial to Preserve the Larynx: Induction Chemotherapy and Radiotherapy Versus Concomitant Chemoradiotherapy Versus Radiotherapy Alone, Intergroup Trial R91–11. Proc Am Soc Clin Oncol. 20, 2001. (Abst. 4)
116 Forastiere AA, Goepfert H, Maor M, et al. Long-term results of Intergroup RTOG 91–11: a phase III trial to preserve the larynx—Induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol. 2006;24(18S):5517.
117 Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091-2098.
118 Pignon JP, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer: individual patient data vs literature data. Br J Cancer. 1995;72(4):1062-1063.
119 Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355(9208):949-955.
120 El-Sayed S, Nelson N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region. A meta-analysis of prospective and randomized trials. J Clin Oncol. 1996;14(3):838-847.
121 Munro AJ. An overview of randomised controlled trials of adjuvant chemotherapy in head and neck cancer. Br J Cancer. 1995;71(1):83-91.
122 Denis F, Garaud P, Bardet E, et al. Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22(1):69-76.
123 Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578.
124 RTOG Website http://www.rtog.org/members/protocols/h0522/h0522.pdf accessed 02/02/2007
125 Harrison LB, Raben A, Pfister DG, et al. A prospective phase II trial of concomitant chemotherapy and radiotherapy with delayed accelerated fractionation in unresectable tumors of the head and neck. Head Neck. 1998;20(6):497-503.
126 Deutsch I, Hu K, Culliney B, et al. Accelerated Radiation Therapy and Concurrent Chemotherapy for Advanced Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys. 2006;66(3S):2445.
127 Bieri S, Allal AS, Dulguerov P, et al. Concomitant boost radiotherapy in oropharynx carcinomas. Acta Oncol. 1998;37(7–8):687-691.
128 Nathu RM, Mendenhall WM, Parsons JT, et al. Induction chemotherapy and radiation therapy for T4 oropharyngeal carcinoma. Radiat Oncol Investig. 1999;7(2):98-105.
129 Vokes EE, Kies M, Haraf DJ, et al. Induction chemotherapy followed by concomitant chemoradiotherapy for advanced head and neck cancer: impact on the natural history of the disease. J Clin Oncol. 1995;13(4):876-883.
130 Kies MS, Haraf DJ, Athanasiadis I, et al. Induction chemotherapy followed by concurrent chemoradiation for advanced head and neck cancer: improved disease control and survival. J Clin Oncol. 1998;16(8):2715-2721.
131 Bensadoun RJ, Etienne MC, Dassonville O, et al. Concomitant b.i.d. radiotherapy and chemotherapy with cisplatin and 5-fluorouracil in unresectable squamous-cell carcinoma of the pharynx: clinical and pharmacological data of a French multicenter phase II study. Int J Radiat Oncol Biol Phys. 1998;42(2):237-245.
132 Machtay M, Rosenthal DI, Algazy KM, et al. Pilot study of organ preservation multimodality therapy for locally advanced resectable oropharyngeal carcinoma. Am J Clin Oncol. 2000;23(5):509-515.
133 Giralt JL, Gonzalez J, del Campo JM, et al. Preoperative induction chemotherapy followed by concurrent chemoradiotherapy in advanced carcinoma of the oral cavity and oropharynx. Cancer. 2000;89(5):939-945.
134 Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338(25):1798-1804.
135 Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol. 2000;18(7):1458-1464.
136 Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy–results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(5):1161-1171.
137 Ryu JK, Swann S, Leveque F, et al. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: A double-blind placebo-controlled prospective Phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys. 2007;67(3):643-650.
138 Bourhis J, Lapeyre M, Tortochaux J, et al. Preliminary results of the GORTEC 96–01 randomized trial, comparing very accelerated radiotherapy versus concomitant radio-chemotherapy for locally inoperable HNSCC. Proc Am Soc Clin Oncol. 21, 2002. (Abs 905)
139 RTOG Website http://www.rtog.org/members/protocols/h0129/h0129.pdf accessed 02/02/2007
140 Rooney M, Kish J, Jacobs J, et al. Improved complete response rate and survival in advanced head and neck cancer after three-course induction therapy with 120-hour 5-FU infusion and cisplatin. Cancer. 1985;55(5):1123-1128.
141 Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890-899.
142 Eisbruch A, Thornton AF, Urba S, et al. Chemotherapy followed by accelerated fractionated radiation for larynx preservation in patients with advanced laryngeal cancer. J Clin Oncol. 1996;14(8):2322-2330.
143 Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24(4):593-598.
144 Adelstein DJ. Induction chemotherapy in head and neck cancer. Hematol Oncol Clin North Am. 1999;13(4):689-698. v–vi
145 Monnerat C, Faivre S, Temam S, et al. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol. 2002;13(7):995-1006.
146 Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86(4):265-272.
147 Zorat PL, Paccagnella A, Cavaniglia G, et al. Randomized phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst. 2004;96(22):1714-1717.
148 Frank DK, Hu KS, Culliney BE, et al. Planned neck dissection after concomitant radiochemotherapy for advanced head and neck cancer. Laryngoscope. 2005;115(6):1015-1020.
149 Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15(8):1179-1186.
150 Argiris A, Haraf DJ, Kies MS, et al. Intensive concurrent chemoradiotherapy for head and neck cancer with 5-fluorouracil- and hydroxyurea-based regimens: reversing a pattern of failure. Oncologist. 2003;8(4):350-360.
151 Vokes EE, Kies MS, Haraf DJ, et al. Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer. J Clin Oncol. 2000;18(8):1652-1661.
152 Adelstein DJ, Saxton JP, Lavertu P, et al. Maximizing local control and organ preservation in stage IV squamous cell head and neck cancer with hyperfractionated radiation and concurrent chemotherapy. J Clin Oncol. 2002;20(5):1405-1410.
153 Haddad R, Tishler R, Wirth L, et al. Rate of pathologic complete responses to docetaxel, cisplatin, and fluorouracil induction chemotherapy in patients with squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2006;132(6):678-681.
154 Hitt R, Paz-Ares L, Brandariz A, et al. Induction chemotherapy with paclitaxel, cisplatin and 5-fluorouracil for squamous cell carcinoma of the head and neck: long-term results of a phase II trial. Ann Oncol. 2002;13(10):1665-1673.
155 Posner MR, Glisson B, Frenette G, et al. Multicenter phase I-II trial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2001;19(4):1096-1104.
156 Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23(34):8636-8645.
157 Remenar E, Van Herpen C, Germa Lluch J, et al. A randomized phase III multicenter trial of neoadjuvant docetaxel plus cisplatin and 5-fluorouracil (TPF) versus neoadjuvant PF in patients with locally advanced unresectable squamous cell carcinoma of the head and neck (SCCHN). Final analysis of EORTC 24971. J Clin Oncol. 2006;24(18S):5516.
158 Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695-1704.
159 Posner M, Hershock D, Le-Lann L, et al. Scientific special session: docetaxel added to induction therapy in head and neck cancer. Proc Am Soc Clin Oncol. 2006.
160 Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705-1715.
161 Mendenhall WM, Parsons JT, Cassisi NJ, et al. Squamous cell carcinoma of the tonsillar area treated with radical irradiation. Radiother Oncol. 1987;10(1):23-30.
162 Kaplan R, Million RR, Cassisi NJ. Carcinoma of the tonsil: results of radical irradiation with surgery reserved for radiation failure. Laryngoscope. 1977;87(4 Pt 1):600-607.
163 Dasmahapatra KS, Mohit-Tabatabai MA, Rush BFJr, et al. Cancer of the tonsil. Improved survival with combination therapy. Cancer. 1986;57(3):451-455.
164 Perez CA, Carmichael T, Devineni VR, et al. Carcinoma of the tonsillar fossa: a nonrandomized comparison of irradiation alone or combined with surgery: long-term results. Head Neck. 1991;13(4):282-290.
165 Spiro JD, Spiro RH. Carcinoma of the tonsillar fossa. An update. Arch Otolaryngol Head Neck Surg. 1989;115(10):1186-1189.
166 Quenelle DJ, Crissman JD, Shumrick DA. Tonsil carcinoma–treatment results. Laryngoscope. 1979;89(11):1842-1846.
167 Puthawala AA, Syed AM, Gates TC. Iridium-192 implants in the treatment of tonsillar region malignancies. Arch Otolaryngol. 1985;111(12):812-815.
168 Leborgne JH, Leborgne F, Barlocci LA, et al. The place of brachytherapy in the treatment of carcinoma of the tonsil with lingual extension. Int J Radiat Oncol Biol Phys. 1986;12(10):1787-1792.
169 Peiffert D, Pernot M, Malissard L, et al. Salvage irradiation by brachytherapy of velotonsillar squamous cell carcinoma in a previously irradiated field: results in 73 cases. Int J Radiat Oncol Biol Phys. 1994;29(4):681-686.
170 Harrison LB, Zelefsky MJ, Armstrong JG, et al. Performance status after treatment for squamous cell cancer of the base of tongue–a comparison of primary radiation therapy versus primary surgery. Int J Radiat Oncol Biol Phys. 1994;30(4):953-957.
171 Dupont JB, Guillamondegui OM, Jesse RH. Surgical treatment of advanced carcinomas of the base of the tongue. Am J Surg. 1978;136(4):501-503.
172 Harrison LB, Zelefsky MJ, Sessions RB, et al. Base-of-tongue cancer treated with external beam irradiation plus brachytherapy: oncologic and functional outcome. Radiology. 1992;184(1):267-270.
173 Han P, Hu K, Culliney B, et al. Concurrent chemoradiation followed by interstitial brachytherapy boost and neck dissection for T4 base of tongue cancer. Int J Radiat Oncol Biol Phys. 2005;63(S1):S358.
174 Harrison LB, Lee H, Kraus DH. Long term results of primary radiation therapy for squamous cancer of the base of tongue. Radiother Oncol. 1996;39:S6. (abst)
175 Harrison LB, Sessions RB, Strong EW, et al. Brachytherapy as part of the definitive management of squamous cancer of the base of tongue. Int J Radiat Oncol Biol Phys. 1989;17(6):1309-1312.
176 Puthawala AA, Syed AM, Eads DL, et al. Limited external beam and interstitial 192iridium irradiation in the treatment of carcinoma of the base of the tongue: a ten year experience. Int J Radiat Oncol Biol Phys. 1988;14(5):839-848.
177 Crook J, Mazeron JJ, Marinello G, et al. Combined external irradiation and interstitial implantation for T1 and T2 epidermoid carcinomas of base of tongue: the Creteil experience 1971–1981. Int J Radiat Oncol Biol Phys. 1988;15(1):105-114.
178 Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73.
179 Vandenbrouck C, Sancho H, Le Fur R, et al. Results of a randomized clinical trial of preoperative irradiation versus postoperative in treatment of tumors of the hypopharynx. Cancer. 1977;39(4):1445-1449.
180 Bernier J, Domenge C, Eschwege F, et al. Chemo-radiotherapy, as compared to radiotherapy alone, significantly increases disease-free and overall survival in head and neck cancer patients after surgery: results of EORTC phase III trial 22931. Int J Radiat Oncol Biol Phys. 2001;51(3(S1)):1.
181 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952.
182 Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944.
183 Cooper JS, Pajak TF, Forastiere AA, et al. Long-term survival results of a phase III intergroup trial (RTOG 95-01) of surgery followed by radiotherapy vs radiochemotherapy for resectable high risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2006;66(3S):S14.
184 Pradhan SA, Rajpal RM, Kothary PM. Surgical management of postradiation residual/recurrent cancer of the base of the tongue. J Surg Oncol. 1980;14(3):201-206.
185 Langlois D, Hoffstetter S, Malissard L, et al. Salvage irradiation of oropharynx and mobile tongue about 192 iridium brachytherapy in Centre Alexis Vautrin. Int J Radiat Oncol Biol Phys. 1988;14(5):849-853.
186 Mazeron JJ, Langlois D, Glaubiger D, et al. Salvage irradiation of oropharyngeal cancers using iridium 192 wire implants: 5-year results of 70 cases. Int J Radiat Oncol Biol Phys. 1987;13(7):957-962.
187 Housset M, Baillet F, Delanian S, et al. Split course interstitial brachytherapy with a source shift: the results of a new iridium implant technique versus single course implants for salvage irradiation of base of tongue cancers in 55 patients. Int J Radiat Oncol Biol Phys. 1991;20(5):965-971.
188 Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120(7):699-702.
189 Armstrong J, Pfister D, Strong E, et al. The management of the clinically positive neck as part of a larynx preservation approach. Int J Radiat Oncol Biol Phys. 1993;26(5):759-765.
190 Bernier J, Bataini JP. Regional outcome in oropharyngeal and pharyngolaryngeal cancer treated with high dose per fraction radiotherapy. Analysis of neck disease response in 1646 cases. Radiother Oncol. 1986;6(2):87-103.
191 Parsons JT, Mendenhall WM, Cassisi NJ, et al. Neck dissection after twice-a-day radiotherapy: morbidity and recurrence rates. Head Neck. 1989;11(5):400-404.
192 Parsons JT, Mendenhall WM, Stringer SP, et al. Twice-a-day radiotherapy for squamous cell carcinoma of the head and neck: the University of Florida experience. Head Neck. 1993;15(2):87-96.
193 Parsons JT, Million RR, Cassisi NJ. Carcinoma of the base of the tongue: results of radical irradiation with surgery reserved for irradiation failure. Laryngoscope. 1982;92(6 Pt 1):689-696.
194 Maccomb WS, Fletcher GH. Planned combination of surgery and radiation in treatment of advanced primary head and neck cancers. Am J Roentgenol Radium Ther Nucl Med. 1957;77(3):397-414.
195 Barkley HTJr, Fletcher GH, Jesse RH, et al. Management of cervical lymph node metastases in squamous cell carcinoma of the tonsillar fossa, base of tongue, supraglottic larynx, and hypopharynx. Am J Surg. 1972;124(4):462-467.
196 Huang DT, Johnson CR, Schmidt-Ullrich R, et al. Postoperative radiotherapy in head and neck carcinoma with extracapsular lymph node extension and/or positive resection margins: a comparative study. Int J Radiat Oncol Biol Phys. 1992;23(4):737-742.
197 Vikram B, Strong EW, Shah JP, et al. Failure in the neck following multimodality treatment for advanced head and neck cancer. Head Neck Surg. 1984;6(3):724-729.
198 Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation therapy: the role of neck dissection for clinically positive neck nodes. Int J Radiat Oncol Biol Phys. 1986;12(5):733-740.
199 Narayan K, Crane CH, Kleid S, et al. Planned neck dissection as an adjunct to the management of patients with advanced neck disease treated with definitive radiotherapy: for some or for all? Head Neck. 1999;21(7):606-613.
200 Ahmed KA, Robbins KT, Wong F, et al. Efficacy of concomitant chemoradiation and surgical salvage for N3 nodal disease associated with upper aerodigestive tract carcinoma. Laryngoscope. 2000;110(11):1789-1793.
201 Corry J, Smith JG, Peters LJ. The concept of a planned neck dissection is obsolete. Cancer J. 2001;7(6):472-474.
202 Boyd TS, Harari PM, Tannehill SP, et al. Planned postradiotherapy neck dissection in patients with advanced head and neck cancer. Head Neck. 1998;20(2):132-137.
203 Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58(5):1418-1423.
204 Kutler DI, Patel SG, Shah JP. The role of neck dissection following definitive chemoradiation. Oncology (Williston Park). 2004;18(8):993-998. discussion 999, 1003–4, 1007
205 Lavertu P, Adelstein DJ, Saxton JP, et al. Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck. 1997;19(7):559-566.
206 Lee HJ, Zelefsky MJ, Kraus DH, et al. Long-term regional control after radiation therapy and neck dissection for base of tongue carcinoma. Int J Radiat Oncol Biol Phys. 1997;38(5):995-1000.
207 McHam SA, Adelstein DJ, Rybicki LA, et al. Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer? Head Neck. 2003;25(10):791-798.
208 Mendenhall WM, Villaret DB, Amdur RJ, et al. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2002;24(11):1012-1018.
209 Roy S, Tibesar RJ, Daly K, et al. Role of planned neck dissection for advanced metastatic disease in tongue base or tonsil squamous cell carcinoma treated with radiotherapy. Head Neck. 2002;24(5):474-481.
210 Somerset JD, Mendenhall WM, Amdur RJ, et al. Planned postradiotherapy bilateral neck dissection for head and neck cancer. Am J Otolaryngol. 2001;22(6):383-386.
211 Stenson KM, Haraf DJ, Pelzer H, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy: the feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg. 2000;126(8):950-956.
212 Chan AW, Ancukiewicz M, Carballo N, et al. The role of postradiotherapy neck dissection in supraglottic carcinoma. Int J Radiat Oncol Biol Phys. 2001;50(2):367-375.
213 Johnson CR, Silverman LN, Clay LB, et al. Radiotherapeutic management of bulky cervical lymphadenopathy in squamous cell carcinoma of the head and neck: is postradiotherapy neck dissection necessary? Radiat Oncol Investig. 1998;6(1):52-57.
214 Peters LJ, Weber RS, Morrison WH, et al. Neck surgery in patients with primary oropharyngeal cancer treated by radiotherapy. Head Neck. 1996;18(6):552-559.
215 Pletcher SD, Kaplan MJ, Eisele DW, et al. Management of cervical metastases in advanced squamous cell carcinoma of the base of tongue. Arch Otolaryngol Head Neck Surg. 2003;129(9):983-986.
216 Sanguineti G, Corvo R, Benasso M, et al. Management of the neck after alternating chemoradiotherapy for advanced head and neck cancer. Head Neck. 1999;21(3):223-228.
217 Wolf GT, Fisher SG. Effectiveness of salvage neck dissection for advanced regional metastases when induction chemotherapy and radiation are used for organ preservation. Laryngoscope. 1992;102(8):934-939.
218 Liauw SL, Mancuso AA, Amdur RJ, et al. Postradiotherapy neck dissection for lymph node-positive head and neck cancer: the use of computed tomography to manage the neck. J Clin Oncol. 2006;24(9):1421-1427.
219 Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27(3):175-181.
220 Ware RE, Matthews JP, Hicks RJ, et al. Usefulness of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with a residual structural abnormality after definitive treatment for squamous cell carcinoma of the head and neck. Head Neck. 2004;26(12):1008-1017.
221 Brkovich VS, Miller FR, Karnad AB, et al. The role of positron emission tomography scans in the management of the N-positive neck in head and neck squamous cell carcinoma after chemoradiotherapy. Laryngoscope. 2006;116(6):855-858.
222 Kubota K, Yokoyama J, Yamaguchi K, et al. FDG-PET delayed imaging for the detection of head and neck cancer recurrence after radio-chemotherapy: comparison with MRI/CT. Eur J Nucl Med Mol Imaging. 2004;31(4):590-595.
223 Rogers JW, Greven KM, McGuirt WF, et al. Can post-RT neck dissection be omitted for patients with head-and-neck cancer who have a negative PET scan after definitive radiation therapy? Int J Radiat Oncol Biol Phys. 2004;58(3):694-697.
224 Gourin CG, Williams HT, Seabolt WN, et al. Utility of positron emission tomography-computed tomography in identification of residual nodal disease after chemoradiation for advanced head and neck cancer. Laryngoscope. 2006;116(5):705-710.
225 Mabanta SR, Mendenhall WM, Stringer SP, et al. Salvage treatment for neck recurrence after irradiation alone for head and neck squamous cell carcinoma with clinically positive neck nodes. Head Neck. 1999;21(7):591-594.
226 Pameijer FA, Mancuso AA, Mendenhall WM, et al. Can pretreatment computed tomography predict local control in T3 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy? Int J Radiat Oncol Biol Phys. 1997;37(5):1011-1021.
227 Pameijer FA, Mancuso AA, Mendenhall WM, et al. Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck. 1998;20(2):159-168.
228 Chua DT, Sham JS, Kwong DL, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997;39(3):711-719.
229 Hermans R, Op de beeck K, Van den Bogaert W, et al. The relation of CT-determined tumor parameters and local and regional outcome of tonsillar cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2001;50(1):37-45.
230 Nathu RM, Mancuso AA, Zhu TC, et al. The impact of primary tumor volume on local control for oropharyngeal squamous cell carcinoma treated with radiotherapy. Head Neck. 2000;22(1):1-5.
231 Aebersold DM, Beer KT, Laissue J, et al. Intratumoral microvessel density predicts local treatment failure of radically irradiated squamous cell cancer of the oropharynx. Int J Radiat Oncol Biol Phys. 2000;48(1):17-25.
232 Grabenbauer GG, Muhlfriedel C, Rodel F, et al. Squamous cell carcinoma of the oropharynx: Ki-67 and p53 can identify patients at high risk for local recurrence after surgery and postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(4):1041-1050.
233 Sittel C, Eckel HE, Damm M, et al. Ki-67 (MIB1), p53, and Lewis-X (LeuM1) as prognostic factors of recurrence in T1 and T2 laryngeal carcinoma. Laryngoscope. 2000;110(6):1012-1017.
234 Jaskulski D, deRiel JK, Mercer WE, et al. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988;240(4858):1544-1546.
235 Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57(1):39-43.
236 Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41(1):31-39.
237 Brizel DM, Sibley GS, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285-289.
238 Vanselow B, Eble MJ, Rudat V, et al. Oxygenation of advanced head and neck cancer: prognostic marker for the response to primary radiochemotherapy. Otolaryngol Head Neck Surg. 2000;122(6):856-862.
239 Overgaard J, Hansen HS, Overgaard M, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother Oncol. 1998;46(2):135-146.
240 Glaser CM, Millesi W, Kornek GV, et al. Impact of hemoglobin level and use of recombinant erythropoietin on efficacy of preoperative chemoradiation therapy for squamous cell carcinoma of the oral cavity and oropharynx. Int J Radiat Oncol Biol Phys. 2001;50(3):705-715.
241 Aebersold DM, Burri P, Beer KT, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61(7):2911-2916.
242 Frisch M, Hjalgrim H, Jaeger AB, et al. Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control. 2000;11(6):489-495.
243 Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125-1131.
244 D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956.
245 Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119(11):2620-2623.
246 Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407-420.
247 Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24(17):2606-2611.
248 Schwartz SR, Yueh B, McDougall JK, et al. Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg. 2001;125(1):1-9.
249 Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736-747.
250 Mellin Dahlstrand H, Lindquist D, Bjornestal L, et al. P16(INK4a) correlates to human papillomavirus presence, response to radiotherapy and clinical outcome in tonsillar carcinoma. Anticancer Res. 2005;25(6C):4375-4383.
251 Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261-269.
252 Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630-5636.
253 Boonstra J. Progression through the G1-phase of the on-going cell cycle. J Cell Biochem. 2003;90(2):244-252.
254 Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747-753.
255 Slebos RJ, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):701-709.
256 Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438.
257 Nasseri M, Gage JR, Lorincz A, et al. Human papillomavirus type 16 immortalized cervical keratinocytes contain transcripts encoding E6, E7, and E2 initiated at the P97 promoter and express high levels of E7. Virology. 1991;184(1):131-140.
258 Schwarz E, Freese UK, Gissmann L, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111-114.
259 Crook T, Morgenstern JP, Crawford L, et al. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. Embo J. 1989;8(2):513-519.
260 Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366(6456):704-707.
261 Cairns P, Mao L, Merlo A, et al. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994;265(5170):415-417.
262 Merlo A, Herman JG, Mao L, et al. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995;1(7):686-692.
263 Reed AL, Califano J, Cairns P, et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56(16):3630-3633.
264 Rhys Evans PH. Complications in head and neck surgery and how to avoid trouble. J Laryngol Otol. 1989;103(10):926-929.
265 Coleman JJ3rd. Complications in head and neck surgery. Surg Clin North Am. 1986;66(1):149-167.
266 Sanders EM, Davis KR, Whelan CS, et al. Threatened carotid artery rupture: a complication of radical neck surgery. J Surg Oncol. 1986;33(3):190-193.
267 Saunders JRJr, Hirata RM, Jaques DA. Considering the spinal accessory nerve in head and neck surgery. Am J Surg. 1985;150(4):491-494.
268 Miles JW, Kagan AR. Head and neck oncology: clinical management. New York: Pergamon Press; 1989. vi, 176
269 Sassler AM, Esclamado RM, Wolf GT. Surgery after organ preservation therapy. Analysis of wound complications. Arch Otolaryngol Head Neck Surg. 1995;121(2):162-165.
270 Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339-3345.
271 Wasserman TH, Brizel DM, Henke M, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and-neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys. 2005;63(4):985-990.
272 Buentzel J, Micke O, Adamietz IA, et al. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys. 2006;64(3):684-691.
273 Bardet E, Martin L, Calais G, et al. Preliminary data of the GORTEC 2000–02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Semin Oncol. 2002;29(6 Suppl 19):57-60.
274 Bardet E, Martin L, Calais G, et al. Subcutaneous (SbQ) versus intravenous (IV) administration of amifostine for head and neck (HN) cancer patients receiving radiotherapy (RT): preliminary results of the GORTEC 2000–02 randomized trial. Int J Radiat Oncol Biol Phys. 2005;63(S1):S127.
275 Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577-587.
206 Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49(4):907-916.
277 Saarilahti K, Kouri M, Collan J, et al. Intensity modulated radiotherapy for head and neck cancer: evidence for preserved salivary gland function. Radiother Oncol. 2005;74(3):251-258.
278 Bussels B, Maes A, Flamen P, et al. Dose-response relationships within the parotid gland after radiotherapy for head and neck cancer. Radiother Oncol. 2004;73(3):297-306.
279 Roesink JM, Moerland MA, Battermann JJ, et al. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001;51(4):938-946.
280 Dawes C. Factors influencing salivary flow rate and composition, 2 ed. In: Edgar WM, O’Mullane DM, editors. Saliva and oral health. London, England: British Dental Journal; 1996:27-41.
281 Saarilahti K, Kouri M, Collan J, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006;78(3):270-275.
282 Seikaly H, Jha N, McGaw T, et al. Submandibular gland transfer: a new method of preventing radiation-induced xerostomia. Laryngoscope. 2001;111(2):347-352.
283 Seikaly H, Jha N, Harris JR, et al. Long-term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Arch Otolaryngol Head Neck Surg. 2004;130(8):956-961.
284 Hassan SJ, Weymuller EAJr. Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15(6):485-496.
285 Rieger J, Seikaly H, Jha N, et al. Submandibular gland transfer for prevention of xerostomia after radiation therapy: swallowing outcomes. Arch Otolaryngol Head Neck Surg. 2005;131(2):140-145.
286 Al-Qahtani K, Hier MP, Sultanum K, et al. The role of submandibular salivary gland transfer in preventing xerostomia in the chemoradiotherapy patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(6):753-756.
287 Pathak KA, Bhalavat RL, Mistry RC, et al. Upfront submandibular salivary gland transfer in pharyngeal cancers. Oral Oncol. 2004;40(9):960-963.
288 RTOG Website http://www.rtog.org/members/protocols/0244/0244.pdf accessed 02/02/2007
289 Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439.
290 Doornaert P, Slotman BJ, Rietveld DH. The mean radiation dose in pharyngeal structures is a strong predictor of acute and persistent swallowing dysfunction and quality of life in head and neck radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(3S):S55.
291 O’Meara EA, Machtay M, Moughan J, et al. Associations between radiation doses to pharyngeal regions and severe late toxicity in head and neck cancer patients treated with concurrent chemoradiotherapy–an RTOG analysis. Int J Radiat Oncol Biol Phys. 2007;69(3S):S54.
292 Caglar HB, Allen AM, Othus M, et al. Dose to the larynx predicts for swallowing complications following IMRT and chemotherapy. Int J Radiat Oncol Biol Phys. 2007;69(3S):S53.
293 Teguh DN, Levendag PC, Noever I, et al. Treatment techniques and site considerations regarding dysphagia-related quality of life in cancer of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 2008.
294 Bedwinek JM, Shukovsky LJ, Fletcher GH, et al. Osteonecrosis in patients treated with definitive radiotherapy for squamous cell carcinomas of the oral cavity and naso- and oropharynx. Radiology. 1976;119(3):665-667.
295 Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
296 Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579.
297 List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564-569.
298 Harrison LB, Zelefsky MJ, Pfister DG, et al. Detailed quality of life assessment in patients treated with primary radiotherapy for squamous cell cancer of the base of the tongue. Head Neck. 1997;19(3):169-175.
299 Jaulerry C, Rodriguez J, Brunin F, et al. Results of radiation therapy in carcinoma of the base of the tongue. The Curie Institute experience with about 166 cases. Cancer. 1991;67(6):1532-1538.
300 Brunin F, Mosseri V, Jaulerry C, et al. Cancer of the base of the tongue: past and future. Head Neck. 1999;21(8):751-759.
301 Spanos WJJr, Shukovsky LJ, Fletcher GH. Time, dose, and tumor volume relationships in irradiation of squamous cell carcinomas of the base of the tongue. Cancer. 1976;37(6):2591-2599.
302 Mak AC, Morrison WH, Garden AS, et al. Base-of-tongue carcinoma: treatment results using concomitant boost radiotherapy. Int J Radiat Oncol Biol Phys. 1995;33(2):289-296.
303 Foote RL, Parsons JT, Mendenhall WM, et al. Is interstitial implantation essential for successful radiotherapeutic treatment of base of tongue carcinoma? Int J Radiat Oncol Biol Phys. 1990;18(6):1293-1298.
304 Gibbs IC, Le QT, Shah RD, et al. Long-term outcomes after external beam irradiation and brachytherapy boost for base-of-tongue cancers. Int J Radiat Oncol Biol Phys. 2003;57(2):489-494.
305 RTOG Website http://www.rtog.org/members/protocols/h0022/h0022.pdf accessed 02/02/2007
306 Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for head-and-neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57(1):49-60.
307 Chao KS, Low DA, Perez CA, et al. Intensity-modulated radiation therapy in head and neck cancers: The Mallinckrodt experience. Int J Cancer. 2000;90(2):92-103.
308 Chao KS, Low D, Perez C, et al. Intensity-modulated radiation therapy in head and neck cancers: the Mallinckrodt experience. Int J Cancer. 2000;90(2):92-103.
309 Eisbruch A, Marsh L, Dawson LA, et al. Recurrences near the base of skull following IMRT of head and neck cancer: implications for target delineation in the high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28-42.
310 Yao M, et al. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59(4):1001-1010.
311 Lawson JD, Otto K, A Chen, et al. Concurrent platinum-based chemotherapy and simultaneous modulated accelerated radiation therapy for locally advanced squamous cell carcinoma of the tongue base. Head Neck. 2008;30(3):327-335.
312 de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64(2):363-373.