29 Cancer of the Oral Cavity
Epidemiology and Genetics
Approximately 19,400 cases of oral cavity cancer (OCC) are seen in the United States each year, with 5200 deaths.1 It is more commonly seen among men than women, with an approximate ratio of 3 : 2.2 It is predominantly a disease of middle-aged men with a history of exposure to various carcinogenic chemicals, including tobacco smoke and alcohol. There is a clear dose-response relationship between the amount and duration of tobacco smoke exposure and the incidence of OCC. In countries such as India where smokeless tobacco (snuff) is popular, oral cancer is also common, although the risk is lower than for smoking (relative risk, 4.2 versus 10 to 15). The use of betel nuts is also related to oral cancer, particularly buccal mucosal cancer.3 Alcohol appears to act as a cocarcinogen and to have a topical effect in that mucosal areas directly exposed to alcohol have a higher incidence of oral cancer.4 Recently, for reasons that are unclear, there is an increasing trend in tongue cancer in young Americans who never had any exposure to tobacco or alcohol.5
The spectrum of molecular changes seen in oral cancer varies by country of origin.6 Mutations of p53 are common in oral cancers in patients from countries with European populations; for example, 47% of Europeans with oral cancer show mutated p53, whereas in patients from India and Southeast Asia, only 7% show mutations. Patients from these areas have tumors with abnormal ras genes, which include mutations, loss of heterozygosity (H-ras), and amplification (K-ras and N-ras). Loss of Rb gene function may be necessary in combination with p53 mutation for oncogenesis. It has been widely observed that oral cancers express transforming growth factor-alpha and epidermal growth factor receptor, which may trigger the autocrine growth pathway.7
Novel markers for the detection of OCC are an area of active research. There have been studies published on novel markers both for patients with a predisposition to OCC resulting from a prior history of oral leukoplakia,8 as well as in an adjunctive setting for diagnosis with messenger ribonucleic acid, using microarray profiling.9 In another study, Sudbφ and colleagues examined cytologic aberrations that persisted in the mucosa of long-term smokers that may predispose to OCC. The authors found that cytogenetic risk factors for OCC are present in smokers, and these findings may normalize after tobacco cessation.10
Anatomy
The oral cavity extends from the skin–vermilion junction of the lips to the junction of the hard and soft palate above, and to the line of circumvallate papillae below. It is divided into the following specific areas (Figs. 29-1 to 29-3).
Staging and Grouping System
The preferred clinical staging is the system found in the sixth edition of the American Joint Committee on Cancer (Table 29-1).11 T4 lesions have been divided into T4a (resectable disease) versus T4b (unresectable disease).
Diagnostic and Staging Studies
Positron emission tomography (PET) scan has also been shown to be of value in OCC staging. A study by Ng and colleagues prospectively evaluated 134 patients with squamous cell OCC and a palpably negative neck with both [18F]fluorodeoxyglucose (FDG) PET scan and CT or MRI. The sensitivity and specificity of PET plus CT or MRI scanning was slightly higher than with a PET scan alone (although not significant), and the PET scan was found to reduce the probability of occult neck metastases to less than 15% in T1 through T3 tumors.12 Another study by Kovacs and colleagues showed that PET scan in combination with sentinel node biopsy reduces the rate of elective node dissections in the treatment of oral and oropharyngeal cancer. Thus, PET scan has become more widely used in the staging of OCC and appears to have an additional benefit when compared with traditional staging with CT and MRI alone.13
General Therapeutic Approaches
Combined Chemotherapy and Radiotherapy for Locoregionally Advanced Disease
Chemotherapy has been combined with radiotherapy in the management of patients with unresectable OCC with the goals of increasing local control and survival as well as decreasing distant metastases. The chemotherapeutic agents most commonly used include cisplatin, paclitaxel (Taxol), fluorouracil (5-FU), methotrexate, and bleomycin. Except for one randomized study reported by Lo and colleagues, which showed increased survival in patients with oral cavity lesions receiving 5-FU concurrently with radiotherapy as compared with patients receiving radiotherapy alone,14 there has been no definitive evidence for improved local control or survival in oral cancer when single-agent chemotherapy is combined with radiotherapy. Two recent randomized studies examined multiagent chemotherapy with consolidative chemoradiation in the treatment of locoregionally advanced head and neck cancer, comparing regimens of cisplatin, 5-FU, and docetaxel with cisplatin and 5-FU alone. One study found improved progression-free survival and overall survival,15 while the other study demonstrated improved locoregional control and overall survival.16 Enhanced radiation mucositis has been commonly observed with concurrent drug and radiation administration, especially when 5-FU is given concurrently with radiation.
Targeted agents are also being used to further improve outcomes. A study by Bonner and colleagues compared patients with advanced head and neck malignancies who were treated with radiation therapy alone versus radiation therapy with cetuximab, and found a median overall survival of 23 months in the control group and 49 months in the experimental arm, changing the treatment approach for patients with advanced disease and creating a pathway for the study of other targeted biologic agents in this disease.17
Postoperative Radiotherapy
The aims of postoperative radiotherapy are (1) to prevent local recurrence when there is known residual gross or microscopical disease after surgery, (2) to prevent recurrence in the neck when there are large or multiple lymph node metastases and tumor invasion of the soft tissues in the neck, and (3) to control occult disease in the cervical lymph nodes that were not resected surgically.18 When radiotherapy is given postoperatively, usually a dose of 60 to 66.6 Gy is delivered in 1.8- to 2.0-Gy daily fractions, five fractions a week, to the primary site and involved neck areas. The clinically and pathologically uninvolved neck areas receive 54 Gy.19 It is generally believed that tumor cells trapped in the surgical scars are less well oxygenated and therefore more radioresistant, and that higher radiation doses are required to eradicate them than are required preoperatively. However, this belief has not been adequately tested. Apparent differences in doses required preoperatively and postoperatively may be due to proliferation of tumor cells in the postoperative period before radiotherapy starts. The volume that is irradiated should include the entire surgical bed and the entire neck. Postoperative radiotherapy can be started as soon as wound healing is complete, usually within 2 to 3 weeks after surgery.
Postoperative Chemotherapy and Radiation
Much of the shift in treatment paradigm over the past several years in the management of OCC has arisen from multiple studies assessing when a postoperative chemoradiation approach achieves better outcomes than postoperative radiation alone. The landmark studies of the Radiation Therapy Oncology Group (RTOG) 9501 and the European Organisation for Research and Treatment of Cancer (EORTC) 22931, followed by the comparative analysis of Bernier and colleagues, demonstrated that all patients with resected head and neck cancer receiving standard fractionated postoperative radiation who are found to have positive margins or extranodal extension should be assigned to a combined chemoradiation approach using concurrent cisplatin (100 mg/m2 on days 1, 22, and 43).20–22 More recently, the German Cancer Society 95-06 trial23 demonstrated that even with hyperfractionated accelerated treatment regimens, patients that received concurrent 5-FU and mitomycin experienced an improvement in locoregional control and overall survival. Finally, a study by Garden and colleagues examined patients with stage III and IV disease who received a concomitant boost treatment regimen and cisplatin on days 1 and 22, and found that 4-year locoregional progression-free survival was 74% and that 4-year overall survival was 54%—results which are quite promising.24 Clearly, the ideal chemotherapeutic regimen in the postoperative setting has not yet been fully elucidated.
Simulation
IMRT now has widespread acceptance in the treatment of OCC. These patients are simulated in the supine position, and surgical scars are wired as described previously. A mask is then generated to encompass the head and neck region and a bite block is used when appropriate. A CT, MRI, or PET simulation scan is taken and an isocenter is set. If a low anterior neck (LAN) field is being generated to treat the low neck lymph nodes, then at our institution we generally set the isocenter at the match line of the IMRT and LAN fields. The isocenter is generally placed directly above the arytenoids. However, when there are bulky lymph nodes that extend inferior to this point, we treat with whole-field IMRT. Fig. 29-4 depicts a three-point mask, in which the head and neck are immobilized. For patients in whom more rigid lower neck and shoulder immobilization is preferred, a five-point mask can be used that extends inferiorly to immobilize these structures.
General Techniques of Radiotherapy and Treatment Plans
Megavoltage External-Beam Radiotherapy
The treatment volume encompassing the primary tumor with adequate margins and cervical lymph nodes of concern is outlined on the treatment simulation films, the skin, or on the relevant imaging slices in the case of IMRT or in 3-D conformal therapy (CT, MRI, or PET scan). When conventional techniques are used, metallic wires, gold seeds, or silver clips placed in the tumor periphery are helpful in localizing the tumor volume on the simulation films. Normal tissues that are in the field but need not be irradiated are shielded with either mounted lead blocks or multileaf collimators. Examples of treatment of an advanced carcinoma of the tongue are shown in Figs. 29-4 to 29-6. Weekly port films are obtained with the patient on the treatment machine to verify the actual volume irradiated. All fields are treated daily. The usual dose fractionation is 1.8 to 2 Gy per day, with five daily treatments per week. The total dose depends on the extent of the disease and whether external-beam radiotherapy is used alone or in combination with interstitial radiotherapy or surgery. When definitive external-beam alone is used, the total dose is usually in the range of 70 to 74 Gy. The spinal cord is either shielded after 44 Gy or constrained in the radiation plan to a dose of 45 Gy or less.
Peroral Cone Radiotherapy
Intraoral cone radiotherapy using 100 to 250 kVp x-rays or 6 meV electron beams is suitable for the treatment of select small lesions, less than 3 cm in diameter, in the floor of the mouth. For the proper administration of this radiotherapy modality, it is necessary that adequate coverage of the lesion is achieved with the intraoral cone and that patient immobilization be maintained during treatment. The use of a special immobilizing chair that locks the patient and applicator in a rigid unit is advised for orthovoltage treatment, as shown in Fig. 29-7. The analysis by Phillips on the time-dose relationships in peroral radiotherapy for oral cancer suggests that surface doses between 55 Gy in 18 days and 60 Gy in 26 days will result in good control rates with acceptable risk of soft-tissue or bone necrosis.19 Wang has described a technique for using peroral cones with electron beams (Fig. 29-8).25
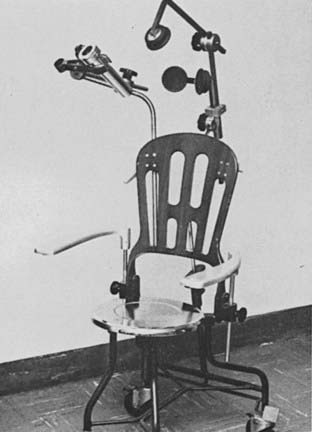
FIGURE 29-7 • Peroral cone for orthovoltage with treatment chair.
(From Phillips TL: Peroral roentgen therapy, Radiology 90:525, 1968.)
Brachytherapy
Interstitial radiotherapy, either alone or in combination with external-beam radiotherapy, is used for selected cases in the treatment of carcinoma of the oral tongue, floor of the mouth, and buccal mucosa.26,27 Radioactive isotopes that have been used in the past in interstitial radiotherapy of oral carcinomas include radium-226, cesium-137, gold-198, and tantalum-182. Iridium-192 used in the form of pins (epingles), wires, or seeds preloaded in a plastic ribbon have the advantage of being suitable for afterloading techniques, and thus is used commonly for temporary implants. Iodine-125 may be substituted and is the isotope of choice for permanent implants. The use of remote afterloading of iridium sources with high-dose rate (HDR) is replacing the use of hand-loaded seeds in ribbons in most institutions. These techniques minimize exposure to personnel and allow optimization of dose distribution.28
When the tumor thickness is 1 cm or less, a single-plane implant is adequate. When the tumor thickness is greater than 1 cm, then a double-plane implant or volume implant is necessary to deliver a relatively uniform dose to the tumor volume. The Patterson-Parker or Quimby tables are often used for treatment planning; however, dosimetry is best done by a computer using localization films of the actual implant.29,30
When interstitial radiotherapy is used alone for patients who present with early-staged T1 to T2-N0 disease, the usual implant dose is 60 to 70 Gy delivered over a period of 6 to 7 days.31,32 For oral tongue lesions, if a combination of external-beam radiotherapy and interstitial implant is considered, several series have reported on the importance of adequate interstitial implant dose. The total implant dose is correlated with local control. Those patients who received an external-beam dose less than 40 Gy along with a higher brachytherapy dose achieved higher local control rates than those who received a lower brachytherapy dose.33–36 If the patients have neck disease, surgery with or without postoperative radiotherapy is recommended over radiotherapy alone.
No adjustment of total dose is made when the dose rate of the minimum tumor dose is between 30 and 60 cGy per hour.35 Examples of a single-plane iridium implant and a volume iridium implant and the isodose distributions are shown in Figs. 29-9 and 29-10.
Carcinoma of the Lip
Clinical Presentation
Second to the skin, carcinoma of the lip is the most common cancer of the head and neck. In the United States, lip cancer accounts for 12.7 per 100,000 annually.37 It is rare among blacks and Asians, and accounts for approximately 25% of all carcinomas of the oral cavity. Approximately 90% of the carcinomas of the lip arise from the lower lip. Elderly men are affected most frequently, particularly in the sixth and seventh decades. The incidence rates are generally stable or falling among men worldwide, but are rising among many female populations.38 Chronic exposure to sunlight appears to be a predisposing factor, as carcinoma of the lip occurs most frequently in light-complected, fair-skinned persons who work outdoors.39 Other possible causal factors are tobacco smoking and viruses.
The majority of carcinomas of the lower lip are moderately to well-differentiated squamous cell carcinomas. Basal cell carcinomas make up the majority of upper lip lesions.40 Basal cell carcinomas arising from the skin that invade the lips secondarily should be considered carcinomas of the skin.
Carcinoma of the lower lip frequently presents as a thickening of the mucous membrane. Later, desquamation and ulceration that does not heal may develop. There may be a history of long-standing keratosis or leukoplakia.41
Routes of Spread
Carcinoma of the lip tends to be localized. It usually spreads by direct invasion of the surrounding soft tissue, skin, and bone, and infrequently metastasizes to regional lymph nodes.42 The incidence of lymph node metastasis varies with the extent of the primary lesion. The average incidence of regional lymph node metastasis is approximately 3% to 8% on presentation, and approximately an equal proportion of patients develop cervical lymph node metastasis subsequent to treatment of the primary lesion. There is a rare incidence of perineural spread, often into the mandibular canal.43
Treatment Approach
Carcinoma of the lip can be treated successfully with either radiotherapy or surgery.44 The choice of treatment depends on the extent of disease, the functional and cosmetic results of the treatment, expediency, the patient’s age, and the available treatment personnel and facilities.45 Radiotherapy is usually indicated for moderately large (>3 cm) infiltrative lesions that would otherwise require a complicated plastic surgery with less satisfactory cosmetic and functional results. Radiation is also indicated for tumors involving the commissure because surgical excision of the commissure would not yield satisfactory cosmetic and functional results.46 Elderly patients who are at a high risk for complications from general anesthesia or who have persistent or recurrent disease after surgery should be considered for radiotherapy. Radiotherapy is also administered postoperatively when the surgical margin is inadequate, there is invasion of the soft tissues of the neck caused by cervical lymph node metastasis, and in combination with surgery for advanced resectable disease. Surgery is usually preferred for small lesions, young patients, very advanced disease with bone invasion, cervical lymph node metastases, and recurrent disease after radiotherapy.
Radiotherapy modalities commonly used for carcinoma of the lip include external-beam radiotherapy with orthovoltage x-rays or electrons,47 and interstitial implants with radium or iridium.27 The “sandwich” technique has also been used in some centers; however, fractionated external-beam radiotherapy usually yields more satisfactory cosmetic results, especially for moderately advanced disease. When interstitial radiotherapy is used, a dose of 60 to 70 Gy in 6 to 8 days is usually adequate.
When external-beam radiotherapy is used, a lead cut-out is usually made to outline the treatment volume. A lead shield can be inserted between the inner surface of the lip and the gingiva to prevent irradiation of the intraoral structures. The treatment volume should include the lesion with adequate margins. Leukoplakic changes on the lip adjacent to the lesion should also be included in the treatment volume. For small superficial tumors, a single field with 100- to 150-kV x-rays is sufficient. For deeper, more advanced tumors, 200- to 250-kV x-rays or electrons are usually used. The optimal dose-fractionation schedule depends on the volume of irradiation. For small lesions (<2 cm in diameter), a dose of 45 Gy to 50 Gy in 15 fractions over 3 weeks is usually adequate, with good cosmetic results. For larger lesions, more protracted treatments with 50 to 70 Gy in 4 to 7 weeks are often used. The base of the skull should be adequately covered in cases with perineural involvement (Fig. 29-11). The neck is usually not irradiated unless cervical lymph node metastasis is present. Acute moist skin and mucous membrane reactions are managed with frequent cleansing and saline soaks. Topical antibiotics are rarely used unless there is an infection.
Treatment Outcome
Results of radiotherapy in select series of patients treated with radiotherapy for carcinoma of the lip are shown in Table 29-2.46,48–50 Control of the primary lesion can be achieved with external-beam irradiation or interstitial implants in the majority of patients. Those with recurrent disease after radiotherapy or cervical lymph node metastasis are usually treated surgically. Death resulting from uncontrolled carcinoma of the lip is uncommon (<10% in most series). The local control rate by interstitial implant is comparable to that achieved with external irradiation, although for larger lesions, better cosmetic results are obtained with protracted external-beam radiotherapy.
Carcinoma of the Oral Tongue
Routes of Spread
Carcinoma of the oral tongue usually spreads by direct invasion into the floor of the mouth, anterior tonsillar pillar, base of tongue, and mandible. Approximately 30% to 40% of the patients have cervical lymph node metastasis on presentation. The thickness as well as the T stage of the tongue lesion is directly correlated with the incidence of lymph nodal involvement.32,51 The subdigastric nodes are most frequently involved. The submandibular and midjugular lymph nodes are less commonly involved, and the submental, lower jugular, and posterior cervical nodes are rarely involved. Distant metastasis is seldom detected before death. The lungs, liver, and bones are the most common distant metastatic sites. Approximately 19% of patients develop a second malignant tumor. The majority of the second cancers are in the upper respiratory-digestive tract. The distribution of lymph nodes reported by Lindberg is shown in Fig. 29-12.52
Treatment Approach
Surgery followed by radiotherapy for both the primary site as well as the bilateral necks is the preferred treatment modality for carcinoma of the oral tongue. Invasive pattern; T1, T2, T3, or T4 lesions; bone invasion; multiple cervical lymph node metastases; close or positive margins; and lymphovascular or perineural invasion are indications for postoperative radiotherapy. In regard to an invasive pattern, multiple studies have demonstrated that the risk of cervical lymph node metastases is much greater for tumors with a depth of invasion of 5 mm or greater.53,54 In patients with positive margins or extranodal extension, a postoperative chemoradiation approach has been shown to be superior to radiation alone in the analysis of pooled RTOG and EORTC data by Bernier and colleagues, and now is the standard of care, albeit with increased toxicity.20–22 Attempts to spare the parotid glands can be made to reduce xerostomia, particularly with the advent of IMRT (Figs. 29-13 and 29-14).55 With the excellent functional outcome of the reconstructed oral tongue after hemiglossectomy, definitive radiotherapy with or without chemotherapy is reserved for selected cases (i.e., if the patient is medically inoperable or if the lesion is unresectable). It must include brachytherapy to be effective.56,57
Surgery is also preferred in elderly patients who tolerate partial glossectomy better than radiotherapy and in whom preservation of speech is not important. Recurrence in the primary lesion after radiotherapy is difficult to salvage by surgery, with one study quoting 5-year actuarial survival rates of 39%, and 27% for locoregional recurrence.58 Local recurrence after treatment with glossectomy alone has a similarly dismal prognosis, with some studies reporting less than 10% of patients being successfully salvaged at 4 years after being diagnosed with recurrent disease.59 Metastatic cervical lymph node metastases that develop after the primary lesion has been controlled can be successfully salvaged, although survival after neck recurrence has been documented to be as low as 18 months.60
When external irradiation is used in combination with interstitial implants in selected cases of carcinoma of the oral tongue, usually 40 Gy are delivered in 2-Gy fractions, five daily fractions per week, before the implant. The implant should deliver at least 40 Gy. Although the primary lesion may shrink after the external irradiation, the treatment volume for the interstitial implant is usually determined before any treatment is initiated. The treatment volume for external irradiation should include the primary lesion as well as the regional lymph nodal areas. The lymph node areas are treated from 54 Gy, for uninvolved lymph nodes being treated electively, to 60 Gy. In patients with unilateral lymph node metastasis, the rate of contralateral occult node metastases has been found to be high in OCC, up to 35% to 40%.61,62 Therefore, we generally advocate contralateral nodal treatment in all patients with carcinoma of the oral tongue because of its aggressive behavior and because it is not a well-lateralized structure such as the buccal mucosa. When lymph nodes are clinically involved, a neck dissection should be considered instead of radiotherapy as it offers better local control. In this clinical scenario, the preference is surgery followed by postoperative radiotherapy when indicated. Table 29-3 depicts nodal treatment guidelines that are used at our institution for oral cavity tumors. Interstitial implant is an integral part of radiotherapy for carcinoma of the oral tongue if definitive radiotherapy is the primary treatment approach.63 The implant dose is directly correlated with local control.33,34,36 External radiotherapy alone is not recommended, as it is rarely successful. Early superficial lesions can be controlled with a single-plane interstitial implant or peroral radiotherapy alone. For lesions greater than 1 cm in thickness, double-plane or volume implants in combination with external irradiation are usually used. Brachytherapy has also been used in the postoperative setting for T1-2-N0 squamous cell carcinomas of the oral tongue and the floor of mouth with close or positive margins. The recommended dose is 60 Gy.64,65 High rates of locoregional control, close to 90%, with low risk of chronic sequelae have been reported.
For interstitial implants of carcinoma of the tongue and floor of the mouth, dose rates in the range of 30 to 100 cGy per hour for the minimum tumor dose have been commonly used when low-dose rate (LDR) brachytherapy is the treatment approach. Although some authors recommend adjustments of the total dose according to the dose rate,29,66 we agree with Mazeron and coworkers that for dose rates between 30 and 100 cGy per hour, no adjustment of total dose is indicated.67 Overcorrection of dose for reduced overall treatment time may lead to a high incidence of local recurrence.68,69
Localization films of the implant are usually taken on the day of the procedure. Computer dosimetry provides detailed information on the dose distribution within the implant volume. The dose distribution in several parallel planes intersecting the tumor volume should be obtained. An isodose curve that encompasses the entire tumor volume on all the planes that intersect the volume should be used to calculate the minimum tumor dose. Sometimes part of the implant may be removed during the course of treatment when there is a volume of overdosage within the implant. The optimal minimum tumor doses for local control vary with the size of the primary lesions. For T1 and select small T2 lesions, the implant alone is used with a dose of 60 to 70 Gy. For larger T2 lesions, a total dose between 75 to 80 Gy is delivered by using both intestitial implant and external-beam radiotherapy, with 30 to 40 Gy being a typical dose delivered by external-beam radiation, as noted previously. The incidence of soft-tissue and bone necrosis depends on the total dose as well as on tumor volume, but is independent of the dose rate.70 It is important to separate the ribbons from the mandible by the use of dental rolls sutured in place.
HDR fractionated interstitial brachytherapy is the preferred treatment approach at our institution for patients undergoing implantation. A phase III trial of high- and low-dose interstitial radiotherapy for early oral tongue cancer showed that HDR can be an alternative to LDR, with similar rates of local control and the advantage of eliminating exposure to the medical staff.28,71 If not using HDR, an afterloading technique using iridium seeds is preferable because of the reduced radiation exposure to the medical and paramedical personnel. The afterloading technique also allows more time for optimal placement of needles, because no radiation exposure occurs during the procedure. The dosing regimen for HDR brachytherapy is typically 54 to 60 Gy in 9 to 10 fractions.
Treatment Outcome
Definitive radiotherapy results for carcinoma of the oral tongue are given in Table 29-4.36,72–76 Most of the patients in the series reported by Wendt and associates75 and Mazeron and associates74 did not have cervical lymph node metastasis at the time of treatment, whereas 50% of the patients in the series reported by Fletcher77 presented with cervical lymph node metastasis. As noted previously, the incidence of occult metastasis in early-stage squamous cell carcinoma of the oral tongue is between 20% and 48%.78
Because interstitial radiotherapy is an integral part of radiotherapy in patients receiving radiotherapy alone for carcinoma of the oral tongue, a small area of the mandible usually receives a dose in excess of 75 Gy. The incidence of osteonecrosis was 19% in the series reported by Delclos and coworkers.79 Most of the necrosis healed with conservative management, although only 35% of the bone necrosis in that series required surgical treatment, for a total of 6% of patients requiring surgical management. Another series reported a 38% complication rate, such as erosive ulcerations and bone exposure, in patients who received brachytherapy for stage I and II disease.80 The patients who also received external-beam radiotherapy in addition to brachytherapy had higher rates of complications than those treated with brachytherapy alone, but the incidence of neck metastases occurred in 31% to 51% of patients in whom the neck was not electively irradiated. Therefore, we still advocate for brachytherapy in combination with external-beam radiation for these patients. There have been multiple studies on the outcomes of patients treated with surgery in carcinoma of the oral tongue. One recent retrospective study from Japan demonstrated a superiority of surgery over brachytherapy in stage I and II tumors, with 5-year overall survival rates for the LDR, HDR, and surgery groups of 84.0%, 72.9%, and 95.4%, respectively.81 Another study by Aksu and colleagues compared definitive radiation with surgery and postoperative radiation in 80 patients with stages I through IVA disease.82 The patients that received surgery and postoperative radiation therapy had a 5-year overall survival advantage versus those treated with definitive radiation alone (49% versus 16%, respectively). Studies such as these confirm that when feasible, surgery should be performed as the definitive treatment modality in patients with oral tongue cancer.
Long-term survival in carcinoma of the oral tongue is directly related to the stage of disease. In a series of 204 patients treated between 1940 and 1971, reported by Fu and associates,70 the overall 5-year actuarial survival was 32% and determinate survival was 47% for all stages. The prognosis for patients who had cervical lymph node metastasis on presentation and persistent or recurrent disease at 3 months after treatment was significantly poorer than the prognosis for patients who had no cervical lymph node metastasis on presentation and those who were free of disease at 3 months after treatment. A recent series of 370 stage T1 and T2 cases reported by Shibuya and associates showed that the 5-year primary tumor control was 85% for superficial lesions, 70% for exophytic lesions, and 45% for infiltrative lesions. The 5-year overall survivals for stages T1, T2a, and T2b were 84%, 78%, and 72%, respectively.80
Carcinoma of the Floor of the Mouth
Clinical Presentation
The majority of the carcinomas of the floor of the mouth are moderately to well-differentiated squamous cell carcinomas. Clinically, they usually present as a growth or an ulcer, most often on one or the other side of the midline. Invasion of the tongue is common, and sometimes it may be difficult to distinguish whether a primary growth is arising from the floor of the mouth or from the oral tongue. The frenulum is frequently involved. With more advanced lesions, patients may complain of otalgia from referred pain, hypersalivation, difficulties in speech, and bleeding. A significant number of patients with carcinoma of the floor of the mouth develop a second primary cancer. In the series reported by Fu and colleagues,70 55 of 153 (36%) of the patients had a second primary cancer. The majority of the second primary sites are in the upper aerodigestive tracts.
Routes of Spread
Carcinomas of the floor of the mouth usually spread by direct invasion into the tongue, the lower alveolar ridge, the mandible, and the submandibular glands. Approximately 30% of the patients have cervical lymph node metastasis on presentation. The submandibular lymph nodes are most frequently involved. Metastasis to subdigastric nodes, midjugular nodes, and submental nodes are less frequent. The incidence of lymph node metastasis is directly proportional to the stage of the primary lesion; 9% for T1 lesions, 25% for T2 lesions, and 68% for T3 and T4 lesions (Fig. 29-15). Similarly, 20% of the patients without initial lymph node metastasis subsequently develop cervical lymph node metastasis. Distant metastasis occurs in approximately 9% of the patients. Lungs, liver, bone, and mediastinal lymph nodes are the most common sites of distant metastasis.
Treatment Approach
For postoperative treatment (with either IMRT or non-IMRT techniques), we recommend 66 Gy in 2-Gy fractions in the case of positive margins, 60 Gy in 2-Gy fractions to the high-risk region, and 54 Gy in 1.8-Gy fractions to the low-risk region. If definitive radiation therapy is used with non-IMRT techniques, for most carcinomas of the floor of mouth without bone invasion, we recommend external-beam irradiation with 50 to 54 Gy delivered at 2 Gy per fraction, five fractions per week, to the primary lesion with adequate margins and the upper neck, including the submandibular and subdigastric nodes, followed by a boost to the primary lesion with 10 to 20 Gy using interstitial implant, peroral cone irradiation, or external-beam irradiation. The boost portion of treatment encompasses any visible tumor plus a margin, and we prefer using 3-D conformal radiotherapy (Fig. 29-16). If IMRT is used as definitive treatment alone, the entire region can be treated using a dose-painting technique, delivering 70 Gy to the primary tumor and involved lymph nodes, 59.4 to 63 Gy in 1.8-Gy fractions or 60 Gy in 2-Gy fractions to the uninvolved but high-risk regions, and 54 Gy in 1.8-Gy fractions to the low-risk region.The lower neck is routinely irradiated when cervical lymph node metastases are present. Similar to carcinoma of the oral tongue, the floor of the mouth is not a well-lateralized structure and thus the bilateral neck is generally treated in all cases. If a LAN field is used, the standard dose is 50 Gy in 2-Gy fractions or 50.4 Gy in 1.8-Gy fractions. For advanced, unresectable lesions not amenable to implantation, patients can be treated with external-beam radiotherapy using accelerated hyperfractionation with a concomitant boost technique.
Similar interstitial radiotherapy techniques for carcinoma of the oral tongue are used for carcinoma for the floor of the mouth, as shown in Fig. 29-10. The iridium seeds in nylon ribbons are afterloaded into nylon tubes extending from the skin of the submental region to the floor of the mouth. The nylon tubes are pulled in behind stainless steel needles, which are then removed. For large lesions, the catheters are usually placed with the use of needles inserted through the submental skin to the dorsum of the tongue, to deliver an adequate dose throughout the tumor volume. If the tubes terminate in the floor of the mouth, the iridium ribbons must protrude at least 1 cm or have double-strength seeds at the tip. Alternately, longer dwell times can be used with HDR. A report from Japan indicated that HDR can achieve high control rates for carcinoma of the floor of mouth and the advantage of HDR versus LDR was the elimination of radiation exposure for the medical staff.83 The recommended HDR dose was 6000 cGy.
Treatment Outcome
Bone and soft-tissue necrosis develop in approximately 10% to 21% of the patients, usually within 2 years after treatment in patients treated with definitive radiation therapy.79,84,85 Most of the necroses heal with conservative management, although some patients require hemimandibulectomy. The results of definitive radiotherapy of carcinoma of the floor of the mouth, as reported in several recent series, are given in Table 29-5.* Most patients were treated with a combination of external irradiation and interstitial implants. Some early lesions were treated with interstitial implants or external irradiation alone. The control of the primary lesion is directly proportionate to the stage of the disease. Control of the cervical lymph node metastasis is also related to the N staging. Approximately two thirds of cases of N1 disease (lesion < 3 cm in diameter) can be controlled with radiation alone or in combination with surgery.
A relatively large series of 160 patients from France showed that brachytherapy alone offers excellent local control of 89% for T1 and T2 lesions with a 5-year survival of 76%.84 In spite of the good local control rate of carcinoma of the floor of the mouth, actuarial survival of these patients is poor, owing to a significant number of deaths from intercurrent disease or a second primary cancer. In the series of 153 patients with carcinoma of the floor of the mouth reported by Fu and colleagues,70 the 5-year actuarial and determinate survival rates were 35% and 60%, respectively, for the entire group of patients. Data from Washington University showed that in a retrospective study of 280 patients treated with surgery or radiation, the overall 5-year disease-specific survival was 56%.86 Recurrence at the primary site (41%) was the most common site of failure followed by neck recurrence of 19%. Metastasis occurred in 30%. Second primaries occurred in 20% of patients, and 53% died of their second primary disease.
Carcinoma of the Buccal Mucosa
Clinical Presentation
After carcinoma of the lip, oral tongue, floor of the mouth, and lower gum, carcinoma of the buccal mucosa is the fifth most common carcinoma of the oral cavity. Squamous cell carcinoma of the buccal mucosa composes approximately 10% of all oral cancers in the United States and western Europe. It is the most common carcinoma of the oral cavity in India, Malaysia, and Taiwan. It usually occurs in the sixth and seventh decades of life, and is more prevalent in men than in women. Tobacco and betel nut chewing appear to play an important role in the cause of these tumors.88,89
Carcinomas of the buccal mucosa often occur in association with pre-existing leukoplakia and tend to have multiple primary sites and recurrence. Excision of the oral leukoplakia may reduce the subsequent development of carcinoma.90 These tumors usually arise in the area adjacent to the lower molars along the occlusal line of the teeth. Clinically, there are three distinct types: exophytic, ulcerative, and verrucous. The patient may present with pain or bleeding, trismus, or cervical lymphadenopathy. In advanced stages, the tumor may destroy the entire cheek and invade the adjacent bones and the neck. Infection is common and mastication becomes difficult. Death usually occurs as a result of poor nutrition and general debilitation.91,92
Routes of Spread
Carcinomas of the buccal mucosa frequently spread by direct invasion into the gingivobuccal sulcus, the upper and lower alveolar ridges, the hard palate, the maxilla, and the mandible. Lymph node metastasis occurs in approximately 9% to 31% of the patients during the course of the disease.91,93 The submandibular lymph nodes are most frequently involved; involvement of the upper cervical and the parotid lymph nodes is less common. The risk of subclinical disease is 16%.92 Distant metastases are rare, as patients often die of uncontrolled local disease before distant metastases are manifested clinically.
Treatment Approach
Radiotherapy alone can be considered in small (T1) lesions involving the commissure or in the mid-cheek without sulcus invasion. Otherwise, surgery followed by postoperative radiotherapy is the recommended treatment modality for all buccal mucosa carcinomas, including T1 lesions. A recent report stated that T classification and negative margins are not adequate predictors of local control, and even early buccal tumors could benefit from adjuvant therapy to enhance local control.94 In another series, local recurrence occurred in all patients with stage I and II disease treated with wide local excision.95 The extent of surgery depends on the degree of locoregional involvement. A marginal or segmental mandibulectomy, or a partial maxillectomy should be performed when tumor invasion to the nearby bony structures is clinically suspected. A radical neck dissection is recommended in all patients with a node-positive neck, whereas a supraomohyoid neck dissection can be considered in the node-negative neck.96 As in other sites of the oral cavity, adjuvant treatment with chemotherapy and radiation is recommended in patients with positive margins or extranodal extension. In cases that are inoperable, radiotherapy in combination with chemotherapy can be used.15,16 Surgery is also considered in patients who recur after radiotherapy as salvage. One series reported a 5-year actuarial disease-free survival after surgical salvage of 59.7%.97
Either 3-D conformal therapy or IMRT should be used to spare the contralateral parotid gland. Because buccal mucosa lesions are lateralized, treatment of the contralateral buccal mucosa is not necessary in T1 and T2 lesions. Representative target delineation and a plan are shown in Figs. 29-17 and 29-18. Postoperative dosing regimens for buccal mucosa cancer are similar to other regions of the oral cavity. We recommend doses of 66 Gy in 2-Gy fractions for positive margins, 60 Gy in 2-Gy fractions or 59.4 to 63 Gy in 1.8-Gy fractions to high-risk regions, and 54 Gy in 1.8-Gy fractions for low-risk regions. An LAN is often used, treated to either 50 Gy in 2-Gy fractions or 50.4 Gy in 1.8-Gy fractions.
Treatment Outcome
Reports of the select retrospective studies of carcinoma of the buccal mucosa can be found in Table 29-6.95,98–102 A prospective, randomized trial from India examined the role of postoperative radiotherapy in stages III and IV carcinoma of the buccal mucosa. The 3-year actuarial disease-free survival was 68% versus 38%, with and without postoperative radiotherapy. The 3-year actuarial overall survival was 94% versus 84%, respectively.103
Surgery can be used to salvage patients that failed radical radiotherapy. One series reported an overall 5-year actuarial disease-free survival after salvage surgery to be 59.7%. At recurrence, T stage and the presence or absence of skin infiltration were the factors that significantly influenced disease-free survival.97
Multiple studies have demonstrated the superiority of multimodality treatment versus that of surgery or radiation alone. A study by Lin and colleagues examined 121 patients with M0 disease treated with either surgery alone, radiotherapy alone, or surgery plus postoperative radiation. The rate of local recurrence in patients with T1-2-N0 disease who received surgery alone was 40%, and for locally advanced disease both locoregional control and overall survival were improved in those patients receiving a combined-modality approach.104 Another study of combined-modality therapy was published by Fang and colleagues, and found that the 3-year locoregional control rate was 64%. Positive surgical margins and skin infiltration were found to be negative prognostic factors.98
Carcinoma of the Upper Gingiva
Treatment Outcome
In the review of the Memorial Sloan-Kettering Cancer Center experience of 61 patients with upper gingival tumor treated mostly with surgery, the 5-year survival rate was 51% (31/61). Clinical stage was the only predicator of survival.105 In another series of 82 patients from Japan, the 5-year local control rate was 61% with a 5-year actuarial survival of 45%.106 A third more recent series from India examined the results of 110 patients with tumors of the superior gingival-buccal complex treated with either surgery alone, radiation alone, or a combined modality approach. Five-year progression-free survival was 49% for patients treated surgically and 0% with a nonsurgical approach. T stage and extracapsular spread were predictors of disease progression.107
Carcinoma of the Lower Gingiva
Routes of Spread
Tumor spreads mainly through the occlusal ridge alone or in combination with penetration of the buccal or lingual plates in edentulous patients.108,109 Lymph node metastasis is present in 13% to 24% of patients with carcinoma of the lower gingiva. The submandibular lymph nodes are most frequently involved. Involvement of the subdigastric and upper cervical nodes is less common. Direct invasion of the mandible may be present in 50% of the patients on initial examination. The actual extent of bone involvement may exceed that shown on radiographs. Therefore, additional imaging is essential to accurate preoperative assessment.
Treatment Approach
Because of frequent bone involvement and lymph node metastasis, surgery has been the primary treatment modality in the management of carcinoma of the lower gingiva. Early superficial lesions can be expeditiously treated by a simple excision or marginal resection with skin graft. In patients with more advanced disease, postoperative radiotherapy can reduce the incidence of local recurrence. Clear margins should be achieved, as inadequate margins result in a high rate of local recurrence. One series showed that the 5-year local control rate in the presence of positive margins was 49% versus 100% with negative margins and survival was 11% versus 91%.110 In addition, patients with advanced T stage, radiologic or histologic evidence of mandibular invasion, tumors involving the symphyseal region, and decreased tumor differentiation have high propensity for regional metastases. Therefore, elective treatment of the neck should be considered.111 One series reported that larger tumor size is more important than mandibular invasion in predicting local control—larger tumors that have a higher propensity for local recurrence require a more extensive surgical resection.112
Treatment Outcome
Local control of carcinomas of the lower gingiva treated with radiotherapy alone was achieved in 71.5% (5/7) of T1 lesions, 70% (7/10) of T2 lesions, 59% (10/17) of T3 lesions, and 29% (4/14) of T4 lesions in the series reported by MacComb.92 The 5-year survival rate of 108 patients treated between 1947 and 1960 at the MD Anderson Cancer Center was 46%.92 Most series report excellent local control with surgery followed by postoperative radiotherapy when indicated. Results from select series are shown in Table 29-7.110,113 In the aforementioned study at MD Anderson Cancer Center112 examining prognostic factors of the disease, the authors found that advanced T stage, positive surgical margins, mandibular invasion, and cervical metastases were all associated with a worse overall survival.
Carcinoma of the Retromolar Trigone
Treatment Approach
Surgical resection has been shown to be the preferred modality over definitive radiation. More advanced tumors, such as those tumors with bone involvement or those with nodal involvement, require surgery and postoperative radiotherapy (Fig. 29-19).
Treatment Outcome
MD Anderson Cancer Center reported results of 159 patients with squamous cell carcinoma of the anterior faucial pillar and retromolar trigone region treated with radiation.114 Local control with radiation alone for T1 lesions was 71% (12/17); for T2 lesions, 70% (51/81); for T3 lesions, 76% (19/25); and three of five T4 lesions were controlled. Most of the failures were treated with surgery, and the ultimate control rate was 100% for T1, 94% for T2, and 92% for T3. The overall N0 neck failure rate was 11%. The authors recommend elective treatment of subdigastric and upper jugular nodes to the level of the hyoid. The 5-year determinate survival rate in the series was 83%. A more recent study at the Notre-Dame Hospital in Quebec demonstrated 5-year regional control rates of 88% after definitive radiation and 67% after salvage surgery.115 See Table 29-8 for select results of treatment for carcinoma of the retro molar trigone.115–117
Although there have been no formal randomized comparisons of radiation alone versus combined surgery and radiation specifically in this setting, reports of institutional experience with the combination treatment versus radiation alone suggest that combined surgery and radiation, particularly with advanced tumors, may yield results superior to radiotherapy alone.116,118 For example, University of Florida investigators noted in a retrospective review of their experience in 99 patients with stage I through III disease that surgery and radiation were associated with higher 5-year survival (56%) than definitive radiation alone (40%).118
Carcinoma of the Hard Palate
Clinical Presentation
Primary malignancies of the hard palate are rare. In one series of 5000 cases of oral cancer, only 25 (0.5%) had squamous cell carcinoma of the hard palate.119 In another study, squamous cell carcinoma accounted for 3% of all oral cancers.120 Patients often complain of ill-fitting dentures, pain, intermittent bleeding, or a sore that does not heal.
Treatment Approach
Very superficial lesions of the hard palate can be treated definitively with surface molds. If definitive external-beam radiation therapy is used with conventional techniques, the patient is treated with opposed-lateral fields or wedge pairs to 66 to 74 Gy in 2-Gy fractions. When IMRT techniques are used, a dose of 70 Gy is used for gross tumor, 59.4 to 63 Gy for high-risk regions, and 54 Gy for low-risk regions. Most tumors of the hard palate and upper gingiva are treated with surgery. Postoperative radiotherapy is added in high-risk patients (i.e., patients with close or positive margins; perineural invasion; lymphovascular invasion; stage T3, T4, or node-positive disease) (Fig. 29-20). In the postoperative setting, doses of 60 to 66 Gy using 1.8 to 2 Gy per fraction are administered, with a dose of 66 Gy being reserved for patients with positive margins. Similar guidelines for nodal coverage are used as has been previously described for carcinomas of the buccal mucosa and retromolar trigone. A prosthesis (i.e., obturator) can be placed over the postsurgical defect during radiotherapy. Alternatively, a balloon filled with water can be used to compensate for postsurgical tissue defects. Postoperative radiotherapy usually begins 2 to 3 weeks after surgery.
Treatment Outcome
Owing to the rarity of this disease, limited information is available. Evans and Shah reported their 15-year experience from the Memorial Sloan-Kettering Cancer Center. For stage I squamous cell carcinoma of the hard palate, the 5-year disease-free survival rate was 75% (6/8); all patients were treated with surgery alone. For stage II disease, 46% (6/13) were disease free at 5 years; postoperative radiotherapy was added in one patient. In stage III disease, the 5-year disease-free survival rate was 40% (4/10); one patient in the group received postoperative radiotherapy. In stage IV patients, 8% (1/12) of patients treated with surgery alone, 25% (1/4) of those treated with radiotherapy alone, and none (0/2) of those treated with the combined modality were disease-free at 5 years.121 In another series of patients with squamous cell carcinoma of the hard palate, from the University of Virginia, select early-stage lesions were equally effectively treated by radiotherapy as surgery during the supervoltage era. Patients with a clear margin after surgical resection had improved local control.122 See Table 29-9 for select results of definitive radiation therapy of the hard palate.122–124
Critical Normal Tissues
Acute and Late Effects
Acute changes occur in the oral mucosa, the salivary glands, and the taste buds during irradiation. Unless the parotid glands are blocked or constrained, xerostomia occurs after a few weeks. With 3-D conformal treatment or IMRT, the restoration of at least partial salivary function can be effectively accomplished with appropriate limits. A study from our institution examined geometric factors influencing dosimetric sparing of the parotid glands during IMRT in head and neck cancer, and found that dosimetric sparing, defined by a mean parotid dose less than or equal to 26.5 Gy, is feasible when the parotid–planning target volume (PTV) overlap is less than 20%. The authors concluded that sparing with additional overlap may lead to inadequate PTV coverage, and thus in some patients dosimetric sparing is not feasible.55 Acute mucositis cannot be avoided. For the definitive treatment of lesions with radiotherapy, the goal is to achieve mucositis that is either patchy (grade 2) or confluent (grade 3). Simple erythema (grade 1) usually means that the protraction of the treatment is too long and tumor proliferation during radiotherapy will be too great. Excellent oral care is required, as detailed in the next section, to avoid grade 4 reactions, which require hospitalization. Nutritional support is also required, as discussed later.
Late effects are seen as bone necrosis in the mandible and the maxilla, which occurs after breakdown of the mucosa and exposure of the bone with ensuing osteomyelitis.125,126 Teeth in poor condition should be extracted prior to radiation therapy. The teeth must be maintained in good condition to avoid extractions, which can lead to infection. Soft-tissue necrosis is frequently seen after both brachytherapy and peroral cone treatment. It is painful, but usually heals without surgery. A necrotic mandible often must be resected.
With the advent of IMRT, there appears to be a reduction in the toxicity associated with treatment of the oral cavity. A study from the University of Michigan demonstrated no cases of osteoradionecrosis in 176 patients treated with head and neck cancer from 1996 to 2005 with at least 6 months of follow-up.127 Of these patients, 75% received greater than or equal to 65 Gy to at least 1% of the mandibular volume, and 50% had received at least 70 Gy to this volume. However, the authors found a high level of falloff across the mandible, with the average gradient being 11 Gy. In a study at Memorial Sloan-Kettering Cancer Center128 examining our experience of patients receiving IMRT in the treatment of postoperative OCC, we found that the rates of grade 3 or higher acute dermatitis, mucositis, and xerostomia were 3%, 23%, and 0%, respectively. Furthermore, limiting the parotid mean dose to 26 Gy or less reduced the rates of grade 2 or higher xerostomia to 11%, a similar relative reduction that has been seen in other studies as well.129 Clearly, more follow-up is needed before more definitively assessing the chronic toxicities of IMRT, but the results at this point are promising, in that local control does not appear to be compromised and short-term toxicity is improved.
Nutrition
In patients with severe pain and dysphagia or problems of mastication or excessive weight loss, tube feeding may be necessary. The aim of a dietary program during head and neck irradiation is to provide adequate nutrition and prevent weight loss. Percutaneous gastrostomy tube placement may be required for patients who lose more than 10% of their body weight. Multiple studies have shown that prophylactic gastrostomy tubes are safe and may limit weight loss in patients receiving radiation for head and neck cancer,130,131 and we strongly consider their placement, particularly in patients receiving chemotherapy in conjunction with radiation.
1 Jemal A, Murray T, Samuels A, et al. Cancer statistics. CA Cancer J Clin. 2003;53(1):5-26.
2 Funk GF, Karnell LH, Robinson RA, et al. Presentation, treatment, and outcome of oral cavity cancer: A national cancer data base report. Head Neck. 2002;24(2):165-180.
3 Chen Y, Huang H, Lin L, et al. Primary oral squamous cell carcinoma: an analysis of 703 cases in southern Taiwan. Oral Oncol. 1999;35(2):173-179.
4 Johnson NW, Warnakulasuriy S, Tavassoli M. Hereditary and environmental risk factors: Clinical and laboratory risk matters for head and neck, especially oral, cancer and precancer. Eur J Cancer Prev. 1996;5(1):5-17.
5 Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128(3):268-274.
6 Paterson IC, Eveson JW, Prime SS. Molecular changes in oral cancer may reflect aetiology and ethnic origin. Eur J Cancer B Oral Oncol. 1996;32B(3):150-153.
7 Wong DT. TGF-alpha and oral carcinogenesis. Eur J Cancer B Oral Oncol. 1993;29B(1):3-7.
8 Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, et al. Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy. J Clin Oncol. 2008;26(3):354-360.
9 Li Y, Elashoff D, Oh M, et al. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol. 2006;24(11):1754-1760.
10 Sudbφ J, Samuelsson R, Risberg B, et al. Risk markers of oral cancer in clinically normal mucosa as an aid in smoking cessation counseling. J Clin Oncol. 2005;23(9):1927-1933.
11 Greene F, Page D, Fleming I, et al. American Joint Committee on Cancer lip and oral cavity. In American Joint Committee on Cancer Staging Handbook, ed 6, New York: Springer; 2002:35-47.
12 Ng SH, Yen TC, Chang JT, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol. 2006;24(27):4371-4376.
13 Kovacs AF, Dobert N, Gaa J, et al. Positron emission tomography in combination with sentinel node biopsy reduces the rate of elective neck dissections in the treatment of oral and oropharyngeal cancer. J Clin Oncol. 2004;22(19):3973-3980.
14 Lo TC, Wiley ALJr, Ansfield FJ, et al. Combined radiation therapy and 5-fluorouracil for advanced squamous cell carcinoma of the oral cavity and oropharynx: a randomized study. AJR Am J Roentgenol. 1976;126(2):229-235.
15 Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695-1704.
16 Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705-1715.
17 Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578.
18 Parsons JT, Mendenhall WM, Stringer SP. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39(1):137-148.
19 Phillips TL. Peroral roentgen therapy. Radiology. 1968;90(3):525-531.
20 Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944.
21 Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005;27(10):843-850.
22 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952.
23 Budach V, Stuschke M, Budach W, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: Final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95–06 Prospective Randomized Trial. J Clin Oncol. 2005;23(6):1125-1135.
24 Garden AS, Harris J, Trotti A, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation therapy oncology group (RTOG 99-14). Int J Radiat Oncol Biol Phys. 2008;71(5):1351-1355.
25 Wang CC, Biggs PJ. Technical and radiotherapeutic considerations of intra-oral cone electron beam radiation therapy for head and neck cancer. Semin Radiat Oncol. 1992;2(3):171-179.
26 Goffinet DR. Brachytherapy for head and neck cancer. Semin Radiat Oncol. 1993;3(4):250-259.
27 Harrison LB. Applications of brachytherapy in head and neck cancer. Semin Surg Oncol. 1997;13(3):177-184.
28 Inoue T, Teshima T, Murayama S, et al. Phase III trial of high and low dose rate interstitial radiotherapy for early oral tongue cancer. Int J Radiat Oncol Biol Phys. 1996;36(5):1201-1204.
29 Quimby E. Dosage table for linear radium sources. Radiology. 1944;43:572.
30 Johns HE, Cunningham JR. The Physics of Radiology. Springfield, Ill.: Charles C. Thomas; 1983.
31 Leung TW, Wong VY, Wong CM, et al. High dose rate brachytherapy for carcinoma of the oral tongue. Int J Radiat Oncol Biol Phys. 1997;39(5):1113-1120.
32 Matsuura K, Hirokawa Y, Fujita M, et al. Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy: Maximum tumor thickness is prognostic of nodal metastasis. Int J Radiat Oncol Biol Phys. 1998;40(3):535-539.
33 Mendenhall WM, Van Cise WS, Bova FJ, et al. Analysis of time-dose factors in squamous cell carcinoma of the oral tongue and floor of mouth treated with radiation therapy alone. Int J Radiat Oncol Biol Phys. 1981;7(8):1005-1011.
34 Chu A, Fletcher GH. Incidence and causes of failures to control by irradiation the primary lesions in squamous cell carcinomas of the anterior two-thirds of the tongue and floor of mouth. Am J Roentgenol Radium Ther Nucl Med. 1973;117(3):502-508.
35 Fu KK, Chan EK, Phillips TL, et al. Time, dose and volume factors in interstitial radium implants of carcinoma of the oral tongue. Radiology. 1976;119(1):209-213.
36 Fu KK, Ray JW, Chan EK, et al. External and interstitial radiation therapy of carcinoma of the oral tongue. A review of 32 years’ experience. AJR Am J Roentgenol. 1976;126(1):107-115.
37 Moore S, Johnson N, Pierce A, et al. The epidemiology of lip cancer: a review of global incidence and aetiology. Oral Dis. 1999;5(3):185-195.
38 Yako-Suketomo H, Marugame T. Comparison of time trends in lip cancer incidence (1973–97) in East Asia, Europe and USA, from Cancer Incidence in five Continents, Vols IV-VIII. Jpn J Clin Oncol. 2008;38(6):456-457.
39 Pogoda JM, Preston-Martin S. Solar radiation, lip protection, and lip cancer risk in Los Angeles County women (California, United States). Cancer Causes Control. 1996;7(4):458-463.
40 Huynh NT, Veness MJ. Basal cell carcinoma of the lip treated with radiotherapy. Australas J Dermatol. 2002;43(1):15-19.
41 Mackay EN, Sellers AH. A statistical review of carcinoma of the lip. Can Med Assoc J. 1964;90:670-672.
42 Cross JE, Guralnick E, Daland EM. Carcinoma of the lip: a review of 563 case records of carcinoma of the lip at Pondville Hospital. Surg Gynecol Obstet. 1948;81:153.
43 Byers RM, O’Brien J, Waxler J. The therapeutic and prognostic implications of nerve invasion in cancer of the lower lip. Int J Radiat Oncol Biol Phys. 1978;4(3–4):215-217.
44 Stranc MF, Fogel M, Dische S. Comparison of lip function: surgery vs radiotherapy. Br J Plast Surg. 1987;40(6):598-604.
45 de Visscher JG, van den Elsaker K, Grond AJ, et al. Surgical treatment of squamous cell carcinoma of the lower lip: Evaluation of long-term results and prognostic factors—a retrospective analysis of 184 patients. J Oral Maxillofac Surg. 1998;56(7):814-820.
46 de Visscher JG, Grond AJ, Botke G, et al. Results of radiotherapy for squamous cell carcinoma of the vermilion border of the lower lip. A retrospective analysis of 108 patients. Radiother Oncol. 1996;39(1):9-14.
47 Sykes AJ, Allan E, Irwin C. Squamous cell carcinoma of the lip: the role of electron treatment. Clin Oncol (R Coll Radiol). 1996;8(6):384-386.
48 Petrovich Z, Parker RG, Luxton G, et al. Carcinoma of the lip and selected sites of head and neck skin. A clinical study of 896 patients. Radiother Oncol. 1987;8(1):11-17.
49 Cerezo L, Liu FF, Tsang R, et al. Squamous cell carcinoma of the lip: analysis of the Princess Margaret Hospital experience. Radiother Oncol. 1993;28(2):142-147.
50 Tombolini V, Bonanni A, Valeriani M, et al. Brachytherapy for squamous cell carcinoma of the lip. The experience of the Institute of Radiology of the University of Rome “La Sapienza.”. Tumori. 1998;84(4):478-482.
51 Yamazaki H, Inoue T, Teshima T, et al. Tongue cancer treated with brachytherapy: is thickness of tongue cancer a prognostic factor for regional control? Anticancer Res. 1998;18(2B):1261-1265.
52 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446-1449.
53 Kane SV, Gupta M, Kakade AC, et al. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32(7):795-803.
54 Fukano H, Matsuura H, Hasegawa Y, et al. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19(3):205-210.
55 Hunt MA, Jackson A, Narayana A, et al. Geometric factors influencing dosimetric sparing of the parotid glands using IMRT. Int J Radiat Oncol Biol Phys. 2006;66(1):296-304.
56 Lyos AT, Evans GR, Perez D, et al. Tongue reconstruction: outcomes with the rectus abdominis flap. Plast Reconstr Surg. 1999;103(2):442-447.
57 Salibian AH, Allison GR, Armstrong WB, et al. Functional hemitongue reconstruction with the microvascular ulnar forearm flap. Plast Reconstr Surg. 1999;104(3):654-660.
58 Yuen AP, Wei WI, Lam LK, et al. Results of surgical salvage of locoregional recurrence of carcinoma of the tongue after radiotherapy failure. Ann Otol Rhinol Laryngol. 1997;106(9):779-782.
59 Yuen AP, Wei WI, Wong SH, et al. Local recurrence of carcinoma of the tongue after glossectomy: patient prognosis. Ear Nose Throat J. 1998;77(3):181-184.
60 Godden DR, Ribeiro NF, Hassanein K, et al. Recurrent neck disease in oral cancer. J Oral Maxillofac Surg. 2002;60(7):748-753.
61 Koo BS, Lim YC, Lee JS, et al. Management of contralateral N0 neck in oral cavity squamous cell carcinoma. Head Neck. 2006;28(10):896-901.
62 O’Brien CJ, Traynor SJ, McNeil E, et al. The use of clinical criteria alone in the management of the clinically negative neck among patients with squamous cell carcinoma of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2000;126(3):360-365.
63 Fujita M, Hirokawa Y, Kashiwado K, et al. Interstitial brachytherapy for stage I and II squamous cell carcinoma of the oral tongue: factors influencing local control and soft tissue complications. Int J Radiat Oncol Biol Phys. 1999;44(4):767-775.
64 Lapeyre M, Hoffstetter S, Peiffert D, et al. Postoperative brachytherapy alone for T1–2 N0 squamous cell carcinomas of the oral tongue and floor of mouth with close or positive margins. Int J Radiat Oncol Biol Phys. 2000;48(1):37-42.
65 Chao KS, Emami B, Akhileswaran R, et al. The impact of surgical margin status and use of an interstitial implant on T1, T2 oral tongue cancers after surgery. Int J Radiat Oncol Biol Phys. 1996;36(5):1039-1043.
66 Paterson R. The treatment of malignant disease by radiotherapy. Baltimore: Williams & Wilkins; 1963.
67 Mazeron JJ, Simon JM, Le Pechoux C, et al. Effect of dose rate on local control and complications in definitive irradiation of T1–2 squamous cell carcinomas of mobile tongue and floor of mouth with interstitial iridium-192. Radiother Oncol. 1991;21(1):39-47.
68 Burgers JM, Awwad HK, van der Laarse R. Relation between local cure and dose-time-volume factors in interstitial implants. Int J Radiat Oncol Biol Phys. 1985;11(4):715-723.
69 Awwad HK, Burgers JM, Marcuse HR. The influence of tumor dose specification on the early clinical results of interstitial radium tongue implants. Radiology. 1974;110(1):177-182.
70 Fu KK, Lichter A, Galante M. Carcinoma of the floor of mouth: an analysis of treatment results and the sites and causes of failures. Int J Radiat Oncol Biol Phys. 1976;1(9–10):829-837.
71 Inoue T, Yoshida K, Yoshioka Y, et al. Phase III trial of high- vs. low-dose-rate interstitial radiotherapy for early mobile tongue cancer. Int J Radiat Oncol Biol Phys. 2001;51(1):171-175.
72 Ichimiya Y, Fuwa N, Kamata M, et al. Treatment results of stage I oral tongue cancer with definitive radiotherapy. Oral Oncol. 2005;41(5):520-525.
73 Fein DA, Mendenhall WM, Parsons JT, et al. Carcinoma of the oral tongue: a comparison of results and complications of treatment with radiotherapy and/or surgery. Head Neck. 1994;16(4):358-365.
74 Mazeron JJ, Crook JM, Benck V, et al. Iridium 192 implantation of T1 and T2 carcinomas of the mobile tongue. Int J Radiat Oncol Biol Phys. 1990;19(6):1369-1376.
75 Wendt CD, Peters LJ, Delclos L, et al. Primary radiotherapy in the treatment of stage I and II oral tongue cancers: importance of the proportion of therapy delivered with interstitial therapy. Int J Radiat Oncol Biol Phys. 1990;18(6):1287-1292.
76 Wang CC, Kelly J, August M, et al. Early carcinoma of the oral cavity: a conservative approach with radiation therapy. J Oral Maxillofac Surg. 1995;53(6):687-690.
77 Fletcher GH. Elective irradiation of subclinical disease in cancers of the head and neck. Cancer. 1972;29(6):1450-1454.
78 Haddadin KJ, Soutar DS, Oliver RJ, et al. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck. 1999;21(6):517-525.
79 Delclos L, Lindberg RD, Fletcher GH. Squamous cell carcinoma of the oral tongue and floor of mouth. Evaluation of interstitial radium therapy. AJR Am J Roentgenol. 1976;126(2):223-228.
80 Shibuya H, Hoshina M, Takeda M, et al. Brachytherapy for stage I & II oral tongue cancer: an analysis of past cases focusing on control and complications. Int J Radiat Oncol Biol Phys. 1993;26(1):51-58.
81 Umeda M, Komatsubara H, Ojima Y, et al. A comparison of brachytherapy and surgery for the treatment of stage I-II squamous cell carcinoma of the tongue. Int J Oral Maxillofac Surg. 2005;34(7):739-744.
82 Aksu G, Karadeniz A, Saynak M, et al. Treatment results and prognostic factors in oral tongue cancer: analysis of 80 patients. Int J Oral Maxillofac Surg. 2006;35(6):506-513.
83 Inoue T, Yamazaki H, Koizumi M, et al. High dose rate versus low dose rate interstitial radiotherapy for carcinoma of the floor of mouth. Int J Radiat Oncol Biol Phys. 1998;41(1):53-58.
84 Marsiglia H, Haie-Meder C, Sasso G, et al. Brachytherapy for T1–T2 floor-of-the-mouth cancers: the Gustave-Roussy Institute experience. Int J Radiat Oncol Biol Phys. 2002;52(5):1257-1263.
85 Lozza L, Cerrotta A, Gardani G, et al. Analysis of risk factors for mandibular bone radionecrosis after exclusive low dose-rate brachytherapy for oral cancer. Radiother Oncol. 1997;44(2):143-147.
86 Sessions DG, Spector GJ, Lenox J, et al. Analysis of treatment results for floor-of-mouth cancer. Laryngoscope. 2000;110(10 Pt 1):1764-1772.
87 Wadsley JC, Patel M, Tomlins CD, et al. Iridium-192 implantation for T1 and T2a carcinoma of the tongue and floor of mouth: retrospective study of the results of treatment at the Royal Berkshire Hospital. Br J Radiol. 2003;76(906):414-417.
88 Lampe I. Radiation therapy of cancer of the buccal mucosa and lower gingiva. Am J Roentgenol Radium Ther Nucl Med. 1955;73(4):628-638.
89 Zain RB, Ikeda N, Gupta PC, et al. Oral mucosal lesions associated with betel quid, areca nut and tobacco chewing habits: consensus from a workshop held in Kuala Lumpur, Malaysia, November 25–27, 1996. J Oral Pathol Med. 1999;28(1):1-4.
90 Saito T, Sugiura C, Hirai A, et al. Development of squamous cell carcinoma from pre-existent oral leukoplakia: With respect to treatment modality. Int J Oral Maxillofac Surg. 2001;30(1):49-53.
91 Conley J, Sadoyama JA. Squamous cell cancer of the buccal mucosa. A review of 90 cases. Arch Otolaryngol. 1973;97(4):330-333.
92 MacComb WS, Fletcher GH, Healey JEJ. Cancer of the head and neck. Baltimore, MD: Williams & Wilkins; 1967.
93 Bloom ND, Spiro RH. Carcinoma of the cheek mucosa. A retrospective analysis. Am J Surg. 1980;140(4):556-559.
94 Sieczka E, Datta R, Singh A, et al. Cancer of the buccal mucosa: are margins and T-stage accurate predictors of local control? Am J Otolaryngol. 2001;22(6):395-399.
95 Strome SE, To W, Strawderman M, et al. Squamous cell carcinoma of the buccal mucosa. Otolaryngol Head Neck Surg. 1999;120(3):375-379.
96 Misra S, Chaturvedi A, Misra N. Management of gingivobuccal complex cancer. Ann R Coll Surg Engl. 2008.
97 Cherian T, Sebastian P, Ahamed MI, et al. Evaluation of salvage surgery in heavily irradiated cancer of the buccal mucosa. Cancer. 1991;68(2):295-299.
98 Fang FM, Leung SW, Huang CC, et al. Combined-modality therapy for squamous carcinoma of the buccal mucosa: treatment results and prognostic factors. Head Neck. 1997;19(6):506-512.
99 Chhetri DK, Rawnsley JD, Calcaterra TC. Carcinoma of the buccal mucosa. Otolaryngol Head Neck Surg. 2000;123(5):566-571.
100 Chaudhary AJ, Pande SC, Sharma V, et al. Radiotherapy of carcinoma of the buccal mucosa. Semin Surg Oncol. 1989;5(5):322-326.
101 Pop LA, Eijkenboom WM, de Boer MF, et al. Evaluation of treatment results of squamous cell carcinoma of the buccal mucosa. Int J Radiat Oncol Biol Phys. 1989;16(2):483-487.
102 Lapeyre M, Peiffert D, Malissard L, et al. An original technique of brachytherapy in the treatment of epidermoid carcinomas of the buccal mucosa. Int J Radiat Oncol Biol Phys. 1995;33(2):447-454.
103 Mishra RC, Singh DN, Mishra TK. Post-operative radiotherapy in carcinoma of buccal mucosa, a prospective randomized trial. Eur J Surg Oncol. 1996;22(5):502-504.
104 Lin CS, Jen YM, Cheng MF, et al. Squamous cell carcinoma of the buccal mucosa: an aggressive cancer requiring multimodality treatment. Head Neck. 2006;28(2):150-157.
105 Soo KC, Spiro RH, King W, et al. Squamous carcinoma of the gums. Am J Surg. 1988;156(4):281-285.
106 Shibuya H, Horiuchi J, Suzuki S, et al. Oral carcinoma of the upper jaw. Results of radiation treatment. Acta Radiol Oncol. 1984;23(5):331-335.
107 Pathak KA, Mathur N, Talole S, et al. Squamous cell carcinoma of the superior gingival-buccal complex. Oral Oncol. 2007;43(8):774-779.
108 Hong SX, Cha IH, Lee EW, et al. Mandibular invasion of lower gingival carcinoma in the molar region: Its clinical implications on the surgical management. Int J Oral Maxillofac Surg. 2001;30(2):130-138.
109 McGregor AD, MacDonald DG. Routes of entry of squamous cell carcinoma to the mandible. Head Neck Surg. 1988;10(5):294-301.
110 Shingaki S, Nomura T, Takada M, et al. Squamous cell carcinomas of the mandibular alveolus: analysis of prognostic factors. Oncology. 2002;62(1):17-24.
111 Eicher SA, Overholt SM, el-Naggar AK, et al. Lower gingival carcinoma. Clinical and pathologic determinants of regional metastases. Arch Otolaryngol Head Neck Surg. 1996;122(6):634-638.
112 Overholt SM, Eicher SA, Wolf P, et al. Prognostic factors affecting outcome in lower gingival carcinoma. Laryngoscope. 1996;106(11):1335-1339.
113 Byers RM, Newman R, Russell N, et al. Results of treatment for squamous carcinoma of the lower gum. Cancer. 1981;47(9):2236-2238.
114 Lo K, Fletcher GH, Byers RM, et al. Results of irradiation in the squamous cell carcinomas of the anterior faucial pillar-retromolar trigone. Int J Radiat Oncol Biol Phys. 1987;13(7):969-974.
115 Ayad T, Gelinas M, Guertin L, et al. Retromolar trigone carcinoma treated by primary radiation therapy: An alternative to the primary surgical approach. Arch Otolaryngol Head Neck Surg. 2005;131(7):576-582.
116 Huang CJ, Chao KS, Tsai J, et al. Cancer of retromolar trigone: long-term radiation therapy outcome. Head Neck. 2001;23(9):758-763.
117 Byers RM, Anderson B, Schwarz EA, et al. Treatment of squamous carcinoma of the retromolar trigone. Am J Clin Oncol. 1984;7(6):647-652.
118 Mendenhall WM, Morris CG, et al. Retromolar trigone squamous cell carcinoma treated with radiotherapy alone or combined with surgery. Cancer. 2005;103(11):2320-2325.
119 New G, Hallberg O. The end results of the treatment of malignant tumors of the palate. Surg Gynecol. 1941;73:520.
120 Chierici G, Silverman SJr, Forsythe B. A tumor registry study of oral squamous carcinoma. J Oral Med. 1968;23(3):91-98.
121 Evans JF, Shah JP. Epidermoid carcinoma of the palate. Am J Surg. 1981;142(4):451-455.
122 Chung CK, Johns ME, Cantrell RW, et al. Radiotherapy in the management of primary malignancies of the hard palate. Laryngoscope. 1980;90(4):576-584.
123 Yorozu a, Sykes AJ, Slevin NJ. Carcinoma of the hard palate treated with radiotherapy: a retrospective review of 31 cases. Oral Oncol. 2001;37(6):493-497.
124 Kovalic JJ, Simpson JR. Carcinoma of the hard palate. J Otolaryngol. 1993;22(2):118-120.
125 Vanderpuye V, Goldson A. Osteoradionecrosis of the mandible. J Natl Med Assoc. 2000;92(12):579-584.
126 Fujita M, Hirokawa Y, Kashiwado K, et al. An analysis of mandibular bone complications in radiotherapy for T1 and T2 carcinoma of the oral tongue. Int J Radiat Oncol Biol Phys. 1996;34(2):333-339.
127 Ben-David MA, Diamante M, Radawski JD, et al. Lack of osteoradionecrosis of the mandible after intensity-modulated radiotherapy for head and neck cancer: Likely contributions of both dental care and improved dose distributions. Int J Radiat Oncol Biol Phys. 2007;68(2):396-402.
128 Gomez DR, Zhung JE, Gomez J, et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys. 2008.
129 Jabbari S, Kim HM, Feng M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: Initial report. Int J Radiat Oncol Biol Phys. 2005;63(3):725-731.
130 Nguyen NP, North D, Smith HJ, et al. Safety and effectiveness of prophylactic gastrostomy tubes for head and neck cancer patients undergoing chemoradiation. Surg Oncol. 2006;15(4):199-203.
131 Wiggenraad RG, Flierman L, Goossens A, et al. Prophylactic gastrostomy placement and early tube feeding may limit loss of weight during chemoradiotherapy for advanced head and neck cancer, a preliminary study. Clin Otolaryngol. 2007;32(5):384-390.


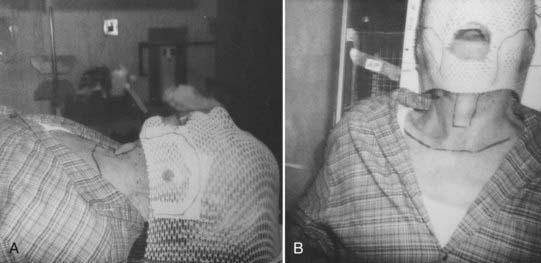
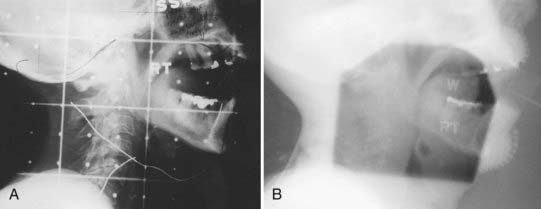
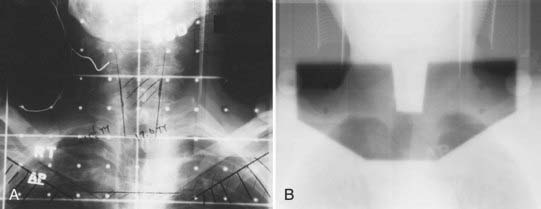
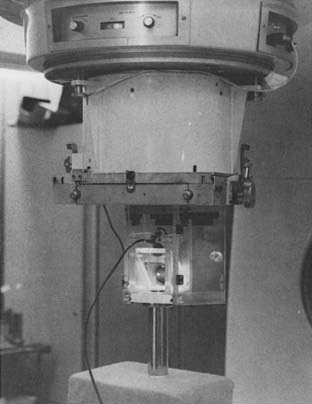
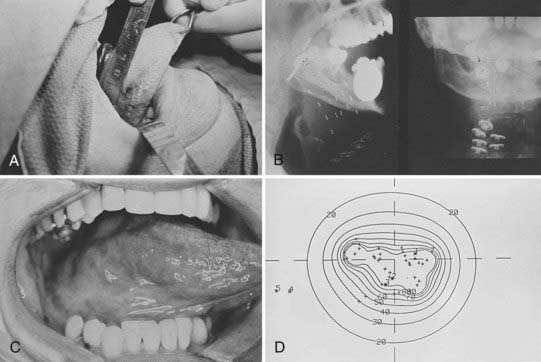
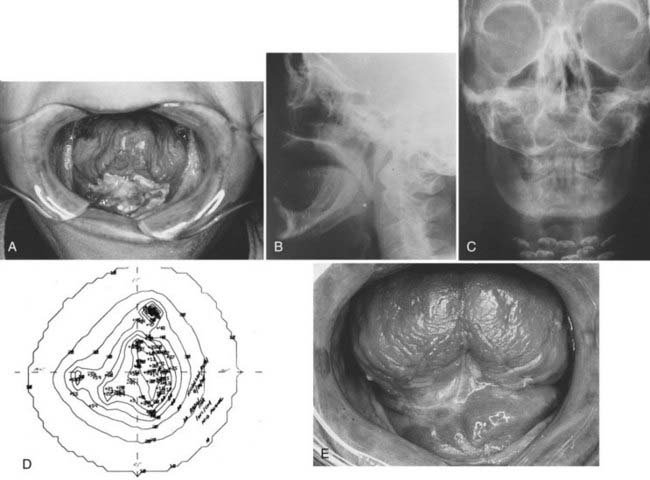
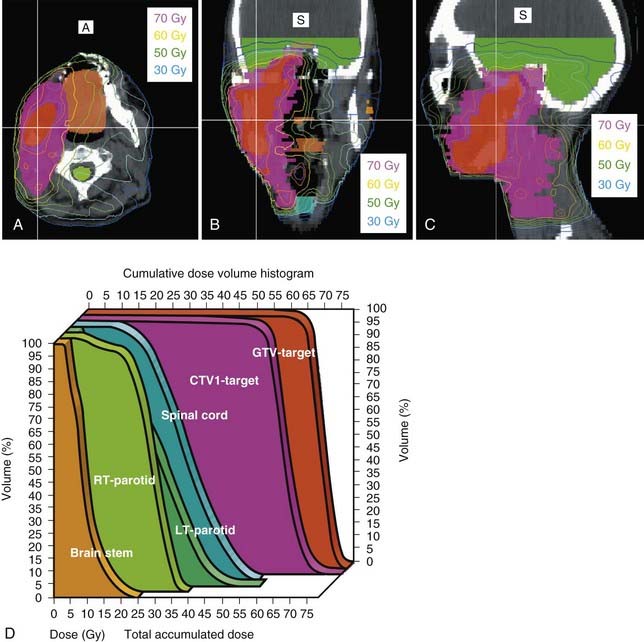
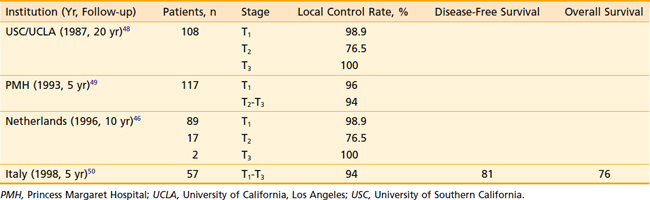
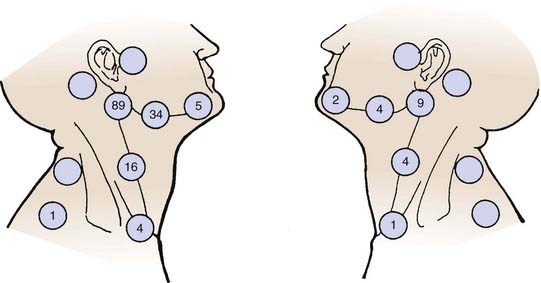
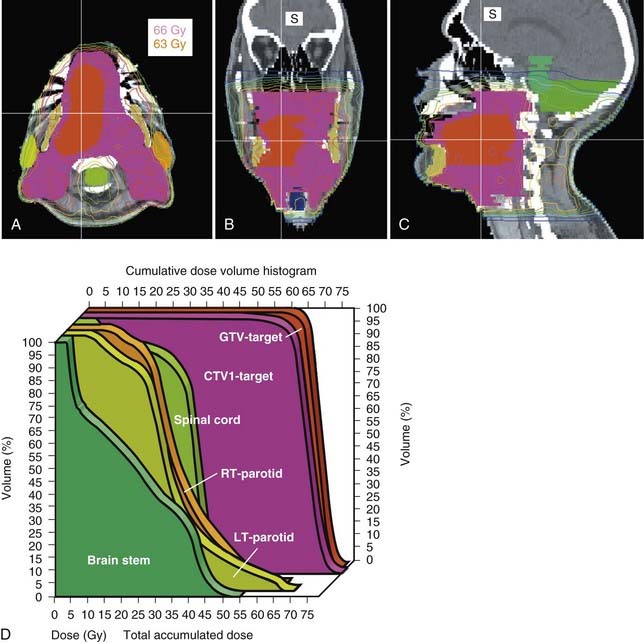
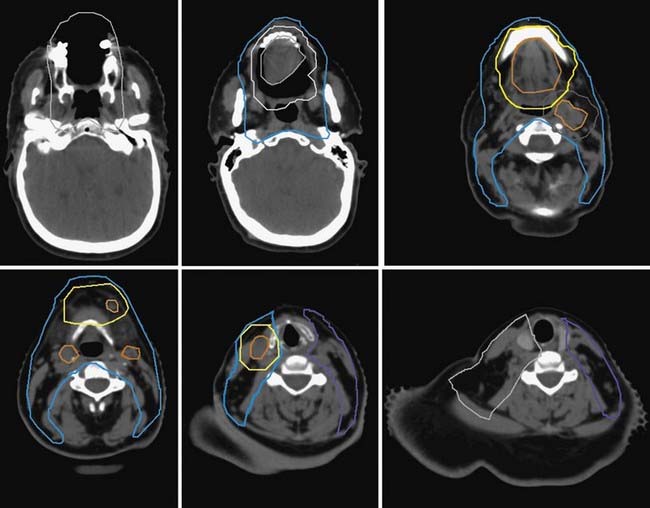
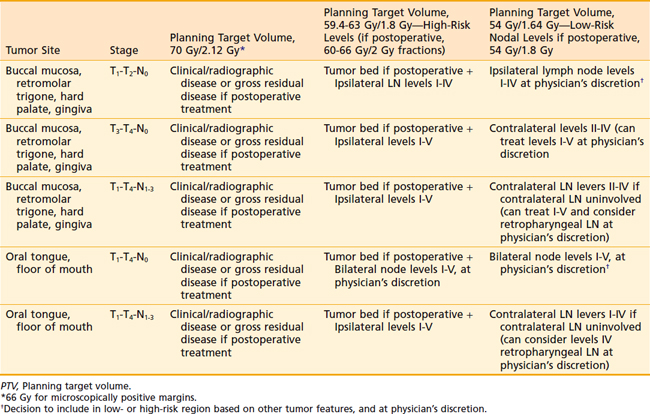
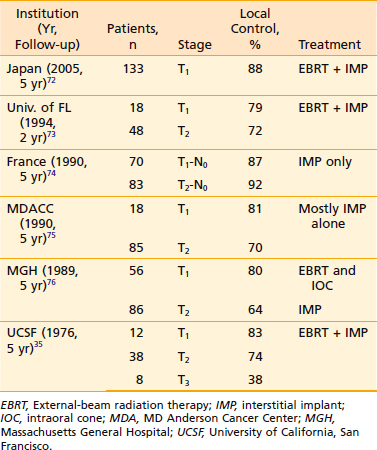
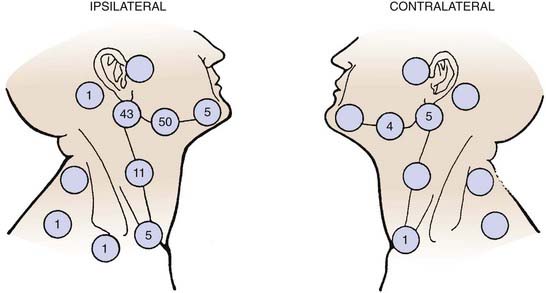
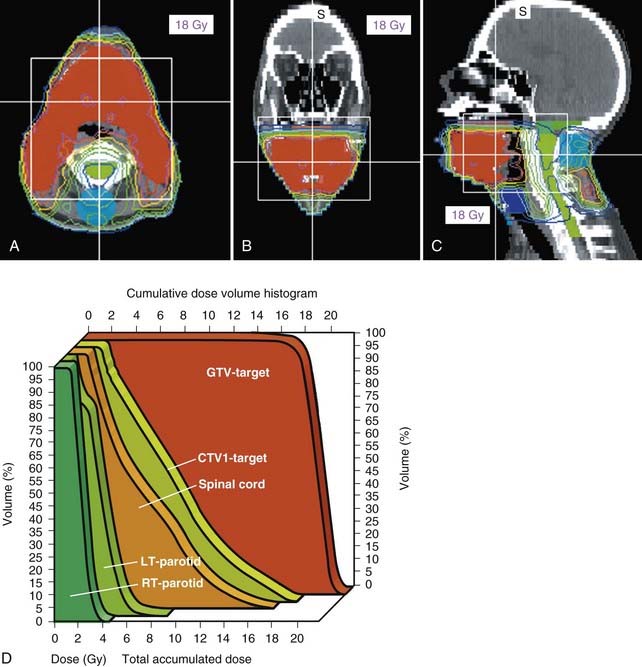
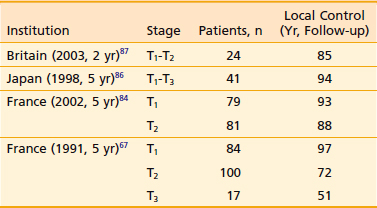
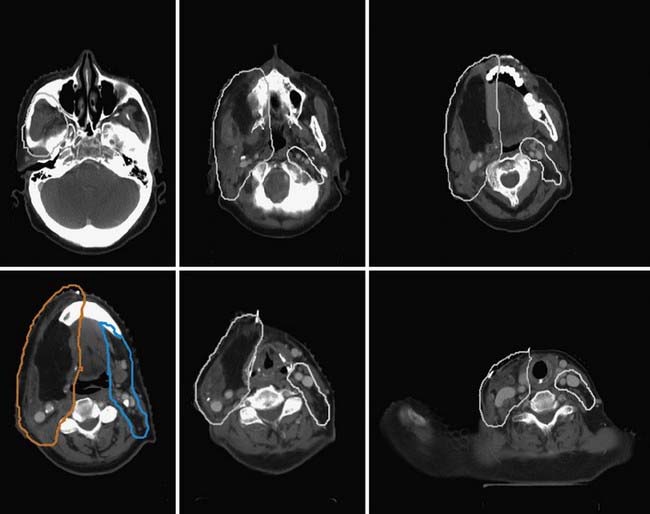
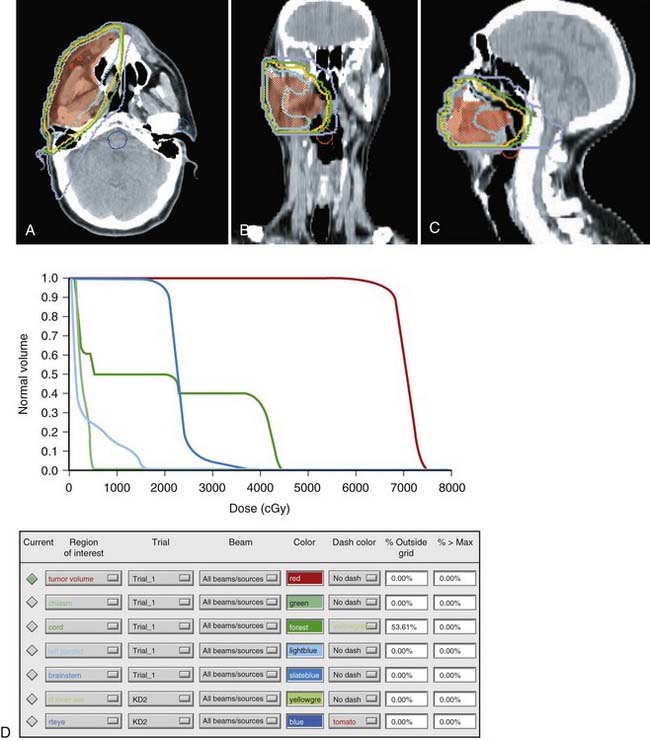
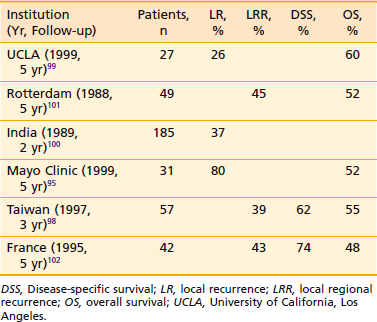
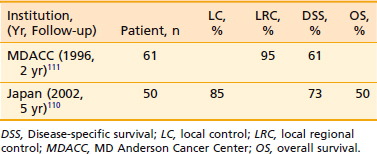
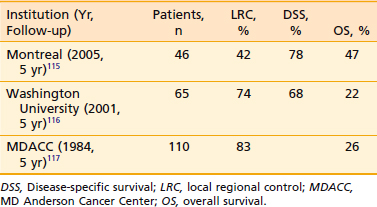
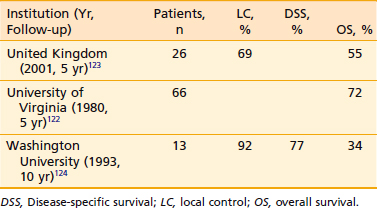
 to 1 teaspoon of salt and
to 1 teaspoon of salt and  to 1 teaspoon of baking soda in 1 quart of warm water) three to four times daily. Moniliasis is a common complication during irradiation of the head and neck. This can be effectively controlled with systemic antifungal agents. Once the patient has been placed on an agent, it is important to maintain its use until the completion of radiotherapy. Otherwise, moniliasis usually recurs if treatment is discontinued during the course of radiotherapy.
to 1 teaspoon of baking soda in 1 quart of warm water) three to four times daily. Moniliasis is a common complication during irradiation of the head and neck. This can be effectively controlled with systemic antifungal agents. Once the patient has been placed on an agent, it is important to maintain its use until the completion of radiotherapy. Otherwise, moniliasis usually recurs if treatment is discontinued during the course of radiotherapy.