27 Cancer of the Nasopharynx
Epidemiology
Nasopharyngeal carcinoma (NPC) is uncommon in most parts of the world. The incidence is highest among people from southern China, particularly those originating from Kwantung Province, followed by the Chinese mixed populations of Southeast Asia and indigenous populations in Alaska.1,2 It is of intermediate incidence in North Africa and in the Philippines and it is rare among whites and Japanese. The age-adjusted incidence rate (per 100,000 population per year) ranges from 28.8 in Hong Kong, 17.2 in indigenous populations in Alaska, 16.8 in Singapore, 4.6 in the Philippines, 2.8 in Algeria, and 0.6 in the United States and Japan.3,4 The peak incidence occurs in the fourth and fifth decade of life, and the male/female ratio is 2 : 1 to 3 : 1.
Causal Factors
The cause of NPC is most likely multifactorial. Current epidemiologic and experimental data suggest at least three major causal factors: (1) viral, (2) genetic, and (3) environmental. Epstein-Barr virus (EBV) has long been associated with NPC. Antibody titers to EBV have been found to be elevated in NPC patients regardless of their ethnic and geographic origin. The EBV genome has been demonstrated by nucleic acid hybridization in biopsies from NPC. Recent studies show that EBV nuclear antigen was expressed in nearly all cases and that latent membrane protein was expressed in approximately two-thirds of cases of EBV-positive NPCs.5 Using real-time polymerase chain reaction technology, Lo6 was able to detect circulating EBV deoxyribonucleic acid (DNA) in 96% of NPC patients in Hong Kong. The high incidence of NPC among southern Chinese and southeast Asian populations of southern Chinese descent suggests a genetically determined susceptibility. Highly significant differences in histocompatibility human leukocyte antigen (HLA) patterns have been found between Chinese NPC patients and control subjects. There is a significant increase of HLA-A2 and HLA-B-SIN2 in Chinese patients.7 However, the high-risk HLA pattern is not present in all NPC patients and such a pattern is present in some individuals with no NPC. The decreased incidence of NPC in successive generations of Chinese-born populations in the United States8 suggests a role for environmental factors in the causal factors of this disease. Various environmental factors such as poor ventilation, occupational exposures to smoke or dusts, and diet have been implicated. The ingestion of salted fish during early childhood has been suggested as the most important environmental factor among the southern Chinese with NPC.2,9,10 Dimethylnitrosamine, a carcinogen found in salted fish, has been shown to induce carcinoma in the upper respiratory tract of rats.9
Anatomy
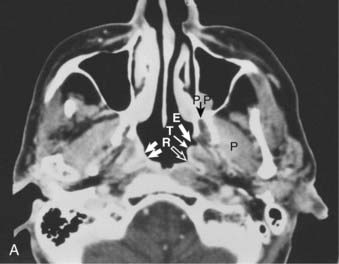
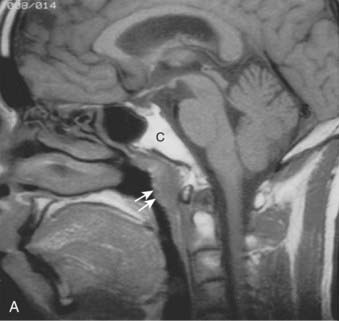
Anteriorly, the nasopharynx is in continuity with the nasal cavity through the posterior choanae, which are separated by the nasal septum. The posterior wall lies at the level of the first two cervical vertebrae and is continuous with the roof. The roof is formed by the basisphenoid, basiocciput, and the anterior arch of the atlas. The lymphoid tissue in this area forms the pharyngeal tonsil (adenoids) (Fig. 27-1). Each of the lateral walls contains the eustachian tube orifice, which is surrounded by the torus tubarius (see Fig. 27-1), a prominence in the cartilaginous portion of the tube. Behind the torus tubarius is a recess called the Rosenmüller fossa (see Fig. 27-1 and 27-2). The lateral walls, including the Rosenmüller fossa, are the most common sites of origin of NPC. The floor of the nasopharynx consists of the upper surface of the soft palate and communicates with the oropharynx via the pharyngeal isthmus. The isthmus is bounded by the uvula anteriorly, the palatopharyngeal arches laterally, and the posterior pharyngeal wall posteriorly.
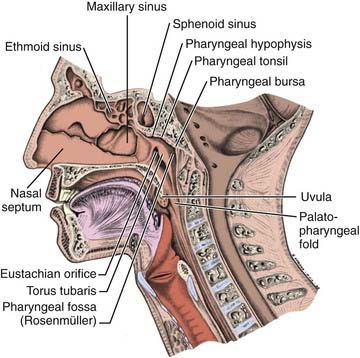
FIGURE 27-1 • Mid-sagittal section of the head showing the nasopharynx and related structures.
(From MacComb WS, Fletcher GH: Cancer of the head and neck, Baltimore, 1967, Williams & Wilkins, p 154.)
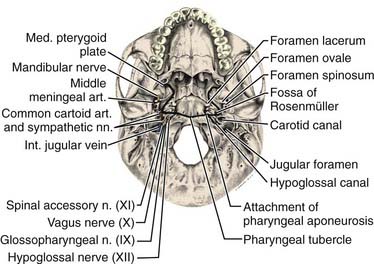
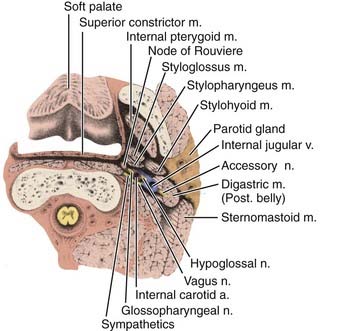
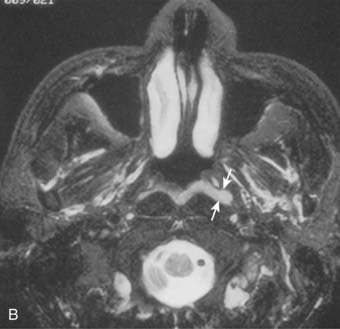
The pharyngeal fascia of the posterior and lateral walls of the nasopharynx is attached to the pharyngeal tubercle on the basiocciput just in front of the foramen magnum. It extends laterally on each side to the ridge on the undersurface of the petrous pyramid, just in front of the carotid canal and anteriorly to the apex of the petrous portion of the temporal bone, and then to the posterior border of the medial pterygoid plate. This fascia is continuous with the fibrous tissue occupying the foramen lacerum, which is separated from the middle cranial fossa only by fibrocartilaginous tissue. Five other foramina are adjacent to the wall of the nasopharynx: the foramen ovale, the foramen spongiosum, the carotid canal, the jugular foramen, and the hypoglossal canal (Fig. 27-3). The foramen lacerum and the foramen ovale constitute the petrosphenoidal crossway, providing an easy pathway into the cranium, and are in close anatomic relationship to the cavernous sinus and hence the II, III, IV, and VI cranial nerves, and the trigeminal ganglion of the fifth nerve and its branches.
Anterior to the eustachian tube, the lateral wall of the nasopharynx is in close relation with the maxillopharyngeal space, which is limited laterally by the ascending ramus of the mandible. In this space is the mandibular nerve descending from the foramen ovale. Posterior to the eustachian tube, Rosenmüller fossa is in close relationship with the lateral pharyngeal or retroparotidian space. The lateral pharyngeal or retroparotidian space is limited anteriorly by the parotid gland and the styloid process and its muscles, posteriorly by the transverse process of the first cervical vertebra, and laterally by the sternocleidomastoid muscle. It contains the lateral pharyngeal nodes, including the lateral retropharyngeal node of Rouvière; the internal carotid artery; the internal jugular vein; and the glossopharyngeal, vagus, spinal accessory, and hypoglossal nerves, as well as the cervical sympathetic nerves as they emerge from the base of skull (Fig. 27-4).
Lymphatic Drainage
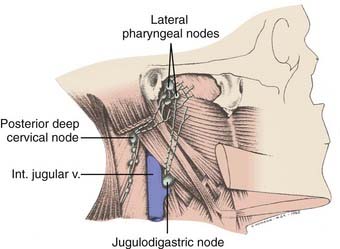
The nasopharynx has a rich lymphatic plexus, particularly in the roof and in the posterior and lateral walls. The lymphatics from the nasopharynx have three major pathways (Fig. 27-5):
Pathologic Conditions
The majority of malignant nasopharyngeal tumors (80% to 99%) arise from the epithelium and should be considered as variants of squamous cell carcinoma. According to the most commonly used World Health Organization (WHO) classifications, carcinomas of the nasopharynx are classified into three histologic types: (1) squamous cell carcinoma, (2) nonkeratinizing carcinoma, and (3) undifferentiated carcinoma.11 In 1991 the WHO reclassified NPC into the following histologic subtypes: (1) keratinizing squamous cell carcinoma, (2a) nonkeratinizing carcinoma, and (2b) undifferentiated carcinoma.12 The histologic distinctions among these three types are by no means sharp. Some lesions share intermediate features and some are histologic hybrids. The term lymphoepithelial carcinoma or lymphoepithelioma is used to describe nonkeratinizing and undifferentiated NPCs in which numerous lymphocytes are found among the tumor cells. The distribution of WHO histopathologic types varies with geography, race, and national origin.13 Asians have the highest proportion of WHO types 2 and 3. In Hong Kong, approximately 3% are type 1, 9% are type 2, and 88% are type 3.14 In North America, approximately 20% of NPCs are type 1, 10% are type 2, and 70% are type 3.15 WHO type 1 includes 75% of the U.S. NPC cases and are found most often in U.S.-born, non-Hispanic whites. WHO types 2 and 3 compose the remaining 25% and are more common in Asians. Other uncommon malignant tumors found in the nasopharynx include adenocarcinoma, lymphoma, plasmacytoma, melanoma, and sarcomas.
Clinical Presentation
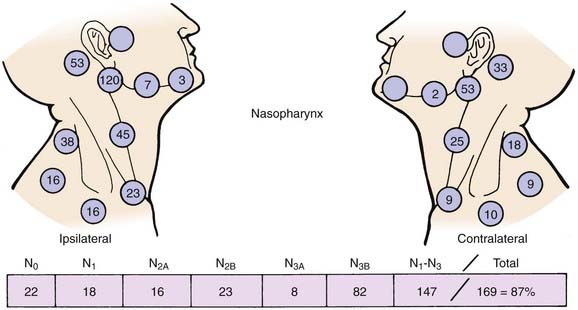
A neck mass is the most frequent presenting symptom and sign in carcinoma of the nasopharynx.16 Other common symptoms include epistaxis, decreased hearing, nasal obstruction, pain, and cranial nerve deficits. Cervical lymphadenopathy is present in 87% of patients.17 Typically, a mass is visible in the upper posterior neck and palpable beneath the superior aspect of the sternocleidomastoid muscle at the tip of the mastoid. This is due to metastasis in the parapharyngeal or superior posterior cervical nodes of the spinal accessory chain or both. Bilateral cervical lymph node metastases occur in approximately 50% of cases. The most frequently involved nodes are the upper posterior cervical, parapharyngeal, and jugulodigastric nodes. The midjugular and midposterior cervical nodes are the next most commonly involved, followed by the lower jugular and supraclavicular nodes. The submental nodes and occipital nodes are occasionally involved, usually in the presence of massive cervical lymphadenopathy in the more commonly involved neck sites. Rarely, patients present with metastatic preauricular nodes. Nasal voice occurs as a consequence of nasal obstruction, loss of nasopharyngeal resonance, and mechanical interference with normal movement of the soft palate. Nasal discharge, epistaxis, and coughing up of blood-tinged sputum from postnasal drip are symptoms due to local tumor effect. Impaired hearing of the conductive type, usually unilateral with or without tinnitus and serous otitis media, occurs as a result of obstruction, usually by compression rather than direct extension, of the eustachian tube orifice by the primary tumor.
Distant metastasis at presentation is detected in approximately 3% of cases.18 The bones, lungs, and liver are the most common distant metastatic sites.
Routes of Spread
Local Extension
Superior and Posterior
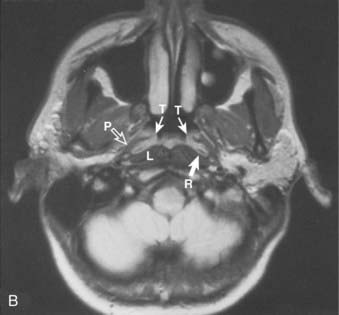
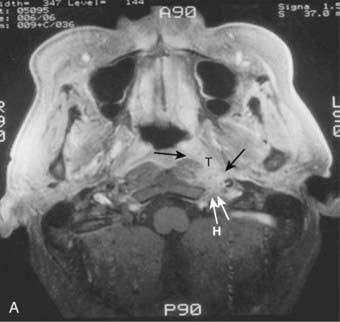
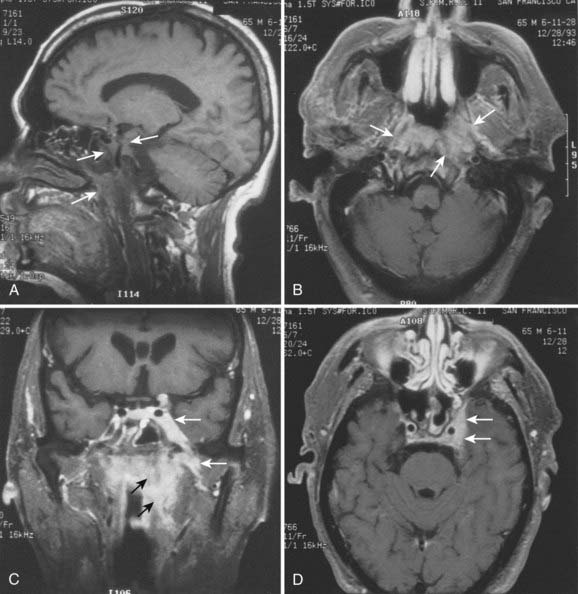
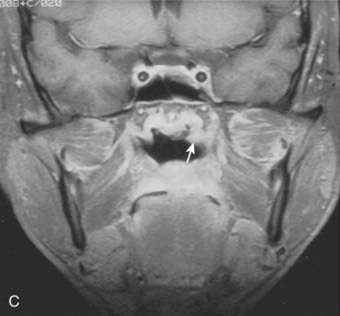
Superiorly and posteriorly, the tumor can directly invade the base of the skull, the sphenoid sinus, and the clivus. The foramen lacerum, located directly above Rosenmüller fossa, is a weak spot in the base of the skull, through which the tumor can gain access into the cavernous sinus and the middle cranial fossa and invade cranial nerves II to VI. Tumor may also invade through the foramen ovale into the middle cranial fossa, the petrous part of the temporal bone, and the cavernous sinus. Invasion of the prevertebral (longus capitus) muscles posteriorly is commonly seen on magnetic resonance imaging (MRI) scans (Fig. 27-6).
Lateral
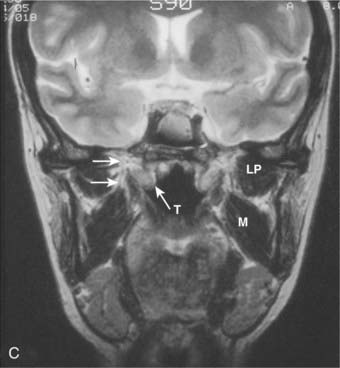
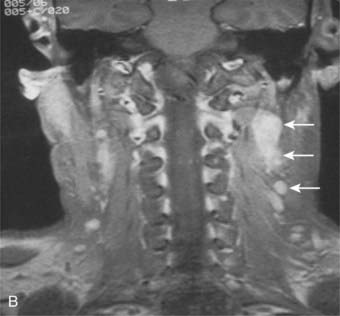
Lateral spread into the lateral parapharyngeal space and invasion of the levator and tensor veli palatini muscles occur early and are commonly seen on MRI scans (see Fig. 27-6 and Fig. 27-7). Invasion of the pterygoid muscles occurs in more advanced disease. Direct tumor extension or lateral retropharyngeal lymph node metastasis in the parapharyngeal space can lead to compression or invasion of cranial nerve XII as it exits through the hypoglossal canal, cranial nerves IX to XI as they emerge from the jugular foramen and the cervical sympathetic nerves. Compression or direct invasion of the internal carotid artery can also occur in advanced disease. Through the eustachian tube, tumor can directly invade the middle ear.
Lymphatic Spread
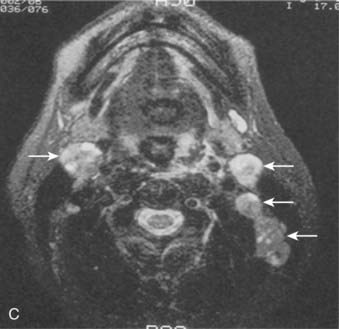
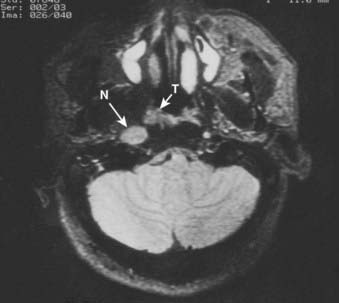
Lymphatic spread to the ipsilateral nodes is common and is present in 85% to 90% of cases.17 Bilateral spread occurs in approximately 50% of cases. Metastasis to the contralateral nodes only is uncommon. The distribution of clinically palpable nodes at diagnosis is shown in Fig. 27-8. Spread to the lateral and posterior retropharyngeal lymph nodes occurs early and is frequently seen on MRI or computed tomography (CT) scans, although the nodes are not palpable (Fig. 27-9). Metastasis to the jugulodigastric and superior posterior cervical nodes is also common. From these first echelon nodes, further metastasis to the midjugular and posterior cervical, lower jugular, and posterior cervical and supraclavicular nodes can occur. Occasionally, spread to the submental and occipital nodes occurs as a result of lymphatic obstruction caused by extensive cervical lymphadenopathy. Metastasis to the mediastinal lymph nodes may occur when supraclavicular lymphadenopathy is present, and occasionally there is metastasis to the axillary nodes as well. Distant lymph node metastasis is often reported in autopsy series, but is rarely apparent clinically at diagnosis.
Hematogeneous Spread
Distant metastasis is present in 3% of the cases at diagnosis and may occur in 18% to 50% or more of cases during the course of the disease.19–23 An 80% incidence rate has been reported in a small autopsy series.24 The incidence of distant metastasis is highest in patients with advanced neck node metastasis,19,25–27particularly in the low neck.28,29 Bone is the most common distant metastatic site followed by the lungs and liver.
Diagnostic and Staging Studies
The following pretreatment diagnostic evaluations are recommended:
Both MRI and CT scans are useful in diagnostic imaging of the nasopharynx, as well as in radiotherapy treatment planning. However, MRI is the imaging technique of choice in the staging evaluation of NPC.30–32 Because the current T classification system requires a search for tumor invasion into the soft tissue, such as parapharyngeal space, and bony structures, MRI may be necessary for proper staging because CT has limitations in accurately defining the tumor extent in these regions.33 MRI is capable of multiplanar display of tumor extent and is superior to CT scans in delineating muscle, other soft tissue involvement, and evaluating the skull base (see Fig. 27-6, Fig. 27-7, and Fig. 27-10).33–35 In post-treatment follow-up examinations, it has the ability to differentiate radiation fibrosis from persistent or recurrent tumor based on T2-weighted signal intensities and with gadolinium enhancement and fat suppression.36 MRI and CT scans can also detect lymph node metastasis that may not be evident on clinical examination37 (see Fig. 27-9). CT scan is superior to MRI in the detection of early bone invasion.32,34 CT scan with bone windows is useful to demonstrate the extent of invasion of the base of the skull or cervical vertebrae, although MRI is preferable. The major limitation of CT is its poor tissue differentiation, which may be problematic in the evaluation of patients after radiotherapy.35
PET CT scanning has been used more frequently recently in the staging of nasopharyngeal cancer patients. Preliminary data suggest that the standardized uptake value (SUV) may be predictive for failure at distant sites.38,39 PET CT is now commonly used in lieu of conventional staging with CT, bone scans, and ultrasound studies, and appears to be at least as sensitive, but large comparative studies of these staging modalities are not available.40,41
The close association between EBV and NPC, regardless of geographic and ethnic background, has provided a tumor marker for the diagnosis of this disease. Both IgA anti-VCA and IgG anti-early antigen (EA) antibodies are sensitive for the diagnosis of NPC, although IgA anti-VCA is more specific.15 Elevated IgA antibody titers have been reported in more than 90% of untreated patients with NPC from Hong Kong, East Africa, and California.42–44 Positive IgA anti-VCA and IgG anti-EA serologies are primarily associated with WHO type 2 and type 3 NPC. Elevated IgA anti-VCA antibody titers were detected in 82%, and IgG anti-EA antibody titers were detected in 86% of patients with nonkeratinizing carcinoma and undifferentiated carcinomas, but in only 16% and 35%, respectively, of patients with squamous cell carcinomas in a North American study.15 Elevated serum IgA anti-VCA antibodies may be demonstrated in patients months before the onset of symptoms and may serve as a screening test in high-risk patients.45,46 Quantification of plasma EBV DNA can be useful at baseline as it appears to add to the prediction of outcome and levels over time can be used as a post-treatment surveillance tool.47,48
Staging Classification
Various staging systems have been formulated for NPC.9,49–55 The most widely used systems in the English literature are the American Joint Committee on Cancer (AJCC), Union International Cancer Centre (UICC), and Ho systems. The latest versions of the AJCC and UICC (2002) systems are virtually identical. Table 27-1 shows the AJCC and Ho systems currently in use. Each system has its limitations. In the AJCC and UICC systems, the designation of T1 or T2, which is based on the extent of involvement within the nasopharynx, is not meaningful and carries no prognostic significance.53,56 In the 2002 version of the AJCC staging system with regard to T2 lesions, it is divided into with or without parapharyngeal involvement, which has prognostic significance.57 Those with parapharyngeal space involvement are at higher risk for local and regional recurrence, as well as a high rate of distant metastases.58 Cranial nerve involvement has been shown to carry a worse prognosis than base of the skull involvement27,28,51,59; however, both are included in the T4 classification in the AJCC (2002) and the UICC systems and the T3 in Ho’s system. With respect to N-stage classification, the Ho system, which is based on the level or location of neck node involvement, appears to be superior to the AJCC and the UICC system, which are based on size, number, and laterality of the lymph nodes involved.10,28,53 Because of these limitations, modifications of both Ho’s and AJCC classification have been proposed and are shown in Table 27-1. Retrospective comparison of the 1997 or the 2002 AJCC with the 1988 AJCC classification in patients staged with CT or MRI scans suggest a more even stage distribution and better correlation with prognosis with the 1997 or the 2002 AJCC classification.60 In the 2010 AJCC staging manual, T1 patients include those who have disease confined to the nasopharynx, or tumor extending to oropharynx and/or nasal cavity without parapharyngeal extension. Tumor with parapharyngeal extension is now staged as T2. There is no longer a subdivision between T2a and T2b disease. The former T2a disease is now down staged as T1 disease in this new staging system.61 With regard to N staging, because retropharyngeal nodes are the first echelons of nodal metastases,62 retropharyngeal lymph node involvement independent of laterality and without cervical lymph node involvement has been proposed as stage N1.61 The adequacy of these new modified staging classifications will require further clinical confirmation.
Standard Therapeutic Approaches
Although surgery is not used in the treatment of the primary tumor, radical neck dissection is the treatment of choice for resectable neck disease that is persistent or recurrent after radiotherapy.63,64 Surgery has also been used to salvage selected patients with locally recurrent disease.65–68
Concurrent chemoradiotherapy using cisplatin (CDDP)-based chemotherapy is the current standard for patients with NPC with stage III or IV (nonmetastatic) disease. Because of the known chemosensitivity of NPC, a series of randomized controlled trials of chemoradiotherapy versus radiotherapy conducted in the 1990s and early 2000s demonstrated that in patients with advanced local or regional disease, the addition of concurrent chemotherapy to radiation was associated with a statistically significant and clinically meaningful survival advantage.69–71 Notably, a phase III trial by the Head and Neck Intergroup in the United States compared radiotherapy alone with radiotherapy plus concurrent and adjuvant chemotherapy for stage III and IV disease.69 Chemotherapy consisted of CDDP 100 mg/m2 every 3 weeks during radiotherapy and CDDP 80 mg/m2 plus 5-fluorouracil (5-FU) infusion 1000 mg/m2/day, for 4 days every 4 weeks after the completion of radiotherapy. Radiotherapy was delivered at 1.8 to 2 Gy per fraction per day, 5 days a week, to a total dose of 70 Gy. An update of the Intergroup trial72 showed that at 5 years, the overall survival was 37% versus 67% (P < 0.001) and progression-free survival was 29% versus 58% in favor of the combined modality arm (P < 0.001). Significant differences were found in the overall survival and progression-free survival between the histologic types for all patients treated. The overall survival was 37% for WHO I, 55% for WHO II, and 60% for WHO III (P < 0.001). A smaller subsequent trial suggested that even in patients with advanced local disease but minimal nodal disease (T3-4N0-1), local control increased with the use of combined chemotherapy and accelerated fractionation radiation versus conventional radiation alone.73 A substantial reduction in both locoregional recurrence and metastatic disease in the combined treatment arms versus radiation alone has been observed in these combined modality trials.
A common theme to all of these chemotherapy regimens is that in all cases the cumulative dose of concurrent CDDP exceeded 180 mg/m2. However, in the U.S. Intergroup trial, although three cycles of 100 mg/m2 of CDDP during radiotherapy were intended, only 63% of the patients received all three cycles. In the trial by Chan et al. in which CDDP was administered weekly to an anticipated total of 240 mg/m295% of the patients were compliant with the plan.74 It appears that weekly dosing is more tolerable and in many cases dosing beyond a cumulative dose of 200-240 mg/m2 is not feasible. In addition, of the three randomized controlled trials discussed, only one, the U.S. Intergroup study, administered adjuvant CDDP and 5-FU. Only approximately half of the patients were able to receive all three cycles, and one-third of the patients randomized to that arm received no adjuvant treatment. Therefore, because all three randomized trials showed an overall survival advantage for patients with advanced disease, it is not clear that adjuvant CDDP and 5-FU has benefit beyond concurrent chemoradiation, nor is it clear that administration of CDDP and 5-FU after chemoradiation is feasible.
A recent study directly compared CDDP versus carboplatin (AUC 5) in the curative setting in 206 patients with NPC who were receiving concurrent radiation.75 In this study, the standard U.S. Intergroup regimen of concurrent plus adjuvant chemoradiation was compared with an identical radiation plan with concurrent AUC 5 100 mg/m2 weekly instead of CDDP, and AUC 5 instead of CDDP in the adjuvant setting.75 There was no difference in overall survival or disease-free survival. Toxicity was markedly less in the AUC 5 arm, and more than twice as many patients in the AUC 5 arm (62% versus 26%) completed all intended chemotherapy treatment. Therefore, although CDDP is still considered the standard, substitution of AUC 5 in patients who are ineligible for CDDP because of renal or neurologic compromise is an alternative treatment approach and warrants further study.
Chemotherapy has been used as induction or neoadjuvant therapy before radiotherapy or as an adjuvant after radiotherapy in the treatment of advanced NPC.76–80 Thus far, prospective randomized trials comparing neoadjuvant or adjuvant chemotherapy combined with radiotherapy to radiotherapy alone have not shown a survival benefit, despite diminution of metastatic relapse rates.81–83 In addition, several meta-analyses did not show a benefit of neoadjuvant chemotherapy in the treatment of NPC.84–86 However, some investigators argue that most of these trials either used treatment regimens regarded as outmoded today or that were underpowered, particularly with respect to induction chemotherapy. There are ongoing large randomized controlled trials designed to definitively answer the question concerning the possible benefit of the addition of CDDP and 5-FU induction chemotherapy to concurrent chemoradiation in patients with NPC. Lee et al.87 of the Hong Kong Nasopharyngeal Cancer Study Group are conducting a trial comparing induction chemotherapy with CDDP and 5-FU versus adjuvant chemotherapy with CDDP and 5-FU in the setting of concurrent chemoradiation as a backbone. This trial, which opened in September 2006, is planning to enroll 798 patients with an estimated completion date of September 2013. Feng et al.,88 of the Taiwan National Health Research Institutes, opened in 2003 a multicenter phase III trial comparing induction chemotherapy with mitomycin, epirubicin, CDDP, fluorouracil, and leucovorin followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in stage IV NPC. This is based on the results of a phase II trial showing a 5-year overall survival of 70% and a 5-year distant metastasis rate of 81% with the induction chemotherapy.89 This trial plans to enroll 480 patients and is expected to be completed in 2013. Therefore, the question of the role of induction plus concurrent chemoradiation using CDDP-based chemotherapy in patients with NPC, although still open, will hopefully be answered in the next 5 years by these two randomized trials, at least for the specific chemotherapy regimens described previously.
Chemotherapy alone or combined with reirradiation is also used in the palliation of recurrent or metastatic disease. Complete response rates of 15% to 44% have been achieved with combination chemotherapy in recurrent or metastatic NPC.76,78,81,90 In general, recurrent NPC is uniformly more responsive to reirradiation when compared with recurrent disease arising from other head and neck cancer sites.91,92
Intensity-Modulated Radiation Therapy
Intensity-modulated radiation therapy (IMRT) has replaced conventional radiotherapy in the treatment of NPC in an increasing number of institutions in the United States as well as throughout the world. With this technique, the intensity of the radiation beams can be modulated so that a high dose can be delivered to the tumor with an improved target volume coverage while significantly reducing the dose to the surrounding normal tissues.93–98 Since the initial publication99 along with the update on the use of IMRT for NPC from the University of California–San Francisco (UCSF),97 several institutions have also implemented this technique as well as reported excellent treatment outcomes.100–106 The following describes the simulation and CT treatment planning procedures.
Simulation and Treatment Planning Computed Tomography Procedures
The patient’s head position should be hyperextended at the initial simulation so that there is adequate separation between the primary and retropharyngeal and the upper neck nodes.97 The tip of the uvula and the base of the occiput should be on a plane parallel to the beam axis. The head, neck, and shoulders are immobilized using a thermoplastic mask with the neck supported on a carbon-fiber head support94,107 (Fig. 27-11). A pair of orthogonal radiographs are taken for isocenter localization at the initial simulation. The isocenter on the initial simulation film is placed at the anticipated treatment isocenter. Treatment planning CT scans are then obtained. In the region that contains the primary gross tumor volume (GTV), CT scan slice thickness should be 3 mm or less. The regions above and below the primary tumor target volume may be scanned with slice thickness of 5 mm.
Treatment Volume
The lymph node groups at risk are outlined on the treatment planning CT scans according to image-based nodal classification.108–111 The surrounding critical normal structures, including the brainstem, spinal cord, optic nerves, chiasm, parotid glands, pituitary, temporomandibular (TM) joints, middle and inner ears, and skin should also be outlined on the treatment planning CT scans.
Treatment Planning and Delivery
Various treatment planning systems are available commercially.112–115 At the Memorial Sloan-Kettering Cancer Center (MSKCC), an in-house treatment planning software is used to plan treatment for all patients undergoing IMRT.116,117 Treatment is delivered using linear accelerators equipped with a computer-controlled auto-sequence multileaf collimator (MLC) system using a step-and-shoot approach, a multivane dynamic MLC (MIMiC), or a dynamic MLC system using a sliding window approach, or helical tomotherapy.115,118 Detailed descriptions of the treatment planning and delivery methods are provided in Chapter 10.
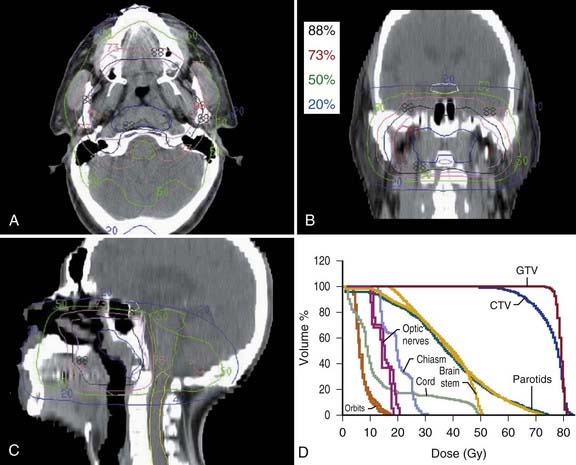
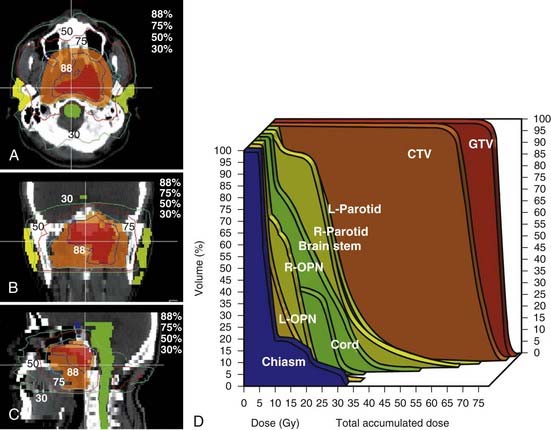
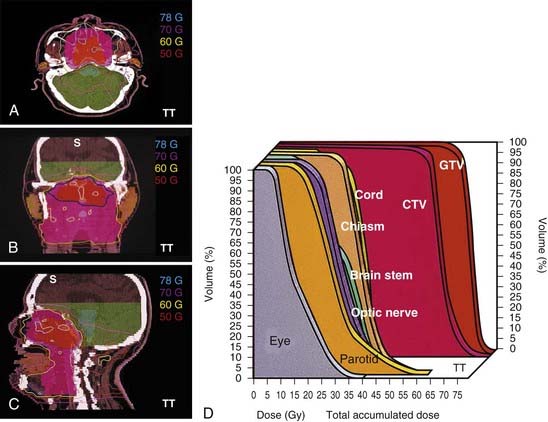
In general, treatment planning is performed with inverse-planning. However, in centers where the expertise is not available, forward planning can also be done.119 Examples of a 10-field forward plan, an inverse peacock plan delivered using the MIMiC collimator, and an inverse corvus plan delivered using MLC are shown in Fig. 27-12, Fig. 27-13, and Fig. 27-14. An example of an MSKCC IMRT plan delivered with the dynamic MLC system using a sliding window approach is shown in Fig. 27-15.
FIGURE 27-15 • An example of a nasopharyngeal cancer patients treated with IMRT delivered with the dynamic MLC system using a sliding window approach.
With increasing use of IMRT in the clinic, questions regarding the optimal treatment technique have been raised. One issue is whether patients should undergo a EWF IMRT technique in which the entire target volume is encompassed in the IMRT plan, or a SF IMRT technique in which the target volumes superior to the vocal cords are treated with an IMRT plan whereas the lower neck nodes are treated with a conventional low-anterior neck field.107,120,121 Debates among radiation oncologists on which IMRT technique is optimal have been on-going. Because of concerns of potential failures at the matchline resulting from underdose or complications resulting from overdose, many centers have implemented EWF IMRT even when no clinically involved lymph nodes are apparent in the region of the matchline. These concerns could be caused by the treatment delivery system that is used at centers where a perfect match between the IMRT fields and the low-anterior neck field is not possible. However, with this technique, an unnecessary dose of radiation is delivered to the normal glottic larynx.122 With the SF IMRT technique, dose to the vocal cords is minimal because they are shielded under the midline Cerrobend block or the MLC. Thus far, no IMRT matchline failures or complications have been reported with the SF IMRT technique.99
Dose and Fractionation
| Brainstem | ≤54 Gy | 60 Gy to 1% of PTV |
| Optic nerves | ≤54 Gy | 60 Gy to 1% of PTV |
| Optic chiasm | ≤54 Gy | |
| Spinal cord | ≤45 Gy | 50 Gy to 1 cc of PTV |
| Mandible | ≤70 Gy | 75 Gy to 1 cc of PTV |
| Temporal lobes | ≤60 Gy | 65 Gy to 1% of PTV |
| Parotid glands | ≤ Mean dose of 26 Gy | |
| Oral cavity | ≤ Mean dose of 40 Gy |
These dose constraints include all scattered and transmitted doses.
Brachytherapy
Brachytherapy with intracavitary insertions123–131 or interstitial implants132–135 has been used as a boost treatment of T1 to T3 carcinoma of the nasopharynx after external-beam irradiation or in the treatment of recurrent disease, either alone or in combination with external-beam irradiation. Because of the rapid falloff of dose with an increase in the distance from the radioactive source, brachytherapy is not suitable for treatment of tumors with intracranial extension. However, since the introduction of IMRT as primary treatment for NPC with excellent local control, the use of brachtherapy as a boost treatment after definitive IMRT has markedly diminished. Various applicators and techniques have been used for the delivery of intracavitary brachytherapy for carcinoma of the nasopharynx.125–127,129,131,136 In the past, intracavitary brachytherapy was delivered using low-dose-rate techniques. More recently, remote afterloading, fractionated high-dose-rate techniques on an outpatient basis are more commonly used.136 In a modification of the technique described by Levendag et al.,137 the mucosa of the nasal cavity and the nasopharynx is first sprayed with a decongestant (0.05% oxymetazoline hydrochloride) and anesthetized with 5% cocaine hydrochloride applied topically with cotton swabs. The soft palate is anesthetized with a spray of benzocaine, tetracaine hydrochloride, butamben, and benzalkonium chloride (Cetacaine). Two 5-French pediatric feeding tubes with an outer diameter of 1.65 mm and an inner diameter of 1.09 mm, respectively, are then introduced into the nasopharynx and withdrawn through the mouth to serve as guide tubes for the Rotterdam nasopharynx applicator. The applicator is then guided intraorally over the pediatric feeding tubes into the nasopharynx and the nasal cavity by pulling the nasal portion of the pediatric feeding tubes. Placement of the applicator into the nasopharynx is facilitated by gentle pushing of the oral portion of the pediatric feeding tubes intraorally with a pair of forceps. After the applicator is positioned in the nasopharynx and the nasal cavity, the pediatric feeding tubes are withdrawn, and the applicator is secured in place with a plastic clamp placed over the external portion of the applicator outside the nose (Fig. 27-16). Dummy iridium-192 sources in afterloading catheters are then inserted into each of the tubes of the applicator. After lead markers are placed on the contralateral outer canthus and tragus, a pair of ortho gonal anterior-posterior and lateral localization x-ray films with the dummy sources in place are obtained (Fig. 27-17). The points of interest, including the nasopharynx, base of skull, node of Rouvière, pituitary, optic chiasm, spinal cord, and the palate points, are marked on the localization films.136 Treatment planning and optimization is then performed with a computer software program (Plato brachytherapy afterloading planning system). A dose of 5 to 6 Gy in two fractions, 5 to 6 hours apart, is prescribed at the isodose curve that passes through the nasopharynx points as a boost treatment for T1 to T2 lesions after 65 Gy of external-beam radiotherapy. The dose for reirradiation of recurrent tumors depends on the previous dose of external-beam radiotherapy. After treatment planning, afterloading catheters are then inserted into the applicator and connected to a remote-controlled, high-dose-rate afterloading machine for treatment delivery. After completion of treatments, the nose clamp and the afterloading catheters are removed. Two 5-French pediatric feeding tubes are reinserted into the applicator and the applicator is withdrawn through the mouth by pushing it into the nose while gently pulling on the feeding tubes exiting the mouth.
Several approaches have been used for permanent interstitial implantation of carcinoma of the nasopharynx at different institutions. They include the transnasal approach133 and the transoral approach132 for lesions in the middle and low posterior wall, implantation of iodine-125 seeds through a transpalatal flap for lesions in the superior or high posterior wall, and the split-palate approach with gold-198 seeds.138
Complications
In the past, because of the large treatment volume and high dose required for NPC in most patients, various complications occurred after conventional radiotherapy.139 The overall complication rate ranged from 31% to 66%.21,27,140,141 Severe complication rates of 6% to 15% and fatal complication rates of 1% to 3% have been reported.21,27,140,141 The complication rates are increased with concurrent chemotherapy141,142 or coexisting medical conditions such as diabetes or hypertension. However, using modern three-dimensional conformal radiotherapy techniques, including IMRT, these complication rates have decreased.96,97,100–102 Xerostomia was by far the most common sequela with conventional radiotherapy. Most patients experience some degree of dry mouth permanently. Dental problems, a consequence of decreased saliva and altered salivary consistency, occurred in 4% to 17% of patients treated with conventional radiotherapy.21,141 Dental prophylaxis with topical fluorides and good dental care before, during, and after radiotherapy can reduce the incidence of dental caries. However, since the introduction of IMRT, most patients recover their salivary gland function more rapidly and in many cases, completely.96,97,100–102 Two recent randomized trials have shown the superiority of IMRT over conventional radiotherapy for early-stage nasopharyngeal carcinoma in terms of improved salivary function as well as patient quality of life.143,144 With time, dental problems should diminish as patients are being treated with IMRT.
Chronic otitis media occurred in 3% to 18% and hearing loss in 6% to 8% of the patients treated with coventional radiotherapy techniques in the past.21,29,141 The incidence of hearing impairment was related to the dose to the inner ear and was significantly higher in patients who received more than 50 Gy to the cochlea.145 Others have suggested that the mean dose to the cochlea should be kept under 48 Gy to minimize sensorineural hearing loss. More recently Honore et al. found that increasing patient age and decreased pretreatment hearing level were associated with increasing risk of sensorineural hearing loss.146
In the past, trismus occurred as a result of fibrosis and contraction of pterygoid muscles or fibrosis of the TM joint in 5% to 10% of patients.21,29,141 Mandible exercise may prevent progression of the trismus and surgical intervention may be necessary occasionally to relieve severe trismus. Severe neck fibrosis usually resulted from the use of a large dose per fraction29 and neck irradiation using anterior-posterior opposed fields,21 especially when compensators were not used. The use of doses less than or equal to 2 Gy/fraction and the use of a combination of photon and electron beam for treatment of posterior cervical nodes should reduce the incidence of this complication.
Soft tissue or bone necrosis or both have been described in 5% to 16%21,29,141 and brain necrosis in 2% to 3% of patients treated with coventional radiotherapy techniques in the past.21,29 Cranial nerve dysfunction, usually affecting cranial nerves IX to XII, and in particular the twelfth nerve, may result from soft tissue fibrosis along the course of the nerves and entrapment in the lateral retroparotid space. The incidence is approximately 1% to 6%.21,27,29,141 Transverse radiation myelitis has been reported in 1% to 4% of patients.21,29,141,147 Improvement in tumor localization and treatment planning with the aid of CT and MRI scans and careful treatment techniques such as IMRT should prevent this devastating treatment complication. Because a portion of the orbit and sometimes the eye, especially in patients with advanced disease, is usually included in the treatment volume, optic nerve injury and radiation retinopathy with impairment of vision can occur in patients after radiotherapy for NPC when using conventional techniques.21,148,149 Retinopathy has occurred after a dose of less than 50 Gy.148,149 Optic nerve injury can occur after 50 Gy, and the incidence increases with increase of fraction size.148 Limiting the fraction size to less than or equal to 2 Gy/fraction per day and a dose of 45 Gy to the retina and 54 Gy or less to the optic nerve should prevent this complication. Hypothalamic-pituitary or thyroid dysfunction or both can occur in 1% to 6% of long-term survivors.29,141,150–153 The true incidence of endocrine dysfunction following radiotherapy of NPC may be higher as most patients have not been routinely evaluated for endocrine function during follow-up examinations. Because most of these endocrine dysfunctions can be corrected medically, periodic evaluation of the hypothalamic, pituitary, and thyroid function may be indicated in the follow-up examination of patients after radiotherapy for NPC.
Carotid stenosis has been reported in patients who undergo irradiation of the head and neck region. Interval from radiotherapy was a significant independent predictor for severe carotid stenosis. Some have recommended a routine duplex ultrasound screening test in these patients.154
Results of Treatment
The local-regional control rates by radiotherapy in select conventional radiotherapy series are shown in Table 27-2 and Table 27-3. Control of the primary lesion using conventional radiotherapy varied with the T classification (see Table 27-2), ranging from 67% to 97% for T1 lesions, 54% to 94% for T2 lesions, 34% to 78% for T3 lesions, and 40% to 71% for T4 lesions.20,21,27,141,155–157 Local control rate increased with increase in dose.20,27,141,156,158 and field size.20,21 Interruption of treatment for 3 weeks or longer adversely affected local control.156 Boost treatment with intracavitary brachytherapy if using non-IMRT treatment techniques126 can also improve local control. Stereotactic radiosurgery boost after external-beam radiotherapy (both conventional and IMRT) has also improved local control.159,160 Nonkeratinizing squamous cell carcinoma and undifferentiated carcinoma had better local control than keratinizing squamous cell carcinoma in T2 to T3 lesions but not in T1 or T4 lesions.27,69,86 Control of cervical lymph node metastasis from carcinoma of the nasopharynx by conventional radiotherapy is excellent even with extensive disease in the neck (see Table 27-3). The control rate of cervical lymph node metastasis ranged from 82% to 100% for N0, 86% to 92% for N1, and 78% to 89% for N2 to N3 disease.21,27,141 Five-year survival rates ranged from 36% to 58% (Table 27-4).* The 5-year survival rate with conventional non-IMRT radiotherapy correlated with the T classification, as well as with the N classification (Table 27-5 and Table 27-6), being 60% to 76% for T1, 48% to 68% for T2, 27% to 55% for T3, and 0% to 29% for T4 lesions21,157; 42% to 78% for N0, 27% to 70% for N1, and 32% to 52% for N2 to N3 disease. The prognosis is poor when nodes in the lower neck or the supraclavicular fossa or both are involved.
Table 27-2 Carcinoma of the Nasopharynx: Control of the Primary Tumor According to 1992 AJCC T-Stage After Conventional Radiotherapy
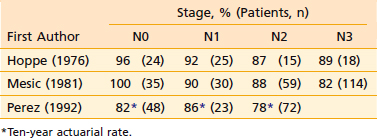
Table 27-3 Carcinoma of the Nasopharynx: Control of Cervical Lymph Node Metastases According to 1992 AJCC N-Stage* After Conventional Radiotherapy
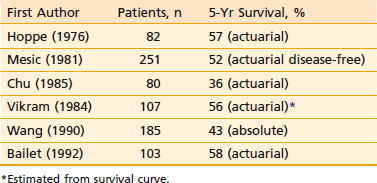
Table 27-4 Carcinoma of the Nasopharynx: 5-Year Survival After Conventional Radiotherapy (1992 AJCC Staging System)*
| First Author | Patients, n | 5-Yr Survival, % |
|---|---|---|
| Hoppe (1976) | 82 | 57 (actuarial) |
| Mesic (1981) | 251 | 52 (actuarial disease-free) |
| Chu (1985) | 80 | 36 (actuarial) |
| Vikram (1984) | 107 | 56 (actuarial)* |
| Wang (1990) | 185 | 43 (absolute) |
| Bailet (1992) | 103 | 58 (actuarial) |
* Estimated from survival curve.
Table 27-5 Carcinoma of the Nasopharynx: 5-Year Survival After Conventional Radiotherapy According to 1992 AJCC T-Classification*
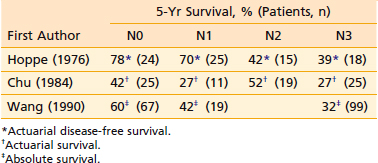
Table 27-6 Carcinoma of the Nasopharynx: 5-Year Survival After Conventional Radiotherapy According to 1992 AJCC N-Classification*
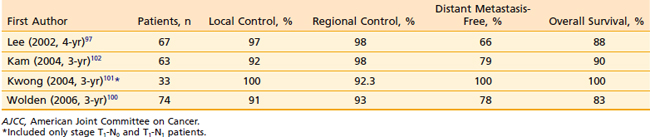
With IMRT, excellent local and regional control was achieved in 97% and 98% of the patients treated at UCSF, respectively (Fig. 27-18 and Fig. 27-19).97 An unpublished update of the UCSF experience continued to show excellent local control of approximately 96%. However, distant metastases remained high; the distant metastases–free rate was 66% at 4 years (Fig. 27-20). The overall survival was 88% (Fig. 27-21). Similar to the UCSF experience, several other institutions also recently published results that continue to show local control rates ranging from 91% to 100% and regional control rates ranging from 92.3% to 98% (Table 27-7). Because of the excellent local control rates from UCSF, the Radiation Therapy Oncology Group (RTOG) conducted a phase II trial using IMRT with or without chemotherapy in the treatment of NPC in which patients with T2b or greater or node-positive disease also received concurrent CDDP followed by adjuvant CDDP and 5-FU chemotherapies. Preliminary results showed that IMRT is feasible in a multi-institutional setting that reproduced the excellent results (local control rate of 92.3%) from single institutions.161 Compliance with concurrent chemotherapy was significantly improved in comparison with the U.S. Intergroup trial in which 88% of the patients underwent three cycles of CDDP. Compliance to adjuvant chemotherapy was slightly decreased; only 46% of the patients received three cycles of CDDP and 5-FU. Although it is encouraging to see the reproducibility of excellent locoregional control rates from single institutions as well as from a cooperative phase II trial, the development of distant metastases after these combined modality treatments is still problematic (21%-34%), which ultimately resulted in patient death (see Table 27-7).97,100,102,160 Patients with NPC with vascular endothelial growth factor (VEGF) overexpression have a higher likelihood of recurrence, distant metastases, and decreased survival.162–165 A recent report showed that overexpression of VEGF was seen in 67% of nasopharyngeal cases and higher expression of VEGF in EBV positive tumors was associated with a higher rate of recurrence, nodal positivity, and lower survival.165 Another report showed that the pre- and post-treatment serum levels of cytokines and angiogenesis factors were predictive markers for outcome in patients with head and neck cancer. With a median follow-up of 37 months, patients were more likely to remain disease-free with the VEGF level decreased post-treatment compared with those who continued to have increased VEGF levels after treatment.166 Given the available data on VEGF overexpression and increased propensity for distant failure in nasopharygneal carcinoma, it seems logical to test the addition of bevacizumab, a monoclonal antibody direacted against VEGF to the present treatment strategy for these patients. This is currently under investigation by the RTOG (protocol 0615).
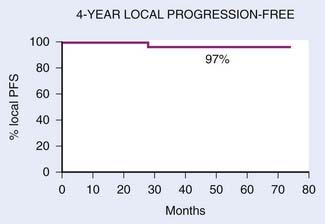
FIGURE 27-18 • Kaplan-Meier estimate of local progression-free probability.
(From Lee N, Xia P, Quivey JM, et al: Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF Experience, Int J Radiat Oncol Biol Phys 53:17, 2002.)
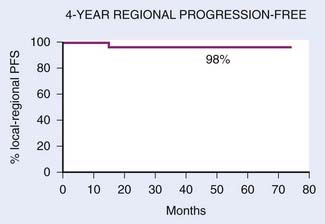
FIGURE 27-19 • Kaplan-Meier estimate of regional progression-free probability.
(From Lee N, Xia P, Quivey JM, et al: Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF Experience, Int J Radiat Oncol Biol Phys 53:18, 2002.)
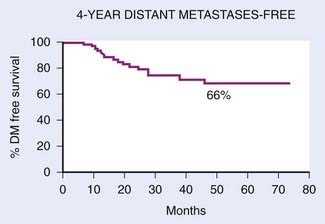
FIGURE 27-20 • Kaplan-Meier estimate of distant-metastasis-free probability.
(From Lee N, Xia P, Quivey JM, et al: Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF Experience, Int J Radiat Oncol Biol Phys 53:18, 2002.)
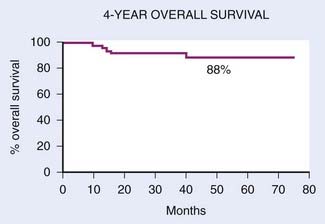
FIGURE 27-21 • Kaplan-Meier estimate of overall survival.
(From Lee N, Xia P, Quivey JM, et al: Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF Experience, Int J Radiat Oncol Biol Phys 53:18, 2002.)
Table 27-7 Carcinoma of the Nasopharynx: Control of Primary Tumor and Regional Lymph Nodes According to the 1997 AJCC T and N staging
In addition to stage, EBV DNA levels, serum VEGF levels, several other factors may influence prognosis: histologic type, age, sex, EBV-specific IgA antibody titer, tumor angiogenesis, HLA antigen pattern, and cell-mediated immune status. Lymphoepitheliomas and undifferentiated carcinomas have been reported to be more radiosensitive and associated with better prognosis than squamous cell carcinoma in some series.20,25,141,157,167–169 Younger patients appear to have better 5-year survival rates than older patients.9,20,27,170 In Ho’s series, the difference in 5-year survival between patients younger than 40 years of age and those 40 years or older was statistically significant (57.8% versus 43.4%, P = 0.001).9 In a retrospective review of 119 patients younger than 30 years of age from the Children’s Cancer Study Group,171 the overall 5-year survival and relapse-free survival rates from diagnosis were 51% and 36%, respectively, and were not significantly different from other series of adult patients.
Better prognosis for women than men has been shown in some series, although the difference is not statistically significant in most series.9,20,21,155,170
EBV antibody titer following treatment may be a significant prognostic factor.42,172,173 In a multicenter follow-up study, increasing titers of IgA anti-EA antibodies in patients in apparent clinical remission 1 year after radiotherapy were highly significant for prediction of relapse.173 In another study from Taiwan, 72% to 100% of the patients who developed recurrent NPC demonstrated elevated IgA, as well as IgG, anti-VCA antibody titers 1 year after radiotherapy, although during the first year following radiotherapy, some decrease and fluctuation of seropositive rates were observed in both the cured and recurrent patients.172 However, in one North American study, sequential measurements of various EBV antibody titers was not useful in post-treatment monitoring of patients.174
Recently circulating EBV DNA concentration has been shown to be an independent prognostic indicator.175 Pre-treatment EBV DNA correlated with disease stage, clinical outcome, and prognosis in patients with endemic NPC.6,175,176 Post-treatment EBV DNA has even a stronger correlation with prognosis and has been used in some centers to monitor patients for recurrence after treatment.47,177,178 Patients with distant failures had significantly higher pretreatment EBV DNA levels than those without distant failures.179 A recently published phase II trial on 31 locoregionally advanced NPC patients who received neoadjuvant chemotherapy followed by concurrent chemoradiation, serum EBV DNA was obtained at baseline, weekly during therapy, 4 to 6 weeks after treatment, and every 2 months for 1 year after treatment.180 Serum EBV DNA level was found to be increased significantly in 8 out of 9 patients who experienced treatment failure versus no evidence of disease if the patients did not have elevated serum EBV DNA levels. Elevated EBV DNA has been shown to predate clinical recurrence by 3 to 7 months.180–182
Reirradiation of Recurrent Carcinoma
Significant palliation and occasional long-term survival can be achieved without excessive risk in patients with recurrent NPC.* Various radiation therapy modalities, including external beam irradiation, intracavitary brachytherapy, interstitial implantation, particle beam radiotherapy, and stereotactic radiosurgery have been used.† More recently, IMRT has also been used with excellent preliminary outcome with control rates as high as 100% for recurrent NPC, namely, rT1-3 in one series.190
The treatment technique and the dose of reirradiation depend on the extent of disease at recurrence and the dose of previous radiotherapy. Superficial recurrent disease limited to the nasopharynx may be treated with intracavitary brachytherapy or interstitial implant alone, or combined with hyperfractionated external-beam radiotherapy with or without concurrent chemotherapy. Given the late complications observed with reirradiation and when the tumor is not amenable to brachytherapy or stereotactic radiotherapy, external-beam radiotherapy using IMRT is preferred for recurrent NPC to minimize the late effects.190–192 Once-a-day fractionation using IMRT to 60 Gy can be used if a desirable plan is achieved with concurrent chemotherapy. High-dose brachytherapy or stereotatctic boost can be considered in patients with residual disease limited to the nasopharynx after external-beam radiotherapy.
Particle beam radiotherapy has a more favorable physical dose distribution than conventional photon irradiation, and heavily charged particle irradiation may have additional biologic advantages. Using heavily charged particle irradiation, a local control rate of 45% and a 5-year survival of 31% was achieved in a small series of patients with recurrent disease involving the base of the skull with or without cranial nerve deficits.186
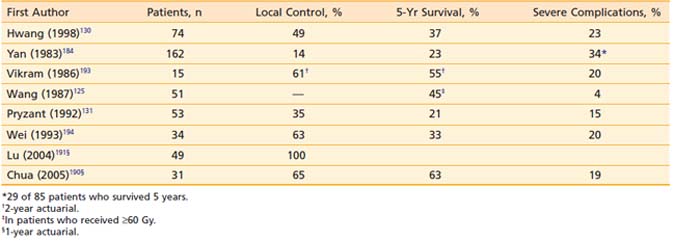
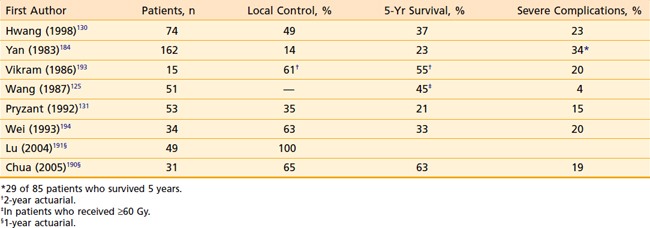
Results of reirradiation of select patients with recurrent NPC are shown in Table 27-8.* However, long-term local control, which depends on the extent of recurrent disease, was possible in only 14% to 60% of the patients retreated with conventional radiotherapy. Survival after retreatment appears to correlate with the time of recurrence after initial radiotherapy,125,130,184 extent of recurrent disease, and dose of reirradiation.125,131,185 Five-year actuarial survival was 13% in patients whose disease recurred 2 years or less after initial radiotherapy and 66% in those with recurrence more than 2 years after initial treatment.125 Five-year actuarial survival was 38% for stage T1 to T2 and 15% for stage T3 to T4 disease at recurrence.125 It was 45% with doses greater than or equal to 60 Gy and 0% with doses less than 60 Gy, although most of the patients who received lower doses had more advanced recurrent disease.125
Following reirradiation, severe complications occurred in 4% to 48% of the patients. The incidence of severe complications of reirradiation increased with the increase of total cumulative dose of external-beam irradiation; it was 4% with doses less than or equal to 100 Gy and 39% with doses greater than 100 Gy.131 Late normal tissue complications of reirradiation should decrease with the use of hyperfractionation and three-dimensional conformal radiotherapy techniques, including IMRT and particle beam radiotherapy.
1 Tai TM. Analytical epidemiology: risk factors for nasopharyngeal carcinoma. Curr Opin Oncol. 2001;8:156.
2 Tai TM. Descriptive epidemiology of nasopharyngeal cancer. Curr Opin Oncol. 2001;8:114.
3 Parkin D, Muir C, Whelan S, et al. Cancer incidence in five continents. Lyon, France: IARC Scientific Publications; 1992.
4 Lanier A, Bender T, Talbot M, et al. Nasopharyngeal carcinoma in Alaskan Eskimos Indians, and Aleuts: a review of cases and study of Epstein-Barr virus, HLA, and environmental risk factors. Cancer. 1980;46:2100-2106.
5 Liebowitz D. Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin Oncol. 1994;21:376-381.
6 Lo YM. Quantitative analysis of Epstein-Barr virus DNA in plasma and serum: applications to tumor detection and monitoring. Ann N Y Acad Sci. 2001;945:68-72.
7 Simons MJ, Wee GB, Singh D, et al. Immunogenetic aspects of nasopharyngeal carcinoma. V. Confirmation of a Chinese-related HLA profile (A2, Singapore 2) associated with an increased risk in Chinese for nasopharyngeal carcinoma. Natl Cancer Inst Monogr. 1977;47:147-151.
8 Buell P. The effect of migration on the risk of nasopharyngeal cancer among Chinese. Cancer Res. 1974;34:1189-1191.
9 Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978;4:182-198.
10 Teo PM, Leung SF, Yu P, et al. A comparison of Ho’s, International Union Against Cancer, and American Joint Committee stage classifications for nasopharyngeal carcinoma. Cancer. 1991;67:434-439.
11 International histological classification of tumors: histological typing of upper respiratory tract tumors. World Health Organization, 1978. 19
12 International histological classification of tumors: Histological typing of upper respiratory tract tumors. World Health Organization, 1991.
13 Marks JE, Phillips JL, Menck HR. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer. 1998;83:582-588.
14 McGuire LJ, Lee JC. The histopathologic diagnosis of nasopharyngeal carcinoma. Ear Nose Throat J. 1990;69:229-236.
15 Neel HB3rd. Nasopharyngeal carcinoma: diagnosis, staging, and management. Oncology (Williston Park). 1992;6:87-95.
16 Skinner DW, Van Hasselt CA. Nasopharyngeal carcinoma: methods of presentation. Ear Nose Throat J. 1990;69:237-240.
17 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
18 Ahmad A, Stefani S. Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J Surg Oncol. 1986;33:194-197.
19 Bedwinek JM, Fineberg B, Lee J, et al. Analysis of failures following local treatment of isolated local-regional recurrence of breast cancer. Int J Radiat Oncol Biol Phys. 1981;7:581-585.
20 Chu AM, Flynn MB, Achino E, et al. Irradiation of nasopharyngeal carcinoma: correlations with treatment factors and stage. Int J Radiat Oncol Biol Phys. 1984;10:2241-2249.
21 Hoppe RT, Goffinet DR, Bagshaw MA. Carcinoma of the nasopharynx. Eighteen years’ experience with megavoltage radiation therapy. Cancer. 1976;37:2605-2612.
22 McNeese MD, Fletcher GH. Retreatment of recurrent nasopharyngeal carcinoma. Radiology. 1981;138:191-193.
23 Moench HC, Phillips TL. Carcinoma of the nasopharynx. Review of 146 patients with emphasis on radiation dose and time factors. Am J Surg. 1972;124:515-518.
24 Lynn TC, Hsu MM, Hsieh T, et al. [The integrated treatment for nasopharyngeal carcinoma]. Taiwan Yi Xue Hui Za Zhi. 1982;81:921-927.
25 Frezza G, Barbieri E, Emiliani E, et al. Patterns of failure in nasopharyngeal cancer treated with megavoltage irradiation. Radiother Oncol. 1986;5:287-294.
26 Johansen LV, Mestre M, Overgaard J. Carcinoma of the nasopharynx: analysis of treatment results in 167 consecutively admitted patients. Head Neck. 1992;14:200-207.
27 Perez CA, Devineni VR, Marcial-Vega V, et al. Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys. 1992;23:271-280.
28 Teo P, Shiu W, Leung SF, et al. Prognostic factors in nasopharyngeal carcinoma investigated by computer tomography—an analysis of 659 patients. Radiother Oncol. 1992;23:79-93.
29 Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976–1985: late complications following megavoltage irradiation. Br J Radiol. 1992;65:918-928.
30 Poon PY, Tsang VH, Munk PL. Tumour extent and T stage of nasopharyngeal carcinoma: a comparison of magnetic resonance imaging and computed tomographic findings. Can Assoc Radiol J. 2000;51:287-295. quiz 286
31 Lanzieri CF, Bangert B. Magnetic resonance imaging of the nasopharynx. Top Magn Reson Imaging. 1990;2:39-47.
32 Mancuso AA, Bohman L, Hanafee W, et al. Computed tomography of the nasopharynx: normal and variants of normal. Radiology. 1980;137:113-121.
33 Sievers KW, Greess H, Baum U, et al. Paranasal sinuses and nasopharynx CT and MRI. Eur J Radiol. 2000;33:185-202.
34 Dillon WP, Harnsberger HR. The impact of radiologic imaging on staging of cancer of the head and neck. Semin Oncol. 1991;18:64-79.
35 Glazer HS, Niemeyer JH, Balfe DM, et al. Neck neoplasms: MR imaging. Part II. Posttreatment evaluation. Radiology. 1986;160:349-354.
36 Gong QY, Zheng GL, Zhu HY. MRI differentiation of recurrent nasopharyngeal carcinoma from postradiation fibrosis. Comput Med Imaging Graph. 1991;15:423-429.
37 Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961-969.
38 Chan SC, Chang JT, Wang HM, et al. Prediction for distant failure in patients with stage M0 nasopharyngeal carcinoma: the role of standardized uptake value. Oral Oncol. 2008;45(1):52-58. –
39 Lee SW, Nam SY, Im KC, et al. Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol. 2008;87:211-216.
40 Gordin A, Golz A, Daitzchman M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography imaging in patients with carcinoma of the nasopharynx: diagnostic accuracy and impact on clinical management. Int J Radiat Oncol Biol Phys. 2007;68:370-376.
41 Liu FY, Lin CY, Chang JT, et al. 18F-FDG PET can replace conventional work-up in primary M staging of nonkeratinizing nasopharyngeal carcinoma. J Nucl Med. 2007;48:1614-1619.
42 Henle W, Ho JH, Henle G, et al. Nasopharyngeal carcinoma: significance of changes in Epstein-Barr virus-related antibody patterns following therapy. Int J Cancer. 1977;20:663-672.
43 Wara WM, Wara DW, Phillips TL, et al. Elevated IGA in carcinoma of the nasopharynx. Cancer. 1975;35:1313-1315.
44 Ho HC, Ng MH, Kwan HC, et al. Epstein-Barr-virus-specific IgA and IgG serum antibodies in nasopharyngeal carcinoma. Br J Cancer. 1976;34:655-660.
45 Zong YS, Sham JS, Ng MH, et al. Immunoglobulin A against viral capsid antigen of Epstein-Barr virus and indirect mirror examination of the nasopharynx in the detection of asymptomatic nasopharyngeal carcinoma. Cancer. 1992;69:3-7.
46 Zeng Y. Seroepidemiological studies on nasopharyngeal carcinoma in China. Adv Cancer Res. 1985;44:121-138.
47 Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461-2470.
48 Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24:5414-5418.
49 AJCC Pharynx; vol 31. 1992; JB Lippincott Company, Philadelphia.
50 Clinical S: Recommendation, nasopharyngeal carcinoma: etiology and control (vol 594), Lyon, France: Scientific Publications No. 20; 1978.
51 Huang SC. Nasopharyngeal cancer: a review of 1605 patients treated radically with cobalt 60. Int J Radiat Oncol Biol Phys. 1980;6:401-407.
52 Huang SC, Lui LT, Lynn TC. Nasopharyngeal cancer: study III. A review of 1206 patients treated with combined modalities. Int J Radiat Oncol Biol Phys. 1985;11:1789-1793.
53 Neel HB3rd, Taylor WF. New staging system for nasopharyngeal carcinoma. Long-term outcome. Arch Otolaryngol Head Neck Surg. 1989;115:1293-1303.
54 Teo PM, Tsao SY, Ho JH, et al. A proposed modification of the Ho stage-classification for nasopharyngeal carcinoma. Radiother Oncol. 1991;21:11-23.
55 UICC Pharynx; vol 20. 1992; Springer-Verlag, Berlin.
56 Sham JS, Wei WI, Nicholls J, et al. Extent of nasopharyngeal carcinoma involvement inside the nasopharynx. Lack of prognostic value on local control. Cancer. 1992;69:854-859.
57 Greene FL, Page DL, ID F. Pharynx. New York: Springer; 2002.
58 Xiao GL, Gao L, Xu GZ. Prognostic influence of parapharyngeal space involvement in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:957-963.
59 Sham JS, Cheung YK, Choy D, et al. Cranial nerve involvement and base of the skull erosion in nasopharyngeal carcinoma. Cancer. 1991;68:422-426.
60 Ozyar E, Yildiz F, Akyol FH, et al. Comparison of AJCC 1988 and 1997 classifications for nasopharyngeal carcinoma. American Joint Committee on Cancer. Int J Radiat Oncol Biol Phys. 1999;44:1079-1087.
61 Lee AWM, Au JS, Teo PM, et al. Staging of nasopharyngeal carcinoma: suggestions for improving the current UICC/AJCC staging system. Clin Oncol. 2004;16:269-276.
62 King AD, Ahuja AT, Leung SF, et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck. 2000;22:275-281.
63 Yen KL, Hsu LP, Sheen TS, et al. Salvage neck dissection for cervical recurrence of nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:725-729.
64 Wei WI, Ho WK, Cheng AC, et al. Management of extensive cervical nodal metastasis in nasopharyngeal carcinoma after radiotherapy: a clinicopathological study. Arch Otolaryngol Head Neck Surg. 2001;127:1457-1462.
65 Fee WEJr, Roberson JBJr, Goffinet DR. Long-term survival after surgical resection for recurrent nasopharyngeal cancer after radiotherapy failure. Arch Otolaryngol Head Neck Surg. 1991;117:1233-1236.
66 Tu GY, Hu YH, Xu GZ, et al. Salvage surgery for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1988;114:328-329.
67 Wei WI. Cancer of the nasopharynx: functional surgical salvage. World J Surg. 2003;27:844-848.
68 Wei WI. Salvage surgery for recurrent primary nasopharyngeal carcinoma. Crit Rev Oncol Hematol. 2000;33:91-98.
69 Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310-1317.
70 Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631-637.
71 Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536-539.
72 Al-Sarraf M, LeBrlan M, Giri PG, et al. Superiority of 5-year survival with chemoradiotherapy vs. radiotherapy in patients with locally advanced nasopharyngeal cancer. Intergroup 0099 Phase III Study: final report. Proc Am Soc Clinc Oncol. 20, 2001.
73 Lee AW, Yau TK, Wong DH, et al. Treatment of stage IV(A-B) nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy and accelerated fractionation. Int J Radiat Oncol Biol Phys. 2005;63:1331-1338.
74 Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038-2044.
75 Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer. 2007;43:1399-1406.
76 Cvitkovic E, Bachouchi M, Armand JP. Nasopharyngeal carcinoma. Biology, natural history, and therapeutic implications. Hematol Oncol Clin North Am. 1991;5:821-838.
77 Dimery IW, Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst. 1993;85:95-111.
78 Fandi A, Altun M, Azli N, et al. Nasopharyngeal cancer: epidemiology, staging, and treatment. Semin Oncol. 1994;21:382-397.
79 Ali H, al-Sarraf M. Chemotherapy in advanced nasopharyngeal cancer. Oncology (Williston Park). 2000;14:1223-1230.
80 Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol. 2002;25:219-223.
81 Orecchia R, Airoldi M, Sola B, et al. Results of chemotherapy plus external reirradiation in the treatment of locally advanced recurrences of nasopharyngeal carcinoma. Eur J Cancer B Oral Oncol. 1992;28B:109-111.
82 Hareyama M, Sakata K, Shirato H, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002;94:2217-2223.
83 Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:1350-1357.
84 Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist. 2004;9:665-672.
85 Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47-56.
86 Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 2004;22:4604-4612.
87 Lee, et al: Hong Kong Nasopharyngeal Cancer Study Group. ClinicalTrials.gov, no. NCT00379262.
88 Feng, et al: Taiwan National Health Research Institutes. ClinicalTrials.gov, no. NCT00201396.
89 Hong RL, Ting LL, Ko JY, et al. Induction chemotherapy with mitomycin, epirubicin, cisplatin, fluorouracil, and leucovorin followed by radiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:4305-4313.
90 Choo R, Tannock I. Chemotherapy for recurrent or metastatic carcinoma of the nasopharynx. A review of the Princess Margaret Hospital experience. Cancer. 1991;68:2120-2124.
91 Lee N, Chan K, Bekelman JE, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:731-740.
92 Dawson LA, Myers LL, Bradford CR, et al. Conformal re-irradiation of recurrent and new primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:377-385.
93 Nutting C, Dearnaley DP, Webb S. Intensity modulated radiation therapy: a clinical review. Br J Radiol. 2000;73:459-469.
94 Xia P, Fu KK, Wong GW, et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:329-337.
95 Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577-587.
96 Eisbruch A, Ship JA, Martel MK, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: techniques and early results. Int J Radiat Oncol Biol Phys. 1996;36:469-480.
97 Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12-22.
98 Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys. 2003;56:145-157.
99 Sultanem K, Shu HK, Xia P, et al. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: the University of California–San Francisco experience. Int J Radiat Oncol Biol Phys. 2000;48:711-722.
100 Wolden SL, Chen WC, Pfister DG, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64:57-62.
101 Kwong DL, Pow EH, Sham JS, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: a prospective study on disease control and preservation of salivary function. Cancer. 2004;101:1584-1593.
102 Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440-1450.
103 Hsiung CY, Ting HM, Huang HY, et al. Parotid-sparing intensity-modulated radiotherapy (IMRT) for nasopharyngeal carcinoma: preserved parotid function after IMRT on quantitative salivary scintigraphy, and comparison with historical data after conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:454-461.
104 McMillan AS, Pow EH, Kwong DL, et al. Preservation of quality of life after intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: results of a prospective longitudinal study. Head Neck. 2006;28:712-722.
105 Chau RM, Teo PM, Kam MK, et al. Dosimetric comparison between 2-dimensional radiation therapy and intensity modulated radiation therapy in treatment of advanced T-stage nasopharyngeal carcinoma: to treat less or more in the planning organ-at-risk volume of the brainstem and spinal cord. Med Dosim. 2007;32:263-270.
106 Taheri-Kadkhoda Z, Pettersson N, Bjork-Eriksson T, et al. Superiority of intensity-modulated radiotherapy over three-dimensional conformal radiotherapy combined with brachytherapy in nasopharyngeal carcinoma: a planning study. Br J Radiol. 2008;81:397-405.
107 Lee N, Mechalakos J, Puri DR, et al. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1299-1309.
108 Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000;174:837-844.
109 Nowak PJ, Wijers OB, Lagerwaard FJ, et al. A three-dimensional CT-based target definition for elective irradiation of the neck. Int J Radiat Oncol Biol Phys. 1999;45:33-39.
110 Gregoire V, Coche E, Cosnard G, et al. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135-150.
111 Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227-236.
112 Lichter AS, Sandler HM, Robertson JM, et al. Clinical experience with three-dimensional treatment planning. Semin Radiat Oncol. 1992;2:257-266.
113 Carol M, Grant WH3rd, Pavord D, et al. Initial clinical experience with the Peacock intensity modulation of a 3-D conformal radiation therapy system. Stereotact Funct Neurosurg. 1996;66:30-34.
114 Boyer AL, Geis P, Grant W, et al. Modulated beam conformal therapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 1997;39:227-236.
115 Welsh JS, Patel RR, Ritter MA, et al. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat. 2002;1:311-316.
116 Spirou SV, Chui CS. Generation of arbitrary intensity profiles by combining the scanning beam with dynamic multileaf collimation. Med Phys. 1996;23:1-8.
117 Chui CS, Spirou SV. Inverse planning algorithms for external beam radiation therapy. Med Dosim. 2001;26:189-197.
118 Xia P, Verhey LJ. Delivery systems of intensity-modulated radiotherapy using conventional multileaf collimators. Med Dosim. 2001;26:169-177.
119 Lee N, Akazawa C, Akazawa P, et al. A forward-planned treatment technique using multisegments in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:584-594.
120 Amdur RJ, Liu C, Li J, et al. Matching intensity-modulated radiation therapy to an anterior low neck field. Int J Radiat Oncol Biol Phys. 2007;69:S46-S48.
121 Dabaja B, Salehpour MR, Rosen I, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: Comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys. 2005;63:1000-1005.
122 Amdur RJ, Li JG, Liu C, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck. 2004;26:257-263.
123 Levendag PC, Schmitz PI, Jansen PP, et al. Fractionated high-dose-rate brachytherapy in primary carcinoma of the nasopharynx. J Clin Oncol. 1998;16:2213-2220.
124 Levendag PC, Lagerwaard FJ, Noever I, et al. Role of endocavitary brachytherapy with or without chemotherapy in cancer of the nasopharynx. Int J Radiat Oncol Biol Phys. 2002;52:755-768.
125 Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma—treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987;13:953-956.
126 Wang CC. Improved local control of nasopharyngeal carcinoma after intracavitary brachytherapy boost. Am J Clin Oncol. 1991;14:5-8.
127 Zhang YW, Liu TF, Fi CX. Intracavitary radiation treatment of nasopharyngeal carcinoma by the high dose rate afterloading technique. Int J Radiat Oncol Biol Phys. 1989;16:315-318.
128 Lee N, Hoffman R, Phillips TL, et al. Managing nasopharyngeal carcinoma with intracavitary brachytherapy: one institution’s 45-year experience. Brachytherapy. 2002;1:74-82.
129 Teo P, Leung SF, Choi P, et al. Afterloading radiotherapy for local persistence of nasopharyngeal carcinoma. Br J Radiol. 1994;67:181-185.
130 Hwang JM, Fu KK, Phillips TL. Results and prognostic factors in the retreatment of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41:1099-1111.
131 Pryzant RM, Wendt CD, Delclos L, et al. Re-treatment of nasopharyngeal carcinoma in 53 patients. Int J Radiat Oncol Biol Phys. 1992;22:941-947.
132 Harrison LB, Sessions RB, Fass DE, et al. Nasopharyngeal brachytherapy with access via a transpalatal flap. Am J Surg. 1992;164:173-175.
133 Vikram B, Permanent iodine-125 (I-125) boost after teletherapy in primary cancers of the nasopharynx is safe and highly effective: long-term results., ” “; Int J Radiat Oncol Biol Phys; 38; 1997:1140.
134 Choy D, Sham JS, Wei WI, et al. Transpalatal insertion of radioactive gold grain for the treatment of persistent and recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1993;25:505-512.
135 Syed AM, Puthawala AA, Damore SJ, et al. Brachytherapy for primary and recurrent nasopharyngeal carcinoma: 20 years’ experience at Long Beach Memorial. Int J Radiat Oncol Biol Phys. 2000;47:1311-1321.
136 Levendag PC, Peters R, Meeuwis CA, et al. A new applicator design for endocavitary brachytherapy of cancer in the nasopharynx. Radiother Oncol. 1997;45:95-98.
137 Levendag PC, Lagerwaard FJ, Noever I, et al. High dose rate brachytherapy for cancer of the head and neck. In: Nag SE, editor. High dose rate brachytherapy: a textbook. Armonk, NY: Futura Publishing Company; 1994:237.
138 Kwong DL, Wei WI, Cheng AC, et al. Long term results of radioactive gold grain implantation for the treatment of persistent and recurrent nasopharyngeal carcinoma. Cancer. 2001;91:1105-1113.
139 Lee AW, Sze WM, Yau TK, et al. Retrospective analysis on treating nasopharyngeal carcinoma with accelerated fractionation (6 fractions per week) in comparison with conventional fractionation (5 fractions per week): report on 3-year tumor control and normal tissue toxicity. Radiother Oncol. 2001;58:121-130.
140 Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261-270.
141 Mesic JB, Fletcher GH, Goepfert H. Megavoltage irradiation of epithelial tumors of the nasopharynx. Int J Radiat Oncol Biol Phys. 1981;7:447-453.
142 Peters LJ, Harrison ML, Dimery IW, et al. Acute and late toxicity associated with sequential bleomycin-containing chemotherapy regimens and radiation therapy in the treatment of carcinoma of the nasopharynx. Int J Radiat Oncol Biol Phys. 1988;14:623-633.
143 Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873-4879.
144 Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981-991.
145 Grau C, Moller K, Overgaard M, et al. Sensori-neural hearing loss in patients treated with irradiation for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:723-728.
146 Honore HB, Bentzen SM, Moller K, et al. Sensori-neural hearing loss after radiotherapy for nasopharyngeal carcinoma: individualized risk estimation. Radiother Oncol. 2002;65:9-16.
147 Tokars RP, Griem ML. Carcinoma of the nasopharynx an optimization of radiotherapeutic management for tumor control and spinal cord injury. Int J Radiat Oncol Biol Phys. 1979;5:1741-1748.
148 Parsons JT, Fitzgerald CR, Hood CI, et al. The effects of irradiation on the eye and optic nerve. Int J Radiat Oncol Biol Phys. 1983;9:609-622.
149 Wara WM, Irvine AR, Neger RE, et al. Radiation retinopathy. Int J Radiat Oncol Biol Phys. 1979;5:81-83.
150 de Schryver A, Ljunggren JG, Baryd I. Pituitary function in long-term survival after radiation therapy of nasopharyngeal tumours. Acta Radiol Ther Phys Biol. 1973;12:497-508.
151 Lam KS, Ho JH, Lee AW, et al. Symptomatic hypothalamic-pituitary dysfunction in nasopharyngeal carcinoma patients following radiation therapy: a retrospective study. Int J Radiat Oncol Biol Phys. 1987;13:1343-1350.
152 Rosenthal MB, Goldfine ID. Primary and secondary hypothyroidism in nasopharyngeal carcinoma. J Am Med Assoc. 1976;236:1591-1593.
153 Samaan NA, Vieto R, Schultz PN, et al. Hypothalamic, pituitary and thyroid dysfunction after radiotherapy to the head and neck. Int J Radiat Oncol Biol Phys. 1982;8:1857-1867.
154 Cheng SW, Ting AC, Lam LK, et al. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:517-521.
155 Bailet JW, Mark RJ, Abemayor E, et al. Nasopharyngeal carcinoma: treatment results with primary radiation therapy. Laryngoscope. 1992;102:965-972.
156 Vikram B, Mishra UB, Strong EW, et al. Patterns of failure in carcinoma of the nasopharynx: I. failure at the primary site. Int J Radiat Oncol Biol Phys. 1985;11:1455-1459.
157 Wang C. Carcinoma of the nasopharynx. In: Wang CC, editor. Radiation therapy for head and neck neoplasms: indications, techniques, and results. Chicago: Year Book Medical Publishers; 1990:261-283.
158 Vikram B, Strong EW, Manolatos S, et al. Improved survival in carcinoma of the nasopharynx. Head Neck Surg. 1984;7:123-128.
159 Le QT, Tate D, Koong A, et al. Improved local control with stereotactic radiosurgical boost in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:1046-1054.
160 Hara W, Loo BW, Goffinet DR. Excellent local control with stereotactic radiotherapy boost after external beam radiotherapy in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2008.
161 Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27(22):3684-3690.
162 Wakisaka N, Wen QH, Yoshizaki T, et al. Association of vascular endothelial growth factor expression with angiogenesis and lymph node metastasis in nasopharyngeal carcinoma. Laryngoscope. 1999;109:810-814.
163 Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434-1440.
164 Qian CN, Zhang CQ, Guo X, et al. Elevation of serum vascular endothelial growth factor in male patients with metastatic nasopharyngeal carcinoma. Cancer. 2000;88:255-261.
165 Krishna SM, James S, Balaram P. Expression of VEGF as prognosticator in primary nasopharyngeal cancer and its relation to EBV status. Virus Res. 2006;115:85-90.
166 Druzgal CH, Chen Z, Yeh NT, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2005;27:771-784.
167 Hoppe RT, Williams J, Warnke R, et al. Carcinoma of the nasopharynx—the significance of histology. Int J Radiat Oncol Biol Phys. 1978;4:199-205.
168 Cammoun M, Ellouz R, Behi J, et al. Histological types of nasopharyngeal carcinoma in an intermediate risk area. IARC Sci Publ. 1978:13-26.
169 Shanmugaratnam K, Chan SH, de-The G, et al. Histopathology of nasopharyngeal carcinoma: correlations with epidemiology, survival rates and other biological characteristics. Cancer. 1979;44:1029-1044.
170 Hsu MM, Huang SC, Lynn TC, et al. The survival of patients with nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 1982;90:289-295.
171 Jenkin RD, Anderson JR, Jereb B, et al. Nasopharyngeal carcinoma—a retrospective review of patients less than thirty years of age: a report of Children’s Cancer Study Group. Cancer. 1981;47:360-366.
172 Lynn TC, Tu SM, Kawamura AJr. Long-term follow-up of IgG and IgA antibodies against viral capsid antigens of Epstein-Barr virus in nasopharyngeal carcinoma. J Laryngol Otol. 1985;99:567-572.
173 De-Vathaire F, Sancho-Garnier H, de-The H, et al. Prognostic value of EBV markers in the clinical management of nasopharyngeal carcinoma (NPC): a multicenter follow-up study. Int J Cancer. 1988;42:176-181.
174 Neel HB3rd, Taylor WF. Epstein-Barr virus-related antibody. Changes in titers after therapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1990;116:1287-1290.
175 Lo YM, Chan AT, Chan LY, et al. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res. 2000;60:6878-6881.
176 Lo YM, Chan LY, Chan AT, et al. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res. 1999;59:5452-5455.
177 Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614-1619.
178 Hong RL, Lin CY, Ting LL, et al. Comparison of clinical and molecular surveillance in patients with advanced nasopharyngeal carcinoma after primary therapy: the potential role of quantitative analysis of circulating Epstein-Barr virus DNA. Cancer. 2004;100:1429-1437.
179 Leung SF, Tam JS, Chan AT, et al. Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating Epstein-Barr virus DNA and anti-Epstein-Barr viral capsid antigen IgA antibody. Clin Chem. 2004;50:339-345.
180 Chan AT, Ma BB, Lo YM, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22:3053-3060.
181 Lo YM, Leung SF, Chan LY, et al. Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. 2000;60:2351-2355.
182 Ngan RK, Lau WH, Yip TT, et al. Remarkable application of serum EBV EBER-1 in monitoring response of nasopharyngeal cancer patients to salvage chemotherapy. Ann N Y Acad Sci. 2001;945:73-79.
183 Hwang HN. Nasopharyngeal carcinoma in the people’s Republic of China: incidence, treatment, and survival rates. Radiology. 1983;149:305-309.
184 Yan JH, Hu YH, Gu XZ. Radiation therapy of recurrent nasopharyngeal carcinoma. Report on 219 patients. Acta Radiol Oncol. 1983;22:23-28.
185 Lee AW, Foo W, Law SC, et al. Reirradiation for recurrent nasopharyngeal carcinoma: factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys. 1997;38:43-52.
186 Feehan PE, Castro JR, Phillips TL, et al. Recurrent locally advanced nasopharyngeal carcinoma treated with heavy charged particle irradiation. Int J Radiat Oncol Biol Phys. 1992;23:881-884.
187 Chua DT, Sham JS, Hung KN, et al. Salvage treatment for persistent and recurrent T1–2 nasopharyngeal carcinoma by stereotactic radiosurgery. Head Neck. 2001;23:791-798.
188 Chua DT, Sham JS, Hung KN, et al. Stereotactic radiosurgery as a salvage treatment for locally persistent and recurrent nasopharyngeal carcinoma. Head Neck. 1999;21:620-626.
189 Kondziolka D, Lunsford LD. Stereotactic radiosurgery for squamous cell carcinoma of the nasopharynx. Laryngoscope. 1991;101:519-522.
190 Chua DT, Sham JS, Leung LH, et al. Re-irradiation of nasopharyngeal carcinoma with intensity-modulated radiotherapy. Radiother Oncol. 2005;77:290-294.
191 Lu TX, Mai WY, Teh BS, et al. Initial experience using intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:682-687.
192 Hsiung CY, Yorke ED, Chui CS, et al. Intensity-modulated radiotherapy versus conventional three-dimensional conformal radiotherapy for boost or salvage treatment of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2002;53:638-647.
193 Vikram V. Permanent iodine-125 implants for recurrent carcinoma of the nasopharynx: early results. Endocurie Hypertherm Oncol. 1986;2:83.
194 Wei WI, Ho CM, Lam KH, et al. Surgical resection for nasopharynx cancer. In: Johnson JT, Didolkar MS, editors. Head and neck cancer. Hong Kong: Elsevier Science; 1993:465.