39 Cancer of the Liver, Bile Duct, and Gallbladder
Anatomy
The liver is divided into two lobes demarcated by the middle hepatic vein. These lobes are further divided into eight segments based on the left and right hepatic veins and the portal vein. Fig. 39-1 demonstrates the Couinaud segments on frontal, posterior, and axial views of the liver. The liver receives a dual blood supply with approximately one-third coming from the portal vein and the remainder from the hepatic arteries. Venous drainage is via the hepatic veins, which empty into the inferior vena cava. The caudate lobe or segment 1 has a separate blood supply, receiving blood from both the right and left hepatic arteries and drainage directly to the inferior vena cava. Because of this division into self-contained units, each segment can be resected without damaging the remainder of the liver. However, there are no true anatomic borders between the segments; thus, tumors can often extend across liver segments.
Bile duct anatomy follows fairly predictable general patterns, with variants that may be important to the surgeon and occasionally of interest to the radiation oncologist. The liver ductules gradually coalesce into right and left hepatic ducts, which then form the common hepatic duct (known as the “upper” or proximal third). Fused with the cystic duct from the gallbladder, they form the common bile duct (the middle third), which, as it draws nearer to the ampulla of Vater, becomes known as the distal common bile duct (the lower or distal third) (Fig. 39-2). This is a lymph-rich system: lymphatic channels course throughout the submucosa of bile ducts and eventually drain into the porta hepatis, the pancreaticoduodenal nodes, and the celiac axis.
Primary Liver Tumors
Epidemiology and Etiology
In the United States, there were an estimated 22,620 new cases of primary liver cancer diagnosed and 18,160 deaths in 2009.1 The worldwide incidence of primary liver cancer is much more significant, with an estimated 1 million patients diagnosed annually.2 Hepatocellular carcinoma (HCC) represents approximately 70% to 85% of the cases. The incidence of HCC has been steadily increasing in the United States with a 75% increase during the past decade.3 The most common cause of HCC worldwide is hepatitis B, with other major causes including hepatitis C, alcoholism, and primary biliary cirrhosis. In addition, HCC has been linked with genetic hemochromatosis, hereditary tyrosinemia, α1-antitrypsin deficiency, aflatoxins, and possibly a promotional relation with alcohol. There is an estimated 0.5% annual risk of developing HCC in chronic hepatitis B patients and 5% in chronic hepatitis C patients.
Primary tumors of the bile ducts, also called cholangiocarcinomas, can be divided into intrahepatic (IHCC) or extrahepatic (EHCC) tumors (including hilar and distal bile duct tumors), and excludes tumors of the ampulla of Vater and gallbladder (see Fig. 39-2). HCCs and IHCCs are often grouped together as primary liver tumors, whereas EHCC and gallbladder tumors are treated as separate tumors of the biliary tree. The incidence and mortality from IHCC has been increasing during the last three decades.4 A host of predisposing factors have been proposed for bile duct tumors; the most widely accepted associations are with liver flukes, ulcerative colitis associated with primary sclerosing cholangitis, and congenital biliary cysts.
Pathologic Conditions
Histologically, early HCCs are generally well differentiated but can eventually show poor differentiation with marked pleomorphism. The only widely agreed-on HCC subcategory is the fibrolamellar type, which tends to occur in younger populations with less pre-existing liver disease and is associated with better outcome. The World Health Organization histologic classification5 recognizes several HCC variants, including childhood, spindle cell, clear cell, combined, carcinosarcoma, and sclerosis; these subcategories are used more, less, or not at all at varying institutions.
IHCCs are primarily adenocarcinomas, and are therefore difficult to distinguish from metastatic lesions from other primary sites involving the liver. There are no pathognomonic immunohistochemical stains distinguishing cholangiocarcinomas, although cytokeratin-7 positivity is often found. Special stains that help in the diagnosis to distinguish IHCC versus HCC include alpha-fetoprotein (AFP), which is positive in 35% to 75% of hepatomas and almost never in IHCC tumors; and CA19-9 or Ca-50, which is positive in 80% to 90% of cholangiocarcinomas and very rarely in hepatomas.6
Clinical Presentation
The presentation of primary liver tumors is often insidious, and clinical symptoms generally herald advanced disease. Patients often complain of antecedent malaise, anorexia, and occasionally abdominal pain. Signs include fever of unknown origin, night sweats, hepatomegaly, or a right-upper-quadrant mass, evidence of portal vein obstruction, bruits, ascites, splenomegaly, weight loss, or findings attributable to metastatic disease. In cirrhotic patients, in whom HCC is more common, unexplained weight loss can be a warning sign and some patients present in florid hepatic failure. Jaundice is less common in patients with primary liver tumors and may be associated with very advanced disease caused by biliary obstruction or hepatic failure resulting from extensive parenchymal destruction. Screening of high-risk patients with ultrasonography and measurement of AFP levels can help to detect small asymptomatic lesions, although the effect of early detection on survival is still unclear.7,8
Diagnostic Studies
The list of imaging studies designed to evaluate local disease is headed by ultrasonography, which, by virtue of its low cost, sensitivity, noninvasive property, and relative simplicity of use, has been effective as both a screening and a diagnostic tool for primary liver tumors. It is useful in determining the number and size of lesions (lesions as small as 1 cm are detectable),7 ductal status, and vessel patency. Contrast-enhanced computed tomography (CT) scans have a somewhat higher sensitivity than ultrasonography (68% versus 60%) based on a pooled analysis of imaging studies for detecting HCC,9 with even higher sensitivity using triple-phase helical CT scans (arterial, portal venous, and delayed phases) of 89%.10 HCC tend to be hypervascular and are therefore best seen on the arterial phase, but appear hypodense or even isodense relative to liver parenchyma during the portal venous phase. IHCC tend to be hypodense during the portal venous phase and often hyperattenuated on delayed images. Peripheral contrast enhancement can also be seen in arterial or portal phase imaging.11 Fig. 39-3 shows an example of HCC and IHCC as seen on CT scan.
CT can also be valuable in evaluating disease beyond the liver and in both the detection of vessel invasion and the identification of vascular variants. Cirrhosis can seriously complicate the picture when evaluating the primary tumor on CT. Recent data suggest that contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive technique for detecting liver nodules, particularly in the setting of cirrhosis; dynamic hepatic arterial-phase contrast material–enhanced imaging is essential with both CT and MRI for visualization of small hepatic nodules in a background of cirrhosis.9,12–16 On MRI, IHCC can look very similar to metastatic colorectal cancer, but is often accompanied by intrahepatic biliary dilatation.17 Magnetic resonance cholangiopancreatography (MRCP) is a newer technique to noninvasively visualize the intrahepatic and extrahepatic bile ducts and the pancreatic duct to determine the extent of disease and potential resectability.
Positron emission tomography (PET) scans using fluorine-18–2-fluoro-2-deoxy-d-glucose (F-18 FDG) have not been routinely used for HCC because of the variable FDG avidity of these tumors; however, they appear to be of more benefit for IHCC, particularly for identifying extrahepatic metastatic lesions.18 Other metastatic studies for both HCC and IHCC include chest CT and bone scan. Of note, IHCC should be regarded as a diagnosis of exclusion because most adenocarcinomas involving the liver are from metastatic disease; thus a work-up to identify a primary site should be performed in the setting of a biopsy-proven adenocarcinoma involving the liver.
The benchmark laboratory study for detection and evaluation of treatment response is the serum AFP; it is elevated in 70% to 80% of all HCC patients. This rate is lower (≅30%) in the United States and Europe19 and in patients with fibrolamellar histologic findings. Overall, AFP, when used with a conventional cutoff point of 500 ng/mL, has a sensitivity of 50% and a specificity of 90% in detecting HCC in a patient with pre-existing liver disease.20 Higher levels are associated with poorer differentiation and shortened survival times.
Histologic confirmation of a hepatic tumor can be obtained through fine-needle aspiration, core biopsy, or open biopsy or resection. For centrally located IHCCs, tissue diagnosis may be a challenge. Endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiogram may be performed to obtain brushings for confirmation of a primary bile duct tumor. Some practitioners also obtain tissue from adjacent liver to determine the extent of coexistent hepatic disease. In patients with underlying liver disease, these procedures can be technically difficult to perform because of coagulopathies and tumor hypervascularity. In the absence of an elevated AFP, it is generally considered crucial to obtain a tissue diagnosis given the large differential and wide range of treatment options and prognoses. However, for tumors larger than 3 cm, with elevated AFP and clinical and radiographic findings suggesting HCC, the false-positive rate for diagnosis of HCC has been shown to be as low as 3%.21
Staging
Several staging systems have been proposed for HCC. Many clinicians categorize tumors as being (1) localized, (2) in liver only but in more than one lobe, or (3) metastatic. Okuda and colleagues13 incorporate tumor size (percentage of liver), ascites, and albumin (<3 g/dL) and bilirubin (>3 mg/dL) levels. The tumor-node-metastasis (TNM) staging system for both HCC and IHCC, as developed by the American Joint Committee on Cancer (AJCC), is presented in Table 39-1.
Therapeutic Modalities
Surgery
Surgery is currently the only potentially curative treatment modality for patients with primary liver tumors. In eligible patients, the surgical options are partial hepatectomy and liver transplantation. Less than 25% of HCC patients are technically and medically surgical candidates.22,23 Severe cirrhosis is often a contraindication to partial hepatectomy and can be best evaluated using Child-Pugh grade, which uses the severity of encephalopathy and ascites, as well as the absolute values for bilirubin, albumin, and PT; all Child C and many Child B patients are not candidates for surgery. Additional contraindications to resection include prolonged bleeding time, vascular invasion (particularly the portal or hepatic vein), and poor overall medical condition. Patients with IHCC are less likely to have underlying liver disease, but still are often unresectable because of other limitations to curative surgical excision, including lymphadenopathy, vascular involvement, metastases, and multilobar involvement.24
Modern series of hepatic resection for HCC report operative mortality rates of less than 5%,22 because of modern diagnostic, surgical, anesthetic, and postoperative monitoring techniques. Chen and associates24 had a 4% operative mortality in 120 patients in China, 46% of whom had cirrhosis (none were classified as Child C). Postoperative morbidity rates have also varied; most modern surveys show severe complication rates of 5% to 10%. These include bleeding, hepatic failure, subphrenic abscess, sepsis, pleural effusion, and bile leakage.24,25
Adverse prognostic factors for recurrence of HCC after liver resection include tumor size, elevated bilirubin or alkaline phosphatase levels, profound weight loss (>25%), ascites, extracapsular extension, positive margins, portal vein involvement, and nonfibrolamellar histologic findings.7,22,24 Ikeda and associates25 reported higher recurrence rates after curative resection if there were multiple nodules, high histologic grade, or negative hepatitis C antibody results.
Okuda and associates13 observed a 7-year overall survival rate of 45% in their select group of HCC patients who underwent resection with curative intent. Ikeda and associates25 reported a 5-year cumulative survival rate of 69% in patients managed very aggressively following recurrence after curative resection. Other surgical survival rates are lower: Chen and associates24 had a 26% 5-year rate, and rates in the United States are generally under 35%.26
Operative therapy for IHCC is often hampered by positive resection margins, even with extensive resection, often including removal of adjacent structures, such as the extrahepatic biliary tree, vascular structures, the diaphragm, or bowel.27 Carpizo and D’Angelica27 cite Lang et al., who evaluated the outcomes of 27 patients who underwent resection for IHCC and demonstrated that achieving an R0 resection was associated with a better survival. The addition of a portal lymph node dissection for IHCC is controversial and may be based on the level of suspicion of metastatic invovlement.27
Liver transplantation can be a curative option for patients with primary liver tumors who fulfill the selection criteria. In addition, transplantation restores liver function in patients with underlying cirrhosis and addresses the potential for multifocal disease.28 Ringe and Iwatsuki’s 1- and 3-year survival rates for liver transplantation were, respectively: stage I, 75% and 75%; stage II, 80% and 60%; stage III, 60% and 40%; and stage IVa, 50% and 15%.29,30 However, given the high-risk of liver transplantation and the limited organs available, strict selection criteria have been established to identify patients most likely to benefit from transplantation. Using the Milan criteria (solitary tumors of less than 5 cm and in those who have up to three tumor nodules, each of which is smaller than 3 cm), excellent results of transplantation for HCC have been reported, with 5-year survival rates exceeding 70%, a rate similar to that in patients who undergo liver transplantation for a nonmalignant disease.31
The role of liver transplantation for IHCC is less well defined than that for HCC. After early enthusiasm, studies of transplant for even the earliest stage IHCC have shown poor outcomes. However, investigators from the Mayo Clinic reported encouraging outcomes with neoadjuvant chemoradiation followed by liver transplantation.32 Eligibility included unresectable IHCC or IHCC in the setting of primary sclerosing cholangitis with no extrahepatic or lymph node metastases as determined by laparotomy. Of 71 patients enrolled, 38 underwent liver transplantation. The 3- and 5-year survival rates for this group were 82% and 82%, respectively. These results compared favorably to another group of patients that underwent resection whose 3- and 5-year survival rates were 48% and 21%, respectively.
Systemic Therapy
The use of chemotherapy for primary liver tumors has been disappointing. For HCC, intravenous agents, both single and multiple, have an overall response rate of 0% to 20% (average around 15%) with virtually all of these being partial responses. Intra-arterial chemotherapy has a higher response range (15% to 70%, average about 50%),22 but has not been proven to improve survival.33
Chemotherapy for IHCC also has a limited role. Historically, fluorouracil (5-FU)–based chemotherapy regimens have had poor response rates; however, with gemcitabine, response rates have improved. Several phase II studies have looked at the combination of gemcitabine and cisplatin for advanced cholangiocarcinomas, showing more promising results with response rates in the range of 17% to 35%.34–37 Regional chemotherapy via a hepatic artery infusion is also being investigated. Hepatic artery infusion with floxuridine and dexamethasone was studied in 34 patients with unresectable primary liver tumors at Memorial Sloan-Kettering Cancer Center. Partial responses were seen in 16 patients (47.1%); time to progression and response duration were 7.4 and 11.9 months, respectively. With median follow-up of 35 months, the median survival was 29.5 months and the 2-year survival was 67%.38
The introduction of targeted agents during the last decade has led to the development of new strategies to treat HCC even in patients with underlying cirrhosis. The Sorafenib Hepatocarcinoma Assessment Randomized Protocol trial was a multicenter, phase III, double-blind, randomized trial demonstrating a significant benefit in overall survival in patients with advanced HCC treated with sorafenib versus placebo.39 Sorafenib is a multikinase inhibitor that simultaneously inhibits molecular components of the Raf-MEK-ERK and the vascular endothelial growth factor receptor (VEGFR)–1, VEGFR-2, VEGFR-3, and platelet-derived growth factor receptor–β signaling pathways.39 Thus, it inhibits both tumor growth and angiogenesis. In this study, patients receiving sorafenib had almost a 3-month longer median survival and time to radiologic progression (10.7 versus 7.9 months and 5.5 versus 2.8 months, respectively). This study was limited to patients with well-preserved liver function (Child-Pugh class A), but appeared to be safe in this population. A second phase III study confirmed these results in Asian patients.40
Based on the results of these trials, sorafenib has become the standard of care for selected patients with Child-Pugh class A liver function with disease that is unresectable, extensive, and not suitable for liver transplantation; local-only disease in patients who are medically inoperable; or metastatic disease.41
Nonsurgical Local Therapy Options
Given that the majority of primary liver tumors are not amenable to surgical therapy, a number of other modalities have been examined. The hepatic artery is a site of considerable interest, as it is generally the principal supply for the major portion, particularly centrally, of HCC tumors.22 Minimally invasive procedures such as hepatic artery ligation or embolization have been used with HCC, but are contraindicated in the presence of severe cirrhosis, portal vein invasion, or thrombosis.7 Embolization can have short-lived effects because of recanalization. Although some studies suggest an improvement in survival with chemoembolization, randomized trials have failed to confirm this.22,42,43 Cryosurgery has been attempted in small series. Ethanol injections, both percutaneously and intraoperatively, have been used for small primary tumors and for recurrent nodules. Necrosis at the site readily occurs, although hepatic recurrences elsewhere remain the rule. It seems to work best for lesions smaller than 3 cm and is a common technique in Japan.7 Hot saline has also been used for intralesional injection. A recent report describes no recurrences (with limited follow-up) and pathologic demonstration of no residual tumor in the few patients who underwent post-treatment biopsies.44
Radiation Therapy Options
Early on, researchers determined that low doses of anterior-posterior–posterior-anterior hepatic irradiation were ineffective in controlling gross HCC disease and that higher doses of whole-liver irradiation resulted in high rates of radiation hepatitis. Stevens45 states that 75% of patients treated with 40 Gy or greater to the whole liver will develop liver dysfunction and reports Finney’s study46 showing that 4 of 52 patients treated with more than 55 Gy had fatal hepatitis. These widely quoted early results have resulted in RT being placed on the “palliative” list of potential HCC therapies. More recent work has emphasized that, although high doses of radiation cannot be administered to the whole liver, this does not necessarily imply that RT has no value, but rather that classic approaches to portals and fractionation must be abandoned and that innovative techniques should be evaluated. These fall into five categories: technical innovations designed to minimize normal hepatic irradiation, new fractionation protocols, new modalities, the use of radioisotopes, and the use of multimodality treatment.
With the development of three-dimensional conformal RT, high-dose, partial liver irradiation was introduced for patients with unresectable liver tumors.47–49 Investigators at the University of Michigan have reported a phase II study of three-dimensional conformal RT and concurrent intra-arterial floxuridine chemotherapy for unresectable hepatobiliary disease. They treated 128 patients with unresectable HCC (n = 35), IHCC (n = 46), or colorectal hepatic metastases (n = 47).50 Doses ranged from 40 to 90 Gy (median, 60.75 Gy) and were based on the amount of normal liver tissue, evaluated by dose-volume histograms, which could be effectively excluded from the fields to a maximum risk of radiation-induced liver disease (RILD) of 10% to 15%. At a median follow-up time of 16 months (26 months in patients who were alive) the median survival was 15.8 months and the actuarial 3-year survival was 17%. These numbers exceed those quoted for radiation or regional chemotherapy alone and match most surgical series, and should serve as the basis for future studies using dose escalation and radiation sensitizers. Overall toxicity was acceptable, with only 38 patients (30%) developing toxicity of grade 3 or higher. Five patients developed RILD, one of whom died.
Alternative fractionation schemes have also been introduced in the treatment of primary liver tumors. Both hyperfractionation (lower dose per fraction, twice-daily treatments) and hypofractionation (high dose per fraction in fewer fractions) have been applied to improve response rates in liver malignanceis. The rationales for hyperfractionation are the rapid doubling time of HCC tumors (estimated at 41 days) and the shortened treatment times. Hyperfractionation was used in the University of Michigan series.47–50 In a nonrandomized study by the Radiation Therapy Oncology Group (RTOG),51 treatment to the whole liver was given to 194 patients with advanced HCC (70% to 80% had metastases or had failed prior treatment). Conventional fractionation (3 Gy four times per week to 21 Gy) was used in 135 patients, whereas 59 received 24 Gy in 1.2-Gy fractions twice a day. Concurrent doxorubicin and 5-FU were given intravenously every other day to both groups. The response rate for both was dismally low (≅20%) and unaffected by fractionation scheme. Both acute esophagitis and thrombocytopenia occurred significantly more often in the group that received twice-daily radiation.
With improved tumor localization techniques and motion-management modalities, hypofractionation using image-guided RT has been evaluated in the treatment of primary liver tumors. Focal, high-dose stereotactic body RT (SBRT) was evaluated in a phase I study of 41 patients with primary liver tumors, the majority of which were HCC. Dawson and colleagues49 prescribed a variable dose (24-54 Gy) given over six fractions; the dose depended on the volume of liver irradiated and the estimated risk of liver toxicity using a normal tissue complication model. Grade 3 elevation of liver enzymes was seen in five patients (12%). No RILD or treatment-related grade 4 or 5 toxicity was seen within 3 months of SBRT, and overall clinical outcomes were better than expected for this high-risk population. This study was limited to patients with Child-Pugh A or less cirrhosis. However, the majority of patients with unresectable HCC have more advanced cirrhosis and the underlying liver dysfunction must be considered when delivering any type of liver irradiation. There are emerging data that dose-volume constraints may be used in cirrhotic patients receiving conformal RT for HCC.52,53 Such guidelines have been shown to allow safe delivery of relatively high doses of conformal RT for HCC.52 These guidelines were used in a phase II study that confirmed three-dimensional conformal RT could be used safely and effectively for patients with tumors smaller than 5 cm and Child-Pugh class A or B cirrhosis.53 SBRT appears to be a promising alternative for patients with unresectable primary liver tumors. This modality may prove to be an option not only for definitive treatment of primary liver tumors, but also as a bridge to transplant for HCC patients.
Hypofractionated, focal RT using charged particles has also been studied, particularly in Asia. In small, nonrandomized studies, high-dose RT delivered with protons has been shown to result in acceptable local control and survival rates for unresectable primary liver tumors.54–56 A recent prospective study of 51 patients treated with proton RT delivered 66 GyE in 10 fractions to patients with one to three HCCs (≤10 cm).55 Of these patients, 20% were in Child-Pugh class B. The 5-year local control and survival were 88% and 39%, respectively. Among patients with solitary tumors and Child-Pugh class A liver function, 5-year survival was 46%. These results are similar to those obtained after surgery. Liver function remained stable or improved in 84% of patients, and no RILD was observed. Investigators at Loma Linda Hospital reported the results of their phase II study in which 34 HCC patients were treated with 63 Cobalt Gy Equivalents (CoGyEq) in 15 fractions using protons, resulting in a 2-year local control rate of 75%.57 Six patients underwent liver transplantation between 6 and 16 months after completion of RT, two of whom had no residual tumor. These newer modalities may be able to minimize normal tissue effects via precise dose localization, and should be investigated further.
Selective internal RT (SIRT) or radioembolization using Yttrium-90 (90Y)–labeled microspheres has gained interest in recent years. 90Y microspheres have several advantages as a potential therapy for liver lesions, including the selective delivery of the radiolabeled microspheres via the hepatic artery as well as the short depth dose afforded by 90Y, a beta emitter. A meta-analysis of SIRT using either resin or glass microspheres demonstrated that, for HCC, a treatment response (complete response, partial response, or stable disease) was seen in 89% for resin microspheres and 78% for glass microspheres.58 In a large, multi-institutional, retrospective review of 515 patients who received 680 treatments of SIRT, stable disease was seen in 76.8%, a partial response in 9.5%, a complete respone in 4.5%, and progressive liver disease in 9%. The treatment was well tolerated with an incidence of RILD in only 4% and non-liver grade 3 or higher toxicity in 6% (gastritis or gastric ulceration).59
Lastly, recent data suggest that the use of combined RT and embolization may give promising results. Seong et al.60 from South Korea evaluated 27 patients who had failed transarterial chemoembolization (TACE; failure judged by incomplete tumor filling of lipiodol adriamycin on angiogram or CT), and went on to receive RT (51.8 Gy ± 7.9 Gy). They found objective responses in 67% of patients, a median survival from RT start of 14 months, and 1-year overall survival of 85%.60 In a separate review, Seong et al.61 evaluated 30 patients treated with TACE followed by planned RT, and noted a 63% objective response rate and a median survival of 17 months. Cheng et al.62 reported similar results, with a median survival of 19.2 months in patients treated with combination radiation and TACE.
Metastases
Radiation offers good palliation for extrahepatic metastases from primary liver tumors. Anecdotal reports have revealed excellent relief of metastatic bone pain (80% to 100%), and Chen and colleagues24 noted that good responses to radiation were seen in 33 patients with metastatic HCC with skin, nodal, pulmonary, and brain lesions, although recurrences were seen.
Follow-Up
After any treatment for primary liver tumors, patients should be closely followed with serial tumor markers (AFP titers or CA19-9), contrast-enhanced CT or MRI, and chest x-ray examinations. Ikeda and co-workers25 make a convincing case for aggressive treatment of recurrences with some of the best survival rates reported in the literature.
Extrahepatic Cholangiocarcinoma
Epidemiology and Etiology
EHCC, or cholangiocarcinoma of the extrahepatic bile ducts, refers to malignancies of the extrahepatic biliary tree, commencing from the biliary confluence (hilum) extending to the common hepatic duct. There are approximately 4500 new cases per year in the United States, with the peak incidence in the eighth decade of life.63,64 EHCC is slightly more common in men, but the exact ratio varies from study to study. A host of predisposing factors have been proposed, as outlined in Table 39-2. The most widely accepted associations are with liver flukes, ulcerative colitis, and congenital (choledochal) biliary cysts. The most common risk factor in the West is primary sclerosing cholangitis, an inflammatory condition often associated with ulcerative colitis.
Table 39-2 Carcinoma of the Bile Duct: Proposed Causal Factors and Associations
Pathologic Conditions
More than 90% of EHCC are adenocarcinomas of varying differentiations. Subclassifications include papillary, nodular, and sclerosing. The World Health Organization histologic classification5 describes three grades and six variations of adenocarcinomas (papillary, intestinal type, mucinous, clear cell, signet ring, and adenosquamous). The remaining 10% of bile duct lesions include squamous cell, mucoepidermoid, granular cell, small cell, adenosquamous carcinoma, leiomyosarcoma, rhabdomyosarcoma, cystadenocarcinoma, carcinoid, Kaposi sarcoma, angiosarcoma, melanoma, and lymphoma.
Clinical Presentation
Initial signs and symptoms include jaundice, pruritus, right upper quadrant or epigastric pain, anorexia, weight loss, nausea, fever, hepatomegaly, and a right upper quadrant mass (Table 39-3). In end-stage disease, patients may have a periumbilical mass, massive ascites and limb edema, a rectal shelf, or supraclavicular lymphadenopathy. When initially diagnosed, EHCC tumors usually appear relatively small and circumscribed; patients rarely present with signs of overt metastatic disease. The bile duct tree is variably involved; 40% to 60% involve the hilus (upper third; Klatskin tumors), 10% to 15% involve the middle third, and 15% to 25% are in the distal third.64,65 Less than 10% of patients present with multifocal or diffuse involvement of the biliary tree.64–66 Although prognosis has historically been considered worse for hilar cholangiocarcinoma than for distal tumors, this is thought to represent the relatively later presentation and failure to institute effective therapy. Recent studies suggest that location in the biliary tree has no effect on survival, provided that complete resection can be performed.65 Approximately 30% of patients have lymph node involvement at presentation65,67; the lymph nodes are a frequent site of recurrence owing to the extensive lymphatics in the bile duct submucosa. Intrahepatic involvement is extremely common at the time of failure, but is usually contiguous with the bile duct lesion itself. Metastases appear in the peritoneum, distant liver, bones, thoracic lymph nodes, and surgical or drain incisions65,68; but often, lethal local recurrence develops before systemic spread is clinically manifested.
| Symptoms |
Diagnostic Studies
Local imaging studies generally begin with ultrasonography to evaluate jaundice, and then proceed to CT scans, which often show dilated intrahepatic ducts and a normal-appearing extrahepatic biliary system. Historically, percutaneous transhepatic cholangiography, ERCP, and angiography were considered standard investigations; currently, however, at Memorial Sloan-Kettering Cancer Center, MRCP, CT, and duplex ultrasonography have replaced these invasive tests and can often provide the same information with less risk to the patient.65 High-quality CT scans can provide indispensable information regarding vascular involvement, level of obstruction, and extent of liver atrophy. Similarly, in the hands of an experienced operator, duplex ultrasonography can provide information not only about vascular invasion, but also about tumor extension within the bile duct and adjacent tissues. MRCP can provide information about the location of the tumor, level of biliary obstruction, presence of isolated ducts, patency of the hilar vascular structures, and the presence of nodal or distant metastases. Invasive cholangiography continues to have multiple uses, however, because it can serve to localize the tumor, act as a biopsy route, and allow for stent placement for biliary drainage or brachytherapy catheters; the need for an invasive procedure needs to be weighed with the risks of biliary intubation. Each modality can serve to pinpoint with considerable accuracy a site of stricture or obstruction, or intraductal disease extent. The primary metastatic study is a chest CT scan.
A tissue diagnosis is preferred, because several other conditions can mimic bile duct cancers, particularly sclerosing cholangitis. Cytologic examination is accurate approximately 60% to 75% of the time.6,65 The optimal method for obtaining tissue is controversial, however. Different institutions prefer brushings, CT-guided needle biopsies, or controlled open procedures. Buskirk and coworkers69 have shown a 37% incidence of peritoneal seeding if there is violation of the lesion, and they recommend the use of transabdominal fine-needle biopsies following stent placement.
Staging
EHCC can be classified by location in the biliary tree as hilar (at or above the bifurcation of the hepatic duct) and distal (below the bifurcation, including the intrapancreatic bile duct).70 Tumors of the hepatic duct bifurcation are classified into four types according to the Bismuth classifciation71 shown in Fig. 39-4.
The AJCC TNM staging classification for extrahepatic bile duct carcinoma is provided in Table 39-4. It has limited applicability in evaluating the older medical literature because of its modified and inconsistent use. In addition, pathologists apply and report these criteria inconsistently; biopsy-only specimens can include insufficient tissue for full T staging; and lymph nodes are often not resected, even during curative procedures. Lastly, because this staging system is based on pathologic criteria, it has little value in preoperative staging and prediction of resectability.
Table 39-4 Classification of Carcinoma of the Bile Duct
Therapeutic Modalities
Surgery
The primary curative modality for EHCC tumors is surgery. However, resectability rates are low and tumor location can be a critical factor in resectability, applicable surgical procedure, and survival. As preoperative diagnostic expertise increases, the ensuing ability to safely perform complete resections grows as patient selection improves, particularly with Klatskin tumors.64,72 Contraindications to “curative” surgical resection include hepatic duct involvement up to secondary biliary radicles bilaterally, encasement or occlusion of the main portal vein proximal to its bifurcation, atrophy of one hepatic lobe with encasement of the contralateral portal venous branch or with contralateral involvement of secondary biliary radicles, distant metastases (peritoneum, liver, lung), and significant medical comorbidities.65 EHCC resections demand considerable skill and experience on the part of the surgeon. Klatskin tumors can require lobectomy. Distal bile duct tumors are often treated with a Whipple procedure (pancreaticoduodenectomy). The primary goal of palliative procedures is to improve the quality of life through resolution of jaundice and pruritus, prevention of infection, and improvement in pain. Options include palliative debulking, external or internal stent placement, and bypass operations, including hepaticojejunostomies for proximal disease. Palliative procedures are accompanied by high 30-day mortality rates and short survivals with inconsistent improvements in quality of life.
Survival rates after any surgical resection, even curative ones, are low. Median survivals after gross total resection and palliative surgery have been remarkably consistent during the past decade, with values of 17 to 42 months versus 7 to 10 months.64,66,72,73 Likewise, Boerma,74 in an analysis of more than 1000 patients treated worldwide, found a mean survival of 21 versus 11 months.
During the past 20 years, increasing extended hepatic resections, especially for patients with hilar cholangiocarcinoma, has led to an increase in the percentage of negative margin resections, and an observed improvement in survival in some series.65 Memorial Sloan-Kettering reported on 225 patients presenting with hilar cholangiocarcinomas. Of these patients, 65 had unresectable disease; 160 patients underwent exploration with curative intent; and 80 patients underwent resection: 62 (78%) had a concomitant hepatic resection and 62 (78%) had an R0 resection (negative histologic margins).75
Wide variations in morbidity and mortality are seen because of variabilities in tumor site, disease extension, medical condition, referral patterns, and institutional expertise. The mortality and morbidity rates after curative resection, however, are relatively high when compared with liver resections performed for other indications. Even in large referral centers, mortality rates can be up to 5% to 10%. Postoperative complication rates are also high (39% at Johns Hopkins,76 25% at the University of California–Los Angeles77), and can include wound infection, liver or subphrenic abscess, cholangitis, pulmonary embolus, gastrointestinal hemorrhage, and small bowel obstruction.
Local recurrence is the most common site of failure and cause of mortality: after potentially curative resections, the local failure rate was 59% in the study by Kopelson and associates78 and 53% in the study by Cameron and associates.76
Liver transplantation has been performed at several centers.79,80 Meyer and colleagues80 reported their experience with 207 patients with unresectable cholangiocarcinomas treated with liver transplantation. The 2- and 5-year survival rates were 48% and 23%, respectively. The Mayo Clinic recently published their results with neoadjuvant chemoradiotherapy followed by orthotopic liver transplantation. Of 148 patients enrolled on the protocol, 90 completed treatemnt. Overall, 1-, 3-, and 5-year patient survival was 82%, 63%, and 55%, respectively; 1-, 3-, and 5-year survival after orthotopic liver transplantation was 90%, 80%, and 71%.79 However, the reported high incidence of recurrence both in the allograft and in distant organs after transplant for EHCC suggests that this should not be considered a standard treatment option.65,80
Chemotherapy
The use of chemotherapy has been described in multiple, small, single-institution studies. A pooled analysis of 112 trial arms and 2810 patients evaluating the activity of systemic chemotherapy in advanced biliary tract cancers was recently published.81 The pooled response rate (complete response or partial response) with all chemotherapy agents was 22.6% and the pooled tumor control rate (complete response, partial response, or stable disease) was 57.3%. Gemcitabine and platinum compounds exhibited the highest activity with response rates of 25% and 30%, respectively. Combinations of chemotherapy seems to be more effective than single agents in the palliative setting. For those patients with completely resected disease, there does not appear to be a role for adjuvant chemotherapy; however, phase III trials are needed to further evaluate the role of adjuvant chemotherapy.82 An ongoing intergroup study is examining the role of adjvuant gemcitabine and capecitabine followed by RT and capecitabine after resection of EHCCs.
Radiation Therapy
Historically, the use of external-beam RT was largely limited to unresectable, recurrent, or gross residual disease treated in the pre-CT era, and this led to the conclusion that extrahepatic bile duct carcinomas were radioresistant. There is, however, no evidence that bile duct tumor cells have an inherent radioresistance, but rather that the doses of radiation generally administered have been limited by the surrounding structures. Indeed, some recent reports indicate that radiation limited to relatively small fields but reaching higher dose levels, combined with more modern surgical approaches and techniques, may have a positive effect on outcome.67,68,76 Generally, this improvement has been found to be most marked in the setting of minimal residual disease,67 although occasionally an advantage is seen in unresectable patients treated with stents and radiation.76
The indications for postoperative RT are not yet clear. Patients with negative resection margins are rarely referred for consideration of RT, although the available data suggests isolated local-regional recurrence rates of up to 60% after curative resection. Investigators at the University of Michigan published their retrospective experience with adjuvant RT after a potentially curative resection in 81 patients with localized EHCC.83 With a median follow-up time of 1.2 years, the median overall survival and progression-free survival were 14.7 and 11 months, respectively. Complete resection (R0) was the only predictive factor significantly associated with increases in both overall and progression-free survival and there was no difference in outcomes between R1 and R2 resections. The first site of failure was locoregional in more than two-thirds of patients. Clearly, local failure plays a significant role in many EHCC deaths, and this is a persistent problem after surgery with or without irradiation, and after irradiation alone.67,68
Prognostic factors that may assist in determining which patients might benefit from adjuvant therapy include age younger than 65 and small tumor size.66 The degree of differentiation, lymph node involvement, local invasiveness, and papillary characteristics may also serve as prognosticators. All of these factors must be weighed with the demands of upper quadrant irradiation, the patient’s overall medical status, and the significant quality-of-life issues raised by this disease. As always, by far the most important prognosticator for survival is the extent of resection.
Brachytherapy has been used at numerous institutions. Overall, there appears to be a small trend toward improved survival with its use as a boost.65 The potential advantages are clear: the area is reasonably accessible via stents placed during surgery or by the interventional radiologist; a high dose can be limited to a few surrounding centimeters; and the risk of serious short-term toxicity is low, although most series report rates of cholangitis of 30% to 50%. Dose distributions can be quite satisfactory with the use of iridium-192 (192Ir) catheters. Ideally, an external-beam dose designed to eradicate microscopic disease (i.e., 45 to 50 Gy) is given first, followed by seed placement designed to give an additional high dose to the site of gross or highly suspect disease. Doses ranging from 15 to 100 Gy have been administered using iodine-192 (192I), iodine-131 (131I), and 198Au. Dose is usually prescribed at 0.5 to 1 cm from the radiation sources.
Long-term complications secondary to the high doses and use of stents are common, and include stricture, bowel obstruction, and bleeding. In addition, the rapid dose falloff means that some of the high-risk area may well lie outside the high-dose area, so brachytherapy should only be used in conjunction with external-beam RT; no study has shown that brachytherapy alone has anything but the briefest of palliative effects. Reports of high-dose-rate brachytherapy are just beginning to appear in the literature.84
Intraoperative RT has been used on EHCCs at several institutions. After promising preliminary work in Japan, a RTOG protocol (85-06) gave 14 to 22 Gy as intraoperative RT before 45 to 50 Gy external beam to eight evaluable patients with unresectable, unresected, or recurrent tumors. Two patients were alive (one with no evidence of disease) at the time of publication with a median follow-up of 10.5 months.85 In their study of 15 patients, Busse and co-workers86 had a median survival of 14 months with a 50% local control rate using 5 to 20 Gy. In the study by Buskirk and co-workers,69 the only long-term survivors had either intraoperative RT or a brachytherapy boost. All of these reports involve the use of both intraoperative and external-beam RT, because the use of the intraoperative RT alone (or brachytherapy alone) has not been shown to have a durable effect. Intraoperative electron doses of 15 to 25 Gy have been given, again before external-beam treatments of approximately 45 to 50 Gy. Buskirk and colleagues69 leave transhepatic catheters in place after this procedure.
Gallbladder Cancer
Epidemiology and Etiology
Gallbladder cancer is the sixth most common cancer of the gastrointestinal tract. It is estimated that gallbladder cancer was diagnosed in 9760 patients in the United States in 2009, of whom 3370 will die of their disease.1 Gallbladder cancer is found almost three times more often in women than in men. High incidences are also seen in American Indians and Americans of Mexican origin.87 The incidence of gallbladder cancer increases steadily with age, and reaches its maximum in the seventh decade,88 with a median age of occurrence of invasive carcinoma of 73 years. During the past several decades, the incidence of gallbladder cancer in Canada, the United Kingdom, and the United States has stabilized or declined; this trend appears to be linked to the rising number of cholecystectomies. Of note, gallbladder cancer is found in approximately 1% of all routine cholecystectomy specimens, and it is estimated that one fewer death from gallbladder cancer occurs for about every 100 cholecystectomies done during the preceding year.89
Although the cause of gallbladder cancer is unknown, a number of risk factors have been proposed, based on epidemiologic data. The most important risk factor for gallbladder cancer is a history of gallstone disease. Of patients with gallbladder cancer, more than 75% have cholelithiasis. Overall, the risk of developing gallbladder cancer is approximately 4 to 5 times higher in patients with gallstones than in patients without gallstones.90 However, in autopsy series, gallbladder cancer is found in only 1% to 3% of patients with gallstones.91
Other variables associated with elevated gallbladder cancer risk include an elevated body mass index, high total energy intake, high carbohydrate intake,92 and anomalous junction of the pancreaticobiliary ducts.93,94 Patients who suffer from Mirizzi syndrome (a rare complication of long-standing cholelithiasis, defined as obstructive jaundice caused by external compression of the common hepatic duct by an impacted stone in the gallbladder neck) may be at significant risk of developing a gallbladder cancer.95 Increasing gallstone size has also been implicated in increasing risk for gallbladder cancer.96 Other risk factors include infection with typhoid,97 ulcerative colitis (relative risk [RR] 5-10),98 porcelain gallbladder (RR 14),99 and family history of gallbladder cancer (RR 3).100
Pathologic Conditions
Multiple genetic changes are associated with development of gallbladder cancer. It is known that many gallbladder cancers are the endpoint of a sequence that starts with dysplasia, then carcinoma in situ, followed lastly by development of frank invasive cancer. This sequence of events is thought to occur during a period of 10 to 15 years. However, in contrast to colonic lesions, based on genetic analysis, adenomas are not thought to be the precursor lesions to gallbladder carcinomas in the majority of cases.101–106
Classification of primary cancers of the gallbladder is provided in Table 39-5. More than 95% of gallbladder cancers are carcinomas. Sarcomas and other unspecified histologic findings occur less than 2% of the time.107–113 Overall, the stage of disease appears to be the most reliable predictor of outcome, regardless of histologic stage or grade.112
Table 39-5 Gallbladder Cancer: Distribution by Histologic Finding*
| Histologic Type | Subtypes, % | 5-Yr OS, % |
|---|---|---|
| Carcinoma | 99.0 | 12.1 |
| Adenocarcinoma (all types) | 89.4 | 13.0 |
| Adenocarcinoma (NOS) | 73.9 | 32.0 |
| Papillary adenocarcinoma | 5.5 | 40.0 |
| Mucinous adenocarcinoma | 5.3 | 7.8 |
| Other adenocarcinomas | 4.7 | 13.0 |
| Other carcinomas | 7.8 | 13.0 |
| Squamous cell carcinoma | 1.8 | 8.8 |
| Sarcoma | 0.2 | NR |
| Carcinoid | <1.0 | NR |
| Primary melanoma | <1.0 | NR |
| Basal cell carcinoma | <1.0 | NR |
NOS, Not otherwise specified; NR, not reported; OS, overall survival.
Clinical Presentation
Carcinoma of the gallbladder occurs rarely, and clinical symptoms often mimic benign gallbladder disease until invasion of surrounding structures causes increasingly severe symptoms. The most frequent presenting symptom is right-upper-quadrant abdominal pain. In advanced disease, jaundice, anorexia, weight loss, nausea and vomiting, and a palpable right-upper-quadrant mass (Courvoisier sign) may be found. Physical examination findings in advanced disease also can include a periumbilical mass (Sister Mary Joseph node), hepatomegaly, a rectal shelf (Blumer shelf, resulting from peritoneal seeding), or supraclavicular lymphadenopathy. Because of the vague symptoms with which it presents, carcinoma of the gallbladder is extremely difficult to diagnose early, and a high index of suspicion is recommended, especially for patients older than 70 years of age who present with recent weight loss and prolonged right-upper-quadrant pain. In the majority of patients at presentation, resection is made impossible by virtue of local invasion of the liver, biliary ducts, and adjacent structures.114–116
Laboratory evaluation should include a complete blood count, screening profile, and liver-function tests. A high alkaline phosphatase with minimal rise in bilirubin can be found with limited obstruction of the intrahepatic bile ducts. Many patients with advanced disease have anemia, hypoalbuminemia, and abnormal liver-function tests.114 If the index of suspicion is high, serum carcinoembryonic antigen or CA19-9 can be considered.117,118
Diagnostic Studies
Ultrasound and CT scan are often the first studies obtained in evaluation of a patient with jaundice or right-upper-quadrant pain or both. These studies may reveal dilated intrahepatic bile ducts, a mass replacing the normal gallbladder, diffuse or focal thickening of the gallbladder wall, or a polypoid mass within the gallbladder lumen. Often, adjacent organ invasion (e.g., the liver) is seen. Because most patients present with advanced disease, it is important to evaluate for periportal and peripancreatic lymphadenopathy, hematogenous metastases, and peritoneal carcinomatosis.119 CT identifies 60% to 80% of primary tumors.120 Advances in MRI may offer more detailed information than either CT scan or ultrasound, and can be used to identify the level of obstruction and the site of underlying tumor in neoplastic pancreaticobiliary duct obstruction.121,122 Endoscopic or percutaneous cholangiograms may be used for evaluation of obstructive jaundice, or in cases in which vague symptoms and abnormal blood work prompt further workup and other imaging modalities are nondiagnostic. A correct preoperative diagnosis is made in fewer than 50% of cases, depending on location and stage of tumor.123 If lesions are not considered amenable to surgical resection, bile cytologic examination can be obtained via ERCP without violating the tumor or risking peritoneal or incisional seeding, with sensitivity in diagnosis of cancer of the gallbladder of up to 90%.124,125 If ERCP is unsuccessful, percutaneous fine-needle aspiration is recommended instead of core needle biopsy, because of the decreased risk of needle-tract seeding. The accuracy of this approach is up to 90%, with a negligible false-negative rate.125,126
Staging
The AJCC/International Union Against Cancer staging system for gallbladder cancer is provided in Table 39-6127; the TNM stage has been found to be a strong predictor of patient outcome in many series. The risk of lymph node metastases increases with increasing depth of tumor invasion. Tsukada et al.128 reviewed their experience with 106 patients with gallbladder cancer treated with radical surgery and found that lymph node metastases were present in 0% of T1 tumors, 48% of T2 tumors, 72% of T3 tumors, and 80% of T4 tumors.128
Overall, according to Surveillance, Epidemiology, and End Results data, 25% of gallbladder cancers are “localized” at diagnosis, and 40% have abdominal or distant metastases.114,129
Therapeutic Modalities
Overall, the curative resection rates for gallbladder cancer range from 10% to 30%.130 Outcome in gallbladder cancer is closely related to tumor stage. According to a National Cancer Database report based on information collected from hospital registries across the United States from 1989 to 1995, the 5-year survival rates for patients with stage 0, I, II, III, and IV tumors were 60%, 39%, 15%, 5%, and 1%, respectively. Overall, 5-year survival was less than 5%, with a median survival of 5 to 8 months. According to this report, many patients diagnosed with gallbladder carcinoma between 1989 and 1995 received no definitive therapeutic intervention because of the advanced stage of disease at presentation.131
AJCC Stage IA and IB
By virtue of its poor prognosis, late presentation, and rarity, there is a lack of consensus about surgical management of gallbladder cancer. In general, simple cholecystectomy is thought to be curative for lesions confined to the mucosal layer of the gallbladder,132,133 but these represent less than 20% of all gallbladder cancers.91 Surgical series suggest that only Tis and T1a disease can be managed by simple cholecystectomy; 5-year survivals after simple cholecystectomy for Tis, T1(a-b), and T2 disease were 93%, 18%, and 10%, respectively.132–135 In contrast, pT1b disease (invasion of the muscularis propria) necessitates extended cholecystectomy.136,137 After simple cholecystectomy, if pathologic resection shows invasion beneath the mucosa, consideration of reoperation and hepatic wedge resection and regional lymphadenectomy is recommended.138 For T2 disease, recent surgical series suggest that aggressive surgical resection can achieve 3-year survival rates of 90% to 100%.139–141 Considering the frequent spread of disease to adjacent liver and regional lymph nodes, many have advocated en bloc resection of the gallbladder, liver resection (ranging from wedge resection of the liver to en bloc resection of segments IVb and V of the liver), and regional lymphadenectomy (including the porta hepatis, posterior pancreaticoduodenal and interaortocaval lymph nodes) in any case for which there is uncertainty about the extent of invasion, or if the tumor is located adjacent to the liver for any stage II or III tumor and for select stage IVa tumors.137 Shirai et al.140,142 have correlated the extent of microscopic angiolymphatic portal tract invasion with the gross depth of direct liver invasion and suggest that this should be used to estimate adequate hepatectomy margins. Similarly, there is disagreement about the extent of lymph node dissection; however, most authors agree that there is little benefit to resection of disease beyond the regional lymph nodes, and significant added morbidity.136,138,140,142–144
AJCC Stage IIA to IV
Management of patients with stage II to IV disease is highly controversial; the benefit of radical surgery is unclear given the dismal long-term survival. However, in select hands, long-term survival has been reported for completely resected stage III and IV disease (5-year survival rates 40%-67% and 19%-35%, respectively).136,141,145 Of note, these series use older versions of the AJCC staging; thus, many of the patients most likely had stage II to III disease, not stage IV disease.
The Role of Adjuvant Radiation Therapy
A review of the literature by Kopelson and Gunderson found that up to 86% of patients with gallbladder cancer treated curatively failed with locoregional disease.78,146 The use of adjuvant RT has been proposed as a method to decrease local-regional failure. Despite a number of largely retrospective series reporting a benefit to adjuvant RT (Table 39-7), the small number of patients with mixed diagnoses, stages, and treatments make it difficult to draw any significant conclusions.134,147–157 Because of the relative rarity of gallbladder cancer, and the difficulty in diagnosis while still locally confined, there are no prospective, randomized studies examining the role of radiation and chemotherapy in this disease. However, given its propensity for local failure, some have suggested that a multimodality approach with effective chemotherapy and RT may provide the best chance for long-term survival. Certainly, the previously mentioned retrospective data, as well as data from gastric and gastroesophageal junction carcinomas158 suggest that there may be a benefit for chemoradiation in these tumors. Therefore, it may not be unreasonable to recommend adjuvant RT for resected, locally advanced gallbladder cancers, or for unresectable disease. However, it is important to note that, in contrast to bile duct tumors, gallbladder cancers are much more likely to fail distantly in addition to locally, thus implying an even greater need for improvements in systemic therapy: Isolated local/regional local failure was seen in only 15% of gallbladder patients after curative resection in one large series, compared with findings of distant failure in 85%.159 Based on this limited data, a prospective multi-institutional phase III study would be of significant value; without this information, no standard treatment recommendations can be given, and treatment decisions must be made on a case-by-case basis.
Radiation Therapy for Unresectable Disease
RT has been used as primary treatment for unresectable gallbladder cancers for relief of jaundice (usually after percutaneous transhepatic biliary drainage has been established) and pain.159–162 It has also been used to prolong stent patency, with rare long-term survivors.163–164 Further trials are needed to define the best local and systemic therapies in this population.165–172
Role of Chemotherapy
The role of chemotherapy in management of gallbladder cancer has not been well defined. Multiple agents have been tried, including 5-FU, adriamycin, l-(2-chloroethyl-3-4-methylcyclohexyl)-l-nitrosourea (methyl-CCNU), irinotecan, mitomycin C, and cisplatin, with few objective responses.91,166–167,173 The Eastern Cooperative Oncology Group conducted a prospective three-arm randomized trial comparing oral 5-FU, oral 5-FU and streptozocin, and oral 5-FU and methyl-CCNU. There was no significant difference among treatments with respect to response or survival.165 Hepatic arterial infusion with 5-FU–based chemotherapy has been used, with mixed results.168,169 Early experience with gemcitabine suggests promising response rates.170–172 Further prospective trials are needed to assess the role of chemotherapy in gallbladder carcinoma.
Hepatic Metastases
The presence of liver metastases poses a common therapeutic problem in the management of multiple malignancies. They not only represent systemic disease, but can adversely affect quality of life and contribute to the cause of death. Liver metastases are frequently seen in colorectal, breast, lung, neuroendocrine, and gastrointestinal malignancies. With the exception of colorectal cancer, most liver metastases develop late in the course of disease. In the United States, the most likely type of metastases to the liver originates from a colorectal primary lesion. Approximately 50% of patients with colorectal cancer eventually develop metastatic disease to the liver.174–176 In 20% of patients with colorectal cancer, the liver is the only site of metastases. Surgical series demonstrate that the completeness of surgical resection of isolated hepatic metastases can vary, depending on patient selection.177 Patients with isolated colorectal lesions and favorable prognostic factors may experience a 5-year survival of 40%-60%.176,178–179 The prognosis of patients with untreated liver metastases, however, can be poor, with a 3-year survival of less than 5%180 and median survival of 5 to 8 months.181,182
Whereas resection of limited number of isolated liver metastases from colorectal cancer has been associated with improved survival,178,183–186 the data demonstrating a survival benefit in the resection of liver metastases from noncolorectal malignancies is scant. However, long-term survival has been reported in select patients with oligometastases from breast, ovarian, renal, and gynecologic cancers as well as melanoma and sarcomas.187–189 The achievement of local control therefore represents an important treatment goal in such patients with a well-defined, isolated number of liver metastases.
Local Treatment Options
Surgical resection is the treatment of choice for isolated liver metastases. Unfortunately, only a minority of patients with liver metastases are candidates for surgery, secondary to patient or tumor-related factors such as size, number, and location of the lesions.177 Local treatments that serve as alternatives to surgery for well-defined liver metastases include hepatic artery infusional, isolated hepatic perfusion chemotherapy, radiofrequency ablation, external-beam RT, or a combination of any of these techniques. Not one of these techniques has been proven to be superior to another. Combination therapies have not yet been demonstrated to be more efficacious than any single modality. The overall treatment of liver metastases must be individualized based on the burden of metastatic disease, status of the primary tumor, previous treatments received by the patient, and performance status and treatment goals of the patient.
Whole-Liver Radiation
The RTOG 7609 study was a prospective, nonrandomized trial that evaluated six different whole-liver dose and fractionation schemes in 109 patients with symptomatic liver metastases, ranging from 21 Gy in 7 fractions to 25.6 Gy in 16 fractions.190 Fifty-five percent of patients reported palliation of pain. No incidences of RILD were reported.23
Additional RTOG trials have attempted to improve the efficacy of whole-liver radiation with either altered hyperfractionated schedules or misonidazole radiosensitization. Although these trials demonstrated that whole-liver radiation improved quality of life (decreased pain, nausea, fever, and other symptoms), it had no effect on survival.190–192 In RTOG 8405, sequential groups of patients with hepatic metastases from various sites received hyperfractionated whole-liver radiation with 1.5-Gy fractions twice a day to total doses of 27, 30, and 33 Gy. No improvement was noted in median survival (4 months) between the different dose regimens. However, an increase in the incidence of radiation hepatitis (clinical or biochemical) was noted in patients treated with 33 Gy compared with the lower doses. In this group, a 10% incidence of grade 3 radiation-induced hepatitis was reported at 6 months, leading to closure of study to patient entry.
Finally, RTOG 8003191 was a randomized, controlled trial in which 187 patients were treated with 21 Gy in seven fractions, with or without concurrent misonidazole. Median response rates of 80% and a median survival of 4.2 months were seen in both arms. However, survival rates varied significantly with the site of the primary cancer, the number of liver lesions, and the status of extrahepatic disease. Misonidazole radiosensitization was not found to improve the therapeutic response of whole-liver radiation for liver metastases.
Stereotactic Radiation for Liver Metastases
Stereotactic external-beam radiation is an increasingly attractive modality for patients with a limited number of unresectable liver metastases requiring treatment, adequate normal liver reserve, and tumors smaller than 8 cm, because many of the same patient- and tumor-related factors that exclude surgery as a first-line treatment for isolated liver metastases can also limit the applicability of alternative treatments. In colorectal patients with focal unresectable liver metastases, dose escalation to these lesions have demonstrated improved response rates, symptom control, and survival rates compared with patients treated with lower doses, independent of tumor size.47,193,194
Several groups have reported results of stereotactic radiation to deliver hypofractionated RT for select liver metastases. The majority of clinical experience with stereotactic radiation for liver metastases has been centered on tumors smaller than 5 cm.195–200 However, tumors as large as 8 to 10 cm have been successfully treated with lower-dose, hypofractionated regimens.201,202 Although patients with liver metastases from primary colorectal cancer constitute the majority of those who received stereotactic radiation in many series, patients with metastases from other primary malignancies such as other gastrointestinal tumors, breast cancer, ovarian cancer, bladder cancer, and renal cell cancer are also included. The highlights of select contemporary studies of stereotactic radiation for liver metastases are summarized in Table 39-8. A typical SBRT treatment plan is shown in Fig. 39-5.
As early as 1995, a study from Blomgren et al.201 reported on 14 patients with 17 metastatic liver lesions (11 colorectal primaries) treated with 7.7 to 45 Gy in 1 to 4 fractions. Reported side effects included transient nausea in the majority of patients in the few hours following treatment, as well as one patient with a history of gastritis who subsequently developed hemorrhagic gastritis after radiation treatment.
In a phase I/II dose-escalation trial, Herfarth et al.195 from the University of Heidelberg, Germany, investigated the use of stereotactic single-dose RT in 60 liver tumors (41 primary liver and 56 liver metastatic lesions) in 37 patients treated with stereotactic radiation for liver metastases. The dose was escalated from 14 to 26 Gy, with the 80% isodose surrounding the planning target volume. Median tumor size was 10 cm3 (range, 1-132 cm3). All patients tolerated the treatment well without any major side effects. Local control rate was 81% at 18 months after treatment. In a subsequent publication including patients accrued after the phase I/II study was closed, a lower control rate was reported (67% at 18 months), which was explained by a high proportion of patients with colorectal liver metastases in which the local control rate was 45%, compared with 91% for metastases of other tumor types.203
Other investigators from Germany, Wulf et al.,204 conducted a phase I/II study, including patients with mostly liver metastases and one cholangiocarcinoma, who received 30 Gy in three fractions. A local control rate of 76% and 61% at 1 and 2 years after stereotactic radiation was reported, and the corresponding overall survival rates were 71% and 43%, respectively. No grade 3 or higher toxicities were reported. In an updated publication including some patients treated with a higher dose of 36 to 37.5 Gy in three fractions (65% isodose) or 26 Gy in one fraction (80% isodose), the actuarial local control observed was 92% and 66% at 1 and 2 years, respectively.200 High radiation dose was the only significant factor for local control in a multivariate analysis. No severe acute or late toxicities greater than RTOG/European Organisation for Research and Treatment of Cancer grade 2 were seen. Overall survival at 1 and 2 years was 72% and 32%, respectively, for all patients.
Mendez Romero et al.198 reported the results of a phase I/II study of 25 patients with 34 liver metastases and 11 HCC tumors. Patients with HCC without cirrhosis and patients with HCCs smaller than 4 cm and cirrhosis received a dose of 37.5 Gy (12.5 Gy in three fractions, prescribed to the 65% isodose) in three fractions. Patients with HCCs 4 cm or larger in the presence of cirrhosis received five 5-Gy or three 10-Gy fractions. With a median follow-up of 12.9 months, actuarial local control was 94% and 82% after 1 and 2 years for the entire group, respectively. Three patients with liver metastases developed acute side effects such as elevated γ-glutamyl transferase (n = 2) and asthenia (n = 1). One patient with liver metastases developed portal hypertension with melena. Another patient with Child-Pugh class B HCC developed liver failure and infection and died within the first month after treatment.
Katz et al.196 from the University of Rochester reports the results of the largest series of patients with liver metastases treated with stereotactic radiation. Sixty-nine patients with 174 metastatic liver lesions were treated with a median dose of 48 Gy (range 30-55 Gy, in 5-15 fractions).196 The mean number of metastatic lesions treated was 2.7 cm (range 1-6). With a median follow-up of 14.5 months, 10- and 20-month local control was 76% and 57%, and the overall median survival was 14.5 months. There were no grade 3 or higher toxicities reported.
In more recent studies published during the past few years, Gunven et al.205 from the Karolinska Institute in Sweden reported on seven patients with nine liver metastases who received 20 to 45 Gy in two to five fractions.205 With a lengthy median follow-up time of 117 months, all patients were reported to have survived 5 to 14 years without local recurrence.
Goodman et al.206 recently published the results of a phase I dose-escalation trial of single-fraction stereotactic radiation for liver malignancies. Twenty-six patients, nine of whom had liver metastases and seven of whom had IHCCs or recurrent HCCs, were treated with 18 to 30 Gy. Dose was escalated in 4-Gy increments, with the maximum dose being 30 Gy. No dose-limiting toxicities (defined as grade 3 or higher) were incurred. After a median follow-up of 17 months, the cumulative 1-year local failure rate was 23%. Median survival was 29 months and 2-year actuarial overall survival was 50.4%.
Rusthoven et al.207 from the University of Colorado reported the results of a multi-institutional phase I/II study of stereotactic radiation for liver metastases. In a study of 47 patients with 63 metastatic liver lesions, 49 lesions were assessible for local control. During the phase I aspect of the study, the dose was escalated from 36 to 60 Gy. The dose used in the phase II part of the study was 60 Gy. The median follow-up for the 49 assessible lesions was 16 months. Actuarial local control was 95% at 1 year and 92% at 2 years. For lesions 3 cm or smaller, the local control was 100% at 2 years. Only one patient experienced a grade 3 or higher toxicity.
Lastly, Lee et al.222 from the Princess Margaret Hospital in Toronto, Canada, published their results following a phase I study of stereotactic radiation for liver metastases. Sixty-eight patients with inoperable liver metastases were treated with stereotactic radiation doses individually prescribed, chosen to maintain the same nominal risk of RILD for three estimated risk levels (5%, 10%, and 20%). Additional patients were treated at the maximal study dose in an expanded cohort. The median stereotactic radiation dose was 41.8 Gy (range 27.7 to 60 Gy). Radiation was delivered in six fractions over 2 weeks. The 1-year local control rate was 71% and the median overall survival was 17.6 months. The highest RILD risk level investigated was safe, with no dose-limiting toxicity. Two grade 3 liver-enzyme changes occurred, but no RILD or other grade 3 to 5 liver toxicity was reported. Six patients (9%) reported acute grade 3 toxicities (two gastritis, two nausea, lethargy, and thrombocytopenia) and one (1%) grade 4 toxicity (thrombocytopenia) was seen.
Radiotherapy Treatment-Planning Considerations
Dose-Limiting Toxicities of Liver Radiation
The most concerning and dose-limiting toxicity of liver radiation is RILD. RILD is a clinical syndrome of fatigue, elevated liver enzymes (particularly alkaline phosphatase over liver transaminases), tender anicteric hepatomegaly, and ascites. Pathologic changes consist of pronounced hyperemia acutely, then veno-occlusive disease, marked central venous congestion, sparing of large veins, and atrophy of adjacent hepatocytes chronically, similar to changes seen following bone-marrow transplant or high-dose chemotherapy.208 Pathophysiologically, deposition of fibrin within the central veins, transforming growth factor-β209 and tumor necrosis factor-α activation210 has been observed, postulating that injury occurs in the endothelial cells rather than hepatocytes.211 RILD can be seen 2 weeks to 8 months following the completion of radiation, but usually occurs within the first 3 months after treatment.212 The treatment for RILD is largely supportive. Although most patients can recover with diuretic and steroid treatment, RILD has the potential to lead to liver failure and death.211
Other acute toxicities include transient increase in liver enzymes, reactivation of hepatitis B, or a general decline in liver function. Notably, these toxicities are more commonly seen in patients with HCC,213 but are rare in patients with liver metastases, unless a history of prior liver radiation or underlying liver disease is present, which can increase the risk of RILD.
Liver Tolerance
Traditionally, the efficacy of radiation has been limited by low liver tolerance for whole-liver irradiation. In the early 1970s, it was discovered that, although 18 Gy in eight fractions of 2.23 Gy to the whole liver was tolerable, escalating the dose to 28 Gy in 3.5-Gy fraction sizes resulted in liver toxicity in 8 of 25 treated patients.214,215 In 1991, Emami et al.216 estimated that RILD would occur in 5% of patients who receive 30 to 35 Gy to the whole liver in 1.8-Gy fractions.
Data establishing partial liver tolerances to radiation has been elucidated in a more detailed fashion during the past decade. Historically, the gross, often multicentric nature of the disease has subsequently prevented high-dose partial liver irradiation. Recently, enhanced diagnostic imaging detecting focal liver lesions and the prospective collection of quantitative data on toxicities seen after partial-liver radiation delivered with three-dimensional conformal techniques have allowed a better understanding of partial-liver radiation tolerance, thereby allowing safe dose escalation to parts of the liver. In addition, the advent of three-dimensional conformal external-beam radiation planning, intensity-modulated RT (IMRT), image-guided RT, and enhanced management of breathing motion have allowed for the delivery of higher focal doses of radiation to the liver than what was traditionally permissible in the treatment of intrahepatic malignancies. Studies of dose escalation with fractionated radiation have shown a clear dose response for intrahepatic tumors, including liver metastases,49 and patients with unresectable focal liver malignancies treated with higher rather than lower doses of radiation have been found to sustain improved symptoms, response rates, and survivals.47,193
In 1986, the first quantitative partial liver tolerance analysis of RILD as a function of dose and volume was published, from the Lawrence Berkeley Laboratories,217 in which 11 patients were treated with heavy ions for pancreatic or biliary carcinoma. The whole liver received 10 to 24 Gy equivalents (GyEq) and tumor doses ranged from 53.5 to 70 GyEq. One case of RILD was reported. The authors concluded that doses larger than 30 to 35 GyEq should be limited to 30% or less of the liver when 18 GyEq of whole-liver radiation is delivered at 2 GyEq per fraction in addition to primary radiation. In 1991, Emami et al.216 also reported on TDs for partial liver, estimating the TDs for 5% (TD 5/5) and 50% (TD 50/5) risk of developing liver toxicity after various total liver doses delivered in 1.8- to 2-Gy fraction sizes. It is important to note that the liver toxicities were ascertained retrospectively in 27 patients in whom dose and volume data were available. Nevertheless, based on his data, Emami estimated that TD 5/5s for one-third, two-third, and whole-liver radiation were 50 Gy, 35 Gy, and 30 Gy and TD 50/5s were 55 Gy, 45 Gy, and 40 Gy.216
The most comprehensive experience on partial liver tolerance is the series from the University of Michigan.23,218 In both series, the Lyman normal tissue complication probability model was used to describe the volume dependence of normal tissue toxicity after radiation. This model assumes a sigmoid relationship between dose of uniform radiation delivered to the volume of liver and the risk of toxicity.219 Three parameters are used in the Lyman model: TD50 (the whole-liver dose associated with a 50% risk of developing toxicity), m (describes the steepness of the dose response at TD50), and n (the volume-effect parameter).
In the first report, published in 1992 by Lawrence et al.,23 79 patients with liver metastases or primary liver malignancy received prescribed doses based on the dose-volume histograms of normal liver. Radiation was delivered with three-dimensional techniques, using 1.5 to 1.65 Gy twice daily with concurrent intra-arterial 5-fluorodeoxyuridine (FuDR) or bromodeoxyuridine (BuDR). All patients had Childs-Pugh class A liver function and were prospectively followed for the development of RILD. Although FuDR did not appear to increase the radiosensitivity of the liver, the 9 of 79 patients who developed RILD were those who received whole-liver radiation as all or part of their treatment that produced a mean dose greater than or equal to 37 Gy. The risk of complication was greatly overestimated among patients receiving a high-dose partial liver radiation. A larger magnitude for the “volume effect parameter” than that quoted in the literature was found.
In 2002, Dawson et al.218 updated the University of Michigan experience in 203 patients with liver malignancies treated with hyperfractionated radiation (37 Gy in 1.5 Gy BID) and FuDR or BuDR. Nineteen patients developed RILD, and it was again found that the volume-effect parameter was higher than previously estimated. Moreover, on multivariate analysis, the mean liver dose, the diagnosis of a primary hepatobiliary carcinoma (as opposed to liver metastases), BuDR chemotherapy, and male gender were found to be statistically significant variables contributing to the development of RILD. Moreover, the tolerance of the liver to radiation for liver metastases was found to be higher than for patients with primary hepatobiliary malignancies. The mean liver dose associated with a 5% risk of developing RILD for patients with liver metastases receiving 1.5 Gy per fraction was 37 Gy, versus 32 Gy for patients with primary liver malignancy. For patients receiving 2 Gy per fraction, the values were 32 Gy and 28 Gy, respectively.
Abiding by certain dosimetric guidelines derived from the studies of high-dose focal radiation to liver metastases may help minimize radiation to the normal surrounding liver parenchyma, and therefore limit the mean dose. Strategies include keeping the mean dose for uninvolved liver to less than 32 Gy in 2 Gy per fraction,218 or the total dose for 700 mL of nontumorous liver to less than 15 Gy in three fractions, or ensuring that no greater than 30% of the liver receives 21 Gy in three fractions or 12 Gy in a single fraction.200 It is important to keep in mind that patient-related factors, such as underlying liver disease, that can subsequently compromise normal liver function is also an important predictor of RILD. As mentioned previously, patients with abnormal liver function may develop different types of radiation toxicity213 and may have a different dose-volume relationship for RILD than those characterized in the models by Dawson et al.218 Therefore, care must be taken before extrapolating these described tolerances to different patient populations.
Knowledge of bile duct tolerance has thus far been limited by the presence of pre-existing ductal injury, the frequency of recurrent or residual disease, and the use of relatively moderate doses of radiation. However, the increasing use of intraoperative RT and brachytherapy has resulted in a heightened understanding of bile duct tolerance. Kopelson160 reported asymptomatic biliary fibrosis following external-beam doses of 60 Gy, but this has not been a problem clinically (or perhaps is masked by recurrent disease). Both animal and human data appear to demonstrate minimal risk to the ducts with single intraoperative RT doses less than 20 Gy. Iwasaki and colleagues220 now keep intraoperative RT doses to less than 20 Gy because of a high incidence of hepatic artery stenosis, obstruction, and aneurysm after doses of 20 to 30 Gy. Buskirk and associates69 recommend long-term stent placement as a precaution based on their experience with temporary fibrosis following high brachytherapy doses.
Duodenal and gastric mucosal TD5 values are reported to be 50 Gy. Virtually all practitioners recommend restriction of dose to these structures to less than 55 Gy, and many restrict doses to less than 50 Gy. The TD5 and TD50 values for kidneys are 23 and 28 Gy, respectively.221 The same values for spinal cord injury (myelopathy) are 50 and 60 Gy.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
2 Cha C, DeMatteo RP, Blumgart LH. Surgery and ablative therapy for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35:S130-S137.
3 El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750.
4 Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357.
5 Albores-Saavedra J, Henson DE, Sobin LH. The WHO histological classification of tumors of the gallbladder and extrahepatic bile ducts. A commentary on the second edition. Cancer. 1992;70:410-414.
6 Lotze M, Flickinger J, Carr B. Hepatobiliary neoplasms. In: DeVita VJr, Hellman S, Rosenberg S, editors. Cancer: principles and practice of oncology. Philadelphia: JB Lippincott; 1993:883.
7 Dusheiko GM, Hobbs KE, Dick R, et al. Treatment of small hepatocellular carcinomas. Lancet. 1992;340:285-288.
8 Wong LL, Limm WM, Severino R, et al. Improved survival with screening for hepatocellular carcinoma. Liver Transpl. 2000;6:320-325.
9 Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-523.
10 Lim JH, Choi D, Kim SH, et al. Detection of hepatocellular carcinoma: value of adding delayed phase imaging to dual-phase helical CT. AJR Am J Roentgenol. 2002;179:67-73.
11 Valls C, Guma A, Puig I, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging. 2000;25:490-496.
12 Heiken JP, Weyman PJ, Lee JK, et al. Detection of focal hepatic masses: prospective evaluation with CT, delayed CT, CT during arterial portography, and MR imaging. Radiology. 1989;171:47-51.
13 Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928.
14 Baron RL, Peterson MS. From the RSNA refresher courses: screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics. 2001;21(Spec No):S117-S132.
15 Rode A, Bancel B, Douek P, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336.
16 Vogl TJ, Hammerstingl R, Schwarz W. Diagnostic imaging of hepatocellular carcinoma. Radiologie. 2001;41:895.
17 Manfredi R, Barbaro B, Masselli G, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164.
18 Li J, Kuehl H, Grabellus F, et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J Surg Oncol. 2008;98:438-443.
19 Oberfield RA, Steele GJr, Gollan JL, et al. Liver cancer. CA Cancer J Clin. 1989;39:206-218.
20 Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145-159.
21 Levy I, Greig PD, Gallinger S, et al. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg. 2001;234:206-209.
22 Kemeny N, Schneider A. Regional treatment of hepatic metastases and hepatocellular carcinoma. Curr Probl Cancer. 1989;13:197-283.
23 Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781-788.
24 Chen MF, Hwang TL, Jeng LB, et al. Hepatic resection in 120 patients with hepatocellular carcinoma. Arch Surg. 1989;124:1025-1028.
25 Ikeda K, Saitoh S, Tsubota A, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer. 1993;71:19-25.
26 El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62-65.
27 Carpizo DR, D’Angelica M. Management and extent of resection for intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18:289-305. viii–ix
28 Kassahun WT, Fangmann J, Harms J, et al. Liver resection and transplantation in the management of hepatocellular carcinoma: a review. Exp Clin Transplant. 2006;4:549-558.
29 Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-228.
30 Ringe B, Pichlmayr R, Wittekind C, et al. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285.
31 Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699.
32 Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458.
33 Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535-1547.
34 Giuliani F, Gebbia V, Maiello E, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Ann Oncol. 2006;17(Suppl 7):vii73-77.
35 Kim ST, Park JO, Lee J, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339-1346.
36 Lee J, Kim TY, Lee MA, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2008;61:47-52.
37 Thongprasert S, Napapan S, Charoentum C, et al. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005;16:279-281.
38 Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589-1595.
39 Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
40 Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
41 Benson AB3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350-391.
42 Pelletier G, Roche A, Ink O, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181-184.
43 A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. N Engl J Med. 1995;332:1256-1261.
44 Honda N, Guo Q, Uchida H, et al. Percutaneous hot saline injection therapy for hepatic tumors: an alternative to percutaneous ethanol injection therapy. Radiology. 1994;190:53-57.
45 Stevens KJr. The liver and biliary systems. In: Cox J, editor. Moss’ radiation oncology: rationale, technique, results. St Louis: Mosby-Year Book; 1994:452.
46 Finney R. An evaluation of postoperative radiotherapy in hypernephroma treatment—a clinical trial. Cancer. 1973;32:1332-1340.
47 Robertson JM, Lawrence TS, Andrews JC, et al. Long-term results of hepatic artery fluorodeoxyuridine and conformal radiation therapy for primary hepatobiliary cancers. Int J Radiat Oncol Biol Phys. 1997;37:325-330.
48 Lawrence TS, Kessler ML, Robertson JM. Conformal high-dose radiation plus intraarterial floxuridine for hepatic cancer. Oncology (Williston Park). 1993;7:51-57.
49 Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210-2218.
50 Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739-8747.
51 Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17:1223-1229.
52 McGinn CJ, Ten Haken RK, Ensminger WD, et al. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16:2246-2252.
53 Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies—mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152-1158.
54 Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799-3805.
55 Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-836.
56 Matsuzaki Y, Osuga T, Saito Y, et al. A new, effective, and safe therapeutic option using proton irradiation for hepatocellular carcinoma. Gastroenterology. 1994;106:1032-1041.
57 Bush DA, Hillebrand DJ, Slater JM, et al. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189-S193.
58 Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951-959.
59 Kennedy AS, McNeillie P, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494-1500.
60 Seong J, Park HC, Han KH, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331-1335.
61 Seong J, Keum KC, Han KH, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393-397.
62 Cheng JC, Chuang VP, Cheng SH, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:435-442.
63 Blumgart LH, Thompson JN. The management of malignant strictures of the bile duct. Curr Probl Surg. 1987;24:69-127.
64 Reding R, Buard JL, Lebeau G, et al. Surgical management of 552 carcinomas of the extrahepatic bile ducts (gallbladder and periampullary tumors excluded). Results of the French Surgical Association Survey. Ann Surg. 1991;213:236-241.
65 Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156-176.
66 Schoenthaler R, Phillips TL, Castro J, et al. Carcinoma of the extrahepatic bile ducts. The University of California at San Francisco experience. Ann Surg. 1994;219:267-274.
67 Schoenthaler R, Castro JR, Halberg FE, et al. Definitive postoperative irradiation of bile duct carcinoma with charged particles and/or photons. Int J Radiat Oncol Biol Phys. 1993;27:75-82.
68 Gonzalez Gonzalez D, Gerard JP, Maners AW, et al. Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor). Semin Liver Dis. 1990;10:131-141.
69 Buskirk SJ, Gunderson LL, Schild SE, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg. 1992;215:125-131.
70 Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473.
71 Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178.
72 Langer JC, Langer B, Taylor BR, et al. Carcinoma of the extrahepatic bile ducts: results of an aggressive surgical approach. Surgery. 1985;98:752-759.
73 Blumgart LH, Hadjis NS, Benjamin IS, et al. Surgical approaches to cholangiocarcinoma at confluence of hepatic ducts. Lancet. 1984;1:66-70.
74 Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery. 1990;108:572-580.
75 Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-517.
76 Cameron JL, Pitt HA, Zinner MJ, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159:91-97.
77 Lai EC, Tompkins RK, Mann LL, et al. Proximal bile duct cancer. Quality of survival. Ann Surg. 1987;205:111-118.
78 Kopelson G, Galdabini J, Warshaw AL, et al. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1981;7:413-417.
79 Isaksson B, Thorell LH, Bengtsson F, et al. Hepatic encephalopathy verified by psychometric testing and EEG in cirrhotic patients: effects of mesocaval interposition shunt or sclerotherapy. HPB (Oxford). 2005;7:65-72.
80 Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637.
81 Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896-902.
82 Huitzil-Melendez FD, O’Reilly EM, Duffy A, et al. Indications for neoadjuvant, adjuvant, and palliative chemotherapy in the treatment of biliary tract cancers. Surg Oncol Clin N Am. 2009;18:361-379. x
83 Ben-David MA, Griffith KA, Abu-Isa E, et al. External-beam radiotherapy for localized extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:772-779.
84 Kurisu K, Hishikawa Y, Taniguchi M, et al. High-dose-rate intraluminal brachytherapy for bile duct carcinoma after surgery. Radiother Oncol. 1991;21:65-66.
85 Wolkov HB, Graves GM, Won M, et al. Intraoperative radiation therapy of extrahepatic biliary carcinoma: a report of RTOG-8506. Am J Clin Oncol. 1992;15:323-327.
86 Busse PM, Stone MD, Sheldon TA, et al. Intraoperative radiation therapy for biliary tract carcinoma: results of a 5-year experience. Surgery. 1989;105:724-733.
87 Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119-2152.
88 Nakayama F. Recent progress in the diagnosis and treatment of carcinoma of the gallbladder—introduction. World J Surg. 1991;15:313-314.
89 Diehl AK, Beral V. Cholecystectomy and changing mortality from gallbladder cancer. Lancet. 1981;2:187-189.
90 Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132-1138.
91 Abi-Rached B, Neugut AI. Diagnostic and management issues in gallbladder carcinoma. Oncology (Williston Park). 1995;9:19-24.
92 Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349-364.
93 Chijiiwa K, Kimura H, Tanaka M. Malignant potential of the gallbladder in patients with anomalous pancreaticobiliary ductal junction. The difference in risk between patients with and without choledochal cyst. Int Surg. 1995;80:61-64.
94 Kobayashi S, Asano T, Yamasaki M, et al. Prophylactic excision of the gallbladder and bile duct for patients with pancreaticobiliary maljunction. Arch Surg. 2001;136:759-763.
95 Redaelli CA, Buchler MW, Schilling MK, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997;121:58-63.
96 Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250:2323-2326.
97 Welton JC, Marr JS, Friedman SM. Association between hepatobiliary cancer and typhoid carrier state. Lancet. 1979;1:791-794.
98 Joffe N, Antonioli DA. Primary carcinoma of the gallbladder associated with chronic inflammatory bowel disease. Clin Radiol. 1981;32:319-324.
99 Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699-703.
100 Fernandez E, La Vecchia C, D’Avanzo B, et al. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:209-212.
101 Albores-Saavedra J, Alcantra-Vazquez A, Cruz-Ortiz H, et al. The precursor lesions of invasive gallbladder carcinoma. Hyperplasia, atypical hyperplasia and carcinoma in situ. Cancer. 1980;45:919-927.
102 Kanthan R, Radhi JM, Kanthan SC. Gallbladder carcinomas: an immunoprognostic evaluation of P53, Bcl-2, CEA and alpha-fetoprotein. Can J Gastroenterol. 2000;14:181-184.
103 Roa I, Araya JC, Villaseca M, et al. Preneoplastic lesions and gallbladder cancer: an estimate of the period required for progression. Gastroenterology. 1996;111:232-236.
104 Wee A, Teh M, Raju GC. Clinical importance of p53 protein in gall bladder carcinoma and its precursor lesions. J Clin Pathol. 1994;47:453-456.
105 WistubaII, Albores-Saavedra J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 1999;6:237-244.
106 Hui AM, Li X, Shi YZ, et al. p27(Kip1) expression in normal epithelia, precancerous lesions, and carcinomas of the gallbladder: association with cancer progression and prognosis. Hepatology. 2000;31:1068-1072.
107 Bartlett D, Fong Y, Tumors of the gallbladder. 3rd ed. Blumgart L, Fong Y, editors. Surgery of the liver and biliary tract; vol 1. 2000; WB Saunders, New York:995.
108 Dong XD, DeMatos P, Prieto VG, et al. Melanoma of the gallbladder: a review of cases seen at Duke University Medical Center. Cancer. 1999;85:32-39.
109 Friedman RB, Anderson RE, Gilchrist KW, et al. Prognostic factors in invasive gallbladder carcinoma. J Surg Oncol. 1983;23:189-194.
110 Gruttadauria S, Doria C, Minervini MI, et al. Malignant fibrous histiocytoma of the gallbladder: case report and review of the literature. Am Surg. 2001;67:714-717.
111 Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493-1497.
112 North JHJr, Pack MS, Hong C, et al. Prognostic factors for adenocarcinoma of the gallbladder: an analysis of 162 cases. Am Surg. 1998;64:437-440.
113 Yokoyama Y, Fujioka S, Kato K, et al. Primary carcinoid tumor of the gallbladder: resection of a case metastasizing to the liver and analysis of outcomes. Hepatogastroenterology. 2000;47:135-139.
114 Arnaud JP, Casa C, Georgeac C, et al. Primary carcinoma of the gallbladder—review of 143 cases. Hepatogastroenterology. 1995;42:811-815.
115 Lillemoe KD. Tumors of the gallbladder, bile ducts, and ampulla. Semin Gastrointest Dis. 2003;14:208-221.
116 Perpetuo MD, Valdivieso M, Heilbrun LK, et al. Natural history study of gallbladder cancer: a review of 36 years experience at M. D. Anderson Hospital and Tumor Institute. Cancer. 1978;42:330-335.
117 Ritts REJr, Nagorney DM, Jacobsen DJ, et al. Comparison of preoperative serum CA19-9 levels with results of diagnostic imaging modalities in patients undergoing laparotomy for suspected pancreatic or gallbladder disease. Pancreas. 1994;9:707-716.
118 Strom BL, Maislin G, West SL, et al. Serum CEA and CA 19-9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer. 1990;45:821-824.
119 Levy AD, Murakata LA, Rohrmann CAJr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295-314.
120 Campbell WL, Peterson MS, Federle MP, et al. Using CT and cholangiography to diagnose biliary tract carcinoma complicating primary sclerosing cholangitis. AJR Am J Roentgenol. 2001;177:1095-1100.
121 Schwartz LH, Coakley FV, Sun Y, et al. Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol. 1998;170:1491-1495.
122 Yoshimitsu K, Honda H, Jimi M, et al. MR diagnosis of adenomyomatosis of the gallbladder and differentiation from gallbladder carcinoma: Importance of showing Rokitansky-Aschoff sinuses. AJR Am J Roentgenol. 1999;172:1535-1540.
123 Chijiiwa K, Sumiyoshi K, Nakayama F. Impact of recent advances in hepatobiliary imaging techniques on the preoperative diagnosis of carcinoma of the gallbladder. World J Surg. 1991;15:322-327.
124 Akosa AB, Barker F, Desa L, et al. Cytologic diagnosis in the management of gallbladder carcinoma. Acta Cytol. 1995;39:494-498.
125 Krishnani N, Shukla S, Jain M, et al. Fine needle aspiration cytology in xanthogranulomatous cholecystitis, gallbladder adenocarcinoma and coexistent lesions. Acta Cytol. 2000;44:508-514.
126 Zargar SA, Khuroo MS, Mahajan R, et al. US-guided fine-needle aspiration biopsy of gallbladder masses. Radiology. 1991;179:275-278.
127 Greene F, Page D, Fleming J, et al, Gallbladder; AJCC Cancer Staging Manual. 6th ed. 2002; Springer, New York
128 Tsukada K, Kurosaki I, Uchida K, et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997;80:661-667.
129 Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(Suppl 1):171-190.
130 Levin B. Gallbladder carcinoma. Ann Oncol. 1999;10(Suppl 4):129-130.
131 Donahue JH. The National Cancer Database report on carcinoma of the gallbladder, 1989-1995. Cancer. 1998;83:2618.
132 Bartlett DL. Gallbladder cancer. Semin Surg Oncol. 2000;19:145-155.
133 Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118-122.
134 Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275-280.
135 Wakai T, Shirai Y, Yokoyama N, et al. Early gallbladder carcinoma does not warrant radical resection. Br J Surg. 2001;88:675-678.
136 Bartlett DL, Fong Y, Tumors of the gallbladder. 3rd ed. Blumgart LH, Fong Y, editors. Surgery of the liver and biliary tract; vol 1. 2000; WB Saunders, New York:1003.
137 Ouchi K, Sugawara T, Ono H, et al. Diagnostic capability and rational resectional surgery for early gallbladder cancer. Hepatogastroenterology. 1999;46:1557-1560.
138 Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer. 1998;83:423-427.
139 Bartlett D, Fong Y, Tumors of the gallbladder. 3rd ed. Blumgart LH, Fong Y, editors. Surgery of the liver and biliary tract; vol 1. 2000; WB Saunders, New York:1002.
140 Shirai Y, Tsukada K, Ohtani T, et al. Hepatic metastases from carcinoma of the gallbladder. Cancer. 1995;75:2063-2068.
141 Tsukada K, Hatakeyama K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery. 1996;120:816-821.
142 Shirai Y, Yoshida K, Tsukada K, et al. Radical surgery for gallbladder carcinoma. Long-term results. Ann Surg. 1992;216:565-568.
143 Donohue JH, Nagorney DM, Grant CS, et al. Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg. 1990;125:237-241.
144 Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639-646.
145 Todoroki T, Kawamoto T, Takahashi H, et al. Treatment of gallbladder cancer by radical resection. Br J Surg. 1999;86:622-627.
146 Kopelson G, Harisiadis L, Tretter P, et al. The role of radiation therapy in cancer of the extra-hepatic biliary system: an analysis of thirteen patients and a review of the literature of the effectiveness of surgery, chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 1977;2:883-894.
147 Bosset JF, Mantion G, Gillet M, et al. Primary carcinoma of the gallbladder. Adjuvant postoperative external irradiation. Cancer. 1989;64:1843-1847.
148 Fields JN, Emami B. Carcinoma of the extrahepatic biliary system—results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1987;13:331-338.
149 Hanna SS, Rider WD. Carcinoma of the gallbladder or extrahepatic bile ducts: the role of radiotherapy. Can Med Assoc J. 1978;118:59-61.
150 Houry S, Haccart V, Huguier M, et al. Gallbladder cancer: role of radiation therapy. Hepatogastroenterology. 1999;46:1578-1584.
151 Kraybill WG, Lee H, Picus J, et al. Multidisciplinary treatment of biliary tract cancers. J Surg Oncol. 1994;55:239-245.
152 Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:167-175.
153 Mahe M, Stampfli C, Romestaing P, et al. Primary carcinoma of the gall-bladder: potential for external radiation therapy. Radiother Oncol. 1994;33:204-208.
154 Sasson AR, Hoffman JP, Ross E, et al. Trimodality therapy for advanced gallbladder cancer. Am Surg. 2001;67:277-283.
155 Todoroki T, Kawamoto T, Otsuka M, et al. Benefits of combining radiotherapy with aggressive resection for stage IV gallbladder cancer. Hepatogastroenterology. 1999;46:1585-1591.
156 Treadwell TA, Hardin WJ. Primary carcinoma of the gallbladder. The role of adjunctive therapy in its treatment. Am J Surg. 1976;132:703-706.
157 De Aretxabala X, Roa I, Burgos L, et al. Preoperative chemoradiotherapy in the treatment of gallbladder cancer. Am Surg. 1999;65:241-246.
158 Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730.
159 Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700.
160 Kopelson G, Gunderson LL. Primary and adjuvant radiation therapy in gallbladder and extrahepatic biliary tract carcinoma. J Clin Gastroenterol. 1983;5:43-50.
161 Pilepich MV, Lambert PM. Radiotherapy of carcinomas of the extrahepatic biliary system. Radiology. 1978;127:767-770.
162 Smoron GL. Radiation therapy of carcinoma of gallbladder and biliary tract. Cancer. 1977;40:1422-1424.
163 Miura Y, Endo I, Togo S, et al. Adjuvant therapies using biliary stenting for malignant biliary obstruction. J Hepatobiliary Pancreat Surg. 2001;8:113-117.
164 Uno T, Itami J, Aruga M, et al. Primary carcinoma of the gallbladder: role of external beam radiation therapy in patients with locally advanced tumor. Strahlenther Onkol. 1996;172:496-500.
165 Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54:965-969.
166 Taal BG, Audisio RA, Bleiberg H, et al. Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol. 1993;4:607-609.
167 Sanz-Altamira PM, O’Reilly E, Stuart KE, et al. A phase II trial of irinotecan (CPT-11) for unresectable biliary tree carcinoma. Ann Oncol. 2001;12:501-504.
168 Ishizaki M, Akiyama N, Tanaka S, et al. [Long-term survival of a patient with postoperative liver metastasis of stage IVa gallbladder cancer responding to hepatic arterial infusion chemotherapy]. Gan To Kagaku Ryoho. 2001;28:395-398.
169 Ohta K, Sawamura A, Miyahara E, et al. [A case of inoperable advanced gall bladder cancer responding to intra-arterial infusion of 5-fluorouracil (5-FU) and leucovorin (LV)]. Gan To Kagaku Ryoho. 2001;28:516-519.
170 Gallardo J, Rubio B, Ahumada M, et al. [Efficacy of gemcitabine in the gallbladder cancer. Initial experience in 4 cases]. Rev Med Chil. 2000;128:1025-1030.
171 Castro MP. Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: A case report. Cancer. 1998;82:639-641.
172 Verderame F, Mandina P, Abruzzo F, et al. Biliary tract cancer: our experience with gemcitabine treatment. Anticancer Drugs. 2000;11:707-708.
173 Gebbia V, Majello E, Testa A, et al. Treatment of advanced adenocarcinomas of the exocrine pancreas and the gallbladder with 5-fluorouracil, high dose levofolinic acid and oral hydroxyurea on a weekly schedule. Results of a multicenter study of the Southern Italy Oncology Group (G.O.I.M.). Cancer. 1996;78:1300-1307.
174 Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203.
175 Sherman DM, Weichselbaum R, Order SE, et al. Palliation of hepatic metastasis. Cancer. 1978;41:2013-2017.
176 Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246.
177 Elaraj DM, Alexander HR. Current role of hepatic artery infusion and isolated liver perfusion for the treatment of colorectal cancer liver metastases. Cancer J. 2004;10:128-138.
178 Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766.
179 Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318.
180 Khatri VP, McGahan J. Non-resection approaches for colorectal liver metastases. Surg Clin North Am. 2004;84:587-606.
181 Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. II. The prognosis for patients with hepatic metastases from gastric carcinoma verified by laparotomy and postmortem examination. Digestion. 1969;2:179-186.
182 Jaffe BM, Donegan WL, Watson F, et al. Factors influencing survival in patients with untreated hepatic metastases. Surg Gynecol Obstet. 1968;127:1-11.
183 Figueras J, Valls C, Rafecas A, et al. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg. 2001;88:980-985.
184 Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825.
185 Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240:438-447.
186 Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460-466.
187 Ravikumar TS, Gallos G. Resection of liver metastases: state of the art. Oncology (Williston Park). 2002;16:1240-1256.
188 Fong Y, Blumgart LH, Cohen AM. Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin. 1995;45:50-62.
189 Bozzetti F, Cozzaglio L, Boracchi P, et al. Comparing surgical resection of limited hepatic metastases from colorectal cancer to non-operative treatment. Eur J Surg Oncol. 1993;19:162-167.
190 Borgelt BB, Gelber R, Brady LW, et al. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7:587-591.
191 Leibel SA, Pajak TF, Massullo V, et al. A comparison of misonidazole sensitized radiation therapy to radiation therapy alone for the palliation of hepatic metastases: results of a Radiation Therapy Oncology Group randomized prospective trial. Int J Radiat Oncol Biol Phys. 1987;13:1057-1064.
192 Russell AH, Clyde C, Wasserman TH, et al. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27:117-123.
193 Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14:722-728.
194 O’Connell MJ, Nagorney DM, Bernath AM, et al. Sequential intrahepatic fluorodeoxyuridine and systemic fluorouracil plus leucovorin for the treatment of metastatic colorectal cancer confined to the liver. J Clin Oncol. 1998;16:2528-2533.
195 Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164-170.
196 Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793-798.
197 Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848-855.
198 Mendez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I-II study. Acta Oncol. 2006;45:831-837.
199 Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579-1584.
200 Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838-847.
201 Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861-870.
202 Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45:856-864.
203 Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: update of the initial phase-I/II trial. Front Radiat Ther Oncol. 2004;38:100-105.
204 Wulf J, Hadinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol. 2001;177:645-655.
205 Gunven P, Blomgren H, Lax I, et al. Curative stereotactic body radiotherapy for liver malignancy. Med Oncol. 2009;26:327-334.
206 Goodman K, Wiegner E, Maturen K, et al: Dose escalation study of stereotactic body radiotherapy for liver malignancies, Int J Radiat Oncol Biol Phys 2010 (in press).
207 Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-1578.
208 Reed GBJr, Cox AJJr. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol. 1966;48:597-611.
209 Seong J, Kim SH, Chung EJ, et al. Early alteration in TGF-beta mRNA expression in irradiated rat liver. Int J Radiat Oncol Biol Phys. 2000;46:639-643.
210 Christiansen H, Saile B, Neubauer-Saile K, et al. Irradiation leads to susceptibility of hepatocytes to TNF-alpha mediated apoptosis. Radiother Oncol. 2004;72:291-296.
211 Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279-283.
212 Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-1248.
213 Cheng JC, Liu HS, Wu JK, et al. Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2005;62:1150-1156.
214 Wharton JT, Delclos L, Gallager S, et al. Radiation hepatitis induced by abdominal irradiation with the cobalt 60 moving strip technique. Am J Roentgenol Radium Ther Nucl Med. 1973;117:73-80.
215 Perez CA, Korba A, Zivnuska F, et al. 60Co moving strip technique in the management of carcinoma of the ovary: analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 1978;4:379-388.
216 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
217 Austin-Seymour MM, Chen GT, Castro JR, et al. Dose volume histogram analysis of liver radiation tolerance. Int J Radiat Oncol Biol Phys. 1986;12:31-35.
218 Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810-821.
219 Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13-S19.
220 Iwasaki Y, Todoroki T, Fukao K, et al. The role of intraoperative radiation therapy in the treatment of bile duct cancer. World J Surg. 1988;12:91-98.
221 Rubin P. Law and order of radiation sensitivity. Absolute versus relative. Front Radiat Ther Oncol. 1989;23:7-40.
222 Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585-1591.
223 Vaittinen E. Carcinoma of the gallbladder: a study of 390 cases diagnosed in Finland 1953-1967. Ann Chir Gynaecol. 1970;59:1.
224 Houry S, Schlienger M, Hunguier M, et al. Gallbladder carcinoma: role of radiation therapy. Br J Surg. 1989;76:1578.

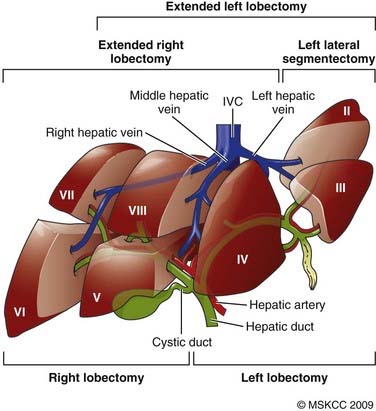
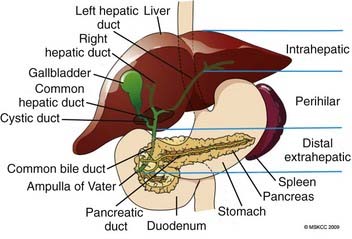
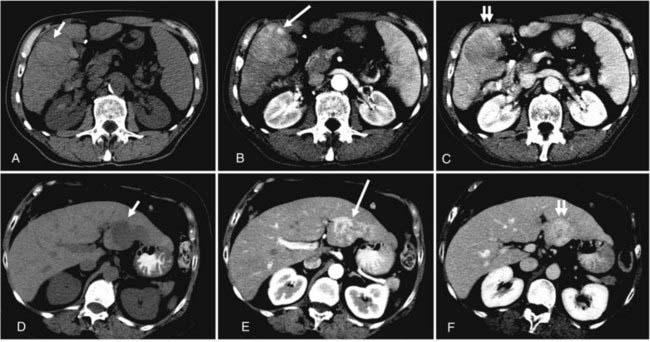
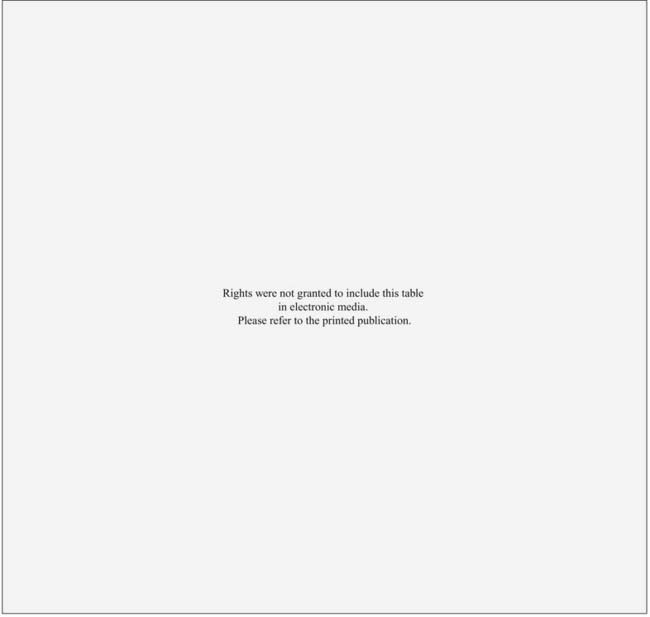
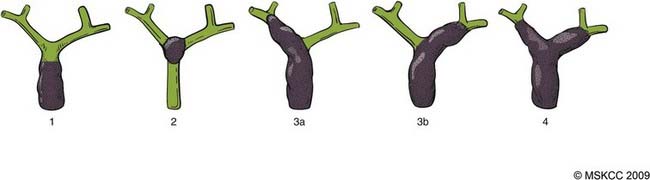
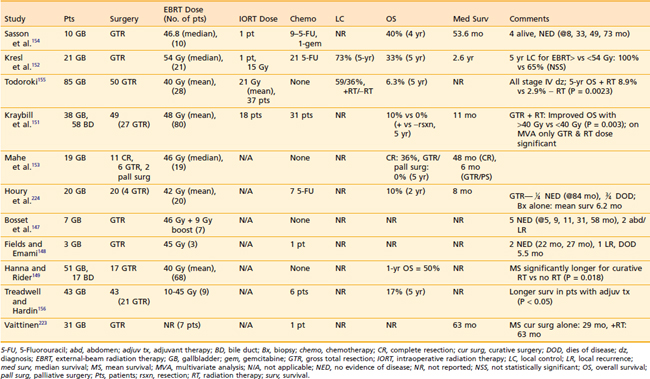
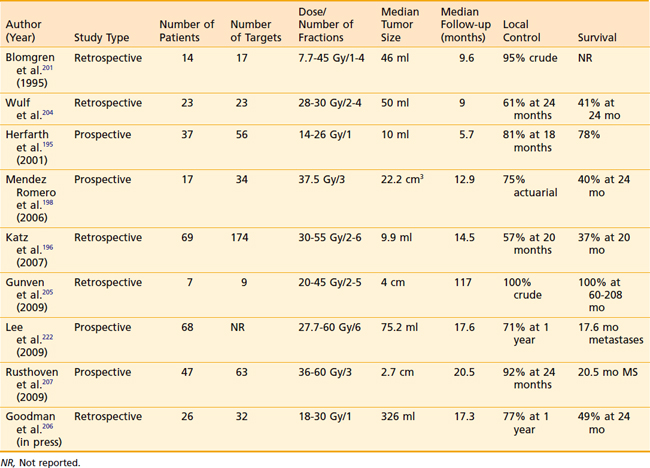
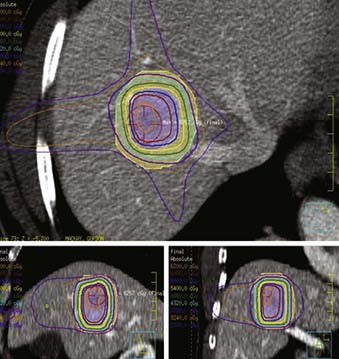
 hour before simulation to allow for visualization of the small bowel and stomach.
hour before simulation to allow for visualization of the small bowel and stomach.




