31 Cancer of the Larynx
Epidemiology, Etiology, and Genetics
Larynx cancers are the most common malignancy of the upper aerodigestive tract; they account for nearly 1% of all malignancies and approximately 25% of head and neck tumors. The estimated number of newly diagnosed larynx cancers in 2008 is 12,250, and the estimated number of deaths from larynx cancer is 3670.1 Primary glottic cancers are approximately three times more common than supraglottic tumors; tumors of the subglottic larynx are exceedingly rare, accounting for approximately 1% to 2% of all larynx cancers. Nearly 80% of larynx cancers occur in men. Larynx cancer is the most curable cancer of the upper aerodigestive tract, but the 5-year survival rate of approximately 65% has remained relatively unchanged during the previous three decades.2 In fact, larynx cancer represents one of the only malignancies for which the 5-year survival has not improved during this period. Great strides, however, have been made with regard to organ preservation in the treatment of this disease, and the majority of patients are now treated with upfront organ preservation protocols.
It is estimated that 75% of larynx cancers are attributable to cigarette smoking and alcohol use. A large international head and neck cancer epidemiology consortium found that the relative risk of smoking is more modest than previously believed.3 After cessation of smoking, the excess risk gradually declines and is no longer evident after 20 years.4 For many years, alcohol and tobacco were thought to act synergistically,5 but more recent data suggests that the two are independent risk factors.3 The effect of smoking and alcohol is greater for supraglottic cancers than glottic cancers.6 People who employ their voices extensively in their work also appear to be at higher risk of developing larynx cancer. In addition, occupational exposure to asbestos, diesel fumes, rubber, and wood dust may contribute to the development of larynx cancer, but a history of tobacco use is also generally present.6 Vitamin and nutrient deficiencies may contribute to the development of larynx cancer.7 Several studies have also implicated gastroesophageal reflux disease (GERD) in the development of larynx cancer, especially in patients without a history of alcohol or tobacco use.8,9 GERD leads to chronic irritation of the larynx, which may lead to the development of larynx cancer. Human papillomavirus is strongly associated with tonsillar cancer, but its role in the etiology of larynx cancer is much less established.10,11
A molecular etiology for larynx cancer is emerging.12,13 Alterations in a variety of chromosomes, oncogenes, tumor suppressor genes, growth factors, and a number of other cell-cycle regulators have been identified. Mutations in p53, Ki-67, Chek-2, EGFR, h-TERT, cyclin D1, cathepsin D, and TGF-B have been identified.14–16 P53 is the most commonly mutated tumor suppressor gene in human cancers. Mutation of the p53 gene is seen in nearly 50% of the patients who are cigarette smokers, but in only 14% of those who are nonsmokers. P53 mutations were identified in 55% of the tumors among drinkers and 20% among nondrinkers.17 Changes in p53 and proliferating cell nuclear antigen expression may be associated with human papillomavirus infection and could play a role in the carcinogenesis of laryngeal cancer.18 Some have advocated that the overexpression of p53 is associated with tumor bulk and poor local control in T1 glottic cancer, whereas others have failed to identify p53 status as a marker for prognosis and clinical outcome in laryngeal carcinoma.19,20 It is becoming increasingly clear that tumors of the larynx may represent a heterogenous group of neoplasms. Two frequently involved alleles are 3p and 9p21; these regions harbor tumor suppressor genes and thus may be involved with malignant transformation. Telomerase activity is often present at high levels in laryngeal cancer and may be an early event in the tumorigenic process.
Anatomy
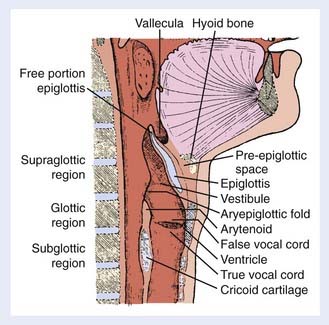
The larynx is a hollow tube lined by mucosa and adapted for protection of the airway and phonation. It is composed of the thyroid, cricoid, and arytenoid cartilages surrounded by connective and muscular tissue. The larynx is subdivided into three anatomical regions: the supraglottis, glottis, and subglottis regions (Fig. 31-1). The supraglottic larynx is composed of the epiglottis, aryepiglottic folds, arytenoids, and false cords (ventricular bands). The epiglottis is divided into a suprahyoid and infrahyoid component based on its relation to the hyoid bone. The supraglottic larynx extends from the tip of the epiglottis superiorly to the superior surface of the true vocal cords. The glottic larynx consists of the true vocal cords and the anterior and posterior commissures (the mucosa between the arytenoids). The true vocal cords are, on average, 2 cm long and are thinnest anteriorly and posteriorly. Most lesions arise on the free edge and upper surface of the anterior two thirds of the vocal cords and frequently extend to the anterior commissure. The lower boundary of the glottis is a horizontal plane 1 cm below the apex of the ventricle, or 0.5 cm below the free edge of the true vocal cords. Head and neck surgeons typically refer to the former definition and radiation oncologists refer to the latter. The subglottis extends down to the inferior margin of the cricoid cartilage and the beginning of the trachea.
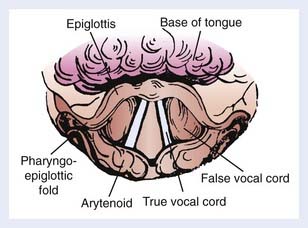
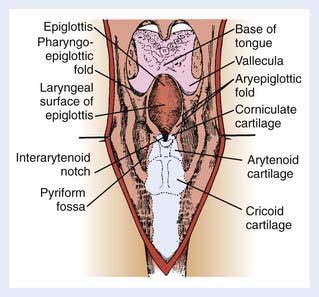
The appearance of the larynx as seen in the indirect mirror examination is shown in Fig. 31-2. The cartilaginous framework of the larynx is important in diagnostic radiology and in evaluating simulation and port films. The relationship of the various cartilages to surface anatomy is shown in Fig. 31-3. The thyroid, cricoid, and the majority of the arytenoid cartilages are composed of hyaline cartilage, which begins to ossify at approximately 20 years of age. The epiglottis, the corniculate and cuneiform cartilages, and the apex and vocal process of the arytenoids are made up of elastic cartilage, which does not ossify and therefore is not radiopaque.
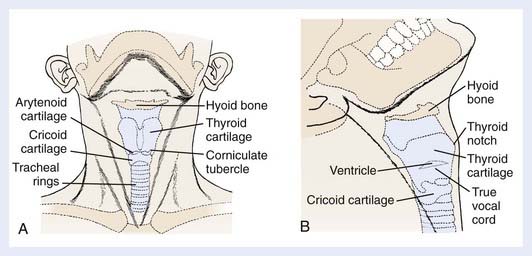
FIGURE 31-3 • A, Anterior view of surface anatomy with cartilages shown. B, Lateral view of surface anatomy with cartilages shown.
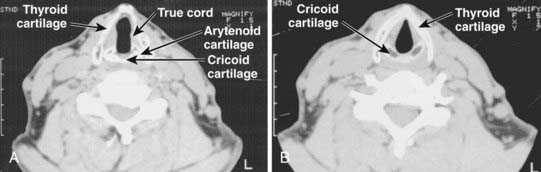
The anterior limits of the larynx consist of the lingual surface of the suprahyoid epiglottis, the thyrohyoid membrane, the anterior commissure, and the anterior wall of the subglottic region, which is composed of the thyroid cartilage, the cricothyroid membrane, and the anterior arch of the cricoid cartilage (see Fig. 31-3B). To avoid underdosing the anterior portion of the larynx when using megavoltage radiation to treat larynx cancer, it is important to remember that the anterior commissure usually lies within 1 cm of the skin surface and that bolus may be required to deliver adequate dose to this area. The posterior and lateral limits include the aryepiglottic folds, the arytenoids, the interarytenoid space, and the posterior surface of the subglottic space formed by the mucous membrane covering the cricoid cartilage. Superiorly, the epiglottis demarcates the boundary with the pharynx, which is usually at the lower border of the C3 vertebra. The inferior extent of the larynx is at the lower margin of the cricoid, which is typically at the level of the C6 vertebra (Fig. 31-4). The anatomy of the larynx can also be appreciated on computed tomography (CT) scans. The key structures are seen in Fig. 31-5.
Routes of Spread
Lymphatics
The supraglottis has a rich lymphatic network. Because of its midline location, it has a high propensity for bilateral lymph node involvement. The lymphatic channels from the supraglottis pass through the thyrohyoid membrane and drain into the jugular chain; thus the primary drainage pattern of supraglottic tumors is the jugular lymph node chain. The distribution of lymph node metastases for supraglottic cancers was identified in a large series of more than 2000 patients with SCC of the head and neck treated at M.D. Anderson Cancer Center and is shown in Fig. 31-6.21 The subdigastric (also known as jugulodigastric, upper jugular or level II) are most commonly involved, followed by the midjugular (jugulocarotid or level III) and lower jugular (jugulo-omohyoid or level IV) nodes. The posterior cervical nodes (level V) are seldom involved, and submandibular (level IB) and submental (level IA) are almost never involved. The overall incidence of clinically involved lymph node metastasis from carcinoma of the supraglottis at the time of diagnosis is 55%, with 16% being bilateral. In addition, up to one half of the remaining clinically node-negative patients have pathologic nodal involvement on elective neck dissection.21–24 The extent of lymph node involvement correlates with tumor size and grade.25 The risk of clinically involved lymph nodes is approximately 40% for T1 and T2 tumors and approximately 60% for T3 and T4 lesions.
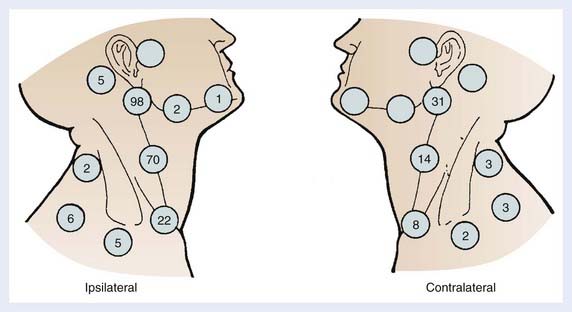
FIGURE 31-6 • Distribution of lymph node metastases from carcinoma of the supraglottic larynx.
(From Lindberg RD: Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts, Cancer 29:1446, 1450, 1992.)
The lymphatic network is less developed in the subglottis and the risk of lymph node metastases is lower compared with supraglottic lesions. The incidence of lymph node metastasis from carcinoma of the subglottis varies from 20% to 50%.26,27 Lymphatic channels from the subglottic area unite to form three lymphatic pedicles, one anteriorly and two posterolaterally. The anterior channels pass through the cricothyroid membrane and drain into the middle and lower jugular nodes or terminate in the prelaryngeal node (Delphian node), from which lymphatics drain into the pretracheal and supraclavicular nodes. The posterolateral lymphatic channels pass through cricotracheal membrane and terminate in the high paratracheal nodes. Mediastinal involvement can occur but is unlikely even for subglottic tumors and when present is considered metastatic disease.
The true vocal cords are nearly devoid of lymphatic capillaries. Thus, for carcinoma of the vocal cords, the incidence of lymph node metastasis at diagnosis approaches zero for T1, 2% for T2, 15% to 20% for T3, and 20% to 30% for T4 lesions.28 In addition, the rate of occult nodal involvement is approximately 16% in patients with clinical T3 and T4 node-negative tumors who undergo elective nodal dissection.23 Lymphatic spread from glottic cancer primarily occurs when there is tumor extension into the supraglottis or the subglottis, where the lymphatic supply is much more extensive. However, the incidence of lymph node metastasis from primary vocal cord carcinomas with supraglottic or subglottic involvement is not as high as the incidence of nodal involvement with primary supraglottic or subglottic carcinomas.
Distant Metastases
The incidence of clinically distant metastasis is relatively low for larynx cancer, but metastases are identified in approximately 10% to 20% of patients, the majority of whom have supraglottic and subglottic primary.29,30 Autopsy studies, however, show that the rate of subclinical metastases is much higher.31 The lung is the most common distant metastatic site and is the first site of presentation in nearly 60% of patients. Bones are the second most common site, as 20% of patients with distant disease develop osseous metastases. Liver metastases are identified clinically in 10% of patients. Brain metastases have been reported but are exceedingly rare.32 Because these patients are usually at high risk for developing a second primary cancer, tissue confirmation should be obtained before they are given the diagnosis of metastatic disease. Lymph node involvement, metastases in the low neck, stage and extranodal extension (ENE) all increase the risk of developing distant metastases in patients with larynx cancer.33 A recent study found that ENE increases the risk of metastases tenfold.34
Diagnostic Evaluation
Initial Evaluation
The combination of information gleaned from cross-sectional imaging with clinical examination allows for the most accurate staging. CT scans with contrast enhancement and thin-cut slices through the larynx and magnetic resonance imaging (MRI) with contrast are useful in the diagnostic imaging of laryngeal cancer. Both modalities are able to provide information that is essential in determining the appropriate treatment for a patient, including the presence or absence of disease in the pre-epiglottis, paraglottis, subglottis, cartilage invasion, extralaryngeal spread of disease, nodal metastases, and tumor volume. These studies are preferably performed before biopsy of the tumor, as postbiopsy edema may cause overestimation of tumor extent. Modern CT scanners provide high spatial resolution images that can be reformatted in any desired plane. The relative usefulness of CT scan versus MRI remains controversial, and in many cases the two modalities are complementary. The staging accuracy of MRI in laryngeal cancer is slightly higher, largely because of more accurate assessment of cartilage involvement and paraglottic and pre-epiglottic extension of tumor.35 MRI is more sensitive but less specific than CT in detecting cartilage invasion.36 With CT scans, correct T classification is possible in 70% to 80% of cases, and N stage in about 80%.37 The limitations of CT include the subtle evaluation of tumor-induced cartilage and bone defects and the detection of superficial tumors. CT evaluation is much faster than MRI and substantially reduces motion artifacts attributable to breathing, swallowing, or coughing. CT is still more commonly used for initial staging because of practical advantages such as cost, speed, and availability.38 According to the National Comprehensive Cancer Network (NCCN) practice guidelines, either study is suitable for staging (see www.nccn.org).
The role of positron-emission tomography (PET) in laryngeal cancer has been growing during the previous decade, and although some have advocated for its routine use in the initial workup, its use in this setting remains investigational.39–41 Given the limited spatial resolution of PET, it is unlikely that it will contribute significantly to assessing the T stage. The benefit of PET is more likely to be in the detection of occult nodal and distant metastases.42 In contrast, the value of PET in post-treatment followup is well established as a method of detecting early recurrences.43,44 Typically, patients who are suspected of having a recurrence because of symptoms or laryngoscopic findings require examination under anesthesia and biopsy. In more than 50% of cases, the biopsies are negative. A study from the Netherlands showed that PET can help distinguish between recurrent disease and post-treatment changes.45 There is an ongoing randomized trial in the Netherlands investigating whether PET can accurately identify patients who require biopsy.46 PET also has a role in the evaluation of the neck after chemoradiation for locally advanced disease and may be able to select patients who do not require a neck dissection.47
Metastatic Workup
To complete the staging workup, imaging of the chest is recommended. A chest x-ray may be sufficient for patients with early stage disease at low risk for pulmonary metastasis. For patients with locally advanced disease at higher risk of metastases, a CT of the chest and possibly the entire torso is advisable. In addition, PET has been gaining popularity in detecting metastatic disease and there is data to suggest that PET can improve the detection of metastatic disease in a percentage of patients with locally advanced disease.42,48
Other Studies
Pulmonary function tests are performed in patients being considered for supraglottic laryngectomy or partial laryngopharyngectomy. Bronchoscopy and esophagoscopy are performed to rule out synchronous tumors. In addition, dental, speech, and swallowing evaluations are often advised. Routine laboratory tests include a complete blood count and liver function tests. If the liver function tests or serum alkaline phosphatase is abnormal, further studies such as liver and bone scans may be indicated. Attention should be given to the hemoglobin level as anemia may be a negative prognostic factor for patients with larynx cancer who receive radiation.49–52
Staging
The tumor-node-metastasis (TNM) staging system of the 2002 American Joint Committee on Cancer for carcinoma of the larynx is shown in Table 31-1.53 Primary tumor (T) classification is based on the extent of involvement within the larynx, extralaryngeal extension, cartilage invasion, and mobility of the vocal cords. In the past, any involvement of the thyroid cartilage was considered T4 disease, but based on the results of the Veterans Affairs (VA) Larynx Trial, minor thyroid cartilage erosion (inner cortex only) is now considered T3 for both glottic and supraglottic tumors. There must be invasion through the thyroid cartilage to be upstaged to T4. T4 lesions have been divided into T4a, or resectable disease, and T4b, or unresectable disease, leading to the division of stage IV into IVA and IVB. Regional lymph node (N) classification is based on size and number, as well as on ipsilateral, bilateral, or contralateral lymph node involvement. T stage is the most important prognostic factor in determining local control and N stage is an important prognostic factor for predicting distant metastasis and survival.
Therapeutic Approaches
Verrucous Carcinoma
Treatment of verrucous carcinoma of the vocal cords has been controversial. Bona fide cases of verrucous carcinoma do not metastasize and are associated with an excellent rate of control. Some have reported that these lesions have limited radioresponsiveness, and anaplastic transformation has been reported to occur after radiation therapy (RT).54–56 However, others have found that RT is quite effective and that anaplastic transformation rarely occurs.56,57 A recent study from Princess Margaret Hospital (PMH) showed that among 62 patients with verrucous carcinoma, none had anaplastic transformation after undergoing radiation.58 However, approximately one third of patients had a local failure after radiation requiring salvage surgery. Nearly all patients can be salvaged successfully, resulting in a 5-year disease-specific survival of 97%. The ultimate rate of larynx preservation in this series was 80%. Treatment for this rare lesion should be based on the extent of the disease. Small tumors can be treated by excision or partial laryngectomy. RT is recommended for large tumors that require total laryngectomy, with surgery reserved for salvage. For more aggressive tumors, concurrent chemotherapy can be considered.59
Carcinoma In Situ
CIS can be treated successfully with surgery or RT. Treatment practices range from observation after biopsy, to surgery (vocal cord stripping, cordectomy, laser excision, open-partial laryngectomy [OPL]), to primary radiation.60–68 Approximately two thirds of patients who pursue a watchful waiting approach develop invasive cancer, and some may no longer be candidates for larynx preserving therapy. Therefore, observation should be used with caution.67,69 Repeated biopsies, strippings, or laser excisions can lead to reduction of voice quality and should be avoided.
RT is an effective treatment modality for CIS of the larynx and there is a slightly higher rate of local control with RT than with vocal cord stripping or laser excision.61 The local control rate with primary RT ranges from 70% to 100% (average 87%). In contrast, the local control rate for laser resections and vocal cord stripping is 83% and 72%, respectively. A 5-year recurrence-free rate of 83% was reported by Elman and associates in a group of 69 patients with CIS of the vocal cords treated with RT.66 Spayne and associates reported the PMH experience of 67 patients treated with moderate-dose RT of 51 Gy in 20 daily fractions for 4 weeks. With a median follow-up of 6.5 years, the 5-year actuarial local control rate was 98%; only one patient developed invasive glottic cancer and eventually underwent salvage laryngectomy.68 A series from the University of Florida showed a 5-year actuarial local control rate of 88% after RT and 100% with salvage surgery.61 For well-defined lesions, stripping or laser resection are reasonable upfront options. For patients who recur, radiation should be considered. Also, for patients with more diffuse lesions or those not suitable for a limited surgical procedure, radiation should be offered. Patients who are not reliable for follow-up should receive radiation. Although doses of less than 60 Gy are often effective for CIS, many prefer to use more than 60 Gy because up to one third of patients have unrecognized invasive disease.69–71
T1 Glottic Carcinoma
The treatment objective for early invasive carcinoma of the larynx is to obtain cure with laryngeal preservation and optimal voice quality with minimal morbidity, expense, and inconvenience. Early stage cancers can effectively be treated with surgery or radiation. All patients should initially be treated with larynx preserving approach. Treatment guidelines emphasize that every effort should be made to avoid combining surgery and radiation because functional outcomes may be compromised. The treatment of choice, however, remains controversial. There is no one modality that has proven superior with regard to all treatment goals.72–78 There are no randomized trials comparing surgery and radiation for the treatment of early stage larynx cancer, and it is unlikely that such a trial will be conducted. A Cochrane review tried to compare the effectiveness of RT and surgery in early laryngeal cancer, but concluded ultimately that there was insufficient evidence to establish one modality as superior to the other.79 Conclusions regarding management must be based on a comparison of nonrandomized studies, and both modalities are currently accepted as standard treatment options.80 Larynx-preserving surgical procedures for early glottic cancers include endoscopic stripping, cordectomy, laser excision, and OPL, but some early stage tumors are not anatomically suitable for these limited surgeries. Selection of a treatment modality for an individual patient depends on a number of factors: location and extent of the tumor, vocal cord mobility, tumor growth characteristics, histology, general medical condition of the patient, occupation, patient compliance, patient preference, cost, availability, and preference of the treating surgical and radiation oncologists. Treatment philosophy varies significantly with geography and institution.81,82 RT is delivered primarily with external-beam irradiation and there is no routine role for brachytherapy in the management of larynx cancer. Patients with medical contraindications or patients who refuse surgery should receive RT. Patients who are not reliable for close follow-up may benefit from upfront surgery.
In general, for T1-N0 lesions, the rates of local control, laryngeal voice preservation, ultimate local control, and survival are comparable for patients treated with transoral laser excision, OPL, and RT. For patients with well-defined, superficial lesions involving one vocal cord, upfront laser resection can be considered. More extensive lesions involving the anterior commissure and both vocal cords have decreased local control after laser resection and upfront radiation is preferred. OPL carries higher morbidity and should be reserved for patients not eligible for either laser resection or RT. Although RT and laser resection offer similar cure rates for most T1 glottic tumors, voice quality is usually better after RT than after surgery, but this issue remains controversial as the literature is not conclusive.83–89 Local control after initial endoscopic resections is lower compared with radiation but local control is similar after salvage therapy.73 For patients whose occupation requires a high-quality, intact voice, RT may be the preferred initial treatment. If RT fails, salvage surgery is successful in 75% to 85% of the patients in whom surgery is attempted. In the past, most patients who failed primary radiation required laryngectomy, but, as the experience with larynx-preserving surgery has grown, up to 50% of patients in the recurrent setting can undergo larynx preserving salvage surgery.90–94
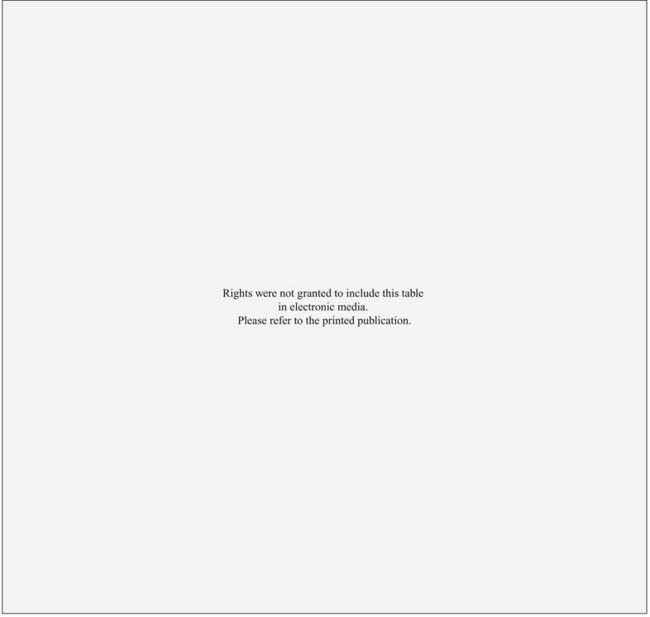
Results of primary RT for T1 carcinoma of the glottis are shown in Table 31-2 and Table 31-3. For T1 lesions, the initial local control rates are in the range of 78% to 95%, and the ultimate local control rates after surgical salvage of failures are in the range of 94% to 99%. The larynx is preserved in 89% to 95% of the irradiated patients. A number of investigators have shown that total treatment time and dose and fraction size can affect local control.95–98 In addition, anterior commissure involvement, subglottic extension, beam type and energy, field size, gender, histologic grade, pretreatment hemoglobin, and total dose may all influence local control rates.52,71,73,95,96,99–103 Higher doses may only be needed for more extensive T1 lesions.73,98 A recent study from Italy found that male gender, greater tumor extent, anterior commissure involvement, and use of electrons or cobalt all were independent predictors on multivariate analysis for local failure.71 Tumor response at 3 to 6 weeks after completion of radiation also significantly affects local control.102
Table 31-3 Stage T1 Carcinoma of the Glottis: Survival After Radiotherapy and Surgical Salvage
| Reference | Patients, n | 5-Yr Survival, % |
|---|---|---|
| Mittal et al.114 | 177 | 97 (determinate) |
| Amornmarn et al.116 | 86 | 96 (determinate disease-free) |
| Mendenhall et al.107 | 291 |
A randomized trial from Japan compared 2 Gy per fraction versus 2.25 Gy per fraction in the treatment of stage 1 glottic cancer.91 In this study, smaller tumors received a slightly lower dose (56.25 Gy in 2.25 Gy fractions or 60 Gy in 2 Gy fractions) than larger tumors (63 Gy in 2.25 Gy fractions or 66 Gy in 2 Gy fractions). Larger fraction size increased local control from 77% to 92%, corroborating the previously published retrospective series that suggested that larger fraction size improves local control. On multivariate analysis, treatment arm was the only significant independent prognostic factor with an odds ratio of 3.38. This study established hypofractionation with 2.25 Gy as a standard fractionation for T1-N0 glottic cancer. At our institution, we treat T1 glottic tumors to 63 Gy in 2.25 Gy fractions regardless of size.
T2 Glottic Carcinoma
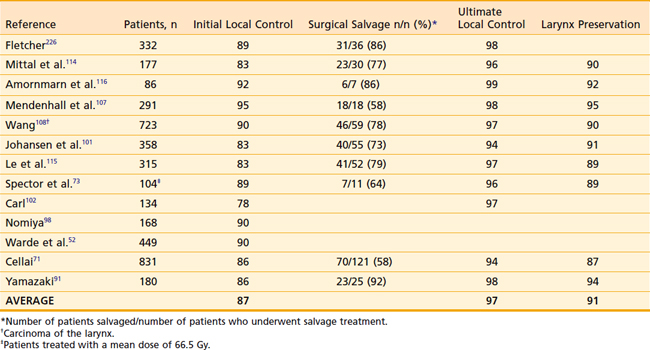
For T2 carcinoma of the glottis treated with primary RT, the initial local control rates are in the range of 67% to 88%. After surgical salvage, the ultimate local control rates are in the range of 85% to 96%. The larynx is preserved in 71% to 88% of the patients irradiated. Results of primary RT for T2 carcinoma of the glottis are shown in Table 31-4 and Table 31-5. Several studies have shown that local control improves with hyper- or hypofractionated radiation.104,105 Garden and colleagues showed that more than 2 Gy per day improved local control whether it was delivered as more than 2 Gy per daily fraction or more than 2 Gy in smaller, twice-daily fractions. This study led to a phase-III Radiation Therapy Oncology Group (RTOG) trial comparing 79.2 Gy in 66 fractions of 1.2 Gy twice-daily fractions to 70 Gy in 35 fractions of 2 Gy daily.106 Preliminary results presented in abstract form showed a nonsignificant improvement from 70% to 79% in local control with hyperfractionation. The outcomes with hypofractionation and hyperfractionation both appear to be superior to conventional fractionation.105,107 Thus the debate regarding the optimal fractionation regimen is ongoing, but it is generally agreed that either hyperfractionation or hypofractionation regimens are favored over conventional fractionation. At Memorial Sloan Kettering Cancer Center (MSKCC), we typically treat T2-N0 glottic tumors to 65.25 Gy in 2.25 Gy fractions.
Table 31-5 Stage T2 Carcinoma of the Glottis: Survival After Radiotherapy and Surgical Salvage
| Reference | Patients, n | 5-Yr Survival, % |
|---|---|---|
| Amornmarn et al.116 | 34 | 88 (determinate disease-free) |
| Karim et al.112 | 156 |
Impaired vocal cord mobility and anterior commissure involvement may be associated with lower local control rate and survival in some series, but the data are inconclusive.28,62,96,97,108–114 Subglottic extension, poorly differentiated histopathology, and male gender have been associated with poorer results in some series.105,108,110,115–117 Paraglottic extension is likely associated with increased tumor volume but, by itself, is not associated with increased local failure.117 Others have noted no significant difference in the outcomes with respect to subglottic extension, gender, or differentiation.111,114
Early Supraglottic Carcinoma
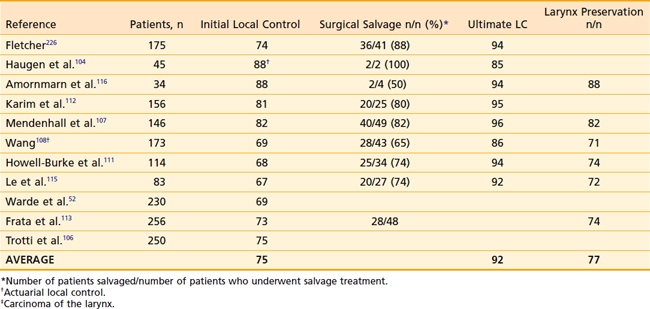
The prognosis for early stage supraglottic cancer is slightly worse than for early glottic cancer. Results of primary RT for supraglottic tumors are shown in Table 31-6 and Table 31-7. As is the case for early stage glottic cancer, all patients with early stage supraglottic larynx cancer should be treated with the intent to preserve the larynx. However, the optimal treatment modality for these tumors is still debated. For early stage, favorable lesions, endoscopic laser resection, open-partial supraglottic laryngectomy, and radiation are all considered standard treatment options. Careful patient selection is critical, as only some patients will be candidates for larynx-preserving surgery because of either tumor location or extent or because of comorbidities. Local control after upfront partial laryngectomy is likely slightly higher than with upfront radiation, but more than 50% of patients will not be candidates for OPL because of comorbid conditions or location and extent of the tumor. Direct comparisons of the two treatment modalities are complicated by the fact that many patients undergoing partial laryngectomy subsequently receive adjuvant RT. Surgical candidates tend to be younger and healthier, further confounding comparisons. In addition, surgical series often use surgical staging, leading a portion of the patients to be upstaged. Supraglottic laryngectomy is contraindicated when there is arytenoid fixation, bilateral arytenoid involvement, involvement of the apex of the pyriform sinus, invasion of the thyroid or cricoid cartilage, involvement of the postcricoid region, impaired vocal cord mobility, extension into the glottic area, and extensive involvement of the base of the tongue. Orus and colleagues showed slightly better initial local control with partial laryngectomy compared with radiation but, with salvage surgery, both resulted in ultimate local control of 90% for T1– and T2-N0 supraglottic tumors.118
Because of the potential morbidity associated with OPL, there has been a growing interest in the treatment of early supraglottic cancer with transoral endoscopic resection. There is emerging data that transoral laser excision in well-selected patients may be equal in outcome to traditional OPL and carries less morbidity.119–123 Table 31-8 and Table 31-9 show a summary of the published literature on the outcomes of laser excision of early stage larynx cancer. Laser partial laryngectomy reduces time required to restore swallowing, tracheostomy rates, aspiration pneumonia rates, and fistula formation, and shortens hospital stays. Preliminary outcomes are encouraging, but more experience is needed with this technique before it can be considered standard therapy.80
Table 31-8 Stage 1 Carcinoma of the Supraglottis: Local Control With Laser Resection
| Reference | Patients, n | Initial LC |
|---|---|---|
| Eckel228 | 9 | 89 |
| Iro et al.229 | 39 | 79 |
| Ambrosch et al.230 | 12 | 100 |
| Rudert et al.231 | 4 | 100 |
| Zeitels et al.232 | 13 | 88 |
LC, Local control.
Table 31-9 Stage 1 Carcinoma of the Supraglottis: Local Control With Laser Resection
| Reference | Patients, n | Initial LC |
|---|---|---|
| Eckel228 | 26 | 92 |
| Iro et al.229 | 54 | 87 |
| Ambrosch et al.230 | 34 | 88 |
| Rudert et al.231 | 10 | 80 |
| Zeitels et al.232 | 6 | 100 |
| Davis et al.233 | 8 | 100 |
LC, Local control.
A large series from Florida showed that 5-year actuarial probability of local control after RT for T1 and T2 supraglottic tumors was 100% and 86%, respectively.124 This cohort included a large percentage of patients who would not be eligible for partial laryngectomy. Other radiation series report similar outcomes. Carl and colleagues showed that tumor grade, size, and patient sex were independent prognostic factors.102 A retrospective series from the United Kingdom for T1 and T2 supraglottic cancers treated with larynx-preserving surgery or standard fractionated radiation showed that control of the primary tumor was equivalent between the two modalities.84 Tumor size has been shown to be a poor prognostic factor in patients receiving radiation. Several studies have shown that large tumors of more than 6 to 8 cm3 on CT have much higher rates of recurrence after radiation.124–126 The presence of lymphadenopathy, especially with lymph nodes greater than 3 cm in diameter, may have an adverse effect on local control as well as survival.127,128 However, Freeman and colleagues found that local control depended on T classification and was not influenced by N stage.129
Locally Advanced Larynx Cancer
Locally advanced glottic and supraglottic larynx cancers (stages III and IV) are treated similarly for the most part and are discussed together. The outcome for patients with advanced disease is much worse than for early stage, which has long-term survival rates ranging from 30% to 60%.130,131 The standard treatment for many years of locally advanced, resectable larynx cancer was laryngectomy and postoperative radiation for surgical candidates and radiation alone for medically inoperable patients. Laryngectomy is widely recognized as one of the most feared surgical procedures by patients. In fact, studies on patient preferences showed that 25% of individuals would be willing to trade a 20% absolute difference in survival for the opportunity to save their voice. As a result of two important randomized trials, there has been a major paradigm shift during the previous two decades for most patients to concurrent chemoradiation. Total laryngectomy should be reserved for patients with T4 lesions with extensive cartilage involvement or as salvage treatment for local failures or nonresponders to chemoradiation. For select T3 and T4 lesions, amenable to larynx-preserving surgery, a conservative surgical approach is an acceptable alternative to chemoradiation. However, postoperative radiation is often needed and voice quality in these cases can be low. Patients with unresectable larynx cancer should also receive concurrent chemoradiation because results with radiation alone are suboptimal.132,133 Primary surgery and concurrent chemoradiation have not been compared exclusively in larynx cancer, but a trial from Singapore compared these two modalities for all SCC of the head and neck (sinonasal and nasopharynx excluded) and found no difference in 3-year disease-free survival. In addition, this study found that larynx preservation was higher for larynx and hypopharynx cancers than other tumor subsites.
Select patients with more favorable Stage III-IV disease or those who are not candidates for chemotherapy, can be treated with definitive radiation alone. These patients should receive altered fractionation regimens. Retrospective comparisons suggest a higher local-regional control rate with hyperfractionated or accelerated hyperfractionated RT.108,134,135 A phase-III RTOG randomized trial showed that hyperfractionation and accelerated fractionation with concomitant boost are more efficacious than standard fractionation for locally advanced head and neck cancer in terms of local control and disease-free survival.136 Whether there is a benefit to using altered fractionation with concurrent chemoradiation is currently being evaluated by the RTOG.
The first trial to demonstrate the feasibility of larynx preservation was the VA Laryngeal Cancer Study.130 This two-arm, randomized trial compared the standard treatment at the time (total laryngectomy and postoperative radiation) against sequential induction chemotherapy followed by definitive radiation in patients who responded to induction chemotherapy. Only patients with resectable, nonmetastatic stage III and IV disease (T1-N1 excluded) who were not candidates for partial laryngectomy were eligible. Patients in the nonsurgical arm received two cycles of induction cisplatin (100 mg/m2) and a 5-day continuous infusion of fluorouracil (1000 mg/m2) on days 1 and 22. Those patients with a partial or complete response in the primary tumor and no disease progression in the neck then received a third cycle of induction chemotherapy followed by definitive radiation with standard fractionation (70 Gy in 35 daily fractions). Nonresponders to induction chemotherapy were taken directly for laryngectomy. Patients in the surgical arm underwent total laryngectomy followed by postoperative radiation. The major finding in this study was that 64% of patients at 2 years in the experimental arm were able to preserve their larynx without any compromise in survival compared with the standard treatment arm. The local failure rate was higher in the nonsurgical arm (12% versus 2%), but the distant metastases rate was slightly lower in this arm (17% versus 11%). The overall survival for patients in both treatment arms was 68% at 2 years. Chemotherapy nonresponders had the same overall survival as all other patients, showing that an attempt at organ preservation does not compromise survival, even in patients who do not respond to chemotherapy. Although the precise contribution of chemotherapy could not be elucidated by the study design, the ability to preserve the larynx without jeopardizing survival was demonstrated. Another important finding of this trial was that patients with T4 disease had a significantly higher rate of local failure (56% versus 29%) and thus were deemed to be poor candidates for larynx preservation. It is debatable whether, with modern surgical techniques, some of the patients enrolled in this trail may have been eligible for larynx-preserving surgery; in this case, outcomes may be inflated.
The VA larynx trial led to a follow-up study, RTOG 91-11. This three-arm trial was designed to identify the optimal organ preserving regimen for patients with potentially resectable, stage III and IV, nonmetastatic, squamous cell carcinoma of the glottic (31%) and supraglottic larynx (69%).137 Patients with T1-N1 disease were excluded. Patients with large-volume T4 lesions (invasion through the thyroid cartilage or extension into more than 1 cm into the base of tongue) were excluded. It is important to note that the patients with T4 tumors that were included in this trial would probably be downstaged to T3 by current American Joint Committee on Cancer (AJCC) classification criteria. Three nonsurgical treatments were compared: radiation alone, sequential chemotherapy and radiation (based on the VA larynx trial), and radiation with concurrent cisplatin (100 mg/m2 on days 1, 22, and 43). The radiation dose and fractionation were identical in all three arms (70 Gy in 35 daily fractions). The 2-year locoregional control rate and larynx preservation rate was significantly higher in the concurrent chemoradiation arm than in the other two arms. There was no difference in either the locoregional control rate or larynx-preservation rate between sequential chemoradiation and RT alone. Interestingly, for the endpoint of laryngectomy-free survival (LFS) (alive and with a larynx) concurrent chemoradiation was significantly better than radiation alone (p = 0.01), but was not significantly better than sequential chemoradiation and sequential chemoradiation was not better than RT alone (p = 0.08). In the 5-year update of this trial, presented at the American Society of Clinical Oncology (ASCO) in 2006, the most notable new finding was that both sequential chemoradiation (44.6%) and concurrent chemoradiation (44.6%) improved LFS compared with RT alone (33.9%) (p = 0.011).138 There was no difference in LFS between the sequential and concurrent arms (p = 0.98). Locoregional control, larynx preservation, and disease-free survival were better with concurrent chemoradiation than with the other two arms. Overall survival was equivalent between the arms at 2 and 5 years. There was a trend (p = 0.06) at 5 years for decreased distant metastases in both chemotherapy arms (14%) compared with the radiation-alone arm (22%). The results of RTOG 91-11 have established concurrent chemoradiation with high-dose cisplatin as the current standard of care for larynx preservation for locally advanced resectable larynx cancer. A follow-up analysis of this trial showed that total laryngectomy after failure was associated with acceptable morbidity and low perioperative mortality, but up to one third of patients developed a pharyngocutaneous fistula. In addition, patients who require salvage surgery have a lower survival than those who remain free of recurrence at the primary site, but this was independent of treatment arm.139 It is important to remember that in addition to showing the advantage of using chemotherapy with radiation, this trial also demonstrated that RT alone is a reasonable treatment option for patients who cannot tolerate chemotherapy. In addition, it is critical to remember that the benefits of adding chemotherapy must be tempered by the higher rates of treatment-related sequelae, some of which can be life-threatening.
The benefit of adding concurrent chemotherapy to radiation for squamous cell carcinomas of all head and neck sites has been shown.140–142 A meta-analysis showed that concurrent chemotherapy offers an 8% survival benefit, with cisplatin-based regimens being effective.143 The benefit of concurrent chemoradiation may disappear in patients older than 70 years.144 In addition, a pooled RTOG analysis showed that older age and larynx primary tumor site were associated with severe late toxicity.145 Given that these patients are at greatest risk of toxicity, the use of chemotherapy in these patients needs to be further investigated.
Many early trials of induction chemotherapy were negative, but the regimens that proved superior to all others was cisplatin and fluorouracil (PF) and a meta-analysis suggested that its use may be associated with a benefit.143 This led to a renewed interest in the role of induction chemotherapy as part of a sequential treatment with chemoradiation for advanced larynx cancer. Phase II data suggested that adding docetaxel (T) to PF was beneficial. Two large randomized trials published in 2007 confirmed that adding Taxotere to PF as established TPF as superior to PF in the neoadjuvant setting. However, before induction chemotherapy can be adopted as a standard treatment, results from ongoing randomized trials comparing induction chemotherapy followed by chemoradiation versus chemoradiation alone are needed.146–148 Response to a single cycle of induction chemotherapy may be a way to select patients who are good candidates for organ-preservation protocols. In a phase-II trial from the University of Michigan, patients who had a greater than 50% response to one cycle of induction cisplatin and 5-FU went on to receive definitive chemoradiation (75% of patients) and patients with less than a 50% response had immediate laryngectomy (25% of patients). In the patients who received chemoradiation, the 3-year overall survival, cause-specific survival, and larynx preservation was 85%, 87%, and 70%, respectively.149 The use of induction chemotherapy as a way to triage patients between organ preservation and surgery can expose patients to excessive toxicity and may limit their ability to receive a more prolonged course of systemic therapy. There has been a growing interest in identifying molecular markers to predict disease behavior and guide individual treatment decisions.
Given the high toxicity rates associated with chemoradiation, interest in targeted agents that can be used in place of chemotherapy with radiation has grown. Epidermal growth factor receptor (EGFR) is abnormally activated in most head and neck cancers.150 Preclinical data showed that blockade of the EGFR pathway sensitizes cells to the effects of radiation. Early clinical reports showed that cetuximab, a monoclonal antibody against the ligand-binding domain of the EGFR, enhances the cytotoxic effects of radiation. As a proof of principle, a phase-III randomized trial from the RTOG showed that adding cetuximab to radiation improves locoregional control and overall survival without increasing the common toxic effects of head and neck radiation.151 This trial led to widespread use of cetuximab with definitive radiation. Unfortunately, cetuximab and cisplatin have not been directly compared and such a comparison is unlikely. Retrospective data is confounded by bias but, thus far, results appear equivalent.152 Some have argued that concurrent cisplatin may be more effective based on a 15% to 20% absolute overall survival benefit in trials comparing radiation to combined radiation and concurrent cisplatin-based chemotherapy, and only a 10% improvement in the landmark Bonner trial. Concurrent cetuximab should be considered for patients whose comorbidities preclude cisplatin-based chemotherapy.
Management of the Neck
The role of post-RT neck dissection in patients who have a complete response after definitive RT is controversial.153,154 In the past, neck dissections were routinely performed after the completion of chemoradiation. With the growing number of pathologically complete responses with the increasing use of concurrent chemotherapy, many have advocated for more selective use of neck surgery to avoid the added morbidity of surgery.155–157 A single institution’s experience of 121 patients with node-positive supraglottic carcinoma who underwent definitive RT and achieved a complete response locoregionally 4 to 6 weeks after treatment resulted in very few isolated neck recurrences. Therefore, post-RT neck dissection is not routinely recommended.158 Several studies have shown that radiographic response on PET and CT scan 8 weeks after completion of RT can be used to direct patients in whom a neck dissection is needed. A study from Canada suggested that a neck dissection is not required for patients with N1 or N2 disease who achieve a complete response (CR) on CT scan. A small series from the University of Iowa showed a 100% negative predictive value for patients with a CR on PET. At our institution, a neck dissection is not typically performed if a clinical CR is achieved after chemoradiation, regardless of the extent of initial neck disease. At most institutions, patients with a CR who had N1 disease are observed, whereas those who presented with N2 or N3 disease undergo neck dissections.
Surgical Management
Because of the high rate of local failure for bulky T4 lesions with extensive thyroid cartilage invasion after chemoradiation, the standard treatment for these tumors remains laryngectomy. In addition to having high rates of local failure after chemoradiation, extensive cartilage invasion causes compromised laryngeal function. Therefore, larynx function will be poor even if larynx preservation is achieved. Thus these patients should typically undergo upfront total laryngectomy. Laryngectomy is also the preferred treatment for more advanced lesions with bilateral vocal cord involvement and compromised airway. Evidence of minimal cartilage invasion (T3 according to current AJCC staging) should be treated with chemoradiation. Indications for postoperative radiation include close or positive margins; multiple lymph nodes; extranodal extension; extension of the primary into the soft tissue of the neck, thyroid, or cricoid cartilage; extensive subglottic extension; perineural, lymphatic, or vascular invasion; or in situations in which emergent tracheostomy is required. In most centers, RT is usually given postoperatively. In a randomized trial by the RTOG comparing preoperative and postoperative RT for locally advanced head and neck cancer, local-regional control was significantly better with postoperative RT, although the difference in survival was not significant.133 Trials investigating altered fractionation and dose escalation in the postoperative setting have been negative.159,160
Carcinoma of the Subglottis
Primary carcinomas of the subglottis are rare. Most of these lesions are relatively advanced at the time of diagnosis and are managed primarily with surgery followed by postoperative RT. Overall survival at 5 years for all patients with subglottic SCC treated at Wake Forest during a 25-year period was 25% because of the high rate of advanced and metastatic disease at presentation.161 RT alone is used for early lesions and for patients who refuse surgery or have medical contraindications to surgery.162,163 Data on the results of RT for carcinoma of the subglottis are sparse. Vermund in 1970 reported a 5-year survival rate of 36% in 127 patients treated with primary RT and 42% in 58 patients treated with primary surgery from pooled data in the literature.164 Warde and colleagues reported on a series of 23 patients treated with initial radical RT. Local control was achieved in 16 patients (70%), and the 5-year actuarial and cause-specific survival rates were 26% and 61%, respectively.165 More recently, the experience at PMH showed that local control was achieved with RT alone in 56% of the patients (n = 43) with an ultimate local control rate after attempted surgical salvage of 81.4%. The 5-year actuarial local relapse-free rate was 52% with an overall and cause-specific actuarial survival rate of 50.3% and 66.9%, respectively.163
Simulation
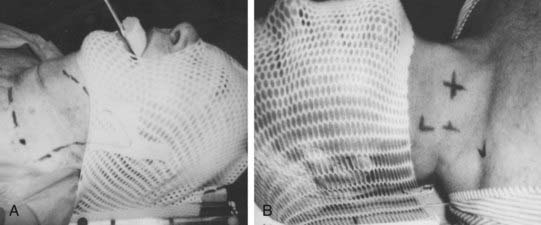
Simulation is performed with the patient in the treatment position: supine with the head hyperextended. The head is immobilized with a face mask to facilitate reproducibility and minimize inadvertent motion (Fig. 31-7). Patients should undergo CT treatment planning with 3-mm cuts to accurately define the gross tumor volume (GTV) unless contraindicated. This has been reported to correlate with local control.126,166–168 Intravenous contrast can be used to help delineate nodal disease. When conventional simulation is used, the anterior skin of the neck at midline and palpable cervical lymphadenopathy is outlined with radiopaque lead foil tape or wire. During fluoroscopy, motion of the larynx should be noted. For postoperative patients, all surgical scars, drain sites, and stoma (if present) should be wired on the skin. The central axis is tattooed on both sides of the neck to ensure a reproducible daily setup (see Fig. 31-7).
Radiotherapy Techniques
Beam Energy
Linear accelerators with 4- to 6-mV photons or 60-Co machines and 6- to 15-meV electrons for supplemental boosting to the nodes are commonly used. Treatment distance should be at least 80 cm source-to-skin distance (SSD). Because of the skin- and lymph node–sparing effects of higher-energy photons, bolus material may be needed with photon energies of 6 mV or more. In a study of patients with glottic carcinoma irradiated with 6-mV x-rays, a lower local control rate was noted in patients with gross involvement of the anterior commissure.99 Other studies have not found anterior commissure involvement to be a poor prognostic factor. This may be the result of technical factors (e.g., the use of wedge filters or understaging of these tumors) because in one series, none of the patients had a pretreatment CT scan.103,169 Some have questioned whether 6-mV photons produce inferior coverage of the anterior larynx,170 but when Sombeck and colleagues compared 60-Co versus 6-mV photons for the treatment of early larynx cancer, they found no difference in the dose to the vocal cords, but did identify an area within 3 mm of the skin that was slightly underdosed with photons.171 Others have shown that excellent outcomes can be obtained with 6-mV photons, but bolus or spoiler is still recommended for thin patients with anterior lesions.172 We routinely use 6-mV photons with 5-mm bolus anteriorly for treating larynx cancer and have not noted an excess of anterior failures. Other institutions only use bolus for patients with very thin anterior necks or anterior commissure involvement.
Treatment Planning and Fractionation
Carcinoma of the Glottis
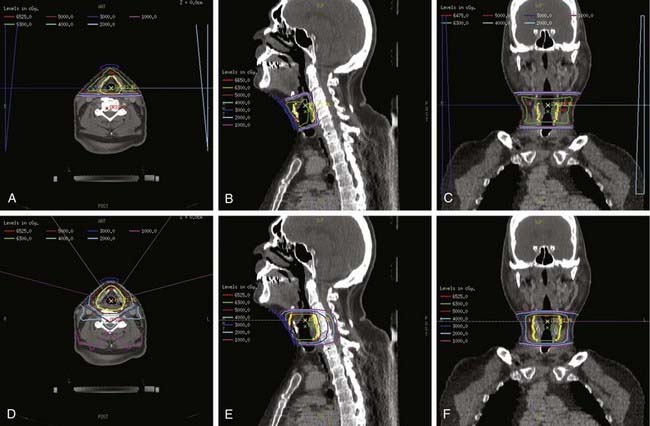
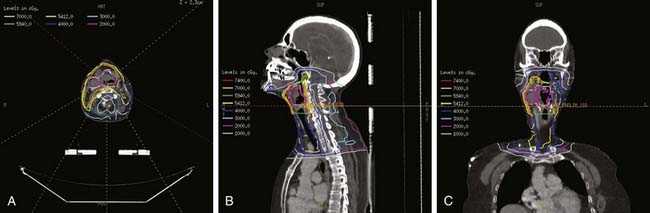
For T1 lesions, two small, opposing lateral photon fields are used to cover the larynx proper (Fig. 31-8). Open fields, wedged fields, or mixed open and wedged lateral fields are used, depending on the shape of the neck in the region of the larynx. Other techniques, including anterior wedge pairs, four-field plans, and three-field plans, have been used at some institutions. The objective is to deliver a uniform dose distribution throughout the target volume and to ensure adequate coverage of the entire larynx. A CT scan of the neck in the treatment position should be used for computerized treatment planning to assess dose distribution (Fig. 31-9). Tissue-equivalent compensatory or wedges, with appropriate angles based on the contours of the neck or CT-scan treatment planning, are used to achieve uniform dose distribution within the target volume. Without wedges, the anterior field can receive up to 15% more than the prescription dose because of differences the amount of tissue present to attenuate the beam between the anterior and posterior parts of the field. With appropriate wedges (usually 15 or 30 degrees) the dose gradient is typically reduced to approximately 5%. The heel of the wedge is placed anteriorly to improve homogeneity. For anterior lesions, a hot spot anteriorly may be preferential. In addition, bolus should be used for anterior lesions to ensure adequate coverage. For patients with a short neck, the beams are given a small inferior tilt to avoid the shoulders, but this can increase the lung dose. At MSKCC, we have started using intensity-modulated radiation therapy (IMRT) for these patients, but the routine use of IMRT for early stage larynx cancer is controversial.173 In addition, we are currently investigating the use of IMRT to improve target coverage and homogeneity, and reduce dose to the carotid arteries. Fig. 31-9 shows the isodose distribution for a conventional wedged plan compared with the isodose distribution for a four-field IMRT plan. It appears that IMRT can decrease dose to the carotid without compromising coverage of the larynx.
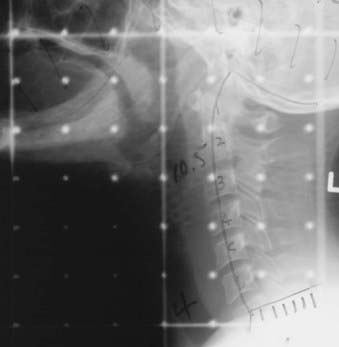
The field is centered on the vocal cords and parallel to the trachea, extending from the upper thyroid notch superiorly to the lower border of the cricoid (at the lower border of C6) inferiorly. The anterior border should be 1 cm anterior to the skin surface at the level of the vocal cords. The posterior border of the field should include the anterior portion of the posterior pharyngeal wall. Common practice is to place the posterior field edge at either the anterior edge of the vertebral bodies or halfway through the vertebral bodies. The collimator is angled to align with the angle of the vertebral bodies. A 5 × 5–cm field is usually sufficient for T1 vocal cord carcinomas (see Fig. 31-8). Inoue and colleagues reported equal local control in 116 patients randomized to receive 60 Gy in 30 fractions for 6 weeks using 4-mV x-rays and wedge filters with a 5 × 5–cm field or a 6 × 6–cm field.174 Of note, the true and false cords and the upper subglottis should be covered by the 95% isodose surface.
For T2-N0 tumors, a 5 × 5–cm to 6 × 6–cm field is usually sufficient when treating just the larynx proper, but sometimes the superior and inferior borders need to be adjusted to ensure adequate coverage. For subglottic extension, the inferior border is lowered to include at least one tracheal ring. The need for elective neck irradiation for T2-N0 vocal cord carcinomas is controversial. Wang recommended elective irradiation of the subdigastric and midjugular nodes when there is impaired vocal cord mobility.108 Harwood and colleagues also recommended elective irradiation of at least the first-echelon lymph nodes for all T2 vocal cord carcinomas.109 However, Mendenhall and associates noted that the risk of occult neck nodes was only 3% when the primary site was controlled, and 22% when there was recurrence at the primary site.175 They concluded that elective irradiation is not indicated for T2-N0 squamous cell carcinoma of the glottis, but recommended that a neck dissection be considered in conjunction with the salvage surgery for local recurrence. A similar treatment policy is recommended by Howell-Burke and coworkers.111 We have not routinely carried out elective neck irradiation when there is minimal extension beyond the vocal cords, but have included at least the first-echelon nodes for the initial 45 to 50 Gy when there is extensive supraglottic and infraglottic extension. For extensive T3 and T4 lesions treated with RT alone, larger lateral fields that include the subdigastric and midjugular nodes and a separate anterior low-neck field that includes the lower jugular nodes are used.
Carcinoma of the Supraglottis
Because of the propensity for lymphatic spread, the treatment volume for carcinoma of the supraglottis should include the primary lesion as well as the regional lymphatics in the neck. Other than T1-N0 lesions, accelerated fractionated RT using a concomitant boost technique is recommended. A pair of lateral opposed fields is used to irradiate the primary tumor and the upper cervical lymph nodes with a minimum 2- to 3-cm margin around the tumor and positive lymph nodes (Fig. 31-10). A single anterior low-neck field is used to irradiate the low neck. A spinal cord block 2 cm in height is placed in the inferior-posterior portion of the lateral upper-neck fields to shield the area of overlap on the spinal cord at the junction for the upper- and lower-neck fields. The upper border of the lateral fields should adequately cover the upper jugular nodes. The lower border of the lateral fields should encompass the larynx, usually at or below the level of C5. If there is involvement of the pyriform sinus, lateral or posterior hypopharyngeal wall, or both, the superior border is placed at the base of the skull (above C1) to include the retropharyngeal lymph nodes. The posterior neck should be included in the treatment volume when there are positive lymph nodes in the anterior neck and for T3 and T4 lesions. The spinal cord should be shielded after 45 Gy, and electrons with appropriate energies are used for supplemental boosting to the posterior cervical lymph nodes. The total dose to these opposed-lateral fields is 54 Gy. A second, smaller boost field is delivered concomitantly after 32.4 Gy. The boost fields starts on day 19 of treatment and includes only the primary tumor volume along with any gross nodal disease with at least a 1- to 1.5-cm margin around the initial primary tumor and the positive lymph nodes. The boost fields receive 1.5 Gy per fraction to a total dose of 18 Gy.
For stage T1-N0 tumors, a dose of 66 to 70 Gy was typically delivered at 2 Gy per fraction to the primary tumor. When fraction size of less than 2 Gy per day was used, local control was lower. An additional 2 to 5 Gy may be used to boost large primary tumors and neck nodes using reduced fields. A dose of 50 Gy is delivered to areas at risk for microscopic disease. The dose to the anterior low neck and supraclavicular fossa is calculated at 3-cm depth and to the upper mediastinum at 5-cm depth. Twice-a-day hyperfractionated RT is recommended for lesions staged at T2 or greater; a total dose of 79.2 to 81.6 Gy at 1.2 Gy/fraction twice daily is delivered to the primary tumor and the upper neck nodes.134 A minimum daily interfraction interval of 6 hours is recommended to minimize late normal tissue toxicity. The spinal cord is shielded after 45.6 Gy. An alternative technique, described earlier, is to use an accelerated fractionated RT using a concomitant boost technique.135
Carcinoma of the Subglottis
Postoperative Radiotherapy
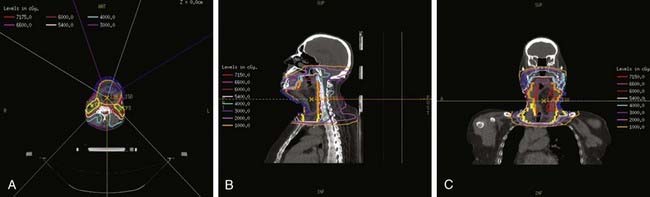
For postoperative RT following total laryngectomy, the standard dose is 57.6 to 60 Gy in 1.8 to 2.0 Gy fractions, 5 days per week. An additional 6 to 10 Gy (for 66 Gy total), at 2 Gy per fraction, is delivered through reduced fields to areas of positive margins, gross residual disease, or for patients with extranodal extension. Treatment is delivered to the tumor bed and the upper neck with two lateral opposed fields and an anterior low-neck field, with the spinal cord shielded after 45 Gy. A dose of 50.4 Gy is delivered to the supraclavicular fossa. The tracheal stoma is usually included in the anterior low-neck field. It is usually not necessary to place a bolus over the stoma site if 60-Co or 4-mV x-rays are used. If higher-energy x-rays are used, bolus of the stomal site is recommended. The standard dose to the tracheal stoma is 50 Gy. Indications of stoma boost include emergency tracheostomy, subglottic extension, extensive involvement of the soft tissues of the neck, or a surgical scar that crosses over the stoma. If a boost is indicated, an additional 10 Gy is delivered through a reduced boost field to the stoma using 9 to 12 meV electrons with 0.5-cm bolus. Postoperative RT is recommended to begin within 3 to 4 weeks after surgery when the wound is healed. The dose to the elective uninvolved elective sites is 50 to 54 Gy. At MSKCC, we have started to use IMRT in the postoperative setting and results have been very encouraging. Figure 31-11 shows the isodose curves using IMRT for a patient after total laryngectomy; because of positive margins, the total dose used was 66 Gy to the tumor bed.
Intensity-Modulated Radiation Therapy and Image-Guided Radiation Therapy for Larynx Cancer
During the preceding decade, IMRT has gained popularity and has proved its value in preserving salivary function and improving quality of life in patients receiving radiation for the treatment of head and neck cancer.176 A phase-III randomized trial from Hong Kong confirmed the ability of IMRT to preserve parotid function.177 In addition, its potential role in reducing treatment-related dysphagia is now being elucidated.178,179 Using computer-optimized planning and computer-controlled delivery systems, IMRT can target irregularly shaped tumors with high precision while minimizing the radiation delivered to nearby critical structures.180 The hallmark of IMRT lies in its ability to use inverse planning to calculate advanced strategies, including dynamic multileaf collimation, to deliver highly conformal RT. As a result, an increase in the therapeutic ratio between tumor and the surrounding normal tissues can occur. The clinical experience with IMRT has expanded greatly during the past few years and reports on nearly all head and neck sites have now been published, including the larynx.181–185 In addition to decreasing xerostomia, IMRT offers another very important advantage compared with conventional techniques. With conventional techniques, it is usually necessary to match fields, which can result in over- or underdosing structures at the match. These hot or cold spots can lead to serious toxicity and underdosing of tumor. Despite the complexity of head and neck IMRT, there is data that shows the feasibility of using IMRT in community practice.186 The benefits of using IMRT, however, should be weighed against the potential for marginal misses resulting from inaccurate targeting or inadequate margins, higher cost, longer treatment times, and limited long-term follow-up.
At MSKCC, we now routinely treat most patients with larynx cancer who require neck irradiation with definitive IMRT to improve target coverage, improve homogeneity, and for parotid sparing.184 In general, for early stage glottic cancer, we still typically use conventional radiation. The use of IMRT for early stage larynx cancer is an area of controversy and one currently under investigation.172 Patients receiving IMRT are immobilized in the supine position with a thermoplastic head, neck, and shoulder mask to ensure daily reproducibility. A CT simulation scans in serial 3-mm axial slices are obtained for treatment planning. A number of commercial IMRT treatment planning softwares are available. Typical plans use 6-mV photons and 5 to 7 fields. An example of a 7-field dose-painting IMRT plan for a patient with locally advanced larynx cancer is shown in Fig. 31-12. IMRT can also be used in the postoperative setting, and an example of a 7-field dose-painting IMRT plan is shown in Fig. 31-11. If chemotherapy was delivered before radiation, the targets should be outlined based on prechemotherapy extent of disease.
At MSKCC, we use dose painting IMRT (simultaneous integrated boost) with three different dose levels delivered during the same length of time with different daily fraction size. Some institutions use conventional fractionation with cone-down PTVs, and some use altered fractionation regimens including concomitant boost and hyperfractionation when using IMRT. The PTV-GTV is usually treated to approximately 70 Gy (2 to 2.12 Gy per fraction), the high-risk subclinical disease to 59 to 63 Gy (1.8 Gy per fraction), and low-risk subclinical disease to approximately 54 Gy (1.6 to 1.8 Gy per fraction). Standard dose constraints should be used on the spinal cord, brainstem, mandible, cochlea, oral cavity, and parotid glands. When using IMRT for the treatment of larynx cancer, we prefer to use an extended, whole-field IMRT plan rather than a split-field IMRT plan with a low anterior neck (LAN) match. A study from MSKCC showed that extended, whole-field IMRT avoids the risk of overlap on the cord and results in a more homogenous plan with excellent target-volume coverage.187 Dramatic differences currently exist in the way institutions report and prescribe dose when using IMRT. Caution must, therefore, be used when comparing clinical outcomes with IMRT and trying to implement new IMRT programs.
Because of the steep dose gradients produced with IMRT, accurate treatment delivery is critical. Imaging technology in RT has evolved to the point at which a variety of systems make it convenient to acquire frequent images of a patient throughout treatment. What has become apparent from these serial images is that the plan that is designed based on the CT scan obtained at simulation may be different than what is delivered because of anatomic and geometric changes that occur during the course of fractionated RT. This causes the potential for normal tissue overdosage and tumor underdosage. The use of image guidance is most relevant for structures that are subject to large interfraction organ motion such as the prostate. For head and neck tumors, which are typically considered to be quite rigid, there is some degree of setup error, which can be significant and the use of frequent in-room CT scanning may decrease error.189 In addition, during the course of fractionated radiation, volumetric and geometric changes can occur. Barker and coworkers examined changes in 14 patients treated for head and neck cancers using repeat in-room CT scans over the treatment course. They observed shrinkage of the primary tumor volume, with a median volume reduction at the end of treatment of 69% (range, 10%-92%). The tumor positions changed with time (median shift 3 mm; range, 0-17 mm). Parotid glands showed a median loss in volume of 28% at the end of treatment and moved medially (median shift 3 mm; range, 0-10 mm). The magnitude of parotid shift was highly correlated with weight loss.190 Kuo and coworkers reported the effects of regression of enlarged neck lymph nodes on parotid dose in IMRT head and neck RT.191 Node regression during treatment caused the parotids to move inward into a high-dose region. The study found that re-planning the patient after delivery of 45 Gy resulted in a mean reduction of approximately 3 Gy to each gland when compared with the initial treatment plan.
Effects of Treatment on Normal Tissues
The acute and late effects of RT or combined RT and surgery for carcinoma of the larynx depend on a number of factors: total dose,97,100,192,193 dose per fraction,97,100,193–195 treatment volume,100,174,193,194 overall time,115 stage of the disease,97,193,195 sequence of RT and surgery (i.e., preoperative versus postoperative RT),196 surgical technique,195 and chemotherapy.137,197 Daily interfraction interval is also a major determinant of late effects with hyperfractionated RT.198 Both acute and late normal-tissue effects may be exacerbated in the presence of other medical conditions such as diabetes, immune suppression, and collagen vascular disease.
Because of the lack of standardized quantitative criteria for scoring normal tissue effects of treatment and heterogeneity in treatment techniques among different centers, the incidence and type of complications reported in different series are quite variable and are not directly comparable.199–201 Concerted efforts are being made to standardize adverse event reporting. Traditionally, radiation side effects have been categorized as either early or late, and for many years the two were thought to be unrelated entities developing via different mechanisms. However, over the past two decades “consequential late effects” have been described.202,203 These were first observed with the introduction of aggressive treatment regimens using altered fractionation and combined-modality treatment protocols. In these situations, acute reactions fail to heal completely and persist into the “late” period. Nonetheless, in general, side effects are divided into acute (during RT or within 90 days of completion) and late (more than 90 days after completion of treatment).
Acute Effects
The acute toxicity of twice-daily hyperfractionated or accelerated fractionated RT are usually more severe than those with once-daily conventional fractionated RT.106,134,204 The use of 2.25 Gy per fraction, however, did not increase the risk of acute skin or mucosal toxicity compared with 2 Gy per fraction.91 The use of concurrent chemotherapy significantly increases the rate of acute toxicity, mostly mucosal reactions. The rate of acute grade 3 or 4 mucosal or pharyngeal toxicity nearly doubles in the patients receiving concurrent chemoradiation.137 The rate of acute toxicity with sequential chemotherapy and radiation is nearly identical to radiation alone, except for myelosuppression, which increases with the use of chemotherapy. In contrast, patients receiving concurrent chemoradiation have a much higher rate of nearly all toxicities, with nearly 80% of patients experiencing some type of grade 3 or 4 adverse effect. The use of concurrent cetuximab with radiation does not increase mucosal toxicity but it is associated with several other adverse effects, including an acneiform rash.151
Late Effects
Laryngeal edema of varying degrees may persist after RT for larynx cancer. In patients irradiated for carcinoma of the glottis, the incidence of mild to moderate laryngeal edema persisting for more than 3 months after RT is about 10% to 25%.100,192,194 The incidence of severe laryngeal edema is about 1.5% to 4.6%.62,97,111,116,174 The incidence of laryngeal edema increases with greater total dose, field size, dose per fraction, and T stage of the lesion.* A randomized study found that persistent laryngeal edema occurred in 4% of the patients treated with 5 × 5–cm2 fields and in 21% of the patients with 6 × 6–cm2 fields and no difference in local control in the two arms.174
Persistent laryngeal edema after RT for carcinoma of the vocal cord often presents a management dilemma to the radiation oncologist and head and neck surgeon. In a series of 247 patients irradiated for carcinoma of the vocal cord, laryngeal edema persisting for more than 3 months following RT developed in 38 patients (15.4%).192 In 17 (44.7%) of these patients, the laryngeal edema was associated with persistent or recurrent disease, although only 25.4% of the patients with uncontrolled disease had laryngeal edema. Our current policy in the management of patients with persistent laryngeal edema following RT for carcinoma of the vocal cord is to adopt initially conservative measures with voice rest; abstinence from alcohol and cigarettes; and careful, close followup examinations, including direct laryngoscopy if necessary. Antibiotics and steroids may be used when there is suspicion of infection or when the edema is severe enough to significantly compromise the airway. If it is mild and stable, if no visible recurrence develops, and especially if it is limited to the arytenoids, no biopsy is attempted because of the risk of inducing laryngeal necrosis. However, if the edema is progressive and unresponsive to conservative measures, and persistent and recurrent disease is strongly suspected, biopsies are carried out to establish the diagnosis. Salvage surgery is performed if biopsies are positive. Imaging studies including MRI and PET may be useful in distinguishing edema from tumor.
The risk of late effects in the larynx depends on the fraction size.100,107,194,205 In a series of 208 patients irradiated for T1 and T2 carcinomas of the vocal cord reported by Deore and associates, moderate to severe late laryngeal edema developed in 44% of the patients who received 50 Gy in 3 weeks at 3.33 Gy/fraction, in 18% of the patients who received 60 Gy in 5 weeks at 2.5 Gy/fraction, and in 17.2% of the patients who received 60.75 Gy in 5.5 weeks at 2.25 Gy/fraction.194 In a series of 303 patients irradiated for T1 or T2 glottic carcinoma reported by Mendenhall and coworkers, the incidence of moderately severe and severe complications was significantly higher in patients who received greater than 2.25 Gy per fraction.107 Yamazaki found that the rate of laryngeal edema at greater than six months after treatment in patients receiving 2.25 Gy or 2 Gy was not different.91 Late laryngeal necrosis following RT is rare, with a reported incidence of approximately 0% to 3% for glottic cancer† and 1% to 2.5% for supraglottic cancer.127,135 When using doses up to 66 Gy in 2 Gy fractions or 63 Gy in 2.25 Gy fractions, Yamazaki and colleagues observed no severe late complications.91
The rate of serious toxicity is greater in patients who receive postoperative radiation after larynx preserving surgery. In a series of 60 patients with intermediate-stage supraglottic carcinoma who underwent supraglottic laryngectomy, 50 (83%) underwent postoperative RT.22 Minor complications occurred in nine patients (14%), including three with vocal cord paralysis, two with dysphagia caused by stricture or esophageal dysmotility, two with fistulas, one with hematoma, and one with wound infection. Significant complications occurred in another nine patients (14%). Seven patients (11%) required gastrostomy for prolonged inability to maintain adequate oral intake; one required intravenous hyperalimentation, and one had a tracheostomy. Major complications included two postoperative deaths and three patients (5%) who underwent total laryngectomy for intractable aspiration. A trial from the RTOG for postoperative radiation for advanced larynx cancer found that only 17 patients of 270 had moderate to severe late complications and there were no treatment related deaths.206
As more patients receive and survive aggressive organ-preservation protocols for head and neck cancers, a better appreciation of the long-term side effects associated with this treatment will be gained. The two treatment-related toxicities that most affect quality of life for patients after radiation for head and neck cancer are swallowing dysfunction and xerostomia.207 It is becoming clear that one of the major late effects of aggressive radiation regimen with or without chemotherapy is prolonged pharyngoesophageal dysfunction resulting in dysphagia and percutaneous endoscopic gastronomy (PEG) dependance. A study from the Netherlands found that swallowing dysfunction has a more marked effect on patient quality of life after treatment that xerostomia. A recent study from M.D. Anderson found that the rate of aspiration on a barium swallow after definitive radiation was greater than 80% (60% received concurrent chemotherapy) and that 50% of patients aspirate silently. Of patients in that study, 38% required a feeding tube long-term.208 The rate of pharyngeal stricture after chemoradiation can be as high as 50%.209,210 An RTOG combined analysis of late toxicity after chemoradiation for head and neck cancers found that nearly 50% of patients will experience severe late pharyngoesophageal toxicity.145 Factors that contribute to the development of late toxicity after chemoradiation are older age, advanced T–stage, and laryngeal/hypopharyngeal tumors.
IMRT and amifostine, a radioprotector, can reduce the rate of xerostomia.176,211 Unfortunately, efforts to reduce mucositis and dysphagia have been less successful, but there is some early data to suggest that by reducing dose to the pharyngeal constrictors, this may be feasible in the future.178,179,212,213
At MSKCC we often place a prophylactic PEG prior to initiation of chemoradiation for patients with locally advanced disease. This improves the nutritional status of our patients. Other groups have also shown that the use of PEG tubes can significantly reduce weight loss and the rate of hospitalization for dehydration and complications of mucositis. Treatment interruptions may also be avoided by the use of PEG tubes in patients with good performance status. The 2-year risk for post-treatment PEG dependency rate after chemoradiation using IMRT for patients with locally advanced larynx cancer was 15%. This compares favorably to other reports using non-IMRT techniques.208 It is extremely important that even patients with PEG tubes continue to swallow throughout the course of radiation; not doing so can significantly increase the risk of long-term swallowing dysfunction and PEG dependence.214
Voice quality after radiation for larynx cancer is typically very good and superior to that achieved after larynx-preserving surgery. Nevertheless, up to 60% of patients can experience some decline in voice function.87,215 Patients who had stripping of the cords prior to radiation and those who continue to smoke after completion of radiation are at increased risk of have poor voice quality.74,216 Intensive voice therapy can improve voice quality in patients who suffer from this.215 In studies that use patient-reported subjective outcomes, up to 80% of patients will return to normal voice quality after radiation. However, objective voice quality is almost always altered.
Emerging data has identified that radiation to the head and neck region increases the risk of carotid artery disease and may increase the risk of stroke.217,218 A large Survival, Epidemiology, and End Results (SEER)-Medicare analysis showed that one third of patients who undergo definitive head and neck radiation will suffer a cerebrovascular event if they do not succumb to other causes within 10 years of treatment. In comparison, only one fourth of patients who undergo surgery will suffer cerebrovascular events during this same period.
Another common side effect of radiation to the head and neck region is hypothyroidism. Approximately 50% to 60% of patients who undergo thyroid-stimulating hormone (TSH) screening will have an elevated TSH level within 5 years of receiving 50 Gy to the low neck. When screening is not routinely used, the rate of hypothyroidism is around 30%.219–221 Because the thyroid gland is typically removed during a laryngectomy, having surgery in addition to neck radiation, not surprisingly, significantly increases the risk of developing hypothyroidism.222 A study from the University of Florida, in which all patients received at least 50 Gy to the thyroid gland, found that 80% of patients receiving postoperative radiation developed hypothyroidism in comparison to only 40% of patients treated with definitive radiation.221 More advanced stage also increases the risk of developing hypothyroidism because patients with more advanced disease receive higher doses to the thyroid gland. The median time to developing hypothyroidism is 6 to 24 months, and the majority of patients develop this complication within 5 years. Patients should be screened for thyroid function levels every 6 months for the first 5 years after treatment and annually thereafter.
Despite the myriad side effects from aggressive organ-preservation protocols, studies show that the quality of life after chemoradiation is higher than after surgery.223,224 However, organ preservation alone is not sufficient to maintain quality of life and the ultimate goal of treatment is cure with preservation of organ function. In cases in which this is not achieved, the quality of life with organ preservation may not be better than after laryngectomy.225
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
2 Cosetti M, Yu GP, Schantz SP. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch Otolaryngol Head Neck Surg. 2008;134(4):370-379.
3 Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777-789.
4 Lewin F, Novell SE, Johansson H, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer. 1998;82(7):1367-1375.
5 Rothman KJ, et al. Epidemiology of laryngeal cancer. Epidemiol Rev. 1980;2(1):195-209.
6 Muscat JE, Wynder EL. Tobacco, alcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer. 1992;69(9):2244-2251.
7 Graham S. Diet and cancer. Am J Epidemiol. 1980;112(2):247-252.
8 Ward PH, Hanson DG. Reflux as an etiological factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98(11):1195-1199.
9 Bacciu A, Mercante G, Ingegnoli A, et al. Effects of gastroesophageal reflux disease in laryngeal carcinoma. Clin Otolaryngol Allied Sci. 2004;29(5):545-548.
10 Hobbs CGL, Sterne JA, Bailey M, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31(4):259-266.
11 Hobbs CGL, Birchall MA. Human papillomavirus infection in the etiology of laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):88-92.
12 Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5(4):311-316.
13 Almadori G, Bussu F, Cadoni G, et al. Molecular markers in laryngeal squamous cell carcinoma: towards an integrated clinicobiological approach. Eur J Cancer. 2005;41(5):683-693.
14 Kapral M, Strzalka B, Kowalczyk M, et al. Transforming growth factor beta isoforms (TGF-beta1, TGF-beta2, TGF-beta3) messenger RNA expression in laryngeal cancer. Am J Otolaryngol. 2008;29(4):233-237.
15 Loyo M, Pai SL. The molecular genetics of laryngeal cancer. Otolaryngol Clin North Am. 2008;41(4):657-672.
16 Yoo SS, Carter D, Turner BC, et al. Prognostic significance of cyclin D1 protein levels in early-stage larynx cancer treated with primary radiation. Int J Cancer. 2000;90(1):22-28.
17 Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332(11):712-717.
18 Jacob SE, Sreevidya S, Chacko E, et al. Cellular manifestations of human papillomavirus infection in laryngeal tissues. J Surg Oncol. 2002;79(3):142-150.
19 Narayana A, Vaughan AT, Kathuria S, et al. P53 overexpression is associated with bulky tumor and poor local control in T1 glottic cancer. Int J Radiat Oncol Biol Phys. 2000;46(1):21-26.
20 Pai HH, Rochon L, Clark B, et al. Overexpression of p53 protein does not predict local-regional control or survival in patients with early-stage squamous cell carcinoma of the glottic larynx treated with radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41(1):37-42.
21 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446-1449.
22 Lee NK, Goepfert H, Wendt CD. Supraglottic laryngectomy for intermediate-stage cancer: U.T. M.D. Anderson Cancer Center experience with combined therapy. Laryngoscope. 1990;100(8):831-836.
23 Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10(3):160-167.
24 Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405-409.
25 Cagli S, Yüce I, Yigilbasi OG, et al. Is routine bilateral neck dissection absolutely necessary in the management of N0 neck in patients with supraglottic carcinoma? Eur Arch Otorhinolaryngol. 2007;264(12):1453-1457.
26 Letterman M. Cancer of the larynx. I: natural history in relation to treatment. Br J Radiol. 1971;44:569.
27 McGavran M, Bauer W, Ogura J. The incidence of cervical lymph node metastasis from epidermoid carcinoma of the larynx and their relationship to certain characteristics of the primary tumor. A study based on the clinical and pathological findings for 96 patients treated by primary en bloc laryngectomy and radical neck dissection. Cancer. 1961;14(55):66.
28 Kaplan MJ, Johns ME, Clark DA, et al. Glottic carcinoma. The roles of surgery and irradiation. Cancer. 1984;53(12):2641-2648.
29 Fu KK, Eisenberg L, Dedo HH, et al. Results of integrated management of supraglottic carcinoma. Cancer. 1977;40(6):2874-2881.
30 Spector GJ. Distant metastases from laryngeal and hypopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63(4):224-228.
31 Kotwall C, Sako K, Razack MS, et al. Metastatic patterns in squamous cell cancer of the head and neck. Am J Surg. 1987;154(4):439-442.
32 Warwick-Brown NP, Cheesman AD. Intracranial metastases from a supraglottic carcinoma (a case report). J Laryngol Otol. 1987;101(6):624-626.
33 Ljumanovic R, Langendik JA, Hoekstra OS, et al. Distant metastases in head and neck carcinoma: identification of prognostic groups with MR imaging. Eur J Radiol. 2006;60(1):58-66.
34 Oosterkamp S, de Jon JM, Van den Ende PL, et al. Predictive value of lymph node metastases and extracapsular extension for the risk of distant metastases in laryngeal carcinoma. Laryngoscope. 2006;116(11):2067-2070.
35 Castelijns JA, Van den Brekel MW, Hermans R. Imaging of the larynx. Semin Roentgenol. 2000;35(1):31-41.
36 Zbaren P, Becker M, Lang H. Staging of laryngeal cancer: endoscopy, computed tomography and magnetic resonance versus histopathology. Eur Arch Otorhinolaryngol. 1997;254(S 1):117-122.
37 Bohndorf K. Assessment of laryngeal carcinoma before therapy: value of computed tomography and magnetic resonance tomography. Strahlenther Onkol. 1991;167(4):239-243.
38 Blitz AM, Aygun N. Radiologic evaluation of larynx cancer. Otolaryngol Clin North Am. 2008;41(4):697-713. vi
39 Fleming AJ, Johansen ME. The clinician’s expectations from the use of positron emission tomography/computed tomography scanning in untreated and treated head and neck cancer patients. Curr Opin Otolaryngol Head Neck Surg. 2008;16(2):127-134.
40 Fleming AJ, Smith SP, Paul CM, et al. Impact of [18F]-2-fluorodeoxyglucose-positron emission tomography/computed tomography on previously untreated head and neck cancer patients. Laryngoscope. 2007;117(7):1173-1179.
41 Connell CA, Corry J, Milner AD, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29(11):986-995.
42 Gourin CG, Watts TL, Williams HT, et al. Identification of distant metastases with positron-emission tomography-computed tomography in patients with previously untreated head and neck cancer. Laryngoscope. 2008;118(4):671-675.
43 Brouwer J, Hooft L, Hoekstra OS, et al. Systematic review: Accuracy of imaging tests in the diagnosis of recurrent laryngeal carcinoma after radiotherapy. Head Neck. 2008;30(7):889-897.
44 Salaun PY, Abgral R, Querellou S, et al. Does 18fluoro-fluorodeoxyglucose positron emission tomography improve recurrence detection in patients treated for head and neck squamous cell carcinoma with negative clinical follow-up? Head Neck. 2007;29(12):1115-1120.
45 Brouwer J, de Bree R, Comans EF, et al. Improved detection of recurrent laryngeal tumor after radiotherapy using (18)FDG-PET as initial method. Radio Ther Oncol. 2008;87(2):217-220.
46 De Bree R, van der Putten L, Hoekstra OS, et al. A randomized trial of PET scanning to improve diagnostic yield of direct laryngoscopy in patients with suspicion of recurrent laryngeal carcinoma after radiotherapy. Contemp Clin Trials. 2007;28(6):705-712.
47 Ong SC, Schöder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for locoregional advanced head and neck cancer. J Nucl Med. 2008;49(4):532-540.
48 Brouwer J, de Bree R, Hoekstra S, et al. Screening for distant metastases in patients with head and neck cancer: Is chest computed tomography sufficient? Laryngoscope. 2005;115(10):1813-1817.
49 Overgaard J, Hansen HS, Jorgensen K, et al. Primary radiotherapy of larynx and pharynx carcinoma—An analysis of some factors influencing local control and survival. Int J Radiat Oncol Biol Phys. 1986;12(4):515-521.
50 Overgaard J, Hansen HS, Andersen AP, et al. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys. 1989;16(4):1065-1068.
51 Cho EI, Sasaki CT, Haffty BG. Prognostic significance of pretreatment hemoglobin for local control and overall survival in T1-T2N0 larynx cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58(4):1135-1140.
52 Warde P, O’Sullivan B, Bristow RG, et al. T1/T2 glottic cancer managed by external beam radiotherapy: The influence of pretreatment hemoglobin on local control. Int J Radiat Oncol Biol Phys. 1998;41(2):347-353.
53 American Joint Committee on Cancer. Larynx. In: Fleming ID, Henson DE, et al, editors. AJCC Cancer Staging Manual. ed 6. New York: Springer Publishing Company; 2002:41.
54 Batsakis JG, Hybels R, Crissman JD, et al. The pathology of head and neck tumors: verrucous carcinoma, Part 15. Head Neck Surg. 1982;5(1):29-38.
55 Kraus FT, Perezmesa C. Verrucous carcinoma. Clinical and pathologic study of 105 cases involving oral cavity, larynx and genitalia. Cancer. 1966;19(1):26-38.
56 O’Sullivan B, Warde P, Keane T, et al. Outcome following radiotherapy in verrucous carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1995;32(3):6111-6117.
57 Ferlito A. Diagnosis and treatment of verrucous squamous cell carcinoma of the larynx: a critical review. Ann Otol Rhinol Laryngol. 1985;94(6 Pt 1):575-579.
58 Huang SH, Lockwood G, Irish J, et al. Truths and myths about radiotherapy for verrucous carcinoma of larynx. Int J Radiat Oncol Biol Phys. 2009;73(4):1110-1115.
59 Strojan P, Smid L, Cizmarevic B, et al. Verrucous carcinoma of the larynx: determining the best treatment option. Eur J Surg Oncol. 2006;32(9):984-988.
60 Ferlito A, Polidoro F, Rossi M. Pathological basis and clinical aspects of treatment policy in carcinoma-in-situ of the larynx. J Laryng Otol. 1981;95(2):141-154.
61 Garcia-Serra A, Hinerman RW, Amdur RJ, et al. Radiotherapy for carcinoma in situ of the true vocal cords. Head Neck. 2002;24(4):390-394.
62 Woodhouse RJ, et al. Treatment of carcinoma of the vocal cord. A review of 20 years experience. Laryngoscope. 1981;91(7):1155-1162.
63 Lillie JC, De Santo LW. Transoral surgery of early cordal carcinoma. Trans Am Acad Opthal Otolaryngol. 1973;77(2):92-96.
64 Bailey B. Management of carcinoma-in-situ and microinvasive carcinoma of the larynx. In: Baily B, Biller H, editors. Surgery of the larynx. Philadelphia: WB Saunders; 1985:229.
65 McGuirt WF, Koufman JA. Endoscopic laser surgery. An alternative in laryngeal cancer treatment. Arch Otolaryngol Head Neck. 1987;113(5):501-505.
66 Elman AJ, Goodman M, Wang CC, et al. In situ carcinoma of the vocal cords. Cancer. 1979;43(6):2422-2428.
67 Hintz BL, Kagan AR, Nussbaum H, et al. A “watchful waiting” policy for in situ carcinoma of the vocal cords. Arch Otolaryngol. 1981;107(12):746-751.
68 Spayne JA, Warde P, O’Sullivan P, et al. Carcinoma-in-situ of the glottic larynx: results of treatment with radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49(5):1235-1238.
69 Pêne F, Fletcher GH. Results in irradiation of the in situ carcinomas of the vocal cords. Cancer. 1976;37(6):2586-2590.
70 Miller AH, Fisher HR. Clues to the life history of carcinoma in situ of the larynx. Laryngoscope. 1971;81(9):1475-1480.
71 Cellai E, Frata P, Magrini SM, et al. Radical radiotherapy for early glottic cancer: results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int J Radiat Oncol Biol Phys. 2005;63(5):1378-1386.
72 Bron LP, et al. Treatment of early stage squamous-cell carcinoma of the glottic larynx: endoscopic surgery or cricohyoidoepiglottopexy versus radiotherapy. Head Neck. 2001;23(10):823-829.
73 Spector JG, et al. Stage I (T1 N0 M0) squamous cell carcinoma of the laryngeal glottis: therapeutic results and voice preservation. Head Neck. 1999;21(8):707-717.
74 Verdonck-de Leeuw IM, et al. Consequences of voice impairment in daily life for patients following radiotherapy for early glottic cancer: voice quality, vocal function, and vocal performance. Int J Radiat Oncol Biol Phys. 1999;44(5):1071-1078.
75 Hocevar-Boltezar I, Zargi M, Honocodeevar-Boltezar I. Voice quality after radiation therapy for early glottic cancer. Arch Otolaryngol Head Neck. 2000;126(9):1097-1100.
76 Johansen LV, Grau C, Overgaard J. Glottic carcinoma—patterns of failure and salvage treatment after curative radiotherapy in 861 consecutive patients. Radiother Oncol. 2002;63(3):257-267.
77 Mortuaire G, Francois J, Wight R, et al. Local recurrence after CO2 laser cordectomy for early glottic carcinoma. Laryngoscope. 2006;116(1):101-105.
78 Kandogan T, Sanal A. Quality of life, functional outcome, and voice handicap index in partial laryngectomy patients for early glottic cancer. BMC Ear Nose Throat Disord. 2005;5(1):3.
79 Dey P, Arnold D, Wight R, et al: Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer, Cochrane Database Syst Rev 2(CD 002027), 2002.
80 American Society of Clinical OncologyPfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704.
81 Groome PA, O’Sullivan B, Irish JC, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the surveillance, epidemiology, and end results areas of the United States. J Clin Oncol. 2003;21(3):496-505.
82 Groome PA, O’Sullivan B, Irish JC, et al. Glottic cancer in Ontario, Canada, and the SEER areas of the United States. Do different management philosophies produce different outcome profiles? J Clin Epidemiol. 2001;54(3):301-315.
83 Epstein BE, Lee DJ, Kashima H, et al. Stage T1 glottic carcinoma: Results of radiation therapy or laser excision. Radiology. 1990;175(2):567-570.
84 Jones AS, Fish B, Fenton JE, et al. The treatment of early laryngeal cancers (T1-T2 N0): surgery or irradiation? Head Neck. 2004;26(2):127-135.
85 Loughran S, Calder N, MacGregor FB, et al. Quality of life and voice following endoscopic resection or radiotherapy for early glottic cancer. Clin Otolaryngol. 2005;30(1):42-47.
86 Goor KM, Peeters AJ, Mahieu HF, et al. Cordectomy by CO2 laser or radiotherapy for small T1a glottic carcinomas: costs, local control, survival, quality of life, and voice quality. Head Neck. 2007;29(2):128-136.
87 Peeters AJ, van Gogh CD, Goor KM, et al. Health status and voice outcome after treatment for T1a glottic carcinoma. Eur Arch Otorhinolaryngol. 2004;261(10):534-540.
88 Mlynarek A, Kost K, Gesser R. Radiotherapy versus surgery for early T1-T2 glottic carcinoma. J Otolaryngol. 2006;35(6):413-419.
89 Brøndbo K, Benninger MS. Laser resection of T1a glottic carcinomas: results and postoperative voice quality. Acta Otolaryngol. 2004;124(8):976-979.
90 Ganly I, Patel SG, Matsuo J, et al. Results of surgical salvage after failure of definitive radiation therapy for early-stage squamous cell carcinoma of the glottic larynx, Arch Otoloaryngol. Head Neck. 2006;132(1):59-66.
91 Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64(1):77-82.
92 Shah JP, Loree TR, Kowalski L. Conservation surgery for radiation-failure carcinoma of the glottic larynx. Head Neck. 1990;12(4):326-331.
93 Nichols RD, Mickelson SA. Partial laryngectomy after irradiation failure. Ann Otol Rhinol Laryngol. 1991;100(3):176-180.
94 Pellini R, Pichi B, Ruscito P, et al. Supracricoid partial laryngectomies after radiation failure: a multi-institutional series. Head Neck. 2008;30(3):372-379.
95 Fein DA, Lee WR, Hanlon AL, et al. Do overall treatment time, field size, and treatment energy influence local control of T1-T2 squamous cell carcinomas of the glottic larynx? Int J Radiat Oncol Biol Phys. 1996;34(4):823-831.
96 Fein DA, Mendenhall WM, Parsons JT, et al. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiotherapy: a multivariate analysis of variables potentially influencing local control. Int J Radiat Oncol Biol Phys. 1993;25(4):605-611.
97 Mendenhall WM, Parsons JT, Million RR, et al. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation therapy: relationship of dose-fractionation factors to local control and complications. Int J Radiat Oncol Biol Phys. 1988;15(6):1267-1273.
98 Nomiya T, Nemoto K, Wada H, et al. Long-term results of radiotherapy for T1a and T1bN0M0 glottic carcinoma. Laryngoscope. 2008;118(8):1417-1421.
99 Akine Y, Tokita N, Ogino T, et al. Radiotherapy of T1 glottic cancer with 6 MeV x-rays. Int J Radiat Oncol Biol Phys. 1991;20(6):1215-1218.
100 Dinshaw KA, et al. Radiation therapy in T1-T2 glottic carcinoma: influence of various treatment parameters on local control/complications. Int J Radiat Oncol Biol Phys. 2000;48(3):723-735.
101 Johansen LV, et al. Primary radiotherapy of T1 squamous cell carcinoma of the larynx: analysis of 478 patients treated from 1963 to 1985. Int J Radiat Oncol Biol Phys. 1990;18(6):1307-1313.
102 Carl J, Andersen LJ, Pedersen M, et al. Prognostic factors of local control after radiotherapy in T1 glottic and supraglottic carcinoma of the larynx. Radiother Oncol. 1996;39(3):229-233.
103 Nozaki M, et al. Radiation therapy for T1 glottic cancer: involvement of the anterior commissure. Anticancer Res. 20(2B), 2000. 1121–114
104 Haugen H, Johansson KA, Mercke C. Hyperfractionated-accelerated or conventionally fractionated radiotherapy for early glottic cancer. Int J Radiat Oncol Biol Phys. 2002;52(1):109-119.
105 Garden AS, Forster K, Wong PF, et al. Results of radiotherapy for T2N0 glottic carcinoma: does the “2” stand for twice-daily treatment? Int J Radiat Oncol Biol Phys. 2003;55(2):322-328.
106 Trotti A, et al. 26: a randomized trial of hyperfractionation versus standard fractionation in T2 squamous cell carcinoma of the vocal cord. Int J Radiat Oncol Biol Phys. 2006;66(3):S15.
107 Mendenhall WM, et al. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19(20):4029-4036.
108 Wang CC. Carcinoma of the larynx. In: Wang CC, editor. Radiation therapy for head and neck neoplasms: indications, techniques and results. Chicago: Year Book Medical Publishers; 1990:223.
109 Harwood AR, et al. T2 glottic cancer: an analysis of dose-time-volume factors. Int J Radiat Oncol Biol Phys. 1981;7(11):1501-1505.
110 Van den Bogaert W, Ostyn F, van der Schueren E. The significance of extension and impaired mobility in cancer of the vocal cord. Int J Radiat Oncol Biol Phys. 1983;9(2):181-184.
111 Howell-Burke D, et al. T2 glottic cancer. Recurrence, salvage, and survival after definitive radiotherapy. Arch Otolaryngol Head Neck. 1990;116(7):830-835.
112 Karim AB, et al. Heterogeneity of stage II glottic carcinoma and its therapeutic implications. Int J Radiat Oncol Biol Phys. 1987;13(3):313-317.
113 Frata P, et al. Radical radiotherapy for early glottic cancer: results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int J Radiat Oncol Biol Phys. 2005;63(5):1387-1394.
114 Mittal B, et al. Role of radiation in the management of early vocal cord carcinoma. Int J Radiat Oncol Biol Phys. 1983;9(7):997-1002.
115 Le QT, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;39(1):115-126.
116 Amornmarn R, et al. A therapeutic approach to early vocal cord carcinoma. Acta Radiol Oncol. 1985;24(4):321-325.
117 Dagan R, et al. Prognostic significance of paraglottic space invasion in T2N0 glottic carcinoma. Am J Clin Oncol Cancer Clin Trials. 2007;30(2):186-190.
118 Orús C, et al. Initial treatment of the early stages (I, II) of supraglottic squamous cell carcinoma: partial laryngectomy versus radiotherapy. Eur Arch Otorhinolaryngol. 2000;257(9):512-516.
119 Rodrigo JP, et al. Transoral laser surgery for supraglottic cancer. Head Neck. 2008;30(5):658-666.
120 Cabanillas R, et al. Oncologic outcomes of transoral laser surgery of supraglottic carcinoma compared with a transcervical approach. Head Neck. 2008;30(6):750-755.
121 Preuss SF, Cramer K, Klussmann JP, et al. Transoral laser surgery for laryngeal cancer: outcome, complications and prognostic factors in 275 patients. Eur J Surg Oncol. 2008;35(3):235-240.
122 Primdahl H, et al. Changes from 1992 to 2002 in the pretreatment delay for patients with squamous cell carcinoma of larynx or pharynx: a Danish nationwide survey from DAHANCA. Acta Oncol. 2006;45(2):156-161.
123 Roh JL, Kim DH, Park CI. Voice, swallowing and quality of life in patients after transoral laser surgery for supraglottic carcinoma. J Surg Oncol. 2008;98(3):184-189.
124 Hinerman RW, et al. Carcinoma of the supraglottic larynx: treatment results with radiotherapy alone or with planned neck dissection. Head Neck. 2002;24(5):456-467.
125 Freeman DE, et al. Irradiation alone for supraglottic larynx carcinoma: can CT findings predict treatment results? Int J Radiat Oncol Biol Phys. 1990;19(2):485-490.
126 Kraas JR, et al. Quantitative analysis from CT is prognostic for local control of supraglottic carcinoma. Head Neck. 2001;23(12):1031-1036.
127 Harwood AR, et al. Supraglottic laryngeal carcinoma: an analysis of dose-time-volume factors in 410 patients. Int J Radiat Oncol Biol Phys. 1983;9(3):311-319.
128 Wall TJ, et al. Relationship between lymph nodal status and primary tumor control probability in tumors of the supraglottic larynx. Int J Radiat Oncol Biol Phys. 1985;11(11):1895-1902.
129 Freeman D, Mendenhall WM, Parsons JT. Does neck stage influence local control probability in squamous cell cancers of the head and neck. Int J Radiat Oncol Biol Phys. 1991;21(suppl 1):138.
130 Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685-1690.
131 Spector GJ, et al. Management of stage IV glottic carcinoma: therapeutic outcomes. Laryngoscope. 2004;114(8):1438-1446.
132 Vokes EE, et al. Head and neck cancer. N Engl J Med. 1993;328(3):184-194.
133 Tupchong L, et al. Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long-term follow-up of RTOG study 73-03. Int J Radiat Oncol Biol Phys. 1991;20(1):21-28.
134 Parsons JT, et al. Twice-a-day radiotherapy for squamous cell carcinoma of the head and neck: The University of Florida experience. Head Neck. 1993;15(2):87-96.
135 Garden AS, et al. Hyperfractionated radiation in the treatment of squamous cell carcinomas of the head and neck: a comparison of two fractionation schedules. Int J Radiat Oncol Biol Phys. 1995;31(3):493-502.
136 Fu KK, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48(1):7-16.
137 Forastiere AA, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091-2098.
138 Forastiere AA, et al. Long-term results of Intergroup RTOG 91-11: a phase III trial to preserve the larynx—Induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol. 2006;24(18S):5517.
139 Weber RS. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group Trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129(1):44-49.
140 Adelstein DJ, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92-98.
141 Al-Sarraf M, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol. 1998;16(4):1310-1317.
142 Denis F, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22(1):69-76.
143 Pignon JP, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355(9208):949-955.
144 Pignon JP, le Maître A, Bourhis J. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S112-S114.
145 Machtay M, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582-3589.
146 Posner M, Vermorken JB. Induction therapy in the modern era of combined-modality therapy for locally advanced head and neck cancer. Semin Oncol. 2008;35(3):221-228.
147 Posner MR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705-1715.
148 Vermorken JB, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695-1704.
149 Urba S, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24(4):593-598.
150 Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21(14):2787-2799.
151 Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578.
152 Caudell JJ, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 2008;71(3):676-681.
153 Pellitteri PK, Ferlito A, Rinaldo A, et al. Planned neck dissection following chemoradiotherapy for advanced head and neck cancer: is it necessary for all? Head Neck. 2006;28(2):166-175.
154 Brizel DM, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58(5):1418-1423.
155 Greven KM, et al. Radiographic complete response on post treatment CT imaging eliminates the need for adjuvant neck dissection after treatment for node positive head and neck cancer. Am J Clin Oncol Cancer Clin Trials. 2008;31(2):169-172.
156 Forest VI, et al. Role of neck dissection following concurrent chemoradiation for advanced head and neck carcinoma. Head Neck. 2006;28(12):1099-1105.
157 Goguen LA, et al. Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck. 2006;132(5):526-531.
158 Chan AW, et al. The role of postradiotherapy neck dissection in supraglottic carcinoma. Int J Radiat Oncol Biol Phys. 2001;50(2):367-375.
159 Sanguineti G, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter phase III study. Int J Radiat Oncol Biol Phys. 2005;61(3):762-771.
160 Ang KK, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571-578.
161 Garas J, McGuirt SWF. Squamous cell carcinoma of the subglottis. Am J Otolaryngol. 2006;27(1):1-4.
162 Guedea F, et al. Primary subglottic cancer: results of radical radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21(6):1607-1611.
163 Paisley S, et al. Results of radiotherapy for primary subglottic squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(5):1245-1250.
164 Vermund H. Role of radiotherapy in cancer of the larynx as related to the TNM system of staging. A review. Cancer. 1970;25(3):485-504.
165 Warde P, Harwood A, Keane T. Carcinoma of the subglottis. Results of initial radical radiation. Arch Otolaryngol Head Neck. 1987;113(11):1228-1229.
166 Hermans R, et al. Value of computed tomography as outcome predictor of supraglottic squamous cell carcinoma treated by definitive radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44(4):755-765.
167 Hermans R, et al. Predicting the local outcome of glottic squamous cell carcinoma after definitive radiation therapy: value of computed tomography-determined tumour parameters. Radiother Oncol. 1999;50(1):39-46.
168 Pameijer FA, et al. Pre- and post-radiotherapy computed tomography in laryngeal cancer: imaging-based prediction of local failure. Int J Radiat Oncol Biol Phys. 1999;45(2):359-366.
169 Maheshwar AA, Gaffney CC. Radiotherapy for T1 glottic carcinoma: impact of anterior commissure involvement. J Laryngol Otol. 2001;115(4):298-301.
170 Million RR. The larynx … so to speak: everything I wanted to know about laryngeal cancer I learned in the last 32 years. Int J Radiat Oncol Biol Phys. 1992;23(4):691-704.
171 Sombeck MD, et al. Radiotherapy for early vocal cord cancer: a dosimetric analysis of 60CO versus 6 MV photons. Head Neck. 1996;18(2):167-173.
172 Foote RL, et al. Radiation therapy for glottic cancer using 6-MV photons. Cancer. 1996;77(2):381-386.
173 Feigenberg SJ, et al. Intensity-modulated radiotherapy for early larynx cancer: is there a role? Int J Radiat Oncol Biol Phys. 2007;68(1):2-3.
174 Inoue T, Chatani M, Teshima T. Irradiated volume and arytenoid edema after radiotherapy for T1 glottic carcinoma. Strahlenther Onkol. 1992;168(1):23-26.
175 Mendenhall WM, et al. Is elective neck treatment indicated for T2N0 squamous cell carcinoma of the glottic larynx? Radiother Oncol. 1989;14(3):199-202.
176 Lin A, et al. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57(1):61-70.
177 Kam MKM, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873-4879.
178 Eisbruch A, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S40-S42.
179 Eisbruch A, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439.
180 Lee NY, Le QT. New developments in radiation therapy for head and neck cancer: intensity-modulated radiation therapy and hypoxia targeting. Semin Oncol. 2008;35(3):236-250.
181 Gomez DR, Zhung JE, Gomez J, et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys. 2009;73(4):1096-1103.
182 Huang K, et al. Intensity-modulated chemoradiation for treatment of stage III and IV oropharyngeal carcinoma: The University of California-San Francisco experience. Cancer. 2008;113(3):497-507.
183 Lee NY, Terezakis SA. Intensity-modulated radiation therapy. J Surg Oncol. 2008;97(8):691-696.
184 Lee NY, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2007;69(2):459-468.
185 Van Dieren EB, et al. Beam intensity modulation using tissue compensators or dynamic multileaf collimation in three-dimensional conformal radiotherapy of primary cancers of the oropharynx and larynx, including the elective neck. Int J Radiat Oncol Biol Phys. 2000;47(5):1299-1309.
186 Seung S, Bae J, Solhjem M, et al. Intensity-modulated radiotherapy for head-and-neck cancer in the community setting. Int J Radiat Oncol Biol Phys. 2008;72(4):1075-1081.
187 Lee N, et al. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;68(5):1299-1309.
188 Das IJ, et al. Intensity-modulated radiation therapy dose prescription, recording, and delivery: Patterns of variability among institutions and treatment planning systems. J Natl Cancer Inst. 2008;100(5):300-307.
189 Zeidan OA, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67(3):670-677.
190 Barker JL, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960-970.
191 Kuo YC, et al. Effect of regression of enlarged neck lymph nodes on radiation doses received by parotid glands during intensity-modulated radiotherapy for head and neck cancer, Am J Clin Oncol. Cancer Clin Trials. 2006;29(6):600-605.
192 Fu KK, et al. The significance of laryngeal edema following radiotherapy of carcinoma of the vocal cord. Cancer. 1982;49(4):655-658.
193 Stewart JG, Jackson AW. The steepness of the dose response curve both for tumor cure and normal tissue injury. Laryngoscope. 1975;85(7):1107-1111.
194 Deore SM, et al. The predictive role of bioeffect dose models in radiation-induced late effects in glottic cancers. Int J Radiat Oncol Biol Phys. 1992;23(2):281-284.
195 Taylor JM, Mendenhall WM, Lavey RS. Dose, time, and fraction size issues for late effects in head and neck cancers. Int J Radiat Oncol Biol Phys. 1992;22(1):3-11.
196 Kramer S, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73–03 of the Radiation Therapy Oncology Group. Head Neck Surg. 1987;10(1):19-30.
197 Caudell JJ, et al: Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer, Int J Radiat Oncol Biol Phys (in press).
198 Cox JD, et al. ASTRO plenary: interfraction interval is a major determinant of late effects, with hyperfractionated radiation therapy of carcinomas of upper respiratory and digestive tracts: results from Radiation Therapy Oncology Group protocol 8313. Int J Radiat Oncol Biol Phys. 1991;20(6):1191-1195.
199 Denis F, et al. Late toxicity results of the GORTEC 94–01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys. 2003;55(1):93-98.
200 Trotti A, et al. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121-5127.
201 Rosenthal DI, et al: The M.D. Anderson symptom inventory—head and neck module, a patient-reported outcome instrument, accurately predicts the severity of radiation-induced mucositis, Int J Radiat Oncol Biol Phys (in press).
202 Denham JW, et al. Do acute mucosal reactions lead to consequential late reactions in patients with head and neck cancer? Radiother Oncol. 1999;52(2):157-164.
203 Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61(3):223-231.
204 Wang CC, et al. Local control of T3 carcinomas after accelerated fractionation: a look at the “gap.”. Int J Radiat Oncol Biol Phys. 1996;35(3):439-441.
205 Maciejewski B, Taylor JM, Withers HR. Alpha/beta value and the importance of size of dose per fraction for late complications in the supraglottic larynx. Radiother Oncol. 1986;7(4):323-326.
206 Peters LJ, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26(1):3-11.
207 Langendijk JA, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776.
208 Hutcheson KA, et al. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck. 2008;134(2):178-183.
209 Nguyen NP, et al. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer. 2002;94(4):1131-1141.
210 Nguyen NP, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol. 2004;15(3):383-388.
211 Brizel DM, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339-3345.
212 Brizel DM, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26(15):2489-2496.
213 Buentzel J, et al. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys. 2006;64(3):684-691.
214 Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24(17):2636-2643.
215 Van Gogh CDL, et al. The efficacy of voice therapy in patients after treatment for early glottic carcinoma. Cancer. 2006;106(1):95-105.
216 Verdonck-de Leeuw IM, et al. Multidimensional assessment of voice characteristics after radiotherapy for early glottic cancer. Laryngoscope. 1999;109(2 Pt 1):241-248.
217 Dorresteijn LDA, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282-288.
218 Smith GL, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;16:6546.
219 Norris AA, et al. Hypothyroidism when the thyroid is included only in the low neck field during head and neck radiotherapy, Am J Clin Oncol. Cancer Clin Trials. 2006;29(5):442-445.
220 Ozawa H, et al. Hypothyroidism after radiotherapy for patients with head and neck cancer. Am J Otolaryngol. 2007;28(1):46-49.
221 Garcia-Serra A, et al. Thyroid function should be monitored following radiotherapy to the low neck. Am J Clin Oncol Cancer Clin Trials. 2005;28(3):255-258.
222 Smolarz K, et al. Hypothyroidism after therapy for larynx and pharynx carcinoma. Thyroid. 2000;10(5):425-429.
223 Boscolo-Rizzo P, et al. Long-term quality of life after total laryngectomy and postoperative radiotherapy versus concurrent chemoradiotherapy for laryngeal preservation. Laryngoscope. 2008;118(2):300-306.
224 Fung K, et al. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63(5):1395-1399.
225 LoTempio MM, et al. Comparison of quality of life outcomes in laryngeal cancer patients following chemoradiation vs. total laryngectomy. Otolaryngol Head Neck Surg. 2005;132(6):948-953.
226 Fletcher GH. Treatment of cancer of the larynx. Ear Nose Throat J. 1977;56(3):91-96.
227 Johansen LV, Grau C, Overgaard J. Supraglottic carcinoma: patterns of failure and salvage treatment after curatively intended radiotherapy in 410 consecutive patients. Int J Radiat Oncol Biol Phys. 2002;53(4):948-958.
228 Eckel HE. Endoscopic laser resection of supraglottic carcinoma. Otolaryngol Head Neck Surg. 1997;117(6):681-687.
229 Iro H, et al. Transoral laser surgery of supraglottic cancer: follow-up of 141 patients. Arch Otolaryngol Head Neck Surg. 1998;124(11):1245-1250.
230 Ambrosch P, Kron M, Steiner W. Carbon dioxide laser microsurgery for early supraglottic carcinoma. Ann Otol Rhinol Laryngol. 1998;107(8):680-688.
231 Rudert HH, Werner JA, Höft S. Transoral carbon dioxide laser resection of supraglottic carcinoma. Ann Otol Rhinol Laryngol. 1999;108(9):819-827.
232 Zeitels SM, et al. Endoscopic treatment of supraglottic and hypopharynx cancer. Laryngoscope. 1994;104(1 Pt 1):71-78.
233 Davis RK, et al. Endoscopic supraglottic laryngectomy with postoperative irradiation. Ann Otol Rhinol Laryngol. 2004;113(2):132-138.