43 Cancer of the Kidney
Epidemiology and Risk Factors
In 2008, there were 54,390 new cases and 13,010 deaths in the United States. According to the statistics reported by the American Cancer Society, cancer of the kidney is the seventh most common cancer in men, and the ninth most common in women.1 More than 85% of these tumors are renal cell carcinomas (RCCs), with the remainder consisting of less common tumors such as transitional cell carcinomas of the renal pelvis and Wilms tumors. Approximately 30% of patients present with metastatic disease; RCC accounts for 3% of all adult malignancies and demonstrates a slight male predominance, with 60% of the diagnoses and deaths occurring in men.
With a peak incidence in the sixth to eighth decades, RCC is a tumor of older adults, although presentation at other ages is not uncommon, especially in cases involving hereditary syndromes. The incidence of RCC is increasing, in part because of the incidental diagnosis of small renal masses (SRMs) (masses ≤4 cm) captured by the increased use of computed tomography (CT) scanning. In the past, treatment of these SRMs has been aggressive, with surgical resection rather than observation. But despite this aggressive treatment, mortality is actually increasing and is greatest in tumors larger than 4 cm. This “treatment disconnect” is likely related to the fact that the majority of these SRMs are benign (20%) or clinically indolent when malignant (70%).2,3 Surveillance of these SRMs has become an acceptable option for the compliant patient.
Numerous risk factors have been associated with the development of RCC, many of which are listed in Table 43-1. Cigarette smoking has a well-established association, with the risk of RCC increasing proportionately with pack-years of use. Patients with end-stage renal failure on long-term hemodialysis are at risk for developing acquired cystic disease of the kidney. It is this latter condition, and not hemodialysis per se, that appears to be the causal factor for the development of RCC in this subpopulation. There is accumulating evidence that chronic diuretic therapy may be a risk factor for development of RCC.2,4,5
| Factor |
|---|
| Cigarette smoking118–120 |
| Obesity118–120 |
| Phenacetin abuse118–120 |
| Asbestos exposure121,122 |
| Leather tanning121,122 |
| Shoe repair121,122 |
| Chronic hemodialysis123–125 |
| Von Hippel-Lindau disease8,10 |
Genetic factors also appear to increase risk. In 1979, Cohen and colleagues6 reported a familial pattern of RCC that appeared to be transmitted via an autosomal dominant gene pattern. A 3;8 translocation was found in numerous family members, and all RCC cases occurred only in those members with the translocation. Pathak and colleagues7 described a second family pedigree with RCC in 1982; however, the translocation occurred between chromosomes 3 and 11 and was present only within tumor tissue. Patients with von Hippel-Lindau (VHL) disease develop retinal angiomas, hemangioblastomas of the central nervous system, and clear cell renal carcinoma. This disease is transmitted in an autosomal dominant pattern and is associated with the development of RCC in 25% to 45% of those afflicted.8–10 Cohen has shown that the human VHL gene maps to chromosome 3p25.11 Furthermore, studies analyzing the polymorphism of restriction fragment lengths have revealed 3p deletions in nonfamilial cases of RCC.
Frequent and early loss of heterozygosity in chromosome 3p has been identified in 90% or more of spontaneous clear cell RCCs.4,5 This led to the identification of a mutation in the VHL tumor suppressor gene, which results in loss of function or reduced levels of the VHL protein. This leads to deregulation of hypoxia-inducible factor (HIF)-1α, allowing it to accumulate and bind with HIF-β.7,11 This transcriptional factor complex induces expression of hypoxia-inducible genes such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which promote angiogenesis, inhibit normal tumor cell apoptosis, and eventually lead to tumor proliferation and survival.12 The identification of this key mutation has led to the development of targeted agents against the VHL pathway in the treatment of metastatic renal cell carcinoma (mRCC). Mutations in either tumor suppressor genes or proto-oncogenes have been identified in almost all histologic subtypes of RCC.8
Although the new targeted agents have greatly increased the treatment arsenal for mRCC, the best chance of cure remains early diagnosis and surgical resection. Patients with locally confined tumors have an expected 5-year disease-specific survival of nearly 90%, and 10% of patients with metastatic disease will be alive at 5 years.13 Unfortunately, the majority of patients who present with localized disease develop metastases during the course of their disease.
Anatomy
The kidneys are paired retroperitoneal organs, the medial edges of which lie parallel to the psoas muscles. Each kidney is typically 11 to 12 cm in length, corresponding radiographically to 3 to 3.5 vertebral bodies. Although there is some variation, the kidney is usually centered at L1 or L2 and extends from T12 to L3 (Fig. 43-1). Because of diaphragmatic contracture, the kidneys may move caudally by as much as 2.5 cm with inhalation. The right kidney is depressed by the liver 1 to 2 cm relative to the left and is in association with the second part of the duodenum, the right colic flexure, and the posterior portions of the right 11th and 12th ribs (Fig. 43-2). The left kidney is adjacent to the spleen, stomach, pancreas, left colic flexure, and left 11th and 12th ribs.
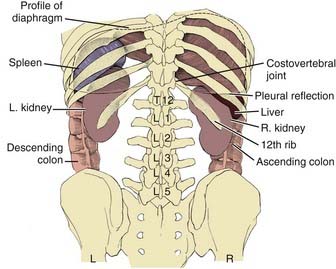
FIGURE 43-1 • Relation of the kidneys to the thorax and vertebral bodies, posterior view.
(From Hinman F Jr: Atlas of urosurgical anatomy, Philadelphia, 1993, WB Saunders, p 260.)
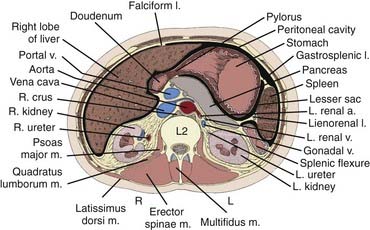
FIGURE 43-2 • Transverse section of the kidney at the L2 level.
(From Hinman F Jr: Atlas of urosurgical anatomy, Philadelphia, 1993, WB Saunders, p 262.)
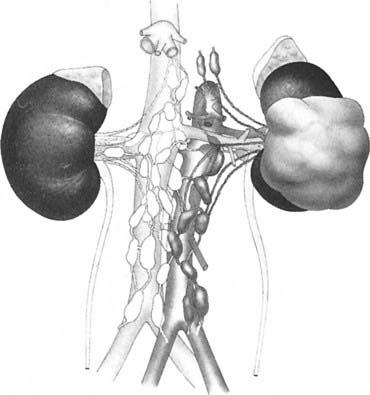
Draining lymph nodes originate within the renal hilus and follow the renal artery to the para-aortic and paracaval chains (Fig. 43-3). Regional nodal drainage extends from the crus of the diaphragm to the bifurcation of the aorta, varying somewhat with tumor laterality. For a right-sided tumor, this specifically includes the paracaval, retrocaval, precaval, interaortocaval, and preaortic lymph nodes. The para-aortic, preaortic, retroaortic, interaortocaval, and precaval lymph nodes are at risk for a left-sided tumor.
Pathologic Conditions
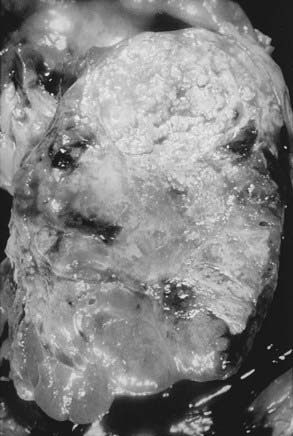
RCC is a neoplasm arising from proximal renal tubular epithelium. Historically, this tumor was erroneously considered to arise from adrenal rests within the kidney and thereby acquired the name hypernephroma, titled by Grawitz in 1883.14 Recent immunohistologic and ultrastructural analysis confirms the true renal origin of RCC,15,16 validating its more common and correct name. Gross sectioning of RCC tumors often reveals the classic yellow-tan fatty appearance with occasional focal areas of hemorrhage and necrosis (Fig. 43-4). Calcifications, fluid-filled cysts, and areas of fibrosis may also be apparent. Although not truly encapsulated, these lesions are often surrounded by a pseudocapsule because of their expansile growth pattern, which results in compression of surrounding renal parenchyma.
There has been a long-standing controversy as to the existence of a benign entity called renal adenoma, defined as lesions less than 3 cm in diameter. Although histologically indistinguishable from RCC, these lesions were considered benign since the 1938 publication of Bell’s classic paper,17 in which he described the absence of metastatic spread in lesions smaller than 3 cm. Although it is not uncommon for such lesions to be detected incidentally or at autopsy without clinical evidence of spread, there is an increased number of reports of tumors fulfilling the size criterion of renal adenoma, but with nodal and distant metastases (DMs) present.18 Furthermore, when one contemplates the natural history of a large RCC lesion, it is illogical to conclude that it had never once measured less than 3 cm. It is generally now advocated that renal neoplasms be defined as malignant or benign based on their histologic appearance, and not because of their size.
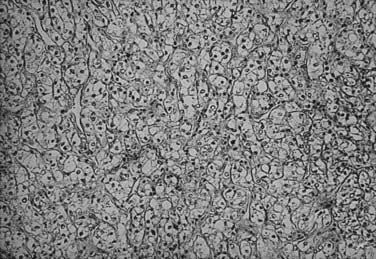
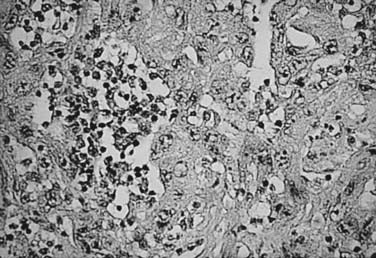
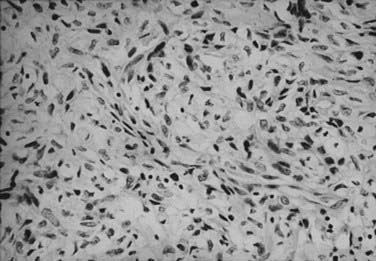
The histologic appearance of RCC is variable, with five main cell types predominating: (1) clear cell, (2) papillary type 1, (3) papillary type 2, (4) chromophobe, and (5) oncocytoma.9 Sarcomatoid can be a variant of any of these subtypes and indicates an aggressive subtype that is associated with a worse prognosis.19 Oncocytomas are considered benign (Figs. 43-5 to 43-7). Clear cell RCC is by far the most common, constituting approximately 75% of all tumors.20 Histologically, these cells contain lightly staining cytoplasm, and are rich in glycogen and lipid material. The cell of origin for clear cell carcinomas is thought to be proximal tubular epithelium. Sarcomatoid or spindle cell tumors resemble mesenchymal cells. They are frequently high grade and demonstrate an aggressive clinical course with 70% to 80% presenting with metastases.21 An individual tumor may demonstrate one or a combination of these cell types.
Clinical Presentation
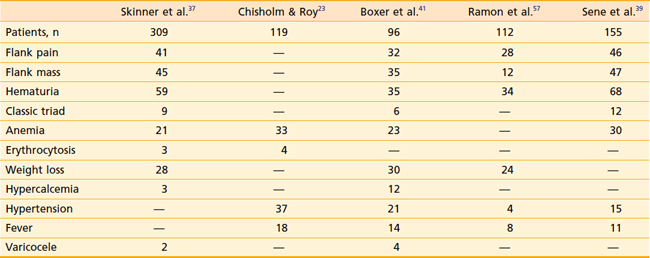
RCC has been described as “the internist’s tumor” because of the myriad of signs and symptoms that may be noted at presentation (Table 43-2), some of which are due to the direct effects of an invasive renal mass, and others of which are considered paraneoplastic in origin. Most RCCs are asymptomatic. Only 30% present with symptoms, and these cases tend to be locally advanced or metastatic. The most common symptoms are gross hematuria, flank pain, or a flank mass. Together, these symptoms compose the classic triad of RCC, which is only seen in approximately 10% of presenting patients. Whether gross or microscopic, hematuria presents primarily when the collecting system has been invaded. Flank masses resulting from RCC are firm, nontender, and homogeneous. The sudden onset of a nondraining varicocele in a male, particularly on the left, should raise the possibility of a renal neoplasm obstructing the gonadal vein at its entry point into the left renal vein.
Paraneoplastic syndromes are commonly associated with RCC.22 Anemia is among the most common, occurring in 30% to 40% of patients, and is generally considered a paraneoplastic process as it is often out of proportion to the degree of hematuria. Conversely, erythrocytosis is also a well-recognized phenomenon; however, it is much less common at around 4% to 5%. Other paraneoplastic syndromes associated with RCC include fever, cachexia, hypercalcemia, hypertension, Stauffer syndrome, amyloidosis, enteropathy, and neuromyopathy.23 Ectopic production of a parathyroid hormone–like material is responsible for the rare finding of hypercalcemia. Paraneoplastic syndromes confer a worse prognosis and do not always improve with removal of the primary tumor.
Routes of Spread
One quarter to one third of all patients are found to have DM at presentation. Furthermore, among patients treated for localized disease, the primary cause of RCC-associated death is metastatic tumor burden and not local failure (LF).24 Approximately 50% of initial metastatic sites are to the lung. The immunoprotected sites of the bone and the liver are the next most common sites of DMs. RCC goes to the brain in 2% to 10% of cases, and patients tend to have associated neurologic symptoms. Local recurrences are less common at approximately 2% or less. The common involvement of the renal vein by this tumor gives direct evidence for the early hematogenous spread commonly seen in this disease.
Staging Workup
The use of 18F-fluorodeoxyglucose positron-emission tomography (PET) in diagnosing and staging primary and mRCC is limited by its low sensitivity.25 However, its high specificity is superior to conventional imaging. Thus, PET may best be used in RCC in combination with CT to make management decisions in which the CT component provides high sensitivity and better anatomic information, whereas PET offers high specificity. This combination is useful to evaluate equivocal findings on CT such as differentiating between response to treatment and recurrence.26
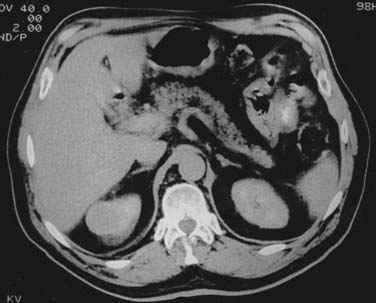
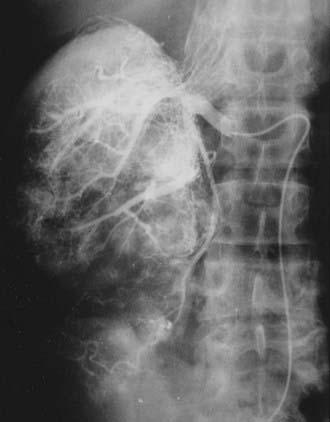
Once the presence of a renal mass has been documented, a chest x-ray film and bone scan (if alkaline phosphatase is elevated) are required. If there is no evidence of DMs or impaired renal function, no further tests are required, and patients are generally brought to the operating room for definitive resection. For patients in whom a partial nephrectomy (PN) is being considered (see later discussion of indications), or a locally advanced lesion is identified, a selective renal arteriogram may be obtained to better delineate tumor extent and vascular anatomy (Fig. 43-8, Fig. 43-9, Fig. 43-10, and Fig. 43-11).27 Inferior venacavography is a useful tool for evaluating renal vein and inferior vena cava extension. Preoperative needle biopsies of the renal mass for the purpose of obtaining a tissue diagnosis may be indicated in patients with evidence of metastatic disease, but they are not required in a patient who is a candidate for definitive resection. Other complementary radiologic tests include ultrasonography, magnetic resonance imaging (MRI), and percutaneous puncture of a renal cyst.
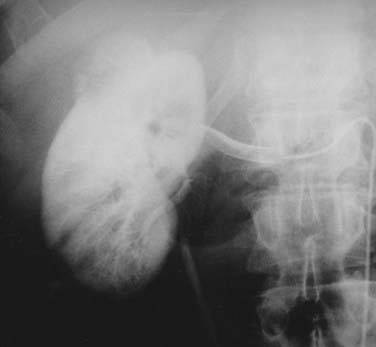
FIGURE 43-9 • Selective renal arteriogram of the patient whose scan is shown in Fig. 43-8. Note the highly vascular appearance of these tumors.
Staging Systems
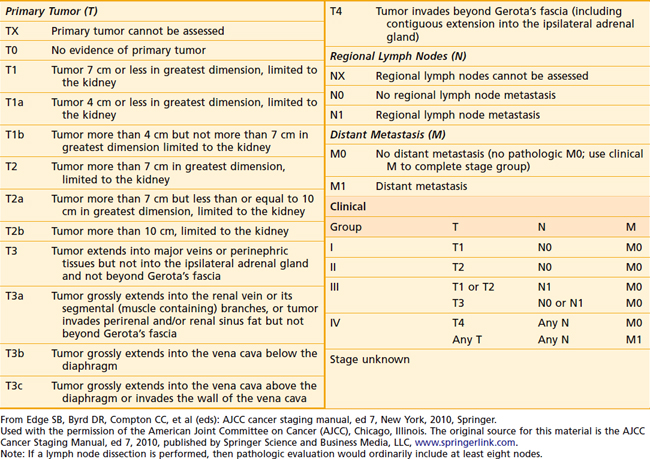
One of the oldest and most commonly used staging systems is the Robson classification system (Table 43-3), which was proposed by Robson28 in 1969 as a modification of the system presented by Flocks and Kadesky in 1958.29 The American Joint Committee on Cancer’s (AJCC’s) latest definition of tumor-node-metastasis (TNM) classification (Table 43-4), provides more detailed information by separating characteristics of the primary lesion from those of regional nodes.30 These definitions can be used as either a clinical or a pathologic staging system. There is very poor correlation between the Robson and AJCC staging systems, as demonstrated in Table 43-5, making comparisons quite confusing.
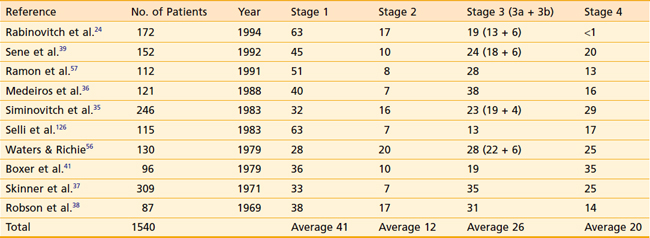
Distribution of patients by Robson stage at presentation is listed in Table 43-6. One must bear in mind that nearly all studies defining tumor stage at presentation are surgical series, resulting in a slight downward stage migration. It must be presumed that many patients with widespread disease at presentation (i.e., stage 4) are not surgical candidates and are therefore excluded from these reports. Overall, approximately 40% of patients have disease confined to the kidney, 12% have perinephric extension, 20% have renal vein extension, 5% have lymph node involvement, and 20% have extension to adjacent organs or distant disease.
TNM staging groups kidney-confined tumors as T1 or T2, and T3a represents involvement confined to the perinephric fat or adrenal gland. T3b and T3c lesions represent disease extending in the venous drainage, and T4 is locally advanced. N1 disease denotes only one regional lymph node, whereas N2 indicates involvement of at least two regional lymph nodes. TNM is also predictive of prognosis. Stage 1 and 2 patients have 5-year survivals of 74% to 96%.31
Prognostic Factors
A myriad of features, both clinical and pathologic, have been analyzed for the determination of their prognostic significance. The great majority of studies analyze individual factors by univariate analysis, ignoring the potential interdependence of factors on patient outcome. A thorough discussion of the relative importance of all such features is beyond the scope of this chapter; however, the interested reader is referred to an exhaustive review by Thrasher and Paulson.32
The most commonly evaluated prognostic factors relate to the gross anatomic extent of the primary lesion at the time of diagnosis; perinephric extension, renal vein extension, lymph node involvement, and metastatic disease have all been demonstrated by numerous authors33–38 to negatively affect patient survival. Pathologic features detectable by light microscopy have been evaluated, and several—tumor histologic type, grade, and cellular pattern—have repeatedly been found to have prognostic import.36,37,39 Patient features, such as age, sex, and race, and performance status have been considered relevant clinical prognostic factors by other authors.40–42
The Fuhrman nuclear grade is a well-established prognostic factor for RCC. Based on the shape and size of the nucleus, as well as the prominence of associated nucleoli, median 5-year disease-specific survivals of 94%, 86%, 59%, and 31% have been noted for grades 1 through 4 respectively.43
In terms of tumor histology, clear cell subtypes have the worst prognosis. However, in the metastatic setting, non–clear cell subtypes appear to do worse.44 Non–clear cell histologies are also more likely to involve lymph nodes.45
Microvascular invasion may also be an important factor in outcome. In a retrospective anlysis of 230 patients, those without evidence for microvascular invasion enjoyed a 5-year median disease-free survival of 87% compared with 27% for those with microvascular invasion (p < 0.001), and a disease-specific survival of 88% versus 40% (p < 0.001).46
Tumor size has also been noted to be an important prognosticator for RCC. When 11 cm was used to classify patients, those with larger lesions had a significantly worse outcome. Patients with larger lesions were more likely to develop DM disease, and 5-year disease-specific survival was 73% versus 57% (p < 0.001) in favor of smaller lesions.47
Molecular markers will likely play a more important role in assessing prognosis. Although data are yet to be validated in larger trials, reports have been published suggesting the utility of markers such as C-reactive protein, B7H1, B7H4, PTEN, P53, gelsolin, vimentin, and Ki67. In particular, P21 may play a role in the transformation of localized disease to metastatic.48,49
Rabinovitch and colleagues24 performed a multivariate analysis on a population of 172 surgically treated patients at Memorial Sloan-Kettering Cancer Center (MSKCC) and demonstrated that only renal vein extension (p = 0.001) and lymph node involvement (p = 0.026) were independent prognosticators for the development of DMs.24 Although positive margins and perinephric extension were considered significant on univariate analysis, these factors lost significance on multivariate analysis. Patient sex, radical versus PN, age, histologic type, and tumor size were not prognosticators on either univariate or multivariate analysis.
Once patients have distant disease, prognostic models can be useful for predicting survival. A commonly used prognostic nomogram was developed at MSKCC, in which Motzer and colleagues retrospectively reviewed the predictive factors for survival in 670 patients who had participated in 24 clinical trials from 1975 to 1996.50 Approximately 8% of patients had had prior cytokine therapy. Absence of nephrectomy, low hemoglobin, high serum-corrected calcium, high lactate dehydrogenase (LDH), and Karnofsky performance status lower than 80% were predictors of poorer survival. Furthermore, based on the number of risk factors, three risk groups were established: good or favorable risk, intermediate risk, and poor risk. If patients had no risk factors, they were a good or favorable risk and their median overall survival (OS) at 5 years was 22 months. If one to two risk factors were present, patients were of intermediate risk with a median OS of 10 months. But if a patient had three or more risk factors, they were deemed a poor risk and had a median OS of 4 months at 5 years. There was a significant difference in survival between the three risk groups (p < 0.0001). Several years later, the same group retrospectively analyzed 463 patients from clinical trials at MSKCC done from 1982 to 1996 who had been treated with interferon (IFN)-α and found that time from diagnosis to the need for initiation of IFN-α of less than 1 year was also a poor prognostic factor.51 The Cleveland Clinic later validated the MSKCC prognostic models in 353 previously untreated mRCC patients.52 They demonstrated that four of the five Motzer prognostic risk factors were independent predictors of survival (time from diagnosis to study entry, hemoglobin, corrected serum calcium, and serum LDH) and expanded the criteria to include prior radiotherapy (RT) (p < 0.001) and number of metastatic sites (more than one).
Surgical Management
Radical Nephrectomy
The RN defines the procedure in which the kidney, along with the Gerota fascia and its contents, are removed en bloc from the retroperitoneum. This procedure, first described by Mortensen38 in 1948, became the standard surgical technique beginning in the following decade, and is the standard of care today. The RN allows a more reliable margin around the known tumor than does a simple nephrectomy. Although the RN has never been compared with the simple nephrectomy in a randomized fashion, Robson in 1963 was the first to propose that the RN confers the best survival results.38
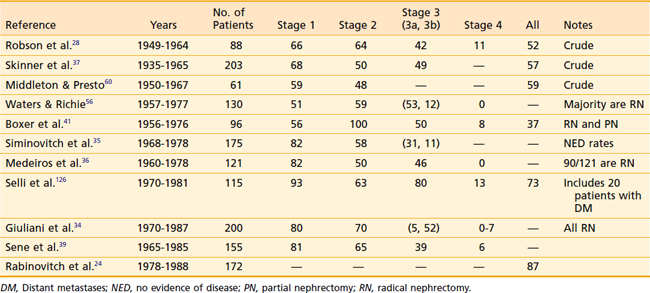
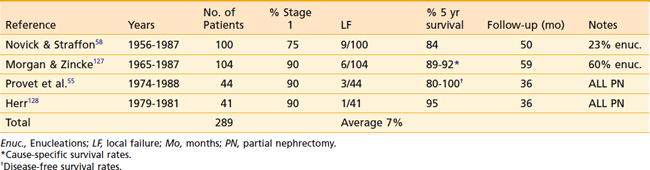
A summary of the results from surgical management of RCC is presented in Table 43-7. Despite the differences among published reports, the overall 5-year survival rates have changed little since Robson’s report in 1963. The relative constancy in outcome reflects not so much any factor regarding surgical management, but rather the lack of progress in any adjuvant treatment modality.
Partial Nephrectomy
Most published series on PN consist of patients with bilateral RCC or unilateral disease in the presence of impaired contralateral renal function. Despite this unfavorable baseline, the overall results are quite acceptable (Table 43-8). LF is a significant concern in this conservative procedure; however, in well-selected cases, recurrence within the residual renal parenchyma is less than 10%. The 5-year cause-specific survival is approximately 85%, although OS is poorer because of the concurrent diseases that necessitate the conservative procedures.
It is reasonable to assume that the PN performed on a healthy group of well-selected patients with peripheral stage 1 lesions, as determined by preoperative evaluation, without contralateral tumors or renal dysfunction, would result in improved OS rates compared with existing PN series. A prospective trial comparing RN to PN is required, although the benefit for renal-conserving surgery in the otherwise healthy patient is unclear. PN is a more complicated surgical procedure than RN, with a greater risk of bleeding complications and longer operative times.40
Patients who develop LF after PN are still eligible for surgical salvage with an RN, assuming the absence of distant disease. Novick and Straffon41 from the Cleveland Clinic have proposed salvage of such patients with a second PN, although longer follow-up in greater numbers of patients is needed.
LF after RN is uncommon, but in the absence of concomitant distant disease, patients should be considered for salvage surgery.42
Patterns of Failure After Surgical Resection
Review of the literature reveals LF rates of 2% to 14% after PN or RN in a widely heterogeneous population of patients.34,53–58 Most of these data are derived from crude rather than actuarial numbers. Only the study from MSKCC presented actuarial LF (Fig. 43-12) and analyzed the pathologic features that predict LF.24 The actuarial LF was only 5% at 7 years among the 172 surgically treated patients. Univariate analysis found LF significantly increased in the presence of either lymph node involvement (21% versus 4%, p = 0.0002) or positive margins (21% versus 4%, p = 0.002).
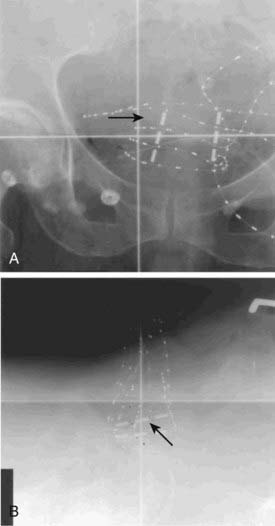
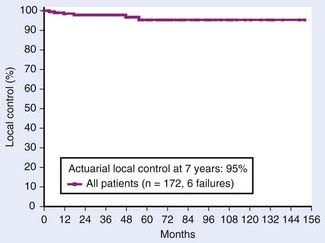
FIGURE 43-12 • Actuarial local control for 172 surgically managed patients from Memorial Sloan-Kettering Cancer Center.
(From Rabinovitch RA, Zelefsky MJ, Gaynor JJ, et al: Patterns of failure following surgical resection of renal cell carcinoma: Implications for adjuvant local and systemic therapy, J Clin Oncol 12:206, 1994.)
The development of DM varies from 9% to 36%,24,56,57,59,60 depending primarily on the stage of disease at presentation, and whether CT technology was available during the study period. Rabinovitch and colleagues24 reported an actuarial incidence of 26% at 7 years (Fig. 43-13). Most importantly, no clear causal relationship has ever been demonstrated between LF and DM. The MSKCC experience found 32 patient-failures among 172 surgically managed patients.24 Of these, only one patient first developed LF and later developed DM. Twenty-six patients were found to have DM without ever having failed locally. Occult DM at the time of presentation is the primary mode of treatment failure, and the lack of causality between local and distant failure argues against the use of local adjuvant treatment (i.e., radiotherapy).
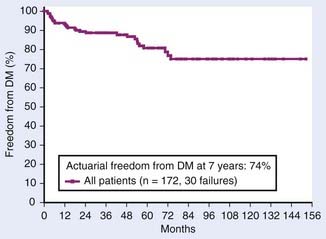
FIGURE 43-13 • Actuarial freedom from distant metastases for 172 surgically managed patients from Memorial Sloan-Kettering Cancer Center.
(From Rabinovitch RA, Zelefsky MJ, Gaynor JJ, et al: Patterns of failure following surgical resection of renal cell carcinoma: Implications for adjuvant local and systemic therapy, J Clin Oncol 12:206, 1994.)
Adjuvant Radiation Therapy
Nonrandomized Data
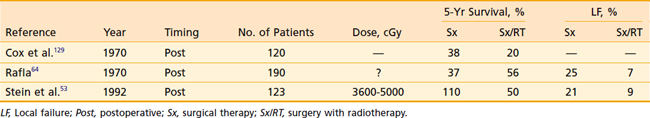
RT has been investigated as an adjuvant treatment for RCC since the 1930s. Several series were published in the 1950s and 1960s comparing nonrandomized groups of patients treated with surgery (Sx) and surgery with adjuvant RT (Sx/RT), most of which suggested a survival advantage to patients treated with adjuvant RT.29,61–63 Several more recent series have suggested a local control advantage (Table 43-9). These studies served as the foundation on which the randomized trials were based (see following text). Rafla64 published the experience of the Winnipeg General Hospital in 1970. He claimed that postoperative RT improved OS at 5 years. Subgroup analysis revealed this survival benefit to be limited only to those patients with “renal pelvis” or “capsular tissue” involvement. Unfortunately, the paper does not include any details of the radiation technique, target volume, total dose delivered, fractionation scheme, beam energy, or treatment toxicity. A significant reduction in the LF rate was obtained in the Sx/RT group: 25% versus 7%.
The most recent retrospective series were published in 1992 by Stein and colleagues53 from the Rambam Medical Center in Israel and in 1997 by Aref and colleagues from Ottawa Regional Cancer Center in Canada.65 In Stein’s series, evaluation of 123 patients treated with and without adjuvant postoperative RT resulted in no significant difference in OS or LF, but there was a trend toward improved outcome in the combined modality patients. Of the 19 LFs among both groups, 3 were scar recurrence. There was, however, a significant decrease in LF for patients with T3 tumors. The authors therefore recommend inclusion of the scar within the treatment volume whenever such patients are treated. Aref evaluated the pattern of failure in patients with resected RCC and found that locoregional failure alone was rare following nephrectomy. DM was the predominant failure and therefore these authors concluded that postoperative RT is not indicated.
Randomized Trials
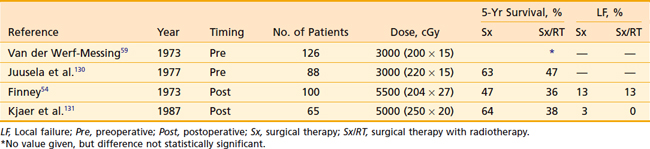
The role of adjuvant RT has been evaluated in four prospective randomized trials (Table 43-10), all conducted in Europe. Two examined preoperative RT, and two examined postoperative RT. The information gleaned from these studies is clouded by various issues. The first three trials were conducted in the 1960s and early 1970s, before the development of CT technology (which is today considered essential for accurate staging of the primary tumor and the regional lymph nodes, and evaluation of suspicious chest x-ray lesions that are suggestive of metastatic disease). These same three studies were also nondiscriminating with regard to which patients were appropriate candidates for study inclusion; that is, even patients with small T1-N0 tumors were eligible for enrollment. With such trial design, it would be difficult to detect a benefit from adjuvant therapy if, for example, only patients with T3 or T4 tumors demonstrated an advantage. Only the most recently published study, by Kjaer and colleagues,55a was performed in the CT era, and it excluded patients with early-stage lesions.
Preoperative Radiotherapy
Both preoperative trials reported no difference in 5-year OS between the two arms of the study.49,56 Neither study, however, used doses considered adequate to sterilize even microscopic disease by current standards. Patients were eligible for participation, regardless of the stage of their lesions. Surprisingly, neither study gives any information regarding LF, a logical endpoint for studies evaluating a local therapy.
Postoperative Radiotherapy
Finney’s study54 is difficult to evaluate because it was published in two journals in the same year and cited different results in each. The results given are crude, not actuarial, further making comparisons between studies difficult. In both postoperative studies, however, doses adequate for microscopic residual disease were delivered. These studies concur with the preoperative studies, demonstrating no survival advantage after adjuvant RT. LF was determined to be unusual regardless of treatment, with no apparent affect on the local treatment.
The postoperative trials both emphasize the toxicity encountered in patients receiving adjuvant RT. Interestingly, the severe complications observed in each study differed. Finney documented four deaths resulting from hepatic toxicity among the 51 patients treated on the experimental arm of the study.46 All four patients were treated for right-sided renal tumors, and no such deaths were encountered in patients treated with surgery alone. Kjaer and colleagues55a describe significant toxicity in 12 of 27 patients treated with postoperative RT. Of these, three were due to hepatic dysfunction; however, all improved with conservative management. The remaining nine patients developed stenosis or bleeding within the duodenum, and five died directly as a result of small-bowel toxicity. This study has been criticized for delivering relatively large fraction sizes (250 cGy) to a total dose of 5000 cGy to volumes that include small bowel. Of the 32 patients randomly assigned to receive postoperative treatment, 4 were never irradiated and 1 had no follow-up.
Conclusions
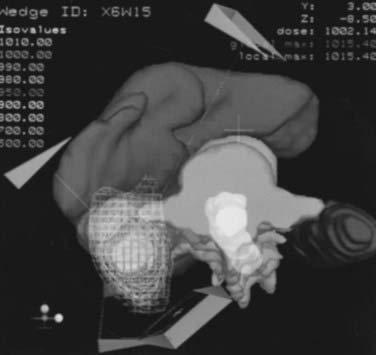
It is unlikely that future trials evaluating adjuvant RT, even if well designed, will be performed. Although it has never proved beneficial in completed randomized trials, a definitive conclusion regarding the use of RT in the adjuvant setting would require a multi-institutional study with hundreds of patients with advanced lesions. These patients would have to be treated with meticulous treatment planning and a well-thought-out fractionation scheme. Clearly, modern three-dimensional conformal radiation therapy and intensity-modulated RT techniques would reduce complications and increase effective dose. Based on several retrospective series, an argument can nevertheless be made for treating positive margins, positive lymph nodes, or unresected disease in medically inoperable patients. As there are no ongoing randomized trials in which to enroll such patients, in these situations it may not be unreasonable to treat with RT. A sample CT-based treatment plan for a patient with a T3a-N0 tumor with positive margins is demonstrated in Fig. 43-14.
Palliative Radiation Therapy
Brain Metastases
Brain metastases occur in approximately 10% of RCC patients and are associated with a poor prognosis. There have been a number of retrospective reports and most conclude that surgical resection is the most effective treatment associated with the best outcome. One such series was reported by Shibui and colleagues in 1990.66 In this series, median survival time from diagnosis of brain metastases was 17 months for patients who underwent resection compared with 4 months for patients who did not undergo surgery. The median survival for patients who received postoperative RT was 20 months, compared with 10.5 months for patients who received RT alone. It is noteworthy that patients who are candidates for craniotomy and whose lesions are resectable are likely to have better outcomes independent of therapeutic modality, and this series, like other retrospective series, has inherent biases associated with selection of patients for specific therapies. Wronski and colleagues reported the MD Anderson Cancer Center experience using whole-brain RT for patients with RCC metastatic to brain.67 Whole-brain RT only was performed on 119 patients, and they form the cohort of study. Median survival for the entire cohort was only 4.4 months. Of these patients, 60% had multiple metastases and 40% had solitary metastases. The authors concluded that these poor results should be impetus to use more aggressive approaches such as surgery and stereotactic radiosurgery. The study period spans almost 2 decades, however, and it is not stated how these patients were selected for whole-brain RT alone. During the study period, 79 patients underwent resection and thus were not included in this report.
Yoshimasa et al. reported their single-institution results using stereotactic radiosurgery for brain metastases from RCC.68 In this study, 35 patients with 52 brain metastases were treated. The median survival was 14 months. The local control rate was 90% for 39 cases evaluated by imaging. These results are comparable to surgical series. There have been no randomized trials comparing surgical metastasectomy with stereotactic radiosurgery. Retrospective reports support comparable outcomes; therefore, selecting one modality over the other should depend on patient- and disease-related factors.
Bone Metastases
The Radiation Therapy Oncology Group showed that higher doses of RT are required for palliation of symptoms related to bone metastases from RCC, but the number of patients with RCC was small.69 Based on the encouraging outcomes demonstrated with stereotactic radiosurgery, the paradigm of high-dose, highly conformal, single-fraction radiation has been applied to spinal metastases. The spine, which is extremely stable in position throughout respirations, is an excellent site for stereotactic or image guided therapy. However, the extreme proximity of most spinal metastases to the spinal cord mandates highly conformal radiation treatment plans. Using intensity-modulated or similar modern radiation treatment planning paradigms, high doses of radiation can be planned to tumors within millimeters of the spinal cord while keeping radiation doses to the spinal cord within safe limits. Image-guided verification, using either two-dimensional or three-dimensional imaging systems incorporated into the treatment unit, allows a high level of accuracy that permits very high doses (18-24 Gy in a single fraction) of highly conformal radation to be delivered to spinal tumors. Local control rates using the high-dose paradigm have been reported to be 90% or greater for RCC.70,71 Using conventional radiation techniques, long-term follow up would be expected to be in the range of less than 30%.72 Hence, in the setting of RCC, the response to radiation may dependent more on the dose per fraction rather than on the total dose administered. It has been suggested that there is a threshold effect on tumor stromal cells, such as the tumor vascular endothelium, at about 8 to 12 Gy, which is not seen when conventional fraction sizes are used.73,74 Hence, based on these and other encouraging reports, high-dose single-fraction or hypofractionated treatment schedules in the management of RCC metastases both intra- and extracranially are increasing in interest.
Renal Radiation Tolerance
The effects of radiation on the kidney were first described in 1952 in a classic paper by Kunkler, Farr, and Luxton,75 analyzing patients treated for testicular seminoma with orchiectomy and abdominal irradiation. A clinical syndrome of radiation-induced nephritis developed in 20 patients, resulting in renal failure and death in 50% of those afflicted. Hypertension, anemia, elevated blood-urea nitrogen levels, and albuminuria were the hallmarks of this syndrome and developed after an average latency period of 8 months. Severe cases included signs and symptoms of severe fluid overload, including peripheral edema, cardiomegaly, and pleural effusions. Death was due to malignant hypertension in six patients, and renal failure in the remaining four. Analysis of the various irradiation techniques in the 93 patients reviewed revealed the greatest risk to those receiving bilateral whole-kidney irradiation to greater than 2300 cGy. The authors proposed, more than 4 decades ago, limiting the renal dose to below this level and excluding at least one third of the total kidney volume from the radiation fields whenever possible.
Whole-abdominal RT has been used in the adjuvant treatment of intermediate-risk ovarian cancer. The total dose that can be given is limited by the radiation tolerance of the kidney. Irwin and colleagues reviewed the Princess Margaret Hospital experience over a 5-year period and identified 60 analyzable patients who had received whole-abdominal RT, had not received chemotherapy, and had at least 5 years of follow-up since therapy.76 The total dose to the kidneys was limited to less than 20 Gy during 4.5 weeks. The following analyses were done: complete blood count, urinalysis, urine microscopy, serum creatinine, urine osmolarity following an overnight fast and 24-hour urine collection for creatinine clearance, phosphorous and uric acid, and quantitative estimation of protein excretion. There was no evidence of renal dysfunction more than 5 years following bilateral kidney irradiation to doses in the 15- to 20-Gy range as a result of whole-abdominal RT for ovarian cancer.
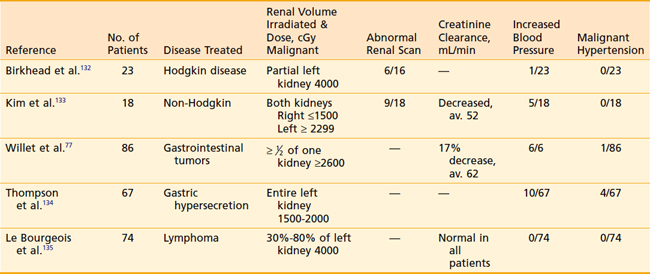
Fortunately, clinical manifestations of renal irradiation are now unusual because of our increased awareness of the marked sensitivity of this organ and subsequent care in treatment planning. Although the syndrome described by Kunkler and colleagues is rarely reported, evidence of renal damage can be documented even when the volume of one kidney or more is excluded from the treatment portals and clinical symptoms are not clinically evident (Table 43-11). Renal scan abnormalities corresponding to the region of irradiated kidney are seen in at least one third of patients when such tests are performed.
Decreases in average creatinine clearance have been documented; however, there have been no reports of such patients requiring dialysis or developing overt renal failure in the absence of malignant hypertension. Willet and colleagues77 nicely demonstrated that the mean percentage decrease in creatinine clearance corresponds to the percentage of kidney irradiated: 10% decrease when 50% of the kidney is irradiated, 19% when 60% to 85% is irradiated, and 24% when 90% to 100% is treated.
Histologically, radiation-induced renal damage is manifested by glomerular endothelial cell swelling, glomerular hyalization, interstitial edema or fibrosis or both, and basement membrane thickening and splitting. Electron microscopy reveals marked subendothelial accumulation of electron-lucent material with deposition of basement membrane material subjacent to the endothelial cells.78–80 In summary, doses to both kidneys that would result in permanent renal function impairment with a probability of 5% and 50% at 5 years are 2000 and 2500 cGy, respectively (tolerance dose [TD]5/5, TD50/5).78 Ideally, every attempt should be made to ensure that at least one third of the total renal mass is shielded from the treatment portals and receives less than 1500 cGy in conventional fractionation.
Total-Body Irradiation
Nonmyeloablative bone marrow or stem cell transplant is showing promise in mRCC and is further discussed in the “Systemic Therapy” section of this chapter. Low-dose total-body irradiation (200 cGy) is being explored as a preparative regimen for miniallogeneic transplant. It is well tolerated and can be given on an outpatient basis.
Systemic Therapy
Chemotherapy
In the past 30 years, no cytotoxic agent or combination of agents with activity against RCC has been identified. Nearly all the reported experience with chemotherapy results from the treatment of patients with measurable metastatic disease, in which activity is measured by complete responses (CRs), partial responses (PRs), and overall response rates (ORRs) (RR = CR PR). More than 40 single agents have been evaluated, all with RRs of 0% to 16%.81
Yagoda and colleagues81 pooled all patients treated since 1983 in trials with single-agent chemotherapy. Of the 3502 patients adequately treated, only 5.6% responded. The authors point out that because of the dismal rate of response with these agents, they have no role as a neoadjuvant or adjuvant treatment. Among RCC, gemcitabine, doxorubicin, and 5-fluorouracil (5-FU) have had the most success in treating metastatic disease.19,82,83 But the main role of these agents is most likely in patients with significant sarcomatoid component. A trial exploring the combination of gemcitabine and doxorubicin in the first-line setting of RCC with sarcomatoid differentiation is ongoing (www.clinicaltrials.gov).
Immunotherapy
Treatment of mRCC with immunotherapy has a more recent history than cytotoxic chemotherapy. The two primary immunotherapeutic agents that have been extensively studied are IFN and interleukin-2 (IL-2). IFN demonstrates antiproliferative, antiviral, immunoregulatory, and antiangiogenic activity. There are three main subtypes of IFN: IFN-α, IFN-β, and IFN-γ. Response to any of the IFNs ranges from 0% to 38%, with an average modest response rate of 14%.84 Response is usually demonstrated within 3 months, and lack of response to treatment within this interval is an indication for cessation of treatment. The duration of response is generally less than 2 years.
Although effecting a similar response rate to IFN-α, high-dose (hd) IL-2 is the only drug to induce durable CRs.85–89 Its use remains limited to younger, more favorable-risk patients who can tolerate its significant toxicities and who have access to the specialized centers with staff expert in administering it.90 In an effort to improve results, IL-2 has been combined with lymphokine-activated killer (LAK) cells, tumor-infiltrating lymphocytes, or IFN-α with differing results. The administration of LAK cells is associated with significant side effects, whereas the administration of IL-2 with IFN-α subcutaneously has resulted in fewer side effects, with the advantage that it can be delivered as an outpatient treatment.90Further attempts at reducing toxicity included lower doses of IL-2 (intravenous or subcutaneous) or combining lower-dose IL-2 with IFN-α or intravenous 5-FU.87,91–96 The dose combinations incurred less toxicity, but also elicited less durable responses.87 Four randomized trials have attempted to elucidate whether combination lower-dose IL-2 or IFN-α is as efficacious as hd IL-2.87,93,97,98 In summary, these trials suggest that hd bolus IL-2 is superior in response rate and quality. Its superiority was especially evident in patients with tumor metastases in immunoprotected sites such as bone or liver, those with primary in situ, and those who are in intermediate or poorer French Immunotherapy risk groups.99
Given the limited response rates in select patients, the future of immunotherapy likely lies in determining predictors of response that will aid in selecting patients with the best chance of response. Currently, the most notable of these markers is carbonic anhydrase IX, a transmembrane glycoprotein involved in cell proliferation and oncogenesis.100 Higher expression is associated with poorer survival in mRCC and better response to hd IL-2.100,101
Nonmyeloablative Bone Marrow or Stem/Hematopoietic Cell Transplant
Metastatic RCC has a poor prognosis, and although responses of approximately 20% have been obtained with IL-2 and IFN-α, there is no associated improvement in survival, and treatment toxicity can be substantial. Allogeneic transplant for hematogenous malignancies can result in profound graft versus tumor effect. Based on the observation that RCC can spontaneously regress, the importance of the immune system was realized. Childs first explored the therapeutic approach of immunosuppressive but nonmyeloablative preparative regimens followed by peripheral–blood stem cell allogeneic transplantation in a patient with mRCC. The goal was to exploit the donor T-cell graft versus tumor effect.102 Childs further reported on 19 consecutive patients with refractory mRCC who were treated with miniallogeneic peripheral stem cell transplant.75 The cumulative probability of response was 53%, and three of 19 patients had a CR that lasted longer than 16 months. The median time to regression was 4 months after transplant. This delay in response is concerning in patients with rapidly progressive metastatic disease who may not live long enough to develop graft versus tumor effect. This approach is still considered experimental, but these early results are promising. Attempts at hematopoietic cell transplant have largely been unsuccessful with high treatment-related mortality.
Targeted Agents
The targeted agents inhibit angiogenesis and cell growth and survival through multiple pathways.103 In the last 3 years, three targeted agents have been approved by the Food and Drug Administration (FDA), including the VEGF tyrosine kinase inhibitors (TKIs), sorafenib and sunitinib, and a mammalian target of rapamycin (mTOR) inhibitor, temsirolimus. The TKIs, sorafenib and sunitinib, block the VEGF receptor tyrosine kinase. These drugs are more promiscuous in nature as each targets multiple receptor tyrosine kinases.104 MTOR inhibitors target the mTOR, a key serine-threonine kinase that is a component of the PI3K/Akt/mTOR (or PTEN) pathway. These agents inhibit tumor progression at multiple levels by causing G1/S cell-cycle arrest, inducing apoptosis, and inhibiting angiogenesis via HIF-1α.
In December 2005, the FDA approved sorafenib for mRCC based on success in the second-line setting. Sunitinib was approved shortly thereafter in January 2006. Sorafenib was approved based on the results of the Treatment Approaches in Renal Cancer global Evaluation Trial (n = 903).105 This phase-III trial evaluated sorafenib in treatment-refractory RCC and demonstrated a significant increase in progression-free survival (PFS) of 5.5 months compared with 2.8 months in the placebo group (HR 0.44, p < 0.01). A 10% PR rate was achieved with sorafenib (p < 0.001). Although the median OS had not been reached at the time of the first analysis, there was a trend toward improved survival in the sorafenib arm. The final OS analysis done at 16 months showed a nonsignificant 13.5% improvement in OS with sorafenib (median 17.8 versus 15.2 months; p = 0.146), but secondary analysis censoring the placebo-arm crossover patients demonstrated a survival advantage of 3 months (17.8 versus 14.3 months, p = 0.0287).105 Thus it is largely thought that crossover likely confounded the survival results.
First-line treatment with sorafenib has been less promising. Szczylik and colleagues compared sorafenib with IFN-α in a phase-II study and found no difference in PFS (5.7 versus 5.6 months) in the first interim analysis.106 Sorafenib did permit better disease control (ORR and stable disease) than IFN-α, 79% versus 64%, but overall in treatment-naïve patients, these results do not favor its use at the FDA-approved dose.
The current first-line treatment of choice for cytokine-ineligible mRCC patients is sunitinib. In a phase-III trial comparing sunitinib with the IFN-α in 750 treatment-naïve mRCC patients, sunitinib significantly improved PFS from 5 months to 11 months (HR 0.42, p < 0.001) and response rate from 6% to 31% (p < 0.001) over IFN-α.107 A recent update of the trial’s survival analysis demonstrated that sunitinib improved OS over IFN-α by 4.6 months (26.4 versus 21.8 months, HR = 0.8, p = 0.0132 using Wilcoxon Rank-Sum test).108 Sunitinib represents the first drug to consistently improve OS well over 2 years in mRCC. Sunitinib is also effective in treatment-refractory mRCC as evidenced by prolongation of PFS to 8.3 months and PR rates of 34% in an open-label, single-arm multicenter clinical trial.109 No randomized prospective study comparing sunitinib versus sorafenib has been performed, but sunitinib’s response and PFS data appear superior.
The intravenous mTOR inhibitor, temsirolimus (CCI-779), was approved by the FDA in May 2007 based on the phase-III trial that showed its impressive efficacy in poorer-risk patients.110 In the Global Advanced Renal Cell Carcinoma trial, 626 patients with poor or intermediate risk were randomized to either temsirolimus alone, IFN-α alone, or a combination of temsirolimus and IFN-α. Single-agent temsirolimus improved OS by 3.6 months (10.9 months versus 7.3 months, p = 0.0069) over the IFN-α arm and PFS by 1.8 months. No significant difference in response rates were seen among the three arms, but the temsirolimus monotherapy arm was thought to be significantly less toxic compared with either of the IFN-containing arms. The lower doses of both temsirolimus and IFN-α and the higher frequency of doses withheld because of the toxicity in the combination arm was thought to contribute to the lack of survival benefit seen with the combination of temsirolimus plus IFN-α.110,111 Based on these results, temsirolimus is the current treatment of choice in poorer-risk mRCC patients, and its efficacy is being investigated in good-risk patients as well. An oral mTOR inhibitor, everolimus (RAD001), has also shown promise in several phase-I and phase-II trials, and is the focus of ongoing investigation.112,113
Other promising agents on the horizon include bevacizumab, a monoclonal VEGF antibody; pazopanib, a multityrosine kinase inhibitor that selectively inhibits all three VEGF receptors, c-Kit, and PDGF-R; axitinib, another VEGF-R and PDGF-R TKI; as well as several others that target angiogenesis and cell growth and survival. The most well-studied investigational agent is bevacizumab. In a phase-II study, bevacizumab monotherapy compared favorably with the historical data of cytokine efficacy in the first-line setting, providing a median PFS of 8.5 months.114 In cytokine-refractory RCC, bevacizumab provided a 10% response rate and a 4.8-month PFS.115 From these studies, bevacizumab monotherapy has shown only modest clinical benefit and its utility likely lies in its use in combination. Although the FDA has not yet approved bevacizumab for use in mRCC, it has been approved in Europe in combination with IFN-α based on the promising results of the AVOREN trial.116 This phase-III study randomized 649 treatment-naïve nephrectomized patients to either IFN-α plus placebo or IFN-α plus bevacizumab. The addition of bevacizumab improved PFS from 5.4 months to 10.2 months (HR = 0.63, p < 0.0001) and ORR by 18% (13% versus 31%). Median OS has not been reached, but there was a trend toward an OS advantage (p = 0.067). A US study similar to the AVOREN trial, Cancer and Leukemia Group B (CALGB) 90206, showed similar promising results but with some notable differences.117 In this phase-III study, 732 treatment-naïve patients were randomized to either IFN-α monotherapy (no placebo arm) or IFN-α plus bevacizumab. As in AVOREN, most patients were good or intermediate risk but nephrectomy was not required. Of these patients, 15% were not nephrectomized. There were also more intermediate-risk patients in this trial compared with the AVOREN trial. This sicker patient population combined with the central independent review likely contributed to the slightly less impressive results in the CALGB trial: PFS was 8.5 months and ORR was 25.6%.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
2 Reddan DN, Raj GV, Polascik TJ. Management of small renal tumors: an overview. Am J Med. 2001;110:558-562.
3 McLaughlin JK, Lipworth L. Epidemiologic aspects of renal cell cancer. Semin Oncol. 2000;27:115-123.
4 Grossman E, Messerli FH, Goldbourt U. Does diuretic therapy increase the risk of renal cell carcinoma? Am J Cardiol. 1999;83:1090-1093.
5 Grossman E, Messerli FH, Goldbourt U. Antihypertensive therapy and the risk of malignancies. Eur Heart J. 2001;22:1343-1352.
6 Cohen AJ, Li FP, Berg S, et al. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979;301:592-595.
7 Pathak S, Strong LC, Ferrell RE, et al. Familial renal cell carcinoma with a 3;11 chromosome translocation limited to tumor cells. Science. 1982;217:939-941.
8 Solomon D, Schwartz A. Renal pathology in von Hippel-Lindau disease. Hum Pathol. 1988;19:1072-1079.
9 Karumanchi SA, Merchan J, Sukhatme VP. Renal cancer: molecular mechanisms and newer therapeutic options. Curr Opin Nephrol Hypertens. 2002;11:37-42.
10 Glenn G, Choryke PL, Zhar B, et al. Von Hippel-Lindau disease: clinical aspects and molecular genetics. Philadelphia: JB Lippincott; 1990.
11 Cohen HT. Advances in the molecular basis of renal neoplasia. Curr Opin Nephrol Hypertens. 1999;8:325-331.
12 Polyzos A. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma and various other solid tumors. J Steroid Biochem Mol Biol. 2008;108:261-266.
13 Drucker BJ. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev. 2005;31:536-545.
14 Grawitz P. Die sogennanten Lipoma der Niere. Virchows Arch Pathol Anat. 1883;93:39.
15 Fisher ER, Horvat B. Comparative ultrastructural study of so-called renal adenoma and carcinoma. J Urol. 1972;108:382-386.
16 Holthofer H. Immunohistology of renal carcinomas. Eur Urol. 1990;18(Suppl 2)):15-16.
17 Bell E. A classification of renal tumors with observations on the frequency of the various types. J Urol. 1938;39:238.
18 Curry NS, Schabel SI, Betsill WLJr. Small renal neoplasms: diagnostic imaging, pathologic features, and clinical course. Radiology. 1986;158:113-117.
19 Nanus DMGA, Milowsky MI, et al. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer. 2004;101:1545-1551.
20 O’Toole KM, Brown M, Hoffmann P. Pathology of benign and malignant kidney tumors. Urol Clin North Am. 1993;20:193-205.
21 Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol. 2002;167:65-70.
22 Vogelzang N, Scardino PT, Shipley WU, et al, editors; Comprehensive textbook of genitourinary oncology; 2000; Lippincott Williams & Wilkins, Philadelphia:112.
23 Chisholm GD, Roy RR. The systemic effects of malignant renal tumours. Br J Urol. 1971;43:687-700.
24 Rabinovitch RA, Zelefsky MJ, Gaynor JJ, et al. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206-212.
25 Kang DE, White RLJr, Zuger JH, et al. Clinical use of fluorodeoxyglucoe F 18 positron emision tomography for detection of renal cell carcinoma. J Urol. 2004;171:1806-1809.
26 Schoder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med 2004. 2004;34:274-292.
27 Mauro MA, Wadsworth DE, Stanley RJ, et al. Renal cell carcinoma: angiography in the CT era. AJR Am J Roentgenol. 1982;139:1135-1138.
28 Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol. 1963;89:37-42.
29 Flocks RH, Kadesky MC. Malignant neoplasms of the kidney: an analysis of 353 patients followed five years or more. J Urol. 1958;79:196-201.
30 Fleming ID, Cooper JS, Henson DE, et al, editors. AJCC cancer staging manual, ed 5, Philadelphia: Lippincott Raven, 1997.
31 Kontak JA, Campbell SC. Prognostic factors in renal cell carcinoma. Urol Clin North Am. 2003;30:467-480.
32 Thrasher JB, Paulson DF. Prognostic factors in renal cancer. Urol Clin North Am. 1993;20:247-262.
33 Golimbu M, Joshi P, Sperber A, et al. Renal cell carcinoma: survival and prognostic factors. Urology. 1986;27:291-301.
34 Giuliani L, Giberti C, Martorana G, et al. Radical extensive surgery for renal cell carcinoma: long-term results and prognostic factors. J Urol. 1990;143:468-473.
35 Siminovitch JM, Montie JE, Straffon RA. Prognostic indicators in renal adenocarcinoma. J Urol. 1983;130:20-23.
36 Medeiros LJ, Gelb AB, Weiss LM. Renal cell carcinoma. Prognostic significance of morphologic parameters in 121 cases. Cancer. 1988;61:1639-1651.
37 Skinner DG, Colvin RB, Vermillion CD, et al. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971;28:1165-1177.
38 Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297-301.
39 Sene AP, Hunt L, McMahon RF, et al. Renal carcinoma in patients undergoing nephrectomy: analysis of survival and prognostic factors. Br J Urol. 1992;70:125-134.
40 Lieber MM, Tomera FM, Taylor WF, et al. Renal adenocarcinoma in young adults: survival and variables affecting prognosis. J Urol. 1981;125:164-168.
41 Boxer RJ, Waisman J, Lieber MM, et al. Renal carcinoma: computer analysis of 96 patients treated by nephrectomy. J Urol. 1979;122:598-601.
42 Chasan SA, Pothel LR, Huben RP. Management and prognostic significance of hypercalcemia in renal cell carcinoma. Urology. 1989;33:167-170.
43 Ficarra V, Richetti R, Martignoni G, et al. Prognostic value of renal cell carcinoma nuclear grading: multivariate analysis of 333 cases. Urol Int. 2001;67:130-134.
44 Tannir NM, Cohen L, Wang X, et al. Improved tolerability and quality of life with maintained efficacy using twice-daily low-dose interferon-alpha-2b: results of a randomized phase II trial of low-dose versus intermediate-dose interferon-alpha-2b in patients with metastatic renal cell carcinoma. Cancer. 2006;107:2254-2261.
45 Kassouf W, Sanchez-Ortiz R, Tamboli P, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma with nonclear cell histology. J Urol. 2007;178:1896-1900.
46 Dall’Oglio MF, Antunes AA, Sarkis AS, et al. Microvascular tumour invasion in renal cell carcinoma: the most important prognostic factor. BJU Int. 2007;100:552-555.
47 Klatte T, Patard JJ, Goel RH, et al. Prognostic impact of tumor size on pT2 renal cell carcinoma: an international multicenter experience. J Urol. 2007;178:35-40.
48 Pantuck AJ, Fang Z, Liu X, et al, J Clin Oncol; 23; 2005, Abstract 4535
49 Weiss RH, Borowsky AD, Seligson D, et al. p21 is a prognostic marker for renal cell carcinoma: implications for novel therapeutic approaches. J Urol. 2007;177:63-69.
50 Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530-2540.
51 Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289-296.
52 Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832-841.
53 Stein M, Kuten A, Halpern J, et al. The value of postoperative irradiation in renal cell cancer. Radiother Oncol. 1992;24:41-44.
54 Finney R. An evolution of post operative radiotherapy in hypernephroma treatment: a clinical trial. Cancer. 1973;32:1322-1339.
55 Provet J, Tessler A, Brown J, et al. Partial nephrectomy for renal cell carcinoma: indications, results and implications. J Urol. 1991;145:472-476.
55a Kjaer M, Frederiksen PL, Engelholm SA. Post operative radiotherapy in stage I and II renal adenocarcinoma: a randomized trial by the Copenhagen Renal Cancer Study Group. Int J Radiat Oncol Biol Phys. 1987;13:665-672.
56 Waters WB, Richie JP. Aggressive surgical approach to renal cell carcinoma: Review of 130 cases. J Urol. 1979;122:306-309.
57 Ramon J, Goldwasser B, Raviv G, et al. Long-term results of simple and radical nephrectomy for renal cell carcinoma. Cancer. 1991;67:2506-2511.
58 Novick AC, Straffon RA. Management of locally recurrent renal cell carcinoma after partial nephrectomy. J Urol. 1987;138:607-610.
59 Van der Werf-Messing B. Proceedings: carcinoma of the kidney. Cancer. 1973;32:1056-1061.
60 Middleton RG, Presto AJ. Radical thoracoabdominal nephrectomy for renal cell carcinoma. J Urol. 1973;110:36-37.
61 Peeling WB, Mantell BS, Shepheard BG. Post-operative irradiation in the treatment of renal cell carcinoma. Br J Urol. 1969;41:23-31.
62 Bratherton DG. Tumours of the kidneys and suprarenals. III. The place of radiotherapy in the treatment of hypernephroma. Br J Radiol. 1964;37:141-146.
63 Riches EW, Griffiths IH, Thackray AC. New growths of the kidney and ureter. Br J Urol. 1951;23:297-356.
64 Rafla S. Renal cell carcinoma. Natural history and results of treatment. Cancer. 1970;25:26-40.
65 Aref I, Bociek RG, Salhani D. Is post-operative radiation for renal cell carcinoma justified? Radiother Oncol. 1997;43:155-157.
66 Shibui S, Nishikawa R, Nomura K. Treatment of metastatic brain tumor from renal cell carcinoma. No Shinkei Geka. 1990;18:935-938.
67 Wronski M, Maor MH, Davis BJ, et al. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997;37:753-759.
68 Yoshimasa M, Kondziolka D, Flickenger J, et al. Stereotactic radiosurgery for brain metastases from renal cell carcinoma. Cancer. 1998;83:344-353.
69 Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: Final results of the study by the Radiation Therapy Oncology Group. Cancer. 1982;50:893-899.
70 Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288-295.
71 Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484-490.
72 Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127-1132.
73 Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89-91.
74 Garcia-Barros M, Lacorazza D, Petrie H, et al. Host acid sphingomyelinase regulates microvascular function not tumor immunity. Cancer Res. 2004;64:8285-8291.
75 Kunkler PB, Farr RF, Luxton RW. The limit of renal tolerance to x-rays: an investigation into renal damage occurring following the treatment of tumours of the testis by abdominal baths. Br J Radiol. 1952;25:192-201.
76 Irwin C, Fyles A, Wong CS, et al. Late renal function following whole abdominal irradiation. Radiother Oncol. 1996;38:257-261.
77 Willett CG, Tepper JE, Orlow EL, et al. Renal complications secondary to radiation treatment of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 1986;12:1601-1604.
78 Rubin P, Cooper K, Phillips TL, Radiation Biology and Radiation Pathology Syllabus, (SET RT1: Radiation Oncology.); 1975; American College of Radiology, Chicago.
79 Pearse H. The kidney. In: Moss WT, Cox JD, editors. Radiation Oncology. 6th ed. St. Louis: Mosby; 1989:416.
80 Keane WF, Crosson JT, Staley NA, et al. Radiation-induced renal disease. A clinicopathologic study. Am J Med. 1976;60:127-137.
81 Yagoda A, Petrylak D, Thompson S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin North Am. 1993;20:303-321.
82 Rini BI, Vogelzang NJ, Dumas MC, et al. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419-2426.
83 Sella A, Logothetis CJ, Ro JY, et al. Sarcomatoid renal cell carcinoma. A treatable entity. Cancer. 1987;60:1313-1318.
84 Wirth MP. Immunotherapy for metastatic renal cell carcinoma. Urol Clin North Am. 1993;20:283-295.
85 Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55-S57.
86 Fyfe GA, Fisher RI, Rosenberg SA, et al. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996;14:2410-2411.
87 McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133-141.
88 Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889-897.
89 Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307-319.
90 Pierce WC, Belldegrun A, Figlin RA. Cellular therapy: scientific rationale and clinical results in the treatment of metastatic renal-cell carcinoma. Semin Oncol. 1995;22:74-80.
91 Tourani JM, Lucas V, Mayeur D, et al. Subcutaneous recombinant interleukin-2 (rIL-2) in out-patients with metastatic renal cell carcinoma. Results of a multicenter SCAPP1 trial. Ann Oncol. 1996;7:525-528.
92 Sleijfer DT, Janssen RA, Buter J, et al. Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal cell cancer on an outpatient basis. J Clin Oncol. 1992;10:1119-1123.
93 Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med. 1998;338:1272-1278.
94 Lopez Hanninen E, Kirchner H, Atzpodien J. Interleukin-2 based home therapy of metastatic renal cell carcinoma: risks and benefits in 215 consecutive single institution patients. J Urol. 1996;155:19-25.
95 Dutcher JP, Atkins M, Fisher R, et al. Interleukin-2-based therapy for metastatic renal cell cancer: The Cytokine Working Group experience, 1989–1997. Cancer J Sci Am. 1997;3(Suppl 1):S73-S78.
96 Atzpodien J, Kirchner H, Hanninen EL, et al. Interleukin-2 in combination with interferon-alpha and 5-fluorouracil for metastatic renal cell cancer. Eur J Cancer. 1993;29A(Suppl 5):S6-S8.
97 Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer. 2007;110:2468-2477.
98 Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127-3132.
99 McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res. 2007;13:716s-720s.
100 Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802-811.
101 Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714-3721.
102 Childs RW, Clave E, Tisdale J, et al. Successful treatment of metastatic renal cell carcinoma with a nonmyeloablative allogeneic peripheral-blood progenitor-cell transplant: evidence for a graft-versus-tumor effect. J Clin Oncol. 1999;17:2044-2049.
103 Brugarolas J. Renal-cell carcinoma—molecular pathways and therapies. N Engl J Med. 2007;356:185-187.
104 Fabian MA, Biggs WH, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329-336.
105 Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125-134.
106 Szczylik C, Demkow T, Staehler M, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon in patients with advanced renal cell carcinoma: final results. J Clin Oncol. 2007;25:5025.
107 Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-124.
108 Figlin R, Hutson TE, Tomczak P, et al. Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2008;26(Suppl):5024.
109 Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295:2516-2524.
110 Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-2281.
111 Garcia JA, Rini BI. Recent progress in the management of advanced renal cell carcinoma. CA Cancer J Clin. 2007;57:112-125.
112 Jac J, Giessinger S, Khan M, et al. A phase II study of RAD001 in metastatic renal cell cancer. ASCO Annu Meet Proc. 2007;25:5107.
113 Porter LL, Burris HA, Jones SF, et al. Summary of results in patients with metastatic renal cell (RCC) from phase I studies of RAD001 (everolimus). ASCO Annu Meet Proc. 24, 2006.
114 Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2006;24:4599.
115 Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427-434.
116 Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103-2111.
117 Rini BI, Halabi S, Taylor J, et al. Cancer and Leukemia Group B 90206: a randomized phase III trial of interferon-alpha or interferon-alpha plus anti-vascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma. Clin Cancer Res. 2004;10:2584-2586.
118 McLaughlin JK, Mandel JS, Blot WJ, et al. A population-based case-control study of renal cell carcinoma. J Natl Cancer Inst. 1984;72:275-284.
119 Yu MC, Mack TM, Hanisch R, et al. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst. 1986;77:351-356.
120 Whittemore AS, Paffenbarger RSJr, Anderson K, et al. Early precursors of urogenital cancers in former college men. J Urol. 1984;132:1256-1261.
121 Malker HR, Malker BK, McLaughlin JK, et al. Kidney cancer among leather workers. Lancet. 1984;1:56.
122 Ross RK, Paganini-Hill A, Landolph J, et al. Analgesics, cigarette smoking, and other risk factors for cancer of the renal pelvis and ureter. Cancer Res. 1989;49:1045-1048.
123 Chung-Park M, Parveen T, Lam M. Acquired cystic disease of the kidneys and renal cell carcinoma in chronic renal insufficiency without dialysis treatment. Nephron. 1989;53:157-161.
124 Matson MA, Cohen EP. Acquired cystic kidney disease: occurrence, prevalence, and renal cancers. Medicine (Baltimore). 1990;69:217-226.
125 Bretan PNJr, Busch MP, Hricak H, et al. Chronic renal failure: a significant risk factor in the development of acquired renal cysts and renal cell carcinoma. Case reports and review of the literature. Cancer. 1986;57:1871-1879.
126 Selli C, Hinshaw WM, Woodard BH, et al. Stratification of risk factors in renal cell carcinoma. Cancer. 1983;52:899-903.
127 Morgan WR, Zincke H. Progression and survival after renal-conserving surgery for renal cell carcinoma: experience in 104 patients and extended followup. J Urol. 1990;144:852-858.
128 Herr HW. Partial nephrectomy for renal cell carcinoma with a normal opposite kidney. Cancer. 1994;73:160-162.
129 Cox CE, Lacy SS, Montgomery WG, et al. Renal adenocarcinoma: 28-year review, with emphasis on rationale and feasibility of preoperative radiotherapy. J Urol. 1970;104:53-61.
130 Juusela H, Malmio K, Alfthan O, et al. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11:277-281.
131 Kjaer M, Frederiksen PL, Engelholm SA. Postoperative radiotherapy in stage II and III renal adenocarcinoma. A randomized trial by the Copenhagen Renal Cancer Study Group. Int J Radiat Oncol Biol Phys. 1987;13:665-672.
132 Birkhead BM, Dobbs CE, Beard MF, et al. Assessment of renal function following irradiation of the intact spleen for Hodgkin disease. Radiology. 1979;130:473-475.
133 Kim TH, Somerville PJ, Freeman CR. Unilateral radiation nephropathy—the long-term significance. Int J Radiat Oncol Biol Phys. 1984;10:2053-2059.
134 Thompson PL, Mackay IR, Robson GS, et al. Late radiation nephritis after gastric x-irradiation for peptic ulcer. Q J Med. 1971;40:145-157.
135 Le Bourgeois JP, Meignan M, Parmentier C, et al. Renal consequences of irradiation of the spleen in lymphoma patients. Br J Radiol. 1979;52:56-60.