30 Cancer of the Hypopharynx
Epidemiology
Approximately 2600 cases of hypopharyngeal cancer are diagnosed in the United States each year, 75% to 80% of which arise in the pyriform sinuses and 15% to 20% from the posterior pharyngeal wall. Postcricoid cancers constitute about 5% of cases. The male-to-female ratio ranges from 5 : 1 to 7 : 1 for pyriform sinus cancer and 3 : 1 to 4 : 1 for pharyngeal wall cancer.1–3 Postcricoid cancers occur predominantly in women. The age-specific incidence rate for White men increases from the 40- to 44-year old group (0.4/100,000) to the 65- to 69-year-old group (9.6/100,000) and then subsequently declines (4.3/100,000) for the 85-year-old group.2 Among African-American men, the incidence continues to increase with age (up to 15.8/100,000 in the 85-year-old group).2 In general, the prognoses for hypopharyngeal tumors are notoriously poor, because of the following associated factors: extensive local-regional spread of disease through the submucosal route, abundant lymphatic drainage of the region with a greater propensity for distant metastatic spread; clinical presentation at an advanced stage; frequent association with nutritional depletion; concurrent medical problems conferred by tobacco and alcohol abuse; and occurrence in patients with a predisposition for the development of second malignancies (secondary to mucosal field cancerization).
Etiology
Tobacco and alcohol use are clearly associated with the development of hypopharyngeal cancer.4–6 Although tobacco alone is known to be carcinogenic, the role of alcohol in the etiology of hypopharyngeal cancer has been underappreciated. A prevailing opinion is that alcohol potentiates carcinogenesis in tobacco-exposed mucosa.7 However, evidence points to an independent role for alcohol.5,8–10 In never smokers, an independent association of heavy alcohol consumption and oropharynx/hypopharynx/larynx cancer was found in a recent pooled analysis by the International Head and Neck Cancer Epidemiology Consortium.10 Other data suggest a synergistic interaction between tobacco and alcohol with regard to the risk of inducing hypopharyngeal cancer, particularly for people with heavy alcohol consumption.5,9,11 Concurrent alcohol consumption and smoking has been found to be associated with a multiplicative increase in risk to 10 to 20 times higher than nonsmokers/nondrinkers.12,13 The risk for hypopharyngeal and oropharyngeal cancer may be especially high in patients with inactive or less active forms of the ADH1B and ALDH2 enzymes.14
Cigarette smoking is clearly a strong risk factor for head and neck cancer independent of alcohol drinking, with a relative risk of 4.0 to 5.0 for oropharyngeal and hypopharyngeal cancers.10 Among cigarette smokers, the use of unfiltered cigarettes or black tobacco (with higher concentrations of carcinogenic N-nitrosamines) increases the risk of cancer.5 For a similar reason, smoking marijuana (with its high tar burden and aromatic hydrocarbon content) appears to confer even greater risk.7 Moist snuff (smokeless tobacco) consumption also exposes the pharynx to carcinogens.15,16 The latter is noteworthy in that snuff consumption increased by 38% in the United States between 1981 and 1993, paralleled by a three-fold increase in snuff sales between 1980 and 2003.17,18 A growing prevalence of snuff dipping among young men, non-Hispanic Whites, people with lower educational attainment, and residents of Southern U.S. states and rural areas has been suggested as a reason for this increase.17,19 Dietary factors predisposing to the development of hypopharyngeal cancer may include a diet low in carotenoids4,7 and iron-deficiency anemia. This anemia has been associated with postcricoid cancers in women in Scandinavia and Great Britain and usually occurs in this setting along with dysphagia (from hypopharyngeal webs) and atrophy of the oral mucosa (manifested by loss of tongue papillae). The triad of iron-deficiency anemia, dysphagia, and glossitis is known as the Plummer-Vinson syndrome in Scandinavia and the Paterson-Brown Kelly syndrome in Great Britain.
Genetic susceptibility of the host influences the likelihood of cancer development.4 DNA repair capability, which can be indirectly measured by assessment of chromosomal breakage induced by in vitro exposure to bleomycin,20 was demonstrated in a case–control study to be a strong predictor of the risk of upper aerodigestive tract cancer.21 However, whereas bleomycin-induced mutagen sensitivity was higher in patients with hypopharynx cancer compared to smoking controls, there was no association between mutagen sensitivity and the risk of squamous cell carcinoma of the hypopharynx. Mutagen sensitivity and its association with cancer risk appeared to decrease from the oral cavity towards the oropharynx and larynx.22 A positive family history of cancer of the respiratory or upper digestive tract among first-degree relatives (especially siblings) also has been associated with an increased risk of head and neck cancer.23
Genetics and Cytogenetics
Genetic abnormalities that appear to be causally linked to the etiology of head and neck cancer are point mutations in the p53 gene24 and bcl-2 overexpression. In the case of bcl-2, the protein encoded by the bcl-2 gene is overexpressed in head and neck cancers among heavy smokers.25 This is hypothesized to result from the chromosomal translocation t(14;18)(q32;q21), which juxtaposes the bcl-2 proto-oncogene with the immunoglobulin heavy-chain gene,26 a juxtaposition that occurs in 85% of non-Hodgkin’s follicular lymphomas.27 It is further hypothesized that carcinogens from cigarette smoke act upon the bcl-2 site and increase the rate of the t(14;18) translocation.25,26
Other oncogenes may be involved in the pathogenesis of head and neck cancer; these include p16 (CDKN2), PRAD1 (cyclin D1), EMS1, the epidermal growth factor (EGF) receptor gene, erb-B-2 (neu), myc, and ras.7,24,28–31 Particular oncogenes are expected to act at distinct points along a multistep pathway in a manner analogous to the colorectal model demonstrated by Vogelstein and associates.31,32
Amplification of the 11q13 region, which encodes for cyclin D1, FGF3, FGF4 and EMS1, has been found to be the most common genetic alteration in hypopharynx carcinomas.33 Cyclin D1 polymorphism has been shown to be associated with poor differentiation and reduced disease-free interval in pharyngeal and laryngeal squamous cell carcinomas.34,35 FGF3 and FGF4 amplification has been correlated with nodal disease, and myc amplification with increasing T-stage. Increased NBS1 expression has been correlated with poor prognosis and decreased overall survival in advanced head and neck squamous cell carcinoma and has been suggested to activate the PI3/Akt pathway and phosphorylation of its downstream target mTOR.36 More recently, hypermethylation has been suggested to represent an important epigenetic alteration in head and neck cancer. One study showed hypermethylation of p16, MGMT, DAP-K, and E-cadherin to be between 27% and 44% in a large group of hypopharynx and larynx cancers. Attempts to correlate hypermethylation with survival have failed to date.37
As a vector of proto-oncogenes, human papillomavirus (HPV) infection may also contribute to the etiology of hypopharyngeal cancer.38 Integration of its DNA into the host genome and expression of early viral proteins, E6 and E7, affects host cellular functions by induction of hyperproliferation, inhibition of apoptosis, altered transcriptional activity and disruption of cell-to-cell interactions.13 HPV-E6 and E7 proteins inactivate p53 and pRb pathways, thereby upregulating p16.39 By contrast, loss of p16 and TP53 gene mutation appear to be early events in tobacco- and alcohol-related squamous cell carcinomas.39 Oral HPV16 infection has been found to be associated with oropharyngeal cancers in patients with and without a strong history of tobacco and alcohol use.40 One study has associated detection of human papillomavirus DNA in laryngeal and hypopharyngeal tumor specimens with both decreased disease-specific survival and decreased local-regional control.38 However, most studies have shown that HPV-associated squamous cell carcinoma carries a favorable prognosis compared to HPV-negative tumors. A favorable prognosis subgroup can be identified using HPV-16 viral load and p16 expression.41,42 Not all studies have detected HPV16 in non-oropharyngeal head and neck subsites.42,43
Anatomy
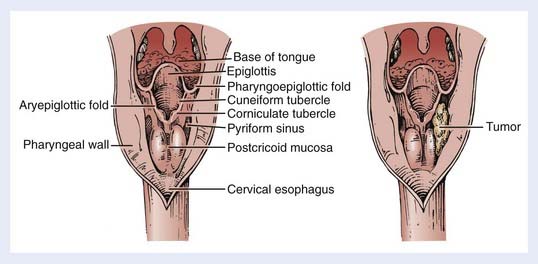
The hypopharynx (the inferior portion of the pharynx) is subdivided into three sites: (1) the pyriform sinuses (the paired recesses lateral to the larynx), (2) the posterior pharyngeal wall, and (3) the postcricoid region. The hypopharynx functions to guide food into the esophagus and away from the larynx, during normal swallowing. The hypopharynx extends from the level of the hyoid bone to the inferior edge of the cricoid cartilage, corresponding to the C4 through C6 vertebral bodies. It envelops the larynx, as shown in Fig. 30-1. The oropharynx lies above, the cervical esophagus below, the larynx anteriorly and medially, and the retropharyngeal space posteriorly.
The bilateral pyriform sinuses (laryngopharyngeal sulci) lie just distal to the glossopharyngeal sulci of the oropharynx. The pharyngoepiglottic fold demarcates each pyriform sinus from the oropharynx (Fig. 30-2). Underlying and inferior to this fold is the pharyngoepiglottic muscle, which constitutes the anterior wall of the pyriform fossa. The medial wall is composed of the mucosal surface overlying the aryepiglottic muscle (lateral to the aryepiglottic fold), as well as the surface lateral to the arytenoid cartilage. Lateral boundaries are the thyrohyoid membrane (upper portion) and thyroid cartilage (lower portion). Inferiorly, the sinus tapers to an apex, the level of which varies among individuals but usually is no lower than the lower border of the cricoid cartilage. The apex is composed of loose, expansile connective tissue. Posteriorly, the pyriform sinus opens to the remainder of the hypopharyngeal lumen.
The posterior pharyngeal wall is continuous with the lateral walls of the two pyriform sinuses (see Fig. 30-1). The wall itself, from the inner to the outer aspect, is composed of the following: mucosal lining, submucosa, fibrous layer (pharyngobasilar fascia), muscular layer, and a deeper layer of fascia (visceral fascia). The muscular layer is composed mainly of the inferior constrictor muscle, consisting of the thyropharyngeal, cricopharyngeal and tracheopharyngeal muscle inferiorly, with contributions from the distal portion of the middle constrictor muscle superiorly. They insert in the pharyngeal raphe posteriorly. These muscles contract in the last phase of deglutition, thus, increasing the pressure on the food and passing it on to the peristaltically-moving esophageal muscles. Behind the posterior pharyngeal wall, loose connective tissue forms a potential space (the retropharyngeal space) between this wall and the prevertebral fascia. Posterolaterally lie the carotid spaces, which contain the neurovascular bundles; these are intimately associated with the deep cervical lymph nodes and vessels.
The postcricoid area is the caudally sloping mucosal surface that overlies the posterior lamina of the cricoid cartilage (see Fig. 30-2). The posterior surfaces of the arytenoid cartilages form the superior boundary of this region. The posterior cricoarytenoid muscles lie immediately underneath the mucosa. Inferiorly, the postcricoid area is continuous with the cervical esophagus.
The hypopharynx receives its arterial supply from pharyngeal branches of the superior and inferior thyroid arteries. Venous drainage occurs through the pharyngeal plexus, posterior to the pharyngeal constrictor muscles. Sensory nerve supply to the hypopharynx is provided through both the internal branch of the superior laryngeal nerve and the recurrent laryngeal nerve. Motor supply is predominantly via the recurrent laryngeal nerve. Both are branches of the vagus nerve (cranial nerve X). The internal branch of the superior laryngeal nerve runs submucosally along the anterolateral wall of the pyriform sinus and exits the laryngeal framework superolaterally through an opening in the thyrohyoid membrane. This branch eventually joins the vagus nerve, which synapses with the auricular nerve of Arnold at the jugular ganglion; this pathway allows for referred otalgia (see Clinical Presentation). Branches of the recurrent laryngeal nerve course submucosally through both the postcricoid region and the pyriform fossa.
Lymphatic Drainage
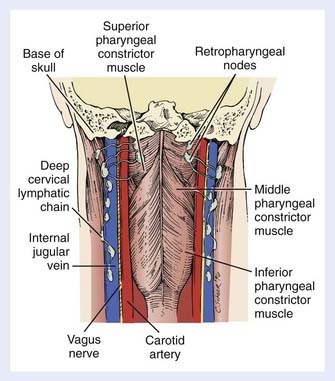
The retropharyngeal lymph nodes can also be accessed from the hypopharynx. These nodes are located high in the retropharyngeal space and are divided into a medial group and a lateral group (Fig. 30-3) (the latter are also called the nodes of Rouvière). Both groups are above the level of the hyoid bone. Cephalad spread results in retropharyngeal nodal involvement up to the base of skull. Below the hyoid, the retropharyngeal space contains fat only. Yet, as there are no fascial barriers within this space, once tumor penetrates it, neoplastic cells can migrate freely throughout. Caudal dissemination allows disease to access the mediastinum, with which the retropharyngeal space is contiguous. This space thus becomes an additional vertical pathway through which tumor cells may travel and develop skip metastases.
Pathology
Squamous cell carcinomas constitute more than 95% of carcinomas of the hypopharynx. Among pyriform sinus carcinomas, approximately one third have been found to be nonkeratinizing and 40% poorly differentiated in one large series.44 The same report noted the tumor growth pattern at the periphery of biopsy specimens to be infiltrating in 80% and pushing in 20%.44 Trends toward increased local-regional relapse were found for keratinizing (vs nonkeratinizing) tumors and for those with infiltrating (vs pushing) margins.44
This information has greater relevance in light of reports from the Department of Veterans Affairs Cooperative Laryngeal Cancer Study Group that demonstrate that the histologic growth pattern of the tumor (for cancer of the larynx) is a significant predictor of both complete tumor response to neoadjuvant chemotherapy45 and disease-free survival.46 Cancers with blunt, thick invading cords of tumor responded less frequently—and were associated with a poorer disease-free survival rate—than those with thin, irregular infiltrating cords. The latter pattern is thought to reflect rapidly growing tumor with a high proliferative rate.45
The basaloid variant of squamous cell carcinoma occasionally arises in the hypopharynx and is associated with an aggressive biologic behavior and poor prognosis.47–49 Other rare tumors of the hypopharynx include minor salivary gland tumors, sarcomas of various types (fibrosarcomas, liposarcomas, synovial cell sarcomas, and malignant fibrous histiocytomas), and lymphomas.
Clinical Presentation
Symptoms
The most common presenting symptoms of cancer of the hypopharynx are sore throat, odynophagia, and a neck mass. As primary hypopharynx tumors remain asymptomatic for a long time and typically present at an advanced stage, a neck mass may be the sole presenting symptom in up to 70% of cases.50–54 Dysphagia and weight loss frequently occur with locally advanced disease but can occur in early stages as well. In very advanced stages, airway obstruction requiring emergency tracheostomy occurs in 5% to 10% of cases. Voice changes may develop in tumors with involvement of the larynx or the recurrent laryngeal nerves. Other symptoms of locally advanced lesions may include otalgia, neck pain, foreign body sensation, mucus retention in the throat, aspiration, and hemoptysis.
The hallmark of postcricoid carcinoma is dysphagia, which is a presenting symptom in more than 90% of cases.55–57 Among posterior wall cancers, dysphagia heralds the disease in 45% to 90% of patients.51,58–60 Other comorbidities may include malnutrition due to alcohol abuse, alcohol-induced liver disease, and chronic obstructive pulmonary disease.
Physical Examination
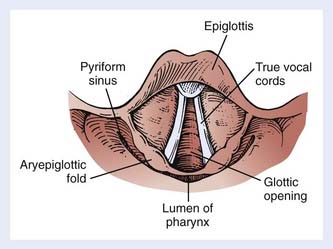
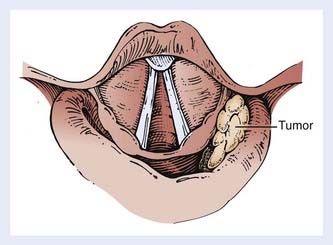
Indirect mirror and direct fiber-optic endoscopic examination of the laryngopharynx are mandatory for evaluation of disease. Schematic endoscopic views of the normal and diseased hypopharynx are shown in Figs. 30-4 and 30-5. Pooling of secretions and arytenoid edema may be prominent findings, especially with tumors of the pyriform apex and postcricoid region. It is not unusual for these findings to obscure visualization of the primary lesion itself. Pyriform sinus or postcricoid lesions usually appear as flat plaques with raised edges and superficial ulceration.52 Tumors of the upper (membranous) portion of the pyriform sinus tend to be locally infiltrating, exophytic lesions, whereas those of the lower (cartilaginous) portion tend to be widely infiltrating, ulcerative lesions. Lesions arising from the cartilaginous portions of the hypopharynx have been associated with a poorer prognosis compared with lesions from the membranous portion of the pyriform region,61–63 and so it is important to note from which portion the tumor arises. It is also important to differentiate a lesion of the medial wall of the pyriform fossa from a lesion arising from the aryepiglottic fold proper, as the latter carries a better prognosis.64 Vocal cord symmetry and mobility should be evaluated with and without phonation in order to rule out endolaryngeal invasion. Posterior wall tumors can present as submucosal bulges with or without ulceration. They tend to be exophytic and are often large (80% >5 cm) at presentation and may extend to the posterior oropharyngeal wall superiorly.52
Routes of Disease Spread
Local
Noteworthy routes of local spread include submucosal extension of disease, not infrequently associated with skip metastases and intervening normal-appearing mucosa.65 Such spread allows for both vertical and horizontal migration of the tumor. Medial extension results in involvement of endolaryngeal structures. The primary tumor can also extend directly into the soft tissues of the neck through the thyrohyoid membrane, through the thyroid cartilage, or around the posterior border of the thyroid ala. In the latter two instances, the thyroid gland may also be invaded, which is a poor prognostic factor.52
Tumors arising in the pyriform apex often invade the thyroid cartilage.66 Tumors originating from the medial wall or the angle of the pyriform sinus commonly invade the paraglottic space, laryngeal cartilages, the apex of the pyriform sinus, and the esophageal verge. Vocal cord fixation may result from involvement of the cricoarytenoid joint, invasion of the posterior cricothyroid muscle, or involvement of the recurrent laryngeal nerve.66–68 Superiorly, pyriform sinus carcinomas commonly extend to the lateral wall of the oropharynx and the base of the tongue.52 Posterior pharyngeal wall carcinomas frequently extend to the oropharynx superiorly52 and appear as an asymmetrical thickening of the posterior pharyngeal wall on imaging.69 Postcricoid tumors commonly spread submucosally toward the cervical esophagus and trachea.69 They also tend to grow anteriorly to involve the posterior cricoarytenoid muscle and may extend through the cricoid cartilage into the trachea.52
Invasion of the carotid artery is suggested when tumor encircles the artery on 3 sides, or 270 degrees or more. Focal stenosis of the carotid artery segment surrounded by tumor also suggests invasion. Invasion of the trachea should be carefully assessed.70,71 Local control of T1-T2 pyriform sinus tumors treated with definitive radiation therapy (RT) has been found to be related to tumor volume more than 6.5 mL and bulky apex disease ≥10 mm.72
Nodal
Nodal involvement is a poor prognostic factor and occurs more commonly in hypopharyngeal cancer than in other head and neck subsites.71,73 Hypopharynx carcinomas most commonly spread to level II nodes, with level III and IV lymph nodes particularly being involved in clinically node-positive patients.52,74 Eleven percent have level V lymph node involvement.74 For pyriform sinus cancers, palpable lymph nodes can be found at presentation in 70% to 75% of patients.53,75–77 Bilateral or contralateral nodes are present in 10% to 14%.75,76,78 Neck dissection increases the yield of node positivity by exposing occult disease. Upon dissection, up to 40% to 50% of patients with clinically N0 necks can be found to harbor nodal metastases.79–81
Posterior wall tumors present with palpable nodes in 40% to 60% of cases.60,82–85 The incidence of bilaterality ranges from 10% to 35%,82,83,85 depending on the location of the primary tumor in the medial-to-lateral dimension. The incidence is higher with more medially located tumors, due to submucosal anastomoses of lymphatic capillaries. Occult metastases have been found in 54% to 66% of N0 patients upon neck dissection.51,86 Between 30% and 40% of patients have retropharyngeal lymph nodes involved with tumor.82,87,88
Postcricoid cancers present with palpable nodes in 33% to 45% of patients.55–57,89–91 Bilateral or contralateral nodes are present in 8% to 18%.56,91 Among patients with T4 tumors, 50% have clinically apparent nodal disease, and 33% have bilateral involvement.57 Occult metastases may frequently be present in paratracheal lymph nodes or the thyroid gland (which is also true for tumors originating in the adjacent apex of the pyriform sinus).86,92–95 Such subclinical disease must be taken into account when the treatment is planned.96–99
Distant
Of patients with hypopharyngeal cancer, 20% to 30% develop clinically overt distant metastases within 2 years, despite treatment. The autopsy incidence of distant metastases among 108 patients treated in the modern era at a single institution was found to be 60%. However, only 13% of these patients had pathologic local-regional control of disease.100 Autopsy studies reporting on heterogeneous cases of head and neck cancer have shown a 19% to 42% incidence of distant metastases among patients who have no evidence of local-regional disease at postmortem examination.101 This suggests the presence of micrometastatic spread of disease at the time of initial diagnosis. Results from a Radiation Therapy Oncology Group (RTOG) analysis of the effect of local-regional control on distant metastatic dissemination for 237 cancers of the hypopharynx support this hypothesis: local-regional control of disease at 6 months was associated with a slight reduction in the incidence of distant metastases at 0.5 to 2.5 years, from 43% to 40% (P = .054).102
The most common metastatic sites, in decreasing order, are lung, mediastinal nodes, liver, and bone.100
Diagnostic and Staging Studies
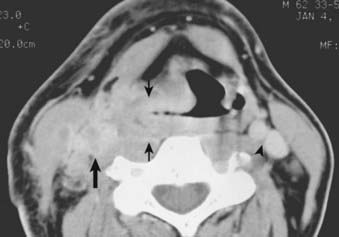
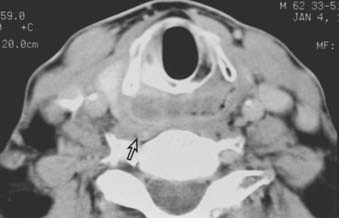
Routine staging studies should include a chest x-ray, blood tests to evaluate liver function, and a computed tomographic (CT) scan with intravenous contrast of the head and neck. The CT scan should be done before biopsy of the tumor to avoid imaging postbiopsy edema, which obscures radiologic definition of tumor extent.103 The CT scan is useful for precise delineation of tumor extent, with particular reference to the following: visualizing inferior extent of tumor (for which fiber-optic endoscopy alone is often inadequate); detecting invasion of the laryngeal framework and extralaryngeal/extrapharyngeal spread into the soft tissues of the neck; detecting submucosal spread toward the midline via the paraglottic space (anteromedial to the pyriform sinus); and detecting posterior spread into the retropharyngeal space (Figs. 30-6, 30-7, and 30-8).104 It is also useful for disclosing clinically occult metastatic lymphadenopathy, particularly positive retropharyngeal nodes, contralateral nodes, and extracapsular extension of disease. Limitations of CT imaging are failure to detect small superficial tumors, overestimating the tumor extent due to inflammatory and edematous changes, early laryngeal cartilage involvement, and distortion of adjacent normal structures that may mimic tumor involvement.71
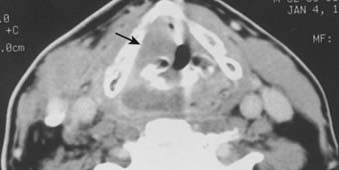
FIGURE 30-7 • Pyriform sinus cancer extending into paraglottic space. Endoscopically, mass effect upon the laryngeal vestibule was apparent.
Helical (spiral) CT provides a rapid and practical means for three-dimensional (3D) imaging of the upper airway.105 It can be used to produce a 3D airway cast—similar to the laryngogram done in years past (but not requiring instillation of foreign material). In demonstrating tumor mass effect upon adjacent airway structures clearly, the 3D laryngogram provides a complementary view of tumor extent.
Magnetic resonance imaging (MRI) is an important adjunctive study in three situations: (1) for determining whether cartilage invasion is present, which is shown by an increase in T2 signal intensity, a low to intermediate signal on T1-weighted images, and postcontrast enhancement69,106,107; (2) for determining the extent of paraglottic space invasion; and (3) for determining esophageal invasion, which is characterized by an increased T2 signal intensity, wall thickening, and effaced fat planes.106,108,109 These are pertinent issues when conservation laryngopharyngeal surgery is being considered, because cartilage invasion and extensive paraglottic space invasion are contraindications to such surgery. MRI is helpful in ruling out esophageal involvement.109 It shows better soft tissue contrast than CT scan and often less artifact from dental fillings than CT scan.71 However, because of the relatively long imaging time, the advantages of soft tissue detail may be outweighed by motion artifacts, especially at the level of the larynx and hypopharynx.106 Newer MRI techniques, including diffusion-weighted imaging, MR spectroscopy, and supermagnetic iron oxide contrast agents, may allow clearer distinction between benign and malignant lymph node involvement, sites of hypoxia, or identification of edema and necrosis.71,110–112
Positron-emission tomography (PET) has seen a rapid rise. It has a detection rate for head and neck primary tumors between 88% and 98%113–115 and, in general, has a higher sensitivity than either CT or MRI.115,116 Limitations include failure to detect very small primary tumors, superficial lesions, and locations near tissues with physiological intense 18-Fluoro-2-deoxy-D-glucose (FDG)-uptake like salivary tissues, Waldeyer’s ring, muscles, and larynx in patients who speak during the FDG uptake phase.117 In evaluating nodal involvement, FDG-PET has a higher sensitivity and specificity than CT, MRI, or ultrasound.118–120 PET/CT as a combined modality appears to be better than PET+CT or CT alone.106,119 However, in clinically N0 necks, despite an approximate incidence of 25% to 50% of occult metastases, PET has failed to show acceptable sensitivity or specificity.121,122 This may in part be related to the spatial resolution of 4 to 5 mm for PET scans.121 PET imaging has been found to alter the TNM score in 15% to 36% of patients compared to CT- or MRI-based staging.123–126 Because of the greater sensitivity for the detection of metastatic lesions and the ability to image the whole body, PET/CT may be used for staging and detection of synchronous second primaries in appropriately selected head and neck patients at >10% risk for distant metastases.116,127–129 These include patients with ≥4 lymph node metastases, level 4, bilateral, or ≥6 cm lymph node metastases, recurrent tumors, or presence of a second primary tumor.130 However, concerns about a false positive rate of up to 20% in some studies131,132 and the relatively small number of patients in available studies to date, require any PET findings to be corroborated by pathologic confirmation.117,133
Biopsy of the primary lesion should be performed under general anesthesia. Because of the risk of synchronous primary malignancies amid vulnerable mucosa, bronchoscopy and esophagoscopy should also be done at this time.134,135 Open biopsy of a neck mass to obtain a tissue diagnosis should be avoided, as this oncologically contaminates the neck.
Staging
The 2010 American Joint Committee on Cancer (AJCC) staging136 for all hypopharyngeal cancers is given in Table 30-1. Note that the staging for T2 lesions maintains the size parameter as well as the proviso that there is no fixation of the hemilarynx. Stage T4a includes invasion of the thyroid gland, cricoid and thyroid cartilage hyoid bone, or central compartment of soft tissue, which includes prelaryngeal strap muscles and subcutaneous fat. Please note that invasion of the esophagus is no longer considered stage T4a, but T3. Stage T4b includes invasion of the prevertebral fascia, encasement of the carotid artery, or invasion of the mediastinal structures.
Standard Therapeutic Approaches
Pyriform Sinus: Early-Stage (T1-T2) Disease
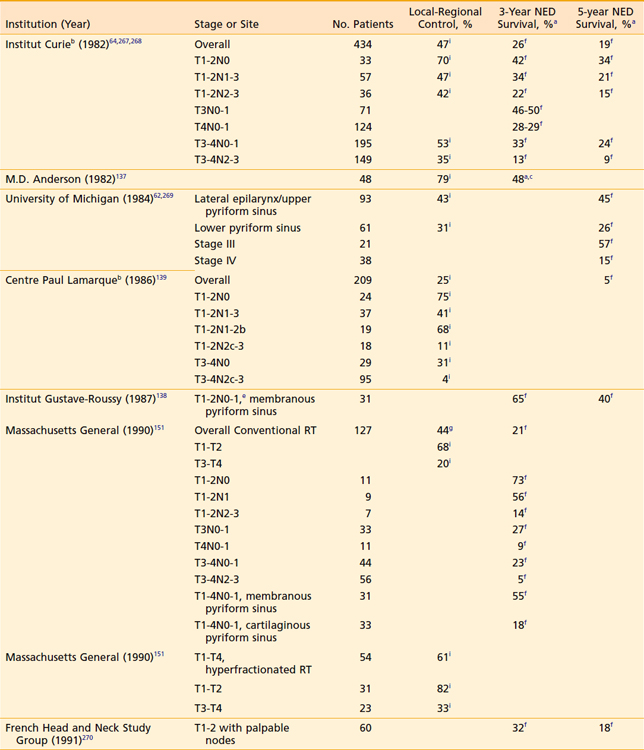
Early-stage favorable disease is not commonly encountered. In each of three separate series of more than 400 patients, T1 and T2 tumors (endophytic included) with nonfixed nodes constituted 12% to 16% of the cases; categorizing disease by the additional features listed previously would further winnow the number of patients actually presenting with favorable disease.64,137–139 Earlier detection of hypopharyngeal cancer, however, may increase the fraction of patients who are eligible for a larynx-conserving approach.
Either a primary conservative surgical approach or primary radiation therapy is a suitable treatment option for early-stage pyriform sinus cancer. Features allowing for conservative therapy are the following: all T1 tumors as well as T2 exophytic tumors of the upper or membranous portion of the pyriform sinus, lack of extension to the apex, lack of disease extension to the arytenoids, intact vocal cord mobility, uncompromised airway, and nonfixed neck nodes.62,63,140–151 Tumors originating in or extending to the apex frequently invade the thyroid cartilage, which often necessitates its removal in surgical management of disease respecting oncologic principles.66 Furthermore, in obtaining clear surgical margins on apical disease, the cricoid cartilage often must be sacrificed, necessitating permanent tracheostomy. In addition, patients require adequate pulmonary function to undergo a conservative hypopharyngeal surgery.
Conservative surgery consists of partial laryngopharyngectomy and ipsilateral modified radical neck dissection (or bilateral for N2c disease).150,152–157 In the setting of an N0 or N1 neck, a lateral neck dissection (involving removal of level II, III, and IV nodes) may be performed instead. Partial laryngopharyngectomy removes the upper portion of the pyriform sinus and adjacent marginal laryngeal structures (false cords, epiglottis, and possibly the ipsilateral arytenoid cartilage). In the setting of positive margins, multiple positive lymph nodes, or extracapsular extension of disease, surgery is followed by postoperative radiotherapy to maximize locoregional control of disease.76,78,80,92,158–160
When primary radiation therapy is decided upon, a planned neck dissection may follow radiotherapy, approximately 3 to 4 weeks after completion of radiotherapy. This approach may be performed if any single lymph node is greater than 2 or 3 cm or if multiple positive lymph nodes are present on initial clinical examination, unless there has been early complete regression of neck disease.63,64,142,149,161,162 A selective or radical neck dissection may be performed for persistent nodal disease after a course of definitive radiation therapy.163,164
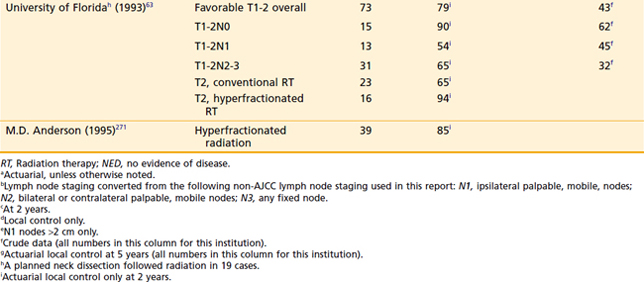
Amdur et al165 reported on 101 patients treated at the University of Florida with radiotherapy alone with or without a planned neck dissection. The 5-year actuarial local control rates for T1 and T2 tumors were 90% and 80%, respectively. After surgical salvage, the ultimate local control was 95% and 91%, respectively. On univariate analysis apical involvement for T1 tumors was associated with an inferior local control compared to patients with no apical involvement (1/3 [33%] versus 14/14 [100%]).
Although the local-regional control and survival outcomes are similar for conservative surgery and radiotherapy (Tables 30-2 and 30-3),142,144,166,167 primary radiation therapy may be the preferable treatment option because of its associated superior functional outcomes involving speech and swallowing. Nevertheless, there is an increased risk of swallowing dysfunction after radiotherapy for early-stage lesions. In 101 patients in the University of Florida series, 6% of patients experienced difficulty swallowing, requiring a permanent gastrostomy tube after radiotherapy alone.165 Although, as noted, there are potential significant complications after radiotherapy alone, the risk of severe toxicity and significant dysfunction is more prevalent after conservation surgery. Furthermore, the risk of chronic aspiration secondary to pharyngolaryngeal dyssynergia (due to sacrifice of the superior laryngeal nerve) after conservation surgery is less often observed after definitive radiation therapy.143,155,156,168,169
Pyriform Sinus: Locally Advanced (T3-T4) Resectable Disease
Postoperative radiation therapy is preferred to preoperative irradiation. A randomized trial by the RTOG, RTOG 73-03, has been the definitive study of this sequencing issue.170,171 Postoperative irradiation was associated with significantly improved local-regional control for all anatomic subsites combined. Among hypopharyngeal cases only, actuarial local-regional control and survival at 4 years were 61% and 28%, respectively, for the postoperative group, compared with 50% and 18% for the preoperative group. Although other studies have suggested an increased perioperative complication rate in particular in patients with hypopharyngeal cancer who are treated with preoperative irradiation,50,147,172,173 this relationship was not confirmed in RTOG 73-03.
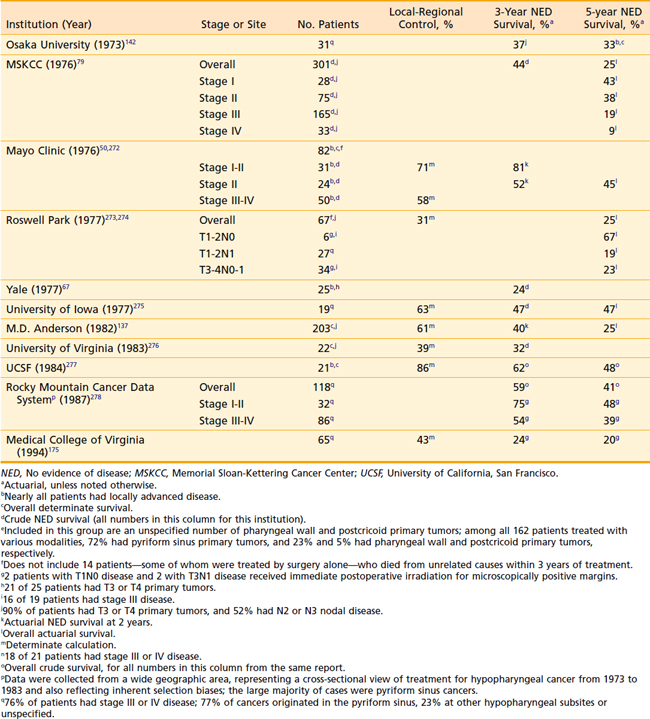
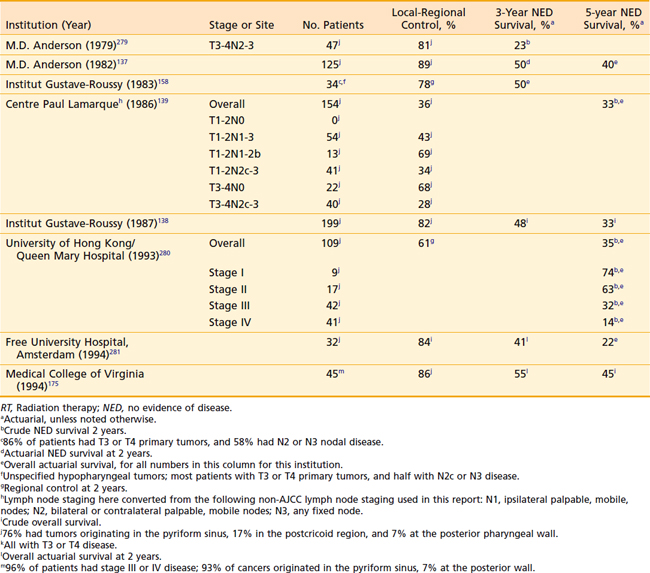
For stage III and IV disease, surgery in combination with postoperative radiation therapy results in better local-regional control rates (and possibly survivorship) than surgery alone. Retrospective data supporting this conclusion are outlined in Tables 30-4 and 30-5. The only randomized trial that compared surgery alone with surgery plus postoperative radiation therapy in the management of locally advanced head and neck cancer, including pharyngeal carcinomas, did not adequately address the question.174 The case mixture was heterogeneous, the sample size small, and the distribution of prognostic factors between groups unequal despite randomization.175 Furthermore, the total dose of radiation administered (50 Gy) was too low to eradicate microscopic disease in an operative bed.
Because distant metastases remain a common reason for treatment failure despite adequate local-regional control of disease,102,138,174–176 systemic therapy is an important consideration in the management of patients with stage III or IV disease. In randomized trials involving heterogeneous cases of advanced head and neck cancers, induction chemotherapy has been shown to affect the incidence of distant metastases.177–179 Adjuvant chemotherapy both after definitive local-regional therapy (as maintenance chemotherapy)180,181 and concurrent with local therapy (in the sequence surgery-chemotherapy-radiation therapy)182 may also reduce the rate of distant failure. Similarly, chemotherapy as part of a larynx-preserving treatment approach may result in decreased failure below the clavicles, as it has been shown in the management of advanced laryngeal cancer.183
Larynx-conserving treatment regimens in which aggressive surgery is avoided have become increasingly common.184–200 Early results from a randomized trial led by the European Organization for Research in Cancer Therapy comparing induction chemotherapy (three cycles of cisplatin and 5-fluorouracil [5-FU]) and radiotherapy with primary surgery and postoperative radiotherapy suggested that larynx preservation might be a viable strategy in managing hypopharyngeal cancer, as survival was not compromised.184 At 3 years, the crude survival rates were 53% for the chemotherapy-radiotherapy approach and 56% for the surgery plus postoperative radiotherapy arm, respectively, and 28% of patients in the chemotherapy-radiotherapy arm were free of disease, with an intact, functioning larynx. Follow-up data from this trial confirm these positive results: Survival rates in the two arms remained equivalent, whereas in the experimental arm, local progression-free survival with a functional larynx was achieved in 35% of patients at 5 years.185
An Intergroup trial (RTOG 95-01, ECOG R9501, SWOG 9515) showed that concomitant postoperative chemotherapy and radiation improves local-regional control and disease-free survival in a high-risk group of squamous cell carcinoma of the head and neck.201 Patients were randomized to postoperative radiotherapy alone (60 to 66 Gy in 30 to 33 fractions) versus concurrent radiotherapy and cisplatin 100 mg/m2 on days 1, 22, and 43. Ten percent of all patients had a primary hypopharyngeal tumor. The high-risk group was defined as: two or more regional lymph nodes involved on histology, extracapsular extension of nodal disease, and microscopically involved mucosal margins of resection. After a median follow-up of 45.9 months, 30% local-regional recurrences were observed with radiotherapy alone versus 19% with the use of concurrent chemotherapy. The two-year rate of local-regional control was 72% for radiotherapy alone and 82% for combined therapy. Disease-free survival was significantly longer after concurrent chemotherapy and radiation compared to radiotherapy alone (hazard ratio 0.78). There was a trend towards improved overall survival with concurrent chemotherapy and radiation. However, the incidence of acute adverse effects of grade 3 or greater substantially increased from 34% with radiation alone to 77% of patients receiving combined therapy.
The EORTC performed a similar trial for stage III or IV head and neck cancer comparing adjuvant concomitant cisplatin 100 mg/m2 (days 1, 22, and 43) and irradiation with radiotherapy alone (66 Gy in 33 fractions in both arms).202 Twenty percent of patients had a hypopharyngeal primary tumor site. At 5 years, local-regional control (31% versus 18%), progression-free survival (47% versus 36%) and overall survival (53% versus 40%) were significantly higher in the combined therapy group. Although there were more severe grade 3 or higher acute adverse effects in the combined therapy group (41% versus 21%), the incidence of severe mucosal and late adverse effects was similar in both groups.
Given the high toxicity of adjuvant chemoradiation, it is recommended only for high-risk, physically fit patients. In a retrospective subgroup analysis of both trials, chemoradiation improved the outcome most significantly in patients that had extracapsular extension and/or microscopically involved surgical margins.203
Pyriform Sinus: Unfavorable, Unresectable Disease
Concomitant chemotherapy and radiation therapy is rapidly becoming the standard management for locally advanced, unresectable head and neck cancer, including hypopharyngeal cancer.200,204–209 Randomized trials involving heterogeneous groups of unresectable, epithelial head and neck cancers have demonstrated the survival benefit of this approach compared with induction chemotherapy with radiotherapy, as well as the use of hyperfractionated radiotherapy regimens without chemotherapy.210,211 Although cisplatin often exacerbates the mucositis caused by radiotherapy, it is the chemotherapeutic agent most commonly used in the concomitant setting, as it is associated with high response rates.212–214 In most series, mucositis is observed in approximately 70% to 80% of treated patients and pretreatment placement of a percutaneous gastrostomy is recommended. Chemotherapy typically is given on day 1, 22, and 43 of a radiotherapy treatment schedule (see Techniques of Radiotherapy).212,213,215
Intensity-modulated radiation therapy with concurrent chemotherapy continues to replace older radiation techniques. Lee et al. reported a 2-year locoregional progression-free survival of 73% and 2-year overall survival rates of 53% for stage III and IV hypopharyngeal cancer.247 Studer et al reported 2-year local control and overall survival rates of 90%.216
Prades et al reported the outcome of patients with pyriform sinus cancers treated with various hyperfractionated radiotherapy and concomitant chemotherapy treatment schedules.186 In one group of 24 patients, carboplatin (days 1 to 16 and 16 to 27) was given in conjunction with hyperfractionated radiotherapy (1.6 Gy twice daily) to a total dose 67.2 Gy. In a second group of 22 patients, carboplatin and 5-FU were given with a delayed concomitant boost approach. For these latter patients, a 1.8-Gy single daily fraction was delivered for the first 2 weeks followed by 1.5 Gy twice-daily for the next 3 weeks to a dose of 63 Gy. The 2-year local control and larynx preservation rates for the first and second groups were 67% and 55%, respectively. In both treatment groups, unplanned hospitalizations due to treatment-related toxicity occurred in 63% and 86%, respectively. No differences in survival outcomes were observed. Budach et al randomized patients to hyperfractionated accelerated radiation alone to a total dose of 77.6 Gy versus (HART) hyperfractioned accelerated radiation to a total dose of 70.6 Gy with concurrent 5-FU/mitomycin-C (C-HART) in locally advanced head and neck cancers.217 Thirty-two percent of patients had hypopharyngeal carcinoma. At 5 years, locoregional control, progression-free and overall survival were superior in the arm using concurrent chemotherapy. Maximum acute reactions were lower with C-HART, and no differences in late reactions was observed between the two groups.
Alternative approaches to the management of unresectable hypopharyngeal cancer include radiation therapy alone, radiation therapy followed by chemotherapy, induction chemotherapy or chemotherapy alone. Induction chemotherapy with docetaxel/cisplatin/fluorouracil (TPF) compared to cisplatin/fluorouracil (PF), both followed by chemoradiotherapy with weekly carboplatin and radiation to a total dose of 70 to 74 Gy has been found to be associated with improved locoregional control, progression-free survival, and overall survival.218 Interestingly, distant-metastases-free survival was not different between the groups. Hematologic toxicity during induction chemotherapy was significantly more common in the TPF arm. The selection of a treatment approach largely depends on the medical condition of the patient, which indicates the level of aggressiveness with which control and cure of disease may be pursued.
Fixed cervical lymph nodes associated with an otherwise resectable primary tumor constitute a separate category of unresectable disease. In this situation, preoperative radiotherapy with or without concomitant chemotherapy may be used for downsizing of neck nodes in an attempt to make neck surgery possible. The usual dose administered is approximately 50 Gy in conventional fractionation to the primary lesion and with a boost to the primary lesion to 70 Gy to 72 Gy depending upon the tumor response at this site. The region of gross neck disease can be boosted to 60 Gy. Neck surgery follows 4 to 8 weeks later if the mass in the neck becomes resectable (i.e., regresses off the neurovascular bundle). Another situation in which preoperative radiotherapy should be considered is when emergency tracheostomy has been performed through obstructing tumor. Because of tumor spillage beyond the usual surgical confines of disease associated with this procedure, radiation therapy should be started as soon as possible.219
Carcinoma of the Posterior Pharyngeal Wall
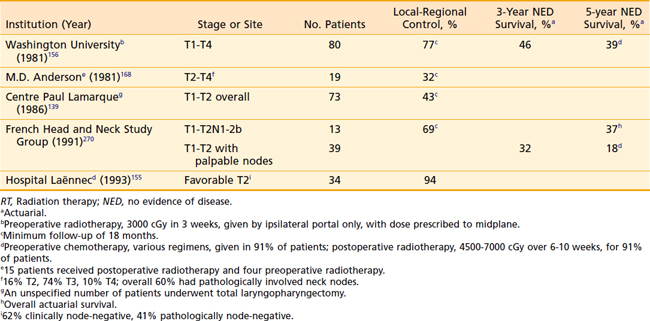
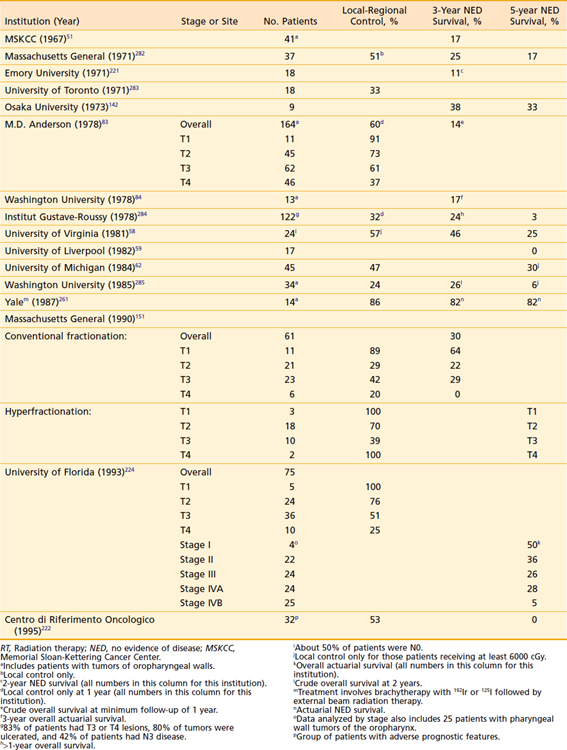
Surgical resection of posterior pharyngeal wall tumors often leaves microscopic disease at the resection margins, because of the difficulty of obtaining a deep margin at the prevertebral fascia and a high incidence of submucosal spread of disease longitudinally.93 Consequently, when surgery is performed as primary therapy, postoperative radiation therapy is usually indicated.220 Occasionally, selected patients may undergo larynx-conserving surgery alone as the only therapy for management of disease.59,60,221–223 Data from series reporting on surgery alone for pharyngeal wall cancer are outlined in Table 30-6.
Table 30-6 Carcinoma of the Posterior Pharyngeal Wall of the Hypopharynx (With/Without Pharyngeal Wall Tumors of the Oropharynx): Results of Surgery Alone
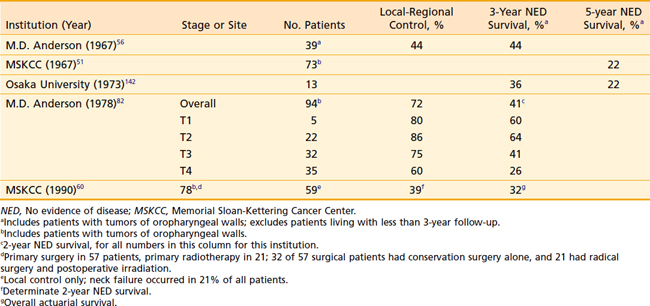
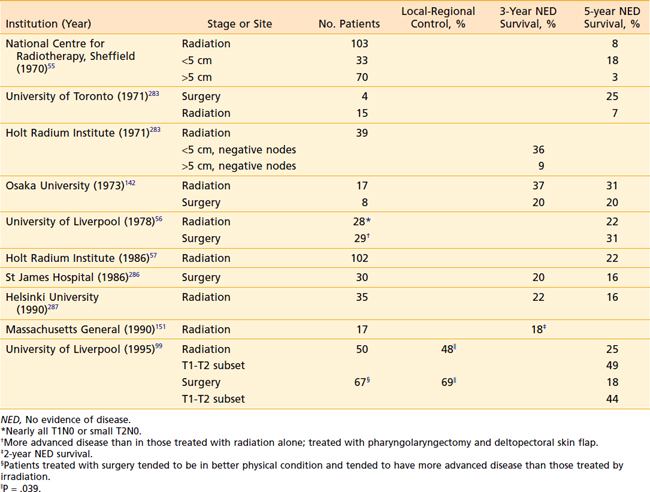
Other investigators advocate that definitive radiation therapy with or without concomitant chemotherapy be the standard management for posterior pharyngeal wall tumors, since the outcomes do not appear to be markedly different from those of surgery alone (Table 30-7), and the morbidity of a major operation can be spared.151,224
Postcricoid Carcinoma
As with posterior pharyngeal wall tumors, inadequate margins of resection are a common problem in the surgical management of this rare cancer. Extensive resections are required not only to encompass potential areas of “skip” metastases resulting from submucosal migration of tumor cells, but also to encompass potential regional spread of disease to paratracheal lymph nodes.94 For these very reasons, postoperative radiotherapy is also indicated—unless primary radiation therapy is given instead. Results of treatment of postcricoid carcinoma are shown in Table 30-8. Definitive radiation therapy appears to confer a survival outcome similar to that of primary surgery. This latter approach, especially for bulky tumors, should be used in conjunction with concomitant chemotherapy.
Recurrent Hypopharyngeal Carcinoma
Diagnosis
With the increased use of definitive chemotherapy and high-dose radiation therapy, the distinction between treatment sequelae and tumor recurrence has become an increasingly frequent and difficult task. Symptoms of radiation injury, such as hoarseness, dysphagia, respiratory distress, and pain may mimic recurrent disease.225 A precise work-up of recurrent tumor is therefore warranted. Direct fiber-optic endoscopy and biopsy under general anesthesia is recommended. However, there remains a relatively high number of false negative biopsies of up to 50%-60%.226,227 This may be due to the great variability in growth pattern of recurrent tumors, often presenting with multiple tumor foci dispersed in different regions and spread underneath an intact mucosa.225,228
Baseline imaging studies following treatment are generally recommended, as they appear to improve detection of recurrent disease on follow-up scans.229 Although anterior dislocation of the arytenoids, collapse of the cartilages, and gas bubbles are classic findings suggestive of chondroradionecrosis, they may not always be present.230 Tumor recurrence and chondroradionecrosis may co-exist, making the diagnosis of tumor recurrence more difficult.225
Pretherapeutic staging of recurrent laryngeal carcinoma by endoscopy, CT, and MRI appears to generally underestimate the true full extent of tumor involvement, with only 50% correct assessment of contralateal and extralaryngeal tumor spread.228
PET scans have been shown to be a sensitive method of detecting local recurrences.227,231–233 In a prospective study, Terhaard et al found that the first PET scan had a sensitivity and specificity of 92% and 63%, respectively.227 The negative and positive predictive value was 89% and 71%, respectively. These were even higher with the use of subsequent PET scans. They suggested that a PET scan should be part of the first diagnostic step after a physical examination and that a negative PET scan would make a biopsy obsolete.227 A positive PET scan and higher standardized uptake value (SUV) have been found to be independent predictors of relapse-free and overall survival.231 Each unit of SUV increase was noted to correlate to an increased relative risk of relapse by 11% and a relative risk of death by 14%. However, whereas the sensitivity was 96% in this study, specificity was only 72% because of false-positive readings due to inflammation.231 The optimal timing for highest accuracy of FDG-PET has been determined to be more than 3-4 months after the end of therapy.115,234 PET scans are therefore a useful tool for monitoring treatment response and restaging recurrences including nodal disease and distant metastases.127
Diffusion-weighted MRI has been demonstrated to have a high sensitivity, specificity, and accuracy for detecting malignancy,235 based on the fact that necrotic tissue shows high apparent diffusion coefficient (ADC) values and highly cellular tumors show diffusion restriction with low ADC values.236,237 Additional work is needed to further evaluate the role of these new imaging modalities for routine use in clinical practice.
Treatment
The standard treatment of resectable recurrent disease is salvage total laryngectomy or pharyngolaryngectomy.225,238 Partial laryngectomy strategies have been explored successfully in laryngeal cancer recurrences.239,240 Surgery may be combined with postoperative radiation therapy and chemotherapy. Alternatively chemotherapy and/or reirradiation may be offered for unresectable disease. Reirradiation using intensity-modulated radiation therapy (IMRT) has been explored and found to be associated with improved locoregional progression-free survival.241 Dose escalation with IMRT and improved chemotherapy may open new opportunities for maximized tumor control in the recurrent setting. Best supportive care is also an option for medically unfit patients.241
Techniques of Radiotherapy
Simulation and Treatment Technique
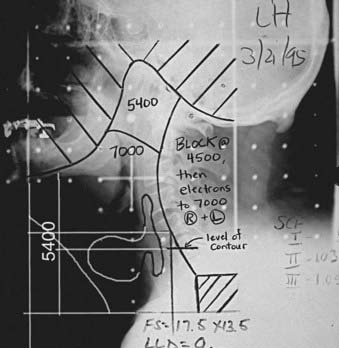
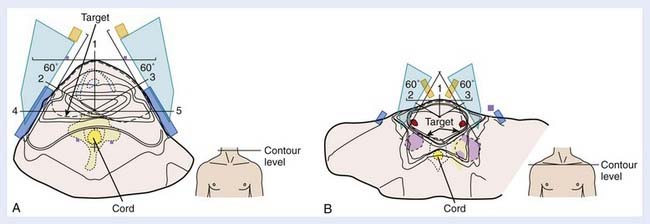
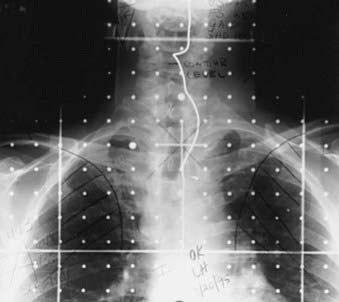
Traditionally, the most commonly used technique for treatment of pyriform sinus, posterior pharyngeal wall, and postcricoid cancers involved the use of opposed lateral photon fields (Fig. 30-9), a low anterior neck (LAN) photon field (Fig. 30-10), and posterior cervical electron fields. General guidelines for the design of these portals are listed in Table 30-9. The gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) are defined in Table 30-10. Topical anatomic features, such as palpable lymphadenopathy and the anterior surface of the neck (and stoma, if postlaryngopharyngectomy), should be outlined on the skin with radiopaque wire so that these structures can be visualized on simulation films.
Table 30-9 Guidelines to Design of Treatment Portals for Radiotherapy of Hypopharyngeal Cancer
| Opposed Lateral Photon Fields | |
| Additional shaping | |
| Superior border | 1.5 cm above the top of C1 to include the retropharyngeal and junctional nodes |
| Inferior border | As low as possible without entrance beam going through the shoulder (as determined by the lightfield on the skin); this border should be at least 2 cm below inferior edge of the cricoid cartilage to include the apex of the pyriform sinus |
| Anterior border | 1.0 to 1.5 cm beyond the skin surface to include thyroid cartilage in its entirety, and anterior soft tissues (for a posterior pharyngeal wall primary tumor, this border may be placed just posterior to the skin surface to exclude a small strip of anterior skin) |
| Posterior border | Up to 4500 cGy—just beyond the posterior spinous process of C2 to include level V lymph nodes |
| After 4500 cGy—curved border along middle of vertebral bodies of cervical spine; if tumor extends to or arises from the posterior pharyngeal wall, the posterior border should be placed at posterior edge of vertebral bodies using a split beam technique with 6 MV photons (see text) | |
| Additional shaping | With Cerrobend blocks to exclude spinal cord at posteroinferior aspect of field, as well as oral cavity, a portion of the mandible, and cerebellum |
| Cone-down field 2 | After 5400 cGy to exclude regions of neck only at risk for having microscopic disease (additional Cerrobend blocking added to superior aspect of field to exclude region of retropharyngeal nodes; occasionally thin strips of blocking are also added to anterior and inferior aspects of field) |
| Low Anterior Neck Field | |
| Superior border | Matched on skin to inferior border of opposed lateral fields; the spinal cord at the junction of fields is blocked in the upper fields |
| Inferior border | With low risk of mediastinal disease—below heads of clavicles, with blocking inferior to inferior aspect of clavicles |
| With high risk of mediastinal disease—5 cm below head of clavicles, with lateral blocking extended inferiorly so that mediastinal portion of field is about 8 cm wide | |
| Lateral borders | Positioned to exclude lateral one third of clavicles |
| Posterior Electron Strips | |
| Matched on skin to off-cord photon fields | |
Table 30-10 Tumor and Target Volume Definitions in the Radiotherapy of Hypopharyngeal Cancer
| Gross Tumor Volume (GTV) | Gross visible, palpable, or demonstrable extent of primary tumor and metastatic lymphadenopathy |
| Clinical Target Volume (CTV) | GTV and regions of potential microscopic disease (includes upper, middle, and lower jugular lymph nodes, spinal accessory lymph nodes, junctional and retropharyngeal lymph nodes, retropharyngeal space, thyroid and cricoid cartilages, pre-epiglottic and paraglottic spaces, paratracheal lymph nodes, parastomal soft tissues if stoma is present, supraclavicular lymph nodes, and occasionally upper mediastinal lymph nodes) |
| Planning Target Volume (PTV) | GTV plus margin (1.5 cm margin for standard three-field technique) to compensate for patient movement and inaccuracies in beam and patient set-up |
Special attention must be given to placement of the posterior border of the off-cord portal for tumors involving the posterior pharyngeal wall, as well as for tumors associated with bulky retropharyngeal lymphadenopathy. Because tumor or lymphadenopathy may extend posterolaterally around the anterior aspect of the vertebral column to make a horseshoe-shaped target volume, optimal inclusion of the CTV within the cone-down field requires that the posterior edge of the field be placed as far posterior as possible without including the spinal cord. This can be achieved by placing the central axis of the cone-down field at the posterior aspect of the vertebral bodies and using a split-beam technique—where the posterior half of the beam is blocked and posterior divergence of the beam is thus avoided.224 Photons of 6 MV should be used for this cone-down field, as the field edge will be sharp, and beam constriction associated with higher energies can be avoided.224,242 Alternatively, conformal radiation therapy—either using an isocentric rotational technique with multileaf collimation243 or a static five-field technique244—may effectively address the problem of a horseshoe-shaped target volume (Fig. 30-11).
Patients with short necks or those unable to displace their shoulders inferiorly pose a separate treatment planning challenge: Their shoulders lie within the same transverse plane as the inferior aspect of the GTV, preventing the use of the standard opposed lateral fields. One solution to this problem involves the use of lateral fields that are angled inferiorly to obtain better inferior coverage of the GTV and CTV, and elimination of the standard LAN field.245,246 Inferior angulation is done by rotating the foot of the treatment table 10 degrees to 20 degrees away from the gantry. Alternating use of no wedges and 15 degrees wedges in these treatment fields allows for requisite homogeneity in the dose distribution. A 3D dose distribution for this technique is shown in Fig. 30-12. (For comparison, a 3D dose distribution for a standard three-field technique is shown in Fig. 30-13.) A customized tissue compensator based on a 3D treatment plan could provide for optimal dose homogeneity.246
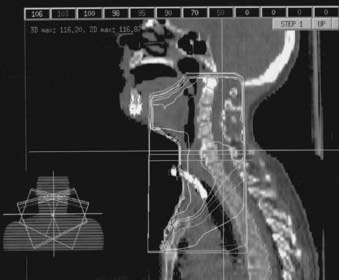
FIGURE 30-12 • Three-dimensional dose distribution for inferiorly angled lateral fields in the treatment of hypopharyngeal cancer.
(Courtesy Scott L. Sailer, MD.)
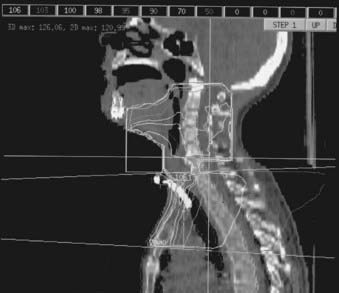
FIGURE 30-13 • Three-dimensional dose distribution for the standard three-field technique fields in the treatment of hypopharyngeal cancer.
(Courtesy Scott L. Sailer, MD.)
Tumors with extension of disease into the cervical esophagus required the use of multiple fields in a complicated treatment plan similar to a thyroid plan; this involved at least one set of wedged anterior oblique fields. An example of such a treatment plan is shown in Fig. 30-14.
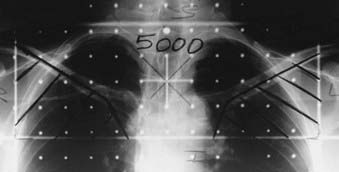
When the upper mediastinum has radiographically evident lymphadenopathy or is deemed to be at significant risk for harboring occult disease, it should be treated in addition to the LAN portal (Fig. 30-15). When an anteroposterior-posteroanterior arrangement was used, a cumulative dose of only 45 Gy (rather than 50 Gy) should be prescribed to protect the spinal cord. Any additional dose, if desired, has to be delivered by a set of off-cord (oblique or lateral) fields; this necessitates the formulation of a 2D treatment plan with isodose distributions at the outset of the treatment course for evaluation of the maximal spinal cord dose.
IMRT has been increasingly used in head and neck cancers including hypopharynx primary sites. IMRT allows the delivery of a highly conformal, more homogenous radiation therapy plan than conventional radiation using dynamic multileaf collimation.247 This may result in a higher therapeutic ratio between tumor and surrounding normal tissue. However, care must be taken to cover all areas of tumor involvement with adequate margins to maintain equivalent tumor control compared to 2D techniques. When substituting 2D techniques with a highly conformal alternative like IMRT, the potential of underdosing high-risk areas, which may result in poorer local control, poses an inherent risk. Detailed knowledge of the natural behavior of hypopharyngeal cancers and their patterns of spread is critical for maintaining excellent local control with IMRT. The great potential of IMRT in sparing normal tissue toxicity is especially apparent in head and neck subsites like the hypopharynx. Several institutional series have reported encouraging early results with the use of IMRT.216,247,248 At Memorial Sloan-Kettering Cancer Center (MSKCC), IMRT became routine practice for all stage III to IV laryngeal and hypopharyngeal cancers in 2004.247 In the absence of contraindications, we use intravenous contrast and a 3-mm slice thicknessn for the stimulation CT. Beams are chosen to ensure that ≥95% dose encompass the target volume. The GTV is defined as any visible tumor on imaging studies or physical examination. The high-risk CTV is defined as a 5 to 10 mm margin around the GTV. We typically include the retropharyngeal region when there are clinically involved neck nodes. Surgical neck levels 1 or 5 are only included when there is gross disease in adjacent levels (e.g., level 2). The planning target volume (PTV) encompasses the GTV and high-risk CTV with a 3-mm margin. Modification based on skin or spinal cord anatomy is done. A second, low-risk PTV may be included, e.g., contralateral neck and base of skull.247 A LAN field is not routinely used with IMRT for hypopharyngeal cancer.
Examples of a typical IMRT plan for a right-sided T3N2b pyriform sinus carcinoma and a T4aN0 posterior pharyngeal wall carcinoma with their respective dose-volume histograms are shown in Figs. 30-16 and 30-17). IMRT plans are carefully evaluated by a series of parameters including adequate coverage of the target structures, conformality, dose homogeneity and gradients, location of hot and cold spots, as well as dose to critical organs. It is critical to assess an IMRT plan on multiple slices, as areas of over- or underdosing may lie outside the section that is examined. Moreover, the dose to 95% of the target volume (D95) should be equal to the prescription dose. Hot spots should ideally be located within the target volume, and no cold spots should be found within the PTV. Organs at risk include the cochleae, submandibular and parotid glands, oral cavity, larynx, esophagus, brainstem, spinal cord, and brachial plexus (Table 30-11). We aim to keep the brainstem at a maximum dose (Dmax) of ≤50 to 54 Gy and the spinal cord ≤45 Gy. Care needs to be taken to avoid any hot spots in the glottic larynx and to keep the mean parotid dose ≤26 Gy whenever possible. It has previously been shown that xerostomia is related to the radiation dose and the volume of the parotid gland within the radiation field.249 Sparing of the parotids can be achieved with IMRT when the volume of overlap between the PTV and parotid is <21%.250 We aim to keep the mean dose to the oral cavity to 30 to 35 Gy to decrease mucositis and increase the patient’s ability to tolerate radiation, especially when given concomitantly with chemotherapy. The rapid decrease in dose towards the spinal cord, brain and oral cavity in Fig. 30-16 is the result of strict application of these dose constraints during treatment planning. Any patients with an emergent tracheostomy are treated with bolus placement around the tracheostomy site to ensure adequate skin and subcutaneous tissue coverage.247 Some limit the dose to the pharyngeal constrictors.251 We have started to determine the dose to the carotid arteries to avoid long-term arteriosclerosis and plaque formation.
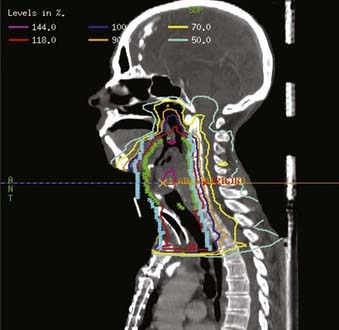
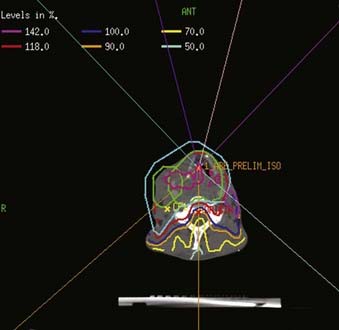
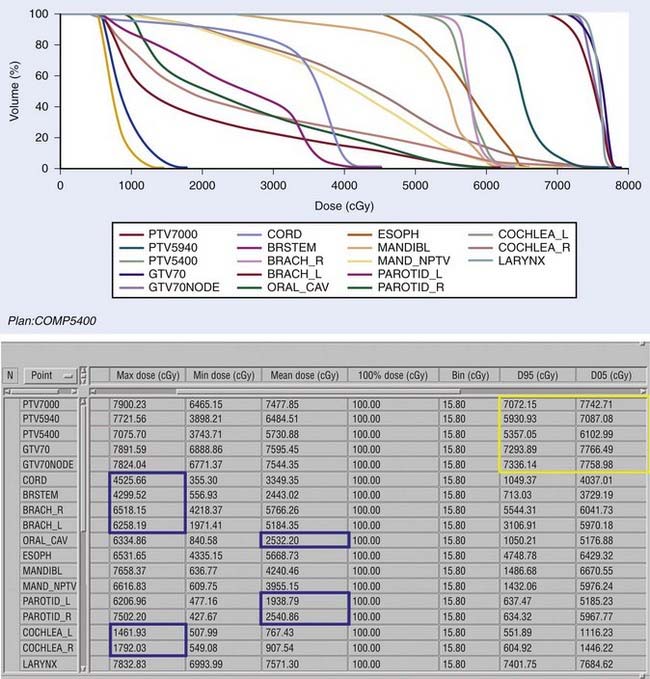
FIGURE 30-16 • Example of an intensity-modulated radiation therapy plan for a piriform sinus carcinoma.
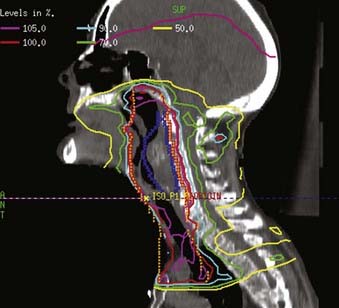
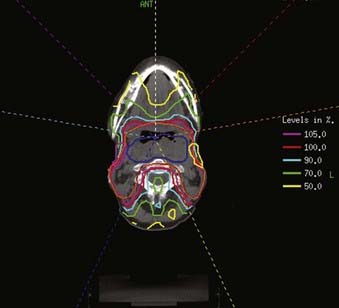
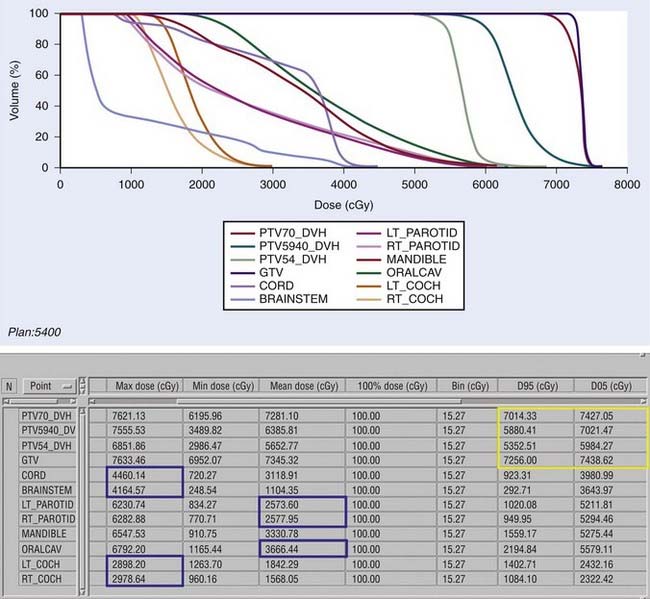
FIGURE 30-17 • An example of an intensity-modulated radiation therapy plan for a posterior pharyngeal wall carcinoma.
Table 30-11 Organs at Risk and Dose Constraints
| Organ at risk | Dose constraint |
| Cochleae | Dmax ≤50 Gy |
| Submandibular glands | Mean dose ≤39 Gy |
| Parotid glands | Mean dose ≤26 Gy (achievable if overlap between PTV and parotid <21%) |
| Oral cavity | Mean dose 30-35 Gy |
| Larynx | Avoid hot spots |
| Esophagus | Avoid hot spots |
| Brainstem | Dmax ≤50 Gy |
| Spinal cord | Dmax ≤45 Gy |
| Brachial plexus | Dmax ≤65 Gy, D05 ≤60 Gy |
Dose and Fractionation for Postoperative Radiotherapy
Postoperative radiotherapy after gastric pull-up reconstruction of the alimentary tract or after microvascular free tissue transfer of a jejunal segment, presents an interesting question: Can the transposed stomach or grafted jejunum tolerate a standard postoperative dose of 63 Gy? Available evidence indicates that this dose appears to be safe, at least after gastric transposition.252,253 In a series of 120 patients at MSKCC, late bleeding manifested by persistently low hemoglobin concentrations with endoscopic evidence of gastritis was found in only four patients, and one additional patient required surgical intervention to control hemorrhage.253 After free jejunal interposition, a mean dose of 56 Gy resulted in no radiation-related complications in a series of 20 patients.254 Nevertheless, caution should be exercised in such clinical situations treating to standard doses due to the lower dose threshold tolerance of jejunal or gastric mucosa.
Dose and Fractionation for Primary Radiotherapy, and Integration of Chemotherapy
At our institution, radiotherapy with a concomitant boost used to involve the delivery of a second daily fraction to the final cone-down field during weeks 5 and 6 of irradiation.255–257 The dose given with this fraction was 1.6 Gy per day. It was delivered in the afternoon, at least 5 hours after the regular daily fraction of 1.8 Gy. A total of 16 Gy was delivered with these boost fractions. When this was added to the 54 Gy delivered over 6 weeks with the regular daily fractions, the cumulative dose was 70 Gy.
The rationale for this fractionation scheme was three-fold: (1) it shortens the overall treatment time of irradiation to increase dose intensity; (2) it concentrates dose delivery during that portion of the radiotherapy schedule when tumor clonogen repopulation accelerates to negate this effect; and (3) it limits twice-a-day irradiation to only a portion of the treatment schedule to minimize the acute toxicity of such fractionation, where it is temporally separated from concomitant chemotherapy administration.258–260
Brachytherapy
Brachytherapy is rarely done for hypopharyngeal cancers. Two techniques have been published.261,262
Critical Normal Tissues
Acute Effects
The major dose-limiting toxicity of radiotherapy for hypopharyngeal cancer is mucositis. This is accompanied by symptoms of sore throat, dysphagia, and hoarseness. Xerostomia and dysgeusia invariably occur with the standard doses administered. As all of these symptoms can lead to significant weight loss, the patient’s nutritional status should be closely monitored by the radiation oncologist. Enteral hyperalimentation with percutaneous endoscopic gastrostomy-tube feeding often is necessary. To ensure adequate nutrition, a prophylactic gastrostomy tube may be placed, having been shown to improve the nutritional status of head-and-neck cancer patients undergoing definitive concurrent chemoradiotherapy with minimal complications.263,264
Laryngeal edema is usually induced by radiotherapy, and this may persist for up to 6 months after completion of treatment. It often is limited to the arytenoids. Biopsy to rule out persistent or recurrent disease should be done only if careful follow-up reveals progressive edema that is unresponsive to conservative measures and if there is a strong index of suspicion for the presence of tumor, as biopsy is associated with the risk of laryngeal necrosis.149
Late Effects
Late laryngeal chondronecrosis in the preserved larynx or soft tissue necrosis of the pharyngeal wall occurs in 2% to 4% of patients.64,83,85,137,147,148 Severe laryngeal edema requiring tracheostomy occurs in 1% to 6% of patients.64,83,85,147,148 Subcutaneous fibrosis of the soft tissues of the neck occurs in up to 11% of patients.137 Permanent gastrostomy for inability to swallow becomes necessary for 2% to 7% of patients treated with radiotherapy alone,64,85,147,148 and up to 31% at 2 years in patients treated with surgery and postoperative irradiation.137,247,265 A hypopharyngeal primary site and combined modality therapy appear to be predictive of stricture formation, the need for feeding tube placement and for pharyngoesophageal dilation.264 The pharyngeal constrictor muscles, supraglottic larynx, and glottic larynx have been found to be the most critical structures leading to swallowing dysfunction. Sparing of these structures can technically be achieved with IMRT, although in hypopharyngeal primary tumors they are often part of the primary target and therefore difficult to spare.251,266 Mortality from irradiation alone is usually secondary to cachexia after pharyngeal/esophageal stricture (or inability to swallow), aspiration pneumonia, asphyxia due to laryngeal edema, or carotid blow-out.64,137,170 The treatment-related mortality rate of radiation therapy alone is 1% to 3%.64,85,137 The figure rises to 5% to 6% when surgical salvage of severe complications is attempted.83,147 This surgical mortality is usually due to wound breakdown, pharyngocutaneous fistula, or hemorrhage from carotid artery rupture. Concomitant chemotherapy and radiation carry a mortality of about 2%.201
Outcome
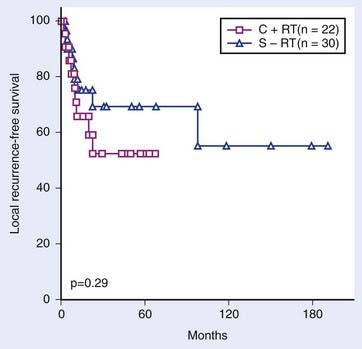
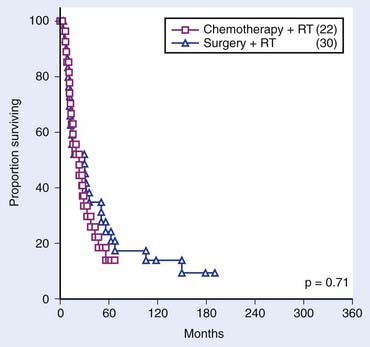
Outcomes of treatment for large series of patients are listed in Tables 30-2 through 30-8. Data on locoregional control, 3-year survival, and 5-year survival have been collated from the published reports indicated. Between 90% and 95% of all recurrences occur within 2 years of treatment. Actuarial data from MSKCC comparing a larynx-preservation treatment approach with standard therapy (surgery and postoperative irradiation) for advanced hypopharyngeal cancer are shown in Figs. 30-18 and 30-19.
1 Marks JE, Spector JG. Hypopharynx. In Perez CA, Brady LW, editors: Principles and practice of radiation oncology, ed 2, Philadelphia: JB Lippincott, 1992.
2 Murthy AK, Galinsky D, Hendrickson FR. Hypopharynx. In: Laramore GE, editor. Radiation therapy in head and neck cancer. Berlin: Springer-Verlag, 1989.
3 Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al, SEER Cancer Statistics Review, 1975–2005, National Cancer Institute, Bethesda, MD; 2008.
4 Spitz MR. Epidemiology and risk factors for head and neck cancer. Semin Oncol. 1994;21(3):281-288.
5 Tuyns AJ, Esteve J, Raymond L, Berrino F, Benhamou E, Blanchet F, et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int J Cancer. 1988;41(4):483-491.
6 Spitz MR, Fueger JJ, Goepfert H, Hong WK, Newell GR. Squamous cell carcinoma of the upper aerodigestive tract: a case comparison analysis. Cancer. 1988;61(1):203-208.
7 Wolf GT, Lippman SM, Laramore GE, et al. Head and neck cancer. In Holland JF, Frei EIII, Bast RCJr, editors: Cancer medicine, ed 3, Philadelphia: Lea & Febiger, 1993.
8 Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282-3287.
9 Day GL, Blot WJ, Austin DF, Bernstein L, Greenberg RS, Preston-Martin S, et al. Racial differences in risk of oral and pharyngeal cancer: alcohol, tobacco, and other determinants. J Natl Cancer Inst. 1993;85(6):465-473.
10 Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium: cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). J Natl Cancer Inst. 2007;99(10):777-789.
11 Menvielle G, Luce D, Goldberg P, Bugel I, Leclerc A. Smoking, alcohol drinking and cancer risk for various sites of the larynx and hypopharynx. A case-control study in France. Eur J Cancer Prev. 2004;13(3):165-172.
12 Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Health. 2006;29(3):193-198.
13 Singh B. Molecular pathogenesis of head and neck cancers. J Surg Oncol. 2008;97(8):634-639.
14 Asakage T, Yokoyama A, Haneda T, Yamazaki M, Muto M, Yokoyama T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases, and drinking, smoking and diet in Japanese men with oral and pharyngeal squamous cell carcinoma. Carcinogenesis. 2007;28(4):865-874.
15 Winn DM, Blot WJ, Shy CM. Snuff dipping and oral cancer among women in the southern United States. NEJM. 1981;304(13):745-749.
16 Mattson ME, Winn DM. Smokeless tobacco: association with increased cancer risk. NCI Monogr. 1989;8:13-16.
17 Hoffmann D, Djordjevic MV, Fan J, Zang E, Glynn T, Connolly GN. Five leading US commercial brands of moist snuff in 1994: assessment of carcinogenic N-nitrosamines. J Natl Cancer Inst. 1995;87(24):1862-1869.
18 Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9(7):667-675.
19 Tomar SL. Trends and patterns of tobacco use in the United States. Am J Med Sci. 2003;326(4):248-254.
20 Hsu TC, Johnston DA, Cherry LM, Ramkissoon D, Schantz SP, Jessup JM, et al. Sensitivity to genotoxic effects of bleomycin in humans: possible relationship to enviromental carcinogenesis. International Journal of Cancer. 1989;43(3):403-409.
21 Spitz MR, Fueger JJ, Beddingfield NA, Annegers JF, Hsu TC, Newell GR, et al. Chromosome sensitivity to bleomycin-induced mutagenesis, an independent risk factor for upper aerodigestive tract cancers. Cancer Res. 1989;49(16):4626-4628.
22 Szekely G, Remenar E, Kasler M, Gundy S. Mutagen sensitivity of patients with cancer at different sites of the head and neck. Mutagenesis. 2005;20(5):381-385.
23 Copper MP, Jovanovic A, Nauta JJP, Braakhuis BJM, De Vries N, Van der Waal I, et al. Role of genetic factors in the etiology of squamous cell carcinoma of the head and neck. Arch Otolaryngol—Head Neck Surg. 1995;121(2):157-160.
24 Sidransky D. Molecular genetics of head and neck cancer. Curr Opin Oncol. 1995;7(3):229-233.
25 Gallo O, Bianchi S, Porfirio B. Bcl-2 overexpression and smoking history in head and neck cancer. J Natl Cancer Inst. 1995;87(13):1024-1025.
26 Bell DA, Liu Y, Cortopassi GA. Occurrence of bcl-2 oncogene translocation with increased frequency in the peripheral blood of heavy smokers. J Natl Cancer Inst. 1995;87(3):223-224.
27 Yunis JJ, Oken MM, Kaplan ME. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin’s lymphoma. NEJM. 1982;307(20):1231-1236.
28 Frank JL, Bur ME, Garb JL, Kay S, Ware JL, Sismanis A, et al. p53 Tumor suppressor oncogene expression in squamous cell carcinoma of the hypopharynx. Cancer. 1994;73(1):181-186.
29 Frank JL, Garb JL, Banson BB, Peterman J, Neifeld JP, Kay S, et al. Epidermal growth factor receptor expression in squamous cell carcinoma of the hypopharynx. Surg Oncol. 1993;2(3):161-167.
30 Issing WJ, Wustrow TPU, Heppt WJ. Oncogenes related to head and neck cancer. Anticancer Res. 1993;13(6 B):2541-2551.
31 Brachman DG. Molecular biology of head and neck cancer. Semin Oncol. 1994;21(3):320-329.
32 Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. NEJM. 1988;319(9):525-532.
33 Rodrigo JP, Gonzälez MV, Lazo PS, Ramos S, Coto E, Alvarez I, et al. Genetic alterations in squamous cell carcinomas of the hypopharynx with correlations to clinicopathological features. Oral Onco. 2002;38(4):357-363.
34 Meredith SD, Levine PA, Burns JA, Gaffey MJ, Boyd JC, Weiss LM, et al. Chromosome 11q13 amplification in head and neck squamous cell carcinoma: association with poor prognosis. Arch Otolaryngol—Head Neck Surg. 1995;121(7):790-794.
35 Holley SL, Parkes G, Matthias C, Bockmühl U, Jahnke V, Leder K, et al. Cyclin D1 polymorphism and expression in patients with squamous cell carcinoma of the head and neck. Am J Path. 2001;159(5):1917-1924.
36 Yang MH, Chiang WC, Chou TY, Chang SY, Chen PM, Teng SC, et al. Increased NBS1 expression is a marker of aggressive head and neck cancer and overexpression of NBS1 contributes to transformation. Clin Cancer Res. 2006;12(2):507-515.
37 Dikshit RP, Gillio-Tos A, Brennan P, De Marco L, Fiano V, Martinez-Penuela JM, et al. Hypermethylation, risk factors, clinical characteristics, and survival in 235 patients with laryngeal and hypopharyngeal cancers. Cancer. 2007;110(8):1745-1751.
38 Clayman GI, Stewart MG, Weber RS, El-Naggar AK, Grimm EA. Human papillomavirus in laryngeal and hypopharyngeal carcinomas: relationship to survival. Arch Otolaryngol—Head Neck Surg. 1994;120(7):743-748.
39 Psyrri A, DiMaio D. Human papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract Oncol. 2008;5(1):24-31.
40 D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. NEJM. 2007;356(19):1944-1956.
41 Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736-747.
42 Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261-269.
43 Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14(2):366-369.
44 Martin SA, Marks JE, Lee JY. Carcinoma of the pyriform sinus: predictors of TNM relapse and survival. Cancer. 1980;46(9):1974-1981.
45 Spaulding MB, Fischer SG, Wolf GT. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. J Clin Oncol. 1994;12(8):1592-1599.
46 Truelson JM, Fisher SG, Beals TE, McClatchey KD, Wolf GT, Waun Ki H, et al. DNA content and histologic growth pattern correlate with prognosis in patients with advanced squamous cell carcinoma of the larynx. Cancer. 1992;70(1):56-62.
47 Banks ER, Frierson HFJr, Mills SE, George E, Zarbo RJ, Swanson PE. Basaloid squamous cell carcinoma of the head and neck: a clinicopathologic and immunohistochemical study of 40 cases. American Journal of Surgical Pathology. 1992;16(10):939-946.
48 Ferlito A, Rinaldo A, Altavilla G, Doglioni C. Basaloid squamous cell carcinoma of the larynx and hypopharynx. Ann Otol Rhinol Laryngol. 1997;106(12):1024-1035.
49 Soriano E, Faure C, Lantuejoul S, Reyt E, Bolla M, Brambilla E, et al. Course and prognosis of basaloid squamous cell carcinoma of the head and neck: a case-control study of 62 patients. Eur J Cancer. 2008;44(2):244-250.
50 Carpenter RJIII, De Santo LW, Devine KD, Taylor WF. Cancer of the hypopharynx. Analysis of treatment and results in 162 patients. Arch Otolaryngol. 1976;102(12):716-721.
51 Cunningham MP, Catlin D. Cancer of the pharyngeal wall. Cancer. 1967;20(11):1859-1866.
52 Helliwell TR. Evidence based pathology: squamous carcinoma of the hypopharynx. J Clin Pathol. 2003;56(2):81-85.
53 Horwitz SD, Caldarelli DD, Hendrickson FR. Treatment of carcinoma of the hypopharynx. Head Neck Surg. 1979;2(2):107-111.
54 Keane TJ. Carcinoma of the hypopharynx. J Otolaryngol. 1982;11(4):227-231.
55 Kalavathi N. Factors influencing cure in the radiotherapy of post cricoid carcinoma. Clin Radiol. 1970;21(3):248-252.
56 Stell PM, Carden EA, Hibbert J, Dalby JE. Post-cricoid carcinoma. Clinical Oncology. 1978;4(3):215-226.
57 Farrington WT, Weighill JS, Jones PH. Post-cricoid carcinoma (a ten-year retrospective study). J Laryngol Otol. 1986;100(1):79-84.
58 Talton BM, Elkon D, Kim JA. Cancer of the posterior hypopharyngeal wall. Int J Radiat Oncol Biol Phys. 1981;7(5):597-599.
59 Raine CH, Stell PM, Dalby J. Squamous cell carcinomas of the posterior wall of the hypopharynx. J Laryngol Otol. 1982;96(11):997-1004.
60 Spiro RH, Kelly J, Vega AL, Harrison LB, Strong EW. Squamous carcinoma of the posterior pharyngeal wall. Am J Surg. 1990;160(4):420-423.
61 Marks JE. The endolarynx and hypopharynx. In Moss WT, Cox JD, editors: Radiation Oncology, ed 6, St. Louis: Mosby, 1989.
62 Ahmad K, Fayos JV. Role of radiation therapy in carcinoma of the hypopharynx. Acta Radiologica. Oncol Radiat Ther Phys Bioly. 1984;23(1):21-26.
63 Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR. Radiotherapy alone or combined with neck dissection for T1-T2 carcinoma of the pyriform sinus: an alternative to conservation surgery. Int J Radiat Oncol Biol Phys. 1993;27(5):1017-1027.
64 Bataini P, Brugere J, Bernier J. Results of radical radiotherapeutic treatment of carcinoma of the pyriform sinus: experience of the Institut Curie. Int J Radiat Oncol Biol Phys. 1982;8(8):1277-1286.
65 Harrison DF. Pathology of hypopharyngeal cancer in relation to surgical management. J Laryngol Otol. 1970;84(4):349-367.
66 Kirchner JA. Pyriform sinus cancer: a clinical and laboratory study. Ann Otol Rhinol Laryngol. 1975;84(6):793-803.
67 Kirchner JA, Owen JR. Five hundred cancers of the larynx and pyriform sinus. Results of treatment by radiation and surgery. Laryngoscope. 1977;87(8):1288-1303.
68 Spector JG, Sessions DG, Emami B, Simpson J, Haughey B, Harvey J, et al. Squamous cell carcinoma of the pyriform sinus: a nonrandomized comparison of therapeutic modalities and long-term results. Laryngoscope. 1995;105(4 I):397-406.
69 Becker M, Burkhardt K, Dulguerov P, Allal A. Imaging of the larynx and hypopharynx. Eur J Radiol. 2008;66(3):460-479.
70 Yousem DM, Hatabu H, Hurst RW, Seigerman HM, Montone KT, Weinstein GS, et al. Carotid artery invasion by head and neck masses: prediction with MR imaging. Radiology. 1995;195(3):715-720.
71 Wycliffe ND, Grover RS, Kim PD, Simental AJr. Hypopharyngeal cancer. Top Magn Reson Imaging. 2007;18(4):243-258.
72 Pameijer FA, Mancuso AA, Mendenhall WM, Parsons JT, Mukherji SK, Hermans R, et al. Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck. 1998;20(2):159-168.
73 Magnano M, Bongioannini G, Lerda W, Canale G, Tondolo E, Bona M, et al. Lymphnode metastasis in head and neck squamous cells carcinoma: multivariate analysis of prognostic variables. Journal of Experimental and Clinical Cancer Research. 1999;18(1):79-83.
74 Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405-409.
75 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446-1449.
76 Bataini JP, Bernier J, Brugere J. Natural history of neck disease in patients with squamous cell carcinoma of oropharynx and pharyngolarynx. Radiother Oncol. 1985;3(3):245-255.
77 Lefebvre JL, Castelain B, De la Torre JC, Delobelle-Deroide A, Vankemmel B. Lymph node invasion in hypopharynx and lateral epilarynx carcinoma: a prognostic factor. Head Neck Surg. 1987;10(1):14-18.
78 Marks JE, Devineni VR, Harvey J, Sessions DG. The risk of contralateral lymphatic metastases for cancers of the larynx and pharynx. Am J Otolaryngol—Head Neck Med Surg. 1992;13(1):34-39.
79 Shah JP, Shaha AR, Spiro RH, Strong EW. Carcinoma of the hypopharynx. Am J Surg. 1976;132(4):439-443.
80 Marks JE, Breaux S, Smith PG, Thawley SE, Spector GG, Sessions DG. The need for elective irradiation of occult lymphatic metastases from cancers of the larynx and pyriform sinus. Head Neck Surg. 1985;8(1):3-8.
81 Buckley JG, MacLennan K. Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck. 2000;22(4):380-385.
82 Guillamondegui OM, Meoz R, Jesse RH. Surgical treatment of squamous cell carcinoma of the pharyngeal walls. Am J Surg. 1978;136(4):474-476.
83 Meoz-Mendez RT, Fletcher GH, Guillamondegui OM, Peters LJ. Analysis of the results of irradiation in the treatment of squamous cell carcinomas of the pharyngeal walls. Int J Radiat Oncol Biol Phys. 1978;4(7–8):579-585.
84 Marks JE, Freeman RB, Lee F, Ogura JH. Pharyngeal wall cancer: an analysis of treatment, results, complications and patterns of failure. Int J Radiat Oncol Biol Phys. 1978;4(7–8):587-593.
85 Mendenhall WM, Parsons JT, Mancuso AA, Cassisi NJ, Million RR. Squamous cell carcinoma of the pharyngeal wall treated with irradiation. Radiother Oncol. 1988;11(3):205-212.
86 Ogura JH, Biller HF, Wette R. Elective neck dissection for pharyngeal and laryngeal cancers. An evaluation. Ann Otol Rhinol Laryngol. 1971;80(5):646-650.
87 Ballantyne AJ. Principles of surgical management of cancer of the pharyngeal walls. Cancer. 1967;20(5):663-667.
88 Ballantyne AJ. Significance of retropharyngeal nodes in cancer of the head and neck. Am J Surg. 1964;108:500-504.
89 Dalley VM. Cancer of the laryngopharynx. J Laryngol Otol. 1968;82(5):407-419.
90 McCrea RS, Dickie WR. Carcinoma of the hypopharynx and cervical oesophagus: a review of sixty-one cases. J Laryngol Otol. 1968;82(5):421-445.
91 Willatt DJ, Jackson SR, McCormick MS. Vocal cord paralysis and tumour length in staging postcricoid cancer. Eur J Surg Oncol. 1987;13(2):131-137.
92 Biller HF, Davis WH, Ogura JH. Delayed contralateral cervical metastases with laryngeal and laryngopharyngeal cancers. Laryngoscope. 1971;81(9):1499-1502.
93 Harrison DF. Surgical management of cancer of the hypopharynx and cervical oesophagus. Br J Surg. 1969;56(2):95-103.
94 Harrison DF. Role of surgery in the management of postcricoid and cervical esophageal neoplasms. Ann Otol Rhinol Laryngol. 1972;81(4):465-468.
95 Harrison DF. Thyroid gland in the management of laryngopharyngeal cancer. Arch Otolaryngol. 1973;97(4):301-302.
96 Harris HH, Butler E. Surgical limits in cancer of the subglottic larynx. Arch Otolaryngol. 1968;87(5):490-493.
97 Harrison DFN. Resection of the manubrium. Br J Surg. 1977;64(5):374-377.
98 Weber RS, Marvel J, Smith P, Hankins P, Wolf P, Goepfert H. Paratracheal lymph node dissection for carcinoma of the larynx, hypopharynx, and cervical esophagus. Otolaryngology—Head Neck Surg. 1993;108(1):11-17.
99 Jones AS, McRae RD, Phillips DE, Hamilton J, Field JK, Husband D. The treatment of node negative squamous cell carcinoma of the postcricoid region. J Laryngol Otol. 1995;109(2):114-119.
100 Kotwall C, Sako K, Razack MS, Rao U, Bakamjian V, Shedd DP. Metastatic patterns in squamous cell cancer of the head and neck. Am J Surg. 1987;154(4):439-442.
101 Zbaren P, Lehmann W. Frequency and sites of distant metastases in head and neck squamous cell carcinoma. An analysis of 101 cases at autopsy. Arch Otolaryngol—Head Neck Surg. 1987;113(7):762-764.
102 Leibel SA, Scott CB, Mohiuddin M, Marcial VA, Coia LR, Davis LW, et al. The effect of local-regional control on distant metastatic dissemination in carcinoma of the head and neck: results of an analysis from the RTOG head and neck database. Int J Radiat Oncol Biol Phys. 1991;21(3):549-556.
103 Silverman PM, Bossen EH, Fisher SR. Carcinoma of the larynx and hypopharynx: computed tomographic-histopathologic correlations. Radiology. 1984;151(3):697-702.
104 Million RR, Cassisi NJ, Mancuso AA. Hypopharynx: pharyngeal walls, pyriform sinus, postcricoid pharynx. In Million RR, Cassisi NJ, editors: Management of head and neck cancer: a multidisciplinary approach, ed 2, Philadelphia: JB Lippincott, 1994.
105 Silverman PM, Zeiberg AS, Sessions RB, Troost TR, Zeman K. Three-dimensional imaging of the hypopharynx and larynx by means of helical (spiral) computed tomography. Comparison of radiological and otolaryngological evaluation. Ann Otol Rhinol Laryngol. 1995;104(6):425-431.
106 Rumboldt Z, Gordon L, Bonsall R, Ackermann S. Imaging in head and neck cancer. Curr Treat Options Oncol. 2006;7(1):23-34.
107 Castelijns JA, Gerritsen GJ, Kaiser MC, Valk J, Van Zanten TEG, Golding RG, et al. Invasion of laryngeal cartilage by cancer: Comparison of CT and MR imaging. Radiology. 1988;167(1):199-206.
108 Wenig BL, Ziffra KL, Mafee MF, Schild JA. MR imaging of squamous cell carcinoma of the larynx and hypopharynx. Otolaryngologic Clin N Am. 1995;28(3):609-619.
109 Roychowdhury S, Loevner LA, Yousem DM, Chalian A, Montone KT. MR imaging for predicting neoplastic invasion of the cervical esophagus. Am J Neuroradiol. 2000;21(9):1681-1687.
110 Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220(3):621-630.
111 Star-Lack JM, Adalsteinsson E, Adam MF, Terris DJ, Pinto HA, Brown JM, et al. In vivo 1H MR spectroscopy of human head and neck lymph node metastasis and comparison with oxygen tension measurements. Am J Neuroradiol. 2000;21(1):183-193.
112 Anzai Y, Prince MR. Iron oxide-enhanced MR lymphography: the evaluation of cervical lymph node metastases in head and neck cancer. J Magn Reson Imaging. 1997;7(1):75-81.
113 Hannah A, Scott AM, Tochon-Danguy H, Chan JG, Akhurst T, Berlangieri S, et al. Evaluation of 18F-fluorodeoxyglucose positron emission tomography and computed tomography with histopathologic correlation in the initial staging of head and neck cancer. Ann Surg. 2002;236(2):208-217.
114 Paulus P, Sambon A, Vivegnis D, Hustinx R, Moreau P, Collignon J, et al. 18FDG-PET for the assessment of primary head and neck tumors: clinical, computed tomography, and histopathological correlation in 38 patients. Laryngoscope. 1998;108(10):1578-1583.
115 Greven KM, Williams DWIII, Frederick McGuirt WSr, Harkness BA, D’Agostino RBJr, Keyes JWJr, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23(11):942-946.
116 Kutler DI, Wong RJ, Kraus DH. Functional imaging in head and neck cancer. Curr Oncol Rep. 2005;7(2):137-144.
117 Greven KM. Positron-emission tomography for head and neck cancer. Semin Radiat Oncol. 2004;14(2):121-129.
118 Kau RJ, Alexiou C, Laubenbacher C, Werner M, Schwaiger M, Arnold W. Lymph node detection of head and neck squamous cell carcinomas by positron emission tomography with fluorodeoxyglucose F 18 in a routine clinical setting. Arch Otolaryngol—Head Neck Surg. 1999;125(12):1322-1328.
119 Adams S, Baum RP, Stuckensen T, Bitter K, Hör G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med. 1998;25(9):1255-1260.
120 Schwartz DL, Ford E, Rajendran J, Yueh B, Coltrera MD, Virgin J, et al. FDG-PET/CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;61(1):129-136.
121 Stoeckli SJ, Steinert H, Pfaltz M, Schmid S. Is there a role for positron emission tomography with 18F-fluorodeoxyglucose in the initial staging of nodal negative oral and oropharyngeal squamous cell carcinoma. Head Neck. 2002;24(4):345-349.
122 Schöder H, Carlson DL, Kraus DH, Stambuk HE, Gönen M, Erdi YE, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med. 2006;47(5):755-762.
123 Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck. 2005;27(6):494-502.
124 Ha PK, Hdeib A, Goldenberg D, Jacene H, Patel P, Koch W, et al. The role of positron emission tomography and computed tomography fusion in the management of early-stage and advanced-stage primary head and neck squamous cell carcinoma. Arch Otolaryngol—Head Neck Surg. 2006;132(1):12-16.
125 Goerres GW, Schmid DT, Grätz KW, Von Schulthess GK, Eyrich GK. Impact of whole body positron emission tomography on initial staging and therapy in patients with squamous cell carcinoma of the oral cavity. Oral Oncol. 2003;39(6):547-551.
126 Veit-Haibach P, Luczak C, Wanke I, Fischer M, Egelhof T, Beyer T, et al. TNM staging with FDG-PET/CT in patients with primary head and neck cancer. Eur J Nucl Med Mol Imaging. 2007;34(12):1953-1962.
127 Agarwal V, Branstetter BFIV, Johnson JT. Indications for PET/CT in the head neck. Otolaryngologic Clin N Am. 2008;41(1):23-49.
128 Schwartz DL, Rajendran J, Yueh B, Coltrera M, Anzai Y, Krohn K, et al. Staging of head neck squamous cell cancer with extended-field FDG-PET. Arch Otolaryngol—Head Neck Surg. 2003;129(11):1173-1178.
129 Teknos TN, Rosenthal EL, Lee D, Taylor R, Marn CS. Positron emission tomography in the evaluation of stage III and IV head and neck cancer. Head Neck. 2001;23(12):1056-1060.
130 De Bree R, Deurloo EE, Snow GB, Leemans CR. Screening for distant metastases in patients with head and neck cancer. Laryngoscope. 2000;110(3 I):397-401.
131 Wax MK, Myers LL, Gabalski EC, Husain S, Gona JM, Nabi H. Positron emission tomography in the evaluation of synchronous lung lesions in patients with untreated head and neck cancer. Arch Otolaryngol—Head Neck Surg. 2002;128(6):703-707.
132 Keyes JWJr, Chen MYM, Watson NEJr, Greven KM, McGuirt WF, Williams DWIII. FDG pet evaluation of head and neck cancer: value of imaging the thorax. Head Neck. 2000;22(2):105-110.
133 Quon A, Fischbein NJ, McDougall IR, Le QT, Loo BWJr, Pinto H, et al. Clinical role of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med. 48(Suppl 1), 2007.
134 McGuirt WF, Matthews B, Koufman JA. Multiple simultaneous tumors in patients with head and neck cancer. A prospective, sequential panendoscopic study. Cancer. 1982;50(6):1195-1199.
135 McGuirt WF. Panendoscopy as a screening examination for simultaneous primary tumors in head and neck cancer: a prospective sequential study and review of the literature. Laryngoscope. 1982;92(5):569-576.
136 American Joint Committee on Cancer. Pharynx (including base of tongue, soft palate and uvula). In AJCC cancer staging manual, ed 7, New York: Springer-Verlag; 2010:45-46.
137 El Badawi SA, Goepfert H, Fletcher GH, Herson J, Oswald MJ. Squamous cell carcinoma of the pyriform sinus. Laryngoscope. 1982;92(4):357-364.
138 Vandenbrouck C, Eschwege F, De la Rochefordiere A, Sicot H, Mamelle G, Le Ridant AM, et al. Squamous cell carcinoma of the pyriform sinus: retrospective study of 351 cases treated at the Institut Gustave-Roussy. Head Neck Surg. 1987;10(1):4-13.
139 Dubois JB, Guerrier B, Di Ruggiero JM, Pourquier H. Cancer of the piriform sinus: treatment by radiation therapy alone and with surgery. Radiology. 1986;160(3):831-836.
140 Baclesse F. Roentgentherapy in cancer of the hypopharynx. JAMA. 1949;140:525-529.
141 Baclesse F. Classification topographique des cancer de l’hypopharynx. In: Baclesse F, editor. Tumeurs malignes du pharynx et du larynx. Paris: Masson & Co; 1960:283.
142 Inoue T, Shigematsu Y, Sato T. Treatment of carcinoma of the hypopharynx. Cancer. 1973;31(3):649-655.
143 Freeman RB, Marks JE, Ogura JH. Voice preservation in treatment of carcinoma of the pyriform sinus. Laryngoscope. 1979;89(11):1855-1863.
144 Million RR, Cassisi NJ. Radical irradiation for carcinoma of the pyriform sinus. Laryngoscope. 1981;91(3):439-450.
145 El Badawi SA, Goepfert H, Fletcher GH. Squamous cell carcinoma of the pyriform sinus. Laryngoscope. 1982;92(4):357-364.
146 Vandenbrouck C, Eschwege F, De la Rochefordiere A, Sicot H, Mamelle G, Le Ridant AM, et al. Squamous cell carcinoma of the pyriform sinus: retrospective study of 351 cases treated at the Institut Gustave-Roussy. Head Neck Surg. 1987;10(1):4-13.
147 Mendenhall WM, Parsons JT, Devine JW, Cassisi NJ, Million RR. Squamous cell carcinoma of the pyriform sinus treated with surgery and/or radiotherapy. Head Neck Surg. 1987;10(2):88-92.
148 Mendenhall WM, Parsons JT, Cassisi NJ, Million RR. Squamous cell carcinoma of the pyriform sinus treated with radical radiation therapy. Radiother Oncol. 1987;9(3):201-208.
149 Fu KK. The endolarynx and hypopharynx. In: Cox JD, editor. Moss radiation oncology: rationale, technique, results. ed 7. St. Louis: Mosby; 1994:214.
150 Dubois JB, Guerrier B, Di Ruggiero JM, Pourquier H. Cancer of the piriform sinus: treatment by radiation therapy alone and with surgery. Radiology. 1986;160(3):831-836.
151 Wang CC. Carcinoma of the hypopharynx. In: Wang CC, editor. Radiation therapy for head and neck neoplasms: indications, techniques, and results. ed 2. Chicago: Year Book Medical Publishers; 1990:207.
152 Freeman RB, Marks JE, Ogura JH. Voice preservation in treatment of carcinoma of the pyriform sinus. Laryngoscope. 1979;89(11):1855-1863.
153 Ogura JH, Jurema AA, Watson RK. Partial laryngopharyngectomy and neck dissection for pyriform sinus cancer. Conservation surgery with immediate reconstruction. Laryngoscope. 1960;70:1399-1417.
154 Ogura JH, Marks JE, Freeman RB. Results of conservation surgery for cancers of the supraglottis and pyriform sinus. Laryngoscope. 1980;90(4):591-600.
155 Laccourreye O, Merite-Drancy A, Brasnu D, Chabardes E, Cauchois R, Menard M, et al. Supracricoid hemilaryngopharyngectomy in selected pyriform sinus carcinoma staged as T2. Laryngoscope. 1993;103(12):1373-1379.
156 Marks JE, Kurnik B, Powers WE, Ogura JH. Carcinoma of the pyriform sinus. An analysis of treatment results and patterns of failure. Cancer. 1978;41(3):1008-1015.
157 Krespi YP, Sisson GA. Voice preservation in pyriform sinus carcinoma by hemicricolaryngopharyngectomy. Ann Otol Rhinol Laryngol. 1984;93(4 I):306-310.
158 Arriagada R, Eschwege F, Cachin Y, Richard JM. The value of combining radiotherapy with surgery in the treatment of hypopharyngeal and laryngeal cancers. Cancer. 1983;51(10):1819-1825.
159 Fletcher GH. Place of irradiation in the management of head and neck cancers. Semin Oncol. 1977;4(4):375-385.
160 Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR. Squamous cell carcinoma of the head and neck treated with irradiation: management of the neck. Semin Radiat Oncol. 1992;2(3):163-170.
161 Jesse RH, Lindberg RD. The efficacy of combining radiation therapy with a surgical procedure in patients with cervical metastasis from squamous cancer of the oropharynx and hypopharynx. Cancer. 1975;35(4):1163-1166.
162 Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation: the role of neck dissection for clinically positive neck nodes. Int J Radiat Oncol Biol Phys. 1986;12(5):733-740.
163 Nikolarakos D, Bell RB. Management of the node-positive neck in oral cancer. Oral Maxillofac Surg Clin N Am. 2008;20(3):499-511.
164 Stenson KM, Haraf DJ, Pelzer H, Recant W, Kies MS, Weichselbaum RR, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy: the feasibility of selective neck dissection. Arch Otolaryngol—Head Neck Surg. 2000;126(8):950-956.
165 Amdur RJ, Mendenhall WM, Stringer SP, Villaret DB, Cassisi NJ. Organ preservation with radiotherapy for T1-T2 carcinoma of the pyriform sinus. Head Neck. 2001;23(5):353-362.
166 Harrison LB, Zelefsky MJ, Armstrong JG, Carper E, Gaynor JJ, Sessions RB. Performance status after treatment for squamous cell cancer of the base of tongue—a comparison of primary radiation therapy versus primary surgery. Int J Radiat Oncol Biol Phys. 1994;30(4):953-957.
167 Million RR. Carcinomas of the larynx and hypopharynx. Curative treatment with preservation of laryngeal function. frontiers of radiation therapy and oncology. 1993;27:31-40.
168 Goepfert H, Lindberg RD, Jesse RH. Combined laryngeal conservation surgery and irradiation: can we expand the indications for conservation therapy. Otolaryngology—Head Neck Surg. 1981;89(6):974-978.
169 Sheed DP, Scatliff JA, Kirchner JA. A cineradiographic study of posteresectional alterations in oral pharyngeal physiology. Surg Gynecol Obstet. 1960;110:69.
170 Kramer S, Gelber RD, Snow JB, Marcial VA, Lowry LD, Davis LW, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73–03 of The Radiation Therapy Oncology Group. Head Neck Surg. 1987;10(1):19-30.
171 Tupchong L, Scott CB, Blitzer PH, Marcial VA, Lowry LD, Jacobs JR, et al. Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: Long-term follow-up of RTOG study 73–03. Int J Radiat Oncol Biol Phys. 1991;20(1):21-28.
172 Vandenbrouck C, Sancho H, Le Fur R. Results of a randomized clinical trial of preoperative irradiation versus postoperative in treatment of tumors of the hypopharynx. Cancer. 1977;39(4):1445-1449.
173 Hamby L, McGrath PC, Luce EA, Meeker W, Kenady DE. Optimizing primary treatment for advanced laryngeal and pyriform sinus carcinoma. Am J Surg. 1992;164(6):629-633.
174 Kokal WA, Neifeld JP, Eisert D, Lipsett JA, Lawrence WJr, Beatty JD, et al. Postoperative radiation as adjuvant treatment for carcinoma of the oral cavity, larynx, and pharynx: preliminary report of a prospective randomized trial. J Surg Oncol. 1988;38(2):71-76.
175 Frank JL, Garb JL, Kay S, McClish DK, Bethke KP, Lind DS, et al. Postoperative radiotherapy improves survival in squamous cell carcinoma of the hypopharynx. Am J Surg. 1994;168(5):476-480.
176 Chiu Ming H, Kam Hing L, Ignace Wei W, Po Wing Y, Lai Kun L. Squamous cell carcinoma of the hypopharynx—analysis of treatment results. Head Neck. 1993;15(5):405-412.
177 Schuller DE, Metch B, Mattox D, Stein DW, McCracken JD. Preoperative chemotherapy in advanced resectable head and neck cancer: Final report of the Southwest oncology group. Laryngoscope. 1988;98(11):1205-1211.
178 Schuller DE, Stein DW, Metch B. Analysis of treatment failure patterns. A southwest oncology group study. Arch Otolaryngol—Head Neck Surg. 1989;115(7):834-836.
179 Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, Sileni VC, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86(4):265-272.
180 Head and Neck Contracts Program. Adjuvant chemotherapy for advanced head and neck squamous carcinoma. Final report of the head and neck contracts program. Cancer. 1987;60(3):301-311.
181 Jacobs C, Makuch R. Efficacy of adjuvant chemotherapy for patients with resectable head and neck cancer: a subset analysis of the head and neck contracts program. J Clin Oncol. 1990;8(5):838-847.
182 Laramore GE, Scott CB, Al-Sarraf M, Haselow RE, Ervin TJ, Wheeler R, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: report on intergroup study 0034. Int J Radiat Oncol Biol Phys. 1992;23(4):705-713.
183 Wolf GT, Waun Ki H, Fisher SG, Urba S, Endicott JW, Close L, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. NEJM. 1991;324(24):1685-1690.
184 Lefebvre JL, Sahmoud T. Larynx preservation in hypopharynx squamous cell carcinoma: preliminary results of a randomized study (EORTC 24891). Proc Am Soc Clin Oncol. 1994;13:283.
185 Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European organization for research and treatment of cancer phase III trial. J Natl Cancer Inst. 1996;88(13):890-899.
186 Prades JM, Schmitt TM, Timoshenko AP, Simon PG, De Cornulier J, Durand M, et al. Concomitant chemoradiotherapy in pyriform sinus carcinoma. Arch Otolaryngol—Head Neck Surg. 2002;128(4):384-388.
187 Zelefsky MJ, Kraus DH, Pfister DG, Raben A, Shah JP, Strong EW, et al. Combined chemotherapy and radiotherapy versus surgery and postoperative radiotherapy for advanced hypopharyngeal cancer. Head Neck. 1996;18(5):405-411.
188 Kraus DH, Pfister DG, Harrison LB, Shah JP, Spiro RH, Armstrong JG, et al. Larynx preservation with combined chemotherapy and radiation therapy in advanced hypopharynx cancer. Otolaryngol Head Neck Surg. 1994;111(1):31-37.
189 Pfister DG, Strong E, Harrison L, Haines IE, Pfister DA, Sessions R, et al. Larynx preservation with combined chemotherapy and radiation therapy in advanced but resectable head and neck cancer. J Clin Oncol. 1991;9(5):850-859.
190 Clayman GL, Weber RS, Guillamondegui O, Byers RM, Wolf PF, Frankenthaler RA, et al. Laryngeal preservation for advanced laryngeal and hypopharyngeal cancers. Arch Otolaryngol—Head Neck Surg. 1995;121(2):219-223.
191 Shirinian MH, Weber RS, Lippman SM, Dimery IW, Earley CL, Garden AS, et al. Laryngeal preservation by induction chemotherapy plus radiotherapy in locally advanced head and neck cancer: the M.D. Anderson Cancer Center experience. Head Neck. 1994;16(1):39-44.
192 Jacobs C, Goffinet DR, Goffinet L, Kohler M, Fee WE. Chemotherapy as a substitute for surgery in the treatment of advanced resectable head and neck cancer. A report from the Northern California Oncology Group. Cancer. 1987;60(6):1178-1183.
193 Vikram B, Bosl GJ, Pfister D, Assad W, Strong EW, Spiro RH, et al. New strategies for avoiding total laryngectomy in patients with head and neck cancer. NCI Monogr. 1988;6(6):361-364.
194 Vikram B, Malamud S, Gold J, Nussbaum M, Kimmelman C, Lucente F, et al. Chemotherapy rapidly alternating with accelerated radiotherapy for advanced carcinomas of the hypopharynx and upper esophagus: a feasibility study. Head Neck. 1991;13(5):415-419.
195 Karp DD, Vaughan CW, Carter R, Willett B, Heeren T, Calarese P, et al. Larynx preservation using induction chemotherapy plus radiation therapy as an alternative to laryngectomy in advanced head and neck cancer. A long-term follow-up report. Am J Clin Oncol: Cancer Clinical Trials. 1991;14(4):273-279.
196 Taylor SG, Murthy AK, Vannetzel JM, Colin P, Dray M, Caldarelli DD, et al. Randomized comparison of neoadjuvant cisplatin and fluorouracil infusion followed by radiation versus concomitant treatment in advanced head and neck cancer. J Clin Oncol. 1994;12(2):385-395.
197 Urba SG, Forastiere AA, Wolf GT, Esclamado RM, McLaughlin PW, Thornton AF. Intensive induction chemotherapy and radiation for organ preservation in patients with advanced resectable head and neck carcinoma. J Clin Oncol. 1994;12(5):946-953.
198 Leyvraz S, Pasche P, Bauer J, Bernasconi S, Monnier P. Rapidly alternating chemotherapy and hyperfractionated radiotherapy in the management of locally advanced head and neck carcinoma: four-year results of a phase I/II study. J Clin Oncol. 1994;12(9):1876-1885.
199 Koch WM, Ding Jen L, Eisele DW, Miller D, Poole M, Cummings CW, et al. Chemoradiotherapy for organ preservation in oral and pharyngeal carcinoma. Arch Otolaryngol—Head Neck Surg. 1995;121(9):974-980.
200 Vokes EE, Weichselbaum RR. Measurable impact: multimodality therapy of head and neck cancer. Int J Radiat Oncol Biol Phys. 1993;27(2):481-482.
201 Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. NEJM. 350(19), 2004.
202 Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefëbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. NEJM. 2004;350(19):1945-1952.
203 Bernier J, Cooper JS, Pajak TF, Van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005;27(10):843-850.
204 Ang KK, Harris J, Garden AS, Trotti A, Jones CU, Carrascosa L, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99-14. J Clin Oncol. 2005;23(13):3008-3015.
205 Garden AS, Harris J, Trotti A, Jones CU, Carrascosa L, Cheng JD, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase ii trial of the radiation therapy oncology group (RTOG 99-14). Int J Radiat Oncol Biol Phys. 2008;71(5):1351-1355.
206 Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. NEJM. 2003;349(22):2091-2098.
207 Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24(17):2636-2643.
208 Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet. 2000;355(9208):949-955.
209 Pignon JP, le Maïtre A, Bourhis J. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an Update. Int J Radiat Oncol Biol Phys. 69(Suppl 2), 2007.
210 Adelstein DJ, Li Y, Adams GL, Wagner HJr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92-98.
211 Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. NEJM. 1998;338(25):1798-1804.
212 Al-Sarraf M, Pajak TF, Marcial VA. Concurrent radiotherapy and chemotherapy with cisplatin in inoperable squamous cell carcinoma of the head and neck: an RTOG study. Cancer. 1987;59(2):259-265.
213 Marcial VA, Pajak TF, Mohiuddin M, Cooper JS, Al Sarraf M, Mowry PA, et al. Concomitant cisplatin chemotherapy and radiotherapy in advanced mucosal squamous cell carcinoma of the head and neck. Long-term results of the Radiation Therapy Oncology Group Study 81-17. Cancer. 1990;66(9):1861-1868.
214 Coughlin CT, Richmond RC. Biologic and clinical developments of cisplatin combined with radiation: concepts, utility, projections for new trials, and the emergence of carboplatin. Semin Oncol. 1989;16(4 Suppl 6):31-43.
215 Harrison LB, Pfister DG, Fass DE, Armstrong JG, Sessions RB, Shah JP, et al. Concomitant chemotherapy-radiation therapy followed by hyperfractionated radiation therapy for advanced unresectable head and neck cancer. Int J Radiat Oncol Biol Phys. 1991;21(3):703-708.
216 Studer G, Luetolf UM, Davis JB, Glanzmann C. IMRT in hypopharyngeal tumors. Strahlentherapie und Onkologie. 2006;182(6):331-335.
217 Budach V, Stuschke M, Budach W, Baumann M, Geismar D, Grabenbauer G, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German cancer society 95–06 prospective randomized trial. J Clin Oncol. 2005;23(6):1125-1135.
218 Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. NEJM. 2007;357(17):1705-1715.
219 Parsons JT. Time-dose-volume relations in radiation therapy. In: Million RR, Cassisi NJ, editors. Management of head and neck cancer: a multidisciplinary approach. ed 2. Philadelphia: JB Lippincott; 1994:203.
220 Amdur RJ, Parsons JT, Mendenhall WM, Million RR, Stringer SP, Cassisi NJ. Postoperative irradiation for squamous cell carcinoma of the head and neck: an analysis of treatment results and complications. Int J Radiat Oncol Biol Phys. 1989;16(1):25-36.
221 Wilkins SA. Carcinoma of the posterior pharyngeal wall. Am J Surg. 1971;122(4):477-481.
222 Barzan L, Barra S, Franchin G, Talamini R, Zanelli G, Caruso G, et al. Squamous cell carcinoma of the posterior pharyngeal wall: characteristics compared with the lateral wall. J Laryngol Otol. 1995;109(2):120-125.
223 McNeill R. Surgical management of carcinoma of the posterior pharyngeal wall. Head Neck Surg. 1981;3(5):389-394.
224 Fein DA, Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR. Pharyngeal wall carcinoma treated with radiotherapy: impact of treatment technique and fractionation. Int J Radiat Oncol Biol Phys. 1993;26(5):751-757.
225 Zbaeren P, Weidner S, Thoeny HC. Laryngeal and hypopharyngeal carcinomas after (chemo)radiotherapy: a diagnostic dilemma. Curr Opinion Otolaryngol Head Neck Surg. 2008;16(2):147-153.
226 Brouwer J, Bodar EJ, De Bree R, Langendijk JA, Castelijns JA, Hoekstra OS, et al. Detecting recurrent laryngeal carcinoma after radiotherapy: room for improvement. European Archives of Oto-Rhino-Laryngology. 2004;261(8):417-422.
227 Terhaard CH, Bongers V, Van Rijk PP, Hordijk GJ. F-18-fluoro-deoxy-glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/pharyngeal cancer. Head Neck. 2001;23(11):933-941.
228 Zbaeren P, Christe A, Caversaccio MD, Stauffer E, Thoeny HC. Pretherapeutic staging of recurrent laryngeal carcinoma: clinical findings and imaging studies compared with histopathology. Otolaryngology—Head Neck Surg. 2007;137(3):487-491.
229 Hermans R, Pameljer FA, Mancuso AA, Parsons JT, Mendenhall WM. Laryngeal or hypopharyngeal squamous cell carcinoma: can follow-up CT after definitive radiation therapy be used to detect local failure earlier than clinical examination alone? Radiology. 2000;214(3):683-687.
230 Hermans R, Pameijer FA, Mancuso AA, Parsons JT, Mendenhall WM. CT findings in chondroradionecrosis of the larynx. Am J Neuroradiol. 1998;19(4):711-718.
231 Wong RJ, Lin DT, Schoeder H, Patel SG, Gonen M, Wolden S, et al. Diagnostic and prognostic value of [18F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol. 2002;20(20):4199-4208.
232 Goerres GW, Schmid DT, Bandhauer F, Huguenin PU, Von Schulthess GK, Schmid S, et al. Positron emission tomography in the early follow-up of advanced head and neck cancer. Arch Otolaryngol—Head Neck Surg. 2004;130(1):105-109.
233 Périé S, Hugentobler A, Susini B, Balogova S, Grahek D, Kerrou K, et al. Impact of FDG-PET to detect recurrence of head and neck squamous cell carcinoma. Otolaryngology—Head Neck Surg. 2007;137(4):647-653.
234 Lonneux M, Lawson G, Ide C, Bausart R, Remacle M, Pauwels S. Positron emission tomography with flourodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope. 2000;110(9):1493-1497.
235 Vandecaveye V, De Keyzer F, Nuyts S, Deraedt K, Dirix P, Hamaekers P, et al. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys. 2007;67(4):960-971.
236 Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43(6):828-836.
237 Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53-60.
238 Wong LY, Wei WI, Lam LK, Yuen APW. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck. 2003;25(11):953-959.
239 Pellini R, Pichi B, Ruscito P, Ceroni AR, Caliceti U, Rizzotto G, et al. Supracricoid partial laryngectomies after radiation failure: a multi-institutional series. Head Neck. 2008;30(3):372-379.
240 Shah JP, Loree TR, Kowalski L. Conservation surgery for radiation-failure carcinoma of the glottic larynx. Head Neck. 1990;12(4):326-331.
241 Lee N, Chan K, Bekelman JE, Zhung J, Mechalakos J, Narayana A, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):731-740.
242 Laughlin JS, Mohan R, Kutcher GJ. Choice of optimum megavoltage for accelerators for photon beam treatment. Int J Radiat Oncol Biol Phys. 1986;12(9):1551-1557.
243 Esik O, Burkelbach J, Boesecke R, Schlegel W, Nemeth G, Lorenz WJ. Three-dimensional photon radiotherapy planning for laryngeal and hypopharyngeal cancers. 2. Conformation treatment planning using a multileaf collimator. Radiother Oncol. 1991;20(4):238-244.
244 Van Mierlo IJM, Levendag PC, Eijkenboom WMH, Jansen PP, Meeuwis CA, Van Assendelft PJ, et al. Radiation therapy for cancer of the piriform sinus: a failure analysis. Am J Clin Oncol: Cancer Clinical Trials. 1995;18(6):502-509.
245 Andrew JW, Eapen L, Kulkarni NS. Homogeneous irradiation of the “short-necked” laryngeal cancer patient. Int J Radiat Oncol Biol Phys. 1984;10(4):549-553.
246 Sailer SL, Sherouse GW, Chaney EL, Rosenman JG, Tepper JE. A comparison of postoperative techniques for carcinomas of the larynx and hypopharynx using 3-D dose distributions. Int J Radiat Oncol Biol Phys. 1991;21(3):767-777.
247 Lee NY, O’Meara W, Chan K, Della-Bianca C, Mechalakos JG, Zhung J, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2007;69(2):459-468.
248 Milano MT, Vokes EE, Kao J, Jackson W, List MA, Stenson KM, et al. Intensity-modulated radiation therapy in advanced head and neck patients treated with intensive chemoradiotherapy: preliminary experience and future directions. Int J Oncol. 2006;28(5):1141-1151.
249 Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577-587.
250 Hunt MA, Jackson A, Narayana A, Lee N. Geometric factors influencing dosimetric sparing of the parotid glands using IMRT. Int J Radiat Oncol Biol Phys. 2006;66(1):296-304.
251 Eisbruch A, Levendag PC, Feng FY, Teguh D, Lyden T, Schmitz PIM, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys. 69(Suppl 2), 2007.
252 Devineni VR, Hayden R, Fredrickson J, Sicard G. Tolerance of gastric mucosal flap to postoperative irradiation. Laryngoscope. 1991;101(5):462-464.
253 Spiro RH, Bains MS, Shah JP, Strong EW. Gastric transposition for head and neck cancer: a critical update. Am J Surg. 1991;162(4):348-352.
254 Petruzzelli GJ, Johnson JT, Myers EN, Shestak K, Jones NF, Cano E, et al. The effect of postoperative radiation therapy on pharyngoesophageal reconstruction with free jejunal interposition. Arch Otolaryngol—Head Neck Surg. 1991;117(11):1265-1268.
255 Knee R, Fields RS, Peters LJ. Concomitant boost radiotherapy for advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1985;4(1):1-7.
256 Ang KK, Peters LJ, Weber RS, Maor MH, Morrison WH, Wendt CD, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19(6):1339-1345.
257 Schmidt-Ullrich RK, Johnson CR, Wazer DE, Masko G, Chasin WD, Karmody CS. Accelerated superfractionated irradiation for advanced carcinoma of the head and neck: concomitant boost technique. Int J Radiat Oncol Biol Phys. 1991;21(3):563-568.
258 Withers HR. Biologic basis for altered fractionation schemes. Cancer. 1985;55(Suppl 9):2086-2095.
259 Peters LJ, Kian Ang K. The role of altered fractionation in head and neck cancers. Semin Radiat Oncol. 1992;2(3):180-194.
260 Dische S. Radiotherapy—New fractionation schemes. Semin Oncol. 1994;21(3):304-310.
261 Son YH, Kacinski BM. Therapeutic concepts of brachytherapy/megavoltage in sequence for pharyngeal wall cancers: Results of integrated dose therapy. Cancer. 1987;59(7):1268-1273.
262 Son YH, Skomro-Dicker C. Crossing and looping methods in radical interstitial therapy for lateral and posterior oro-, hypopharyngeal tumors. Int J Radiat Oncol Biol Phys. 1983;9(3):401-405.
263 De Arruda FF, Puri DR, Zhung J, Narayana A, Wolden S, Hunt M, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64(2):363-373.
264 Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91(9):1785-1790.
265 Lee NK, Goepfert H, Wendt CD. Supraglottic laryngectomy for intermediate-stage cancer: M.D Anderson Cancer Center experience with combined therapy. Laryngoscope. 1990;100(8):831-836.
266 Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439.
267 Bataini JP, Brugere J, Jaulerry CH. Radiation treatment of lateral epilaryngeal cancer. Prognostic factors and results. Am J Clin Oncol: Cancer Clinical Trials. 1984;7(6):641-645.
268 Bataini JP. Head and neck cancer and the radiation oncologist (The ESTRO Regaud Lecture). Radiother Oncol. 1991;21(1):1-10.
269 Ahmad K, Fayos JV. High-dose radiation therapy in carcinoma of the pyriform sinus. Cancer. 1984;53(10):2091-2094.
270 Brugere J. Early pharyngolaryngeal carcinomas with palpable nodes. Am J Surg. 1991;162(4):377-380.
271 Garden AS, Morrison WH. Hyperfractionated radiation in the treatment of squamous cell carcinomas of the head and neck: a comparison of two fractionation schedules. Int J Radiat Oncol Biol Phys. 1995;31(3):493-502.
272 Carpenter RJIII, DeSanto LW. Cancer of the hypopharynx symposium on head neck surg II. Surg Clin N Am. 1977;57(4):723-735.
273 Razack MS, Sako K, Marchetta FC. Carcinoma of the hypopharynx: success and failure. Am J Surg. 1977;134(4):489-491.
274 Razack MS, Sako K, Kalnins I. Squamous cell carcinoma of the pyriform sinus. Head Neck Surg. 1978;1(1):31-34.
275 Eisbach KJ, Krause CJ. Carcinoma of the pyriform sinus a comparison of treatment modalities. Laryngoscope. 1977;87(11):1904-1910.
276 Driscoll WG, Nagorsky MJ, Cantrell RW, Johns ME. Carcinoma of the pyriform sinus: analysis of 102 cases. Laryngoscope. 1983;93(5):556-560.
277 Yates A, Crumley RL. Surgical treatment of pyriform sinus cancer: a retrospective study. Laryngoscope. 1984;94(12 I):1586-1590.
278 Pingree TF, Davis RK, Reichman O, Derrick L. Treatment of hypopharyngeal carcinoma: a 10-year review of 1,362 cases. Laryngoscope. 1987;97(8):901-904.
279 Byers RM, Krueger WWO, Saxton J. Use of surgery and postoperative radiation in the treatment of advanced squamous cell carcinoma of the pyriform sinus. Am J Surg. 1979;138(4):597-599.
280 Ho CM, Lam KM, Wei WI, Yuen PW, Lam LK. Squamous cell carcinoma of the hypopharynx—analysis of treatment results. Head Neck. 1993;15(5):405-412.
281 Slotman BJ, Kralendonk JH, Snow GB, Tiwari RM, Karim ABMF. Surgery and postoperative radiotherapy and radiotherapy alone in T3-T4 cancers of the pyriform sinus—treatment results and patterns of failure. Acta Oncologica. 1994;33(1):55-60.
282 Wang CC. Radiotherapeutic management of carcinoma of the posterior pharyngeal wall. Cancer. 1971;27(4):894-896.
283 Bryce D. The conventional surgical management of carcinoma of the hypopharynx. J Laryngol Otol. 1971;85(12):1221-1226.
284 Pene F, Avedian V, Eschwege F. A retrospective study of 131 cases of carcinoma of the posterior pharyngeal wall. Cancer. 1978;42(5):2490-2493.
285 Marks JE, Smith PG, Sessions DG. Pharyngeal wall cancer. A reappraisal after comparison of treatment methods. Arch Otolaryngol. 1985;111(2):79-85.
286 Hennessy TPJ, O’Connell R. Carcinoma of the hypopharynx, esophagus and cardia. Surg Gyneco Obstet. 1986;162(3):243-247.
287 Kajanti M, Mantyla M. Carcinoma of the hypopharynx. A retrospective analysis of the treatment results over a 25-year period. Acta Oncologica. 1990;29(7):903-907.