36 Cancer of the Esophagus
Epidemiology, Etiology, Genetics, and Cytogenetic Abnormalities
The epidemiology of esophageal cancer has changed during the past decade. There is an increasing incidence of adenocarcinoma, most commonly occurring at the gastroesophageal (GE) junction.1 In 2008, there were an estimated 16,479 new cases of esophageal cancer in the United States, with 12,970 occurring in men and 3500 occurring in women. Approximately 14,280 deaths are expected.2
Squamous Cell Cancer
In African-American men, the incidence is higher than the incidence in White men (16.8 versus 1.0 per 100,000).3 In most countries, the primary contributing factor is tobacco and alcohol abuse.4 Diet can have a protective benefit.5 For example, high intake of raw vegetables and fresh fruit (especially citrus) is associated with a decreased risk.
Adenocarcinoma
In most large referral centers, the incidence of adenocarcinoma of the gastroesophageal (GE) junction represents 60% to 80% of newly diagnosed patients, compared with 10% to 15% only 10 years ago.6 Data from the Surveillance, Epidemiology and End Results (SEER) Program database reveal that the incidence of adenocarcinoma in White men doubled between the early 1970s and the late 1980s. By contrast with squamous cell cancer, the male to female ratio is 7 : 1 and there is an increased incidence in Whites versus African Americans. In a Swedish population-based case–control study, Lagergren et al. reported a strong association between symptomatic gastroesophageal reflux and adenocarcinoma.7
Barrett’s esophagus is a metaplastic change of the lining of the esophagus whereby normal squamous epithelium is replaced by columnar epithelium.8 The pathophysiology, biology, and possible molecular events associated with Barrett’s has been extensively reviewed.9,10 There is strong evidence to support the theory that most adenocarcinomas develop from areas of Barrett’s esophagus.11 Antireflux medications, which relax the lower esophageal sphincter, may be a contributing factor. For example, Lagergren et al. reported that patients who used these agents for more than 5 years had an increased risk compared to those who never used them.12 Interestingly, only 10% of patients who have Barrett’s develop adenocarcinoma; however, most patients with adenocarcinoma have a history of Barrett’s. In fact, most patients with Barrett’s will die of other causes.
Although not as prevalent as reported in patients with squamous cell cancer, tobacco and alcohol abuse also increases the risk of adenocarcinoma.11 A multicenter case-controlled study identified excess weight (body mass index) as a risk factor.13 Likewise, a diet high in calories and fat is associated with an increased risk.14 Although Helicobacter pylori is a risk factor for gastric cancer, it does not appear to be associated with adenocarcinoma of the GE junction.15
Emerging data suggest that the development of esophagus cancer, as with many other epithelial-derived carcinomas, is a multistep process that involves a successive activation or deletion of genes and their protein products. This is mediated by regulatory genes.16–19 Markers such as cyclin D1 are associated with more aggressive disease.20 Activated transcription factor nuclear factor kB is associated with chemoresistance and poor prognosis.21 A comprehensive discussion of molecular genetics is beyond the scope of this chapter.
Anatomy
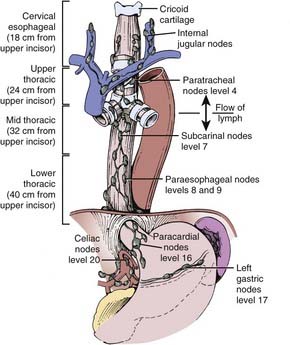
The esophagus is generally divided into three segments: cervical, thoracic, and abdominal (GE junction). The esophagus can also be classified as the upper third (proximal), middle third, and lower third (distal). Not only is there is no consensus as to the delineation between these segments, but there is overlap between the various definitions.22 Some definitions use the distance from the incisors (Fig. 36-1), whereas others use anatomic criteria such as the thoracic inlet and carina.
Pathology
As seen in Table 36-1, a variety of histologic types of tumors arise in the esophagus.23 The most common are squamous cell and adenocarcinoma. The location of the tumor also influences the histologic type. In the upper and middle third, the majority of cancers are squamous cell, whereas in the lower third they are usually adenocarcinomas. The treatment results discussed in this chapter will be limited to these two histologies. Given the increasing incidence of adenocarcinoma of the esophagus compared with squamous cell carcinoma, treatment results need to be examined by histology. At the time of writing, the data are conflicting, with some series reporting different results by histology whereas other series report no difference. Fortunately, the National Cancer Institute (NCI) Intergroup randomized trials are now stratified by histology. Until these stratified results are available, the impact of histology cannot be adequately assessed and it is reasonable to treat both histologies in a similar fashion.
NOS, Not otherwise specified.
Routes of Spread
The incidence of lymph node involvement increases with T-stage. The incidence is also related to the site of the primary tumor in the esophagus. Metastases from the cervical esophagus to the abdominal lymph nodes are unusual, whereas from the upper thoracic esophagus, metastases may occur in greater than 30% of patients. The relative incidence of metastasis is summarized in Fig. 36-2.24 Hematogenous metastases may occur in 25% to 30% of patients at the time of presentation and in as high as 50% of patients at the time of autopsy. The most common sites of metastasis are lung, liver, pleura, bone, kidney, and adrenal gland. Regardless of the anatomic location or histologic subtype, the median survival of patients with distant metastatic disease is 6 to 12 months.
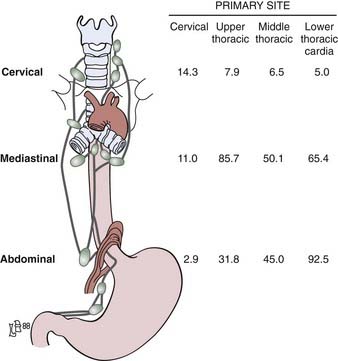
FIGURE 36-2 • Location of the lymph node involvement (%) by esophageal carcinoma is determined by site of primary tumor.
(From Sons HU, Borchard F: Cancer of the distal esophagus and cardia, Ann Surg 203:188, 1986; Akiyama H, Tsurumaro M, Kawamura T, et al: Principles of surgical treatment for carcinoma of the esophagus, Ann Surg 194:438, 1981.)
Diagnostic and Staging Studies
Diagnosis
The initial diagnosis is often made on upper endoscopy, which allows for delineation of the proximal and distal extent of the tumor and biopsy. The barium esophagram can also demarcate the proximal and distal margins as well as identify a tracheoesophageal fistula. It is helpful for correlation with simulation films. Computed tomography (CT) scan of the chest and abdomen is essential for staging, because it can identify extension beyond the esophageal wall, enlarged lymph nodes, and visceral metastases. Endoscopic ultrasound is highly accurate in determining depth of invasion as well as lymph node involvement.25 Patients with tumors arising or extending above the level of the carina should also be referred for a bronchoscopy to evaluate for a tracheoesophageal fistula. For cervical primaries, a head and neck examination should include palpation of the Level I to IV lymph nodes as well as visualization of all possible mucosal sites of a synchronous primary. A neck CT should be performed to evaluate for cervical lymph node involvement.
Recent studies have examined the effectiveness of 18fluorodeoxyglucose positron-emission tomography (FDG-PET) in the staging of esophageal cancer. Following standard staging for esophageal cancer (including CT and endoscopy) undetected metastatic disease was detected by PET in 15% of patients in the series by Flamen et al.26 and 20% in the series by Downey and associates.27 Others have confirmed the utility of PET in staging, response to preoperative therapy, and prognosis.28–30 and should be included in the standard work-up.
Staging
The most accurate staging is pathologic.31 The American Joint Commission on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC) TNM staging systems are similar. Although the length of the primary tumor (greater than or less than 5 cm) is prognostic, the 5th edition of the AJCC staging system no longer includes length (Table 36-2). Similar to the staging system used for gastric and colorectal cancers, the T stage is based on the extension of the primary tumor through the wall.
Since many patients are treated with combined modality therapy (CMT) alone or preoperatively, clinical staging, although less accurate, may understage patients. Rizk et al. report that the AJCC staging system does not accurately predict survival in patients receiving neoadjuvant therapy.32
One of the most controversial areas is the delineation between adenocarcinomas of the GE junction and the stomach. These cancers commonly involve both the esophagus and stomach. There is no standard approach how to define the organ of origin. The most common (and practical) definition is based on where the majority of the tumor is. Whichever organ has the largest percentage of tumor is considered the primary site. Siewert and colleagues proposed a topographic anatomic classification system to differentiate three types of tumors arising in this region. They distinguish an adenocarcinoma of the distal esophagus (AEG Type I) which may infiltrate the GE junction from above from an adenocarcinoma of the gastric cardia (AEG Type II) and both of these are distinct from a subcardial gastric carcinoma the infiltrates the GE junction from below (AEG Type III).33
Standard Therapeutic Approaches
There is considerable controversy as to the ideal therapeutic approach. In the 1996 to 1999 U.S. Patterns of Care Study, 414 patients who received radiation therapy as part of definitive or adjuvant management at 59 institutions were surveyed.34,35 Overall, 51% had adenocarcinoma and 49% squamous cell carcinoma. With a median follow-up of 8 months, multivariate analysis revealed that patients who received CMT followed by surgery had a significant decrease in locoregional recurrence (HR: 0.40, P < .0001) and survival (HR: 0.32, P < .001) compared with those who did not undergo surgery. A similar significant decrease in locoregional recurrence (HR: 1.36, P = .01) and survival (HR: 1.32, P < .03) was seen in those patients who received their care at large radiation oncology centers (those that treat ≥500 new cancer patients per year) compared with small centers (those that treat <500 new cancer patients per year). In a similar pattern of care study of 767 patients treated in Japan from 1998 to 2001, 220 (28%) received preoperative or postoperative radiation or both, with or without chemotherapy.35 The National Comprehensive Cancer Network (NCCN) offers general guidelines for therapy.36
Techniques of Radiation Therapy
General Techniques
Although CT can identify adjacent organs and structures, it may be limited in defining the extent of the primary tumor. To assess the consistency of target volume delineation, Tai and colleagues sent sample cases with CT scans to 48 radiation oncologists throughout Canada and asked them to complete questionnaires regarding treatment techniques as well as outline the boost target volumes.37 There was substantial inconsistency in defining the planning target volume, both in the transverse and longitudinal dimensions. The integration of other imaging modalities in radiation treatment planning such as esophageal ultrasound, PET scan, and magnetic resonance imaging (MRI) are under active investigation.
Leong and colleagues from the Peter MacCallum Cancer Center have demonstrated that the addition of PET/CT information for treatment planning improved the identification of the gross tumor volume (GTV).38 The GTV based on CT information alone excluded PET-avid disease in 11 of 16 patients (69%), five of whom would have had a geographic miss of the gross tumor.
The standard radiation dose for patients selected for CMT is 50.4 Gy at 1.8 Gy per fraction.39 Randomized data from France reveal a higher local control (57% versus 29%) and 2-year survival rate (37% versus 23%) with continuous-course compared with split-course radiation.40 The radiation field should include the primary tumor with 5-cm superior and inferior margins and 2-cm lateral margins. The primary local/regional lymph nodes should receive the same dose. For cervical (proximal) primary tumors (defined as at or proximal to the carina) the treatment volume includes the bilateral supraclavicular nodes and for GE junction (distal) primaries the celiac axis nodes need to be included.
Treatment by Primary Site
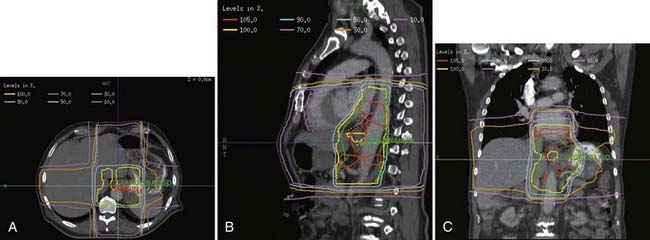
For cervical primaries, patients are placed supine. Various field designs are possible and their choice depends on the geometry of the primary tumor in relation to the spinal cord. The ideal design is a three-field technique (two anterior obliques and a posterior). However, since the primary tumor is rarely limited to the midline, the most common approach is anteroposterior (AP)/posteroanterior (PA) to 39.6 to 41.4 Gy (Fig. 36-3) followed by a left or right opposed oblique pair with photons to 50.4 Gy (Fig. 36-4A-C). Since this technique will exclude the ipsilateral supraclavicular fossa, a separate electron field is added (commonly to a depth of 2 to 3 cm depending on the patient’s anatomy) thereby bringing the total dose to 50.4 Gy.
FIGURE 36-3 • Contours for a proximal esophageal cancer. Planning target volume (PTV) (blue) and gross tumor volume (red).
For distal (GE junction) primaries, patients are treated supine using the same four-field technique. With a CT simulation, sometimes it is difficult to distinguish normal stomach from the primary tumor. In this setting, the extent of tumor involvement on the staging PET/CT scan as well as the endoscopy report may help to define the caudal extent of the tumor. The greater curvature of the stomach is commonly used for the anastomosis. Therefore, for patients receiving preoperative treatment, care should be taken to exclude as much of the normal stomach as possible. An example of this technique is seen in Fig. 36-5A-B.
Investigators at the M.D. Anderson performed retrospective treatment planning studies on 10 patients and found that IMRT decreased the dose-volume of exposed normal lung but had no clinically meaningful differences on the irradiated volumes of spinal cord, heart, liver, or total body integral doses.41 The impact of respiratory and organ movement on defining target volumes is just starting to be identified.42 Hong et al. provide a comprehensive review of technical considerations in treatment planning.43 Examples are seen in Fig. 36-6A-C.
Critical Normal Tissues
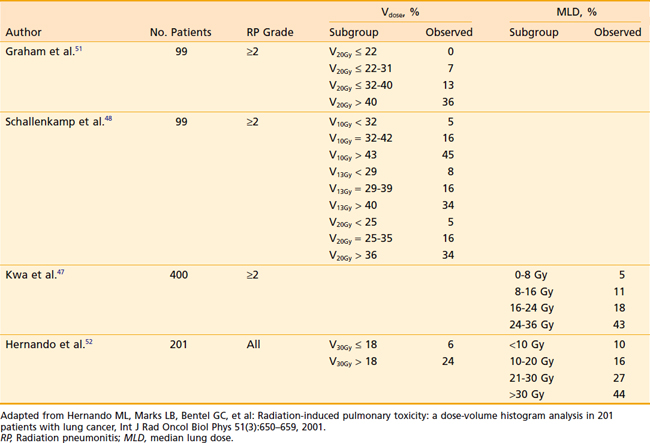
Acute and subacute toxicity due to thoracic radiotherapy are clearly linked to the dose and volume of lung treated. However, the acceptable level of dose to the lung in the combined modality setting has not been defined. Various single parameters have been proposed to estimate the probability of developing radiation pneumonitis after radiotherapy 44–52 Investigators from the Netherlands47 compared different normal tissue complication probability (NTCP) models to predict radiation pneumonitis. Using the observed incidence of radiation pneumonitis among breast cancer, malignant lymphoma, and inoperable non–small cell lung cancer (NSCLC) patients, they found that the underlying local dose–effect relation for radiation pneumonitis was linear. This was better represented by the mean lung dose (MLD) model than by a step-function model represented by a threshold dose such as the volume receiving 20 Gy (V20). In their patient population, the MLD was the most accurate predictor for the incidence of radiation pneumonitis. Willner and colleagues performed an analysis of pneumonitis risk from DVH parameters among patients treated with three-dimensional conformal radiotherapy (3DCRT).45 Their data indicated that it is reasonable to disperse the dose outside the target volume over large areas in order to reduce the volumes of lung receiving >40 Gy. They found that reducing the high-dose volume reduces the pneumonitis rate more than a corresponding reduction in the low-dose regions of the DVH. Investigators from Memorial Sloan-Kettering Cancer Center found that grade 3+ radiation pneumonitis was significantly correlated with MLD and Fdam (an estimate of the fractional volume of lung damaged during treatment) for patients treated to higher doses (range: 57.6 Gy to 81 Gy) for NSCLC.46 Graham et al. reported that among patients treated with radiation therapy alone for NSCLC, the risk of grade 2+ pneumonitis was 0% when the V20 was <22%, 7% when the V20 was 22% to 31%, 13% when the V20 was 32% to 40%, and 36% when V20 was >40%.50 Table 36-3 summarizes the radiation pneumonitis risks associated with dosimetric criteria, V20 and MLD in several studies. Based on these studies and others, the Radiation Therapy Oncology Group (RTOG) adopted the cut-offs of a V20 ≤37% and MLD ≤20 Gy to identify patients at high risk for radiation pneumonitis in studies of NSCLC.
Most of these parameters are derived from studies of definitive radiation CMT rather than pre- or postoperative treatment. Postoperative pulmonary complications may be an even more relevant endpoint for patients with esophageal cancer, because the impact of radiotherapy on lung function may be greater in patients who undergo a thoracotomy and further insult to the lungs during surgical resection. One recent study showed that dosimetric differences impact clinical outcomes in the combined modality setting, with preoperative lung dose an important predictor of postoperative pulmonary complications. In the treatment of esophageal cancer using a neoadjuvant regimen of 45 Gy with concurrent chemotherapy, a lung V10 of 40% or greater, and a V15 of 30% or greater, was shown to be predictive of significantly greater pulmonary complications.53 Several other publications have evaluated the effect of low dose radiotherapy on the risk of pneumonitis and postoperative pulmonary complications. It appears likely that normal tissue tolerance in neoadjuvant therapy (i.e., preoperative CMT followed by surgical resection) may differ from that seen with definitive radiation alone, and even relatively low doses when delivered to a sufficient percentage of lung volume may be less tolerable when given preoperatively with concurrent chemotherapy.
Outcome
Surgery
Surgery is one of the two standard therapies for clinically resectable esophageal cancer. Two randomized trials of preoperative chemotherapy (fluorouracil [5-FU]/cisplatin over two cycles) have been performed. The INT 0113 trial revealed no survival advantage.54,55 Although the Medical Research Council (MRC) trial did show a 10% survival advantage, 9% of patients received chemotherapy plus radiation.56 In the surgical control arm from INT 0113, the median survival was 15 months and the 5-year survival was 20%. Major surgical controversies include the operative approach (transthoracic or Ivor-Lewis esophagectomy versus transhiatial esophagectomy) and the extent of resection. Because of high complication rates, three-field dissection is rarely performed in Western countries.57 Likewise, a minority of surgeons from Western countries advocate en bloc resections. Although some phase II trials suggest improved survival with an en bloc resection, this may be due to selection bias, since this operation allows more-pathologically-accurate staging. In a recent series of 111 patients (of which 10% also had preoperative adjuvant therapy), the 5-year survival of the 67 patients with node-positive disease was 26%, which is similar to the surgical control arm of INT 0113.58
Nonsurgical Therapy
Radiation Therapy Alone
Many historical series have reported results of external beam radiation therapy (EBRT) alone. Most include patients with unfavorable features, such as clinical T4 disease. Overall, the 5-year survival rate for patients treated with conventional doses of radiation therapy alone is 0% to 10%.59–61 Shi and colleagues reported a 33% 5-year survival rate with the use of late course accelerated fractionation to a total dose of 68.4 Gy.62 However, in the “radiation therapy alone” arm of the RTOG 85-01 trial, in which patients received 64 Gy at 2 Gy/day with conventional techniques, all patients were dead of disease by 3 years.63,64 There is limited experience with the use of radiation in the curative setting for patients with cT1N0 disease. Sai and colleagues from Kyoto University treated 34 patients who were either medically inoperable or refused surgery with either EBRT alone (64 Gy) or EBRT (52 Gy) plus 8 to 12 Gy with brachytherapy.65 With a median follow-up of 61 months, 5-year results were 59% survival, 68% local-relapse-free survival, and 80% cause-specific survival. Treating a similar population of 63 patients with CMT plus brachytherapy, Yamada et al. reported 66% survival, 64% disease-free survival, and 76% cause-specific survival at 5 years.66
Combined Modality Therapy
Six randomized trials compare radiation therapy alone with CMT.63,67–73 Of the six trials, five used suboptimal doses of radiation and three have used inadequate doses of systemic chemotherapy. The only trial which was designed to deliver adequate doses of systemic chemotherapy with concurrent radiation therapy was the RTOG 85-01 trial reported by Herskovic et al. (Fig. 36-7).63,70,73 This intergroup trial primarily included patients with squamous cell carcinoma. Patients received four cycles of 5-FU (1000 mg/m2/day during 4 days) and cisplatin (75 mg/m2 on day 1). Radiation therapy (50 Gy at 2 Gy/day) was given concurrently with day 1 of chemotherapy. Curiously, cycles 3 and 4 of chemotherapy were delivered every 3 weeks (weeks 8 and 11) rather than every four weeks (weeks 9 and 13). This intensification may explain, in part, why only 50% of the patients finished all four cycles of the chemotherapy. The control arm was radiation therapy alone, albeit a higher dose (64 Gy) than the CMT arm.
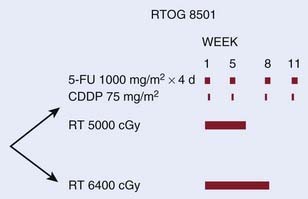
FIGURE 36-7 • Phase III Intergroup trial RTOG 8501 for patients with squamous cell and adenocarcinoma of the esophagus selected for a nonoperative approach.46,56,59
Patients who were randomized to receive CMT had a significant improvement in both median (14 months versus 9 months), and 5-year survival (27% versus 0%, P < .0001).70 With a minimum follow-up of 5 years, the 8-year survival was 22%.73 Histology did not significantly influence the results with 21% of the 107 patients with squamous cell carcinomas alive at 5 years compared with 13% of the 23 patients with adenocarcinoma (P = NS). The incidence of local failure as the first site of failure (defined as local persistence plus recurrence) was also lower in the CMT arm (47% versus 65%). The protocol was closed early because of the positive results. Although African Americans had larger primary tumors of which all were squamous cell cancers, there was no difference in survival compared with Whites.74
Intensification of the Radiation Dose
Brachytherapy
Brachytherapy has been used both as primary therapy (usually as a palliative modality)75–79 as well as boost following EBRT or CMT.75,80–83 It can be delivered by high dose rate or low dose rate.84 Although there are technical and radiobiologic differences between the two dose rates, there are no clear therapeutic advantages.
Series that combine brachytherapy with EBRT or CMT report results similar to those for conventional CMT. Yorozu et al. reported a local failure rate of 57% and a 5-year actuarial survival of 28% in 46 patients with stage T2-3N0-1M0 disease.85 Even for a more favorable subset of patients with clinical T1-2 disease, Yorozu et al. reported a local failure rate of 44% and a 5-year survival of 26%,86 and in the series by Pasquier and associates, local failure was 23% and 5-year survival was 36%.87
Brachytherapy alone is as a palliative modality and results in a local control rate of 25% to 35% and a median survival of approximately 5 months.75–78 In the randomized trial from Sur et al., there was no significant difference in local control or survival with high dose rate brachytherapy compared with EBRT.76
A major limitation of brachytherapy is the effective treatment distance. The primary isotope is iridium-192 (192Ir), which is usually prescribed to treat to a distance of 1 cm from the source. Therefore, any portion of the tumor which is >1 cm from the source will receive a suboptimal radiation dose. This limitation has been confirmed by pathologic analysis of treated specimens.88
Series which combine brachytherapy with external beam or CMT report similar results to conventional CMT. Calais et al. reported a local failure rate of 43% and a 5-year actuarial survival of 18%.80 Even with the more favorable subset of patients with clinical T1-2 disease Yorozu et al. reported a local failure rate of 44% and a 5-year survival of 26%.86
In the RTOG 92-07 trial, 75 patients with squamous cell cancers (92%) or adenocarcinomas (8%) of the thoracic esophagus received the RTOG 85-01 CMT regimen (5-FU/cisplatin/50 Gy) followed by a boost during cycle 3 of chemotherapy with either low dose rate or high dose rate intraluminal brachytherapy.89 The choice of the dose rate was at the discretion of the investigator. Due to low accrual, the low dose rate option was discontinued and the analysis was limited to patients who received the high dose rate treatment. High dose rate brachytherapy was delivered in weekly fractions of 5 Gy during weeks 8, 9, and 10. Following the development of several fistulas, the fraction delivered at week 10 was discontinued.
Although the complete response rate was 73%, with a median follow-up of only 11 months, local failure as the first site of failure was 27%. Acute toxicity included 58% grade 3, 26% grade 4, and 8% grade 5 (treatment-related death). The cumulative incidence of fistula was 18%/year and the crude incidence was 14%. Of the six treatment-related fistulas, three were fatal. Given the significant toxicity this treatment approach should be used with caution. A recursive partitioning analysis of pretreatment variables of 416 patients treated on RTOG 85-01 and 92-07 did not identify any novel prognostic information which could be used as stratification variables in future CMT trials.90
The American Brachytherapy Society has developed guidelines for esophageal brachytherapy.91 Their recommendations include the following: for patients treated in the curative setting brachytherapy should be limited to tumors ≤10 cm with no evidence of distant metastasis. Contraindications include tracheal or bronchial involvement, cervical esophagus location, or stenosis which cannot be bypassed. The applicator should have an external diameter of 6 to 10 cm. If CMT is used (defined as 5-FU based chemotherapy plus 45 to 50 Gy) the recommended doses of brachytherapy are 10 Gy in 2 weekly fractions of 5 Gy each for high dose rate and 20 Gy in a single fraction at 4 to 10 Gy/hour for low dose rate. The doses should be prescribed to 1 cm from the source. Lastly, brachytherapy should be delivered after the completion of external beam and not concurrently with chemotherapy.
External Beam Radiation
Based on the tolerability of higher doses of external beam radiation (64.8 Gy) in the Intergroup 0122 trial, this dose was used in the experimental arm of the intergroup esophageal trial INT 0123 (RTOG 9405).39 INT 0123 was the follow-up trial to RTOG 8501. In this trial, patients with either squamous cell (85%) or adenocarcinomas (15%) selected for a nonsurgical approach were randomized to a slightly modified RTOG 85-01 combined modality regimen with 50.4 Gy versus the same chemotherapy with 64.8 Gy (Fig. 36-8).
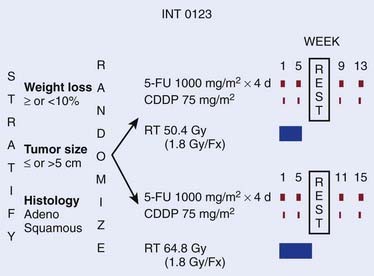
FIGURE 36-8 • Phase III Intergroup trial 0123 (RTOG 94-05) for patients with squamous cell or adenocarcinoma of the esophagus.36
For the 218 eligible patients, there was no significant difference in median survival (13.0 months versus 18.1 months), 2-year survival (31% versus 40%), or local/regional failure and/or local/regional persistence of disease (56% versus 52%) between the high dose and standard dose arms.39 Although 11 treatment-related deaths occurred in the high dose arm compared with two in the standard dose arm, seven of the 11 occurred in patients who had received ≤50.4 Gy.
In addition to increasing the total dose, radiation can be intensified by accelerated or hyperfractionation. Zhao and associates treated 201 patients with squamous cell cancer using 41.4 Gy followed by late-course accelerated hyperfractionation to 68.4 Gy.92 The results were similar to RTOG 85-01 (38% local failure and 26% 5-year survival). Choi et al. treated 46 patients with 5-FU/cisplatin and twice-daily radiation using a concurrent boost technique and reported a 37% 5-year survival.93 Although these approaches are reasonable, most series report an increase in acute toxicity without any clear therapeutic benefit. These regimens remain investigational.
Despite retrospective data from the M.D. Anderson Cancer Center suggest a positive correlation between radiation dose and locoregional control.94 Based on results of the INT 0123 trial, the standard dose of external-beam radiation remains 50.4 Gy.
Neoadjuvant chemotherapy before radiation therapy or CMT has also been examined. Brenner and colleagues reported results of neoadjuvant CPT-11/cisplatin in weeks 1, 2, 3, and 5 before CMT, with the majority of patients having relief of dysphagia.95 Another potential advantage of neoadjuvant chemotherapy is the early identification of those patients who may or may not respond to the chemotherapeutic regimen being delivered. Ilson et al. showed that the change in standard uptake value (SUV) on FDG-PET scan was able to predict which patients showed a response to the full course of chemotherapy.96 Although this approach is investigational, if the nonresponders can be identified early, changing the chemotherapeutic regimen may be helpful. Weider and associates reported similar findings in 38 patients with squamous cell cancers.97
Palliation of Dysphagia With Radiation Therapy
Dysphagia is a common problem in patients with esophageal cancer. A major weakness of the series examining palliation is they are retrospective and most do not use objective criteria to define and assess dysphagia (Table 36-4). There are a number of options for palliation such as stents, feeding tubes, chemotherapy, and EBRT or brachytherapy (or both). The selection of the technique is variable and is commonly based on physician preference. In a randomized trial from the Dutch SIREC Study Group of stent versus 1 × 12 Gy fraction of brachytherapy, dysphagia, as measured by a variety of quality of life scales, improved more rapidly after stent placement. However, long-term relief was superior after brachytherapy.98 Median survivals were similar (145 versus 155 days).
Table 36-4 Randomized Trials of Preoperative Combined Modality Therapy for Esophageal Cancer
| Trial | Significant Survival Benefit | Comments |
|---|---|---|
| University of Michigan131 | No | 15% survival benefit but not significant |
| Walsh et al.129 | Yes | Only 6% 5-yr survival with surgery alone |
| EORTC130 | No (+disease-free survival) | Split-course radiation and unconventional chemotherapy schedule |
| Australasian133 | No | Only 35 Gy |
| Seoul134 | No | 1.2 Gy twice-daily radiation |
| CALGB 9781135 | Yes | Only 56 of the planned 475 patients entered |
EORTC, European Organization for Research and Treatment of Cancer; CALGB, Cancer and Leukemia Group B.
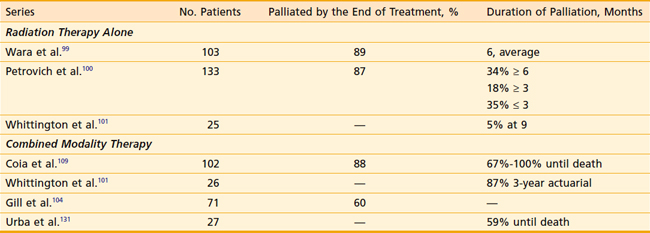
As seen in Table 36-5, a limited number of series have examined the palliative benefits with either radiation alone68,99–102 or CMT.101,103–108 Overall, EBRT alone offers palliation of dysphagia in approximately 70% to 80% of patients.
The most comprehensive analysis of swallowing function in 102 patients receiving CMT is from Coia et al.109 Before the start of therapy, 95% of patients had some degree of dysphagia. Within 2 weeks following the start of treatment, 45% had improvement in dysphagia, and by the completion of the 6-week therapy, 83% had improvement. Overall, 88% had an improvement in dysphagia. The median time to maximum improvement was 4 weeks (range: 1 to 21 weeks) and all but two patients could swallow at least soft or solid foods at the time of maximum symptomatic improvement. Even in patients treated in the noncurative setting, 91% had an initial improvement in swallowing and 67% were palliated until the time of their death. Harvey and colleagues treated 106 patients and reported that 78% had improvement of at least one grade in their dysphagia score and 51% maintained swallowing improvement until the time of last follow-up.108
Intraluminal brachytherapy is also an effective albeit more limited method of palliation. It achieves palliation of dysphagia in 40% to 90% of patients.75–78 As previously discussed, since it is usually prescribed to 1 cm from the source it may underdose gross disease. There is a selection bias against brachytherapy since it is commonly used for patients who have either failed EBRT or who are medically unable to travel for daily outpatient treatment. Even accounting for these selection biases, given its limited effective range, it is usually not as successful as EBRT beam in treating the entire tumor volume.
Acute and Long-Term Toxicity of Radiation Therapy
The most carefully documented acute radiation-related toxicity data are from the control arm of RTOG 85-01, where patients received radiation therapy alone to a dose of 64 Gy.63,70. The incidence of acute grade 3 toxicity was 25% and grade 4 toxicity was 3%. There were no treatment-related deaths. The incidence of long-term grade 3+ toxicity was 23% and grade 4+ was 2%.73 In the control arm of INT 0123 (which was similar to the CMT arm of RTOG 85-01), the incidence of acute grade 3 toxicity was 43% and grade 4 toxicity was 26%. There were no treatment-related deaths. The incidence of long-term grade 3+ toxicity was 24% and grade 4+ was 13%.39 As with surgery, radiation therapy can produce esophageal strictures. The incidence of benign stricture (in the absence of local recurrence) was 12%.109
Treatment-Related Deaths
The incidence of treatment-related death with modern CMT was only 2% in RTOG 850163 and INT 0123 trials.39 This compares favorably with the 6% reported for the surgery-alone control arm in INT 0113.54
Comparison of Radiation and Surgical Therapies
Although there are a number of trials comparing preoperative CMT with surgery alone, only two trials directly compare nonoperative treatment with surgery. One randomized trial compared surgery with radiation alone110 and one compared surgery with CMT.111 Both series have small numbers of patients and limited follow-up, and neither report a difference in survival.
Necessity for Surgery after Chemoradiation
Two randomized trials examine whether surgery is necessary after CMT. In the Federation Francaise de Cancerologie Digestive (FFCD) 9102 trial, 445 patients with clinically resectable T3-4N0-1M0 squamous cell or adenocarcinoma of the esophagus received initial CMT.112 Patients initially received two cycles of 5-FU, cisplatin, and concurrent radiation (either 46 Gy at 2 Gy/day or split-course 15 Gy weeks 1 and 3).112 The 259 patients who had at least a partial response were then randomized to surgery versus additional CMT, which included three cycles of 5-FU, cisplatin, and concurrent radiation (either 20 Gy at 2 Gy/day or split-course 15 Gy). There was no significant difference in 2-year survival (34% versus 40%, P = .56) or median survival (18 months versus 19 months) in patients who underwent surgery versus additional CMT. These data suggest that for patients who initially respond to CMT, they should complete CMT rather than stop and undergo surgery. Using the Spitzer index, there was no difference in global quality of life. However, a significantly greater decrease in quality of life was observed in the surgery arm during the postoperative period (7.52 versus 8.45, P < .01, respectively).113
The German Oesophageal Cancer Study Group compared preoperative CMT followed by surgery versus CMT alone.114 In this trial, 172 eligible patients <70 years old with uT3-4N0-1M0 squamous cell cancers of the esophagus were randomized to preoperative therapy (three cycles of 5-FU, leucovorin, etoposide, and cisplatin, followed by concurrent etoposide, cisplatin, plus 40 Gy) followed by surgery versus CMT alone (the same chemotherapy but the radiation dose was increased to 60 to 65 Gy with or without brachytherapy). The pathologic complete response (PCR) rate was 33%. Despite a decrease in 2-year local failure (36% versus 58%, respectively; P = .003) there was no significant difference in 3-year survival (31% versus 24%, respectively) for those who were randomized to preoperative CMT followed by surgery versus CMT alone.
In summary, although there is good rationale for its use, it is not clear that the combination of surgery and CMT, regardless of the sequence, improves the survival results of either treatment alone. An alternative approach is to use “selective” surgery after preoperative CMT. Swisher et al. reported a retrospective analysis of patients who underwent a salvage compared with a planned esophagectomy at the M.D. Anderson Cancer Center from 1987 to 2000.115 The operative mortality was higher in those who underwent salvage versus planned surgery (15% versus 6%, respectively) but there was no difference in survival (25%). Since only 13 patients were identified who had salvage, the results need to be interpreted with caution. The RTOG 0241 phase II trial is prospectively examining the approach of preoperative paclitaxel/CDDP and 50.4 Gy followed by selective surgery.
Treatment in the Setting of a Tracheoesophageal Fistula
The presence of a malignant tracheoesophageal fistula is an unfavorable prognostic feature. Although the survival of such patients is poor, occasionally they may survive for a prolonged period of time. In the historical literature, radiation therapy was considered contraindicated for fear of exacerbating the fistula as the tumor responded. More recently there have been reports to the contrary. In the Mayo Clinic series, ten patients with a malignant tracheoesophageal fistula received 30 to 66 Gy EBRT, and the median survival was 5 months.116 Most importantly, none of the patients experienced an enlarging or more-debilitating fistula following radiation. In a case report, a patient who developed a fistula while receiving EBRT continued treatment to a total dose of 56.5 Gy, and it healed 2 months following the completion of radiation.117 Although the experience is very limited, the data suggest that radiation does not necessarily increase the severity of a malignant tracheoesophageal fistula, and it may be administered safely. Due to the poor prognosis of this group of patients, it is unclear whether it improves outcome.
Adjuvant Therapy
Adjuvant Radiation Therapy Without Chemotherapy
Preoperative Radiation Therapy
There are six randomized trials of preoperative radiation therapy for patients with clinically resectable disease.69,118–122 Overall, preoperative radiation therapy did not increase the resectability rate. The only series to show a significant improvement in survival was from Nygaard and associates; however, patients also received chemotherapy.69 A recent meta-analysis from the Oesphageal Cancer Collaborative Group also showed no clear evidence of a survival advantage with preoperative radiation.123
Postoperative Radiation Therapy
Despite encouraging nonrandomized reports of postoperative radiation therapy,124 there are only two randomized trials, which were limited to patients treated in the adjuvant setting.125,126 In the series from Teniere et al., there was a significant decrease in local failure in patients with negative nodes.125 However, neither reported a survival advantage. Although there are not clear data to support it, the only role for postoperative radiation therapy is for patients with positive margins. In this setting, it is reasonable to combine systemic chemotherapy with radiation.
Preoperative Combined Modality Therapy
Nonrandomized Trials
Most of the trials report PCR rates of approximately 25%. Furthermore, the mortality rate is not increased compared with surgery alone.127,128 Some report higher rates as well as 3-year survival rates superior to surgery. Despite these encouraging results, this approach needs to be confirmed in randomized trials.
Randomized Trials
Six randomized trials compare preoperative CMT with surgery alone in patients with clinically resectable disease (see Table 36-4).129–131 The series from Le Prise et al. is not included, because patients received sequential rather than concurrent chemotherapy plus radiation.132
Urba and associates from the University of Michigan randomized 100 patients (75% with adenocarcinoma) to preoperative cisplatin, vinblastine, 5-FU, and concurrent radiation therapy (1.5 Gy twice daily to 45 Gy) followed on day 42 by a transhiatial esophagectomy versus surgery alone. In the most recent update there was a significant decrease in local recurrence (19% versus 42%, respectively); however, the survival advantage at 3 years (30% versus 15%, respectively) did not reach statistical significance.131
In the series from Walsh et al., 113 patients with adenocarcinoma of the mid or distal esophagus (including the cardia) were randomized to two cycles of 5-FU/cisplatin plus concurrent preoperative radiation therapy (2.67 Gy/day to 40 Gy) versus surgery alone.129 There was a significant improvement in both median survival (16 months versus 11 months, P = .01) and 3-year survival (32% versus 6%, respectively; P = .01). The major criticism of this trial is the high operative mortality rate of 9% and the low 3-year survival (6%) in the surgical control arm.
The third randomized trial of preoperative CMT was reported by Bosset et al. from the European Organization for Research and Treatment of Cancer (EORTC).130 A total of 282 patients with clinically resectable squamous cell carcinomas were randomized to preoperative CMT versus surgery alone. The unconventional preoperative regimen included 3.7 Gy × 5 followed by a 2-week rest (split-course) and another 3.7 Gy/day × 5. Chemotherapy was limited to low-dose cisplatin 0 to 2 days before (not concurrent with) radiation therapy. Patients who received preoperative CMT had a significantly greater 3-year disease-free survival (40% versus 28%, respectively) and local-disease-free survival rates (relative risk: 0.6); however, they had no improvement in median survival (19 months) or overall 3-year survival (36%) compared with surgery alone.
The Australasian gastrointestinal trials group randomized 256 patients (61% with adenocarcinoma) to preoperative cisplatin day 1, followed by 5-FU days 2 to 5 with concurrent 35 Gy at 2.33 Gy/day) versus surgery alone.133 Comparing preoperative CMT versus surgery alone, the median overall survival was 22 months versus 19 months, respectively (P = .38). For patients with squamous cell cancer, there was a significant increase in relapse-free survival, but that did not translate into a survival advantage. There was no advantage for adenocarcinomas.
A similar trial was reported from Korea. A total of 102 patients with squamous cell cancer were randomized to surgery alone versus preoperative therapy with 45.6 Gy (1.2 Gy twice daily) plus 5-FU/cisplatin.134 There was no difference in median survival (28 versus 27 months, respectively).
The CALGB 9781 trial was designed to accrue 475 patients. A total of 56 patients (75% adenocarcinomas) were randomized to surgery versus preoperative therapy with 50.4 Gy plus 5-FU/cisplatin.135 Despite the small numbers, there was a significant improvement in 5-year survival with preoperative therapy (39% versus 16%, respectively).
In summary, the majority of the trials do not suggest a benefit in survival with preoperative CMT. However, all have limited patient numbers and heterogeneous treatment regimens, and in some the dose of radiation may be insufficient, based on a dose–response analysis by Geh et al.136 Despite all of these limitations, a recent meta-analysis does suggest a survival benefit.137 Overall, there may be a 5% to 10% survival benefit; however, this would require a much larger randomized trial to prove.
It would be helpful to predict tumors that have a higher likelihood of responding to radiation or CMT. Data from both Berger et al.138 and Rohatgi et al.139 suggest that patients who achieve a PCR had an improvement in survival compared to those who do not (5-year survival: 48% versus 15%, respectively; median: 133 months versus 34 months, respectively). However, the ability to predict a PCR before surgery is variable. Yang and associates at the M.D. Anderson Cancer Center found that patients with a negative posttreatment biopsy had a higher chance of achieving a PCR (33%) compared with those who did not have a negative biopsy (7%). A multivariate analysis by Gaca and colleagues reported that posttreatment nodal status (P = .03) but not the degree of primary tumor response predicted disease-free survival.140
Posttreatment imaging does not consistently identify response. Ultrasound following CMT does not accurately predict a PCR.141 By contrast, Levine et al. reported that percent decrease in SUV measured by 18FDG-PET predicted response21 and Brucher and colleagues found that it correlated with survival.142
The predictive ability of molecular markers have been examined. In 38 patients with squamous cell carcinoma who received CMT with or without surgery, tumors with lymphocytic infiltration had a significantly lower survival compared to those patients whose tumors did not have lymphocytic infiltration.143 Understanding the mechanism of radioresistance through identification and targeting of molecular pathways by serum protein profiling,144 and identification of genes involved in apoptosis,145 activated transcription factor nuclear factor kB,21 microvascular density,146 may offer new opportunities for therapeutic advances.
New Chemotherapeutic Agents
Phase I/II trials have been developed examining the use of newer chemotherapeutic agents such as paclitaxel,147–149 capecitabine,147 docetaxel,150 irinotecan,95,151 herceptin,152 oxaliplatin,153,154 bevacizumab,155 and cetuximab,156 and are being used as the foundations for new regimens. The RTOG randomized phase II trial E-0113 compared paclitaxel plus cisplatin, with or without 5-FU.157 Whether these investigational approaches offer improved results compared to conventional CMT regimens based on 5-FU and cisplatin is not known. The development of the ideal regimens and schedules remains an active area of clinical investigation.
New Radiation Treatment Modalities
There is very limited experience with intraoperative radiation as an alternative to EBRT.158 The impact of protons has been presented by Japanese investigators. Sugahara and colleagues treated 46 patients with squamous cell cancers using protons with or without photons to a median total dose of 76 Gy.159 The 5-year local control rate was T1: 83%; T2-4:29%; and survival T1:55%; T2-4:13%. Koyama and Tsujii reported mean actuarial survival rates of 60% for patients with superficial and 39% for those with advanced disease treated to mean total doses of 78 to 81 Gy.160 The incidence of esophageal ulcer was 67%. These approaches remain investigational.
1 Daly JD, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562-583.
2 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
3 Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289.
4 Brown LM, Hoover R, Silverman D. Excess incidence of squamous cell esophagus cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001;153:114-122.
5 Launoy G, Milan C, Day NE, et al. Diet and squamous cell cancer of the oesophagus: a French multicenter case-control study. Int J Cancer. 1998;76:7-12.
6 Pera M, Pera M. Recent changes in the epidemiology of esophageal cancer. Surg Onc. 2001;10:81-90.
7 Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New Engl J Med. 1999;340:825-831.
8 Spechler SJ, Goyal RK. The columnar-lined esophagus, intestinal metaplasia, and normal Barrets. Gastroenterology. 1996;110:614-621.
9 DeMeester TR. Clinical biology of Barrett’s metaplasia, dysplasia to carcinoma sequence. Surg Onc. 2001;10:91-102.
10 Wijnhoven BP, Tilanus HW, Dinjens WN. Molecular biology of Barrett’s adenocarcinoma. Ann Surg. 2001;233:322-337.
11 Gammon MD, Schoenberg JB, Ahsan H. Tobacco, alcohol, and socioeconomic status and adenocarcinoma of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277-1284.
12 Lagergren J, Bergstrom R, Adami HO, et al. Association between medications that relax the lower esophageal sphincter and risk for esophageal cancer. Ann Int Med. 2000;133:227-229.
13 Chow WH, Blot WJ, Vaughan TL. Body mass index and risk of adenocarcinoma of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;87:104-109.
14 Zhang ZF, Kurtz RC, Yu GP, et al. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutrit Cancer. 1997;27:298-309.
15 Huang JQ, Subbaramiah S, Chen Y, et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169-1179.
16 Stein HJ, Feith M. Cancer of the esophagus. In: Gospodarowicz M, editor. Prognostic factors in cancer. New York: Wiley-Liss; 2001:211-221.
17 Stein HJ, Brucher BLDM, Sendler A, et al. Esophageal cancer: patient evaluation and pre-treatment staging. Surg Onc. 2001;10:103-111.
18 Ireland AP, Shibata DK, Chandrasoma P, et al. Clinical significance of p53 mutations in adenocarcinoma of the esophagus and cardia. Ann Surg. 2000;231:179-187.
19 Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554-568.
20 Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317-324.
21 Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kB with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748-754.
22 Stein HJ, Sendler A, Fink U, et al. Multidisciplinary approach to esophageal and gastric cancer. Surg Clin North Am. 2000;80:659-682.
23 Begin LR. The pathobiology of esophageal cancer. In: Roth JA, Ruckdeschel JC, Weisenburger TH, editors. Thoracic oncology. Philadelphia: W.B. Saunders; 1995:288-335.
24 Sons HU, Borchard F. Cancer of the distal esophagus and cardia. Ann Surg. 1981;203:188-200.
25 Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479.
26 Flamen P, van Cutsem E, Lerut T, et al. The utility of positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) to predict the pathologic response and survival of esophageal cancer after preoperative chemoradiation therapy (CRT). Proc ASCO. 2001;20:127a.
27 Downey RJ, Ilson D, Koong H, et al. 18FDG PET measures the pathologic response of esophageal cancer to induction therapy. Proc ASCO. 2001;20:127a.
28 Blackstock AW, Farmer MR, Lovato J, et al. A prospective evaluation of the impact of 18-F-fluoro-deoxy-d-glucose positron emission tomography staging on survival for patients with locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2006;64:455-460.
29 Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagus. J Clin Oncol. 2006;24:4692-4698.
30 Konski A, Doss M, Milestone B, et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1123-1128.
31 American Joint Committee on Cancer. Esophagus. In: Fleming ID, Cooper JS, Henson DE, et al, editors. AJCC cancer staging manual. Philadelphia: Lippincott-Raven; 1997:65-70.
32 Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507-512.
33 Siewert JR, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction. Results of surgical therapy based on anatomic-topographic classification in 1002 consecutive patients. Ann Surg. 2000;232:353-361.
34 Suntharalingam M, Moughhan J, Coia LR, et al. Outcome results of the 1996–1999 patterns of care survey of the national practice for patients receiving radiation therapy for carcinoma of the esophagus. J Clin Oncol. 2005;23:2325-2331.
35 Kenjo M, Oguchi M, Gomi K, et al. Radiation therapy for esophageal cancer: results of the patterns of care study in Japan 1995–1997. Esophagus. 2005;2:77-83.
36 Ajani J, Bekaii-Saab T, D’Amico TA, et al. Esophageal cancer. J Natl Comp Canc Netw. 2006;4:328-347.
37 Tai P, van Dyk J, Yu E, et al. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;42:277-288.
38 Leong T, Everitt C, Yuen K, et al. A prospective study to evaluate the impact of FDG-PET on CT-based radiotherapy treatment planning for oesophageal cancer. Radiother Oncol. 2006;78:254-261.
39 Minsky BD, Pajak T, Ginsberg RJ, et al. INT 0123 (RTOG 94-05) phase III trial of combined modality therapy for esophageal cancer: high dose (64.8 Gy) vs. standard dose (50.4 Gy) radiation therapy. J Clin Oncol. 2002;20:1167-1174.
40 Jacob JH, Seitz JF, Langlois C, et al. Definitive concurrent chemo-radiation therapy (CRT) in squamous cell carcinoma of the esophagus (SCCE): preliminary results of a French randomized trial comparing standard vs. split course irradiation (FNCLCC-FFCD 9305). Proc ASCO. 1999;18:270a.
41 Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol. 2005;77:247-253.
42 Lorchel F, Dumas JL, Noel A, et al. Esophageal cancer: determination of internal target volume for conformal radiotherapy. Radiother Oncol. 2006;80:327-332.
43 Hong TS, Crowley EM, Killoran J, et al. Considerations in treatment planning for esophageal cancer. Sem Radiat Oncol. 2006;17:53-61.
44 Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys. 2003;55:724-735.
45 Willner J, Jost A, Baier K, et al. A little to a lot or a lot to a little? An analysis of pneumonitis risk from dose-volume histogram parameters of the lung in patients with lung cancer treated with 3-D conformal radiotherapy. Strahlenther Onkol. 2003;179:548-556.
46 Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:329-339.
47 Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1-9.
48 Schallenkamp J, Miller R, Brinkmann D, et al. Incidence of radiation pneumonitis after thoracic irradiation; dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410-416.
49 Wang S, Liao Z, Wej X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66:1399-1407.
50 Kong F, Hayman J, Griffith K, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075-1086.
51 Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329.
52 Hernando ML Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Rad Oncol Biol Phys. 2001;51(3):650-659.
53 Lee HK, Vaporciyan A, Cox JD, et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys. 2003;57:1317-1322.
54 Kelsen DP, Ginsberg R, Pajak T, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. New Engl J Med. 1998;339:1979-1984.
55 Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719-3725.
56 Clark PI. Medical Research Council randomised trial of surgery with or without pre-operative chemotherapy in resectable cancer of the oesophagus (MRC Upper GI Tract Cancer Group). Ann Oncol. 2000;11:4.
57 Nishimaki T, Suzuki T, Suzuki S, et al. Outcomes of extended radical esophagectomy for thoracic esophageal cancer. J Am Coll Surg. 1998;186:306-312.
58 Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581-587.
59 De-Ren S. Ten-year follow-up of esophageal cancer treated by radical radiation therapy: analysis of 869 patients. Int J Radiat Oncol Biol Phys. 1989;16:329-334.
60 Newaishy GA, Read GA, Duncan W, et al. Results of radical radiotherapy of squamous cell carcinoma of the esophagus. Clin Radiol. 1982;33:347-352.
61 Okawa T, Kita M, Tanaka M, et al. Results of radiotherapy for inoperable locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 1989;17:49-54.
62 Shi X, Yao W, Liu T. Late course accelerated fractionation in radiotherapy of esophageal carcinoma. Radiother Oncol. 1999;51:21-26.
63 Herskovic A, Martz LK, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. New Engl J Med. 1992;326:1593-1598.
64 Al-Sarraf M, Martz K, Herskovic A, et al. Superiority of chemo-radiotherapy (CT-RT) vs radiotherapy (RT) in patients with esophageal cancer. Final report of an Intergroup randomized and confirmed study. Proc ASCO. 1996;15:206.
65 Sai H, Mitsumori M, Arai K, et al. Long-term results of definitive radiotherapy for stage I esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;62:1339-1344.
66 Yamada K, Murakami M, Okamoto Y, et al. Treatment results of chemoradiotherapy for clinical stage I (T1N0M0) esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:1106-1111.
67 Araujo CMM, Souhami L, Gil RA, et al. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67:2258-2261.
68 Roussel A, Jacob JH, Jung GM, et al. Controlled clinical trial for the treatment of patients with inoperable esophageal carcinoma: a study of the EORTC gastrointestinal tract cancer cooperative group. In: Schlag P, Hohenberger P, Metzger U, editors. Recent results in cancer resecrch. Berlin: Springer-Verlag; 1988:21-30.
69 Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104-1110.
70 Al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277-284.
71 Slabber CF, Nel JS, Schoeman L, et al. A randomized study of radiotherapy alone versus radiotherapy plus 5-fluorouracil and platinum in patients with inoperable, locally advanced squamous cell cancer of the esophagus. Am J Clin Oncol (CCT). 1998;21:462-465.
72 Smith TJ, Ryan LM, Douglass HO, et al. Combined chemoradiotherapy vs. radiotherapy alone for early stage squamous cell carcinoma of the esophagus: a study of the Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:269-276.
73 Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer. Long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA. 1999;281:1623-1627.
74 Streeter OE, Martz KL, Gaspar LE, et al. Does race influence survival for esophageal cancer patients treated on the radiation and chemotherapy arm of RTOG # 85-01? Int J Radiat Oncol Biol Phys. 1999;44:1047-1052.
75 Moni J, Armstrong JG, Minsky BD, et al. High dose rate intraluminal brachytherapy for carcinoma of the esophagus. Dis Esoph. 1996;9:123-127.
76 Sur RK, Singh DP, Sharma SC. Radiation therapy of esophageal cancer: role of high dose rate brachytherapy. Int J Radiat Oncol Biol Phys. 1992;22:1043-1046.
77 Jager J, Langendijk H, Pannebakker M, et al. A single session of intraluminal brachytherapy in palliation of esophageal cancer. Radiother Oncol. 1995;37:237-240.
78 Sur RK, Donde B, Levin VC, et al. Fractionated high dose rate intraluminal brachytherapy in palliation of advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;40:447-453.
79 Maingon P, d’Hombres A, Truc G, et al. High dose rate brachytherapy for superficial cancer of the esophagus. Int J Radiat Oncol Biol Phys. 2000;46:71-76.
80 Calais G, Dorval E, Louisot P, et al. Radiotherapy with high dose rate brachytherapy boost and concomitant chemotherapy for stages IIB and III esophageal carcinoma: results of a pilot study. Int J Radiat Oncol Biol Phys. 1997;38:769-775.
81 Akagi Y, Hirokawa Y, Kagemoto M, et al. Optimum fractionation for high-dose-rate endoesophageal brachytherapy following external irradiation of early stage esophageal cancer. Int J Radiat Oncol Biol Phys. 1999;43:525-530.
82 Schraube P, Fritz P, Wannenmacher MF. Combined endoluminal and external irradiation of inoperable oesophageal carcinoma. Radiother Oncol. 1997;44:45-51.
83 Okawa T, Dokiya T, Nishio M, et al. Multi-institutional randomized trial of external radiotherapy with and without intraluminal brachytherapy for esophageal cancer in Japan. Int J Radiat Oncol Biol Phys. 1999;45:623-628.
84 Caspers RJL, Zwinderman AH, Griffioen G, et al. Combined external beam and low dose rate intraluminal radiotherapy in oesophageal cancer. Radiother Oncol. 1993;27:7-12.
85 Yorozu A, Toya K, Dokiya T. Long-tern results of concurrent chemoradiotherapy followed by high dose rate brachytherapy for T2-3N0-1M0 esophageal cancer. Esophagus. 2006;3:1-5.
86 Yorozu A, Dokiya T, Oki Y, et al. Curative radiotherapy with high-dose-rate brachytherapy boost for localized esophageal carcinoma: dose-effect relationship of brachytherapy with the balloon type applicator system. Radiother Oncol. 1999;51:133-139.
87 Pasquier D, Mirabel X, Adenis A, et al. External beam radiation therapy followed by high-dose-rate brachytherapy for inoperable superficial esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:1456-1461.
88 Sur M, Sur R, Cooper K, et al. Morphologic alterations in esophageal squamous cell carcinoma after preoperative high dose rate intraluminal brachytherapy. Cancer. 1996;77:2200-2205.
89 Gaspar LE, Qian C, Kocha WI, et al. A phase I/II study of external beam radiation, brachytherapy and concurrent chemotherapy in localized cancer of the esophagus (RTOG 92-07): preliminary toxicity report. Int J Radiat Oncol Biol Phys. 1997;37:593-599.
90 Thomas CR, Berkey BA, Minsky BD, et al. Recursive partitioning analysis of pretreatment variables of 416 patients with locoregional esophageal cancer treated with definitive concomitant chemoradiotherapy on Intergroup and Radiation Therapy Oncology Group trials. Int J Radiat Oncol Biol Phys. 2004;58:1405-1410.
91 Gaspar LE, Nag S, Herskovic A, et al. American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Int J Radiat Oncol Biol Phys. 1997;38:127-132.
92 Zaho KL, Shi XH, Jiang GL, et al. Late course accelerated hyperfractionated radiotherapy for localized esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2004;60:123-129.
93 Choi N, Park SD, Lynch T, et al. Twice-daily radiotherapy as concurrent boost technique during chemotherapy cycles in neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: mature results of a phase II study. Int J Radiat Oncol Biol Phys. 2004;60:111-122.
94 Zhang Z, Liao Z, Jin J, et al. Dose response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:656-664.
95 Brenner B, Ilson DH, Minsky BD, et al. Phase I trial of combined modality therapy for localized esophageal cancer: escalating doses of continuous infusion paclitaxel with cisplatin and concurrent radiation therapy. J Clin Oncol. 2004;22:45-52.
96 Ilson DH, Bains M, Rizk NP, et al. Phase II trial of preoperative cisplatin-irinotecan followed by concurrent cisplatin-irinotecan and radiotherapy: PET scan after induction therapy may identify early treatment failure. Proc ASCO. 2006;24:184s.
97 Wieder HA, Brucher BLDM, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900-908.
98 Homs MYV, Essink-Bot ML, Borsboom GJJM, et al. Quality of life after palliative treatment for oesophageal carcinoma—a prospective comparison between stent placement and single dose chemotherapy. Eur J Cancer. 2004;40:1862-1871.
99 Wara WM, Mauch PM, Thomas AN, et al. Palliation for carcinoma of the esophagus. Radiology. 1976;121:717-720.
100 Petrovich Z, Langholz B, Formenti S, et al. Management of carcinoma of the esophagus: the role of radiotherapy. Am J Clin Oncol (CCT). 1991;14:80-86.
101 Whittington R, Coia LR, Haller DG, et al. Adenocarcinoma of the esophagus and esophago-gastric junction: the effects of single and combined modalities on the survival and patterns of failure following treatment. Int J Radiat Oncol Biol Phys. 1990;19:593-603.
102 Caspers RJL, Welvaart K, Verkes RJ, et al. The effect of radiotherapy on dysphagia and survival in patients with esophageal cancer. Radiother Oncol. 1988;12:15-23.
103 Algan O, Coia LR, Keller SM, et al. Management of adenocarcinoma of the esophagus with chemoradiation alone or chemoradiation followed by esophagectomy: results of sequential nonrandomized phase II studies. Int J Radiat Oncol Biol Phys. 1995;32:753-761.
104 Gill PG, Denham JW, Jamieson GG, et al. Patterns of treatment failure and prognostic factors associated with the treatment of esophageal carcinoma with chemotherapy and radiotherapy either as sole treatment of followed by surgery. J Clin Oncol. 1992;10:1037-1043.
105 Urba SG, Turrisi AT. Split-course accelerated radiation therapy combined with carboplatin and 5-fluorouracil for palliation of metastatic or unresectable carcinoma of the esophagus. Cancer. 1995;75:435-439.
106 Seitz JF, Giovannini M, Padaut-Cesana J, et al. Inoperable nonmetastatic squamous cell carcinoma of the esophagus managed by concomitant chemotherapy (5-fluorouracil and cisplatin) and radiation therapy. Cancer. 1990;66:214-219.
107 Izquierdo MA, Marcuello E, Gomez de Segura G, et al. Unresectable nonmetastatic squamous cell carcinoma of the esophagus managed by sequential chemotherapy (cisplatin and bleomycin) and radiation therapy. Cancer. 1993;71:287-292.
108 Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esoph. 2004;17:260-265.
109 Coia LR, Soffen EM, Schultheiss TE, et al. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer. 1993;71:281-286.
110 Yu J, Ren R, Sun X, et al. A randomized clinical study of surgery versus radiotherapy in the treatment of resectable esophageal cancer. Proc ASCO. 2006;24:181s.
111 Chiu PWY, Chan ACW, Leung SF, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE). J Gastrointest Surg. 2005;9:794-802.
112 Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cell cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-1168.
113 Bonnetain F, Bedenne L, Michel P, et al. Definitive results of a comparative longitudinal quality of life study using the Spitzer index in the randomized multicentric phase III trial FFCD 9102 (surgery vs. radiochemotherapy in patients with locally advanced esophageal cancer). Proc ASCO. 2003;22:250.
114 Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-2317.
115 Swisher SG, Hofsetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005;241:810-820.
116 Gschossmann JM, Bonner JA, Foote RL, et al. Malignant tracheoesophageal fistula in patients with esophageal cancer. Cancer. 1993;72:1513-1521.
117 Arlington A, Bohorquez J. Irradiation of carcinoma of the esophagus containing a tracheoesophageal fistula. Cancer. 1993;71:3808-3812.
118 Launois B, Delarue D, Campion JP, et al. Preoperative radiotherapy for carcinoma of the esophagus. Surg Gynecol & Obstet. 1981;153:690-692.
119 Arnott SJ, Duncan W, Kerr GR, et al. Low dose preoperative radiotherapy for carcinoma of the oesophagus: results of a randomized clinical trial. Radiother Oncol. 1993;24:108-113.
120 Huang GJ, Gu XZ, Wang LJ, et al. Combined preoperative irradiation and surgery for esophageal carcinoma. In: Delarue NC, editor. International trends in general thoracic surgery. St. Louis: C.V. Mosby; 1988:315-318.
121 Mei W, Xian-Zhi G, Weibo Y, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: report on 206 patients. Int J Radiat Oncol Biol Phys. 1989;16:325-327.
122 Gignoux M, Roussel A, Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study of the E.O.R.T.C. World J Surg. 1987;11:426-432.
123 Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy in esophageal carcinoma: a meta-analysis using individual patient data (oesophageal cancer collaborative group). Int J Radiat Oncol Biol Phys. 1998;41:579-583.
124 Yamamoto M, Yamashita T, Matsubara T, et al. Reevaluation of postoperative radiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:75-78.
125 Teniere P, Hay J-M, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. Surg Gynecol & Obstet. 1991;173:123-130.
126 Fok M, Sham JST, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled trial. Surg. 1993;113:138-147.
127 Ruol A, Portale G, Castoro C, et al. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2007;14:3243-3250.
128 Rizk NP, Bains M, Flores R, et al. Impact of pre-operative chemoradiotherapy on post-esophagectomy morbidity and mortality. Proc ASCO. 2006;24:184s.
129 Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. New Engl J Med. 1996;335:462-467.
130 Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous cell cancer of the esophagus. New Engl J Med. 1997;337:161-167.
131 Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313.
132 Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779-1784.
133 Burmeister BH, Smithers BM, Fitzgerald L, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668.
134 Lee JL, Kim SB, Jung HY, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947-954.
135 Tepper JE, Krasna MJ, Niedzwieki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092.
136 Geh JI, Bond SJ, Bentzen SM, et al. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: evidence of a radiation and chemotherapy dose response. Radiother Oncol. 2006;78:236-244.
137 Urschel JD, Vasan H. A meta-analysis of randomized controlled clinical trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2002;185:538-543.
138 Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330-4337.
139 Rohatgi PR, Swisher SG, Correa AM, et al. Histologic subtypes as determinants of outcome in esophageal carcinoma patients with pathologic complete response after preoperative chemoradiotherapy. Cancer. 2005;106:552-558.
140 Gaca JG, Petersen RP, Peterson BL, et al. Pathologic nodal status predicts disease-free survival after neoadjuvant chemoradiation for gastroesophageal junction carcinoma. Ann Surg Oncol. 2006;13:340-346.
141 Kalha I, Kaw M, Fukami N, et al. The accuracy of endoscopic ultrasound for restaging esophageal carcinoma after chemoradiation therapy. Cancer. 2004;101:940-947.
142 Brucher BLDM, Becker K, Lordick F, et al. The clinical impact of histopathologic response assessment by residual tumor cell quantification in esophageal squamous cell carcinomas. Cancer. 2006;106:2119-2127.
143 Morita M, Kuwano H, Araki K, et al. Prognostic significance of lymphocytic infiltration following preoperative chemoradiotherapy and hyperthermia for esophageal cancer. Int J Radiat Oncol Biol Phys. 2001;49:1259-1266.
144 Hayashida Y, Honda K, Osaka Y, et al. Possible prediction of chemoradiosensitivity of esophageal cancer by serum protein profiling. Clin Cancer Res. 2005;11:8042-8047.
145 Chang JY, Zhang X, Komaki R, et al. Tumor-specific apoptotic gene targeting overcomes radiation resistance in esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2006;64:1482-1494.
146 Nomiya T, Nemoto K, Miyachi H, et al. Relationships between radiosensitivity and microvascular density in esophageal carcinoma: significance of hypoxic fraction. Int J Radiat Oncol Biol Phys. 2005;58:589-596.
147 Czito BG, Kelsey CR, Hurwitz HI, et al. A phase I study of capecitabine, carboplatin, and paclitaxel with external beam radiation therapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:1002-1007.
148 Henry LR, Goldberg M, Scott W, et al. Induction cisplatin and paclitaxel followed by combination chemoradiotherapy with 5-fluorouracil, cisplatin, and paclitaxel before resection in localized esophageal cancer: a phase II report. Ann Surg Oncol. 2006;13:214-220.
149 Roof KS, Coen J, Lynch TL, et al. Concurrent cisplatin, 5-FU, paclitaxel, and radiation therapy in patients with locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1120-1128.
150 Font A, Garcia-Alfonso P, Arellano A, et al. Preoperative combined multimodal therapy with docetaxel plus 5-fluorouracil and concurrent hyperfractionated radiotherapy for locally advanced esophageal cancer. Proc ASCO. 2002;21:128.
151 Ilson DH, Bains M, Kelsen DP, et al. Phase I trial of escalating-dose irinotecan given weekly with cisplatin and concurrent radiotherapy in locally advanced esophageal cancer. J Clin Oncol. 2003;21:2926-2932.
152 Safran H, DiPetrillo T, Nadeem A, et al. Neoadjuvant herceptin, paclitaxel, cisplatin, and radiation for adenocarcinoma of esophagus: a phase I study. Proc ASCO. 2002;21:141a.
153 O’Connor B, Chadha MK, Pande A, et al. Concurrent oxaliplatin, 5-fluorouracil, and radiotherapy in the treatment of locally advanced esophageal carcinoma. Cancer J. 2007;13:119-124.
154 Chiarion-Sileni V, Corti L, Innocente R, et al. Oxaliplatin (OX) and leucovorin (L) combined with protracted- infusion fluorouracil (F) and radiation (XRT) in locally advanced esophageal cancer (LAEC): a multicentric phase II study. Proc ASCO. 2005;22:325s.
155 Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or esophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206.
156 Safran H, Suntharalingam M, DiPetrillo T, et al. Cetuximab with concurrent chemoradiation for esophogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys. 2008;70:391-395.
157 Komaki R, Winter K, Ajani J, et al. A randomized phase II study of two paclitaxel-based chemoradiotherapy regimens for patients with the non-operative esophageal carcinoma (RTOG 0113). Int J Radiat Oncol Biol Phys. 2006;66:174.
158 Hosokawa M, Shirato H, Ohara K, et al. Intraoperative radiation therapy to the upper mediastinum and nerve-sparing three-field lymphadnectomy followed by external beam radiotherapy for patients with thoracic esophageal carcinoma. Cancer. 1999;86:6-13.
159 Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys. 2005;61:76-84.
160 Koyama S, Hirohiko T. Proton beam therapy with high dose irradiation for superficial and advanced esophageal carcinomas. Clin Cancer Res. 2003;9:3577-3582.