Chapter 43 Cancer of the Esophagus
The diagnosis and management of esophageal cancer is currently the focus of considerable clinical investigation. In the presence of localized disease, advances in the operative and postoperative management and rational applications of multimodality therapy have failed to significantly improve survival rates over the last 25 years.1 In this chapter, we will review recent changes in the histologic composition of esophageal cancers and how this may relate to tumor biologic and epidemiologic factors. We will also discuss the evolving impact of 18-fluorodeoxyglucose positron emission tomography (FDG-PET) for the staging and treatment of esophageal cancer. We will review the rationale for novel combined-modality approaches and the results of clinical trials testing both single-modality and combined-modality therapies, as well as the use of palliative therapies for disease that cannot be cured. We will conclude with a summary of the data and the current treatment recommendations and a discussion of several important clinical trials in progress.
Etiology and Epidemiology
Esophageal cancer is a highly aggressive neoplasm. In 2010, 16,640 Americans were diagnosed with esophageal cancer, representing a 14.6% increase in incidence over the previous 5 years. Approximately 87% of patients died of their disease; this fact indicates that no significant improvement in outcome has been seen over the same time period.1,2 Local invasion and early metastases are common in newly diagnosed esophageal cancers because the esophagus has a rich lymphatic and vascular supply. Approximately half of newly diagnosed patients will present with locally advanced disease, with a 20% to 30% 5-year survival rate after surgical resection or multimodality therapy.3 Cure rates of 60% to 80% can be achieved in only the 10% or so of patients with node-negative disease confined to the esophagus that has been treated with surgical resection.4
The incidence of adenocarcinoma of the distal esophagus and gastroesophageal junction is increasing in the Western world and currently represents approximately 60% of all esophageal carcinomas in the United States, but squamous cell carcinoma remains dominant in underdeveloped parts of the world.5,6,7 Data from the Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute (1975 to 2004) demonstrate that in the United States, the incidence of esophageal cancer in white males has steadily risen from 5.76 per 10,000 person-years during the years 1975 to 1979 to 8.34 per 10,000 person-years during the years 2000 to 2004. The adenocarcinoma incidence rate has increased by 463%, from 1.01 to 5.69 per 10,000 person-years, but the incidence rate of squamous cell cancers has declined by 50%, from 3.81 to 1.90 per 10,000 person-years.8 Among white females, the incidence of esophageal cancer has remained constant at 2 per 10,000 person-years, with a 29% decrease in squamous cell cancers (0.98 per 10,000 person-years) and a 335% increase in adenocarcinomas (9.74 per 10,000 person-years from 1975 to 2004).8,9 Similarly, the incidence of squamous cell carcinoma has shown a significant decline in black males beginning in 1992,10 but it remains the most common cell type among blacks in the United States,11 despite the twofold to threefold increase in incidence of adenocarcinomas in black males and females.
Data reported by Hesketh12 in a review of the New England tumor registries show that the incidence of adenocarcinoma has increased even when tumors located at the esophagogastric junction have been excluded. Table 43-1 reflects recent changes in the prevalence of adenocarcinoma of the esophagus over the span of several decades. Pohl and colleagues7,13,14,15 extracted information from the National Cancer Institute’s SEER database from 1973 to 2001 to counter a number of suggested possibilities for the recent increase in adenocarcinomas of the esophagus, and found no evidence for either histologic reclassification of esophageal squamous cancers or anatomic reclassification of adenocarcinomas of the gastric cardia.
| Investigator | Years | Adenocarcinoma (%) |
|---|---|---|
| Smithers211 | 1936-1951 | 7.3 |
| Hesketh/Conn. Register12 | 1983-1986 | 22.9 |
| Birgisson212 | 1987-1994 | 73.5 |
| Steyerberg16 | 1991-1999 | 52.0 |
| Brown8 | 2000-2004 | 61.1* |
* White males and females only.
Esophageal cancer is more commonly seen in men than women; of the more than 16,000 cases of esophageal cancer diagnosed in the United States in 2010, approximately 13,000 were found in men.1,2 Esophageal cancer is the seventh leading cause of cancer deaths among males in the United States. Black males are more likely to present with advanced and/or metastatic disease, resulting in a survival rate that is 60% of that for white males.1,2 In a multivariate regression analysis controlling for age, gender, marital status, tumor histologic type, and tumor location, black race was associated with worse survival rates. In this study analyzing SEER data, when the tumor status, surgical technique, and radiotherapeutic modality were added to the model, race was no longer significantly associated with survival rates.11 Provocative data from Steyerberg and associates16 also suggested that the underuse of potentially curative surgery may, in part, explain the poorer survival rates observed for black patients with locally advanced disease; in a population-based analysis, the authors observed that once the data had been corrected for treatment received, there was no difference in survival rates between white and black patients. It has been postulated that the increased incidence of disease and increased mortality rates observed in black patients are reflections of socioeconomic status and dietary risk factors but not ethnicity. Squamous cell carcinoma remains the most common histologic type among black males and may be associated with worse outcomes, facts that could explain some of the observed racial disparities in survival rates.11 Data from Brown and colleagues17 support a correlation between an increased rate of squamous cancers in black males with known risk factors. In this population-based case-control study, there appeared to be a risk for developing esophageal cancer in black patients beyond what could be attributed to alcohol and tobacco use.17 The reasons for the apparent racial difference in risk from the same level of alcohol and tobacco use could be associated, in part, with increased mutations in the TP53 gene in esophageal cancers found in black patients, as reported by Baron and colleagues.18
Prevention and Early Detection
Smoking is an established risk factor for squamous cell carcinoma of the esophagus and also for adenocarcinoma but to a lesser extent; smoking cessation reduces the risk of only squamous cell carcinoma, however.19 Chronic esophagitis is also thought to be a precursor for the development of squamous cell cancers.20 Other environmental factors associated with the development of squamous cell cancers include thermal injury and exposure to nitrates and potentially carcinogenic nitrosamines, asbestos fibers, or water contaminated with petroleum products.21–23 Data from several laboratories have implicated genital-mucosal strains of human papillomaviruses (HPV-16 and HPV-18) as risk factors for the development of cancers of the esophagus.24,25
Alcohol consumption has been shown to be a risk factor for squamous cell carcinoma but not adenocarcinoma.19 Gastroesophageal reflux disease (GERD) predisposes to Barrett’s esophagus and is associated with an increased risk of adenocarcinoma of the esophagus, with severe long-standing disease.19 It has been postulated that the incidence of GERD is increasing in the United States; however, it is not clear whether this increase is contributing to the increase in adenocarcinomas.19 An increasing body mass index is also strongly associated with adenocarcinoma risk.26 Diet has been demonstrated to affect both types of esophageal carcinoma, with increased intake of fruits and vegetables associated with a reduced incidence of cancer.27 Lifestyle changes, including weight loss and exercise, postulated to reduce the risk of developing esophageal adenocarcinoma, are currently being investigated.
Barrett’s esophagus is known to be associated with GERD and is a precursor lesion for esophageal adenocarcinoma, although most Barrett’s lesions do not proceed to carcinoma. Although families with Barrett’s esophagus have been described, most cases are sporadic and thought to be caused by chronic gastroesophageal reflux. Suleiman and associates28 have postulated that the use of pharmaceutical agents for the treatment of gastroesophageal reflux (i.e., antisecretory agents and therapies to relax the lower esophageal sphincter) may preclude the surveillance required for the detection and management of Barrett’s esophagus and that their use is therefore related to the recent increase in the incidence of Barrett’s esophagus–associated adenocarcinomas.
Although the molecular understanding of the premalignant conditions associated with Barrett’s esophagus continues to expand, efforts to improve early detection and incorporation of chemoprevention efforts are under investigation. Nguyen29 reported the results of a retrospective observational study of 344 patients with Barrett’s esophagus that examined the association between prescription use of proton pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin, or statins and the risk of developing esophageal dysplasia or adenocarcinoma. In this study, PPI use was associated with a reduced risk of high-grade dysplasia or cancer with adjustment for gender, age, and the length of duration of Barrett’s esophagus. Use of NSAIDs or aspirin resulted in a nonsignificant trend toward a lower incidence of high-grade dysplasia and cancer, whereas statin use was not significantly associated with the risk of developing neoplasia.29 A meta-analysis supports these findings, demonstrating that the use of NSAIDs decreases the risk of both squamous cell carcinoma and adenocarcinoma, with odds ratios of 0.58 and 0.67, respectively.30 Vaughan and associates31 demonstrated similar results in a prospective cohort study, and currently, there is a large multicenter, randomized controlled clinical trial (AspECT) evaluating the long-term chemopreventive effect of esomeprazole with or without aspirin.32
Adenocarcinomas and, more recently, squamous cancers of the esophagus have been shown to overexpress the cyclooxygenase-2 (COX-2) enzyme.33,34 Data from Shamma and colleagues35 demonstrates that COX-2 expression is correlated with the proliferative activity in dysplastic lesions of the esophagus. In this study, the COX-2 level was found to be increased, in a stepwise fashion, with the transition from normal esophageal tissue to low-grade dysplasia to high-grade dysplasia, suggesting that COX-2 is involved in the early stages of carcinogenesis and that interruptions in the dysplasia-carcinoma sequence could prove to be an important part of a chemoprevention strategy. In addition, the HER2/neu oncogene is overexpressed and/or amplified in preneoplastic lesions and in adenocarcinoma of the esophagus and has been associated with a poor prognosis.36 Translational approaches with targeted therapies, such as trastuzumab, are being investigated based on preclinical evidence.37 Precancerous lesions of the esophagus have also been shown to overexpress TP53,36,38,39 cyclin D1,40 and P16,41 which may be targeted by chemoprevention agents in the future.
Molecular Characteristics of Esophageal Cancer
Mutations in the TP53 gene are present in up to 80% of esophageal cancers. Interestingly, the mutations for squamous tumors, which are int A-T base pairs, are very different from the mutations usually seen in adenocarcinomas.42 Data from Montesano42 indicate that the mutations common to squamous tumors are correlated with smoking. Mutations in the TP53 gene are an early event in the carcinogenic process from Barrett’s esophagus mucosa to high-grade dysplasia and esophageal adenocarcinoma. In data from Coggi and associates,43 TP53 gene mutations in exons 5 to 8 were detected in 53% of 74 esophageal cancers. Interestingly, there was no concordance between TP53 gene mutations and the accumulation of the TP53 protein nor were the mutations in the TP53 gene independently predictive of clinical outcome. In vitro data evaluating the sensitivity of esophageal adenocarcinoma cell lines to chemotherapeutic agents (e.g., 5-FU; mitomycin C, and cisplatin) showed that wild-type TP53 protein levels increased after treatment with each of the agents via post-translational and or translational processes. Schrump and collaborators44 also confirmed a positive correlation with drug sensitivity and the increased expression of wild-type TP53 protein versus a negative in vitro sensitivity effect with deficient TP53 protein expression or expression of mutated TP53 protein.
Additional molecular abnormalities include cyclin D1, a cell cycle-regulating protein involved in the G1 phase to S phase transition. Overexpression of cyclin D1 has been observed in approximately 30% to 40% of esophageal adenocarcinomas and squamous cell carcinomas.45 Inactivation of the P16 gene, a tumor supressor gene, occurs in a significant number of esophageal cancers,41 and restoring P16 expression appears to markedly inhibit the proliferation and tumorgenicity of esophageal cancers.44 These represent but a few of the potential molecular targets for directing therapies in future clinical studies.
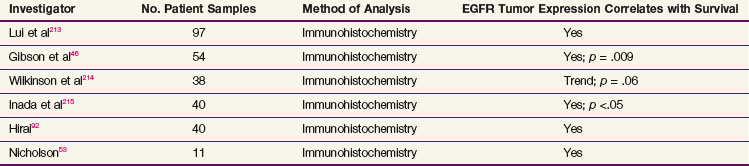
Overexpression of the epidermal growth factor receptor (EGFR) is found in both esophageal squamous cell carcinomas and esophageal adenocarcinomas, as well as their precursor lesions, such as squamous dysplasia and Barrett’s esophagus.46–48 Overexpression of EGFR on immunohistochemical testing occurs in approximately 80% of patients with esophageal adenocarcinoma and squamous cell carcinoma.49 Additionally, amplification of the EGFR gene has been detected by fluorescence in situ hybridization (FISH) analysis in 8% to 30% of esophageal adenocarcinomas.49–52 Clinical studies have shown that increased EGFR expression is associated with an overall decrease in survival rates in patients with esophageal cancer53 (Table 43-2).
Pathologic Findings and Pathways of Spread
The predominant histologic types of esophageal cancer are squamous cell carcinomas and adenocarcinomas. Approximately 20% of esophageal squamous tumors involve the upper “cervical” esophagus, while the majority (50%) are found in the middle esophagus, defined as the segment of esophagus from the aortic arch to the inferior pulmonary vein and usually representing the segment at 25 to 32 cm from the incisors. The remaining 30% of squamous tumors are found in the distal esophagus (at 33 to 42 cm from the incisors). In contrast, over 90% of adenocarcinomas are found in the distal esophagus and gastroesophageal junction. Other malignant histologic types are unusual but include adenosquamous, mucoepidermoid, and adenoid cystic tumors and malignant tumors with endocrine differentiation (small cell cancers). Histologic review of esophagectomy specimens with early-stage disease revealed little difference between the rate of submucosal spread and the rate of lymph node metastasis for squamous cell carcinomas and adenocarcinomas.54 In addition, no difference in survival rates has been correlated with histologic subtype for patients who undergo surgery for esophageal cancer.54,55,56
Clinical Manifestations, Patient Evaluation, and Staging
Box 43-1 lists the most common clinical symptoms associated with esophageal cancer at presentation. Over 90% of patients present with progressive and worsening dysphagia often resulting in significant weight loss. Other findings include odynophagia, chest pain, cough, and fever associated with possible respiratory fistulas, hoarseness associated with tumor involvement of the recurrent laryngeal nerve, and melena resulting from intraluminal bleeding. Recommended staging tests are outlined in Box 43-2.
The use of 18-fluorodeoxy-D-glucose (FDG) positron emission tomography (PET) or PET-CT for staging in esophageal cancer continues to evolve. The addition of PET to standard staging techniques has improved the detection of occult metastatic disease57–60,61 and can result in up to 15% upstaging from M0 to M1 compared with CT and endoscopic ultrasonography.58 In a meta-analysis reported by van Westreenen and colleagues,62 PET showed limited sensitivity (0.51) and reasonable specificity (0.81) for the detection of locoregional metastases. These statistics were improved, however, for detecting distant lymphatic and hematogenous metastasis, with a sensitivity and specificity of 0.67 and 0.97, respectively.62 Taken in total, these data are compelling and have resulted in an increasing use of PET for the routine staging of patients with esophageal cancer.
The initial PET maximum standardized uptake value (SUVmax) has not proven to be predictive of survival times in patients with locally advanced esophageal adenocarcinoma who receive preoperative chemoradiotherapy; patients with a high initial SUVmax demonstrate a better response to preoperative therapy, however.63 The use of PET to provide an early assessment of response and response-adapted treatment has been under investigation. In the MUNICON trial, locally advanced distal esophageal adenocarcinoma or gastric cardial adenocarcinoma patients were assigned to 2 weeks of platinum- and fluorouracil-based induction chemotherapy. After completion of induction chemotherapy, patients found to have an SUV decrease of 35% or more on PET imaging, who were defined as metabolic responders, continued to receive additional 5-FU-based neoadjuvant chemotherapy for 12 weeks, followed by surgery. Metabolic nonresponders discontinued chemotherapy and proceeded directly to surgery. Of the 49% of patients who were metabolic responders, median overall survival was not reached by 2.3 years, whereas median overall survival was 25.8 months in the nonresponders. Major histologic remissions (<10% of residual tumor) were noted in the 58% who were metabolic responders, but no histologic response was noted in metabolic nonresponders.64 A similar adaptive treatment strategy has been adopted by the Cancer and Leukemia Group B (CALGB) for investigation.
The TNM staging system for esophageal cancer according to the American Joint Committee on Cancer (AJCC) staging manual, seventh edition, is shown in Box 43-3. As reflected in the staging, patients with regional and/or celiac axis lymphadenopathy should not necessarily be considered to have metastatic disease. Some difficulties have arisen because this is a surgical staging system and many patients are now treated initially without resection, making accurate pathologic staging impossible. Esophageal cancers are staged according to the depth of invasion, presence of regional lymph nodes or distant metastatic disease, and grade and location of tumor. Diagnostic tools relevant to determining the locoregional stage include endoscopic ultrasound and thoracoscopic staging.
Box 43-3
Staging and Prognosis for Esophageal Cancer
TNM Staging of Esophageal Cancer
| Primary Tumor | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | High-grade dysplasia |
| T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
| T1a | Tumor invades lamina propria or muscularis mucosae |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4 | Tumor invades adjacent structures |
| T4a | Resectable tumor invading pleura, pericardium, or diaphragm |
| T4b | Unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea |
| Regional Lymph Nodes | |
| Nx | Regional nodes not assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-2 regional lymph nodes* |
| N2 | Metastasis in 3-6 regional lymph nodes* |
| N3 | Metastasis in 7 or more regional lymph nodes* |
| Distant Metastasis | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Stage and Prognostic Groupings for Esophageal Cancer
| Adenocarcinoma | |
| Stage 0 | Tis, N0, M0, grade 1 or X |
| Stage I A | T1, N0, M0, grade 1-2 or X |
| Stage I B | T1, N0, M0, grade 3 |
| T2, N0, M0, grade 1-2 or X | |
| Stage IIA | T2, N0, M0, grade 3 |
| Stage IIB | T3, N0, M0, any grade |
| T1-2, N1, M0, any grade | |
| Stage IIIA | T1-2, N2, M0, any grade |
| T3, N1, M0, any grade | |
| T4a, N0, M0, any grade | |
| Stage IIIB | T3, N2, M0, any grade |
| Stage IIIC | T4a, N1-2, M0, any grade |
| T4b, any N, M0, any grade | |
| Any T, N3, M0, any grade | |
| Stage IV | Any T, any N, M1, any grade |
| Squamous Cell Carcinoma | |
| Stage 0 | Tis, N0, M0, grade 1 or X, any location |
| Stage I A | T1, N0, M0, grade 1 or X, any location |
| Stage I B | T1, N0, M0, grade 2 or 3, any location |
| T2-3, N0, M0, grade 1 or X, lower esophagus or X | |
| Stage IIA | T2-3, N0, M0, grade 1 or X, upper and middle esophagus |
| T2-3, N0, M0, grade 2 or 3, lower esophagus or X | |
| Stage IIB | T2-3, N0, M0, grade 2 or 3, upper and middle esophagus |
| T1-2, N1, M0, any grade, any location | |
| Stage IIIA | T1-2, N2, M0, any grade, any location |
| T3, N1, M0, any grade, any location | |
| T4a, N0, M0, any grade, any location | |
| Stage IIIB | T3, N2, M0, any grade, any location |
| Stage IIIC | T4a, N1-2, M0, any grade, any location |
| T4b, any N, M0, any grade, any location | |
| Any T, N3, M0, any grade, any location | |
| Stage IV | Any T, any N, M1, any grade, any location |
*Regional lymph nodes extend from cervical nodes to celiac nodes.
From the American Joint Committee on Cancer: AJCC Cancer Staging Manual, ed 7. New York, 2009, Springer-Verlag.
Hiele and associates,65 in a prospective review, found that preoperative endoscopic ultrasound reflected an accurate T stage in 59% of patients subsequently taken to surgery and an accuracy of 82% in patients with transmural tumor extension. Natsugoe and colleagues66 found the accuracy of endoscopic ultrasound to be 87% for detecting mediastinal nodal disease. The CALGB, in a multi-institutional trial of thoracoscopic staging, found thoracoscopic determination of tumor penetration and lymph node staging to be accurate in 88% of patients later taken to resection. The accuracy of overall staging further improved with concomitant laparoscopic lymph node staging.67 Although endoscopic ultrasound is recommended for the staging of esophageal cancers by current National Comprehensive Cancer Network (NCCN) guidelines, the precise role of endoscopic ultrasound is not well defined, because the results often do not affect the clinical management. Similarly, thoracoscopic staging studies were done before the common use of PET scans, and thoracoscopy and laparoscopy are now infrequently used. Studies have demonstrated that the diagnostic accuracy of re-endoscopy with rebiopsy and endoscopic ultrasound is inadequate for objective pathologic response evaluation after neoadjuvant chemoradiation.68,69
Cancers confined to the epithelium and muscularis mucosa and without nodal involvement are considered stage I and II tumors. In a clinicopathologic review of 165 patients with esophageal cancer treated with resection only, Holscher and colleagues70 observed 0% lymph node metastases in patients with disease confined to the mucosa compared with 18% for tumors with submucosal spread. The 5-year overall survival rate reported for patients with node-negative disease was 63%. Tumors invading the adventitia or surrounding structures have a worse outcome than more limited disease, and there is almost always associated nodal involvement.
Primary Therapy
Single-Modality Therapy
Surgery
In 1913, Dr. Franz Torek71 used a transpleural approach to perform the first successful resection of an esophageal carcinoma, reconstructed with an external rubber esophagus. With improvements in anesthesia, subsequent surgeons used mainly a transthoracic approach with primary esophagogastric anastomosis, and in 1933, Ohsawa72 reported extended survival rates in 8 of 20 patients who had a resection. In 1938, Adams and Phernister73 were the first Western surgeons to successfully adopt the Japanese transthoracic technique. The results of these studies, however, revealed a discouraging 5-year survival rate of 5% to 10%.
Despite recent advances in surgical and anesthetic techniques, the results with surgery alone have produced only modest improvements in outcome. Moertel74 reviewed 18 surgical series involving 4109 patients treated with resection and found an 5-year overall survival rate of 9.6% (range, 3% to 20%). Our review of more recently published surgical series reveals slightly more encouraging results, but there has been careful patient selection in some of these trials. Mariette and colleagues,75 in a series of 179 patients with stage 0 to II esophageal cancer treated with surgery alone between 1982 and 2002, observed an overall actuarial survival rate at 5 years of 59%. The investigators report, however, that no long-term survivors were observed among a subset of patients with locally advanced disease.
Analysis of surgical pathology shows that a significant number of patients present with disease involving the regional lymph nodes and that such involvement results in a worse outcome. In 156 patients evaluated by Frunberger and colleagues,76 53% had nodal disease at surgery. Sun and associates77 found that of 474 operable patients, 211 (44.5%) had involved regional lymph nodes at the time of surgery. The overall survival rate for the entire group was 31%; but only 13% of patients with nodal disease were alive at 5 years versus 44% for node-negative patients. Similar results have been reported by Collard and associates78; the 5-year survival rate after surgery alone fell from 57% for node-negative patients to 15% in patients with positive nodes. Holscher and colleagues,70 in an evaluation of 165 patients treated with en bloc esophagectomy, found that no patient with more than 30% regional nodal tumor involvement was alive at 5 years, whereas 45% of patients with less than 30% nodal disease were alive at 5 years.
The optimum number of lymph nodes to be removed and examined at the time of resection remains unclear. A recent retrospective analysis performed on SEER data demonstrated that the number of lymph nodes removed at the time of surgery was an independent predictor of survival time and that the optimal threshold predicted by Cox regression analysis for this survival benefit was removal of 23 nodes.79 Another recent study suggested that 18 lymph nodes should be removed.80
At present, there are no definitive data to suggest that one technique is superior to the others. Fok and colleagues,81 in a review of 210 patients treated with either a transthoracic resection (n = 172) versus a transhiatal resection (n = 38), reported an increased incidence of tumor perforation (18%) and injuries to the recurrent laryngeal nerve (13%) following the transhiatal approach. In a retrospective study of 238 patients, Pac and associates82 observed increased rates of wound infections, pneumothorax, and hospital mortality for patients treated with transthoracic resection. In contrast, Stark and associates83 observed increased rates of respiratory complications and hospital mortality following transhiatal resection. Others have identified little or no difference in overall complication rates or operative mortality rates between the two surgical approaches.84,85 These findings are consistent with a meta-analysis reported by Hulscher and colleagues,86 who observed that the 5-year survival rate with surgery alone was approximately 20% after both transthoracic and transhiatal resections.
Esophagectomy remains the standard of care for high-grade dysplasia and superficial cancers; surgical morbidity and mortality may be substantial for patients who are medically unfit, however. More recently, minimally invasive esophagectomy (MIE) and endoscopic treatments have become treatment options for selected patients with early-stage localized esophageal cancer. A recent meta-analysis using data from 10 studies to evaluate the effects of MIE versus open esophagectomy on outcome demonstrated trends in favor of MIE, with reductions in morbidity, pulmonary complications, anastomotic leakage, mortality, length of hospital stay, operating time, and blood loss.87 Similarly, laparoscopic inversion esophagectomy (LIE) has been shown to be comparable to open transhiatal esophagectomy (THE), with potential benefits related to the use of minimally invasive techniques, and is associated with less blood loss and a shorter operative time and length of hospital stay without increased morbidity or mortality.88,89 It must be recognized, however, that not all patients are candidates for minimally invasive procedures.
Photodynamic therapy has been reported to provide local control in early esophageal cancers arising from Barrett’s esophagus, ranging from 17% to 100%, and high-grade dysplasia, ranging from 75% to 100%.90,91 Complete remission rates of more than 90% have also been reported with endoscopic mucosal resection.91 Recurrence rates are higher than with esophagectomy, however, and require close endoscopic surveillance and retreatment in some patients.
Chemotherapy
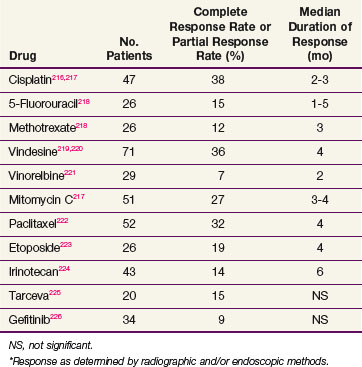
It has been observed in several autopsy series that esophageal cancer has a distant failure rate of more than 70%. A large number of chemotherapy agents have been evaluated for response in cancer of the esophagus (Table 43-3), but unfortunately, the response rates remain very low, with only about 20% of patients having an objective response to a variety of single agents. Combination chemotherapeutic regimens have shown a higher response rate, the best responses being seen in patients receiving combinations with cisplatin (Table 43-4). Although the increased response rate has been gratifying, the duration of response has been short and comparable to that seen with single-agent chemotherapy. Current drugs under intense investigation include oxaliplatin, irinotecan, and gemcitabine in combination with cisplatin and the targeted therapies.
TABLE 43-4 Combination-Agent Activity in Chemotherapy of Esophageal Cancer*
| Combination | No. Patients | Overall Response Rate (%) |
|---|---|---|
| Cisplatin + bleomycin227 | 61 | 15 |
| Cisplatin + vindesine + bleomycin228 | 68 | 53 |
| Taxol + cisplatin229 | 20 | 40 |
| 5-Fluorouracil + cisplatin + doxorubicin230 | 21 | 33 |
| Cisplatin + etoposide231 | 73 | 48 |
| 5-Fluorouracil + cisplatin232 | 88 | 35 |
| Bleomycin + doxorubicin233 | 16 | 19 |
| Irinotecan + cisplatin174 | 35 | 57 |
| Gemcitabine + cisplatin234 | 36 | 41 |
| Gemcitabine + irinotecan235 | 61 | NS |
| Bevacizumab + irinotecan + cisplatin114 | 47 | 65 |
| Trastuzumab + 5-fluorouracil + cisplatin104 | 594 | 47.3 |
NS, not significant.
* Response as determined by radiographic and/or endoscopic methods.
Targeted Biologic Therapy
It is estimated that up to 80% of esophageal adenocarcinomas and squamous cell carcinomas demonstrate increased EGFR expression, which is associated with a decrease in overall survival rates in patients with esophageal cancer. Investigations looking into molecular predictors of sensitivity to EGFR inhibitors for patients with esophageal and gastroesophageal junction cancers are under investigation.92 Multiple phase II trials have been reported for the tyrosine kinase inhibitors erlotinib and gefitinib and for the anti-EGFR antibody cetuximab. These agents appear to have minimal activity in adenocarcinomas and limited activity in squamous cell carcinomas.92–99 Furthermore, there has been no correlation of change in expression of EGFR or downstream markers with response.94,95,97,99 There is preclinical evidence that EGFR inhibition may enhance radiosensitivity of esophageal cancers.100 Clinical trials evaluating the efficacy of adding EGFR inhibition during preoperative chemotherapy and irradiation are under way. One phase II multicenter trial using cetuximab with paclitaxel, carboplatin, and irradiation for patients with esophageal cancer has demonstrated tolerability without increased radiation toxicity and a 70% complete clinical response rate. The pathologic response rate has not yet been reported.101
Overexpression of HER2/neu ranges from 0% to 52% in squamous cell carcinomas of the esophagus and 0% to 73% in adenocarcinomas.102 Although there is some suggestion that HER2/neu overexpression is important in the progression of dysplasia to cancer, resistance to therapy, and extramucosal invasion, its value as a prognostic factor is uncertain.103 The ToGa trial screened tumors from 3807 patients with advanced esophagogastric disease, and 22.1% were found to be HER2/neu-positive.104 Patients with HER2/neu-positive tumors were randomized to receive cisplatin and 5-FU with or without trastuzumab. The addition of trastuzumab resulted in an increase in progression-free survival rates.104,105 A phase I and II study of full-dose trastuzumab, paclitaxel, cisplatin, and irradiation for locally advanced, stage II to III or higher, HER2-overexpressing esophageal adenocarcinomas reported a median survival time of 24 months and a 2-year survival rate of 50%, similar to prior studies and without an increase in toxicity.105 Further evaluations of trastuzumab for esophageal cancer are needed to clarify the utility of targeting HER2/neu in this setting.
Vascular Endothelial Growth Factor (VEGF)
Data suggest a role for vascular endothelial growth factor (VEGF), an agiogenic factor, in the development of esophageal cancer. VEGF expression levels and microvessel density are significantly higher in cancerous tissues compared with normal tissues and Barrett’s dysplastic tissues.106 VEGF is overexpressed in 30% to 60% of esophageal cancers, and studies have demonstrated a correlation between high levels of VEGF expression, advanced stage, and poor overall survival rates in esophageal cancer patients.101,107–112,113 Trials combining VEGF-targeted therapy are ongoing in the treatment of locally advanced esophageal cancer. A recent phase II trial combined bevacizumab with irinotecan and cisplatin in metastatic gastroesophageal junction and gastric cancers, resulting in a response rate of 65% with 8.9 months as the median time to progression, significantly better results than those with historical controls.114 Further phase II and III trials of bevacizumab in esophageal cancer are under way. There are no clinical data on the use of oral tyrosine kinase inhibitors that target VEGF (sorafenib, sunitinib, and AG13736) in esophageal cancer to date. Similar to EGFR, VEGF inhibition has demonstrated enhanced radiosensitivity in preclinical trials.115,116 The impact of VEGF inhibition in combination with chemotherapy and irradiation in patients with esophageal cancer is currently being investigated.
Cyclooxygenase-2 (COX-2)
COX-2 is another molecular target that has been shown to have significance in cancer development and progression. Selective inhibition of COX-2 has been shown to alter the development and progression of cancer in clinical trials.117 In addition, inhibition of COX-2 activity results in enhanced radiosensitization of tumor tissue but not normal tissue.118,119 Selective COX-2 inhibition has also been shown to directly inhibit tumor neovascularization.118,119,120,121–123 The combination of these effects suggests potential enhancement of selective tumor targeting by COX-2 inhibition. Studies combining COX-2 inhibition with neoadjuvant chemotherapy and radiation have demonstrated tolerability but fail to demonstrate an improvement in the pathologic response over standard neoadjuvant combined-modality treatment.124,125 Pretreatment VEGF expression does not correlate with treatment response, and pretreatment COX-2 expression has been shown to correlate with treatment response only in the subset of patients with squamous cell carcinoma, although patients whose tumors expressed high levels of VEGF and COX-2 tended to have shorter overall survival times.125
Radiation Therapy
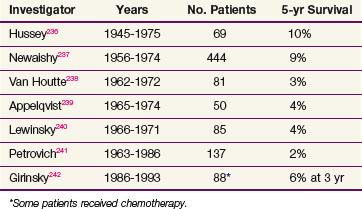
Early attempts to cure esophageal cancer with irradiation were generally restricted to patients with middle and upper esophageal lesions, whereas lesions of the distal esophagus were managed with surgical resection. In the 1950s, Buschke126 reported a 5-year survival rate of 5% for patients with lesions of the mid-esophagus treated with irradiation, similar to the surgical results being reported at that time. The outcome following primary radiation therapy alone in the treatment of clinically localized esophageal cancer still remains poor, with a 2-year survival rate of approximately 10% to 20% and a 5-year survival rate of approximately 5% (Table 43-5). Several autopsy studies demonstrate that 50% to 89% of patients harbor both local and undetected distant disease.127 Review of the literature examining patterns of failure following irradiation alone demonstrate local failure rates of 50% to 91% following doses greater than 5000 cGy128,129,130 and a distant failure rate of 23% to 66%.128
Radiation Therapy Followed by Surgery
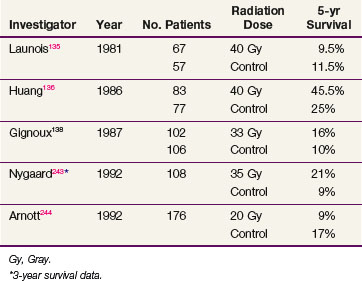
Early attempts to improve local control and survival rates combined radiation and surgery. At the Memorial Sloan-Kettering Cancer Center from 1956 through 1966, 85 patients were treated with preoperative radiation and surgery, with 47 patients ultimately going on to resection. The overall crude 5-year survival rate was 6 % and the median survival rate was 14 months. No tumor was seen in the surgical specimen in 7 (14%) of the tumors resected.131 Nakayama and colleagues132 compared the outcomes for patients who underwent staging laparotomy, gastrostomy, and nutritional supplementation prior to preoperative radiation (20 to 25 Gy in 4 to 5 fractions) followed by a total esophagectomy with the outcomes for patients who underwent either irradiation or surgery alone. The 3-year survival rate was 27% for patients treated with combined therapy versus 22% for those treated with surgery alone and 6% with irradiation alone,132 but updated data showed a 5-year survival rate of 13% for the combined-modality cohort.133 Others report a 5-year survival rate of 25% for patients receiving 50 Gy preoperatively versus 14% for patients treated with surgery alone.134 These studies, however, were all retrospective reviews that did not allow direct comparisons of treatments. Prospective randomized trials evaluating outcomes for patients treated with either surgery alone or 40 Gy of preoperative irradiation demonstrated no benefit to the addition of preoperative radiotherapy; reported 5-year survival rates ranged from 9.5% to 45% for the irradiation and surgery group and from 11.5% to 25% for patients treated with surgery alone.135,136,137,138 Table 43-6 summarizes the results of EBRT alone followed by surgical resection.
Combined-Modality Therapy
Sequential Chemotherapy and Irradiation
The usefulness of sequential chemotherapy followed by a course of definitive radiation therapy alone has been evaluated on a limited scale. Izquierdo and colleagues139 treated patients with sequential cisplatin/bleomycin chemotherapy for three courses followed by definitive thoracic irradiation and reported a partial response rate of 52% and a complete response rate of 16% as determined by CT scanning and endoscopy. The 1-year and 4-year survival rates were disappointing at 20% and 8%, respectively.139 Valerdi and colleagues140 reported the results of two cycles of induction cisplatin-vindesine-bleomycin chemotherapy followed by definitive radiotherapy and found a discouraging 15% overall survival rate at 5 years. A more recent phase II sequential study reported by Sharma and associates,141 testing multiple cycles of 5-FU plus cisplatin followed by a definitive course of irradiation (60 Gy) was equally disappointing, with a median survival time of 39 weeks. This approach has generated relatively little interest because it does not take advantage of the probable benefit of chemotherapy and irradiation given in combination.
Induction and Concurrent Chemotherapy and Irradiation
A number of investigators have tested the use of induction chemotherapy followed by a course of definitive irradiation delivered concurrently with chemotherapy. Stahl and associates,142 in a phase II study of 90 patients with locally advanced esophageal cancer, recently reported their results with three cycles of induction 5-FU, leucovorin, etoposide, and cisplatin followed by irradiation to 40 Gy and concurrent cisplatin and etoposide. Of the 72 evaluable patients, 44 underwent resection, with a pathologic complete response rate of 22%. Unfortunately, the operative mortality rate was 15%. The median survival time for evaluable patients was 17 months and the overall 3-year survival rate was 33%.142 Minsky and colleagues,143,144 in a study of 45 patients with locally advanced disease, evaluated three cycles of induction 5-FU and cisplatin followed by two additional cycles of chemotherapy delivered concurrently with irradiation to 64.8 Gy. Although the median survival time was an encouraging 20 months, six patients died from treatment-related toxicity.143,144 A renewed interest in this approach has come about recently with exploration of early PET scans to assess the response.64 Currently, the CALGB is developing a response-adapted strategy using neoadjuvant chemotherapy with early PET imaging followed by chemoirradiation, with concurrent chemotherapy selection based on the response to initial chemotherapy.
Twice-a-Day Chemoradiation
Building on the encouraging data of twice-daily radiotherapy in other tumor sites, a number of researchers have investigated its usefulness in esophageal cancer. Kikuchi and associates145 reported in 1991 on 60 patients receiving twice-a-day concomitant boost radiation therapy alone, and observed an encouraging 5-year cause-specific survival rate of 31.5%. Zhao and colleagues146 reported on the feasibility of late-course accelerated hyperfractionated radiotherapy for early-stage esophageal carcinoma. Disease was treated with conventional fractionation during the first two-thirds of the treatment period to 41.4 Gy in 5 weeks, followed by a 2-week course of twice-daily irradiation (1.5 Gy per fraction) using reduced fields, for a cumulative dose of 67 to 70 Gy. The investigators observed an encouraging 5-year local control rate of 85%.146
Girvin and colleagues147 treated patients with twice-a-day preoperative irradiation plus concurrent 5-FU, cisplatin, and vinblastine and observed a remarkable complete response rate of 79%. Data from a larger series using a similar concurrent regimen of 5-FU plus cisplatin and twice-daily irradiation did not confirm the findings of Girvin, however. Adelstein and associates148 observed a complete response rate of 27% and an operative mortality rate of 18%. Yu and colleagues149 have reported their results with an alternating schedule of cisplatin and 5-FU with twice-daily irradiation on alternating weeks (1.8 to 2 Gy to 60 Gy). Although a local tumor control rate of 94% was reported, the regimen was associated with significant grade 5 acute toxicity.149 The use of concurrent chemotherapy and twice-daily irradiation is currently restricted to clinical trials.
Concurrent Chemotherapy and Irradiation
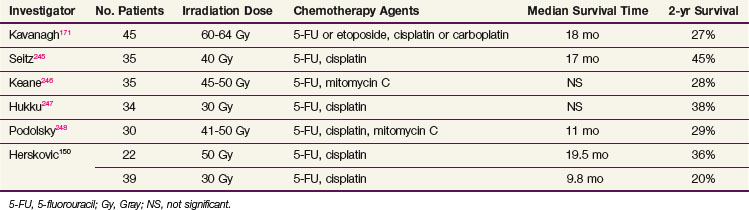
Table 43-7 reports the results of several nonrandomized trials employing concomitant chemotherapy and radiation therapy for the definitive treatment of esophageal cancer. Generally, the most successful treatment regimens have combined infusional 5-FU with either mitomycin C or cisplatin. Data from Wayne State University employing a 5-FU plus cisplatin regimen given concurrently with irradiation (5000 cGy) resulted in a median survival time of 19 months.150 For patients treated with the same chemotherapy regimen but a radiation dose of 3000 cGy, the median survival time was 9.8 months, suggesting a dose-response effect. In a phase II trial reported by Coia and colleagues,151 57 patients were treated with curative intent to 6000 cGy combined with 5-FU (1 g/m2/24 hours) as a continuous 4-day infusion during the first and fifth weeks of irradiation and mitomycin C (10 mg/m2) on day 2, resulting in a median survival time of 18 months and a 3-year survival rate of 29%.
TABLE 43-7 Results from Combined Chemotherapy and Radiotherapy as Primary Therapy for Patients with Esophageal Cancer
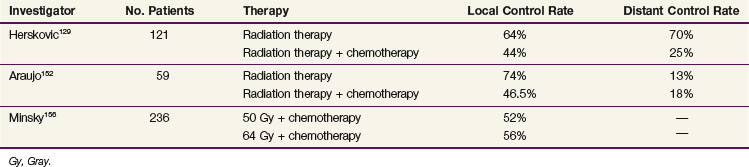
Subsequent randomized data (Table 43-8) from other investigators have shown benefit in patients treated with concurrent therapy with local control rates ranging from 24% to 67% (Tables 43-9 and 43-10). Araujo and colleagues152 randomly assigned 59 patients to irradiation alone (50 Gy at 2 Gy per day) versus the same dose of radiation delivered concurrently with 5-FU as a continuous infusion for 72 hours plus mitomycin C and bleomycin. The local complete response rate was 58% for irradiation alone versus 75% for the chemoradiation group, and the overall 5-year survival rates were 6% and 16%, respectively.152 Sischy and associates153 reported data from a study that randomized patients to 6000 cGy of radiation versus 6000 cGy with a concurrent bolus of mitomycin C and two cycles of infusional 5-FU. A median survival and 2-year survival advantage was observed in the combined-modality arm of the trial (14.9 months vs. 9.3 months and 30% vs. 12%).153
TABLE 43-8 Randomized Studies Comparing Irradiation Alone with Combined Irradiation and Chemotherapy
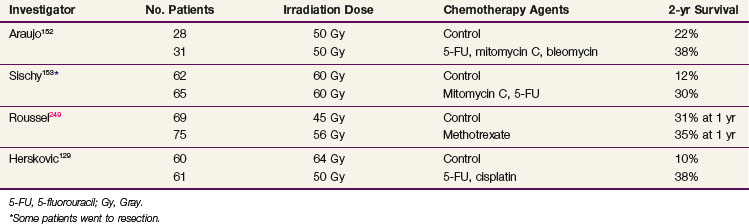
The most important contemporary trial is that of Herskovic and associates129 (RTOG 85-01). One hundred and twenty-one patients were randomized to either 5000 cGy with concurrent chemotherapy with 5-FU (1000 mg/m2 for 4 days) and cisplatin (75 mg/m2) versus 6400 cGy alone. At 5 years, 27% of the combined-modality patients were alive versus none of the patients in the irradiation-only group.154 The median survival time for the combined-modality arm was 14.1 months versus 9.3 months for the irradiation-alone arm.155 These results are important because they not only demonstrate a survival advantage with the combined-modality therapy but they also show that the survival advantage cannot be obtained by simply increasing the radiation dose, as might be the case if chemotherapy were acting as a pure radiation sensitizer.
The improvement in survival rates in the combination trials discussed above is related at least in part to an improvement in local control rates. Tables 43-12 and 43-13 show the crude patterns of failure from several chemoradiation trials. These data suggest that combined-modality therapy produced a shift in the pattern of failure, from a local failure rate of 50% to 90% for irradiation alone to 20% to 50% with combined-modality therapy. Herskovic and colleagues129 reported a crude local persistence or recurrence rate of 44% for patients treated with chemoradiation versus 64% for the irradiation alone group. The 2-year actuarial local failure rate was reduced from 68% to 43% and the rate of distant metastases was reduced from 70% to 25% with combined-modality therapy.
Nonetheless, because the higher radiation dose was well tolerated and did not seem to be the cause of the higher mortality rate, it was used in the high-dose arm (64.8 Gy) of the subsequent randomized trial. In the Intergroup 0123 study, patients were randomized to receive combined-modality therapy consisting of four monthly cycles of 5-FU (1000 mg/m2 per 24 hours for 4 days) and cisplatin (75 mg/m2 as a bolus on day 1), with concurrent irradiation to 64.8 Gy versus the same chemotherapy schedule but with the radiation dose limited to 50.4 Gy. Unfortunately, the trial was stopped after an interim analysis.156 For the 218 eligible patients, there was no significant difference in median survival times (13 months vs. 18.1 months), 2-year survival rate (31% vs. 40%), or locoregional failure rate (56% vs. 52%) between the high-dose and standard-dose treatment arms, respectively. Although 11 treatment-related deaths occurred in the high-dose arm compared with 2 in the standard-dose arm, 7 of the 11 deaths occurred in patients who had received 50.4 Gy or less. The reason for the lack of benefit in the high-dose arm is unclear. When comparing the high-dose arm with the low-dose arm, there was a significant prolongation of the treatment time because of toxicity breaks when correcting for the number of radiation treatments as well as a significantly lower actual dose of 5-FU as a percentage of the protocol dose.
An important concern regarding the use of combined-modality therapy is treatment toxicity. In the Wayne State University series, 5 of 20 patients required hospitalization for intravenous hydration and nutritional support, and 38% developed pulmonary compromise (thought to be secondary to receiving bleomycin plus irradiation) that required corticosteroids.150 The overall irradiation dose appears to be important, as has been reflected in data from Sauter and associates.157 In a study of 30 patients receiving concurrent chemotherapy and irradiation (60 Gy), only 67% of patients were able to complete the irradiation schedule as planned and only 18 of the 30 patients were able to proceed to resection.157 Coia and collaborators129,151,158 reported a 56% incidence of moderate to severe acute toxicities, whereas the researchers of RTOG 85-01 found that side effects were severe in 44% of patients and life-threatening in 20% of patients in the combined-modality arm versus 25% and 3% in the radiation only group, respectively. Most of the toxicity was hematologic, along with significant reactions (esophagitis and stomatitis) in the oral cavity, pharynx, and esophagus. Although only 1 of 61 patients in the combined-modality group died of acute toxicity, only 50% of patients actually completed all four cycles of chemotherapy.
Although the magnitude of the benefit obtained with irradiation and concurrent 5-FU infusion and cisplatin or mitomycin C is unclear, there seems little doubt that this combination represents a therapeutic advance over single-modality radiation therapy or chemotherapy. Other agents have been investigated in an attempt to improve outcomes. RTOG 0113 has reported the results of a randomized phase II study for patients who were unwilling to have surgery or were medically unfit for surgery, who were randomly assigned to receive either induction treatment with 5-FU, cisplatin, and paclitaxel and then 5-FU plus paclitaxel with 50.4 Gy of irradiation or induction with paclitaxel plus cisplatin and then the same chemotherapy with 50.4 Gy of irradiation. Unfortunately, both arms were associated with high morbidity rates, and the study did not meet its 1-year survival end point.159 The optimum regimen has not been established, but at present an irradiation dose of 5000 cGy combined with infusional 5-FU (1000 mg/m2/day for 4 days) and cisplatin (100 mg/m2), with the drugs given twice during radiation therapy, remains a reasonable standard.
Neoadjuvant or Adjuvant Chemotherapy and Surgery
Although a number of studies evaluating preoperative chemotherapy have demonstrated good response rates, only a few randomized studies have been reported. The most robust experience comes from the Medical Research Council Oesophageal Cancer Working Group.160 In this study, 802 previously untreated patients with resectable esophageal cancer were randomized to receive either two cycles of cisplatin plus 5-FU followed by surgery or surgery alone. The overall survival rate was better in the patients who received chemotherapy (hazard ratio, 0.79; 95% CI, 0.67 to 0.93; p = .004). The median survival time was 16.8 months for patients randomized to chemotherapy compared with 13.3 months for patients who underwent surgery alone. The 2-year survival rate was also improved for patients randomized to chemotherapy (43%) versus patients who underwent surgery alone (34%). Lesser improvements in median survival times have been demonstrated in other randomized studes.161–163
In striking contrast are the data from Kelsen and colleagues164 from the intergroup trial. Four hundred sixty-seven patients were entered into this randomized trial comparing surgery alone versus induction chemotherapy (three cycles of cisplatin/5-FU chemotherapy) followed by surgery in patients with operable disease. With a median follow-up time of 55.4 months, there were no significant differences between the two groups in median survival time: it was 14.9 months for the patients who received preoperative chemotherapy and 16.1 months for those who underwent immediate surgery (p = .53). At 1 year, the survival rate was 59% for those who received chemotherapy and 60% for those who had surgery alone; at 2 years, the survival rate was 35% and 37%, respectively.
The utility of postoperative chemotherapy alone has also been studied in a limited fashion. Armanios and associates165 in a multicenter phase II trial, evaluated four cycles of adjuvant paclitaxel and cisplatin chemotherapy; the 3-year survival rate was encouraging at 42%, but most patients (76%) had failure at distant sites, confirming the need for more effective systemic agents. In a randomized trial reported by Ando and collaborators,166 242 patients with squamous cell carcinoma of the esophagus underwent transthoracic esophagectomy with lymphadenectomy and were then randomized to either observation or two courses of cisplatin plus 5-FU chemotherapy. Although an improvement in the 5-year disease-free survival rate was seen for patients receiving the adjuvant chemotherapy (55% vs. 45%), the slight improvement in the overall survival rate was not statistically significant (61% vs. 52%). These results were not different from those observed in a 205-patient phase III trial showing no benefit with the addition of adjuvant cisplatin and vindesine chemotherapy to surgery.167 Because of the conflicting data, we currently recommend that patients receive neoadjuvant or adjuvant chemotherapy only as part of a clinical trial.
Preoperative Chemoradiation Therapy
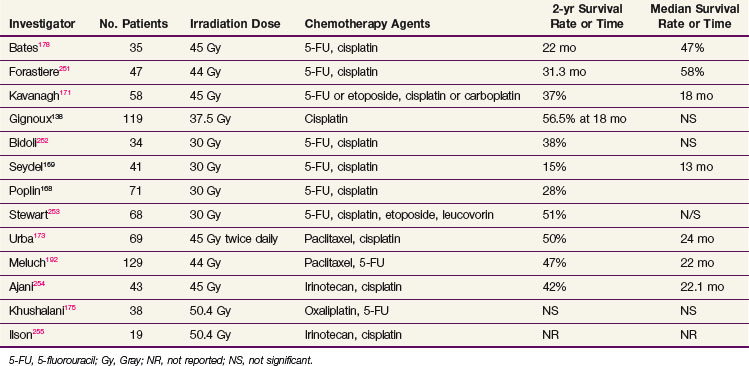
The Southwestern Oncology Group (SWOG) and the Radiation Therapy Oncology Group (RTOG), based on pilot data from Wayne State University, performed two very similar phase II trials using concurrent chemotherapy and irradiation followed by planned resection. In the SWOG study, 113 patients with initially resectable tumors received treatment with an infusional 5-FU plus cisplatin chemotherapy regimen with concurrent irradiation to a total dose of 3000 cGy. The overall operability rate was 63%, and the overall resectability rate was 49%. The median survival time was 12 months, with 16% of patients alive at 3 years.168 Of the 41 patients entered in the RTOG preoperative trial of concurrent irradiation plus 5FU and cisplatin, 7.5% were alive at 3 years.169 The data from the University of Michigan and Duke University are more positive, with 34% and 27% of patients, respectively, alive at 5 years.170,171 The results from several nonrandomized trials employing platinum- plus 5-FU–based preoperative chemoradiation strategies are shown in Table 43-11.
The next generations of chemoradiation trials attempted to incorporate more novel chemotherapeutic agents. Choi and colleagues,172 in a 46-patient phase II study of irradiation and concurrent cisplatin, 5-FU, and paclitaxel, observed a pathologic complete response rate of 45% for the 40 patients able to undergo resection. The overall median survival time was 34 months, with 37% of patients alive at 5 years. Urba and associates173 reported results from a phase II trial using preoperative cisplatin, paclitaxel, and twice-daily irradiation, with 90% of patients able to undergo surgery and a 19% complete pathologic response rate. Survival data compared favorably with other previously reported combinations, with a median survival time of 24 months.173 Ilson and collaborators174 have reported that the combination of cisplatin and irinotecan possesses activity in this setting, with a 57% response rate in 35 patients with metastatic or advanced esophageal cancer. The median duration of response was 4.2 months, and the toxicity was mild.
Oxaliplatin has also been evaluated in combination with 5-FU-based chemotherapy with or without irradiation in the neoadjuvant setting. Data reported by Khushalani and colleagues175 suggest that a course of preoperative oxaliplatin and concurrent chemotherapy with protracted venous infusion of 5-FU with irradiation (50.4 Gy) is an active regimen in locally advanced esophageal cancer, resulting in rates of 81% for complete radiologic response and 38% for complete pathologic response. More recent studies demonstrated promising complete pathologic response rates of up to 63%,176 but excessive treatment-related deaths were encountered. Currently, it is thought that concurrent irradiation and oxaliplatin-containing regimens are not acceptable because of excess toxicity.176,177
Data reported from the University of North Carolina revealed an encouraging disease-free survival rate of 33 months and an overall 3-year survival rate of 36% in a cohort of 35 patients receiving preoperative chemoradiation. Of perhaps more importance, this study found that the use of preresection esophagogastroduodenoscopy was not useful for determining the tumor response; although 77% of patients were reported to have had a clinical complete response (seen visually by endoscopy) preoperatively, 41% of these patients had residual tumor in the pathologic specimen.178 The inaccuracy of endoscopy, further confirmed in a study from Roswell Park,179 in discerning the response after preoperative chemoradiation is important because it suggests that a strategy of initial chemotherapy plus radiation therapy with surgery saved for patients who do not have a complete clinical response, may not be successful.
PET as an indicator of complete response has also been evaluated.63,180 Tumors with low pretreatment standard uptake unit values, defined as less than 4.5, are less likely to show evidence of treatment response after chemoradiation. A higher likelihood of nodal and pathologic complete response has been demonstrated in tumors with pretreatment SUVmax values greater than 4.5. Patients with tumors in both low and high pretreatment SUVmax groups demonstrate similar survival rates.63 Although the initial PET SUVmax did not predict survival rates, it may be used to better select esophageal cancer patients for combined-modality treatment.
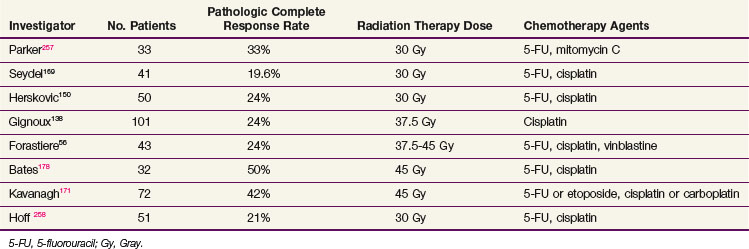
Compared with historical unimodality series, failures locally and distantly have markedly improved with the advent of multimodality therapy (Table 43-12). In general, preoperative irradiation and chemotherapy result in a 30% to 50% incidence of no pathologic evidence of tumor at the time of resection (Table 43-13). This finding predicts a favorable outcome. In the SWOG study, patients who had no evidence of disease at the time of surgery, when compared with all patients who had resection, had a projected 3-year survival rate of 45% versus 14%, and a median survival time of 32 months versus 14 months.168 In data from the University of North Carolina, the median survival time for patients with no evidence of disease at resection was 37 months versus 13 months for patients with residual tumor.178 Similarly, in the University of Michigan series,56 the median survival time was improved for patients without evidence of residual disease at the time of surgery compared to the entire cohort (70 vs. 29 months, respectively). Although survival rates were higher in patients who had no disease in the surgical specimen in the University of Michigan and University of North Carolina series, there were long-term survivors among patients with residual disease, suggesting that the addition of surgery, at least in a subset of patients, can produce additional cures after chemoradiation therapy.
TABLE 43-12 Patterns of Failure in Concurrent Chemotherapy and Radiation Therapy Followed by Surgical Resection

Recently, a retrospective review was performed using a recursive partitioning analysis for 276 patients who received chemoradiation before esophagectomy. These results supported the idea that a pathologic complete response predicted for improved survival rates compared with patients with residual disease, but was less prognostic than the presence of nodal disease and metastases.181 Nodal status following neoadjuvant chemoradiation has also been demonstrated to be a predictive factor for survival rates. Gaca and colleagues182 performed a retrospective analysis of 28 patients with a complete pathologic response and found that node-negative patients, regardless of T stage, experienced improved median disease-free survival rates compared with patients with stage N1 nodes. They also found that preoperative tumor stage, patient age, tumor location, or the presence of Barrett’s esophagus did not independently predict overall survival rates on univariate analysis. Multivariate analysis showed that only post-treatment nodal status, and not post-treatment tumor status, predicted disease-free survival rates.182
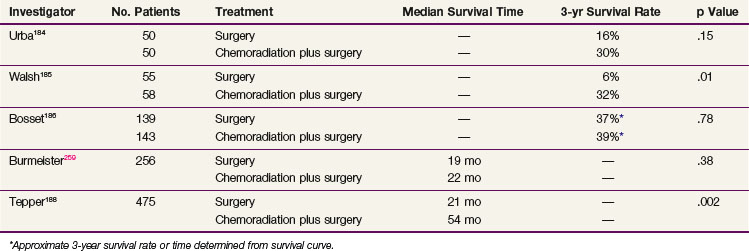
Despite the encouraging results from the preoperative chemoradiation studies, controversy exists over whether this approach is superior to that of surgery alone (Table 43-14). In a nonrandomized study of preoperative chemoradiation and surgery versus surgery alone, Vogel and associates183 reported an overall survival advantage for patients receiving multimodality therapy, with 36% alive at 5 years versus 11% for surgery alone. Randomized data from Urba and associates,184 in patients receiving preoperative concurrent chemoradiation compared with surgery alone, observed an overall nonstatistically significant survival advantage for patients in the multimodality arm; 30% were alive at 3 years compared with 16% in the surgery only arm. Walsh and coauthors185 conducted a randomized study of patients treated with two 5-day courses of 5-FU given on weeks 1 and 6 and cisplatin with concurrent (40 Gy) irradiation, delivered prior to surgery compared with patients who underwent surgery alone. The reported (actuarial) survival rates favored patients receiving combined-modality therapy, with 32% alive at 3 years versus 6% who underwent surgery only.185 In contrast, a randomized study reported by Bosset and colleagues186 discerned no survival advantage for patients receiving preoperative chemoradiation versus surgery alone. This study, however, used a unique irradiation and chemotherapy schedule: irradiation was delivered via a split course and, over the span of 2 weeks, a total dose of 37 Gy in 3.7-Gy daily fractions was delivered. The chemotherapy consisted of cisplatin given prior to each week of irradiation. Patients were taken to surgery 2 to 4 weeks after completing the preoperative regimen.186 The lack of benefit and increased toxicity observed for the combined-modality arm may have been predicted with the application of large radiation fractions, the inclusion of a planned treatment break, an inadequate recovery period between the preoperative therapy and surgery, and the use of single-agent chemotherapy. A recently reported meta-analysis of over 1000 patients enrolled in nine randomized trials evaluating preoperative chemoradiation versus surgery alone revealed a 0.66 survival odds ratio at 3 years in favor of patients receiving preoperative therapy.187
The U.S. Gastrointestinal Intergroup trial testing preoperative chemoradiation therapy versus surgery alone was stopped before completion because of poor accrual, and it is not likely that U.S. studies will pursue this question. Nonetheless, Tepper and associates188 reported the results from this trial (CALGB 9781) for the 56 patients who were enrolled before the trial closed. A median survival time of 4.48 years versus 1.79 years in favor of trimodality therapy and a 5-year survival rate of 39% versus 16% in favor of trimodality therapy were demonstrated on an intent-to-treat analysis.188
Preoperative Chemoradiation versus Definitive Chemoradiation
Bedenne and collaborators189,190 have reported the results of a study comparing preoperative chemoradiation followed by surgery versus definitive chemoradiation. In this randomized phase III trial, patients with locally advanced esophageal cancer received two cycles of induction 5-FU plus cisplatin with radiotherapy, either delivered via standard fractionation (46 Gy in 4.5 weeks) or over a split course. Patients were then randomized between surgical resection versus completion of definitive chemoradiation to 61 Gy. The reported 2-year survival rates and median survival times were not different, at 34% and 17.7 months for patients in the preoperative arm versus 40% and 19.3 months for patients randomized to definitive chemoradiation (p = .56). In a related German study, patients with locally advanced squamous carcinoma of the esophagus received induction chemotherapy followed by a randomization to either chemoradiotherapy (40 Gy) followed by surgery or definitive chemoradiotherapy.191 The analysis of the 172 randomized patients showed the overall survival rates to be equivalent; the survival rate at 3 years was 31% for patients randomized to combined-modality therapy versus 24% for patients treated with definitive chemoradiation. A study from the Minnie Pearl Cancer Research Network reflects the difficulties associated with studies that randomize patients away from a surgical intervention.192 In this study, patients with locally advanced esophageal cancer were to receive a course of preoperative chemoradiation followed either by surgery or by completion of the definitive chemoradiation regimen. One-hundred and ninety-four patients were entered, but only 57 patients actually proceeded with the treatment to which they were randomized. When all patients were considered, the survival rates were similar; the 3-year survival rate for patients undergoing resection was 35% versus 31% for patients receiving definitive chemoradiation.192 The data, taken in aggregate, suggest that the results with definitive chemoradiation are comparable to those observed with treatment strategies that incorporate preoperative chemoradiation and surgery.
Data regarding local relapse following definitive chemoradiation for localized esophageal cancer rather than surgery requiring salvage esophagectomy have provided additional information regarding the importance of adjuvant surgery. Compared with data from historical control patients undergoing planned esophagectomy 4 to 6 weeks after completion of chemoradiation, patients undergoing salvage esophagectomy following definitive chemoradiation experienced increased rates of operative death and complications, including the need for prolonged intubation or longer stays in the intensive care unit and hospital and increased anastomotic leaks. Salvage esophagectomy, however, resulted in a 25% 5-year survival rate in a subset of patients and seemed to benefit patients with stage T1to 2, N0 tumors, those who had undergone R0 resection, and those with a prolonged time to recurrence.193
Palliation
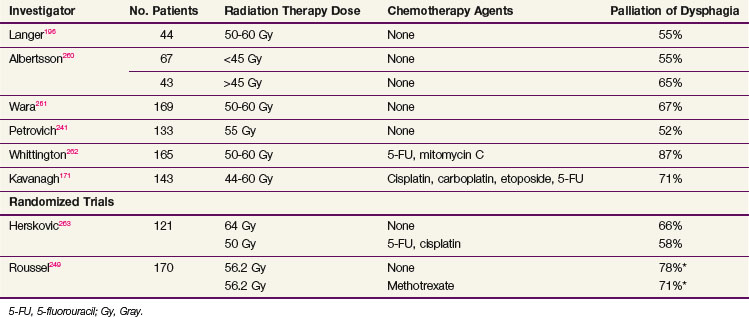
Oncologists can produce palliation with irradiation alone by using EBRT doses ranging from 30 Gy in 2 weeks to 5000 to 6000 cGy over 6 weeks (Table 43-15). Wara and colleagues194 reported on 103 patients who completed therapy with 5000 to 6000 cGy, with 89% having symptomatic improvement and 66% maintaining relief for 2 months or more. The average duration of palliation was 6 months. The dysphagia usually improved near the end of therapy, and almost all patients reported an arrest of their previous symptom progression.194 Similar data report a 60% to 88% improvement in dysphagia with radiation doses of 5000 cGy or higher.195,196 Concurrent chemoradiation may also be used for palliation of dysphagia. Studies demonstrate a rate of up to 88% in improvement in swallowing, with the median time to improvement being 2 weeks with durable relief of symptoms.158 Aggressive concurrent irradiation potentially increases complications in patients with incurable disease and a limited life span, but it may offer a more effective means of palliation.
Benign Strictures Resulting From Irradiation
Unfortunately, benign strictures can result from irradiation of esophageal cancers, causing a worsening of symptoms. The incidence of benign stricture is estimated at 12% to 30% in patients treated with irradiation alone. Strictures usually develops 4 to 6 weeks after therapy.158,197,198 In 25 patients treated with concurrent irradiation and chemotherapy, Coia and colleagues158 observed a 12% incidence of benign strictures that responded to one or two dilations.
Tracheoesophageal Fistula
The development of a malignant fistulous tract between the esophagus and airway (trachea or bronchus) is not uncommon because of the anatomic location of the two structures. Most fistulae involve the trachea, but they can also involve either a main stem, lobar, or segmental bronchus. Involvement of the trachea with tumor can lead to fistula formation during irradiation because of necrosis of the tumor or the natural progression of the disease. The middle-third lesions are most commonly involved. It is estimated that the incidence of this complication is 5% to 10% of patients with esophageal cancer. In general, excision, bypass, or intubation has been recommended in an attempt to prevent further contamination of the airway. The median survival time following these limited measures can be as brief as 6 to 10 weeks, with the procedures themselves resulting in a mortality rate of 10% to 32%.199
Many oncologists accept the fact that irradiation of a fistula worsens the condition because healing may be compromised by irradiation. However, Burt and associates200 found that the survival rate for patients with an untreated fistulous tract was 4% at 6 months and 1% at 1 year versus 15% and 5% in patients treated with irradiation, respectively. Yamado and collaborators201 reported on 14 patients with fistulae secondary to esophageal cancer who were treated with primary irradiation. Closure of the fistula occurred in 5 of 8 patients whose fistulae developed before or during irradiation. In two of these cases, the closure lasted over the long term.201 For patients who developed fistulae during irradiation, resolution or closure was less likely. Gschossmann and colleagues202 reported on 10 patients with fistulae treated with irradiation at the Mayo Clinic and observed that the severity of the fistulae did not increase with therapy and the median survival time was 4.8 months. There is also a growing body of data supporting the safe use of chemotherapy with or without irradiation in managing patients with a tracheoesophageal fistula. Malik and colleagues203 observed an objective response and closure of the fistulae in two patients treated with chemoradiation and concluded that the presence of a fistula should not exclude a patient from receiving combined-modality therapy. At present, it is difficult to determine whether aggressive combined-modality therapy will increase treatment-related morbidity in patients who present with airway or esophageal fistulae or develop them shortly after starting therapy. Once the diagnosis of a fistulous tract into the airway is documented and the process is stabilized, we recommend proceeding with planned curative therapy for selected patients with localized disease, especially if the fistula is relatively small.
Brachytherapy
Several small series have demonstrated that intracavitary irradiation in the appropriately selected patient is a safe and effective method of palliation. Harvey and coworkers204 reviewed 22 patients treated with either 2000 cGy in three fractions of low-dose-rate brachytherapy versus 1250 cGy in one fraction of high-dose-rate brachytherapy and found that both modalities resulted in equally effective palliation of dysphagia. Accelerated treatments are especially suitable for patients in poor physical condition or with a short life expectancy; however, the incidence of stricture and fistula formation increased with the brachytherapy fraction size (9.5% for fractions <500 cGy, 20% for fractions between 500 and 800 cGy, and 38% for fractions >800 cGy). In data reported by Gava and associates,205 the use of intraluminal brachytherapy in conjunction with either chemotherapy or EBRT resulted in superior palliation when compared with brachytherapy alone, at rates of 89%, 88%, and 71%, respectively, without an increase in treatment-related morbidity.
irradiation Techniques
The difficulty in controlling esophageal tumors with irradiation relates to the frequent extension of tumor through the thin esophageal wall, the involvement of vital mediastinal structures, including large vessels and the trachea, and the threat of perforation; the frequent spread of tumor through submucosal lymphatics, ultimately involving long segments of the esophagus; the spread of tumor to regional lymph nodes; the presence of metastatic disease in a high percentage of patients (either occult or clinically apparent disease); and the generally poor nutritional status of patients at presentation. Esophageal tumors can extend submucosally in the cephalocaudad direction for a significant distance from the primary tumor. In 1962, Miller206 reported a 15% incidence of longitudinal microscopic tumor spread at greater than 6 cm from the primary lesion and an incidence of regional nodal disease of approximately 40% to 70%. In an autopsy series reported by Bloedorn and Kasdorf,13 the presence of nodal disease was 70%.
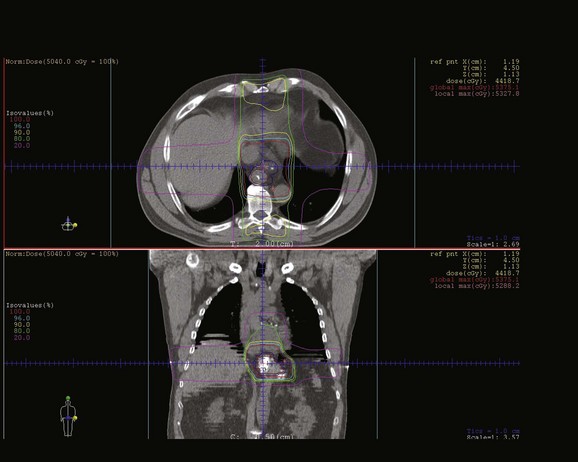
The typical irradiation field extends approximately 5 cm above and below gross tumor, with a field width of approximately 8 cm. The size of the tumor dictates the exact field width needed to obtain adequate mediastinal nodal coverage and to adequately cover the primary tumor mass. Figure 43-1 represents a three-dimensional CT-based plan demonstrating radiation treatment for a distal esophageal tumor.
Intensity-modulated radiation therapy (IMRT) carries the potential of reducing the amount of radiation delivered to surrounding normal tissues. However, much remains unknown about the routine use of IMRT for esophageal cancer because there is little literature regarding its use. Wu and coworkers207 compared IMRT plans with forward and inverse three-dimensional conformal irradiation plans for 15 patients with mid-esophageal cancers and demonstrated that the IMRT plans generated the most conformal high-dose distribution around the planning target volume while delivering a lower mean lung dose and mean heart dose. Others have demonstrated similar findings comparing the ability of three-dimensional therapy and IMRT to deliver simultaneous integrated boost, dose-escalated plans in upper esophageal locations. Lower V20 and V30 volumes in the lung were consistently demonstrated in the IMRT plans.208 One additional study explores the potential advantage of IMRT in distal esophageal cancers, resulting in a significant reduction in the mean lung dose, V10, and V20, without a significant reduction in the cardiac dose from the three-dimensional conformal plans.209 Because the actual use of IMRT plans has not been well studied, care needs to be taken to avoid the risk of unexpected normal tissue toxicities resulting from the low-dose regions being widely spread into normal tissue. Figure 43-2 is a dose-volume histogram demonstrating the dose distribution differences for surrounding normal structures between a three-dimensional treatment plan and an IMRT treatment plan for the same patient.
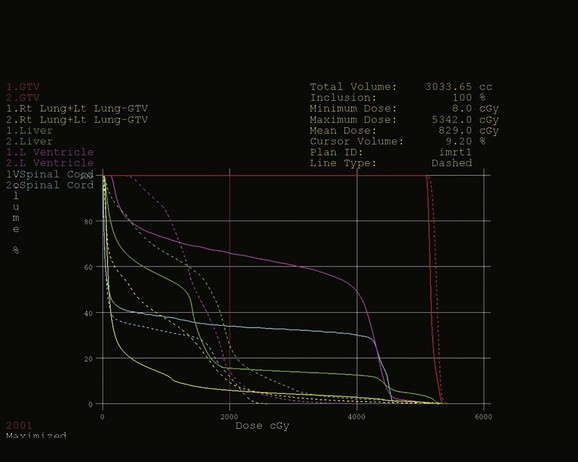
Figure 43-2 Dose-volume histograms compare dose delivered to surrounding normal tissues resulting from the three-dimensional treatment plan illustrated in Figure 43-1 (solid lines) and a seven-field intensity-modulated radiation therapy (IMRT) treatment plan (dashed lines) designed to treat the same volumes to the same dose (45 Gy to the initial clinical target volume, followed by a boost to the tumor plus 2 cm to 50.4 Gy). Note that mean doses of normal tissue are reduced, whereas the volumes of normal tissue receiving lower radiation doses (V10) increase for the IMRT plan compared with the three-dimensional conformal plan for all structures.
Other changes in treatment planning are being pursued with improved tumor localization. One study compared CT-based tumor volumes to PET/CT-based tumor volumes and demonstrated a change in target volumes for 84% of patients, with 48% with minor differences and 36% with major differences. These discrepancies were mostly in celiac or distant mediastinal lymph node involvement, resulting in a change in length of the tumor volume in 56% percent of patients.210 Although the routine use of PET/CT in treatment planning is still under investigation, it should be recognized that significant changes in treatment volumes may occur that will not only improve tumor targeting but may, in fact, increase the irradiation volume and irradiation-induced toxicity. Caution should be exercised in determining the exact size of a tumor from a PET scan, as the apparent size on PET imaging is determined heavily by the settings on the computer. PET is perhaps most useful in finding areas of unexpected disease and determining the precise location of known lesions.
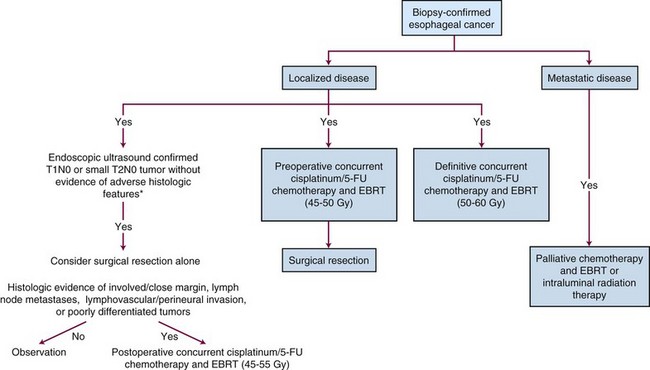
Future Possibilities and Treatment Algorithm
Until very recently, patients with esophageal cancer had little reason for optimism because treatment successes were rare. Although surgery alone remains the standard of care in the management of early-stage localized esophageal cancer, combined-modality approaches appear to have an important role for the typical patient with a locally advanced tumor. Figure 43-3 describes an algorithm for the treatment of esophageal cancer. Today, the treatment of this disease requires a multimodality approach by the surgeon, radiation oncologist, and medical oncologist and a clear understanding of the interactions and toxicities associated with these modalities. The results using combined chemoradiation either as definitive therapy or in the preoperative setting are encouraging but remain controversial because local control rates appear to be improved but the effect on survival time has not been well demonstrated. The complications associated with multimodality therapy is significant and must be considered. Newer, minimally invasive techniques are promising options in selected patients; they may reduce the complications associated with surgery. IMRT and improved tumor targeting using PET/CT imaging may assist in reducing treatment-related morbidity. The evolving role of newer chemotherapeutic agents such as paclitaxel, oxaliplatin, and the molecular targeted compounds and attempts at altering irradiation schedules and doses are currently are being studied. The optimal combinations of chemotherapy, irradiation, and/or surgery are not known but also remain under active investigation.
1 Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
2 Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30.
4 Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143:1198-1203. discussion Arch Surg 143:1203, 2008
5 Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
6 Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2-8.
8 Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184-1187.
9 Younes M, Henson DE, Ertan A, et al. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359-1365.
11 Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol. 2008;15:881-888.
14 Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289.
56 Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus. Final report. J Clin Oncol. 1993;11:1118-1123.
61 Sihvo EI, Rasanen JV, Knuuti MJ, et al. Adenocarcinoma of the esophagus and the esophagogastric junction. Positron emission tomography improves staging and prediction of survival in distant but not in locoregional disease. J Gastrointest Surg. 2004;8:988-996.
63 Rizk NP, Tang L, Adusumilli PS, et al. Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol. 2009;4:875-879.
64 Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction. The MUNICON phase II trial. Lancet Oncol. 2007;8:797-805.
69 Cerfolio RJ, Bryant AS, Ohja B, et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241.
79 Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer. An international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556.
80 Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239-1246.
87 Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir. 2009;64:121-133.
89 Perry KA, Enestvedt CK, Pham T, et al. Comparison of laparoscopic inversion esophagectomy and open transhiatal esophagectomy for high-grade dysplasia and stage I esophageal adenocarcinoma. Arch Surg. 2009;144:679-684.
100 Taira N, Doihara H, Oota T, et al. Gefitinib, an epidermal growth factor receptor blockade agent, shows additional or synergistic effects on the radiosensitivity of esophageal cancer cells in vitro. Acta Med Okayama. 2006;60:25-34.
101 Safran H, Suntharalingam M, Dipetrillo T, et al. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys. 2008;70:391-395.
104 VanCutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial. A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol. 2009;27(Suppl; Abstract LBA4509):18s.
105 Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2/neu-positive advanced gastric or gastro-oesophogeal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697.
113 Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161-1168.
120 Milas L. Cyclooxygenase-2 (COX-2) enzyme inhibitors as potential enhancers of tumor radioresponse. Semin Radiat Oncol. 2001;11:290-299.
125 Kulke MH, Odze RD, Mueller JD, et al. Prognostic significance of vascular endothelial growth factor and cyclooxygenase 2 expression in patients receiving preoperative chemoradiation for esophageal cancer. J Thorac Cardiovasc Surg. 2004;127:1579-1586.
128 Aisner JF, Forestiere A, Aroney R. Patterns of recurrence for cancer of the lung an esophagus. Cancer Treat Symp. 1983;2:87-105.
129 Herskovic AM, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598.
138 Gignoux M, Rousell A, Paillot B. The value of preoperative radiotherapy in esophageal cancer. Results of a study of the EORTC. World J Surg. 1987;11:426-432.
143 Minsky B, Neuberg D, Kelsen D, et al. Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90-12). Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 1999;43:517-523.
150 Herskovic A, Leichman L, Lattin P, et al. Chemo/radiation with and without surgery in the thoracic esophagus. The Wayne State experience. Int J Radiat Oncol Biol Phys. 1988;15:655-662.
151 Coia LR, Engstrom PF, Paul AR, et al. Long-term results of infusional 5-FU, Mitomycin C., and radiation as primary management of esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:29-36.
152 Araujo C, Souhami L, Gil R, et al. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67:2258-2261.
153 Sischy B, Ryan L, Haller D, et al. Interim report of EST 1282 phase III protocol for the evaluation of combined modalities in the treatment of patients with carcinoma of the esophagus, stage I and II (meeting abstract). Proc Am Soc Clin Oncol. 1990;9:105.
154 Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer. Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627.
155 al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer. An intergroup study. J Clin Oncol. 1997;15:277-284.
156 Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer. High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174.
159 Ajani JA, Winter K, Komaki R, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551-4556.
160 Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer. A randomised controlled trial. Lancet. 2002;359:1727-1733.
164 Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984.
168 Poplin E, Fleming T, Leichman L, et al. Combined therapies for squamous cell carcinoma of the esophagus. A Southwest Oncology Group Study (SWOG-8037). J Clin Oncol. 1990;5:622-628.
169 Seydel HG, Leichman L, Byhardt R, et al. Preoperative radiation and chemotherapy for localized squamous cell carcinoma of the esophagus. A RTOG Study. Int J Radiat Oncol Biol Phys. 1988;14:33-35.
170 Forastiere A, Orringer MB, Perez-Tamayo C, et al. Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local-regional cancer of the esophagus. J Clin Oncol. 1990;8:119-127.
181 Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507-512.
184 Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313.
185 Walsh TN, Noonam N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467.
186 Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous cell cancer of the esophagus. N Engl J Med. 1997;337:161-167.
187 Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-543.
188 Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092.
189 Bedenne L, Michel P, Bouche O, et al. Randomized phase III trial in locally advanced esophageal cancer. Radiochemotherapy followed by surgery versus radiochemotherapy alone (FFCD 9102). Proc Am Soc Clin Oncol. 2002;21(Abstract 519):130a.
190 Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus. FFCD 9102. J Clin Oncol. 2007;25:1160-1168.
1 Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
2 Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30.
3 Kelsen D. Preoperative chemoradiotherapy for esophageal cancer. J Clin Oncol. 2001;19:283-285.
4 Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143:1198-1203. discussion Arch Surg 143:1203, 2008
5 Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
6 Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2-8.
7 Devesa SS, Blot WJ, Fraumeni JFJr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053.
8 Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184-1187.
9 Younes M, Henson DE, Ertan A, et al. Incidence and survival trends of esophageal carcinoma in the United States. Racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359-1365.
10 Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99:582-588.
11 Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol. 2008;15:881-888.
12 Hesketh PJ, Clapp RW, Doos WG, et al. The increasing frequency of adenocarcinoma of the esophagus. Cancer. 1989;64:526-530.
13 Bloedorn FG, Kasdorf H. Radiotherapy in squamous cell carcinoma of the esophagus. In: Clark RL, Cumley RW, McCay JE, et al, editors. Oncology. Chicago: Year Book; 1971:111-120.
14 Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289.
15 Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146.
16 Steyerberg EW, Earle CC, Neville BA, et al. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer. A population-based analysis of elderly patients. J Clin Oncol. 2005;23:510-517.
17 Brown LM, Hoover RN, Greenberg RS, et al. Are racial differences in squamous cell esophageal cancer explained by alcohol and tobacco use? J Natl Cancer Inst. 1994;86:1340-1345.
18 Baron PL, Gates CE, Reed CE, et al. p53 overexpression in squamous cell carcinoma of the esophagus. Ann Surg Oncol. 1997;4:37-45.
19 Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9.
20 Gore RM. Esophageal cancer. Radiol Clin North Am. 1997;35:243-263.
21 Auerbach O, Stout AP, Hammond EC, et al. Histologic changes in esophagus in relation to smoking habits. Arch Environ Health. 1965;11:4-15.
22 Sons HU. Etiologic and epidemiologic factors of carcinoma of the esophagus. Surg Gynecol Obstet. 1987;165:183-190.
23 Stellman JM, Stellman SD. Cancer and the work place. CA Cancer J Clin. 1996;46:70-92.
24 He D, Zhang DK, Lam KY, et al. Prevalence of HPV infection in esophageal squamous cell carcinoma in Chinese patients and its relationship to the p53 mutation. Int J Cancer. 1997;72:959-964.
25 Togowa K, Rustigi A. Human papillomavirus DNA sequences in esophagus squamous cell carcinoma. Gastroenterology. 1994;107:128-136.
26 Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150-155.
27 Navarro S, Mayne ST, Risch H. Dietary and lifestyle patterns and risk of subtypes of esophageal and gastric cancer. Factor analysis. J Nutr. 2004;134:3521-3547.
28 Suleiman UL, Harrison M, Britton A, et al. H2-receptor antagonists may increase the risk of cardio-oesophageal adenocarcinoma. A case-control study. Eur J Cancer Prev. 2000;9:185-191.
29 Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7:1299-1304.
30 Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer. A systematic review and meta-analysis. Gastroenterology. 2003;124:47-56.
31 Vaughan TL, Dong LM, Blount PL, et al. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus. A prospective study. Lancet Oncol. 2005;6:945-952.
32 Das D, Chilton AP, Jankowski JA. Chemoprevention of oesophageal cancer and the AspECT trial. Recent Results Cancer Res. 2009;181:161-169.
33 Wilson K, Fu S, Ramanujam K, et al. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929-2934.
34 Katja C, Mario S, Artur-Aron W, et al. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204.
35 Shamma A, Yamamoto H, Doki Y, et al. Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res. 2000;6:1229-1238.
36 Rossi E, Villanacci V, Bassotti G, et al. Her-2/neu in Barrett esophagus. A comparative study between histology, immunohistochemistry, and fluorescence in situ hybridization. Diagn Mol Pathol. 2006;15:125-130.
37 Villanacci V, Rossi E, Grisanti S, et al. Targeted therapy with trastuzumab in dysplasia and adenocarcinoma arising in Barrett’s esophagus. A translational approach. Minerva Gastroenterol Dietol. 2008;54:347-353.
38 Casson AG, Manolopoulos B, Troster M, et al. Clinical implications of p53 gene mutation in the progression of Barrett’s epithelium to invasive esophageal cancer. Am J Surg. 1994;167:52-57.
39 Casson AG, Mukhopadhyay T, Cleary KR, et al. p53 gene mutations in Barrett’s epithelium and esophageal cancer. Cancer Res. 1991;51:4495-4499.
40 Arber N, Lightdale C, Rotterdam H, et al. Increased expression of the cyclin D1 gene in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 1996;5:457-459.
41 Barrett MT, Sanchez CA, Galipeau PC, et al. Allelic loss of 9p21 and mutation of the CDKN2/p16 gene develop as early lesions during neoplastic progression in Barrett’s esophagus. Oncogene. 1996;13:1867-1873.
42 Montesano R, Hollstein M, Hainaut P. Molecular etiopathogenesis of esophageal cancers. Ann Ist Super Sanita. 1996;32:73-84.
43 Coggi G, Bosari S, Roncalli M, et al. p53 protein accumulation and p53 gene mutation in esophageal carcinoma. A molecular and immunohistochemical study with clinicopathologic correlations. Cancer. 1997;79:425-432.
44 Schrump DS, Chen GA, Consuli U, et al. Inhibition of esophageal cancer proliferation by adenovirally mediated delivery of p16INK4. Cancer Gene Ther. 1996;3:357-364.
45 Jiang W, Kahn SM, Tomita N, et al. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992;52:2980-2983.
46 Gibson MK, Abraham SC, Wu TT, et al. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9:6461-6468.
47 Itakura Y, Sasano H, Shiga C, et al. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994;74:795-804.
48 Yacoub L, Goldman H, Odze RD. Transforming growth factor-alpha, epidermal growth factor receptor, and MiB-1 expression in Barrett’s-associated neoplasia. Correlation with prognosis. Mod Pathol. 1997;10:105-112.
49 Takaoka M, Harada H, Andl CD, et al. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64:7711-7723.
50 al-Kasspooles M, Moore JH, Orringer MB, et al. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer. 1993;54:213-219.
51 Miller CT, Moy JR, Lin L, et al. Gene amplification in esophageal adenocarcinomas and Barrett’s with high-grade dysplasia. Clin Cancer Res. 2003;9:4819-4825.
52 Rygiel AM, Milano F, Ten Kate FJ, et al. Gains and amplifications of c-myc, EGFR, and 20.q13 loci in the no dysplasia-dysplasia-adenocarcinoma sequence of Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2008;17:1380-1385.
53 Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9-S15.
54 Holscher AH, Bollschweiler E, Schneider PM, et al. Prognosis of early esophageal cancer. Comparison between adeno and squamous cell carcinoma. Cancer. 1995;76:178-186.
55 Dumont P, Wihlm JM, Roeslin N, et al. Results of surgery of esophageal cancer. Analysis of a series of 349 cases based on resection methods. Ann Chir. 1993;47:773-783.
56 Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus. Final report. J Clin Oncol. 1993;11:1118-1123.
57 Block MI, Patterson GA, Sundaresan RS, et al. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg. 1997;64:770-776. discussion Ann Thorac Surg 64:776-777, 1997
58 Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202-3210.
59 Flanagan FL, Dehdashti F, Siegel BA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol. 1997;168:417-424.
60 Luketich JD, Schauer PR, Meltzer CC, et al. Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg. 1997;64:765-769.
61 Sihvo EI, Rasanen JV, Knuuti MJ, et al. Adenocarcinoma of the esophagus and the esophagogastric junction. Positron emission tomography improves staging and prediction of survival in distant but not in locoregional disease. J Gastrointest Surg. 2004;8:988-996.
62 Westreenen HV, Westerterp M, Bossuyt P, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805-3812.
63 Rizk NP, Tang L, Adusumilli PS, et al. Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol. 2009;4:875-879.
64 Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction. The MUNICON phase II trial. Lancet Oncol. 2007;8:797-805.
65 Hiele M, De Leyn P, Schurmans P, et al. Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc. 1997;45:381-386.
66 Natsugoe S, Yoshinaka H, Morinaga T, et al. Ultrasonographic detection of lymph-node metastasis in superficial carcinoma of the esophagus. Endoscopy. 1996;28:674-679.
67 Krasna MJ, Reed CE, Jaklitsch MT, et al. Thoracoscopic staging of esophageal cancer. A prospective, multi-institutional trial. Cancer and Leukemia Group B Thoracic Surgeons. Ann Thorac Surg. 1995;60:1337-1340.
68 Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer. Implications for response classification. Ann Surg. 2005;242:684-692.
69 Cerfolio RJ, Bryant AS, Ohja B, et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241.
70 Holscher AH, Bollschweiller E, Bumm R, et al. Prognostic factors of resected adenocarcinoma of the esophagus. Surgery. 1995;118:845-855.
71 Torek F. The first successful case of resection of the thoracic portion of the oesphagus for carcinoma. Surg Gynecol Obstet. 1913;16:614-617.
72 Ohsawa T. The surgery of the esophagus. Arch Jpn Chir. 1933;10:605.
73 Adams W, Phernister DP. Carcinoma of the lower thoracic esophagus. Report of a successful resection and esophagogastrostomy. J Thorac Surg. 1938;7:621-632.
74 Moertel CG. Carcinoma of the esophagus. Is there a role for surgery? The case against surgery. Am J Dig Dis. 1978;23:735-736.
75 Mariette C, Piessen G, Balon JM, et al. Surgery alone in the curative treatment of localised oesophageal carcinoma. Eur J Surg Oncol. 2004;30:869-876.
76 Frunberger L, Kraus B, Dworak O. Distribution of lymph nodes and lymph node metastasis in esophageal carcinoma. Zentralbl Chir. 1996;121:102-105.
77 Sun K, Zhang R, Zhang D, et al. Prognostic significance of lymph node metastasis in surgical resection of esophageal cancer. Chinese Med J. 1996;109:89-92.
78 Collard JM, Otte JB, Reynaert MS, et al. Extensive lymph node clearance for cancer of the esophagus or cardia. Merits and limits in reference to 5 year absolute survival. Hepatogastroenterology. 1995;42:619-627.
79 Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer. An international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556.
80 Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239-1246.
81 Fok M, Siu KF, Wong J. A comparison of transhiatal and transthoracic resection for carcinoma of the thoracic esophagus. Am J Surg. 1989;158:414-419.
82 Pac M, Basoglu A, Kocak H, et al. Transhiatal versus transthoracic esophagectomy for esophageal cancer. J Thorac Cardiovasc Surg. 1993;106:205-209.
83 Stark SP, Romberg MS, Pierce GE, et al. Transhiatal versus transthoracic esophagectomy for adenocarcinoma of the distal esophagus and cardia. Am J Surg. 1996;172:478-481.
84 Chu KM, Law SY, Fok M, et al. A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg. 1997;174:320-324.
85 Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy. A prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114-1120.
86 Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus. A meta-analysis. Ann Thorac Surg. 2001;72:306-313.
87 Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer. A systematic review and meta-analysis. Minerva Chir. 2009;64:121-133.
88 Perry KA, Enestvedt CK, Diggs BS, et al. Perioperative outcomes of laparoscopic transhiatal inversion esophagectomy compare favorably with those of combined thoracoscopic-laparoscopic esophagectomy. Surg Endosc. 2008;23:2147-2154.
89 Perry KA, Enestvedt CK, Pham T, et al. Comparison of laparoscopic inversion esophagectomy and open transhiatal esophagectomy for high-grade dysplasia and stage I esophageal adenocarcinoma. Arch Surg. 2009;144:679-684.
90 Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma. Long-term results. Gastrointest Endosc. 2003;58:183-188.
91 Tokar JL, Haluszka O, Weinberg DS. Endoscopic therapy of dysplasia and early-stage cancers of the esophagus. Semin Radiat Oncol. 2007;17:10-21.
92 Hirai T, Kuwahara M, Yoshida K, et al. Clinical results of transhiatal esophagectomy for carcinoma of the lower thoracic esophagus according to biological markers. Dis Esophagus. 1998;11:221-225.
93 Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas. SWOG 0127. J Clin Oncol. 2006;24:4922-4927.
94 Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol. 2006;24:1612-1619.
95 Ferry DR, Anderson M, Beddard K, et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma. Evidence of gene expression, cellular, and clinical response. Clin Cancer Res. 2007;13:5869-5875.
96 Gold P, Goldman B, Iqbal S: Cetuximab as second-line therapy in patients with metastatic esophageal cancer. A phase II Southwest Oncology Group Study. Proceedings of the American Society of Clinical Oncology Annual Meeting Abstract no. 4536, 2008.
97 Ku G, Shah M, Tang L: Cetuximab (C225) plus irinotecan/cisplatin (CPT/Cis) for CPT/Cis-refractory esophageal cancer. Proceedings of the American Society of Clinical Oncology Annual Meeting Abstract no. 15580, 2008.
98 Pinto C, Defabio F, Siena S. Phase II study of cetuximab plus FOLFIRI as first-line treatment in patients with unresectable or metastatic gastric or gastroesophageal junction cancer. Preliminary results. J Clin Oncol. 2006;24(Abstract 4031):186s.
99 Rojo F, Tabernero J, Albanell J, et al. Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol. 2006;24:4309-4316.
100 Taira N, Doihara H, Oota T, et al. Gefitinib, an epidermal growth factor receptor blockade agent, shows additional or synergistic effects on the radiosensitivity of esophageal cancer cells in vitro. Acta Med Okayama. 2006;60:25-34.
101 Safran H, Suntharalingam M, Dipetrillo T, et al. Cetuximab with concurrent chemoradiation for esophagogastric cancer. Assessment of toxicity. Int J Radiat Oncol Biol Phys. 2008;70:391-395.
102 Aklilu M, Ilson DH. Targeted agents and esophageal cancer—the next step? Semin Radiat Oncol. 2007;17:62-69.
103 Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554-568.
104 VanCutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial. A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol. 2009;27(Suppl; Abstract LBA4509):18s.
105 Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2/neu-positive advanced gastric or gastro-oesophogeal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697.
106 Lord RV, Park JM, Wickramasinghe K, et al. Vascular endothelial growth factor and basic fibroblast growth factor expression in esophageal adenocarcinoma and Barrett esophagus. J Thorac Cardiovasc Surg. 2003;125:246-253.
107 Inoue A, Moriya H, Katada N, et al. Intratumoral lymphangiogenesis of esophageal squamous cell carcinoma and relationship with regulatory factors and prognosis. Pathol Int. 2008;58:611-619.
108 Inoue K, Ozeki Y, Suganuma T, et al. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206-213.
109 Kitadai Y, Amioka T, Haruma K, et al. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001;93:662-666.
110 Kitadai Y, Haruma K, Tokutomi T, et al. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4:2195-2200.
111 Kleespies A, Bruns CJ, Jauch KW. Clinical significance of VEGF-A, -C and -D expression in esophageal malignancies. Onkologie. 2005;28:281-288.
112 Kleespies A, Guba M, Jauch KW, et al. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol. 2004;87:95-104.
113 Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161-1168.
114 Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206.
115 Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374-3378.
116 Nomiya T, Nemoto K, Miyachi H, et al. Relationships between radiosensitivity and microvascular density in esophageal carcinoma. Significance of hypoxic fraction. Int J Radiat Oncol Biol Phys. 2004;58:589-596.
117 Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors. A rational advance? J Natl Cancer Inst. 2003;95:1440-1452.
118 Kishi K, Petersen S, Petersen C, et al. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:1326-1331.
119 Milas L. Cyclooxygenase-2 (COX-2) enzyme inhibitors and radiotherapy. Preclinical basis. Am J Clin Oncol. 2003;26:S66-S69.
120 Milas L. Cyclooxygenase-2 (COX-2) enzyme inhibitors as potential enhancers of tumor radioresponse. Semin Radiat Oncol. 2001;11:290-299.
121 Milas L, Mason KA, Crane CH, et al. Improvement of radiotherapy or chemoradiotherapy by targeting COX-2 enzyme. Oncology (Williston Park). 2003;17:15-24.
122 Petersen C, Petersen S, Milas L, et al. Enhancement of intrinsic tumor cell radiosensitivity induced by a selective cyclooxygenase-2 inhibitor. Clin Cancer Res. 2000;6:2513-2520.
123 Raju U, Nakata E, Yang P, et al. In vitro enhancement of tumor cell radiosensitivity by a selective inhibitor of cyclooxygenase-2 enzyme: mechanistic considerations. Int J Radiat Oncol Biol Phys. 2002;54:886-894.
124 Govindan R, McLeod H, Mantravadi P, et al. Cisplatin, fluorouracil, celecoxib, and RT in resectable esophageal cancer. Preliminary results. Oncology (Williston Park). 2004;18:18-21.
125 Kulke MH, Odze RD, Mueller JD, et al. Prognostic significance of vascular endothelial growth factor and cyclooxygenase 2 expression in patients receiving preoperative chemoradiation for esophageal cancer. J Thorac Cardiovasc Surg. 2004;127:1579-1586.
126 Buschke F. Surgical and radiological results in the treatment of esophageal carcinoma. AJR Am J Roentgenol. 1954;71:9-21.
127 Kelsen D. Neoadjuvant therapy for gastrointestinal cancers. Oncology. 1993;7:25-32.
128 Aisner JF, Forestiere A, Aroney R. Patterns of recurrence for cancer of the lung and esophagus. Cancer Treat Symp. 1983;2:87-105.
129 Herskovic AM, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598.
130 John MF, Mowr P, Podolsky W, et al. Radiotherapy alone and chemoradiation for nonmetastatic esophageal carcinoma. A critical review of chemoradiation. Cancer. 1989;63:2397-2403.
131 Goodner JT. Surgical and radiation treatment of cancer of the thoracic esophagus. AJR Am J Roentgenol. 1969;105:523-528.
132 Nakayama K, Orihata H, Yamaguchi K. Surgical treatment combined with preoperative concentrated irradiation for esophageal cancer. Cancer. 1967;20:778-788.
133 Isono K, Onoda S, Ishikawa T, et al. Studies on the causes of deaths from esophageal carcinoma. Cancer. 1982;49:2173-2179.
134 Akakura I, Nakamura Y, Kakegawa T, et al. Surgery for carcinoma of the esophagus with preoperative irradiation. Chest. 1970;57:47.
135 Launois B, DeLarue D, Campion JP, et al. Preoperative radiotherapy for carcinoma of the esophagus. Surg Gynecol Obstet. 1981;153:690.
136 Huang G, Gu X, Wang I. Experience with combined preoperative irradiation and surgery for carcinoma of the esophagus. Gann Monogr Cancer Res. 1986;31:159-164.
137 Wang LJ, Huang GJ: Combined preoperative irradiation and surgery versus surgery alone for carcinoma of the midthoracic esophagus. A prospective randomized study in 360 patients (Abstract). Presented at the Fourth World Congress of the International Society for Diseases of the Esophagus. Rennes, France, 1989.
138 Gignoux M, Rousell A, Paillot B. The value of preoperative radiotherapy in esophageal cancer. Results of a study of the EORTC. World J Surg. 1987;11:426-432.
139 Izquierdo M, Marcuello E, Gomez de Segura G, et al. Unresectable nonmetastatic squamous cell carcinoma of the esophagus managed by sequential chemotherapy (cisplatin and bleomycin) and radiation therapy. Cancer. 1993;71:287-292.
140 Valerdi JJ, Tejedor TM, Illarramendi JJ, et al. Neoadjuvant chemotherapy and radiotherapy in locally advanced esophageal carcinoma. Long-term results. Int J Radiat Oncol Biol Phys. 1993;27:843-847.
141 Sharma D, Krasnow SK, Davis EB, et al. Sequential chemotherapy and radiotherapy for squamous cell esophageal carcinoma. Am J Clin Oncol. 1997;20:151-153.
142 Stahl M, Wilke H, Fink U, et al. Combined preoperative chemotherapy and radiotherapy in patients with locally advanced esophageal cancer. Interim analysis of a phase II trial. J Clin Oncol. 1996;14:829-837.
143 Minsky B, Neuberg D, Kelsen D, et al. Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90-12). Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 1999;43:517-523.
144 Minsky BD, Neuberg D, Kelsen DP, et al. Neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. A preliminary analysis of the phase II intergroup trial 0122. J Clin Oncol. 1996;14:149-155.
145 Kikuchi Y. Study on clinical application of multiple fractions per day radiation therapy with concomitant boost technique for esophageal cancer. Hokkaido Igaku Zasshi. 1993;68:537-556.
146 Zhao KL, Wang Y, Shi XH. Late course accelerated hyperfractionated radiotherapy for clinical T1-2 esophageal carcinoma. World J Gastroenterol. 2003;9:1374-1376.
147 Girvin GW, Matsumoto GH, Bates DM, et al. Treating esophageal cancer with combination of chemotherapy, radiation and excision. Am J Surg. 1995;169:557-559.
148 Adelstein DJ, Rice TW, Becker M, et al. Use of concurrent chemotherapy, accelerated fractionation radiation, and surgery for patients with esophageal carcinoma. Cancer. 1997;80:1011-1020.
149 Yu L, Vikram B, Malamud S, et al. Chemotherapy rapidly alternating with twice-a-day accelerated radiation therapy in carcinomas involving the hypopharynx or esophagus. An update. Cancer Invest. 1995;13:567-572.
150 Herskovic A, Leichman L, Lattin P, et al. Chemo/radiation with and without surgery in the thoracic esophagus. The Wayne State experience. Int J Radiat Oncol Biol Phys. 1988;15:655-662.
151 Coia LR, Engstrom PF, Paul AR, et al. Long-term results of infusional 5-FU, Mitomycin C, and radiation as primary management of esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:29-36.
152 Araujo C, Souhami L, Gil R, et al. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67:2258-2261.
153 Sischy B, Ryan L, Haller D, et al. Interim report of EST 1282 phase III protocol for the evaluation of combined modalities in the treatment of patients with carcinoma of the esophagus, stage I and II (meeting abstract). Proc Am Soc Clin Oncol. 1990;9:105.
154 Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer. Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627.
155 al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer. An intergroup study. J Clin Oncol. 1997;15:277-284.
156 Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer. High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174.
157 Sauter ER, Coia LR, Keller SM. Pre-operative high dose radiation and chemotherapy in adenocarcinoma of the esophagus and esophagogastric junction. Ann Surg Oncol. 1994;1:5-10.
158 Coia L, Soffen E, Schultheiss T, et al. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer. 1993;71:281-286.
159 Ajani JA, Winter K, Komaki R, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551-4556.
160 Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer. A randomised controlled trial. Lancet. 2002;359:1727-1733.
161 Kok TC, Lanschot J, Siersema PD, et al. Neoadjuvant chemotherapy in operable esophageal squamous cell cancer. Final report of a phase III multi-center randomized controlled trial. Proc Am Soc Clin Oncol. 1997;16:277a.
162 Law S, Fok M, Chow S, et al. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus. A prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210-217.
163 Roth JA, Pass HI, Flanagan MM, et al. Randomized clinical trial of pre-operative and post-operative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1988;96:242-248.
164 Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984.
165 Armanios M, Xu R, Forastiere AA, et al. Adjuvant chemotherapy for resected adenocarcinoma of the esophagus, gastro-esophageal junction, and cardia. Phase II trial (E8296) of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:4495-4499.
166 Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus. A Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol. 2003;15:4592-4595.
167 Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus. The Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg. 1997;114:205-209.
168 Poplin E, Fleming T, Leichman L, et al. Combined therapies for squamous cell carcinoma of the esophagus. A Southwest Oncology Group Study (SWOG-8037). J Clin Oncol. 1990;5:622-628.
169 Seydel HG, Leichman L, Byhardt R, et al. Preoperative radiation and chemotherapy for localized squamous cell carcinoma of the esophagus. A RTOG Study. Int J Radiat Oncol Biol Phys. 1988;14:33-35.
170 Forastiere A, Orringer MB, Perez-Tamayo C, et al. Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local-regional cancer of the esophagus. J Clin Oncol. 1990;8:119-127.
171 Kavanagh B, Ancher M, Leopold K, et al. Patterns of failure following combined modality therapy or esophageal cancer, 1984-1990. Int J Radiat Oncol Biol Phys. 1992;24:633-642.
172 Choi N, Park SD, Lynch T, et al. Twice-daily radiotherapy as concurrent boost technique during two chemotherapy cycles in neoadjuvant chemoradiotherapy for resectable esophageal carcinoma. Mature results of phase II study. Int J Radiat Oncol Biol Phys. 2004;60:111-122.
173 Urba SG, Orringer MB, Ianettonni M, et al. Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer. 2003;98:2177-2183.
174 Ilson DH, Saltz L, Enzinger P, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol. 1999;17:3270-3275.
175 Khushalani NI, Leichman CG, Proulx G, et al. Oxaliplatin in combination with protracted-infusion fluorouracil and radiation. Report of a clinical trial for patients with esophageal cancer. J Clin Oncol. 2002;20:2844-2850.
176 Lorenzen S, Brücher B, Zimmermann F, et al. Neoadjuvant continuous infusion of weekly 5-fluorouracil and escalating doses of oxaliplatin plus concurrent radiation in locally advanced oesophageal squamous cell carcinoma. Results of a phase I/II trial. Br J Cancer. 2008;99:1020-1026.
177 Burmeister BH, Walpole ET, D’Arcy N, et al. A phase II trial of chemoradiation therapy with weekly oxaliplatin and protracted infusion of 5-fluorouracil for esophageal cancer. Invest New Drugs. 2009;27:275-279.
178 Bates BA, Detterbeck FC, Bernard SA, et al. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol. 1996;14:156-163.
179 Shaukat A, Mortazavi A, Demmy T, et al. Should preoperative endoscopy (EGD) be performed routinely for esophageal cancer patients after chemoradiation treatment? Proc Am Soc Clin Oncol. 2003;22:365.
180 Kato H, Fukuchi M, Miyazaki T, et al. Prediction of response to definitive chemoradiotherapy in esophageal cancer using positron emission tomography. Anticancer Res. 2007;27:2627-2633.
181 Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507-512.
182 Gaca JG, Petersen RP, Peterson BL, et al. Pathologic nodal status predicts disease-free survival after neoadjuvant chemoradiation for gastroesophageal junction carcinoma. Ann Surg Oncol. 2006;13:340-346.
183 Vogel SB, Mendenhall WM, Sombeck MD, et al. Downstaging of esophageal cancer after pre-operative radiation and chemotherapy. Ann Surg. 1995;221:685-693.
184 Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313.
185 Walsh TN, Noonam N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467.
186 Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous cell cancer of the esophagus. N Engl J Med. 1997;337:161-167.
187 Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-543.
188 Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer. CALGB 9781. J Clin Oncol. 2008;26:1086-1092.
189 Bedenne L, Michel P, Bouche O, et al. Randomized phase III trial in locally advanced esophageal cancer. Radiochemotherapy followed by surgery versus radiochemotherapy alone (FFCD 9102). Proc Am Soc Clin Oncol. 2002;21(Abstract 519):130a.
190 Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus. FFCD 9102. J Clin Oncol. 2007;25:1160-1168.
191 Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-2317.
192 Meluch AA, Greco FA, Gray JR, et al. Preoperative therapy with concurrent paclitaxel/carboplatin/infusional 5-FU and radiation therapy in locoregional esophageal cancer. Final results of a Minnie Pearl Cancer Research Network phase II trial. Cancer J. 2003;9:251-260.
193 Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002;123:175-183.
194 Wara WM, Mauch PM, Thomas AN, et al. Palliation for carcinoma of the esophagus. Radiology. 1976;121:717-720.
195 Caspers R, Welvaart K, Verkes R. The effect of radiotherapy on dysphagia and survival in patients with esophageal cancer. Radiother Oncol. 1988;12:15-23.
196 Langer M, Choi NC, Orlow E, et al. Radiation therapy alone or in combination with surgery in the treatment of carcinoma of the esophagus. Cancer. 1986;58:1208-1213.
197 O’Rourke IC, Tiver K, Bull C, et al. Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer. 1988;61:2022-2026.
198 Beatty JD, DeBoer G, Rider WD. Carcinoma of the esophagus—pretreatment assessment, correlation of radiation treatment parameters with survival and identification and management of radiation treatment failure. Cancer. 1979;43:2254-2267.
199 Little AG, Ferguson MK, Demeester TR, et al. Esophageal carcinoma with respiratory tract fistula. Cancer. 1984;53:1322-1328.
200 Burt M, Diehl W, Martini N, et al. Malignant esophagorespiratory fistula. Management options and survival. Ann Thorac Surg. 1991;52:1222-1228.
201 Yamado S, Takai Y, Ogawa Y, et al. Radiotherapy for malignant fistula to other tract. Cancer. 1989;64:1026-1028.
202 Gschossmann JM, Bonner JA, Foote RL, et al. Malignant tracheoesophageal fistula in patients with esophageal cancer. Cancer. 1993;72:1513-1521.
203 Malik SM, Krasnow SH, Wadleigh RG. Closure of tracheoesophageal fistulas with primary chemotherapy in patients with esophageal cancer. Cancer. 1994;73:1321-1323.
204 Harvey JC, Fleishman EH, Bellotti JE, et al. Intracavitary radiation in the treatment of advanced esophageal carcinoma. A comparison of high dose rate vs. low dose rate brachytherapy. J Surg Oncol. 1993;52:101-104.
205 Gava A, Fontan L, Bolner A, et al. High-dose-rate brachytherapy in esophageal carcinoma. The Italian experience. Radiol Med (Torino). 1996;91:118-121.
206 Miller C. Carcinoma of thoracic oesophagus and cardia. A review of 405 cases. Br J Surg. 1962;49:507-522.
207 Wu VW, Sham JS, Kwong DL. Inverse planning in three-dimensional conformal and intensity-modulated radiotherapy of mid-thoracic oesophageal cancer. Br J Radiol. 2004;77:568-572.
208 Fu WH, Wang LH, Zhou ZM, et al. Comparison of conformal and intensity-modulated techniques for simultaneous integrated boost radiotherapy of upper esophageal carcinoma. World J Gastroenterol. 2004;10:1098-1102.
209 Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol. 2005;77:247-253.
210 Hong TS, Killoran JH, Mamede M, et al. Impact of manual and automated interpretation of fused PET/CT data on esophageal target definitions in radiation planning. Int J Radiat Oncol Biol Phys. 2008;72:1612-1618.
211 Smithers DW. Adenocarcinoma of the oesophagus. Thorax. 1956;11:257-267.
212 Birgisson S, Rice TW, Easley KA, et al. The lack of association between adenocarcinoma of the esophagus and gastric surgery. A retrospective study. Am J Gastroenterol. 1997;92:216-221.
213 Lui KE, Panchal AS, Santhanagopal A, et al. Epidermal growth factor stimulates proton efflux from chrondrocytic cells. J Cell Physiol. 2002;192:102-112.
214 Wilkinson NW, Black JD, Roukhadze E, et al. Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. J Gastrointest Surg. 2004;8:448-453.
215 Inada S, Koto T, Futami K, et al. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor). Surg Today. 1999;29:493-503.
216 Davis S, Shanmugathasa M, Kessler W. Cis-dichlorodiammine platinum (II) in the treatment of esophageal carcinoma. Cancer Treat Rep.. 1980;64:709-711.
217 Engstrom P, Lavin P, Lassen D. Evaluation of mitomycin and cisplatinum in advanced esophageal carcinoma. Cancer Treat Rep. 1983;67:709-711.
218 Ezdinli E, Gelber R, Desai D, et al. Chemotherapy of advanced esophageal carcinoma. Cancer. 1980;46:2149-2153.
219 Bezwoda W, Derman D: Treatment of advanced oesophageal cancer with vindesine. Proceedings of the 13th International Cancer Congress, Abstract 41, 1983.
220 Kelsen D, Bains D, Cvitkovic E. Vindesine in the treatment of esophageal carcinoma. A phase II study. Cancer Treat Rep. 1979;63:2019-2021.
221 Kulke MH, Muzikansky A, Clark J, et al. A Phase II trial of vinorelbine in patients with advanced gastroesophageal adenocarcinoma. Cancer Invest. 2006;24:346-350.
222 Ajani J, Ilson D, Daugherty K. Taxol (Paclitaxel) is active against carcinoma of the esophagus. Report of a phase II trial. Proc Am Soc Clin Oncol. 1994;13:192.
223 Harstrick A, Bokemeyer C, Preusser P, et al. Phase II study of single-agent etoposide in patients with metastatic squamous-cell carcinoma of the esophagus. Cancer Chemother Pharmacol. 1992;29:321-322.
224 Enzinger PC, Kulke MH, Clark JW, et al. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci. 2005;50:2218-2223.
225 Tew W, Shah M, Schwartz G, et al. Phase II trial of erlotonib for second-line treatment in advanced esophageal cancer. Proc Am Soc Clin Oncol. 2005;Abstract:10247.
226 VanGroeningen C, Richel D, Giaccone G. Gefitinib phase II study in second-line treatment of advanced esophageal cancer. Proc Am Soc Clin Oncol. 2004;22:4022.
227 Kelsen K, Cvitkovic E, Bains M. Cis-Dichlorodiammineplatinum(II) and bleomycin in the treatment of esophageal carcinoma. Cancer Treat Rep. 1978;62:1041-1046.
228 Kelsen K, Coonley C, Hilaris B. Cisplatinum, vindesine, and bleomycin combination chemotherapy of local-regional and advanced esophageal carcinoma. Am J Med. 1983;75:639-652.
229 Petrasch S, Welt A, Reinacher A, et al. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br J Cancer. 1998;78:511-524.
230 Grisselbrecht C, Calvo F, Mignot L. Fluorouracil, adriamycin, and cisplatinum combination chemotherapy of advanced esophageal carcinoma. Cancer. 1983;52:974-977.
231 Kok TC, Van der Gaast A, Dees J, et al. Cisplatin and etoposide in oesophageal cancer. A phase II study. Rotterdam Oesophageal Tumour Study Group. Br J Cancer. 1996;74:980-984.
232 Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216-1220.
233 Kolaric K, Maricic Z, Roth A, et al. Chemotherapy versus chemoradiotherapy in inoperable esophageal cancer. Results of three controlled studies. Oncology (Europe). 1980;37(Suppl 1):77-82.
234 Kroep JR, Pinedo HM, Giaccone G, et al. Phase II study of cisplatin preceding gemcitabine in patients with advanced oesophageal cancer. Ann Oncol. 2004;15:230-235.
235 Williamson SK, McCoy SA, Gandara DR, et al. Phase II trial of gemcitabine plus irinotecan in patients with esophageal cancer. A Southwest Oncology Group (SWOG) trial. Am J Clin Oncol. 2006;29:116-122.
236 Hussey DH, Barkley HT, Bloedorn FG. Carcinoma of the esophagus. In: Fletcher GH, editor. Textbook of Radiotherapy. ed 3. Philadelphia: Lea and Febiger; 1980:688-703.
237 Newaishy GA, Read GA, Duncan W, et al. Results of radical radiotherapy of squamous cell carcinoma of the oesophagus. Clin Radiol. 1982;33:347.
238 Van Houtte P. Radiotherapie du cancer l’oesophage. Acta Gastroenterol Belg. 1977;40:121.
239 Appelqvist O, Silvo J, Rissanen P. The results of surgery and radiotherapy in the treatment of small cell carcinomas of the thoracic oesophagus. Ann Clin Res. 1979;11:184.
240 Lewinsky BS, Annes GP, Mann SG. Carcinoma of the esophagus. An analysis of results and of treatment techniques. Radiol Clin North Am. 1975;44:192.
241 Petrovich Z, Langholz B, Formenti S, et al. Management of carcinoma of the esophagus. The role of radiotherapy. Am J Clin Oncol. 1991;14:80-86.
242 Girinsky T, Auperin A, Marsiglia H, et al. Accelerated fractionation in esophageal cancers. A multivariate analysis on 88 patients. Int J Radiat Oncol Biol Phys. 1997;38:1013-1018.
243 Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma. A randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104-1109. discussion World J Surg 16:1110, 1992
244 Arnott SJ, Duncan W, Kerr GR, et al. Low dose preoperative radiotherapy for carcinoma of the oesophagus. Results of a randomized clinical trial. Radiother Oncol. 1992;24:108-113.
245 Seitz J, Giovannini M, Paduat-Cesana J, et al. Inoperable non-metastatic squamous cell cancer of the esophagus managed by concomitant chemotherapy (5-fluorouracil and cisplatin) and radiation therapy. Cancer. 1990;66:214-219.
246 Keane TJ, Harwood AR, Elhakim T, et al. Radical radiation therapy with 5-fluorouracil and mitomycin C for oesophageal squamous cell cancer. Radiother Oncol. 1985;4:205-210.
247 Hukku S, Gernades P, Vasishta S, et al. Radiation therapy alone and in combination with bleomycin and 5-FU in advanced carcinoma of the esophagus. Indian J Med. 1989;26:131-136.
248 Podolsky WJ, Xavier AM, Wittlinger PS, et al. Radiotherapy alone and chemoradiation for non-metastatic esophageal carcinoma. Cancer. 1989;63:2397-2403.
249 Roussel A, Bleiberg H, Dalesio O, et al. Palliative therapy of inoperable esophageal carcinoma with radiotherapy and methotrexate. Final results of a controlled clinical trial. Int J Radiat Oncol Biol Phys. 1989;16:67-72.
250 Chan A, Wong A, Arthur K. Concomitant 5-fluorouracil infusion, mitomycin C and radical radiation therapy in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 1989;16:59-65.
251 Forastiere AA, Heitmiller RF, Lee DJ, et al. Intensive chemoradiation followed by esophagectomy for squamous cell and adenocarcinoma of the esophagus. Cancer J Sci Am. 1997;3:144-152.
252 Bidoli P, Spinazze S, Valente M, et al. Combined chemotherapy (CT) radiotherapy (RT) +/− esophagectomy (E) in squamous cell cancer of the esophagus (SCCE) (Abstract). Proc Am Soc Clin Oncol. 1990;9:110.
253 Stewart JR, Hoff SJ, Johnson DH, et al. Improved survival with neoadjuvant therapy and resection for adenocarcinoma of the esophagus. Ann Surg. 1993;218:571-576.
254 Ajani JA, Walsh G, Komaki R, et al. Preoperative induction of CPT-11 and cisplatin chemotherapy followed by chemoradiotherapy in patients with locoregional carcinoma of the esophagus or gastroesophageal junction. Cancer. 2004;100:2347-2354.
255 Ilson DH, Bains M, Kelsen DP, et al. Phase I trial of escalating-dose irinotecan given weekly with cisplatin and concurrent radiotherapy in locally advanced esophageal cancer. J Clin Oncol. 2003;21:2926-2932.
256 MacFarlane SD, Hill LD, Jolly PC, et al. Improved results of surgical treatment for esophageal and gastroesophageal junction carcinomas after preoperative combined chemotherapy and radiation. J Thorac Surg. 1988;95:415-423.
257 Parker EF, Gregorie HB, Prioleau WH, et al. Carcinoma of the esophagus. Observations of 40 years. Ann Surg. 1982;195:618.
258 Hoff J, Stewart R, Sawyers L. Preliminary results with neoadjuvant therapy and resection for esophageal carcinoma. Ann Thorac Surg. 1993;56:282-286.
259 Burmeister B, Smithers S, Fitzgerald L, et al. A randomized phase III trial of preoperative chemoradiation followed by surgery (CR-S) versus surgery alone (S) in localized resectable cancer of the esophagus. Proc Am Soc Clin Oncol. 2002;21:130A.
260 Albertsson M, Ewers SB, Widmark H, et al. Evaluation of the palliative effect of radiotherapy for esophageal carcinoma. Acta Oncol. 1989;28:267-270.
261 Wara WM, Mauch PM, Thomas AN, et al. Palliation for carcinoma of the esophagus. Radiology. 1976;121:717.
262 Whittington R, Coia L, Haller D, et al. Adenocarcinoma of the esophagus and esophago-gastric junction. The effects of single and combined modalities on the survival and patterns of failure following treatment. Int J Radiat Oncol Biol Phys. 1990;19:593-603.
263 Herskovic AM, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598.