49 Cancer of the Endometrium
Epidemiology
Endometrial cancer is the most common gynecologic cancer and the fourth most frequently diagnosed cancer in women in the United States. According to the 2009 cancer statistics, the estimated number of newly diagnosed cases is 42,160.1 Endometrial cancer occurs most commonly in postmenopausal women, although 25% of cases occur in women before menopause and 5% in patients younger than 40 years of age.2 Worldwide, the incidence of endometrial cancer is higher in North America and Western Europe than in developing countries and Japan. Immigrant populations generally assume the risk of native ones, reflecting the importance of environmental factors in this disease. In the United States, Caucasian women have a twofold higher risk of developing this disease than African-American women, and it is more common among urban than rural residents. The expected number of deaths from endometrial cancer in 2009 is 7780, making it the eighth-leading cause of death from cancer in women.1
Anatomy
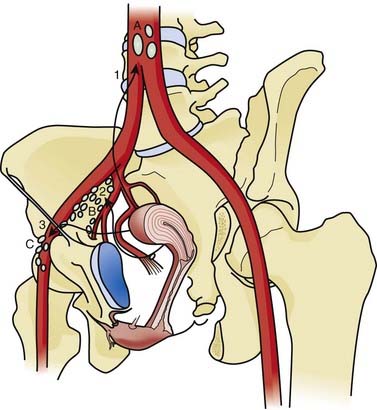
The main supports of the uterus are fibromuscular condensations of tissue in the pelvic fascia, namely the broad ligaments, the round ligaments, the uterosacral ligaments, and the transverse or Mackenrodt cardinal ligaments. The arterial supply is from the paired uterine arteries, which usually arise from the anterior division of the internal iliac arteries. The uterine artery enters the uterine wall at the isthmus, after crossing the ureter, and has free anastomoses with the ovarian and vaginal arteries. The venous drainage follows the arterial supply. There are relatively few lymphatics in the endometrium, but the myometrium and the subserosa have a rich lymphatic network. The uterine body drains primarily to the external and internal iliac lymph nodes, which then drain to the lateral aortic nodes. When the cervix is involved by disease, the pattern of spread of cancer parallels that of cervical cancer. Anastomoses between lymphatics from the body of the uterus and those in the round ligament provide a pathway for the rare metastasis to the inguinal lymph nodes. Similarly, there is a direct pathway for the lymphatics from the fundus of the uterus to the lateral aortic nodes, bypassing the iliac nodes (Fig. 49-1).
Risk Factors
Hormones
Alterations in the hormonal milieu are closely associated with an increased risk of developing endometrial cancer. The interaction between estrogens and endometrial cancer has been discussed for several decades.3 The coexistence of hyperplasia and adenocarcinoma and the apparent evolution of adenomatous hyperplasia into florid adenocarcinoma have been recognized by pathologists for more than 3 decades,4 and cytopathologists have long reported on the increased prevalence of well-cornified vaginal epithelium, indicating high levels of estrogens, in women with endometrial cancer.5 The clinical counterpart of these pathologic findings is the increased risk for endometrial cancer in several conditions associated with abnormally high levels of estrogens. Thus, Stein-Leventhal syndrome, or polycystic ovarian syndrome, has traditionally been associated with a very high risk (up to 25%) of endometrial cancer. Although this number may be somewhat of an overestimate, these cancers account for a large proportion of cases in young women.6 Similarly, a fairly large number of patients with granulosa or theca cell tumors of the ovary, or both, also develop a concomitant endometrial carcinoma. This association was first described by Novak and Yui7 in 1936, and it is now recognized that the probability of developing endometrial carcinoma is related to the predominance of theca cells in the ovarian tumor, because these are the important source of feminizing estrogens.3 It is also interesting to note that the prognosis of endometrial cancer in either of these settings is more favorable than for endometrial cancer in general.8
Late menopause, nulliparity, and obesity are classically associated with the development of endometrial cancer. Thus, delayed menarche is associated with a lower risk of endometrial cancer, whereas menopause after 52 years of age results in a 2.5-fold increase in the risk. The effect of delayed menopause (and to a lesser extent, early age at menarche) may reflect prolonged exposure of the endometrium to unopposed estrogen stimulation in the presence of anovulatory cycles.9 Similarly, nulliparous women have the highest risk, with 25% to 30% of all endometrial cancers occurring in nulliparous women. Having one child reduces this risk by half, and quintipara have less than 20% the risk faced by nulliparous women.10 This adverse effect of nulliparity may also be a manifestation of prolonged periods of infertility.11 In one study, nulliparous women who sought advice for infertility were at an almost eightfold excess risk compared with nulliparous women without an infertility problem.12 A number of biologic mechanisms have been proposed for this increased risk with infertility: anovulatory menstrual cycles resulting in prolonged exposure to estrogens without sufficient progesterone, high serum levels of androstenedione, lower levels of serum sex-hormone binding globulin (with resultant high levels of free estrogen), and the absence of the monthly sloughing of the endometrial lining (allowing the residual tissue to become hyperplastic).13 Finally, obesity is a well-recognized risk factor for endometrial cancer, possibly accounting for up to 25% of occurrences of the disease.14,15 Obesity affects the incidence of both premenopausal and postmenopausal endometrial cancer.14–16 Being 21 to 50 pounds overweight increases the risk by almost threefold, and women who are more than 50 pounds overweight may have as high as a tenfold increase in risk of developing endometrial cancer.17 The increased peripheral conversion of androstenedione to estrone may be the underlying pathophysiologic mechanism for this risk. Apart from the absolute increase in weight, the duration of obesity15 and the distribution of body fat may also play an important role.15,18,19 Conversely, physical activity may exert a protective effect on the risk of endometrial carcinoma.20,21 This association is biologically quite feasible, because physical activity is well known to effect changes in hormone levels and menstrual cycles.
Exogenous estrogens, in the form of estrogen replacement therapy, are also implicated in endometrial carcinoma. Several studies have looked at this correlation, and case reports have existed in the literature for 40 years.22 These studies have been performed in at least eight countries, under a wide spectrum of medical care systems and conventions, in urban and rural settings, in high-usage and low-usage populations, using cohort and case-control designs, and with population-based and hospital-based comparison groups. Most of the studies have shown a strong association between past estrogen use and the development of endometrial cancer. The biologic plausibility of this association is further enhanced by the positive correlations between the dose of estrogen used, the duration of use, and the risk of subsequent endometrial cancer. At least 2 to 3 years’ use was required to develop an increased risk, and the highest relative risks of 10 to 20 are observed after at least 10 years of estrogen use.23–25 The relative risk is highest among thin, nondiabetic, normotensive women.23,25,26 This may hint at a difference in estrogen metabolism in this group of patients, or that the risk in the typical obese, diabetic, hypertensive patient is already high enough as to not be affected by additional exogenous estrogens. Like the tumors arising in the setting of high levels of endogenous estrogen, most of these tumors are also relatively nonaggressive: They are generally diagnosed at an early stage, are well differentiated, and have little myometrial penetration26,27 and a relatively small effect on mortality.28,29
Further evidence for the role of estrogens comes from the studies evaluating the effects of oral contraceptives. These studies have demonstrated significantly higher risks in users of the sequential oral contraceptives—which have a relatively high dose of estrogen with a weak progestin—and substantially lower risks of endometrial cancer in the women using estrogen-progestin combination pills, which have a higher dose of progestins.11,16,30,31 The protective effect of the combination “pill” may be most marked in nulliparous women and in nonobese patients who have not been exposed to noncontraceptive estrogens.32
Progesterone is known to produce regressive changes in endometrial hyperplasia, and recently there has been an upsurge in enthusiasm for combining estrogen therapy with progestin to counteract the carcinogenic effects.23,33,34 The recently published Women’s Health Initiative randomized trial of healthy, postmenopausal women showed that estrogen and progestin did not increase the risk of endometrial or colorectal cancers, but it did significantly increase the risk of invasive breast cancer.35
The risk of endometrial cancer from tamoxifen use has been the subject of many studies in light of its widespread use for breast cancer treatment and prevention. The mechanism of action is competition with endogenous estrogen for estrogen receptors. In premenopausal women, tamoxifen has an antiestrogenic effect, but in postmenopausal women it has a weak estrogenic effect because of the up-regulation of estrogen receptors. In an overview of the breast cancer prevention trials, Cuzick et al.36 reported that the rates of endometrial cancer were increased with tamoxifen in all prevention trials (relative risk 2.4, 95% confidence interval [CI]: 1.5-4; P = 0.0002). Most of the excess risk was seen in women 50 years old or older.36 Most authors37–39 believe that there is no real difference in the stage, grade, or prognosis of endometrial cancers associated with tamoxifen, although there are emerging data to the contrary.40
Diet
In contrast to cervical cancer, uterine cancer is a disease of the Western countries; even there, it is more common in the urban population belonging to the higher socioeconomic strata of society. The rates are highest in North America and Northern Europe; intermediate in Israel, Southern Europe, and Latin America; and lowest in Africa and Asia.41 This geographic difference may be a reflection of the high content of animal fat in the Western diets,42 because both observational and interventional studies have shown a higher level of plasma estrone, estradiol, and prolactin among women consuming a high-fat diet.43,44 Diet probably plays a multifaceted role in the risk of developing endometrial cancer, as other studies have shown a protective effect of high levels of micronutrients45 and diets high in fruits and vegetables.46
Other Comorbidities
Endometrial carcinoma is also known to be associated with several other medical conditions. Diabetes and hypertension have long been related to a significant increase in the endometrial cancer risk, and recent reports confirmed that this risk persisted over time and was independent of the patient’s weight.47,48 Other interesting correlations include the interrelationship with smoking. The bulk of evidence from epidemiologic studies suggests that smoking reduces the risk of developing endometrial cancer. The exact cause is not clear, but mechanisms such as the antiestrogenic effect of smoking on circulating estrogen concentrations, a reduction in relative body weight, and earlier age at menopause have been suggested.49 Alcohol also has been associated with a reduction in the risk of endometrial cancer, perhaps by attenuating the endogenous estrogens.50
Genetics
Genetic predisposition to endometrial carcinoma occurs primarily within the context of hereditary nonpolyposis colorectal cancer (HNPCC), which probably accounts for less than 5% of all endometrial cancer cases. In patients with HNPCC there is increased risk of developing not only colon cancer but other cancers as well. The latter (Lynch syndrome II) includes endometrial cancers; ovarian cancers; gastric adenocarcinomas; carcinomas of the small bowel, pancreas, and biliary tree; transitional cell carcinomas of the ureters and renal pelvis; and tumors of the skin (Muir-Torre syndrome).51 The lifetime risk estimates for endometrial carcinoma range from 40% to 60%, corresponding to a relative risk of 13 to 20. These estimates are not population-based, but are derived from analyses of HNPCC families and thus may be higher than expected in the population generally. The genes involved in HNPCC include MSH2, MLH1, and MSH6. Tumors from patients with HNPCC syndrome are characterized by genetic instability that leads to error-prone deoxyribonucleic acid (DNA) replication-RER+ phenotype, also referred to as microsatellite instability.52 Apart from the special considerations of colorectal cancer, screening for endometrial and ovarian cancers should also be carried out. Currently for endometrial cancer, this consists of assessment of the endometrial stripe at the time of transvaginal ultrasonography, as well as endometrial aspiration for cytologic and histologic examinations. With respect to risk-reducing surgery, there are no data on the efficacy of hysterectomy and bilateral salpingo-oophorectomy (BSO) for cancer prevention in women at risk for HNPCC, and no recommendation was made for or against risk-reducing surgery by the Cancer Genetics Studies Consortium that addressed this issue.53 Nevertheless, it is reasonable to assume that hysterectomy would be completely preventive for endometrial carcinoma, and there are no published case reports to the contrary.
Pathologic Conditions
Hyperplasia
On gross inspection, hyperplastic endometrium has no distinctive features. There is a diffuse thickening resulting in an increase in the amount of endometrial tissue. The endometrium appears pale and knobby, and has a spongy, velvety feel. Microscopically, the diagnostic criterion for hyperplasia is an increase in the number and size of proliferating glands. The International Society of Gynecologic Pathologists has standardized the subclassification of endometrial hyperplasia.54 In simple hyperplasia, there is only glandular proliferation and enlargement with increased stromal cellularity. This rarely progresses to carcinoma (<1%). Complex hyperplasia is characterized by back-to-back proliferation of glands with intraluminal papillae, epithelial pseudostratification, and few mitotic figures. If there is no cytologic atypia, the risk of malignant degeneration is again quite low, on the order of 3%. Any proliferation demonstrating cytologic abnormalities (in cellular or nuclear morphology) is classified as atypical hyperplasia. Atypical hyperplasia has a much higher risk of progression to an invasive carcinoma—8% for simple atypical hyperplasia, increasing to almost 30% for complex hyperplasia associated with atypia. Based on the histologic type and the patient’s age and general medical condition, treatment options for endometrial hyperplasia include periodic endometrial curettage, high-dose progestin, ovulation induction, total abdominal hysterectomy (TAH) with BSO, and even intracavitary radiotherapy.
Carcinomas
On gross inspection, most endometrial cancer involves the endometrial surface diffusely and is found frequently in the upper part of the uterine body, especially on the posterior wall. Only 40% of endometrial cancers are localized, which may take the form of a polyp or a localized, flattened, pyramidal thickening involving a part of the endometrial surface. The tumor obtained from endometrial curettage has a typical firm feel with a dry, granular, and often friable appearance. Necrotic areas may be yellow, white, or red. The International Society of Gynecologic Pathologists has proposed a classification for endometrial carcinomas (Table 49-1). Although the typical carcinoma is easy to diagnose, it may not always be easy to distinguish between a well-differentiated adenocarcinoma and an advanced atypical hyperplasia.55,56 The chief feature that helps in this distinction is the extent of the proliferative changes: To diagnose a specimen as well-differentiated adenocarcinoma, the proliferative changes must be widespread and involve at least one half of a low-power microscopic field, 4.2 mm in diameter. Nuclear atypia is not required for this distinction.
Endometrioid Adenocarcinoma
Most of the endometrioid adenocarcinomas are designated not otherwise specified. Foci of squamous differentiation are often found with endometrioid adenocarcinoma. The squamous component could be benign with the designation of adenoacanthoma or malignant in cases in which it is called adenosquamous carcinoma. But such designations have not been very useful because the degree of differentiation of the squamous component parallels that of the glandular architectural grading. Therefore, most gynecologic pathologists use the term adenocarcinoma with squamous differentiation. Other subtypes of endometrioid adenocarcinoma include the relatively common villoglandular carcinoma, which grows in papillary fashion. The prognosis of this subtype is similar to that of low-grade endometrioid cancer and must not be confused with serous carcinoma because of its papillary features. Secretory carcinoma, which represents less than 2% of all endometrial carcinomas, is characterized by a very well-differentiated glandular pattern with a lot of intracellular glycogen, thus resembling early secretory endometrium. Although the cells have clear cytoplasm, their histologic and cytologic features are different from clear cell carcinoma. Included is also ciliated carcinoma, which is a very rare subtype, characterized by the presence of ciliated cells composing more than 75% of the tumor specimen.57 It is usually associated with a history of prior estrogen usage, and the prognosis is quite good, because most are well differentiated.
Mucinous Carcinoma
Composing approximately 9% of all endometrial cancers, the mucinous carcinoma designation requires more than 50% of the tumor cells to be mucinous. These cells are carcinoembryonic antigen–positive, and are laden with mucin that stains positively with mucicarmine and periodic acid–Schiff stains but is diastase-resistant. Because this subtype is more commonly seen as an endocervical primary tumor, it is essential to establish the endometrial origin of the tumor. This is done by excluding an endocervical tumor on endocervical curettage. Mucinous carcinomas are usually well differentiated and have the same prognosis as ordinary endometrioid carcinomas.58
Serous Carcinomas
Serous carcinomas, also known as papillary serous (PS) cancer, resemble ovary cancer in terms of histologic characteristics and to some extent in terms of behavior. The mere presence of papillary structure is not diagnostic because other histologic types may have papilla as well. But the presence of marked cellular atypia in addition to papilla distinguishes serous carcinoma from others. Psammoma bodies are found in up to 33% of cases. In most single large institution series, the prevalence is approximately 10%, but in a population-based study from Norway it was only 1%, reflecting perhaps the patterns of referrals.59 This is a very aggressive subtype with a high propensity for early lymphatic and intra peritoneal dissemination, often despite little myometrial penetration.60 The overall relapse rates are very high—up to 50%, even in stage I to II tumors.61
Clear Cell Carcinoma
Clear cell carcinoma of the endometrium resembles renal carcinoma. Previously believed to arise from the mesonephric duct remnants (hence the synonyms mesonephroma or adenocarcinoma of mesonephric origin), its origin from müllerian structures is now well established.62 Unlike vaginal and cervical clear cell carcinoma, it is not related to intrauterine diethylstilbestrol exposure. The microscopic structure may vary from solid patterns to glandular differentiation. In the latter pattern, the small cells resembling “hobnail” cells line spaces and glands. These are cells that have extruded their cytoplasm, leaving bare nuclei that protrude into the glandular lumens. The prognosis of this cancer is somewhat similar to that of serous cancer.63
Simultaneous Tumors
Cancers of an identical type may be discovered in the ovary and endometrium simultaneously.64 Usually, the site of the largest tumor is assigned the primary origin, but occasionally true primary endometrial and ovarian malignancies may coexist. This field effect of the müllerian system may occur in as much as 15% to 20% of the ovarian endometrioid tumors.65 If the endometrial tumor is less than 5 cm in diameter, is well differentiated, has no vascular invasion, is limited to less than the middle third of the myometrium, and the ovarian lesions are bilateral, it is more likely that there are two concomitant primary tumors.66
Molecular Biology
Several investigators in the past pointed out that there are two distinct types of endometrial cancer.67,68 In type I endometrial cancer there is strong correlation with prior estrogen stimulation. The cancers in this category are often indolent in nature, with minimal myometrial invasion and low-grade histologic findings. They affect premenopausal and perimenopausal women. Type II endometrial cancer often affects postmenopausal women with no prior history of estrogen stimulation. The histologic characteristics of the tumors is often high grade, such as serous or clear cell cancers with deep invasion, and at a more advanced stage at the time of presentation. What is intriguing is the fact that at the molecular level, the existence of two distinct types of endometrial cancer seems to be validated.69
Inactivation of the p53 tumor suppressor gene is seen in almost 90% of cases of serous carcinoma.70,71 Mutation in the p53 gene, however, is encountered in only 10% of endometrioid adenocarcinoma, with most occurring in International Federation of Gynecology and Obstetrics (FIGO) grade 3 tumors. Several studies have also demonstrated that p53 mutation is an independent predictor of poor outcome.72,73 In contrast, mutation in the PTEN tumor suppressor gene is found in 30% to 50% of endometrioid adenocarcinoma.74,75 PTEN mutation is also associated with early-stage, nonmetastatic disease and more favorable survival.76 Microsatellite instability, which is found in patients with HNPCC, is also seen in approximately 20% of “sporadic” endometrial cancers.77 Data in the literature suggest a favorable survival associated with microsatellite instability in endometrioid endometrial cancers.78 Mutation in k-ras proto-oncogene is seen in 10% to 30% of endometrial cancer patients,79 but it seems that there is no correlation between k-ras mutation and clinical outcome. Over expression of HER-2/NEU is also seen in 10% to 15% of endometrial cancers and is associated with more advanced disease and poor outcome.80,81 Other oncogenes82–85 that have been found to be overexpressed or amplified include c-myc, bcl-2, c-fms, and B-catenin. Microarray analysis has further revealed distinct gene expression profiles among different histologic types of endometrial cancer.86
Mode of Spread
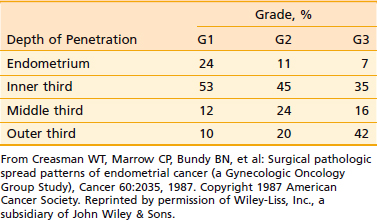
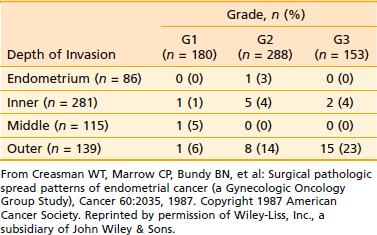
Endometrial cancer tends to remain confined to the body of the uterus for a long time. In most cases, the initial spread is by local extension, along the endometrial surface with or without enlargement of the uterus. Subsequent growth continues in radial and longitudinal directions. Longitudinal growth results in cervical involvement, initially through the openings of the endocervical glands, and later with frank cervical stromal infiltration. The tumor can also extend along the cornua to involve the fallopian tubes (although ovarian involvement is more often embolic in nature). As the tumor grows radially, it penetrates the myometrium and makes its way toward the subserosa and serosa. In a small number of cases, following extensive penetration through the myometrium or the cervix, the tumor involves the pelvic soft tissues and continues to reach the pelvic sidewall; the tumor may also directly invade the bladder or the rectum. Once the tumor breaches the serosa, transperitoneal dissemination can occur, resulting in a pattern of dissemination much like that of ovarian cancer. Overall, of the clinical stage I tumors, 15% are limited to the endometrium, 45% penetrate the inner third of the myometrium, 20% reach the middle third of the myometrium, and only approximately 20% reach the subserosa. The extent of myometrial penetration correlates very well with the histologic grade of the tumor (Table 49-2). Peritoneal seeding can result from transmural spread of the tumor once the serosa is breached, or because of transtubal spillage of tumor cells into the general peritoneal cavity.
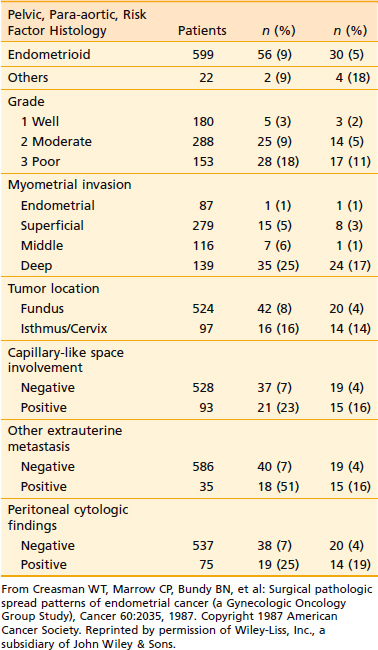
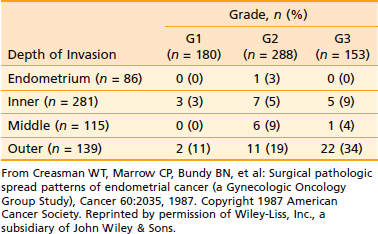
The endometrium proper has few lymphatics. However, once the tumor penetrates the myometrium, and especially when it reaches the lymphatic-rich subserosa, spread via lymphatic embolization is common. Overall, only approximately 11% of patients with clinical stage I and occult stage II endometrial carcinoma have pelvic or para-aortic lymph node involvement.87 However, a number of adverse pathologic factors can affect this number, as shown in Table 49-3. There is a close correlation between the tumor grade and the depth of myometrial invasion, as discussed earlier. This risk of pelvic and para-aortic lymph node involvement as related to grade and depth of myometrial invasion is given in Table 49-4 and Table 49-5. Lymph node involvement also increases with increasing surgical stages. Thus, approximately 10% to 15% of all patients with stage I disease have pelvic lymph node involvement; this rises to 30% to 40% in stage II.
Clinical Presentation
Early endometrial cancer has few symptoms, and a high index of suspicion is necessary to avoid any delay in diagnosis. The perimenopausal phase has only one normal pattern: menstrual periods that get progressively lighter and further apart. Any variant of this should always sound a warning note in the treating clinician. Although postmenopausal bleeding can have several nonmalignant causes,88 abnormal bleeding is the presenting symptom in more than 80% of cases of endometrial cancer. Another fairly frequent (10%), but rather nonspecific, symptom is that of leukorrhea. Pain or other symptoms of pelvic pressure are rarely present in early-stage disease.
Several imaging studies are being evaluated for their ability to accurately define the disease extent preoperatively. The typical finding on ultrasonogram is a thickened endometrium. It is suggested that less-differentiated tumors may be hypoechoic89 or that distinct sonographic patterns allow one to distinguish among hyperplasia, polyps, and carcinoma.90 In this respect, transvaginal ultrasonography (TVS) is believed to be superior to conventional transabdominal sonography.91 TVS is also useful in determining the depth of myometrial invasion.92 Its overall accuracy in determining invasion is 79% and its accuracy is in determining the degree of myometrial invasion is 60% to 76%. Good-quality pelvic computed tomography (CT) scans obtained with oral and intravenous contrast can demonstrate the extent of the endometrial tumor. The endometrial carcinoma is a hypodense mass relative to the normal myometrium93 and may be seen as a diffuse, circumscribed vegetative or polypoidal mass within the uterine cavity. If myometrial invasion is seen, it usually implies involvement of greater than one third to one half of the myometrial thickness.94 Endometrial involvement of the cervix is seen on CT as cervical enlargement greater than 3.5 cm in diameter with heterogeneous low-attenuation areas within the fibromuscular stroma.95 Parametrial or sidewall extension is seen by the loss of periureteral fat in the former and less than 3 mm of intervening fat between the soft tissue mass and the pelvic sidewall in the latter.96 Involvement of the fallopian tubes, ovaries, and lymph nodes is detected in the usual fashion.
Although the largest study looking at the ability of CT to detect myometrial invasion by dynamic and delayed images reported an overall accuracy of approximately 76%, the authors noted that CT did provide clinically useful information in almost 90% of cases.94 The major limitation of CT is the failure to detect microscopic parametrial, lymph nodal, bladder, or rectal invasion.95,97 Other problems include an unreliability in assessing myometrial invasion in the atrophic uteri of postmenopausal women and an occasional difficulty in differentiating a uterine from an adnexal mass.93,94,97 Because of these problems, and because surgical staging is the standard approach in most patients, CT is not routinely recommended in clinical stage I or II disease. Its greatest effect may lie in clinical stage III patients, in whom it may confirm extrauterine spread, detect pelvic lymphadenopathy, or reclassify the disease as stage IV by detecting extrapelvic involvement.95,97 It may also help in staging a patient with an equivocal pelvic examination or a contraindication to surgery and in confirming advanced stage III or IV disease.93–9597 CT scanning is also useful in the evaluation of recurrent disease by helping to define the extent of disease at relapse and in facilitating a directed biopsy for histologic confirmation of relapse.95,97,98
Magnetic resonance imaging (MRI) is increasingly being recommended as part of the preoperative evaluation of endometrial carcinoma.99–102 Because unenhanced T1- and T2-weighted images lack specificity in diagnosing endometrial cancer, use of intravenous contrast is necessary.91,103 Following contrast injection, even small tumors contained within the endometrium can be diagnosed by their enhancement relative to the normal endometrium, and dynamic MRI with rapid administration of gadolinium diethylenetriaminepentaacetic acid using the turbo–fast, low-angle shot technique can delineate the inner myometrium from the outer layers even in postmenopausal women.
MRI scans have been correlated to the FIGO subdivisions of stage I endometrial cancer.104 Stage IA tumors typically show a clear junctional zone or preservation of a sharp delineation between the tumor and the myometrium; IB disease is characterized by disruption of the junctional zone or increased signal-intensity tumor in the inner half of the myometrium with preservation of the outer myometrium, or both. Finally, in stage IC patients, there is extension of the high-signal-intensity tumor into the outer myometrium with preservation of a peripheral rim of normal, intact myometrium.105 MRI also helps delineate stage II disease by showing an expansion of the cervical canal with stromal heterogeneity. Similarly, imaging of extrauterine spread is facilitated, although lymph node involvement is limited to the criterion of increased size. State-of-the-art MRI using gadolinium chelates is probably accurate in more than 90% of cases.106,107 It definitely outperforms CT in determining myometrial invasion103,107,108 and is possibly better than TVS.109,110
Tumor markers such as CA-125 have been shown to be elevated in 63% to 67% of patients with advanced or metastatic disease compared with 10% to 19% in those with early-stage disease. Whether CA-125 is a useful tool in screening for endometrial cancer is not determined, but most authors agree about its potential in patients with aggressive histologic findings, advanced disease, or pretreatment elevated levels.111,112
Staging and Prognostic Factors
The Gynecologic Oncology Group (GOG) conducted two large prospective trials87,113 in 1984 and 1987. The major prognostic factors in endometrial cancer were defined from these studies, and surgical staging has gradually become the standard in this disease. This surgical approach led FIGO, in 1988, to design a surgical staging scheme for endometrial cancer, incorporating most of the known prognostic factors (Table 49-6).114 For the small minority of patients who are not surgical candidates and are treated with primary radiotherapy, the earlier clinical staging system is still applied (Table 49-7).115
| Stage | Definition | |
|---|---|---|
| IA | G123 | Tumor limited to the endometrium |
| IB | G123 | Invasion to <50% of the myometrium |
| IC | G123 | Invasion to >50% of the myometrium |
| IIA | G123 | Cervical involvement restricted to the endocervical glands |
| IIB | G123 | Cervical stromal invasion |
| IIIA | G123 | Tumor invades uterine serosa, or adnexae, or positive peritoneal cytology |
| IIIB | G123 | Tumor involving the vagina |
| IIIC | G123 | Pelvic or para-aortic lymph nodal involvement |
| IVA | G123 | Invasion of bladder, bowel mucosa, or both |
| IVB | Distant metastases, including intra-abdominal spread or inguinal lymph nodes | |
FIGO, International Federation of Gynecology and Obstetrics.
Table 49-7 Clinical Staging of Endometrial Cancer (FIGO 1971)
| Stage | Definition |
|---|---|
| I | Carcinoma confined to the uterus |
| IA | Length of uterine canal is ≤8 cm |
| IB | Length of uterine canal is >8 cm |
| Histologic Subtypes of Adenocarcinoma | |
| G1 | Highly differentiated adenomatous carcinoma |
| G2 | Differentiated adenomatous carcinoma with partly solid areas |
| G3 | Predominantly solid or entirely undifferentiated carcinoma |
| II | Carcinoma involves the corpus and the cervix |
| III | Carcinoma extends outside the uterus but is confined to the true pelvis |
| IV | Carcinoma extends outside the true pelvis or involves the bladder, rectum, or both |
FIGO, International Federation of Gynecology and Obstetrics.
Several clinicopathologic factors have been identified in patients with endometrial carcinoma to help predict the prognosis and individualize the treatment plan. Based on clinical experiences, Nolan and Huen116 developed a mathematical model for prognostication, wherein patient prognosis is directly proportional to host resistance, inversely related to tumor virulence, and positively affected by appropriate treatment factors.
Host Resistance Factors
Age
The importance of age to endometrial cancer has been well documented. Frick and associates,117 in 1973, reported a better outcome for patients younger than the age of 59 with stage I endometrial cancer, even after correcting for comorbid medical conditions. This was subsequently confirmed by other investigators.118–120 The adverse effect of advanced age on outcome persists even when elderly patients are treated as aggressively as their younger counterparts.121 The improved survival in younger patients may reflect the tendency toward earlier, better-differentiated tumors with less myometrial invasion in these patients; in older postmenopausal women with atrophic uteri, relatively little myometrial penetration brings the tumor close to the lymphatic-rich subserosa, with a correspondingly high risk of lymph node metastases.122 Better host immunocompetence may be an additional factor in the younger patients.
Race
Caucasian women tend to fare better than African Americans independent of other prognostic factors.123 It is important to note that although the prevalence of endometrial cancer is lower in African American women, the incidence of high-risk tumors in this group is higher.124 The influence of histologic type and differentiation on outcome was discussed earlier.
Tumor Virulence Factors
Stage at Diagnosis
Because the very concept of stage grouping is based on creating groups with similar prognoses, it is not surprising that the outcome gets less favorable with advancing stage. Reported results of stage-based survival are remarkably consistent, with stages I, II, III, and IV disease having 5-year survival rates of 92%, 80%, 40%, and 5% respectively.125
Size of the Uterus
Uterus size has long been recognized as a predictor of survival and was incorporated in the earlier clinical staging for endometrial cancer. Creasman and associates showed a direct correlation between uterine size and the incidence of para-aortic and pelvic nodal metastases.87 It has been pointed out, however, that uterine size may not always reflect tumor volume, because a number of benign conditions can cause enlargement of the uterus.126 Furthermore, because the size of the uterus correlates with tumor grade and myometrial penetration, it is very possible that size merely acts as a surrogate for these far more significant prognostic factors.
Lower Uterine Segment Involvement
Although 92% of stage I endometrial carcinomas lie in the fundus, it has been proposed that cancers involving the isthmus or lower uterine segment may have a worse prognosis.127 The GOG study of surgical-pathologic spread patterns found a doubling of the incidence of pelvic nodal involvement from 8% to 16% and an increase in para-aortic nodal involvement from 4% to 14% when the tumor arose from or involved the isthmus.87 Mayr and associates128 found that lower uterine segment involvement, in the absence of any other adverse prognostic factors, resulted in an almost 50% local failure rate when postoperative radiotherapy was omitted; in patients receiving postoperative radiotherapy, the relapse rates dropped to 3%.
Cervical Involvement
It is important to separate pathologic (occult) from clinical involvement of the cervix in endometrial cancer because only occult cervical involvement is recognized in the 1988 FIGO staging system. Prognosis for patients with cervical involvement (stage II) is much poorer than for those with earlier-stage lesions. Surwit and associates122 reported that cervical stromal involvement dropped the survival rate to less than 50%, as compared with almost 75% with tumor involvement limited to the endocervical glands; access to the cervical stroma probably increases the propensity for lymphatic dissemination, as positive pelvic lymph nodes are seen in approximately 35% of the patients.129 Similarly, Rubin and associates reported on the Memorial Sloan-Kettering Cancer Center experience with stage II endometrial cancer.130 At 60 months, the actuarial disease-free survival rate was 39% in patients with gross cervical involvement, 62% in those with clinically occult stromal involvement (detected only on a fractional D&C), and 88% in the subset having only detached tumor fragments on fractional D&C.
Histologic Subtypes
Although the most common subtype—endometrioid adenocarcinoma—has a worse prognosis with increasing grade,87,131 there are cell types that have a high propensity to early systemic dissemination with a uniformly bad prognosis, regardless of the pathologic grade. These include the serous, clear cell, squamous, and undifferentiated subtypes. The overall survival rate in this group is less than 35%.132,133
Tumor Grade
Tumor grade is one of the most sensitive indicators of prognosis. Grade directly affects the depth of myometrial penetration and the frequency of lymph node involvement (see Table 49-3).87,131,134,135 Thus, most grade 1 tumors are limited to the endometrium or have superficial myometrial penetration, and the overall risk of pelvic and para-aortic lymph nodal metastases is 3% and 1.5%, respectively; however, 10% of grade 1 tumors have deep myometrial penetration, and the pelvic and para-aortic lymph nodal involvement in these are 12% and 6%, respectively. Similarly, more than 50% of grade 3 tumors have a greater than 50% myometrial penetration, and these have pelvic and para-aortic nodal involvement on the order of 30% and 20%, respectively.87
Myometrial Invasion
Myometrial invasion is probably the factor most consistently identified with treatment failures. Conventionally, the depth of invasion has been reported as inner, middle, or outer thirds of the myometrium. The FIGO staging system, however, refers only to inner and outer halves, and the pathologic reporting should change to incorporate this recommendation. In patients with stage IB disease (<50% invasion), it seems that invasion to less than one-third versus greater than one-third is not a significant predictor of outcome.136 Lutz and associates also noted that proximity to the serosa may be more important than absolute depth of penetration.126 They found a 65% survival rate for patients whose tumors had invaded to within 5 mm of the serosa, compared with a 97% survival rate for patients with tumors more than 10 mm from the serosa. Both grade and depth of penetration are predictive for lymph nodal involvement; however, depth is more influential in determining lymphatic and extrauterine spread, treatment failure, and relapse. Thus, regardless of grade, only 1% of tumors limited to the endometrium had lymph nodal involvement, as compared with 25% pelvic and 17% para-aortic involvement with deep penetration.87
Vascular or Capillary-Like Space Invasion
Vascular or capillary-like space (CLS) invasion is seen in approximately 15% of the cases of endometrial cancer.87,137,138 The GOG study found that CLS-positive tumors were associated with a 27%, or fourfold, increase in the pelvic lymph nodal metastases, and a 19%, or sixfold, increase in para-aortic nodal metastases.87 This translates into more frequent relapses and a poorer survival for the CLS-positive patients.137
Adnexal Involvement
Approximately 5% of patients with stage I and occult stage II disease have adnexal involvement.87 This is associated with a fourfold increase in lymph node metastases; thus, pelvic lymph nodal positivity rises to 32% (as compared with 8% without adnexal spread) and para-aortic nodal involvement is seen in 20% (as opposed to only 5% in patients with no adnexal spread). The depth of myometrial invasion is still an important factor: Patients with adnexal spread but less than 50% myometrial involvement have a 5-year survival rate of approximately 80%, as compared with a 45% 5-year survival rate when adnexal involvement is associated with deep myometrial invasion. The extent of adnexal involvement is also of prognostic significance. Patients with occult, microscopic invasion do far better (5-year survival rate >80%) than those with gross adnexal involvement (5-year survival rate <40%).139
Peritoneal Cytologic Findings
Peritoneal fluid positive for malignant cells is found in 12% to 15% of all patients undergoing surgical staging. This is associated with 25% pelvic lymph nodal involvement and 19% para-aortic nodal involvement. However, only approximately 5% of patients with positive peritoneal cytologic finding have no extrauterine disease,87 and the literature regarding the true effect of positive peritoneal cytology is mixed. Several small series indicate that a positive peritoneal cytologic finding has no independent prognostic value.140–142 On the other hand, two relatively large series show a distinct adverse effect from positive peritoneal cytology.143,144 One confounding factor, mainly in patients with no other risk factors, is whether all endometrial cancer cells that gained access to the peritoneal cavity are capable of independent growth. The meta-analysis by Milosevic and associates concluded that, although malignant cytologic findings were strongly associated with disease recurrence (odds ratio of 4.7 with 95% CI of 3.5-6.3), this was largely due to the association of malignant cytologic findings with other adverse prognostic factors, which dominate the clinical presentation.145 In the absence of any other adverse features, the true incidence of malignant cytologic findings is quite low and is unlikely to act as an independent predictor for relapse. Recent data suggest a higher rate of positive cytologic findings for patients undergoing laparoscopic-vaginal hysterectomy, in which there is manipulation of the uterine cavity, compared with TAH, in which there is no such manipulation.146
Serosal Involvement
This is the least-studied feature of all stage III-A subsets. Its incidence ranges from 7% to 16% and it is often associated with other poor prognostic factors.147 But even when it is isolated, the 5-year disease-free survival is only 41.5%.
Pelvic and Para-Aortic Lymph Nodes
Overall, approximately 11% of patients with stage I and occult stage II endometrial cancer have pelvic nodal involvement.87 This increases to 25%, 30%, and 50% with deep myometrial penetration, adnexal involvement, and extrauterine spread, respectively. Lymph nodal involvement is a major predictor for distant disease: the 5-year disease-free survival rates drop to 65% to 70% in patients with pelvic lymph nodal involvement as their only risk factor.148 Similarly, although para-aortic nodal metastases are seen in approximately 5% of all patients with stage I and occult stage II disease, the biggest risk factor for para-aortic node involvement is the presence of pelvic nodal metastases; more than 30% of patients with pelvic nodal involvement have para-aortic disease. The pattern of lymphatic spread in endometrial cancer is different than in cervical cancer. In endometrial cancer, a simultaneous spread to both pelvic and para-aortic nodes could occur, whereas in cervical cancer the spread to para-aortic nodes is almost always secondary to pelvic lymph nodal involvement. Despite optimal debulking and radiation, 5-year DFS rates drop to approximately 30% in this subpopulation.
Other Significant Prognostic Factors
Ploidy
There are several studies now to indicate that aneuploid tumors correlate with advanced surgical stage, higher grade, and a poorer clinical outcome. Multivariate analysis has identified ploidy as an independent risk factor. In fact, a recent review on this subject notes that “DNA ploidy is an independent, broadly applicable, quantifiable predictor of progression-free survival in patients with endometrial cancer and, therefore, warrants designation as a major prognostic factor or therapeutic determinant.”149 There appears to be an association between DNA ploidy and the overexpression of several regulatory genes, such as c-fms, k-ras, HER-2/neu, and p53. Although overexpression of these oncogenes and tumor suppressor genes harbors prognostic significance in endometrial cancer, the ploidy status of the tumor appears to represent the most objective prognostic variable.149–158 In fact, there are data to suggest that ploidy may be an independent prognosticator with greater predictive power than tumor grade.159,160 As a variant of ploidy, the DNA index (DI) has also been evaluated for prognostic importance in endometrial cancer. A DI greater than 1.2 was found to be strongly predictive of recurrent disease, independent of other conventional risk factors, including histologic subtype, depth of myometrial penetration, and lymphovascular invasion.161
S Phase
As a marker of tumor proliferation, the fraction of tumor cells in S phase has been extensively studied. The results so far are mixed; at one extreme, S phase is believed to have a very high prognostic value, although other investigators claim that S phase is not shown to add to the risk-modeling based on conventional surgicopathologic factors.151,156,159,162,163
Hormone Receptors
The physiology of the normal endometrium is closely regulated by estrogens and progesterone, and it is not surprising that expression of hormone receptors by endometrial carcinoma is of prognostic value. In general, patients with receptor-positive tumors have a significantly better DFS rate, with receptor status acting as an independent risk factor.150,164–167 The receptor status is also important in predicting the likelihood of response to hormonal therapy in patients who have relapsed.
Prognostic Information from Molecular Biology
The application of molecular biology tools to endometrial cancer has provided insights into the pathophysiology of the disease and may reveal new avenues for early diagnoses as well as development of therapeutic strategies based on newer models of risk stratification. Mutations and overexpression of the tumor suppressor gene p53 have been most extensively studied. There is a consistent observation linking the overexpression of p53 with advanced stage and a poorer outcome. In most studies, p53 maintained an independent prognostic value separate from the conventional clinicopathologic risk factors.149,151,152,168–176 A study from Duke University also reported an increased frequency of p53 overexpression in African-American women, which may account for the racial disparity in survival.168 Hamel and associates,155 from the Mayo Clinic, reported on a retrospective analysis of 221 patients managed surgically for endometrial carcinoma. Their multivariate analysis identified four independent prognostic factors for progression-free survival: intense p53 expression (defined as staining of 66% or more of the nuclear area), histologic subtype, DNA ploidy status, and HER-2/neu expression. When none of these were present, the 4-year progression-free survival rate was 96%; in contrast, it dropped to 63% in the presence of one of these factors and was only 40% when two or more factors were present.
Several other prognostic factors have been identified, including c-fms,84 HER-2/neu,* epidermal growth factor receptor,152,171,172,179,180 k-ras activation,181 Nm23,182,183 and macrophage colony-stimulating factor 1.184 Although a lot of the information regarding these newer prognostic factors is preliminary, it is likely that molecular abnormalities are the fundamental mechanisms underlying malignant transformation and further progression of the established neoplasm-tumor grade. Myometrial penetration and the other gross prognostic factors may be mere epiphenomena, that is, the secondary results of the molecular changes. As the knowledge regarding the molecular biology of endometrial cancer matures, the risk stratification may soon be based on cytogenetic and molecular abnormalities rather than on pathologic variables. This may be particularly useful in devising treatment strategies for patients who would currently be classified as belonging to the intermediate-risk group.
Treatment-Related Prognostic Factors
Residual Tumor after Preoperative Radiotherapy
In their study of 91 patients with early endometrial cancer treated with two intracavitary insertions, Macasaet and associates found residual tumor in almost 50% of cases.185 These patients had a 19% relapse rate and a 23% probability of dying from their tumor, compared with only 6% in patients without residual cancer.
Delay in Initiating Postoperative Adjuvant Therapy
A recent report from the Fox Chase Cancer Center concluded that prolonging the surgery-to-radiation interval beyond 6 weeks has an adverse effect on disease-specific survival and may also influence local control.186 In light of this, it was surprising that overall treatment time and external-beam treatment time did not emerge as significant prognostic factors.
Treatment
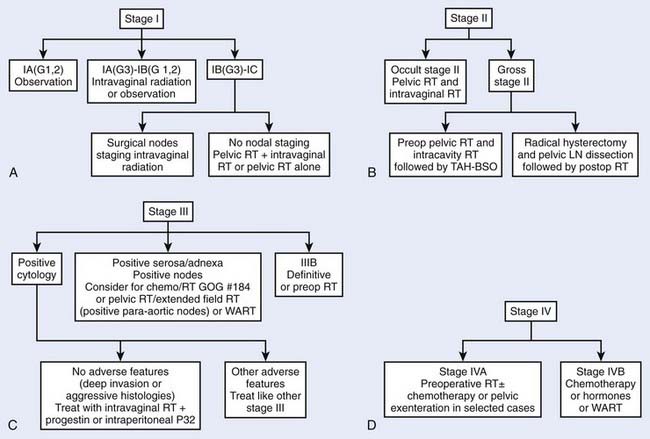
Endometrial cancer is perhaps unique in having more treatment options advocated by different clinicians than any other malignancy of the female pelvis. This is especially the case for stage I/II tumors, which constitute more than 75% of all endometrial cancers. A summary of the current approach to managing the various stage presentations of endometrial carcinoma is outlined in the critical pathway flow diagrams (Fig. 49-2).
Surgery
Surgery is the mainstay treatment for most patients with endometrial cancer. In a small subset of patients who present with unresectable disease or those whose disease is resectable but medically inoperable, definitive radiation is used instead as the primary treatment. The surgical management of endometrial cancer often involves simple hysterectomy and BSO with or without lymph node staging. However, pelvic and para-aortic lymph nodal sampling and dissection (as part of a surgical staging) has become routine in the North America and Australia, especially for patients operated on by gynecologic oncologists. Patients with endometrial cancer often have a multitude of comorbid conditions that put them at a higher-than-normal risk for complications related to major or prolonged surgery, such as an extended lymphadenectomy. Pelvic radiation following lymph nodal sampling has been shown to be associated with a higher than expected (7% versus 1%) risk of severe complications.187 Surgical staging also significantly influenced the likelihood of patients being referred for postoperative radiotherapy. The knowledge of pathologically negative lymph nodes has reduced the likelihood of patients being referred for postoperative radiotherapy, especially in the subgroup with 50% to 66% myometrial invasion.188,189 This is unfortunate, because the adequacy of a lymph node dissection varies greatly among different surgeons. Whether the procedure is being performed by a gynecologist or a gynecologic oncologist, the patient’s general condition, the habitus of the patient, and several other factors affect the thoroughness of the lymphadenectomy. The minimum number of lymph nodes that need to be retrieved for the dissection to be representative has yet to be determined, and the final evaluation of a lymphadenectomy specimen will also depend on how meticulously the specimens are examined by the pathologist. Also, pelvic lymph nodal involvement is likely a marker for a biologically aggressive disease with a propensity for distant failures; any pelvic radiation offered in that setting is aimed at essentially improving local-regional control.148 On the other hand, patients with high-risk uterine factors without lymph nodal involvement may be the very subpopulation at greatest risk for an isolated local relapse, and radiation could have the maximum curative potential in this subgroup. Thus, it is imperative that the role of extensive surgical staging procedures be critically evaluated in well-conducted trials. Such a pivotal trial was completed and reported in Lancet in 2008. A Study in the Treatment of Endometrial Cancer (ASTEC) randomized 1408 patients with endometrial cancer from four countires (United Kingdom, South Africa, Poland, and New Zealand) to hysterectomy and BSO with or without lymphadenectomy.190 If the patient was found to have intermediate-risk or high-risk early-stage disease, the patient was then randomized to observation or external radiotherapy, both of which might also include brachytherapy. The study was powered to detect an improvement of 5-year overall survival from 80% in the standard group to 90% in the lymphadenectomy group. The study was conducted between July 1, 1998, and March 31, 2005. In the lymphadenectomy group 12% had 1 to 4 nodes removed, 65% had more than 10 nodes removed, and the median number of nodes removed was 12. The risk of developing short-term major surgical complications was generally low in both groups, but more women in the lymphadenectomy group developed ileus (3% versus 1%), deep-vein thromboiss (1% versus 0.1%), lymphocyst (1% versus 0.3%), and major wound dehiscence (1% versus 0.3%). Approximately half the women with intermediate-risk or high-risk early-stage disease were randomized and treated with radiotherapy. In patients found to have advanced disease, a higher proportion of women in the standard surgery group than the lymphadenectomy group had radiotherapy (61% versus 53%). After adjuvant treatment, including postoperative radiotherapy in participants who received it, more women in the lymphadectomy group than in the standard surgery group reported moderate or severe morbidity or treatment-related complications (17% versus 12%). However, there was no clear evidence of an association between external-beam radiotherapy and lymphoedema. With a median follow up of 37 months, the overal survival curves showed no evidence of a difference between the two groups (P = 0.31). The 5-year overall survival was 81% in the standard surgery group and 80% in the lymphadenectomy group. The recurrence-free survival rates favor the standard surgery group, with 5-year recurrence-free survival of 79% versus 73% (P = 0.017). A subset analysis of number of nodes showed that the higher the number of lymph nodes removed in a lymphadenectomy, the worse the outcome. ASTEC is one the largest surgical gynecological cancer trials. This trial and another smaller randomized trial191 has shown no evidence of benefit for systematic lymphadenectomy for endometrial cancer. Pelvic lymphadenectomy should not be recommeded as routine procedure for therapeutic purposes outside of clincial trials.
Surgical Technique
Hysterectomy
TAH and BSO (TAH-BSO) is still considered the standard operative procedure for patients with endometrial cancer. The first successful TAH for this disease was performed by W. A. Freund192 in 1878. Usually, an adequate midline incision is employed to allow thorough intra-abdominal exploration and lymph nodal sampling. Upon entry into the peritoneal cavity, fluid samples are obtained for cytologic examination. This is followed by a thorough intra-abdominal exploration with a biopsy or excision of any suspicious areas. The uterus is then evaluated for any breach in the serosa, the distal ends of the fallopian tubes are ligated, and an extrafascial hysterectomy with BSO is performed. The operative plane of dissection lies outside the pubocervical fascia and does not require any unroofing of the ureters. The excised uterus is then opened away from the surgical field and an assessment is made regarding the depth of myometrial penetration.193 Frozen sections may be employed in doubtful cases.194 A new approach that is gaining popularity is the laparoscopically assisted vaginal hysterectomy and BSO (LAVH-BSO). The use of laparoscopy enables the surgeon to have a thorough intra-abdominal exploration and to be able to perform BSO, which is difficult to perform with just a vaginal hysterectomy. The potential advantages of this approach include significantly shorter hospitalization stays and fewer complications, resulting in fewer overall hospital charges when compared with patients treated by TAH.195 Currently, the GOG is conducting a prospective randomized trial comparing TAH-BSO to LAVH-BSO in early-stage endometrial cancer. Radical hysterectomy is not routinely performed in endometrial cancer because of the low incidence of parametrial involvement. There is no evidence to show that the cure rates are any better with such radical operations.196 The possible exception to this might be in patients with gross cervical involvement.197
Lymph Nodal Staging
The result of ASTEC trial leaves the issue of which patients need lymph nodal staging as a matter of great debate. Some advocate full nodal staging for all patients regardless of stage or grade, whereas others do not employ it even for high-risk patients because they will be getting radiation anyhow. The middle-of-the-road approach includes lymph nodal sampling for patients with aggressive histologic findings or more than 50% myometrial invasion (Table 49-8). The degree of lymph nodal staging also varies, but at a minimum it should include inspection and removal of any enlarged pelvic or para-aortic nodes. Pelvic and para-aortic sampling includes removal of the fat pads surrounding the major vessels in the abdomen and pelvis without skeletonizing them. According to the GOG surgical guidelines, for the sampling to be adequate, five lymphatic stations need to be removed. These include para-aortic nodes, common iliac, internal iliac, external iliac, and obturator. Lymph nodal dissection involves not only removal of the fat but also skeletonization of the vessels in similar fashion to pelvic lymph nodal dissection in cervical cancer. The surgical approach for pelvic and para-aortic lymph nodal sampling or dissection is still an intra-abdominal approach, but there is growing interest in evaluation of a laparoscopic retroperitoneal approach in an attempt to reduce morbidity.
Table 49-8 Indications for Lymph Node Sampling in Endometrial Cancer
Adjuvant Radiation Therapy
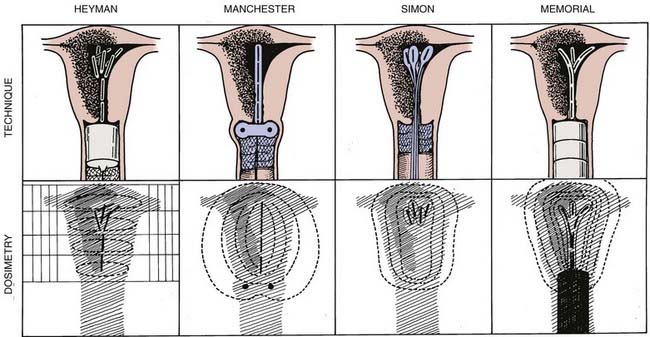
Historically, radiation therapy was given preoperatively using various techniques (Fig. 49-3) followed by an extrafascial hysterectomy. Supplemental postoperative radiotherapy was added in select cases based on the pathologic risk factors. Proposed advantages to the use of preoperative radiotherapy include (1) delivery of radiation to a well-oxygenated tumor, (2) cytoreduction (especially of the superficial tumor mass) to facilitate surgical resection and reduce the likelihood of intraoperative implantation, (3) minimizing the risk of tumor embolization, (4) eradication of microscopic tumor extensions to ensure a pathologically clear resection margin, and (5) potentially lowering the overall complication rate, resulting in better patient outcome. This approach198 has yielded excellent long-term results, with 5-year survival rates of 85% to 90%. The major disadvantages of this approach include the unnecessary treatment of patients who are at a low risk for recurrence and would have been cured by surgery alone. There is also the concern about downstaging the tumor and thus losing valuable pathologic prognostic information on which further adjuvant treatment would be based. Current treatment policies for endometrial carcinoma aim to minimize the use of preoperative radiation. All patients’ disease is preferably surgically staged and postoperative treatment is employed selectively for those patients deemed to be at a significant risk for relapse based on clinicopathologic prognostic factors (Table 49-9).
| Low Risk | Intermediate Risk | High Risk |
|---|---|---|
| IA (Grades 1 & 2) | IA, G3 | IIIA, IIIB, IIIC (All grades) |
| IB, IC (All grades) | IVA, IVB (All grades) | |
| IIA, IIB (All grades) | ||
| IIIA (Positive cytologic finding) |
The low-risk group includes patients with stage IA G1, G2 tumors. The risk of pelvic lymph nodal positivity87 is less than or equal to 3%, and the 5-year progression-free survival rate in this group is on the order of 90% to 96%. It is unlikely that postoperative pelvic external-beam radiation would add anything to the final outcome, and therefore radiation is not routinely recommended to this group of patients.199–202 The role of intravaginal radiation in these patients is also of questionable benefit because of a very low rate of vaginal recurrence with surgery alone.
The intermediate-risk group has been defined by the GOG as those with pathologic stage IB to IIB (occult cervical involvement). It is in this risk category in which most of the controversy resides about the indication and type of radiation needed. With regard to the indication of adjuvant radiation in intermediate-risk patients, the controversy relates to the lack of improvement in overall survival with the use of adjuvant radiation compared with surgery alone. The two recently completed randomized trials demonstrating lack of survival advantage leads to the conclusion on the part of many gynecologic oncologists that adjuvant radiation is not needed. The first trial was GOG protocol 99 in which 392 patients with stage IB to IIB endometrial cancer who underwent TAH-BSO and pelvic and para-aortic lymph nodal sampling were randomized to observation (N = 202) or postoperative pelvic radiation (N = 190) to a total dose of 50.4 Gy in 28 fractions.203 The study was designed to have an 80% chance of detecting a 58% decrease in the recurrence hazard rate and a 56% decrease in death hazard rate. The primary outcome was recurrence-free interval and is defined as the time from study entry to clinical, histologic, or radiographic evidence of disease recurrence. With a median follow-up of 69 months, the 2-year cumulative incidence of recurrence was 12% in the observation group and 3% in the adjuvant radiotherapy group. This was statistically significant (P = 0.007). The estimated 4-year survival rate was 86% in the observation group, compared with 92% in the irradiated group. This was not a statistically significant difference (P = 0.557). The second trial was the Postoperative Radiation in Endometrial Cancer (PORTEC) trial in which 714 patients with stage IB G2,3 and IC G1,2 were randomized after TAH-BSO and without lymph nodal sampling to observation (N = 360) or pelvic radiation (N = 354) to a total dose of 46 Gy in 23 fractions.120 With a median follow-up of 52 months, the 5-year locoregional control was 96% in the radiation arm compared with 86% in the observation arm (P < 0.001). The corresponding 5-year survival rates were 81% and 85% respectively (P = 0.37). There is a third study, the ASTEC-EN5 trial, which is a pooled meta-analysis of two randomized trials (ASTEC and EN.5) that tried to address the same issue.204 Between 1996 and 2005, 905 patients with intermediate-risk or high-risk endometrial cancer were randomly assigned to obervation or external-beam radiotherapy. Vaginal brachytherapy was allowed based on each center’s policy. As a result, 51% of the observation group received vaginal brachytherapy. Not surprisingly, the analysis did not find any evidence of overall survival benefit with external-beam radiotherapy.
With regard to the type of radiation to be used, there are three options: intravaginal brachytherapy, pelvic external-beam radiation, or a combination of both. Only one prospective randomized trial has tried to determine the optimal type of postoperative radiation in this group of patients.205 Aalders et al.205 reported on 540 patients with stage IB to IC endometrial cancer who underwent TAH-BSO without lymph nodal sampling, and postoperative intravaginal brachytherapy to 60 Gy to vaginal mucosa. Then the patients were randomized to observation (N = 277) or to supplemental pelvic radiation to 40 Gy (N = 263). A significant reduction in local recurrence rates was seen with the addition of pelvic radiation (1.9% versus 6.9%, P < 0.01). With regard to overall survival, there was no significant difference between the two arms of the study, but in the subset of patients with grade 3 disease and deep myometrial penetration, there was a survival advantage (cause-specific survival) of 18% versus 7% in favor of the pelvic radiation arm.
The data from this trial contributed somewhat to the shift in treatment policies from intravaginal brachytherapy alone to external-beam pelvic radiation alone. But whether pelvic radiation alone is equivalent to a combination of pelvic and intravaginal radiation has never been compared in a randomized fashion. Greven et al.206 reviewed the experience of two institutions to compare the outcome of the two approaches. In that study, there were 270 patients with stage I to II endometrial cancer—173 were treated with postoperative pelvic radiation alone and 97 with a combination of intravaginal and pelvic radiation.206 The corresponding 5-year pelvic control and disease-free survival rates were 96% versus 93% (P = 0.32) and 88% versus 83% (P = 0.41). This study and others called into question whether the addition of vaginal radiation is needed.207,208 A number of other reports,201,209 however, suggest that vaginal vault radiation can be added to pelvic radiation with minimal morbidity and a very low rate of recurrence (Fig. 49-4).
Although such debate is intriguing to radiation oncologists, the changes in the management of these patients made this debate less pressing. The two randomized trials120,210 comparing surgery with adjuvant radiation both employed pelvic radiation alone with a local recurrence of only 2% to 4%. On the other hand, with the increase of surgical lymph nodal staging, the use of postoperative intravaginal brachytherapy alone regained its appeal, the rationale being that full surgical lymph nodal staging could potentially eliminate the need for pelvic radiation while vaginal brachytherapy could still address the risk of vaginal cuff recurrence. PORTEC-2 is a multi-institutional randomized trial comparing external-beam radiation (46 Gy) versus vaginal brachytherapy (high dose rate [HDR] 7 Gy × 3, or low dose rate [LDR] 30 Gy). So far this study has been reported in abstract form only. The study randomized 427 patients. The pelvic radiotherapy decreased pelvic relapse (3.6% versus 0.7%). There was no signficant difference in 3-year vaginal cuff recurrence, overall survival, or relapse-free survival.211
Stage IA G3
Even though this group of patients was not included in the GOG definition of intermediate risk, from a treatment point of view this group should be treated in a similar fashion. These patients constituted less than 10% of all grade 3 patients in the GOG study,87 and while they have a very low risk of lymph nodal metastasis (see Table 49-5), there were no relapses in the three patients receiving postoperative radiation as compared with one failure out of the five patients who received no postoperative therapy. These patients could be offered either intravaginal brachytherapy alone or observation.
Stage IB G1,2
This group of patients constitutes the most common stage subgroup of all endometrial cancers. The outcome of patients who had lymph nodal dissection and no adjuvant radiation seems to be very good. Straughn et al.212 reported on 296 patients with IB G1,2 and found only 9 (3%) vaginal recurrences and 1 (0.3%) pelvic recurrence. In comparison, data from Memorial Sloan-Kettering Cancer Center on 233 patients with IB G1,2 showed a vaginal recurrence rate of only 1% and pelvic recurrence of 2% using postoperative intravaginal brachytherapy alone without routine surgical lymph nodal staging.213 Other investigators reported a vaginal recurrence rate of 0% to 1% and pelvic recurrence rate of 1% to 2%, also using intravaginal brachytherapy.214–216 Horowitz et al.217 reported on 62 patients who had surgical lymph nodal staging and received adjuvant intravaginal brachytherapy. There was one (1.6%) vaginal recurrence and zero pelvic recurrence.217 Based on data from the Aalders randomized trial, it seems that pelvic radiation is not needed in this group of patients, even without surgical lymph nodal staging. When the subset of patients with stage IB G1,2 was evaluated (n = 257), the rate of local recurrence either in the vagina or pelvis was 4% (5/126) in those treated with intravaginal brachytherapy compared with 2.3% (3/131) for those treated with brachytherapy and external radiation.205
Thus it seems reasonable to suggest that either observation or intravaginal brachytherapy are acceptable options. But when deciding whether adjuvant radiation is needed, it is important to address two issues. First, older patients tend to have higher rates of relapse. In the study by Straughn et al.,212 8 of the 10 vaginal and pelvic recurrences were in patients 60 years old or older. Second, often the indications for adjuvant radiation are rather arbitrarily based on the depth of myometrial invasion defined in thirds and on grade 1 versus 2. Yet the amount of myometrial invasion in this group of patients and whether an endometrial cancer is assigned to grade 1 as opposed to 2 in general does not appear to be a significant predictor of outcome.136,218
Stage IB G3-IC G1,2,3
Most data in the literature on this group of patients are based on pelvic radiation either alone or in combination with intravaginal brachytherapy.199,207,209,219,220 Weiss et al.221 reported on 61 patients with stage IC endometrial cancer who were treated with postoperative pelvic radiation alone. With a median follow-up of 69.5 months, there was only one recurrence in the pelvis (1.6%). Their review of the published data from the literature on patients with IC showed a pelvic recurrence of 1.04% in 240 patients treated with pelvic radiation alone compared with 0.97% in 301 patients treated with pelvic and intravaginal vaginal radiation. Their conclusion was that pelvic radiation alone is sufficient and effort should focus instead on trying to reduce the risk of distant relapse in this group of patients.221
With the increase in surgical lymph nodal staging, a shift is occurring with regard to the role of radiation even in stage IC disease. A recent multi-institutional review of 220 patients with stage IC endometrial cancer by Straughn et al.222 compared adjuvant radiation to no radiation in patients with negative nodes on surgical staging. The authors concluded that adjuvant radiation is not needed even though the 5-year disease-free survival was 74.5% for those treated with surgery alone compared with 92.5% for those treated with adjuvant radiation (P = 0.0134). It is unlikely that observation alone, even in those patients with full surgical staging, will be accepted by the radiation oncology community or even the patients when they see an 18% statistically significant difference in disease-free survival from a retrospective study in which, most likely, those patients with the worst prognostic features were the ones who received radiation.
Perhaps a reasonable alternative to observation or even pelvic radiation is intravaginal brachytherapy. In the Aalders randomized trial, the rate of local recurrence in the subset of patients with IB G3 to IC G1,2,3 was 9.3% (13/137) for those treated with brachytherapy alone compared with 1.3% (2/146) for those treated with brachytherapy and external radiation.205 Although subset analysis should be interpreted with caution, it seems that the addition of external-beam radiation therapy did have an effect, in terms of local recurrence, on patients with stage IB G3 to stage IC. This is not surprising because no lymph nodal sampling was done in that trial. But if one evaluates the data on patients with surgical lymph nodal staging and who received intravaginal brachytherapy alone, it becomes evident that the rate of local recurrence is lower. Horowitz et al. reported on 81 patients who were IB G3-IC. Only two patients (2.4%) had vaginal recurrences and one (1%) had a pelvic recurrence.217 Similarly, Chada et al.223 and Fanning224 reported no vaginal or pelvic recurrence in 38 and 39 patients, respectively.
Stage II
There are very little data regarding the optimal treatment of stage II patients. It is important to recognize the distinction between gross and occult cervical involvement and that these patients have a risk of parametrial involvement as well as a pelvic lymph nodal positivity of 25% to 50%. Preoperative radiation is generally preferred in patients with gross cervical involvement. This is usually given as a combination of external-beam pelvic radiotherapy and intracavitary radiotherapy, followed by an extrafascial TAH and BSO. An alternative approach involves surgery upfront: A modified or radical hysterectomy with BSO and pelvic lymph nodal dissection is first employed and is followed by postoperative pelvic and intravaginal radiation based on pathologic findings. For occult cervical involvement, the combination of preoperative radiation and surgery may still be employed, although most centers would prefer a formal surgical staging followed by postoperative pelvic and intravaginal brachytherapy.209,225 A review of the literature fails to support any one approach over the other (Table 49-10). Survival rates at 5 years in the range of 70% to 80% can be obtained if these principles are adhered to. Data are emerging on the role of intravaginal brachytherapy alone in patients with occult cervical involvement who also had surgical lymph nodal staging. The rate of pelvic recurrence in four such series ranged from 0% to 6%, but the data need confirmation on a larger number of patients and longer follow-up.217,216,224,236
High Risk
The high-risk group consists of all patients with stage III and IV disease, regardless of grade, with the possible exception of patients who are stage III (positive peritoneal cytologic findings) but without deep invasion or aggressive histologic findings. Although the overall outcome is far from satisfactory, this is a very heterogeneous group and the therapy has to be individualized according to the patient’s presentation. In general, for stage III patients, the outcome is much better for patients presenting with pathologic stage III disease than for those who present with clinical stage III disease.237 Although vascular space involvement, tumor grade, and myometrial invasion are still important prognostic factors, the number and site of extrauterine involvement, the ability to maximally debulk, and the presence or absence of nodal metastases have far greater prognostic importance.199,237–239
Stage III
In patients with stage IIIA disease (positive cytologic findings), the presence of other adverse features should be determined. If they are present, then the patients should be considered truly high-risk and be treated as such. On the other hand, if they are absent, then the true prognostic value of positive peritoneal cytologic findings is still unclear.145 The literature regarding the benefits of treatment in this setting is mixed; even if treatment is beneficial, the appropriate modality still must be defined. Based on the concept that the entire peritoneal cavity is at risk, intraperitoneal radioactive colloidal P32 has been used by some with results that were better than in historic controls.144 One of the important lessons learned early in the experience with P32 was that its use should not be combined with that of external-beam radiation because of a high complication rate. Soper and associates240 reported 29% bowel complications requiring surgical intervention in such patients, with two patients dying of operative complications. In a similar setting of positive peritoneal cytologic findings, Piver and associates241 have employed systemic therapy with megestrol acetate (Megace) in 45 patients. The treatment was given for 1 year, and only 2 of the 36 patients who underwent a second-look laparotomy had persistence of positive peritoneal cytologic findings. These two patients underwent continued therapy for another year and both were negative at a third-look laparotomy. However, even when no therapy was given, disease recurred in only 10% of similar patients with a minimum follow-up of 10 years; thus, the true status of adjuvant treatment in this subgroup is still a subject of considerable debate.
Patients with stage IIIA disease (positive adnexa or serosa) usually undergo surgical staging as the initial part of their therapy. Postoperative pelvic treatment with or without intravaginal radiation is offered to patients with disease limited to the adnexa; these form the most favorable subset, with a 5-year survival rate of approximately 80% (Table 49-11). Stage IIIB is a very uncommon presentation; these patients are not surgical candidates and are generally treated with a combination of external-beam and intracavitary-interstitial radiotherapy tailored to the extent of their disease. Following a good response to radiation, the treatment may be consolidated with an extrafascial hysterectomy. Patients with stage IIIC disease usually have the staging established at surgery when they are discovered to have positive pelvic or para-aortic nodes. If pelvic nodal involvement is the only major risk factor, treatment with postoperative pelvic radiotherapy can yield a 60% to 72% long-term survival rate in these patients.199,239
Patients with stage IIIC disease, by virtue of para-aortic nodal involvement, represent a particularly high-risk group. Following optimal debulking, these patients are generally treated with extended-field radiation to encompass the pelvis and the para-aortic regions (with a carefully designed boost to any gross residual disease). With this aggressive approach, several authors have reported 30% to 40% survival rates in small patient populations.199,251–257
The recognition that a significant number of patients with stage III disease fail in the abdomen237,258 has prompted numerous investigators to evaluate whole abdominal radiotherapy in these patients.259,260 The GOG also did a pilot study, No. 94, on patients with maximally debulked stages III and IV disease using whole-abdominal radiotherapy to a total dose of 30 Gy at 1.5 Gy per fraction followed by a pelvic boost for additional 19.8 Gy at 1.8 Gy per fraction. The 3-year disease-free and overall survival rates for the 58 patients with stage III endometrioid histologic findings were 42.2% and 34.5%, respectively. The incidence of late gastrointestinal morbidity was 6%.261 Using a different technique capable of delivering higher doses of radiation to the areas at risk for relapse in the abdomen, Stewart et al.262 reported 5-year disease-free and overall survival rates of 62% and 67%, respectively, in 62 patients with stage III endometrioid adenocarcinoma.
Stage IV
Stage IV disease usually has a dismal outcome, with survival rates in the range of 5% to 15%. Even in patients with tumors that can be thoroughly and optimally debulked, the results of whole-abdominal radiation are still much inferior to those with stage III disease.259 In the previously mentioned GOG trial, 19 patients with stage IV endometrioid adenocarcinoma were treated with whole-abdomen radiation after maximal debulking. The 3-year disease-free and overall survival rates were 12.5% and 21.1%, respectively.261
Radiation Therapy Techniques
External-Beam Radiation
Pelvic Radiation
Most patients are treated in the postoperative setting. The clinical target volume consists of the areas at risk, including the pelvic lymph nodes (external, internal, and lower common iliac groups) and the proximal two thirds of the vagina (because the treatment is generally delivered in the adjuvant setting without any residual tumor, there is no real gross tumor volume). The planning target volume is derived by ensuring an appropriate margin beyond the anatomic location of these structures to allow for variability in daily setup and beam penumbra. High-energy linear accelerators are preferred, and the four-field pelvic-box technique is employed (Fig. 49-5 and Fig. 49-6). The conventional field borders are as follows:
Extended Field
Intensity-modulated radiotherapy (IMRT) has become the standard radiotherapeutic modality for endometrial cancer. IMRT uses treatment beams of varying intensity, allowing the high-dose region to be very conformal to the shape of the target volume. It can also create dose heterogeneity to allow concurrent boost of targets within target volume. IMRT is particular desirable in endometrial cancer, because it can decrease treatment-related toxicity in postoperative patients and patients receiving chemotherapy. Compared with three-dimensional conformal radiotherapy, IMRT improved sparing of small bowel.263–266 This is a particularly important for extended-field radiotherapy. IMRT also improves sparing of bone marrow and improved tolerance of large-field radiotherapy and chemotherapy.267,268 Based on the same principles, there is growing interest in application of IMRT for whole-abdominal radiotherapy.269–271 In 2007 a consensus guidelines for delineation of clinical target volume for IMRT was published.272 The guidelines has been adapted in ongoing Radiation Therapy Oncology Group and GOG protocol.
Whole-Abdomen Radiation
The standard approach to whole-abdomen radiation therapy is anterior-posterior–posterior-anterior (AP–PA) open fields with five half-value layer kidney blocks placed over the PA field only (if the patient is lying supine) from the start of the treatment. The dose is usually 30 Gy at 1.5 Gy per fraction followed by 19.8 Gy boost to the pelvis at 1.8 Gy per fraction. The upper border is usually placed 1 cm above the diaphragm, and the lateral borders should extend beyond the peritoneal reflections. The lower border is usually at the bottom of the obturator foramen. The alternative approach is advocated by Martinez et al.273 Treatment starts with 9 Gy to the true pelvis via anterior and posterior open fields at daily fractions of 1.5 to 1.8 Gy. Subsequently, 30 Gy are given at this fractionation scheme to a classic whole-abdominal field, and then 12 Gy through a T-shaped field, which includes the para-aortic and the medial diaphragmatic regions.
Intravaginal Brachytherapy
HDR remote brachytherapy offers several advantages over the conventional LDR treatments in that the entire course of therapy can be delivered on an outpatient basis, the patients are far more comfortable during the short treatment time at each session, and there is a much better optimized dose distribution. The dose distribution with the LDR applicators often shows a relatively cold spot at the apex and at the distal end of the treatment volume caused by anisotropy.274 The intravaginal brachytherapy is usually delivered approximately 4 to 6 weeks postoperatively or a week after completion of the external-beam radiotherapy. This is best delivered with a vaginal cylinder rather than the conventional colpostats—the geometry of the cylinder conforms much better to the posthysterectomy vagina than the colpostat; the cylinder also gives a better dose distribution in this setting. The largest possible cylinder that fits the vagina snugly is selected. This allows for uniform mucosal apposition and ensures an optimal depth-dose distribution without overdosing the vaginal mucosa. An empirical study of different fraction sizes showed that a dose per fraction of greater than 7.5 Gy prescribed at 0.5 cm was associated with an unacceptable level of both early and late bladder and bowel toxicity.275 Dose prescriptions at greater depths (with the same, or even smaller, fraction sizes) result in much higher vaginal mucosal doses, with a much higher incidence of late vaginal stenosis and other side effects.275 The HDR monotherapy as fractionation is evolving and there are probably multiple fractionation schemes that can be adapted. However, when adapting to a new HDR fractionation technique, similar type and size of applicator should be used along with the same prescribed point or volume.
Adjuvant Systemic Therapy
Endocrine Therapy
Several hormonal agents have been studied in patients with endometrial carcinoma. Initial studies showing tumor response to alpha-hydroxy-progesterone stimulated the development of a number of clinical trials. Although early, uncontrolled trials using progestational agents in an adjuvant setting suggested a reduction in the relapse rates in patients with stages I and II disease, these results have not been borne out in the three randomized trials that studied progesterone in the adjuvant setting.276–278 Two of these failed to show any advantage for progesterone,276,277 and one showed a reduction in cancer-related deaths at the expense of an increase in non–cancer-related deaths, resulting in no net benefit from the treatment.278 The problems of obtaining statistically meaningful data from randomized trials in the adjuvant setting are highlighted by the fact that these patients have an approximately 75% cure rate, and more than 40% of the deaths are from nonmalignant causes. Thus, detection of a treatment-related benefit of even 5% would require more than 2000 patients, a goal that has not been met in any of the studies.279 Thus, adjuvant progestin therapy cannot be recommended as a standard treatment. The situation can only be clarified by further trials focusing on high-risk patients, and stratifying for receptor status so that we can define a subpopulation that may benefit from this therapy.
Chemotherapy
Although radiotherapy can help patients with stage III disease, and even cure select subgroups (such as those with only adnexal involvement, or with microscopic disease limited to a few extrauterine sites that are all optimally debulked), advances in systemic therapy may hold the key to improving cures in these patients. A series of recently completed randomized clinical trials has demonstrated the potential benefit of adjuvant chemotherapy in management of stage III and IV endometrial adenocarcinoma. The GOG launched study No. 122 in 1992. This study involved 396 patients with stage III and IV endometrial cancer (all histologic findings) with maximal residual disease of 2 cm or smaller who were randomized to whole-abdominal radiotherapy (30 Gy + 15-Gy boost) and doxorubicin and cisplatin. The primary end point of the study was progression-free survival and the initial accural goal was 240 patients; however, there was overestimation of hazard and the sample size was then increased. With a median follow-up time of 74 months, the hazard ratio for progression was 0.71 favoring the chemotherapy arm (P = 0.007). Both the 5-year overall survival and progression-free survival favored the chemotherapy arm (53% versus 42%, 42% versus 38%).280 Another randomized trial comparing adjuvant radiotherapy versus adjuvant chemotherapy was conducted in Italy. Between 1990 and 1997, 491 patients with high-risk endometrial carcinoma (stage IC G3, stage II G3 with >50% myometrial invsion, and stage III) were randomized between radiotherapy of 45 to 50 Gy to the pelvis and para-aortic node for node-positive patients, versus five cycles of cyclophosphamide (600 mg/m2), adriamycin (45 mg/m2), and cisplatin (50 mg/m2). The primary end point for the trial was overall survival. At the median follow-up time of 95.5 months, there was no difference in progression-free and overall survival. There was less local regional recurrences in the radiotherapy arm and less distant relapses in the chemotherapy arm.281 A similar study was done by the Japanese GOG. Between 1994 and 2000, 475 patients with stage IC through IIIC endometrial carcinoma with deeper than 50% myometrial invasion were randomized to pelvic radiotherapy of 45 to 50 Gy versus three or more courses of cyclophosphamide (333 mg/m2), doxorubicin (40 mg/m2), and cisplatin (40 mg/m2). The primary end point for the trial was overall survival. At the median follow-up time of 60 months, there was no significant difference in progression-free survival and overall survival. In a subset analysis, chemotherapy improved the higher-risk group (stage IC in patients older than 70 years old or with G3 endometrioid adenocarcinoma or stage II or III), and radiotherapy decrased vaginal recurrences.282
Recurrent Endometrial Cancer
Despite the fact that most patients with endometrial cancer are cured with standard therapy, disease does recur both locally and systemically. Of the patients with stage I disease treated with a combination of surgery and postoperative radiotherapy, approximately 3% have an isolated pelvic relapse, 7% have only distant disease, and another 2% have both. For stage II patients who are optimally treated, pelvic (including cuff) failures occur in approximately 3%, isolated distant failures occur in 10.5%, and another 8% have a component of both. Overall, recurrent endometrial carcinoma is confined to the pelvis in approximately half of the patients, with half of these having disease limited to the vagina. Factors predictive of salvage include vaginal vault versus pelvic relapse, lack of prior radiation, tumor size greater than 2 cm, and longer disease-free intervals.283,284 There is also a suggestion that apical vaginal relapses fare better than distal relapses, although there are reports to the contrary.285 Depending on the location of the recurrence, the extent of disease, and prior therapy, patients may be treated for palliation or with a curative intent. The treatment plan may include irradiation, surgery, endocrine therapy, or cytotoxic chemotherapy.
Radiation Therapy
Radiation therapy can be curative in a select group of patients with small vaginal recurrences who have not received prior radiation. The 5-year local control rate ranges from 42% to 65% and the 5-year overall survival rate from 31% to 53%.283,286,287 The cause of vault recurrences is still uncertain. Upper vaginal recurrences have been variously attributed to spill or implantation at the time of surgery or positive surgical margins, whereas lower vaginal relapses are often believed to represent retrograde lymphatic or vascular embolization and are especially associated with high-grade tumors. Almost 90% of the relapses occur within the first 3 years after hysterectomy. The vault and the upper third of the vagina are twice as often involved as the distal vagina, and vaginal relapses account for 20% to 25% of all failures in patients treated with surgery alone.195
With the impetus to try to eliminate any adjuvant radiation for early-stage endometrial cancer, the issue of radiation for salvage has become a very intriguing topic. Creutzberg et al.288 reported on survival after relapse in the PORTEC randomized trial. In patients who were initially randomized to surgery alone, the 5-year survival after vaginal relapse was 65%. Thus, the conclusion that some are drawing is that we should accept the higher rate of relapse with surgery alone because the majority of patients could be salvaged with radiation.
But before adopting such an approach, it is important to address certain pertinent issues. First, this high rate of salvage pertains only to isolated vaginal recurrence and not pelvic recurrence. In fact, the rate of survival for pelvic recurrence in the PORTEC trial was 0% at 3 years. On the one hand, we are being asked to omit adjuvant radiation despite its significant improvement in local control because overall survival was not improved (i.e., we need to look at the big picture), yet on the other hand we are being asked to focus only on the high rate of salvage in those with isolated vaginal recurrence and not pelvic recurrences. Second, there is no mention in the trial of how salvage radiation was given and what the potential complications were. In addition to the lack of survival advantage, the high rate of complications from adjuvant radiation in the PORTEC trial was the second impetus to recommend omitting adjuvant radiation. It is not unreasonable to expect that the rate of complications from the definitive radiation used for salvage is much higher than just adjuvant radiation. Third, the extent of vaginal recurrence is a significant predictor of outcome.286 The question then becomes how comparable this salvage rate is in the setting of a randomized trial in which patients are vigorously followed and recurrences are detected early to that in a routine clinical setting in which patients are seen by their general gynecologists perhaps once or twice a year. Fourth, as alluded to by Cardenes and Randall289 in their editorial, many advocates of omitting any type of adjuvant radiation in patients who are surgically staged use the data from the PORTEC trial to justify their conclusion. But if we were to accept the findings of this trial, then why recommend surgical lymph nodal staging? None of the patients in the PORTEC trial had it.289
Surgery
The rare patient who has an isolated central recurrence after prior irradiation may benefit from pelvic exenteration. One of the largest series of pelvic exenterations for endometrial carcinoma comes from Barakat and associates,290 who reviewed the Memorial Sloan-Kettering Cancer Center experience from 1947 to 1994. Of the 44 patients so treated, 20% were long-term survivors, with a median survival of only 10.2 months. Almost half the patients needed a total exenteration, and the complication rate was very high (>80% major complications). However, the procedure remains the only potentially curative option for patients with a central pelvic recurrence following prior surgery and radiotherapy.
Hormonal Therapy
Progestational agents have been extensively used in recurrent disease. If objective and rigorous criteria are used to evaluate the response, response rates range from 15% to 20%.291,292 The average time to progression following an initial response is approximately 4 months; patients achieving a partial or complete response may remain stable for 6 months to several years. The overall survival is approximately 1 year, although there are reports of up to 15% 5-year survival rates.291–293 Patients with well-differentiated tumors that are receptor-positive and who have a long disease-free interval (2 to 3 years) appear to have a higher response rate and a better overall outcome.293–295
Cytotoxic Chemotherapy
A number of single agents and combination regimens show objective responses in endometrial carcinoma; however, most responses are partial and last only an average of 3 to 6 months, with a median survival of only 7 to 10 months. The rare patient who attains a complete remission may have a progression-free interval of 1 to 2 years, but that is more a reflection of the biology of the disease process than an attribute of the therapy. Doxorubicin, cisplatin, carboplatin, and taxol are the main single agents with activity in endometrial carcinoma.296–299 The response rates range from 20% to 36%. Among the combinations used, regimens of doxorubicin-cisplatin and doxorubicin-cisplatin-cyclophosphamide have response rates varying from 30% to 80%. This variation in response rates is probably affected by the patient’s performance status, prior therapy, extent of disease, and the criteria used to evaluate response. Most of the responses are partial, with durations ranging from 4 to 8 months, and a median survival of less than 12 months. Although several agents are being evaluated by the GOG, the European Organisation for the Research and Treatment of Cancer (EORTC), and others, the doxorubicin-cisplatin-taxol carboplatin and taxol combinations are probably the most active regimens available today. The addition of hormones to chemotherapy does not seem to improve the outcome. Also, prior hormonal therapy does not influence the response rates to subsequent chemotherapy; this means that all patients should have a trial of hormonal therapy first, reserving the more toxic chemotherapy for endocrine treatment failures.
Treatment of Medically Inoperable Patients
Almost 10% of patients with endometrial cancer are not surgical candidates because of major comorbid medical conditions. These patients can be successfully treated with radiotherapy alone.300,301 Patients with clinical stage I disease are treated with a curative intent using a combination of external-beam therapy and intracavitary brachytherapy. The outcome of curative radiotherapy in these patients depends on the tumor-related prognostic factors as well as the severity of the underlying medical conditions. Although it is generally believed that radiation alone may yield slightly lower local control rates, a significant proportion of the mortality in this population is due to the patients’ intercurrent medical problems.302,303 Grigsby et al.302 reported that the 5-year progression-free survival rate of patients with clinical stage I disease treated with a combination of external and intracavitary radiotherapy was 94% for grade 1 disease, 92% for grade 2 disease, and 78% for grade 3 disease. At the University of California–San Francisco, the external-beam component is delivered as in the adjuvant setting, and we prefer HDR intracavitary brachytherapy, delivered using tandems and vaginal cylinders (the Martinez-Y applicator) or an ultrasound-guided intracavitary or interstitial approach for the “boost” to the site of initial disease. A CT or MRI scan is used to determine the myometrial thickness for treatment planning. Because these patients usually have intercurrent medical problems that make them a poor anesthetic risk, the intracavitary portion of the therapy may be delivered in three fractions of 6 Gy each, covering the appropriate myometrium up to the serosal surface using CT- or MRI-based planning and inverse optimization. For clinical stage II presentations, the parametria also need to be adequately treated. We deliver the intracavitary portion using a tandem and ovoids, prescribing the dose to clinical target volume. The results in stage II disease are less encouraging: almost 25% of patients have a component of pelvic failure, whereas distant metastases are seen in approximately 35% of cases.
Poor-Prognosis Histologic Subtypes
Given the aggressive nature and the relapse patterns of PS and clear cell cancers, both systemic chemotherapy and whole-abdominal radiation have been tried, following maximal surgical debulking.262,304–306 Lim et al.306 reported on 78 patients with stage I to IIIA PS carcinoma, 58 of whom were treated with whole-abdomen radiation and 20 of whom were not. The corresponding 5-year disease-specific survival rates were 74.9% and 41.3%, respectively (P = 0.04). In GOG No. 94, the 3-year disease-free and overall survival rates for the 60 patients with stage III PS or clear cell carcinomas were 40.9% and 45%, respectively.261 The data on whole-abdomen therapy are somewhat encouraging, but the rate of relapse is still substantial, indicating the need for effective systemic therapy.
The important role of adjuvant radiotherapy after conservative surgery in management of soft tissue sarcoma of the extremity has been documented in two randomized clinical trials in the 1990s.300,301,307,308 Retrospective studies in sarcoma have suggested a similar benefit of radiotherapy in uterine sarcoma. However, it was not until 2008 that the benefit of adjuvant radiotherapy was documented by a prospective randomized trial.309 In EORTC 55874, 224 women with stage I and II of all uterine sarcoma subtypes after TAH and BSO with optional nodal sampling were randomized to observation versus postoperative radiotherapy to the whole pelvis (50.4 Gy).302 The primary objective of the study was to detect a decrease in pelvic recurrence rate from 70% to 50%. The study took 13 years instead of the planned 3 years to reach accrual. At a median follow-up of 6.8 years, adjuvant radiotherapy statistically significantly reduced locoregional recurrence (22% versus 40%, P = 0.004), but had no effect on overall survival. It took a great deal of resources and time to demonstrate the benefit of adjuvant radiotherapy in this high-risk group of patients, so the conclusion from this study should not be overlooked in favor of other unproven approaches.
Sequelae of Treatment
Pelvic external-beam radiotherapy may cause transient, self-limiting diarrhea or cystitis, but these are rarely a problem if megavoltage units and judicious field-shaping are employed.310 Late (chronic) small-bowel toxicity, proctitis, or cystitis are also uncommon (less than 5%) with modern techniques and standard dose/fractionation regimens. In the PORTEC randomized trial,303 the overall (grades 1 to 4) rate of late complications was 26% in the radiotherapy group compared with 4% in the observation group (P < 0.0001). Most of the late complications in the radiotherapy group were grades 1 to 2 (22%) and only 3% were grades 3 to 4. It is important to note that many patients in this trial were treated with AP–PA fields, in which the overall rate of complications was 30% compared with 21% for those treated with a four-field box (P = 0.06). Whole-abdomen radiation toxicity is more pronounced than pelvic radiation, but not as high as expected. In GOG study No. 94, the rate of grade 4 gastrointestinal toxicity was seen in six patients (3.8%). Severe liver toxicity was seen in 3 of 158 evaluable patients (2%), with two-thirds recovering without sequelae. And there was no grade 3 to 4 genitourinary toxicity.261
Intravaginal brachytherapy’s main advantage is its ability to deliver a relatively high dose of radiation to the vagina while limiting the dose to the surrounding normal structures such as bowels and bladder.311 This advantage is manifested with the low rate of severe late toxicity seen with this treatment technique, ranging from 0% to 1%.213,214,217 But such very low rates of severe complications cannot be taken for granted because special attention must be paid to the depth of prescription, dose per fraction, the length of vagina treated, and the diameter of the cylinder used. Sorbe and Smeds311 reported a 15% late complication rate and a very high incidence of vaginal stenosis following postoperative HDR intravaginal irradiation. This was attributed to the high dose per fraction of 6 to 9 Gy; moreover, this dose was prescribed at a depth of 10 mm from the surface of the cylinder, resulting in very high vaginal mucosal, bladder, and rectal doses.
1 Jemal A, Siegal R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225.
2 Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417.
3 Lucas WE. Causal relationships between endocrine-metabolic variables in patients with endometrial carcinoma. Obstet Gynecol Survey. 1974;29:507.
4 Sommers SC, Meissner WA. Endocrine abnormalities accompanying human endometrial cancer. Cancer. 1957;10:516.
5 Rosenblum JM, Hendricks CH. Estrogenated vaginal epithelium. Obstet Gynecol. 1954;3:535.
6 Farhi DC, Nosanchuk J, Silverberg SG. Endometrial adenocarcinoma in women under 25 years of age. Obstet Gynecol. 1986;68:741.
7 Novak E, Yui E. Relation of endometrial hyperplasia to adenocarcinoma of the uterus. Am J Obstet Gynecol. 1936;32:674.
8 McDonald TW, Malkasian GE, Gaffey TA. Endometrial cancer associated with feminizing ovarian tumor and polycystic ovarian disease. Obstet Gynecol. 1977;49:654.
9 Elwood JM, Cole P, Rothman KJ, et al. Epidemiology of endometrial cancer. J Natl Cancer Inst. 1977;59:1055.
10 MacMahon B. Risk factors for endometrial cancer. Gynecol Oncol. 1974;2:122.
11 Henderson BE, Casagrande JT, Pike MC, et al. The epidemiology of endometrial cancer in young women. Br J Cancer. 1983;47:749.
12 Brinton LA, Berman RJ, Mortel R, et al. Reproductive, menstrual and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167:1317.
13 Brinton LA, Hoover RN. Epidemiology of gynecologic cancers. In: Hoskins WJ, Perez CA, Young RC, editors. principles and practice of gynecologic oncology. 2nd ed. Philadelphia: Lippincott-Raven; 1997:3.
14 Swanson CA, Potischman N, Wilbanks GD, et al. Relation of endometrial cancer risk to past and contemporary body size and body fat distribution. Cancer Epidemiol Biomark Prev. 1993;2:231.
15 La Vecchia C, Parazzini F, Negri E. Anthropometric indicators of endometrial cancer risk. Eur J Cancer. 1991;27:487.
16 Kelsey JL, LiVolsi VA, Holford TR, et al. A case-control study of cancer of the endometrium. Am J Epidemiol. 1982;116:333.
17 Wynder EL, Escher GC, Mantel N. An epidemiological investigation of cancer of the endometrium. Cancer. 1966;19:489.
18 Shu XO, Brinton LA, Zheng W, et al. Relation of obesity and body fat distribution to endometrial cancer in Shanghai, China. Cancer Res. 1992;52:3865.
19 Schapira DV, Kumar NB, Lyman GH, et al. Upper body fat distribution and endometrial cancer risk. JAMA. 1991;266:1808.
20 Sturgeon SR, Brinton LA, Berman ML, et al. Past and present physical activity and endometrial cancer risk. Br J Cancer. 1993;68:585.
21 Shu XO, Hatch MC, Zheng W, et al. Physical activity and risk of endometrial cancer. Epidemiology. 1993;4:342.
22 Gusberg SB, Hall RE. Precursors of corpus cancer: III. The appearance of cancer of the endometrium in estrogenically conditioned patients. Obstet Gynecol. 1961;17:397.
23 Brinton LA, Hoover RN. The Endometrial Cancer Collaborative Group: estrogen replacement therapy and endometrial cancer risk: unresolved issues. Obstet Gynecol. 1993;81:265.
24 Hulka BS, Fowler WC, Kaufman DG, et al. Estrogen and endometrial cancer: cases and two control groups from North Carolina. Am J Obstet Gynecol. 1980;137:92.
25 Weiss NS, Szeleky DR, English DR, et al. Endometrial cancer in relation to patterns of menopausal estrogen use. JAMA. 1979;242:261.
26 Mack TM, Pike MC, Henderson BE, et al. Estrogens and endometrial cancer in a retirement community. N Engl J Med. 1976;294:1262.
27 Underwood PB, Miller MC, Kreutner A, et al. Endometrial carcinoma: the effect of estrogens. Gynecol Oncol. 1979;8:60.
28 La Vecchia C, Franceschi S, Gallus G, et al. Prognostic features of endometrial carcinoma in estrogen users and obese women. Am J Obstet Gynecol. 1982;144:387.
29 Chu J, Schweid AI, Weiss NS. Survival among women with endometrial cancer: a comparison of estrogen users and non-users. Am J Obstet Gynecol. 1982;143:569.
30 Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to use of oral contraceptives. N Engl J Med. 1980;302:551.
31 Voigt LF, Deng Q, Weiss NS. Recency, duration and progestin content of oral contraceptives in relation to the incidence of endometrial cancer (Washington, USA). Cancer Causes Control. 1994;5:227.
32 Cancer and Steroid Hormone. Study of the Centers for Disease Control and The National Institute of Child Health and Human Development: combination oral contraceptive use and the risk of endometrial cancer. JAMA. 1987;257:796.
33 Gambrell RDJr, Bagnell CA, Greenblatt RB. Role of estrogens and progesterone in the etiology and prevention of endometrial cancer: review. Am J Obstet Gynecol. 1983;146:696.
34 Voigt LF, Weiss NS, Chu J, et al. Progestagen supplementation of exogenous estrogens and the risk of endometrial cancer. Lancet. 1991;338:274.
35 Writing Group for the Women’s Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321.
36 Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296.
37 Fisher B, Constantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the national surgical adjuvant breast and bowel project (NSABP)-B14. J Natl Cancer Inst. 1994;86:527.
38 Barakat RR, Wong G, Curtin JP, et al. Tamoxifen use in breast cancer patients who subsequently develop corpus cancer is not associated with a higher incidence of adverse histological features. Gynecol Oncol. 1994;55:164.
39 Fornander T, Hellstrom A-C, Moberger B. Descriptive clinicopathologic study of 17 patients with endometrial cancer during or after adjuvant tamoxifen in early breast cancer. J Natl Cancer Inst. 1993;85:1850.
40 Bergman L, Beelen ML, Gallee MP, et al. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000;356:881.
41 Parker SL, Tong T, Bolden S, et al. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5.
42 Potischman N, Swanson CA, Brinton LA, et al. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. 1993;4:239.
43 Barbosa JC, Shultz TD, Fillery SJ, et al. The relationship among adiposity, diet and hormone concentrations in vegetarian and nonvegetarian postmenopausal women. Am J Clin Nutr. 1990;51:798.
44 Prentice R, Thompson D, Clifford C, et al. Dietary fat reduction and plasma estradiol concentration in healthy postmenopausal women. J Natl Cancer Inst. 1990;82:129.
45 Barbone F, Austin H, Partridge EE. Diet and endometrial cancer: a case-control study. Am J Epidemiol. 1993;137:393.
46 Levi F, Franceschi S, Negri E, et al. Dietary factors and the risk of endometrial cancer. Cancer. 1993;71:3575.
47 Parazzini F, La Vecchia C, Negri E, et al. Diabetes and endometrial cancer: an Italian case-control study. Int J Cancer. 1999;81:539.
48 Soler M, Chatenoud L, Negri E, et al. Hypertension and hormone-related neoplasms in women. Hypertension. 1999;34:320.
49 Terry PD, Rohan TE, Franceschi S, et al. Cigarette smoking and the risk of endometrial cancer. Lancet Oncol. 2002;3:470.
50 Webster LA, Weiss NS. The Cancer and Steroid Hormone Study Group: alcoholic beverage consumption and the risk of endometrial cancer. Int J Epidemiol. 1989;18:786.
51 Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993;104:1535.
52 Parsons R, Li G-M, Longley MJ, et al. Hypermutability and mismatch repair deficiency in RER? tumor cells. Cell. 1994;75:1227.
53 Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA. 1997;277:915.
54 Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403.
55 Norris HJ, Tavassoli FA, Kurman RJ. Endometrial hyperplasia and carcinomas, diagnostic considerations. Am J Surg Pathol. 1988;7:839.
56 Silverberg SG. Hyperplasia and carcinoma of the endometrium. Semin Diagn Pathol. 1988;5:135.
57 Ng AB, Reagan JW, Hawliczek S, et al. Significance of endometrial cells in the detection of endometrial carcinoma and its precursors. Acta Cytol. 1974;18:356.
58 Ross JC, Eifel PJ, Cox RS, et al. Primary mucinous adenocarcinoma of the endometrium: a clinicopathologic and histochemical study. Am J Surg Pathol. 1983;7:715.
59 Abeler VM, Kjorstad KE. Endometrial adenocarcinoma in Norway: a study of a total population. Cancer. 1991;67:3093.
60 Cirisano FDJr, Robboy SJ, Dodge RK, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999;74:385.
61 Hendrickson M, Ross J, Eifel P, et al. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93.
62 Kurman RJ, Scully RE. Clear-cell carcinoma of the endometrium: an analysis of 21 cases. Cancer. 1976;37:872.
63 Cirisano FDJr, Robboy SJ, Dodge RK, et al. The outcome of stage I-II clinically and surgically staged papillary serous and clear cell endometrial cancers when compared with endometrioid carcinoma. Gynecol Oncol. 2000;77:55.
64 Piura B, Glezerman M. Synchronous carcinomas of the endometrium and ovary. Gynecol Oncol. 1989;33:261.
65 Scully RE, Tumors of the ovary and maldeveloped gonad, AFIP pamphlet No. 16; 1982; Armed Forces Institute of Pathology, Washington, DC. 92
66 Ulbright T, Roth L. Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Human Pathol. 1985;16:28.
67 Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10.
68 Deligdisch L, Holinka CF. Endometrial carcinoma: two diseases? Cancer Detect Prev. 1987;10:237.
69 Lax SF, Kendall B, Tashiro H, et al. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814.
70 Tashiro H, Isacson C, Levine R, et al. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997;150:177.
71 Moll UM, Chalas E, Auguste M, et al. Uterine papillary serous carcinoma evolves via a p53-driven pathway. Hum Pathol. 1996;27:1295.
72 Sung CJ, Zheng Y, Quddus MR, et al. p53 as a significant prognostic marker in endometrial carcinoma. Int J Gynecol Cancer. 2000;10:119.
73 Geisler JP, Geisler HE, Wiemann MC, et al. p53 expression as a prognostic indicator of 5-year survival in endometrial cancer. Gynecol Oncol. 1999;74:468.
74 Tashiro H, Blazes MS, Wu R, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935.
75 Risinger JI, Hayes AK, Berchuck A, et al. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736.
76 Risinger JI, Hayes K, Maxwell GL, et al. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res. 1998;4:3005.
77 Duggan BD, Felix JC, Muderspach LI, et al. Microsatellite instability in sporadic endometrial carcinoma. J Natl Cancer Inst. 1994;86:1216.
78 Maxwell GL, Risinger JI, Alvarez AA, et al. Favorable survival associated with microsatellite instability in endometrioid endometrial cancers. Obstet Gynecol. 2001;97:417.
79 Boyd J, Risinger JI. Analysis of oncogene alterations in human endometrial carcinoma: prevalence of ras mutations. Mol Carcinog. 1991;4:189.
80 Berchuck A, Rodriguez G, Kinney RB, et al. Overexpression of HER-2/neu in endometrial cancer is associated with advanced stage disease. Am J Obstet Gynecol. 1991;164:15.
81 Hetzel DJ, Wilson TO, Keeny GL, et al. HER-2/neu expression: a major prognostic factor in endometrial cancer. Gynecol Oncol. 1992;47:179.
82 Berchuck A, Boyd J. Molecular basis of endometrial cancer. Cancer. 1995;76(suppl 10):2034.
83 Taskin M, Lallas TA, Barber HR, et al. bcl-2 and p53 in endometrial adenocarcinoma. Mod Pathol. 1997;10:728.
84 Leiserowitz GS, Harris SA, Subramaniam M, et al. The proto-oncogene c-fms is overexpressed in endometrial cancer. Gynecol Oncol. 1993;49:190.
85 Kobayashi K, Sagae S, Nishioka Y, et al. Mutations of the beta-catenin gene in endometrial carcinomas. Jpn J Cancer Res. 1999;90:55.
86 Risinger JI, Maxwell GL, Chandramouli GV, et al. Microarray analysis reveals distinct gene expression profiles among different histologic types of endometrial cancer. Cancer Res. 2003;63:6.
87 Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer (a Gynecologic Oncology Group Study). Cancer. 1987;60:2035.
88 Caspi E, Perpinial S, Reif A. Incidence of malignancy in Jewish women with post-menopausal bleeding. Israel J Med Sci. 1977;13:299.
89 Fleisher AC, Dudley BS, Entman SS, et al. Myometrial invasion by endometrial carcinoma: Sonographic assessment. Radiology. 1987;162:307.
90 Hulka CA, Hall DA, McCarthy K, et al. Endometrial polyps, hyperplasia, and carcinoma in post-menopausal women: differentiation with endovaginal sonography. Radiology. 1994;191:755.
91 Mendelson EB, Bohm-Velez M, Joseph N, et al. Endometrial abnormalities: evaluation with transvaginal sonography. Am J Roentgenol. 1989;150:139.
92 DelMaschio A, Vanzulli A, Sironi S, et al. Estimating the depth of myometrial involvement of endometrial carcinoma: efficacy of transvaginal sonography vs. MR imaging. Am J Roentgenol. 1993;160:533.
93 Hamlin DJ, Burgener FA, Belcham JB. CT of intramural carcinoma: contrast enhancement is essential. Am J Roentgenol. 1981;137:551.
94 Dore R, Moro B, D’Andrea F, et al. CT evaluation of myometrial invasion in endometrial carcinoma. J Comput Assist Tomogr. 1987;11:282.
95 Balfe DM, Van Dyke J, Lee JKT, et al. Computerized tomography in endometrial neoplasms. J Comput Assist Tomogr. 1982;7:677.
96 Walsh JW, Vick CW. Staging of female genital tract cancer. In: Walsh JW, editor. computerized tomography of the pelvis. New York: Churchill Livingstone; 1985:163.
97 Walsh JW, Goplerud DR. Computerized tomography of primary, persistent and recurrent endometrial malignancy. Am J Roentgenol. 1982;139:1149.
98 Franchi M, LaFianza A, Babilonti L, et al. Clinical value of computerized tomography (CT) in assessment of recurrent uterine cancers. Gynecol Oncol. 1989;35:31.
99 Chen SS, Rumanik WM, Spiegel G. Magnetic resonance imaging in stage I endometrial carcinoma. Obstet Gynecol. 1990;75:274.
100 Hricak H, Rubinstein LV, Gherman GM, et al. MR imaging evaluation of endometrial carcinoma: results of an NCI cooperative study. Radiology. 1991;179:829.
101 Pellorito JS, Taylor KJ, Quedenas-Case C, et al. Endovaginal color flow scoring system: a sensitive indicator of pelvic malignancy [abstract]. Radiology. 1994;193(P):276.
102 Weber TM, Sostman DH, Spritzer CE, et al. Cervical carcinoma: determination of recurrent tumor versus radiation changes with MR imaging. Radiology. 1995;194:135.
103 Hricak H, Stern JL, Fisher MR, et al. Endometrial carcinoma staging by MR imaging. Radiology. 1987;162:297.
104 Joja I, Asakawa M, Nakagawa T, et al. Endometrial carcinoma: dynamic MRI with turbo-FLASH technique. J Computer Assist Tomogr. 1996;20:878.
105 Carrington B, Hricak H. The uterus and vagina. In: Hricak H, Carrington BM, editors. MRI of the pelvis: a text atlas. London: Martin Dunitz; 1991:93.
106 Hirano Y, Kubo K, Hirai Y, et al. Preliminary experience with gadolinium-enhanced dynamic MR imaging for uterine neoplasms. Radiology. 1992;12:243.
107 Sironi S, Mellone R, Venzulli A, et al. Assessment of the myometrial infiltration of endometrial carcinoma (FIGO stage I-II): the accuracy of magnetic resonance. Radiol Med (Torino). 1989;77:386.
108 Fishman-Javitt MC, Stein HL, Lovecchio JL. MRI in staging endo-metrial and cervical carcinoma. Magn Reson Imaging. 1987;5:83.
109 DelMaschio A, Vanzulli A, Sironi S, et al. Estimating the depth of myometrial involvement of endometrial carcinoma: efficacy of transvaginal sonography vs. MR imaging. Am J Roentgenol. 1993;160:533.
110 Gordon AN, Fleisher AC, Dudley BS. Preoperative assessment of myometrial invasion of endometrial adenocarcinoma by sonography (US) and magnetic resonance imaging. Gynecol Oncol. 1989;34:175.
111 Berchuck A, Soisson AP, Clarke-Pearson DL, et al. Immunohistochemical expression of CA 125 in endometrial adenocarcinoma: Correlation of antigen expression with meta static potential. Cancer Res. 1989;49:2091.
112 Rose PG, Sommers RM, Reale FR, et al. Serial serum CA 125 measurements for evaluation of recurrence in patients with endometrial carcinoma. Obstet Gynecol. 1994;84:12.
113 Boronow RC, Morrow CP, Creasman WT, et al. Surgical staging in endometrial cancer: Clinicopathological findings of a prospective study. Obstet Gynecol. 1984;63:825.
114 International Federation of Gynecology and Obstetrics. corpus cancer staging. Int J Gynecol Obstet. 1989;28:190.
115 International Federation of Gynecology and Obstetrics. classification and staging of malignant tumors in the female pelvis. Int J Gynecol Obstet. 1971;9:172.
116 Nolan JF, Huen A. Prognosis in endometrial cancer. Gynecol Oncol. 1976;4:384.
117 Frick HC, Munnell EW, Richart RM, et al. Carcinoma of the endometrium. Am J Obstet Gynecol. 1973;115:663.
118 Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. J Epidem Biostat. 2001;6:45.
119 Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973–1987 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31.
120 Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomized trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404.
121 Alektiar KM, Abu-Rustum N, Venkatraman E, et al: Is endometrial cancer intrinsically more aggressive in elderly patients? Cancer 98:2368–2377, 2003.
122 Nori D, Hilaris B. Principles of radiation therapy in the treatment of carcinoma of the endometrium. In: Nori D, Hilaris B, editors. radiation therapy of gynecologic cancer. New York: Alan R. Liss; 1987:121.
123 Connell PP, Rotmensch J, Waggoner SE, et al. Race and clinical outcome in endometrial carcinoma. Obstet Gynecol. 1999;94:713.
124 Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base report on endometrial carcinoma in African-American women. Cancer. 1998;83:2629.
125 Nori D, Hilaris BS, Batata MA, et al. Clinical application of remote afterloaders with special reference to endometrial cancer. In: Brachytherapy Oncology, Memorial Hospital Annual Brachytherapy Proceedings. New York: MSKCC Publishers; 1983:101-118.
126 Lutz MH, Underwood PBJr, Kreutner AJr, et al. Endometrial carcinoma: a new method of classification of therapeutic and prognostic significance. Gynecol Oncol. 1978;6:83.
127 Boente MP, Yordan ELJr, McIntosh DG, et al. Prognostic factors and long-term survival in endometrial adenocarcinoma with cervical involvement. Gynecol Oncol. 1993;51:316.
128 Mayr NA, Wen BC, Benda JA, et al. Postoperative radiotherapy in clinical stage I endometrial cancer—corpus, cervical and lower uterine segment involvement: patterns of failure. Radiology. 1995;196:323.
129 Surwit EA, Fowler WCJr, Rogoff EE, et al. Stage II adenocarcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 1979;5:323.
130 Rubin SC, Hoskins WJ, Saigo PE, et al. Management of endometrial adenocarcinoma with cervical involvement. Gynecol Oncol. 1992;45:294.
131 Chambers SK, Kapp DS, Peschel RE, et al. Prognostic factors and sites of failure in FIGO stage I, grade 3 endometrial cancer. Gynecol Oncol. 1987;27:180.
132 Wilson TO, Podratz KC, Gaffey TA, et al. Evaluation of unfa vorable histologic subtypes in endometrial adenocarcinoma. Am J Obstet Gynecol. 1990;162:418.
133 Nicklin JL, Copeland LJ. Endometrial papillary serous carcinoma: patterns of spread and treatment. Clin Obstet Gynecol. 1996;39:686.
134 Sutton GP, Geiser HE, Stehman FB, et al. Features associated with survival and disease-free survival in early endometrial cancer. Am J Obstet Gynecol. 1989;160:1385.
135 Wharton JT, Kikuta JJ, Mettlin C, et al. Risk factors and current management in carcinoma of the endometrium. Surg Gynecol Obstet. 1986;162:515.
136 Alektiar KM, McKee A, Lin O, et al. The significance of the amount of myometrial invasion in patients with stage IB endometrial carcinoma. Cancer. 2002;95:316.
137 Hanson MB, van Nagell JRJr, Powell DE, et al. The prognostic significance of lymph-vascular space invasion in stage I endometrial cancer. Cancer. 1985;55:1753.
138 Sivridis E, Buckley CH, Fox H. The prognostic significance of lymphatic vascular space invasion in endometrial adenocarcinoma. Br J Obstet Gynecol. 1987;94:991.
139 Nori D, Hilaris B. Principles of radiation therapy in the treatment of carcinoma of the endometrium. In: Radiation therapy of gynecologic cancer. New York: Alan R. Liss; 1987:122.
140 Konski A, Poulter C, Keys H, et al. Absence of prognostic significance, peritoneal dissemination and treatment advantage in endometrial cancer patients with positive peritoneal cytology. Int J Radiat Oncol Biol Phys. 1988;14:49.
141 Lurain JR, Ramsey NK, Schink JC, et al. Prognostic significance of positive peritoneal cytology in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1989;74:175.
142 Yazigi R, Piver MS, Blumenson L. Malignant peritoneal cytology as prognostic indicators in stage I endometrial cancer. Obstet Gynecol. 1983;62:359.
143 Turner DA, Gershenson DM, Atkinson N, et al. The prognostic significance of peritoneal cytology for stage I endometrial cancer. Obstet Gynecol. 1989;74:775.
144 Creasman WT, DiSaia PJ, Blessing J, et al. Prognostic significance of peritoneal cytology in patients with endometrial cancer and preliminary data concerning therapy with intraperitoneal radio-pharmaceuticals. Am J Obstet Gynecol. 1981;141:921.
145 Milosevic MF, Dembo AJ, Thomas GM. The clinical significance of malignant peritoneal cytology in stage I endometrial carcinoma. Int J Gynecol Cancer. 1992;2:225.
146 Sonoda Y, Zerbe M, Smith A, et al. High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy. Gynecol Oncol. 2001;80:378.
147 Ashman JB, Connell PP, Yamada D, et al. Outcome of endometrial carcinoma patients with involvement of the uterine serosa. Gynecol Oncol. 2001;82:338.
148 Descamps P, Calais G, Moire C, et al. Predictors of distant recurrence in clinical stage I or II endometrial carcinoma treated by combination surgical and radiation therapy. Gynecol Oncol. 1997;64:54.
149 Evans MP, Podratz KC. Endometrial neoplasia: prognostic significance of ploidy status. Clin Obstet Gynecol. 1996;39:696.
150 Lindahl B, Ranstam J, Norgren A, et al. Identification of high-risk groups in endometrial carcinoma stage I-II: a combination of DNA- and steroid-receptor measurements identifies early deaths from the disease. Anticancer Res. 1995;15:1095.
151 Pisani AL, Barbuto DA, Chen D, et al. HER-2/neu, p53 and DNA analyses as prognosticators for survival in endometrial carcinoma. Obstet Gynecol. 1995;85:729.
152 Lukes AS, Kohler MF, Pieper CF, et al. Multivariate analysis of DNA ploidy, p53 and HER-2/neu as prognostic factors in endometrial cancer. Cancer. 1994;73:2380.
153 Coleman RL, Schink JC, Miller DS, et al. DNA flow cytometric analysis of clinical stage I endometrial carcinoma with lymph node metastasis. Gynecol Oncol. 1993;50:20.
154 Melchiorri C, Chieco P, Lisignoli G, et al. Ploidy disturbances as an early indicator of intrinsic malignancy in endometrial carcinoma. Cancer. 1993;72:165.
155 Hamel NW, Sebo TJ, Wilson TO, et al. Prognostic value of p53 and proliferating cell nuclear antigen expression in endometrial carcinoma. Gynecol Oncol. 1996;62:192.
156 Kaleli S, Kosebay D, Bese T, et al. A strong prognostic variable in endometrial carcinoma: flow cytometric S-phase fraction. Cancer. 1997;79:944.
157 Nordstrom B, Strang P, Lindgren A, et al. Endometrial carcinoma: the prognostic impact of papillary serous carcinoma (UPSC) in relation to nuclear grade, DNA ploidy and p53 expression. Anticancer Res. 1996;16:899.
158 Ferrara F, De Santis L, Mangili F, et al. Role of DNA ploidy and ERB-B2 oncogene expression in the prognosis of endometrial carcinoma. Pathol Res Pract. 1994;190:1039.
159 Friberg LG, Noren H, Delle U. Prognostic value of DNA ploidy and S-phase fraction in endometrial cancer stage I and II: a prospective 5-year survival study. Gynecol Oncol. 1994;53:64.
160 Nazeer T, Ballouk F, Malfetano JH, et al. Multivariate survival analysis of clinicopathologic features in surgical stage I endometrial carcinoma including analysis of HER-2/neu expression. Am J Obstet Gynecol. 1995;173:1829.
161 Geisler JP, Weimann MC, Zhou Z, et al. DNA index by image analysis in advanced endometrial carcinoma. J Surg Oncol. 1996;63:91.
162 Nordstrom B, Strang P, Bergstrom R, et al. A comparison of proliferation markers and their prognostic value for women with endometrial carcinoma: Ki-67, proliferating nuclear antigen and flow cytometric S-phase fraction. Cancer. 1996;78:1942.
163 Pfisterer J, Kommoss F, Sauerbrei W, et al. The prognostic value of DNA ploidy and S-phase fraction in stage I endometrial carcinoma. Gynecol Oncol. 1995;58:149.
164 Creasman WT. Prognostic significance of hormone receptors in endometrial cancer. Cancer. 1995;71(4 suppl):1467.
165 Nyholm HC, Christensen IJ, Nielsen AL. Progesterone receptor levels independently predict survival in endometrial adenocarcinoma. Gynecol Oncol. 1995;59:347.
166 Vecek N, Nola M, Marusic M, et al. Prognostic value of steroid hormone receptors concentration in patients with endometrial carcinoma. Acta Obstet Gynecol Scand. 1994;73:730.
167 Wang D, Konishi I, Koshiyama M, et al. Expression of c-erb-2 protein and epidermal growth receptor in endometrial carcinomas: correlation with clinicopathologic and sex steroid receptor status. Cancer. 1993;72:2628.
168 Kohler MF, Carney P, Dodge R, et al. p53 Overexpression in advanced stage endometrial adenocarcinoma. Am J Obstet Gynecol. 1996;175:1246.
169 Kohlberger P, Gitsch G, Loesch A, et al. p53 Protein overexpression in early stage endometrial cancer. Gynecol Oncol. 1996;62:213.
170 Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268.
171 Reinartz JJ, George E, Lindgren BR, et al. Expression of p53, transforming growth factor alpha, epidermal growth factor receptor, and c-erb-2 in endometrial carcinoma and correlation with survival and known predictors of survival. Hum Pathol. 1994;25:1075.
172 Khalifa MA, Mannel RS, Haraway SD, et al. Expression of EGFR, HER-2/neu, p53 and PCNA in endometrioid, serous papillary, and clear-cell endometrial adenocarcinomas. Gynecol Oncol. 1994;53:84.
173 Ito K, Watanabe K, Nasim S, et al. Prognostic significance of p53 overexpression in endometrial cancer. Cancer Res. 1994;54:4667.
174 Berchuck A, Boyd J. Molecular basis of endometrial cancer. Cancer. 1995;76:2034. Review
175 Kihana T, Hamada K, Inoue Y, et al. Mutation and allelic loss of the p53 gene in endometrial carcinoma: incidence and outcome in 92 surgical patients. Cancer. 1995;76:72.
176 Honda T, Kato H, Imamura T, et al. Involvement of p53 gene mutations in human endometrial carcinomas. Int J Cancer. 1993;53:963.
177 Saffari B, Jones LA, el-Naggar A, et al. Amplification and overexpression of HER-2/neu in endometrial cancers: correlation with overall survival. Cancer Res. 1995;55:5693.
178 Kohlberger P, Loesch A, Koelbl H, et al. Prognostic value of immunohistochemically detected HER-2/neu oncoprotein in endometrial cancer. Cancer Lett. 1996;98:151.
179 Jassoni VM, Amadori A, Santini D, et al. Epidermal growth factor receptor (EGF-R) and transforming growth factor alpha (TGFA) expression in different endometrial cancers. Anticancer Res. 1995;15:1327.
180 Watanabe J, Sato Y, Kuramoto H, et al. Expression of nm23-H1 and nm23-H2 protein in endometrial carcinoma. Br J Cancer. 1995;72:1469.
181 Ito K, Watanabe K, Nasim S, et al. K-ras point mutations in endometrial carcinoma: effect on outcome is dependent on the age of the patient. Gynecol Oncol. 1996;63:238.
182 Marone M, Scambia G, Ferrandina G, et al. Nm23 expression in endometrial and cervical cancer: inverse correlation with lymph node involvement and myometrial invasion. Br J Cancer. 1996;74:1063.
183 Niikura H, Sasano H, Matsunaga G, et al. Prognostic value of epidermal growth factor receptor expression in endometrioid endometrial carcinoma. Hum Pathol. 1995;26:892.
184 Hakala A, Kacinski BM, Stanley ER, et al. Macrophage colony-stimulating factor 1, a clinically useful tumor marker in endometrial adenocarcinoma: comparison with CA 125 and the aminoterminal propeptide of type III procollagen. Am J Obstet Gynecol. 1995;173:112.
185 Macasaet M, Brigati D, Boyce J, et al. The significance of residual disease after radiotherapy in endometrial carcinoma: clinico-pathologic correlation. Am J Obstet Gynecol. 1980;138:557.
186 Ahmad NR, Lanciano RM, Corn BR, et al. Postoperative radiation therapy for surgically staged endometrial cancer: impact of time factors (overall treatment time, and surgery-to-radiation interval) on outcome. Int J Radiat Oncol Biol Phys. 1995;33:837.
187 Corn BW, Lanciano RM, Greven KM, et al. Impact of improved irradiation technique, age and lymph node sampling on the severe complication rate of surgically staged endometrial cancer patients: a multivariate analysis. J Clin Oncol. 1994;12:510.
188 Gretz HF3rd, Economos K, Husain A, et al. The practice of surgical staging and its impact on adjuvant treatment recommendations in patients with stage I endometrial carcinoma. Gynecol Oncol. 1996;61:409.
189 Fanning J, Nanavati PJ, Hilgers RD. Surgical staging and high dose-rate brachytherapy for endometrial cancer: limiting external radiotherapy to node-positive patients. Obstet Gynecol. 1996;87:1041.
190 Efficacy of Systematic Pelvic Lymphadenectomy in Endometrial Cancer (MRC ASTEC Trial): a Randomized Study. Lancet. 2009;373:125.
191 Panici PB, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707-1716.
192 Freund WA. A new method for extirpation of the whole uterus. Berl Klin Wochenschr. 1878;18:275.
193 Doering DL, Barnhill DR, Weiser EB, et al. Intraoperative evaluation of depth of myometrial invasion in stage I endometrial adenocarcinoma. Obstet Gynecol. 1989;74:930.
194 Malviya VK, Deppe G, Malone JMJr, et al. Reliability of frozen section examination in identifying poor prognostic indicators in stage I endometrial adenocarcinoma. Gynecol Oncol. 1989;34:299.
195 Gemignani ML, Curtin JP, Zelmanovich J, et al. Laparoscopic-assisted vaginal hysterectomy for endometrial cancer: clinical outcomes and hospital charges. Gynecol Oncol. 1999;73:5.
196 Homesley HF, Boronow RC, Lewis JL. Treatment of adenocarcinoma of the endometrium at Memorial-James Ewing Hospitals: 1946–1965. Obstet Gynecol. 1976;47:100.
197 Mariani A, Webb MJ, Keeney GL, et al. Role of wide/radical hysterectomy and pelvic lymph node dissection in endometrial cancer with cervical involvement. Gynecol Oncol. 2001;83:72.
198 Grigsby PW, Perez CA. Clinical stage I endometrial cancer: results of adjuvant irradiation and patterns of failure. Int J Radiat Oncol Biol Phys. 1991;21:379.
199 Morrow CP, Bundy BN, Kumar RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stages I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55.
200 Carey M, O’Connell G. Good outcome associated with a standardized treatment protocol using selective post-operative radiation with clinical stage I adenocarcinoma of the endometrium. Gynecol Oncol. 1995;57:138.
201 Kucera H, Vaura N, Weghoupt K. Benefit of external irradia- tion in pathologic stage I endometrial carcinoma: a prospective clinical trial of 605 patients who received postoperative vaginal irradiation and additional pelvic irradiation in the presence of unfavorable prognostic factors. Gynecol Oncol. 1990;38:99.
202 Elliot P, Green D. The efficacy of postoperative vaginal irradiation in preventing vaginal recurrence in endometrial cancer. Int J Gynecol Cancer. 1994;4:84.
203 Keys H, Roberts J, Brunetto V, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study. Gynecol Oncol. 2004;92:744.
204 Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137-146.
205 Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419.
206 Greven KM, D’Agostino RBJr, Lanciano RM, et al. Is there a role for a brachytherapy vaginal cuff boost in the adjuvant management of patients with uterine-confined endometrial cancer? Int J Radiat Oncol Biol Phys. 1998;42:101.
207 Rush S, Gal D, Potters L, et al. Pelvic control following external beam radiation for surgical stage I endometrial adenocarcinoma. Int J Radiat Oncol Biol Phys. 1995;33:851.
208 Randall ME, Wilder J, Greven K, et al. Role of intracavitary cuff boost after adjuvant external irradiation in early endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:49.
209 Nori D, Merimsky O, Batata M, et al. Postoperative high dose-rate intravaginal brachytherapy combined with external irradiation for early stage endometrial cancer: a long-term followup. Int J Radiat Oncol Biol Phys. 1994;30:831.
210 Roberts JA, Brunetto VL, Keyes HM, et al. A phase III randomized study of surgery vs. surgery plus adjunctive radiation therapy in intermediate-risk endometrial carcinoma (GOG 99) abstract 35. Proc Soc Gynecol Oncol. 70, 1998.
211 Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Vaginal brachytherapy versus external beam pelvic radiotherapy for high-intermediate risk endometrial cancer: results of the randomized PORTEC-2 trial [abstract]. J Clin Oncol. 2008;26:LBA5503.
212 Straughn JMJr, Huh WK, Kelly FJ, et al. Conservative management of stage I endometrial carcinoma after surgical staging. Gynecol Oncol. 2002;84:194.
213 Alektiar KM, McKee A, Venkatraman E, et al. Intravaginal high-dose-rate brachytherapy for stage IB (FIGO grade 1, 2) endometrial cancer. Int J Radiat Oncol Biol Phys. 2002;53:707.
214 Petereit DG, Tannehill SP, Grosen EA, et al. Outpatient vaginal cuff brachytherapy for endometrial cancer. Int J Gynecol Cancer. 1999;9:456.
215 Anderson JM, Stea B, Hallum AV, et al. High-dose-rate postoperative vaginal cuff irradiation alone for stage IB and IC endometrial cancer. Int J Radiat Oncol Biol Phys. 2000;46:417.
216 MacLeod C, Fowler A, Duval P, et al. High-dose-rate brachytherapy alone post-hysterectomy for endometrial cancer. Int J Radiat Oncol Biol Phys. 1998;42:1033.
217 Horowitz NS, Peters WAIII, Smith MR, et al. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235.
218 Scholten AN, Creutzberg CL, Noordijk EM, et al. Long-term outcome in endometrial carcinoma favors a two-instead of a three-tiered grading system. Int J Radiat Oncol Biol Phys. 2002;52:1067.
219 Irwin C, Levin W, Fyles A, et al. The role of adjuvant radiotherapy in carcinoma of the endometrium—results in 550 patients with pathologic stage I disease. Gynecol Oncol. 1998;70:247.
220 Piver M, Hempling R. A prospective trial of post-operative vaginal radium/cesium for grade 1–2 less than 50% myometrial invasion and pelvic radiation therapy for grade 3 or deep myometrial invasion in surgical stage I endometrial adenocarcinoma. Cancer. 1990;66:133.
221 Weiss MF, Connell PP, Waggoner S, et al. External pelvic radiation therapy in stage IC endometrial carcinoma. Obstet Gynecol. 1999;93:599.
222 Straughn JM, Huh WK, Orr JWJr, et al. Stage IC adenocarcinoma of the endometrium: survival comparisons of surgically staged patients with and without adjuvant radiation therapy. Gynecol Oncol. 2003;89:295.
223 Chadha M, Nanavati PJ, Liu P, et al. Patterns of failure in endometrial carcinoma stage IB grade 3 and IC patients treated with postoperative vaginal vault brachytherapy. Gynecol Oncol. 1999;75:103.
224 Fanning J. Long-term survival of intermediate risk endometrial cancer (stage IG3, IC, II) treated with full lymphadenectomy and brachytherapy without teletherapy. Gynecol Oncol. 2001;82:371.
225 Pitson G, Colgan T, Levin W, et al. Stage II endometrial carcinoma: prognostic factors and risk classification in 170 patients. Int J Radiat Oncol Biol Phys. 2002;53:862.
226 Lanciano RM, Curran WJ, Greven KM, et al. Influence of grade, histologic subtype and timing of radiotherapy on the outcome among patients with stage II carcinoma of the endometrium. Gynecol Oncol. 1990;39:368.
227 Podczaski ES, Kaminski P, Manetta A, et al. Stage II endometrial carcinoma treated with external beam radiotherapy, intracavitary application of cesium, and surgery. Gynecol Oncol. 1989;35:251.
228 Ahmad K, Kim YH, Deppe G, et al. Radiation therapy in stage II carcinoma of the endometrium. Cancer. 1989;63:854.
229 Larson DM, Copeland LJ, Gallagher HS, et al. Stage II endometrial carcinoma: Results and complications of a combined radiotherapeutic-surgical approach. Cancer. 1988;61:1528.
230 Greven K, Olds W. Radiotherapy in the management of endometrial carcinoma with cervical involvement. Cancer. 1987;60:1737.
231 Calkins AR, Stehman FB, Sutton GP, et al. Adenocarcinoma corpus et colli: analysis of diagnostic variables. Int J Radiat Oncol Biol Phys. 1986;12:911.
232 Grigsby PW, Perez CA, Camel HM, et al. Stage II carcinoma of the endometrium: results of therapy and prognostic factors. Int J Radiat Oncol Biol Phys. 1985;11:1915.
233 Onsrud M, Aalders J, Abeler V, et al. Endometrial carcinoma with cervical involvement (stage II): prognostic factors and value of combined radiological-surgical treatment. Gynecol Oncol. 1982;13:76.
234 Kinsella TJ, Bloomer WD, Lavin PT, et al. Stage II endometrial carcinoma: 10 year followup of combined radiation and surgical treatment. Gynecol Oncol. 1980;10:290.
235 Bruckman JE, Goodman RL, Murthy A, et al. Combined irradiation and surgery in the treatment of stage II carcinoma of the endometrium. Cancer. 1978;42:1146.
236 Ng TY, Nicklin JL, Perrin LC, et al. Postoperative vaginal vault brachytherapy for node-negative stage II (occult) endometrial carcinoma. Gynecol Oncol. 2001;81:193.
237 Greven K, Corn B, Lanciano RM. Pathologic stage III endometrial carcinoma. Cancer. 1993;71:3697.
238 Mariani A, Webb MJ, Keeney GL, et al. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol. 2002;87:112.
239 Nelson G, Randall M, Sutton G, et al. FIGO stage IIIC endometrial carcinoma with metastases confined to pelvic lymph nodes: analysis of treatment outcomes, prognostic variables, and failure patterns following adjuvant radiation therapy. Gynecol Oncol. 1999;75:211.
240 Soper JT, Creasman WT, Clarke-Pearson DL, et al. Intraperi-toneal chromic phosphate P-32 suspension therapy of malignant peritoneal cytology in endometrial carcinoma. Am J Obstet Gynecol. 1985;153:191.
241 Piver MS, Recio FO, Baker TL, et al. A prospective trial of progesterone therapy for malignant peritoneal cytology in patients with endometrial cancer. Gynecol Oncol. 1993;47:373.
242 Antoniades J, Brady LW, Lewis GC. The management of stage III carcinoma of the endometrium. Cancer. 1976;38:1838.
243 Bruckman JE, Bloomer WD, Marck A, et al. Stage III adenocarcinoma of the endometrium: two prognostic groups. Gynecol Oncol. 1980;9:12.
244 Danoff BF, McDay J, Louka M, et al. Stage III endometrial carcinoma: analysis of patterns of failure and therapeutic implications. Int J Radiat Oncol Biol Phys. 1980;6:1491.
245 Greven KM, Curran WJ, Whittington R, et al. Analysis of failure patterns in stage III endometrial carcinoma and therapeutic implications. Int J Radiat Oncol Biol Phys. 1989;17:35.
246 Mackillop WJ, Pringle JF. Stage III endometrial carcinoma: a review of 90 cases. Cancer. 1985;56:2519.
247 Milton PJ, Metters J. Endometrial carcinoma: an analysis of 355 cases treated at St. Thomas’ Hospital, 1945–1969. J Obstet Gynecol Br Commonwealth. 1972;79:455.
248 Greer BE, Hamberger AD. Treatment of intraperitoneal metastatic adenocarcinoma of the endometrium by the whole abdomen moving-strip technique and pelvic boost irradiation. Gynecol Oncol. 1983;16:365.
249 Potish RA, Twiggs LB, Adcock LL, et al. Role of whole abdominal radiation therapy in the management of endometrial cancer: prognostic importance of factors indicating peritoneal metastases. Gynecol Oncol. 1985;21:80.
250 Nori D, Hilaris BS, Tome M, et al. Combined surgery and radiation in endometrial carcinoma: an analysis of prognostic factors. Int J Radiat Oncol Biol Phys. 1987;13:489.
251 Potish RA, Twiggs LB, Adcock LL, et al. Para-aortic lymph node radiotherapy in cancer of the uterine corpus. Obstet Gynecol. 1985;654:251.
252 Komaki R, Mattingly RF, Hoffman RG. Irradiation of para-aortic lymph node metastases from carcinoma of the cervix or endometrium: preliminary results. Radiology. 1983;147:245.
253 Blythe JG, Hodel KA, Wahl TB, et al. Para-aortic lymph node biopsy in cervical and endometrial cancer: does it affect survival? Am J Obstet Gynecol. 1986;155:306.
254 Feuer GA, Calnog A. Endometrial carcinomas: treatment of positive para-aortic nodes. Gynecol Oncol. 1987;27:106.
255 Corn BW, Lanciano RM, Greven KM, et al. Endometrial carcinoma with para-aortic lymphadenopathy: patterns of failure and opportunity for cure. Int J Radiat Oncol Biol Phys. 1992;24:223.
256 Hicks ML, Piver S, Jeffrey LP, et al. Survival in patients with paraaortic lymph node metastases from endometrial adenocarcinoma clinically limited to the uterus. Int J Radiat Oncol Biol Phys. 1993;26:607.
257 Rose PG, Cha SD, Tak WK, et al. Radiation therapy for surgically proven para-aortic node metastasis in endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1992;24:229.
258 Mariani A, Webb MJ, Keeney GL, et al. Endometrial cancer: predictors of peritoneal failure. Gynecol Oncol. 2003;89:236.
259 Martinez A, Podratz K. Results of whole abdomino-pelvic radiation with nodal boost for patients with endometrial cancer at high risk of failure in the peritoneal cavity. Hematol Oncol Clin North Am. 1988;2:431.
260 Gibbons S, Martinez A, Schary M, et al. Adjuvant whole abdominopelvic irradiation for high-risk endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:1019.
261 Sutton G, Axelrod JH, Bundy BN, et al: Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a Gynecologic Oncology Group study, Gynecol Oncol 97:755–763, 2005.
262 Stewart KD, Martinez AA, Weiner S, et al. Ten-year outcome including patterns of failure and toxicity for adjuvant whole abdominopelvic irradiation in high-risk and poor histologic feature patients with endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:527.
263 Roeske JC, Lujan A, Rotmensch J, et al. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2000;48:1613.
264 Portelance L, Chao KS, Grigsby PW, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51:261.
265 Heron DE, Gerszten K, Selvaraj RN, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: A comparative dosimetric study of dose-volume histograms small star, filled. Gynecol Oncol. 2003;91:39.
266 Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330.
267 Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54:1388.
268 Lujan AE, Mundt AJ, Yamada SD, et al. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:516.
269 Kim YB, Kim JH, Jeong KK, et al. Dosimetric comparisons of three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy in whole abdominopelvic radiotherapy for gynecologic malignancy. Technol Cancer Res Treat. 2009;8:369.
270 Rochet N, Jensen AD, Sterzing F, et al. Adjuvant whole abdominal intensity modulated radiotherapy (IMRT) for high risk stage FIGO III patients with ovarian cancer (OVAR-IMRT-01)—pilot trial of a phase I/II study: study protocol. BMC Cancer. 2007;7:227.
271 Hong L, Alektiar K, Chui C, et al. IMRT of large fields: whole-abdomen irradiation. Int J Radiat Oncol Biol Phys. 2002;54:278.
272 Small WJr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428.
273 Martinez A, Schray MF, Howes AE, et al. Postoperative radiation therapy for epithelial ovarian cancer: the curative role based on a 24-year experience. J Clin Oncol. 1985;3:901.
274 Gore G, Gillin MT, Albano K. Comparison of high dose-rate and low dose-rate dose distributions for vaginal cylinders. Int J Radiat Oncol Biol Phys. 1995;31:165.
275 Nori D. High dose rate brachytherapy in endometrial cancer. Selectron Brachyther J. 1991;2:5.
276 Lewis GCJr, Slack NH, Mortel R, et al. Adjuvant progestogen therapy in the primary definitive treatment of endometrial cancer. Gynecol Oncol. 1974;2:368.
277 MacDonald RR, Thorogood J, Mason MK. A randomized trial of progestogens in the primary treatment of endometrial carcinoma. Br J Obstet Gynecol. 1988;95:166.
278 Vergote I, Kjorstad K, Abeler V, et al. A randomized trial of adjuvant progestogen in early endometrial cancer. Cancer. 1989;64:1011.
279 Kneale BL, Quinn MA, Rennie GC, et al. A randomized trial of progestogens in the primary treatment of endometrial cancer. Br J Obstet Gynecol. 1988;95:828. Letter
280 Marcus E, Randall VL, Filiaci HM, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:1.
281 Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Brit J Cancer. 2006;95:266.
282 Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226.
283 Sears J, Greven K. Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer. 1994;74:1303.
284 Aalders J, Abeler V. Recurrent adenocarcinoma of the endometrium: a clinical and histopathological study of 379 patients. Gynecol Oncol. 1984;17:85.
285 Ackerman I, Malone S, Thomas G, et al. Endometrial carcinoma: relative effectiveness of adjuvant irradiation vs therapy reserved for relapse. Gynecol Oncol. 1996;60:177.
286 Curran WJ, Whittington R, Peters AJ, et al. Vaginal recurrences of endometrial carcinoma: the prognostic value of staging by a primary vaginal carcinoma system. Int J Radiat Oncol Biol Phys. 1988;15:803.
287 Wylie J, Irwin C, Pintilie M, et al. Results of radical radiotherapy for recurrent endometrial cancer. Gynecol Oncol. 2000;77:66.
288 Creutzberg CL, van Putten WL, Koper PC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecol Oncol. 2003;89:201.
289 Cardenes H, Randall ME. Is observation and salvage (when necessary) an appropriate approach to intermediate risk endometrial cancer? Gynecol Oncol. 2003;89:199.
290 Barakat RR, Goldman NA, Patel DA, et al. Pelvic exenteration for recurrent endometrial cancer. Gynecol Oncol. 1999;75:99.
291 Thigpen JT, Blessing J, DiSaia P, Oral medroxyprogesterone acetate in advanced or recurrent endometrial carcinoma: results of therapy and correlation with estrogen and progesterone levels, The Gynecologic Oncology Group experience; Baulieu EE, Slacobelli S, McGuire WL, editors; Endocrinology of malignancy; 1986; Parthenon, Park Ridge, NJ:446.
292 Thigpen JT, Homesley HD. A randomized study of medroxy progesterone acetate (MPA) 200 mg vs 1000 mg in the treatment of advanced, persistent or recurrent carcinoma of the endometrium. In: Gynecologic Oncology Group Statistical Report. Buffalo, NY: Gynecologic Oncology Group; 1990:177.
293 Reifenstein ECJr. The treatment of advanced endometrial cancer with hydroxyprogesterone caproate. Gynecol Oncol. 1974;2:377. Review
294 Piver MS, Barlow JJ, Lurain JR, et al. Medroxyprogesterone acetate (depo-Provera) vs hydroxyprogesterone caproate (Delalutin) in women with metastatic endometrial adenocarcinoma. Cancer. 1980;45:268.
295 Podratz KC, O’Brien PC, Malkasian GDJr, et al. Effects of progestational agents in the treatment of endometrial carcinoma. Obstet Gynecol. 1985;66:106.
296 Thigpen JT, Buchsbaum HJ, Mangan C. Phase II trial of Adriamycin in the treatment of advanced or recurrent endometrial carcinoma: a gynecologic oncology group study. Cancer Treat Rep. 1979;63:21.
297 Thigpen JT, Blessing JA, Homesley H, et al. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 1989;33:68.
298 Long HJ, Pfeifle DM, Wieand HS, et al. Phase II evaluation of carboplatin in advanced endometrial carcinoma. JNCI. 1988;80:276.
299 Ball HG, Blessing JA, Lentz SS, et al. A phase II trial of paclitaxel in patients with advanced and recurrent adenocarcinoma of the endometrium: a gynecologic oncology group study. Gynecol Oncol. 1996;62:278.
300 Knocke TH, Kucera H, Weidinger B, et al. Primary treatment of endometrial carcinoma with high-dose-rate brachytherapy: results of 12 years of experience with 280 patients. Int J Radiat Oncol Biol Phys. 1997;37:359.
301 Nguyen TV, Petereit DG. High-dose-rate brachytherapy for medically inoperable stage I endometrial cancer. Gynecol Oncol. 1998;71:196.
302 Grigsby P, Kuske R, Perez CA, et al. Medically inoperable stage I adenocarcinoma of the endometrium treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1986;13:483.
303 Rose P, Baker S. Primary radiation therapy for endometrial carcinoma: a case-controlled study. Int J Radiat Oncol Biol Phys. 1993;27:585.
304 Smith RS, Kapp DS, Chen Q, et al. Treatment of high-risk uterine cancer with whole abdominopelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:767.
305 Levenback C, Burke T. Uterine papillary serous carcinoma: treatment with cisplatin, doxorubicin and cyclophosphamide. Gynecol Oncol. 1992;46:317.
306 Lim P, Al Kushi A, Gilks B, et al. Early stage uterine papillary serous carcinoma of the endometrium: effect of adjuvant whole abdominal radiotherapy and pathologic parameters on outcome. Cancer. 2001;91:752.
307 Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197.
308 Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859.
309 Reed NS, Mangioni C, Malmstro HM, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an european organisation for research and treatment of cancer gynaecological cancer group study. Eur J Cancer. 2008;44:808.
310 Creutzberg CL, van Putten WL, Koper PC, et al. The postoperative radiation therapy in endometrial carcinoma. The morbidity of treatment for patients with stage I endometrial cancer: results from a randomized trial. Int J Radiat Oncol Biol Phys. 2001;51:1246.
311 Sorbe BG, Smeds AC. Postoperative vaginal irradiation with high dose-rate afterloading technique in endometrial carcinoma Stage I. Int J Radiat Oncol Biol Phys. 1990;18:305.