40 Cancer of the Colon
Epidemiology, Etiology, Genetics, and Cytogenetic Abnormalities
Epidemiology
In 2007, there will be an estimated 112,340 new cases of colon cancer in the United States, with 55,290 occurring in men and 57,050 occurring in women. Approximately 52,180 deaths are expected.1 The median age at diagnosis is 62 years. Patients under 40 years have a less favorable prognosis. However, it is unclear if this is independent of stage.
It has been postulated that colorectal cancer is caused or promoted by environmental factors and especially by dietary factors that affect the enteric milieu.2 A number of studies reveal an association of human colorectal cancer with certain diets (such as those rich in animal fats and meat and poor in fiber) and certain high-risk populations.3
In general, the traditional Western diet (high fat, low fiber, high phosphate, and low calcium) is associated with an increased risk of colorectal cancer. However, there have not been any clear studies demonstrating whether dietary factors increase the risk in patients with an underlying genetic susceptibility. There are a variety of risk factors for colorectal cancer (Table 40-1). Approximately 75% of colorectal cancers are sporadic.
| General |
| Age older than 40 |
| Genetic syndromes |
| Peutz-Jeghers |
| Familial adenomatous polyposis |
| Gardner’s |
| Turcot’s |
| Oldfield’s |
| Familial |
| Family history |
| Lynch I: Familial colorectal cancer syndrome |
| Lynch II: Hereditary adenocarcinomatosis syndrome |
| Other diseases |
| Prior colorectal cancer |
| Malignant colorectal polyps |
| Inflammatory bowel disease |
Molecular Genetics
Only recently has the genetic basis of colorectal cancer and its precursors begun to be understood.4,5 The data suggest that the development of colorectal cancer is a multistep process that involves a successive activation or deletion of genes and their protein products.6 This process is mediated by regulatory genes. Advances in molecular biology techniques have allowed characterization of the genetic changes thought to be responsible for this multistep process.7
Numerous molecular markers examined to date, such as microsatellite instability (MSI),8 18q,9 TP53,10 and others such as proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression.11 However, they are not currently incorporated in the staging system and T and N classification remain the most predictive factors. But this is rapidly changing, and US intergroup trials now prospectively collect tissue. The phase III ECOG 5202 trial for patients with high-risk stage II colon cancer assigns treatment based on MSI/18q status. In the near future, more-individualized therapeutic recommendations based on genetic markers may be possible.12
Anatomy
The large bowel is divided into the colon and rectum.13,14 The cecum, transverse colon, and sigmoid loop are mobile structures that lie free in the peritoneal cavity and are completely covered with serosa (visceral peritoneum). The dorsal or posterior aspect of the ascending and descending colon, and both flexures are frequently without serosa. Tumor spread from these segments may involve the retroperitoneal soft tissues, kidney, ureter, and pancreas. Although the rectum is frequently considered to be extraperitoneal, the anterior surface of the proximal third of the rectum is covered with serosa and is therefore intraperitoneal. Anatomically, the transition from sigmoid colon to rectum is marked by the fusion of the tenia of the sigmoid colon to the circumferential longitudinal muscle of the rectum at the level of the sacral promontory. This occurs approximately 12 to 15 cm from the dentate line. Patterns of recurrence of proximal rectal cancer may depend upon whether the location of the tumor is anterior or posterior.
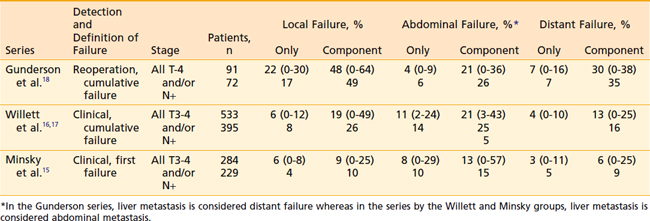
The overall rationale of the use of adjuvant radiation therapy in colon cancer is based on the patterns of failure following potentially curative surgery. The primary determinant of failure patterns in colorectal cancer is the location of the tumor in reference to the posterior peritoneal reflection. In contrast to tumors located at or below the posterior peritoneal reflection (rectosigmoid and rectum), most tumors located above the peritoneal reflection (cecum-sigmoid), have a higher incidence of failure within the abdominal cavity.15–18 This is because colon tumors have easier access to a free peritoneal surface.
Representative series examining the patterns of failure after potentially curative surgery are seen in Table 40-2. There is much variation as to what defines failure, the method by which failure is determined, the staging system used, and whether patients with metastatic disease are excluded from analysis. Failure patterns will be lowest in series which use clinical and/or radiographic evidence of first failure,15 whereas they will be highest in series which use reoperation and/or autopsy evidence of cumulative failure.18
Pathology
Histologic Subtypes
The most common histologic type of large bowel cancer is adenocarcinoma, which accounts for 90% to 95% of all large bowel tumors.13 It is the only histologic type further classified by grade. A number of histologic types of large bowel cancer have been identified. The World Health Organization has developed a classification of both benign and malignant tumors. Colloid or mucinous adenocarcinoma represent approximately 17% of large bowel tumors.19 These adenocarcinomas are defined by large amounts of extracellular mucin retained within the tumor. A separate classification is the rare signet-ring cell carcinoma (2% to 4% of mucinous carcinomas), which contains abundant intracellular mucin, pushing the nucleus to one side. Some signet-ring tumors form a linitis plastica–type appearance, tend to present at later stages, and, thus, have a poor prognosis.20,21
Other rare variants of epithelial tumors include squamous cell carcinomas, adenosquamous carcinoma (adenoacanthoma), undifferentiated carcinomas, small cell,22 and neuroendocrine cancers.23 Gastrointestinal stromal tumors should be treated as sarcomas.24 Sarcomas account for 0.1 to 0.3% of all malignancies of the colorectum.25 The most common type is leiomyosarcoma. It may arise from the smooth muscle of the muscularis propria, muscularis mucosa, or blood vessels.26 Primary colorectal malignant lymphomas are usually diffuse large B-cell lymphomas.27 Squamous cell cancers of the rectum should be treated as an anal cancer with fluorouracil (5-FU)/mitomycin-C and radiation, reserving surgery for salvage.28
Grade
Broders classified adenocarcinomas by their degree of differentiation. He designated four grades based on the percentage of differentiated tumor cells. Well-differentiated in Broders’ system meant well-formed glands resembling an adenoma. Broders included the mucinous carcinomas in his system. Dukes considered mucinous carcinomas separately.29 There is no uniform agreement on the grading criteria, but most investigators agree on the use of a three-grade system similar to that described in other adenocarcinomas.
Other Clinicopathologic Factors
The influence of clinical and pathologic factors on the patterns of failure and survival following surgery colorectal cancer has been the subject of numerous investigations. By univariate analysis, a number of factors have been reported to be of prognostic importance. Many of these factors are interrelated and merely reflections of the same overall characteristic of the cancer. Using a multivariate (proportional hazards) analysis, a variety of independent factors for survival have been reported. However, the majority of investigators agree that the most important independent pathologic factor for survival and/or recurrence following surgery is stage (depth of penetration through the bowel wall and the presence and number of positive lymph nodes).30,31 Prognosis is related to the number of tumors rather than the volume of tumor present in the nodes.32 The presence of lymphatic vessel (L) or venous (V) invasion is now indicated as an additional descriptor in the TNM staging system.
Selected adverse clinical factors include: age <40 years, blood transfusions, long duration of symptoms, obstruction or perforation, ulceration, and various primary tumor locations. Selected adverse pathologic/molecular factors include: perineural invasion, high grade, colloid, signet ring cell, blood vessel invasion, lymphatic vessel invasion, aneuploidy, elevated carcinoembryonic antigen (CEA) levels, collagen, local immune response, cell surface antigen 19-9, and various growth factors, receptors, oncogenes, and blood group antigens.13 Except for CEA, none of these tumor markers have independently been shown to be helpful in prospective follow-up following treatment.33
Routes of Spread
Lymph Nodes
The risk of lymph node metastases increases with increasing tumor grade. In addition, there is a clear relationship between lymphatic vessel invasion and the incidence and number of lymph node metastases in colorectal cancer.34,35 At least 12 to 14 lymph nodes must be examined for an accurate classification of nodal status.36,37 Although they may be confused with lymph nodes, pericolonic tumor deposits are a harbinger of intra-abdominal metastasis in colon cancer.38 In the sixth edition of the American Joint Commission on Cancer (AJCC) TNM Staging System, smooth metastatic nodules in the pericolic or perirectal fat are considered lymph node metastasis, whereas irregularly contoured metastatic nodules in the fat are considered vascular invasion.39
In colon cancer, the normal lymphatic flow is through the lymphatic channels along the major arteries, with three echelons of lymph nodes: pericolic, intermediate, and principal lymph nodes (Table 40-3).13 If tumors lie between two major vascular pedicles, lymphatic flow may drain in either or both directions. If the central lymph nodes are blocked by tumor, lymphatic flow can become retrograde along the marginal arcades both proximally and distally.
| Site | Regional Nodes |
|---|---|
| Cecum | Anterior and posterior cecal, ileocolic, right colic |
| Ascending colon | Ileocolic, right and middle colic |
| Hepatic flexure | Middle and right colic |
| Transverse colon | Middle colic |
| Splenic flexure | Middle and left colic, inferior mesenteric |
| Descending colon | Left colic, inferior mesenteric, sigmoid |
| Sigmoid loop | Inferior mesenteric, superior rectal (hemorrhoidal), sigmoid, sigmoid mesenteric |
In rectal cancer, the disease metastasizes to the perirectal nodes at the level of the primary tumor, immediately above it and up to 5 cm distal to the primary tumor.40,41 Then, the chain accompanying the superior hemorrhoidal vessels is involved. Discontinuous or skip metastases are rare. The pericolic lymph nodes along the mesenteric border of the pelvis usually are not involved by these rectal tumors unless there is very extensive tumor with lymphatic blockage.
Diagnostic and Staging Studies
The work-up for colon carcinoma is seen in Table 40-4. Fluorodeoxyglucose-positron emission tomography (FDG-PET) scanning shows promise, but remains investigational.43,44 The staging of colorectal carcinoma has been complicated by the fact that it has evolved over a century and various authors have developed systems that use the same terms to represent different stages. Because of these discrepancies in coding for the same stages, the comparison of clinical studies reported in the literature is often impossible.
The Astler-Coller staging system allowed separation of wall penetration and nodal status. The Gunderson-Sosin modification of the Astler-Coller staging system subdivided T3 tumors into those with microscopic (B2m or C2m) or gross (B2m+g or C2m+g) penetration of tumor through the bowel wall.45,18 It also defined tumors adherent to or invading an adjacent organ or structure as B3 if the nodes were negative and C3 if the nodes were positive. Several studies have analyzed both local failure and survival using the modified Astler-Coller staging system15,16,18,45–47 Most have confirmed the predictive capability of this staging system.
In 1988, the AJCC48 and the Union Internationale Contre le Cancer (UICC)49 proposed a joint TNM staging system. This was based on the fifth edition of the AJCC TNM staging system. The sixth edition of the AJCC TNM staging system is shown in Table 40-5,39 with four major changes from the fifth edition. These include: (1) a revised description of the anatomy of the colon and rectum which better delineates the data regarding the boundaries between the colon, rectum, and anus. Adenocarcinomas of the veriform appendix are classified with the TNM system, but should be recorded separately. (2) Smooth metastatic nodules in the pericolic or perirectal fat are considered nodal metastasis. By contrast, irregularly contoured metastatic nodules in the peritumoral fat are considered vascular invasion and are coded as a subcategory of the T stage as V1 (microscopic vascular invasion) if microscopically visible or V2 (macroscopic vascular invasion) is grossly visible. (3) stage group II is subdivided into IIA (T3 disease) and IIB (T3 disease) and (4) stage group III is subdivided into IIIA (T1-2N1M0 disease), IIIB (T3-4N1M0 disease), and IIIB (TanyN2M0 disease). There are also additional descriptors which, although they do not affect the stage grouping, do indicate cases needing separate analysis. The AJCC TNM staging system should be used routinely for staging and treatment purposes. Although CEA has prognostic importance, it was not added to the staging system.50
An elevated CEA level at the time of presentation has an adverse impact on survival independent of tumor stage.51 If the pretreatment CEA is >100 and the abdominal/pelvic CT does not reveal evidence of metastatic disease, a liver MRI should be performed to exclude liver metastasis. Preoperative CEA is recommended however there is insufficient evidence at this time for the routine use of molecular markers.52
The role of the pathologist is critical to proper staging. At least 12 pelvic nodes must be examined to obtain an accurate N classification.36
Standard Therapeutic Approaches
Surgery
Surgery is the standard treatment for both colon and rectal cancer. The successful management of this disease is dependent on a thorough preoperative evaluation, careful surgical technique,53 and the experience of the surgeon.54,55 Surgical resection can be performed either open or laproscopically. An 872-patient randomized trial reported by Nelson et al. comparing open versus laparoscopic techniques in colon cancer revealed no significant differences in recurrence or survival.56 Laparoscopic colectomies for cancer can result in less postoperative pain and shorter lengths of stay.57 However, the improvement is modest at best, and thus has not replaced open surgery. It is important that surgery, whether done open or through minimally invasive techniques, be performed using an oncologic resection (high ligation of the blood supply and minimal tumor handling).
When patients present with bowel obstruction, self-expanding stents should be considered as a bridge to surgery, because emergent procedures are associated with higher rates of stoma creation and higher morbidity rates.58 Patients who undergo emergency surgery usually present with more advanced disease and thus have lower resectability rates than those undergoing elective resection.59 Patients with metastatic disease may require palliative resection to avoid and/or alleviate obstructive symptoms. Depending on the size and location of metastases in the liver and lung, these may be amenable for resection during the same procedure when the colon is resected.
Chemotherapy
The standard adjuvant therapy for node positive (T1-4N1-2M0) resectable colon cancer is 12 cycles (6 months) of postoperative FOLFOX chemotherapy.60 Capecitabine is an option for patients unable to tolerate FOLFOX.61 The role of adjuvant therapy in a patient with high risk T3N0M0 disease remains controversial.61,62 For patients with metastatic disease the standard initial therapy is a combination of a fluropyrimidine and either oxaliplatin or irinotecan. Depending upon comorbidities and the goals of therapy, bevacizumab or cetuximab may also play roles. The concept of “lines” of therapy has been superseded by a continuum-of-care strategy which may soon be driven by biomarkers. The National Comprehensive Cancer Network (NCCN) offers general guidelines for therapy.63
Radiation Therapy
Local/Regional Radiation Therapy
Although the overall incidence of local failure is relatively low in colon cancer, data suggest that, depending upon the anatomic location and selected pathologic features, there are subsets of patients in whom the incidence of local failure is increased. However, there is not uniform agreement as to how to define these subsets. Gunderson et al18 divided the colon into two regions. Anatomically immobile (or mainly retroperitoneal) included the ascending colon, hepatic flexure, splenic flexure, and descending colon. Anatomically mobile (or mainly intraperitoneal) included the cecum and transverse colon. The highest incidence of local failure occurred in the cecum (30%), whereas the other intraperitoneal site, the transverse colon, had one of the lowest rates of local failure (13%).
By contrast, Minsky et al.15 reported a general trend of increased local failure with more distal colon sites. Patients with cecal cancer had a significantly lower incidence of local failure (3%) compared to those with cancer of the transverse (15%) or descending colon (25%). These data do not support the notion that bowel mobility is predictive of local failure.
Willett et al.16,17 divided the colon into two groups. Group 1 included the cecum, ascending, midsigmoid, transverse, and descending colon. Group 2 included the high and low sigmoid colon, the splenic flexure, and the hepatic flexure. In T1-2N0M0 disease, there was a higher incidence of local failure in Group 1 tumors (16% to 24%) compared with Group 2 tumors (0% to 11%).
Techniques of Radiation Therapy
Introduction
The biologic mechanisms of acute and delayed toxicity as well as dietary interventions have been previously published.64,65 This review will be limited to the technical aspects of the design of local/regional radiation therapy fields for colon cancer. It will include idealized treatment fields for a variety of common clinical presentations. These techniques are modified from Gunderson, Martenson, and Willett.
Outcomes
Adjuvant Local/Regional Radiation Therapy
Although all the series are retrospective, the most comprehensive series examining the role of local/regional radiation in colon cancer is from the Massachusetts General Hospital (MGH).66,67 Following potentially curative surgery for T3-4N0-2M0 colon cancer, 203 patients received postoperative adjuvant radiation therapy. Eligibility included the following patients: T4N0-2M0 regardless of anatomic site, T3N1-2M0 excluding mid sigmoid and transverse colon, and selected high risk T3N0M0 tumors with close margins. Patients received 45 Gy to the primary tumor bed with a 5-cm margin as well as the primary draining lymph nodes. This was followed by a shrinking field technique to 50.4 to 55 Gy, depending on the volume of small bowel which could be excluded from the high dose field. Of the 203, 173 were treated in the adjuvant setting and 30 after a subtotal resection. Sixty-three received bolus 5-FU with a variety of doses and schedules.
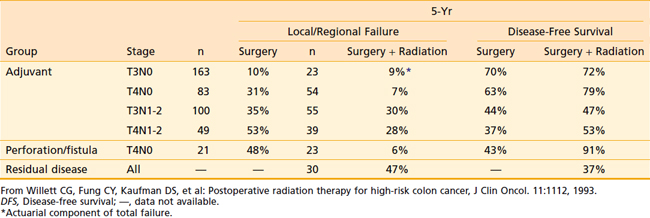
The results at 5 years were compared with a historical control group of 395 patients who underwent surgery only.66 As seen in Table 40-6, three groups of patients appeared to benefit from postoperative radiation therapy. First, there was a significant improvement in local control and disease-free survival for patients with stages T4N0M0 or T4N1-2M0 disease. Second, patients with stage T4N0M0 disease with a perforation or fistula had improved local control and disease-free survival. Third, radiation therapy salvaged some patients with residual disease-following subtotal resection (37% 5-year disease free survival). There was no benefit in local control or disease-free survival in patients with stage T3N0M0 or T3N1-2M0 disease. It must be emphasized that the results may be biased against radiation therapy, because many patients were high-risk patients referred because of concerns about margins.
With 10-year follow-up, local control in patients with T4 disease was 78% with negative margins and 53% with positive margins.67 For the 42 patients with positive margins, local control was 56% for the 30 with microscopically positive and 42% for the 12 with grossly positive margins.
Complications of treatment were acceptable. For the total patient group, the incidence of Grade 3+ acute bowel toxicity was 8%. Grade 3+ long-term bowel toxicity was 4.5%. These toxicity results are very similar to those reported from the Mayo Clinic/North Central Cancer Treatment Group postoperative adjuvant rectal trial 79-41-51.31 Therefore, with careful treatment techniques, the acute and long term bowel toxicity of postoperative local/regional radiation therapy (with or without chemotherapy) for colon cancer are comparable to those reported for rectal cancer.
On the basis of the retrospective data from the MGH, a randomized phase III intergroup trial coordinated by the Mayo/North Central Cancer Treatment Group (INT 0130) was developed. Patients with T4 or selected T3N1-2 colon tumors were randomized to 12 cycles of bolus 5-FU plus levamisole with or without local/regional radiation (45 to 50.4 Gy in 25 to 28 fractions) beginning with cycle 2 of chemotherapy. The trial was closed early because of poor accrual with only 222 of the anticipated 400 patients randomized, leaving 189 eligible patients.68 Grade 3+ toxicity was modestly but not significantly higher in the combined modality therapy arm (43% versus 35%). With a median follow-up of 35 months, there was no significant difference in survival between the two arms. The patterns of failure data have not been reported.
In a phase I trial of 21 patients (four with colon cancers) who received upper abdominal radiation plus continuous infusion 5-FU, Martenson and associates reported a 40% grade 3+ toxicity rate.69 Because of the large radiation field sizes, the dose of continuous infusion 5-FU needs to be attenuated in patients receiving combined modality therapy for upper abdominal malignancies.
Palermo and associates treated 45 patients with intraperitoneal 5-FU plus radiation to the tumor bed and para-aortic nodes.70 Although local failure was only 11%, the 10-year survival was 53% which is not superior to conventional approaches.
Given the significant advances in adjuvant chemotherapy for colon cancer as well as the negative results of the INT 0130 trial, the role of radiation therapy in colon cancer is limited. Therefore, the details of the radiation fields which appeared in the colon cancer chapter from the second edition of this textbook will not be reproduced in this edition.71 However, there are two clinical situations where it is reasonable to use radiation for colon cancers. The first is in patients with close or positive resection margins; the second is in a patient who presents with a T4 colon cancer adherent to pelvic structures. These cancers (most commonly sigmoid or cecal primaries) have local failure rates similar to rectal cancers and it is reasonable to treat them as such preferably with preoperative combined modality therapy, surgery, and postoperative chemotherapy.72
Whole Abdominal Radiation Therapy
The use of whole abdominal radiation therapy is limited by dose considerations. To treat the volume at risk with a potentially curative dose of radiation required for microscopic disease, the whole abdomen would need to receive 45 Gy. Although limited portions of the abdomen can tolerate this dose, the tolerance of the whole abdomen with conventional fractionation is 30 Gy. On the basis of the high incidence of abdominal failure in some colon cancers, a number of phase II adjuvant trials were designed to examine the efficacy of whole abdominal radiation therapy.73–77
In general, patients received 20 to 30 Gy to the whole abdomen with or without a boost to the primary tumor bed. In three of the series, 5-FU was delivered with a variety of doses and schedules. The combined results revealed an in-field (abdominal) failure rate of 12% to 50%. Significant toxicity varied from 5% to 38%. Although the initial phase II results appeared promising, three of the series (Brenner et al.74 Meek et al.75 and Wong et al.73) have not been updated.
The most encouraging data were reported by Fabian et al. from the Southwest Oncology Group (SWOG 8572).76 In this phase II adjuvant pilot trial, 41 patients with T3N1-2M0 disease received whole abdominal radiation therapy plus continuous infusion 5-FU followed by nine monthly cycles of continuous infusion 5-FU. Because of unacceptable toxicity in the first six patients, the protocol was modified such that (1) the 5-FU was started on day 1 and radiation began concurrently on day 8, and (2) a 1-week treatment break from both the 5-FU and radiation was required at day 42. The tumor bed boost (1.6 Gy × 10 fractions) was delivered first followed by whole abdominal radiation therapy (1 Gy/day × 30 fractions) for a total dose of 30 Gy to the whole abdomen and 46 Gy to the tumor bed.
Radiation for Recurrent/Unresectable Disease
If a patient has suspected or confirmed invasion into peritoneal, retroperitoneal structures, and/or pelvic structures, IORT followed by postoperative radiation should be considered as an adjunct of treatment, especially if the margins are close or positive. In a retrospective series of 100 patients with recurrent or locally advanced extrapelvic colon cancer, all underwent IORT.78 Radiation fields were designed according to preoperative computed tomography (CT) scans and intraoperative findings. Radiation doses ranged from 7.5 to 30 Gy per field. IORT dose was determined by the amount of residual tumor. Patients treated for locally advanced cancer and those treated for recurrent disease had 5-year survival rates of 49% and 25%, respectively. In the recurrent disease group, overall survival was improved when the resection was complete.
Follow-Up for Colorectal Cancer
These is significant controversy as to the recommended follow-up following treatment for patients with colorectal cancer. Evidence-based clinical practice guidelines have been published by the American Society of Clinical Oncology.79 However, these can be modified based on risk and individual patient and physician requirements. Isolated liver, lung, and pelvic recurrences should be considered for aggressive treatment.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66.
2 Weisburger JH, Wynder EL. Etiology of colorectal cancer with emphasis on mechanism of action and prevention. In: DeVita V, Hellman S, Rosenberg SA, editors. Important advances in oncology. Philadelphia: JB Lippincott; 1987:197-221.
3 Michels KB, Giovannucci E, Joshipura KJ, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740-1752.
4 Nicholl ID, Dunlop MG. Molecular markers of prognosis in colorectal cancer. J Natl Cancer Inst. 1999;91:1267-1269.
5 Ogunbiyi OA, Goodfellow PJ, Herfarth K, et al. Confirmation that chromosome 18q allelic loss in colon cancer is a prognostic indicator. J Clin Oncol. 1998:427-433.
6 Leslie A, Carey FA, Pratt NR, et al. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845-860.
7 Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23:11-27.
8 Kohonen-Corish MRJ, Daniel JJ, Chan C, et al. Low microsatellite instability is associated with poor prognosis in stage C colon cancer. J Clin Oncol. 2005;23:2318-2324.
9 Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. New Engl J Med. 2001;344:1196-1206.
10 Westra JL, Schaapveld M, Hollema H, et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J Clin Oncol. 2005;23:5635-5643.
11 Garrity MM, Burgart LJ, Mahoney MR, et al. Prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with resected Dukes’ B2 or C colon cancer: a North Central Cancer Treatment Group Study. J Clin Oncol. 2004;22:1572-1582.
12 Liersch T, Langer C, Ghadimi BM, et al. Lymph node status and TS gene expression are prognostic markers in stage II/III rectal cancer after neoadjuvant fluorouracil-based chemoradiotherapy. J Clin Oncol. 2006;24:4062-4068.
13 Skibber JM, Minsky BD, Hoff PM. Cancer of the colon. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: Lippincott, Williams and Wilkins; 2001:1216-1270.
14 Skibber JM, Hoff PM, Minsky BD. Cancer of the Rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: Lippincott, Williams and Wilkins; 2001:1271-1318.
15 Minsky BD, Mies C, Rich TA, et al. Potentially curative surgery of colon cancer: 1. Patterns of failure and survival. J Clin Oncol. 1988;6:106-118.
16 Willett CG, Tepper JE, Cohen AM, et al. Failure patterns following curative resection of colonic carcinoma. Ann Surg. 1984;200:685-690.
17 Willett C, Tepper JE, Cohen AM, et al. Local failure following curative resection of colonic adenocarcinoma. Int J Radiat Oncol Biol Phys. 1984;10:645-651.
18 Gunderson LL, Sosin H, Levitt S. Extrapelvic colon–areas of failure in a reoperation series: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1985;11:731-741.
19 Minsky BD. Clinicopathologic impact of colloid in colorectal carcinoma. Dis Colon Rectum. 1990;33:714-719.
20 Psathakis D, Schiedeck THK, Krug F, et al. Ordinary colorectal adenocarcinoma vs. primary colorectal signet-ring cell carcinoma. Study matched for age, gender, grade, and stage. Dis Colon Rectum. 1999;42:1618-1625.
21 Fu KI, Sano Y, Kato S, et al. Primary signet-ring cell carcinoma of the colon at early stage: a case report and a review of the literature. World J Gastroenterol. 2006;12:3446-3449.
22 Brenner B, Shah MA, Gonen M, et al. Small cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer. 2004;90:1720-1726.
23 Bernick PE, Klimstra DS, Shia J, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163-169.
24 Pidhorecky I, Cheney RT, Kraybill WG, et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712.
25 Hatch KF, Blanchard DK, Hatch GF, et al. Tumors of the rectum and anal canal. World J Surg. 2000;24:437-443.
26 Grann A, Paty PB, Cohen AM, et al. Sphincter preservation of leiomyosarcoma of the rectum and anus with local excision and brachytherapy. Dis Colon Rectum. 1999;42:1296-1299.
27 Doolabh N, Anthony T, Simmang C, et al. Primary colonic lymphoma. J Surg Oncol. 2000;74:257-262.
28 Nahas CSR, Shia J, Joseph R, et al. Squamous cell carcinoma of the rectum: a rare but curable tumor. Dis Colon Rectum. 2007;50:1-8.
29 Dukes CE. The classification of cancer of the rectum. J Pathol. 1932;35:323-332.
30 Minsky BD, Mies C, Rich TA, et al. Potentially curative surgery of colon cancer. The influence of blood vessel invasion. J Clin Oncol. 1988;6:119-127.
31 Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-715.
32 Wong JH, Steinemann S, Tom P, et al. Volume of lymphatic metastasis does not independently influence prognosis in colorectal cancer. J Clin Oncol. 2002;20:1506-1511.
33 Bast RC, Ravdin P, Hayes DF, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865-1878.
34 Davis NC, Evans EB, Cohen JR, et al. Staging of colorectal cancer: The australian Clinico-Pathological Staging (ACPS) System compared with the Dukes’ system. Dis Colon Rectum. 1984;27:707-713.
35 Minsky BD, Mies C, Rich TA, et al. Lymphatic vessel invasion is an independent prognostic factor for survival in colorectal cancer. Int J Radiat Oncol Biol Phys. 1989;17:311-318.
36 Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157-163.
37 Wong JH, Severino R, Honnebier MB, et al. Number of nodes examined and staging accuracy in colorectal cancer. J Clin Oncol. 1999;17:2896-2900.
38 Goldstein NS, Turner JR. Pericolonic tumor deposits in patients with T3N+M0 colon adenocarcinomas. Markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer. 2000;88:2228-2238.
39 Colon and rectum. In: Green FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. AJCC cancer staging manual. New York: Springer; 2002:113-124.
40 Cohen AM, Minsky BD, Friedman MJ. Cancer of the rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: J.B. Lippincott; 1993:978-1005.
41 Scott N, Jackson P, Al-Jaberi T, et al. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031-1033.
42 Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203.
43 Larson SM, Schoder H, Yeung H. Positron emission tomography/computerized tomography functional imaging of esophageal and colorectal cancer. Cancer J. 2004;10:243-250.
44 Desai DC, Zervos EE, Arnold MW, et al. Positron emission tomography affects surgical management in recurrent colorectal cancer patients. Ann Surg Oncol. 2003;10:59-64.
45 Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum: clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278-1292.
46 Minsky BD, Mies C, Recht A, et al. Resectable adenocarcinoma of the rectosigmoid and rectum: 1. Patterns of failure and survival. Cancer. 1988;61:1408-1416.
47 Rich T, Gunderson LL, Lew R, et al. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317-1329.
48 Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Cancer Res. 1998;4:343-348.
49 Obrand DI, Gordon PH. Continued change in the distribution of colorectal carcinoma. Br J Surg. 1998;85:246-248.
50 Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer prognostic factors consensus conference. Colorectal Working Group. Cancer. 2000;88:1739-1757.
51 Meling GI, Rognum TO, Clausen OP. Serum carcinoembryonic antigen in relation to survival, DNA ploidy pattern, and recurrent disease in 406 colorectal carcinoma patients. Gastroenterol. 1992;27:1061-1068.
52 Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor makers in gastrointestinal cancer. J Clin Oncol. 2006;33:5313-5327.
53 Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal surgery. J Natl Cancer Inst. 2001;93:583-596.
54 Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. J Amer Med Assoc. 2000;284:3028-3035.
55 Stocchi L, Nelson H, Sargent DJ, et al. Impact of surgical and pathologic variables in rectal cancer: A United States community and cooperative group report. J Clin Oncol. 2001;19:3895-3902.
56 Nelson H, Sargent D, Weiand H, et al. Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. New Engl J Med. 2004;350:2050-2059.
57 Steele SR, Brown TA, Rush RM, et al. Laparoscopic vs open colectomy for colon cancer: results from a large nationwide population-based analysis. J Gastrointest Surg. 2008;12:583-591.
58 Ng KC, Law WL, Lee YM, et al. Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: a case-matched study. J Gastrointest Surg. 2006;10:798-803.
59 Pavlidis TE, Marakis G, Ballas K, et al. Does emergency surgery affect resectability of colorectal cancer? Acta Chir Belg. 2008;108:219-225.
60 Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. New Engl J Med. 2005;352:2696-2704.
61 Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and \leucovorin as adjuvant treatment for colon cancer. New Engl J Med. 2004;350:2343-2351.
62 Lembersky BC, Wieand HS, Petrelli NJ, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from a National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24:2059-2064.
63 Engstrom PF, Benson ABIII, Chen YJ, et al. Colon cancer: clinical practice guidelines. J Natl Comp Cancer Network. 2005;3:468-490.
64 Kinsella TJ, Bloomer WD. Tolerance of the intestine to radiation therapy. Surg Gynecol Obstet. 1980;151:273-284.
65 Klimberg VS, Souba WW, Dolson DJ, et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62-68.
66 Willett CG, Fung CY, Kaufman DS, et al. Postoperative radiation therapy for high-risk colon cancer. J Clin Oncol. 1993;11:1112-1117.
67 Willett CG, Goldberg S, Shellito PC, et al. Does postoperative irradiation play a role in the adjuvant therapy of stage T4 colon cancer. Cancer J Sci Am. 1999;5:242-247.
68 Martenson JA, Willett CG, Sargent DJ, et al. Phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer: results of Intergroup protocol 0130. J Clin Oncol. 2004;22:3277-3288.
69 Martenson JA, Swaminathan R, Burch PA, et al. Pilot study of continuous-infusion 5-fluorouracil, oral leucovorin, and upper-abdominal radiation therapy in patients with locally advanced residual or recurrent upper gastrointestinal or extrapelvic colon cancer. Int J Radiat Oncol Biol Phys. 1997;37:615-618.
70 Palermo JA, Richards F, Lohman KK, et al. Phase II trial of adjuvant radiation and intraperitoneal 5-fluorouracil for locally advanced colon cancer: results with 10 year follow-up. Int J Radiat Oncol Biol Phys. 2000;47:725-733.
71 Minsky BD. Cancer of the Rectum. In: Leibel SA, Phillips TL, editors. Textbook of Radiation Oncology. Philadelphia: W.B.Saunders; 2004:897-912.
72 Sauer R, Becker H, Hohenberger P, et al. Preoperative chemoradiotherapy as compared with postoperative chemoradiotherapy for locally advanced rectal cancer. New Engl J Med. 2004;351:11-20.
73 Wong CS, Harwood AR, Cummings BJ, et al. Total abdominal irradiation for cancer of the colon. Radiother Oncol. 1984;2:209-214.
74 Brenner HJ, Bibi C, Chaitchik S. Adjuvant therapy for Dukes’ C adenocarcinoma of the colon. Int J Radiat Oncol Biol Phys. 1983;8:1789-1792.
75 Meek AG, Lam WC, Order SE. Carcinoma of the colon: irradiation by delayed split whole-abdominal technique. Radiology. 1983;148:845-849.
76 Fabian C, Shankar S, Estes N, et al. Adjuvant continuous infusion 5-FU, whole-abdominal radiation, and tumor bed boost in high-risk stage III colon carcinoma; a Southwest Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1995;32:457-464.
77 Ben-Joseph E, Court WS. Whole abdominal radiotherapy and concomitant 5-fluorouracil as adjuvant therapy in advanced colon cancer. Dis Colon Rectum. 1995;38:1088-1092.
78 Taylor WE, Donohue JH, Gunderson LL, et al. The Mayo Clinic experience with multimodality therapy of locally advanced or recurrent colon cancer. Ann Surg Oncol. 2002;9:177-185.
79 Desch CE, Benson ABIII, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology Guideline. J Clin Oncol. 2005;23:8512-8519.