58 Cancer of the Breast
Breast cancer accounts for 26% of all malignancies in women and is the second most common cause of cancer death in women.1 The last decade has witnessed major changes in our understanding of the biology and heterogeneity of the disease. This knowledge has resulted in the development of new therapies and treatment strategies with an emphasis on tailored therapy (i.e., that which is designed specifically for the individual patient based on tumor biology and extent of disease). This approach has been implemented in the surgical management of breast cancer, with sentinel node biopsy replacing axillary dissection as the initial staging procedure and skin-sparing or total skin-sparing mastectomy as an alternative to total mastectomy. Systemic therapies targeting molecular pathways or receptors that have proven effective in the metastatic setting are currently being evaluated in the adjuvant and neoadjuvant setting. Therapeutic interventions to manipulate the tumor stroma or microenvironment include the use of antiangiogenesis factors. Decisions for adjuvant chemotherapy may now be based on gene expression profiles and molecular subtypes of the tumor. Pathologic complete response to neoadjuvant chemotherapy has become an intermediate surrogate for disease-free and overall survival, and factors predicting for a complete pathologic response have become the focus of a number of clinical trials. The impact of systemic therapy on local control has received increasing attention with the recent meta-analysis of randomized trials demonstrating that one breast cancer death will be avoided for every four local recurrences prevented.
Breast Anatomy and Routes of Spread
The lymphatic channels are present in the subareolar skin and follow the duct and lobular complexes and most frequently drain into the lymph node chains located in the axillary basin. Breast lymphatics can also directly communicate with the infraclavicular/supraclavicular or internal mammary lymph node chains. Intramammary nodes are located within the breast parenchyma and can contain the metastatic tumor from the primary site. The axillary nodes are divided into three levels (I-III) based on their relationship with the pectoralis minor muscle (Fig. 58-1). Level I is located caudal and lateral, level II nodes are beneath the muscle, and level III (infraclavicular) nodes are located cranial and medial to the pectoralis minor. Rotter nodes or intrapectoral nodes are sandwiched between the pectoralis major and minor muscles. Orderly spread of tumor cells from the primary breast tumor most commonly starts at the level I axillary nodes and then proceed to level II and level III lymph nodes. “Skip” metastases that defy this order can occur but are less likely and a standard axillary dissection for sentinel positive disease requires pathologic analysis of level I and level II axillary nodes.
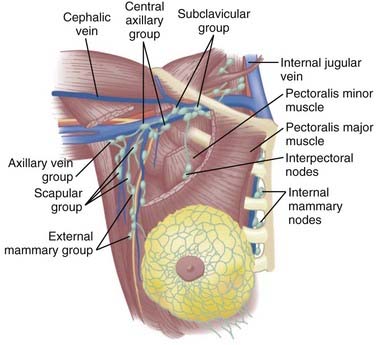
FIGURE 58-1 • Schematic representation of the regional nodes for breast cancer.
(From Donegan WL, Spratt JS: Cancer of the breast, ed 3, Philadelphia, 1988, Saunders, p 19.)
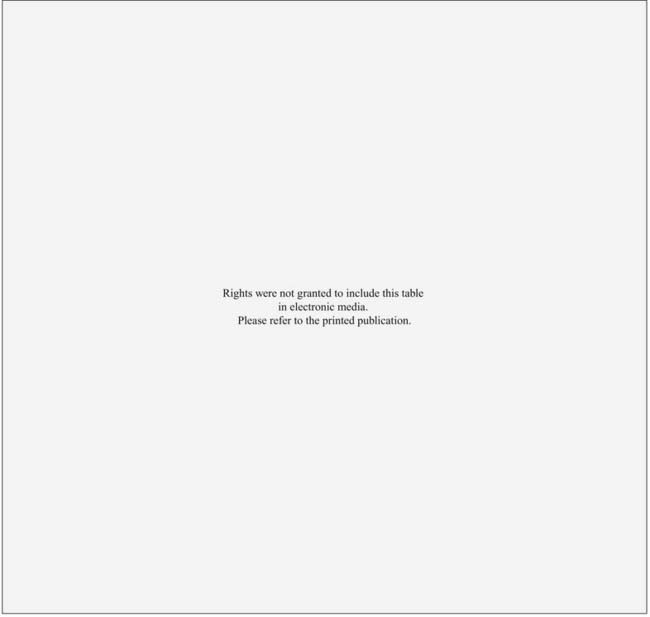
The supraclavicular lymph nodes are located within the space defined by the omohyoid muscle and tendon (lateral and superior borders), the internal jugular vein (medial border), and the clavicle and subclavian vein (inferior border).2 The internal mammary lymph node chain is encased within the endothoracic fascia in the parasternal space and runs alongside the corresponding artery and vein. The nodes located in the first through third intercostal spaces are the most common lymphoscintigraphic sites of drainage. Table 58-1 presents the location of the sentinel nodes as identified on lymphoscintigrams following peritumoral or intratumoral injection of technetium-99 sulfur colloid in women with early stage breast cancer. In the NSABP B-32 trial and the multicenter validation study, lymphoscintigrams were not required and the location of the sentinel nodes was determined by a hand-held gamma detector.3,4 This may account for the lower reported incidence of internal mammary nodes in these two studies. Overall, level I-II axillary nodes were the most common location for sentinel nodes regardless of tumor location. Internal mammary nodes were identified in less than 5% of women when lymphoscintigrams were not performed. Internal mammary nodes are not identified if the injection is intradermal.6 Estourgie et al.5 reported localization to the internal mammary node (IMN) node in 21.8% of all patients with the use of lymphoscintigrams. Inner quadrant tumors had a higher incidence of hot spots in the IMN nodes with the highest frequency in tumors in the lower inner quadrant. Upper quadrant lesions are more commonly localized to the second, third, and fourth interspaces while central and lower quadrant lesions are more commonly localized to the second, third, fourth, and fifth interspaces. Drainage to the supraclavicular nodes occurred in less than 1% of patients except for those with a central primary tumor. It should be noted that localization to a nodal region does not mean nodal positively. Biopsy of hot internal mammary nodes has demonstrated nodal positively in 8% to 27% of women.7
Epidemiology
Breast cancer is a major public health problem throughout the world. In 2007,8 it was estimated that 1.3 million women were diagnosed with new cases of breast cancer and 465,000 deaths from breast cancer were expected. Breast cancer remains the most frequently diagnosed cause of cancer death in women worldwide, only slightly surpassed by cervical cancer in economically underdeveloped countries. In the last 25 years, overall breast cancer rates have risen, including in Africa and Asia, continents with very low rates of breast cancer. The highest incidence of breast cancer occurs in North America, Northern and Western Europe, and Australia.8–10 The San Francisco Bay area has the highest rate of breast cancer in the United States.11 Currently a woman living in the United States has a 12% or a 1 in 8 lifetime risk of developing breast cancer. In 2009 in the United States, an estimated 192,370 new cases of invasive breast cancer will be diagnosed among women along with an estimated 62,280 additional cases of in situ breast cancer.1 Approximately 40,930 women are expected to die from breast cancer. About 1910 cases of breast cancer are expected to occur among men with 450 deaths.
From 1980 to 2001, breast cancer incidence rates initially increased rapidly followed by a slow increase after 1987. From 2001 through 2004, the incidence rates dropped 3.5% per year. The increase in the 1980s is largely attributed to the introduction and routine use of screening mammography and is reflected by the shift in the increase in detection of smaller previously nonpalpable breast tumors (2 cm) and a decrease in tumors of 3 cm by nearly a third.12 The increase has also been attributed to the popular use of hormone replacement therapy (HRT) and lifestyle choices that changed reproductive patterns. After 2000, the incidence rates began to drop in the United States, due largely in part to the declining use of HRT following the publication of the Women’s Health Initiative randomized trial in 200213–16 and a measurable reduction in the number of annual screening mammograms.17 Among women younger than 50, incidence rates have remained unchanged since 1986. Early detection by screening mammography also brought an increased incidence of in situ breast tumors in the 1980s and 1990s, particularly in women 50 years and older, but has since leveled off in this population. However, the incidence of in situ breast cancer continues to rise in young women.18
Epidemiologic studies of breast cancer focusing on age, race, ethnicity, and geographical location have shown disparities.19 For example, although whites have a higher incidence of breast cancer than African American women, African American women have a higher incidence before the age of 40 years and a higher mortality rate at any age.9,20
Risk Factors
Aside from being female, age is the single most important breast cancer risk factor. According to the National Cancer Institute, the risk between ages 30 and 39 is 0.43% (1 in 233), 40 and 49 is 1.44% (1 in 69), 50 and 59 is 2.63% (1 in 38), and between 60 and 69 is 3.65% (1 in 27) based on probabilities for the whole population and not individual risk factors.10
A family history of breast cancer particularly in a first-degree relative is a significant risk factor, and the risk escalates with the number of relatives affected and younger age at diagnosis. This pattern suggests an inherited genetic mutation that predisposes to the development of breast cancer. Approximately 5% to 10% of breast cancer patients have a familial form of the disease.21 Many of these cases contain an alteration in the breast cancer genes, BRAC1 and BRAC2. More than 100 distinct mutations have been identified in high-risk families and it is not clear if all carry an equal cancer risk. Some populations have a higher likelihood of carrying germline mutations such as family members of Ashkenazi Jewish (Eastern European) heritage and families with multiple cases of breast and/or ovarian cancers. The estimated lifetime risk of developing a breast cancer is up to 80% (36% to 85%), with a near 40% risk of developing a contralateral breast cancer. The risk of developing an ovarian cancer is 40% in BRCA1 carriers and 20% for BRAC2 carriers.22,23 Genetic counseling should be offered to these patients including those of young age at diagnosis, two primary breast cancers (ipsilateral or contralateral) or with breast and ovarian cancer and male breast cancer.24 There are other rare familial genetic syndromes that display a predisposition to breast cancer, the most well-known are Li-Fraumeni syndrome with germline mutations in TP53 and Cowden and Bannayan-Riley-Ruvalcaba syndrome due to PTEN mutations.
The absolute risk of a contralateral breast cancer in women with a personal history is 0.5% to 1% per year or up to 10% during the 10 years following diagnosis.25,26 Biopsy-proven atypical proliferative disorders, including atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), and atypical ductal hyperplasia (ADH), may increase the risk by a range of fourfold to tenfold with a further increase in a patient with a family history.27–30
Mammographic density is a strong independent risk factor with a fourfold to sixfold increase for postmenopausal women with high breast density compared with those with least dense breasts.31–36 Breast density refers to the amount of white area (fibrous and glandular tissue) on a black (primarily fat tissue) mammogram. While methods of measurement and definitions of breast density vary among studies, women with breast densities of more than 60% to 75% have been found to have an increased risk of breast cancer.37 Higher breast density is more common in Caucasian women and younger women and decreases during menopause. Hereditary factors may account for the majority of highly dense breasts. Several studies have identified genes that influence mammographic density.38–40 Tamoxifen has been shown to decrease breast density especially during the first 18 months of treatment.41
The risk of developing breast cancer after exposure to ionizing radiation is dose and age dependent and has been demonstrated from data collected from the Japanese atomic bomb survivors and patients exposed to radiation for nonmalignant conditions, such as thymus enlargement, multiple chest fluoroscopies for tuberculosis, and mastitis examinations.42–45 Particularly susceptible are adolescents who demonstrate the greatest risk of breast carcinogenesis over a lifetime. Secondary breast cancer has been described in young women who underwent mantle irradiation for Hodgkin disease with doses ranging from 20 to 44 Gy.46 In a multi-institutional review of 1380 adolescents treated before age 16 years old, a cumulative probability of breast cancer at age 40 years was 35%. This cohort of survivors had a risk of breast cancer 75 times higher than that of the general population.47 The risk of breast cancer significantly increases 15 to 30 years after treatment for those women exposed between the ages of 10 to 30 years; however, relative risk begins to increase several years following radiation exposure.48 Current practice using lower doses of radiation and limited fields for Hodgkin disease may decrease the risk of breast cancer for these patients.49
A moderate relative risk is associated with factors which affect circulating hormone levels such as delayed childbirth, nulliparity, early or late menarche and exogenous hormones. Body mass index (BMI) or postmenopausal obesity, has clearly been associated with breast cancer risk likely due to higher estradiol levels associated with aromatase in adipose tissue, which converts androgens to estradiol. A pooled analysis of prospective studies demonstrated a 30% higher risk in postmenopausal woman with a BMI more than 31 kg/m2 compared with a BMI of less or equal to 20 kg/m2.50 Weight gain at menopause is associated with increased risk; however, weight loss with maintenance is associated with a substantial lowering of breast cancer risk.51
Alcohol consumption increases the risk of breast cancer. In the Oxford meta-analysis of 53 epidemiologic studies, 58,515 patients with breast cancer and 95,067 women without breast cancer demonstrated that two drinks a day (defined as 24 g of alcohol) can increase breast cancer risk by 21%.52 The relative risk of breast cancer was dose-dependent and increased with daily amount. Another analysis of 184,418 postmenopausal women showed that moderate intake of one to two drinks a day can increase risk by about 32% and three or more by 51%.53
Two commonly used models to estimate an individual’s breast cancer risk are the Gail and Claus models which heavily weigh family history with a combination of other risk factors.54,55 A breast cancer risk assessment tool is available on the National Cancer Institute website based on the Gail model (cancer.gov/bcrisktool). Other decision models have been developed to estimate the likelihood of a BRCA mutation include the BRCAPRO.56,57 and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA).58
Clinical Presentation and Detection
The most frequent presentation of early stage breast cancer is an asymptomatic, nonpalpable mass, which is detected as an abnormality on screening mammogram. The most common physical sign is a nonpainful mobile mass.59 A detailed physical examination includes evaluation of the ipsilateral and contralateral breast tissue and regional lymph nodes (bilateral axillae, supraclavicular, infraclavicular, anterior cervical/neck, and submental and submandibular lymph nodes chains). The treatment approach is determined, in part, by the clinical presentation such as tumor size, location, and skin involvement. A large tumor can be a contraindication to breast conservation; inner quadrant tumors require lymphoscintigraphy to identify sentinel nodes located in the internal mammary chain.
Mammographic signs of cancer consist of two primary findings: (1) a mass with ill-defined, irregular, or spiculated edges and/or (2) irregular, pleomorphic calcifications. Further diagnostic imaging including targeted ultrasonography and mammographic magnification and compression views of all suspicious areas should be completed before a biopsy procedure. The imaging reports should include size and location of the primary tumor and a description of the findings in accordance with the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) guidelines.60 A pathologic diagnosis of cancer is necessary before proceeding to definitive surgery. Fine-needle aspiration of a palpable mass or lymph node can be performed at the time of the initial evaluation and before a core needle biopsy. A benign FNA is an incomplete evaluation if discordant with the clinical findings and must be followed with an ultrasound-guided core needle biopsy, stereotactic biopsy, or an excisional biopsy. Clinically enlarged lymph nodes are also evaluated by FNA or core needle biopsy and computerized tomography (CT)/positron emission tomography (PET). Once a breast cancer diagnosis has been made, magnetic resonance imaging (MRI) can be a diagnostic tool for assessing the extent and pattern of cancer in the breast parenchyma and detecting occult contralateral cancers.61,62
Mammography as a screening tool has been proven to reduce mortality from early stage breast cancer.63–65 Additional imaging tools, such as breast ultrasound and dynamic/contrast-enhanced MRI, have not been shown to impact mortality, and given the technical and practical limitations have no universal screening role but are useful supplemental techniques. MRI has been shown to have superior sensitivity for detecting breast cancers in high-risk populations over mammography, clinical examination, and ultrasound. The cost of higher sensitivity is the variable specificity that is lower than mammography in all studies.66
In woman with a high risk of breast cancer due to a strong family history or a BRCA mutation, routine surveillance with clinical breast examination and mammography is suboptimal compared with MRI. Six prospective nonrandomized studies have demonstrated the role of MRI screening in this high-risk population.67–72 One of these studies followed 236 women with BRCA mutations with breast examination, mammography, ultrasound, and MRI. More than twice as many cancers were detected by MRI (77%) than by mammography (36%), and the combined sensitivity of all four modalities was 95% compared with the 45% from breast examination and mammography alone.68 Similar results were obtained from a larger study, which surveyed 1909 high-risk women (15% or more cumulative lifetime breast cancer risk) with annual breast examination, mammography, and MRI. With a median follow-up of 2.9 years, 51 tumors were detected with a sensitivity of 18%, 33%, and 79%, respectively, and specificity of 98%, 95%, and 90%, respectively.67
The American Cancer Society published guidelines for the use of screening magnetic resonance imaging (MRI) as an adjunct to mammography in 2007.66 Annual screening MRI was recommended for proven carriers of a BRCA mutation, untested first-degree relatives of a proven mutation carrier and women with a lifetime risk of breast cancer at 20% as determined by risk assessment models such as BRCAPRO. It was also recommended for women with a prior history of chest radiation between the ages of 10 to 30 years and for women with Li-Fraumeni syndrome or other rare genetic syndromes and their first-degree relatives. The role of annual MRI screening in women with a 15% to 20% lifetime risk for breast cancer (i.e., those with a personal history or extremely dense breasts on mammography) was not clarified. Annual MRI screening was not recommended for women with less than 15% lifetime risk of breast cancer.
Pathology
The American Joint Committee on Cancer classifies breast cancers into the following histopathologic categories73,74 in situ cancers and invasive cancers. The in situ cancers include those that are not otherwise specified (NOS), ductal carcinoma in situ (DCIS, intraductal cancer), and Paget disease with DCIS. The invasive cancers include those that are not otherwise specified, ductal, lobular, medullary NOS, or medullary with lymphoid stroma, mucinous, tubular, papillary (predominantly micropapillary pattern), Paget with invasive cancer, inflammatory, undifferentiated, squamous cell, adenoid cystic, secretory, and cribriform.
The term lobular carcinoma in situ (LCIS) is a misnomer in that LCIS is considered a marker for an increased risk for the development of breast cancer and not a cancer in itself. LCIS was first described by Foote and Stewart in 1941.75 It is characterized by the presence of large, nonuniform in size, discohesive epithelial cells that fill the acinar spaces to varying degrees.76,77 Mastectomy specimens have demonstrated LCIS to be multicentric in 90% of cases and bilateral in 35% to 59%.76,77 LCIS is usually estrogen receptor-positive. C-erbB-2 and p53 are infrequently overexpressed.77,78 Loss of E-cadherin (CDH1), an adhesion molecule, is found in 95% of LCIS.79 LCIS, when not associated with an invasive cancer, is usually detected as an incidental finding at the time of a benign biopsy. It is found more frequently in premenopausal women with an average age of 45 years.77 The invasive cancer, which develops subsequent to a diagnosis of LCIS, is most often an invasive ductal cancer, which can occur in either breast.76,80 In the National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized trial for DCIS, 182 patients were inadvertently entered with LCIS. Five percent of these women who had excision alone developed a subsequent invasive cancer in the index breast compared with 5.6% in the contralateral breast.81 The respective rates for DCIS were 9% for the index breast and 1.2% for the contralateral breast. The median follow-up was 12 years. It was of interest that all of the cancers that developed in the index breast occurred in the vicinity of the LCIS. Treatment options for LCIS in the absence of an invasive cancer range from bilateral mastectomy to excision alone. Because of the propensity for the bilateral risk, unilateral mastectomy is not appropriate. The most commonly accepted approach is excision alone with close follow-up. The role of negative resection margins for LCIS in diminishing the subsequent risk is unknown. Tamoxifen has been shown to decrease (but not eliminate) the risk of cancer following a diagnosis of LCIS and has been used as a prevention strategy. In the NSABP P-1 prevention trial, tamoxifen resulted in a 56% decrease in the risk of a cancer in women with LCIS.82,83
Pleomorphic LCIS is a variant of LCIS. The majority of reported cases of pleomorphic LCIS have occurred in association with pleomorphic invasive lobular cancer.84 Pleomorphic LCIS has been found to have a higher proliferation rate and greater percentage of p53 positivity when compared with classic LCIS and is estrogen-receptor-positive, HER-2/neu negative, and E-cadherin negative.84 Necrosis may be seen in 40%.84 Recent studies have suggested that pleomorphic LCIS is more closely related to LCIS than DCIS.84,85 The biologic course of pleomorphic LCIS is unknown. In the past many of these lesions were classified as DCIS due to the presence of necrosis and calcifications. Sneige et al.84 reported outcome in 10 cases. None of the seven cases who underwent adequate excision or simple mastectomy recurred.
Ductal carcinoma in situ by definition is carcinoma confined to the pre-existing duct system of the breast without penetration of the basement membrane by light microscopy. Pathologically, DCIS may be characterized by its architectural pattern (comedo, solid, cribriform, papillary, and micropapillary), nuclear grade (low, intermediate, high), and the presence or absence of comedonecrosis. Less common architectural patterns include apocrine, clinging, signet-cell cystic, hypersecretory, and neuroendocrine.86 It is not uncommon for more than one architectural pattern to be present in a single lesion. DCIS currently represents approximately 21% of all breast cancers and 20% to 30% of those that are mammographically detected.
The natural history of DCIS is characterized by a low risk for axillary metastases (<5%) and an extremely low breast cancer mortality (<5%). DCIS is detected as a mammographic abnormality (usually calcifications) in 80% to 90% of women with 10% to 20% having a physical finding (palpable mass, bloody nipple discharge). The extent of DCIS may be underestimated by the area of calcification on the mammogram, especially if magnification views are not obtained.87–89 In addition, pathologic examinations of mastectomy specimens in women with DCIS not infrequently reveal discontinuous extension in the duct system.90 Contiguous extension has been described in two directions: one centrally towards the nipple and one peripherally and laterally.91
This somewhat unpredictable pattern of spread contributes to the difficulty in assessing the pathologic extent of DCIS. Micropapillary and low-grade lesions tend to have a more diffuse and discontinuous pattern of spread.92 In contrast to high-grade DCIS, two thirds of low-to intermediate grade DCIS have been reported to have a multifocal disease with distances between foci of up to 1 cm.87,90 More recently, MRI has been used to evaluate the extent of DCIS.93,94 In a study of 45 patients from the University of California, San Francisco, MRI overestimated the pathologic extent of disease more than twofold in 23% and underestimated it in 9%.93 A discrepancy between the pathologic size and MRI extent was more frequently observed with the MRI patterns of regional or multiregional enhancement and in estrogen receptor-positive DCIS.
Foci of occult invasion are more often observed with high-grade DCIS, those with comedonecrosis, or with a size greater than 2.5 cm88,92,95 The estrogen receptor is positive in 90% of low-grade DCIS and 25% of high-grade lesions. Overexpression of c-erBB-2 and P53 is present in less than 20% of low-grade lesions but in two thirds of high-grade lesions.96 DCIS is considered a precursor for the development of an invasive ductal cancer in the index breast. However, it is not an obligate precursor in that not all DCIS progresses to an invasive cancer. In a study of women who had undergone a biopsy for what was interpreted at the time as benign disease and on subsequent review was found to be low-grade DCIS, 39% 15 to 42 years later developed an invasive cancer in the index breast.97,98
Pathologic classification systems have been proposed to predict the clinical behavior of DCIS in terms of ipsilateral breast tumor recurrence (IBTR), either invasive or noninvasive after breast-conserving surgery and breast cancer specific survival. A system which stratifies risk for recurrence is useful in defining the roles of radiation and mastectomy. A 1997 international consensus conference99 recommended that a number of features should be described in the pathology report including nuclear grade, necrosis, polarization, architectural pattern, margin status, size, presence of absence of calcifications, and relationship of calcifications to the DCIS. Silverstein100 proposed a classification system (the University of Southern California/Van Nuys Prognostic Index-USC-VNPI) based on clinical and pathologic features to predict biologic behavior. The scoring system has four categories (age, size, margins, and nuclear grade combined with necrosis) and within each category there are three scores (1, 2, 3). The age scores are 1 greater than or equal to 60 years, 2 = 40 to 60 years, and 3 ≤ 40 years. The size scores are 1 less than or equal to 1.5 cm, 2 = 1.6-4 cm, and 3 greater than or equal to 4 cm. The margin scores are 1 greater than or equal to 1 cm, 2 = 1 to 9 mm, and 3 less than or equal to 1 mm. The pathologic scores are 1 = low grade and no necrosis, 2 = low grade with necrosis, and 3 = high grade. Summing each of the category scores results in total scores ranging from 4 to 12. These four factors were culled from a number of factors evaluated in a multivariate analysis of prognostic factors for IBTR in patients with DCIS treated in their practice. It should be noted that their work was based on detailed clinical (mammographic and operative findings) and pathologic correlation and required processing of the entire surgical specimen with a uniform thickness of 2 to 3 mm and sequential analysis, thereby providing the most accurate assessment of size and margin status. The reproducibility of this system has been questioned by a number of investigators in retrospective and prospective studies and their detailed pathologic evaluation is not commonly used in clinical practice.101–104 At the present time, there is no one system which best predicts outcome and multiple factors should be considered.
Paget disease of the breast consists of 1% to 3% of all breast cancers.105–107 It is characterized by the presence of neoplastic cells in the epidermis of the nipple. There is no penetration of the dermal basement membrane and therefore Paget is a form of in situ cancer. HER-2/neu overexpression is common in Paget disease.108,109 Paget is frequently associated with underlying ductal carcinoma in-situ or an invasive cancer or both,105,107,110 although the process may be isolated to the nipple. Chen et al.111 reported a decrease in the overall incidence of Paget disease in the Surveillance, Epidemiology and End Results (SEER) registry from 1988-2002. However, no underlying breast cancer was identified in only 13% of the 1704 women with Paget disease. Paget disease has erythema and an eczematous scaling of the nipple skin. It may progress to crusting and frank ulceration. The prognosis of Paget disease is related to that of the underlying invasive cancer, if present.
Invasive ductal cancer is the most common invasive cancer.112 Approximately 50% of invasive ductal cancers will have an associated DCIS component. The term extensive intraductal component (EIC) was first described by Schnitt et al. from Harvard.113 By definition, the entity consists of the simultaneous presence of DCIS comprising 25% or more of the primary tumor and its presence in the normal surrounding breast tissue. The definition also includes DCIS with focal areas of invasion (i.e., microinvasive cancer). While there have been various definitions of microinvasive cancer, it is currently defined by the AJCC staging manual as foci of invasion no greater than 0.1 cm.73,74 In instances where there are multiple foci of invasion, only the largest focus of invasion should be considered. The clinical presentation of EIC positive tumors is usually the presence of mammographic calcifications without an associated mass.114 The clinical significance of an extensive intraductal component is its association with an increased risk of ipsilateral breast tumor recurrence after breast-conserving surgery with or without radiation.115–122 This observation has been explained in part by the pathologic studies of Holland et al.123 In a serial subgross and correlated mammographic examination of 217 mastectomy specimens, they correlated the presence of an extensive intraductal component in an invasive ductal cancer with the incidence of residual tumor following an excisional biopsy. EIC positive tumors were significantly more likely to have residual tumor (predominantly DCIS) and at greater distances from the primary than EIC negative tumors. At a distance of 6 cm from the edge of the invasive cancer, 21% of EIC positive tumors had residual cancer compared with 8% of EIC negative tumors. The presence of EIC has not been associated with an increased risk of chest wall recurrence after mastectomy.124 EIC positive tumors have also not been found to have a higher risk of distant metastases113 and in some series have been reported to have a better prognosis.124,125
Invasive cancers can be further classified by their histologic grade. A modification of the Scharf Richardson Bloom system is commonly used. The total score for grade is based on adding the individual scores for differentiation, mitoses, and nuclear pleomorphism with each having a score of 1 to 3 and total scores of 3 to 9. Low-grade tumors have scores of 3 to 5, intermediate grade scores of 6 to 7, and high grade of 8 to 9.126 The Nottingham grading system127 assigns scores of one to three for the percentage of tubule formation, the degree of nuclear pleomorphism and mitotic count in a defined field area. The sum of the three scores determines the final grade with three grade divisions. Histologic grade is a prognostic factor for local-regional recurrence following mastectomy128–130 and breast-conserving surgery with or without radiation131–138 and has been correlated with breast cancer specific survival.134,139
The presence of lymphovascular invasion (LVI) has been associated with an increased risk of local-regional recurrence following breast-conserving surgery with or without radiation and mastectomy, and with an overall worse prognosis, especially in axillary node-negative women.115,140–150 Colleoni et al.150 found extensive lymphovascular invasion to be associated with a significant decrease in disease-free and overall survival in axillary node-negative women.
Invasive lobular cancers represent 5% to 10% of all breast cancers and represent the second most common type of breast cancer. They arise from the lobules and are often characterized by multifocality and a higher incidence of bilaterality in some series.151,152 There are five subtypes of invasive lobular cancers (classic, tubulolobular, solid, alveolar, and pleomorphic). Classic invasive lobular cancers are characterized by the presence of small relatively uniform cells that invade the stroma in a single-file pattern with little or no desmoplastic reaction. The pleomorphic variant has a similar pattern of invasion but the cells are larger and have more nuclear variation. The different subtypes of invasive lobular cancer have been associated with different prognoses.153 The pleomorphic variant has been associated with a larger primary tumor size, a greater frequency of positive nodes and a worse prognosis.154,155 Invasive lobular cancers are usually estrogen and progesterone receptor-positive and HER-2/neu negative.156,157
Several of the more uncommon histologic subtypes of breast cancer have been associated with a very favorable prognosis. These include tubular and mucinous or colloid cancers. Axillary nodal metastases are less common and they tend to occur in older women.158–161 In contrast, invasive micropapillary carcinoma is associated with a worse prognosis due to its frequent involvement of axillary nodes and larger primary tumor size.162 Medullary carcinomas are associated with a prominent lymphocytic infiltrate and are well circumscribed grossly and microscopically. While more often high grade, they tend to be associated with a better prognosis. They occur more frequently in younger women and have been associated with BRCA1 mutation carriers.163
Guidelines for the basic elements of a pathology report for breast cancer have been established by the College of American Pathologists.164 The following is a list of information that is pertinent for the radiation oncologist to consider in the decision-making process:
| Partial Mastectomy | Size of specimen |
| DCIS (with or without an invasive component)—architectural pattern, nuclear grade, presence or absence of necrosis, calcifications in benign disease or DCIS, size and extent of DCIS, multifocality, estrogen and progesterone receptor status, HER-2/neu (investigational) | |
| Invasive cancer—size and extent, histology, grade (nuclear grade, mitotic count, tubule/papilla formation), presence or absence of LVI, perineural invasion, or extensive intraductal component, presence or absence of LCIS (pleomorphic or classic), calcifications in benign disease or invasive cancer, multifocality. | |
| Estrogen and progesterone status, HER-2/neu (IHC or FISH), invasive cancer. | |
| Margins of resection—width of negative margin for DCIS and invasive cancer (anterior, posterior, superior, inferior, lateral, medial), extent of positive margin and location. | |
| Mastectomy | Type of procedure (total mastectomy, skin-sparing, total skin-sparing [i.e., nipple preserved]) |
| Skin ellipse size and size of specimen | |
| Location of primary tumor(s) | |
| DCIS (with or without an invasive component)—architectural pattern, nuclear grade, presence or absence of necrosis, calcifications in benign disease or DCIS, size and extent of DCIS, multifocality, estrogen receptor status, HER-2/neu (investigational) | |
| Invasive cancer—size and extent, histology, grade (nuclear grade, mitotic count, tubule/papilla formation) presence or absence of LVI, perineural invasion, EIC, presence or absence of LCIS (pleomorphic or classic), calcifications in benign disease or invasive cancer, multifocality, extension to muscle | |
| Estrogen and progesterone status, HER-2/neu (IHC or FISH), invasive cancer | |
| Mastectomy margins, deep, anterior superior and anterior inferior | |
| Axillary Nodes | Number of sentinel and nonsentinel nodes |
| Number of positive nodes | |
| Method of detection of positive nodes (H+E, IHC, RT-PCR) | |
| Size of nodal metastasis | |
| Presence or absence of extracapsular extension and, if present, extent |
Staging
The current staging system for breast cancer is presented in Table 58-2, A and can be found in the seventh edition of the AJCC Cancer Staging Manual.74 The prior staging system is presented in Table 58-2, B.73 The staging system relies on primary tumor size, the presence and extent of regional node involvement, and distant metastases to define prognostic categories. The clinical classification (cTNM) is based on findings from the physical examination, imaging studies including mammography, ultrasound, MRI, CT scans, but not lymphoscintigraphy and pathologic examination of breast and/or regional node biopsies. Dimpling of the skin or nipple retraction does not imply T4 disease. Extensive imaging is not required to designate a case as M0 provided the clinical history and examination do not suggest the presence of distant metastases. The term M0 (i+) has been introduced to denote patients with circulating tumor cells in the blood or bone marrow.
Table 58-2B TNM Classification for Breast Cancer from the AJCC Staging Manual, 6th Edition
Rights were not granted to include this table in electronic media. Please refer to the printed book.
Pathologically positive nodes are classified as macrometastases, if the metastatic deposit is more than 2 mm. Micrometastases are defined as deposits more than 0.2 mm and less than 2 mm. T1N1miM0 tumors are now classified as stage IB. Isolated tumor cells are defined as clusters of 0.2 mm or not exceeding 200 cells in a single cross section of a node (pN0i+). They are usually detected by immunohistochemistry or molecular methods (pN0mol+) but can be verified by hematoxylin and eosin stains. Intramammary nodes are considered axillary nodes for the staging system and metastatic deposits in the axillary fat are classified as positive nodes. The number of positive nodes and their location further defines the staging groups (Table 58-2, B). The modifier (sn) is used for patients who have sentinel node biopsies only or when the total number of nodes removed is less than 6 nodes.
Surgical Considerations
The goal of breast-conserving surgery (BCS) is to eradicate both invasive and in situ disease and to achieve a desirable cosmetic result, which can be appreciated by both the surgeon and patient. Breast-conserving surgery has been shown to be an effective treatment when compared with mastectomy, yet BCS lacks the psychological impact and impaired body image that may accompany mastectomy.165,166 The decision to undergo BCS should be a carefully thought out plan that incorporates information from pathology and radiology and patient preference in an attempt to individualize care.
Once a patient is determined to be a candidate for BCS, attention turns to the technique for surgical excision. Localization of the tumor is performed either by palpation or placement of a wire under mammography, ultrasound, or MRI guidance. New techniques for localization include placement of a radioactive seed at the tumor, which can be identified intraoperatively using a hand-held gamma probe. However, only a few institutions have incorporated this into surgical practice.167–169 The size of excised breast tissue depends on multiple factors, which include tumor size and location, breast size, and radiographic findings. Complete excision of the tumor and any suspicious radiologic findings should be done in the most cosmetically appreciated way. Some surgeons advocate removing additional margins at the time of surgery to decrease the incidence of positive or close margins at final pathology. It is not necessary to remove the skin overlying the tumor except in cases where the skin is tethered or in intimate contact with the cancer.
Standard wound closure is a two-layer closure at the dermal/epidermal level. Generally, the cavity is left alone to fill with seroma fluid so as to maintain the shape of the breast. Recently, new techniques of wound closure have incorporated breast-flap advancement for volume replacement following large partial mastectomy resections.170–173 In an attempt to offer breast-conserving surgery, patients who had previously been destined for mastectomy are now assessed for “oncoplastic” surgery. The approach to oncoplastic surgery requires the surgical team to rethink the traditional curvilinear incisions in the upper pole and radial incisions in the lower pole, and focus on an approach that allows incorporation of fibroglandular rearrangement. These newer techniques have broadened the surgical choices for many patients who have concerns regarding mastectomy; however, there are inherent risks that cannot be overlooked.174 Further excision for compromised margins could be very difficult with this volume-replacement technique, and disruption of lymphatic channels may have consequences as it pertains to future lymphatic mapping in cases of local recurrence. Planning for radiation therapy must also be considered in terms of the identification of the primary tumor site for delineation of the boost volume. Placement of surgical clips in the tumor bed is especially important in these women. There have been no randomized controlled trials involving oncoplastic surgery and the risk of a local recurrence. Small cohorts have shown promising results for both aesthetics and local control, but careful consideration must be taken into account when planning oncoplastic techniques until better, long-term data are available to support this technique for routine use.175–177
If a patient desires BCS but has a tumor/breast ratio that initially would not be amendable to lumpectomy, there is the possibility of tumor “downstaging” with neoadjuvant therapy. Chemotherapy has been shown to be equally effective whether it is given presurgery (neoadjuvant) or postsurgery (adjuvant).178,179 The discussion of neoadjuvant therapy should be a multidisciplinary approach to ensure appropriate recommendations from medical, radiation, and surgical oncology. Magnetic resonance imaging (MRI) has become a valuable tool for predicting BCS success and is rapidly gaining wide use in this setting.180–182 The MRI images can direct the surgeon to the volume of breast tissue that should be removed in relation to response to therapy. However, mammography is equally important in assessing the extent of residual calcifications that need to be excised at the time of lumpectomy. At the start of neoadjuvant therapy, the patient should have a titanium clip placed under the core biopsy of the tumor to mark the location. The titanium clip can be used for localization in cases where a complete radiographic response is achieved.183 Clip placement has been associated with improved local control in patients receiving neoadjuvant chemotherapy and undergoing breast-conserving surgery.184 The volume of breast tissue excised after neoadjuvant chemotherapy is generally the residual nidus and not the original volume. However, all malignant appearing calcifications must be excised before radiation. Margin assessment may be more difficult in tumors that regress in a multifocal pattern.
Mastectomy
Not all patients will be candidates for BCS even after neoadjuvant therapy, and many will choose mastectomy regardless for personal reasons. Mastectomy offers an excellent oncologic treatment for breast cancer, and with new surgical techniques, the cosmetic results have greatly improved if the patient desires breast reconstruction. The most recent advance in surgical technique has been the introduction of the total skin-sparing mastectomy or nipple-sparing mastectomy.185,186 Leaving the nipple-areolar complex (NAC) intact has led to considerable improvements in cosmetic results without compromising the oncologic procedure itself. The tissue directly underneath the areola is sharply dissected and the nipple is inverted to core out all ductal tissue with an approximate 5% rate of nipple loss secondary to ischemia. Contraindications to nipple-sparing mastectomy are inability to achieve acceptable margins at the NAC or direct involvement of the nipple tissue itself. Patients can choose either autologous tissue transfer or implant reconstruction following nipple-sparing mastectomy.
It is generally accepted that immediate reconstruction after mastectomy is safe in terms of local recurrence rates and does not significantly prolong the start of adjuvant therapy.187,188 Most plastic surgeons agree that immediate reconstruction offers a better aesthetic outcome in conjunction with the psychologic benefit of awakening from surgery with an intact reconstructed breast.189,190 However, there is considerable debate surrounding immediate reconstruction for those patients who are at a higher risk of requiring postmastectomy radiation therapy (PMRT) with concern for an increased rate of complications. Numerous studies have shown that radiation increases the rate of TRAM flap complications, namely fat necrosis and flap necrosis.191,192 These complications can occur both with immediate reconstruction and delayed reconstruction. Some would argue that the immediate reconstruction with skin-sparing or nipple-sparing mastectomy leads to a superior cosmetic result and that it is beneficial in light of the potential for volume loss and fibrosis that can accompany PMRT. In an attempt to avoid these complications, but also to achieve excellent cosmesis, some have implemented a “two-stage” approach.193 If the patient is deemed high risk for PMRT, they undergo either a skin-sparing mastectomy or nipple-sparing mastectomy with placement of a subpectoral tissue expander to preserve the skin envelope. After review of the final pathology, patients who do not require PMRT can undergo immediate reconstruction, while those who require PMRT can have this treatment with the expander in place. Following radiation the patient can then undergo delayed reconstruction with the preserved skin envelope.
Axilla
Sentinel lymph node biopsy has proven to be an accurate technique for staging the axilla in breast cancer patients and is associated with less morbidity when compared with complete axillary node dissection. Several prospective trials in the United States and Europe have randomized women to sentinel node biopsy and axillary dissection versus sentinel node biopsy and axillary dissection only if the sentinel node is positive. Veronesi et al.194 reported the results of the European Institute of Oncology trial for stage I breast cancer. The false-negative rate was 8.8% and the negative predictive value was 95.4%. There were no axillary recurrences in the women who had sentinel node biopsy only. In the NSABP B-32 trial of 5611 women, sentinel nodes were identified in 97.1%, the false-negative rate was 9.8%, and the negative predictive value was 96.1%.4 When performed by experienced hands, the false-negative rate has been shown to be less than 5%, with long-term recurrence rates in sentinel node-negative patients approaching 0.3%.195
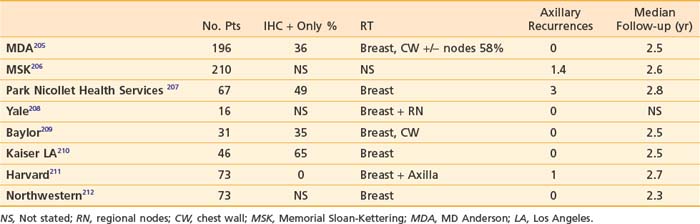
Completion axillary dissection is recommended for patients with positive sentinel nodes.196 This recommendation is based on the finding from a meta-analysis of 69 trials of sentinel node biopsy of 48% nonsentinel node positivity in women with positive sentinel nodes.195 However, the role of completion axillary dissection in women with immunohistochemical (IHC) only positive sentinel nodes or micrometastases (>0.2 mm and <2 mm) is unknown. A number of predictive models have been developed to estimate the risk of nonsentinel node positivity in patients with positive sentinel nodes. These include three nomograms197–199 and three scoring systems.200–202 Factors evaluated in these models include method of detection (frozen section, routine H+E, serial H+E, or IHC), primary tumor size, nuclear grade, multifocality, presence of lymphovascular invasion, estrogen receptor status, size of sentinel node metastasis, number of positive sentinel nodes, and number of sentinel nodes removed. It has been suggested by some investigators that women with a less than 10% risk of nonsentinel node positivity may forego completion axillary dissection.203,204 The role of axillary irradiation in patients with positive sentinel nodes who do not undergo an axillary dissection has been evaluated in eight single institution studies (Table 58-3).
It should be noted that in a significant number of patients, the sentinel node had isolated tumors cells or was IHC positive only. Overall axillary recurrence rates are 3% or less with median follow-up times of 2.5 years. Axillary recurrence appears to be low whether the low axilla is included in the tangential fields or with axillary radiation. This issue is being investigated in an EORTC phase III randomized trial213 in which sentinel node-positive patients with primary tumors 0.5 to 3 cm are randomized to axillary dissection or axillary radiation.213 Accrual is estimated to be complete in 2010. The ACOSOG Z0011 trial was designed to ask a similar question but closed prematurely due to poor accrual in the United States.214
Sentinel lymph node biopsy, which began as a staging tool in early stage primary breast cancer, has now been expanded to patients undergoing neoadjuvant therapy and patients undergoing a reoperation for recurrent breast cancer with a previous negative sentinel node biopsy.215 Patients with an ipsilateral recurrence or a new primary breast cancer after previous SLNB may be spared a complete axillary node dissection by undergoing remapping with lymphoscintigraphy. Of course, in the 20% to 25% of patients who fail mapping in this setting, the axilla should be dissected for complete staging and eradication of potential disease.
The use of SLNB in the neoadjuvant setting has become more widespread with increased use of preoperative chemotherapy, leading to controversy surrounding the issue of timing of the sentinel node procedure. Proponents of performing SLNB after neoadjuvant therapy argue that complete axillary response is possible in 20% to 40% of women harboring lymph node metastases.216,217 Eradication of this disease allows the woman to undergo SLNB only, foregoing the morbidity associated with a complete axillary node dissection. Other advantages to SLNB following neoadjuvant therapy include limiting surgery to one combined procedure and avoiding a delay in starting neoadjuvant therapy secondary to an axillary node dissection in the event a positive SLN is identified in a preadjuvant setting.
Those who advocate performing the SLNB before initiation of neoadjuvant therapy would argue that the procedure’s accuracy is compromised if done at completion of neoadjuvant therapy. The false-negative rate for SLNB following neoadjuvant therapy has been shown to vary from 9% to 30% in published series with a recent meta-analysis of 21 published studies investigating more than 1200 women reporting a false-negative rate of 12% and an identification rate of 90%.218,219 Some would argue that these latter statistics are within the expected range for SLNB accuracy in a nonneoadjuvant setting; however, with more experience now, these numbers are more likely to be in the 2% and 98% range, respectively. Another reason to argue for SLNB upfront involves accurate staging of the axilla to be used in determining the extent of radiation therapy. Decisions on treatment rely on knowing the number of positive nodes, and this information could be lost if axillary staging is done after neoadjuvant therapy.
One issue that most surgeons agree on is the use of SLNB only in patients who are deemed clinically positive in the axilla at the time of diagnosis, or have a biopsy proven positive axillary node. The false-negative rate for SLNB following neoadjuvant therapy for these patients is unacceptably high, and should only be done within the confines of a clinical trial.220 Axillary dissection with or without a SLNB is recommended in this setting. Whether to perform SLNB before or after neoadjuvant therapy is surgeon dependent, but should be discussed within a multidisciplinary team to achieve the best treatment plan for the patient.
Adjuvant Systemic Therapy Breast Cancer
Risk Stratification for Adjuvant Therapy
Oncotype DX
Oncotype DX is a diagnostic assay that quantifies the likelihood of distant breast cancer recurrence in women with newly diagnosed, early stage breast cancer. The assay is performed using formalin-fixed, paraffin-embedded (FFPE) tumor tissue and analyzes the expression of a panel of 21 genes that were selected through analysis of three different clinical trial cohorts. These included patient samples from the tamoxifen-treated arm of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 trial, and from other trials with node-positive and hormone receptor-negative patients. The results are provided as a Recurrence Score (RS) measured on a scale of 0 to 100 with a RS of 17 being low risk; 18 to 30 being intermediate risk, and more than 30 being high risk. Among the genes assessed by the assay, the proliferation and estrogen receptor (ER) pathways and HER-2 expression have the greatest impact on the RS calculation. The Oncotype DX was validated on 668 ER-positive, lymph node-negative cases of tamoxifen-only treated breast cancer patients enrolled in the NSABP B-14.221 In this validation cohort, only 6.8% of patients with tumors with a low RS recurred in 10 years, whereas 30.5% of patients with a high RS recurred at 10 years. A subsequent analysis of samples from the NSABP B-20 trial in which patients were randomized to receive either tamoxifen alone or tamoxifen plus cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) demonstrated that the assay predicted significant benefit from chemotherapy in patients with tumors having a high RS, whereas those with a low or intermediate RS did not derive significant benefit from chemotherapy. Data supporting the use of the Oncotype assay in patients with node-positive disease has also recently been published. In a sample of 465 patients with hormone receptor-positive breast cancer with zero to three positive axillary nodes treated with chemotherapy and hormonal therapy, RS was a more accurate predictor of relapse than standard pathologic staging features such as tumor size and lymph node involvement.222 Oncotype DX is currently included in the ASCO 2007 Update of Recommendations for the Use of Tumor Markers in Breast Cancer and the NCCN 2008 Clinical Practice Guidelines in Oncology Breast Cancer, and the test is accepted by many third-party payers, Kaiser Permanente, Medicare, and Medicaid for select patients with hormone receptor-positive node-negative disease.
MammaPrint
MammaPrint is a commercialized microarray-based multigene prognostic assay for breast cancer that has received 510(k) clearance from the FDA. This test cannot currently be performed on FFPE tissues and requires either fresh-frozen tumor samples or tissues collected into an RNA preservative solution. The assay was developed at the Netherlands Cancer Institute using DNA microarray analysis on primary breast tumors of 117 lymph node-negative patients, and then applying supervised classification to identify a gene expression signature strongly predictive of a short interval to distant metastases (defined as a “poor prognosis” signature).223 The 70 genes that comprise the MammaPrint assay are focused primarily on proliferation, with additional genes associated with invasion, metastasis, stromal integrity, and angiogenesis. The assay was used to classify a consecutive series of 151 lymph node-negative and 144 lymph node-positive breast cancer patients younger than 53 years old.224 Among the 295 patients, 180 were found to have a poor prognosis signature with a mean (±SE) overall 10-year survival rate of 54.6% ± 4.4% and 115 had a good-prognosis signature with a mean survival of 94.5% ± 2.6%. At 10 years, the probability of remaining free of distant metastases was 50.6% ± 4.5% in the poor-prognosis signature group and 85.2% ± 4.3% in the good prognosis signature group. Multivariable Cox regression analysis showed that the prognosis profile was a more powerful predictor of the outcome of disease in young patients with breast cancer than standard systems based on clinical and histologic criteria. The test was then validated by the TRANSBIG Consortium of European Cancer Centers. In this multicenter study, 307 patients with a median follow-up of 13.6 years were divided into high- and low-risk groups based on the gene signature classification and on clinical risk classifications.225 Patients were assigned to the gene signature low-risk group if their 5-year distant metastasis-free survival as estimated by the gene signature was greater than 90%. In this study, the MammaPrint signature was shown to provide further risk stratification and independent prognostic information within the Adjuvant! Online clinicopathologic risk categories.
Benefit of Adjuvant Chemotherapy and Hormonal Therapy—Evidence from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)
The most recent EBCTCG results published in 2005 combined six meta-analyses: anthracycline-based versus no chemotherapy; CMF-based versus no chemotherapy; anthracycline-based versus CMF-based chemotherapy; 5 years of tamoxifen versus none; 1 to 2 years of tamoxifen versus none; and 5 years versus 1 to 2 years of tamoxifen.226 Treatment with approximately 6 months of anthracycline-based chemotherapy with a regimen such as 5-fluorouracil, Adriamycin, and cyclophosphamide (FAC) reduces the annual breast cancer death rate by about 38% for women younger than 50 years of age and by about 20% for those of age 50 to 69. This meta-analysis also confirmed a moderate but highly significant advantage for anthracycline-based regimens (those including Adriamycin or epirubicin) to regimens consisting of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) in trials involving more than 14,000 patients. Since few women greater than 70 years of age participated in chemotherapy trials in the past, the benefits of chemotherapy in this patient population are not as well defined.
The Evolution of Adjuvant Chemotherapy—Practice Changing Trials
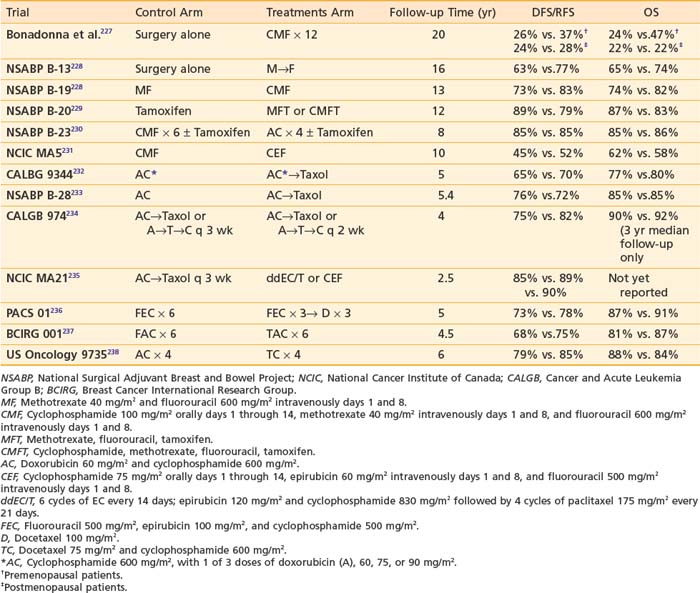
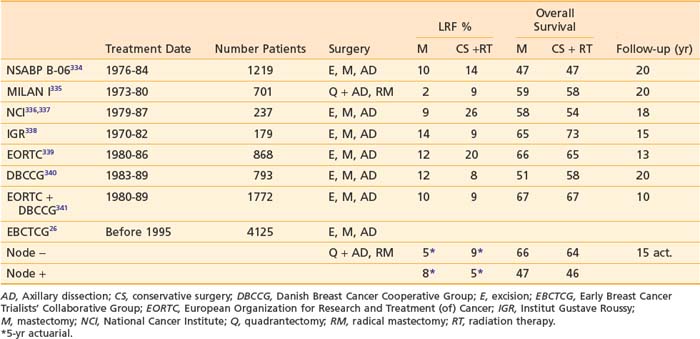
Systemic chemotherapy has become an integral component of the adjuvant treatment of early stage breast cancer since investigators began reporting significant improvements in disease-free survival (DFS) with single-agent chemotherapy after radical mastectomy in the 1970s. Polychemotherapy was first evaluated by Bonadonna, who randomized women with node-positive breast cancer to 12 monthly cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy or no further therapy after radical mastectomy227 (Table 58-4).
After more than 19 years of follow-up, significant improvements in relapse-free survival (RFS, relative risk 0.65) and overall survival (OS, relative risk 0.76) were observed.239 The benefit of postoperative chemotherapy for ER-negative, node-negative breast cancer patients was further reinforced through a series of clinical trials by the National Surgical Adjuvant Breast and Bowel Project (NSABP). The B-13 trial randomized 760 patients to surgery alone or methotrexate and 5-fluorouracil (MF), and with 16 years of follow-up, an overall benefit was seen with MF relative to surgery alone (RFS: HR = 0.59, P < .001; OS: HR = 0.75, P = .03).228 The B-19 trial randomized 1095 patients to MF or cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), and with 13 years of follow-up, an overall benefit was seen for CMF relative to MF (RFS: HR = 0.59, P < .001; OS: HR = 0.71, P = .01).228 In the NSABP B-20 study, 2306 ER-positive, node-negative women were randomized to adjuvant tamoxifen alone, MF plus tamoxifen (MFT) or CMF plus tamoxifen (CMFT).240,241 The study was updated after 12 years of follow-up revealing an RFS of 89% versus 79% and an OS of 87% versus 83%, demonstrating a benefit for the addition of chemotherapy (either MF or CMF) to tamoxifen.242
A number of trials have compared anthracycline-based to CMF-based regimens. The NSABP B-15 trial compared 2 months of doxorubicin and cyclophosphamide (AC) with 6 months of CMF in 2194 patients with ER-negative breast cancer. This trial also evaluated whether AC followed in 6 months by intravenous (IV) CMF was more effective than AC without reinduction therapy. Through 3 years of follow-up, there were no significant differences in disease-free survival (DFS), distant disease-free survival (DDFS), or overall survival among the three groups.241 In NSABP B-23, 2008 ER-negative breast cancer patients were assigned to CMF administered every 4 weeks for six cycles or doxorubicin and cyclophosphamide (AC) administered every 3 weeks for four cycles.230 Patients were also randomized to receive tamoxifen to determine if there was any benefit from hormonal therapy in this ER-negative patient population. With 8 years of follow-up, there were no statistically significant differences between the CMF and AC groups (RFS: HR = 1.00, P = .97; OS, HR = 0.92, P = .51). As expected, tamoxifen with either chemotherapy regimen resulted in no significant advantage over that achieved from chemotherapy alone. The National Cancer Institute of Canada (NCIC) MA5 trial compared the efficacy of an intensive cyclophosphamide, epirubicin, and fluorouracil (CEF) adjuvant chemotherapy regimen with CMF in 710 node-positive premenopausal patients. With a median follow-up of 10 years, RFS was 52% for patients who received CEF compared with 45% for CMF patients (HR for CMF versus CEF = 1.31; P = .007), and the 10-year OS for patients who received CEF and CMF were 62% and 58%, respectively (HR for CMF vs. CEF = 1.18; P = .085).231
The Role of Taxanes
The next steps in the evolution of breast cancer adjuvant chemotherapy were studies with paclitaxel or docetaxel demonstrating the superiority of taxane containing regimens. The CALGB 9344 study randomized 3121 women who had node-positive breast cancer to four cycles of AC or four cycles of AC followed by four cycles of paclitaxel (AC-T) with dose-escalated doxorubicin.232 The addition of paclitaxel was associated with significant 5-year DFS (70% versus 65%) and OS (80% versus 77%) benefits. There was no benefit observed with dose escalation of doxorubicin beyond 60 mg/m2/dose. In the NSABP B-28 trial, 3060 patients were randomly assigned to four cycles of AC or four cycles of AC followed by four cycles of paclitaxel (AC-T).233 With a median follow-up of 64.6 months, 5-year DFS was 76% for patients receiving AC-T compared with 72% for those receiving AC. Five-year OS was 85% for both groups. Several explanations have been put forth to explain the lack of survival benefit in the NSABP trial. Patients in the NSABP trial were older and had lower-risk disease compared with those in the CALGB trial. Additionally, hormone receptor-positive women received tamoxifen concurrently rather than sequentially with their chemotherapy in the NSABP study, which may have decreased the expected benefits of adding paclitaxel.
The efficacy of adjuvant docetaxel has also been evaluated in several clinical trials. The BCIRG 001 study randomized 1491 women with axillary node-positive breast cancer to six cycles of treatment with either docetaxel, doxorubicin, and cyclophosphamide (TAC) or six cycles of 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC) as adjuvant chemotherapy after surgery.237 With a median follow-up of 55 months, the estimated DFS at 5 years was 75% for patients receiving TAC and 68% for those receiving FAC, representing a 28% reduction in the risk of relapse (P = .001) in the TAC group. The estimated rates of overall survival at 5 years were 87% for TAC and 81% for FAC, representing a 30% reduction in the risk of death with TAC (P = .008). Grade 3 or 4 neutropenia, febrile neutropenia, and grade 3 or 4 infections were significantly higher in the TAC group but no deaths occurred as a result of infection. Adjuvant docetaxel was also evaluated in the PACS 01 trial, which compared six cycles of fluorouracil, epirubicin, and cyclophosphamide (FEC) with a sequential regimen of three cycles of FEC followed by three cycles of docetaxel (FEC-D) as adjuvant treatment in 1999 women with node-positive early breast cancer.236 With a median follow-up of 60 months, 5-year DFS rates were 73.2% with FEC and 78.4% with FEC-D (P = .012). Five-year OS rates were 86.7% with FEC and 90.7% with FEC-D, demonstrating a 27% reduction in the relative risk of death (P = .017).
The US Oncology 9735 trial randomized women to four cycles of the nonanthracycline-containing docetaxel-cyclophosphamide (TC) regimen or four cycles of AC. The initial publication of the trial after a median follow-up of 5.5 years demonstrated an improvement in 5-year DFS for TC over AC (86% versus 80%, respectively; hazard ratio [HR] = 0.67; P = .015).238 Data subsequently presented at the San Antonio Breast Cancer Symposium in 2007 showed that with further follow-up to 7 years, TC is associated with a statistically significant improvement in OS (6-year OS 88% versus 84%, HR = 0.73, P = .045).243 This trial has established the TC regimen as a reasonable option for women with low-to-intermediate risk disease who would have previously been considered good candidates for four cycles of AC.
Dose Density
Dose density, another strategy to maximize benefit from adjuvant chemotherapy, has been tested in a number of studies and is based on the Norton-Simon model of tumor kinetics. Rooted in the Gompertzian model of tumor growth, in which smaller tumors grow faster and tumor regrowth between treatment cycles is more rapid when cell kill is greatest, dose density reduces the time available for tumor regrowth.244 Dose dense chemotherapy was tested in the CALGB 9741 trial in which 2005 female patients were randomly assigned to receive one of the following regimens: sequential Adriamycin for four doses followed by paclitaxel for four doses followed by cyclophosphamide for four doses with cycles either every 2 weeks or every 3 weeks, or concurrent Adriamycin and cyclophosphamide for four doses followed by paclitaxel for four doses with cycles every 2 weeks or every 3 weeks. Patients randomized to “dose-dense” (every 2 week) cycles also received filgrastim support. With a median follow-up of 36 months, dose-dense treatment improved DFS (RR = 0.74; P = .010), and OS (RR = 0.69; P = .013).234 Four-year DFS was 82% for the dose-dense regimens and 75% for the others. There was no difference in either DFS or OS between the concurrent and sequential schedules. Severe neutropenia was less frequent in patients who received the dose-dense regimens, likely due to support with filgrastim. However, anemia requiring transfusion was greater in the dose-dense arms.
The benefits of taxane chemotherapy and dose density were also investigated in the National Cancer Institute of Canada (NCIC) MA.21 trial. This trial randomized 2014 women with lymph node-positive disease to six cycles of cyclophosphamide-epirubicin 5-fluorouracil (CEF), four cycles of doxorubicin-cyclophosphamide, followed by four cycles of paclitaxel every 3 weeks (AC-T) or four cycles of dose-dense epirubicin-cyclophosphamide followed by paclitaxel every 3 weeks for four cycles (ddEC/T). At a median follow-up of 30 months, the standard every 3 week taxane-containing AC-T regimen was inferior to both CEF and ddEC/T for disease-free survival (DFS).235 The 3-year DFS was 85% for AC-T versus 89% for ddEC/T (HR = 1.49, P = .0005) and versus 90% for CEF (HR = 1.68, P = .0006). There was no difference in DFS between the ddEC/T and CEF arms and the follow-up time is too short to evaluate a survival difference between the three arms of the study.
In summary, polychemotherapy has been consistently shown to be beneficial in the treatment of many breast cancer patients. Anthracycline-based chemotherapy has been the backbone for most chemotherapy regimens used in the adjuvant setting. While the addition of taxanes appears to improve outcomes, some studies have suggested that the benefit from taxanes may be limited to certain subsets of patients.245 However, more recently, certain taxane-based regimens have proven to be equally effective or more effective than the comparator regimen containing an anthracycline.243,246
Adjuvant Hormonal Therapy
The NSABP B-14 investigators first demonstrated the role of tamoxifen in the management of ER-positive, node-negative breast cancer when they randomized approximately 3000 women to receive either adjuvant tamoxifen or a placebo.247 The study was updated in 2004 after 15 years of follow-up, with a recurrence-free survival (78% versus 65%) and overall survival (71% versus 65%) benefit with the addition of tamoxifen.242 In the updated EBCTG analysis with 15 years of follow-up time, 5 years of adjuvant tamoxifen reduces the risk of breast cancer recurrence by 41% and the annual breast cancer death rate by 33% in estrogen receptor-positive breast cancer. Furthermore, 5 years of tamoxifen treatment was shown to be significantly more effective than 1 to 2 years of tamoxifen.226
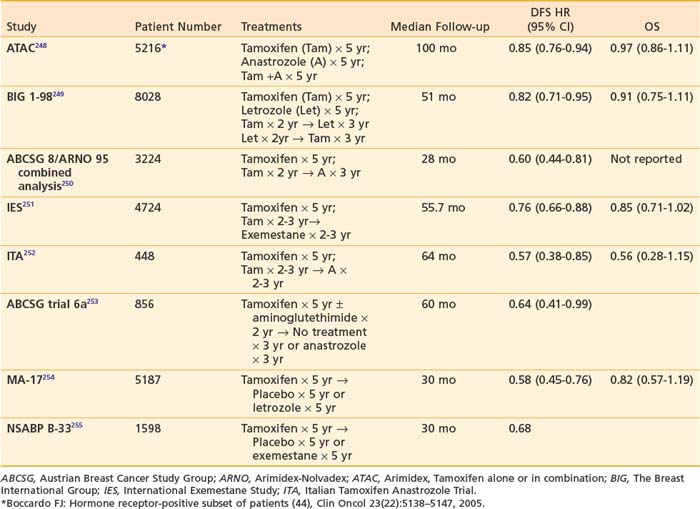
While tamoxifen remains the appropriate adjuvant hormonal treatment for premenopausal patients, a number of large, randomized trials have evaluated the role of the aromatase inhibitors (AIs) in postmenopausal women with hormone receptor-positive breast cancer (Table 58-5). These studies have compared tamoxifen to AIs in a number of treatment approaches. Two large trials have reported outcomes comparing initial treatment with tamoxifen for 5 years versus an AI for 5 years. The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial randomized more than 9000 postmenopausal women to receive tamoxifen, anastrozole, or the combination. The trial results were first presented in 2002 at which time treatment with 5 years of anastrozole was superior to tamoxifen in terms of disease-free survival.256 When anastrozole was administered concurrently with tamoxifen, the benefit of the AI was lost so subsequent follow-up data have focused on the AI versus tamoxifen arms of the trial. Additionally, since a significant percentage (16%) of patients on this trial were estrogen receptor-negative or unknown, analyses of treatment benefit have been presented for both the intent to treat population and for the estrogen receptor-positive population for whom the benefit of hormonal therapy is proven. With a median follow-up of 100 months, disease-free survival was improved by 15%, time to recurrence by 24%, time to distant recurrence by 16%, and contralateral breast cancer by 40% in the patients receiving an AI.248 Despite the fact that absolute differences in time to recurrence increased and recurrence rates remained significantly lower on anastrozole compared with tamoxifen after treatment completion, there was no difference in overall survival between tamoxifen and the AI. The second trial that compared upfront AI to 5 years of tamoxifen is the Breast International Group (BIG) 1-98 study in which women were randomly assigned to tamoxifen for 5 years, letrozole for 5 years, 2 years of tamoxifen followed by 3 years of letrozole or 2 years of letrozole followed by 3 years of tamoxifen.257 At a median follow-up time of 51 months, the use of upfront letrozole for 5 years resulted in a significant reduction in the risk of an event (HR.82; P = .007) compared with upfront tamoxifen for 5 years.249 Preliminary data from the arms comparing sequencing of tamoxifen and letrozole (2 years of tamoxifen followed by 3 years of letrozole or vice versa) to 5 years of letrozole were recently reported at the 2008 San Antonio Breast Cancer Symposium. Due to a significant portion of patients in the tamoxifen only arm crossing over and starting letrozole, a comparison of 5 years of tamoxifen to the sequential arms was not performed in this analysis. With a median follow-up of 71 months, 5-year DFS was 87.9% for the patients receiving 5 years of letrozole, 87.6% for patients who received letrozole for 2 years followed by tamoxifen for 3 years, and 86.2% for patients who received 2 years of tamoxifen followed by 3 years of letrozole.258 Statistically, there was no difference in DFS, OS, or time to distant recurrence for these three arms.
Several trials have reported more mature outcomes from switching strategies. The largest of the switching trials is the Intergroup Exemestane Study (IES), which randomly assigned 4724 postmenopausal women who were disease free after 2 to 3 years of tamoxifen to continue tamoxifen or switch to exemestane for a total duration of 5 years of endocrine therapy. After a median follow-up of 55.7 months, switching to exemestane was associated with a significant improvement in DFS (HR = 0.76; P = .0001) and when hormone receptor-negative patients were excluded, an improved overall survival (HR = 0.83; P = .05).251 The Austrian Breast and Colorectal Cancer Study Group (ABCSG) trial 8 and the Arimidex-Nolvadex (ARNO 95) are two multicenter, open-label trials that randomized postmenopausal women with hormone-sensitive early breast cancer who had completed 2 years of adjuvant oral tamoxifen (20 or 30 mg daily) to receive 1 mg oral anastrozole (n = 1618) or 20 or 30 mg tamoxifen (n = 1606) daily for the remainder of their adjuvant therapy. At a median follow-up of 28 months, a 40% reduction in the risk for an event was observed in the anastrozole group as compared with the tamoxifen group (HR = 0.60; P = .0009).250
Data from the EBCTG meta-analysis reveals that more than half of all recurrences occur in years 6 to 15 after diagnosis226 and therefore interventions to reduce the risk of late recurrences have been explored. Studies extending the course of tamoxifen beyond 5 years have yielded inconclusive results. In the NSABP B-14 study,242 where patients were reassigned to either continuation of tamoxifen beyond 5 years or a placebo, a slight advantage was observed in patients who discontinued tamoxifen relative to those who continued to receive it: DFS = 82% versus 78% (P = .03), RFS = 94% versus 92% (P = .13), and survival = 94% versus 91% (P = .07), respectively. Two small combined ECOG studies of patients with node-positive breast cancer (E4181 and E5181) found no statistically significant differences in time to relapse or survival between women continuing to receive tamoxifen beyond 5 years and those on observation. With a median follow-up of 5.6 years since randomization to continue tamoxifen, 85% of the women receiving tamoxifen were disease free compared with 73% of those on observation (P = .10); survival was 86% for those continuing to receive tamoxifen and 89% for those on observation (P = .52).259 Patients with estrogen receptor-positive tumors appeared to experience a longer time to relapse with continued tamoxifen therapy (P = .014), but there was no the survival difference for this subgroup. Lastly, in the Scottish Adjuvant Tamoxifen Trial patients who were disease free at 5 years were randomly assigned either to stop taking tamoxifen or to continue taking it indefinitely until relapse or death. With a median follow-up of 15 years, no additional benefit was observed in those randomly assigned to continue taking tamoxifen beyond 5 years.260 Despite the preponderance of evidence suggesting that extension of tamoxifen therapy beyond 5 years is not beneficial, two large ongoing trials, ATLAS (Adjuvant Tamoxifen-Longer Against Shorter) and aTTom (Adjuvant Tamoxifen Treatment-Offer More) may offer new insight into the optimal duration of tamoxifen therapy.
In contrast to the trials evaluating prolonged duration of tamoxifen, several other trials have shown that extension of hormonal therapy by switching to an aromatase inhibitor can be beneficial. The National Cancer Institute of Canada (NCIC) MA-17 was a double-blind, placebo-controlled trial evaluating the effectiveness of 5 years of letrozole therapy in postmenopausal women with breast cancer who completed 5 years of tamoxifen therapy.261 At the first interim analysis, the estimated 4-year disease-free survival rates were 93% for the letrozole group and 87% for the placebo group (P = .001). These findings led to study termination and unblinding. With a median follow-up of 30 months, women in the letrozole arm had statistically significantly better DFS and distant DFS than women in the placebo arm. Overall survival was not different for both arms, but among lymph node-positive patients, overall survival was statistically significantly improved with letrozole (HR = 0.61, 95% CI = 0.38 to 0.98; P = .04).254 In a similar design, the NSABP B-33 trial evaluated the steroidal aromatase inhibitor exemestane as extended adjuvant therapy in postmenopausal breast cancer patients who were disease free after 5 years of tamoxifen. Due to the unblinding of MA-17, patients in the placebo arm of B-33 were offered exemestane, and 44% of these patients elected to receive exemestane. With 30 months of median follow-up and despite early closure of the study, patients originally assigned to exemestane experienced a borderline statistically significant improvement in 4-year DFS and in a statistically significant improvement in 4-year relapse-free survival compared with patients originally assigned to the placebo group.255 The Austrian Breast and Colorectal Cancer Study Group (ABCSG) Trial 6a evaluated 3 years of anastrozole or no further treatment among women who were disease free at the end of the ABCSG Trial 6, in which they had received 5 years of adjuvant tamoxifen, with or without 2 years of the aromatase inhibitor aminoglutethimide. At a median follow-up of 62.3 months, women who received anastrozole had a statistically significantly reduced risk of recurrence compared with women who received no further treatment (HR = 0.62; 95% CI = 0.40 to 0.96, P = .031).253 Taken together, these three trials provide strong support for extending adjuvant tamoxifen therapy beyond 5 years with an AI in postmenopausal patients. The optimum length of extended adjuvant therapy requires further research, and patients who have completed 5 years of letrozole on the MA-17 study are being rerandomly assigned to continuation of letrozole versus a placebo. This study will provide evidence of safety for extension of treatment with AIs beyond 5 years.
Biologic Therapies—Trastuzumab
The NSABP B-31 trial compared four cycles of doxorubicin and cyclophosphamide (AC), followed by four cycles of every-3-week paclitaxel (P) (AC→P, arm 1) with AC followed by P with 52 weeks of trastuzumab beginning with the first cycle of paclitaxel (AC→PH→H, arm 2) . The N9831 trial was a three-arm study comparing four cycles of AC followed by 12 weekly doses of paclitaxel (AC→P, arm A) with AC→P followed by 52 weeks of H beginning after P (AC→P →H, arm B) and AC→P plus 52 weeks of H beginning with the first P cycle (AC→PH →H, arm C). Because arm 1 and arm 2 of the B-31 trial are similar to arms A and C of the N9831 trial, the studies were amended to include a joint statistical analysis combining arm 1 and arm A for comparison with arm 2 and arm C. Arm B of the N9831 trial was excluded from the analysis because H was not given concurrently with P. In both trials, only patients with tumors scored as with 3+ or more staining for HER-2 by immunohistochemistry (IHC) or gene amplification by fluorescence in situ hybridization (FISH) were eligible. In an intent to treat analysis with a combined median follow-up of 2 years (2.4 years for B-31 and 1.5 years for N-9831), there was a highly significant 52% reduction in the risk of disease recurrence with sequential trastuzumab (DFS 87% versus 75%, HR 0.48), and despite the short follow-up, a 33% reduction in the risk of death (3-year OS 94.3 versus 91.7%, HR 0.67).262
A different design was employed in the HERA trial, which compared 1 year or 2 years of trastuzumab given every 3 weeks with observation (no trastuzumab) in patients with HER-2/neu-positive early breast cancer who had completed local-regional therapy and at least four cycles of neoadjuvant or adjuvant chemotherapy. The results of 1 year of trastuzumab versus observation were reported and included 1698 patients receiving only chemotherapy and 1703 patients treated with 1 year of trastuzumab after chemotherapy who were followed for an average of 24 months.263 The addition of trastuzumab after chemotherapy resulted in a statistically significant 36% reduction in disease recurrence (HR 0.64, 3-year DFS of 81% versus 74%) and a significant improvement in overall survival (HR 0.66, 92% versus 90% in the trastuzumab and nontrastuzumab groups, respectively).
Patients enrolled in the smaller FinHer trial were randomized to three cycles of docetaxel or vinorelbine followed by three cycles of fluorouracil, epirubicin, and cyclophosphamide. The primary aim of that trial was to compare treatment using docetaxel with treatment using vinorelbine. The subset of women with HER-2-positive tumors (n = 232) was further randomized to either receive or not receive trastuzumab for 9 weeks together with the first three cycles of docetaxel or vinorelbine. Within this subgroup, DFS was significantly better among those who received trastuzumab (89% versus 78%, P = .01) and there was a trend toward better OS (96% versus 90%, P = .07).264 Surprisingly, the magnitude of those benefits is similar to those seen in the other trials that used 1 or 2 years of trastuzumab treatment.
The BCIRG-006 trial evaluated the efficacy and safety of three regimens as adjuvant systemic therapy: (1) doxorubicin and cyclophosphamide followed by trastuzumab plus docetaxel chemotherapy (AC-TH), (2) docetaxel and carboplatin plus trastuzumab (TCH), and (3) AC followed by docetaxel alone (AC-T) as the control arm. The results of the first interim analysis of 3222 patients was presented at the San Antonio Breast Cancer Symposium in 2005.265 At a median of 23 months of follow-up, DFS was significantly improved in patients treated with any trastuzumab-containing regimen, compared with chemotherapy alone, with a hazard ratio of 0.49 for AC-TH compared with AC-T (P < .0001) and 0.61 for TCH (P = .0002). No significant difference in DFS was found between the two treatment arms that included trastuzumab (P = .16). However, the absolute benefit in DFS from an anthracycline plus trastuzumab compared with TCH was 4%. The second planned interim analysis was presented in 2006, and with median follow-up at 36 months, both AC-TH and TCH significantly improved the DFS and OS over the control (relative reduction risk of relapse 39% (P < .0001) and 33% (P = .0003) respectively, for AC-TH and TCH versus control).246 Relative reduction in the risk of death was 41% (P = .0041) and 34% (P = .017), respectively, for AC-TH and TCH versus control. At both time points, there were fewer symptomatic cardiac events and a lower incidence of asymptomatic left ventricular ejection fraction decline with TCH compared with either anthracycline group.
At the 2007 SABCS, however, the first trial that did not demonstrate a benefit for adjuvant trastuzumab therapy in HER-2-positive disease was presented. The PACS 04 study randomized 3010 women with node-positive disease to either six cycles of epirubicin and docetaxel (ED) or six cycles of 5-FU-epirubicin-cyclophosphamide (FEC-100). The 526 women with HER-2-positive disease underwent a second randomization to either 1 year of adjuvant trastuzumab or no adjuvant trastuzumab after the completion of their primary chemotherapy. After a median follow-up of 40 months, there was no difference in either DFS or OS for the trastuzumab versus the nontrastuzumab containing arms (HR for DFS 0.86, P = .41).266 It is not clear why the PACS 04 trial did not demonstrate a benefit similar to the other adjuvant trastuzumab trials, although it is important to note that 10% of women randomized to the trastuzumab arm were never treated with adjuvant trastuzumab therapy.
Other Biologic Therapies and Future Directions of Adjuvant Therapy
Despite significant reduction in recurrences with adjuvant chemotherapy, hormonal therapy, and trastuzumab, many women still experience distant recurrence and ultimately die of metastatic disease. Recent molecular data including expression and genomic profiling have confirmed that breast cancer consists of distinct phenotypes, including luminal, HER-2, and basal-like subtypes. Tumors that lack expression of the estrogen receptor, progesterone receptor, and HER-2/neu (so called “triple negative” breast cancer) represent a distinct challenge. Triple negative tumors most commonly correspond to the basal-like subtype of breast cancer. Since triple negative tumors are not amenable to hormonal or HER-2-targeted therapies, they typically require chemotherapy-based treatment. Specific targets associated with basal-like or triple negative breast cancer are under investigation. The use of inhibitors of poly (ADP-ribose) polymerase in triple negative metastatic breast cancer has been reported in a randomized phase II trial of gemcitabine/carboplatin with or without PARP inhibition.267 In an analysis of the first 86 of the 120 patients enrolled on the trial, the addition of the PARP inhibitor resulted in a significant improvement in median progression free and overall survival. Fong et al.268 reported the results of a phase 1 trial evaluating olaparib (AZD2281), an oral PARP inhibitor in the treatment of patients with metastatic previously treated breast (nine patients), ovarian, prostate or other cancers of which one third had BRCA 1 or 2 mutations. Dosing ranged from 10 mg daily for 2 out of 3 weeks to 600 mg twice daily. Antitumor activity was demonstrated only in the mutation carriers and toxicity was minimal. The investigators subsequently enrolled only mutation carriers who took olaparib 200 mg/day. The PARP inhibitors represent another example of targeted therapy. The underlying mechanism of action is a synthetic lethality in which inhibition of PARP1 activity, which is responsible for base-excision repair results in the elimination of the only remaining way in which the cancer cells of mutation carriers can repair DNA damage. These cancer cells have lost all BRCA 1 or 2 function and therefore do not have the ability to repair DNA damage by homologous recombination. The result is cell death. The study by O’Shaughnessy et al.267 suggest that the benefit of PARP inhibitors may not be limited to mutation carriers but may include triple negative nonmutation carriers. A number of ongoing trials are evaluating the role or PARP inhibitors in the metastatic setting and ultimately the adjuvant setting.
Targeting angiogenesis is one strategy that is being explored in the adjuvant setting to improve outcomes. Bevacizumab, a monoclonal antibody to the vascular endothelial growth factor, has been approved for use in a variety of tumor types, including colorectal cancer, non-small cell lung cancer, and metastatic breast cancer. Compared with paclitaxel alone, the combination of paclitaxel and bevacizumab improved overall response rates and prolonged progression-free survival and in patients with breast cancer receiving their first line of chemotherapy for metastatic disease.269 Based on this trial result, bevacizumab ECOG 5103 was designed as a randomized phase III trial studying doxorubicin, cyclophosphamide, and paclitaxel with or without bevacizumab in the adjuvant treatment of patients with HER-2-negative lymph node-positive or high-risk lymph node-negative breast cancer. Also based on results in the metastatic setting, lapatinib appears to be another promising agent among patients with HER-2-positive disease. The addition of lapatinib to capecitabine extended time to progression in patients who experienced disease progression after trastuzumab-based therapy.270 The Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) trial is an international research study that will randomize more than 8000 patients to receive trastuzumab alone for 52 weeks, lapatinib alone for 52 weeks, trastuzumab for 12 weeks, followed by a 6-week break, followed by lapatinib for 34 weeks, or lapatinib in combination with trastuzumab for 52 weeks.
Ductal Carcinoma In Situ
Treatment
Ductal carcinoma in situ (DCIS) currently represents approximately 25% of all breast cancers and 20% to 30% of those that are mammographically detected. The American Cancer Society estimates that there will be 67,770 new cases of in situ cancer of the breast in the United States in 2008, of which the majority will be DCIS.1 Local treatment options for ductal carcinoma in situ include mastectomy with or without reconstruction and breast-conserving surgery with or without radiation. Patterns of care studies suggest that approximately two thirds of women will undergo breast-conserving surgery and half of these will receive radiation.271 Systemic options include tamoxifen or aromatase inhibitors.
The goals of treatment for DCIS include the eradication of the initial cancer, prevention of the appearance of an invasive or noninvasive cancer in the ipsilateral breast, and the ability to minimize the risk of death from breast cancer. Endpoints commonly used in assessing the outcome of cancer treatment include survival (cause-specific, i.e., death due to cancer or overall, i.e., deaths from all causes) and recurrence (local and/or regional and distant). Mortality from DCIS is related to the presence of a clinically occult invasive cancer or the subsequent development of an ipsilateral invasive cancer, which becomes the source of distant metastases. Breast cancer mortality from DCIS is less than 5% and does not vary significantly with initial treatment (Tables 58-6 and 58-7). This lack of variation is related to the finding that 50% of ipsilateral breast tumor recurrences after breast-conserving surgery are ductal carcinoma in situ and 50% are invasive cancers. Salvage of a noninvasive recurrence is almost 100% and salvage of an invasive recurrence is approximately 80%.272,273 Breast cancer mortality following breast-conserving surgery for DCIS is approximately 10% of the overall ipsilateral breast tumor recurrent rate (IBTR). For example, if the ipsilateral breast tumor recurrence rate was 30% following conservative surgery alone and 15% following conservative surgery and radiation, the respective breast cancer mortality rates would be 3% and 1.5%. Following mastectomy for DCIS, chest wall recurrences are usually invasive cancers and salvage is approximately 50%. Therefore, breast cancer mortality following mastectomy for DCIS is one half the local recurrence rate. Since breast cancer mortality for DCIS is low regardless of the treatment, the primary consideration is the risk of an ipsilateral breast tumor recurrence and, in particular, an invasive recurrence. However, any local recurrence is significant in that the individual patient must face cancer again and subsequent treatment may not include breast-conserving surgery. An invasive recurrence has the potential to be life threatening. Treatment decisions may reflect the individual patient’s acceptance of the risk of recurrence and the perceived benefit of treatment.
Information regarding the results of treatment for DCIS may be obtained from prospective randomized trials, single-arm perspective studies, and retrospective series. There have been no prospective randomized trials comparing mastectomy to breast-conserving surgery for DCIS. However, 76 patients with DCIS were inadvertently entered into the National Surgical Adjuvant Breast and Bowel Project (NSABP) Trial B-06 for invasive cancer.274,275 Ipsilateral breast tumor recurrence was reported in 43% of the 21 women who underwent conservative surgery alone and 24% of these were invasive cancers. Seven percent of the women who received radiation experienced an ipsilateral breast tumor recurrence and 4% of these were invasive cancers. There were no chest wall recurrences in the women who underwent mastectomy. Similar results have been obtained from retrospective series; however, they are clearly subject to treatment selection bias. The results of six of these series are presented in Tables 58-6 and 58-7.
Routine sentinel node biopsy and/or axillary dissection is not recommended for women with DCIS. Axillary node metastases are infrequent in DCIS (i.e., less than 3%).281 Sentinel node metastases were identified in 3.1% of 223 women with DCIS in a European Institute of Oncology study.282 However, selective use of sentinel node biopsy has been recommended for DCIS more than 4 cm, palpable tumors, high-grade disease, and patients undergoing mastectomy. These features have been associated with an increased risk of occult invasive disease92,95,283,284 and therefore an increased risk of positive axillary nodes.285–287
Five prospective randomized trials have compared breast-conserving surgery to breast-conserving surgery and radiation. Descriptions of the trials and their results are presented in Tables 58-8 and 58-9. These trials have been criticized for their lack of detailed pathologic and clinical correlation. There was considerable variation among the participating institutions in specimen processing and pathologic assessment. Central pathology review was not performed for all slides in each individual case. The trials were designed and initiated at a time when the importance of various factors (margin width, size, nuclear grade) was not well appreciated. In addition, there were variations in surgical and radiotherapy technique. Despite these limitations, the trials are fairly consistent in their results. The addition of radiation decreased the risk of an ipsilateral breast tumor recurrence by 50% to 60% with an absolute benefit of 9%-16%. Invasive recurrences were decreased by 40% to 50% with an absolute benefit of 2%-9%. There were no significant differences in survival. It should be noted that the majority of tumors were low-grade or noncomedo DCIS in the EORTC trial and the United Kingdom-Australian-New Zealand Trial was a trial primarily of women greater than 50 years of age, with only 10% less than 50. A Cochrane review295 of four randomized trials of breast-conserving surgery with or without radiation (3925 women) reported a 51% decrease in ipsilateral breast tumor recurrence (invasive or DCIS) with the addition of radiation. There was no increase in non-breast cancer death with radiation and overall survival was similar for the two treatments.
The rationale for breast conservation therapy for ductal carcinoma in situ was based on the demonstrated effectiveness of the conservative approach for early stage invasive cancer. However, not all women are candidates for breast-conserving surgery. The current challenge is to identify women with DCIS whose risk of an ipsilateral breast tumor recurrence (primarily invasive) with breast-conserving surgery, with or without radiation, is significant enough to prompt a recommendation for a mastectomy and to identify women whose risk of an ipsilateral breast tumor recurrence following conservative surgery alone may be low enough to avoid radiation. A number of factors have been analyzed for their ability to predict an ipsilateral breast tumor recurrence following conservative surgery with or without radiation. These include the clinical factors of patient age, family history, method of detection, size, and residual calcifications on a postsurgery mammogram. Pathologic factors include architectural pattern, nuclear grade, necrosis, tumor size, margins of resection, angiogenesis, fibrosis, lymphocyte infiltrate, calcifications, multifocality, S phase fraction, estrogen and progesterone receptor and HER-2/neu. Table 58-10 presents outcomes reported from the prospective randomized trials of conservative surgery with or without radiation related to some of these factors. The relative importance of each factor has not been established. Difficulties in assessing the size and extent of DCIS, resection margin status and discordance among pathologists for the classification of DCIS and the reproducibility of any classification system have resulted in variations in the significance of each of these factors and the magnitude of risk of an ipsilateral breast tumor recurrence.
The single factor that is not subject to interpretation is patient age. Women 40 years of age or younger with DCIS have been reported to have ipsilateral breast tumor recurrence rates of approximately 50% in retrospective series100,277,296–298 and the EORTC prospective randomized trial.290 The addition of radiation to conservative surgery in the EORTC trial resulted in an ipsilateral breast tumor recurrence rate of 23% in women 40 years of age or younger. In the French Regional Cancer Center experience, the addition of radiation to conservative surgery decreased the ipsilateral breast tumor recurrence rate from 43% to 24% in women 40 years of age or younger with a median follow-up of 7.8 years.277 In the Rare Cancer Network study,296 the addition of radiation to conservative surgery decreased the ipsilateral breast tumor recurrence rate from 54% to 14% in women 45 years of age or younger with a median follow-up of 6 years. In addition, young age has been associated with an increased risk of an invasive recurrence following conservative surgery with or without radiation.276,299 Therefore, conservative surgery alone does not appear to be a treatment option associated with a reasonable risk of ipsilateral breast tumor recurrence in young women. An additional consideration in young women is the possibility of a BRCA1 or BRCA2 mutation. The prevalence of DCIS in mutation carriers has been reported to be similar to noncarriers. Hwang et al.205 and Claus et al.300 reported a greater percentage of high-grade DCIS in BRCA1 carriers when compared with BRCA2 carriers or sporadic breast cancer. Currently, there are no studies reporting outcome of treatment for DCIS in women with BRCA1 or BRCA2 mutations. Mutation carriers with invasive cancers have been reported to have an ipsilateral breast tumor recurrence rate at 10 years of 34% when treated with conservative surgery alone301 compared with 13% when treated with conservative surgery and radiation. Pierce et al.302 noted that the addition of oophorectomy or tamoxifen to conservative surgery and radiation resulted in a decreased risk of ipsilateral breast tumor recurrence and contralateral breast cancer. Treatment options, therefore, for women with BRCA1- or BRCA2-linked DCIS include unilateral mastectomy versus bilateral mastectomy or conservative surgery and radiation, combined with tamoxifen or oophorectomy.
Most of the prospective randomized trials (see Table 58-10) suggest that increasing age is associated with a decreased risk of ipsilateral breast tumor recurrence in patients treated with conservative surgery alone or conservative surgery and radiation. However, for each of the age groups, the addition of radiation decreased the risk of an ipsilateral breast tumor recurrence when compared with conservative surgery alone. In the South Sweden trial,291 the benefit of radiation appeared greatest in women over 50 years of age. In a recent analysis of the Surveillance Epidemiology and End Results (SEER)303 Medicare database of women 66 years of age or older with DCIS, the addition of radiation decreased the 8-year ipsilateral breast tumor recurrence rate from 11% to 3% and the 8-year risk of an ipsilateral invasive recurrence from 7% to 2%. A low-risk group was identified, being age 70 years or older, tumor size less than 2.5 cm, noncomedo histology and nuclear G2. In this low risk group, the risk of an ipsilateral breast tumor recurrence at 5 years was 8% without radiation compared with 1% with radiation. Therefore, even in the most favorable subgroup based on age, the addition of radiation decreases the risk of an ipsilateral breast tumor recurrence, although the magnitude of the benefit may be small.
In each of the randomized trials, the detection of DCIS solely by mammography was associated with a lower risk of ipsilateral breast tumor recurrence when compared with clinical detection, with symptoms such as a palpable mass or bloody nipple discharge (see Table 58-10). An emerging area of interest is breast density and its association with an increased risk of ipsilateral breast tumor recurrence in women with DCIS. Habel et al.304 reviewed the mammograms of 392 of the 818 patients entered into the NSABP B-17 trial. The median follow-up was 11 years. Thirty-three percent of the women were 50 years of age or younger and 52% received radiation. A highly dense breast was seen on mammography (highly dense ≥ 75%) in 15.6% of the women less than 50 years of age compared with 2% of the women over 50. Women with mammographic density greater than or equal to 75% had a 40% ipsilateral breast tumor recurrence rate compared with 18% for mammographic density less than or equal to 25%, 27% for mammographic density 25% to 49% and 24% for mammographic density 50% to 74%.
The extent of DCIS impacts on the decision for breast-conserving surgery versus mastectomy. The extent can be assessed radiographically or by pathologic examination. Radiographic assessment includes the extent of calcifications on a mammogram; however, this may underestimate the pathologic extent of DCIS, especially for low-grade lesions.87,305 More recently, MRI has been used to evaluate the extent of DCIS. In a study of 45 patients from the University of California, San Francisco, MRI overestimated the pathologic extent of disease more than twofold in 23% of patients and underestimated it in 9%.93 The discrepancy between the pathologic size and MRI extent was more frequently observed in the MRI patterns of regional or multi-regional enhancement and in estrogen-receptor-positive DCIS. Clinical assessment of tumor size includes measurements of a palpable mass, the dimensions of the mammographic abnormalities, including calcifications and/or a mass. In the EORTC290 and South Sweden291 prospective randomized trials, increasing clinical size was associated with an increased risk of ipsilateral breast tumor recurrence in patients treated with conservative surgery alone but not those treated with conservative surgery and radiation. In the NSABP B-17 randomized trial,288 the ipsilateral breast tumor recurrence rate was correlated with the extent of calcifications on the mammogram, both in women treated with conservative surgery alone or conservative surgery and radiation. It is of note that the appearance of scattered calcifications was associated with an ipsilateral breast tumor recurrence rate of 37% in patients treated with conservative surgery alone and 27% in those treated with conservative surgery and radiation.
In patients having calcifications, a postexcision mammogram before radiation or observation is essential to assure the removal of all malignant-appearing calcifications. If calcifications are not identified on routine views, magnification views of the surgical site should be performed. A specimen x-ray is not adequate proof that calcifications have been removed. All of the five patients reported in the literature306,307 in whom residual malignant calcifications were not removed before radiation have reportedly recurred. Standards for the management of ductal carcinoma in situ have been developed by the American College of Radiology, the Society of Surgical Oncology, the American College of Surgeons, and the College of the American Pathologists308 and emphasize the importance of obtaining a postexcision mammogram.
The pathologic extent of DCIS can be assessed by gross examination, although most DCIS is not grossly evident. It can be determined by measuring the area on a given slide, the number of blocks with DCIS, or complete tissue processing with uniform section thickness and sequential analysis as performed by Silverstein et al. This latter method is not commonly employed in clinical practice. In the NSABP B-17 and B-24 trials,288,293,309 size of DCIS as measured by the area on a given slide did not significantly correlate with ipsilateral breast tumor recurrence rates in patients treated with conservative surgery or conservative surgery and radiation. In a single-arm prospective study of conservative surgery alone,102 there was no correlation with ipsilateral breast tumor recurrence rates when size and extent were measured by the number of blocks with DCIS. MacDonald et al.310 reported 8-year ipsilateral breast tumor recurrence rates of 14% for DCIS less than or equal to 1.5 cm treated with conservative surgery alone compared with 23% for those with DCIS more than 1.6 to 3 cm and 30% for those greater than 3 cm treated with conservative surgery alone (median follow-up 4.8 years). For patients undergoing conservative surgery and radiation, the ipsilateral breast tumor recurrence rate was 13% for DCIS less than or equal to 1.5 cm, 28% for DCIS 1.6 to 4 cm, and 32% for DCIS more than 4 cm, with a median follow-up at 8.6 years.311 In these series, pathologic size was assessed by detailed processing and analysis of sequential sections. The size was then computed by knowing the number of sections with DCIS, the location of the sections and the width of the blocks. For example, if DCIS appeared on 10 consecutive sections and the blocks were 3 mm in thickness, the size of the DCIS would be 3 cm.
Multifocal DCIS has been defined as multiple foci separated by intervening normal breast tissue. Its presence has been associated with an increased risk of ipsilateral breast tumor recurrence in the NSABP B-17,289 B-24,292 and South Sweden291 prospective randomized trials when compared with unifocal disease in patients treated with conservative surgery alone or conservative surgery and radiation (see Table 58-10). The presence of multifocal disease may be a contraindication to conservative surgery alone. In a retrospective series reported from the University of Toronto,312 the 10-year ipsilateral breast tumor recurrence rate for women undergoing conservative surgery alone was 20% for unifocal disease compared with 41% for multifocal DCIS. For women treated with conservative surgery and radiation, ipsilateral breast tumor recurrence rates at 10 years were 13% for unifocal disease compared with 20% for multifocal disease. In the NSABP B-24 trial, the presence of multifocal DCIS was a significant factor predicting for ipsilateral breast tumor recurrence rates in patients treated with conservative surgery and radiation with or without tamoxifen.292
A recent study from Memorial Sloan-Kettering Cancer Center reported an increased risk of IBTR in women with DCIS and atypical lobular hyperplasia (ALH) or LCIS undergoing breast-conserving surgery.313 Fourteen percent of the 294 patients had associated ALH or LCIS. Eighty percent did not receive radiation and only 10% had tamoxifen. The 10-year cumulative incidence of an IBTR with LCIS was 47% and 21% for ALH compared with 19% for those with DCIS only. The presence of lobular neoplasia at an excision margin was associated with a 46% IBTR rate (12/26) compared with 25% (3/12) without lobular neoplasia at an excision margin. However, two additional series have not reported an increased risk of IBTR in women with DCIS and LCIS undergoing breast-conserving surgery.314,315
Based on the above considerations of size and extent of DCIS, mastectomy is the recommended treatment for extensive areas of ductal carcinoma in situ (i.e., those manifesting as diffuse areas of malignant appearing calcification or evidence of DCIS in more than one quadrant in a single breast). Women with DCIS extending over a distance of more than 4 cm generally are not considered for breast conservation therapy, based on the outcome data from Silverstein et al.311
Resection margin status is a complex issue. Positive margins of resection have been associated with an increased risk of ipsilateral breast tumor recurrence in the NSABP B-17, EORTC, and the NSABP B-24 trials (see Table 58-10) in patients undergoing conservative surgery or conservative surgery and radiation. However, the magnitude of the risk of ipsilateral breast tumor recurrence in patients with positive margins has varied and reflects difficulties in assessing margins in the absence of a standardized method for assessing and reporting margins, especially in terms of the width of a negative margin. Margins cannot be assessed if the specimen is submitted in fragments, if it is not adequately sampled, if it is not oriented by the surgeon, and if it is not inked. There are six surgical margins (anterior, posterior, superior, inferior, medial, and lateral). The location of the positive margin influences recommendations for further surgery. A positive posterior margin usually does not require further surgery if the initial excision extended down to the pectoralis fascia. Similarly, a positive anterior margin does not require additional surgery if the excision extended to the skin. Ipsilateral breast tumor recurrence rates may be influenced by the extent of margin positivity and the number of positive margins and there appears to be an interaction between patient age and margin positivity. In a multi-institution study of breast-conserving surgery and radiation for mammographically detected DCIS, Solin et al.316 reported ipsilateral breast tumor recurrence rates of 50% for women 39 years of age or younger with close margins compared with 7% for women 50 years of age or older. Women with positive margins of resection 50 years of age or older experienced a 13% ipsilateral breast tumor recurrence rate compared with 24% for those 40 to 49 years of age. The width of the negative margin that best minimizes the risk of an ipsilateral breast tumor recurrence following conservative surgery or conservative surgery and radiation is controversial. A recent report from the NSABP B-24 trial292 reported no differences in ipsilateral breast tumor recurrence rates when comparing a negative margin width of less than or equal to 1 mm to more than 1 mm in patients undergoing conservative surgery and radiation with or without tamoxifen. However, in a series from Tufts New England Medical Center, negative margins more than 10 mm were associated with a 0% risk of an ipsilateral breast tumor recurrence in patients receiving conservative surgery and radiation.317 Solin et al.316 reported 10-year ipsilateral breast tumor recurrence rates of 8% for women with DCIS and more than 2 mm negative margins treated with conservative surgery and radiation. Dunne et al.318 performed a meta-analysis of 21 retrospective studies and one randomized trial of DCIS in which the status of the excision margin was reported. The meta-analysis included 4660 women treated with breast-conserving surgery and radiation. Negative margins greater than or equal to 2 mm were associated with a decreased risk of ipsilateral breast tumor recurrence when compared with those less than 2 mm. Silverstein et al. have suggested that a 1 cm or greater negative margin is sufficient to prevent radiation in women undergoing breast-conserving surgery. The 8-year actuarial risk of ipsilateral breast tumor recurrence in 197 women who underwent conservative surgery alone with greater than or equal to 1 cm negative margins was 8%.310 Margins less than 1 cm were associated with ipsilateral breast tumor recurrence rates ranging from 61% for a positive margin to 22% to 51% for negative margins in the less than 1 mm to 9.9 mm range. In a retrospective multi-institutional study of wide excision alone for DCIS, Kerlikowske et al.299 reported a 9%, 5-year ipsilateral breast tumor recurrence rate for women with negative margins greater than or equal to 1 cm. As width of the negative margin increased, the risk of an ipsilateral breast tumor recurrence with DCIS decreased but the risk of an ipsilateral breast tumor recurrence with an invasive cancer was not significantly changed. Therefore, negative margins of resection may have a greater impact on diminishing the risk of a noninvasive ipsilateral breast tumor recurrence rather than an invasive recurrence.
High nuclear grade and the presence of necrosis have been associated with an increased risk of ipsilateral breast tumor recurrence in patients undergoing conservative surgery in the NSABP B-17288,289 and EORTC290 randomized trials (see Table 58-10). These factors have had less of an impact on ipsilateral breast tumor recurrence rates in patients undergoing conservative surgery and radiation. In the NSABP B-24 trial, high nuclear grade was not associated with an increased risk of ipsilateral breast tumor recurrence in patients undergoing conservative surgery and radiation with or without tamoxifen. However, the presence of a micropapillary pattern of DCIS and comedo necrosis were significant factors associated with an increased risk of ipsilateral breast tumor recurrence in these women.292
Silverstein et al.,100 employing a multivariable analysis of prognostic factors for ipsilateral breast tumor recurrence in patients with DCIS treated in their practice, identified age, size of margins, nuclear grade, and necrosis as the most significant factors. Their work was based on detailed clinical (mammographic and operative) findings and pathologic correlation and required processing of the entire surgical specimen with a uniform thickness of 2 to 3 mm and sequential analysis, thereby providing the most accurate assessment of size and margin status. This analysis formed the basis for the University of Southern California/Van Nuys Prognostic Index (USC-VPNI), which is a scoring system with four categories: age, size, margins, and nuclear grade combined with necrosis. Within each category there are three scores (1, 2, 3). The age scores are 1 = more than 60 years, 2 = 40 to 60 years, and 3 = less than 40 years. The size scores are 1 = less than or equal to 1.5 cm, 2 = 1.6 to 4 cm, 3 = more than 4 cm. The margin scores are 1 = greater than or equal to 1 cm, 2 = 1 to 9 mm and 3 = less than 1 mm. The pathologic scores are 1= low grade and no necrosis, 2 = low grade with necrosis, and 3 = high grade. Summing each of the category scores results in total scores ranging from 4 to 12. The 12-year actuarial ipsilateral breast tumor recurrence rates were 4% for scores of 4 to 6, 40% for scores of 7 to 9, and 87% for scores of 10 to 12 in patients treated with conservative surgery alone, and 4% for scores of 4 to 6, 30% for scores 7 to 9, and 38% for scores of 10 to 12 in patients treated with conservative surgery and radiation.100 The investigators concluded that patients with scores of 4 to 6 were candidates for wide excision alone; scores of 7 to 9, for excision and radiation; and scores of 10 to 12, mastectomy. Recognizing that their degree of pathologic processing and analysis was not routinely performed in clinical practice and that there was discordance among the pathologists in the classification of DCIS, the investigators ultimately concluded that margin status was the most important factor for ipsilateral breast tumor recurrence and represented a surrogate for the USC-VNPI index. The reproducibility of this system has been questioned by a number of investigators in retrospective and prospective studies.101–104 Inaccuracies in calculating the score could result in overtreatment or undertreatment. Use of margin width as the sole prognostic factor may be an oversimplification of a complex issue especially in young women.
The relationship between the resection margin status and chest wall recurrences in patients with DCIS undergoing mastectomy has received little attention. Rashtian et al.319 reported a 7.5% local recurrence rate in 80 women with DCIS and a less than 10 mm mastectomy margin. The median follow-up was 6.1 years. All six local recurrences were invasive. Local recurrence was 12% for less than 60 years compared with 0% for women 60 years old. Margin status of 2 mm with comedonecrosis or nuclear G3 DCIS was associated with a 25% local recurrence. Local recurrence was 6% for total mastectomy compared with 11% for modified radical mastectomy. Carlson et al.320 reported a 3.3% recurrence rate in 223 women who had undergone skin-sparing mastectomy and immediate reconstruction. The median follow-up was 6.8 years. The local recurrence rate was 11% for margins of 1 mm, 6% for age 50 years old, 6% for nuclear G3 DCIS, and 6% for DCIS of 4 cm. In contrast, Chan et al.321 reported a local recurrence rate of 1.7% in 59 women with DCIS and a 5 mm mastectomy margin. Median follow-up was 8 years (range 1 to 21 years) and the median pathologic size of DCIS was 4.5 cm. The local recurrence rate was 3% for high-grade DCIS and 5% for skin-sparing mastectomy. None of the four patterns with a positive margin experienced a local recurrence. There were no chest wall recurrences in patients who underwent total mastectomy or total skin-sparing mastectomy. The single chest wall recurrence occurred in of the 14 patients who underwent skin-sparing mastectomy. The differences in the reported outcome in these series may reflect variations in surgical technique. Outcome of empiric treatment with radiation postmastectomy of 14 patients with DCIS with close or positive margins (1 mm) has been reported in a single series.322 There were no local recurrences with a median follow-up of 4.8 years.
Two prospective single-arm studies were conducted in an attempt to identify a low-risk group of women for whom conservative surgery alone may be an acceptable treatment. The Dana Farber Cancer Center102 initiated a single-arm prospective study of conservative surgery alone for women with DCIS less than or equal to 2.5 cm (mammographic size), nuclear G1-G2, and margins greater than or equal to 1 cm. The study was terminated after 158 patients were enrolled because the observed ipsilateral breast tumor recurrence rate had exceeded the expected. With a median follow-up of 3.3 years, the crude ipsilateral breast tumor recurrence rate was 9% and the 5-year actuarial rate was 12.5% with a projected rate of 25% at 10 years and 30% at 12 years. The investigators were unable to duplicate the low ipsilateral breast tumor recurrence rate (8% at 8 years) reported by MacDonald et al.323 in a somewhat similar group of 212 with greater than or equal to 1 cm margins and no radiation. Not all of the patients in the Harvard study were required to have a postexcision mammogram to exclude residual calcifications and only 38% of the patients actually had one. It is possible that some of the recurrences were persistent disease, which was not completely resected. In addition, 40% of the 10 patients with G3 tumors experienced an ipsilateral breast tumor recurrence compared with 6% of the G1-G2 tumors. The Eastern Cooperative Oncology Group324 completed a single-arm prospective study of conservative surgery alone with or without tamoxifen for nuclear G1 or G2 DCIS less than or equal to 2.5 cm, with margins of resection greater than or equal to 3 mm and DCIS less than or equal to 1 cm nuclear G3 with necrosis and with margins greater than or equal to 3 mm. Size was defined as the largest dimension of the area of the DCIS histologically. The median follow-up was 6.2 years. For the 565 low-grade lesions, median lesion size was only 6 mm (only 24% >1 cm) and surgical margins were 5 to 10 mm in 21% and more than 1 cm in 49%. At 5 years the ipsilateral breast tumor recurrence rate was 6.1%, with 53% of these recurrences being invasive cancers. The contralateral breast cancer rate was 3.7%. For the 102 high-grade lesions, median lesion size was 5 mm with surgical margins 5 to 10 mm in 30% and more than 1 cm in 53%. The ipsilateral breast tumor recurrence rate at 5 years was 15.3% and 35% were invasive cancers. The contralateral breast cancer rate was 3.9%. For the low-grade lesions, ipsilateral breast tumor recurrence rates were not significantly influenced by age, lesion size, or margin widths. For high-grade DCIS, the ipsilateral breast tumor recurrence rate for the 11 women less than 45 years of age was 54.4% compared with 10.3% for those 45 years of age or older. Margin width greater than or equal to 1 cm was not associated with a decreased ipsilateral breast tumor recurrence rate (IBTR). The 5-year IBTR rate for 1-cm high-grade DCIS was 32.9% compared with 12.7% for size less than 1 cm. Therefore both the Dana Farber and the ECOG studies suggest that high-grade DCIS, even with small size and widely negative margins, may be associated with an ipsilateral breast tumor recurrence rate sufficient enough to recommend radiation after local excision.
The role of tamoxifen in the treatment of ductal carcinoma in situ has been evaluated in two prospective randomized trials (see Tables 58-8 and 58-9). In the NSABP B-24 trial, women with DCIS were randomized to radiation with or without tamoxifen. In contrast to the NSABP B-17 trial, the B-24 trial included patients with positive margins (16%) and diffuse disease.293 The United Kingdom Coordinating Committee on Cancer Research DCIS Working Group (UK/ANZ) trial randomized patients to conservative surgery with or without tamoxifen or conservative surgery and radiation with or without tamoxifen.294 The addition of tamoxifen to radiation significantly decreased ipsilateral breast tumor recurrence rates in the NSABP B-24 trial but not in the UK/ANZ trial. The addition of tamoxifen to conservative surgery alone had no significant impact on ipsilateral breast tumor recurrence rates in the UK/ANZ trial. Tamoxifen was associated with a reduction in contralateral breast cancers in both trials and in both trials women 50 years of age or younger appeared to have a greater benefit from tamoxifen. In the NSABP B-24 trial, 33% of the women were 50 years of age or younger compared with only 10% in the UK/ANZ trial. This difference in the age of the patient populations may account in part for the different results. More recently, the NSABP B-24 investigators have reported that the benefit of tamoxifen was limited to ER-positive DCIS.325 This finding has prompted the routine performance of estrogen and progesterone receptors in DCIS. The NSABP B-35 trial evaluates the role of aromatase inhibitors in the treatment of ductal carcinoma in situ. The trial randomizes postmenopausal women with DCIS and negative margins and estrogen and progesterone receptor-positive DCIS by immunohistochemistry to radiation and tamoxifen for 5 years or radiation and anastrozole for 5 years.
Several investigators have initiated trials of neoadjuvant therapy for DCIS. Hwang and Esserman326 at UCSF are evaluating neoadjuvant tamoxifen. Preliminary results suggest that response as measured by MRI and the subsequent excision pathology appears greater in ER-positive disease. Neoadjuvant lapatinib for HER-2/neu or epidermal growth factor receptor-positive DCIS is being evaluated at Baylor College of Medicine327 and neoadjuvant trastuzumab is under investigation at MD Anderson Cancer Center. An NSABP trial will randomize patients with HER-2/neu overexpressing DCIS with negative excision margins to radiation with or without concurrent trastuzumab. HER-2/neu overexpression is more common in high-grade DCIS or comedo DCIS and ER-negative DCIS.328,329
Given the heterogeneity of DCIS, it is unlikely that a single treatment will be appropriate for all women. The management of DCIS requires a multidisciplinary approach with careful and detailed clinical and pathologic correlation. DCIS represents a spectrum of disease. Local treatment options include mastectomy with or without reconstruction, or breast-conserving surgery with or without radiation. For appropriately selected patients, differences in local treatment result in minor differences in breast cancer survival. For inappropriately selected patients, differences in local treatment significantly impact on recurrence and ultimately breast cancer survival. Mastectomy is the recommended treatment in patients with multicentric DCIS, (i.e., two or more separate quadrants of the breast) and those with extensive or diffuse malignant-appearing calcifications. Patients in whom negative margins of resection cannot be obtained after reasonable surgical attempts are advised to undergo mastectomy. Young women, (i.e., 40 years of age or younger) and those with multifocal DCIS have a significantly increased risk of ipsilateral breast tumor recurrence when treated with conservative surgery alone. Treatment options in these women include conservative surgery and radiation or mastectomy. Women with BRCA1– or BRCA2-linked DCIS may elect bilateral mastectomy given their increased risk of contralateral breast cancer and the increased risk of an ipsilateral breast tumor recurrence, which has been reported with invasive cancers treated with conservative surgery and radiation without tamoxifen or oophorectomy. For patients undergoing breast-conserving surgery, negative margins minimize the risk of an ipsilateral breast tumor recurrence, although the width of the negative margin that best achieves this goal remains controversial. Nuclear grade appears more important in predicting the risk of an ipsilateral breast tumor recurrence in patients undergoing conservative surgery alone. The presence of high nuclear grade or necrosis may prompt a recommendation for postoperative radiation. The addition of radiation to conservative surgery has not resulted in a significant increase in second non-breast cancer malignancies in the NSABP B-17 or B-24 trial, nor an increased risk in cardiovascular death in the EORTC or NSABP B-17 trials.288,290,293 A Cochrane review of four randomized trials of breast-conserving surgery with or without radiation in 3925 women reported no significant long-term toxicity from radiotherapy.330
Paget disease of the nipple has traditionally been treated by mastectomy. However, the demonstrated success of breast-conserving surgery in the treatment of ductal carcinoma in situ prompted interest in this treatment for Paget disease. There are no randomized trials comparing breast-conserving surgery to mastectomy for Paget disease and because the overall incidence of Paget disease is low, the reported series have small numbers of patients. Ipsilateral breast tumor recurrence rates following conservative surgery alone for Paget disease were reported by Polgar et al.331 in a series of 33 women with Paget disease, with or without an underlying ductal carcinoma in situ. With a median follow-up of 6 years, the crude local recurrence rate was 33% in this group of women. Sixty-one patients were treated with excision and radiation and the crude local recurrence rate was 7%. Bijker et al.332 reported a 5-year local recurrence rate of 5% in 61 patients registered in the European Organization for Research and Treatment of Cancer Study 10873. Surgery consisted of complete excision of the nipple-areolar complex with negative margins, followed by radiation to the entire breast, consisting of 5000 cGy in 25 fractions. The authors concluded that breast-conserving surgery with radiation was associated with a low ipsilateral breast tumor recurrence rate at 5 years in patients with Paget disease with limited extent of underlying DCIS. Complete excision of the nipple-areolar complex was recommended. Marshall et al.333 reported results of 38 patients with Paget disease having a palpable mass or mammographic density in a multi-institution study of surgery consisting of complete or partial excision of the nipple-areolar complex followed by postoperative radiation to the entire breast, consisting of 5000 CGy, with a majority of patients receiving an additional boost to the tumor bed. The median follow-up was 9.4 years and the crude ipsilateral breast tumor recurrence rate was 11%. The local control rate at 10 and 15 years was 87%. These studies suggest that selected patients with Paget disease may be candidates for breast-conserving surgery with radiation and that excision of the nipple-areolar complex is recommended with negative microscopic margins. The removal of all malignant-appearing calcifications should be documented on a postsurgery mammogram before the initiation of radiation.
Stage I-II Invasive Breast Cancer Treatment
Breast-Conserving Surgery and Radiation versus Mastectomy
Six prospective randomized trials have compared conservative surgery and radiation with mastectomy for the treatment of stage I-II breast cancer. The published results of these trials are presented in Table 58-11. The trials have demonstrated no significant difference in terms of local regional recurrence, distant metastases, or long-term survival. In particular, patients with histologically positive axillary nodes have not been found to have an improved survival when treated with mastectomy. Both the NSABP B-06 and Milan I trials demonstrated a somewhat more favorable outcome in patients with positive nodes treated with breast-conserving surgery and radiation, although the differences were not statistically significant.342,343
The Early Breast Cancer Trialists’ Collaborative Group (EBCTG)26 reported a meta-analysis of seven prospective randomized trials comparing mastectomy and axillary dissection versus breast-conserving surgery, axillary dissection, and radiation. For axillary node-negative patients, the 5-year actuarial risk of an isolated local recurrence in mastectomy patients was 5.3% compared with 8.6% in the breast-conserving surgery arm. For node-positive patients, the 5-year actuarial risk of an isolated local recurrence was 7.9% in the mastectomy patients compared with 4.7% in patients undergoing breast-conserving surgery. Breast cancer mortality at 15 years for the node-negative patients was 26% with mastectomy and 27.3% for breast-conserving surgery and radiation. For the node-positive patients, breast cancer mortality was 47.4% with mastectomy and 49.3% with breast-conserving surgery and radiation. These differences were not statistically significant. Overall survival of the node-negative patients with mastectomy was 65.6% compared with 64.5% with breast-conserving surgery and radiation. For the node-positive patients, overall survival at 15 years was 46.7% with mastectomy and 46.2% for breast-conserving surgery and radiation. Therefore, the equivalence of breast-conserving surgery with radiation to mastectomy in terms of local-regional recurrence and overall survival in appropriately selected patients with stage I-II breast cancer has been demonstrated by the randomized trials with results now out to 20 years.
Cosmesis
Cosmetic considerations include primary tumor size and location and the overall breast size or total body weight. In patients with very small breasts, complete excision of the primary tumor may result in an unacceptable cosmetic result. Patients with large pendulous breasts or obese patients are less likely to achieve a good-to-excellent cosmetic result because of an increased incidence of breast fibrosis and retraction. However, reconstruction following mastectomy in these patients may be more complex and may require reduction mammoplasty of the contralateral breast. Bilateral reduction mammoplasty may be considered before radiation to diminish breast size and facilitate the administration of treatment. Breast size may be less important with improved radiotherapy techniques (to be discussed later in the chapter) such as the use of higher energy photon beams with or without beam spoilers. Tumors in the subareolar region may require resection of the nipple or areola complex for complete excision. The subsequent appearance of the breast may be unacceptable for some patients; however, it is likely to be as good as or better than that of the reconstructed breast following mastectomy. Experience with conservative surgery and radiation in the aesthetically augmented breast is limited. The primary concern is cosmetic and is related to the development of significant capsular contracture. The rate of acceptable cosmesis ranges from 32% to 100% with an average of 63%.344 Capsular contracture has been reported more frequently for subglandular implants as opposed to submuscular implants. Submuscular implantation may not only decrease the risk of late capsular contracture, but it also enhances visibility on mammography.
Complications
Patients who may be at a significant risk for developing complications with radiation following conservative surgery include those with a pre-existing history of collagen-vascular disease, or a prior history of chest or mantle irradiation. It is generally accepted that scleroderma is an absolute contraindication to radiation345 due to the development of severe acute and late effects including marked fibrosis. Patients with rheumatoid arthritis have not been reported to have a significantly increased risk of acute or late complications when treated with radiation.346,347 The role of radiotherapy in patients with systemic or discoid lupus remains controversial. The data for the most part are limited. With three individual case studies348–350 and three case control studies346,350,351 and a single large retrospective study,347 some patients with lupus have been reported to have exaggerated acute and late responses to radiation while others have not. A pre-existing history of lupus may be a relative contraindication to radiation. Patients with mixed connective tissue disorders and multiple collagen-vascular diseases have been reported to have exaggerated reactions to radiation.352,353 Therefore, radiation should be avoided in patients with mixed connective tissue disorder, multiple collagen-vascular diseases, scleroderma, and probably Crest syndrome (calcinosis, Reynaud phenomenon, decreased esophageal motility, sclerodactyly, and telangiectasia) and lupus.
The role of conservative surgery and radiation for breast cancer in a patient who has received prior chest or mantle irradiation is controversial. Because of the potential for severe complications from reirradiation of the soft tissues and ribs and the possible development of long-term radiation-induced sarcoma, mastectomy is the preferred treatment. One of two patients treated at Stanford University with breast-conserving surgery and radiation after prior chest irradiation developed soft-tissue necrosis in the lateral breast and chest wall354,355 reported no significant acute or late effects in 12 women treated with excision and radiation, 10 to 29 years after prior radiation for the treatment of Hodgkin disease or non-Hodgkin lymphoma.
Risk of Ipsilateral Breast Tumor Recurrence
In the majority of prospective randomized trials comparing conservative surgery and radiation to mastectomy, the incidence of local-regional recurrence has been almost identical for the two treatments (see Table 58-11).
Low-Risk IBRT
Factors associated with a low-risk of ipsilateral breast tumor recurrence include tumor size and tumor location. In general, there is no increased risk of ipsilateral breast tumor recurrence in T2 tumors provided that an excision with negative margins has been performed.356,357 Similarly there is no increased risk of ipsilateral breast tumor recurrence with subareolar tumors treated conservatively.358 Sacrifice of the nipple areolar complex may not always be necessary in these patients if a complete excision can be performed with negative margins; however, a central lumpectomy with sacrifice of the nipple areolar complex is often preferable to mastectomy. Invasive lobular cancers do not appear to have an increased risk of ipsilateral breast tumor recurrence359,360 provided that the primary tumor can be excised with negative margins. Data for histologies such as medullary, colloid, or tubular carcinomas are limited but would suggest that these patients are candidates for conservative surgery and radiation.158,160,163,361,362 Patients with positive axillary nodes do not have an increased risk of breast recurrence when treated with conservative surgery and radiation and this may be in part due to the combination of adjuvant systemic therapy and radiation (Table 58-11).356 In contrast, for patients undergoing mastectomy without radiation, the number of positive nodes correlates with the incidence of local-regional recurrence.26 Treatment-related factors that are associated with a low risk of recurrence include the use of quadrantectomy and the addition of chemotherapy or tamoxifen.363–366 In the NSABP B-14 trial for node-negative estrogen receptor-positive patients, the addition of tamoxifen decreased the 10-year cumulative risk of an ipsilateral breast tumor recurrence from 11% to 3.6%.365 In the NSABP B-13 node-negative trial for estrogen receptor-negative patients, the addition of methotrexate and 5-fluorouracil decreased the 10-year cumulative incidence of ipsilateral breast tumor recurrence from 15.3% with no chemotherapy to 2.6% with chemotherapy.365
Intermediate-Risk IBRT
Factors associated with an intermediate risk of ipsilateral breast tumor recurrence following conservative surgery and radiation include young age, the presence of lobular carcinoma in situ in association with an invasive cancer, lymphovascular invasion, and histologic G3 tumors. Multiple retrospective series from North America and Europe have reported increased ipsilateral breast tumor recurrence rates in young women (35 to 40 years of age) when compared with older women.367–377 In these series, ages 35 to 40 years have been associated with ipsilateral breast tumor recurrence rates of 20% to 30% compared with 10% to 15% in women less than 35 to 40 years of age. Ipsilateral breast tumor recurrence rates related to age have been reported in two of the prospective randomized trials comparing conservative surgery and radiation to mastectomy. In the combined results of the EORTC and Danish Breast Cancer Group trials, women 35 years of age had a 35% ipsilateral breast tumor recurrence rate compared with 9% in women 36 to 40 years of age with a median follow-up of 9.8 years.341 In the NSABP B-06 trial, women 35 years of age experienced a 24% ipsilateral breast tumor recurrence rate compared with 7% in women more than 35 years of age with a median follow-up of 8.1 years.378 In a multivariate analysis of risk factors for ipsilateral breast tumor recurrence in 488 patients treated with conservative surgery and radiation in the NSABP B-06 trial, age less than 35 years was the only significant factor.378 The factors analyzed included patient age, tumor size, histology, histologic and nuclear grade, the presence or absence of an extensive intraductal component, lobular carcinoma in situ, lymphovascular invasion, cellular reaction, nodal status, and extracapsulary extension.
Multiple factors may contribute to the increased risk of ipsilateral breast tumor recurrence observed in young women. These factors include a greater prevalence of EIC-positive (extensive intraductal component) tumors, the presence of adverse histopathologic factors such as high nuclear and histologic grade, multicentricity, the presence of a major mononuclear cell reaction, the presence of lymphovascular invasion, and a higher probability of a significant residual tumor burden after excision, which is not controlled with moderate doses of radiation. The 10-year actuarial ipsilateral breast tumor recurrence rate has ranged from 25% to 54% in young women with positive resection margins compared with 3% to 38% with negative margins.122,136,375,379–383 Women with EIC-positive tumors and close or positive margins treated with conservative surgery and radiation have been reported to have ipsilateral breast tumor recurrence rates ranging from 7% to 66% compared with 0% to 16% with negative margins.115,118,384–388 Therefore, wider surgical excisions appear to diminish risk of an ipsilateral breast tumor recurrence in young women treated with conservative surgery and radiation. An additional treatment modification, which may decrease the risk of ipsilateral breast tumor recurrence in young women treated with conservative surgery and radiation, is the addition of a boost dose of radiation. In the EORTC randomized trial of boost versus no boost, women 40 years of age had a 10-year ipsilateral breast tumor recurrence rate of 23% when the boost was omitted compared with a 12% rate with the addition of the boost.389
Several studies have suggested that adjuvant systemic therapy will decrease the risk of an ipsilateral breast tumor recurrence in young women treated with breast-conserving surgery and radiation. The Early Breast Cancer Trialists’ Collaborative Group meta-analysis226 of adjuvant systemic therapy trials reported a 37% decrease in local-regional recurrence in women less than 50 years of age with chemotherapy (HR 0.63; 95% CI 0.52-0.76) and a 53% decrease in women of all ages with adjuvant hormone therapy (HR 0.47; 95% CI 0.38-0.59). In the EORTC trial, a single course of perioperative chemotherapy consisting of 5-fluorouracil, doxorubicin, and cyclophosphamide resulted in a decrease in the ipsilateral breast tumor recurrence rate from 31% to 15% in women 43 years of age.390 Buchholz et al.364 reported a decrease in the ipsilateral breast tumor recurrence rate in node-negative women 40 years of age, from 26% at 8 years to 10% with the addition of adjuvant chemotherapy. In a separate study from MD Andersen Cancer Center, Beadle et al.391 reported a decrease in local-regional recurrence rates from 27.9% to 13.5% (P = .04) with the addition of chemotherapy to breast-conserving surgery and radiation in 53 women 35 years of age. The addition of tamoxifen to conservative surgery and radiation in women 35 years of age resulted in a decrease in the ipsilateral breast tumor recurrence rate from 22% to 0% at 8 years in a series from Memorial Sloan-Kettering.368 However, the addition of adjuvant chemotherapy was not found to decrease ipsilateral breast tumor recurrence rates in women 40 years of age in a series reported by the Curie Institute.375 Freedman et al.118 suggested that adjuvant systemic therapy may merely delay the appearance of an ipsilateral breast tumor recurrence rather than preventing it. The median interval to an ipsilateral breast tumor recurrence in this series was 3.7 years without adjuvant therapy, 5 years for chemotherapy with or without tamoxifen, and 6.7 years for patients receiving tamoxifen. With longer follow-up, the addition of chemotherapy and/or tamoxifen did not appear to decrease the risk of an ipsilateral breast tumor recurrence in patients with close or positive resection margins. In the NSABP B-21 trial (Tla or Tlb node negative) of conservative surgery with or without radiation and/or tamoxifen, an initial report with a median follow-up was 7.2 years, noted an ipsilateral breast tumor recurrence rate of 9% in the patients treated with conservative surgery and radiation compared with 3% in those treated with tamoxifen and radiation.392 However, with longer follow-up the benefit of tamoxifen decreased. With a median follow-up of 11.2 years, the ipsilateral breast tumor recurrence rate was 11% in patients treated with radiation compared with 10% in those treated with radiation and tamoxifen.393 Singletary,394 in a review of 34 studies evaluating the outcome of conservative surgery and radiation related to surgical margin status, noted that as follow-up increased, ipsilateral breast tumor recurrence rates continued to increase in positive-margin patients. However, in patients with negative margins (>2 mm) increasing follow-up was associated with a decrease in the risk of ipsilateral breast tumor recurrence. A study from the Netherlands380 reported an ipsilateral breast tumor recurrence at 10 years of 65% in women 40 years of age with a positive margin for invasive cancer compared with 32% for positive margin for DCIS. In contrast, young women with negative margins had a 15%, 10-year ipsilateral breast tumor recurrence rate. It is unclear as to whether young women with negative resection margins and the absence of an extensive intraductal component, high histologic or nuclear grade, or lymphovascular invasion will continue to have an increased ipsilateral breast tumor recurrence rate when compared with their older counterparts.
Multiple genes have been associated with a hereditary predisposition to breast cancer with the BRCA1/2 genes being the most common. These account for 3% to 5% of all breast cancers and 10% of breast cancers in women less than 40 years old and 50% of women with a strong family history of breast cancer, ovarian cancer, or both. The majority of BRCA1 carriers have triple negative breast cancer (i.e., estrogen and progesterone receptor-negative and HER-2/neu negative).395 In contrast, approximately 25% of BRCA2 carriers have triple negative cancers, which appear to develop at a later age. Ninety-four percent of the triple negative cancers in BRCA1 carriers are high grade compared with 75% in the nontriple negative BRCA1 carriers. The role of breast conservation therapy in the treatment of the BRCA1/2-associated invasive breast cancer has been questioned. In a large, multi-institution study, Pierce et al.302 reported a 10-year actuarial ipsilateral breast tumor recurrence rate of 12.5% in 170 women with BRCA1 or BRCA2 mutations, compared with 8.6% in 299 women with sporadic breast cancer. However, the risk was 31% at 15 years in the mutation carriers who did not receive tamoxifen or undergo bilateral oophorectomy. The addition of oophorectomy decreased this risk to 18% and tamoxifen decreased it to 22% compared with 17% in the sporadic group. There were no increased complications related to radiation in the mutation carriers; however, there was an increased incidence of contralateral breast cancer, which for all patients was 39% at 15 years compared with 7% in the sporadic group. Metcalfe et al.301 reported no increase in contralateral breast cancer in BRCA1 or BRCA2 carriers who received radiation. However, Broeks et al.396 reported an increased frequency of mutation carriers with BRCA1-2, CHEK2, and ATM in 169 patients with contralateral breast cancer who received radiation when compared with 78 patients with contralateral breast cancer who did not receive radiation. This later study suggests that radiation may contribute to an increased risk of contralateral breast cancer above their baseline in these women. There is an ongoing European study to evaluate the risk of breast cancer from medical irradiation in these gene carriers.
There are conflicting data as to whether young women with early stage invasive breast cancer have an improved outcome when treated with mastectomy as compared with breast-conserving surgery. Arriagada et al.397 reported a 36%, 15-year local-regional failure in women 40 years of age treated with conservative surgery and radiation compared with a 12% risk in women treated with mastectomy. However, a series from the British Columbia Cancer Agency398 reported no increased risk of local-regional failure or decreased cause-specific survival in women age 20 to 39 treated with breast-conserving therapy or mastectomy. In a combined analysis of the EORTC and Danish Breast Cancer Group randomized trials of conservative surgery and radiation versus mastectomy, age 35 was associated with an increased risk of local failure and distant metastasis in women treated with conservative surgery and radiation when compared with mastectomy.341 For women age 36 to 40, the results were similar. Beadle et al.383,399 reported local-regional recurrence rates of 16% in women 35 years of age with T1-T2 tumors with one to three positive nodes treated with conservative surgery and radiation compared with 21% treated with mastectomy and 6% treated with mastectomy and radiation. For young women with stage II disease, mastectomy and radiation resulted in the lowest risk of local-regional recurrence (5.7% mastectomy and radiation, versus 22.8% mastectomy, versus 17.7% breast-conserving surgery and radiation, P = .02) and an improved 10-year overall survival. There were no significant differences in local-regional recurrence rates, distant metastasis, or overall survival when comparing mastectomy to breast-conserving surgery and radiation in these young women with stage I disease.
Histopathologic factors that have been associated with an intermediate risk of ipsilateral breast tumor recurrence include: high histologic or nuclear grade134–138,400,401 and the presence of lymphovascular invasion.115,140,402 Voogd et al.341 reported 10-year local failure rates of 15% for histologic G3 tumors compared with 7% for histologic G1-G2 when treated with conservative surgery and radiation. The presence of lymphovascular invasion was associated with a 15%, 10-year local recurrence rate compared with 8% in the absence of lymphovascular invasion.
The impact of the presence of lobular carcinoma in situ on ipsilateral breast tumor recurrence rates has varied. Three series403–405 have reported an increased risk of ipsilateral breast tumor recurrence in patients with lobular carcinoma in situ in association with an invasive cancer when treated with conservative surgery and radiation. Two series have found no increased risk.406,407 In the series from Fox Chase Cancer Center, the risk was greatest in women 50 years of age who did not receive tamoxifen.404
High-Risk IBTR
The primary factor that is associated with a high-risk of ipsilateral breast tumor recurrence following conservative surgery and radiation is the resection margin status. A positive margin is one in which either invasive cancer or ductal carcinoma in situ is present at the inked surface of the specimen. If the shaved margin technique is employed, the presence of tumor anywhere in the shaved margin is considered a positive margin. The impact of a positive resection margin on ipsilateral breast tumor recurrence rates has varied in the literature. In a review of 34 series published by Singletary,394 ipsilateral breast tumor recurrence rate for positive margin patients ranged from 0% to 29%. As previously noted, the risk is influenced by the length of follow-up, with series with longer follow-up reporting higher risks. The extent of margin positivity also correlates with ipsilateral breast tumor recurrence rates. DiBiase et al.408 reported that a single positive margin was associated with a 10-year ipsilateral breast tumor recurrence rate of 26% compared with 37% for two or more margins positive. Park et al.409 reported an 8-year ipsilateral breast tumor recurrence rate of 14% for patients with focal margin involvement defined as DCIS or invasive cancer of three low-power microscopic fields compared with 27% for patients with extensive margin involvement. Cowen et al.136 reported a 14% ipsilateral breast tumor recurrence rate for single margin positivity compared with 36% for multiple-involved margins in node-negative patients. Additional factors that influence outcome in positive margin patients include young age, the presence of an extensive intraductal component (EIC), invasive lobular cancer, and sequencing of chemotherapy and radiotherapy. As previously noted, young women with positive resection margins treated with conservative surgery and radiation have been reported to have ipsilateral breast tumor recurrence rates ranging from 25% to 54%.122,136,375,379–383 The presence of an extensive intraductal component with a positive resection margin has been associated with ipsilateral breast tumor recurrence rates ranging from 25% to 66%.118,122,384–388 Bouvet et al.410 reported ipsilateral breast tumor recurrence rates of 33% in women with invasive lobular cancers and positive or close (≤1 mm) resection margins treated with conservative surgery and radiation. In the randomized trial from Harvard of chemotherapy first or radiation first in patients treated with conservative surgery and radiation, patients with close margins of resection experienced a 32% ipsilateral breast tumor recurrence rate with chemotherapy first compared with 4% with radiation first. In contrast, for the 51 patients with positive margins, the ipsilateral breast tumor recurrence rate was 20% for those treated with radiation first compared with 23% for chemotherapy first.411,412
The Early Breast Cancer Trialists’ Collaborative Group meta-analysis226 of adjuvant systemic therapy trials reported a 37% decrease in local-regional recurrence in women less than 50 years of age with chemotherapy (HR 0.63; 95% CI 0.52–0.76) and a 53% decrease in women of all ages with adjuvant hormone therapy (HR 0.47; 95% CI 0.38-0.59). Retrospective studies and prospective studies have demonstrated a decrease in the risk of ipsilateral breast tumor recurrence with the addition of tamoxifen or chemotherapy after breast-conserving surgery and radiation.* However, it is uncertain as to whether adjuvant tamoxifen or chemotherapy decreases or delays recurrence in patients with close or positive margins. In the study from Fox Chase Cancer Center and the review by Singletary,118,394 adjuvant systemic therapy appears to delay the appearance of an ipsilateral breast tumor recurrence rather than preventing it. Three studies have also demonstrated that increasing the radiation dose does not decrease the ipsilateral breast tumor recurrence rate in patients with close or positive surgical margins384,417,418 The EORTC initiated a phase III randomized trial evaluating a higher boost dose of radiation at the excision site (26 Gy) for patients with positive resection margins.419 The trial did not meet its accrual goal of the 251 patients with microscopically incomplete tumor excision; 126 were randomized to a boost of 10 Gy and 125 to 26 Gy. The median follow-up was 11.1 years. The 10-year cumulative incidence of an IBTR was 17.5% in the lower dose boost group compared with 10.8% in the higher dose boost group (difference not statistically significant). Moderate or severe fibrosis was more common in the high-dose group. Therefore, patients with close or positive resection margins who are young, have an extensive intraductal component, invasive lobular cancer, or multiple margin positivity have a significant risk of an ipsilateral breast tumor recurrence that does not appear to be modified by the addition of adjuvant systemic therapy or increasing the dose of radiation.
The width of the negative margin that best minimizes the risk of an ipsilateral breast tumor recurrence is controversial. While some series have suggested that a 1-mm margin is adequate,409 others have suggested that it should be more than 2 mm118,385,408,420 For the most part, margins more than 2 mm are recommended, especially in women with high-risk features, such as young age, an extensive intraductal component, and invasive lobular cancer. The location of the positive margin may also influence outcome. A positive deep margin may be of less concern if the excision has extended to the chest wall, and similarly, a positive anterior margin may not be of concern if the excision has extended anteriorly to the skin surface.421
The presence of gross multicentric disease defined as clinical and/or mammographic evidence of more than one malignant area in separate quadrants of the breast, has been associated with a high risk of ipsilateral breast tumor recurrence in patients undergoing conservative surgery and radiation. The risk of ipsilateral breast tumor recurrence in these patients has ranged from 36% to 40%.422–424 The increased risk of breast recurrence in these patients appears to be related to a significant residual tumor burden. This has been confirmed in pathologic studies from the University of Pennsylvania.425 In 57 patients with gross multicentric disease who underwent mastectomy, a residual tumor was identified in 46% in two or more quadrants of the breast. Similar findings were noted for patients with diffuse microcalcifications. Of the 21 patients who underwent mastectomy, 43% had a residual tumor in more than one quadrant of the breast. The role of conservative surgery and radiation in the treatment of patients with gross multifocal disease (i.e., more than one clinical area of malignancy in a single quadrant) is controversial. It has been suggested that these patients may be candidates for breast-conserving therapy, provided that negative margins of resection can be achieved with reasonable surgical excision and acceptable cosmesis.426 Microscopic multifocality is not a contraindication to breast-conserving surgery and radiation.
Mammographic breast density has been correlated with ipsilateral breast tumor recurrence in patients in the NSABP B-17 trial of conservative surgery with or without radiation for ductal carcinoma in situ304; 15.6% of the women less than 50 years of age were considered to have highly dense breasts upon mammography (i.e., 75% compared with 2.4% of the women aged 50 to 59 and 2.2% of the women 60). The ipsilateral breast tumor recurrence rate was 40% in women with breast density of 75%, with a median follow-up of 11 years. It should be noted that only 392 of the 818 patients entered into the study were reviewed for mammographic density and 52% of these patients received radiation. Park et al.427 reported, in a small nested case-control study of 136 women with invasive cancer treated with breast-conserving surgery and radiation, a correlation between high mammographic breast density (>75%) and local-regional recurrence. With a median follow-up of 7.7 years, the local-regional recurrence was 27% in the 15 women with breast density greater than 75% compared with 12% in the 121 women with lower breast densities. However, the strongest correlation was with obesity defined as body mass index of 30. The local-regional recurrence rate was 54% in the obese women compared with 9% in those with body mass index less than 30. None of the obese women had a high breast density. The authors postulated that the breast microenvironment may influence the likelihood of a local-regional failure. Anderson et al.365 in an analysis of ipsilateral breast tumor recurrences in 3799 axillary node-negative women treated with breast-conserving surgery and radiation on 5 NSABP adjuvant therapy protocols noted no significant association with body mass index and IBTR.
IBTR and Molecular Subtypes and Genomic Profiles
Genomic profiling of breast cancers has been used to predict relapse (distant and local-regional) in addition to the standard clinical and pathologic factors. The 21-gene recurrence score (Oncotype DX, Genomics Health Inc., Redwood City, Calif.) includes 16 cancer-related genes (proliferation, estrogen, invasion, HER-2, GSTM 1, CD68, and BAG I and 5 reference genes). The score has been correlated with the risk of distant metastasis in node-negative, ER-positive women treated with tamoxifen in the NSABP trials.221 More recently the score has been correlated with ipsilateral breast tumor recurrence in these women treated with conservative surgery and radiation. Ipsilateral breast tumor recurrence rates more than 25% have been reported in women less than 50 years of age with intermediate to high-risk score, (i.e., 18%).428 However, Nuyten et al.429 reported no correlation with the 70-gene prognosis profile (proliferation, invasion, metastases, stromal integrity, angiogenesis) and ipsilateral breast tumor recurrence rates in 161 women less than 53 years of age with invasive cancers treated with conservative surgery and radiation. The median follow-up was 7.7 years. A wound response signature (65 activated wound response and 96 quiescent wound response) correlated significantly with ipsilateral breast tumor recurrence. Patients with a high-risk wound response signature had a 29% ipsilateral breast tumor recurrence at 10 years compared with a 5% risk for a low-risk signature. In a subsequent study,430 the investigators compared gene expression profiles of 56 primary invasive cancer patients, who developed an IBTR within 10 years after breast-conserving surgery and radiation, to the profiles of 109 tumors from patients without a recurrence. Twenty-seven percent of the cases had positive margins compared with 17% of the controls. All patients were less than 51 years of age. Gene expression profiles examined included the 70-gene and the 76-gene profiles, wound response, hypoxia, 52-gene radioresistance, 45-gene proliferation, and the Sweden 81 gene. Young age was the only significant factor predicting IBTR in multivariate analysis. There was an association between IBTR and the 45-gene proliferation profile, which was not captured by histologic grading. Further studies are required to assess the role of gene expression signatures in predicting ipsilateral breast tumor recurrence.
Recent interest has also focused on breast cancer intrinsic subtypes identified by immuno-histochemistry with clinical approximation by receptor status. Triple negative breast cancers have not been found to have a significantly increased risk of ipsilateral breast tumor recurrence when treated with conservative surgery and radiation in three of five published studies.401,431–434 However, triple negative breast cancers have a shorter interval to local failure.433 HER-2+, estrogen receptor-negative patients have been noted to have an increased risk of local-regional failure in some series when treated with conservative surgery and radiation432 or postmastectomy radiation without trastuzumab.435 In an analysis of the Danish Breast Cancer Group’s studies 82b and c, HER-2/neu positive, estrogen receptor-and progesterone receptor-negative patients had a 15-year local-regional failure rate of 21% with postmastectomy radiation compared with 3% in ER+ HER-2 negative or ER+, HER-2-positive patients. The 15-year local-regional failure rate was 15% for the triple negative patients. In contrast to these series, Harris et al.436 reported no correlation with HER-2 status and local-regional recurrence in women treated with conservative surgery and radiation. It should be noted that none of these patients received trastuzumab, and local-regional recurrence has not been correlated with HER-2 status in women treated with conservative surgery and radiation. Buchholz et al.437 reported a similar lack of correlation in patients treated with mastectomy and radiation.
Integration of Multiple Factors Associated With IBTR
Of the multiple factors that may influence ipsilateral breast tumor recurrence rates, age appears to be the most important. In a recursive partitioning analysis of ipsilateral breast tumor recurrence following conservative surgery and radiation for stage I-II breast cancer, Freedman et al.122 identified the first category as age, 55 versus more than 55. For women more than 55, ipsilateral breast tumor recurrence was influenced by the addition of tamoxifen. For women less than 55 years of age, EIC was the most important factor. For EIC-negative patients, age 35 and margin status were the most important factors. For age 36-55, estrogen receptor status and the addition of tamoxifen were most important. This analysis noted that margin status was less important in women more than 55 years of age and it is possible that an older woman with a focally positive margin and the absence of an extensive intraductal component or invasive lobular cancer may not require re-excision before the initiation of radiation.
IBTR! is a web-based tool developed by investigators at Tufts University to estimate the risk of an ipsilateral beast tumor recurrence following breast-conserving surgery with or without radiation.438 The nomogram includes seven prognostic factors (age, tumor size, tumor grade, lymphovascular invasion, margin status, and the use of chemotherapy and/or hormonal therapy) and information to calculate the risk was obtained from nine prospective randomized trials of breast-conserving surgery with or without radiation, the meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group, and selected single institution retrospective studies. It should be noted that data for certain factors were not available in the randomized trials. Databases from the Massachusetts General Hospital (MGH) and British Columbia Cancer Agency have been used to validate the nomogram primarily in patients receiving radiation.439,440 IBTR! was relatively accurate in predicting ipsilateral breast tumor recurrence rates in women with favorable factors associated with a low risk. However, in both data sets, the model overestimated the risk in women with unfavorable factors, (i.e., young age, positive margins). Its clinical usefulness will require further evaluation.