44 Cancer of the Bladder
Epidemiology and Etiology
Bladder cancer is estimated to have an annual incidence in the United States of 68,810 cases, accounting for 5% of all newly diagnosed cancers. Approximately 14,100 people per year will die of this disease, accounting for 2.5% of all cancer-related mortality in the United States and 3% of all cancer deaths in men.1 The incidence of bladder cancer varies considerably among countries; the highest incidence rates are in Western countries. At diagnosis, approximately 75% of patients have localized disease only, 20% have regional disease, and 5% present with distant metastases. Two thirds of all bladder cancer diagnoses occur among persons 65 years of age or older. The male/female ratio is 3 : 1. In the United States, the risk for white men is approximately twice that for African-American or Hispanic men.2 From the early 1970s to 2004 the incidence of this cancer rose slightly while mortality fell. This phenomenon is likely attributable to the increased detection of localized lesions and improved therapies.3
Cigarette smoking is the single most significant known cause of bladder cancer, as demonstrated by both cohort and case-control studies.3–8 The risk of bladder cancer appears to be related to the number of pack-years and, as such, an individual’s risk of bladder cancer declines if smoking is ceased.9 It is estimated that 50% of bladder cancers diagnosed in the United States can be linked to a smoking history.10 In a case-controlled study of more than 1500 patients with bladder cancer and a large control group matched for age, sex, and race, there was a statistically higher risk of bladder cancer observed in female smokers compared with male smokers with similar smoking habits.11 In addition, younger age at initiation of tobacco use correlates to a higher risk of developing bladder cancer.12 It appears that the relationship of smoking to bladder cancer development stems from exposure to selected arylamines. Studies have demonstrated that smokers have higher levels of 3- and 4-aminobiphenyl hemoglobin adducts, which are increased 10-fold and 3-fold, respectively, compared with nonsmokers.11 Cytogenetic analyses indicate a direct relationship between smoking and suppressor gene p53 nuclear overexpression.13
Exposure to industrial chemicals and high rates of bladder cancer in specific occupations have been a major focus of epidemiologic investigation. Individuals in contact with chemicals necessary for the production of dyes, rubber, plastics, and synthetic materials are at increased risk for development of bladder cancer.3,13–17 Dye workers may be particularly at risk owing to exposure to aromatic amines, specifically 2-naphthylamine and benzidine, which have been shown to be bladder carcinogens.18 One study demonstrated an additive effect for bladder cancer development among smokers who use hair dyes.19 There have been recent changes in hair dye formulations that appear to have decreased this risk.20 Two recent meta-analyses did not demonstrate a link to personal hair dye users and the subsequent development of bladder cancer.21,22 Rubber workers are also exposed to 2-naphthylamine, and benzidine is found in paints.3 Dry cleaners may have an increased risk of bladder cancer from exposure to organic solvents, including perchloroethylene.23
Schistosoma haematobium is presumed to be the primary cause of endemic squamous cell carcinoma common in Egypt.24 This form of bladder cancer is recognized to occur in patients 10 to 20 years younger than patients with typical cases of transitional cell carcinoma diagnosed in the United States.25 It has been hypothesized that the parasite may induce an inflammatory response that includes the release of reactive oxygen radicals, which results in genetic damage, leading to carcinogenesis.26 Patients with chronic urinary tract infections or with the need to frequently self-catheterize after spinal cord injury are also predisposed to squamous cell carcinomas.27–29 Mechanisms proposed include obstructive uropathy with recurrent bacterial superinfections leading to urine acidification, nitrosamine production, and bacteria-induced oxidative damage.30
Patients with a history of upper tract urothelial cancer are at high risk for the subsequent development of synchronous or metachronous cancers of the urinary bladder as well as contralateral upper urinary tract malignancies. The incidence of developing a bladder cancer is 20% to 50% regardless of the treatment modality and typically occurs within the first 2 years after treatment of the upper tract; however, lifelong surveillance is necessary in these patients.31
The role of diet in the predisposition for, or reduction of risk of, developing bladder cancer remains unclear. There have been conflicting data about the role of coffee consumption and an association with bladder cancer development. A summary of 35 case-control studies of the causal role of coffee showed no evidence of a relationship between the beverage and the development of bladder cancer.32,33 On the other hand, a recent meta-analysis for urinary tract cancers estimated a 20% increase of bladder cancers among coffee drinkers irrespective of dose or duration of consumption.34 It is now believed that there is no relationship between artificial sweeteners and the development of bladder cancer.35 Some studies have shown a minimal protective effect with vitamin A, fruits, and vegetables.36,37 People who drink fresh well water were found to have lower rates of bladder cancer than those drinking chlorinated municipal water.38 This may be related to trihalomethanes, by-products of chlorine or bromine disinfection processes, and currently most governments place restrictions on these chemicals. A higher incidence of bladder cancer has been noted in agricultural areas where fertilizers seep into water supplies, raising the level of nitrates in drinking water.39
Iatrogenic causes of bladder cancer include radiation exposure, chronic phenacetin usage, and administration of cyclophosphamide.3 Several reports have noted an increase in bladder cancer in patients who received pelvic radiation for cervical, prostate, and testicular cancers40–42; however, other studies have not established a clear causative relationship.43,44 Patients with rheumatoid arthritis were shown to have an increased rate of bladder cancer for as long as 17 years after taking oral cyclophosphamide.45
A genetic predisposition to bladder cancer in certain families has been described. It is likely that there is an interaction between hereditary predisposition of oncogenes and tumor suppressor genes that are amplified or deleted because of environmental stimuli.3 Acetylation of aromatic amines, an important mechanism of detoxification of these compounds, may be interrupted in individuals with mutations of the N-acetyltransferase genes, particularly NAT2. These “slow acetylators” have been reported to have an increased risk of bladder cancer, particularly in individuals exposed to aromatic amines and with a higher relative risk compared with “rapid acetylators” (i.e., individuals without NAT2 mutations).46 The interplay between environment and genetic predisposition is also exemplified by the reduction of glutathione S-transferase activity with the loss of the GSTM1 gene. The glutathione enzyme appears to play a role in modulating the carcinogenic effects of tobacco smoke.47,48
Cytogenetics
Scientific inquiry has concentrated on the molecular biology and cytogenetics of bladder cancer, with the expectation that insight into the natural history of the disease will translate into clinical advances. An attempt is under way to define independent prognostic factors that predict which of two similar superficial lesions is destined to relapse as muscle-invasive disease. Flow cytometry has shown that lesions with an increased chromosome number and aneuploidy were associated with higher-grade tumors destined to be invasive.49–51 One study showed that the percentages of hyperdiploid cells for chromosomes 7, 11, and 17 were predictive of grade and stage.52 Other studies have shown that FGFR3 mutation is strongly associated with low tumor grade and low risk of progression to more advanced bladder cancer.53 A model of an orderly progression of alteration in genetic material involving oncogenes and tumor suppressor genes is being elucidated.54
An early occurrence that may be critical in distinguishing Tis and T1 from normal bladder epithelium and the less aggressive papillary tumors (Ta) appears to involve loss of a tumor suppressor gene on chromosome 9.54–57 Progression to muscle-invasive disease appears to require loss of the tumor suppressor gene p53 on chromosome 17, shown to be present in 65% of Tis compared with 3% of Ta tumors.58 Other intermediate events in the progression to invasive disease likely involve deletions in 5q, 3p, and 17p, which are not present in Ta lesions.59,60 No single oncogene has been found in the majority of tumors, although c-erb B2, H-ras, c-src, and c-jun have been expressed and likely play a role in the intermediate progression to muscle-invasive lesions.61–63 Late events may involve loss of suppressor genes on 11p, 6q, 13q, and 18q.48,54,64 In particular, the loss of the retinoblastoma gene on chromosome 13 likely has a role in bladder cancer similar to its role in other malignancies.65,66 Investigators have also suggested additional loci of tumor-suppressor genes, including 4p, 8p, 14q, and 12q.67,68 Others have recently demonstrated a strong association with 6p+ and high tumor cell proliferation rate, which was noted to be an independent variable in addition to grade and tumor stage.69
A prognostic factor for transitional cell carcinoma confined to the bladder independent of grade, stage, and lymph node status is the accumulation of the p53 protein in the nucleus. With mutation of the wild-type p53, the protein product has a longer half-life and can be detected by immunohistochemistry.70 After radical cystectomy, patients with stages pT1, pT2, and pT3a who had p53 detected by immunohistochemistry had a shorter relapse-free and overall survival.71 Similarly, an analysis of p53 nuclear overexpression in 33 patients with carcinoma in situ (CIS) showed disease progression in 13 of 15 p53-positive patients compared with 3 of 18 p53-negative patients.72 The potential clinical implications of using the p53 status as a marker are far reaching and include the selection of lower-risk patients for bladder conservation and patients with early-stage disease who may benefit from adjuvant radiotherapy or chemotherapy.73–77
Anatomy
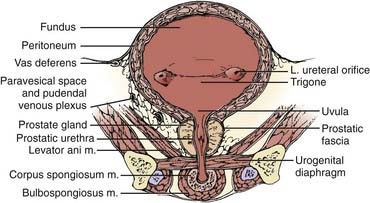
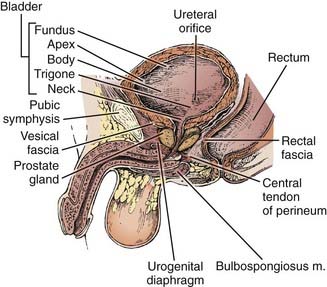
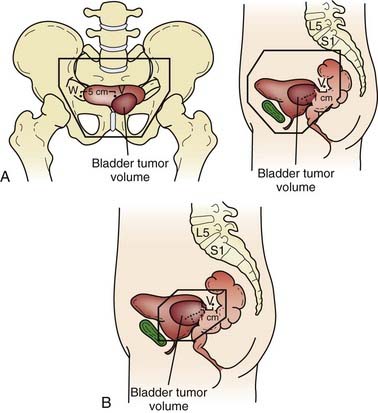
Both for the radiation oncologist and for the surgeon, the relationship between the bladder and neighboring structures is critical in treatment planning. The bladder functions as a muscular reservoir and is anatomically divided into a fundus, apex, body, trigone, and neck. Empty, the bladder is confined to the true pelvis. As the organ fills, it expands anterosuperiorly into the abdominal cavity. The fundus or base faces posteriorly and in the male is separated from the rectum superiorly by the rectovesical pouch and inferiorly by the seminal vesicles (Fig. 44-1 and Fig. 44-2). In the female, the bladder faces the uterus and the anterior vaginal wall. The neck is located 3 to 4 cm behind the symphysis pubis. This lowest and most fixed part of the bladder is pierced by the urethra and in the male approximates the prostate. A remnant of the median umbilical ligament called the urachus links the apex of the bladder (which lies posterior to the cranial section of the symphysis pubis) to the umbilicus. The superior surface of the bladder is covered by peritoneum and may approximate the sigmoid colon or ileum and the uterus in the female. Fascia forms the pubovesical ligaments that suspend the bladder between the pubic bones, anterior abdominal wall, and pelvic side walls. An empty bladder is characterized by a mucosa with folds except for a smooth triangular region called the trigone. The superior border of the trigone is characterized by its appearance as a pale band on cystoscopy and is called the interureteral crest. This band separates the ureteral openings. At the trigonal apex is the internal urethral orifice completing the triangle.78
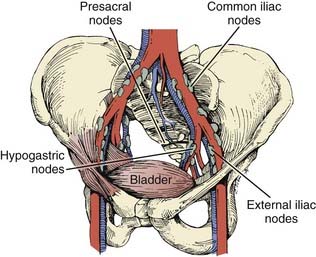
The arterial supply to the bladder arises from the anterior division of the hypogastric artery. Branches from the obturator and inferior gluteal arteries contribute to the rich vascular supply. In the female, additional vessels include the uterine and vaginal arteries. An important venous plexus exists between the bladder wall and the adventitial layer that drains into the hypogastric veins.78 Lymphatics from the mucosa and muscular layer drain to the surface of the bladder and form three collecting ducts at the trigone, anterior, and posterior walls. The trigone collecting duct drains into the external iliac nodes (Fig. 44-3). Hypogastric and common iliac lymph nodes are the destination of lymph collected in the anterior and posterior ducts. Some of the lymph also flows through the route of the anterior bladder wall collecting ducts to external iliac nodes.79
Innervation of the bladder can be divided into a parasympathetic nerve supply arising from S2-4 and a sympathetic supply that originates mainly in T11-12 and L1-2. Sympathetic nerves are found primarily in the trigone, and in the male these nerves stimulate closure of the bladder neck sphincter to prevent reflux ejaculation. Parasympathetic nerves play a large role in detrusor muscle contraction. A small group of autonomic nerves can be found scattered in the bladder wall. The adult male bladder has a normal capacity of 120 to 320 ml, with pain due to stretch sensations mediated by parasympathetic nerve fibers when distention beyond 500 ml occurs.78,80
Several anatomic variations are important with respect to a predilection to develop bladder cancer. One such variation is the persistence of a patent urachal remnant. The urachus is an embryologic canal connecting the urinary bladder of the fetus with the allantois, a structure that contributes to the formation of the umbilical cord. The lumen of the urachus is normally obliterated during embryonic development, transforming the urachus into a solid cord; however, in some children this closure fails to occur and remnants can take the form of a cyst, sinus, diverticulum, or patent urachus.81 Urachal adenocarcinoma can arise in these retained remnants. The location of these tumors are typically midline, either anteriorly or in the dome, and although often discrete, can form a large mass on the anterior abdominal wall. Urachal adenocarcimona is rare and accounts for approximately 0.35% to 0.7% of all bladder cancers.82 Extroversion of the bladder is one of the most common congenital bladder anomalies leading to a portion of the bladder, typically the mucosal surface of the fundus, protruding from the abdominal wall surface. This defect is also associated with epispadias and pelvic bone development abnormalities, and predisposes patients to bladder cancers of variable histologic anatomies.83 Adults with a history of exstrophy have a 694-fold increase in the risk of bladder cancer by the age of 40 years. Etiologic factors remain unclear, but may be related to resconstructive techniques.84 Bladder tumors are often multifocal, reflecting a “field cancerization” defect. Of the tumors that have a subsite listed at diagnosis, the majority occur on the bladder walls. The lateral walls account for 40% of malignancies, with posterior involvement of 11% and anterior involvement of 3%. As many as 17% of tumors are known to arise adjacent to the ureteral orifice, whereas the trigone, dome, and neck account for 13%, 9%, and 7%, respectively, of sites from which the cancers arise.3
Pathologic Findings
Transitional cell carcinomas account for approximately 93% of all bladder cancers in the United States.3 Pattern of tumor growth can be either papillary or flat. Papillary lesions can be either benign (i.e., inverted papilloma) or malignant. Flat lesions can be either dysplasia, CIS, or invasive cancer. In 1998, the World Heath Organization and the International Society of Urologic Pathologists published a consensus classification for urothelial malignancies that has been accepted as the standard classification schema.85 According to this schema, urothelial malignancies are classified as either low- or high-grade depending on the degree of anaplasia and architectural features. The vast majority of invasive cancers are high-grade. Noninvasive lesions are either flat or papillary.
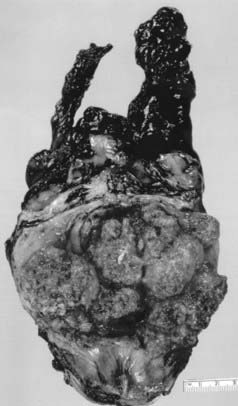
CIS on cystoscopy often appears as multiple, flat, erythematous patches without a gross structural component. Histologic examination reveals an increase in the number of cell layers, lack of cohesion between cells that results in shedding into the urine, disordered architecture, and an increased number of mitotic figures. The basement membrane confines the tumor to the mucosa. CIS associated with an invasive tumor has been associated with a worse prognosis. Papillary lesions have exophytic stalks protruding into the lumen of the bladder. Invasive lesions show involvement of the muscularis propria layer and are often palpable on examination.86,87 The predominant histologic findings associated with invasive carcinomas are transitional cell or urothelial carcinomas. Transitional cell carcinomas often contain squamous or glandular components and are classified as transitional tumors with a natural history similar to those of pure histology. Immunohistochemical analysis of microvessel density has been shown to be an independent prognostic indicator demonstrating a relationship between tumor angiogenesis, nodal metastasis, and survival.88,89
Pure squamous cell carcinomas comprise 5% to 8% of primary bladder malignancies in the United States and Europe; however, in Egypt and other areas endemic with Schistosoma haematobium, squamous cell tumors are the predominant histologic finding. Most squamous cell carcinomas are bulky, polypoid, solid masses and often present at an advanced stage. Necrotic material and keratin debris on the surface is typical. Pure adenocarcinomas account for 1% to 2% of bladder tumors. Most often, these tumors are derived from the urothelium of the bladder (“nonurachal adenocarcinoma”) and less frequently from a urachal remnant (“urachal adenocarcinoma”). They predominantly arise in the base of the bladder or trigone, but may arise at any location.87,90 A variety of rare histologic findings account for less than 2% of all primary bladder cancers. Extrapulmonary small cell carcinoma can occasionally originate in the bladder and, because of its aggressive and high metastatic potential, it is often treated with chemotherapy generally used for small cell carcinomas of the lung in addition to local therapy.91 These patients may be younger (median 40-60 years); however, similar to other histologic findings, small cell carcinoma of the bladder is associated with smoking.92 Because of the rarity of this finding, there are no prospective trials designed to define the best management of these patients. Poor long-term survival has been seen with single-modality therapy; therefore, combined-modality regimens are typically used.92 Rhabdomyosarcoma of the bladder occurs in children but rarely presents in adults.93 Other rare histologic findings include lymphoma, leiomyosarcoma, melanoma, and primary bladder pheochromocytoma.94–97 In addition, adenocarcinomas can metastasize to the bladder from the colon, rectum, endometrium, prostate, ovary, breast, and lung.98
Clinical Presentation and Routes of Spread
Gross or microscopic hematuria is a presenting sign in 75% of patients with bladder cancer. Approximately 25% of bladder cancer patients present with symptoms of bladder irritation (i.e., urinary frequency, urgency, dysuria), especially patients with CIS. Typically, symptoms are intermittent and diagnosis is often delayed as these may be similar to benign disorders such as cystitis, prostatitis, or bladder stones. Advanced tumors may present as pelvic pain, ureteral obstruction, hydronephrosis, or rectal obstruction.99,100
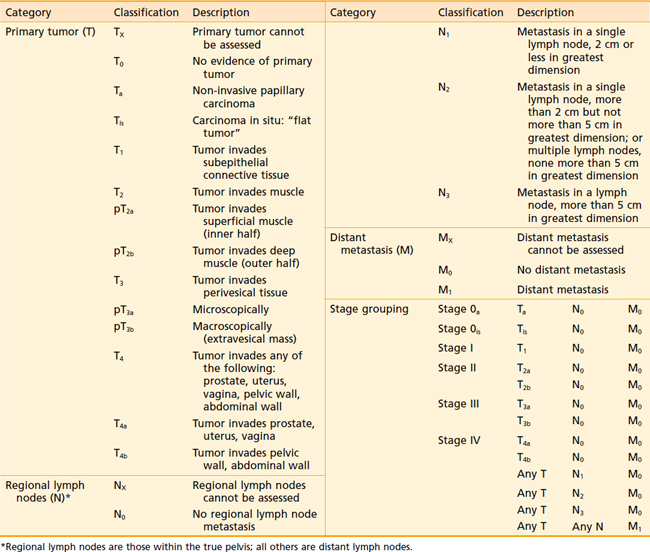
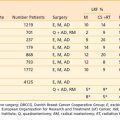
The regional lymph nodes at risk as defined by the current American Joint Committee on Cancer (AJCC) 2002 staging system are those in the true pelvis below the bifurcation of the common iliac arteries. These lymph nodes include the hypogastric, obturator, internal and external iliac, perivesical, pelvic, sacral, and presacral chains. Staging depends on the size and number of involved nodes, but not on whether they are unilateral or ipsilateral (Table 44-1). Spread to the common iliac nodes is considered a manifestation of distant metastases and not regional disease.101 Recent surgical series, however, have demonstrated long-term disease control in patients undergoing cystectomy with positive lymph nodes from extended lymph node dissections including the periaortic, common iliac, and presacral chains (see Surgical Treatment of Muscle-Invasive Bladder Cancer).102,103 The extent of nodal dissection currently remains controversial and the staging system has not yet been altered. Skinner and colleagues104 correlated the depth of bladder wall invasion to involvement of lymph nodes. In their study, 5% of patients with pT1, 30% with pT2, 31% with pT3a, 64% with pT3b, and 50% with pT4 disease had nodal metastases. The most common sites of distant metastatic disease are the lung, bone, and liver.
Diagnostic and Staging Studies
The initial formulation of an organized staging system reproducible in predicting survival dates back to the work of Jewett and Strong in the 1940s. Primary bladder tumors were classified as stage A when involvement was limited to the submucosa, as stage B when tumor penetrated into the muscularis, and as stage C when there was evidence of perivesical fat involvement. Stage A tumors had no evidence of metastases compared with 13% of stage B tumors and 74% of stage C tumors. In 1951, Jewett proposed a division of stage B into B1 and B2 to correspond with superficial and deep muscle penetration. The following year, Marshall added a stage 0 to include tumors not infiltrating the lamina propria and stage D for metastatic disease.105
The current tumor-node-metastasis (TNM) staging system standardized by the AJCC and the TNM Committee of the International Union Against Cancer continues to reflect the importance of depth of bladder wall invasion. The T stands for depth of tumor in relation to the mucosa, muscularis, and the perivesical fat. Regional nodes include those of the true pelvis, whereas M disease involves nodes above the bifurcation of the common iliac arteries or distant visceral metastasis.101
The current TNM staging system (see Table 44-1) recognizes that Ta papillary growths convey a significantly better prognosis than Tis (CIS) or T1 lesions. Also recognized is that either microscopic (T3a) or gross involvement (T3b) of perivesical fat is more important than the distinction between superficial (T2a) or deep muscle invasion (T2b). Stage T4 includes locally advanced tumors that invade the prostate, uterus, or vagina (T4a) or directly adhere to the pelvic or abdominal wall (T4b).101
The presence of gross hematuria in a patient older than 40 should raise suspicion for urothelial carcinoma until proven otherwise, particularly in a patient with known risk factors. Approximately 20% of all patients presenting with gross hematuria and 5% to 10% of all patients with microscopic hematuria will ultimately be diagnosed with bladder cancer.106,107 A history and physical examination should be followed by a urinalysis with urine cytologic study to detect the presence of suspicous cells. A cystoscopic examination includes documentation on a bladder diagram of tumor location, number of lesions, pattern of growth, and tumor size. A bimanual examination should be performed before and after endoscopic resection and is an important part of clinical staging. A mobile bladder mass indicates a T3 tumor, whereas a fixed mass suggests T4 disease. A transurethral resection of a bladder tumor (TURBT) is diagnostic and often therapeutic in superficial lesions. Muscle must be seen by the pathologist in a biopsy or transurethral resection for it to be an adequate indicator of prognosis. Biopsy specimens of surrounding and apparently unaffected areas, including the prostatic urethra, are taken because CIS may be visibly indistinguishable from normal mucosa. Persistence of a palpable mass on examination under anesthesia after TURBT identifies a poor prognostic group, usually with T3b disease.108 Patients with muscle-invasive disease require a screening workup for metastatic disease that includes chest imaging by either computed tomography (CT) or plain films as well as pelvic imaging to identify nodal involvement using CT or magnetic resonance imaging (MRI) of the pelvis. Imaging should include the abdomen if there is pelvic nodal disease or abnormal results of liver biochemistry studies. The upper urinary tract should be imaged with intravenous urography or contrast CT urogram to rule out synchronous upper tract lesions. In patients with invasive or locally advanced disease or in patients with elevation of the serum alkaline phosphatase level or bone pain, a bone scan should be performed to rule out metastases.108,109
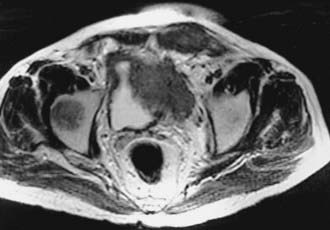
Imaging with CT or MRI has an important role in defining the extent of disease for locally advanced bladder lesions. Mural and mucosal lesions are distinguishable from perivesical fat and urine, allowing visualization of papillary and extravesical lesions (Fig. 44-4). Important diagnostic information that can be demonstrated with CT is the presence of gross perivesical extension or lymphadenopathy.110 Understaging of local disease, however, remains an important problem with clinical staging evaluations. Although not always as available as CT, MRI may be more accurate in determining the T stage of bladder tumors.111 MRI has many advantages over other modalities for detecting and staging bladder neoplasms because of its intrinsic high soft tissue contrast, direct multiplanar imaging capabilities, and the availability of a non-nephrotoxic, renally excreted contrast agent. Tachibana and coworkers112 correlated imaging with histologic staging for 57 bladder tumors. The sensitivity and specificity for differentiating superficial and invasive tumor were 96% and 83% for gadolinium-enhanced MRI, 96% and 58% for CT, and 88% and 67% for transurethral ultrasonography. The contrast between tumor and fat is critical for evaluating locally advanced lesions and is best detected on T1-weighted images. It is important to recognize in the post-treatment management of the patient that changes in signal intensity of normal bladder wall 3 to 4 months after irradiation limit the usefulness of MRI in detecting tumor recurrences.113 Reports indicate improved accuracy in staging muscle-invasive tumors when MRI is performed with gadolinium-enhanced images. Tanimoto and associates114 showed that for 86 tumors, MRI with gadolinium enhancement had a staging accuracy of 85%, in comparison with 55% for CT scan and 58% for conventional MRI. Detection of superficial lesions was improved and there was a decrease in the overstaging of T1 tumors. Sensitivity and specificity of MRI for staging bladder tumors have been improved with the advent of imaging with oblique planes, smaller pixel size, and three-dimensional (3-D) technology. Applying 3-D technology to MRI was associated with improved accuracy of local staging from 78% to 93% and nodal staging from 86% to 93%.113,115,116 The detection of nodal involvement with clinical staging has obvious treatment-planning implications for the radiation oncologist. One report from the Christie Hospital in the United Kingdom demonstrated that preradiotherapy staging with MRI was helpful in providing more anatomic detail and clarifying the extent of disease. In that series, 39 of 71 patients with clinical T2a tumors were upstaged. Among the 27 patients in that series upstaged to T3b, this group experienced a significantly worse local control and overall survival outcomes. A multivariate analysis demonstrated that, among other variables, tumor stage based on MRI was a significant factor predicting for treatment failure and overall survival.117 MRI may be particularly helpful in the evaluation of direct prostatic extension of tumor (T4a).118
The role of fluorodeoxyglucose (FDG)–positron emission tomography (PET) remains controversial in patients with superficial and locally advanced bladder cancer. The presence of radiotracer in the urine makes interpretation of early stage disease problematic. For the early detection of locally advanced bladder cancer with lymph node involvement, FDG-PET shows promise. FDG-PET imaging prior to cystectomy had a sensitivity of 67% and a specificity of 86% for detection of lymph node metastases, which compares favorably with conventional CT.119 FDG-PET appears able to detect metastatic lesions with high frequency as well.120 The full role of FDG-PET and other PET technology has yet to be realized in bladder cancer.
Pathologic staging requires either a partial or total cystectomy and lymph node dissection. Suspected metastatic disease on imaging studies can often be confirmed by CT-guided biopsies. Staging error between clinical and pathologic stages has been shown to be as high as 50%.101 Survival is lower for each stage when grouped clinically as opposed to pathologically. It is for this reason that comparing outcomes of radiotherapy series based on clinical staging with surgical series based on pathologic staging is fraught with difficulties. Nevertheless, with both modalities, survival decreases with increasing stage. This provides the clinician with the necessary prognostic information to formulate a treatment approach.108
Identification of prognostic factors allows the clinician to select those patients who would potentially benefit from bladder conservation approaches or, conversely, patients better managed with upfront radical cystectomy. Advanced-stage disease has been shown to be an accurate predictor for a high risk of developing early distant metastases.109 In addition to the depth of invasion and the presence or absence of nodal involvement reflected in the stage, other prognostic variables have been defined. Low-grade and papillary growth have been shown to represent good prognostic indicators for survival.121 Tumors characterized as larger than 5 cm, with associated CIS, with evidence of vascular invasion, and with failure to obtain a complete response to chemoradiation have been shown to have a poor outcome and these patients may be better suited for cystectomy.122 The presence or absence of hydronephrosis has also been correlated with prognosis in both cystectomy and radiation therapy series.123,124 Researchers have indicated that a tumor with a high degree of angiogenesis may have a poorer prognosis.89 It is likely that in the future prognostic factors will be increasingly identified at the cytogenetic level.
Treatment of Superficial Bladder Carcinoma
Three fourths of bladder carcinomas present as Ta, T1, or Tis superficial lesions that do not extend deeper than the lamina propria. Although the majority of such tumors are controlled by transurethral resection, an estimated 40% to 80% will recur within 6 to 12 months and 10% to 25% will progress to muscle-invasive disease. Prognostic factors for recurrence include grade, depth of penetration, multifocality, and presence of CIS. The management of superficial bladder carcinoma involves TURBT and, in many cases, adjuvant therapy to prevent recurrence and potential development of muscle-invasive disease.99,125,126
Ta papillary tumors typically present as low-grade lesions that frequently recur multiple times prior to progressing to invasive tumors. Low-grade Ta papillary lesions without multifocality and without associated CIS can be treated with a diagnostic and therapeutic resection using a resectoscope, followed by close monitoring and repeat cystoscopies. Muscle must be seen in this specimen before ruling out invasive disease and biopsies of apparently uninvolved urothelium should be obtained to rule out occult Tis. Approximately 5% to 30% of patients will ultimately progress to a higher-grade lesion or require more aggressive therapy.127,128 For patients with persistent tumor cells on urine cytology after TURBT, multifocal lesions, grades II or III histologic findings, or evidence of CIS, adjuvant intravesical therapy is indicated. If all visualized superficial lesions are not excised, there is also a need for further therapy. Adjuvant therapy is given in the form of intravesical administration of immunotherapy or chemotherapy. Bacille Calmette-Guérin (BCG) is administered adjuvantly weekly for 6 weeks. BCG, a live attenuated form of Mycobacterium bovis, has an unknown exact mechanism of action; however, it triggers an immune-response cascade including mononuclear cells and cytokines such as interleukin (IL)-1, IL-2, IL-6, IL-8, interferon-γ, and tumor necrosis factor-α. In addition, a direct tumor-cell suppression may be involved. The chemotherapeutic agents of greatest known efficacy are mitomycin C, doxorubicin, and thiotepa. These three agents have all demonstrated efficacy in the reduction of disease recurrence.126 Thiotepa causes the most systemic toxicity, including myelosuppression in 20% of patients, owing to its low molecular weight, which allows absorption. Doxorubicin creates the greatest local reaction, which may predispose the patient to urinary urgency. The three drugs have nearly identical efficacy in prolonging the time to recurrence.99
A randomized trial of intravesical doxorubicin versus BCG in 262 patients with superficial transitional cell carcinoma showed an advantage in the complete response and 5-year disease-free survival rates for those treated with adjuvant BCG.99 A classic series of 86 patients with recurrent superficial bladder cancer prospectively randomized to TURBT plus BCG or TURBT alone was reported by Herr and colleagues.129 Patients treated with BCG had a prolonged median disease-free interval of 24 months compared with 8 months for those patients treated with TURBT alone.129 An update of the data has revealed a 10-year disease-specific survival rate of 75% in the group that received TURBT plus BCG compared with 55% in those treated with TURBT alone. The majority of recurrences were discovered within the first 5 years. Of the 61 patients who received BCG, 33 (54%) were disease free with their bladders preserved.130,131
In a recent review of 11 clinical trials consisting of 2749 patients treated with BCG or mitomycin C, a greater incidence of tumor recurrence was observed for patients treated with mitomycin C compared with BCG (46% versus 36%, respectively). This review also demonstrated improved results with BCG maintenance therapy for reducing the risk of tumor recurrence; however, this is currently controversial because of the potential increased toxicity with BCG maintenance. The incidence of associated cystitis related to intravesical therapy was more prevalent among BCG treated patients (54% versus 39%, respectively).132
The standard of care for superficial bladder cancer with a high risk of recurrence has been established as intravesical BCG. Histologic persistence after 6 months indicates the need to change the intravesicular agent. Disease beyond 1 year indicates the need for a different treatment approach to prevent progression to muscle-invasive cancer. Investigators are testing the application of interferon α.126,133 The role of external-beam radiation in the treatment of CIS is unclear. Especially among patients previously treated with multiple resections and intravesical therapy, the tolerance of external irradiation may be poor because of the pre-existing chronic irritation within the bladder mucosa. Van der Werf-Messing et al.134 have reported excellent control rates (>90%) for limited T1 tumors using interstitial therapy through an open cystotomy approach. In that report, interstitial radium-226 was used with an intended dose of 60 Gy delivered to the visualized bladder tumor. This approach, however, is generally not used in the United States. Patients with persistent CIS or refractory Ta/T1 disease ultimately require cystectomy.
Surgical Treatment of Muscle-Invasive Bladder Cancer
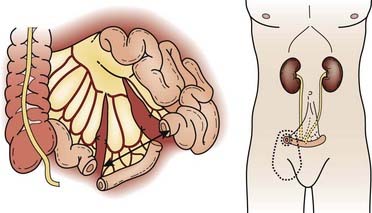
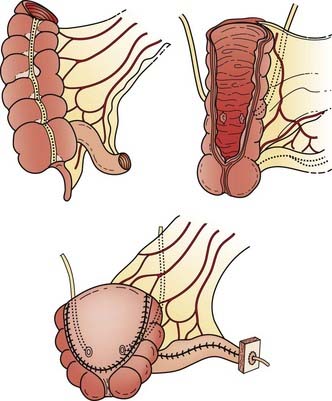
Radical cystectomy in the male implies the en bloc removal of the bladder (with its peritoneal covering), perivesical adipose tissue, lower ureters, prostate gland and seminal vesicles, pelvic vas deferens, proximal urethra, and pelvic lymph nodes (Fig. 44-5). A total urethrectomy is performed for patients with CIS, multicentric tumors, and involvement of the bladder neck or prostatic urethra. A radical cystectomy in female patients is an anterior exenteration with sacrifice of the bladder (with its peritoneal covering), entire urethra, uterus, fallopian tubes, ovaries, anterior vaginal wall, and pelvic lymph nodes. A standard pelvic lymph node dissection generally includes removal of the bilateral external iliac, obturator, internal iliac (hypogastric), and common iliac lymph node chains. The dissection extends from the aortic bifurcation superiorly to the inguinal ligament and node of Cloquet inferiorly and the genitofemoral nerve laterally.135,136 An extended dissection includes all nodes from the standard template plus paracaval, interaortocaval, para-aortic, and presacral lymph nodes. Herr reported on the prognostic effect of nodal status in 637 patients who underwent radical cystectomy and pelvic lymph node dissection.137 For both node-negative and node-positive patients, improved survival and reduced local recurrence related to negative surgical margins and the number of lymph nodes removed at the time of the node dissection. The 5-year survival rates for patients who had 0 to 5, 6 to 10, 11 to 14, and more than 14 lymph nodes removed were 79%, 73%, 44%, and 33%, respectively. The corresponding local relapse rates in these aforementioned nodal groups were 4%, 7%, 8.5%, and 17%, respectively. A multivariate analysis demonstrated that the number of nodes removed was an independent predictor of survival outcome. This observation has been observed in other series and clinical trials.102,103,138 One caveat, however, is that it is not clear if this improved outcome is related to therapeutic removal of occult nodal disease or if it represents a surrogate for the quality of surgery.
Attempts to reduce the morbidity of radical cystectomy have focused on preserving potency in the male and developing urinary diversion alternatives that maximize continence and cosmesis. A nerve-sparing radical cystectomy requires preservation of the neurovascular bundles and the dorsal-venous complex surrounded by the lateral prostatic fascia located posterolateral to the prostatic capsule and membranous urethra.136,139
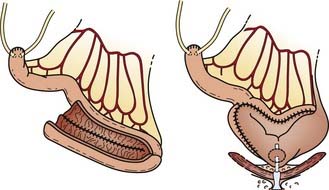
Reconstructive techniques following radical cystectomy generally fall into two categories: incontinent or continent diversions. The standard urinary diversion, an incontinent diversion, is accomplished with a conduit derived from 15 cm of distal ileum to which the ureters are anastomosed (Fig. 44-6). An ostomy is created by attachment of the distal end of the ileum to the defect in the anterior abdominal wall.136 More recently, a second type of urinary diversion, the creation of internal reservoirs, has become available to allow the patient to retain continence. Two classes of continent diversions of the urinary flow are (1) stomal reservoirs that require intermittent catheterization and (2) orthotopic neobladders anastomosed to the remaining distal urethra (Fig. 44-7). There are several techniques for creating a continent cutaneous diversion that can be catheterized including the cecoileostomy (Indiana pouch [Fig. 44-8]) and the ileostomy (Koch pouch). All involve constructing a detubularized pouch from intestine that becomes a spherical reservoir with a constructed sphincter to maintain continence. Because these reservoirs are composed of portions of bowel, metabolic and absorptive disturbances vary depending on the segments and lengths of bowel used.
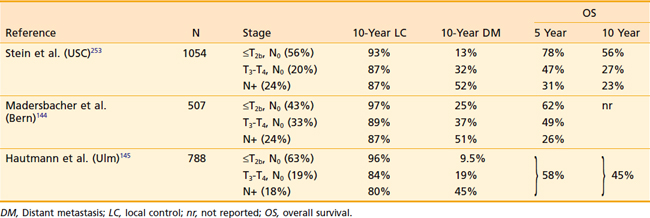
Because of the longer operative times and complexity of these procedures, a higher risk of acute postoperative complications including urinary leak are noted as compared with ileal conduits. By 3 to 4 months the average catheterization interval is decreased to 6 to 8 hours. Nevertheless, the pouch is associated with a 2% mortality, a 5% incidence of serious complications, and a reoperative rate of 30%. Over the past decade, orthotopic neobladder reconstruction techniques have become the preferred method of urinary diversion in appropriately selected patients. Because this reconstruction leaves intact the external urinary sphincter, patients are able to void by performing the Valsalva maneuver. Patients must be carefully chosen for the orthotopic neobladders to avoid incontinence, particularly during sleep, because the guarding reflex is lost with the native bladder. Rates of nocturnal incontinence range from 15% to 40%.140 Five-year survival rates for early stage T2 tumors were often reported to be as low as 17% to 36% before 1980. Patients with stage T3a and T3b tumors had 5-year rates ranging from 18% to 31% and 20% to 25%, respectively. However, more recent studies of cystectomy using modern surgical techniques report survival rates as high as 50% to 88% for T2, 36% to 69% for T3a, and 11% to 47% for T3b tumors.135,141 A summary of recently reported outcomes in large surgical series is summarized in Table 44-2. The overall local control rates with radical cystectomy alone have been reported to be as high as 95%142,143; however, in particular, patients with locally advanced or node-positive disease treated with radical cystectomy alone do not fare as well. Madersbacher et al.144 reported in a recent series of patients treated with radical cystectomy alone isolated local recurrence rates of 9% to 20% for non–organ-confined (T3b-T4) node-negative disease and 13% for node-positive disease. Another surgery-only series reported by Hautmann et al.145 demonstrated local recurrence rates of 16% and 20% in non–organ-confined and node-positive patients, respectively. Of note, this is likely an underestimate of the true local recurrence rates as patients with simultaneous local and distant recurrences are typically included in the metastatic cohort. The overall outcomes of these patients remain suboptimal even in series including neoaduvant and adjuvant therapies. Distant metastases occur in 20% to 50% of patients with locally advanced disease.144,145 For patients with node-positive disease, long-term disease-free survival rates are poor at 25% to 35%.
Preoperative Radiation Therapy
In an effort to improve the outcome for patients with locally advanced bladder cancers treated with surgery, preoperative radiotherapy was commonly used from 1960 to 1980. The rationale for using this approach was to reduce the incidence of tumor bed failures and potential seeding of malignant cells from the operation. Although some retrospective studies have suggested that the addition of preoperative radiotherapy improves the overall outcome,146,147 other series have found no benefit when results are compared with modern surgical series.142,148,149 Several randomized prospective trials that compared preoperative radiotherapy plus radical cystectomy to radical cystectomy alone have demonstrated no significant differences between the treatment arms.150–152 Nevertheless, it must be pointed out that these trials were performed before the improvement in surgical techniques and suffered as well from limited accrual, compromising their power to detect significant differences between the study arms. A review from the M.D. Anderson Hospital supports the notion that preoperative irradiation improves the outcome in a select cohort of patients.153 In this report, 338 patients treated with preoperative radiotherapy between 1960 and 1983 were retrospectively compared with 232 patients treated at the same institution between 1985 and 1990. The majority of the latter group were treated with neoadjuvant or adjuvant chemotherapy. Despite this apparent advantage, there was a significant improvement in local control among patients with T3b disease who received preoperative irradiation. Within this cohort of patients, there was a trend for improved disease-free survival and overall survival, although no significant differences were seen. The use of preoperative radiotherapy in the modern surgical era remains unclear, and at this time is not a standard approach in the United States.
Neoadjuvant/Adjuvant Chemotherapy
Various neoadjuvant regimens have been evaluated in a number of prospective randomized trials. The Nordic Cystectomy I trial evaluated neoadjuvant chemotherapy in combination with low-dose irradiation and cystectomy.154 Patients with T1G3, T2-T4a NXM0 tumors were randomly assigned to either two cycles of chemotherapy with cisplatin and doxorubicin or no chemotherapy before cystectomy. In addition, all patients in this study received radiotherapy (4 Gy/day × 5 days) following the second cycle of chemotherapy. Initial results appeared to show a benefit for neoadjuvant chemotherapy. However, longer follow-up failed to demonstrate a statistically significant difference in overall survival between the two groups (59% versus 51%, P = 0.1).155 Subgroup analysis also demonstrated no survival benefit for patients with T1 and T2 tumors, although patients with T3 and T4a tumors did show a 5-year survival benefit of 52% versus 37% (P = 0.03).
The largest neoadjuvant trial to date was a European study involving more than 900 patients. Between 1989 and 1995, patients with T2G3, T3, and T4a, N0 to NX, M0 tumors were randomized to receive either three cycles of cisplatin, methotrexate, and vinblastine (CMV) neoadjuvant chemotherapy (n = 491) or no chemotherapy (n = 485) before planned cystectomy or definitive radiation therapy (chosen at the discretion of the local treating institution).156 Chemotherapy-associated mortality was 1% and cystectomy operative mortality was 3.7%. Approximately 42% of patients in each arm received radiotherapy as the primary therapy. Although at 4 years no significant difference in overall survival was seen, after longer follow-up (median 7.4 years), a statistically significant 6% absolute survival benefit was found in favor of the chemotherapy-treated patients.157 In addition, the rate of pathologic complete response was found to be dramatically higher in the primary tumors of chemotherapy-treated patients (33% versus 13%).
A similar smaller randomized phase III trial of neoadjuvant methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (MVAC) plus cystectomy (n = 153) versus cystectomy alone (n = 154) was conducted by the U.S. Intergroup in patients with T2 to T4a N0M0 bladder cancer. After a median follow-up of 8.5 years, survival in the MVAC arm was marginally superior, with a hazard ratio of 0.74 (95% CI 0.55-0.99, P = 0.06). The estimated median survival of the MVAC arm was 77 months, while the no-MVAC arm was 46 months.158
Two meta-analyses have been conducted to evaluate the effect of neoadjvuant chemotherapy on overall survival. One meta-analysis of individual patient data consisting of 98% of patients from known randomized trials using cisplatin combination chemotherapy has shown that neoadjuvant therapy provides a moderate but significant benefit in patients with T2-T4a disease. Patients who received neoadjuvant cisplatin combination chemotherapy experienced a 14% reduction in the risk of death (hazard ratio = .86 [CI = .70-.95]) and an absolute improvement in survival of 5% at 5 years.159 Single-agent cisplatin chemotherapy was not shown to provide any survival benefit. A second meta-analysis was conducted that confirmed these results.160 Neoadjuvant cisplatin combination chemotherapy prior to definitive local chemoradiotherapy has been tested in only one study, and did not appear to demonstrate a benefit in favor of chemotherapy. Several other neoadjuvant studies allowed either radiotherapy or cystectomy as local therapy following neoadjuvant chemotherapy. All of these studies have been included in the meta-analyses cited previously, and there was no significant association between the type of local therapy (cystectomy versus radiotherapy) and outcome.
There is no randomized data regarding the use of adjuvant chemotherapy following primary radiotherapy or chemoradiation for advanced bladder cancer. Several phase II studies of chemoradiation have been reported that include adjuvant combination chemotherapy. These studies have promising response rates and survival data. Radiation Therapy Oncology Group (RTOG) 97-06 tested three cycles of methotrexate, cisplatin, and vinblastine (MCV) chemotherapy in complete responders following induction and consolidation chemoradiation using concurrent cisplatin. Only 45% of patients completing chemoradiation were able to complete all three cyles of MCV adjuvant chemotherapy, although overall results of the strategy seemed promising.161 Another study evaluated two cycles of concurrent 5-fluorouracil (5-FU) and cisplatin during radiotherapy followed by two additional cycles of adjuvant 5-FU and cisplatin. Protocol therapy on this trial was completed by only 57% of patients enrolled.162 However, 5-year survival from both of these studies was 45% to 49% in patients who were cystectomy candidates, comparing favorably with large cystectomy series. Other phase II studies are underway to test the use of newer agents such as the taxanes and gemcitabine in this setting.
Several prospective randomized trials have attempted to address whether adjuvant chemotherapy improves survival after cystectomy. An early trial using single-agent cisplatin was unable to detect any advantage for adjuvant therapy.163 Subsequently, a prospective randomized trial using combination chemotherapy was undertaken in patients with pT3, pT4, or node-positive transitional cell carcinoma of the bladder. Between 1980 and 1988, 91 patients were randomly assigned to adjuvant chemotherapy or observation. CISCA was the predominant chemotherapy administered on this trial, although some patients received other chemotherapy agents and regimens. This trial showed a significant increase in time to progression in favor of adjuvant chemotherapy (6.58 versus 1.92 years, P = 0.011), although overall survival was not significantly different at 5 years. In retrospective subgroup analyses, patients with a single positive lymph node appeared to derive the most benefit from adjuvant chemotherapy. Interestingly, patients with two or more positive lymph nodes did not appear to benefit from adjuvant chemotherapy.164 This study has been criticized for the lack of standard treatment, as well as the failure to receive assigned therapy in a significant proportion of the patients.
In a study from Stanford University, 55 patients who underwent cystectomy and were found to have pT3 and T4 tumors with or without lymph node involvement were randomized to four cycles of CMV chemotherapy or observation. This study was halted early because of the observation of significant benefit in favor of patients receiving chemotherapy. With a median follow-up of 62 months, freedom from progression was superior in patients receiving adjuvant chemotherapy (37 versus 12 months, P = 0.01), although overall survival was not significantly different (63 versus 36 months, p = 0.32). Patients who developed progressive disease and who had not received adjuvant therapy subsequently received systemic therapy, perhaps accounting for the fact that overall survival was not significantly improved by the use of adjuvant therapy.165
A third prospective randomized trial involved 49 patients with pT3b, pT4a, or pelvic lymph node involvement without any evidence of tumor remaining after cystectomy. These patients were randomized to three cycles of adjuvant MVAC or methotrexate, vinblastine, epirubicin, cisplatin (26 patients) or observation (23 patients). Only 18 of the 26 patients randomized to adjuvant chemotherapy actually went on to receive adjuvant chemotherapy. The study was designed to show a 35% improvement in disease-free survival at 5 years. However, an interim analysis showed a significant increase in disease-free survival among patients in the chemotherapy arm, resulting in early stopping of the study. In an intent-to-treat analysis, at 3.5 years, 63% of patients in the chemotherapy arm were disease free, compared with 13% in the observation arm. However, overall survival was not significantly improved in this study. The benefit of adjuvant therapy appeared strongest for those patients with lymph node involvement.166
Because of the lack of statistical power and early closing of trials, it is difficult to reach conclusions regarding the true efficacy of adjuvant chemotherapy for advanced bladder cancer. A meta-analysis of data from six randomized controlled clinical trials of cisplatin-based adjuvant chemotherapy evaluated updated patient data for 491 patients. This analysis was limited in its power because of the relatively small sample size, but suggested that adjuvant chemotherapy was associated with a 25% reduction in the risk of death compared with controls.167
Definitive Radiotherapy for Muscle-Invasive Bladder Cancer
Radiation therapy has been used in the management of bladder cancer for more than 50 years.168 As a single modality, however, results were disappointing, with high local failure rates and poor survival.169,170 In addition to suboptimal radiation techniques, poor patient selection and lack of systemic therapy to address micrometastatic disease contibuted to these poor results. Proper patient selection and the use of chemotherapy in combination with modern radiation therapy have improved these results considerably. There are, however, no reported modern prospective randomized studies directly comparing definitive chemoradiation with surgery. An ongoing randomized trial in the UK is designed to address this issue (Selective Bladder Preservation against Radical Excision trial) (public.ukcrn.org.uk). Patients offered definitive irradiation have typically been nonsurgical candidates with various comorbidities and more advanced disease, contributing to poorer prognoses than those selected for surgery. In addition, surgical series have the benefit of accurate pathologic staging, whereas radiation series rely on clinical staging with its intrinsic inaccuracies related to understaging. Comparison of cystectomy to radiotherapy series must be interpreted with the understanding that there are inherent selection-bias and stage-migration differences between these groups of patients as well as the differences in overall performance status. The primary goal of bladder preservation strategies remains disease control, with organ preservation a secondary albeit important treatment goal, and hence, depite these difficulties with data comparison, radical cystectomy outcomes remain the standard with which other therapies should be compared. In Great Britain, Canada, and Europe, external-beam radiotherapy is often first-line therapy, with salvage cystectomy reserved for treatment failures.
The 5-year survival rate for muscle-invasive bladder cancer treated with definitive external-beam radiotherapy alone is 20% to 40%. Most series report complete response rates of approximately 50%. Similar to surgical series, survival rates following definitive radiotherapy have been higher in patients with invasion limited to the bladder compared with those with extravesical (T3b or T4) disease.142,171,172 Analysis of clinical characteristics has defined several prognostic factors that predict for local control, freedom from metastasis, and survival in patients treated with definitive irradiation. Most series show that depth of tumor invasion defined by stage is an important prognostic indicator.172–175 Patients with stage T4 disease have been shown to have 5-year survival rates often lower than 10%, indicating irradiation alone is likely to benefit only a minority of those with advanced disease. In addition to advanced T stage, other poor prognostic signs include tumors greater than 5 cm, residual disease after TURBT, and radiographic evidence of ureteral obstruction with hydronephrosis.175 A multivariate analysis of 116 patients treated at Fox Chase Cancer Center showed that both local control and survival depended on the pretreatment clinical T stage and hemoglobin levels.173 Grade was also shown to influence prognosis, with a 5-year survival of 43% in patients with grade I or II carcinoma versus 27% in those with grade III or IV histologic findings.173 Patients with a papillary or mixed-tumor morphology have been shown to have higher local control rates and survival than those with solid lesions.169 A report from M.D. Anderson Hospital of 135 patients, of whom the majority underwent an attempt at complete transurethral resection of tumor before definitive irradiation, indicated the importance of a cystoscopically verified complete response 2 to 6 months after the completion of therapy.176 Multivariate analysis showed that a clinically complete response was the most important independent prognostic factor for survival and local pelvic control.176,177 A study from Scotland of 333 patients with stage T3 disease confirmed the correlation of a documented complete response to improved survival.177 The presence or absence of histologic evidence of vascular invasion has not been shown to influence prognosis in the majority of series.173–175
In an effort to improve local control rates with external-beam radiotherapy for patients with invasive bladder cancers, hyperfractionated treatment schedules have been used.146,171,178–180 The rationale behind this approach is derived from the recognition of tumor repopulation. Tumor repopulation by surviving clonogenic cells is believed to be one mechanism of treatment failure following fractionated radiotherapy and this phenomenon is increased with more protracted radiation schedules.181 Edsmyr and coworkers179 reported a Swedish trial of 168 patients with T2 to T4 disease randomly assigned to receive either 1 Gy three times a day to a total of 84 Gy or 2 Gy once a day to a total of 64 Gy. Both regimens were given over 8 weeks, with an imposed mid-course 2-week treatment interruption. The 5-year survival for the group receiving 84 Gy was 37%, and for those receiving 64 Gy it was 16%.171 Patients with T3 disease appeared to benefit most from the hyperfractionated schedule. An update with a follow-up period of at least 10 years shows that the benefit in survival for the entire group, and specifically for those with T3 lesions, has persisted. To further evaluate the relationship of dose and overall treatment time, Majewski et al.182 conducted a retrospective study of 480 patients with T2-T3 bladder cancer treated with four different fractionation regimens: (1) conventional fractionation (1.8-2.5 Gy/day), (2) protracted radiation (1.6-1.7 Gy/day pelvic radiotherapy plus boost of 2.0 Gy/day), (3) accelerated hyperfractionation boost (2.0 Gy/day pelvic radiotherapy plus boost of 1.3-1.4 Gy/twice daily), and (4) accelerated hyperfractionation (1.2-1.5 Gy/twice daily pelvis plus boost radiotherapy). Although clinical prognostic factors including T stage were the most significant predictors of outcome, an increase in radiation dose was associated with improved local control (P = 0.011), although overall treatment time did not reach significance (P = 0.077). Horwich et al.183 reported a prospective trial conducted at the Royal Marsden Hosptial comparing an accelerated hyperfractionated regimen to conventionally fractionated treatment in 229 patients with muscle-invasive bladder cancer (T2-T3) and minimal nodal disease. Patients were randomized to receive either 64 Gy in daily 2-Gy fractions to the whole bladder with margin or twice-daily radiation (1.8 Gy am dose and 2.0 Gy pm dose) to a total dose of 60.4 Gy over a 26-day period. Although the initial results of this study appeared promising, with longer follow-up there was no significant difference in local control or overall survival with the accelerated regimen. In addition, acute grade 2 and 3 bowel toxicity was higher in the accelerated arm, 40% versus 26% and 3% versus 0%, respectively. Acute grade 2 and 3 bladder toxicity appeared similar, at 16% versus 17% and 18% versus 19%, respectively. Treatment with accelerated and hyperfractionated schedules remains investigational and should be conducted in a protocol setting (see Multimodality Bladder-Preservation Therapy).
Multimodality Bladder-Preservation Therapy
In an effort to improve disease outcomes with radiation therapy, multimodality bladder-preserving approaches have been developed and typically include cystoscopic extirpation, radiation therapy, and concurrent chemotherapy. Several reports now substantiate the efficacy of combined chemotherapy and radiotherapy as an organ-preservation alternative for well-selected patients with localized transitional cell carcinoma of the bladder.184–193 Current results suggest that this chemoradiation approach provides comparable survivorship to that expected for similar patients treated with radical cystectomy with a high rate of bladder preservation. The success of this treatment approach is predicated on the meticulous selection of patients with known favorable prognostic factors that are associated with improved response rates. This section summarizes the experience with combined modality therapy and discusses current trials that attempt to further explore this approach.
One of the earliest reports using combined modality therapy as a means for bladder preservation was conducted by the National Bladder Cancer Cooperative Group.192 From 1981 to 1986, 70 patients with T2 to T4 bladder cancer were enrolled in a prospective study, all with medical contraindications for radical cystectomy. Cisplatin was given at a dose of 70 mg/m2 every 3 weeks concurrent with external-beam radiotherapy to a total dose of 64.8 Gy. Initially, 45 Gy was delivered to the entire bladder, perivesicular tissues, and adjacent lymph nodes using a four-field plan followed by a boost to the tumor within the bladder for an additional 19.8 Gy in 11 fractions delivered in 2 weeks. The overall complete response rate was 70%, and the 4-year survival among complete responders was 57%, compared with 11% among patients who did not achieve a complete response. Long-term survival rates were 64% for T2 patients and 22% for T3 patients. A subsequent randomized trial conducted by the National Cancer Institute of Canada between 1985 and 1989 comparing preoperative or definitive radiation therapy with or without concurrent cisplatin chemotherapy reported a significant improvement in local control in patients treated with cisplatin (67% versus 47%) without an effect on distant metastases or overall survival.194 Of note, this trial is the only prospective, randomized trial to date comparing radiation alone with chemoradiation. Several single-institution studies have been performed demonstrating that a complete transurethral resection also affects local control prior to radiation therapy alone or concurrent with chemotherapy.195,196
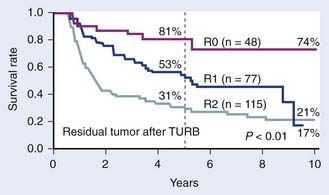
In Europe, similar studies were being conducted. Housset et al.188 at the University of Paris reported results of a prospective study of 54 patients with operable T2-T4 bladder cancer treated with transurethral resection followed by 5-FU/cisplatin with concommitant twice-daily split-course radiotherapy to the whole pelvis (24 Gy in eight fractions over 17 days). A restaging cystoscopy with deep biopsies performed at 6 weeks postinduction demonstrated a 74% pathologic complete response rate. These complete responders were then treated with either radical cystectomy or definitive chemoradiation with an additional 20 Gy bladder boost (2.5 Gy twice-daily split course) concurrent with 5-FU/cisplatin. Among an initial 18 complete responders who underwent planned surgery after chemoradiation, no residual tumor was found in the cystectomy specimens in all cases. The 3-year disease-free survival rate was significantly better in responders compared with nonresponders (77% versus 23%). However, overall survival rates were similar (81% versus 77%), demonstrating that there was no negative survival impact on bladder-preservation attempts. Importantly, treatment was well tolerated. At the University of Erlangen, Dunst et al.196 reported their experience using conservative surgery followed by radiation therapy with or without chemotherapy in a series of 245 patients treated bewtween 1982 through 1991 on a prospective protocol. Patients with high-risk T1-T3 bladder cancer were treated with a maximal transurethral resection followed by definitive radiotherapy consisting of 46 Gy to the pelvis and a whole-bladder boost to 54 Gy. After 1985, patients were treated with concurrent cisplatin or carboplatin routinely. A restaging cystoscopy and transurethral resection were performed 6 to 8 weeks following radiation, with salvage cystectomy reserved for persistent or recurrent disease. With a median follow-up of 5.9 years, the 5- and 10-year overall survival was 47% and 26%, respectively. The bladder preservation rate in 5-year survivors was 83%. This study also demonstrated the importance of the amount of residual tumor after transurethral resection with 5-year survival rates of 80% after R0, 53% after R1, and 31% after R2 resection (P < 0.01). Rodel and colleagues recently updated the University of Erlangen experience.187 In that report, 289 patients with locally advanced tumors were treated with transurethral resection followed by concurrent platin-based chemotherapy and pelvic radiotherapy plus a tumor boost for a cumulative dose of 54 Gy. Chemotherapy was administered during the first and fifth week of radiotherapy and consisted of cisplatin 25 mg/m2 daily for 5 days. Carboplatin was used for those patients with creatinine clearance less than 60 mL per minute or congestive heart disease. In 68% the full-dose chemotherapy was able to be administered, whereas in 32% the doses needed to be curtailed secondary to hematologic toxicity, gastrointestinal toxicity, or nephrotoxicity. The median follow-up in the study was 60 months. The complete response rates at the 6 week restaging TURBT for patients treated with radiotherapy in combination with cisplatin and 5-FU, cisplatin alone, and carboplatin was 87%, 82%, and 66%, respectively. These rates of response were significantly higher than the complete response rate (61%) observed among 126 patients who were treated with radiotherapy alone (P = 0.001). Among all patients who achieved a complete response, the 10-year local control rate was 64%. The overall likelihood of developing distant metastases and disease-specific survival was 35% and 42%, respectively, at 10 years. Of long-term survivors, 80% maintained functioning bladders, and cystectomy was only required in 2% of patients secondary to a contracted bladder after radiotherapy. In a multivariate analysis, early tumor stage and the performance of a complete TURBT before radiochemotherapy were predictors of a higher complete response rate and improved overall survival. The 5-year survival rates among patients who had a complete TURBT, microscopic residual disease, and gross residual disease after resection were 76%, 52%, and 34%, respectively (Fig. 44-9). The data also suggested that adjuvant radiotherapy is of benefit even among selected patients who had complete TURBT. In this latter group, the incidence of bladder preservation was 85%, compared with 75% reported by Herr197 for highly selected patients with muscle-invasive tumors treated with TURBT alone.
These highly encouraging results were the impetus for a phase II chemoradiation bladder-preservation study for patients with localized bladder cancer who were otherwise eligible for radical cystectomy conducted at Massachusetts General Hospital.193 Fifty-three patients were enrolled in this study, of whom 15 had clinical T2, 29 had T3, and 9 had T4 disease. Treatment consisted of a transurethral resection followed by two cycles of neoadjuvant MCV and then 45 Gy to the pelvis with two concomitant doses of cisplatin. The 36 patients with a complete response at 40 Gy (as demonstrated by repeat urologic examination including cystoscopy, examination under anesthesia, and urine cytology) were allowed to complete the pelvic radiation and proceed to a 24.8 Gy tumor boost with a final dose of cisplatin. Radical cystectomy was performed for 15 patients who could not tolerate the combined chemoradiation, had an incomplete response at the repeat cystoscopy, or required salvage after completion of the entire treatment plan. This study demonstrated that a multimodality approach resulted in a 5-year survival rate of 48%, comparable with the expected outcome after radical cystectomy based on historic controls. There were no major complications involving the bladder or rectum, with only two patients experiencing intermittent hematuria. Toxicity associated with chemotherapy included nausea and vomiting (73%), stomatitis (24%), and diarrhea (10%). A 58% rate of bladder conservation was achieved at median follow-up of 48 months without an apparent compromise in survival. Of 27 patients who were complete responders after neoadjuvant chemotherapy and 40 Gy of radiation, 81% maintained a normal, functioning bladder.193 Shipley et al.185 recently updated the results of 190 patients treated on various institutional protocols at Massachusetts General Hospital from 1986 to 1997 with a combined modality bladder-preservation approach. With a median follow-up time of 6.7 years, the 5- and 10-year overall survival rates were 54% and 36%, respectively. For patients with clinically staged T2 disease, the 5- and 10-year survival rates were 62% and 41%, respectively. For patients with clinically staged T3 to T4 disease, the 5- and 10-year survival rates were 47% and 31%, respectively. Disease-free survival outcome with an intact bladder was also excellent in this group of selected patients. The 5- and 10-year disease-free survival rates with an intact bladder were 57% and 50%, respectively. For patients with clinically staged T3 to T4 disease, the 5- and 10-year disease-free survival with intact bladder rates were 35% and 34%, respectively. Bladder contractures requiring cystectomy were not observed in these patients.
RTOG Experience
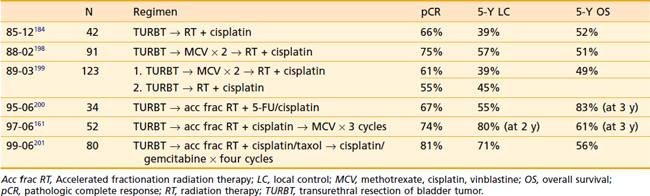
A series of phase I-III studies have been performed by the RTOG during the last 20 years, including 415 patients treated with combined-modality regimens; these are summarized in Table 44-3. All of these trials use a trimodality approach including maximal transurethral resection and combined cisplatin-based chemoradiation with frequent cystoscopic evaluation and salvage cystectomy at the earliest sign of recurrence. RTOG 85-12 demonstrated the safety and efficacy of trimodality therapy with complete response rates of 66% and encouraging 4-year survival rates of 64% for T2 disease and 24% for T3-4 disease.184 RTOG 88-02 was a phase II study (based on an Massachusetts General Hospital pilot study) designed to evaluate the addition of two cycles of MCV followed by radiotherapy 39.6 Gy and concurrent cisplatin, with complete responders continuing to a consolidation phase consisting of a 25.2 Gy bladder boost with an additional dose of cisplatin. Treatment was well tolerated with a 75% complete response rate and 51% 5-year survival.198 In RTOG 89-03, the only phase III protocol to date, patients were randomized to trimodality therapy with or without neoadjuvant MCV.199 This study was stopped prior to accrual goals with 123 of 174 planned patients because of poor tolerance of the MCV regimen, including leukopenia, sepsis, and three treatment-related deaths. With a median follow-up of 60 months, there was no difference in complete response, bladder preservation, distant metastases, or overall survival in the neoadjuvant arm. In 1995, further RTOG protocols used accelerated fractionation regimens based on data suggesting an improvement in local disease control including the University of Paris series. RTOG 95-06, using a regimen similar to that of Housset et al.188 reported results in 34 patients, including a 67% complete response rate and an overall survival rate of 83% at 3 years.200 This regimen was abandoned in subsequent studies because of high-grade 3/4 hematologic toxicity (21%). RTOG 97-06 explored the combination of twice-daily radiation therapy concurrent with cisplatin 30 mg/m2 given on the first 3 days of each week in addition to three cycles of adjuvant chemotherapy.161 Radiation consisted of 1.8 Gy to the pelvis and a 1.6-Gy concomitant boost to the tumor delivered daily 4 to 6 hours apart. In the induction phase, the cumulative tumor dose was 40.8 Gy and pelvic nodes received 21.6 Gy. Restaging cystoscopy was performed after a 3-week interval; complete responders continued to consolidation (1.5 Gy twice daily for an additional 24 Gy) and patients with less than a complete response underwent cystectomy. All patients received adjuvant chemotherapy. Although only 11% of patients undergoing chemoradiation developed acute grade 3 or 4 toxicity, there was significant difficulty with completion of adjuvant MCV and 36% of patients developed grade 4 hematologic toxicity during adjvuant therapy. The complete response rate was similar to other RTOG studies at 74%, as were the 2-year actuarial locoregional control, bladder-intact survival, and overall survival rates. In RTOG 99-06, the adjuvant regimen was changed to cisplatin and gemcitabine, which has demonstrated similar efficacy to MCV but with a lower toxicity profile.201,202 In addition, paclitaxel was added to cisplatin in the induction and consolidation phases. The results of this study have recently been reported, including an 81% postinduction complete response rate, 5-year disease-free survival of 71%, and a 5-year overall survival rate of 56%.201 Although more grade 3-4 acute toxicity (26%) was noted in this study compared with prior trials, most were transient and late toxicities appeared similar. RTOG 0233, which recently completed accrual, is examining the role of accelerated twice-daily radiation therapy in combination with either paclitaxel/cisplatin or 5-FU/cisplatin. Both cohorts will also receive four cycles of adjuvant gemcitabine/paclitaxel/cisplatin chemotherapy. RTOG 0524 is ongoing and will evaluate the role of concurrent trastuzumab/paclitaxel in patients with human epidermal growth factor receptor 2 (her2)/neu overexpression.
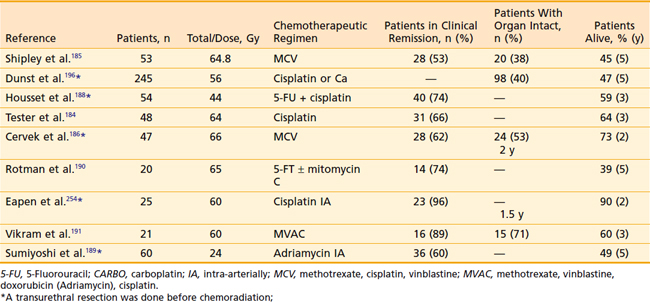
Additional phase II studies and retrospective reports of patients with invasive bladder cancer treated conservatively with combined modality therapy have been reported (Table 44-4). Identification of prognostic factors is important for the selection of patients who are most likely to benefit from this multimodality approach. The Massachusetts General Hospital group analyzed clinicopathologic characteristics in 40 patients treated with their combined modality bladder-preservation program.125 Based on a multivariate analysis, tumor stage and absence of CIS were significant predictors of complete response. Failure to obtain a complete response to therapy was strongly predictive of distant metastastic development and a short survival duration. For this reason, repeat cystoscopy to evaluate for complete response has been routinely incorporated into bladder-preservation treatment programs to select out, before completion of chemoradiation, those patients likely to suffer recurrence. High-risk patients should proceed directly to cystectomy without the added toxicity that would result from completion of the chemoradiation therapy.
The optimal chemotherapy regimen and the sequencing of radiation to maximize bladder-preservation rates while minimizing toxicity are not defined. One of the most potent single agents in bladder cancer is cisplatin, which is also an attractive drug to use for bladder-preservation therapy because of its radiation-enhancing properties. In patients with advanced and metastatic bladder cancer, results of randomized trials have demonstrated superior response rates with combination regimens such as CMV or MVAC compared with cisplatin alone.203–205 As noted earlier, excellent responses have also been reported with cisplatin and 5-FU in combination with radiotherapy for patients with muscle-invasive disease.188 Cisplatin carries a significant risk of nephrotoxicity, which is partially minimized with pre- and post-treatment hydration. However, some patients have limited renal function related to disease-related hydronephrosis or other unrelated conditions. These patients may not be good candidates for cisplatin-containing regimens. Limited data employing alternative drug regimens and routes of delivery are described in the bladder-conservation literature. Rotman and colleagues have used 5-FU infusion alone concurrent with irradiation and reported excellent long-term bladder-preservation rates.190 In a larger Japanese study with 60 patients, 60% achieved a complete response with TURBT, intra-arterial doxorubicin, and irradiation.189 A femoral artery catheter delivered three doses of doxorubicin within 48 hours in 3- to 4-week intervals for four cycles. Pelvic radiation was 6 Gy in three fractions per chemotherapy cycle. Tumor stage, size, and grade significantly predicted response. Use of oral capecitabine, a 5-FU, was tested in one small pilot study of 21 elderly patients resulting in 43% of patients with a complete response to radiotherapy.206 Additional retrospective data suggest this agent may be worthy of further evaluation.207 The taxanes have been used as alternative radiation-sensitizing agents and in particular paclitaxel has been safely used in two completed trials (RTOG 0233 and RTOG 9906) and one ongoing trial (RTOG 0524). Paclitaxel is metabolized in the liver, which makes it a particularly attractive alternative, because impaired renal function does not contraindicate its use. In a small pilot study conducted in 36 patients, paclitaxel (30 mg/m2 twice weekly) combined with radiation (1.8 Gy daily to 56.0 ± 3.7 Gy) was reasonably well tolerated, with 19.4% requiring dose reductions or treatment discontinuation because of toxicity, mostly related to gastrointestinal toxicity. In 26 evaluable patients, response rates appeared similar to prior cisplatin-based studies. Gemcitabine, a nucleoside analog, has demonstrated activity in transitional cell carcinoma. Several phase I and II trials have demonstrated the radiosensitizing properties of gemcitabine in other tumor types as well.208,209 Kent et al.210 reported the results of a phase I pilot study in 24 operable stage T2-T3 bladder-cancer patients treated with conventional fractionation radiotherapy (2 Gy daily to 60 Gy to the bladder and extravesical tumor with 1 cm margin) with a twice-weekly gemcitabine dose escalating from 10 to 30 mg/m2.210 The maximum tolerated dose was found to be 27 mg/m2 with systemic dose-limiting toxicity of elevated liver enzymes, malaise, and edema. Genitourinary and bowel toxicity was minimal, with a bladder preservation rate of 65%. Despite the limited target volume, pelvic recurrences have not been identified at a median follow-up of 43 months.
The optimal sequence and integration of chemotherapy with radiotherapy is also not established. RTOG 89-03 evaluated neoadjuvant CMV chemotherapy for two cycles followed by the bladder-preservation regimen compared with concurrent cisplatin and radiotherapy without neoadjuvant chemotherapy in 123 paitents with muscle-invasive bladder cancer. At a median follow-up of 5 years, there was no difference in overall survival (48% with neoadjuvant chemotherapy and 49% without neoadjuvant chemotherapy) or intact-bladder survival (36% for neoadjuvant chemotherapy versus 40% for no neoadjuvant chemotherapy).199 Preliminary results of alternate fractionation schedules of radiotherapy have been reported by Vikram and colleagues.191 In their report, 21 patients with T2 or T3 disease were treated with three cycles of MVAC, rapidly alternating with three cycles of accelerated irradiation. Three cycles of twice-a-day irradiation of 20 Gy in 10 fractions was administered with the first two cycles to the true pelvis and the last with a cone-down approach. At a median follow-up of 2 years, the observed survival rate is 72%, with normal bladder function in 84% of evaluable patients. Given the high risk of distant metastases as the most common mode of treatment failure, adjuvant chemotherapy has been incorporated into current RTOG trials. The optimal regimen, cycle number, and patient selection for adjuvant chemotherapy following combined modality bladder preservation is currently undefined.
Bladder preservation accomplished with combined-modality therapy is an acceptable treatment option for selected patients with muscle-invasive bladder cancer. Critics of this approach have raised concerns about the possible risks of a greater number of deaths associated with this treatment program compared with patients treated with up-front cystectomy. However, there is no evidence that there is any diminution in survival rates from any of the published studies among patients who underwent radiotherapy and subsequently required a salvage cystectomy. Although concerns have also been raised regarding the extended treatment time and morbidity associated with combined-modality chemotherapy, these are unfounded as the long-term toxicity outcomes have been minimal. In fact, it is rare for patients to require a cystectomy for treatment-related morbidity without evidence of recurrence. Several reports evaluating long-term bladder function demonstrate excellent bladder function based on a patient questionnaire.211,212 In a report by Zietman et al.,212 71 patients were asked to undergo urodynamic testing and complete a quality-of-life questionnaire, of whom 69% complied, with a median time from treatment of 6.3 years. Of these patients, 75% had normally functioning bladders on urodynamics. Reduced bladder compliance was seen in 22% but in only one-third of these was it reflected by symptomatology. Difficulty with bladder control was seen in 19% with 11% wearing pads (all women). The majority of men retained sexual function and global health-related quality of life remained high.
Simulation and Treatment
During simulation and treatment, the patient is supine and the bladder is emptied to ensure reproducibility. CT simulation is the gold standard, as two-dimensional simulation using a cystogram has a risk of geographic miss. Visualization of the pelvic vessels and bladder tumor may be enhanced using intravenous contrast. At the time of simulation, a Foley catheter may be inserted and a small volume (25 to 30 ml) of radiopaque contrast material may be instilled with or without 10 to 15 ml of air for bladder visualization. One must be cautious to ensure that the bladder volume is not significantly increased such that daily reproducibility will be compromised. A rectal tube with or without barium contrast medium is placed to visualize the rectum. If using contrast, similar care regarding rectal volume reproducibility should be used. CT planning involves the delineation of normal tissue and target structures on CT images using treatment-planning software. Various approaches to defining the clinical target volume exist. The most common approaches are (1) a two-phase approach to encompass the whole pelvis (including pelvic nodes) followed by a boost to the bladder or involved bladder wall or (2) a single-phase approach encompassing the bladder alone to full dose. Alternatives may also include treatment to the involved bladder wall alone in an effort to spare uninvolved bladder and bowel. Proponents of bladder-only treatment cite no significant increase in pelvic recurrence in these patients and no negative effect on survival; however, direct comparisons are difficult.213 A recently closed UK phase II trial, BC2001, is designed to address target volume and the results are anticipated.214
The classic radiation fields include elective pelvic nodal irradiation in addition to the bladder and proximal urethra followed by a boost to the involved bladder (Fig. 44-10A). In general, a four-field box approach is used (anterior, posterior, and lateral fields) delivered through 15- to 25-MV x-rays. The initial fields encompass the entire bladder and the first-echelon draining lymph nodes. The superior border of the anterior-posterior–posterior-anterior fields is the L5 to S1 interspace. The inferior border of these fields is at the level of the bottom of the obturator foramina, and the lateral fields generally extend 1.5 to 2 cm beyond the widest portion of the bony pelvis. The anterior border of the lateral fields extends to the anterior bladder wall with a 1.5- to 2-cm margin. Often, the anterior border may extend several centimeters beyond the symphysis pubis. The posterior border of the lateral fields can be placed with a 2- to 3-cm margin posterior to the bladder, which will incorporate the presacral lymph nodes.
To properly plan the boost portion of therapy, which is typically limited to the involved area within the bladder, the radiation oncologist will need to know the location of the tumor based on the pretreatment cystoscopy with a detailed bladder map and findings from the examination under anesthesia or from pre-TURBT radiographic imaging and any extravesical disease should be included in the boost target volume. A treatment-planning CT scan can be obtained and target volumes delineated. Often, a full bladder is helpful during the boost simulation and treatment to minimize the irradiated bowel and uninvolved bladder mucosa in the high-dose volume. Care must be taken to ensure similar degrees of bladder filling during the planning process and during treatment, and ultrasound verification may be adviseable. Although opposed lateral fields (see Fig. 44-10B) have been used on prior RTOG protocols, a multifield technique may be used that minimizes the volume of normal bladder exposed to the prescription radiation dose. In some cases based on the location of the bladder tumor (i.e., limited to the dome or anterior-posterior wall), lateral fields alone may be sufficient for this phase of the therapy. More recently, the authors have incorporated 3-D treatment planning for planning the cone-down phase of therapy (Fig. 44-11). A multifield plan can be used in an effort to minimize the dose to uninvolved regions of the bladder as well as other normal tissue structures such as the rectum, bowel, and femoral heads.
Intensity-modulated radiation therapy has been implemented in many disease sites in an effort to improve treatment results and reduce toxicity. For bladder cancer treatment, this approach is particularly appealing, given the difficulty in delivering tumoricidal radiation doses in the pelvis adjacent to radiosensitive bowel and other organs. The steep dose gradients achievable with intensity-modulated radiation therapy, however, mean that organ-motion uncertainties may lead to significant tumor underdosage or normal tissue overdosage. Several studies show significant bladder motion during a course of standard radiotherapy.215 Muren et al.215 found bladder wall motion of greater than 15 mm in up to 40% of patients with maximal displacement of 29 to 36 mm as well as significant variations in rectal volumes and small bowel position. Pos et al.216 measured organ motion from four weekly repeat CT scans and found that, despite treatment margins of 1.5 to 2 cm, the gross tumor volume was outside of the planning tumor volume at least once in 65% of patients treated with a full bladder. Most studies are in agreement that the predominant bladder motion is seen in the superior and anterior directions and the least motion noted in the inferior direction.
Image-guided radiation therapy may be the most promising step toward more precise radiation delivery. Using daily imaging, the radiation oncologist may be able to adapt treatment delivery to account for these large variations in target motion. This could potentially allow a reduction of the large treatment margins currently necessary, thus allowing safe dose escalation. Several different approaches are currently being explored, including fiducial marker placement and daily two-dimensional kV imaging217 or 3-D cone-beam CT imaging,218 which allows soft-tissue visualization immediately prior to treatment while the patient is on the treatment couch. One additional benefit of cystoscopically placed markers is the added ability to accurately define the tumor bed and reduce uncertainties in target definition.
Acute and Late Toxicity
Radiotherapy is generally well tolerated; however, typical acute radiation-induced side effects include dysuria, urgency, urinary frequency, and diarrhea as acute self-limiting symptoms. Typically, these symptoms increase over the course of therapy and following therapy slowly resolve. Some patients are predisposed to more acute reactions if they have had multiple prior TURBTs and intravesical therapies. The incidence of long-term complications is acceptable when proper treatment techniques are used. In general, such complications become clinically manifest within the first 3 years after radiotherapy and include chronic irritative cystitis and hemorrhagic cystitis. In a reported series from Germany on 112 patients treated with chemoradiation to the whole bladder to 55.8 to 59.4 Gy, 79% of patients who retained their bladders were delighted or pleased with their urinary function as assessed by the International Prostate Symptom Score sheet. The incidence of grade 3 Late Effects in Normal Tissues—Subjective, Objective, Management and Analytic Scales toxicity (i.e., reduced bladder capacity [100-200 ml] with micturition at less than 2-hour intervals), was 9% with only 0.9% requiring salvage cystectomy because of bladder contracture. Bowel obstruction requiring surgical intervention occurred in 1.4%. A range of toxicities was reported in a series of 135 patients treated at M.D. Anderson Hospital between 1960 and 1984 in which the majority were treated to a dose of 40 to 50 Gy with a cone-down technique for a median total dose of 66 Gy.176,177 Late complications included hematuria, bladder contracture, bladder and rectal ulceration, rectal stricture, and small bowel obstruction. Severe complications requiring surgery occurred in 12%. Analysis indicated that patients treated with a four-field technique versus arc rotation, higher energy machines as opposed to cobalt, and less than 70 Gy had significantly lower rates of severe complications. A measurement of quality of bladder function on a more modern series of patients was performed by Lynch and colleagues219 at the Royal London Hospital. The 72 patients who showed an initial complete response to radiotherapy for muscle-invasive bladder cancer were assessed for hematuria, frequency, incontinence, and rectal symptoms. The bowel and bladder symptomatology scores were found to be similar to those of a group of 55 inpatients in other surgical wards who did not have a history of urinary or bowel disease. Cox and coworkers171 reported the results of a phase I study in which patients were treated with 1.2 Gy fractions twice daily to a cumulative dose of 60 to 69.6 Gy. Treatment was well tolerated, whereas the incidence of grade III or IV late toxicity was 11% for patients treated at the highest dose level. Most recently, investigators from the Massachusetts General Hospital reported the results of a retrospective quality-of-life evaluation performed on 21 women who underwent bladder-preservation therapy for invasive bladder cancer.220 Of 21 patients, 19 reported no change or improvement of their baseline bladder capacity and function. No patient reported any symptoms of bowel incontinence. With the advent of new imaging modalities and 3-D conformal treatment-planning technologies that allow for enhanced accuracy of radiation delivery and further limit the volume of normal organs exposed to the doses of therapy, improved results are likely with a further reduction in treatment-related toxicity.
Brachytherapy
Brachytherapy for bladder cancer was originally developed in the early twentieth century and typically consisted of permanent radon seed implantation or temporary radium needle insertion.221 Because of crude techniques causing high complication rates and high radiation exposure to staff, brachytherapy for the treatment of bladder cancer fell out of favor. With the subsequent development of more precise techniques and afterloading capability, there has been renewed interest in this approach for bladder-preserving treatment. As with other bladder-sparing approaches, there is general agreement that small (<5 cm), solitary, organ-confined tumors are most amenable to brachytherapy-based treatment.
Although the experience with brachytherapy for localized bladder cancer has been limited in the United States, an extensive experience with radium needle implants has been reported by Van der Werf-Messing and colleagues from Rotterdam. The Rotterdam series established the importance of preoperative external-beam radiation prior to brachytherapy, as this significantly decreased scar metastases, which were a frequent component of treatment failure prior to its use. In the most recent Rotterdam report, 328 patients with T2 disease and 63 patients with T3 muscle-invasive bladder cancer were treated with preoperative radiation of three fractions each at a dose of 3.5 Gy followed by radium implantation.222 Local recurrence at 5 years was 16% for T2 tumors and 28% for T3 tumors. Survival was similar to historic controls undergoing definitive external-beam radiotherapy or cystectomy, with 56% of patients with T2 disease and 37% of those with T3 disease alive at 5 years. In addition to stage, other poor prognostic factors were identified, including grade, vascular invasion, pathologic findings on intravenous pyelography, and more than one transurethral resection.
The Netherlands and France have the largest reported experiences establishing indications and techniques for bladder brachytherapy. Most series incorporate a suprapubic cystotomy with a unilateral or bilateral pelvic lymphadenectomy approximately 1 to 2 weeks following completion of preoperative radiation. Typically, a partial cystectomy is subsequently performed, removing the residual disease with a 1-cm margin. In some series, only a cystotomy is performed. A target area is identified and can be marked with radio-opaque markers to assist in treatment planning. In the afterloading technique, afterloading catheters are placed into the bladder wall target area in a parallel arrangement 1 to 1.5 cm apart (Fig. 44-12). The catheters are brought to the abdominal wall surface and secured. Treatment planning can currently be accomplished using 3-D CT-based dosimetry. Dose prescriptions vary considerably (30-60 Gy) with higher doses given in patients with T1 and favorable T2 tumors with shorter courses of external-beam radiation; however, there is currently no established consensus. Following treatment, catheters can be removed, generally with minimal anesthesia.
Since the introduction of afterloading techniques, most current series use iridium.223–226 Mazeron et al.225 treated 30 patients with T2 disease and 5 with T3 disease using a single fraction of 8.5 Gy, followed by partial cystectomy and iridium implantation, and attained excellent local control. Rozan and colleagues226 reported a multicenter French experience that included 98 patients with pT1-stage disease, 66 with pT2, 26 with pT3a, 9 with pT3b, and 1 with pT4 treated by preoperative external-beam irradiation, partial cystectomy with lymphadenectomy, and placement of an iridium implant into the tumor bed. Preoperative radiation was targeted to the bladder in 44 patients and to the pelvis in 161 patients for a mean total dose of 11 Gy and a mean dose per fraction of 5.4 Gy. Of the 36 tumor recurrences, 9 were located at the original site and 19 occurred in a different site. They reported an overall 5-year survival of 67%. Survival was 77% for pT1, 63% for pT2, and 47% for pT3a disease. Serious complications included 8 patients with hematuria, 17 patients with chronic cystitis, and 11 with fistulas. Blank et al.227 reported results of 122 patients treated with external-beam radiation followed by partial cystectomy (n = 37) or cystotomy (n = 85) and an iridium-192 implant. Brachytherapy consisted of either pulsed-dose-rate (n = 23) or continuous low-dose rate (n = 99) with doses varied from 20 to 50 Gy, depending on the extent of surgery and external-beam radiation dose. At 5 years, the local relapse-free survival was 76% and distant-metastasis-free survival was 83%. The overall survival rate was 49% at 10 years. Complications included two patients with ileus managed conservatively and three patients with crippled bladder function.
Salvage Therapy for Local Recurrence
The key to the success of bladder-preservation strategies with respect to outcomes is close cystoscopic surveillance following therapy. A significant concern is the development of recurrent disease. For muscle-invasive recurrence, prompt salvage cystectomy should be performed. As previously discussed, survival for these patients does not appear compromised compared with patients undergoing immediate cystectomy. The management of superficial recurrences is also important, because some of these lesions, even in a de novo setting, may exhibit aggressive behavior despite aggressive surgical management. Several reports have demonstrated that superficial recurrences in a retained bladder following multimodality bladder-preservation therapy may be managed as de novo disease.228,229 Weiss et al.228 reported results on 531 consecutive patients treated with combined-modality bladder preservation and found that 17% (68/389) of patients achieving a complete response to therapy developed superficial recurrences (≤T1). Of the 64 patients who underwent conservative management, consisting of a transurethral resection with or without intravesical therapy, 48% had no further recurrence, 33% experienced additional superficial recurrences, and 19% progressed to invasive disease. Compared with patients without bladder relapse, there was no difference in disease-specific survival (72% with superficial recurrence versus 79% without recurrence; P = 0.78). Zietman et al.229 reported results for 190 patients treated with chemoradiation for bladder cancer and found 57 separate superficial recurrences in 32 patients. Of the superficial recurrences, 67% occurred within the same subregion as the original invasive tumor. Of the 28 conservatively treated patients, 18 (64%) had no further recurrence during the period of observation. Seven had additional superficial recurrences, of which three were managed conservatively and four with cystectomy. Three of the 28 patients ultimately developed invasive tumors requiring cystectomy. Only 1 of the 32 patients with superficial relapse developed uncontrolled pelvic disease. The response of these superficial tumors to conservative therapy is similar to results in patients with primary superficial disease.
Chemotherapy for Metastatic Disease
Reported survival for patients with metastatic bladder cancer treated on chemotherapy trials varies widely. This wide variation can be explained by pretreatment disease and patient-related factors. In an effort to define the effect of pretreatment patient characteristics on clinical outcome, Bajorin and colleagues conducted a multivariate analysis evaluating 18 variables in 203 patients treated with MVAC.230 In this analysis, the only variables that were prognostic of survival were Karnofsky performance status (KPS) lower than 80% and the presence of visceral metastases. Patients with a KPS lower than 80% and visceral metastases had a median survival of 9.3 months, compared with patients who had no adverse prognostic factors and whose median survival was 33 months.230 Prognostic factors for survival were also evaluated in a 405-patient randomized trial of combination cisplatin-based chemotherapy. This analysis concluded that improved overall survival was associated with a KPS greater than 70%, absence of distant metastases, low or normal alkaline phosphatase, fewer than three sites of disease, and the absence of visceral metastases.231 An understanding of prognostic features of patients enrolled in prospective clinical trials may allow more sophisticated and accurate interpretation of outcomes reported in phase 2 clinical trials, allowing for improved selection of treatments for phase III testing. Identification of additional pretreatment prognostic factors will allow more accurate identification of patients at high risk for death from their cancer.
Historically, MVAC had been considered the standard therapy for treating advanced bladder-cancer patients. The efficacy of MVAC was first reported in 1989 when Sternberg and colleagues treated 121 patients with advanced urothelial tract cancers and demonstrated a 72% response rate.232 MVAC was subsequently compared with single-agent cisplatin and shown to be superior in terms of response rate and overall survival in patients with advanced bladder cancer.233 MVAC has also been compared with the previously used multiagent regimen CISCA: 110 patients were randomized to either MVAC or CISCA. MVAC was found to have both a higher objective response rate and a longer median survival.234 Despite superior outcome with MVAC, significant limitations include its severe toxicity (mucositis, infectious complications, and a 3% toxic death rate are consistently seen in randomized trials). In addition, despite high objective response rates, only a very small percentage of patients with metastatic bladder cancer have long-term disease-free survival (3.7% at 6 years).233
MVAC has been administered in a dose-intense fashion with the incorporation of prophylactic filgrastim to prevent excessive toxicity. A randomized phase III study was conducted comparing dose-intense MVAC administered every 2 weeks to standard MVAC in 263 patients with untreated metastatic transitional cell carcinoma. The dose-intense MVAC arm was associated with objective responses in 64% of patients compared with 50% with standard-dose MVAC. Long-term follow-up of this trial demonstrated that 24.6% of patients on the dose-intense arm were alive at 7.3 years, compared with 13.2% on the standard-dose MVAC arm, although the median survival was similar between the two regimens.235 The hazard ratio for survival was 0.76 (95% CI = 0.58-0.99). Toxicity with dose-intense MVAC treatment appears to be similar to or better than standard-dose MVAC treatment. Dose-intense MVAC remains a standard treatment option for patients with advanced transitional cell carcinoma.
The GC regimen has largely replaced MVAC as the standard of care based on a randomized trial comparing the two regimens.169 This trial was designed to demonstrate superior efficacy for GC. Although the trial was not sufficiently powered to prove equivalence of GC to MVAC, this 405-patient trial showed similar anti-tumor efficacy (equal complete response rate and similar partial response rate) and a hazard ratio for survival approaching 1.0. An overall response proportion of approximately 50% was observed with both regimens. Long-term follow-up showed no difference in survival (13% in patients treated with GC, 15.3% in patients treated with MVAC). However, toxicity in GC-treated patients was substantially lower than observed in patients treated with MVAC, including a lower death rate, fewer infections, and less mucositis. This markedly superior therapeutic index has led to fairly wide acceptance of GC as the standard first-line therapy for metastatic bladder cancer.
Taxanes have significant activity in advanced transitional carcinoma. Paclitaxel has been tested in combination with gemcitabine and cisplatin and demonstrated significant activity in phase II testing.236 Phase III evaluation of the triplet regimen compared with GC alone has been reported in abstract form, and suggests that there is no significant improvement with the addition of a third agent to GC chemotherapy.237
Unfortunately, many patients with advanced transitional cell carcinoma have renal insufficiency and are unable to undergo therapy with cisplatin or other potentially nephrotoxic agents. Consequently, regimens with less nephrotoxic drugs have been developed. These have included single-agent treatments with drugs such as the taxanes, gemcitabine, and carboplatin.238–247 The median survival reported for patients with renal insufficiency (in predominantly single-institution studies) ranges from 8 to 10 months, and is generally inferior to the results of the large multicenter trial (n = 405) comparing GC to MVAC in patients with nonimpaired renal function, which found median survivals of 13.8 and 14.8 months, respectively.202 Whether this is attributable to a fundamentally worse prognosis in patients with renal insufficiency or to the use of less effective regimens is not known. Frequently-used combinations in metastatic transitional cell carcinoma patients with renal insufficiency include gemcitabine/carboplatin and carboplatin/paclitaxel. Optimal chemotherapy for patients with renal insufficiency remains undefined.
Palliative Radiotherapy
Bladder cancer can metastasize to various visceral organs, causing symptoms that require palliation. Radiation therapy is an effective therapy for pain or hematuria associated with advanced disease. The treatment approach in advanced disease is dictated by a desire to deliver therapy for rapid relief of pain or hematuria with minimal inconvenience to the patient. This can be accomplished with a short course of radiation with a high dose per fraction. Srinivasan and associates248 compared in 41 patients a conventional regimen of 45 Gy in 12 fractions over 26 days to a hypofractionated treatment of 17 Gy in 2 fractions over 3 days.248 In patients receiving the hypofractionated regimen, 59% had a clearance of hematuria and 73% had an improvement in pain. This was superior to the conventional treatment that resulted in a 16% clearance of hematuria and a 37% improvement in pain. Median survival was higher at 14 months for those treated conventionally compared with 10 months for those receiving the hypofractionated treatment.
A palliative regimen of 30 Gy in six fractions of two fractions per week was reported by Salminen249 in 94 patients. Forty attained complete palliation and 27 had partial improvement, although many suffered acute side effects, including diarrhea in nearly 60% of patients. A regimen of 21 Gy in three fractions over 5 days resulted in palliation of 75 of 102 patients but at a cost of significant acute toxicity.250 External-beam irradiation has had mixed results in relieving ureteral obstruction.251 The obstruction was relieved for 20%, unchanged in 24%, and worse in 56% after a 60-Gy course in 2-Gy fractions for 55 patients with 68 obstructed kidneys. This limited success was at a cost of vesicovaginal fistulas in 1 patient, contracted bladders in 22 patients, and diarrhea in 25 patients. Median survival was 11 months, with a median of 9.5 months spent at home. A randomized trial conducted by the Medical Research Council demonstrated that 21 Gy in 3 fractions were as efficacious as 35 Gy in 10 fractions for palliation of local bladder symptoms.252 Symptomatic improvement was reported in 64% of patients in the short-course arm and 71% in the longer course but this did not reach statistical significance. Toxicity was modest and similar in both arms. Shorter courses of palliative radiation therapy are ideal for rapid palliation in poor-prognosis, ill patients whereas longer courses of therapy with or without chemotherapy may be appropriate for patients with a good performance status and a lower disease burden. When chemotherapy is used, lower daily doses (1.8-2.5 Gy) should be used with total doses of 45 to 50 Gy. The optimal regimen should be tailored to the individual patient.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
2 Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107:1711-1742.
3 Silverman DT, Hartge P, Morrison AS, et al. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 1992;6:1-30.
4 Anton-Culver H, Lee-Feldstein A, Taylor TH. The association of bladder cancer risk with ethnicity, gender, and smoking. Ann Epidemiol. 1993;3:429-433.
5 Chyou PH, Nomura AM, Stemmermann GN. A prospective study of diet, smoking, and lower urinary tract cancer. Ann Epidemiol. 1993;3:211-216.
6 Momas I, Daures JP, Festy B, et al. Bladder cancer and black tobacco cigarette smoking. Some results from a French case-control study. Eur J Epidemiol. 1994;10:599-604.
7 Siemiatycki J, Dewar R, Krewski D, et al. Are the apparent effects of cigarette smoking on lung and bladder cancers due to uncontrolled confounding by occupational exposures? Epidemiology. 1994;5:57-65.
8 Sorahan T, Lancashire R, Sole G. Urothelial cancer and cigarette smoking: findings from a regional case-controlled study. Br J Urol. 1994;74:753.
9 Salminen E, Pukkala E, Teppo L. Bladder cancer and the risk of smoking-related cancers during followup. J Urol. 1994;152:1420-1423.
10 Yu MC, Skipper PL, Tannenbaum SR, et al. Arylamine exposures and bladder cancer risk. Mutat Res. 2002;506–507:21-28.
11 Esteban Castelao J, Yuan JM, Skipper PL, et al. Gender- and smoking-related bladder cancer risk. J Nat Cancer Inst. 2001;93:538-545.
12 Bjerregaard BK, Raaschou-Nielsen O, Sorensen M, et al. Tobacco smoke and bladder cancer—in the European prospective investigation into cancer and nutrition. Int J Cancer. 2006;119:2412-2416.
13 Kunze E, Chang-Claude J, Frentzel-Beyme R. Life style and occupational risk factors for bladder cancer in Germany, a case-control study. Cancer. 1992;69:1776-1790.
14 Bi W, Hayes RB, Feng P, et al. Mortality and incidence of bladder cancer in benzidine-exposed workers in China. Am J Ind Med. 1992;21:481-489.
15 Zheng W, McLaughlin JK, Gao YT, et al. Bladder cancer and occupation in Shanghai, 1980–1984. Am J Ind Med. 1992;21:877-885.
16 Barbone F, Franceschi S, Talamini R, et al. Occupation and bladder cancer in Pordenone (north-east Italy): a case-control study. Int J Epidemiol. 1994;23:58-65.
17 Tremblay C, Armstrong B, Theriault G, et al. Estimation of risk of developing bladder cancer among workers exposed to coal tar pitch volatiles in the primary aluminum industry. Am J Ind Med. 1995;27:335-348.
18 Vineis P. Epidemiology of cancer from exposure to arylamines. Environ Health Perspect. 1994;102(Suppl 6):7-10.
19 Gago-Dominguez M, Castelao JE, Yuan JM, et al. Use of permanent hair dyes and bladder-cancer risk. Int J Cancer. 2001;91:575-579.
20 Czene K, Tiikkaja S, Hemminki K. Cancer risks in hairdressers: assessment of carcinogenicity of hair dyes and gels. Int J Cancer. 2003;105:108-112.
21 Takkouche B, Etminan M, Montes-Martinez A. Personal use of hair dyes and risk of cancer: a meta-analysis. JAMA. 2005;293:2516-2525.
22 Kelsh MA, Alexander DD, Kalmes RM, et al. Personal use of hair dyes and risk of bladder cancer: a meta-analysis of epidemiologic data. Cancer Causes Control. 2008;19:549-558.
23 Weiss NS. Cancer in relation to occupational exposure to perchloroethylene. Cancer Causes Control. 1995;6:257-266.
24 Badawi AF, Mostafa MH, Probert A, et al. Role of schistosomiasis in human bladder cancer: evidence of association, aetiological factors, and basic mechanisms of carcinogenesis. Eur J Cancer Prev. 1995;4:45-59.
25 Warren W, Biggs PJ, el-Baz M, et al. Mutations in the p53 gene in schistosomal bladder cancer: a study of 92 tumours from Egyptian patients and a comparison between mutational spectra from schistosomal and non-schistosomal urothelial tumours. Carcinogenesis. 1995;16:1181-1189.
26 Rosin MP, Anwar WA, Ward AJ. Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res. 1994;54:1929s-1933s.
27 Bickel A, Culkin DJ, Wheeler JSJr. Bladder cancer in spinal cord injury patients. J Urol. 1991;146:1240-1242.
28 Gonzalez CA, Errezola M, Izarzugaza I, et al. Urinary infection, renal lithiasis and bladder cancer in Spain. Eur J Cancer. 1991;27:498-500.
29 Shirai T. Etiology of bladder cancer. Semin Urol. 1993;11:113-126.
30 Magee PN, Barnes JM. Carcinogenic nitroso compounds. Adv Cancer Res. 1967;10:163-246.
31 Kauffman EC, Raman JD. Bladder cancer following upper tract urothelial carcinoma. Expert Rev Anticancer Ther. 2008;8:75-85.
32 Viscoli CM, Lachs MS, Horwitz RI. Bladder cancer and coffee drinking: a summary of case-control research. Lancet. 1993;341:1432-1437.
33 Stensvold I, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 1994;5:401-408.
34 Zeegers MP, Dorant E, Goldbohm RA, et al. Are coffee, tea, and total fluid consumption associated with bladder cancer risk? Results from the Netherlands Cohort Study. Cancer Causes Control. 2001;12:231-238.
35 Elcock M, Morgan RW. Update on artificial sweeteners and bladder cancer. Regul Toxicol Pharmacol. 1993;17:35-43.
36 Nomura AM, Kolonel LN, Hankin JH, et al. Dietary factors in cancer of the lower urinary tract. Int J Cancer. 1991;48:199-205.
37 Vena JE, Graham S, Freudenheim J, et al. Diet in the epidemiology of bladder cancer in western New York. Nutr Cancer. 1992;18:255-264.
38 McGeehin MA, Reif JS, Becher JC, et al. Case-control study of bladder cancer and water disinfection methods in Colorado. Am J Epidemiol. 1993;138:492-501.
39 Morales Suarez-Varela M, Llopis Gonzalez A, Tejerizo Perez ML, et al. Concentration of nitrates in drinking water and its relationship with bladder cancer. J Environ Pathol Toxicol Oncol. 1993;12:229-236.
40 Kleinerman RA, Boice JDJr, Storm HH, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76:442-452.
41 Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89:1429-1439.
42 Boorjian S, Cowan JE, Konety BR, et al. Bladder cancer incidence and risk factors in men with prostate cancer: results from Cancer of the Prostate Strategic Urologic Research Endeavor. J Urol. 2007;177:883-887.
43 Chrouser K, Leibovich B, Bergstralh E, et al. Bladder cancer risk following primary and adjuvant external beam radiation for prostate cancer. J Urol. 2005;174:107-110.
44 Muller A, Ganswindt U, Bamberg M, et al. Risk of second malignancies after pelvic irradiation? Strahlenther Onkol. 2007;183:605-609.
45 Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum. 1995;38:1120-1127.
46 Marcus PM, Vineis P, Rothman N. NAT2 slow acetylation and bladder cancer risk: a meta-analysis of 22 case-control studies conducted in the general population. Pharmacogenetics. 2000;10:115-122.
47 Bell DA, Taylor JA, Paulson DF, et al. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993;85:1159-1164.
48 Wijkstrom H, Naslund I, Ekman P, et al. Short-term radiotherapy as palliative treatment in patients with transitional cell bladder cancer. Br J Urol. 1991;67:74-78.
49 Sandberg AA, Berger CS. Review of chromosome studies in urological tumors. II. Cytogenetics and molecular genetics of bladder cancer. J Urol. 1994;151:545-560.
50 Schapers RF, Smeets AW, Pauwels RP, et al. Cytogenetic analysis in transitional cell carcinoma of the bladder. Br J Urol. 1993;72:887-892.
51 Lipponen PK, Eskelinen MJ, Nordling S. Progression and survival in transitional cell bladder cancer: a comparison of established prognostic factors, S-phase fraction and DNA ploidy. Eur J Cancer. 1991;27:877-881.
52 Nemoto R, Nakamura I, Uchida K, et al. Numerical chromosome aberrations in bladder cancer detected by in situ hybridization. Br J Urol. 1995;75:470-476.
53 Knowles MA. Role of FGFR3 in urothelial cell carcinoma: biomarker and potential therapeutic target. World J Urol. 2007;25:581-593.
54 Borland RN, Brendler CB, Isaacs WB. Molecular biology of bladder cancer. Hematol Oncol Clin North Am. 1992;6:31-39.
55 Sauter G, Moch H, Carroll P, et al. Chromosome-9 loss detected by fluorescence in situ hybridization in bladder cancer. Int J Cancer. 1995;64:99-103.
56 Matsuyama H, Bergerheim US, Nilsson I, et al. Nonrandom numerical aberrations of chromosomes 7, 9, and 10 in DNA-diploid bladder cancer. Cancer Genet Cytogenet. 1994;77:118-124.
57 Cairns P, Shaw ME, Knowles MA. Initiation of bladder cancer may involve deletion of a tumour-suppressor gene on chromosome 9. Oncogene. 1993;8:1083-1085.
58 Spruck CH3rd, Ohneseit PF, Gonzalez-Zulueta M, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994;54:784-788.
59 Voorter C, Joos S, Bringuier PP, et al. Detection of chromosomal imbalances in transitional cell carcinoma of the bladder by comparative genomic hybridization. Am J Pathol. 1995;146:1341-1354.
60 Dalbagni G, Presti J, Reuter V, et al. Genetic alterations in bladder cancer. Lancet. 1993;342:469-471.
61 Tiniakos DG, Mellon K, Anderson JJ, et al. C-jun oncogene expression in transitional cell carcinoma of the urinary bladder. Br J Urol. 1994;74:757-761.
62 Burchill SA, Neal DE, Lunec J. Frequency of H-ras mutations in human bladder cancer detected by direct sequencing. Br J Urol. 1994;73:516-521.
63 Lipponen P. Expression of c-erbB-2 oncoprotein in transitional cell bladder cancer. Eur J Cancer. 1993;29A:749-753.
64 Presti JCJr, Reuter VE, Galan T, et al. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991;51:5405-5409.
65 Kubota Y, Miyamoto H, Noguchi S, et al. The loss of retinoblastoma gene in association with c-myc and transforming growth factor-beta 1 gene expression in human bladder cancer. J Urol. 1995;154:371-374.
66 Dalbagni G, Cordon-Cardo C, Reuter V, et al. Tumor suppressor gene alterations in bladder carcinoma. Translational correlates to clinical practice. Surg Oncol Clin N Am. 1995;4:231-240.
67 Chang WY, Cairns P, Schoenberg MP, et al. Novel suppressor loci on chromosome 14q in primary bladder cancer. Cancer Res. 1995;55:3246-3249.
68 Kallioniemi A, Kallioniemi OP, Citro G, et al. Identification of gains and losses of DNA sequences in primary bladder cancer by comparative genomic hybridization. Genes Chromosomes Cancer. 1995;12:213-219.
69 Tomovska S, Richter J, Suess K, et al. Molecular cytogenetic alterations associated with rapid tumor cell proliferation in advanced urinary bladder cancer. Int J Oncol. 2001;18:1239-1244.
70 Esrig D, Spruck CH3rd, Nichols PW, et al. p53 Nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol. 1993;143:1389-1397.
71 Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259-1264.
72 Sarkis AS, Dalbagni G, Cordon-Cardo C, et al. Association of p53 nuclear overexpression and tumor progression in carcinoma in situ of the bladder. J Urol. 1994;152:388-392.
73 Uchida T, Wada C, Ishida H, et al. p53 Mutations and prognosis in bladder tumors. J Urol. 1995;153:1097-1104.
74 Kusser WC, Miao X, Glickman BW, et al. p53 Mutations in human bladder cancer. Environ Mol Mutagen. 1994;24:156-160.
75 Lianes P, Orlow I, Zhang ZF, et al. Altered patterns of MDM2 and TP53 expression in human bladder cancer. J Natl Cancer Inst. 1994;86:1325-1330.
76 Dalbagni G, Presti JCJr, Reuter VE, et al. Molecular genetic alterations of chromosome 17 and p53 nuclear overexpression in human bladder cancer. Diagn Mol Pathol. 1993;2:4-13.
77 Fujimoto K, Yamada Y, Okajima E, et al. Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res. 1992;52:1393-1398.
78 Williams P, Warwick R, Dyson M, et al. Gray’s anatomy. New York: Churchill Livingstone; 1989.
79 Lerner S. Lymphadenectomy for bladder cancer atlas of the urologic clinics of North America: lymphadenectomy for urologic cancers. Philadelphia: WB Saunders; 1995. p. 52
80 Tanagho E. Anatomy of the lower urinary tract. In: Walsh P, editor. Campbell’s urology. Philadelphia: WB Saunders; 1992:40.
81 Blichert-Toft M, Nielsen OV. Congenital patient urachus and acquired variants. Diagnosis and treatment. Review of the literature and report of five cases. Acta Chir Scand. 1971;137:807-814.
82 Johnson DE, Hodge GB, Abdul-Karim FW, et al. Urachal carcinoma. urology. 1985;26:218-221.
83 Smeulders N, Woodhouse CR. Neoplasia in adult exstrophy patients. BJU Int. 2001;87:623-628.
84 Woodhouse CR, North AC, Gearhart JP. Standing the test of time: long-term outcome of reconstruction of the exstrophy bladder. World J Urol. 2006;24:244-249.
85 Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435-1448.
86 Kissane J. Anderson’s pathology. St Louis: CV Mosby; 1990.
87 Someren A. Urologic pathology with clinical and radiologic correlations. New York: Macmillan; 1989.
88 Dickinson AJ, Fox SB, Persad RA, et al. Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br J Urol. 1994;74:762-766.
89 Jaeger TM, Weidner N, Chew K, et al. Tumor angiogenesis correlates with lymph node metastases in invasive bladder cancer. J Urol. 1995;154:69-71.
90 Burnett AL, Epstein JI, Marshall FF. Adenocarcinoma of urinary bladder: classification and management. Urology. 1991;37:315-321.
91 Holmang S, Borghede G, Johansson SL. Primary small cell carcinoma of the bladder: a report of 25 cases. J Urol. 1995;153:1820-1822.
92 Pan CX, Zhang H, Lara PNJr, et al. Small-cell carcinoma of the urinary bladder: diagnosis and management. Expert Rev Anticancer Ther. 2006;6:1707-1713.
93 Aydoganli L, Tarhan F, Atan A, et al. Rhabdomyosarcoma of the urinary bladder in an adult. Int Urol Nephrol. 1993;25:159-161.
94 Suzuki Y, Nakada T, Suzuki H, et al. Primary bladder pheochromocytoma without hypertension. Int Urol Nephrol. 1993;25:153-158.
95 Bitker MO, Bagnis C, Mouquet C, et al. [Primary bladder lymphoma in kidney transplant recipients: report of a case]. Prog Urol. 1992;2:908-912.
96 Kawamura J, Sakurai M, Tsukamoto K, et al. Leiomyosarcoma of the bladder eighteen years after cyclophosphamide therapy for retinoblastoma. Urol Int. 1993;51:49-53.
97 Lange-Welker U, Papadopoulos I, Wacker HH. Primary malignant melanoma of the bladder. A case report and literature review. Urol Int. 1993;50:226-228.
98 Williams JR, Stott MA, Moisey CU. Bilateral hydronephrosis secondary to breast carcinoma metastasizing to the bladder. Br J Urol. 1992;69:97-98.
99 Fair W, Fuks Z, Scher H. Cancer of the bladder. In: Devita V, Hellman S, Roenberg S, editors. Cancer: principles and practice of oncology. Philadelphia: JB Lippincott; 1993:1052.
100 Parsons J, Million R. Bladder. In: Perez C, Brady L, editors. Principles and practice of radiation oncology. Philadelphia: JB Lippincott; 1992:1036.
101 American Joint Committee on Cancer, Urinary bladder. Greene F, Page D, Fleming I, et al, editors; AJCC cancer staging manual; 2002:367.
102 Stein JP, Cai J, Groshen S, et al. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol. 2003;170:35-41.
103 Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol. 1998;160:2015-2019.
104 Skinner DG, Tift JP, Kaufman JJ. High dose, short course preoperative radiation therapy and immediate single stage radical cystectomy with pelvic node dissection in the management of bladder cancer. J Urol. 1982;127:671-674.
105 Liewskovsky G, Ahlering T, Skinner D. Diagnosis and staging of bladder cancer. In: Skinner DG, Lieskovsky G, editors. Diagnosis and management of genitourinary cancer. Philadelphia: WB Saunders, 1988.
106 Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163:524-527.
107 Sultana SR, Goodman CM, Byrne DJ, et al. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol. 1996;78:691-696.
108 Gospodarowicz M. Staging of bladder cancer. Semin Surg Oncol. 1994;10:51.
109 Denis LJ. Clinical staging: Its importance in therapeutic decisions and clinical trials. Hematol Oncol Clin North Am. 1992;6:41-58.
110 Goldman S, Gatewood O. CT and MRI of the genitourinary tract. New York: Churchill Livingstone; 1990.
111 Kim B, Semelka RC, Ascher SM, et al. Bladder tumor staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology. 1994;193:239-245.
112 Tachibana M, Baba S, Deguchi N, et al. Efficacy of gadolinium-diethylenetriaminepentaacetic acid-enhanced magnetic resonance imaging for differentiation between superficial and muscle-invasive tumor of the bladder: a comparative study with computerized tomography and transurethral ultrasonography. J Urol. 1991;145:1169-1173.
113 Hawnaur JM, Johnson RJ, Read G, et al. Magnetic resonance imaging with Gadolinium-DTPA for assessment of bladder carcinoma and its response to treatment. Clin Radiol. 1993;47:302-310.
114 Tanimoto A, Yuasa Y, Imai Y, et al. Bladder tumor staging: comparison of conventional and gadolinium-enhanced dynamic MR imaging and CT. Radiology. 1992;185:741-747.
115 Narumi Y, Kadota T, Inoue E, et al. Bladder tumors: staging with gadolinium-enhanced oblique MR imaging. Radiology. 1993;187:145-150.
116 Maeda H, Kinukawa T, Hattori R, et al. Detection of muscle layer invasion with submillimeter pixel MR images: staging of bladder carcinoma. Magn Reson Imaging. 1995;13:9-19.
117 Robinson P, Collins CD, Ryder WD, et al. Relationship of MRI and clinical staging to outcome in invasive bladder cancer treated by radiotherapy. Clin Radiol. 2000;55:301-306.
118 Pagano F, Bassi P, Ferrante GL, et al. Is stage pT4a (D1) reliable in assessing transitional cell carcinoma involvement of the prostate in patients with a concurrent bladder cancer? A necessary distinction for contiguous or noncontiguous involvement. J Urol. 1996;155:244-247.
119 Bachor R, Kotzerke J, Reske SN, et al. [Lymph node staging of bladder neck carcinoma with positron emission tomography]. Urologe A. 1999;38:46-50.
120 Kosuda S, Kison PV, Greenough R, et al. Preliminary assessment of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with bladder cancer. Eur J Nucl Med. 1997;24:615-620.
121 Angulo JC, Lopez JI, Flores N, et al. The value of tumour spread, grading and growth pattern as morphological predictive parameters in bladder carcinoma. A critical revision of the 1987 TNM classification. J Cancer Res Clin Oncol. 1993;119:578-593.
122 Fung CY, Shipley WU, Young RH, et al. Prognostic factors in invasive bladder carcinoma in a prospective trial of preoperative adjuvant chemotherapy and radiotherapy. J Clin Oncol. 1991;9:1533-1542.
123 Canter D, Guzzo TJ, Resnick MJ, et al. Hydronephrosis is an independent predictor of poor clinical outcome in patients treated for muscle-invasive transitional cell carcinoma with radical cystectomy. Urology. 2008;72:379-383.
124 Fokdal L, Hoyer M, von der Maase H. Treatment outcome and prognostic variables for local control and survival in patients receiving radical radiotherapy for urinary bladder cancer. Acta Oncol. 2004;43:749-757.
125 Itoku KA, Stein BS. Superficial bladder cancer. Hematol Oncol Clin North Am. 1992;6:99-116.
126 Herr HW. Intravesical therapy. Hematol Oncol Clin North Am. 1992;6:117-127.
127 Holmang S, Andius P, Hedelin H, et al. Stage progression in Ta papillary urothelial tumors: Relationship to grade, immunohistochemical expression of tumor markers, mitotic frequency and DNA ploidy. J Urol. 2001;165:1124-1128.
128 Bostwick DG. Natural history of early bladder cancer. J Cell Biochem Suppl. 1992;16I:31-38.
129 Herr HW, Laudone VP, Badalament RA, et al. Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J Clin Oncol. 1988;6:1450-1455.
130 Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: Ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404-1408.
131 Herr HW, Wartinger DD, Fair WR, et al. Bacillus Calmette-Guerin therapy for superficial bladder cancer: a 10-year followup. J Urol. 1992;147:1020-1023.
132 Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90-95.
133 Molto L, Alvarez-Mon M, Carballido J, et al. Use of intracavitary interferon-alpha-2b in the prophylactic treatment of patients with superficial bladder cancer. Cancer. 1995;75:2720-2726.
134 Van der Werf-Messing BH, Friedell GH, Menon RS, et al. Carcinoma of the urinary bladder T3NxMo treated by preoperative irradiation followed by simple cystectomy. Int J Radiat Oncol Biol Phys. 1982;8:1849-1855.
135 Freiha F. Open bladder surgery. In: Walsh P, editor. Campbell’s urology. Philadelphia: WB Saunders; 1992:2750.
136 Richie JP. Surgery for invasive bladder cancer. Hematol Oncol Clin North Am. 1992;6:129-145.
137 Herr HW. Extent of surgery and pathology evaluation has an impact on bladder cancer outcomes after radical cystectomy. Urology. 2003;61:105-108.
138 Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22:2781-2789.
139 Brendler CB, Steinberg GD, Marshall FF, et al. Local recurrence and survival following nerve-sparing radical cystoprostatectomy. J Urol. 1990;144:1137-1140.
140 Parekh DJ, Donat SM. Urinary diversion: options, patient selection, and outcomes. Semin Oncol. 2007;34:98-109.
141 Wang C. Clinical radiation oncology: Indications, techniques, and results. Littleton: Wright-PSG; 1988.
142 Montie JE, Straffon RA, Stewart BH. Radical cystectomy without radiation therapy for carcinoma of the bladder. J Urol. 1984;131:477-482.
143 Wishnow KI, Dmochowski R. Pelvic recurrence after radical cystectomy without preoperative radiation. J Urol. 1988;140:42-43.
144 Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today—a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690-696.
145 Hautmann RE, Gschwend JE, de Petriconi RC, et al. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176:486-492.
146 Boileau MA, Johnson DE, Chan RC, et al. Bladder carcinoma: results with preoperative radiation therapy and radical cystectomy. Urology. 1980;16:569-576.
147 Herr HW. Preoperative irradiation with and without chemotherapy as adjunct to radical cystectomy. Urology. 1985;25:127-134.
148 Skinner DG, Lieskovsky G. Contemporary cystectomy with pelvic node dissection compared to preoperative radiation therapy plus cystectomy in management of invasive bladder cancer. J Urol. 1984;131:1069-1072.
149 Blackard CE, Byar DP. Results of a clinical trial of surgery and radiation in stages II and 3 carcinoma of the bladder. J Urol. 1972;108:875-878.
150 Anderstrom C, Johansson S, Nilsson S, et al. A prospective randomized study of preoperative irradiation with cystectomy or cystectomy alone for invasive bladder carcinoma. Eur Urol. 1983;9:142-147.
151 Crawford ED, Das S, Smith JAJr. Preoperative radiation therapy in the treatment of bladder cancer. Urol Clin North Am. 1987;14:781-787.
152 Slack NH, Bross ID, Prout GRJr. Five-year follow-up results of a collaborative study of therapies for carcinoma of the bladder. J Surg Oncol. 1977;9:393-405.
153 Cole CJ, Pollack A, Zagars GK, et al. Local control of muscle-invasive bladder cancer: preoperative radiotherapy and cystectomy versus cystectomy alone. Int J Radiat Oncol Biol Phys. 1995;32:331-340.
154 Rintala E, Hannisdahl E, Fossa SD, et al. Neoadjuvant chemotherapy in bladder cancer: a randomized study, Nordic Cystectomy Trial I. Scand J Urol Nephrol. 1993;27:355-362.
155 Malmstrom PU, Rintala E, Wahlqvist R, et al. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. The Nordic Cooperative Bladder Cancer Study Group. J Urol. 1996;155:1903-1906.
156 Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533-540.
157 Hall R. Updated results of a randomised controlled trial of neoadjuvant cisplatin (C), methotrexate (M) and vinblastine (V) chemotherapy for muscle-invasive bladder cancer (abstr 710). Proc Am Soc Clin Oncol. 2002:21.
158 Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859-866.
159 Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202-205.
160 Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171:561-569.
161 Hagan MP, Winter KA, Kaufman DS, et al. RTOG 97–06: initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:665-672.
162 Hussain MH, Glass TR, Forman J, et al. Combination cisplatin, 5-fluorouracil and radiation therapy for locally advanced unresectable or medically unfit bladder cancer cases: a Southwest Oncology Group Study. J Urol. 2001;165:56-60.
163 Studer UE, Bacchi M, Biedermann C, et al. Adjuvant cisplatin chemotherapy following cystectomy for bladder cancer: results of a prospective randomized trial. J Urol. 1994;152:81-84.
164 Skinner DG, Daniels JR, Russell CA, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol. 1991;145:459-464.
165 Freiha F, Reese J, Torti FM. A randomized trial of radical cystectomy versus radical cystectomy plus cisplatin, vinblastine and methotrexate chemotherapy for muscle invasive bladder cancer. J Urol. 1996;155:495-499.
166 Stockle M, Meyenburg W, Wellek S, et al. Advanced bladder cancer (stages pT3b, pT4a, pN1 and pN2): Improved survival after radical cystectomy and 3 adjuvant cycles of chemotherapy. Results of a controlled prospective study. J Urol. 1992;148:302-306.
167 Adjuvant chemotherapy for invasive bladder cancer (individual patient data), Cochrane Database Syst Rev CD006018, 2006.
168 Cuccia CA, Jones S, Crigler CM. Clinical impressions in 100 consecutive cases of carcinoma of the urinary bladder treated by supervoltage. J Urol. 1958;79:99-109.
169 Gospodarowicz MK, Hawkins NV, Rawlings GA, et al. Radical radiotherapy for muscle invasive transitional cell carcinoma of the bladder: failure analysis. J Urol. 1989;142:1448-1453.
170 Duncan W, Quilty PM. The results of a series of 963 patients with transitional cell carcinoma of the urinary bladder primarily treated by radical megavoltage x-ray therapy. Radiother Oncol. 1986;7:299-310.
171 Cox JD, Guse C, Asbell S, et al. Tolerance of pelvic normal tissues to hyperfractionated radiation therapy: Results of Protocol 83–08 of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1988;15:1331-1336.
172 Gospodarowicz MK, Warde P. The role of radiation therapy in the management of transitional cell carcinoma of the bladder. Hematol Oncol Clin North Am. 1992;6:147-168.
173 Cole DJ, Durrant KR, Roberts JT, et al. A pilot study of accelerated fractionation in the radiotherapy of invasive carcinoma of the bladder. Br J Radiol. 1992;65:792-798.
174 Bessell EM, Taylor J, Moloney AJ, et al. Regression of transitional cell carcinoma of the bladder with radiotherapy: progression-free control in the bladder at 5 years. Radiother Oncol. 1993;29:344-346.
175 Shipley WU, Prout GRJr, Kaufman SD, et al. Invasive bladder carcinoma. The importance of initial transurethral surgery and other significant prognostic factors for improved survival with full-dose irradiation. Cancer. 1987;60:514-520.
176 Pollack A, Zagars GK, Swanson DA. Muscle-invasive bladder cancer treated with external beam radiotherapy: prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30:267-277.
177 Pollack A, Zagars GZ. Radiotherapy for stage T3b transitional cell carcinoma of the bladder. Semin Urol Oncol. 1996;14:86-95.
178 Quilty PM, Duncan W. Primary radical radiotherapy for T3 transitional cell cancer of the bladder: an analysis of survival and control. Int J Radiat Oncol Biol Phys. 1986;12:853-860.
179 Edsmyr F, Andersson L, Esposti PL, et al. Irradiation therapy with multiple small fractions per day in urinary bladder cancer. Radiother Oncol. 1985;4:197-203.
180 Naslund I, Nilsson B, Littbrand B. Hyperfractionated radiotherapy of bladder cancer. A ten-year follow-up of a randomized clinical trial. Acta Oncol. 1994;33:397-402.
181 Wither H. The four Rs of radiotherapy. Adv Radiat Biol. 1975;5:241-271.
182 Majewski W, Maciejewski B, Majewski S, et al. Clinical radiobiology of stage T2-T3 bladder cancer. Int J Radiat Oncol Biol Phys. 2004;60:60-70.
183 Horwich A, Dearnaley D, Huddart R, et al. A randomised trial of accelerated radiotherapy for localised invasive bladder cancer. Radiother Oncol. 2005;75:34-43.
184 Tester W, Porter A, Asbell S, et al. Combined modality program with possible organ preservation for invasive bladder carcinoma: results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys. 1993;25:783-790.
185 Shipley WU, Kaufman DS, Zehr E, et al. Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology. 2002;60:62-67.
186 Cervek J, Cufer T, Kragelj B, et al. Sequential transurethral surgery, multiple drug chemotherapy and radiation therapy for invasive bladder carcinoma: initial report. Int J Radiat Oncol Biol Phys. 1993;25:777-782.
187 Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061-3071.
188 Housset M, Maulard C, Chretien Y, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol. 1993;11:2150-2157.
189 Sumiyoshi Y, Yokota K, Akiyama M, et al. Neoadjuvant intra-arterial doxorubicin chemotherapy in combination with low dose radiotherapy for the treatment of locally advanced transitional cell carcinoma of the bladder. J Urol. 1994;152:362-366.
190 Rotman M, Macchia R, Silverstein M, et al. Treatment of advanced bladder carcinoma with irradiation and concomitant 5-fluorouracil infusion. Cancer. 1987;59:710-714.
191 Vikram B, Malamud S, Silverman P, et al. A pilot study of chemotherapy alternating with twice-a-day accelerated radiation therapy as an alternative to cystectomy in muscle infiltrating (stages T2 and T3) cancer of the bladder: preliminary results. J Urol. 1994;151:602-604.
192 Shipley WU, Prout GRJr, Einstein AB, et al. Treatment of invasive bladder cancer by cisplatin and radiation in patients unsuited for surgery. JAMA. 1987;258:931-935.
193 Kaufman DS, Shipley WU, Griffin PP, et al. Selective bladder preservation by combination treatment of invasive bladder cancer. N Engl J Med. 1993;329:1377-1382.
194 Coppin CM, Gospodarowicz MK, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14:2901-2907.
195 Shipley WU, Rose MA, Perrone TL, et al. Full-dose irradiation for patients with invasive bladder carcinoma: Clinical and histological factors prognostic of improved survival. J Urol. 1985;134:679-683.
196 Dunst J, Sauer R, Schrott KM, et al. Organ-sparing treatment of advanced bladder cancer: a 10-year experience. Int J Radiat Oncol Biol Phys. 1994;30:261-266.
197 Herr HW. Conservative management of muscle-infiltrating bladder cancer: prospective experience. J Urol. 1987;138:1162-1163.
198 Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol. 1996;14:119-126.
199 Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576-3583.
200 Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471-476.
201 Shipley WU, Kaufman DS, Tester WJ, et al. Overview of bladder cancer trials in the Radiation Therapy Oncology Group. Cancer. 2003;97:2115-2119.
202 Von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068-3077.
203 Khandekar JD, Elson PJ, DeWys WD, et al. Comparative activity and toxicity of cis-diamminedichloroplatinum (DDP) and a combination of doxorubicin, cyclophosphamide, and DDP in disseminated transitional cell carcinomas of the urinary tract. J Clin Oncol. 1985;3:539-545.
204 Loehrer PJSr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10:1066-1073.
205 Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer. 1983;52:767-772.
206 Minea L, Angehel R, Oprea L, et al. Definitive radiochemotherapy with capecitabine for elderly patients with bladder cancer (abstract 322). Proc Genitourinary Cancers Symposium. 2008.
207 Patel B, Forman J, Fontana J, et al. A single institution experience with concurrent capecitabine and radiation therapy in weak and/or elderly patients with urothelial cancer. Int J Radiat Oncol Biol Phys. 2005;62:1332-1338.
208 Pipas JM, Mitchell SE, Barth RJJr, et al. Phase I study of twice-weekly gemcitabine and concomitant external-beam radiotherapy in patients with adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2001;50:1317-1322.
209 Epelbaum R, Rosenblatt E, Nasrallah S, et al. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J Surg Oncol. 2002;81:138-143.
210 Kent E, Sandler H, Montie J, et al. Combined-modality therapy with gemcitabine and radiotherapy as a bladder preservation strategy: results of a phase I trial. J Clin Oncol. 2004;22:2540-2545.
211 Fokdal L, Hoyer M, Meldgaard P, et al. Long-term bladder, colorectal, and sexual functions after radical radiotherapy for urinary bladder cancer. Radiother Oncol. 2004;72:139-145.
212 Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772-1776.
213 Cowan RA, McBain CA, Ryder WD, et al. Radiotherapy for muscle-invasive carcinoma of the bladder: results of a randomized trial comparing conventional whole bladder with dose-escalated partial bladder radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:197-207.
214 University of Binghampton. BC2001 Trial. http://www.bc2001.bham.ac.uk/index.shtml.
215 Muren LP, Smaaland R, Dahl O. Organ motion, set-up variation and treatment margins in radical radiotherapy of urinary bladder cancer. Radiother Oncol. 2003;69:291-304.
216 Pos FJ, Koedooder K, Hulshof MC, et al. Influence of bladder and rectal volume on spatial variability of a bladder tumor during radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:835-841.
217 Mangar S, Thompson A, Miles E, et al. A feasibility study of using gold seeds as fiducial markers for bladder localization during radical radiotherapy. Br J Radiol. 2007;80:279-283.
218 Burridge N, Amer A, Marchant T, et al. Online adaptive radiotherapy of the bladder: small bowel irradiated-volume reduction. Int J Radiat Oncol Biol Phys. 2006;66:892-897.
219 Lynch WJ, Jenkins BJ, Fowler CG, et al. The quality of life after radical radiotherapy for bladder cancer. Br J Urol. 1992;70:519-521.
220 Kachnic LA, Shipley WU, Griffin PP, et al. Combined modality treatment with selective bladder conservation for invasive bladder cancer: long-term tolerance in the female patient. Cancer J Sci Am. 1996;2:79-84.
221 Barringer B. Twenty five years of radon treatment of cancer of the bladder. JAMA. 1947;135:616-618.
222 Van der Werf-Messing BH, van Putten WL. Carcinoma of the urinary bladder category T2,3NXM0 treated by 40 Gy external irradiation followed by cesium137 implant at reduced dose (50%). Int J Radiat Oncol Biol Phys. 1989;16:369-371.
223 Grossman HB, Sandler HM, Perez-Tamayo C. Treatment of T3a bladder cancer with iridium implantation. Urology. 1993;41:217-220.
224 Straus KL, Littman P, Wein AJ, et al. Treatment of bladder cancer with interstitial iridium-192 implantation and external beam irradiation. Int J Radiat Oncol Biol Phys. 1988;14:265-271.
225 Mazeron JJ, Crook J, Chopin D, et al. Conservative treatment of bladder carcinoma by partial cystectomy and interstitial iridium 192. Int J Radiat Oncol Biol Phys. 1988;15:1323-1330.
226 Rozan R, Albuisson E, Donnarieix D, et al. Interstitial iridium-192 for bladder cancer (a multicentric survey: 205 patients). Int J Radiat Oncol Biol Phys. 1992;24:469-477.
227 Blank LE, Koedooder K, van Os R, et al. Results of bladder-conserving treatment, consisting of brachytherapy combined with limited surgery and external beam radiotherapy, for patients with solitary T1-T3 bladder tumors less than 5 cm in diameter. Int J Radiat Oncol Biol Phys. 2007;69:454-458.
228 Weiss C, Engehausen DG, Krause FS, et al. Radiochemotherapy with cisplatin and 5-fluorouracil after transurethral surgery in patients with bladder cancer. Int J Radiat Oncol Biol Phys. 2007;68:1072-1080.
229 Zietman AL, Grocela J, Zehr E, et al. Selective bladder conservation using transurethral resection, chemotherapy, and radiation: management and consequences of Ta, T1, and Tis recurrence within the retained bladder. Urology. 2001;58:380-385.
230 Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173-3181.
231 Von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602-4608.
232 Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer. 1989;64:2448-2458.
233 Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15:2564-2569.
234 Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8:1050-1055.
235 Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50-54.
236 Bellmunt J, Guillem V, Paz-Ares L, et al. Phase I-II study of paclitaxel, cisplatin, and gemcitabine in advanced transitional-cell carcinoma of the urothelium. Spanish Oncology Genitourinary Group. J Clin Oncol. 2000;18:3247-3255.
237 Bellmunt J, von der Maase H, Mead G, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine (PCG) and gemcitabine/cisplatin (GC) in patients with locally advanced (LA) or metastatic (M) urothelial cancer without prior systemic therapy; EORTC30987/Intergroup Study. J Clin Oncol. 2007;25:LBA5030.
238 Petrioli R, Frediani B, Manganelli A, et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer. 1996;77:344-351.
239 Waxman J, Barton C. Carboplatin-based chemotherapy for bladder cancer. Cancer Treat Rev. 1993;19(Suppl C):21-25.
240 Raabe NK, Fossa SD, Paro G. Phase II study of carboplatin in locally advanced and metastatic transitional cell carcinoma of the urinary bladder. Br J Urol. 1989;64:604-607.
241 Small EJ, Fippin LJ, Ernest ML, et al. A carboplatin-based regimen for the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer. 1996;78:1775-1780.
242 Dreicer R, Gustin DM, See WA, et al. Paclitaxel in advanced urothelial carcinoma: its role in patients with renal insufficiency and as salvage therapy. J Urol. 1996;156:1606-1608.
243 Dimopoulos MA, Deliveliotis C, Moulopoulos LA, et al. Treatment of patients with metastatic urothelial carcinoma and impaired renal function with single-agent docetaxel. Urology. 1998;52:56-60.
244 Yang MH, Yen CC, Chang YH, et al. Single agent paclitaxel as a first-line therapy in advanced urothelial carcinoma: its efficacy and safety in patients even with pretreatment renal insufficiency. Jpn J Clin Oncol. 2000;30:547-552.
245 Bellmunt J, de Wit R, Albanell J, et al. A feasibility study of carboplatin with fixed dose of gemcitabine in “unfit” patients with advanced bladder cancer. Eur J Cancer. 2001;37:2212-2215.
246 Carles J, Nogue M, Domenech M, et al. Carboplatin-gemcitabine treatment of patients with transitional cell carcinoma of the bladder and impaired renal function. Oncology. 2000;59:24-27.
247 Vaughn DJ. Paclitaxel and carboplatin in bladder cancer: recent developments. Eur J Cancer. 2000;36(Suppl 2):7-12.
248 Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clin Oncol (R Coll Radiol). 1994;6:11-13.
249 Salminen E. Unconventional fractionation for palliative radiotherapy of urinary bladder cancer. A retrospective review of 94 patients. Acta Oncol. 1992;31:449-454.
250 Aass N, Fossa SD. Aims and results of palliative radiotherapy in urological cancer. Curr Opin Oncol. 1994;6:308-312.
251 Honnens de Lichtenberg M, Miskowiak J, Rolff H. Results of radiotherapy on ureteric obstruction in muscle-invasive bladder cancer. Br J Urol. 1995;75:197-199.
252 Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47:379-388.
253 Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666-675.
254 Eapen L, Stewart D, Danjoux C, et al. Intraarterial cisplatin and concurrent radiation for locally advanced bladder cancer. J Clin Oncol. 1989;7:230-235.