Chapter 52F Cancer and the Nervous System
Nervous System Metastases
Brain Metastases
Epidemiology
Parenchymal brain metastases are the most common direct neurological complication of systemic cancer. Their precise incidence is unknown, but they outnumber primary tumors by a 10 : 1 ratio (Sawaya et al., 1994). Current estimates range from 20% to 40% of those dying with cancer (Posner, 1995). Among 1.3 million Americans with cancer, 100,000 to 170,000 will develop brain metastases (Landis et al., 1998). For comparison, 35,000 new patients with primary brain tumors are diagnosed each year in the United States.
The incidence of brain metastases varies with the tumor type. For example, the chance of developing brain metastases is 1% in men with prostate cancer, whereas the figure is 3% in women with ovarian cancer. In contrast, the likelihood of developing brain metastases with melanoma ranges from 18% to 90%, whereas corresponding figures for lung cancer are 18% to 63% and for breast cancer, 20% to 30%. However, a population-based analysis of patients diagnosed with a single primary lung, melanoma, renal, or colorectal cancer between 1973 and 2001 suggests that the true incidence percentages are lower than previously estimated (Barnholtz-Sloan et al., 2004). Overall, lung cancer accounts for 40% to 50% of all patients with brain metastases, and breast cancer accounts for 15% to 20% (Fig. 52F.1). Melanoma, renal cell carcinoma, and gastrointestinal tumors each account for an additional 5% to 10% of cases (Lassman and DeAngelis, 2003). The overall incidence of brain metastases appears to be rising. One possible explanation is improved survival of systemic disease, allowing more time for development of brain metastases. Additionally, the blood-brain barrier prevents systemic chemotherapeutic agents from treating brain metastases.
Although most patients develop brain metastases in the setting of known cancer, brain metastases are the initial manifestation of an underlying primary tumor in 10% to 30% of cases. Less than one-fourth of such patients have clinical features pointing to the location of the primary tumor. Nonetheless, 80% will eventually have the primary site of tumor identified during their lifetime. Lung cancer is the most common cause of brain metastases presenting without a known primary, accounting for two-thirds of cases. Among lung tumor metastases, two-thirds are from non–small cell lung cancer (NSCLC) (Le Chevalier et al., 1985). Gastrointestinal primaries account for an additional 10%. A retrospective analysis of 176 patients with newly diagnosed brain masses concluded that chest computed tomography (CT) and brain magnetic resonance imaging (MRI), if used in concert as initial diagnostic studies, would have identified a biopsy site in 97% of patients with a newly detected intracranial mass (Mavrakis et al., 2005). The high likelihood of a primary lung tumor and the fact that many patients with other primary tumors have lung metastases by the time they develop brain metastases makes restricting initial radiological studies to the chest the more cost-effective approach. Because most brain metastases are multiple, and most patients with brain metastases have a known cancer, only 15% of solitary intracranial masses in patients not known to have cancer turn out to be metastatic tumors (Voorhies et al., 1980).
Clinical Presentations
Symptoms of brain metastases may arise as long as 20 years after discovery of the primary tumor, or may even antedate discovery of the underlying systemic cancer. The latter is common with lung cancer, whereas patients with systemic breast cancer and melanoma may enjoy years of apparent freedom from systemic cancer prior to discovery of cerebral metastasis (Henson and Urich, 1982).
The presenting features are usually progressive over days to weeks, although occasional patients present acutely with seizures or stroke-like syndrome in the setting of intratumoral hemorrhage. Half of all patients complain of headache, and a third have mental status changes. Most headaches are indistinguishable from tension headache (Cavaliere, 2008). The “classic” brain tumor headache, which is worse in the mornings or awakens the patient from sleep, is uncommon, and its absence does not preclude the diagnosis of a brain tumor. Headache in the absence of other symptoms is more likely to be due to multiple metastases than a single metastasis. Over time, headache from brain metastasis becomes progressively more severe and may be accompanied by nausea, vomiting, and drowsiness. Unilateral weakness and gait disturbances are other common presenting complaints. Seizures are present at diagnosis in 18% of patients with brain metastases (Cohen et al., 1988).
Mental status changes and hemiparesis are the most common findings on neurological examination; each is present in approximately 60% of patients (Posner, 1995). Despite the frequent occurrence of increased intracranial pressure, papilledema is detectable in only 10% of patients.
Differential Diagnosis
Several neurological conditions may mimic brain metastases both clinically and radiographically. A primary brain tumor must be a consideration, especially in patients with a single brain mass. This is a particularly important consideration in patients with breast cancer and a dural-based tumor (Schoenberg et al., 1975). Abscess, demyelination, progressive multifocal leukoencephalopathy, cerebrovascular disease, and the effects of radiation or chemotherapy also simulate brain metastases. Although the clinical syndrome and the neuroimaging studies usually provide a diagnosis, brain biopsy is sometimes required.
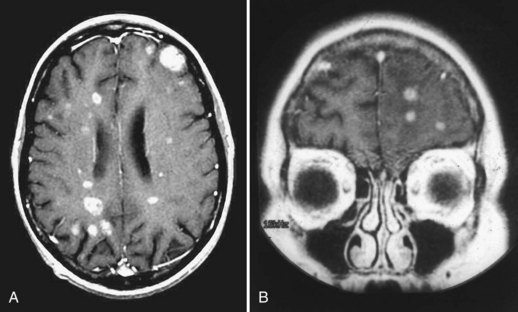
Neuroimaging
Neuroimaging advances since the early 1970s have made the diagnosis of brain metastases relatively easy in almost all cases. Complete coverage of this topic is elsewhere in this section; discussion here is limited to recent comparisons of various imaging modalities. Noncontrasted MRI is as sensitive as contrast-enhanced CT for detection of brain metastases. Use of gadolinium-containing contrast agents dramatically improves the sensitivity of MRI, making it markedly superior to contrast-enhanced CT scanning. Triple-dose administration of contrast further improves the sensitivity of MRI. Although the two dosages are equivalent for detecting metastases larger than 1 cm in diameter, triple-dose studies demonstrate three times as many metastases smaller than 0.5 cm in diameter (Yuh et al., 1995). Delayed imaging after standard dose contrast is intermediate in sensitivity between single- and triple-dose gadolinium.
Management
Supportive Care
Although other chapters address corticosteroids and antiepileptic drug (AED) usage, a few comments pertinent to their rational use in brain metastases are appropriate. Corticosteroids improve symptoms associated with brain metastases in two-thirds of patients. Their use improves median survival in otherwise untreated patients from 1 to 2 months. One randomized controlled trial examined different doses in patients with brain metastases. These patients all had a Karnofsky Performance Score (KPS) less than or equal to 80 (Table 52F.1). All patients received standardized whole-brain radiotherapy (WBRT) after receiving dexamethasone for 1 week. In the first part of the trial, with patients randomized to receive either 8 or 16 mg daily in divided doses, the two groups did equally well, with slightly less toxicity related to steroids in the lower-dose group. Other patients were then randomized to either 4 or 16 mg daily. The lower-dose group did slightly less well at 7 days (although the difference was not significant) and better at 28 days, with significantly less toxicity than the high-dose group. The authors concluded that unless patients were in danger of herniation, 2 mg twice daily was an appropriate starting dose (Vecht et al., 1994).
| KPS 100 | Normal; no complaints, no evidence of disease |
| KPS 90 | Able to carry on normal activity; minor signs or symptoms of disease |
| KPS 80 | Normal activity with effort; some signs or symptoms of disease |
| KPS 70 | Cares for self; unable to carry on normal activity or do active work |
| KPS 60 | Requires occasional assistance but is able to care for most personal needs |
| KPS 50 | Requires considerable assistance and frequent medical care |
| KPS 40 | Disabled; requires special care and assistance |
| KPS 30 | Severely disabled; hospitalization is indicated, although death not imminent |
| KPS 20 | Very sick; hospitalization necessary, active support treatment necessary |
| KPS 10 | Moribund; fatal processes progressing rapidly |
| KPS 0 | Dead |
Approximately 10% to 20% of patients with brain metastases present with seizures and require treatment with standard anticonvulsants. The use of prophylactic anticonvulsants in patients who have not had seizures is controversial. Fewer than 20% of these patients experience seizures later in the course of their illness, and this risk does not appear to be reduced with prophylactic anticonvulsants. Consequently, the American Academy of Neurology has issued a practice parameter recommending against the prophylactic use of anticonvulsants in patients with brain metastases who have not had a seizure (Glantz et al., 2000). Potential exceptions to this guideline include patients with metastases from melanoma (which may be more epileptogenic because of multiplicity or hemorrhage), tumors in motor cortex, or concomitant parenchymal and leptomeningeal brain metastases. Although the efficacy is similar among the available anticonvulsants, differences in pharmacokinetic profile may influence which agent is used. Anticonvulsants metabolized by the P450 system interact with corticosteroids and many common antineoplastic therapies such as irinotecan and erlotinib. Consequently, the effectiveness of a given dosage of dexamethasone may be decreased, and tumor exposure to an antineoplastic agent may be reduced. Examples of enzyme-inducing anticonvulsants include phenytoin and carbamazepine. Alternatively, non–enzyme inducing agents such as levetiracetam and pregabalin do not interact with other medications administered concurrently.
Radiation Therapy
The goals of radiation therapy (RT) are to alleviate neurological deficits due to tumor and to shrink the tumors and prolong survival. In terms of meeting the first goal, approximately three-fourths of patients undergoing RT experience symptom palliation, and two-thirds maintain this improvement (Cairncross et al., 1980). With respect to tumor shrinkage, this depends on the size and radiosensitivity of the metastasis. With standard doses of WBRT, 37% of patients with SCLC, 35% with breast cancer, 25% with squamous cell cancer, and 14% with non-breast adenocarcinomas achieve a complete response (CR) (Nieder et al., 1997). Tumors with a pretreatment volume of less than 0.5 mL had a 100% CR rate, whereas tumors with a pretreatment volume larger than 10 mL had little likelihood of disappearing in the same study. These results highlight the unfortunate fact that CR of brain metastasis to WBRT is the exception, not the rule.
The large RTOG database has permitted the application of statistical techniques such as recursive partitioning analysis (RPA) to separate patients with brain metastases treated with WBRT into different prognostic classes based on clinical features at presentation. Patients with brain metastases can be divided into three classes. Class 1 consists of patients with a KPS greater than or equal to 70 (see Table 52F.1), age younger than 65 years, primary site of tumor resected or controlled with treatment, and no extracranial sites of metastatic tumor. Such patients have a median survival of 7.1 months. Class 3 is composed of all patients whose KPS is less than 70; the median survival in this group is only 2.3 months. Class 2 contains all patients who do not fall into classes 1 and 3; class 2 patients have a median survival of 4.2 months (Gaspar et al., 1997). Subsequent studies have validated these results (Gaspar et al., 2000). The limited number of patients with KPS less than 70 in the RTOG dataset precluded further analysis of this population. Yet it is recognized that other factors may predict outcome. Lutterbach et al. performed a single institutional analysis that not only validated the original RPA classification but also broke down RPA class 3 patients with KPS below 70 into three groups based on age (<65 versus ≥ 65), primary tumor status, and number of lesions (single versus multiple). Survival differences among the groups were statistically significant and did not overlap with class 2 (Lutterbach et al., 2002). Sperduto et al. reanalyzed the RTOG database that included patients from a study completed after the initial RPA review. In addition to age, performance status, and the presence of extracranial metastases, they identified the number of cerebral lesions to be predictive of survival. Four distinct groups were defined whose survival was statistically different (11, 6.9, 3.8, and 2.6 months) (Sperduto et al., 2008).
Radiation sensitizers with selective tumor uptake offer the theoretical promise of increasing the efficacy of WBRT. A recent randomized clinical trial of motexafin gadolinium administered prior to each radiation treatment did not find any prolongation of survival or time to intracranial tumor progression. However, patients with brain metastases from NSCLC had significantly prolonged time to tumor progression with this agent. A confirmatory study of patients with NSCLC failed to show a statistically significant benefit in outcome, although statistical factors and differences in treatment among the participating institutions may have negatively influenced the results. The future of motexafin gadolinium remains uncertain (Mehta et al., 2009). RSR-13, an allosteric modifier of hemoglobin that allows more oxygen to be released to hypoxic tissue and may thereby sensitize brain metastases to radiation, has shown promising results in phase II studies. A phase III trial of WBRT plus supplemental oxygen with or without RSR-13 failed to demonstrate improvement in overall or progression-free survival in patients with brain metastases (Suh et al., 2006). However, among the subgroup with breast cancer, the addition of RSR-13 reduced death rate and improved quality-adjusted survival (Scott et al., 2007). Caution must be used in interpreting the results, as they were derived from subgroup analysis and confirmation is required.
Radiation Toxicity
With standard fractionation schemes, WBRT is tolerated well. Patients should expect temporary alopecia and fatigue. Headache and nausea occasionally occur but are generally alleviated with corticosteroids and antiemetics. In poor-prognosis patients, the acute side effects of WBRT must be considered, however, as they may have a significant impact on the quality of the remaining short predicted lifespan (Komosinska et al., 2010).
Long-term survivors of brain metastases are at risk of suffering late complications of WBRT. Of WBRT recipients for brain metastases, as many as 10% to 30% develop cognitive impairment by 1 year if radiation doses per fraction exceed 300 cGy (Behin and Delattre, 2002). Symptoms commonly include poor short-term memory, abulia, gait unsteadiness, and urinary urgency. MRI frequently reveals extensive symmetrical periventricular white matter changes termed radiation leukoencephalopathy, ventriculomegaly, and sometimes cortical atrophy. The clinical picture may resemble normal-pressure hydrocephalus, but a positive durable response to ventriculoperitoneal shunt is uncommon (DeAngelis et al., 1989). Because the risk of this complication is greater with larger fraction sizes, many radiation oncologists treat patients with good prognosis with 20 fractions of 200 cGy or similar regimens.
Prophylactic Cranial Irradiation
Brain metastases are extremely common in SCLC, being present in 10% of patients at diagnosis, increasing to 20% during therapy, and 35% at time of autopsy (Jeyapalan and Henson, 2002). At 2 years post diagnosis, the cumulative risk of brain metastasis is 47% for patients with limited disease and 69% for those with extensive disease. Presumably the brain is a pharmacological sanctuary for microscopic tumor against systemic chemotherapy, which does not penetrate the intact blood-brain barrier. This has led to numerous trials designed to test whether prophylactic cranial irradiation (PCI) would decrease the incidence of brain relapse and improve survival in patients who achieved systemic CR. A consistent finding was that PCI significantly decreased the risk of cerebral metastases. An often-cited metaanalysis of these studies indicated that PCI reduced the risk of subsequent brain metastasis (59% versus 33% at 3 years) and modestly increased 3-year survival from 15.3% to 20.7% (P = .01) (Auperin et al., 1999). There was also a suggestion of a dose response, although this was not confirmed in a recent randomized study in which patients were randomized to 25 or 36 Gy (Le Pechoux et al., 2009). The role of PCI in patients with incomplete response to treatment or with extensive small-cell lung cancer remains unclear. The poor prognosis of these patients (median survival of 9 months) brings into question the utility of PCI. Slotman et al. randomized patients with extensive small-cell lung cancer that responded to treatment to observation or PCI. Patients treated with PCI had a cumulative risk of symptomatic brain metastases of 14.6% compared to 40.4% among observation patients. Patients treated with PCI also had a significantly longer overall survival (Slotman et al., 2007). Controversy still remains over whether the benefits of PCI outweigh its toxicities, particularly leukoencephalopathy. Two large prospective randomized trials of PCI did not document increased neuropsychological deficits among PCI recipients. Others argue that the small numbers of long-term survivors in these trials precluded accurate assessment of the risk of leukoencephalopathy, and that because PCI benefited only about a fourth of its recipients, it should not be considered standard therapy.
Surgery
In determining whether or not surgery is appropriate, the patient’s performance status and extent of extracranial disease are the most important considerations. For several decades, neurosurgeons resected single brain metastases in selected patients and argued that surgery produced better results than radiotherapy alone, particularly noting improvement in the percentage of long-term survivors. In 1990, a randomized controlled trial verified the neurosurgeons’ contention. In this study, eligible patients had a single surgically accessible metastasis identified by contrast CT or MRI scan. Patients with highly radiosensitive primary tumors were excluded. Enrolled patients were randomized to biopsy followed by WBRT (36 Gy in 12 fractions) versus resection and WBRT. Patients who underwent surgical resection of the metastasis followed by RT developed fewer local recurrences (20% versus 52%) and significantly improved survival (40 weeks versus 15 weeks) compared to those patients who received only a biopsy and RT. Patients who underwent surgical resection also had improved performance status and a reduced risk of dying as a result of neurological causes. Multivariate analysis showed that surgery and longer time between diagnosis of the primary tumor and the development of brain metastases were associated with increased survival, whereas disseminated disease and increasing age were associated with decreased survival (Patchell et al., 1990). Thus in patients with surgically accessible single brain metastases and absent or controlled systemic cancer, surgical resection became the standard of care.
The role of WBRT following resection of a single metastasis is uncertain. In one trial, patients with a single metastasis on gadolinium MRI scan who underwent complete resection were randomized to receive either 50.4 Gy in 28 fractions or no radiation (Patchell et al., 1998). The recurrence rate either locally or distantly in the brain was significantly reduced in the radiation group (18%) compared to the observation group (70%). However, overall survival did not differ significantly between the two groups. Radiation substantially reduced the death rate resulting from neurological causes; however, patients in the observation group who did not die of neurological causes appeared to live longer than similar patients in the radiotherapy group. There was no difference in how long patients maintained functional independence. In the absence of survival benefit to postoperative WBRT, its use must be decided on an individual case basis. For some patients and physicians, the reduction in recurrent brain metastasis and neurological death will outweigh the potential side effects of radiotherapy. Alternatively, focal radiation to the surgical bed may reduce the risk of local recurrence. Several retrospective studies examining the role of postoperative radiosurgery to the surgical bed found that focused radiation is efficacious in controlling local tumor growth and maintaining long-term quality of life (Jagannathan et al., 2009).
Stereotactic Radiosurgery
Numerous single-institution experiences with radiosurgery for single or oligometastatic brain lesions have been published. In a review summarizing published series comprising more than 2000 patients treated over 8 years in the 1990s, Loeffler found that SRS achieved permanent local control in more than 80% of patients, with complications in fewer than 10% (mainly radiation necrosis). Outcome appeared independent of the number of metastases treated (Loeffler et al., 1999). Median survival following SRS is approximately 9 to 10 months, very similar to surgical series. Radiosurgical treatment is also effective for metastases that have recurred following fractionated radiotherapy.
One consistent and remarkable finding across numerous SRS series is that metastases from highly radio-resistant tumors like melanoma and renal cell carcinoma, which respond very poorly to fractionated radiotherapy, respond virtually as well to SRS as tumors far more sensitive to conventional radiation. However, intracranial failure rates of highly radioresistant tumors without WBRT were 25.8% and 48.3% at 3 and 6 months, respectively, according to a recent phase II trial conducted by the Eastern Cooperative Oncology Group. Therefore, delaying adjuvant WBRT may be appropriate for some subgroups of patients with radioresistant tumors, but routine avoidance of WBRT should be approached judiciously (Manon et al., 2005).
According to an evidence-based review by the American Society for Therapeutic Radiation and Oncology (ASTRO), in selected patients with small (<4 cm) brain metastases (up to three in number and four in one randomized trial), radiosurgery boost with WBRT improves local brain control compared to WBRT alone. Similarly, in patients with a single brain metastasis, radiosurgery boost to WBRT improves survival. In selected patients treated with radiosurgery alone for newly diagnosed brain metastases, overall survival is not altered. However, omission of up-front WBRT was associated with markedly poorer local and distant brain control (Mehta et al., 2005).
Because the results for SRS appear similar to those from surgery, one might ask whether SRS has been proven to improve the patient’s outcome, as has surgery when administered with fractionated radiation. An RTOG clinical trial has affirmed this hypothesis by randomizing patients with one to three brain metastases to WBRT with or without radiosurgery. Patients with a single brain metastasis had a significant survival benefit as well as improved performance status from the addition of radiosurgery, as did patients younger than 50 and those in RPA class 1 (Andrews, 2004).
The relative effectiveness of radiosurgery versus surgery in patients with brain metastases has never been ascertained. A retrospective review from the Mayo Clinic compared the efficacy of neurosurgery versus radiosurgery in local tumor control and patient survival in patients with solitary brain metastases. There was no significant difference in patient survival (P = 0.15) between the 74 neurosurgery patients and the 23 radiosurgery patients. The 1-year survival rates for the neurosurgery and radiosurgery groups were 62% and 56%, respectively. There was a significant (P = 0.020) difference in local tumor control, but none of the radiosurgery group had local recurrence compared with 19 (58%) in the neurosurgery group (O’Neill et al., 2003). Although surgeons occasionally remove two or even three brain metastases, surgery is generally restricted to single lesions, whereas multiple lesions generally present no problems for radiosurgery. Radiosurgery appears more cost-effective than surgery, although surgery alleviates symptoms of mass effect much more rapidly and reliably than SRS. Phase III trials comparing these two modalities have been proposed, but no major multicenter study has yet been undertaken.
The role of WBRT following radiosurgery for brain metastases is also uncertain. Two retrospective cohort studies have examined this issue. Pirzkall et al. (1998) compared outcomes in 158 patients treated with radiosurgery alone versus 78 receiving radiosurgery plus fractionated WBRT. All patients had three or fewer brain metastases. The overall median survival was 5.5 months, with no difference between treatment groups. However, median survival in patients without extracranial tumor was increased in patients getting both forms of radiation (15.4 versus 8.3 months, P = .08). A trend existed for superior local control in patients getting combined therapy. A similar smaller study also found no difference in median survival (11 months) or 1-year progression-free survival. Brain relapse was significantly more common in patients receiving radiosurgery alone; however, most patients who did relapse could still be salvaged. In a prospective randomized Japanese study of 132 patients comparing SRS alone with SRS plus WBRT there was increased risk of tumor recurrence both locally (30% versus 14%) and distantly in the brain (52% versus 18%) in those who did not receive up-front WBRT. However, increased risk of intracranial failure was not associated with decreased survival, increased risk of neurological death, or worse neurocognitive performance (Aoyama et al., 2006). Similarly, Chang et al. randomized patients with three or less cerebral metastases to radiosurgery with or without WBRT. The primary endpoint in this study, however, was neurocognitive outcome at 4 months as assessed by Hopkins Learning Verbal Test-Revised. The study was halted by the data-monitoring committee after only 58 patients were enrolled according to the early stopping rules because outcomes were inferior in the combined group (Chang et al., 2009). A more recent study by the European Organization of Research and Treatment of Cancer of 359 patients with one to three brain metastases randomized to adjuvant WBRT or observation after radiosurgery or surgical resection evaluated duration of functional independence (primary endpoint), which did not differ between the two groups. Overall survival and global quality of life were also similar between two groups. The incidence of and time to intracranial progression, however, was significantly greater in the observation group, as was rate of neurological death. Neurocognitive endpoints were not included in this study. Thus, the issue of WBRT remains unresolved, although it is clear that cognitive and quality-of-life endpoints, rather than survival, may be the determinant of whether or not to add WBRT for patients receiving SRS in oligometastatic brain disease. An ongoing U.S. trial will further compare WBRT plus SRS with SRS alone and explore neurocognitive endpoints.
The role of WBRT in patients with oligometastases (four or less lesions) treated with radiosurgery is perhaps one of the greatest debates in neuro-oncology. At the center of the uncertainty is what has a more deleterious effect on neurological status and cognition, CNS disease progression or treatment toxicity. It is recognized that the risk of CNS disease progression is greater among patients treated with focal therapeutics alone. Brain tumor burden has been associated with cognitive impairment. Aoyama et al. (2007) noted that at baseline prior to treatment, MMSE scores correlated with the extent of brain edema and total volume of brain metastases. Among patients treated with WBRT, lesion volume was the only predictor of global neurocognitive impairment prior to therapy (Meyers et al., 2004). Therefore, by extension, progressive disease and the associated increase in tumor volume may lead cognitive and neurological deterioration. In the randomized study reported by Aoyama et al., patients receiving radiosurgery alone experienced a higher rate of neurological decline than those treated with whole-brain radiation. The difference was attributed to a higher rate of brain tumor recurrence (Aoyama et al., 2007). Similarly, in an analysis of patients enrolled in RTOG 91-04, all of whom were treated with WBRT, clinically significant decline in MMSE was noted only among those with uncontrolled brain metastases (Regine et al., 2001). In a retrospective review of patients with cerebral metastases treated with SRS alone, Regine et al. (2002) noted that tumor recurrence was associated with neurological symptoms and deficits in 71% and 59% of patients, respectively; 17% of patients were too impaired to undergo salvage therapy. Consequently, the prevention of progressive brain disease may preserve a patient’s neurological status. Alternatively, with vigilant radiographic surveillance, progressive disease may be captured in a presymptomatic state, allowing patients to be salvaged prior to neurological decline. In a prospective study of patients with up to three brain metastases treated with SRS followed by vigilant observation, only 37% of recurrences were symptomatic. In the remaining patients, recurrence was diagnosed radiographically before symptom onset. Ninety-one percent of patients received salvage treatment, and 58% of symptomatic patients improved neurologically (Luterbach et al., 2003). In addition, deferring WBRT preserves this therapeutic as a future salvage option. A significant limitation of the data is the lack of long-term assessments. While tumor progression may impact cognitive status in the short term (Aoyama et al., 2007; Regine et al., 2001), survivors may experience the chronic adverse effects of treatment. Despite initially being stable, MMSE among patients treated with WBRT was noted to decline after 2 years (Aoyama et al., 2007). Although this exceeds survival in the majority of patients, it may become more of an issue as therapeutics improve in the future.
Treatment Options for Recurrent Brain Metastases
Re-Irradiation
Several case series have examined the safety and efficacy of administering a second course of fractionated WBRT to patients with recurrent or progressive brain metastases. In general, this option is considered only for patients who had a good and relatively durable response to their prior course of WBRT. Sometimes the entire brain is re-irradiated, but if metastases are not widespread, fractionated radiation may be delivered to a limited portion of the brain. A majority of patients achieve symptom palliation that lasts a median of 3 months. Because survival under these circumstances is generally limited (median survival 4 months), clinical radiation-induced leukoencephalopathy is relatively uncommon (Wong et al., 1996).
Spinal Cord Compression
Epidemiology
Epidural spinal cord compression (ESCC) refers to compression of the spinal cord or cauda equina from a neoplastic lesion outside the spinal dura. This complication of systemic cancer is estimated to affect approximately 25,000 Americans each year (Schiff, 2003). Although every type of cancer is capable of producing ESCC, several tumors predominate. A population-based study of malignant spinal cord compression (MSCC) in Ontario concluded that there is a 40-fold variation in the cumulative incidence of MSCC among different types of cancer (Loblaw et al., 2003). Thus in most series, breast, lung, and prostate cancer each account for about 20%, whereas renal cell carcinoma, non-Hodgkin lymphoma (NHL), and multiple myeloma typically account for 5% to 10% each (Schiff, 2002). Ewing sarcoma and neuroblastoma are particularly common causes in children. Of all ESCCs, 20% occur in patients not previously known to have cancer; among this group, lung cancer, lymphoma, and multiple myeloma are the typical underlying tumors.
Pathophysiology and Pathology
A protective ring of bones encloses the spinal cord. The ring is composed of the vertebral body anteriorly, and the lamina, pedicles, and spinous processes posteriorly. The thecal sac lies within this bony ring. The outermost layer of the thecal sac is composed of dura mater that is continuous with cranial dura mater and fuses with periosteum of the sacrum at S2. The spinal epidural space lies between the periosteum of the vertebral bones and the dura and normally contains fat, connective tissue, and a venous plexus. Tumor cells generally seed the vertebral bones (usually the vertebral body) through hematogenous dissemination via arteries or the valveless low-pressure venous plexus of Batson. The tumor gradually expands and forms an epidural mass, producing compression of the spinal cord. The tumor takes the path of least resistance, frequently encircling the spinal cord. Collapse of the vertebral body may occur, exacerbating the severity of spinal cord compression. Less commonly, primary or metastatic tumor in the paravertebral region grows through the neural foramen to produce ESCC. Animal models suggest that both demyelination, produced with slowly expanding masses and often reversible, and venous infarction, play a role in spinal cord dysfunction. Roughly 60% of ESCCs arise in the thoracic spine and 30% in the lumbar spine (Schiff, 2002).
Clinical Presentations
Back pain, present in 83% to 95% of patients at the time of diagnosis, is usually the first symptom of ESCC (Schiff, 2002). On average, the pain precedes the development of neurological symptoms by approximately 2 months. The duration of pain is often related to the rate of tumor growth, with slower-growing tumors presenting with longer duration of pain. Most patients have local pain related to disruption and stretching of cortical bone and periosteum. Cough, movement, and recumbency commonly exacerbate the pain. Eventually, the pain takes on a radicular distribution, which in the thoracic spine may produce a bilateral gripping sensation. Pain is often quite severe by the time ESCC is diagnosed.
Motor involvement, the most dreaded of ESCC manifestations, is present in 80% of patients (Helweg-Larsen and Sorensen, 1994). Once weakness is present, progression is often rapid, and diagnostic workup and therapy must proceed expeditiously. This is particularly important because pretreatment neurological function is a major predictor of posttreatment outcome. Approximately 50% of patients with ESCC are ambulatory, 35% are paraparetic, and 15% are paraplegic at diagnosis. Weakness is typically bilateral and symmetrical; the iliopsoas muscles may be disproportionately affected.
Sensory loss is detectable in about 75% of patients at diagnosis, typically reflecting the extent of weakness. Patients frequently experience ascending numbness and paresthesias. A correlation exists between the extent of sensory loss and the inability to ambulate (Schiff, 2002). Radicular sensory loss may help localize the level of ESCC, but the spinal sensory level detected on examination is often several levels below the responsible lesion (Posner, 1995). Lesions above the cauda equina generally result in sparing of saddle sensation, whereas cauda equina lesions may produce saddle sensory loss. Occasionally, ESCC in the cervical cord may be associated with the Lhermitte sign, which is characterized by an electrical sensation in the spine and extremities when the neck is flexed.
Differential Diagnosis
The differential diagnosis of ESCC in cancer patients is broad and can be separated into neoplastic and non-neoplastic causes (Box 52F.1). Spine metastases not impinging on the thecal sac can produce severe pain mimicking ESCC. These may be clinically indistinguishable from ESCC. Patients with radiographically high-grade ESCC may not show features of radiculopathy and myelopathy if cord compression has developed slowly. Intramedullary spinal cord metastases are rare compared to ESCC. The clinical features are similar to ESCC, although many patients pass through a stage in which spinal cord dysfunction is unilateral or at least very asymmetrical, unusual with ESCC. Leptomeningeal metastases (LMs), discussed later, occasionally produce a cauda equina syndrome which may resemble ESCC. Radicular pain predominates over local pain in this condition, and bowel and bladder involvement may be particularly prominent compared to ESCC. Neoplastic masses in the brachial or lumbosacral plexus may also simulate ESCC, although unilateral findings are the rule. Malignant plexopathy often coexists with ESCC, given the proximity to the spinal cord (Fig. 52F.2).
Box 52F.1 Differential Diagnosis of Epidural Spinal Cord Compression
Two rare neurological conditions related to, but not a direct result of, cancer are radiation myelopathy and paraneoplastic myelopathy. Radiation myelopathy may occur if the spinal cord was included in prior radiotherapy treatment ports (Behin and Delattre, 2002). The most common form, chronic progressive radiation myelopathy, generally develops 1 to 2 years after radiotherapy. Ascending numbness and pyramidal tract dysfunction often occur in an asymmetrical fashion, suggesting the Brown-Séquard syndrome. The risk of myelopathy is increased by high radiation doses, long length of irradiated spinal cord, and a large radiation fraction size. Paraneoplastic disorders are a rare cause of myelopathy. The most common clinical syndrome is a painless myelopathy accompanied by encephalitis or sensory neuropathy. A necrotizing myelopathy without associated antibody or other serum markers is also described with cancer.
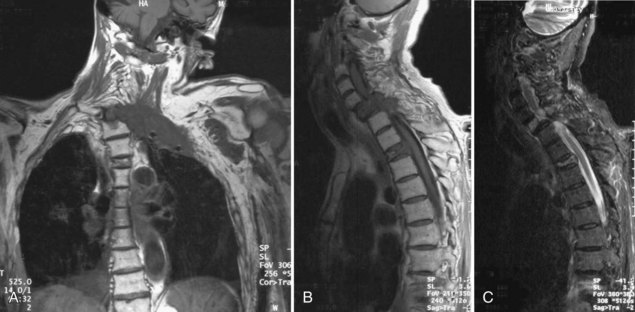
Neuroimaging
The availability of MRI has revolutionized the definitive diagnosis of ESCC (see Fig. 52F.2, B and C). Prior to MRI, myelography with or without CT scanning was the gold standard. Before proceeding to myelography, patients were often screened with plain radiographs or bone scans. Plain radiographs demonstrating vertebral collapse or pedicle erosion at a level corresponding to clinical findings were highly predictive of ESCC. However, false-negative studies were common. Radionuclide bone scanning was more sensitive, although this test could be normal in neoplasms without increased blood flow or new bone formation like multiple myeloma. Moreover, abnormal studies were frequently related to benign causes.
Management
Corticosteroids
A few years after the beneficial effect of corticosteroids on brain tumors was established, corticosteroids were observed to ameliorate the clinical features of ESCC. Since then, corticosteroids have been routinely used in patients with ESCC. Subsequently, rodent models of ESCC confirmed a benefit with dexamethasone. One randomized controlled trial of dexamethasone in patients with carcinomatous ESCC undergoing radiation showed that the use of dexamethasone increased the likelihood of patients remaining ambulatory (Sorensen et al., 1994). The optimal dose of corticosteroids for ESCC remains unresolved. Based on extrapolation from a dose that was demonstrated to be effective in rodent models, some protocols have suggested an initial dose of 100 mg dexamethasone, followed by 24 mg 4 times daily. Retrospective cohort studies suggest that substantially lower doses (e.g., 10 mg initially, then 4 mg 4 times daily) achieve equivalent results, with fewer serious corticosteroid complications such as gastrointestinal bleeding (Heimdal et al., 1992). If the higher dosage is used, one reasonable strategy is to taper the dose by 50% every 3 days, so long as the patient is stable and definitive treatment is underway.
Radiotherapy
Radiotherapy is the treatment of choice for most patients with ESCC. By stopping tumor growth and sometimes shrinking the tumor, radiotherapy usually alleviates pain and stabilizes or improves neurological function. Radiation ports are created on the basis of the radiological studies, and traditionally extend one vertebral level above and below the site of epidural tumor. Ports are widened when a paravertebral mass is known to be present. Radiation dose represents a compromise among tumor control, risk of radiation myelopathy, and treatment duration compatible with the patient’s condition. A variety of dose-fractionation schemes have been used. In a recent prospective multicenter trial comparing 30 Gy in 10 fractions and 40 Gy in 20, there were no significant differences in posttreatment motor function or overall percentage of patients regaining ambulation (Rades et al., 2004). A retrospective analysis of over 1300 patients looked at 5 regimens ranging from 8 Gy to a total of 40 Gy in 20 fractions; all regimens yielded similar functional results. However, the more protracted courses were associated with lower risk of recurrence within the radiation field (Rades et al., 2005).
Recently, neurosurgeons and radiation oncologists have been working to modify radiation equipment to deliver SRS to the spine. Advantages of this approach include maximizing dose delivery to the tumor while minimizing exposure to the neighboring spine (which has a lower tolerance than brain tissue), reducing exposure of overlying skin thereby reducing risk of surgical wound breakdown, and treating anterior spinal elements following posterior decompression with or without instrumentation, consequently avoiding more extensive surgery. Furthermore, spinal radiosurgery allows for the safe delivery of higher doses of radiation compared to conventional treatment. This may be especially useful when treating radioresistant histologies. Spine radiosurgery may be an effective salvage option in patients with recurrent disease within a previously treated volume in whom further conventional therapy or open surgery are not appropriate (Gerszten et al., 2009). Although few data have been reported in detail, spinal radiosurgery may alter the treatment paradigm for epidural spinal cord compression as radiosurgery already has in the treatment of cerebral metastases.
Surgery with Radiation Therapy
A recent randomized clinical trial examined whether aggressive debulking surgery prior to radiation improved the outcome compared to RT alone. Enrolled patients were required to have a life expectancy greater than 3 months and a single level of cord compression. Patients with lymphoma and primary spine tumors were excluded, as were patients with paraplegia for more than 48 hours. Both groups received high-dose steroids on presentation and underwent surgery or began RT within 24 hours of presentation. The radiation consisted of 30 Gy given over a 10-day period beginning within 14 days of surgery. Patients treated with surgery retained the ability to walk significantly longer than those treated with radiotherapy alone (median, 126 days versus 35 days, P = 0.006). Surgically treated patients also maintained continence and functional Frankel and American Spinal Injury Association scores significantly longer than patients in the radiation group (Patchell, 2005). Thus, the combination of surgery and RT appears to be superior to RT alone in preserving ambulatory status in selected patients with cord compression.
Intramedullary Spinal Cord Metastases
Metastases are capable of spreading to the substance of the spinal cord, either by hematogenous spread or secondary to leptomeningeal invasion and subsequent centripetal growth. Intramedullary spinal cord metastases cause progressive myelopathy, which often initially takes the form of a hemicord syndrome (Brown-Séquard syndrome) with ipsilateral pyramidal weakness and posterior column sensory loss, and contralateral spinothalamic sensory loss. Lung cancer accounts for about half of all cases, with small-cell histology being particularly common. Melanoma, lymphoma, and renal cell carcinoma are other common causes. MRI scanning generally reveals a contrast-enhancing mass with a larger surrounding region of T2 signal abnormality (edema). Most patients with this complication either have concurrent or prior brain metastases. Treatment generally consists of corticosteroids and fractionated radiotherapy, which usually stabilize neurological function for several months (Schiff and O’Neill, 1996).
Leptomeningeal Metastases
Involvement of the leptomeninges by tumor is an increasingly common problem in patients with cancer and leads to significant morbidity and mortality (Kesari and Batchelor, 2003; Mason, 2002). The factors that have contributed to the increased incidence are a greater awareness of the condition among oncologists, improved diagnostic tests, and longer survival among patients with systemic malignancies. Survival in this setting is usually short and averages 3 to 4 months (Jaeckle, 2006). Only seven randomized prospective trials of neoplastic meningitis have been conducted, some with conflicting results, so no standard therapeutic guidelines exist at this time. However, prognostic factors have become more apparent. Good prognosis was associated with female sex, history of intraparenchymal tumor, short duration of symptoms, and controlled systemic disease. Negative prognosis was associated with age older than 55 years, increased CSF protein, hypoglycorrhachia, poor KPS, cranial nerve palsy, carcinomatous encephalopathy, concomitant bulky CNS metastases, poorly controlled systemic disease, and persistent CSF block (Gleissner, 2006).
Epidemiology
Approximately 5% of cancer patients have LM (Posner, 1995). The incidence varies with different tumor types. LM occurs in up to 8% of patients with solid tumors, 5% to 29% of patients with NHL, and 11% to 70% of leukemias (Kesari and Batchelor, 2003; Wen and Fine, 1997).
Among solid tumors, adenocarcinomas have a particular propensity to metastasize to the leptomeninges. Among lung tumors, patients with SCLC have a greater tendency to develop LM than those with other lung cancer subtypes. In several large series, breast cancer accounted for 11% to 64% of patients with LM, followed by lung cancer (14%-29%), melanoma (6%-18%), and gastrointestinal cancers (4%-14%). Primary brain tumors, especially medulloblastoma and high-grade gliomas, also have a tendency for CSF spread. Some solid tumors (e.g., head and neck cancer, thyroid cancer, prostate cancer, carcinoid, bladder cancer) rarely seed the leptomeninges (Kesari and Batchelor, 2003; Wen and Fine, 1997).
LM associated with acute lymphoblastic leukemia (ALL) in children was previously common, but CNS prophylaxis has reduced the incidence from 66% to 5%. The incidence of LM in adults with ALL remains high despite similar prophylactic measures. Patients with acute myelogenous leukemia have a 5% risk of LM with CNS prophylaxis and 10% without it (Drappatz and Batchelor, 2005). LM is uncommon in patients with chronic myelogenous leukemia and hairy cell leukemia. Leptomeningeal involvement is present in up to 50% of patients with chronic lymphocytic leukemia at autopsy, although it is almost always asymptomatic during life (Grossman and Moynihan, 1991).
Seeding of the leptomeninges occurs in approximately 6% of patients with NHL. The highest risk occurs in those with diffuse, lymphoblastic, or Burkitt histology, or those who have involvement of the bone marrow, testes, or extranodal sites. Leptomeningeal disease is rare in patients with Hodgkin disease, mycosis fungoides, and multiple myeloma (Grossman and Moynihan, 1991).
Pathogenesis
Tumor cells usually reach the leptomeninges by direct extension from preexisting tumor in the brain parenchyma or epidural space or by hematogenous spread (Posner, 1995). Tumor cells may also spread along spinal nerve roots from a paraspinal mass or along cranial nerves from a head and neck tumor. Once tumor cells reach the leptomeninges, they spread along the surface of the brain, spinal cord, and nerve roots (Fig. 52F.3). The flow of CSF carries exfoliated tumor cells to other parts of the neuraxis, especially to the basal cisterns and cauda equina, where they tend to settle as a result of gravity and slow CSF flow.
The presence of tumor cells in the leptomeninges produces multifocal neurological dysfunction in several ways. Direct invasion of spinal and cranial nerves can cause demyelination and subsequent axonal degeneration of the nerves. Tumor cells may also grow along the Virchow-Robin spaces and directly invade the brain or spinal cord, producing symptoms of confusion and seizures (Wasserstrom, 1995). LM may also produce CNS dysfunction by causing hydrocephalus. Tumor cells can occlude the CSF outflow foramina of the fourth ventricle or impede the reabsorption of CSF through the arachnoid granulations, leading to hydrocephalus. Occasionally, intracranial pressure may increase without enlargement of the ventricles, and (rarely) herniation occurs. Tumor cells may interfere with the blood supply, decreasing cerebral blood flow and even producing transient ischemic attacks and strokes. Tumor cells may also directly compete with neurons for oxygen and essential metabolites such as glucose. This is the mechanism proposed for the weight gain seen in children with leukemic meningitis and infiltration of the hypothalamus (Posner, 1995).
Clinical Features
LM is usually a late complication of systemic cancer, occurring 6 months to 3 years after the diagnosis of the primary tumor (Wasserstrom, 1995). In rare patients with breast carcinoma and melanoma, an interval of up to 10 years may exist between the diagnosis of the primary tumor and the leptomeningeal relapse. LM usually occurs in the setting of active disease outside the nervous system, although as systemic therapy improves, increasing numbers of patients are developing LM as the sole site of relapsed disease. In 5% of patients, leptomeningeal involvement is the initial presentation of a neoplasm (Posner, 1995).
Cerebral symptoms occur in up to 50% of patients (Kaplan et al., 1990; Wasserstrom, 1995). The most common are headaches, which can be nonspecific or have features suggestive of increased intracranial pressure. Other symptoms include nausea, vomiting, cognitive changes, and occasionally seizures. Focal cerebral symptoms are relatively rare. Papilledema may occasionally be present in patients with hydrocephalus. Rarely, LM may produce a diencephalic syndrome, diabetes insipidus, central hypoventilation, cerebral infarction, and complex partial status epilepticus (Wen and Fine, 1997).
Diagnostic Tests
Table 52F.2 summarizes the most useful tests for the diagnosis of LM.
| Test | Measurement | Positive Findings |
|---|---|---|
| Lumbar puncture | Lymphocytic pleocytosis | >70% |
| Elevated opening pressure | 50% | |
| Elevated protein | 75% | |
| Reduced glucose | 30%-40% | |
| Cytology after 1 LP | 50% | |
| Cytology after 3 LPs | 90% | |
| CSF markers | Variable | |
| Immunohistochemistry | Variable | |
| PCR | Variable | |
| Brain MRI | Meningeal enhancement | >50% |
| Enlarged ventricles | <50% | |
| Spine MRI/myelogram | Subarachnoid masses | <25% |
| Meningeal enhancement | >50% |
CSF, Cerebrospinal fluid; LP, lumbar puncture; MRI, magnetic resonance imaging; PCR, polymerase chain reaction.
Cerebrospinal Fluid Examination
Examination of the CSF is the most important test for the diagnosis of LM. The finding of malignant cells in the CSF is the definitive diagnosis. However, the CSF is almost always abnormal even if the cytological examination result is negative. These abnormalities may include lymphocytic pleocytosis, elevated opening pressure, increased CSF protein, and a decreased glucose concentration (Kesari and Batchelor, 2003; Posner, 1995). In 3% of patients, the CSF is normal.
Low CSF glucose (hypoglycorrhachia) is present in approximately 30% of patients with LM (Wasserstrom et al., 1982). The precise cause of the low CSF glucose is unknown. Possible reasons include impaired carrier mediated transport of glucose across the blood-CSF barrier and increased utilization of glucose by tumor cells and reactive lymphocytes.
The high rate of false-negative cytological findings may reflect the manner of spinal fluid collection and management. The sensitivity of cytology is dependant on the volume of CSF collected. Glantz et al. (1998), in their unique prospective study of CSF collection among patients with suspected carcinomatosis meningitis, reported a false-negative rate of a 3.5 mL sample of 32%, which decreased to 10% and 3% for 7.0 mL and 10.5 mL samples, respectively. Yet the mean CSF volume submitted to the cytology laboratory was 2.9 mL, with 97% of specimens less than 10.5 mL. In addition, the efficiency of processing collected CSF samples influenced outcomes. The false-negative rate after a 48-hour delay in processing the spinal fluid was 36%. The site from which CSF is collected, from the lumbar or cervical cisterns or from the ventricles, may also influence the sensitivity of cytology. Several authors have noted a higher rate of positive cytological findings when collecting fluid from sites that approximate clinical or radiographic disease (Chamberlain et al., 2001; Glantz et al., 1998). Repeat CSF analysis may further reduce false negatives when suspicion remains after a negative cytological study. In the often-cited study by Wasserstrom et al. (1982), cytological findings were positive in 54% at first lumbar puncture, which increased to 86% at the third sample. Other authors have reported similar findings in subsequent studies (Glantz et al., 1998). Despite repeated samples, 8% to 10% of patients with carcinomatosis meningitis have persistently negative cytology results.
When the CSF cytological examination result is negative, biochemical markers can sometimes be useful in assisting the diagnosis of LM and monitoring the response to therapy (Kesari and Batchelor, 2003; Wen and Fine, 1997). Table 52F.3 lists some of the more commonly used markers. Although some of these markers are fairly specific, their lack of sensitivity often limits their usefulness. Occasionally, parenchymal metastases adjacent to leptomeningeal or ependymal surfaces falsely elevate the levels of biochemical markers in the CSF.
Table 52F.3 Cerebrospinal Fluid Tumor Markers in Leptomeningeal Metastases
| Marker | Tumor |
|---|---|
| RELATIVELY SPECIFIC | |
| AFP | Teratocarcinoma, yolk sac tumor, ECC, endodermal sinus tumor |
| CA-125 | Ovarian cancer |
| CA-15-3 | Breast cancer |
| CA19-9 | Adenocarcinoma, biliary disease |
| Creatine kinase BB | Small-cell lung cancer |
| hCG β-subunit (β-hCG) | Choriocarcinoma, ECC, germ cell tumor |
| 5-HIAA | Carcinoid |
| HPAP | Germinoma |
| IgM | Myeloma |
| Melanin | Melanoma |
| PSA | Prostate cancer |
| Tissue polypeptide antigen | Breast cancer |
| NONSPECIFIC | |
| β2-microglobulin | Lymphoma, infection, other tumors |
| β-glucuronidase | Nonspecific |
| CEA | Colon, ovarian, breast, bladder, lung |
| HMFG1 mAb | Nonspecific |
| LDH isoenzymes | Carcinoma, nonspecific |
| Telomerase | Nonspecific |
| VEGF | Nonspecific |
AFP, Alpha fetoprotein; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; ECC, embryonal cell carcinoma; hCG, human chorionic gonadotropin; HIAA, hydroxyindoleacetic acid; HMFG, human milk fat globule; HPAP, human placental alkaline phosphatase; IgM, immunoglobulin M; LDH, lactate dehydrogenase; PSA, prostate specific antigen; VEGF, vascular endothelial growth factor.
In some patients, immunocytochemistry can increase the sensitivity for detecting malignant cells. Monoclonal antibodies against surface markers on lymphocytes can be especially useful in distinguishing lymphoma cells (which are usually monoclonal B cells) from reactive T lymphocytes. However, because malignant lymphocytes can mix with reactive T cells, the presence of polyclonality does not exclude tumor. When the diagnosis of leptomeningeal lymphoma is difficult, polymerase chain reaction (PCR) technique is useful to determine whether the Ig gene rearrangement is identical in all the lymphocytes, suggesting a neoplastic process (Thomas et al., 2000).
Neuroimaging
MRI is the most sensitive neuroimaging test for detecting LM. Meningeal enhancement is present in half of patients with LM. Also observed are small enhancing cortical nodules and communicating hydrocephalus from impaired CSF absorption. Rarely, focal LM may mimic a meningioma. Whenever possible, perform MRI before lumbar puncture. Lumbar puncture occasionally results in intracranial hypotension and meningeal enhancement, which leads to an erroneous diagnosis of LM. Myelography shows thickening and nodularity of nerve roots in 25% to 33% of patients with LM and may be helpful when MRI is not appropriate. Increasingly, the diagnosis of LM is established in patients with characteristic clinical and radiological findings, even if the CSF cytological examination result is negative (Freilich et al., 1995).
Diagnosis
The basis for the diagnosis of LM is the combination of characteristic clinical, radiological, and CSF features, and ideally, positive CSF cytological findings. Other conditions causing subacute neurological deficits at multiple sites in the neuraxis may mimic LM (Box 52F.2). These include parenchymal and epidural tumor deposits and subacute and chronic meningitides such as syphilis, sarcoidosis, Lyme disease, and fungal and tuberculous meningitis. Routine imaging, CSF studies, and positive cytology results usually distinguish other entities from LM. Specific serological tests are also useful. These include Lyme antibody titers; serum angiotensin-converting enzyme (sarcoidosis); fluorescent treponemal antibody (syphilis); cryptococcal antigen; fungal cultures, and PCR for herpes simplex, varicella-zoster, and tuberculosis. A meningeal biopsy may be useful when the diagnosis remains in doubt.
Treatment
The goal of therapy is to improve or stabilize the patient’s neurological status and prolong survival. Specific treatment depends on tumor type, site of the leptomeningeal tumor, and clinical condition of the patient. Without treatment, the median survival of patients with LM is 4 to 6 weeks. Death usually results from progressive neurological dysfunction. With treatment, median survival increases to 3 to 6 months. The response rate is 50% to 60% for carcinomas and more than 80% for NHL (Wen and Fine, 1997). Although treatment often provides effective local control, LM usually occurs in the setting of systemic relapse, and most patients who survive beyond the first month (two-thirds of the total) eventually die of their systemic disease. Patients who benefit most from treatment are those with minimal neurological deficits, good performance status, slowly progressive systemic disease with little or no systemic metastases, and a diagnosis of NHL or breast cancer (Grossman and Moynihan, 1991).
Because tumor cells disseminate throughout the subarachnoid space, the entire neuraxis requires treatment if therapy is to be effective. If only symptomatic sites are treated, early relapse is likely secondary to seeding from residual tumor cells in untreated areas of the leptomeninges. Currently, standard therapy for LM involves radiation to sites of symptomatic and bulky disease and regions of CSF flow interruption, intraventricular administration of chemotherapy via an Ommaya or Rickham reservoir, and optimal treatment of systemic disease (Box 52F.3).
Box 52F.3 Treatment of Leptomeningeal Metastases
Radiation Therapy
RT is limited to symptomatic areas and sites of bulky disease where the penetration of intrathecal drugs is limited. Although RT is more effective than chemotherapy in treating tumor cells in the Virchow-Robin spaces and nerve root sleeves, the use of craniospinal radiation to the entire neuraxis is rare because of bone marrow suppression. This is of particular concern in patients with LM, because many have systemic metastases that require treatment with chemotherapy. A commonly administered dose of radiation is approximately 3000 cGy administered over 2 weeks. When effective at restoring normal CSF circulation, radiation to sites of CSF flow obstruction may also improve outcome (Chamberlain and Kormanik, 1996).
Chemotherapy
Administer intrathecal therapy, the delivery of chemotherapy directly into the CSF compartment, via repeated lumbar punctures or into the ventricles though an intraventricular cannula with a subcutaneous reservoir (Ommaya or Rickham reservoirs). Normal CSF circulation carries fluid preferentially out of the ventricles. Consequently, a uniform distribution of drug may be achievable when injected into the ventricle rather than the lumbar cistern. This is particularly true of drugs with a short half-life (Glantz et al., 2010). In addition, it is less uncomfortable and avoids epidural and subdural leakage of the drug. In many patients with extensive LM, obstruction of CSF flow in the subarachnoid space decreases the effectiveness of intrathecal chemotherapy and increases the likelihood of toxicity. In these patients, 111Indium-DTPA CSF flow studies may be helpful in defining the CSF blocks (Mason et al., 1998). These blocks require treatment with RT and may influence outcome.
Only a limited number of chemotherapeutic agents are available for intrathecal (IT) administration (Kesari and Batchelor, 2003; Mason, 2002; Wen and Fine, 1997). Methotrexate (MTX) is the most widely used drug. It is an S-phase-specific antimetabolite and interferes with DNA synthesis by inhibiting dihydrofolate reductase. It is active against leukemia, lymphoma, breast cancer, and other solid tumors to a much lesser extent. The typical treatment is 10 to 12 mg twice weekly for 5 to 8 treatments, or until the CSF clears. Weekly and then monthly maintenance therapy follows. The most effective duration of treatment is unclear, but standard recommendations suggest treatment for at least 3 to 6 months, and perhaps indefinitely. MTX successfully clears malignant cells from the CSF in 20% to 61% of cases (Glantz et al., 1998). Therapeutic concentrations (>10–6 molar) are attained in the CSF for 48 hours by administration of 12 mg of MTX. The MTX is gradually reabsorbed into the blood stream by bulk flow, and transport via the choroid plexus results in a low systemic concentration (peak systemic concentration of > 10–7 molar), which may cause myelosuppression and mucositis. To reduce these systemic side effects, administer leucovorin (10 mg orally twice daily) for 3 to 4 days after administration of MTX. Complications of IT MTX include aseptic meningitis, leukoencephalopathy, mucositis, myelosuppression, encephalopathy, and opportunistic infections (Kesari and Batchelor, 2003).
Cytosine arabinoside (ara-C) is a synthetic pyrimidine nucleoside analog with activity against leukemias and lymphomas but not most solid tumors. The half-life of ara-C is very short in the serum but significantly longer in the CSF because of low levels of cytidine deaminase. The standard IT dose of ara-C is 50 mg twice a week. Intrathecal ara-C has relatively little systemic toxicity because of the rapid deamination of any drug reaching the systemic circulation. Neurological complications associated with IT ara-C include transverse myelopathy, aseptic meningitis, encephalopathy, headaches, and seizures (Kesari and Batchelor, 2003).
A slow-release, liposomal formulation of cytarabine (DepoCyt) was approved for IT use in patients with lymphomatous meningitis (Glantz, Jaeckle et al., 1999; Glantz, LaFollette et al., 1999). An important advantage of this drug is that cytotoxic concentrations of cytarabine (>0.1 µg/mL) are maintained in the CSF for 2 weeks. In a randomized study of 28 patients with lymphomatous meningitis treated with either cytarabine (every 2 weeks) or ara-C (twice a week) for 1 month, the response rate in the cytarabine-treated patients was significantly higher compared with the ara-C treated patients (71% versus 15%) (Glantz, LaFollette et al., 1999). Patients treated with cytarabine also had increased time to neurological progression and improvement in KPS compared with those treated with ara-C. A second phase III study compared the efficacy of DepoCyt to IT MTX in the treatment of LM from solid tumors. Cytarabine was slightly more effective with respect to cytological response (26% versus 20%, P = .76) and median survival (105 days versus 78 days, P = .15). Cytarabine treatment was significantly better in delaying the time to neurological progression (58 days versus 30 days, P = 0.007) (Glantz, Jaeckle et al., 1999; Kesari and Batchelor, 2003). Arachnoiditis can occur in up to 60% of patients receiving cytarabine. Oral dexamethasone (4 mg twice daily for 5 days) reduces the frequency of arachnoiditis significantly.
Thiotepa (N,N′,N″-triethylenethiophosphoramide) is an alkylating agent with activity against a variety of tumors including leukemia and breast cancer. The dosage is 10 mg administered twice weekly. The rapid clearance of thiotepa from the CSF potentially limits its usefulness. However, in one randomized trial of IT chemotherapy in patients with LM, thiotepa was as effective as MTX and less toxic (Grossman et al., 1993).
Although most regard IT chemotherapy as standard treatment for LM in conjunction with radiotherapy, its usefulness has been challenged (Siegal et al., 1994). A study to assess the benefit of intraventricular chemotherapy in addition to systemic treatment and involved field RT in patients with LM from breast cancer showed no survival benefit or improved neurological response. Furthermore, IT was associated with increased risk of neurotoxicity (Boogerd et al., 2004). Further studies will be required to define the precise role of IT chemotherapy.
Systemic and Hormonal Therapy
High-dose intravenous methotrexate achieves potentially cytotoxic CSF levels, and some evidence supports its utility in neoplastic meningitis. In one study, the treatment of patients with LM from solid tumors was with HD MTX with leucovorin rescue. Cytological clearing of CSF occurred in 13 of 16 patients, compared with 9 of 15 retrospective controls treated with IT MTX (Glantz et al., 1998). High-dose systemic ara-C (3 g/m2 every 12 hours) penetrates well into the CNS and used sometimes in patients with leukemia or NHL who have both systemic and CNS disease. Recent data suggest that the 5-fluorouracil oral prodrug, capecitabine, may be useful in meningeal seeding from breast cancer. A recent case study demonstrated a durable response to capecitabine monotherapy in a patient with LM from breast cancer. However, further clinical studies are required to further evaluate the role of this drug in the treatment of LM (Rogers et al., 2004). Hormonal therapy may occasionally be of benefit in patients with LM due to hormone-sensitive tumors such as breast cancer and prostate cancer (Wen and Fine, 1997). Anecdotally, small-molecule tyrosine kinase inhibitors such as erlotinib have shown some activity in selected cases of carcinomatosis meningitis.
Prognosis
Without treatment, patients with LM usually survive only 1 to 2 months, with occasional long-term survivals. The presence of encephalopathy in the setting of LM is associated with poor survival. In a cohort study of 40 patients, 20 had LM-related encephalopathy. Median survival in the group with encephalopathy was 10 weeks, compared to 24 weeks in the group without LM-associated encephalopathy (Chamberlain et al., 2004). Poor survival is also associated with CSF flow blocks in the setting of LM. Flow blocks culminate in non-uniform distribution of intrathecal chemotherapy, which in turn creates regions of low and high concentrations of chemotherapy, resulting in tumor sanctuaries and increased toxicity, respectively. CSF flow blocks identified on CSF flow studies are often correctable with radiation. Uncorrectable flow blocks have been associated with significantly shorter survival (0.7 months) compared to normal CSF flow (6.9 months) and abnormal but correctable flow blocks (13.0 months) (Glantz et al., 1995). Other prognostic factors include performance status, activity, and extent of systemic cancer, concomitant parenchymal metastases, and possibly histology. With treatment, the median survival increases to 3 to 6 months (Posner, 1995). Patients with NHL and breast cancer tend to respond better to treatment than those with other cancers, and the percentage of long-term survivors is increased. The 1-year survival rate for breast cancer is approximately 11%, whereas that for NHL is 6% to 23%. In general, fixed neurological deficits such as cranial nerve palsies or paraplegia do not improve significantly with therapy, but encephalopathy may improve dramatically.
Other Therapies
More effective treatment programs for LM are under investigation. The strategies being evaluated include IT administration of drugs such as busulfan, gemcitabine, and mafosfamide; radiolabeled monoclonal antibodies; immunotoxins; interleukin 2; and viral-mediated gene therapy. Treatment options for intracranial hypertension associated with LM are presently under consideration. In a study of 37 patients with LM, 27 experienced improvement in symptoms related to increased intracranial pressure after ventriculoperitoneal shunt placement (Omuro et al., 2005).
Skull and Dural Metastases
As with other nervous system metastases, the incidence of skull and dural metastases appears to increase with prolonged patient survival and improved neuroimaging (Jansen and Sillevis Smitt, 2002; Posner, 1995). Skull and dural metastases may be asymptomatic and detected incidentally on neuroimaging studies or produce symptoms by compression of adjacent neural structures (see Fig. 52F.3). Treatment of these metastases is usually effective.
Skull Metastases
Skull metastases occur in 15% to 25% of all cancer patients, usually in the setting of bony metastases elsewhere in the body. At least half are asymptomatic. Skull metastases usually arise from hematogenous spread via either the arterial circulation or Batson plexus. The most common primary tumors that metastasize to the skull base and calvarium are breast, lung, and prostate, followed by renal, thyroid, and melanoma (Jansen and Sillevis Smitt, 2002). Renal cell and thyroid carcinoma may produce solitary calvarial metastasis. Rarely, extracranial tumors may extend centrally along cranial nerve branches and enter the skull through foramina. Tumors with a predilection for perineural growth include squamous cell carcinoma of the nasopharynx, esthesioneuroblastoma, lymphoma, nerve sheath tumors, and skin cancers.
Skull-base metastases often present with characteristic clinical features (Greenberg et al., 1981) (Table 52F.4). The differential diagnosis includes leptomeningeal and parenchymal metastases, as well as benign conditions such as granulomatous or infectious diseases. Biopsy is a consideration if the diagnosis is inconclusive following clinical and radiological evaluation and CSF examination (to exclude leptomeningeal disease).
Table 52F.4 Classification of Clinical Syndromes Caused by Skull Base Metastasis
| Site of Skull Base Metastasis | Symptoms and Signs |
|---|---|
| Orbital | Local pain, proptosis, sensory loss (V1), diplopia, decreased vision (late) |
| Parasellar/cavernous sinus | Unilateral frontal headache, oculomotor palsies (III, IV, VI), sensory loss (V1) |
| Middle cranial fossa | Facial numbness or pain (V2,3), sometimes abducens or facial nerve palsy (VI, VII) |
| Jugular foramen | Unilateral postauricular pain, hoarseness, dysphagia (IX, X), sternocleidomastoid or trapezius weakness (XI) |
| Occipital condyle | Unilateral occipital pain, stiff neck, unilateral tongue weakness (XII) |
From Greenberg, H.S., Deck M.D.F., Vikram, B., et al., 1981. Metastasis to the base of the skull: clinical findings in 43 patients. Neurology 31, 530-537.
Calvarial metastases appear as irregular lucencies on skull radiographs. In 90% of patients, boney metastases are present elsewhere in the body. CT and MRI usually show lesions involving all three tables of the skull bone and provide information regarding the extent of intracranial extension and relation to venous sinuses (Jansen and Sillevis Smitt, 2002). The differential diagnosis of multiple skull defects includes normal structures such as venous lakes, pacchionian granulations, and parietal foramina. Pathological conditions include Langerhans cell histiocytosis, hyperparathyroidism, osteomyelitis, and radiation necrosis. For single calvarial lesions, the differential diagnosis includes meningiomas (including primary intraosseous meningioma), hemangiomas, epidermoid cysts, leptomeningeal cysts (in children), Langerhans cell histiocytosis, Paget disease, postsurgical defect, and osteomyelitis (Jansen and Sillevis Smitt, 2002). In patients not known to have cancer, comparison with prior imaging studies may be helpful.
The prognosis of skull metastases depends on systemic tumor control as well as local factors including invasion of venous sinus or dura, leptomeninges, and brain parenchyma (Jansen and Sillevis Smitt, 2002). In general, better outcomes are associated with starting treatment less than 1 month after diagnosis.
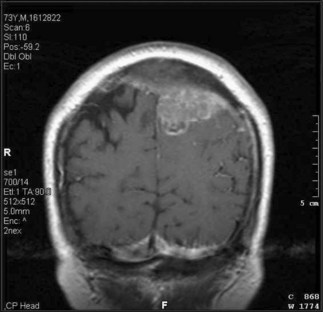
Dural Metastases
Dural metastases occur in up to 20% of patients in autopsy studies (Posner, 1995), but symptomatic lesions are much less frequent. The most common tumors giving rise to dural metastases are NSCLC, prostate cancer, and breast cancer. Less commonly, melanoma, gastric, colon, SCLC, and renal cell carcinoma, pleural mesothelioma, carcinoid tumors, and lymphoma may be responsible (Rodas and Greenberg, 1997). Metastases usually reach the dura by invasion from tumors in the adjacent skull or brain parenchyma, or by hematogenous spread.
Dural metastases cause symptoms by compressing or invading the underlying brain, obstructing adjacent venous sinuses, or producing subdural fluid collections and hematomas (Rodas and Greenberg, 1997). The subdural collections are often indistinguishable from subdural hematomas and hygromas from benign causes. The relation of the subdural collections to an underlying neoplasm may only be determined by finding malignant cells at surgery.
MRI usually establishes the diagnosis. However, small dural metastases are often difficult to distinguish from meningiomas. This is especially important in patients with breast cancer, who may have an increased incidence of meningiomas (Schoenberg et al., 1975).
The prognosis for patients with dural metastases is slightly better than for parenchymal metastases (median survival of 24 weeks versus 18 weeks). The most important variable for prognosis of dural metastases is the extent of systemic disease (Jansen and Sillevis Smitt, 2002).
Plexus Metastases
Brachial Plexopathy
Invasion of the brachial plexus is by local spread of tumor from lung or breast carcinoma or axillary lymph nodes (Briemberg and Amato, 2003; Kori, 1995; Kori et al., 1981) (see Fig. 52F.2, A). Rarely, tumor may reach the brachial plexus by hematogenous or lymphatic spread. The lower trunk of the brachial plexus is usually involved. Most patients experience constant severe pain radiating from the shoulder down the medial aspect of the arm into the fourth and fifth digits. Numbness and paresthesias may be associated. Examination shows weakness, atrophy, and sensory loss in the distribution of the lower trunk of the brachial plexus (C8-T1 nerve roots). Horner syndrome is present in 50% of patients and reflects involvement of the stellate ganglion. The main differential diagnosis is epidural and leptomeningeal disease affecting cervical nerve roots, and radiation plexopathy. Radiation plexopathy usually occurs 1 year or more (median 40 months) after RT with doses of 6000 cGy or more. Features suggesting radiation injury rather than tumor are the relative absence of pain, involvement of the upper trunk or entire brachial plexus, severe lymphedema, slow progression or stabilization, absence of Horner syndrome or associated epidural disease, and myokymic discharges (spontaneous rapid semirhythmic bursts of potentials) on electromyography (EMG) (Kori, 1995). CT or MRI of the brachial plexus usually establishes the diagnosis. Occasionally, MRI of the cervical spine may be necessary to exclude epidural disease. When standard imaging studies and positron-emission tomography cannot establish the diagnosis, surgical exploration and biopsy of the brachial plexus may be necessary. The treatment for neoplastic brachial plexopathy is generally RT, with chemotherapy reserved for patients unable to receive further RT to the plexus. Approximately half of patients experience amelioration of pain, but neither strength nor sensory symptoms improve.
Lumbosacral Plexopathy
Lumbosacral plexopathy usually results from direct extension of tumor or from metastases to local lymph nodes or bone. The most common tumors associated with lumbosacral plexopathy are colorectal cancer, lymphoma, cervical carcinoma, and sarcomas (Jaeckle et al., 1985). The initial features are pain, numbness, paresthesias, weakness, and edema of the leg. Impotence and incontinence may occur. Examination findings may include weakness, sensory loss, reflex asymmetry, leg edema, and (rarely) a pelvic mass on rectal examination. Bilateral lumbosacral plexopathies occur in 25% of patients; the usual cause is metastatic breast cancer. Occasionally, patients develop isolated obturator neuropathy. MRI or CT scan of the lumbosacral spine and pelvis establish the diagnosis. A significant minority of patients have spread of tumor to the epidural space. Radiation injury to the lumbosacral plexus is less common than radiation injury to the brachial plexus but is in the differential diagnosis. Radiation injuries tend to be less painful than tumor infiltration and EMG may show myokymia. Treatment of neoplastic lumbosacral plexopathy involves RT and occasionally surgery.
Peripheral Nerve Metastases
Very rarely, mononeuropathies, mononeuropathy multiplex, and even a symmetrical polyneuropathy may result from direct nerve infiltration by tumor (Briemberg and Amato, 2003). Lymphomas and leukemias, particularly chronic lymphocytic leukemia, have been associated with peripheral neuropathy due to malignant infiltration of nerve (Amato and Dumitru, 2002).
Amato A.A., Dumitru D. Acquired neuropathies. Dumitru D., Amato A.A., Zwarts M.J. Electrodiagnostic Medicine, second ed, Philadelphia: Hanley and Belfus, 2002.
Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
Aoyama H., Shirato H., Masao T., et al. Sterotactic radiosurgery plus whole-brain radiation therapy vs sterotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
Aoyama H., Tago M., Kato N., et al. Neurocognitive function of patients with brain metastases who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388-1395.
Auperin A., Arriagada R., Pignon J.P., et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. New Engl J Med. 1999;341:476-484.
Barnholtz-Sloan J.S., Sloan A.E., Davis F.G., et al. Incidence proportions of brain metastases in patients diagnosed (1973-2001) in the Metropolitan Detroit Surveillance System. J Clinical Oncol. 2004;22:2865.
Behin A., Delattre J.Y. Neurologic sequelae of radiotherapy on the nervous system. In: Schiff D., Wen P.Y. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press, 2002.
Boogerd W., van den Bent M.J., Koehler P.J., et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40:2726-2733.
Briemberg H.R., Amato A.A. Neuromuscular complications of cancer. Neurol Clin. 2003;21(1):141-165.
Cairncross J.G., Kim J.H., Posner J.B. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529-541.
Cavaliere R. Headache associated with intracranial neoplasms. In: Schiff D., Kesari S., Wen P.Y. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press, 2008.
Chamberlain M.C., Kormanik P.A. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996;46:1674-1677.
Chamberlain M.C., Kormanik P.A., Glantz M.J. A comparison between ventricular and lumbar cerebrospinal cytology in adult patients with leptomeningeal metastases. Neuro Oncol. 2001;3:42-45.
Chamberlain M.C., Tsao-Wei D., Groshen S. Neoplastic meningitis-related encephalopathy: prognostic significance. Neurology. 2004;63(11):2159-2161.
Chang E.L., Wefel J.S., Hess K.R., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole brain radiotherapy: A randomized controlled trial. Lancet Oncol. 2009;10:1037-1044.
Cohen N., Strauss G., Lew R., et al. Should prophylactic anticonvulsants be administered to patients with newly-diagnosed cerebral metastases? A retrospective analysis. J Clin Oncol. 1988;6:1621-1624.
DeAngelis L.M., Delattre J.Y., Posner J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
Drappatz J., Batchelor T.T. Leptomeningeal neoplasms. Curr Treat Options Neurol. 2007;9(4):283-293.
Freilich R.J., Krol G., De Angelis L.M. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastases. Ann Neurol. 1995;38:51-57.
Gaspar L., Scott C., Rotman M., et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
Gaspar L.E., Scott C., Murray K., et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001-1006.
Gerszten P.C., Mendel E., Yamamda Y. Radiotherapy and radiosurgery for metastatic spine disease. Spine. 2009;34:s78-s92.
Glantz M.J., Cole B.F., Forsyth P.A., et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886-1893.
Glantz M.J., Cole B.F., Recht L., et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561-1567.
Glantz M.J., Cole B.F., Glantz L.K., et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false negative results. Cancer. 1998;15:733-739.
Glantz M.J., Hall W.A., Cole B.F., et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995;75(12):2919-2931.
Glantz M.J., Jaeckle K.A., Chamberlain M.C., et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394-3402.
Glantz M.J., LaFollette S., Jaeckle K.A., et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17:3110-3116.
Glantz M.J., Van Horn A., Fisher R., et al. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer. 2010;116:1947-1952.
Gleissner B., Chamberlain M.C. Neoplastic meningitis. Lancet Neurol. 2006;5:443-452.
Greenberg H.S., Deck M.D.F., Vikram B., et al. Metastasis to the base of the skull: Clinical findings in 43 patients. Neurology. 1981;31:530-537.
Grossman S.A., Moynihan T.J. Neoplastic meningitis. Neurol Clin. 1991;3:729-750.
Grossman S.A., Finkelstein D.M., Ruckdeschel J.C., et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:561-569.
Heimdal K., Hirschberg H., Slettebo H., et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neuro Oncol. 1992;12:141-144.
Helweg-Larsen S., Sorensen P.S. Symptoms and signs in metastatic spinal cord compression: A study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer. 1994;30:396-398.
Henson R.A., Urich H. Cancer and the Nervous System. Oxford: Blackwell Scientific; 1982.
Jaeckle K.A. Neoplastic meningitis from systemic malignancies: diagnosis, prognosis and treatment. Semin Oncol June. 2006;33(3):312-323.
Jaeckle K.A., Young D.F., Foley K.M. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985;35:8-15.
Jagannathan J., Yen C., Ray D.K., et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009;111:431-438.
Jansen B.P.W., Sillevis Smitt P.A.E. Cancer Neurology in Clinical Practice. In: Schiff D., Wen P.Y. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press, 2002.
Jeyapalan S.A., Henson J.W. Neuro-oncologic complications of lung cancer. In: Schiff D., Wen P.Y. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press, 2002.
Kaplan J.G., DeSouza T.G., Farkash A., et al. Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neuro Oncol. 1990;9:225-229.
Kesari S., Batchelor T.T. Leptomeningeal metastases. Neurol Clin. 2003;21(1):25-66.
Komosinska K., Kepka L., Niwinska A., et al. Prospective evaluation of palliative effect of whole brain radiotherapy in patients with brain metastases and poor performance status. Acta Oncol. 2010;49:382-388.
Kori S.H., Foley K.M., Posner J.B. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31:45-50.
Kori S.H. Diagnosis and management of brachial plexus lesions in cancer patients. Oncology (Huntingt). 1995;9(8):756-760.
Landis S.H., Mrray T., Bolden S., et al. Cancer statistics. CA Cancer J Clin. 1998;48:6-29.
Lassman A., DeAngelis L.M. Brain metastases. Neurol Clin. 2003;21(1):1-23.
Le Chevalier T., Smith F.P., Caille P., et al. Sites of primary malignancies in patients presenting with cerebral metastases. A review of 120 cases. Cancer. 1985;56:880-882.
Le Pechoux C., Dunant A., Senan S., et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomized clinical trial. Lancet Oncol. 2009;10:467-474.
Loeffler J.S., Barker F.G., Chapman P.H. Role of radiosurgery in the management of central nervous system metastases. Cancer Chemother Pharmacol. 1999;43:S11-S14.
Loblaw D.A., Laperriere N.J., Mackillop W.J. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15:211-217.
Lutterbach J., Bartelt S., Stancu E., et al. Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol. 2002;63:339-345.
Lutterbach J., Cyron D., Henne K., et al. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003;52:1066-1073.
Manon R., O’Neill A., Werner, et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol. 2005;23:8870-8876.
Mason W.P., Yeh S.D., DeAngelis L.M. 111Indium-diethylenetriamine pentaacetic acid cerebrospinal fluid flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology. 1998;50:438-444.
Mason W.P. Leptomeningeal metastases. In: Schiff D., Wen P.Y. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press, 2002.
Mavrakis A.N., Halpern E.F., Barker F.G.2nd, et al. Diagnostic evaluation of patients with a brain mass as the presenting manifestation of cancer. Neurology. 2005;65:908-911.
Mehta M.P., Shapiro W.R., Phan S.C., et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurological progression in non-small cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73:1069-1076.
Mehta M.P., Tsao M.N., Whelan T.J., et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63:37-46.
Meyers C.A., Smith J.A., Bezjak A., et al. Neurocognitive function and progression in patients with brain metastases treated with whole brain radiation and motexafin gadolinium: results of a phase III trial. J Clin Oncol. 2004;22:157-165.
Nieder C., Berberich W., Schnabel K. Tumor-related prognostic factors for remission of brain metastases after radiotherapy. Int J Radiat Oncol Biol Phys. 1997;39:25-30.
Omuro A.M., Lallana E.C., Bilsky M.H., et al. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64:1625.
O’Neill B.P., Iturria N.J., Link M.J., et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169.
Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
Patchell R.A., Tibbs P.A., Regine W.F., et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
Patchell R.A., Tibbs P.A., Walsh J.W., et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322:494-500.
Pirzkall A., Debus J., Lohr F., et al. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol. 1998;16:3563-3569.
Posner J.B. Neurologic Complications of Cancer. Philadelphia: F A Davis; 1995.
Rades D., Fehlauer F., Stalpers L.J., et al. A prospective evaluation of two radiotherapy schedules with 10 versus 20 fractions for the treatment of metastatic spinal cord compression: final results of a multicenter study. Cancer. 2004;101:2687.
Rades D., Stalpers L.J., Veninga T., et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23:3366.
Regine W.F., Scott C., Murray K., et al. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated hyperfractionated radiotherapy: an analysis from RTOG study 91-04. Int J Radiat Oncol Biol Phys. 2001;51:711-717.
Regine W.F., Huhn J.L., Patchell R.A., et al. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: results and implications. Int J Radiat Oncol Biol Phys. 2002;52:333-338.
Rodas R.A., Greenberg H.S. Dural, calvarial and skull base metastasis. In: Neuro-Oncology. Part III. Neurologic Disorders in Systemic Cancer. Handbook of Clinical Neurology. Amsterdam: Elsevier; 1997.
Rogers L.R., Remer S.E., Tejwani S. Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro Oncol. 2004;6:63-64.
Sawaya R., Ligon B.L., Binda lR.K. Management of metastatic brain tumors. Ann Surg Oncol. 1994;1:169-178.
Schiff D. Spinal metastases. In: Schiff D., Wen P.Y. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press, 2002.
Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86.
Schiff D., O’Neill B.P. Intramedullary spinal cord metastases: clinical features and treatment outcome. Neurology. 1996;47:906-912.
Schoenberg B.S., Christine B.W., Whisnant J.O. Nervous system neoplasms and primary malignancy of other sites: the unique association between meningiomas and breast cancer. Neurology. 1975;25:705.
Scott C., Suh J., Stea B., et al. Improved survival, quality of life, and quality adjusted survival in breast cancer patients treated with efaproxiral plus whole brain radiation therapy for brain metastases. Am J Clin Oncol 2007. 2007;30L:580-587.
Siegal T., Lossos A., Pfeffer M.R. Leptomeningeal metastases: analysis of 31 patients with sustained off-therapy response following combined-modality therapy. Neurology. 1994;4:1463-1469.
Slotman B., Faivre-Finn C., Gijs K., et al. Prophylactic cranial irradiation in extensive small cell lung cancer. N Engl J Med. 2007;357:664-672.
Sorensen P.S., Helweg-Larsen S., Mouridsen H., et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30:22-27.
Sperduto P.W., Berkey B., Gaspar L.E., et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510-514.
Suh J.H., Stea B., Nabid A., et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106-114.
Thomas J.E., Falls E., Velasco M.E., et al. Diagnostic value of immunocytochemistry in leptomeningeal tumor dissemination. Arch Path Lab Med. 2000;124:759-761.
Vecht C.J., Hovestadt A., Verbiest H.B., et al. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44:675-680.
Voorhies R.M., Sundaresan N., Thaler H.T. The single supratentorial lesion. An evaluation of preoperative diagnostic tests. J Neurosurg. 1980;53:364-368.
Wasserstrom W.R., Glass J.P., Posner J.B. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759-772.
Wasserstrom W.R. Leptomeningeal metastases. In: Wiley R.G., editor. Neurological Complications of Cancer. New York: Marcel Dekker, 1995.
Wen P.Y., Fine H.A. Leptomeningeal metastases. In: Black P.McL, Loeffler J.S. Cancer of the Nervous System. Oxford, United Kingdom: Blackwell Scientific, 1997.
Wong W.W., Schild S.E., Sawyer T.E., et al. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34:585-590.
Yuh W.T., Tali E.T., Nguyen H.D., et al. The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. Am J Neuroradiol. 1995;16:373-380.