Calculation of Medications Used Intravenously
• Interpret orders for volume of medications to be administered as intravenous (IV) fluids
• Calculate dosages of IV solutes in IV fluids including those expressed as percentages
• Calculate IV flow rates in drops per minute (gtts/min)
• Calculate time needed to infuse an ordered volume of IV fluids
Introduction
Intravenous solutions are available in 50-mL to 1000-mL containers (commonly in flexible plastic bags) with each containing solutes and solvents prescribed for the patient’s specific needs. The reason for therapy dictates the type of fluid and rate of infusion ordered. Fluids given to keep a vein open are given slowly, whereas replacement fluids are usually given at a rate that will provide the necessary fluids while preventing an overload on the vascular system. The rate of flow depends on the patient’s physical condition. Most IV fluids are found in percentages of solutes (solids) in the total volume of solvent (solution) such as D-5-W, which is 5% dextrose in water. As learned in Chapter 11, this means that 5 g of dextrose are dissolved in every 100 mL of sterile water, or 50 g of dextrose are found in 1000 mL of water. While most IV fluids are infused by pump today, it is still important to be conscious of selecting the correct fluids for the IV infusion and ensuring an infusion is administered at the prescribed rate.
Interpreting Amounts of Solutes in Intravenous Fluids
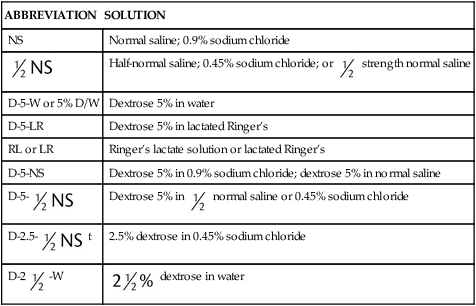
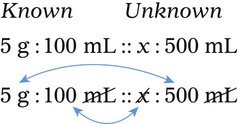
In most cases the physician will use common abbreviations for ordering IV fluids. These are shown in Table 12-1. Notice fluids are labeled with a percentage of solute in the container of fluids or the amount of solute found in the solvent. D-5-W indicates that 5% dextrose is available in the solvent (sterile water). Remember that percentage is based on 100. So if the percentage is shown in 100 mL of solution, the 5% indicates that 5 g of dextrose is found in 100 mL of fluid. However, if the 5% is shown in 500 mL of a solvent such as water, then the total weight of the solute must be calculated. The easiest method for calculating the weight of solute is ratio and proportion (Known solute : Known volume :: Desired-to-know solute : Desired-to-know volume). To review percentage and ratio and proportion, see Chapter 2.
TABLE 12.1
Abbreviations for Common Intravenous Solutions
| ABBREVIATION | SOLUTION |
| NS | Normal saline; 0.9% sodium chloride |
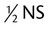 |
Half-normal saline; 0.45% sodium chloride; or 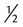 strength normal saline strength normal saline |
| D-5-W or 5% D/W | Dextrose 5% in water |
| D-5-LR | Dextrose 5% in lactated Ringer’s |
| RL or LR | Ringer’s lactate solution or lactated Ringer’s |
| D-5-NS | Dextrose 5% in 0.9% sodium chloride; dextrose 5% in normal saline |
D-5-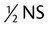 |
Dextrose 5% in 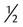 normal saline or 0.45% sodium chloride normal saline or 0.45% sodium chloride |
D-2.5-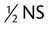 t t |
2.5% dextrose in 0.45% sodium chloride |
D-2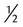 -W -W |
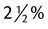 dextrose in water dextrose in water |
Calculating Intravenous Flow Rates in Drops Per Minute
This section covers calculation of the drops or milliliters per minute (called flow rate). An IV infusion set (or equipment) includes the sealed container of sterile fluids and tubing connected to the needle for insertion into the vein for administration of the fluids. Equipment for IV infusions includes tubing for carrying fluid from the container to the person (Figure 12-1). The packaging for the tubing provides information on the number of drops per milliliter to be supplied. The rate of flow or the infusion rate in drops per minute is adjusted by the clamp on the tubing and the size of the drop from the drip chamber or the drop factor or by the use of infusion pump that calculates drops/minute based on the size of the drop.
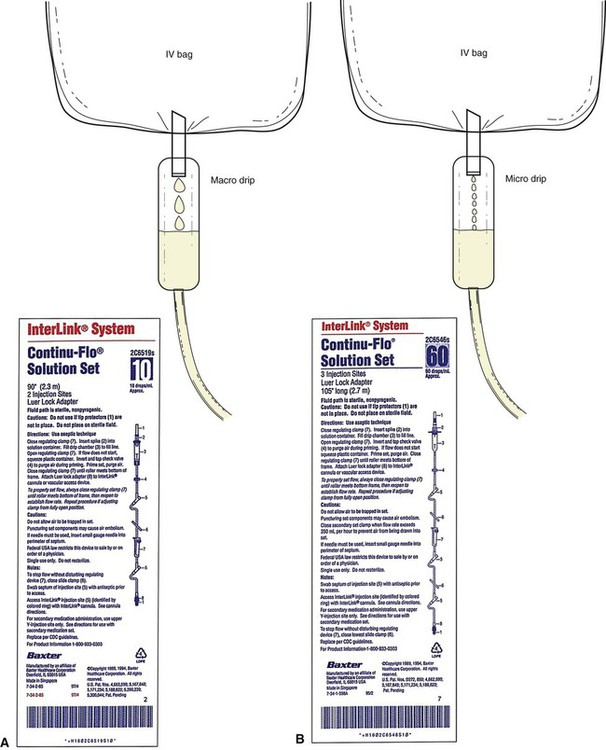
The most common drop factors for IV fluids are 10, 15, 20, and 60 gtts/mL (Figure 12-2, A). Drop factors that deliver fewer and larger drops per milliliter such as 10, 15, or 20 gtts/mL are called macrodrip infusion sets. Macrodrip sets are used when large volumes of fluids must be administered or if fluids must be administered at a rapid rate. Tubing that delivers 60 drops per milliliter is called a microdrip infusion set. These sets are used for slower delivery of fluids such as at rates of 50 mL per hour (see Figure 12-2, B).
• The total amount of fluids to be administered in milliliters (mL)
• The calibration of the administration set in drops/milliliter (gtts/mL)
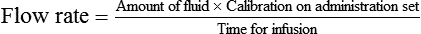
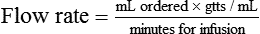
If dimensional analysis is the preferred means of calculating dosage, see Example 12-5.
Review of Rules
Interpreting the Amounts of Solutes in Intravenous Fluids
• Remember that 1 mL of water equals 1 g in weight.
• Percentage is based on 100, so 5% indicates that 5 g solute is found in 100 mL of solvent.
• The easiest method for calculating weight of solute by ratio and proportion is as follows:
Known solute : Known volume :: Desired-to-know solute : Desired-to-know volume
Calculating Intravenous Flow Rates in Drops per Minute
• Tubing used for the IV infusion set provides information for the number of drops per milliliter to be supplied to the patient.
• The drop factor is found on the packaging for the infusion set and may be either microdrops or macrodrops.
• To calculate the flow rate, consider the following factors:
The total amount of fluid to be administered in milliliters
The calibration of the administration set in drops/milliliter
• The flow rate of IV fluids may be calculated using the following formula:
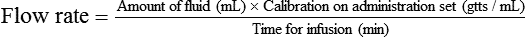
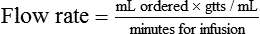
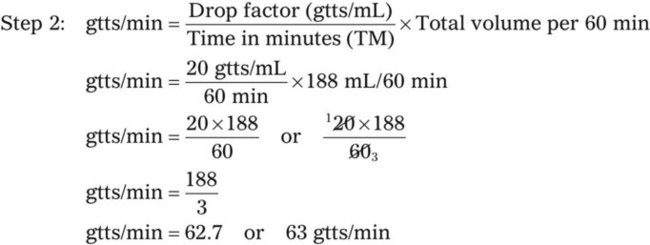
• This calculation may also be accomplished using two steps:
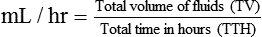
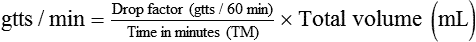
• If dimensional analysis is used, the formula is based on the following: DF = Drip factor; CFV = Conversion factor volume; DV = Dose volume; DT = Dose time; CFT = Conversion factor time; FR = Flow rate in gtts/min.
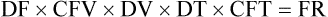
Calculating Intravenous Infusion Times
• If a physician orders fluids without a specific running time, the problem is to decide the total time of the infusion. This calculation provides availability of fluids for follow-up infusions at the appropriate time to meet physician’s order.
• The formula for calculating the infusion time is as follows:
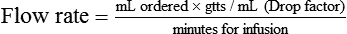
• In this case the minutes for infusion becomes x, and the given amounts are placed in the equation.
• If using dimensional analysis, use the same abbreviations found earlier. The formula is as follows:
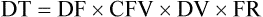










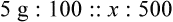
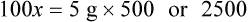
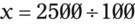
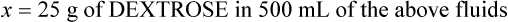
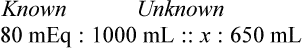
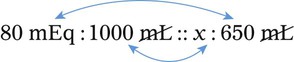
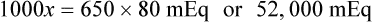
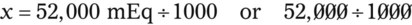
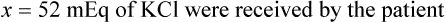





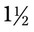 hours, how many milligrams of medication would the patient receive? ____________________
hours, how many milligrams of medication would the patient receive? ____________________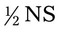 to infuse over 10 hours.
to infuse over 10 hours.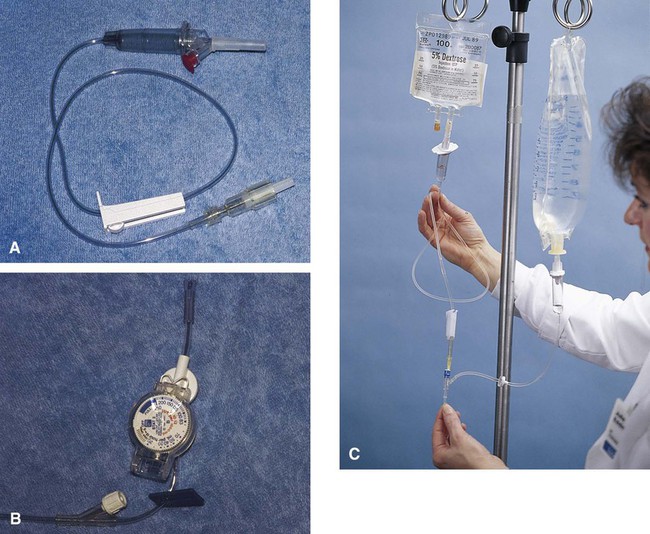
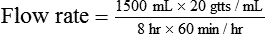
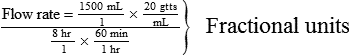
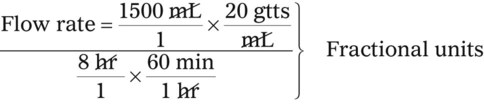
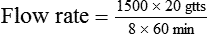
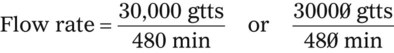
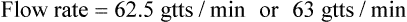

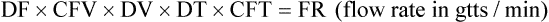
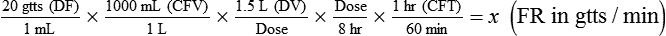
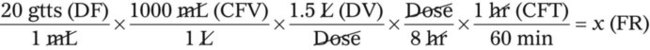
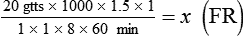
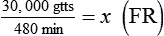
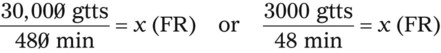
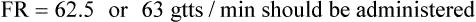
 hours. The drop factor is 15 gtts/mL.
hours. The drop factor is 15 gtts/mL.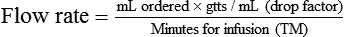
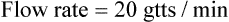
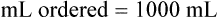
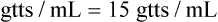
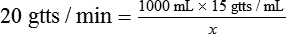
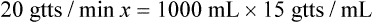

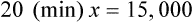
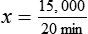
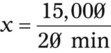
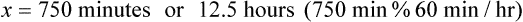
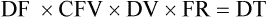
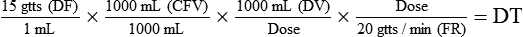
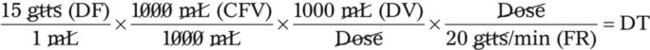
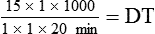
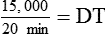
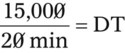
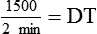
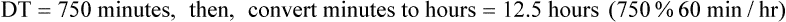
 NS to be infused at 30 gtts/min. The drop factor is 10 gtts/mL.
NS to be infused at 30 gtts/min. The drop factor is 10 gtts/mL.








 NS 100 mL as an IVPB. The drop factor is 60 gtts/mL. The physician wants the INSULIN to infuse at 2.5 units/hr. Use the following label for your calculations.
NS 100 mL as an IVPB. The drop factor is 60 gtts/mL. The physician wants the INSULIN to infuse at 2.5 units/hr. Use the following label for your calculations.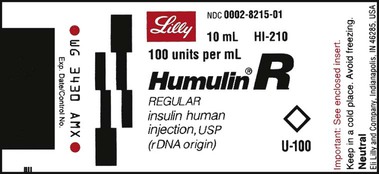



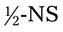 1000 mL for a child who is dehydrated. The physician wants the patient to receive 90 mL the first hour and the remainder over 12 hours. The drop factor is 60 gtts/mL.
1000 mL for a child who is dehydrated. The physician wants the patient to receive 90 mL the first hour and the remainder over 12 hours. The drop factor is 60 gtts/mL.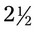 hours. The drip factor is 20 gtts/mL.
hours. The drip factor is 20 gtts/mL.
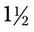 hours. The medication is available as DOXYCYCLINE 100 mg/10 mL. The drop factor is 20 gtts/mL.
hours. The medication is available as DOXYCYCLINE 100 mg/10 mL. The drop factor is 20 gtts/mL.