CHAPTER 79 BURNS
The frequency of burn injury and its subsequent multisystem effects make the treatment of burn patients a commonly encountered management challenge for the trauma/critical care surgeon. The emergency surgery components of initial burn care include fluid resuscitation and ventilatory support, as well as preservation and restoration of remote organ function. Following appropriate resuscitation, burn patient management is focused on wound care and provision of the necessary metabolic support. The involvement of the emergency/trauma surgeon in burn wound management is dependent on the extent and depth of the wound and the rapid identification of those patients who are best cared for at a burn center.
INCIDENCE
The precise number of burns that occur in the United States each year is unknown because only 21 of 50 states mandate the reporting of burn injury. An estimated total number of burns has been obtained by extrapolation of those data. At present, 1.25 million is regarded as a realistic estimate of the annual incidence of burns in the United States, 80% of which involve less than 20% of the total body surface. Approximately 190–263 patients per million population are estimated to require admission to a hospital for burn care each year. In the population of burn patients requiring hospital care, there is a smaller subset of approximately 20,000 burn patients who, as defined by the American Burn Association (Table 1), are best cared for in a burn center each year. This subset consists of 42 patients per million population with major burns, and 40 patients per million population having lesser burns but a complicating cofactor.
|
Burns in patients with pre-existing medical conditions that can complicate management, prolong recovery, or affect mortality
|
a If the mechanical trauma poses the greater immediate risk, the patient may be stabilized and receive initial care at a trauma center before transfer to a burn center.
Adapted with permission from Stabilization, Transfer and Transport, Chapter 8. In Advanced Life Burn Support Course Instructors Manual. Chicago, American Burn Association, 2001, pp. 73–78.
PATHOPHYSIOLOGY
Local Effects
Burn injury causes three zones of damage. Centrally located is the zone of coagulation. In a full-thickness burn, the zone of coagulation involves all layers of the skin, extending down through the dermis and into the subcutaneous tissue. In partial-thickness injuries, this zone extends down only into the dermis, and there are surviving epithelial elements capable of ultimately resurfacing the wound. Surrounding the zone of coagulation is an area of lesser cell injury, the zone of stasis. In this area, blood flow is altered but is restored with time as resuscitation proceeds. If patients are inadequately resuscitated, thrombosis can occur and the zone of stasis can be converted to a zone of coagulation. The most peripheral zone is an area of minimally damaged tissue, the zone of hyperemia, which abuts undamaged tissue. The zone of hyperemia is best seen in patients with superficial partial-thickness injuries as occur with severe sun exposure.
Systemic Response
Burn injury affects the hematopoietic system, resulting in the loss of balance in both leukocyte and erythrocyte production and function. Burns of greater than 20% of total body surface area are associated with both alterations in red cell production and increases in red cell destruction at the level of the cutaneous circulation, resulting in anemia. Such anemia can be further compounded by frequent phlebotomy, surgical blood loss, hemodilution due to resuscitation, and transient alterations in erythrocyte membrane integrity. Longer-term changes appear to be related to hyporesponsiveness of the erythroid progenitor cells in the bone marrow to erythropoietin. During the early stages of resuscitation, reductions in platelet number, depressed fibrinogen levels, and alterations in coagulation factors return to normal or near normal values with appropriate resuscitation. Changes in white cell number occur early, with an increase in neutrophils due to demargination and accelerated bone marrow release. With uncomplicated burn injury, bone marrow myelopoiesis is preserved.
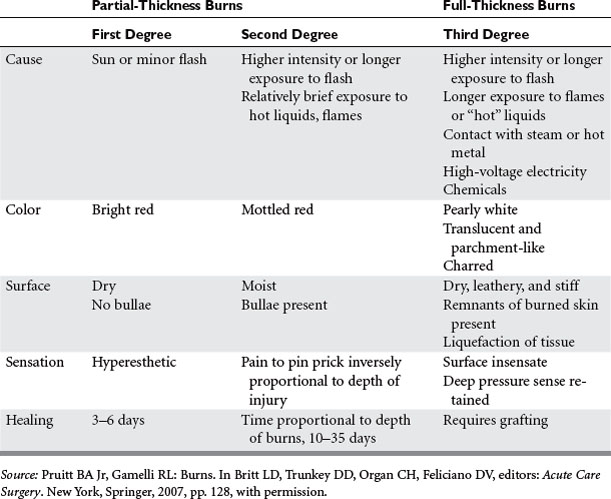
GRADING OF BURN WOUND DEPTH
The injuries that will be apparent on examination are the consequences of the level of tissue destruction. Wounds that are superficial are associated with hyperemia, fine blistering, increased sensation, and exquisite pain upon palpation. The wounds are hyperemic, warm, and readily blanch. These types of injuries represent firstdegree burns or are alternatively termed superficial partial-thickness injuries. With a second degree or deeper partial-thickness burn, the wound presents with intact or ruptured blisters or is covered by a thin coagulum termed “pseudoeschar.” The key physical finding is preservation of sensation in the burned tissue, although it is reduced (Table 2). With proper care, superficial and even deeper partial-thickness injuries are capable of spontaneous healing without grafting. The risk of infection in deep partial-thickness wounds is significant, and if an infection develops it can lead to a greater depth of skin loss. A full-thickness wound occurs when the injury penetrates all layers of the skin or extends into the subcutaneous or deeper tissues. These wounds will appear pale or waxy, be anesthetic, dry, and inelastic, and contain thrombosed vessels. Occasionally in children or young women, the initial appearance of a wound may be more that of a brick red coloration. Such wounds will have significant edema and are inelastic and insensate. Full-thickness wounds are infection-prone wounds, as they no longer provide any viable barrier to invading organisms and if left untreated become rapidly colonized and a portal for invasive burn wound sepsis.
RESUSCITATION PRIORITIES
Fluid Administration
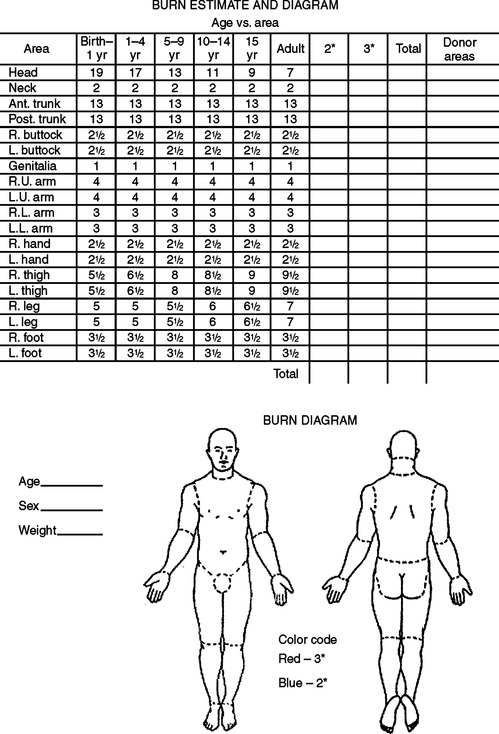
Resuscitation fluid needs are proportional to the extent of the burn (combined extent of partial- and full-thickness burns expressed as a percentage of total body surface area) and are related to body size (most readily expressed as body weight) and age (the surface area per unit of body mass is greater in children than in adults). The patient should be weighed on admission and the extent of partial- and full-thickness burns estimated according to standard nomograms (Figure 1). The fluid needs for the first 24 hours can be estimated on the basis of the Advanced Burn Life Support and Advanced Trauma Life Support consensus formula (Table 3).
BW, Body weight; LR, lactated Ringer’s; TBSAB, total body surface area burned.
The limited glycogen stores in a child may be rapidly exhausted by the marked stress hormone response to burn injury. Serum glucose levels in the burned child should be monitored, and 5% dextrose in lactated Ringer’s administered if serum glucose decreases to hypoglycemic levels. In the case of small children with small burns, the resuscitation fluid volume as estimated on the basis of burn size may not meet normal daily metabolic requirements. In such patients, maintenance fluids should be added to the resuscitation regimen.
Ventilatory Support
Presenting signs and symptoms of an inhalation injury are stridor, hypoxia, and respiratory distress. The probability that a patient has suffered an inhalation injury is highly correlated with being burned in an enclosed space, having burns of the head and neck, and having elevated carbon monoxide levels. The extent and severity of the inhalation injury are directly related to the duration of exposure and the types of toxins contained within the smoke, and exacerbates the ensuing host inflammatory response. Activation of the inflammatory cascade results in the recruitment of neutrophils and macrophages which propagate the injury. Altered surfactant release causes obstruction and collapse of distal airway segments. As part of the response to injury, there is a marked and near-immediate increase in bronchial artery blood flow, which is associated with marked alterations in vascular permeability within the lung. The net effect is that extensive destruction and inflammation reduce pulmonary compliance and impair gas exchange, resulting in altered pulmonary blood flow patterns and ventilation perfusion mismatches.
Initial Wound Care
Mafenide acetate was one of the first effective topical agents introduced for the management of the burn wound. It was initially available as Sulfamylon® Burn Cream, which is highly effective against Gram-positive and Gram-negative organisms but provides little antifungal activity. Mafenide acetate readily diffuses into the eschar and is the agent of choice for significant burns of the ears because it is also capable of penetrating cartilage. Drawbacks with the use of mafenide acetate include pain on application to partial-thickness burns, and limited activity against methicillin-resistant S. aureus. Mafenide acetate also inhibits carbonic anhydrase and may cause a self-limiting hyperchloremic acidosis. Mafenide acetate has more recently become available as a 5% aqueous solution and is an excellent agent to use on freshly grafted wounds and is not associated with the problems found with the cream formulation.
Burn Wound Excision and Grafting
The blood loss occurring with burn wound excision is related to the time of excision post-burn, the area to be excised, the presence of infection, and the type of excision. Donor sites can also represent a significant portion of the blood loss. The quantity of blood loss has been estimated to range from 0.45 to 1.25 ml/cm2 of burn area excised. Adjunctive measures that can be used to control blood loss include elevation of limbs undergoing excision, applications of topical thrombin and/or vasoconstrictive agents in solutions to the excised wound and donor site, clysis of skin graft harvest sites and/or the eschar prior to removal, and application of tourniquets. Spray application of fibrin sealant can also reduce bleeding from the excised wound after release of the tourniquet. Blood loss will be compounded if the patient becomes coagulopathic, hypothermic, or acidotic during the procedure, a triad that can be avoided by partnership with an experienced anesthesiologist.
Specialized Injuries: Cold Injuries
Injuries occurring secondary to environmental exposure can result in local injuries, frostbite, or systemic hypothermia. The pathophysiology of frostbite is crystal formation due to freezing of both extracellular and intracellular fluids. Patients presenting with frostbite will have coldness of the injured body part, with loss of sensation and proprioception. On initial examination, the limb may well appear pale, cyanotic, or have a yellow-white discoloration. During rapid rewarming at 40°–42° C in water for 15–30 minutes, hyperemia will occur followed by pain, paresthesias, and sensory deficits. Greater than 1 week may pass before a true determination of the depth and extent of the injury can be obtained. The injured extremity should be elevated in an attempt to control edema and padded to avoid pressure-induced ischemia as a secondary insult. Frostbite wounds are tetanus-prone wounds, and therefore tetanus toxoid should be administered based on the patient’s immunization status.
MORBIDITY AND COMPLICATIONS MANAGEMENT
Early Complications
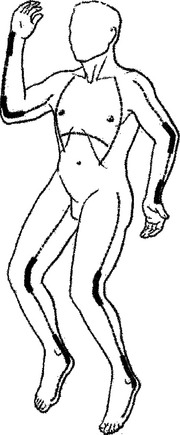
As resuscitation proceeds and edema forms beneath the inelastic eschar of encircling full-thickness burns of a limb, blood flow to underlying and distal unburned tissue may be compromised. Cyanosis of distal unburned skin and progressive paresthesias, particularly unrelenting deep tissue pain, which are the most reliable clinical signs of impaired circulation, may become evident only after relatively long periods of relative or absolute ischemia. Since the full-thickness eschar is insensate, the escharotomy can be performed as a bedside procedure without anesthesia, using a scalpel or an electrocautery device. On an extremity, the escharotomy incision, which is carried only through the eschar and the immediately subjacent superficial fascia, is placed in the mid-lateral line and must extend from the upper to the lower limit of the burn wound (Figure 2). The circulatory status of the limb should then be reassessed. If that escharotomy has not restored distal flow, another escharotomy should be placed in the mid-medial line of the involved limb. A fasciotomy may be needed when there has been a delay in restoring the patient’s limb circulation and in particular if the patient is receiving a massive fluid load.
Edema formation beneath encircling full-thickness truncal burns can restrict the respiratory excursion of the chest wall. If the limitation of chest wall motion is associated with hypoxia and elevated peak inspiratory pressure, chest escharotomy is indicated to restore chest wall motion and improve ventilation. These escharotomy incisions are placed in the anterior axillary line bilaterally, and if the eschar extends onto the abdominal wall, the anterior axillary line incisions are joined by a costal margin escharotomy incision (see Figure 2).
Metabolic and Nutritional Support
Burn injury induces insulin resistance, which may lead to hyperglycemia. The maintenance of blood glucose values below 120 mg/dl with aggressive insulin infusion has been demonstrated to have a favorable impact on the outcome of critically ill patients. Potassium and phosphorous must also be given to meet the patient’s needs, which often exceed initial estimates, particularly when large loads of glucose are being given with exogenous insulin. Over the course of the patient’s care, as the open wound area decreases and the hypermetabolic state slowly resolves, the nutrient load should be adjusted so that balance is maintained between metabolic needs and substrate delivery, preventing overfeeding.
TRANSPORTATION AND TRANSFER
Many important advances have been made in the care and management of burn-injured victims during the past 50 years. One of the more significant advances has been the recognition of the benefits of a team approach in the care of critically injured burn patients. The American College of Surgeons and the American Burn Association have developed optimal standards for providing burn care and a burn center verification program that identifies those units that have undergone peer review of their performance and outcomes. Patients with burns and/or associated injuries and conditions listed in Table 1 should be referred to a burn center.
MORTALITY
Early post-burn renal failure as a consequence of delayed and/or inadequate resuscitation has been eliminated, and inhalation injury as a comorbid factor has been tamed. Invasive burn wound sepsis has been controlled, and early excision with prompt skin grafting and general improvements in critical care have reduced the incidence of infection, eliminated many previously life-threatening complications, and accelerated the convalescence of burn patients. Mortality for various ages and burn sizes is reported in Table 4. Not only has survival improved, but the elimination of many life-threatening complications and advances in wound care have improved the quality of life of even those patients who have survived extensive, severe thermal injuries.
Table 4 Changes in Burn Patient Mortality at U.S. Army Burn Center, 1945–1991
| Age Group | Percentage of Body Surface Burn Causing 50% Mortality (LA50) | |
|---|---|---|
| 1945–1957 | 1987–1991 | |
| Children (0–14) | 51 | 72 a |
| Young adults (15–40) | 43 | 82 b |
| 73 c | ||
| Older adults | 23 | 46 d |
Source: Pruitt BA Jr, Gamelli RL: Burns. In Britt LD, Trunkey DD, Organ CH, Feliciano DV, editors: Acute Care Surgery. New York, Springer, 2006, p. 155, with permission.
American Burn Association. Advanced Life Burn Support Course Instructors Manual. Chicago: American Burn Association, 2001.
American Burn Association. Burn Care Resources in North America. Chicago: American Burn Association, 2004.
Asch MJ, Fellman RJ, Walker HL, Foley FD, Popp RL, Mason ADJr, Pruitt BAJr. Systemic and pulmonary hemodynamic changes accompanying thermal injury. Ann Surg. 1973;178:218-221.
Bennett B, Gamelli RL. Profile of an abused child. J Burn Care Rehabil. 1998;19:88-94.
Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339:1603-1608.
Heinrich JJ, Brand DA, Cuono CB. The role of topical treatment as a determinant of an infection in outpatient burns. J Burn Care Rehabil. 1988;9:253-257.
Kowal-Vern A, McGill V, Gamelli R. Ischemic necrotic bowel disease in thermal injury. Arch Surg. 1997;132:440-443.
Martin RR, Becker WK, Cioffi WG, Pruitt BPJr. Thermal injuries. In: Wilson RF, Walt AJ, editors. Mangement of Trauma: Pitfalls and Practice. 2nd ed. Baltimore: Williams and Wilkins; 1996:760-771.
McManus WF, Mason ADJr, Pruitt BAJr. Excision of the burn wound in patients with large burns. Arch Surg. 1989;124:718-720.
Mochizuki H, Trocki O, Dominion L, Brackett KA, Joffe SN, Alexander JW. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984;200:297-300.
Peck M. Practice guidelines for burn care: nutritional support. J Burn Care Rehabil. 2001;12:59S-66S.
Pruitt BAJr, Gamelli RL. Burns. In: Britt LD, Trunkey DD, Organ CH, Feliciano DV, editors. Acute Care Surgery: Principles and Practice. New York: Springer; 2007:125-160.
Pruitt BAJr, Goodwin CWJr. Critical care management of the severely burned patient. In: Parrillo JE, Dellinger RP, editors. Critical Care Medicine. 2nd ed. St. Louis: Mosby; 2001:1475-1500.
Rico RM, Ripamonti R, Burns AL, Gamelli RL, DiPietro LA. The effect of sepsis on wound healing. J Surg Res. 2002;102(2):193-197.
Tasaki O, Goodwin CW, Saitoh D, Mozingo DW, Ishihara S, Brinkley WW, Cioffi WGJr, Pruitt BAJr. Effects of burns on inhalation injury. J Trauma. 1997;43:603-607.