206 Burns
Over the past few decades, survival and quality of life have improved markedly for victims of serious burns. A better understanding of injury physiology and realization that the natural history of burns can be changed by prompt surgery led to these improvements.1 Maintenance of patients with serious burns through the physiologic trial of staged wound closure is an essential component of this success. Many aspects of burn critical care are unique to this disease process.2
 Phases of Burn Care
Phases of Burn Care
Successful management of patients with serious burns requires both effective initial resuscitation and development of an overall plan for acute-phase hospitalization. Commonly, this overall plan can be considered to have four phases (Table 206-1).3 The first phase, from day 1 through 3, the initial evaluation and resuscitation phase, focuses on complete evaluation and accurate fluid resuscitation. The second phase, initial wound excision and biological closure, describes changes in the natural history of the disease, which include progressive wound sepsis and systemic inflammation and infection. This phase entails a series of staged operations that are completed during the first few days after injury. The third phase, definitive wound closure, requires that temporary wound covers be replaced with definitive covers and that small complex wounds such as those of the face and hands are addressed. The final stage of care is rehabilitation and reconstruction. Although rehabilitation begins during resuscitation, it becomes much more time consuming and involved near the end of the acute stay. Return to work, school, and community is the major objective of the entire acute hospitalization.
TABLE 206-1 The Four Phases of Burn Care, with Physiologic Changes and Objectives
| Phase and Timing | Physiologic Changes | Objectives |
|---|---|---|
| 1: Initial evaluation and resuscitation,0 to 72 h | Massive capillary leak and burn shock | Accurate fluid resuscitation and thorough evaluation |
| 2: Initial wound excision and biological closure, days 1-7 | Hyperdynamic and catabolic state with high risk of infection | Accurately identify and remove all full-thickness wounds and achieve biological closure |
| 3: Definitive wound closure, day 7 to week 6 | Continued catabolic state and risk of non-wound septic events | Replace temporary with definitive covers, and close small complex wounds |
| 4: Rehabilitation, reconstruction, and reintegration, day 1 through discharge | Waning catabolic state and recovering strength | Initially to maintain range of motion and reduce edema; subsequently to strengthen and facilitate return to home, work, school |
 Physiology of Burn Injury
Physiology of Burn Injury
Serious burns are associated with a stereotypical sequence of physiologic changes. Anticipation of these metabolic aberrations facilitates optimal support (see Table 206-1). During the first 1 or 2 days after a serious burn, patients require substantial hemodynamic support.4 If the patient is successfully resuscitated, a hyperdynamic and hypermetabolic state typically ensues. This later phase, characterized by high cardiac output, reduced afterload, fever, and muscle catabolism, must be supported by provision of adequate quantity and quality of substrates.
Resuscitation Phase
The massive fluid resuscitation required by burn patients is unique in medicine. It is secondary to a diffuse but transient capillary leak driven by poorly characterized mediators.5 The clinical result is extravasation of fluids, electrolytes, and even moderate-sized colloid molecules into both burned and unburned soft tissues to a degree not seen in other disease processes. Since the 1930s, a variety of resuscitation formulas have been developed based on burn and patient size. However, this remains an area of clinical art, with no formula being reliably accurate for all patients.2 Besides burn size and patient size, a variety of other factors have an impact on resuscitation requirements. These include delay in initiation of resuscitation, inhalation injury, patient age, baseline cardiovascular health, and the depth and vapor transmission characteristics of the wound itself.6
Burns under 15% generally do not require a formal fluid resuscitation program. As burn size increases, physiologic aberrations increase in intensity, explaining escalating volume requirements. Formulas do not accurately predict the needs of individual patients. Optimal burn resuscitation requires hourly reevaluation of resuscitation endpoints, with titration of volume infusions. In essence, the formula chosen will only help initiate resuscitation and roughly guide planning of volume needs. Of the many resuscitation formulas available, the modified Brooke protocol (Box 206-1) is representative. All formulas have their adherents, and all are useful if employed as rough guidelines only while monitoring physiologic resuscitation endpoints. The role of colloid is expanding in burn resuscitation, although there is no uniform agreement. Many providers, the author included, begin 5% albumin at a maintenance rate immediately during resuscitation and find it reduces the incidence of edema-related complications, including abdominal compartment syndrome.7
Box 206-1
Modified Brooke Resuscitation Formula
24-48 Hours
Hyperdynamic Phase
Typically there is a very noticeable decline in intravenous volume requirements 18 to 30 hours after injury. It is assumed that this is because the capillary leak has “sealed” in well-resuscitated patients. After this hypodynamic period, a systemic hypermetabolic state predictably develops and is sustained in surviving patients until it slowly regresses, well after wound closure.8 This state is characterized by high cardiac output, low peripheral vascular resistance, fever, and increased protein flux. In patients not well supported with protein substrate, this increased protein flux will be associated with significant muscle catabolism. Although the basic biology is not well understood, the postresuscitation physiologic state is assumed to be caused by inflammatory mediators and augmented release of the counterregulatory hormones, cortisol, catecholamines, and glucagon.9 These hormonal changes are triggered by a combination of wound- and gut-released bacteria and their byproducts, pain, foci of infection, and some degree of evaporative heat loss.
A central component of burn critical care is to ensure adequate support of the hypermetabolic state. This is done by providing accurate fluid repletion, adequate supplies of metabolic substrates, control of environmental temperature, and competent pain control. Early identification and excision of necrotic skin and soft tissue with immediate biological closure of the resulting wounds truncates the hypermetabolic physiologic state and is the most effective way to avoid the deleterious consequences of prolonged hypermetabolism.10
Burn critical care requires control of the patient’s environmental temperature. Burn patients have enormous and invisible evaporative water and energy losses if they are maintained in the typical cool dry air of a general hospital.11 Burn units and burn operating rooms must be engineered to maintain high ambient temperature and humidity to avoid the difficult problem of hypothermia and excessive energy loss.
 Initial Evaluation and Burn-Specific Secondary Survey
Initial Evaluation and Burn-Specific Secondary Survey
Burn patients often spend many hours in transport before reaching the location of definitive care, and their initial evaluation and management must be completed outside the burn unit setting. Often when patients arrive in the intensive care unit (ICU) where definitive care will be rendered, a complete burn-specific secondary survey has not been completed.12 It is essential for the intensivist to have a familiarity with these issues so burn-related pathology and coexisting injuries are not overlooked. Evaluations should follow the format taught by the Advanced Trauma Life Support course. All seriously burned patients should be approached as having potential multiple trauma.13
Initial Evaluation
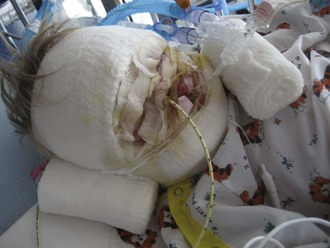
The primary survey of the burn patient is similar to that of the trauma patient, although there are a few important differences worthy of emphasis. First among these is the progressive mucosal edema that may compromise airway patency in the early hours after burns. This is especially true in young children because of their much smaller airway.14 Progressive stridor or hoarseness should prompt visualization and/or intubation of the airway. Ideally, this need is anticipated before the crisis stage so proper equipment and personnel can be gathered, facilitating smooth tube placement. The facial and airway edema that is so common makes the burn patient’s airway among the most challenging to control. Reintubation can be exceedingly difficult if not impossible after airway edema has progressed, making accidental extubation a potentially lethal complication. Security of the endotracheal tube should be regularly assessed. A twill-tie harness is a reliable method of securing the endotracheal tube (Figure 206-1).
Burn-Specific Secondary Survey
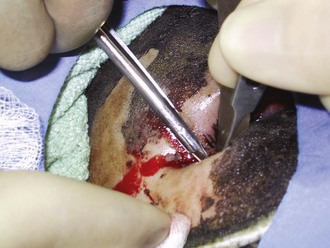
The ocular and otolaryngologic examination should begin with palpation of the head and face for signs of coincident blunt or penetrating trauma. The globes should be examined early, prior to the development of facial and eyelid edema, which will limit examination (Figure 206-2).15 Serious globe burns impart a clouded appearance to the cornea, and fluorescein staining will detect more subtle injuries. Tarsorrhaphy is virtually never indicated acutely, because lid edema will generally provide excellent globe coverage even in the presence of serious lid burns. Pressure on the burned ear and occiput is avoided. Topical mafenide acetate is applied, as it will penetrate the relatively avascular underlying cartilage.16 Signs of inhalation injury, such as carbonaceous debris and singed nasal hairs, are noted on examination of the nose and throat. Ties securing endotracheal and nasogastric tubes should be checked so that pressure on the nasal septum or oral commissures is avoided.
The initial neurologic evaluation centers on exclusion of coincident neurologic injury and control of pain and anxiety. Even if they arrive alert and oriented, patients with serious burns typically become obtunded over the succeeding hours and days, if only because of the effects of pain medications and sleep deprivation. It is therefore important to exclude central nervous system trauma if the mechanism of injury is either unknown or consistent with such trauma. There should be a low threshold for ordering a computed tomographic scan of the head and spine, based on mechanism of injury. Pain and anxiety management should begin during the initial evaluation, within limits of safety.17 Good pain control may have physiologic as well as the obvious psychological benefits. In the emergency setting, this is best done with incremental administration of small doses of narcotics and benzodiazepines. When caring for paralyzed or obtunded patients, it is important to make sure there is no pressure on peripheral nerves, so that neuropathies are avoided. Finally, those burned in structural fires should be assessed for carbon monoxide (CO) exposure by history, neurologic examination, and determination of a carboxyhemoglobin level, because selected patients with significant exposure may benefit from hyperbaric oxygen treatment.18
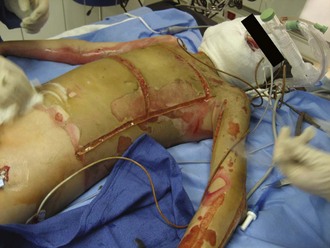
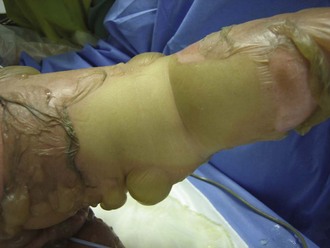
The chest wall should be assessed for compliance and symmetrical air movement. Patients with deep near-circumferential or circumferential chest wall burns may require escharotomy to facilitate ventilation (Figure 206-3). If properly performed, escharotomy of the torso markedly enhances compliance.
Most patients are hypovolemic at the time of presentation and respond promptly to volume administration. Some patients, especially the elderly, will have previously unsuspected myocardial disease that may become clinically important during the stress of resuscitation. Some data also exist to support the existence of a myocardial depressant factor in some patients with very extensive injuries.19 Patients who do not respond as expected to calculated resuscitation volumes may benefit from invasive monitoring, pulmonary artery catheterization, or cardiac ultrasonography.
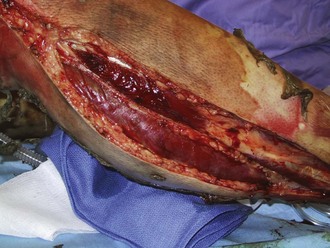
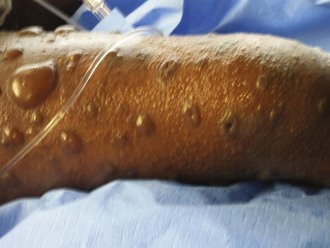
Perhaps the most important component of evaluating the extremities is to identify areas at risk for loss of perfusion with progressive edema during resuscitation and to develop an effective monitoring plan. Resuscitation-associated edema can cause profound limb ischemia secondary to swelling under a circumferential eschar or within inelastic muscle compartments. This complication is seen in patients who have suffered deep extremity burns (especially if circumferential) or high-voltage electrical injuries. Low-pressure flow in the extremity should be monitored, commonly using a Doppler probe to demonstrate flow in the palmar arch or digital vessels, because capillary perfusion pressure is only one-third the mean arterial pressure monitored in larger vessels. Prompt identification of ischemic extremities is essential so that escharotomy (Figure 206-4) or fasciotomy (Figure 206-5) can be effected in a timely manner.20
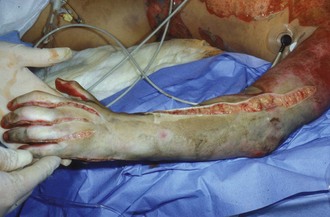
Figure 206-4 Properly performed escharotomy will result in immediate improvement in extremity blood flow.
Abuse or neglect should be considered when evaluating all burns, not just those in young children. Approximately 20% of burns in young children are reported to state authorities for investigation, but abuse occurs in all age groups.21 Burns can also be a result of domestic violence or other interpersonal assaults. Often this determination is not made until the patient has been admitted to the ICU. Suspicious cases should be filed with appropriate state agencies. Documentation of the stated injury circumstances and of the wounds is essential. Wound photography is ideal (Figure 206-6).
 Fluid Resuscitation
Fluid Resuscitation
In the first 1 or 2 hours after a large burn, patients experience little change in intravascular volume or hemodynamics. In fact, patients are often remarkably alert during this period. In the hours that follow, however, the wound releases mediators that are absorbed into the systemic circulation. In addition, stress-related hormones are secreted, and reactive oxygen species are formed on reperfusion of marginally perfused tissues. These and perhaps other factors trigger a diffuse loss of capillary integrity, resulting in extravasation of fluids, electrolytes, and even moderate-sized colloid molecules into soft tissues, including tissues distant from the burn. This remarkable physiologic phenomenon, the so-called capillary leak, abates 18 to 24 hours later and explains the unique resuscitation needs of patients who have sustained large burns. Predicting resuscitation requirements of specific patients involves multiple variables besides burn size: burn depth, vapor transmission characteristics of the wound, patient age and cardiovascular health, resuscitation delay, environmental temperature and humidity, and presence or absence of concomitant inhalation injury. Numerous formulas have been promulgated to roughly guide resuscitation efforts, but none is accurate in every patient.6,22 A common consensus formula is the modified Brooke formula summarized in Box 206-1. The role of colloid is expanding in burn resuscitation, although there is no uniform agreement. Many providers, the author included, begin 5% albumin at a maintenance rate immediately during resuscitation of patients with larger injuries and find it reduces the incidence of edema-related complications including abdominal compartment syndrome.7 Patients not responding as predicted to resuscitation efforts should have serum levels of cortisol checked, particularly if cryptic hypotension, hypernatremia, and/or hypokalemia are also in evidence. Inaccurate fluid resuscitation will cause significant morbidity. Formulas can only help determine initial volume infusion rates and roughly predict 24-hour volume requirements; they are so inherently inaccurate that resuscitation should be guided by hourly reevaluation of clinical endpoints. Resuscitation endpoints are summarized in Table 206-2. Measured oxygen delivery and consumption have been used as adjunctive resuscitation guides but are not necessary in the vast majority of patients.
| Resuscitation Endpoint | Resuscitation Target |
|---|---|
| Sensorium | Comfortable, arousable |
| Physical examination | Warm extremities, full peripheral pulses |
| Urine output | Infants: 1-2 mL/kg/h; children: 0.5-1 mL/kg/h; all others: 0.5 mL/kg/h |
| Base deficit | Less than 2 |
| Systolic blood pressure | Infants: 60-70 mm Hg |
| Children: 70-90 + (twice age in years) mm Hg | |
| Adolescents and adults: 90-120 mm Hg |
Note: Age-specific resuscitation endpoints should be assessed regularly throughout burn resuscitation and infusions adjusted up or down in 10% to 20% increments to meet needs of the individual patient.
 Burn Critical Care Issues
Burn Critical Care Issues
Airway Issues
Dangerous and frightening emergencies in the seriously burned involve the airway. Although evaluation and control of the airway are part of the initial evaluation, concerns extend throughout the period of intensive care. Endotracheal tube security should be part of the regular reevaluation of every patient in the burn ICU, because facial and hypopharyngeal edema can make reintubation after unplanned extubation incredibly difficult.23
Inhalation Injury
Inhalation injury remains a clinical diagnosis.24 A history of closed-space fire, the presence of singed nasal hairs and facial burns, and carbonaceous sputum support the diagnosis of inhalation injury. Fiberoptic bronchoscopy can be useful in equivocal cases, as can technetium scanning. However, in the large majority of patients, the diagnosis is made by history and physical examination. The initial chest radiograph is almost always normal, as are gas exchange and compliance until the endobronchial mucosa sloughs several days later, occluding small airways and leading to subsegmental atelectasis and respiratory insufficiency.
Five clinical consequences commonly occur in patients with inhalation injury: acute upper airway obstruction, bronchospasm, small airway occlusion, pulmonary infection, and respiratory failure.25 Airway obstruction and bronchospasm are early complications, typically appearing the first day. Airway edema and obstruction are managed with endotracheal intubation. Bronchospasm from aerosolized irritants can be particularly intense during the first 24 to 48 hours and is managed with in-line nebulization β-adrenergic agonists, with infrequent use of intravenous bronchodilators such as terbutaline or low-dose epinephrine infusions. Ventilatory strategies should be designed to minimize automatic positive end-expiratory pressure in this setting.
Respiratory failure is unfortunately common in patients with inhalation injury and can be well managed with a pressure-limited ventilation strategy based on permissive hypercapnia.26 Patients who fail this can sometimes benefit from investigational modes of support such as inhaled nitric oxide or extracorporeal oxygenation, although the utility of the latter is quite limited in burn patients, owing to the need for anticoagulation.27,28
Carbon Monoxide and Cyanide Exposure
Patients injured in structural fires are commonly exposed to high levels of CO. Although an obtunded state in this clinical setting can be due to other causes such as intoxication, trauma, or anoxia, hyperbaric oxygen (HBO) has been reported to improve the prognosis of patients who have suffered very severe CO exposure.18 There are controlled data both supporting29 its use and refuting the utility of HBO,30 so clinical judgment must be brought to bear in the decision whether to use this form of therapy in individual patients.
CO binds and inactivates heme-containing enzymes such as hemoglobin and the cytochromes. The binding of CO and hemoglobin forms carboxyhemoglobin, which does not deliver oxygen, resulting in acute physiologic anemia, much like an isovolemic hemodilution. A serum carboxyhemoglobin level of 50% is similar to an isovolemic hemodilution to 50% of the baseline hemoglobin concentration. This level of carboxyhemoglobin results in unconsciousness, implying that other mechanisms are also involved in the pathophysiology of CO injury. CO binding to the cytochrome system in the mitochondria probably interferes with oxygen utilization. Approximately 10% of patients with severe CO exposure have been reported to develop severe delayed neurologic sequelae.31
There are two practical treatment options: 100% normobaric oxygen or HBO. There are well-designed clinical studies both supporting and refuting the utility of HBO for CO poisoning.29,30 Proponents cite a decreased incidence of delayed neurologic sequelae in those treated with HBO. In patients with very severe CO poisoning with either very high carboxyhemoglobin levels or neurologic impairment not otherwise explainable, HBO is probably warranted if it can be safely administered.
Commonly recommended HBO treatment is 2 or 3 atm for 90 minutes, with three 10-minute “air breaks” (breathing of pressurized room air rather than pressurized oxygen) to decrease the incidence of seizures. Most treatments are delivered in monoplace chambers, making it more risky to attempt treatment in unstable patients. Other relative contraindications are wheezing or air trapping, which increase the risks of pneumothorax or gas embolism, and high fever, which increases the risk of seizures. Before placement in the chamber, endotracheal tube balloons should be filled with saline to avoid balloon compression–associated air leaks, and upper body central venous cannulation should be avoided if possible to avoid sudden enlargement of an occult pneumothorax during decompression.32
Hydrogen cyanide is detected in the smoke from many structural fires and in the serum of some burn patients. At a high enough concentration, cyanide causes failure of oxygen utilization at the cytochrome level, with a secondary unexplained metabolic acidosis. Cyanide poisoning can be treated with amyl nitrate and sodium thiosulfate.33 However, cyanide is rapidly metabolized in resuscitated patients, making specific treatment generally not necessary or useful.
Pain and Anxiety Management
Undertreatment of pain and anxiety was very common in the past, and burn intensivists need to pay particular attention to this issue. Reasons for undertreatment are related to the extraordinary drug doses required to adequately address pain in seriously burned patients and consequent fear of respiratory depression, addiction, and litigation. The opiate and benzodiazepine tolerance of patients with large open wounds is truly remarkable.34 Once wounds are closed, drug needs rapidly decrease, and addiction is rare. The best way to eliminate burn pain is prompt wound closure.
Unfortunately, control of pain and anxiety is very difficult in burn patients. Successful management is greatly aided by a set of guidelines. One such program addresses four clinical states: intubated acute, nonintubated acute, chronic acute, and reconstructive patients.35 Within each clinical state are separate guidelines for background pain, background anxiety, procedural pain, procedural anxiety, and transition to the next clinical state. Guidelines seem most effective when they use a limited formulary and emphasize dose ranging based on regular assessment of objective efficacy. Attention to the issue has physiologic as well as the obvious psychological benefits. Reduced secretion of catecholamines may decrease systemic hypermetabolism, and treatment-related acute stress is reduced.36
Ocular Exposure
Contraction of burned eyelids and facial skin can cause exposure of the globe in the days or weeks after burns.15 If unchecked, this will result in exposure and then desiccation of the globe, with secondary keratitis and corneal ulceration. Infected corneal ulcers rapidly lead to globe perforation because the cornea is almost avascular and tolerates desiccation and infection very poorly. When minimal or moderate, globe exposure can be managed with frequent ocular lubrication. Acute eyelid release should be done promptly if exposure is severe or keratitis does not resolve with lubrication over a few days.
Peripheral Neuropathies
Peripheral neuropathies are more common than is usually appreciated in burn patients.37,38 They can be caused by direct thermal damage to peripheral nerves or by the many metabolic disturbances seen during acute burn care. A minority of these lesions are caused by constricting eschar, compartment syndrome, or improperly filled splints. Extremities at risk should be monitored for compartment syndrome and constricting eschar. These issues are best addressed surgically as early as possible. Heavily sedated patients or those under general anesthesia in the operating room should be examined to make sure that traction and pressure injuries are avoided.
Gastrointestinal Issues
Curling’s ulcers were a common cause of massive upper gastrointestinal bleeding in the past. This is now an infrequent occurrence with better resuscitation, which decreases splanchnic ischemia. Routine use of prophylactic gastric alkalinization also has been important. Patients with serious burns should be treated with empirical histamine-receptor blockers, proton-pump inhibitors, and/or antacids until they are tolerating tube feedings and are at low enough risk that this therapy can reasonably be stopped. Calculous or acalculous cholecystitis in the critically ill burn patient is easily missed and can be the cause of significant illness. Fevers are often assumed to be secondary to the wound. Cholestatic blood chemistry values and modest clinical jaundice are identical to the changes that typify hepatic insufficiency. If untreated, gangrenous cholecystitis associated with peritonitis and sepsis can result. Diagnosis is easily made by bedside ultrasonography. Treatment can be either laparoscopic or open cholecystectomy. In the critically ill patient, percutaneous transhepatic drainage is a very reasonable alternative.39
Although uncommon, pancreatitis is a reported complication seen in patients with very large burns.40 Like cholecystitis, it is easily missed until the condition is far advanced. Abdominal distention and ileus, with tenderness in those who are conscious, should prompt measurements of serum amylase and lipase concentrations as well as appropriate abdominal imaging in selected cases. Most patients can be treated with bowel rest, although pseudocysts and abscesses have been reported in this population.
Bowel ischemia and necrosis are complications seen generally in those with prolonged burn shock, often part of a delayed resuscitation syndrome. These complications present as ileus and then peritonitis. Bowel necrosis is lethal unless operated on promptly. It is a frequently reported autopsy finding in patients dying of burns.41,42
Superior mesenteric artery syndrome is a rare occurrence but should be seriously considered in patients with major weight loss during the acute phase of injury who develop intractable vomiting in the recovery phase of their illness. It is due to compression of the duodenum in the angle between the aorta and superior mesenteric artery.43 Diagnosis is by barium swallow, and treatment is a combination of parenteral nutrition and tube feedings past the point of obstruction if possible.
Nutritional Support
Burn patients need accurate energy and protein support. Underfeeding and overfeeding have adverse sequelae. Ideally, tube feedings are begun during resuscitation.44 Most patients do well with continuous intragastric tube feedings, although some require postpyloric feedings.45 Enteral nutritional support can be started through a nasogastric sump tube so that gastric residuals can be used to help determine tolerance of the feedings initially.46 Parenteral support is useful during periods when ileus is likely, such as during septic episodes or periods when high-dose vasopressor support is needed, or during the perioperative period. Transient parenteral support can be particularly important in hypermetabolic young children who are very catabolic and do not tolerate prolonged periods of fasting.
Goals for nutritional support for burned patients are controversial. There are a variety of formulas designed to predict these needs, but actual requirements vary widely and unpredictably in individual patients. Consensus recommendations are as follows: approximately 2.5 g/kg/d of protein should be provided, and the caloric load should be between 1.5 and 1.7 times the calculated basal metabolic rate or 1.3 to 1. 5 times the measured (by indirect calorimetry) resting energy expenditure.47,48 Nutritional support should be adjusted throughout the illness, based on specific endpoints. Serial physical examination, quality of wound healing, nitrogen balance, and indirect calorimetry can be integrated to assess the adequacy of support and help fine-tune the predictions of nutritional equations.
Infectious Disease Issues
Historically, wound sepsis has been the great killer in burn units, and burn wound infections remain surprisingly common today.49 Diagnosis of wound sepsis is generally clinical, based on signs and symptoms of systemic infection along with changes in wound appearance. The diagnosis can be supported by wound biopsy and quantitative cultures, but both of these diagnostic techniques are infamously inaccurate, making a clinical diagnosis the most reliable.50
The best way to prevent wound sepsis is to identify and excise deep burns within the first few days after injury and to close the resulting wounds. Topical agents are only an adjunct to this effort and cannot on their own be relied upon to prevent wound sepsis but can delay the onset of wound sepsis in deep wounds. They can also serve to minimize desiccation and colonization of healing wounds. There are several agents in wide general use; the most common are listed in Table 206-3. All have specific advantages and disadvantages. Use of aqueous silver nitrate commonly promotes development of hyponatremia and hypokalemia. Use of mafenide acetate, which inhibits carbonic anhydrase, leads to development of metabolic acidosis, making it more difficult to use permissive hypercapnia for the management of patients with severe respiratory failure. Silver sulfadiazine application leads to large losses of free water across the burn wound eschar.
| Agent | Characteristics |
|---|---|
| Silver sulfadiazine | Painless on application, fair to poor eschar penetration, no metabolic side effects, broad antibacterial spectrum |
| Mafenide acetate | Painful on application, excellent eschar penetration, carbonic anhydrase inhibitor, broad antibacterial spectrum |
| 0.5% Silver nitrate | Painless on application, poor eschar penetration, leaches electrolytes, broad spectrum (including fungi) |
Infection-control practices should be routine and relatively rigid in burn units. This patient population has a high incidence of infection in general, and resistant bacterial species are very common. Universal precautions should be practiced in all patients. The use of prophylactic antibiotics is not advised.51
Prevention and Recognition of Complications
Some common burn complications are itemized in Box 206-2. Optimally, complications should be diagnosed early through regular careful physical examinations, aided by a high degree of suspicion.
Box 206-2
Common Complications in Burn Patients
Cardiovascular
Pulmonary
Neurologic
Hematologic
Otolaryngologic
Gastrointestinal
Ophthalmologic
Genitourinary
Musculoskeletal
Soft Tissue
 Rehabilitation Therapy in the Burn Intensive Care Setting
Rehabilitation Therapy in the Burn Intensive Care Setting
Good burn care is extremely multidisciplinary. Physical and occupational therapists should be involved from the outset and strategies implemented to avoid common contractures that will otherwise interfere with recovery later (Table 206-4). Typically, physical therapy includes passive movement of all joints through an appropriate range of motion and static positioning in ways that minimize the risk of deformity. Involvement of physical and occupational therapists escalates as patients progress toward recovery; many hours of treatment are required each day after wound closure. Burn patients will have a much harder time with subsequent rehabilitation if therapeutic efforts are ignored during the period of protracted critical illness.12,52
TABLE 206-4 Common Contractures and Prevention Strategies Useful in the ICU
| Anatomic Area | Common Contracture | ICU Preventive Splinting and Positioning Strategy |
|---|---|---|
| Neck | Flexion | Daily range-of-motion exercises and extension splinting and conformers; split mattress |
| Shoulder | Adduction | Daily range-of-motion exercises and abduction splinting with axillary splints or troughs |
| Elbow | Flexion and extension | Daily range-of-motion exercises and alternating extension and flexion splints |
| Wrist | Flexion and extension | Daily range-of-motion exercises and splinting in functional position (20 degrees of extension) |
| Metacarpophalangeal joints | Extension | Daily range-of-motion exercises and splinting in functional position (metacarpophalangeal joints at 70 to 90 degrees of flexion, all interphalangeal joints in extension, first web space open, wrist at 20 degrees of extension) |
| Hips | Flexion | Daily range-of-motion exercises and extension splints and prone positioning (if tolerated) |
| Knees | Flexion | Daily range-of-motion exercises and knee splints and knee immobilizers |
| Ankles | Extension | Daily range-of-motion exercises and neutral splints |
| Metatarsophalangeal joints | Extension | Daily range-of-motion and splinting in functional position; rocker-bottom shoes |
 Special Injury Considerations
Special Injury Considerations
Electrical Injury
Exposures can be somewhat arbitrarily divided into low voltage ([household] 110-220 volts), intermediate voltage (220-1000 volts), and high voltage (>1000 volts). Patients with good contact to low and intermediate voltages commonly have severe local wounds but rarely suffer systemic consequences such as compartment syndromes or rhabdomyolysis.53 Patients with good contact to high voltages commonly have compartment syndromes, myocardial injury, fractures of the long bones and spine, and free pigment in the plasma that may cause renal failure if not promptly cleared.54,55 These patients also suffer from electrical soft-tissue burns, flash burns, and burns from clothing ignition. Many such patients have also suffered blunt trauma during the incident.
Cold Injury
Cold injuries often generate wounds best cared for in the burn unit; initial management is usually conservative. Necrotic tissue is excised when demarcation is clear, and the resulting wounds are grafted. These patients very often suffer coincident hypothermia which must be managed, often in the ICU. If patients present with ischemic extremities with less than 24 hours of thawed warm ischemia time, diagnostic angiography may be considered in selected stable patients. If there is no flow despite intraarterial vasodilators, thrombolytic therapy may be appropriate.57
Chemical and Tar Injury
Chemical injuries can be associated with both local and systemic effects. Poison control centers should be consulted, particularly as regards systemic toxicities. It is essential to protect staff from exposure to the chemicals during removal. Most agents can be irrigated off with tap water for 30 minutes, although some, particularly alkaline substances, may take longer. When the “soapy feeling” alkaline substances often impart to the gloved finger is gone, or when litmus paper indicates a neutral pH, irrigation may be stopped. Hydrofluoric acid, especially in concentrated form, may result in severe acute hypocalcemia, because the fluoride anion strongly binds divalent cations.56 Subeschar injection of 10% calcium gluconate and/or immediate excision of the wound may be lifesaving. Elemental metals such as solid lithium or sodium can ignite on contact with water or air and should therefore be covered with oil. White phosphorus, a component of many munitions, will also ignite on contact with air, and wound particles are ideally covered with wet cloth or gauze. A number of road-surfacing materials are viscous and heated up to 700°F for application. They are designed to stay solid in the hot sun on dark pavement. When these materials splash road workers, the wounds should be quickly cooled by tap water irrigation. Wounds should be soaked in a lipophilic solvent after cooling and then be débrided and grafted as indicated by burn depth, which is often quite deep.
Toxic Epidermal Necrolysis
The cause of toxic epidermal necrolysis remains a mystery. For unclear immunologic reasons, epidermal-dermal bonding is disrupted to a variable degree. Frequently there is an influenza-like prodrome, and usually there is a drug exposure that is believed to trigger the syndrome (commonly anticonvulsants or nonsteroidal antiinflammatory agents). All mucosal surfaces are affected to some degree. Most patients are only affected slightly, but those affected to a large degree have a life-threatening condition and are often referred to burn units for care.58
Seriously affected patients with toxic epidermal necrolysis have both cutaneous and visceral wounds—slough of the skin as well as surfaces lined by mucosa or conjunctiva (Figure 206-7). The severity of the skin and mucosal sloughs are not directly related, but the cutaneous wound usually begins first. Although the skin slough is what heralds the disease and what brings most patients to the attention of burn programs, it is generally the easier of the two wounds to manage, with topical antimicrobials and biological dressings.59 The visceral wound is much more problematic. Conjunctival sloughing threatens the globe.60 Pulmonary involvement leads to respiratory failure.61 Gastrointestinal sloughing may lead to bleeding and bacterial translocation. Clinical outcomes have been best when these patients are managed in burn units.62 The utility of intravenous gamma globulin in toxic epidermal necrolysis remains controversial and is not considered a standard of care.63
Purpura Fulminans
Patients with meningococcal and other bacterial septic lesions may develop a syndrome in which soft tissues are rendered ischemic by spotty small-vessel thrombosis. This has become less common where vaccination programs have been successful. It has been theorized that thrombosis is due to transient protein C deficiency, which occurs when the liver ceases production of clotting proteins secondary to the septic event.64 Protein C is an anticoagulant protein that helps maintain control of the process of clotting and has the shortest half-life of the clotting factors (about 6 hours). Patients with purpura fulminans present with organ failures and acute new deep wounds, often heralded by an ominous rash in the affected distribution. These patients are often referred promptly to burn units for care of organ failures associated with large soft-tissue wounds.65
 Conclusion
Conclusion
Serious burns present a unique set of challenges to the ICU team that crosses multiple disciplines. However, outcome data support the contention that most survivors of serious burns can have a very satisfying long-term quality of life.66 A successful outcome requires a coordinated effort by intensive care, surgical, nursing, and rehabilitation therapy professionals during what is often a technically demanding but ultimately rewarding ICU stay.
Key Points
Zonies D, Mack C, Kramer B, Rivara F, Klein M. Verified centers, nonverified centers, or other facilities: a national analysis of burn patient treatment location. J Am Coll Surg. 2010;210:299-305.
Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg Infect (Larchmt). 2009;10:389-397.
Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110-117.
Sheridan RL. Burn care: results of technical and organizational progress. JAMA. 2003;290:719-722.
Sheridan RL, Hinson MI, Liang MH, et al. Long-term outcome of children surviving massive burns. JAMA. 2000;283:69-73.
1 Thompson P, Herndon DN, Abston S, Rutan T. Effect of early excision on patients with major thermal injury. J Trauma. 1987;27:205-207.
2 Sheridan RL. Burns. Crit Care Med. 2002;30(11 Suppl):S500-S514.
3 Sheridan RL. Burn care: Results of technical and organizational progress. JAMA. 2003;290:719-722.
4 Cuthbertson DP. Second annual Jonathan E. Rhoads Lecture. The metabolic response to injury and its nutritional implications: Retrospect and prospect. JPEN J Parenter Enteral Nutr. 1979;3:108-129.
5 Youn YK, LaLonde C, Demling R. The role of mediators in the response to thermal injury. World J Surg. 1992;16:30-36.
6 Cartotto RC, Innes M, Musgrave MA, et al. How well does the Parkland formula estimate actual fluid resuscitation volumes? J Burn Care Rehabil. 2002;23:258-265.
7 Lawrence A, Faraklas I, Watkins H, Allen A, Cochran A, Morris S, et al. Colloid administration normalizes resuscitation ratio and ameliorates “fluid creep.”. J Burn Care Res. 2010;31(1):40-47.
8 Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology. 2003;189:75-88.
9 Demling RH, DeSanti L. Increased protein intake during the recovery phase after severe burns increases body weight gain and muscle function. J Burn Care Rehabil. 1998;19:161-168. discussion 160
10 Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345(17):1223-1229.
11 Gwosdow AR, Cunningham JJ, Lydon M, et al. Evaporative water losses through a temporary wound dressing under simulated wound conditions. J Burn Care Rehabil. 1993;14:450-454.
12 Sheridan RL. Comprehensive treatment of burns. Curr Probl Surg. 2001;38:657-756.
13 Rosenkranz KM, Sheridan R. Management of the burned trauma patient: Balancing conflicting priorities. Burns. 2002;28:665-669.
14 Sheridan RL. Recognition and management of hot liquid aspiration in children. Ann Emerg Med. 1996;27:89-91.
15 Malhotra R, Sheikh I, Dheansa B. The management of eyelid burns. Surv Ophthalmol. 2009;54:356-371.
16 Mills DC2d, Roberts LW, Mason ADJr, et al. Suppurative chondritis: Its incidence, prevention, and treatment in burn patients. Plast Reconstr Surg. 1988;82:267-276.
17 Stoddard FM, Martyn JJ, Sheridan RL. Psychiatric issues in pain of burn injury: Controlling pain and improving outcomes. Curr Rev Pain. 1997;1:130-136.
18 Sheridan RL, Shank ES. Hyperbaric oxygen treatment: A brief overview of a controversial topic. J Trauma. 1999;47:426-435.
19 Cioffi WG, DeMeules JE, Gamelli RL. The effects of burn injury and fluid resuscitation on cardiac function in vitro. J Trauma. 1986;26:638-642.
20 Sheridan RL, Tompkins RG, McManus WF, Pruitt BAJr. Intracompartmental sepsis in burn patients. J Trauma. 1994;36:301-305.
21 Peck MD, Priolo-Kapel D. Child abuse by burning: a review of the literature and an algorithm for medical investigations. J Trauma. 2002 Nov;53(5):1013-1022.
22 Holm C. Resuscitation in shock associated with burns: Tradition or evidence-based medicine? Resuscitation. 2000;44:157-164.
23 Fidkowski CW, Fuzaylov G, Sheridan RL, Coté CJ. Inhalation burn injury in children. Paediatr Anaesth. 2009 Jul;19(Suppl 1):147-154.
24 Hollingsed TC, Saffle JR, Barton RG, et al. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166:592-596.
25 Sheridan R. Specific therapies for inhalation injury. Crit Care Med. 2002;30:718-719.
26 Sheridan RL, Kacmarek RM, McEttrick MM, et al. Permissive hypercapnia as a ventilatory strategy in burned children: Effect on barotrauma, pneumonia, and mortality. J Trauma. 1995;39:854-859.
27 Sheridan RL, Zapol WM, Ritz RH, Tompkins RG. Low-dose inhaled nitric oxide in acutely burned children with profound respiratory failure. Surgery. 1999;126:856-862.
28 Sheridan RL, Hess D. Inhaled nitric oxide in inhalation injury. J Burn Care Res. 2009 Jan-Feb;30(1):162-164.
29 Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057-1067.
30 Scheinkestel CD, Bailey M, Myles PS, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: A randomised controlled clinical trial [see comments]. Med J Aust. 1999;170:203-210.
31 Thom SR, Taber RL, Mendiguren II, et al. Delayed neuropsychologic sequelae after carbon monoxide poisoning: Prevention by treatment with hyperbaric oxygen [see comments]. Ann Emerg Med. 1995;25:474-480.
32 Grube BJ, Marvin JA, Heimbach DM. Therapeutic hyperbaric oxygen: Help or hindrance in burn patients with carbon monoxide poisoning? J Burn Care Rehabil. 1988;9:249-252.
33 Barillo DJ, Goode R, Esch V. Cyanide poisoning in victims of fire: Analysis of 364 cases and review of the literature [review]. J Burn Care Rehabil. 1994;15:46-57.
34 Sheridan R, Stoddard F, Querzoli E. Management of background pain and anxiety in critically burned children requiring protracted mechanical ventilation. J Burn Care Rehabil. 2001;22:150-153.
35 Sheridan RL, Hinson M, Nackel A, et al. Development of a pediatric burn pain and anxiety management program. J Burn Care Rehabil. 1997;18:455-459. discussion 453-454
36 Stoddard FJJr, Sorrentino EA, Ceranoglu TA, Saxe G, Murphy JM, Drake JE, et al. Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. J Burn Care Res. 2009 Sep-Oct;30(5):836-843.
37 Dagum AB, Peters WJ, Neligan PC, Douglas LG. Severe multiple mononeuropathy in patients with major thermal burns. J Burn Care Rehabil. 1993;14:440-445.
38 Marquez S, Turley JJ, Peters WJ. Neuropathy in burn patients. Brain. 1993;116(pt 2):471-483.
39 Sheridan R, Schulz J, Ryan C, et al. Percutaneous endoscopic gastrostomy in burn patients. Surg Endosc. 1999;13:401-402.
40 Ryan CM, Sheridan RL, Schoenfeld DA, et al. Postburn pancreatitis. Ann Surg. 1995;222:163-170.
41 Iliopoulou E, Markaki S, Poulikakos L. Autopsy findings in burn injuries. Arch Anat Cytol Pathol. 1993;41:5-8.
42 Desai MH, Herndon DN, Rutan RL, et al. Ischemic intestinal complications in patients with burns. Surg Gynecol Obstet. 1991;172:257-261.
43 Lescher TJ, Sirinek KR, Pruitt BAJr. Superior mesenteric artery syndrome in thermally injured patients. J Trauma. 1979;19:567-571.
44 Montecalvo MA, Steger KA, Farber HW, et al. Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. The Critical Care Research Team [see comments]. Crit Care Med. 1992;20:1377-1387.
45 Kadillack P, Prelack K, Sheridan R. Gastric tube feedings with supplemental parenteral nutrition in children with large burns. J Burn Care Rehabil. 1999;20:S248.
46 Scaife CL, Saffle JR, Morris SE. Intestinal obstruction secondary to enteral feedings in burn trauma patients. J Trauma. 1999;47:859-863.
47 Sheridan RL, Yu YM, Prelack K, et al. Maximal parenteral glucose oxidation in hypermetabolic young children: A stable isotope study. JPEN J Parenter Enter Nutr. 1998;22:212-216.
48 Prelack K, Cunningham JJ, Sheridan RL, Tompkins RG. Energy and protein provisions for thermally injured children revisited: An outcome-based approach for determining requirements. J Burn Care Rehabil. 1997;18:177-181. discussion 176
49 Peck MD, Weber J, McManus A, et al. Surveillance of burn wound infections: A proposal for definitions. J Burn Care Rehabil. 1998;19:386-389.
50 Sheridan RL. Sepsis in pediatric burn patients. Pediatr Crit Care Med. 2005 May;6(3-Suppl):S112-S119.
51 Sheridan RL, Weber JM, Pasternack MM, Tompkins RG. Admission streptococcal screening allows elimination of burn wound prophylaxis. J Burn Care Rehabil. 1999;20:S146.
52 Tyack ZF, Ziviani J. What influences the functional outcome of children at 6 months post-burn? Burns. 2003;29:433-444.
53 Rabban JT, Blair JA, Rosen CL, et al. Mechanisms of pediatric electrical injury: New implications for product safety and injury prevention. Arch Pediatr Adolesc Med. 1997;151:696-700.
54 Rosen CL, Adler JN, Rabban JT, et al. Early predictors of myoglobinuria and acute renal failure following electrical injury. J Emerg Med. 1999;17:783-789.
55 Moran KT, Munster AM. Low voltage electrical injuries: The hidden morbidity. J R Coll Surg Edinb. 1986;31:227-228.
56 Sheridan RL, Ryan CM, Quinby WCJr, et al. Emergency management of major hydrofluoric acid exposures. Burns. 1995;21:62-64.
57 Sheridan RL, Walker TG. A young man with severe hypothermia and frostbite. N Engl J Med. 2009 Dec 31;361(27):2654-2662.
58 Palmieri TL, Greenhalgh DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002;23:87-96.
59 Goyal S, Gupta P, Ryan CM, Kazlas M, Noviski N, Sheridan RL. Toxic epidermal necrolysis in children: medical, surgical, and ophthalmologic considerations. J Burn Care Res. 2009 May-Jun;30(3):437-449.
60 Haus C, Paquet P, Marechal-Courtois C. Long-term corneal involvement following drug-induced toxic epidermal necrolysis (Lyell’s disease). Ophthalmologica. 1993;206:115-118.
61 Sheridan RL, Weber JM, Schulz JT, et al. Management of severe toxic epidermal necrolysis in children. J Burn Care Rehabil. 1999;20:497-500.
62 Sheridan RL, Schulz JT, Ryan CM, et al. Long-term consequences of toxic epidermal necrolysis in children. Pediatrics. 2002;109:74-78.
63 Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: A prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. 2003;139:33-36.
64 Warner PM, Kagan RJ, Yakuboff KP, et al. Current management of purpura fulminans: A multicenter study. J Burn Care Rehabil. 2003;24:119-126.
65 Sheridan RL, Briggs SE, Remensnyder JP, Tompkins RG. Management strategy in purpura fulminans with multiple organ failure in children. Burns. 1996;22:53-56.
66 Sheridan RL, Hinson MI, Liang MH, et al. Long-term outcome of children surviving massive burns. JAMA. 2000;283:69-73.