Burn Care Procedures
Two million people suffer a burn-related injury every year in the United States. The American Burn Association (ABA) estimates that approximately 450,000 of these patients received medical evaluation and treatment in 2011 and approximately 10% (45,000) required hospitalization.1 Patients who suffer burn injuries are predominately male (70%), and their mean age is 32 years old. Children younger than 5 years account for 18% of burns, and patients older than 60 years account for an additional 12%. Seventy percent of all burns involve less than 10% of the total body surface area (TBSA). Nearly 80% of all burns are caused by flame or fire or by scalds, with scald injury occurring most in children younger than 5 years.
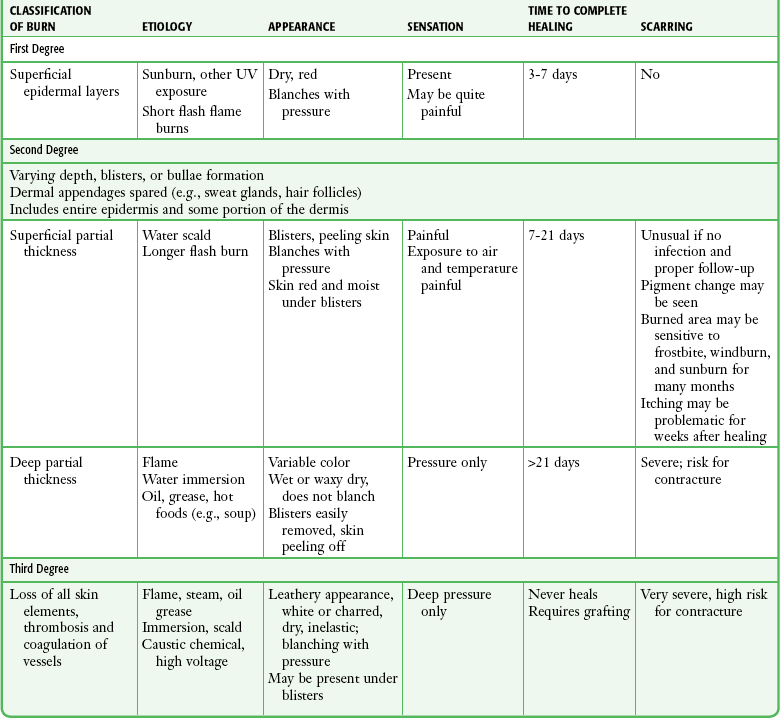
The classification of burns is based on three criteria2: depth of skin injury, percentage of TBSA involved, and source of injury (thermal, chemical, electrical, or radiation). The seriousness of a burn injury is determined by the characteristics and temperature of the burning agent, the duration of exposure, the location of the injury, the presence of associated injuries, and the age and general health of the victim (Table 38-1).
TABLE 38-1
Characteristics of Burns, by Depth
Modified after Clayton MC, Solem LD. No ice, no butter: advice on management of burns for primary care physicians. Postgrad Med. 1995;97:151; and Morgan ED, Bledsoe SC, Barker J. Ambulatory management of burns. Am Fam Physician. 2000;62:2015.
The ABA defines minor burns as uncomplicated partial-thickness burns involving less than 5% TBSA in children (<10 years old) or the elderly (>50 years old), less than 10% TBSA in adults, or full-thickness burns less than 2% TBSA.3 Moderate or major burns include injuries that involve a greater TBSA, as well as burns in areas of specialized function, such as the face, hands, feet, and perineum. More serious burns also include those caused by a high-voltage electrical injury or those with associated inhalation injuries or other major trauma.
Wound Evaluation
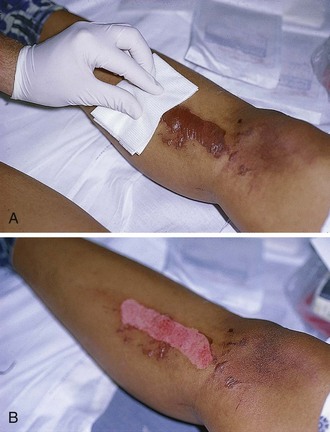
Emergency clinicians should be aware that the depth of a burn wound cannot always be determined accurately on clinical grounds alone at initial evaluation and that burn injury is a dynamic process that may change over time, particularly during the 24 to 48 hours after the burning process has been arrested. It is common, for example, for a seemingly minor or superficial burn to appear deeper on the second or third return visit (Fig. 38-1). This phenomenon is not a continuation of the burning process that can be altered by clinician intervention but is considered to be a pathophysiologic event related to tissue edema, dermal ischemia, or desiccation.4
Estimating Burn Depth
The depth of a burn is commonly classified by degree.5 First degree involves the epidermis only, second-degree (or partial-thickness) burns extend into the dermis, and third-degree (or full-thickness) burns destroy the entire skin. An additional fourth degree is sometimes used to describe injuries to the underlying muscle, tendon, or bone (Fig. 38-2).
First-degree burns involve the epidermis only (Fig. 38-3A). The skin is reddened but is intact and not blistered. This injury ranges from mildly irritating or even pruritic to exquisitely painful. Minor edema may be noted. Causes include ultraviolet light (as in sunburn) and brief thermal “flash” burns. First-degree burns may blister within 24 to 36 hours, and the patient should be warned about this possibility. Frequently, the epidermis flakes or peels within 5 to 10 days. Healing occurs spontaneously, usually without scarring.
Second-degree burns involve the epidermis and extend into the dermis to include the sweat glands and hair follicles. Superficial second-degree burns involve only the papillary dermis (see Fig. 38-3B). These burns are pink, moist, and extremely painful. Blisters are common and the skin may slough. The burn blanches with pressure, and mild to moderate edema is common. Hair follicles are often intact. Superficial second-degree burns are the most common burns seen in the emergency department (ED). The usual causes are scalds, contact with hot objects, or exposure to chemicals. Barring infection or repeated trauma, these burns heal spontaneously and without scarring in about 2 weeks. These areas may be sensitive to sunburn, windburn, and skin irritation for months after the original injury has healed.
Deep second-degree burns involve the reticular dermis and appear mottled white or pink (see Fig. 38-3C). There is obvious edema and sloughing of the skin, and any blisters are usually ruptured. Blanching is absent. These burns are not generally painful initially and may have decreased sensation, but pressure may be perceived. Within a few days, however, these burns can become exquisitely painful. This type of burn may be converted to a full-thickness injury by further trauma or infection.
Third-degree burns result from complete loss of the dermis and may extend into subcutaneous (SQ) tissue (see Fig. 38-3D). These burns usually appear dry, pearly white, or charred. They are initially painless, with a leathery texture. Circumferential third-degree burns on an extremity or the torso cause a loss of elasticity that may impair the circulation or ventilation and necessitate an escharotomy.
Fourth-degree burns extend deeply into SQ tissue, muscle, fascia, or bone (see Fig. 38-3E). These burns are characteristically caused by contact with molten metal, flame, or high-voltage electricity.
A more practical method of classifying burns is to describe them as either superficial or deep because this approach defines both treatment and prognosis. Superficial burns involve the papillary dermis, with its rich vascular plexus, and the epidermis, which permits spontaneous healing by reepithelialization from the dermal appendages, including hair follicles, sebaceous glands, and sweat glands. This usually occurs within 2 weeks with minimal scarring. Superficial burns appear wet, pink, and blistered and blanch with pressure. They are painful. Deep burns involve the reticular dermis and SQ fat and generally lack sufficient epithelial appendages for spontaneous healing. If healing does occur, it will occur slowly and produce unstable skin, hypertrophic scarring, and contracture. Deep burns are best treated by excision and skin grafting. The initial appearance of deep burns ranges from cherry red, mottled, white, and nonblanching to leathery, charred, brown, and insensate (Table 38-2).
TABLE 38-2
American Burn Association’s Grading System for Burn Severity and Disposition of Patients*
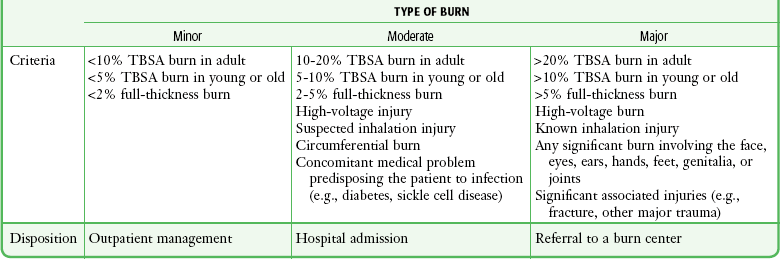
TBSA, total body surface area (percentage) affected by the injury.
*Burn, partial-thickness or full-thickness burn, unless specified; young, patient younger than 10 years; adult, patient 10 to 50 years of age; old, patient older than 50 years.
Adapted with permission from hospital and prehospital resources for optimal care of patients with burn injury: Guidelines for development and operation of burn centers. American Burn Association. J Burn Care Rehabil. 1990;11:98; with additional information from Hartford CE. Care of outpatient burns. In: Herndon DN, ed. Total Burn Care. Philadelphia: Saunders; 1996:71.
Although bedside evaluation of very superficial or deep wounds presents little diagnostic difficulty, clinical assessment of a mid-dermal or “indeterminate” burn is accurate only about two thirds of the time.6 Even though it is useful to initially characterize the extent of the burn, it must be noted that the early appearance of a burn wound may not accurately reflect the true extent of the soft tissue injury. Reexamination and follow-up are critical because burn wounds may change during the 24 to 72 hours following injury. Indeterminate burns may eventually heal spontaneously, or they may convert to deeper wounds requiring excision and skin grafting (see Fig. 38-1).
Estimating Burn Size
Estimating burn size assists practitioners in determining the initial fluid requirements and prognosis. Several formulas are available to estimate TBSA in burn patients. In 1944, Lund and Browder published the now famous Lund-Browder chart (Fig. 38-4).7,8 In their landmark paper, they used direct measurements and body surface area formulas to produce a chart that clinicians can use to estimate %TBSA. The initial Lund-Browder chart was developed from human anatomic studies derived from 11 adults (3 women and 8 men) and produced a unisex chart. A more recent study involving 60 volunteers determined that the Lund-Browder chart significantly underestimates the size of chest burns in large-breasted women. The investigators developed a table that incorporates a correction using brassiere cup size.9
The simplest method for estimating TBSA in adults is the “rule of nines.” This formula was developed in the late 1940s by Pulaski and Tennison, who observed that the percentage of each body segment was approximately a multiple of nine (Fig. 38-5).10 Similar formulas for children adjust estimates for their disproportionately large head surface area.
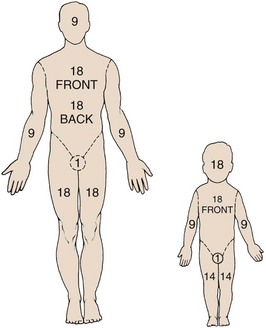
Figure 38-5 The “rule of nines.” The rule of nines is a rough estimate of the total body surface area (TBSA) burned. Note that adults and children are different. This formula frequently overestimates the extent of a burn in clinical practice. As a rough guide, the area covered by the individual’s palm is approximately 1.25% TBSA. See Figure 38-4 for a more accurate method of determining TBSA burned in children.
The size of a burn can also be estimated by using the patient’s hand as representing approximately 1% TBSA. With this method, the hand is a rectangle. However, two studies using planimetry have determined that the hand actually represents from 0.5% to 0.78% of a patient’s TBSA.11
Histopathology of Burns
One thermal wound theory describes three zones of injury in burns (Fig. 38-6)12:
1. Zone of coagulation: dead, avascular tissue that must be débrided.
2. Zone of stasis: injured tissue in which blood flow is impaired. Desiccation, infection, or mechanical trauma may lead to cell death.
3. Zone of hyperemia: minimally injured, inflamed tissue that forms the border of the wound. The hyperemia usually resolves within 7 to 10 days but may be mistaken for cellulitis.
Histologically, full-thickness burns are characterized by confluent vascular thrombosis involving arterioles, venules, and capillaries. Edema secondary to loss of microvascular integrity results not only from the effects of direct thermal injury but also from the release of vasoactive mediators. The increase in vascular permeability is linked to activation of complement and release of histamine. Histamine increases catalytic activity of the enzyme xanthine oxidase, with resultant production of hydrogen peroxide and hydroxyl radicals. These by-products increase the damage to dermal vascular endothelial cells and result in progressive vascular permeability.13
Outpatient Versus Inpatient Care
One of the first steps in minor burn care is to select patients for whom outpatient care is appropriate. For patients determined to require inpatient care, the admit or transfer decision depends on the burn care capabilities of the initial treating facility. Guidelines set forth by the ABA14 regarding criteria for referral to a burn center are listed below. Burn injuries that should be referred to a burn center include the following:
1. Partial thickness burns greater than 10% TBSA
2. Burns involving the face, hands, feet, genitalia, perineum, or major joints
3. Third-degree burns in any age group
4. Electrical burns, including lightning injury
7. Burn injury in patients with preexisting medical disorders that could complicate management, prolong recovery, or affect mortality
8. Any patient with burns and concomitant trauma in which the burn injury poses the greatest risk for morbidity or mortality after emergency or surgical stabilization of the traumatic injuries
9. Burned children in hospitals without qualified personnel or equipment for the care of children
10. Burn injury in patients who will require special social, emotional, or rehabilitative intervention
Poor candidates for outpatient care of even minor burns include patients with concomitant medical problems such as diabetes mellitus, peripheral vascular disease, congestive heart failure, and end-stage renal disease; patients taking steroids or other immunosuppressive agents; patients who are very young or very old; those who are mentally impaired or have drug and alcohol dependency; homeless persons; those who are malnourished; and patients without a sufficient home support system. Inpatient treatment should be considered in these circumstances even though the burn might be considered “minor” by ABA criteria. Other admission considerations include pain control, the ability to return for follow-up care, the degree of incapacity, the ability to receive wound care at home, and the overall social situation—all should influence the final decision of whether admission is warranted.15
1. Patients requiring intravenous (IV) access. Following a burn there is an immediate capillary leak of plasma-like fluid that can last for 18 to 24 hours. In burns involving greater than 20% TBSA, the leak occurs in both burned and nonburned tissues. If not replaced, this fluid loss can lead to hypovolemic shock and renal failure. IV fluid resuscitation is indicated for all patients with second- and third-degree burns greater than 10% TBSA, in patients younger than 10 or older than 50 years, and for burns greater than 20% TBSA in all other age groups.
2. Anticipated surgery. Deep burns are best treated by early surgical excision and skin grafting. This permits faster wound healing, provides more stable skin, and reduces contractures. Hospital admission facilitates wound care and preparation for surgery.
3. Respiratory problems. Patients with respiratory distress requiring oxygen therapy and those suspected of inhaling toxic fumes or vapors should be admitted for observation or intubation and mechanical ventilation (Box 38-1). Direct bronchoscopic evaluation of the airway may assist in the evaluation and diagnosis of tracheobronchitis or pneumonitis from a smoke inhalation injury.
4. Need for special nursing care. Specialized wound care, dressings, and nursing care are often required for burns involving the face, hands, feet, perineum, and genitalia and are best treated on an inpatient basis. Patients unable to care for themselves or those lacking family and friends able to assist them may also require admission.
5. Special burn injuries. Chemical injuries are often more severe than the initial examination would suggest. Unlike thermal burns, tissue destruction may continue many hours after injury. Patients with chemical injuries should be admitted whenever the injuries are of indeterminate depth, affect a large area, or are deep and require surgical excision or if there are systemic manifestations of chemical toxicity or when the chemical responsible for injury requires a specific antidote. Swelling from deep circumferential burns may constrict the chest or limbs and result in compartment syndrome. Such burns should monitored frequently to determine the need for escharotomy or fasciotomy (Fig. 38-7). Whenever the patient’s condition prevents a reliable clinical examination, direct measurement of compartment pressures can provide an objective measurement of intracompartmental pressure and assist in the decision to perform these surgical procedures (see Chapter 54). Patients with extensive burn injuries that require fluid resuscitation with large volumes of crystalloid should be monitored closely for the development abdominal compartment syndrome. Patients with mechanical burns involving large areas of skin loss and with significant frostbite injuries are generally admitted for specialized wound care and parenteral pain management. Patients with electrical injuries are often admitted for cardiac monitoring, specialized wound care, or both.
Procedure
Cooling is most beneficial for small burns if started within 3 minutes of injury and possibly of additional benefit if continued for the first few hours after the burn. Doing so has been shown to reduce pain significantly and can limit tissue damage by decreasing thromboxane production. When cooling a burn wound, it is important to avoid hypothermia or freezing of tissue because this may deepen the injury.16 At home or in the field, room-temperature or cold tap water irrigation, immersion, or compresses (20°C to 25°C) will provide some pain relief without the risk of further injuring burned tissues and inducing hypothermia, which can occur with iced solutions (Fig. 38-8).17,18 Placing ice on a burn should not be done. Sterile dressings are not required for field treatment; a moist towel or nonadherent sheet may be used. Nonmentholated shaving cream makes an excellent temporary covering for out-of-hospital use if a dressing is not available.19 Home remedies, such as butter or Vaseline, are best avoided but are probably benign.20 Remove jewelry and gross debris in the field if possible.
Initial Care of Major Burns
Major burns require the specialized resources of a burn center. Emergency physicians should initiate the resuscitation, consult the burn center for referral, and transfer the patient as soon as practically possible. Initial resuscitation should follow standardized trauma protocols, including a primary and secondary survey, and provide immediate interventions directed at airway management, breathing, and cardiovascular support, as needed. IV catheters can be inserted initially through burned skin when unburned sites are unavailable. Early IV access permits the administration of resuscitative fluids, medications, and parenteral narcotics to relieve the pain. Patients with burns exceeding 20% TBSA should receive IV fluid resuscitation with lactated Ringer’s solution based on the Parkland or Brooke formulas (Box 38-2). More recently, the U.S. Army Institute of Surgical Research has advocated a simpler formula for estimating hourly fluid requirements in burn patients. This simpler formula may be more useful for prehospital providers or ED resuscitation (Box 38-3). The formulas estimate hourly fluid requirements and must be adjusted up or down to achieve a urine output of 0.5 to 1.0 mL/hr. Insertion of a Foley catheter is usually necessary to accurately measure hourly urine output.
Patients exposed to carbon monoxide should have carboxyhemoglobin levels measured and empirically receive 100% oxygen for 6 hours. Once considered a traditional empirical treatment, there is no evidence-based proven benefit from hyperbaric oxygen therapy for carbon monoxide poisoning. A recent Cochrane review (http://summaries.cochrane.org/CD002042) concluded that there is insufficient evidence to support the use of hyperbaric oxygen for the treatment of patients with carbon monoxide poisoning. The Cochrane review of published trials found conflicting, potentially biased, and generally weak evidence regarding the usefulness of hyperbaric oxygen for the prevention of neurologic injury. Per an evidence-based analysis, existing randomized trials do not establish whether the administration of hyperbaric oxygen to patients with carbon monoxide poisoning reduces the incidence of adverse neurologic outcomes. Because there may still be advocates of hyperbaric oxygen therapy, consultation with a local hyperbaric center is reasonable, but it is not standard that this intervention be routinely implemented. Critically ill and pregnant patients are still often offered hyperbaric treatment, but controversy over the efficacy and safety persists even for these subgroups.
Initial Care of Minor Burns
Prompt cooling of the burned part is an almost instinctive response and is one of the oldest recorded burn treatments, having been recommended by Galen (ad 129-199) and Rhazes (ad 852-923).4 In the ED, room-temperature or cold tap water irrigation, immersion, or compresses (20°C to 25°C) are optimal in obtaining pain relief and providing some measure of protection for burned tissues without the problems of hypothermia that iced solutions can cause.8,9 If not done before ED treatment, immediate cold water immersion may still have some ability to limit the extent of a burn and will provide significant pain relief. It is acceptable to add a few ice chips to the water, but packing the wound in ice must be avoided.
All involved clothing and jewelry (such as rings), along with any gross debris, should be removed from the burned area. Chemical burns involving the skin or eyes require prolonged tap water irrigation. The burn should otherwise be covered with a moist, sterile dressing. In the ED and prehospital phase, appropriate analgesics, usually narcotics, are the best way to control pain and should not be forgotten in the initial phase of burn care. The burned area may be immersed immediately in room-temperature water or covered with gauze pads soaked in room-temperature water or saline (see Fig. 38-8). The gauze may be kept cool and moist to provide continued pain relief; the patient will let the clinician know when additional cooling is desired. Many clinicians use sterile saline for cooling, but it has no proven benefit over tap water, even when the skin is broken. It is acceptable to add ice chips to water or saline to lower the temperature. As stated previously, immersion of burned tissue in ice or ice water should be avoided because ice immersion increases pain and risks frostbite injury or systemic hypothermia.
The potential benefits of burn cooling are listed in Box 38-4. Because most patients with minor burns seek medical attention after initial self-instituted prehospital cooling, it is unlikely that the clinician can favorably affect the burned tissue with any intervention in the ED. With the exception of pain relief and removal of debris, the primary benefits of burn cooling are probably experienced only if the burn is cooled promptly, within the first 3 minutes after injury, thus making home care important.21,22 Minor burns are considered tetanus prone, and tetanus toxoid should be administered if patients are unsure of their tetanus immunization status or when it has been more than 10 years since the last immunization. Nonimmunized patients should receive human tetanus immune globulin, 250 units intramuscularly, along with tetanus toxoid and a booster injection of toxoid in about 3 weeks.
Outpatient Care of Minor Burns
Minor burns are generally those that will heal spontaneously and do not require surgery or specialized wound care. These wounds are not associated with immunosuppression or hypermetabolism, nor are they highly susceptible to infection, a quality associated with larger burns.23,24 Treated conservatively, most minor burns will heal without significant scarring. Many complications seen with minor burns are thought to result from overtreatment rather than undertreatment. Examples include the use of dry dressings that can adhere to newly forming skin and secondary infections from the overzealous use of topical or systemic antibiotics.
The most important characteristic of a dressing is that it controls fluids within the wound. Burn dressings that keep the surface of the wound moist and avoid pooling of fluids will speed healing.25 The best material for this purpose is a generous amount of simple dry gauze applied over a nonadherent dressing or topical preparation. The outer layer of dressing should be porous to permit evaporation of water from the absorbent dressing material. Some clinicians prefer to eschew a nonadherent portion of the dressing so that subsequent dressing removal aids in minor débridement. Wound preparation and basic bandaging should include the following steps (Fig. 38-9):
1. Cleanse the burned area gently with a clean cloth or gauze and a mild antibacterial wound cleaner such as chlorhexidine, and irrigate the wound with saline or water. It is not necessary to shave the hair in or around the wound. There is no benefit to vigorously washing a minor wound with strong antiseptic preparations (such as povidone-iodine [Betadine] and others).24
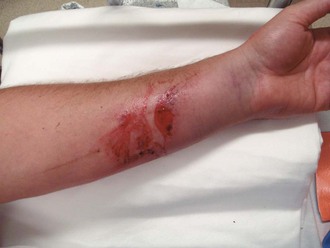
2. Débride blisters and sloughed skin initially by peeling the devitalized skin from the wound (Fig. 38-10A-C). Blisters can be opened with scissors and forceps. If necessary, analgesia should be provided for any painful débridement. The initial débridement should attempt to remove only grossly devitalized tissue. Additional débridement of the wound can take place, if needed, during subsequent follow-up visits when the wound has matured.
3. Consider applying a layer of antibiotic cream or ointment such as 1% silver sulfadiazine (Silvadene) or bacitracin directly to the wound (see Fig. 38-10D).
4. Apply fine-mesh gauze or commercial nonadherent gauze such as Adaptic or petrolatum gauze impregnated with 3% bismuth tribromophenate (Xeroform) to the burn wound.
5. Cover and pad the wound with loose gauze fluffs. If fingers and toes are involved, pad the web spaces and the digits individually and separate them with strips of gauze (see Fig. 38-9D and 38-10E and F). The entire dressing is wrapped snugly (but not tightly) with an absorbent, slightly elastic material such as Kerlix.
6. The patient should be instructed to elevated the affected limb to prevent swelling, which may cause delayed burn conversion or wound infection.
Burn Dressings
Biologic Dressings: Biologic dressings are natural tissues, including skin, that consist of collagen sheets containing elastin and lipid. They are not routinely used in the emergency care of minor wounds. The benefits of biologic dressings include a reduction in surface bacterial colonization, diminished fluid and heat loss, avoidance of further wound contamination, and prevention of damage to newly developed granulation tissue. Examples of biologic dressings include cadaveric human skin and commercially available porcine xenograft or collagen sheets.
Synthetic Dressings: Synthetic dressings are manufactured in various forms. Film-type dressings have a homogeneous structure and are usually polymers. Because these dressings are nonpermeable, problems with retention of wound exudates have occurred. Some second-generation dressings have been developed to address these problems. These products include Tegaderm, Vigilon, DuoDERM, Biobrane, Op-Site, Omniderm, and Sildimac.25,26 These preparations have theoretical benefits under certain circumstances, but none has proved to have superior performance over simple gauze dressings for minor outpatient burns. These products are most often used by burn centers and have little applicability for minor burns in patients discharged from the ED. For patients admitted or transferred to a burn center, simple gauze dressings are appropriate. Some burn centers prefer that topical agents not be applied before transfer so that the full extent of the burn can be assessed immediately.
Specific Clinical Issues in Minor Burn Care
Analgesia: Pain is a critical feature of any burn injury. Relief of pain by the appropriate and judicious use of narcotic analgesics is of the utmost importance in the initial care of all burn patients. Prehospital narcotics are very appropriate when standard contraindications do not exist. Analgesia should be provided before extensive examination or débridement is performed. Inadequate analgesia is probably the most common ED error in the treatment of burn injuries, especially when burns occur in children. Parenteral narcotic analgesics have been erroneously relegated to pain control only for major burns, but it is suggested that narcotics be generously administered in the initial treatment of even minor painful burns.
Parenteral opioids (fentanyl, 1 to 2 µg/kg, or morphine, 0.1 to 0.2 mg/kg) are usually required, especially if painful procedures such as débridement and dressing changes are planned. We prefer to use IV opioids (occasionally supplemented with a short-acting benzodiazepine such as midazolam) for all painful procedures. For complicated débridement or dressing changes, adequate analgesia and sedation (see Chapter 33) is strongly advocated.
Pruritus: Postburn pruritus is one of the most common and distressing complications of burn injury and is estimated to affect 87% of burns.27 It typically develops in the subacute phase and is therefore not a common issue for the emergency clinician in the acute treatment phase. Despite the limited literature on the treatment of postburn pruritus, available therapies include oral antihistamines, topical antihistamines, and topical moisturizers. The use of topical therapies should be withheld until sufficient wound healing has occurred.
Edema: Minor burns lead to immediate inflammation mediated by the release of histamine and bradykinins, which cause localized derangements in vascular permeability with resultant burn wound edema. This edema may be harmful in several ways. First, the increase in interstitial fluid increases the diffusion distance of oxygen from capillaries to cells and thereby increases hypoxia in an already ischemic wound. Second, the edema may produce untoward hemodynamic effects by a purely mechanical mechanism: compression of vessels in muscular compartments. Third, edema has been associated with the inactivation of streptococcicidal skin fatty acids, thus predisposing the patient to burn cellulitis.28,29
Successful management of burn edema hinges on immobilization and elevation. Most patients are unfamiliar with the medical definition of elevation and are not aware or convinced of its value. Patient education in this regard is critical; however, certain burns (e.g., burns in dependent body areas) are prone to edema despite everyone’s best intentions. It is for this reason that lower extremity burns in general and foot burns in particular are prone to problems. Major burns of the hand should be elevated while the patient is still in the ED. This is most readily accomplished by hanging the injured hand from an IV pole with a stockinette to support the bandaged hand (Fig. 38-11).
Use of Topical Preparations and Antimicrobials: Minor burns result in insignificant impairment of normal host immunologic defenses, and burn wound infection is not usually a significant problem. Topical antimicrobials are often used; however, some believe that these agents may actually impair wound healing.29 Although the procedure is of unproven value, many clinicians routinely use antibiotic creams or ointments on even the most minor burns. Most patients expect some type of topical concoction, so a discussion of their use—or nonuse—is prudent.
Silver Sulfadiazine (Silvadene).: This poorly soluble compound is synthesized by reacting silver nitrate with sodium sulfadiazine. It is the most commonly used topical agent for outpatients, and it is well tolerated by most patients (Fig. 38-12A; also see Fig. 38-10D). It has virtually no systemic effects and moderate eschar penetration, and it is painless on application. Although silver sulfadiazine is commonly used, many burn specialists prefer plain bacitracin ointment as the topical of choice because of its cost, equal efficacy, and good patient acceptance.
Other Topical Preparations.: Mafenide acetate (Sulfamylon), gentamicin, chlorhexidine, povidone-iodine, and silver nitrate are products that have been replaced with newer topicals, but they are mentioned for historical interest. These products are not used in modern burn therapy, although they are generally acceptable alternatives.
Broad-Spectrum Antibiotic Ointments.: Many nonprescription topical antimicrobials are used for minor burn therapy despite a paucity of data attesting to specific benefits. Included are bacitracin zinc ointment, polymyxin B–bacitracin (Polysporin), and nitrofurazone (Furacin). These are all soothing, cosmetically acceptable for open treatment (such as on the face), and effective antiseptics under burn dressings. Some researchers caution against agents containing neomycin because of a potential for sensitization (see Fig. 38-12B). Though commonly applied by patients without adverse effects, we advise against the use of topicals that contain neomycin (Neosporin) because of the potential for contact dermatitis. The authors suggest plain bacitracin ointment as the routine topical agent, although Silvadene is a very acceptable, albeit more expensive alternative.
Aloe Vera Cream.: Aloe vera cream is commercially available in a 50% or higher concentration with a preservative. It exhibits antibacterial activity against at least four common burn wound pathogens: Pseudomonas aeruginosa, Enterobacter aerogenes, S. aureus, and Klebsiella pneumoniae. Heck and coworkers and others30,31 compared a commercial aloe vera cream with silver sulfadiazine in 18 patients with minor burns. Healing times were found to be similar, and there was no increase in wound colonization in the aloe vera group in comparison to patients treated with silver sulfadiazine. Other authors have promulgated the use of aloe gel preparations for minor burns.31 Aloe vera cream is an acceptable, inexpensive option for open or dressed outpatient care of minor burns.
Honey.: Honey has long been advocated as an inexpensive and effective topical for minor outpatient burns. The physicochemical properties of honey (osmotic effect, pH) give this substance the antibacterial and antiinflammatory properties that support its use. It may be superior to silver sulfadiazine with regard to minor burn wound healing. Honey is not widely used, but it has been promulgated as a safe, effective, and inexpensive dressing for the outpatient management of burn wounds.32–34
Follow-Up Care of Minor Burns
If a topical antibiotic agent is used, the dressing should be changed daily with removal and reapplication of the topical preparation. The wound should be rechecked by a clinician after 2 to 3 days and periodically thereafter, depending on wound size, compliance, healing, and other social issues. If a dry dressing is chosen, follow-up every 3 to 5 days is usually adequate. The purpose of any burn dressing changes or home care regimen is defeated if the patient cannot afford the material or is not instructed in the specifics of burn care. Many EDs supply burn dressing material on patient release. A complete pack includes antibiotic ointment or cream, gauze pads (fluffs), an absorbent gauze roll, a sterile tongue blade to apply the cream, and tape (Fig. 38-13). Providing limited supplies of the items necessary for dressing changes may enhance compliance with follow-up if the patient has to return for additional supplies. Writing a prescription and merely stating that the dressing should be changed daily may not be sufficient.
Daily home care can be performed by the patient with help from a family member or visiting nurse (Box 38-5). The dressing may be removed each day and gently washed with a clean cloth or a gauze pad, tap water, and a bland soap. Sterile saline and expensive prescription soaps are not required. A tub or shower is an ideal place to gently wash off burn cream. The affected area may be put through a gentle range of motion during dressing changes. After the burn is cleaned, the patient inspects it in the hope that complications can be recognized and prompt further follow-up. After complete removal of the old cream, a new layer is applied with a sterile tongue blade and covered with absorbent gauze.
In the postacute phase, dryness in healing skin may be treated with mild emollients such as Nivea (Beiersdorf, Inc., Norwalk, CT), Vaseline Intensive Care Lotion (Chesebrough Ponds, Inc., Greenwich, CT), or other readily available over-the-counter skin care moisturizing lotions. Natural skin lubrication mechanisms usually return by 6 to 8 weeks.23 Excessive sun exposure should be avoided during wound maturation because this may lead to hyperpigmentation. When the patient is outdoors, sun avoidance strategies should be used, or at the very least, a commercially available sunblock should be applied. Exposure of the recently healed burned area to otherwise minor trauma (chemicals, heat, sun) may result in an exaggerated skin response. Pruritus is common and may be treated with oral antihistamines or a topical moisturizing cream.
Burn Wound Healing
Burn wound healing differs from healing of other soft tissue wounds.2 The duration is variable but is often proportional to burn depth. Within 1 to 3 weeks and following the initial inflammatory response, neovascularization of the burn occurs and is accompanied by fibroblast migration. Collagen production begins but it is often deposited randomly, thereby leading to scar formation. Reepithelialization follows collagen deposition. The persistence of necrotic tissue and eschar in the wound will impede all aspects of healing. The extent of scar formation is related directly to healing time. Wound healing that occurs in fewer than 16 days often results in decreased scar formation.2 Proper wound débridement is also associated with faster wound healing and minimizes scar formation.
Special Minor Burn Care Circumstances
Management of blisters in minor burns is controversial. In reality, there is little one can do wrong when it comes to a clinical approach to blisters in minor burns (Fig. 38-14). Management arguments are generally theoretical or based on local tradition; the ultimate outcome of a minor burn is rarely determined by how one treats blisters. Intact blisters do offer a physiologic dressing that rarely becomes infected; however, most large blisters spontaneously rupture after 3 to 5 days and eventually require débridement. When the integrity of the blister is breached, the fluid becomes a potential culture medium. Clinical choices for blister management include débridement, aspiration, or simply leaving the blister intact.
Some studies suggest that intact burn blisters may allow reversal of capillary stasis and less tissue necrosis.2 Madden and colleagues36 showed that burn exudate (as contained within intact blisters) is beneficial in stimulating epidermal cell proliferation.
Swain and associates37 demonstrated that the density of wound colonization with microorganisms was much lower in minor burns with blisters left intact. They also found that 37% of patients with aspirated blisters experienced a reduction in pain versus none of those whose blisters were unroofed. Other investigators believe that undressed wounds with débrided blisters are subject to additional necrosis secondary to desiccation, which can convert a partial-thickness burn to a full-thickness injury.3 Finally, intact blisters clearly provide some pain relief, as evidenced by the sudden increase in pain immediately after débridement. Increased pain should be anticipated and analgesia offered as appropriate when débridement is necessary. We suggest the guidelines in Box 38-3 as a general approach to burn blisters 2,3,36–38
Minor Burn Infections
Prophylactic systemic antibiotics are not warranted in the routine treatment of outpatient burns. It may be difficult to separate the erythema of the injury or healing process from cellulitis, but minor burns rarely become infected, with infection rates being well under 5%.39 There are bacteria on the skin at all times—normal skin usually harbors nonvirulent pathogens such as S. epidermidis and diphtheroids. Therefore, all burns are contaminated but not necessarily infected. Because thermal trauma results in coagulative necrosis, burn wounds contain a variable amount of necrotic tissue, which if infected, acts much as an undrained abscess and prevents access of antibiotics and host defense factors.
The microbial flora of outpatient burns varies with time after the burn. Shortly after injury, the burn becomes colonized with gram-positive bacteria such as S. aureus and S. epidermidis. After this period there is a gradual shift toward inclusion of gram-negative organisms, 80% of which originate from the patient’s own gastrointestinal tract.4 Common organisms seen on days 1 to 3 include S. epidermidis, β-hemolytic streptococci, Bacillus subtilis, S. aureus, enterococci, Mima polymorpha, Enterobacter spp., Acinetobacter spp., and C. albicans. One week after the burn these organisms may be seen along with E. coli, P. aeruginosa, Serratia marcescens, K. pneumoniae, and Proteus vulgaris.
Anaerobic colonization of burn wounds is rare unless there is excessive devitalized tissue, as occurs with a high-voltage electrical injury.40 For this reason, routine anaerobic cultures are generally unnecessary in an assessment of infective organisms that produce minor infections.
Effective topical treatment at the time of initial burn care and subsequent dressing changes is meant to delay bacterial colonization, maintain the bacterial density of wounds at low levels, and produce a less diverse wound flora. Because outpatient management of burns should be attempted only when the risk for infection is minimal, the use of systemic antibiotics is unnecessary for minor burns, even in the setting of delayed treatment, diabetes, and steroid use.41 Unnecessary antibiotic use may select for resistant organisms. Antibiotics in the management of minor burns have been recommended for patients undergoing an autograft procedure.42 There are no data on the use of antibiotics as prophylaxis for patients with burns in the setting of valvular heart disease.
In minor burn care, wound cultures are not required or recommended. It is useless, for example, to culture blister fluid in a patient who arrives for emergency care immediately after a thermal injury. Cultures are necessary only when overt infection develops, especially when it occurs while a topical or systemic antibiotic is being used. Cultures may also be of benefit when the infected wound is old, when hygiene is poor, or when there are preexisting abrasions nearby.43 Although they may adequately reflect wound flora, falsely sterile cultures are relatively frequent. In general, superficial cultures do not reflect deep burn flora and provide no quantitative information.
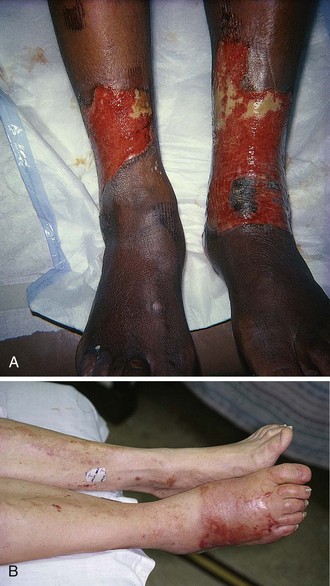
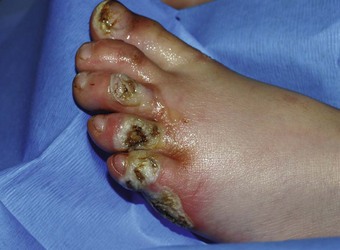
Foot Burns
Despite their relatively small surface area, foot burns tend to heal poorly, usually because of excessive edema; therefore, they are generally considered major burns. Foot burns are the most common burn category to fail outpatient therapy and subsequently require admission and inpatient care (Fig. 38-15). Zachary and coworkers44 reported on a series of 104 patients with foot burns. In no patient admitted on the day of injury did burn cellulitis develop; in contrast, 27% of delayed-admission patients had cellulitis. Their study also noted a higher incidence of hypertrophic scarring and need for skin grafting in the delayed-admission group. Overall, fewer days of hospitalization were required for the initially admitted group.
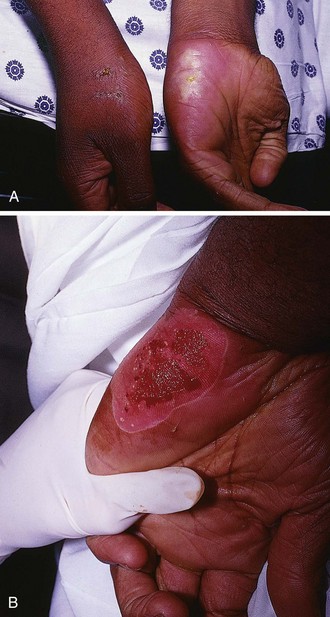
Hand Burns
Because of its functional significance, burns involving the hand can result in significant functional loss even when the TBSA burned is small. Losing the use of one or both hands can become seriously disabling and affect the patient’s activities of daily living regardless of whether the cause of the loss is a burn dressing, the late onset of scar contractures, or loss as a result of amputation.45
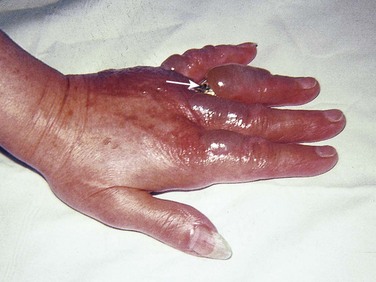
Many of the issues complicating outpatient management of foot burns are relevant to the care of hand burns. After initial burn cooling, the wound should be gently cleansed with mild soap. Any loose skin or ruptured blisters should be gently débrided, rinsed, patted dry, and covered with a topical antimicrobial agent and a nonadherent, bulky gauze dressing. The fingers should be carefully separated and bandaged individually. Small, intact blisters that do not interfere with hand function should be left intact to serve as a biologic dressing. Elevation of the hand is very important in the first few days after a burn injury to minimize edema. Deep partial- or full-thickness burns on the dorsum of the hand should be splinted46,47 after bandaging to avoid the development of contractures or a boutonnière deformity.
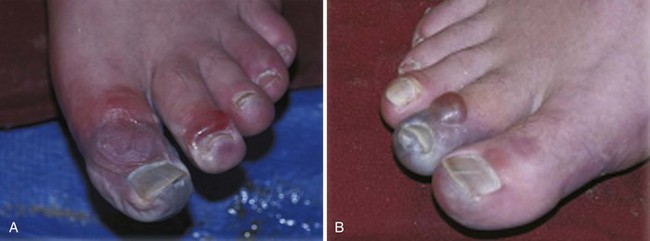
Hospital admission or burn center referral should be considered for all significant hand burns, particularly full-thickness injuries and circumferential burns involving the digits (Fig. 38-16). If outpatient treatment is attempted, the patient must be given comprehensive instructions and should have the resources available to perform daily dressing changes and range-of-motion exercises of the fingers and wrist during these dressing changes. An initial follow-up visit should be arranged in 48 to 72 hours, but the patient should be encouraged to return if burn cellulitis, worsening pain, fever, or lymphangitis develops. Ideally, the patient should be seen twice in the first week after injury and then once a week until the burn is healed.
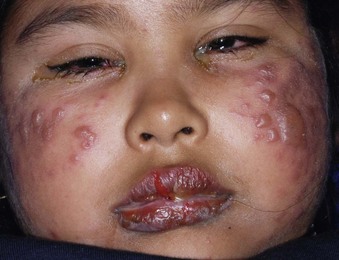
Facial Burns
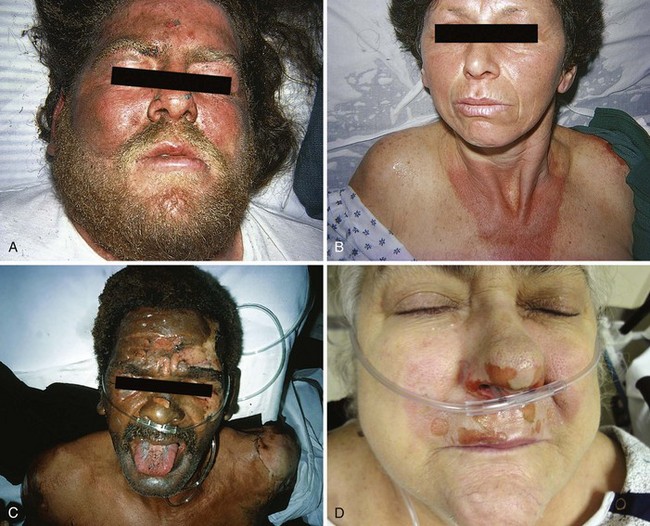
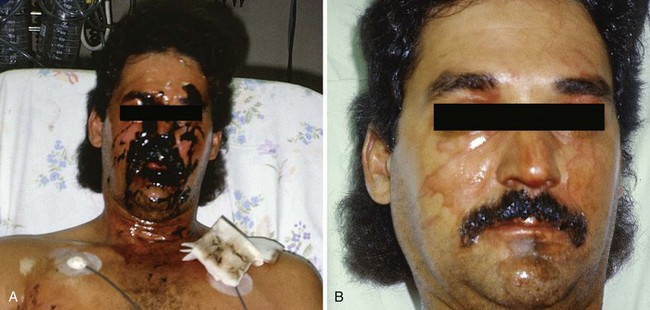
Facial burns commonly result from unexpected ignition flash burns (e.g., from a stove, oven, or charcoal grill) or from car radiator accidents (Fig. 38-17A and B).48,49 Facial burns from these sources usually do well, but singeing of facial hair, significant edema, and pain often result. However, facial burns from these causes may produce airway problems and might require skin grafting. Singed nasal hairs or any sign of significant heat exposure to the face should prompt an evaluation of airway injury, which may result in airway compromise at a time later than the initial incident (see Fig. 38-17C).
Concurrent globe or corneal injury is quite rare because of protective blinking reflexes. If the eye is burned, it is usually in the setting of a life-threatening concomitant burn injury.50 Burns involving the eyelids can cause significant scarring. Fluorescein staining and slit-lamp examination should be performed to confirm or exclude the diagnosis of corneal injury (Fig. 38-18). Treatment of a corneal injury can involve irrigation, topical ophthalmic antibiotics, and consideration of eye patching versus protective soft contact lens (see Chapter 62). Referral to an ophthalmologist is usually prudent. Facial burns are otherwise treated in the usual fashion and with an open (no dressing) technique. Patients are instructed to wash their face two or three times a day with a mild soap and then apply a thin layer of antibiotic ointment, such as bacitracin zinc. Car radiator burns result from the combination of a hot liquid and steam burn (see Fig. 38-17B). Antifreeze does not produce a caustic injury, nor is it systemically absorbed. Neck burns are treated similarly.
A flash burn in a patient smoking cigarettes while using nasal oxygen causes a facial burn that is not uncommonly seen in the ED (see Fig. 38-17D). These burns may involve the nose and lips, may have melted plastic particles onto the skin, and can be quite painful. Although such patients generally do well, facial burns can make the continued use of nasal cannula oxygen problematic until healing takes place. Though not generally an inhalation burn issue, careful evaluation of the upper airways and assessment of lung function are prudent. Many patients using oxygen are immunocompromised. They cannot tolerate even minor physical insults and have fragile conditions that require careful evaluation, short-term observation in some cases, and close follow-up for delayed healing and infection. Hospitalization for burn care and general supportive measures are suggested for all but minor burns. Minor burns can be treated on an outpatient basis in an open fashion with topical ointments, such as bacitracin.
All patients with head or neck burns should be evaluated carefully for a concomitant inhalation injury. Such patients may have direct evidence of injury, such as oral burns, blisters, soot, or hyperemia and a history of being in an enclosed space, or have indirect evidence, such as dyspnea, wheezing, arterial hypoxemia, or an elevated carboxyhemoglobin level. The definitive diagnostic test for inhalation injury is fiberoptic bronchoscopy.51 Flash ignition burns involving the face do not pose a problem with carbon monoxide poisoning, and although inhalation injuries generally do not occur with minor flash ignition burns, airway management should remain a consideration.
Corneal contact burns, as from accidental contact with a curling iron, are often manifested rather dramatically as opacified, “heaped-up” corneal epithelium (see Fig. 38-18). Despite their appearance, the end result is usually excellent. Treatment is the same as for a corneal abrasion.52
Abuse of Children and Elderly Individuals
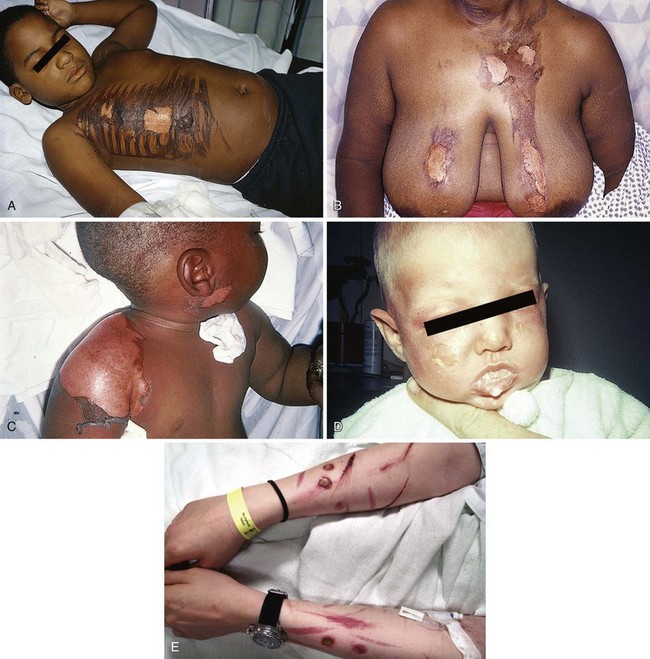
Recognition of the possibility of deliberate abuse by burning in the pediatric and geriatric populations is essential. In addition, children younger than 2 years have a thinner dermis and a less well-developed immune system than adults do. Elderly patients (>65 years) likewise tolerate burns poorly. These two populations are the most prone to abuse, often by family members (Fig. 38-19). For these reasons, both these groups of patients often require inpatient care.18
The majority of abused children are 18 to 36 months old, and for unknown reasons, the majority are male.29 Immersion burns are a common type of abuse and are characterized by circumferential, sharply demarcated burns on the hands, feet, buttocks, and perineum. Cigarette burns and burns from hot objects such as irons should be obvious. Contact burns on “nonexploring” parts of the child also warrant suspicion. A delay in seeking treatment may be a tip-off that a burn resulted from abuse. In older populations, the presence of confirmed self-inflicted burns such as cigarette burns suggests psychiatric disease (see Fig. 38-19E).
Burns in Pregnancy
There is little information in the literature concerning the special problems of pregnant burn victims. Ying-bei and Ying-jie53 reported on 24 pregnant burn patients representing a wide range of burn severity. Complications of the burn injuries included abortion and premature labor, although all patients in this series with burns covering less than 20% TBSA did well and delivered living full-term babies.
Specific Burning Agents
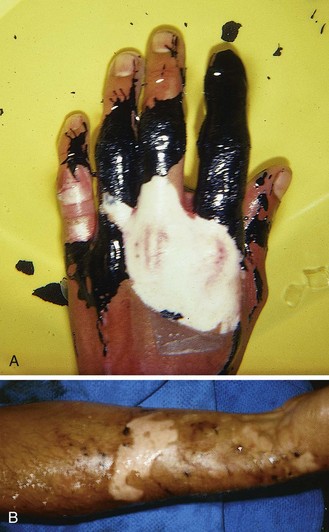
Asphalt is a product of the residues of coal tar and is commonly used in roofing and road repair. It is kept heated to approximately 450°F. When spilled onto the skin, the tar cools rapidly, but the retained heat is sufficient to produce a partial-thickness burn. Fortunately, full-thickness burns are unusual. Cooled tar is nonirritating and does not promote infection. When cooled tar is physically removed, the adherent skin is usually avulsed (Fig. 38-20). Careless removal of the tar may inflict further damage on burned tissues. Agents such as alcohol, acetone, kerosene, or gasoline have been used to remove the tar, but these are flammable and may cause additional skin damage or a toxic response secondary to absorption.
There is no great need to meticulously remove all tar at the first visit. Obviously devitalized skin can be débrided, but adherent tar should be emulsified or dissolved rather than manually removed (Fig. 38-21). Polyoxyethylene sorbitan (Tween 80 or polysorbate 80) is the water-soluble, nontoxic, emulsifying agent found in Neosporin and several other topical antibiotic creams. Note that the cream formulations, not the ointments, contain the most useful tar dissolvers. The creams contain a complex mixture of ethers, esters, and sorbitol anhydrides that possess excellent hydrophilic and lyophilic characteristics when used as nonionic, surface-active emulsifying agents. With persistence, most tar can be removed (emulsified) on the initial visit.
Another household product (De-Solv-It multiuse solvent) also appears logical for topical ED use.43,54 De-Solv-It has a surface-active moiety that wets the chemical’s surface and emulsifies tar and asphalt. Because De-Solv-It is itself a petroleum-based solvent, it should be applied only briefly, and the operator should wear gloves and protective eyewear during application. It should be used only for external exposure to tar or asphalt.
Many clinicians instead prefer to emulsify the majority of tar on an outpatient basis. A generous layer of polysorbate-based ointment can be applied under a bulky absorbent gauze dressing. The patient is then released home, and the residual tar is easily washed off after 24 to 36 hours (see Fig. 38-20B). A number of dressing changes may be required. Once the residual tar is removed, the wound is treated as any other burn.
Chemical Burns
Chemical burns generally occur in the workplace, and the offending substance is usually well known. More than 25,000 chemicals currently in use are capable of burning the skin or mucous membranes. Commonly used chemical agents capable of producing skin burns are shown in Box 38-6.
Injury is caused by a chemical reaction rather than a thermal burn.55 Reactions are classified as oxidizing, reducing, corrosive, desiccant, or vesicant or as protoplasmic poisoning. The injury to skin continues until the chemical agent is physically removed or exhausts its inherent destructive capacity. The degree of injury is based on the strength, concentration, and quantity of the chemical; duration of contact; location of contact; extent of tissue penetration; and mechanism of action.
Immediate flushing with water is recommended for all chemical burns, with the exception of those caused by alkali metals. Flushing serves to cleanse the wound of unreacted surface chemical, dilute the chemical already in contact with tissue, and restore lost tissue water. Leonard and colleagues56 clearly demonstrated that patients receiving immediate copious water irrigation for chemical burns had less full-thickness burn injury and a 50% or greater reduction in hospital stay.
Acid and Alkali Burns
Strong alkali burns may require irrigation for 1 to 2 hours before tissue pH returns to normal. Some recommend that if the burn continues to feel “slippery” or tissue pH has not returned to normal after extensive irrigation, chemical neutralization may be helpful.57,58 Given that any heat of neutralization will be carried away with the irrigation solution,59 prompt irrigation with a dilute acid (e.g., vinegar, or 2% acetic acid) may hasten neutralization and patient comfort.
Contact Burns from Wet Cement
The major constituent of Portland cement, an alkaline substance, is calcium oxide (64%), combined with oxides of silicon, aluminum, magnesium, sulfur, iron, and potassium. There is considerable variability in the calcium oxide content of different grades of cement, with concrete having less and fine-textured masonry cement having more.56 The addition of water exothermically converts the calcium oxide to calcium hydroxide (Ca[OH]2), a strongly corrosive alkali with a pH of 11 to 13. As the cement hardens, the calcium hydroxide reacts with ambient carbon dioxide and becomes inactive.
Both the heat and the Ca(OH)2 produced in this exothermic reaction can result in significant burns. Because of its low solubility and consequent low ionic strength, long exposure to Ca(OH)2 is required to produce injury. This usually occurs when workers spill concrete into their boots or kneel in it for a prolonged period. The burn wound and the resultant protein denaturation of tissues produce a thick, tenacious, ulcerated eschar. Concrete burns are insidious and progressive. What may appear initially as a patchy, superficial burn might in several days become a full-thickness injury requiring excision and skin grafting.60 The pain associated with these burns is often severe and more intense than the appearance of the wound might suggest (Fig. 38-22). Interestingly, many workers are not warned of the dangers of prolonged contact with cement, and because the initial contact with cement is usually painless, exposure may not be realized until the damage is done.
Air Bag Keratitis and Thermal Burns
Safety legislation has mandated increased use of air bags to protect automobile occupants in the event of collision (Fig. 38-23). Burns from air bags can be thermal, friction, or chemical. The automobile air bag is a rubberized nylon bag that inflates on spark ignition of sodium azide to yield nitrogen gas, ash, and a small amount of sodium hydroxide. Within seconds, the superheated air is vented, and this can produce a thermal burn if it contacts an extremity, the face, or the upper part of the torso.5,61,62 If the air bag ruptures, the alkaline contents of the bag are dispersed as a fine, black powder that usually causes no problems unless the eyes are exposed. Patients with eye exposure exhibit clinical evidence of a chemical keratoconjunctivitis, including photophobia, tearing, redness, and decreased visual acuity. Tear pH is usually elevated, and there may be a small amount of particulate material in the fornices.63
The severity of an ocular alkaline burn is related to the duration of exposure and the concentration and pH of the chemical. For this reason, prompt, copious irrigation of the eyes with frequent assessment of tear pH is essential to prevent or minimize the injury (see Chapter 62). A rising pH suggests that trapped particulate matter is releasing additional chemical. Corneal edema and conjunctival blanching are signs of serious injury and can necessitate immediate ophthalmologic consultation.
Hydrocarbon Burns
Hydrocarbons are capable of causing severe contact injury by virtue of their irritant, fat-dissolving, and dehydrating properties. Cutaneous absorption may cause even more dangerous systemic effects. Gasoline, the usual agent involved, is a complex mixture of C4 to C11 alkane hydrocarbons and benzene; the hydrocarbons appear to be the major toxic agent. Lead poisoning caused by either absorption through intact skin or burns from exposure to leaded gasoline have been reported previously but are currently quite rare because unleaded gasoline has virtually replaced the leaded version for most purposes.64
The depth of injury is related to the duration of exposure and the concentration of the chemical agent. Gasoline immersion injuries resemble scald burns and are usually partial thickness.65 Occasionally, gasoline-injured skin exhibits a pinkish brown discoloration, possibly related to dye additives. A common source of gasoline exposure is motor vehicle collisions in which a comatose patient has been lying in a pool of gasoline.
Phenol Injury
Phenol is a highly reactive aromatic acid alcohol that acts as a corrosive. Carbolic acid, an earlier term for phenol, was noted to have antiseptic properties and was used as such by Joseph Lister in performing the first antiseptic surgery. Hexylresorcinol, a phenol derivative, is in current use as a bactericidal agent. Phenols, in strong concentrations, cause considerable eschar formation, but skin absorption also occurs and can result in systemic effects such as central nervous system depression, hypotension, hemolysis, pulmonary edema, and death. Interestingly, phenol acts differently from other acids in that it penetrates deeper when in a dilute solution than when in a more concentrated form.55 Therefore, irrigation with water is suboptimal for phenol burns, but because water is commonly readily available, it is frequently used for irrigation.
Hydrofluoric Acid Injury
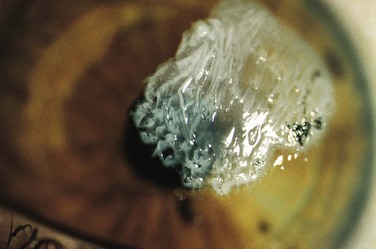
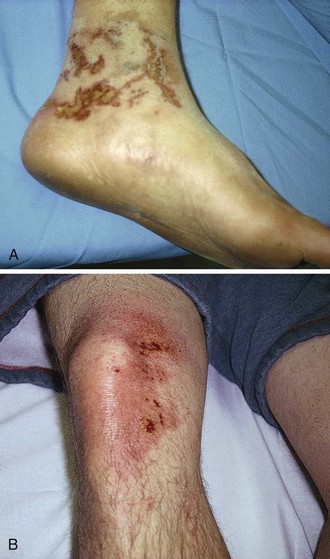
Hydrofluoric acid (HFA) is one of the strongest inorganic acids known; it has been widely used since its ability to dissolve silica was discovered in the late 17th century.66 Currently, HFA is used in masonry restoration, glass etching, and semiconductor manufacturing; for control of fermentation in breweries; and in the production of plastics and fluorocarbons. It is also used as a catalyst in petroleum alkylating units. It is available in industry as a liquid in varying concentrations up to 70%. It is also readily sold in home improvement and hardware stores. Significant concentrations of HFA are present in many home rust removal products, aluminum brighteners, automobile wheel cleaners, and heavy-duty cleaners in concentrations of less than 10%. Despite its ability to cause serious burns, unregulated and poorly labeled HFA products are recklessly used on a regular basis in the home and in small businesses. The public and many clinicians are generally unaware of the potential problems with this acid (Fig. 38-24).
In high concentrations, the fluoride ions may penetrate to bone and produce demineralization. Exposure of skin to concentrated HFA involving as little as 2.5% TBSA can lead to systemic hypocalcemia and death from intractable cardiac arrhythmias; it has been calculated that exposure to 7 mL of anhydrous HFA (HFA gas) is capable of binding all the free calcium in a 70-kg adult.67,68 Hyperkalemia and hypomagnesemia can also develop. If the hands are exposed, the acid characteristically penetrates the fingernails and injures the nail bed and cuticle area. As with most caustics, the pain is generally out of proportion to the apparent external physical injury. HFA burns produce variable areas of blanching and erythema, but rarely are blisters or skin sloughing seen initially. Skin necrosis and cutaneous hemorrhage may be noted in a few days.
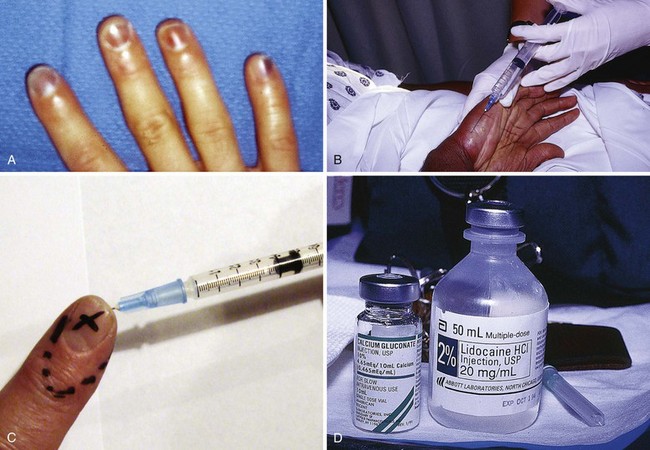
Initial treatment of a more concentrated exposure begins as described earlier and includes immediate débridement of necrotic tissue to remove as much fluoride ion as possible.69 After débridement, a 10% solution of calcium gluconate (note: avoid calcium chloride for tissue injections) is injected intradermally and subcuticularly with a 30-gauge needle about the exposed area, with about 0.5 mL per square centimeter of burn being used. Pain relief should be almost immediate if this therapy is adequate. Because the degree of pain is a measure of the effectiveness of treatment, the use of anesthetics, especially by local infiltration, may be deleted if the burn is on the arm or leg. HFA can penetrate fingernails without damaging them. Soft tissue can be injected without prior anesthesia, but if the fingertips or nail beds are involved, they may be injected after a digital nerve block has been performed (Fig. 38-25). Before anesthesia and injection of calcium, the patient can outline the affected areas with a pen to ensure accurate injection of the antidote (see Fig. 38-25C). Although some investigators recommend that the fingernails be removed routinely, we strongly advise against this unless the nails are very loose or there is obvious necrosis of the nail bed. Fingers are best injected with a 25- or 27-gauge needle (a tuberculin syringe works well).58 Nails frequently become loose in a few days, but they often return to normal and do not require removal, particularly when lower-concentration nonindustrial products are involved.
Several authorities have advocated intraarterial calcium infusions in the treatment of serious HFA burns of the extremities.70,71 Though very effective, this technique is not recommended for burns secondary to dilute HFA (i.e., concentrations <10%) because the morbidity is usually quite mild. When using this technique, 10 mL of 10% calcium gluconate is diluted in 50 mL of a 5% dextrose and water solution. The dilute solution is administered by slow infusion into an arterial catheter. It is unclear which artery best delivers the calcium to injured tissues. If only the radial three digits are involved, probably only the radial artery need be cannulated. Otherwise, a percutaneous catheter is inserted into the brachial artery. However, some investigators have advocated use of the radial artery in all cases, and because the arterial supply of the hand is interconnected, this may be a reasonable recommendation.72 The radial artery is usually more easily cannulated than the brachial artery. When arterial access has been accomplished, the solution is infused slowly over a 4-hour period. At this point the catheter is left in place and the patient is observed. If pain returns at any time over the next 4 hours, the infusion is repeated. If the patient is pain free over the 4-hour observation period, the burn is dressed and the patient is released home. This technique may be initiated in the ED, but many clinicians are reluctant to cannulate an artery and infuse calcium in the ED. Such patients require hospitalization or burn center referral for further evaluation and observation.
Infusing calcium into the general venous circulation is of no benefit for HFA burns. Some authors have advocated the use of regional IV calcium gluconate, similar to the method used with the Bier block for regional anesthesia (see Chapter 32).73 Case reports have noted variable success, but this technique has neither been well studied nor rigorously compared with other options. This method would be useful only for upper extremity burns. To perform regional calcium therapy, an IV catheter is placed in the dorsum of the hand on the involved extremity. The arm is partially exsanguinated by elevation, wrapping with an elastic bandage, or both. A Bier block tourniquet or a heavy-duty blood pressure cuff is applied proximal to the burn and inflated 20 to 30 mm Hg above systolic pressure to stop blood flow to and from the arm. Because slow deflation of a regular blood pressure cuff may thwart success of the procedure, use of a specialized tourniquet is recommended. Then, 10 mL of 10% calcium gluconate, diluted with 30 to 40 mL of saline, is infused into the venous catheter, and the solution is kept in the arm by the tourniquet for 20 to 30 minutes. Some patients cannot tolerate arm ischemia for this period, thus limiting the effectiveness of this procedure. Theoretically, the calcium diffuses out of the venous system and into the injured tissues. After 20 to 30 minutes, the cuff is deflated and normal circulation to the extremity is restored. It may require 10 to 20 minutes after deflation of the tourniquet before the patient experiences relief of pain. This procedure is safe, but its efficacy is variable.
HFA burns involving the eye are potentially devastating injuries that deserve special mention. Ophthalmologist referral is mandatory. Ocular exposure to liquid or gaseous HFA will result in severe pain, tearing, conjunctival inflammation, and corneal opacification or erosion. Complications include decreased visual acuity, globe perforation, uveitis, glaucoma, conjunctival scarring, lid deformities, and keratitis sicca. Optimal therapy for ocular HFA burns, other than initial irrigation, is unknown. Irrigation may be performed with water, isotonic saline, or magnesium chloride.74 We advise copious saline irrigation. Topical antibiotics and cycloplegics, along with light pressure patching, are also recommended. The use of topical steroids has been advocated by some to lessen corneal fibroblast formation, but other attempted therapies such as subconjunctival injections of calcium gluconate and ocular irrigation with quaternary ammonium compounds have been associated with additional injury.75
Chromic Acid Injury
Treatment includes immediate excision of the burned tissues to lessen the total body dichromate burden. Wounds should be washed with a 1% sodium phosphate or sulfate solution and dressed with bandages soaked in 5% sodium thiosulfate solution. These actions reduce the hexavalent chromium ion to the less well absorbed trivalent form.76
Phosphorus Burns
Phosphorus causes both thermal burns from the flaming pieces and acid burns from the oxidation of phosphorus to phosphoric acid. The burns classically emit a white vapor with a characteristic garlic odor.77
Electrical Burns
Electrical burn wounds occur when energy traveling through the body across a potential difference is converted to heat. This energy has the ability to destroy deep tissues, including muscles, tendons, and nerves (Fig. 38-26). In addition, electrical injuries can arise from the arc produced when electricity passes through the air and from flames caused by the ignition of clothing. Electrical injuries from high-voltage or high-current sources (>1000 V and >5000 mA) are more likely to result in deep soft tissue damage, whereas low voltage or low current (<1000 V and 5 to 60 mA) causes less soft tissue damage but is more likely to result in cardiac arrhythmias.78
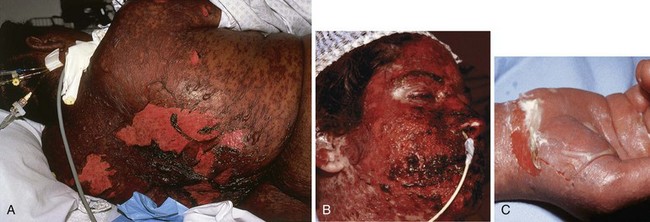
TEN and SJS
The distinction between TEN and SJS is one of extent, with lesions occupying less than 10% TBSA qualifying as SJS (Fig. 38-27) and lesions involving greater than 30% TBSA being called TEN (Fig. 38-28); when the extent of involvement lies between 10% and 30%, an intermediary term is coined, SJS-TEN overlap. These disorders are rare with an annual incidence of 0.4 to 1.2 and 1.2 to 6.0 per million persons, respectively, and are more common in females and the elderly.79 Patients at risk are those who are severely immunocompromised (e.g., human immunodeficiency virus infection, lymphoma). Death occurs on average in every third patient with TEN. More than 100 drugs, including antibiotics (particularly sulfonamides), nonsteroidal antiinflammatory drugs, and anticonvulsants, have been implicated.
Various theories exist to explain the precise sequence of molecular and cellular events responsible for the development of TEN. The 1- to 3-week interval between the onset of TEN and the commencement of drug therapy favors an immune etiology. Cytotoxic T cells are seen in cutaneous lesions, and it is hypothesized that necrolysis is due to their recognition of complexes between drug metabolites and major histocompatibility complex class I molecules on the surface of keratinocytes. Exfoliation is due to the death of keratinocytes via apoptosis, and recent data suggest that the latter is mediated by interaction of the death receptors, transmembrane proteins, Fas, and its ligand FasL. This activates the proteolytic cascade (caspases), which leads to cellular disintegration.80 Evidence has shown upregulation of FasL in patients with TEN.
Management
Initial management of TEN requires immediate discontinuation of all medications, including antibiotics, if there are no signs of infection. Supportive care is similar to that required in the treatment of thermal burns. Attention is therefore paid to correction of fluid and electrolyte abnormalities, renal insufficiency, nutrition, and sepsis. Involvement of respiratory mucosa may warrant intubation and ventilation. The wounds are carefully débrided and a biopsy specimen is taken to confirm the diagnosis. Regular hydrotherapy and topical antimicrobials are used to decrease infection. Silvadene and mafenide are best avoided because they may be implicated in causing the disorder, but alternative dressings include the various silver products (e.g., Acticoat), as well as bacitracin and Xeroform. Synthetic dressings such as Biobrane and biologic materials, including allograft, have all been used with various success.81
Regular examination by an ophthalmologist is also recommended. The eyelids should be cleansed daily and followed with a daily application of antibiotic ointment. Attention to oral hygiene is also mandatory because oral lesions are common. Several case reports and uncontrolled series suggest the utility of specific therapies for the treatment of TEN/SJS; however, thus far there are no strong evidence-based standards to promote any particular therapy. Promising studies have included cyclosporine, cyclophosphamide, plasmapheresis, and N-acetylcysteine, but these therapies are not established standard of care.82 The use of systemic corticosteroids remains controversial, and they may even increase mortality. Promising results were shown with the use of IV immunoglobulins that contain antibodies against Fas that can block the binding of Fas with FasL.83
Frostbite
Frostbite is the result of exposure to low environmental temperatures (Fig. 38-29). The formation of ice crystals within extracellular fluid causes direct cellular injury and cellular dehydration through transmembrane osmotic shifts. In addition, a secondary vascular effect of cooling leads to endothelial damage and progressive dermal ischemia.84
Radiation Burns
Accidents involving ionizing radiation are not common, but when they occur, the clinical findings may range from erythema to charring of the superficial layers of skin. Whole-body exposure of more than 100 rad causes acute radiation syndrome within hours of exposure. This is characterized acutely by nausea, vomiting, diarrhea, fever, fatigue, and headache. The symptoms may resolve transiently during a latent period only to recur as hematopoietic, gastrointestinal, or vascular complications.85,86
Emergency Escharotomy
On occasion, because of high-volume fluid resuscitation, noncircumferential and deep partial-thickness burns require surgical decompression to prevent the complications of nerve or muscle damage. Once signs and symptoms of vascular impairment are present, the clinician must act quickly to prevent tissue hypoxia and cellular death. This pathophysiology may be manifested within a time frame that requires an emergency clinician to intervene. Clinical assessment of tight compartments may be aided by measurements such as capillary refill, Doppler signals, pulse oximetry, and direct measurement of compartment pressures. Escharotomy, when required, is usually performed within the first 2 to 6 hours of a burn injury. The need for non–burn specialists to identify the need for and perform an adequate escharotomy is illustrated by the report of Brown and associates,87 who found that 44% of pediatric burn cases were inadequately decompressed before arrival at a referral burn unit.
Indications
The indications for escharotomy are based on clinical examination, compartment pressure, or both. A high index of suspicion and a low threshold for intervention are essential for a successful outcome. Skin temperature and palpation of pulses are unreliable and imprecise indicators of the adequacy of circulation because of peripheral vasoconstriction and local edema. A patient with circulatory embarrassment significant enough to warrant escharotomy may complain of deep aching pain, progressive loss of sensation, or paresthesias, but these parameters are difficult to quantitate in a severely burned, sedated, or mechanically ventilated patient. However, motor activity and peripheral pulses may remain intact despite severe underlying muscle ischemia. In the series by Brown and associates,87 peripheral pulses were present in 74% of the limbs that required decompression. Muscle compartments with pressures in excess of 30 mm Hg should be decompressed. Measurements should be taken before and after escharotomy to ensure adequate decompression.
In a patient with absent distal arterial flow (as determined with a Doppler ultrasonic flow meter) but otherwise adequate blood pressure, immediate escharotomy is indicated. Bardakjian and coworkers88 suggested that an oxygen saturation below 95% in the distal end of the extremity as demonstrated by pulse oximetry (in the absence of systemic hypoxia) is also a reliable indicator of the need for emergency escharotomy.
Technique of Escharotomy
Limbs
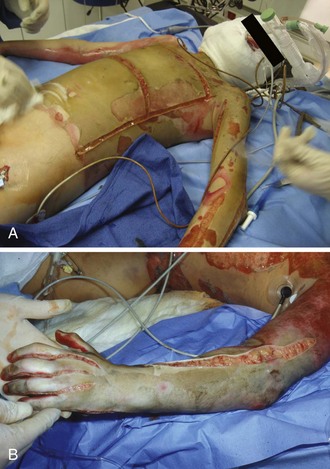
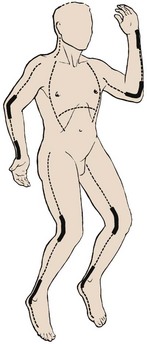
Under sterile conditions, the lateral and medial aspects of the involved extremity are incised with a scalpel or electrocautery 1 cm proximal to the burned area and 1 cm distal to the involved area of constricting burn (Fig. 38-30). The incision is carried through the full thickness of skin only and should result in immediate separation of the constricting eschar to expose SQ fat. Because joints are areas of tight skin adherence and potential vascular impingement, incisions should cross these structures (Fig. 38-31). Care must be taken to avoid vital structures, such as the ulnar nerve at the elbow, the radial nerve at the wrist, the superficial peroneal nerve near the fibular head, and the posterior tibial artery at the ankle. In circumferential burns of the feet, if escharotomy is indicated, the incision should extend to the great toe medially and the little toe laterally. In circumferential burns of the hands in which escharotomy is indicated, the incisions should extend to the thenar and hypothenar aspects of the hands (see Figs. 38-30 and 38-31). Softening of the compartment, improved distal tissue perfusion, return of sensation, Doppler flow signal strength, and oximetry values indicate adequate release.88
Chest
Full-thickness circumferential chest or upper abdominal burns may impair respiration. Nearly all these patients would expected to be intubated and mechanically ventilated. Evidence of the need to release the eschar is increasing airway pressure or an inability to ventilate. Escharotomy of the chest wall should extend from the clavicle to the costal margin in the anterior axillary line bilaterally while avoiding breast tissue in females, and it may be joined by transverse incisions to result in a chevron-shaped subcostal incision (see Fig. 38-30).
Acknowledgment
The significant contributions of Courtney A. Bethel, MD, to earlier editions are appreciated.
References
1. ABA Burn Incidence Report. Available at http://www.ameriburn.org/resources_factsheet.php.
2. Baxter, CR, Waeckerle, JF. Emergency treatment of burn injury. Ann Emerg Med. 1988;17:12.
3. American Burn Association. Hospital and prehospital resource for optimal care of patients with burn injury: guidelines for development and operation of burn centers. J Burn Care Rehabil. 1990;11:98.
4. Kagan, RJ, Warden, GD. Management of the burn wound. Clin Dermatol. 1994;12:47.
5. Kao, CC, Garner, WL. Acute burns. Plast Reconstr Surg. 2000;105:2482–2492.
6. Heimbach, D, Engrav, L, Grube, B, et al. Burn depth: a review. World J Surg. 1992;16:10–15.
7. Lund, C, Browder, N. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352–358.
8. Berkow, S. A method of investigating the extensiveness of lesions (burns and scalds) based on surface area proportions. Arch Surg. 1924;8:8–48.
9. Hidvegi, N, Nduka, C, Myers, S, et al. Estimation of breast burn size. Plast Reconstr Surg. 2004;6:1591–1597.
10. Knaysi, GA, Criklair, GF, Cosman, B. The rule of nines in history and accuracy. Plast Reconstr Surg. 1968;41:560–563.
11. Amirsheybani, HR, Crecelius, GM, Timothy, N, et al. The natural history of the growth of the hand: 1 Hand area as a percentage of body surface area. Plast Reconstr Surg. 2001;107:726–733.
12. Monafo, WW, Ayvazian, VH. Topical therapy. Surg Clin North Am. 1978;58:1157.
13. Ward, PA, Till, GO. Pathophysiologic events related to thermal injury of skin. J Trauma. 1990;30:S75.
14. American Burn Association/American College of Surgeons. Guidelines for the operation of burn centers. J Burn Care Res. 2007;28:134–141.
15. Morgan, ED, Bledsoe, SC, Barker, J. Ambulatory management of burns. Am Fam Physician. 2000;62:2015.
16. Sawada, Y, Urushidate, S, Yotsuyanagi, T, et al. Is prolonged and excessive cooling of a scalded wound effective? Burns. 1997;23:55–58.
17. Davies, JWL. Prompt cooling of burned areas: a review of benefits and the effector mechanisms. Burns. 1983;9:1.
18. Trott AT, ed. Wounds and Lacerations: Emergency Care and Closure. Mosby–Year Book: St. Louis, 1991:260.
19. Yarbrough, DR. The history of burn treatment. Emerg Med Serv. 1988;17:21.
20. Clayton, MC, Solem, LD. No ice, no butter: advice on management of burns for primary care physicians. Postgrad Med. 1995;97:151.
21. Demling, RH, Mazess, RB, Wolbert, W. The effect of immediate and delayed cold immersion on burn edema formation and resorption. J Trauma. 1979;19:56.
22. Phillips, LG, Robson, MC, Heggers, JP. Treating minor burns: ice, grease, or what? Postgrad Med. 1989;85:219.
23. Warden, GD. Outpatient care of thermal injuries. Surg Clin North Am. 1987;67:147.
24. Shuck, JM. Outpatient management of the burned patient. Surg Clin North Am. 1978;58:1107.
25. Quinn, KJ. Design of a burn dressing. Burns. 1987;13:377.
26. Demling, RH, DeSanti, L. Management of partial thickness facial burns: comparison of topical antibiotics and bio-engineered skin substitutes. Burns. 1999;25:256.
27. Bell, PL, Gabriel, V. Evidence based review of the treatment of post-burn pruritus. J Burn Care Res. 2009;30:55–61.
28. Ricketts, CR, Squires, JR, Topley, E, et al. Human skin lipids with particular reference to the self-sterilizing power of the skin. Clin Soc Mol Med. 1951;10:89.
29. Stuart, JD, Kenney, JG, Morgan, RF. Pediatric burns. Am Fam Physician. 1987;36:139.
30. Heck, E, Head, M, Nowak, D, et al. Aloe vera (gel) cream as a topical treatment for outpatient burns. Burns. 1980;7:291.
31. Rodriguez-Bigas, M, Cruz, NI, Suarez, A. Comparative evaluation of aloe vera in the management of burn wounds in guinea pigs. Plast Reconstr Surg. 1988;81:286.
32. Subrahmanyam, M. A prospective randomized clinical trial and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24:157.
33. Lusby, PE, Coombes, A, Wilkerson, JM. Honey: a potent agent for wound healing? J Wound Ostomy Continence Nurs. 2002;29:295.
34. Subrahmanyam, M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22:491.
35. Singer, AJ, McClain, SA. The effects of a high potency topical steroid on cutaneous healing of burns in pigs. Acad Emerg Med. 2002;9:977.
36. Madden, MR, Nolan, E, Finkelstein, JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29:924.
37. Swain, AH, Azadian, BS, Wakeley, CJ, et al. Management of blisters in minor burns. BMJ. 1987;295:181.
38. Sargent, RJ. Management of blisters in the partial-thickness burn: an integrative research review. J Burn Care Res. 2006;27:66–81.
39. Boss, WK. Effectiveness of prophylactic antibiotics in the outpatient treatment of burns. J Trauma. 1985;25:224.
40. Monafo, WW, Freedman, B. Topical therapy for burns. Surg Clin North Am. 1987;67:133.
41. Boss, WK, Brand, DA, Acampora, D, et al. Effectiveness of prophylactic antibiotics in the outpatient treatment of burns. J Trauma. 1985;25:224.
42. O’Neill, JA, Jr. Burns: office evaluation and management. Prim Care. 1976;3:531.
43. Shuck, JM. Current practices in burn management. Am Surg. 1974;40:145.
44. Zachary, LS, Heggers, JP, Robson, MC, et al. Burns of the feet. J Burn Care Rehabil. 1987;8:192.
45. Drueck, C. Emergency department treatment of hand burns. Emerg Med Clin North Am. 1993;11:797.
46. Coppard, BM, Lohman, H. Introduction to Splinting: A Critical-Thinking and Problem-Solving Approach. St. Louis: Mosby; 1996.
47. Falkenstein, N, Weiss-Lessard, S. Hand Rehabilitation: A Quick Reference Guide and Review. St. Louis: Mosby; 1999.
48. Al-Baker, AA, Attalla, MF, El-Ekiabi, SA. Car radiator burns: a report of 72 cases. Burns. 1989;15:265.
49. O’Neal, N, Purdue, G, Hunt, J. Burns caused by automobile radiators: a continuing problem. J Burn Care Rehabil. 1992;13:422.
50. Lipshy, KA, Wheeler, WE, Denning, DE. Ophthalmic thermal injuries. Am Surg. 1996;62:481.
51. Jordan, MH. Management of head and neck burns. Ear Nose Throat J. 1992;71:219.
52. Bloom, SM, Gittinger, JW, Jr., Kazarian, EL. Management of corneal contact thermal burns. Am J Ophthalmol. 1986;100:536.
53. Ying-bei, Z, Ying-jie, Z. Burns during pregnancy: an analysis of 24 cases. Burns. 1981;8:286.
54. Tsou, TJ, Hutson, HR, Bear, M, et al. De-Solv-It for hot paving asphalt burn: case report [letter]. Acad Emerg Med. 1995;2:88.
55. Stewart, CE. Chemical skin burns. Am Fam Physician. 1985;31:149.
56. Leonard, LG, Scheulen, JJ, Munster, AM. Chemical burns: effect of prompt first aid. J Trauma. 1982;22:420.
57. Jelenko, C, III. Chemicals that burn. J Trauma. 1974;14:65.
58. Arena, JM. Treatment of caustic alkali poisoning. Mod Treat. 1971;8:613.
59. Homan, CS, Maitra, SR, Lane, BP, et al. Effective treatment for acute alkali injury to the esophagus using weak-acid neutralization therapy: an ex-vivo study. Acad Emerg Med. 1995;2:952.
60. Wilson, GR, Davidson, PM. Full thickness burns from ready-mixed cement. Burns. 1985;12:139.
61. Hendrickx, I, Mancini, LL, Guizzardi, M, et al. Burn injury secondary to air bag deployment. J Am Acad Dermatol. 2002;46:S25.
62. Vitello, W, Kim, M, Johnson, RM. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212.
63. Ingraham, HJ, Perry, HD, Donnenfeld, ED. Air-bag keratitis [letter]. N Engl J Med. 1991;324:1599.
64. Williams, JB, Ahrenholz, DH, Solem, LD, et al. Gasoline burns: the preventable cause of thermal injury. J Burn Care Rehabil. 1990;11:446.
65. Hansbrough, JF, Zapata Sirvent, R, Dominic, W, et al. Hydrocarbon contact injuries. J Trauma. 1985;25:250.
66. Mistry, DG, Wainwright, DJ. Hydrofluoric acid burns. Am Fam Physician. 1992;45:1748.
67. Vance, MV, Curry, SC, Kunkel, DB, et al. Digital hydrofluoric acid burns: treatment with intra-arterial calcium infusion. Ann Emerg Med. 1986;15:890.
68. Rubinfeld, RS, Silbert, DI, Arentsen, JJ, et al. Ocular hydrofluoric acid burns. Am J Ophthalmol. 1992;114:420.
69. Dibbell, DG, Iverson, RE, Jones, W, et al. Hydrofluoric acid burns of the hand. J Bone Joint Surg Am. 1970;52:931–936.
70. Roberts, JR, Merigian, KM. Acute hydrofluoric acid exposure. Am J Emerg Med. 1988;7:125.
71. Pegg, SP, Siu, S, Gillett, G. Intra-arterial infusions in the treatment of hydrofluoric acid burns. Burns. 1985;11:440.
72. Lin, TM, Tsai, CC, Lin, SD. Continuous intra-arterial infusion therapy in hydrofluoric acid burns. J Occup Environ Med. 2000;42:892.
73. Graudins, A, Burns, MJ, Aaron, CK. Regional intravenous infusion of calcium gluconate for hydrofluoric acid burns of the upper extremity. Ann Emerg Med. 1997;30:604.
74. Bertolini, JC. Hydrofluoric acid: a review of toxicity. J Emerg Med. 1992;10:163.
75. Bentur, Y, Tennenbaum, S, Yaffe, Y, et al. The role of calcium gluconate in the treatment of hydrofluoric acid eye burns. Ann Emerg Med. 1993;22:1488.
76. Wang, XW, Davies, JWL, Zapata Sirvent, RL, et al. Chromic acid burns and acute chromium poisoning. Burns. 1985;11:181.
77. Konjoyan, TR. White phosphorus burns: case report and literature review. Mil Med. 1983;148:881.
78. Arnoldo, B, Klein, M, Gibran, NS. Practice guidelines for the management of electrical injuries. J Burn Care Res. 2006;25:439–447.
79. Roujeau, JC, Guillaume, JC, Fabre, JP, et al. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981-1985. Arch Dermatol. 1990;126:37–42.
80. Abe, R, Shimizu, T, Shibak, A, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand. Am J Pathol. 2003;162:1515–1520.
81. Bradley, T, Brown, RE, Kucan, JO, et al. Toxic epidermal necrolysis: a review and report of the successful use of Biobrane for early wound coverage. Ann Plast Surg. 1996;36:224.
82. French, LE, Prins, C, Toxic epidermal necrolysis. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology, 3rd ed. St. Louis, Saunders, 2012. Available at, http://www.dermtext.com/content.
83. Viard, I, Wehrli, P, Bullani, R, et al, Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulins. Science 1998;282:490–493. Available at, http://www.dermtext.com.
84. Robson, MC, Heggers, JP. Evaluation of hand frostbite blister fluid as a clue to pathogenesis. J Hand Surg [Am]. 1981;6:43–47.
85. Milner, SM, Herndon, DN. Radiation injury, vesicant burns and mass casualties. In: Herndon DN, ed. Total Burn Care. 2nd ed. Philadelphia: Saunders; 2001:481–492.
86. Glasstone, S, Dolan, PJ, The Effects of Nuclear Weapons. Prepared and published by the United States Department of Defense and the Energy Research and Development Administration, 3rd ed. 1977. [541-628].
87. Brown, RL, Greenhalgh, DG, Kagan, RJ, et al. The adequacy of limb escharotomies-fasciotomies after referral to a major burn center. J Trauma. 1994;37:916.
88. Bardakjian, VB, Kenney, JG, Edgerton, ML, et al. Pulse oximetry for vascular monitoring in burned upper extremities. J Burn Care Rehabil. 1988;9:63.

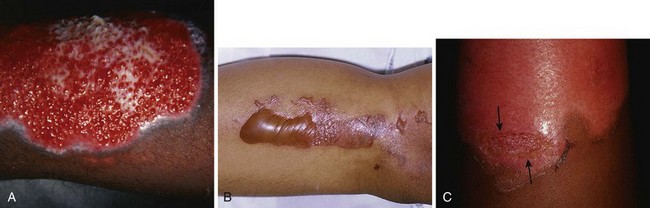
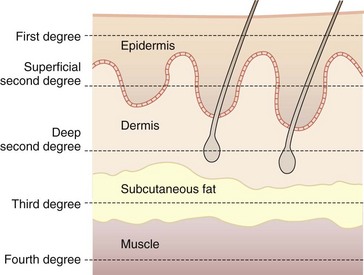
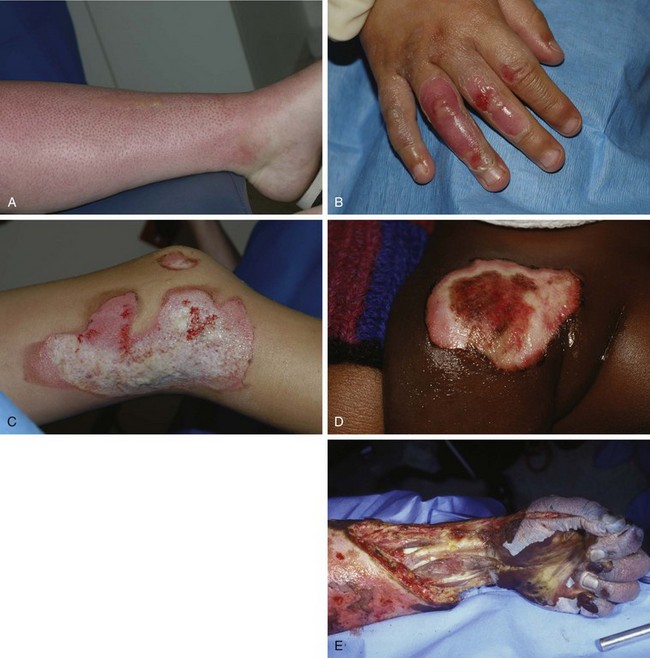
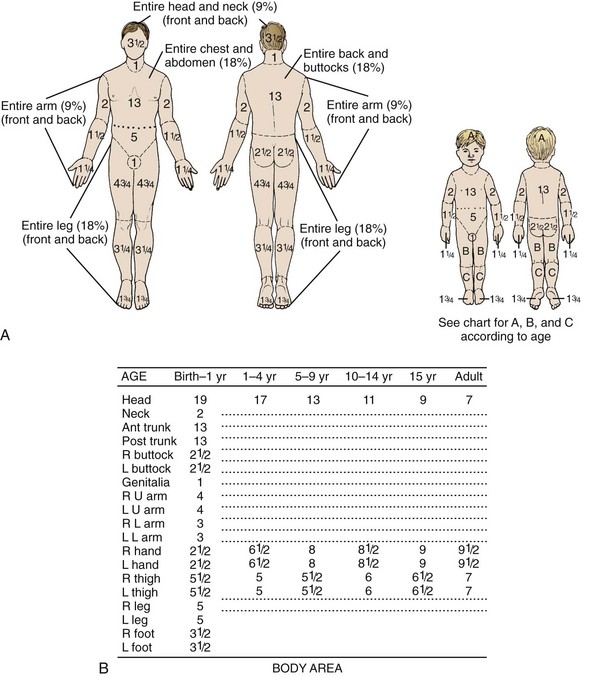
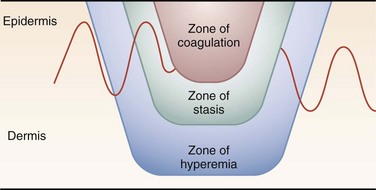
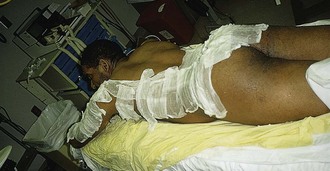
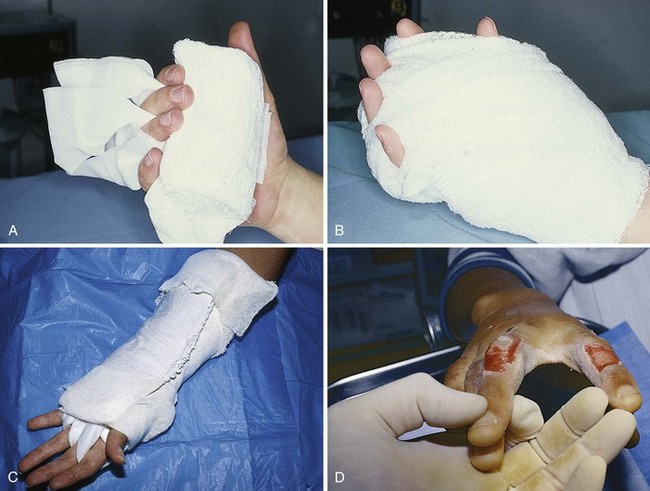
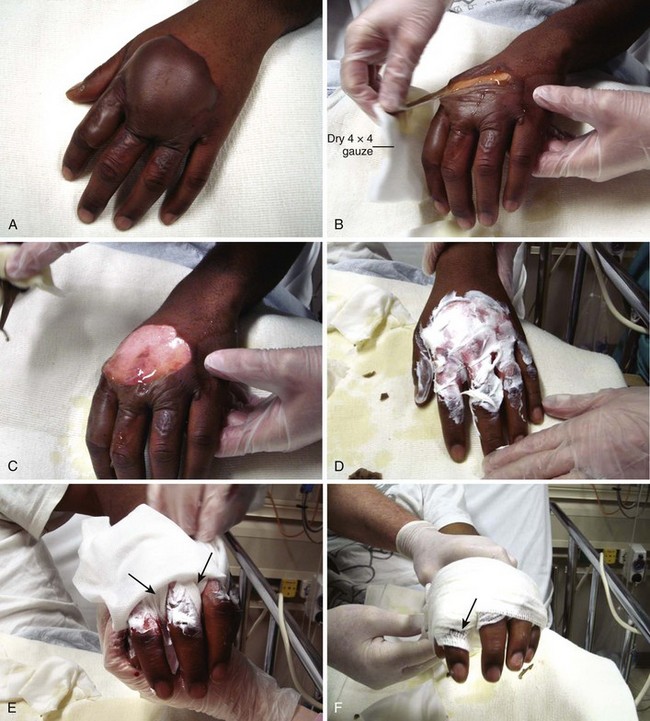
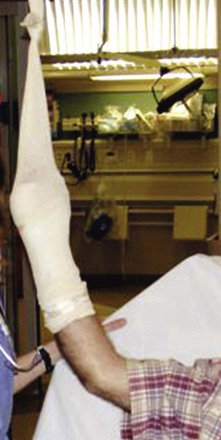
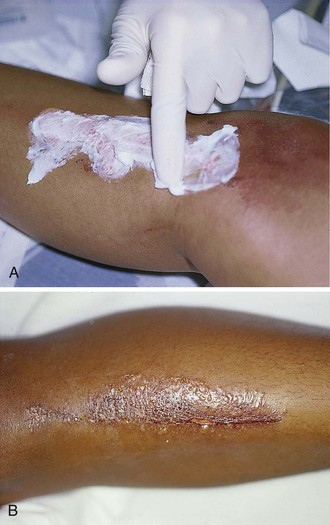
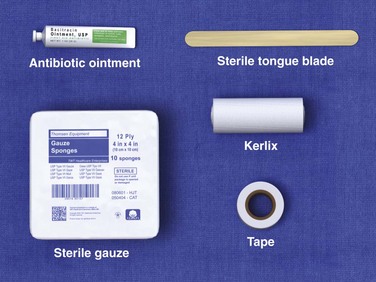
 hour before dressing change if you find dressing changes to be painful.
hour before dressing change if you find dressing changes to be painful.