Bundle Branch Reentry Tachycardia
Bundle branch reentry (BBR) ventricular tachycardia (VT), first elucidated by Guerot et al. in 1974, is a unique, fast (200 to 300 beats/min), monomorphic tachycardia associated with hemodynamic collapse, syncope, and/or cardiac arrest and caused by a macroreentry circuit involving the right and left bundle branches, an upper common pathway, and septal ventricular muscle.1–3 BBR occurs in patients who have dilated cardiomyopathy and in those with coronary artery disease, valvular heart disease, myotonic dystrophy, or even no heart disease with associated His-Purkinje system disease.4–9 The incidence is reported to be 3.5% and 6% of ventricular tachycardias in separate series and 20% in a series of patients with nonischemic cardiomyopathy alone undergoing evaluation for ablation.10–12 Likely, BBR is underrecognized.
Electrocardiographic Appearance
The morphology of the tachycardia can have a typical left bundle branch block (LBBB)13 pattern (Figure 83-1, A) or right bundle branch block (RBBB) pattern (Figure 83-1, B). Some patients have both counterclockwise and clockwise BBR, causing an LBBB and RBBB morphology, respectively.14 It is possible for LBBB morphology BBR to have several morphologies presumably as the result of extensive right bundle branch (RBB) disease in patients with dilated heart.13 Multiple monomorphic morphologies can occur, as anterograde conduction via the left anterior or left posterior fascicle can be present along with retrograde activation of the RBB.15 BBR-VT with multiple morphologies has also been described in Ebstein’s anomaly.7
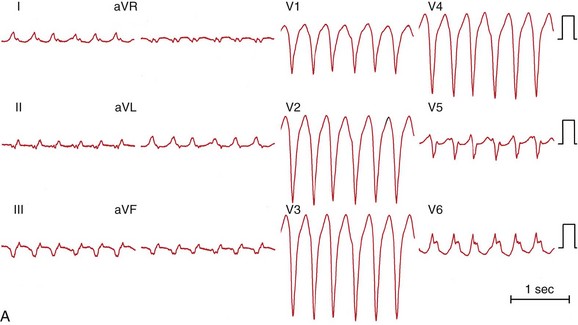
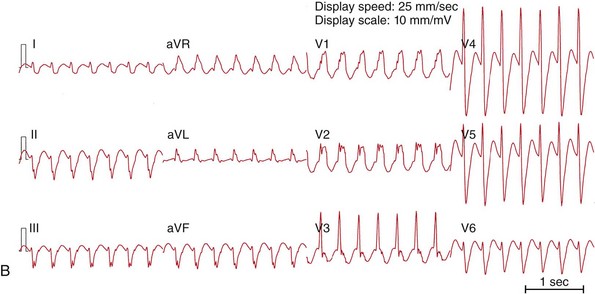
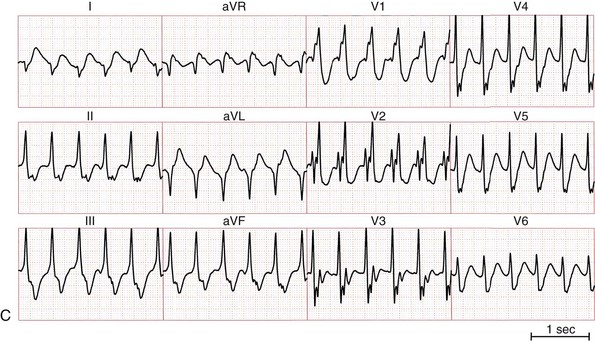
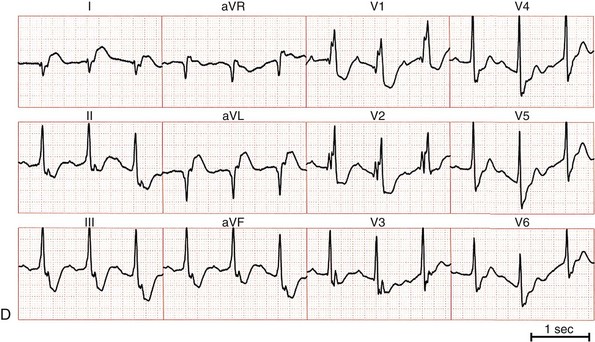
Figure 83-1 Twelve-Lead ECGs During Bundle Branch Reentry Tachycardias
A, ECG of a bundle branch reentry with left bundle branch block morphology (type A). B, ECG of a bundle branch reentry with right bundle branch block morphology and left-axis deviation (type C). C, ECG of an interfascicular reentry with right bundle branch block morphology and right-axis deviation (type B). D, ECG during sinus rhythm of the patient with interfascicular reentry in (C), showing PR prolongation, right bundle branch block, and right-axis deviation. Note that QRS morphology in tachycardia is identical to that in sinus rhythm.
BBR can present with a similar or identical QRS complex morphology to that present in normal sinus rhythm. Although the QRS complex morphology in VT can be identical to that in sinus rhythm in all 12 electrocardiographic (ECG) leads,16 not all such VTs are necessarily BBR.17 An RBBB and left hemiblock pattern is more consistent with an interfascicular tachycardia (Figure 83-1, C, D).
Mechanisms
Attempts have been made to model the His-Purkinje system and to define the reentrant circuit(s) responsible for BBR.18 Under normal conditions, sustained reentry cannot occur, but under conditions of slowed conduction in the His-Purkinje system and ventricular muscle, the path length can be increased such that sustained anatomical reentry does occur using these structures and with an excitable gap (Figure 83-2).
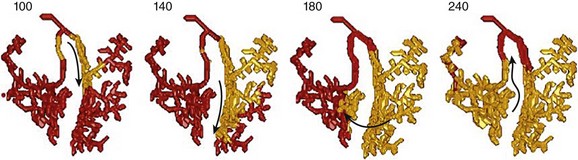
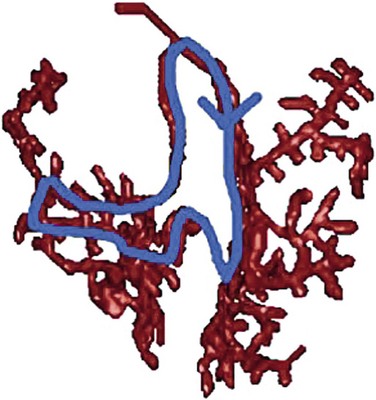
Figure 83-2 Successful Attempt to Stimulate Bundle Branch Reentry in a Model of Ventricular Conduction System
Activation sequence in the Purkinje system under simulated temporal conduction block in the right Purkinje branch. Arrows indicate the direction of wave propagation. Excitation goes from the LBB to the septum, up the RBB, toward the LBB, where it runs into the previous excitation wave. To attain sustained BBR-VT, the effective path length of the reentry circuit needs to be increased (such as slowing conduction sufficiently). (Modified from Tusscher KH, et al: Modeling of the ventricular conduction system. Prog Biophys Mol Biol 96:152-170, 2008.18)
During BBR-VT, activation proceeds anterograde via the RBB through the septum and retrograde via the LBB to activate the septal summit/His bundle and reactivate the RBB sequentially (“counterclockwise” reentry). The ECG morphology of counterclockwise BBR is a typical LBBB with an R-wave transition between leads V4 and V5. LBBB morphology BBR is the cause of 98% of episodes of BBR-VT.19 This may be due to the fact that the LBB is the preferred retrograde route of activation.20 Alternatively, activation can proceed anterograde via the LBB, through the septum, and retrograde via the RBB, creating an RBBB morphology (“clockwise” reentry).10,20 Anterograde activation via the RBB and retrograde via the left posterior fascicle (LPF) or the left anterior fascicle (LAF) alone is another possibility.
Conduction delay, critical for initiation and perpetuation of BBR, may be present in connections between the bundles and even within the His bundle itself. Anisotropic conduction in the common His bundle, potentially critical for initiation and maintenance of BBR,21 may explain the greater degree of H-V delay often noted during tachycardia than in sinus rhythm. Other mechanisms potentially explaining the observed H-V prolongation during tachycardia could be due to progressive distal Purkinje system conduction delay in diseased bundles, whose slowing is more apparent at rapid rates (phase 3 block).
BBR can occur in patients who have apparently normal His-Purkinje conduction during sinus rhythm but who have evidence of a functional conduction impairment at faster rates.22 Li et al.22 reported a series of 178 patients with VT, of whom 13 had BBR-VT. Of those 13, 6 had an H-V interval ≤55 ms during sinus rhythm (i.e., normal). In those BBR-VT patients with a normal H-V interval at baseline, the H-V interval was prolonged during BBR-VT (73 ± 18 vs. 47 ± 7 ms; P = .007). Functional His-Purkinje delay was present with rapid atrial pacing or premature extrastimuli in this group but not in those with prolonged H-V intervals in sinus rhythm who had BBR-VT. The explanation for BBR-VT in these patients may be the higher turnaround point in the BBR circuit in the proximal portion of the His bundle or in the N-H region of the AV node. Additionally, functional block initiating BBR may be perpetuated by persistent “linking.”23
BBR-VT is distinguished from interfascicular VT, in which the tachycardia depends on activation via the LPF and the LAF alone. His-bundle or RBBB activation is not necessary for the tachycardia to propagate. During interfascicular tachycardia, the morphology is an RBBB with right or left axis deviation. Right bundle reentry has been reported.24
Initiation of Tachycardia
BBR-VT has several modes of spontaneous initiation. Ventricular premature beats are often the trigger. Occasionally, premature atrial beats or atrial fibrillation25 can initiate BBR. Bradycardia-dependent LBBB initiation of BBR, without obvious intra-myocardial conduction delay, is possible spontaneously during sinus bradycardia.26 BBR has also been described in a patient with cycles of AV block and in association with bradycardia-dependent phase 4 bundle branch block.27 BBR-VT storm has been related to ventricular pacing that causes retrograde activation through the His-Purkinje system.28
In the electrophysiology laboratory, ventricular extrastimuli delivered with long-short coupling intervals (pacing train of 400 milliseconds with a delay of 600 or 800 milliseconds before the short-coupled premature coupling intervals are introduced) tend to cause unidirectional block or sufficient conduction delay in a bundle branch to initiate BBR. Long-short coupling intervals are specific for initiation of BBR.29
Atrial pacing and/or isoproterenol may be required.30 In one report, incremental atrial pacing during isoproterenol infusion initiated BBR with an RBBB pattern in four of six patients with inducible BBR-VT.31 Class IA or IC antiarrhythmic drugs may slow conduction sufficiently in one of the bundles to allow BBR to occur (and thus may be proarrhythmic for this condition32). Rarely are such drugs used to initiate BBR in the clinical context.
Diagnosis of Bundle Branch Reentry
Several criteria help in the diagnosis of BBR-VT: (1) The 12-lead ECG appearance during tachycardia is a typical LBBB or RBBB morphology. (2) A critical delay in His-Purkinje system conduction is needed to initiate tachycardia. (3) Although atrioventricular (AV) dissociation may be present, there is persistent 1 : 1 His-bundle/QRS activation with the H-V interval during the tachycardia equal to or longer than the H-V interval in sinus rhythm (and no H-V dissociation). (4) Tachycardia stops and cannot be reinitiated if conduction in one of the bundle branches is disrupted. In some instances, temporary disruption of conduction via one bundle may occur purposefully, or unintentionally, with a catheter bump.33 (5) His-bundle, RBB, and LBB activation sequences during tachycardia recorded by point-by-point mapping are consistent with the presence of bundle branch block (i.e., if there is an LBBB morphology tachycardia, activation is in the order of His bundle, RBB, and then LBB), and the activation relationship remains stable. (6) A predictable change in the V-V interval occurs after the H-H interval if rate fluctuations exist. H-H interval timing changes before timing of the V-V interval. The H-V timing remains constant. If no perturbation in the H-H interval is present, atrial pacing can advance to atrial then ventricular activation with a similar QRS complex morphology and H-V interval. (7) Entrainment with constant fusion (manifest entrainment) is present during pacing from the right ventricular apex.34 However, pacing from the right ventricular apex during BBR-VT results in a postpacing interval similar to the length of the tachycardia cycle (9 ± 11 ms), and never longer than 30 ms.35 These findings serve as evidence of macro-reentry within the His-Purkinje system.36 During resetting, when pacing from the right ventricular apex, an increasing reset response is noted (i.e., return cycle length is progressively longer), whereas recordings with respect to the H-V interval show a flat response consistent with a reentry circuit that has an excitable gap and involves the bundle branches but not the ventricular muscle.37 (8) Entrainment with concealed fusion is present during atrial pacing if AV nodal conduction allows faster pacing than is seen with tachycardia.
Activation Patterns
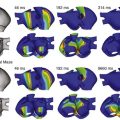
Tchou et al. described three categories of BBR-VT (Table 83-1; Figure 83-3).38 Type A and type C are the classic counter-clockwise and clockwise BBR-VTs circuits. Type B is reentry within the LBB fascicles (interfascicular reentry).
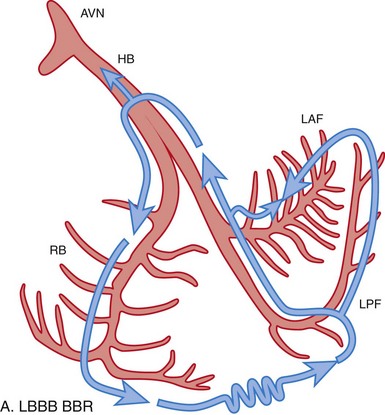
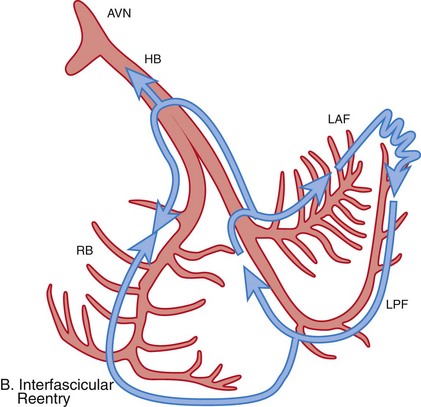
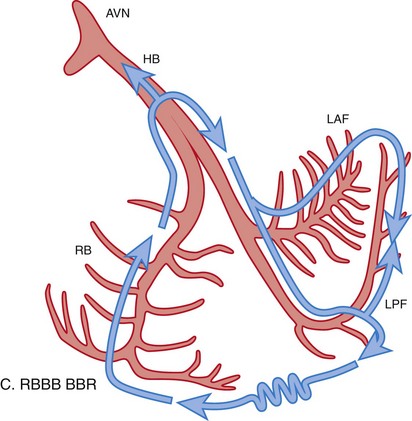
Figure 83-3 Schematic Illustrations of Reentrant Circuits for Bundle Branch Reentry and Interfascicular Reentry
A, Typical type of BBR in which retrograde conduction occurs via the left bundle (LBB) branch and anterograde conduction over the right bundle (RBB) branch. B, Interfascicular reentry with anterograde and retrograde conduction over opposing fascicles of the LBB. C, Reversal of the circuit depicted in (A). AVN, Atrioventricular node; BBR, bundle branch reentry; HB, His bundle; LAF, left anterior fascicle; LPF, left posterior fascicle; RBB, right bundle branch. (Modified from Nogami A: Purkinje-related arrhythmias. Part I: Monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 34: 624-650, 2011.46)
In types A and C BBR-VT, the onset of ventricular depolarization is preceded by His-bundle, RBB, or LBB potentials with an appropriate sequence of His-bundle->RBB>LBB activation and relatively stable H-V, RBB-V, or LBB-V intervals. Spontaneous variations in V-V intervals are preceded by similar changes in H-H/RBB-RBB/LBB-LBB intervals (Figure 83-4).
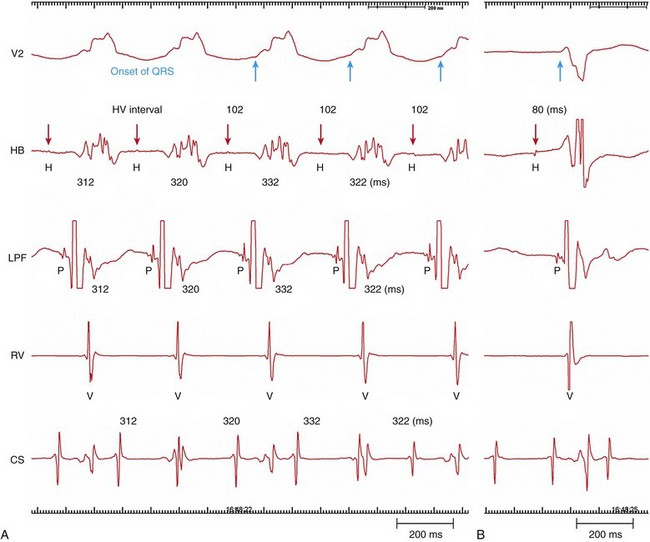
Figure 83-4 Intracardiac Recording During Bundle Branch Reentry (Type C)
A, During bundle branch reentry (BBR), each His (H) activation precedes the ventricular (V) depolarization. Changes in the H-H interval precede any changes in the V-V interval. The H-V interval during the BBR was longer than the H-V interval during sinus rhythm (B). (Modified from Machino T, et al: Three-dimensional visualization of the entire reentrant circuit. Heart Rhythm 10:459-460, 2013.39)
A characteristic feature of classical BBR-VT (types A and C) is that the H-V interval during tachycardia is longer than H-V during sinus rhythm. Prolongation of the H-V interval during tachycardia is speculated to be caused by anisotropic conduction seen in the distal His bundle at the upper turnaround point of the tachycardia circuit.21 In the interfascicular variant (type B), the His bundle is activated retrograde concurrently during anterograde conduction over the fascicle (of the LBB), giving rise to a shorter H-V than during sinus rhythm.
Recording from both sides of the septum may help in identification of the BBR mechanism. Documentation of a typical H-RBB-V-LBB (during VT with LBBB morphology) or H-LBB-V-RBB (during VT with RBBB morphology) activation sequence would further support a BBR-VT diagnosis. In addition, during BBR-VT with LBBB morphology, right ventricular excitation must precede left ventricular excitation. The opposite is true for a BBR-VT with an RBBB morphology.38 Three-dimensional electroanatomical mapping is also valuable in demonstrating the entire reentrant circuit.
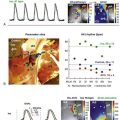
Machino et al. reported type C BBR-VT using three-dimensional electroanatomical propagation mapping (Figure 83-5).39 Sequential activation of the His-Purkinje system accounted for the entire tachycardia cycle length. Anterograde activation occurred via the LAF and the LPF, resulting in collision at the middle portion of the LAF, a bystander of the reentrant circuit.
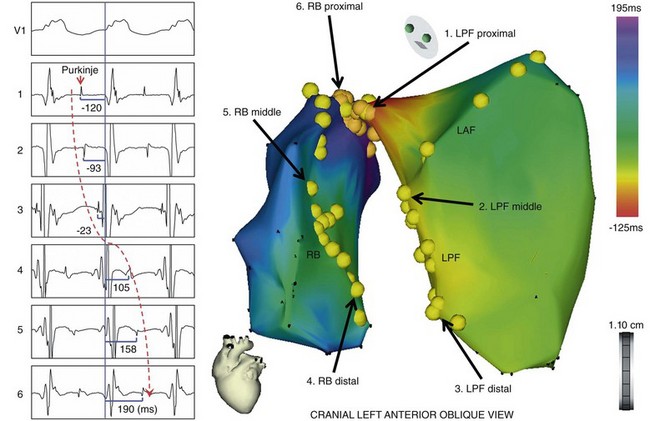
Figure 83-5 Three-Dimensional Electroanatomical Mapping During Bundle Branch Reentry
The activation map of the BBR-VT descending the left posterior fascicle (LPF) and ascending the right bundle branch (RBB), with the earliest activation displayed in red (LPF proximal) and the latest in violet (RBB proximal). The yellow dots and the numbered sites with arrows indicate fascicular potentials and local activations of the His-Purkinje system (left boxes), respectively. Sequential activations of the local His-Purkinje system accounted for the entire tachycardia cycle length. The propagation map of the BBR-VT also demonstrated that anterograde activation through the left anterior fascicle and LPF resulted in a collision at the middle portion of the left anterior fascicle—a bystander of the reentrant circuit (see the Online Supplemental Movie: doi:10.1016/j.hrthm.2011.11.001) (From Machino T, et al: Three-dimensional visualization of the entire reentrant circuit. Heart Rhythm 10:459-460, 2013.39)
Interfascicular tachycardia has been reported less commonly.3,30,40–42 In this tachycardia, one of the fascicles serves as the anterograde limb and the other as the retrograde circuit. The distal link between fascicles occurs through ventricular myocardium. The LAF is usually the anterograde limb and the LPF the retrograde limb.5,42 BBR-VT and interfascicular tachycardia may be present in the same patient,2,43 or the interfascicular tachycardia may be inducible after ablation of the right bundle branch to stop BBR.42 It may even become incessant after RBB ablation.44
Interfascicular VT usually has RBBB morphology. The orientation of the frontal plain axis is variable and may depend on the direction of the reentrant circuit. Anterograde activation over the LAF and retrograde activation through the LPF would be associated with right axis deviation and the reverse activation sequence with left axis deviation. In contrast to BBR, the H-V interval during interfascicular tachycardia is usually shorter by more than 40 milliseconds than that recorded in sinus rhythm.45 This occurs because the upper turnaround point of the circuit (the left bundle branching point) is relatively far from the His bundle activated in the retrograde direction. During interfascicular tachycardia, the LBB potential is inscribed before the His potential.45
Differential Diagnosis
VT caused by myocardial reentry needs to be excluded in every case because most patients with BBR also have the clinical substrate for developing myocardial VT. If the myocardial VT has a passive conduction into the His-Purkinje system, the morphology becomes similar to that during basal rhythm.17 With myocardial reentry, the His potential usually does not precede the QRS complex, and variations in the H-H interval follow changes in the V-V interval if there is retrograde passive activation of the His-Purkinje system. The postpacing interval after entrainment of tachycardia from the right ventricular apex may differentiate BBR from myocardial VT.3,35 The difference between the postpacing interval and the tachycardia cycle length with BBR is short (<30 ms) because the RBB-VT inserts into the ventricular apex. For myocardial VT (unless originating in the apex), the difference greatly exceeds 30 milliseconds.
The possibilities of Purkinje-related VTs,46 such as idiopathic fascicular VT,47 fascicular VT postmyocardial infarction,48,49 automatic His-Purkinje VT, focal Purkinje VT,50 AV node/His-Purkinje reentry,51 should also be considered. Intrafascicular VT has a negative or short H-V interval during tachycardia and a normal baseline QRS and H-V interval.46 As with interfascicular VT, His activation follows activation of the left fascicles, which is inconsistent with an RBBB pattern BBR. VT arising from myocardial foci rarely produces entirely typical RBBB or LBBB patterns on the surface 12-lead ECG, as is expected with BBR. In addition, His activation usually occurs late in the QRS complex and may be dissociated from the tachycardia.
Catheter Ablation
Right Bundle Branch Ablation
The technique of choice is ablation of the RBB.3,43,52 BBR-VT may be prevented by ablation of the right or left main bundle branch.4,38 Even though most patients demonstrate increased conduction system disease in the LBB, the RBB is typically the target for ablation. This is so because of the technical ease of ablation of the RBB, in contrast to the difficulties involved in ablation of the LBB. With this technique, the catheter initially is placed at the His-bundle area and then is gradually advanced toward the anterior-superior ventricular septum. To ensure catheter stability, slight clockwise torque is applied to improve contact with the RBB (Figure 83-6).
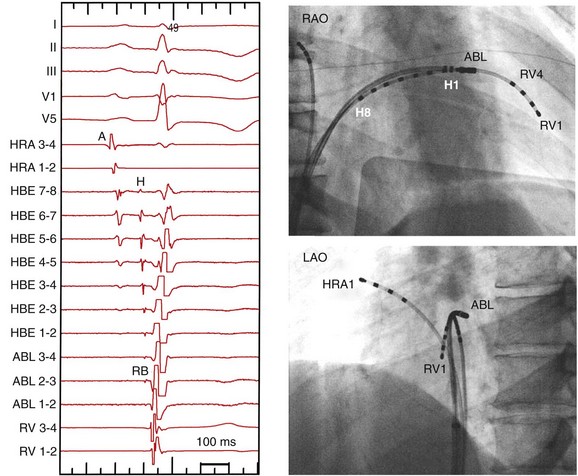
Figure 83-6 Recording of the Right Bundle Branch
Right and left anterior oblique (RAO and LAO) views of the 8-polar His and ablation (ABL) catheters are shown. His and RBB potentials are recorded continuously. The RBB recording occurs more than 10 milliseconds later than the typical His potential with a large atrial electrogram, and no atrial electrogram is recorded at the RBB recording site.
The RBB potential is identified by the following characteristics: (1) a sharp deflection inscribed 10 to 15 milliseconds after the typical His potential, and (2) the absence of an atrial electrogram on the same recording. The RBB-V interval value of less than 30 milliseconds may not be a reliable marker of RBB potential in these patients because of His-Purkinje system disease that can cause prolongation of the RBB-V conduction time (Figure 83-7, A).43,52,53 If any RBB conduction delay is present, the RBB potential may be obscured by local ventricular activation in sinus rhythm.
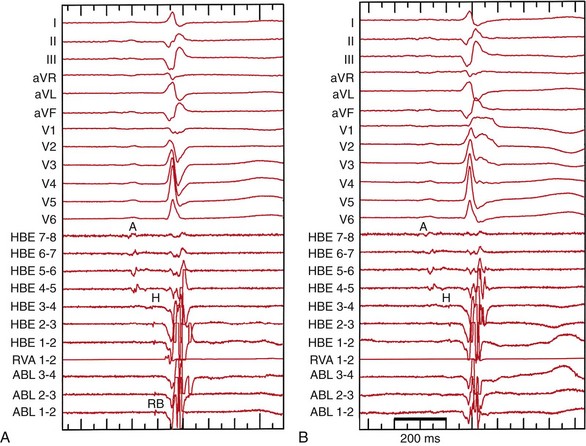
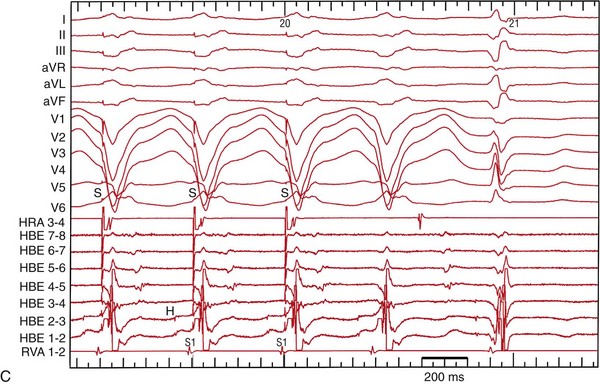
Figure 83-7 Recordings Before and After Right Bundle Ablation During Sinus Rhythm and Atrial Pacing
A, Before ablation of the right bundle branch (RBB). The RBB recording occurs more than 10 milliseconds later than the His potential, and no atrial electrogram is recorded at the RBB recording site. The RBB-V interval is 32 milliseconds. B, After ablation of the RBB, the surface ECG shows complete RBBB and the RBB potential at the ablation site has disappeared. C, Before ablation, burst atrial pacing at a cycle length of 400 ms exhibits a complete LBBB configuration that is identical to clinical tachycardia. D, After ablation of the RBB, burst atrial pacing shows 2 : 1 H-V block.
Although it is important to obtain a stable catheter position to minimize the risk of AV block, in some cases, the RBB potential may not be identifiable in sinus rhythm and may be evident only during retrograde conduction during sustained BBR-VT or BBR echo beats. If the RBB is not well recorded at the basilar septum, mapping of the apical course of the RBB may be effective. Because of the superficial nature of the His-Purkinje system in most patients, standard 4-mm-tip RF ablation catheters are sufficient.3,42
The need for cooled ablation systems has not been described. Energy settings of 20 to 60 W with target temperatures of 60° C are reported.3,42 In sinus rhythm, complete RBB or LBB develops with successful ablation (Figure 83-7, B), although QRS changes may be subtle in patients with preexisting conduction abnormalities. Electrical axis changes may be the only manifestation of fascicular ablation. Elimination of retrograde V-H conduction has been used as a marker of successful ablation.3
After successful RBB ablation, programmed electrical stimulation is performed at baseline and on isoproterenol. This should include pacing from two different sites at two different cycle lengths and with the use of extrastimuli with a short-long-short sequence. In patients with complete anterograde block in the LBB, RBB ablation necessitates permanent pacing. The reported incidence of clinically significant conduction system impairment requiring implantation of a permanent pacemaker varies from 0% to 30%.3,6,9,22,29,42,43 In patients with His-Purkinje disease, it is important to ascertain the need for permanent pacing by atrial stimulation (Figure 83-7, C, D). It is advisable to stress the His-Purkinje system with intravenous class IA or IC antiarrhythmic drugs and to ensure that the anterograde conduction is preserved.
Left Bundle Branch Ablation
For many patients with BBR-VT who have LBBB during sinus rhythm, anterograde slow conduction over the LBB is present,39 or the LBB is activated retrograde (transseptal).5 In patients with a complete LBBB pattern during sinus rhythm, anterograde ventricular activation occurs solely via the RBB. These latter patients are at risk of developing complete AV block with RBB ablation. To avoid this potential complication, the LBB might be targeted in such patients with LBBB during sinus rhythm, and BBR-VT as an alternative approach (Figure 83-8).5 However, LBB ablation is technically difficult because it is a broad structure and usually requires arterial access; a catheter placed at the left ventricular septum may be unstable when the transseptal approach is used, even with a deflectable sheath. The risk for procedural complications (e.g., damage to the femoral, coronary and carotid arteries) is increased.
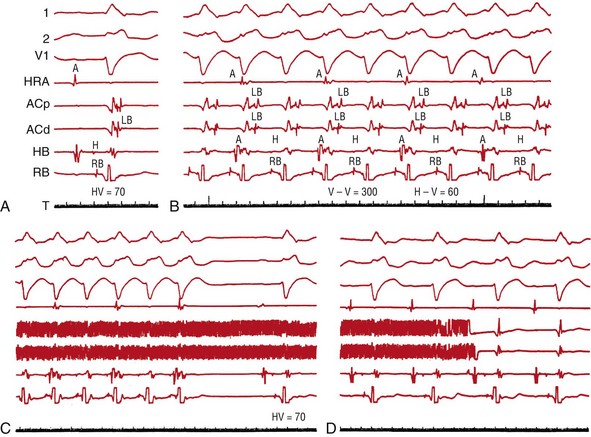
Figure 83-8 Radiofrequency Catheter Ablation of the Left Bundle Branch in a Patient With Left Bundle Branch Block During Sinus Rhythm and a Bundle Branch Reentry Tachycardia
A, Retrograde (transseptal) activation of the LBB is recorded during sinus rhythm. B, During an induced BBR-VT, the activation propagates down the right bundle branch (RBB) and up the LBB. The sequence of activation of the left bundle recordings during sinus rhythm and the tachycardia is identical. C, Catheter ablation of the LBB terminated the tachycardia. D, After the ablation, QRS duration and configuration remain unchanged, and the left bundle potential is no longer recordable. (From Blanck Z, et al: Catheter ablation of the left bundle branch for the treatment of sustained bundle branch reentrant ventricular tachycardia. J Cardiovasc Electrophysiol 6:40-43, 1995.5)
The LBB arises as a broad band of fibers directed inferiorly from the bundle of His. The left main bundle is typically 1 to 3 cm long and 1 cm wide but shows great individual variation. For LBB ablation, the ablation catheter is advanced across the aortic valve and is positioned in the left ventricle along the interventricular septum. The LBB potential is recorded beneath the aortic valve, typically 1 to 1.5 cm inferior to the optimal His-bundle recording site.38,52 The LBB potential is identified by a potential-to-ventricular electrogram interval of ≤20 ms and an A/V electrogram ratio of ≤1 : 10.38 Delivery of RF energy at this level results in complete LBBB and successfully interrupts the tachycardia circuit. However, LBB ablation usually requires more extensive ablation to transect this broad structure. After LBB ablation, routine follow-up is recommended even if the anterograde conduction via the RBB appears preserved. The long-term risk of complete heart block and progressive heart failure is unclear.
Ablation of Interfascicular Tachycardia
In interfascicular reentry, an RBB ablation will not cure the tachycardia because the RBB is a bystander. Similarly, ablation of the main LBB would not be expected to terminate the tachycardia because the circuit is distal to this point. Catheter ablation of the LAF or the LPF will result in termination of the tachycardia.41,42 Ablation of one of the left-sided fascicles would not be expected to prevent BBR-VT in a patient with both interfascicular VT and BBR-VT. In this situation, both of the left-sided fascicles of the RBB and one of the left fascicles must be ablated.
Clinical Outcomes
In the two largest series reported, acute success rates for BBR-VT and interfascicular reentry were 100%.3,42 After ablation, BBR-VT recurrence is uncommon but has not been thoroughly documented with follow-up testing.3,42 Despite the success of ablation of the RBB branch in eliminating BBR-VT, patients with cardiomyopathy and heart failure continue to have a high mortality rate. Despite the impressive success of ablation of BBR-VT, progressive heart failure is a common cause of death.3,9,10,19,22,43,52,53 Furthermore, patients with cardiomyopathy remain at high risk for sudden cardiac death. A small risk of progressive AV block is present as well.
Therefore, after ablation, the need for a permanent pacemaker or an implantable cardioverter-defibrillator (ICD), with or without cardiac resynchronization capabilities, should be considered based on the status of the residual conduction system and the severity of the underlying structural heart disease. Furthermore, VT of myocardial origin may be induced in 36% to 60% of patients after successful ablation of one of the limbs of the BBR reentrant circuit.19,42 Cardiac resynchronization therapy should be considered based on standard recommendations even after ablation is performed for patients with severe left ventricular dysfunction and an intraventricular conduction disturbance.
The management strategy for patients with BBR-VT and preserved left ventricular function who have no other risk markers for sudden death is less clear. Very limited data suggest that these patients have a favorable long-term prognosis after successful ablation of the RBB to cure BBR-VT.3,4,43 However, they still have long-term risks of complete heart block and progressive heart failure when undergoing LBB ablation to cure BBR-VT. After ablation, patients with Mobitz II second-degree or third-degree AV block at physiologic heart rates or a markedly prolonged H-V interval (>100 ms) but with preserved ventricular function and no inducible tachycardia should be considered for a dual-chamber pacemaker. Patients with myotonic dystrophy should be considered for prophylactic permanent pacemaker (or ICD) implantation because of the progressive nature of their conduction system disease.6
Complications
The most common complication of the ablation of BBR-VT is high-grade AV block, which is reported to occur in 0% to 30% of patients after a successful ablation.3,6,9,22,29,42,43 In most of these patients, the RBB was ablated to cure BBR-VT. In a series of 20 patients with BBR-VT, 15 had permanent pacemakers or ICDs at the time of the ablation, and two of the remaining five patients had indications for ICD implantation after the ablation as the result of myocardial VT.42 Only two patients underwent pacemaker implantation for impaired AV conduction. Incessant interfascicular tachycardia after successful RBB ablation for the treatment of BBR-VT has been reported.44 The long-term risk of complete heart block and progressive heart failure after LBB ablation remains uncertain.
References
1. Guerot, C, Valere, PE, Castillo-Fenoy, A, et al. [Tachycardia by branch-to-branch reentry]. Arch Mal Coeur Vaiss. 1974; 67:1–11.
2. Akhtar, M, Damato, AN, Batsford, WP, et al. Demonstration of re-entry within the His-Purkinje system in man. Circulation. 1974; 50:1150–1162.
3. Blanck, Z, Dhala, A, Deshpande, S, et al. Bundle branch reentrant ventricular tachycardia: Cumulative experience in 48 patients. J Cardiovasc Electrophysiol. 1993; 4:253–262.
4. Blanck, Z, Jazayeri, M, Dhala, A, et al. Bundle branch reentry: A mechanism of ventricular tachycardia in the absence of myocardial or valvular dysfunction. J Am Coll Cardiol. 1993; 22:1718–1722.
5. Blanck, Z, Deshpande, S, Jazayeri, MR, et al. Catheter ablation of the left bundle branch for the treatment of sustained bundle branch reentrant ventricular tachycardia. J Cardiovasc Electrophysiol. 1995; 6:40–43.
6. Merino, JL, Carmona, JR, Fernandez-Lozano, I, et al. Mechanisms of sustained ventricular tachycardia in myotonic dystrophy: Implications for catheter ablation. Circulation. 1998; 98:541–546.
7. Andress, JD, Vander Salm, TJ, Huang, SK. Bidirectional bundle branch reentry tachycardia associated with Ebstein’s anomaly: Cured by extensive cryoablation of the right bundle branch. Pacing Clin Electrophysiol. 1991; 14:1639–1647.
8. Eckart, RE, Hruczkowski, TW, Tedrow, UB, et al. Sustained ventricular tachycardia associated with corrective valve surgery. Circulation. 2007; 116:2005–2011.
9. Narasimhan, C, Jazayeri, MR, Sra, J, et al. Ventricular tachycardia in valvular heart disease: Facilitation of sustained bundle-branch reentry by valve surgery. Circulation. 1997; 96:4307–4313.
10. Caceres, J, Jazayeri, M, McKinnie, J, et al. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation. 1989; 79:256–270.
11. Delacretaz, E, Stevenson, WG, Ellison, KE, et al. Mapping and radiofrequency catheter ablation of the three types of sustained monomorphic ventricular tachycardia in nonischemic heart disease. J Cardiovasc Electrophysiol. 2000; 11:11–17.
12. Cantillon, DJ, Bianco, C, Wazni, OM, et al. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm. 2012; 9:859–864.
13. Wang, CW, Sterba, R, Tchou, P. Bundle branch reentry ventricular tachycardia with two distinct left bundle branch block morphologies. J Cardiovasc Electrophysiol. 1997; 8:688–693.
14. Wang, PJ, Friedman, PL. “Clockwise” and “counterclockwise” bundle branch reentry as a mechanism for sustained ventricular tachycardia masquerading as supraventricular tachycardia. Pacing Clin Electrophysiol. 1989; 12:1426–1432.
15. Enjoji, Y, Mizobuchi, M, Shibata, K, et al. Bundle brunch reentrant ventricular tachycardia with two distinct conduction patterns in a patient with complete right bundle branch block. Pacing Clin Electrophysiol. 2006; 29:1438–1441.
16. Olshansky, B. Ventricular tachycardia masquerading as supraventricular tachycardia: A wolf in sheep’s clothing. J Electrocardiol. 1988; 21:377–384.
17. Guo, H, Hecker, S, Levy, S, et al. Ventricular tachycardia with QRS configuration similar to that in sinus rhythm and a myocardial origin: Differential diagnosis with bundle branch reentry. Europace. 2001; 3:115–123.
18. Tusscher, KH, Panfilov, AV. Modelling of the ventricular conduction system. Prog Biophys Mol Biol. 2008; 96:152–170.
19. Blanck, Z, Akhtar, M. Ventricular tachycardia due to sustained bundle branch reentry: Diagnostic and therapeutic considerations. Clin Cardiol. 1993; 16:619–622.
20. Mehdirad, AA, Keim, S, Rist, K, et al. Asymmetry of retrograde conduction and reentry within the His-Purkinje system: A comparative analysis of left and right ventricular stimulation. J Am Coll Cardiol. 1994; 24:177–184.
21. Fisher, JD. Bundle branch reentry tachycardia: Why is the HV interval often longer than in sinus rhythm? The critical role of anisotropic conduction. J Interv Card Electrophysiol. 2001; 5:173–176.
22. Li, YG, Gronefeld, G, Israel, C, et al. Bundle branch reentrant tachycardia in patients with apparent normal His-Purkinje conduction: The role of functional conduction impairment. J Cardiovasc Electrophysiol. 2002; 13:1233–1239.
23. Lehmann, MH, Denker, S, Mahmud, R, et al. Linking: A dynamic electrophysiologic phenomenon in macroreentry circuits. Circulation. 1985; 71:254–265.
24. Littmann, L, Tenczer, J, Svenson, RH. Demonstration of reentry within the right bundle branch in man. Pacing Clin Electrophysiol. 1984; 7:861–866.
25. Blanck, Z, Jazayeri, M, Akhtar, M. Facilitation of sustained bundle branch reentry by atrial fibrillation. J Cardiovasc Electrophysiol. 1996; 7:348–352.
26. Morgera, T, Zecchin, M, Camerini, F. [Bundle-branch reentry ventricular tachycardia induced by sinus beat]. G Ital Cardiol. 1996; 26:1295–1301.
27. Fedgchin, B, Pavri, BB, Greenspon, AJ, et al. Unique self-perpetuating cycle of atrioventricular block and phase IV bundle branch block in a patient with bundle branch reentrant tachycardia. Heart Rhythm. 2004; 1:493–496.
28. Reithmann, C, Hahnefeld, A, Oversohl, N, et al. Reinitiation of ventricular macroreentry within the His-Purkinje system by back-up ventricular pacing: A mechanism of ventricular tachycardia storm. Pacing Clin Electrophysiol. 2007; 30:225–235.
29. Akhtar, M, Denker, S, Lehmann, MH, et al. Macro-reentry within the His Purkinje system. Pacing Clin Electrophysiol. 1983; 6:1010–1028.
30. Simons, GR, Sorrentino, RA, Zimerman, LI, et al. Bundle branch reentry tachycardia and possible sustained interfascicular reentry tachycardia with a shared unusual induction pattern. J Cardiovasc Electrophysiol. 1996; 7:44–50.
31. Mizusawa, Y, Sakurada, H, Nishizaki, M, et al. Characteristics of bundle branch reentrant ventricular tachycardia with a right bundle branch block configuration: Feasibility of atrial pacing. Europace. 2009; 11:1208–1213.
32. Chalvidan, T, Cellarier, G, Deharo, JC, et al. His-Purkinje system reentry as a proarrhythmic effect of flecainide. Pacing Clin Electrophysiol. 2000; 23:530–533.
33. Gossinger, HD, Siostrzonek, P, Wagner, L, et al. Inadvertent catheter-induced right bundle branch block in a patient with preexistent left bundle branch block and recurrent macroreentrant ventricular tachycardia. Pacing Clin Electrophysiol. 1989; 12:1857–1862.
34. Merino, JL, Peinado, R, Fernandez-Lozano, I, et al. Transient entrainment of bundle-branch reentry by atrial and ventricular stimulation: Elucidation of the tachycardia mechanism through analysis of the surface ECG. Circulation. 1999; 100:1784–1790.
35. Merino, JL, Peinado, R, Fernandez-Lozano, I, et al. Bundle-branch reentry and the postpacing interval after entrainment by right ventricular apex stimulation: A new approach to elucidate the mechanism of wide-QRS-complex tachycardia with atrioventricular dissociation. Circulation. 2001; 103:1102–1108.
36. Kitazawa, H, Washizuka, T, Uchiyama, H, et al. Fusion with postpaced return cycle identical to tachycardia cycle length during transient entrainment of ventricular tachycardia and its implications. Jpn Heart J. 1997; 38:369–378.
37. Sarter, BH, Schwartzman, D, Callans, DJ, et al. Bundle branch reentry ventricular tachycardia: An investigation of the circuit with resetting. J Cardiovasc Electrophysiol. 1996; 7:1082–1085.
38. Tchou, P, Mehdirad, AA. Bundle branch reentry ventricular tachycardia. Pacing Clin Electrophysiol. 1995; 18:1427–1437.
39. Machino, T, Tada, H, Sekiguchi, Y, et al. Three-dimensional visualization of the entire reentrant circuit of bundle branch reentrant tachycardia. Heart Rhythm. 2013; 10:459–460.
40. Berger, RD, Orias, D, Kasper, EK, et al. Catheter ablation of coexistent bundle branch and interfascicular reentrant ventricular tachycardias. J Cardiovasc Electrophysiol. 1996; 7:341–347.
41. Crijns, HJ, Smeets, JL, Rodriguez, LM, et al. Cure of interfascicular reentrant ventricular tachycardia by ablation of the anterior fascicle of the left bundle branch. J Cardiovasc Electrophysiol. 1995; 6:486–492.
42. Lopera, G, Stevenson, WG, Soejima, K, et al. Identification and ablation of three types of ventricular tachycardia involving the His-Purkinje system in patients with heart disease. J Cardiovasc Electrophysiol. 2004; 15:52–58.
43. Cohen, TJ, Chien, WW, Lurie, KG, et al. Radiofrequency catheter ablation for treatment of bundle branch reentrant ventricular tachycardia: Results and long-term follow-up. J Am Coll Cardiol. 1991; 18:1767–1773.
44. Blanck, Z, Sra, J, Akhtar, M. Incessant interfascicular reentrant ventricular tachycardia as a result of catheter ablation of the right bundle branch: Case report and review of the literature. J Cardiovasc Electrophysiol. 2009; 20:1279–1283.
45. Crijns, HJ, Kingma, JH, Gosselink, AT, et al. Comparison in the same patient of aberrant conduction and bundle branch reentry after dofetilide, a new selective class III antiarrhythmic agent. Pacing Clin Electrophysiol. 1993; 16:1006–1016.
46. Nogami, A. Purkinje-related arrhythmias part I: Monomorphic ventricular tachycardias. Pacing Clin Electrophysiol. 2011; 34:624–650.
47. Nogami, A. Idiopathic left ventricular tachycardia: Assessment and treatment. Card Electrophysiol Rev. 2002; 6:448–457.
48. Morishima, I, Nogami, A, Tsuboi, H, et al. Verapamil-sensitive left anterior fascicular ventricular tachycardia associated with a healed myocardial infarction: Changes in the delayed Purkinje potential during sinus rhythm. J Interv Card Electrophysiol. 2008; 22:233–237.
49. Hayashi, M, Kobayashi, Y, Iwasaki, YK, et al. Novel mechanism of postinfarction ventricular tachycardia originating in surviving left posterior Purkinje fibers. Heart Rhythm. 2006; 3:908–918.
50. Metzner, A, Ouyang, F, Wissner, E, et al. Monomorphic and polymorphic ventricular tachycardias arising from the His-Purkinje system: What do we know? Future Cardiol. 2011; 7:835–846.
51. Markowitz, SM, Stein, KM, Engelstein, ED, et al. AV nodal-His-Purkinje reentry: A novel form of tachycardia. J Cardiovasc Electrophysiol. 1995; 6:400–409.
52. Mehdirad, AA, Keim, S, Rist, K, et al. Long-term clinical outcome of right bundle branch radiofrequency catheter ablation for treatment of bundle branch reentrant ventricular tachycardia. Pacing Clin Electrophysiol. 1995; 18:2135–2143.
53. Tchou, P, Jazayeri, M, Denker, S, et al. Transcatheter electrical ablation of right bundle branch: A method of treating macroreentrant ventricular tachycardia attributed to bundle branch reentry. Circulation. 1988; 78:246–257.