CHAPTER 57 Breast reconstruction
Physical evaluation
• Cancer stage, status, and planned adjuvant therapy.
• Functional status (ambulation, weight, BMI).
• Medical history: specifically (diabetes, vascular disease and smoking) and other co-morbidities.
• Quantity and quality of remaining tissue (laxity, thickness, and condition of pectoralis major and serratus anterior muscles).
• Body and breast morphology (breast dimensional analysis).
• Size of opposite breast/plan of opposite breast (prophylactic mastectomy).
• Availability of flap donor sites (evaluating scars, i.e. proximity of axillary dissection).
Anatomy
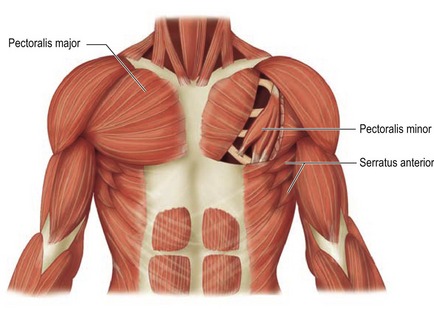
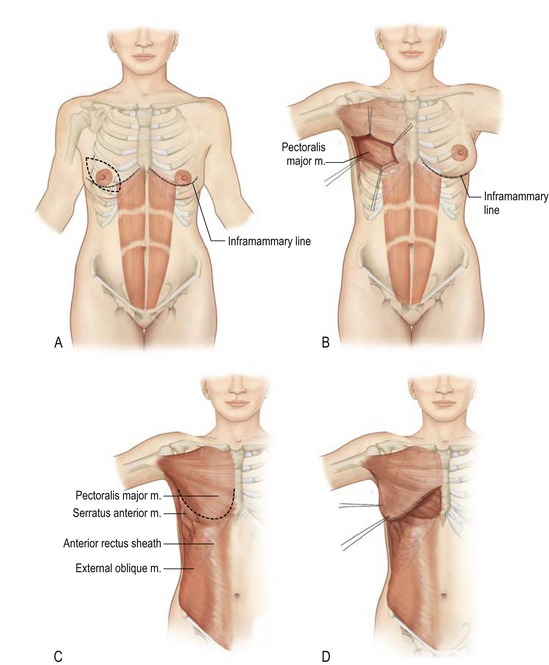
The breast is composed of both fatty tissue and glandular milk-producing tissues, and with age, the amount of fatty tissue increases as the glandular tissue diminishes. The soft tissues are supported by Cooper’s ligaments. They originate from the deep fascia and attach to the dermis and are considered to be the suspensory ligaments of the breast. The primary blood supply to the breast comes from the perforating branches of the internal mammary artery and all secondary blood supply come from the lateral thoracic, pectoral branches and the highest thoracic arteries. In addition lateral branches form the third, fourth and fifth posterior intercostal arteries. Sensory innervation of the breast is mainly derived from the anterolateral and anteromedial branches of thoracic intercostal nerves T3–T5. Supraclavicular nerves from the lower fibers of the cervical plexus also provide innervation to the upper and lateral portions of the breast. This overlap is thought to make the upper outer quadrant of the breast as the most sensitive portion. The lateral cutaneous branch of T4 is believed to be the primary innervation to the nipple. The breast lies over the musculature that encases the chest wall. The muscles involved include the pectoralis major, serratus anterior, external oblique, and rectus abdominus fascia. These muscles are very important both in aesthetic and reconstructive breast surgery since they provide additional soft tissue coverage.
Pectoralis major
The pectoralis major muscle originates from the medial clavicle and lateral sternum and inserts on the humerus (Fig. 57.1). The major blood supply is thoracoacromial artery in addition to the intercostal perforators. The medial and lateral anterior thoracic nerves provide innervation for the muscle. The action of the pectoralis major is to flex, adduct, and rotate the arm medially. This muscle becomes important when additional soft tissue coverage is needed for an implant both in an aesthetic as well as a reconstructive setting.
Serratus anterior
The serratus anterior muscle runs along the anterolateral chest wall. Its origin is the outer surface of the upper borders of the first through eighth ribs and inserts on the deep surface of the scapula (see Fig. 57.1). Its vascular supply is derived equally from the lateral thoracic artery and branches from the thoracodorsal artery. The long thoracic nerve serves to innervate the serratus anterior, which acts to rotate the scapula, raising the point of the shoulder and drawing the scapula forward toward the body. To completely cover the implant with muscle in reconstructive surgery, often the serratus anterior must be elevated sharply to obtain the necessary coverage.
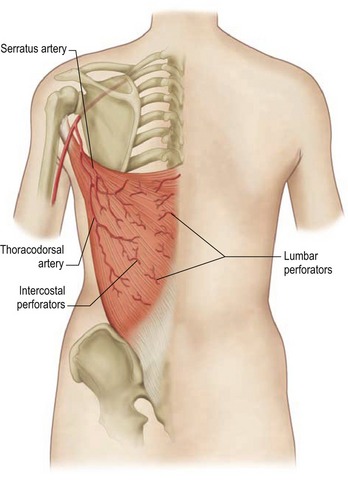
Latissimus dorsi
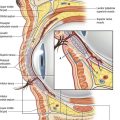
The latissimus dorsi muscle, which is a mirror image of the pectoralis major, arises from the lower thoracic vertebrae, the lumbar and sacral spinal processes, and the iliac crest. Its large, flat muscle belly converges into a flat tendon that inserts into the lesser tubercle of the humerus. The thoracodorsal artery provides the dominant vascular supply to the flap (Fig. 57.2). The subscapular artery originates from the axillary artery, gives off the circumflex scapular artery, and terminates in the thoracodorsal artery. This vessel, located in the posterior aspect of the axilla, gives off one or two serratus branches before entering the anterior undersurface of the latissimus dorsi muscle. The thoracodorsal artery is accompanied on its course by the thoracodorsal nerve and veins. The structures can be injured during an aggressive axillary dissection and therefore assessment of the pedicle in these patients is important in the preoperative planning.
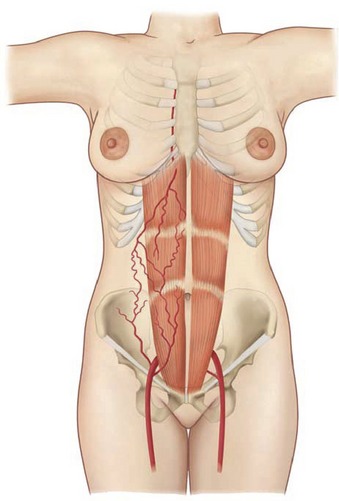
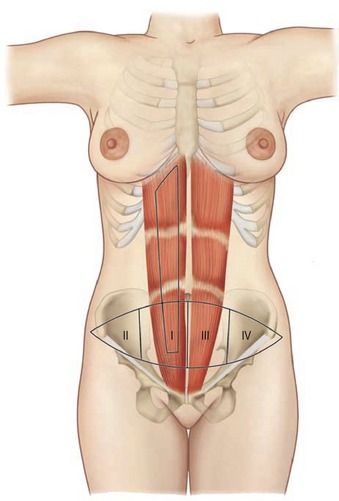
Rectus abdominus
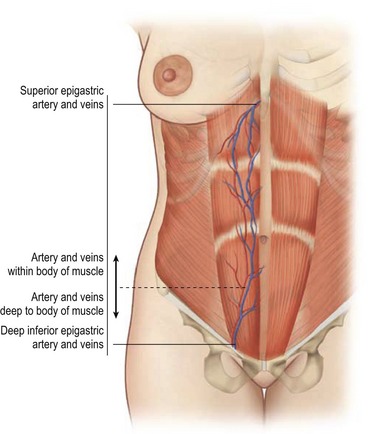
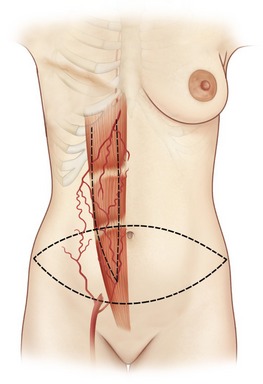
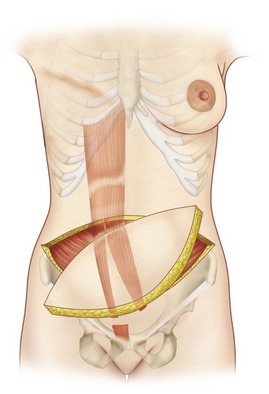
The paired rectus abdominis muscles originate from the cartilage of the sixth, seventh, and eighth ribs and insert into the pubic tubercle and pubic crest (Fig. 57.3). The blood supply comes from the superior epigastric artery above and deep inferior epigastric artery below (Fig. 57.4). Within the muscle these main vascular sources may not have direct anastomoses with each other under normal physiologic conditions. Although the deep inferior epigastric system has been shown to be the primary vascular pedicle for this skin territory, the superior epigastric artery and vein are able to carry the lower abdominal soft tissue and comprise the vascular basis for the transverse rectus abdominis flap (Fig. 57.5). Additional blood supply is from the posterior perforating vessels accompanying the eighth through twelfth sensory and motor nerves. The musculocutaneous perforators that supply the flap are centered in the perumbilical area and emerge through the medial half of the rectus muscle to supply the subdermal plexus superficial to it (Fig. 57.6). Patient selection is important for this operation. Patients with an obese, pendulous abdominal panniculus, diabetics, and smokers carry a much higher risk of flap loss.
Technical steps
The indications for breast reconstruction have been liberalized as a result of increasing experience with the various procedures and recognition of the beneficial psychological effects of breast reconstruction. The most frequent reasons given for the reconstruction include: (1) to get rid of the need for an external breast prosthesis; (2) to be able to wear many different types of clothing; (3) to regain femininity; and (4) to feel whole again. The major reasons for not having reconstruction were a fear of complications and the perception of being too old for the procedure. Not surprisingly, the reconstructive cohort was the younger cohort. Clearly age and the lack of information may be significant factors in women’s reluctance to undergo breast reconstruction. The authors urge improved patient education on the risks and benefits of post-mastectomy reconstruction.
The current surgical options for post-mastectomy/lumpectomy reconstruction are as follows:
• Temporary skin expander subsequently exchanged for a permanent implant.
• Permanent expander implant requiring only valve removal.
• Permanent implant reconstruction.
• Latissimus dorsi musculocutaneous flap with implant or expander.
• Autogenous latissimus dorsi musculocutaneous flap.
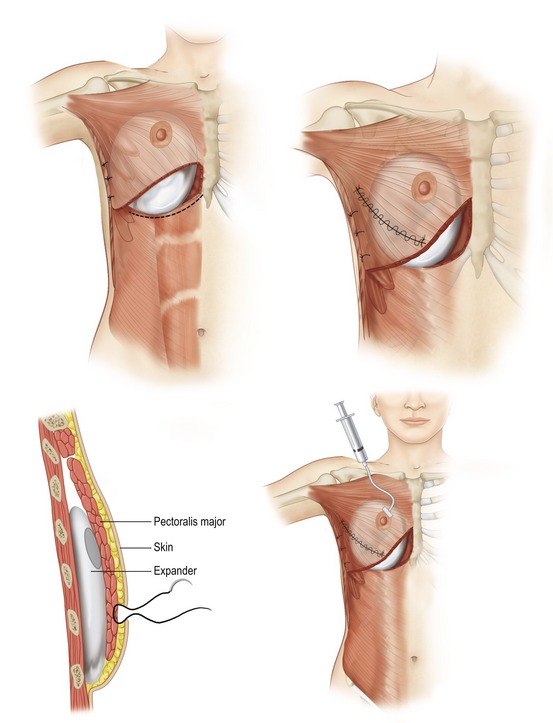
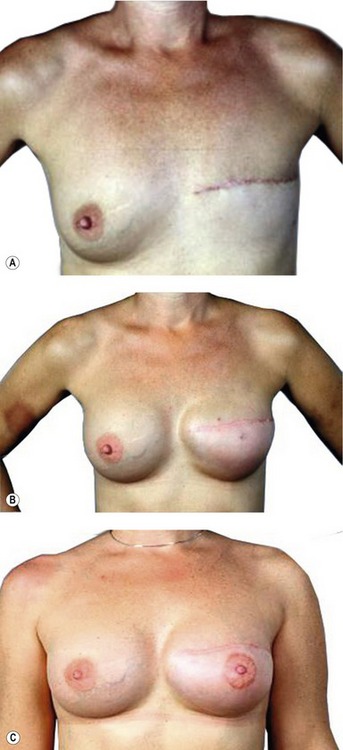
Expander/implant reconstruction
Simple placement of an implant beneath the soft tissues of the chest can provide a breast mound of acceptable shape and volume in women with small to moderate sized breasts. By placement of the implant beneath the muscles of the chest wall, an additional layer of healthy tissue is interposed between implant and the mastectomy flaps. This serves to soften the contours of the reconstructed breast and decrease the incidence of complications. When planning a submuscular reconstruction, the inframammary fold on the contralateral side is marked and the mark is transposed to the side of the reconstruction. A second mark is made approximately 2 cm below the first mark, which becomes the lower extent of the pocket dissection. If the submuscular pocket is not made lower initially, the implant generally ends up too high. A true submuscular pocket consists of pocket being dissected deep to the pectoralis major, serratus anterior laterally to the level of the anterior axillary line and the rectus abdominis fascia (Fig. 57.7).
When the total muscle coverage of the prosthesis is not possible, the use of allogenic tissue supplements avoids the problems of autogenous tissue coverage and provides camouflage, thus decreasing rippling, reinforcing the capsulectomy or capsulotomy, and increasing soft tissue padding (Fig. 57.8).
The use of acellular dermal matrix (ADM) in breast reconstruction is of particular interest to plastic surgeons, especially in patients at high-risk for post-mastectomy complications. The rising demand has spurred tremendous growth in the number of available ADMs. Published research regarding the use and efficacy of acellular dermal matrices in immediate breast reconstruction is growing but hasn’t kept pace with the market explosion. The many features and indications of all ADMs can confound the decision making process for surgeons who want to incorporate ADMs in their treatment armamentarium. From our extensive experience using multiple products it has become clear to us that the use of ADM in breast reconstruction has several advantages. There is less rippling due to additional soft tissue coverage that is achieved with ADMs. In addition, a controlled pocket can be created with complete device coverage and full control of the inframammary fold position. From our revisionary breast surgery data we have found that ADMs play an important role in the management of capsular contracture. Changing the implant interface environment is going to be one of the critical points in ameliorating capsular contracture.
Following complete coverage of expander with ADM or autologous tissue, intra-operative fill is enough to flatten the base of the expander, remove any wrinkles, and in general gain as much ground as possible without putting undue pressure on skin flaps or wound closure. Isotonic saline is used to fill the expander (Fig. 57.9). Percutaneous expansion is begun one to two weeks postoperatively or as soon as wound appears ready. Expansion can be done every week based on tissue and patient tolerance. Although there is a variation in injecting volumes, the average is around 50–100 mL per expansion. The expander is usually filled to a volume 10%–30% greater than that which seems necessary. The total expansion time is usually a period of 2–6 months. Recently we have started utilizing the use of botulism toxin to paralyze the pectoralis major to decrease pain and facilitate expansion. This study is currently in progress and we are seeing early encouraging results with decrease visits to the office, less narcotic use and enhanced expansion in a period of 4–8 weeks.
Autogenous reconstruction
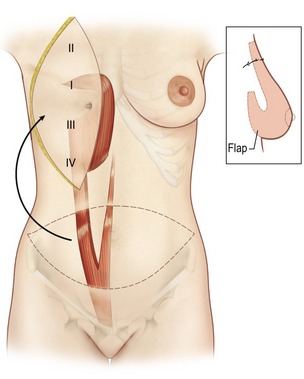
The pedicled and free TRAM flaps have the tremendous theoretical advantage of creating a breast mound composed of autogenous tissue without the need of prostheses and producing an abdominal lipectomy in the donor area (Fig. 57.10). Its disadvantage is that it is an operation of greater magnitude with a longer postoperative recuperation time than other techniques.
The procedure is begun in the supine position and measurement of the tissue deficits and necessary flap dimensions are helpful. The inferior abdominal incision is made and carried to the level of rectus fascia. An incision in the anterior rectus sheath (on the side of the flap) is made and the deep inferior epigastric vessels are identified and tied off near their origin (in case need for conversion to free flap or supercharging). Whether a contralateral or ipsilateral flap is chosen, this is left to the surgeon’s preference and the patients underlying surgical history. The upper incision of the transverse skin island is made, and the upper abdominal panniculus is elevated above the costochondral cartilages (Fig. 57.11). The arterialized side of the flap is then elevated to halfway across the anterior rectus fascia. The muscle is then identified through the fascial incision and divided and care is again taken to preserve length on the deep inferior epigastric vessels. A sterile Doppler is then utilized to determine the location of the superior epigastric blood vessels along the deep surface of the rectus muscle. The lateral 40% or more of the rectus muscle and fascia can be preserved as well as a portion medial to the umbilicus. The lateral muscle slip may remain innervated. After incision around the umbilical stalk, the remaining central portion of the rectus abdominis muscle with its overlying fascia is delivered from the sheath to allow the flap to be suspended on its superior epigastric blood supply. Some surgeons prefer to remove the entire muscle. The chest skin flaps are elevated to create and adequate pocket and a connecting wide tunnel is made medially with the abdominal dissection over the sternum. The flap is carefully passed through this tunnel to the chest wall (Fig. 57.12). Care is taken to avoid any undue tension or torque on the vascular pedicle. Abdominal reconstruction can be accomplished by primary closure of the remaining medial anterior rectus muscle and sheath to the remaining lateral muscle and sheath. Additional options for reconstruction of the abdomen may be with marlex or praline mesh or acellular human dermis as either onlay or inlay depending on the condition of the fascia and whether additional reinforcements are necessary. The table is then flexed and procedure is completed as in abdominal lipectomy.
After preoperative markings in the upright position the procedure is begun with the patient in the lateral decubitus position. The flap is elevated from distal to proximal. The latissimus dorsi tendinous insertion into the humerus can be left or severed (Fig. 57.13). A tunnel is made across the apex of the axilla to pass the flap from the back to the front. Drains are inserted in the back and after donor site closure the patient is turned to the supine position. A subpectoral pocket is then created and the flap is redraped in the desired position. The upper latissimus muscle is sutured to the lower pectoralis major muscle (Fig. 57.14). The skin island is carefully tailored and the inframammary fold formed by suturing it in position (Fig. 57.15). An appropriately sized implant, expander-implant, or expander is selected and placed submuscular followed by suction drains.
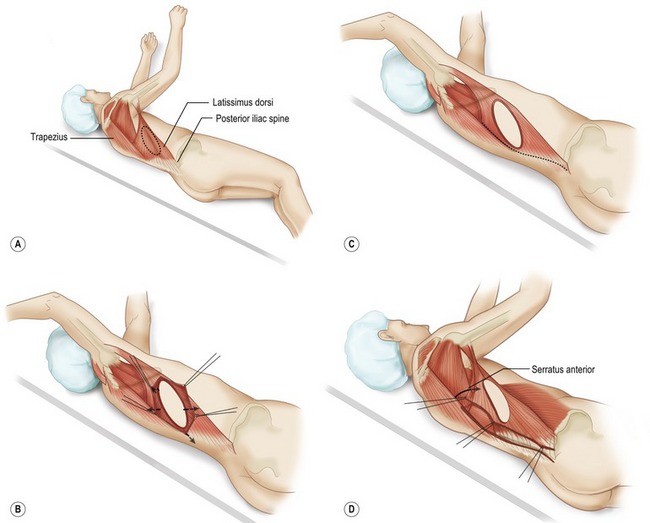
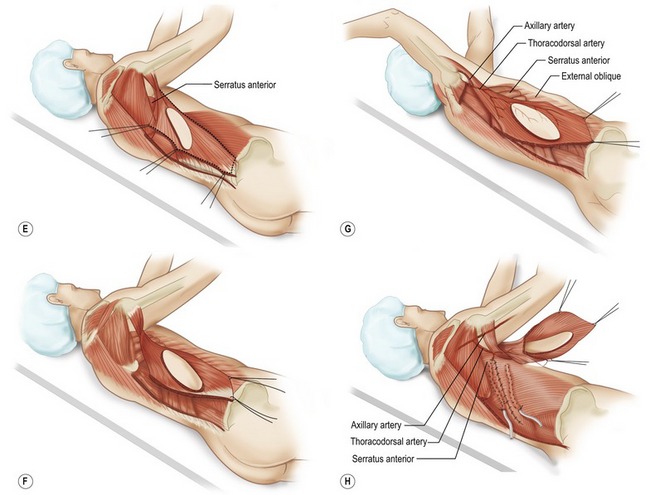
Fig. 57.13 Technique for latissimus dorsi musculocutaneous flap. A, Elevation of the latissimus dorsi musculocutaneous; Patient positioned in lateral decubitus position. Posterior chest (flap donor site) and anterior chest (breast mound reconstruction site) are included in the operative field. B, Incision around skin island allows dissection to expose anterior surface of latissimus dorsi muscle. C, Latissimus dorsi fibers of origin from lumbosacral fascia and vertebrae are divided. D, Deep surface of muscle is exposed. Care is taken to avoid flap dissection deep to the serratus anterior muscle. The latissimus muscle is separated from the underlying serratus anterior, with dissection starting posterior where the muscle plane is easily identified. E, Final mobilization of the latissimus dorsi muscle is separation from congenital adhesions with adjacent musculature at the anterior axillary line. F, Proximal dissection toward latissimus dorsi insertion required for identification and preservation of the thoracodorsal artery, veins, and nerve. A lighted retractor allows visualization of the pedicle while dissection is performed to muscle insertion. G, Complete elevation of the latissimus dorsi muscle with skin island. Thoracodorsal branch to serratus can generally be left intact. H, Incision is made adjacent to inframammary line, and remaining skin envelope and pectoralis muscle are elevated together. A tunnel is made in the superior axilla, and the flap is transposed to the anterior chest. The donor site is closed, and the patient is ready for repositioning in the supine position for flap inset.
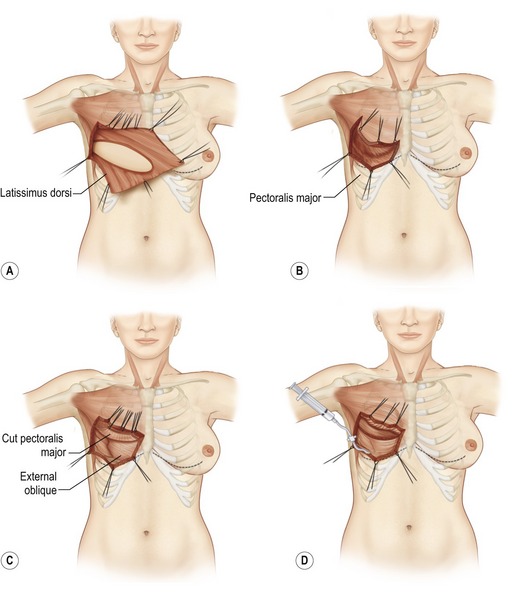
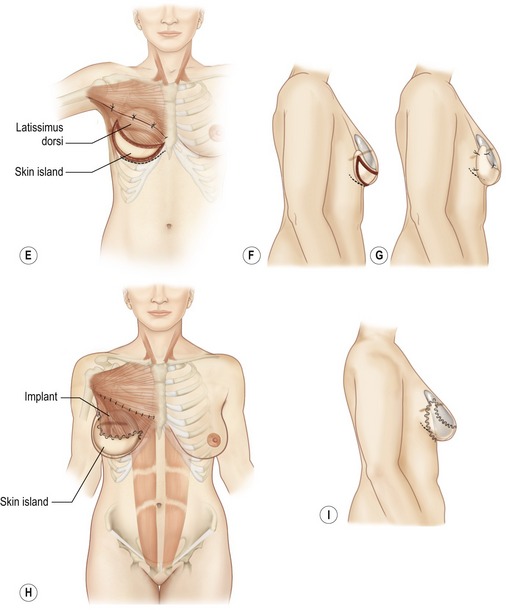
Fig. 57.14 Latissimus dorsi musculocutaneous flap inset for delayed breast reconstruction. A, Patient is reprepared and draped in supine position. Flap is placed in axilla while recipient site is prepared. B, Inset of latissimus dorsi musculocutaneous flap for delayed breast reconstruction. Skin envelope is elevated with underlying pectoralis major muscle. Care is required to preserve vascular connection between skin envelope and pectoralis muscle. If the pectoralis muscle is absent (radical mastectomy), skin is elevated from ribs and costal muscles. C, Pocket is formed between pectoralis major muscle and chest wall. Pectoralis major fibers of origin are divided along the inframammary line and at the sternal edge between fourth and sixth intercostal spaces. D, Latissimus dorsi skin island is placed over defect. The patient is placed in the sitting position for final inset of the flap and selection of appropriately sized implant. Implant sizer is beneath latissimus muscle inferiorly and pectoralis major muscle superiorly. E, Lateral mastectomy scar is released and skin island inset to provide inferior skin envelope. The permanent implant is selected on the basis of the sizer and placed in a pocket beneath the pectoralis and latissimus dorsi muscles. F, Release of lateral mastectomy scar will allow skin envelope to drape and demonstrate slight ptosis. G, After release of mastectomy scar laterally, skin island is inset. The distal skin envelope below the mastectomy scar is resected if adequate skin envelope is provided by latissimus skin island. H, Permanent implant is positioned behind latissimus and pectoralis muscles. Superior edge of latissimus muscle is loosely sutured to edge of pectoralis muscle. I, Permanent implant size should be selected on the basis of symmetry to contralateral breast. If the skin envelope appears inadequate despite addition of latissimus dorsi skin island, a tissue expander should be selected with plans to expand the region.
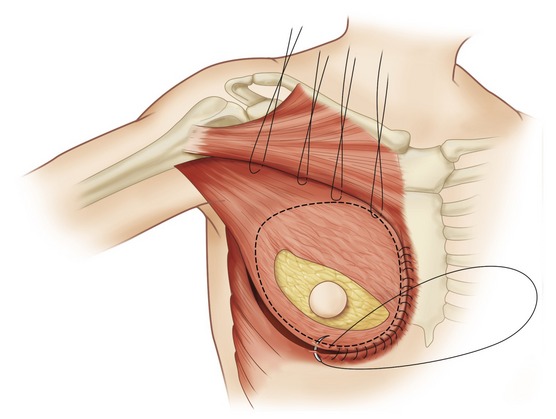
Fig. 57.15 Transposition of flap. The expander pocket is created by anchoring to the latissimus flap.
Postoperative care
The care is routine for all of the patients starting with immediate ambulation, TED hose, sequential stockings and heparin 5000 unit/mL subcutaneously every 8 hours to prevent deep vein thrombosis. It is important to remember that these patients are in a hypercoagulable state due to their history of cancer and all measures should be taken to ensure safe recovery. The usage of incentive spirometer to prevent atelectasis is also important as these patients can be under general anesthesia for prolonged periods of time with decreased work of breathing postoperatively. This is important in all TRAM, latissimus and free flap reconstruction, as patients may be bedridden for one or more days. Antimicrobial therapy generally is given to all patients for 5 days since they have either implants or drains or both in place; these are considered foreign bodies and should receive prophylaxis. Generally drains remain in place until the output is less than 30 mL in a 24-hour period. Expansion is started one week after surgery depending on patient and tissue status and can be expanded every week until the desired fill is reached.
Complications
Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32(1):32–38.
Bindingnavele V, Gaon M, Ota KS, Kulber DA, Lee DJ. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218.
Bostwick J, 3rd., Nahai F, Wallace JG, Vasconez LO. Sixty latissimus dorsi flaps. Plast Reconstr Surg. 1979;63(1):31–41.
Bradley P, Bengtson BW, Van Natta DK, Murphy AS, Maxwell GP. Style 410 highly cohesive silicone breast implant. Core study results at 3 years. Plast Reconstr Surg 2007;7:405–485.
Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116(3):768–779.
Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg. 2006;56(1):22–25.
Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69(2):216–225.
Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13(3):423–427.
Maxwell GP, Falcone PA. Eighty-four consecutive breast reconstructions using a textured silicone tissue expander. Plast Reconstr Surg. 1992;89(6):1022–1034.
Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69(2):195–208.