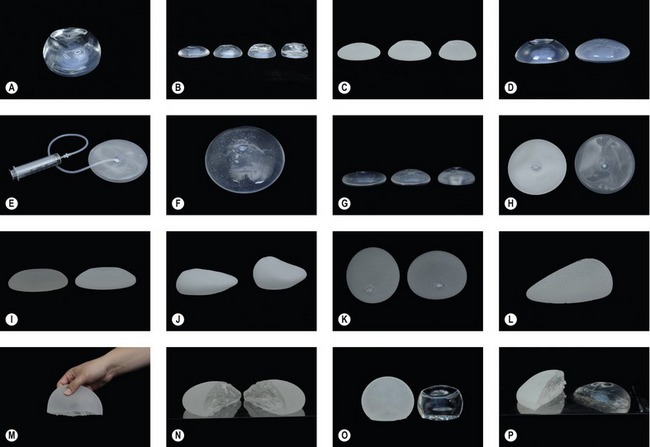
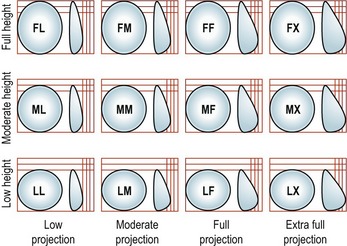
CHAPTER 52 Breast implants: background, safety and general considerations
History/implant development
Implant historical timeline
1. 1962 – The first silicone gel implant introduced by Cronin & Gerow, and manufactured by Dow Corning.
2. 1965 – The first inflatable saline implant introduced by Anon. Manufactured by Simiplast Corporation, France. High initial deflation rate of up to 75%. Withdrawn from market.
3. 1968 – Heyer–Schulte Co. (later purchased by Mentor) – more durable inflatable saline implant (Mentor 1800) in the United States.
4. 1974 – McGhan Medical Corporation incorporated, founded to manufacture silicone products for plastic and reconstructive surgery.
5. 1976 – FDA enacts the Medical Devices Amendment to the Federal Food, Drug and Cosmetic Act. FDA now has authority to review and approve new medical devices. Silicone breast implants have been on the market for 15 years and are “grandfathered”. Manufacturers will be required to provide safety and effectiveness data.
6. 1977 – Houston attorney wins first lawsuit against Dow Corning.
7. 1977 – Minnesota Mining and Manufacturing Company (3M) acquires McGhan Medical. In 1984, McGhan Medical is spun off.
8. 1984 – Stern vs. Dow Corning, San Francisco. Internal Dow Corning documents discovered in a Dow storage area by attorney Dan Bolton. First introduced idea of silicone-induced complications.
9. 1984 – Mentor acquires the Heyer-Schulte division of American Hospital Supply and its breast implant product line.
10. 1986 – McGhan changes its name to Inamed.
11. 1988 – FDA classifies implants as Class III. Premarket Approval Applications from silicone breast implant manufacturers must be submitted. The PMAs must prove that devices are safe and effective in order to keep them on the market.
12. November 1991 – FDA convenes the General and Plastic Surgery Devices Panel to review all safety data from PMAs. Panel concludes that data was inadequate but recommends the devices stay on the market while clinical trials are carried out.
13. January 1992 – FDA Commissioner goes against the panel recommendations and calls for a voluntary moratorium on the use of silicone breast implants until new data available. The manufacturers agree.
14. April 1992 – The advisory panel reconvenes and concludes that no causal link has been established between autoimmune disease and silicone breast implants. Further use of implants is limited for reconstruction and women receiving the implants must participate in scientific protocols.
15. March 1992 – Dow Corning, Bristol-Myers Squibb and Bioplasty stop manufacturing silicone breast implants. McGhan and Mentor are remaining manufacturers.
16. March 1994 – Class action suit finalized by manufacturers. Dow Corning is largest contributor. Other contributors include Baxter, Bristol-Myers Squibb and 3M. No requirements are needed to prove implants are the cause of patient ailments.
17. June 1994 – The Mayo Clinic epidemiologic study published in the New England Journal of Medicine finds no increased risk of connective-tissue disease in women with silicone implants.
18. May 1995 – Dow Corning files for bankruptcy.
19. September 1997 – The Journal of the National Cancer Institute publishes a review of numerous medical studies and concludes that breast implants do not cause breast cancer.
20. July 1998 – Plaintiffs agree to Dow Corning’s offer to settle tens of thousands of claims of injury from silicone breast implants. The agreement will let Dow Corning emerge from bankruptcy proceedings.
21. June 1998 – FDA approved Inamed’s investigational device exemption (IDE) study (i.e. Core Study) for its silicone gel-filled breast implants. This is the Core Study for P020056.
22. June 1999 – The Institute of Medicine releases a 400-page report concluding that silicone breast implants do not cause any systemic diseases such as connective tissue diseases.
23. August 2000 – FDA approved Mentor’s IDE study (i.e. Core Study) for its silicone gel-filled breast implants. This is the Core Study for P0300053.
24. December 2001 – Pre-Market Approval application for silicone gel implants was resubmitted by Inamed Corporation.
25. October 2003 – FDA expert advisory panel reviews PMA application. Finds no evidence to support the hypothesis that silicone gel implants cause disease.
26. January 2004 – The FDA commissioner again overrules the panel’s recommendation and requests additional data with longer follow-up from manufacturers.
27. April 2005 – Additional data presented to FDA advisory panel in support of Mentor and Inamed PMA.
28. March 2006 – Allergan acquires Inamed Corporation.
29. November 17, 2006 – U.S. Food and Drug Administration approves both Mentor’s and Allergan’s application to market silicone filled breast implants.
30. 2006 – Mentor begins marketing its silicone gel-filled implants under the Trademark “Memory Gel”.
31. June 2007 – Allergan begins marketing the Inamed line of implants under the product name Natrelle.
Implant type
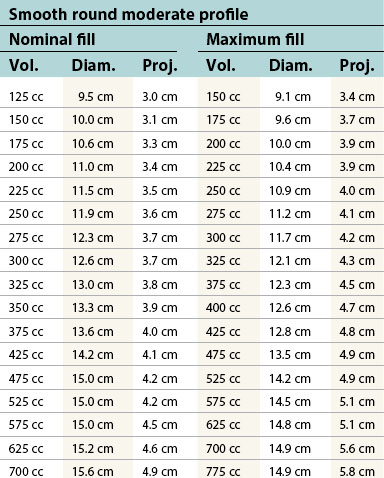
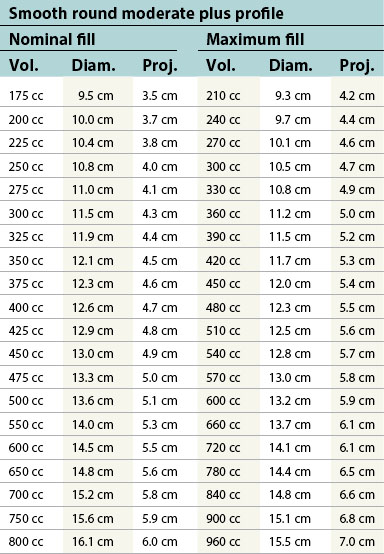
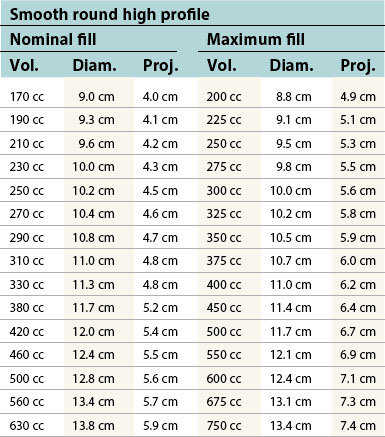
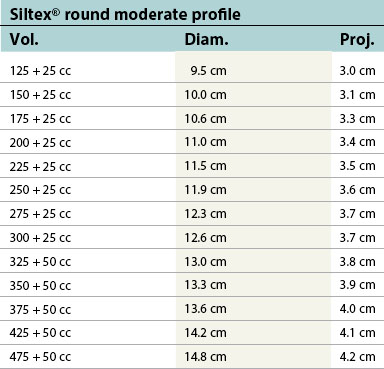
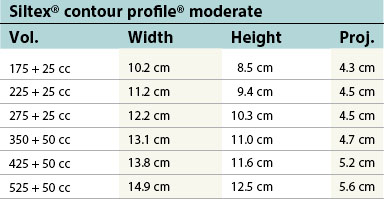
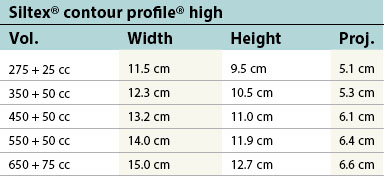
Table 52.1B Mentor silicone implants (MemoryGel™), smooth and textured (Siltex®)
| Smooth round low profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 100 cc | 9.4 cm | 1.9 cm |
| 125 cc | 10.1 cm | 2.0 cm |
| 150 cc | 10.7 cm | 2.2 cm |
| 175 cc | 11.3 cm | 2.3 cm |
| 200 cc | 11.7 cm | 2.4 cm |
| 225 cc | 12.3 cm | 2.5 cm |
| 250 cc | 12.7 cm | 2.6 cm |
| 275 cc | 13.1 cm | 2.7 cm |
| 300 cc | 13.5 cm | 2.7 cm |
| 325 cc | 13.9 cm | 2.8 cm |
| 350 cc | 14.2 cm | 2.9 cm |
| 375 cc | 14.5 cm | 2.9 cm |
| 400 cc | 14.8 cm | 3.0 cm |
| 450 cc | 15.4 cm | 3.1 cm |
| 500 cc | 16.0 cm | 3.3 cm |
| 550 cc | 16.5 cm | 3.4 cm |
| 600 cc | 17.0 cm | 3.5 cm |
| Smooth round moderate profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 100 cc | 9.3 cm | 2.1 cm |
| 125 cc | 10.0 cm | 2.2 cm |
| 150 cc | 10.3 cm | 2.3 cm |
| 175 cc | 11.2 cm | 2.4 cm |
| 200 cc | 11.7 cm | 2.5 cm |
| 225 cc | 12.2 cm | 2.6 cm |
| 250 cc | 12.3 cm | 2.8 cm |
| 275 cc | 13.2 cm | 2.9 cm |
| 300 cc | 13.5 cm | 3.0 cm |
| 325 cc | 13.9 cm | 3.0 cm |
| 350 cc | 14.2 cm | 3.1 cm |
| 375 cc | 14.4 cm | 3.2 cm |
| 400 cc | 14.5 cm | 3.2 cm |
| 450 cc | 14.9 cm | 3.4 cm |
| 500 cc | 15.2 cm | 3.6 cm |
| 550 cc | 15.9 cm | 3.6 cm |
| 600 cc | 16.5 cm | 3.7 cm |
| 700 cc | 17.4 cm | 3.9 cm |
| 800 cc | 18.2 cm | 4.1 cm |
| Smooth round moderate plus profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 100 cc | 8.2 cm | 2.7 cm |
| 125 cc | 8.9 cm | 2.8 cm |
| 150 cc | 9.5 cm | 2.9 cm |
| 175 cc | 10.0 cm | 3.1 cm |
| 200 cc | 10.5 cm | 3.2 cm |
| 225 cc | 10.9 cm | 3.3 cm |
| 250 cc | 11.3 cm | 3.4 cm |
| 275 cc | 11.7 cm | 3.5 cm |
| 300 cc | 12.0 cm | 3.6 cm |
| 325 cc | 12.3 cm | 3.7 cm |
| 350 cc | 12.5 cm | 3.9 cm |
| 375 cc | 12.8 cm | 4.0 cm |
| 400 cc | 13.1 cm | 4.0 cm |
| 450 cc | 13.6 cm | 4.2 cm |
| 500 cc | 14.1 cm | 4.3 cm |
| 550 cc | 14.6 cm | 4.5 cm |
| 600 cc | 15.0 cm | 4.6 cm |
| 700 cc | 15.8 cm | 4.9 cm |
| 800 cc | 16.5 cm | 5.1 cm |
| Smooth round moderate plus profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 125 cc | 8.3 cm | 3.5 cm |
| 150 cc | 8.8 cm | 3.7 cm |
| 175 cc | 9.3 cm | 3.9 cm |
| 200 cc | 9.7 cm | 4.0 cm |
| 225 cc | 10.1 cm | 4.2 cm |
| 250 cc | 10.5 cm | 4.3 cm |
| 275 cc | 10.8 cm | 4.4 cm |
| 300 cc | 11.1 cm | 4.5 cm |
| 325 cc | 11.4 cm | 4.6 cm |
| 350 cc | 11.7 cm | 4.8 cm |
| 375 cc | 12.0 cm | 4.8 cm |
| 400 cc | 12.2 cm | 5.0 cm |
| 425 cc | 12.5 cm | 5.0 cm |
| 450 cc | 12.8 cm | 5.1 cm |
| 500 cc | 13.2 cm | 5.3 cm |
| 550 cc | 13.6 cm | 5.5 cm |
| 600 cc | 14.0 cm | 5.6 cm |
| 650 cc | 14.4 cm | 5.7 cm |
| 700 cc | 14.8 cm | 5.8 cm |
| 800 cc | 15.5 cm | 6.0 cm |
| Siltex® round moderate profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 100 cc | 8.8 cm | 2.5 cm |
| 125 cc | 9.3 cm | 2.8 cm |
| 150 cc | 10.2 cm | 2.7 cm |
| 175 cc | 10.7 cm | 2.8 cm |
| 200 cc | 11.2 cm | 2.8 cm |
| 225 cc | 11.4 cm | 3.0 cm |
| 250 cc | 11.5 cm | 3.3 cm |
| 275 cc | 12.4 cm | 3.4 cm |
| 300 cc | 12.6 cm | 3.5 cm |
| 325 cc | 12.9 cm | 3.6 cm |
| 350 cc | 13.4 cm | 3.7 cm |
| 375 cc | 13.4 cm | 3.8 cm |
| 400 cc | 13.5 cm | 3.9 cm |
| 450 cc | 13.9 cm | 4.1 cm |
| 500 cc | 14.2 cm | 4.3 cm |
| 550 cc | 14.8 cm | 4.4 cm |
| 600 cc | 15.4 cm | 4.5 cm |
| 700 cc | 16.8 cm | 4.3 cm |
| 800 cc | 17.2 cm | 4.6 cm |
| Siltex® round moderate plus profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 100 cc | 8.1 cm | 2.7 cm |
| 125 cc | 8.8 cm | 2.9 cm |
| 150 cc | 9.4 cm | 3.0 cm |
| 175 cc | 10.0 cm | 3.2 cm |
| 200 cc | 10.5 cm | 3.3 cm |
| 225 cc | 10.9 cm | 3.5 cm |
| 250 cc | 11.3 cm | 3.6 cm |
| 275 cc | 11.7 cm | 3.7 cm |
| 300 cc | 12.0 cm | 3.7 cm |
| 325 cc | 12.3 cm | 3.8 cm |
| 350 cc | 12.6 cm | 3.8 cm |
| 375 cc | 12.9 cm | 3.9 cm |
| 400 cc | 13.2 cm | 4.0 cm |
| 450 cc | 13.7 cm | 4.1 cm |
| 500 cc | 14.1 cm | 4.2 cm |
| 550 cc | 14.4 cm | 4.4 cm |
| 600 cc | 14.7 cm | 4.5 cm |
| 700 cc | 15.7 cm | 4.8 cm |
| 800 cc | 16.6 cm | 5.0 cm |
| Siltex® round high profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 125 cc | 8.4 cm | 3.6 cm |
| 150 cc | 8.9 cm | 3.8 cm |
| 175 cc | 9.4 cm | 4.0 cm |
| 200 cc | 9.9 cm | 4.1 cm |
| 225 cc | 10.2 cm | 4.3 cm |
| 250 cc | 10.5 cm | 4.5 cm |
| 275 cc | 10.9 cm | 4.6 cm |
| 300 cc | 11.1 cm | 4.7 cm |
| 325 cc | 11.5 cm | 4.8 cm |
| 350 cc | 11.7 cm | 4.9 cm |
| 375 cc | 12.0 cm | 5.0 cm |
| 400 cc | 12.3 cm | 5.1 cm |
| 425 cc | 12.5 cm | 5.2 cm |
| 450 cc | 12.7 cm | 5.2 cm |
| 500 cc | 13.2 cm | 5.4 cm |
| 550 cc | 13.5 cm | 5.6 cm |
| 600 cc | 14.0 cm | 5.7 cm |
| 650 cc | 14.3 cm | 5.8 cm |
| 700 cc | 14.7 cm | 6.0 cm |
| 800 cc | 15.4 cm | 6.3 cm |
| Siltex® round ultra high profile, cohesive I™ | ||
|---|---|---|
| Vol. | Diam. | Proj. |
| 135 cc | 8.0 cm | 4.3 cm |
| 160 cc | 8.4 cm | 4.5 cm |
| 185 cc | 8.6 cm | 4.6 cm |
| 215 cc | 8.9 cm | 4.7 cm |
| 240 cc | 9.2 cm | 4.9 cm |
| 270 cc | 9.5 cm | 5.0 cm |
| 295 cc | 9.9 cm | 5.2 cm |
| 320 cc | 10.0 cm | 5.3 cm |
| 350 cc | 10.3 cm | 5.4 cm |
| 375 cc | 10.5 cm | 5.5 cm |
| 400 cc | 10.8 cm | 5.6 cm |
| 430 cc | 11.1 cm | 5.8 cm |
| 455 cc | 11.3 cm | 5.9 cm |
| 480 cc | 11.6 cm | 6.0 cm |
| 535 cc | 12.1 cm | 6.3 cm |
| 590 cc | 12.6 cm | 6.5 cm |
| 640 cc | 13.1 cm | 6.8 cm |
| 695 cc | 13.6 cm | 7.0 cm |
Table 52.1C Allergan saline implants (Natrelle™ Collection), smooth round: style 68 and textured (Biocell®): style 168, 468, 163, and 363
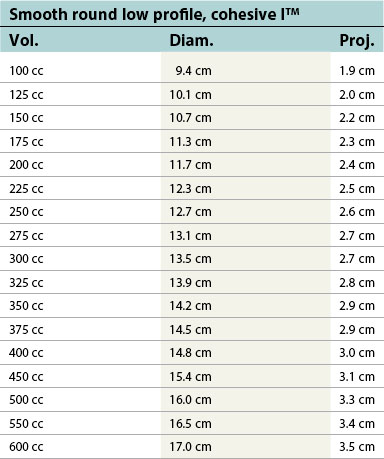
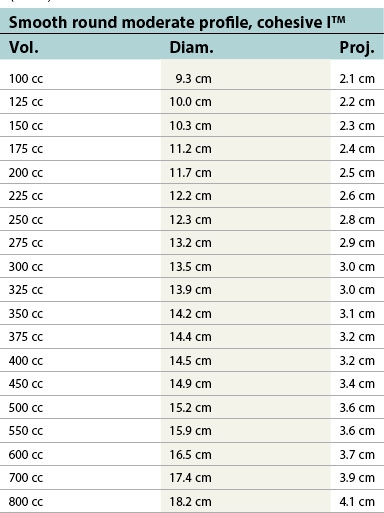
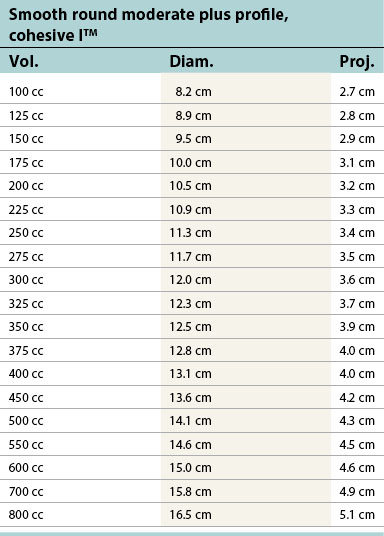
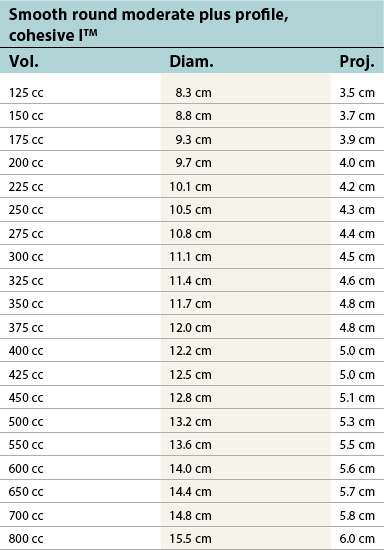
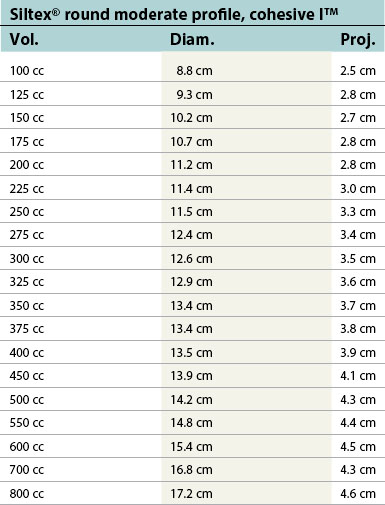
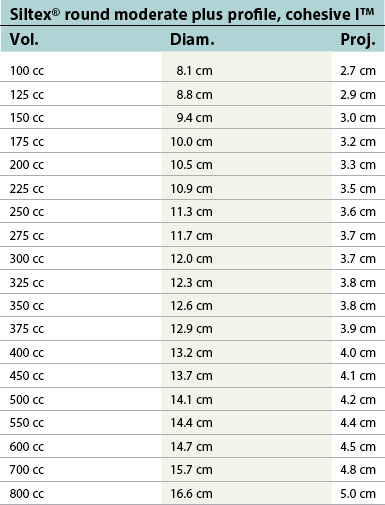
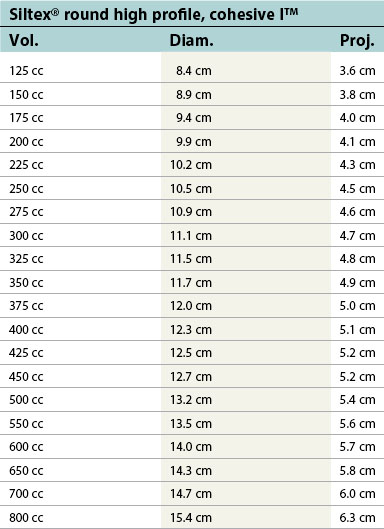
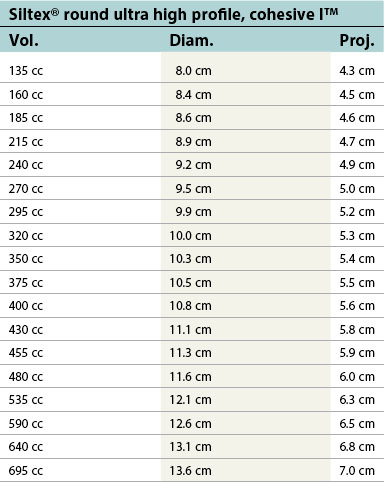
Table 52.1D Allergan silicone implants (Natrelle™ Collection), smooth round: style 10 – style 45. Textured (Biocell®): style 110 – style 120
| Style 10 moderate profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 120 cc | 9.4 cm | 2.5 cm |
| 150 cc | 10.1 cm | 2.7 cm |
| 180 cc | 10.7 cm | 2.9 cm |
| 210 cc | 11.5 cm | 3.0 cm |
| 240 cc | 11.7 cm | 3.2 cm |
| 270 cc | 12.2 cm | 3.3 cm |
| 300 cc | 12.6 cm | 3.5 cm |
| 330 cc | 13.0 cm | 3.6 cm |
| 360 cc | 13.4 cm | 3.7 cm |
| 390 cc | 13.6 cm | 3.8 cm |
| 420 cc | 14.0 cm | 3.8 cm |
| 450 cc | 14.4 cm | 3.9 cm |
| 480 cc | 14.8 cm | 3.9 cm |
| 510 cc | 15.1 cm | 4.0 cm |
| 550 cc | 15.4 cm | 4.0 cm |
| 600 cc | 15.8 cm | 4.3 cm |
| 650 cc | 16.0 cm | 4.5 cm |
| 700 cc | 16.4 cm | 4.6 cm |
| 750 cc | 16.8 cm | 4.8 cm |
| 800 cc | 17.2 cm | 4.9 cm |
| Style 15 midrange profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 158 cc | 9.5 cm | 3.2 cm |
| 176 cc | 9.9 cm | 3.3 cm |
| 194 cc | 10.3 cm | 3.4 cm |
| 213 cc | 10.6 cm | 3.5 cm |
| 234 cc | 10.9 cm | 3.6 cm |
| 265 cc | 11.4 cm | 3.7 cm |
| 286 cc | 11.7 cm | 3.8 cm |
| 304 cc | 11.9 cm | 4.0 cm |
| 339 cc | 12.4 cm | 4.0 cm |
| 397 cc | 13.1 cm | 4.2 cm |
| 421 cc | 13.3 cm | 4.3 cm |
| 457 cc | 13.7 cm | 4.5 cm |
| 492 cc | 14.0 cm | 4.6 cm |
| 533 cc | 14.4 cm | 4.7 cm |
| 575 cc | 14.8 cm | 4.8 cm |
| 616 cc | 15.2 cm | 4.9 cm |
| 659 cc | 15.4 cm | 5.0 cm |
| 700 cc | 15.8 cm | 5.1 cm |
| 752 cc | 16.2 cm | 5.2 cm |
| Style 20 high profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 120 cc | 9.0 cm | 2.7 cm |
| 140 cc | 9.1 cm | 3.3 cm |
| 160 cc | 9.4 cm | 3.5 cm |
| 180 cc | 9.6 cm | 3.8 cm |
| 200 cc | 9.7 cm | 4.0 cm |
| 230 cc | 10.0 cm | 4.2 cm |
| 260 cc | 10.4 cm | 4.3 cm |
| 280 cc | 10.6 cm | 4.5 cm |
| 300 cc | 10.9 cm | 4.5 cm |
| 325 cc | 11.2 cm | 4.6 cm |
| 350 cc | 11.4 cm | 4.9 cm |
| 375 cc | 11.7 cm | 4.9 cm |
| 400 cc | 11.9 cm | 5.0 cm |
| 425 cc | 12.0 cm | 5.2 cm |
| 450 cc | 12.4 cm | 5.2 cm |
| 475 cc | 12.6 cm | 5.5 cm |
| 500 cc | 13.0 cm | 5.2 cm |
| 550 cc | 13.5 cm | 5.6 cm |
| 600 cc | 13.8 cm | 5.7 cm |
| 650 cc | 14.2 cm | 5.9 cm |
| 700 cc | 14.5 cm | 6.2 cm |
| 750 cc | 15.0 cm | 6.0 cm |
| 800 cc | 15.3 cm | 6.1 cm |
| Style 40 moderate profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 80 cc | 8.8 cm | 1.7 cm |
| 100 cc | 8.9 cm | 2.2 cm |
| 120 cc | 9.1 cm | 2.5 cm |
| 140 cc | 9.4 cm | 2.7 cm |
| 160 cc | 9.7 cm | 3.1 cm |
| 180 cc | 10.0 cm | 3.3 cm |
| 200 cc | 10.2 cm | 3.5 cm |
| 220 cc | 10.5 cm | 3.6 cm |
| 240 cc | 10.9 cm | 3.7 cm |
| 260 cc | 11.2 cm | 3.8 cm |
| 280 cc | 11.4 cm | 3.8 cm |
| 300 cc | 11.7 cm | 3.9 cm |
| 320 cc | 12.0 cm | 3.9 cm |
| 340 cc | 12.3 cm | 4.0 cm |
| 360 cc | 12.5 cm | 4.1 cm |
| 400 cc | 12.7 cm | 4.2 cm |
| 460 cc | 13.8 cm | 4.2 cm |
| 500 cc | 14.2 cm | 4.3 cm |
| 560 cc | 14.7 cm | 4.6 cm |
| Style 45 full profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 120 cc | 7.4 cm | 3.6 cm |
| 160 cc | 8.2 cm | 3.8 cm |
| 200 cc | 9.0 cm | 4.1 cm |
| 240 cc | 9.6 cm | 4.3 cm |
| 280 cc | 10.0 cm | 4.6 cm |
| 320 cc | 10.4 cm | 4.8 cm |
| 360 cc | 10.8 cm | 5.1 cm |
| 400 cc | 11.2 cm | 5.1 cm |
| 460 cc | 11.4 cm | 5.9 cm |
| 500 cc | 11.9 cm | 5.7 cm |
| 550 cc | 12.4 cm | 6.0 cm |
| 600 cc | 12.8 cm | 6.1 cm |
| 650 cc | 13.2 cm | 6.2 cm |
| 700 cc | 13.5 cm | 6.4 cm |
| 800 cc | 14.2 cm | 6.7 cm |
| Style 110 moderate profile | ||
|---|---|---|
| Implant | A Diam. | B Proj. |
| 90 cc | 8.7 cm | 2.0 cm |
| 120 cc | 9.0 cm | 2.4 cm |
| 150 cc | 9.7 cm | 2.5 cm |
| 180 cc | 10.3 cm | 2.7 cm |
| 210 cc | 11.1 cm | 2.8 cm |
| 240 cc | 11.7 cm | 2.9 cm |
| 270 cc | 12.3 cm | 3.0 cm |
| 300 cc | 12.6 cm | 3.1 cm |
| 330 cc | 12.8 cm | 3.1 cm |
| 360 cc | 13.5 cm | 3.2 cm |
| 390 cc | 13.7 cm | 3.2 cm |
| 420 cc | 13.9 cm | 3.3 cm |
| 450 cc | 14.3 cm | 3.3 cm |
| 480 cc | 15.1 cm | 3.3 cm |
| 510 cc | 15.5 cm | 3.4 cm |
| Style 115 midrange profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 150 cc | 9.7 cm | 3.1 cm |
| 167 cc | 10.0 cm | 3.2 cm |
| 185 cc | 10.4 cm | 3.3 cm |
| 203 cc | 10.7 cm | 3.3 cm |
| 222 cc | 11.0 cm | 3.5 cm |
| 253 cc | 11.6 cm | 3.6 cm |
| 272 cc | 11.8 cm | 3.7 cm |
| 290 cc | 12.0 cm | 3.8 cm |
| 322 cc | 12.5 cm | 3.9 cm |
| 354 cc | 13.0 cm | 4.0 cm |
| 378 cc | 13.2 cm | 4.1 cm |
| 401 cc | 13.4 cm | 4.2 cm |
| 435 cc | 13.8 cm | 4.3 cm |
| 469 cc | 14.1 cm | 4.4 cm |
| 507 cc | 14.5 cm | 4.5 cm |
| 547 cc | 14.9 cm | 4.5 cm |
| 586 cc | 15.3 cm | 4.6 cm |
| 627 cc | 15.6 cm | 4.8 cm |
| 666 cc | 16.0 cm | 4.9 cm |
| 716 cc | 16.4 cm | 5.0 cm |
| Style 120 high profile | ||
|---|---|---|
| Vol. | A Diam. | B Proj. |
| 180 cc | 9.4 cm | 3.3 cm |
| 220 cc | 9.9 cm | 3.7 cm |
| 260 cc | 10.6 cm | 4.0 cm |
| 300 cc | 11.0 cm | 4.2 cm |
| 340 cc | 11.5 cm | 4.3 cm |
| 400 cc | 12.1 cm | 4.5 cm |
| 440 cc | 12.7 cm | 4.6 cm |
| 500 cc | 13.5 cm | 4.7 cm |
| 550 cc | 13.9 cm | 4.8 cm |
| 600 cc | 14.5 cm | 4.9 cm |
| 650 cc | 15.5 cm | 5..0 cm |
Implant generations
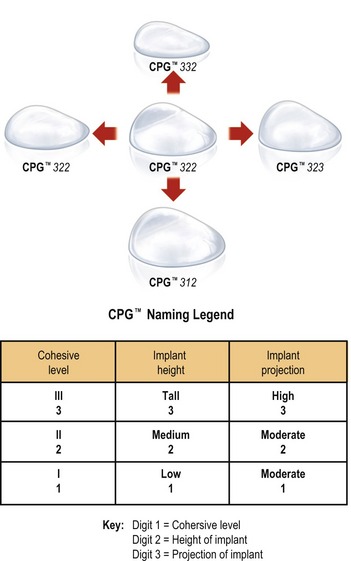
A new category or 5th generation includes the highly cohesive form-stable silicone implants that are currently under investigational use. These so-called “gummy bear” implants are enhanced cohesive silicone gel-filled breast implants with higher cross-linking of the silicone molecules. This form-stable gel helps maintain the shape of the implant. They include Allergan’s style 410 implant and Mentor’s 300 series CPG (Contoured Profile Gel – Cohesive III) implants. At this time, these breast implants are available only through clinical studies being conducted by Mentor and Allergan (Table 52.2, Figs 52.1–52.3).
| Implant design has advanced in landmark shifts that denote clear generational demarcation. Current generation implants have thick multilayered shells, barrier layers, more cohesive silicone gels, and are available in numerous textures, and shapes. |
| Implant generation | Time period | Characteristics |
|---|---|---|
| First generation | 1960s |
Safety and efficacy
Breast implant rupture, connective tissue disease and breast cancer
The moratorium on silicone breast implant use instituted in January 1992 against the advice of the FDA expert panel demonstrated the contentious nature of the controversy surrounding silicone implants and associated disorders such as connective tissue diseases and breast cancer. Because of the scale of this controversy, breast implants have become the single most investigated medical implant device. Numerous large-scale epidemiologic studies have shown no statistically significant relevant increased risk of connective tissue disease in women who have undergone breast augmentation or reconstruction with silicone gel implants.
Questions about the relationship of implants to breast cancer have also been raised over the years and anecdotal reports have suggested possible links. Delayed detection and decreased survival rates in breast cancer are common concerns for women contemplating breast augmentation. Deapen (2007) methodically reviewed 21 cohort and case–control studies, representing nine different populations from around the world. The data from numerous locations in the United States as well as Denmark, Sweden, Finland, Canada, Australia and Scotland consistently demonstrated no increased risk of cancer. In fact in many studies the relative risk was decreased. Furthermore, the risk of delayed detection and poorer prognosis was not borne out with any consistent evidence.
Just as reassuring are the findings by the Institute of Medicine of the National Academy of Sciences which, in 1999, was commissioned through congressional legislation to study the safety of silicone breast implants. The Institute of Medicine’s 400-page report by an independent committee of 13 scientists demonstrated no causal link between silicone implants and systemic diseases. Similarly, implants were not felt to be the cause of any connective tissue disorder or cancer. They did, however, conclude that breast implants were responsible for localized problems such as capsular contracture.
Complications
The Inamed and Mentor Core pre-market approval (PMA) studies were designed to address the issue of local complications associated with these devices. The recent reintroduction of silicone implants to the US market is based on data provided by these ten-year long studies. Most recently the six-year Inamed data and three-year Mentor data have become available. These data relate the most current rates of complications associated with current generation silicone implants. Similar data on saline implants have been available for some time.
In addition to local complications, the rate of revision surgery is also a telling criterion when evaluating the efficacy of breast implants. In the first six years of the Inamed study, 28% of women required revision surgery. The primary reason for revision was capsular contracture. The second most popular reason was patient choice and desire for size or implant change. Despite the complication rates, the Inamed data reported a 95% satisfaction rate at 6 years (Tables 52.3 and 52.4).
Table 52.3 Mentor and Allergan silicone breast implant core studies
| This and the following table demonstrate the most common complications of breast implants and their associated incident rates. These data are from published reports of studies sponsored by the implant manufacturers for both saline and silicone implants. The silicone implant core studies represent the most recently published reports of available data submitted by the manufacturers for the PMA. These studies are ongoing and therefore complication rates may very well rise as implant life progresses. |
| Complication | Mentor silicone (3 years) | Inamed silicone (6 years) |
|---|---|---|
| Reoperation | 15.4% | 28% |
| Capsular contracture Baker grade III/IV |
8.1% | 14.8% |
| Removal with replacement | 2.8% | 10.0% |
| Removal without replacement | 2.3% | 2.8% |
| Breast pain | 1.7% | 9.6% |
| Rupture | 0.5% | 3.5% |
Table 52.4 Mentor and Inamed (Allergan A95) saline implant studies
| Complication | Mentor saline (5 years) | Inamed silicone (5 years) |
|---|---|---|
| n= 1264 patients | n= 901 patients | |
| Reoperation | 20% | 26% |
| Capsular contracture Baker grade III/IV |
10% | 11% |
| Implant removal | 14% | 12% |
| Implant deflation | 10% | 7% |
| Breast pain | 7% | 17% |
Diagnosis of silicone breast implant rupture
Although current data indicate that the risk of silicone gel implant rupture in the latest generation implants is relatively low (0.5–3.5%), past studies have included earlier generation implants and have indicated rupture rates ranging from 0 to 69%. Later generation implant rupture rates tend to range in the single digit range with one study by Heden et al. demonstrating a 1% rupture rate in the Inamed silicone style 410 implants at six years. The ability to properly diagnose implant rupture is critical for surgical management and patient reassurance. Furthermore, the reintroduction of silicone implants onto the US market has come with specific guidelines set by the FDA for surveillance and tracking of their use. All patients must be informed that the FDA recommends MRI surveillance every two years starting three years after breast augmentation.
Physical evaluation
Implant position
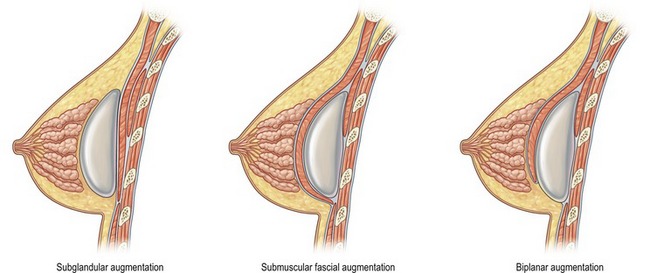
When choosing the optimal implant pocket location, there are certain factors to keep in mind. Most important is achieving adequate soft tissue coverage. The options available include subglandular, submuscular or biplanar (dual-plane) (Fig. 52.4). Most early breast augmentation was subglandular. Benefits of this position include anatomic placement that is not affected by pectoral muscle contraction and a more natural appearance under certain circumstances. Poor soft tissue coverage, especially in the upper pole, however can lead to implant palpability as well as rippling.
Access incision
Pearls & pitfalls
Pearls
• Implant selection and operative technique must be based on the patient’s tissue characteristics. A properly sized and placed implant will lead to fewer complications and an improved long-term aesthetic result.
• No matter which preop measuring and sizing system the surgeon uses, the most important thing is use it consistently. By doing so, the surgeon can continue to refine his or her technique and become more able to produce reliably good results. Careful documentation of physical exam, measurement, and sizing may also help to protect a surgeon medicolegally.
• Informed consent should introduce the idea that breast implants may not last forever. There is a strong likelihood that a patient may need additional surgeries over her lifetime.
• A goal of every surgeon should be to decrease reoperation rates. The way to do this is obvious: improve sterile technique of the entire team, focus on factors to decrease contracture, practice and improve measuring and sizing to reliably deliver good results the first time, and most importantly, thorough preoperative patient screening and counseling.
• Always have a chaperone when examining and measuring the patient, period; even if a family member is in the room. Your chaperone should record your measurement so the patient doesnt feel she is being stared at.
• Try educating the patient on the pros and cons of saline vs. silicone, but in the end the choice must be on made by the patient and family. Of course, fight the temptation to steer patients to one or the other if the patient or family members have strong biases about implant safety.
• Routine mammograms to screen for breast cancer may be more involved with breast implants. Attempt to enlist the patient in a positive way to see this as an opportunity to be more vigilant about breast health and cancer screening in order to possibly catch small problems earlier.
• Use materials in the office that accurately give the rates of complications with augmentation. This demonstrates an informed surgeon and makes the patient realistically understand the risks. Make it part of your routine and document that patient is aware of these rates.
• Review on a semiannual basis, the measuring, sizing, and implant selection for augmentation cases and correlate them with the short and long-term results photographic record. This will allow the surgeon to make modifications in his or her technique.
Pitfalls
• It is imperative to review preoperative photos with the patient and to point out asymmetries to her. Tell every patient, “Breasts are like snowflakes, no two are the same, not even on your own body.”
• Some slight asymmetries in breast mound size or volume may be improved with differential filling with saline or different implant selection. However, many asymmetries, particularly in nipple–areolar complex position may, in fact, become more exaggerated with augmentation. The patient must be counseled about this preoperatively.
• Be careful with the infra-mammary incision. Err on the side of the having it at the level of the IMF or slightly above. If it is too low, it may slip under the bikini at the beach.
• If a silicone gel-filled breast implant ruptures, the patient may have no symptoms and may not know that the implant has ruptured. The MRI is still the gold standard.
• Avoid a surgeon’s headache: make sure the option to perform a mastopexy, if needed, is available on the consent. Be careful about the patient refusing to allow a surgeon that option. Despite a great deal of preoperative counseling, quite frequently these patients, their families, or interested friends may end up unhappy and in the end the patient will require the mastopexy the surgeon wanted in the first place.
Summary of steps
• Although breast augmentation can be one of the most rewarding procedures available to both the patient and the surgeon, the complexities and complications associated with the procedure must not be underestimated or overlooked. During the preoperative consultations with the patient a thorough informed consent must be obtained.
• A knowledgeable surgeon will discuss the relevant data and history with the patient and evaluate the patient’s tissue and body characteristics critically in order that the best decisions regarding operative planning and implant selection are made.
• Given the complex medical, legal and political history of breast implants as well as their technical evolution and ongoing scientific evaluation, it is difficult for any patient to be fully aware of the many choices available to them regarding implant selection, surgical technique and potential complications. It should be the surgeon’s goal, by having the available knowledge and informing a patient adequately, to foster the type of relationship that will lead to better patient choices and aesthetic results.
• With the reintroduction of silicone gel-filled breast implants onto the market and the rising popularity of breast augmentation, there is a new sense of enthusiasm in this field. However, the lessons of the last breast implant crisis must not be forgotten. Despite the weight of evidence that debunks the past association of silicone implants with systemic diseases, the rate of local complications and reoperations in women undergoing breast augmentation remains high. If we do not use the wealth of data that is available to properly inform patients and carefully plan the optimal surgical procedure, we risk new breast implant crises as a result of high rates of local complications.
• The next phase of improvement in the quality of care relating to augmentation involves our ultimate goal of decreasing reoperation rates nationally. This can be achieved by absolute sterile technique, practicing more reliable ways of assessing and predicting parenchyma and skin movement postoperatively, attention to decreasing incidence of capsular contracture and most importantly, thorough preoperative counseling and education of the patient with the ultimate goal of having realistic expectations.
American Society of Plastic Surgeons, Procedural statistics press kit. 2007. www.plasticsurgery.org/media/press_kits/procedural_statistics.html
Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116(3):768–779.
Deapen D. Breast implants and breast cancer: a review of incidence, detection, mortality, and survival. Plast Reconstr Surg. 120(7 Suppl 1), 2007.
FDA, Saline Implant PMA. www.fda.gov/cdrh/ode/guidance/1239.html
Gorczyca DP, Gorczyca SM, Gorczyca KL. The diagnosis of silicone breast implant rupture. Plast Reconstr Surg. 120(7 Suppl 1), 2007.
Holmich LR, et al. Breast implant rupture and connective tissue disease: a review of the literature. Plast Reconstr Surg. 120(7 Suppl 1), 2007.
Maxwell GP, Baker MB. Augmentation mammaplasty: general considerations. In: Spear SL, ed. Surgery of the breast, principles and art. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:1237–1260.
National Academy Press, Washington, DC, 1999.
PBS, Breast Implants on Trial, in Frontline, PBS. www.pbs.org/wgbh/pages/frontline/implants
Release FP. FDA Moratorium, FDA, Editor, 1992
Sanchez-Guerrero J. Silicone breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med. 1995;332(25):1666–1670.
Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process [reprint in Plast Reconstr Surg 2006;118(7 Suppl):35S–45S; PMID: 17099482]. Plast Reconstr Surg. 2005;116(7):2005–2016.
Tebbetts JB. A system for breast implant selection based on patient tissue characteristics and implant–soft tissue dynamics [see comment]. Plast Reconstr Surg. 2002;109(4):1396–1409.
Tebbetts JB. Patient evaluation, operative planning, and surgical techniques to increase control and reduce morbidity and reoperations in breast augmentation. Clin Plast Surg. 2001;28(3):501–521.
American Society of Plastic Surgeons. Procedural statistics press kit. www.plasticsurgery.org/Media/Press_Kits/Procedural_Statistics.html. Accessed Feb 2009.