Chapter 62 Breast Cancer
Postmastectomy Radiation Therapy, Locally Advanced Disease, and Inflammatory Breast Cancer
Locally advanced breast cancer remains a therapeutic challenge for oncology specialists, but treatment advances continue to improve the outlook for patients with this disease. It is clear that patients who present with large primary tumors and/or extensive regional disease are at high risk for both distant and locoregional disease recurrence. Fortunately, improvements in systemic and locoregional treatments help decrease these risks, and with aggressive multidisciplinary management, many patients can be cured. The administration of systemic and locoregional treatments requires careful coordination among all disciplines involved in oncology. Previous data have indicated that the degree to which this coordination exists, and the experience of the treating center, can affect the treatment outcome. In a study of breast cancer patients in California, those treated at large teaching hospitals had significantly better survival rates than those treated at small community or health maintenance organization hospitals.1 Multidisciplinary care is of particular importance for patients with advanced disease because the optimal sequencing of various therapies is less straightforward than for patients with early-stage breast cancer.
Locally advanced breast cancer was once considered a fatal disease. Prior to the routine use of chemotherapy, the vast majority of patients developed distant metastases and died.2–4 In the past, locoregional treatments (i.e., mastectomy alone, radiation therapy alone, or combined surgery and radiation therapy) were attempted to prevent uncontrolled locoregional disease progression. With such strategies, the outcome of patients was poor.2–4 Fortunately, the current outlook for patients with locally advanced breast cancer is much more optimistic. Although distant metastases remain a persistent problem, long-term DFS have significantly improved with systemic treatments.5 This improvement in metastatic disease control has also heightened the importance of achieving locoregional control. Arguably, the success of chemotherapy in eradicating micrometastatic disease has allowed the advances achieved in surgery and radiation therapy to also contribute to improved patient survival rates.
Epidemiology
The incidence of locally advanced breast cancer is decreasing in the United States, despite the fact that the overall incidence of breast cancer has remained relatively stable. In the most recent estimates of cancer staging provided by the American Cancer Society, the percentage of patients with lymph node involvement at the time of original diagnosis has decreased at a rate of 2.8% per year since 2001. Tumors have also become smaller over time. The incidence rates of tumors 3 cm or larger decreased 27% between 1980 and 1987, and between 1997 and 2000. they continued to decline 1.9% annually.6,7 In the year 2000, it was estimated that only 6% of breast cancers were larger than 5 cm at the time of diagnosis. Given this trend, it is reasonable to estimate that 12,000 patients will be diagnosed with T3 or T4 breast cancer in the coming year. The use of mammography screening has decreased during the last decade, however, and, accordingly, the most recent data have shown a slight increase in the percentage of tumors over 5 cm.8 Still, stage III breast cancer represents a small percentage of the total breast cancers diagnosed in the Unites States each year. In developing countries and in countries with a lack of screening programs and public education about the disease, the percentage of patients diagnosed with stage III disease is much higher.
There are still subgroups of patients in the United States for whom locally advanced breast cancer remains an epidemiologic problem. For example, black women are more often diagnosed with locally advanced disease than white women.6,7 This finding was first noted during the 1980s and continues to be true today. There are many possible reasons for the higher incidence of locally advanced breast cancer in blacks, including decreased access to medical care, less use of screening mammography, and biologic differences in the cancer. Specifically, breast cancers in black women tend to be of higher grade and more often lack estrogen receptors compared with those in white women.9,10
Biologic Characteristics And Molecular Biology
Histopathologic findings of inflammatory breast cancer are most commonly characterized by a high histologic grade, a high percentage of cells in the S phase, aneuploidy, absent estrogen receptor expression, and high expression of TP53 and epidermal growth factor.11 Some authors have found that HER2/neu is not more commonly overexpressed in inflammatory breast cancer than in noninflammatory advanced disease,11,12 although more recent studies have reported higher rates of HER2/neu overexpression.13 The potential higher rate of both HER2/neu and epidermal growth factor receptor (EGFR) has permitted new opportunities for clinical trials investigating the targeting of these tyrosine kinase receptors.
Recent work has also suggested that many of these tumors overexpress RHOC guanosine triphosphatase (GTPase) and have loss of expression of the WIPS3 tumor suppressor gene. If confirmed and validated, these markers may prove to be useful for diagnosis and may be future therapeutic targets.14,15 Another biomarker with a unique expression pattern in inflammatory breast cancer is E-cadherin. E-cadherin is thought to play an important role in cellular adhesion and has been noted to be overexpressed in a high percentage of cases of inflammatory breast cancer and may contribute to the tumor emboli formation within the dermal lymphatic channels.16,17
One of the pathologic hallmarks of inflammatory breast cancer is invasion into the dermal lymphatics and erythema of the skin, which may in part be representative of tumor angiogenesis. Shirakawa and colleagues18 found inflammatory breast cancer xenografts to have a particular pattern of neovascular growth in which blood vessels within cancer tissue did not have a lining of endothelial cells. In addition, human inflammatory breast cancer specimens have been noted to have a higher microvessel density pattern compared with noninflammatory breast cancer.16,19 These data suggest that the angiogenesis pathway may be a therapeutic target for treatments in inflammatory breast cancer.
Pathology And Pathways Of Spread
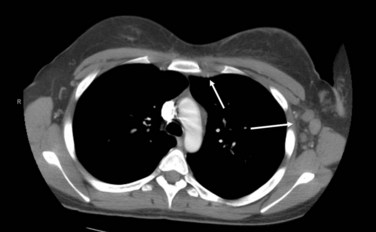
The pathways of spread of locally advanced breast cancer are most commonly through the regional lymphatics. Involvement of the axillary level I to II nodes is common in patients presenting with a T3 or T4 primary tumor. In addition, such patients are at risk for disease in the level III axillary, supraclavicular, and internal mammary lymph nodes. Figure 62-1 shows an axial CT image of a patient with a locally advanced breast cancer that has involved the internal mammary and axillary lymph nodes. Patients with locally advanced disease are also at risk of hematogenous spread, most commonly to the lung, liver, bone, and brain.
Clinical Manifestations, Patient Evaluations, And Staging
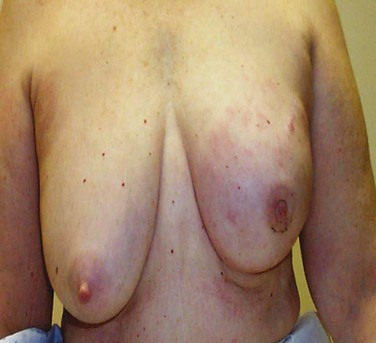
Patients with inflammatory breast cancers most frequently describe a rapid onset of breast erythema and increasing breast size and/or heaviness. Often, these changes progress over days or weeks and are frequently initially misdiagnosed as breast cellulitis. Figure 62-2 shows a photograph of the presenting appearance of the breast in a patient with inflammatory breast cancer. The history of presenting signs and symptoms is the critical determinant in classifying a breast cancer as inflammatory.
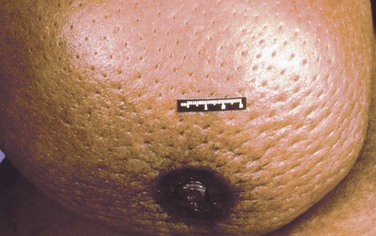
The initial physical examination is of critical importance in patients with locally advanced breast cancer. Unlike patients with early-stage disease, the majority of patients with T3 or T4 tumors are treated with neoadjuvant chemotherapy. This treatment significantly shrinks the tumor in most patients, and careful documentation of the pretreatment extent of disease is very important for determination of subsequent locoregional therapies. The breast examination should document breast asymmetry, mass size and whether the mass is freely mobile, skin erythema or edema, and skin or nipple retraction. Mobility of the primary mass should be tested with the pectoralis major muscle contracted and relaxed to distinguish chest wall invasion from invasion into the pectoralis muscle. Skin edema is best assessed with the patient in the supine position. Edema of the skin causes thickening, which makes the hair follicle more prominent, resulting in the classic “peau d’orange” appearance. This can best be appreciated by gently pinching the affected skin between the thumb and first finger. Figure 62-3 shows a patient who presented with T4 primary disease and was found to have peau d’orange dimpling on initial examination. The axilla and supraclavicular fossa should be examined with the patient upright, and regional adenopathy should be described and include the size, consistency, and mobility of axillary, infraclavicular, and supraclavicular lymph nodes. The internal mammary lymph nodes are infrequently palpable, even when involved with disease. Despite this, the parasternal area should be examined for asymmetric prominence with the patient supine.
The use of magnetic resonance imaging (MRI) and positron emission tomography (PET) continues to be investigated, and these techniques are not currently considered to be the standard of care. As refinements of these modalities improve their positive predictive value, however, they will likely play important roles in the staging evaluation. Preliminary data from our institution suggest that both PET/CT and breast MRI techniques provide additional clinically useful staging information for patients with inflammatory breast cancer.20 Finally, serum tumor markers such as carcinoembryonic antigen (CEA), CA 15.3, and CA 27-29 are used by some practitioners but are currently not recommended as the standard of care.21
Treatment Overview
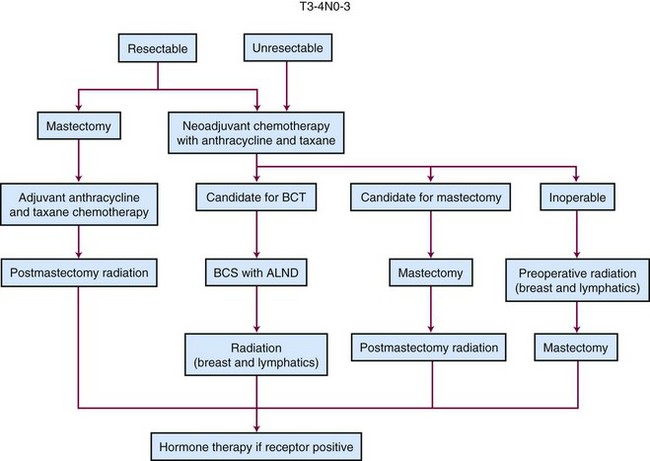
Patients with stage III disease are optimally treated with a combined-modality approach. The incorporation of systemic treatments into the management of locally advanced disease, along with improvements in surgical and radiation therapies, has significantly improved the outcomes of patients with advanced disease. For example, early studies in which patients with advanced disease were treated with mastectomy and/or radiation therapy reported 5-year survival rates of only 25% to 45%.2–4 In contrast, more recent data have shown that patients with advanced-stage disease, when treated with a combination of surgery, radiation therapy, and chemotherapy, have 5-year survival rates approaching 80% for stage IIIA disease and 45% for stage IIIB disease.5
Treating patients with advanced primary tumors with chemotherapy prior to surgery has several potential advantages. One advantage is that it gives selected patients with T3 or T4 tumors the option of being treated with breast-preservation surgery. Indeed, numerous studies have indicated that neoadjuvant chemotherapy achieves a substantial reduction in the size of the primary tumor and nodal metastases in more than 80% of cases, which often permits a breast-conserving operation that achieves an acceptable aesthetic outcome.22–25 A second advantage is that neoadjuvant chemotherapy allows physicians to assess the primary tumor’s response to a particular chemotherapy regimen. For the minority of patients whose disease does not respond or even progresses, this assessment allows for the change to a different regimen so that the most effective chemotherapy can be given as early as possible and unnecessary toxicity from an ineffective therapy can be avoided. A final advantage of neoadjuvant chemotherapy is that its use permits clinical trials that test new systemic treatments to be conducted and reported in a much more expeditious fashion.
Data from multiple clinical trials indicate that pathologic complete remission (pCR) (i.e., no residual cancer is found in the breast or lymph nodes after neoadjuvant chemotherapy) is associated with an excellent long-term prognosis.22,26 Data from our institution show a 5-year survival rate of 89% for patients with relatively advanced breast cancer in whom a pCR was achieved, versus 64% for patients in whom a pCR was not achieved (p <.01).26 Because higher pCR rates correlate with excellent long-term survival rates, phase III clinical trials have been designed using pCR as the primary endpoint.
One desired advantage of neoadjuvant chemotherapy that has not been realized is the hope that earlier administration of chemotherapy would increase the probability of eradicating preexisting micrometastatic disease. Unfortunately, the two largest trials that compared neoadjuvant and adjuvant chemotherapy found that sequencing chemotherapy before surgery provided no overall survival advantage. Specifically, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 randomized 1523 patients with early-stage, operable breast cancer to receive four cycles of doxorubicin/cyclophosphamide (AC) either before or after surgical treatment. After a long follow-up, the overall survival (OS) and disease-free survival (DFS) rates were nearly identical between the two groups.27 Interestingly, there appeared to be an advantage for neoadjuvant chemotherapy for younger patients and an advantage for adjuvant chemotherapy in older patients. A second large, randomized, prospective trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) also demonstrated equivalent rates of survival and distant metastases between preoperative and postoperative chemotherapy with four cycles of fluorouracil, epirubicin, and cyclophosphamide (FEC).24,28
Locoregional Treatment Of Patients With Advanced Primary Disease
Breast Conservation after Neoadjuvant Chemotherapy
Historically, all patients with advanced primary breast cancers underwent mastectomy and postmastectomy radiation therapy as the preferred locoregional treatment. However, the high response rates associated with neoadjuvant chemotherapy have allowed for breast conservation in selected patients with advanced disease. The NSABP and EORTC trials comparing neoadjuvant chemotherapy with adjuvant chemotherapy in patients with stage II or III breast cancer found that breast-conservation rates were higher in the neoadjuvant chemotherapy arms.22,24 This increase was directly due to a greater percentage of patients with T3 disease being offered breast conservation after first responding to chemotherapy. One concern over using chemotherapy before breast-conserving surgery is that many advanced breast cancers do not shrink concentrically to a solitary nidus in response to neoadjuvant chemotherapy. In such cases, surgery directed at the primary core may leave a high burden of microscopic disease around the tumor bed site, which may be associated with higher rates of breast cancer recurrence.
Some clinical data indeed indicate that patients with advanced tumors who have been treated with breast-conservation surgery after neoadjuvant chemotherapy have higher rates of breast cancer recurrence. For example, in the NSABP B-18 trial, the local cancer recurrence rate in patients with large primary tumors in whom a response to neoadjuvant chemotherapy allowed breast-conservation surgery was twice that in patients with smaller tumors who were treated with surgery first (15.7% vs. 7.6%, respectively).22 Other series have also shown relatively high breast cancer recurrence rates in patients who receive neoadjuvant chemotherapy.29,30 From these data, it is clear that breast-conservation procedures after neoadjuvant chemotherapy are more complex, that multidisciplinary coordination is important, and that proper patient selection for breast-conservation procedures is critical.
With appropriate patient selection and multidisciplinary management, the outcome of breast conservation for selected patients with locally advanced breast cancer can be excellent.31 Investigators from the M.D. Anderson Cancer Center reported on 340 carefully selected patients who were treated with breast-conserving therapy after showing a favorable response to chemotherapy. Patient selection criteria for a breast-conserving approach included no postoperative residual malignant calcifications, no residual T4 breast skin abnormalities, negative surgical margins, no multicentric disease, and a willingness and ability to undergo both surgery and radiation therapy. With such criteria, the outcome was favorable, with 5- and 10-year local recurrence rates of 5% and 10%, respectively, despite the fact that 72% of patients in the study had clinical stage IIB or III disease.
Chen and associates31 identified the following four factors associated with breast cancer recurrence and locoregional recurrence: (1) clinical N2 or N3 disease, (2) lymphovascular space invasion, (3) a multifocal pattern of residual disease (defined as noncontiguous foci of disease interspersed among areas of fibrosis or necrosis, granulomas, and giant cells within the resected specimen), and (4) residual disease larger than 2 cm in diameter. Eighty-four percent of patients had either just one of these factors or none of the factors, and this group had only a 4% breast cancer recurrence rate at 10 years.32 In contrast, the 4% of patients with three of these factors had a 45% breast cancer recurrence rate. Having a T3 or T4 tumor did not correlate with breast cancer recurrence in most cases in which the primary tumor shrank to become a solitary nidus or no residual disease was found in the breast after chemotherapy. The breast cancer recurrence rate was 20% in patients with T3 or T4 tumors that broke up and left a multifocal pattern of residual disease, however.31 Improvements in breast imagining, such as the use of MRI, may prove to be of value in detecting this pattern of residual disease prior to a surgical intervention.33
Mastectomy and Postmastectomy Radiation Therapy
Although postmastectomy irradiation has been evaluated in more than 25 trials conducted over 40 years, some significant controversies over its use remain. Historically, it was questioned whether the benefits of postmastectomy irradiation were offset by its associated toxicity. Specifically, in 1987, Cusik and colleagues34 published the first meta-analysis of data from postmastectomy radiation therapy trials and reported that radiation use was associated with a poorer OS. In a subsequent analysis, Cusik and associates35 reported that postmastectomy radiation therapy decreased the breast cancer death rate but increased the death rate from causes other than breast cancer, a finding that resulted in equivalent OS between the two groups.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has performed a more recent and comprehensive meta-analysis of trials investigating postmastectomy radiation therapy.36 The 2005 publication of this meta-analysis included data from 9933 patients treated in clinical trials with mastectomy and axillary clearance with or without postmastectomy irradiation. The results of this study clearly demonstrated that postmastectomy radiation therapy reduced the isolated locoregional recurrence rate for patients with lymph node—positive disease treated with mastectomy. Specifically, postmastectomy radiation therapy decreased the 15-year isolated locoregional recurrence rate for patients with lymph node–positive disease from 29% to 8%.36 The more revolutionary finding was that this significant absolute improvement in locoregional control reduced the 15-year breast cancer mortality rate from 60% (no irradiation) to 55% (irradiation). This finding was particularly important in that previous meta-analyses suggested that the benefit of adjuvant irradiation was limited to locoregional control.
The benefits of adjuvant irradiation for patients with locally advanced disease may be greater than those reported in meta-analyses. Many of the trials begun before the 1980s included patients at low risk for locoregional recurrence, and many used unconventional radiation doses, fractionation patterns, and radiation field designs. To minimize these confounding effects, Van de Steene and colleagues37 conducted a similar meta-analysis but excluded trials that began before 1970, trials with small sample sizes, trials with relatively poor survival rates, and trials that used radiation fractionation schedules that are no longer standard practice. When these less-than-optimal studies were excluded, postmastectomy radiation therapy significantly improved OS, with an odds reduction for death of 12.4%. In addition, Whelan and colleagues38 performed a meta-analysis of the published postmastectomy irradiation trials that included systemic therapy in both treatment arms. In this analysis, the addition of irradiation after mastectomy was also shown to reduce the risk of any recurrence (odds ratio, 0.69) and mortality (odds ratio, 0.83).38
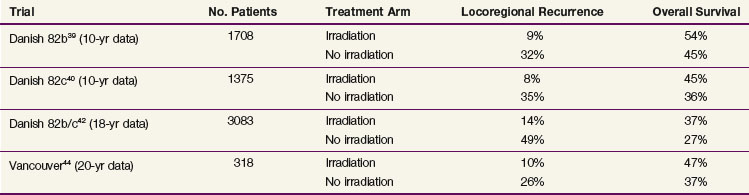
The most recent phase III randomized trials investigating postmastectomy radiation therapy were initiated almost three decades ago but remain relevant in that they used more modern radiation treatment techniques and dose fractionation and included some form of systemic therapy for all patients. Table 62-1 displays the results from these trials. The Danish Breast Cancer Cooperative Group (DBCCG) 82b trial randomized 1708 premenopausal women with stage II or III breast cancer to receive mastectomy followed by nine cycles of CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy or mastectomy, radiation therapy, and eight cycles of CMF chemotherapy.39 The simultaneously run DBCCG 82c study randomized over 1300 postmenopausal women to receive mastectomy and tamoxifen or mastectomy, tamoxifen, and radiation therapy.40 Patients randomized to receive radiation therapy in these studies had a lower 18-year rate of locoregional recurrence and a higher 18-year OS.41,42 A smaller trial, conducted in Vancouver, British Columbia, randomized 318 premenopausal women with lymph node–positive disease to receive mastectomy and CMF chemotherapy with or without postmastectomy radiation therapy.43,44 Again, patients who underwent radiation therapy had lower 20-year rates of locoregional recurrence and similar improvement in the 20-year OS.
TABLE 62-1 Ten-Year Locoregional Recurrence and Overall Survival Rates in Randomized Trials Investigating Irradiation after Mastectomy and Systemic Treatments
The data from the EBCTG and the three trials referenced above were obtained from patients treated in an earlier era, and significant changes have occurred in imaging and staging, surgical management, and systemic treatments since these studies were conducted. These changes have the potential to improve the locoregional outcome for patients with advanced disease. Accordingly, a number of groups have investigated patients with stage III disease and found that they have a high risk of locoregional recurrence after a standard modified radical mastectomy and chemotherapy (without irradiation). The data from these studies, which are displayed in Table 62-2, indicate that patients with stage III disease (defined by the presence of four or more positive lymph nodes) have clinically relevant locoregional recurrence rates that warrant postmastectomy radiation treatments. The 20% to 30% risk of locoregional recurrence without irradiation is similar to that reported in the EBCTCG meta-analysis, and one would predict, therefore, that such patients would receive a survival advantage from the use of postmastectomy irradiation. Accordingly, consensus statements from both the American Society for Radiation Oncology (ASTRO) and the American Society of Clinical Oncology (ASCO) recommend postmastectomy irradiation for patients with stage III disease.45,46
TABLE 62-2 Ten-Year Locoregional Recurrence Rates in Clinical Trials That Used Mastectomy and Systemic Treatments without Irradiation
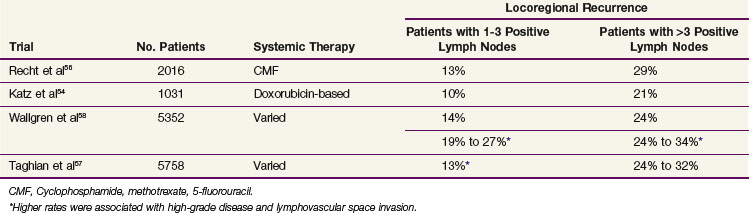
Consensus statements and data from the EBCTCG meta-analysis indicate that women with stage T1N0 or T2N0 disease have a low risk of recurrence after mastectomy and, therefore, are not recommended to receive postmastectomy radiation therapy. Data from two retrospective studies, however, have suggested that young breast cancer patients with lymph node–negative disease that is of a high grade with lymphovascular space invasion have a locoregional recurrence risk without irradiation of up to 20% or 30%.47,48 These data have yet to be confirmed in a prospective trial dataset.
There is significant controversy over whether postmastectomy radiation therapy is indicated for patients with stage II breast cancer with one to three positive lymph nodes. Most of the patients enrolled in the Danish and British Columbia randomized trials had one to three positive lymph nodes, and based on the positive findings from these studies, many think that all patients with lymph node–positive disease should received radiation treatments. The difficulty in interpreting these data, however, is that many patients in the Danish trial did not undergo a formal level I or II axillary dissection. The median number of axillary lymph nodes resected in the trial was only seven, with 76% of the patients having fewer than ten lymph nodes removed and 15% having three or fewer lymph nodes removed.39 Accordingly, some of the patients reported to have three or fewer positive lymph nodes likely would have had four or more positive lymph nodes if a more extensive surgical procedure had been performed. Correspondingly, their risk of a chest wall or supraclavicular recurrence would be higher than that usually estimated for patients with one to three positive nodes. In addition, failure to remove these additional involved axillary lymph nodes would predispose patients to axillary recurrence, which could be avoided by a more complete axillary dissection. Indeed, in an earlier publication of the Danish 82b trial, it was reported that 45% of all locoregional recurrences occurred in the axilla.49 The Danish group have reanalyzed their data and evaluated only the patients with one to three positive lymph nodes who had at least eight lymph nodes removed at the time of mastectomy. This analysis still demonstrated a significant reduction in locoregional recurrence with the use of irradiation (27% to 4%) and also continued to demonstrate an absolute 9% survival advantage for irradiation in this cohort.50
The most recent analyses from the EBCTCG comparing mastectomy with axillary clearance with or without adjuvant radiation therapy also evaluated the benefit of irradiation according to the number of positive lymph nodes.51 This analysis included data from approximately 11,000 patients treated in 26 trials that were started between 1961 and 1984 and demonstrated a significant benefit in locoregional recurrence (3% vs. 25% at 15 years) and survival (56% vs. 50% at 15 years) for the patients with one to three positive lymph nodes, in addition to noting benefits for the patients with four or more positive lymph nodes. Based on these data, it would appear that postmastectomy irradiation is warranted for all patients with lymph node–positive disease. However, a number of studies performed in the United States suggest that patients with one to three positive lymph nodes have a much lower risk of locoregional recurrence after mastectomy and chemotherapy. These data are summarized in Table 62-2. In general, these data suggest that the 10-year locoregional recurrence risk for patients with one to three positive lymph nodes is approximately 12%, which is almost three times lower than the locoregional recurrence rate in the arm of the Danish trials that did not receive irradiation. Correspondingly, the benefit gained from the addition of postmastectomy radiation therapy would be expected to be much less.
Two nonrandomized institutional studies suggest this. An analysis from the M.D. Anderson Cancer Center found that the use of postmastectomy irradiation reduced the 10-year locoregional recurrence rate in patients with stage II disease and one to three positive lymph nodes from 13% to 3%.52 Similarly, investigators from the Massachusetts General Hospital found that postmastectomy irradiation reduced the 10-year risk of locoregional recurrence from 11% to 0% for patients with one to three positive lymph nodes.53 Whether this degree of benefit is clinically meaningful with respect to patient survival is unknown. Another important consideration is that locoregional recurrences after mastectomy can develop after 10 years of follow-up. Therefore the 10-year data presented in Table 62-2 may underestimate the true percentage of patients with persistent locoregional disease after surgery and chemotherapy. Specifically, in the 20-year update of the Vancouver, British Columbia, randomized trial, approximately 20% of the total number of locoregional recurrences in the group randomized to not receive postmastectomy irradiation developed after 10 years of follow-up.44
Some series have identified subgroups of patients with stage II breast cancer and one to three positive lymph nodes that have higher risks of locoregional recurrence.52,54–58 Factors that identify these subgroups include young age, the presence of extracapsular extension greater than 2 mm, tumor size larger than 4 cm, positive or close (2 mm) surgical margins, lymphovascular space invasion, invasion of the skin, nipple, or pectoralis muscle, fewer than 10 lymph nodes resected, and a 20% or higher rate of lymph node involvement. These factors were identified in retrospective subset analyses and have yet to be validated.
The ASTRO and ASCO consensus statements regarding postmastectomy radiation therapy recommended that an additional trial be performed to further clarify the benefit of postmastectomy radiation therapy for women with stage II disease and one to three positive lymph nodes.45,46 Unfortunately, an intergroup trial designed to determine the benefits of postmastectomy radiation therapy for these patients closed owing to poor accrual, but a similarly designed trial is currently being conducted in the United Kingdom and internationally.
Postmastectomy Radiation Therapy after Neoadjuvant Chemotherapy
As previously highlighted, the use of neoadjuvant chemotherapy has significantly increased in recent years, particularly for patients with advanced primary tumors. One of the most important questions raised by this increased use concerns the indications for postmastectomy radiation therapy. In patients treated with mastectomy first, the decision to administer radiation therapy is made on the basis of the pathologic extent of disease. This is because pathologic quantification of disease is much more precise than clinical and radiographic examinations. Accordingly, the ASTRO and ASCO consensus statements regarding postmastectomy radiation therapy have recommended that treatments be based in large part on the pathologic determination of disease extent in lymph nodes.45,46 Neoadjuvant chemotherapy changes the extent of pathologic disease in 80% to 90% of cases, and it is unclear how the post-treatment pathologic information should guide decisions regarding radiation treatments. One study compared how the pathologic extent of disease correlated with locoregional recurrence after mastectomy for patients treated either with surgery first or chemotherapy first.59 Not surprisingly, this study found that the locoregional recurrence rate associated with a particular pathologic extent of disease was higher in patients treated with chemotherapy first than in patients treated with surgery first. These data imply that patients treated with neoadjuvant chemotherapy have a risk of locoregional recurrence that is affected by both the pretreatment clinical stage and the extent of pathologically residual disease after chemotherapy.
To date, only one published study has evaluated the efficacy of postmastectomy radiation therapy in patients treated with neoadjuvant chemotherapy. This study compared the outcomes of 579 patients who received neoadjuvant chemotherapy, mastectomy, and radiation therapy with those of 136 patients who were treated with neoadjuvant chemotherapy and mastectomy alone.60 All of the patients included in this study were treated in prospective trials at the M.D. Anderson Cancer Center, but it is important to recognize that radiation therapy was not a randomized variable in any of these trials. Therefore, there were significant imbalances in the prognostic factors between the two groups, with those being referred for radiation therapy having more extensive disease. Despite the imbalances in both T and N clinical stage, the locoregional recurrence rate was significantly lower in the patients treated with postmastectomy radiation therapy than in those treated with neoadjuvant chemotherapy and mastectomy alone (the 10-year locoregional recurrence rates were 8% and 22%, respectively; p = .001). More importantly, as indicated in Table 62-3, treatment with radiation therapy was associated with better overall and cause-specific survival rates in selected groups of patients with high-risk disease. In addition, a multivariate analysis for the endpoint of cause-specific survival showed that treatment with radiation therapy was independently associated with a better outcome, with the hazard ratio in patients who did not receive radiation therapy being 2.03 (95% CI, 1.41 to 2.92; p <.0001). The hazard ratio for not receiving radiation therapy for the endpoint of LRR was 4.7 (95% CI, 2.7 to 8.1; p <.0001).
TABLE 62-3 Ten-Year Locoregional Recurrence and Overall Survival for Selected Subgroups of Patients in the M.D. Anderson Cancer Center Series Investigating the Efficacy of Postmastectomy Irradiation after Neoadjuvant Chemotherapy60
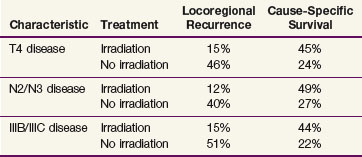
That paper also addressed the two aforementioned clinically important questions. The data indicated that patients with locally advanced clinical disease who had favorable responses to chemotherapy and were treated with mastectomy still benefited from the addition of radiation therapy.60 For patients with stage III disease who achieved a pCR, the locoregional recurrence rate for those treated with radiation therapy was 3% versus 33% for those who did not receive radiation therapy (p = .006).61 In addition, using a broader definition of favorable response as having less than 5 cm of disease and fewer than four positive lymph nodes, similar benefits were noted. For those with stage III disease who achieved a favorable response using these criteria, the locoregional recurrence rates were 9% for those receiving radiation therapy versus 20% for those not receiving radiation therapy.60
A second paper from this group specifically attempted to address the question of which patients treated with neoadjuvant chemotherapy for clinical stage II breast cancer should receive radiation therapy. The authors examined 132 such patients who had not received radiation therapy and found that the small number of patients with clinical T3N0 disease and those with four or more positive lymph nodes had high rates of locoregional recurrence.62 However, the 42 patients with clinical stage II disease who had one to three positive lymph nodes after neoadjuvant chemotherapy had a relatively low rate of locoregional recurrence (5-year rate of 8%). An update of these data from the M.D. Anderson Cancer Center continued to find that patients with clinical T1 to T2, N0 to N1 disease who have one to three positive lymph nodes have low locoregional recurrence risks without irradiation but patients with clinical stage T3N0 disease treated with neoadjuvant chemotherapy maintain a higher locoregional recurrence risk.63,64 Investigators for the NSABP have also retrospectively analyzed data from their B-18 and B-27 trials for patients treated with neoadjuvant chemotherapy, mastectomy, and no irradiation. They noted that patients with negative lymph nodes after neoadjuvant chemotherapy had 8-year locoregional recurrence rates of less than 10%, but this rate was 15% for those with residual positive lymph nodes.65 Clearly, prospective data are needed to better define the risk in these patients.
On the basis of these data, it is reasonable to recommend postmastectomy radiation therapy for all patients with clinical T3 or T4 tumors or clinical stage III disease regardless of their response to the chemotherapy regimen. In terms of clinical stage I or II breast cancer, postmastectomy radiation therapy should be recommended for patients with four or more positive lymph nodes after chemotherapy and for the unusual patient in whom the disease progresses and the primary tumor exceeds 5 cm in diameter. It is clear, however, that additional studies are needed to quantify the locoregional recurrence risk in patients who present with T1 or T2 disease and have one to three positive lymph nodes after neoadjuvant chemotherapy. These recommendations are also articulated in a National Cancer Institute Statement of Science publication on this topic.66
Irradiation Techniques/TOLERANCE
Radiation treatment plays a critical role in the management of locally advanced breast cancer, and as such the design of treatment fields is of critical importance. For patients with stage III disease, the initial target volume should include the breast/chest wall and draining lymphatic area. At a minimum, the undissected axillary apex and the supraclavicular fossa lymph nodes should be included. In our institution, we also include the internal mammary lymph nodes as a therapeutic target. One rational for including these structures is that the internal mammary lymph nodes frequently contain disease in patients with locally advanced disease, particularly those with medial/central tumors.67
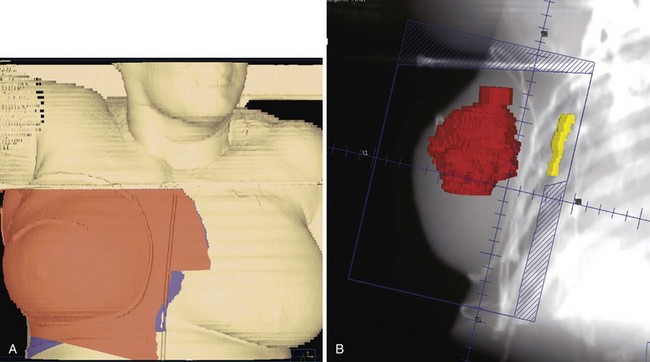
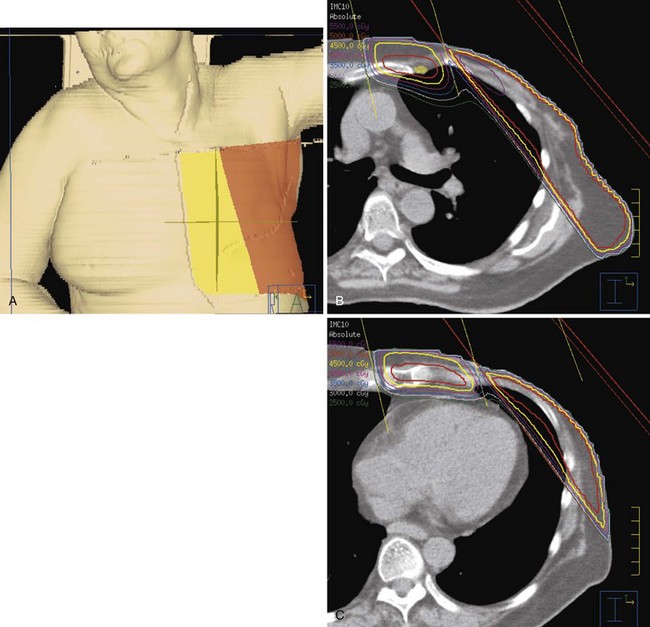
For patients treated with breast-conservation surgery, the breast is typically treated with medial and lateral tangential photon fields. These fields are created with matched nondivergent deep and cranial borders. A nondivergent cranial border is created through rotation of the couch. The nondivergent deep border is typically achieved by overrotating the gantry or a half-beam block. The collimators are rotated to match the chest wall slope, and any volume that extends into the supraclavicular field is blocked. For patients with advanced disease and tumor locations in the central or medial breast, we attempt to include the contoured internal mammary structures by widening the deep border in the upper half of the field (often called deep tangents or half-wide tangents). CT simulation allows physicians to assess a variety of gantry angles for these tangents and pick the optimal angle that minimizes lung volume while avoiding irradiation of the contralateral breast. Figure 62-4 shows an example of a “deep” tangent field that includes the upper internal mammary lymph nodes. A supraclavicular/axillary apex field is matched to the tangents with a nondivergent half-beam block. The gantry of this field is rotated 15 degrees to be off the spinal cord. For patients with advanced disease, we typically place the border lateral to the humeral head to cover the anterior soft tissues of the breast/chest wall and axilla. Figure 62-5 shows an example of such a field. We use a posterior axillary boost only when there is gross disease present in the midaxilla or in instances where the axillary dissection was omitted or inadequate.
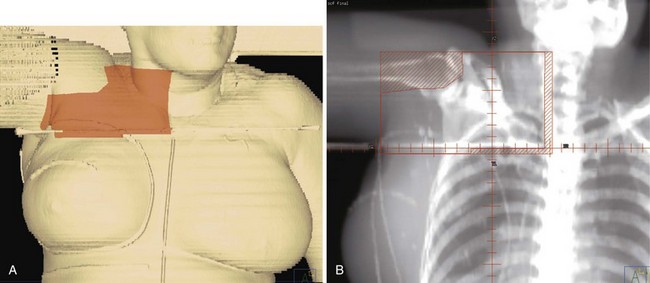
Figure 62-5 An example of a field used to treat the supraclavicular and axillary apex lymph nodes and the superior/lateral breast and chest wall. In this case, the tumor bed was high in the breast and approached the junction of this field with those in Figure 62-4. Therefore a 5-mm overlap of the field was purposefully designed in this region. A, A skin surface rendering of the treatment fields as projected on the reconstructed images from the CT treatment planning study. B, Beams-eye-view, reconstructed radiograph of the field.
For patients treated after mastectomy, we target the chest wall and draining lymphatics with a four-field technique. The supraclavicular/axillary apex field is identical to that described above. The lateral chest wall is treated with an opposed tangent technique similar to that used for a breast. The medial chest wall and internal mammary lymph nodes are treated with a medial electron field (that includes the contoured internal mammary vessels) that is angled 15 degrees. This field is matched to the tangents on the skin. Figure 62-6 shows examples of such fields. One advantage of this technique is that it provides relatively broad circumferential coverage of the chest wall, something that is important, particularly for patients with T4 tumors. In addition, the technique allows for avoidance of heart irradiation.
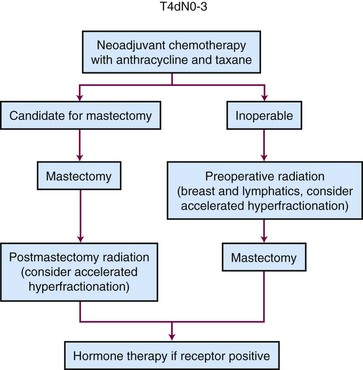
We treat our initial targets to a total dose of 50 Gy in 25 fractions over 5 weeks. For patients treated with mastectomy, we use a 3- to 5-mm bolus over the chest wall every other day for 2 weeks and then as needed to ensure that a brisk radiation dermatitis develops. This dermatitis should not lead to a treatment interruption, however. For patients with T4 tumors involving the skin, we slightly overlap the junctions of the matched fields. After completing a course of treatment to 50 Gy, we treat the chest wall with a generous boost of electron fields (5 to 10 cm beyond the mastectomy scar and covering the tumor bed location of the original primary tumor) for an additional 10 Gy in five fractions over 1 week. In addition, we treat all sites of unresected but initially involved adenopathy in the internal mammary, infraclavicular, and supraclavicular regions with a radiation boost. If ultrasonography has shown a resolution of disease in these areas down to 1 cm or less, we treat this region with an additional boost of 10 Gy. If more than 1 cm of disease exists, we increase the boost treatment to 16 Gy. In our institution, we treat most patients with inflammatory cancer on an accelerated irradiation schedule, based on a retrospective study that suggested that this schedule improved the outcome over comparison with a historical dataset.68 The initial target volumes are treated to 51 Gy in 1.5-Gy fractions given twice a day for 17 treatment days. Subsequently, the boost fields are treated with an additional 15 Gy in 1.5-Gy twice-daily fractions over 5 treatment days. We have found that this fractionation schedule is associated with an improved rate of locoregional control and has an acceptable toxicity profile.
Incorporation Of Postmastectomy Irradiation And Breast Reconstruction
One important consideration is the timing of breast reconstruction. Immediate breast reconstruction has the advantage of permitting a skin-sparing mastectomy, which can preserve the native scaffolding of the breast contour and enhance the final cosmetic outcome. However, skin-sparing mastectomy may be contraindicated for some cases of locally advanced disease, such as inflammatory breast cancer, where the skin of the breast is at risk of having disease. Moreover, radiation treatments of a reconstructed breast have been shown by numerous authors to adversely affect the cosmetic outcome. A series from the M.D. Anderson Cancer Center reported a 42% rate of implant failures that were treated with postmastectomy irradiation compared with a 10% failure rate in patients who did not require irradiation.69 Another series noted a 5-year complication rate of 27% in patients who had their tissue expanders or implants irradiated.70 These complication rates are higher with longer follow-up times. Irradiation also has adverse effects on autologous tissue reconstruction. In a series of 41 patients who underwent an immediate transverse rectus abdominis myocutaneous (TRAM) flap reconstruction and then received irradiation, 78% experienced a volume loss, 56% had firmness within their flap, and 24% required an additional flap.71
Immediate reconstruction also can affect the radiation field design. An interesting study evaluated postmastectomy radiation fields in a cohort of 106 patients who had undergone immediate reconstruction and compared the field design with that of a matched control set of patients without a reconstruction at the time of treatment.72 The authors reported that in 52% of the reconstruction cohort patients, a compromise had to be made in the treatment planning process compared with only 7% of the control group patients. These compromises included inferior coverage of the chest wall, increased volume of lung or heart included in the radiation field, or inability to cover the internal mammary lymph nodes.
Accordingly, some patients with locally advanced disease may be best served by having a delayed reconstruction. Following irradiation of the chest wall, it is often difficult—if not impossible—for patients to undergo tissue expansion and placement of an implant; in such cases, most patients who undergo a delayed reconstruction require an autologous tissue flap. For selected patients who are candidates for a skin-sparing mastectomy, one can also consider a delayed-immediate reconstruction. In this procedure, a skin-sparing mastectomy is performed with placement of a tissue expander. The expander is then deflated during the course of radiation therapy to allow for optimal geometric design of the fields and is subsequently reexpanded and replaced with an autologous tissue flap. Early results using this approach have been promising.73
Outcome
The routine use of chemotherapy and hormonal therapy has significantly improved the outcomes for patients with stage III breast cancer. Before the use of systemic treatments, very few patients survived for 10 years after surgery and/or radiation therapy.2–4 In comparison, 67% of patients with stage IIB or IIIA disease and 33% of patients with stage IIIB or C disease survive for 10 years if treated with anthracycline chemotherapy, surgery, radiation therapy, and hormonal treatments.5 Patients currently diagnosed with locally advanced disease are likely to have even better long-term survival rates than these statistics indicate. This is because these data were obtained before the more recent incorporation of taxanes, other new chemotherapeutics, aromatase inhibitors, and improvements in radiation therapy and surgery.
Multimodality treatment has also been able to improve long-term locoregional recurrence rates. For patients treated with chemotherapy, appropriate surgery, and radiation therapy, the locoregional recurrence rates are predicted to be less than 15%.60
The subgroup of patients with locally advanced breast cancer that responds poorly to initial neoadjuvant chemotherapy remains a therapeutic challenge. It is clear that patients with a significant volume of residual primary disease and/or four or more lymph nodes after neoadjuvant chemotherapy have a poor prognosis, with DFS of only 25% and locoregional recurrence rates as high as 25%.60 New therapeutic approaches are needed for this cohort.
Inflammatory Breast Cancer
Inflammatory breast cancer makes up only 1% to 6% of primary breast cancers.74 Interestingly, data from the Surveillance, Epidemiology, and End Results (SEER) database indicate that the incidence of inflammatory breast cancers increased from 0.3 to 0.7 cases per 100,000 person-years between 1975 to 1977 and 1990 to 1992. This increase was much larger than that noted for noninflammatory forms of breast cancer during the same period.75 This study also found that inflammatory breast cancer was associated with a statistically significant lower OS (p = .0001) than that of noninflammatory breast cancer.
There are no randomized prospective clinical trials that have specifically evaluated treatments in patients with inflammatory breast cancer. The M.D. Anderson Cancer Center has one of the largest single-institution experiences in the treatment of inflammatory breast cancer. In this institution, six prospective inflammatory breast cancer trials were conducted between 1974 and 2001, and 242 patients were treated in these trials. In summary, these data suggest that aggressive trimodality treatment and the incorporation of taxanes into the chemotherapy regimen have had a favorable impact on the outcomes of patients in that there has been a significant improvement in the 3-year OS (71% vs. 53%; p = .12) and the progression-free survival rate (46% vs. 39%; p = .19).76
The current standard of care for patients with inflammatory breast cancer is initial neoadjuvant chemotherapy followed by mastectomy, if feasible, and postmastectomy radiation therapy. In our institution, we have used an accelerated radiation schedule, giving patients twice-daily treatments (1.5 Gy twice daily to a total dose of 51 Gy to the chest wall and draining lymphatics, followed by a boost to the chest wall of an additional 15 Gy). In an initial retrospective analysis, this twice-daily schedule to a 66-Gy total dose was associated with a locoregional recurrence rate of 16%, which was much lower than the 42% rate during the era when treatment was given to 60 Gy or when once-a-day radiation schedules were used.68 An update of this experience reported that the majority of patients continued to have a benefit from the higher 66-Gy dose but that this dose was also associated with a 17% rate of grade III or greater toxicity. Patients who were over 45 years of age who had favorable pathologic responses to neoadjuvant chemotherapy and negative surgical margins were found to do equally well with the 60-Gy dose and as such, in this selected cohort, the higher toxicity with the 66-Gy dose does not appear to be warranted.77
Treatment Algorithms
Figures 62-7 and 62-8 show treatment algorithms for patients with locally advanced breast cancer and patients with inflammatory breast cancer, respectively.
New Therapeutic Approaches
The current era is arguably the most exciting in the history of breast cancer therapeutics. During the 1990s, the U.S. Food and Drug Administration (FDA) approved twice the number of drugs for breast cancer treatment than it had during the 1980s.78 More exciting is the move from the development of additional nonselective cytotoxic chemotherapies toward the development of new biologic therapies designed to act against tumor-specific genetic targets. The clearest example of such a strategy has been the successful use of trastuzumab for patients with tumors that overexpress the HER2/neu receptor. Incorporation of this targeted therapy has significantly improved the outcomes of patients with metastatic disease, has shown initial promise as a neoadjuvant treatment, and is under active investigation as a component of adjuvant therapies.
Advances in molecular biology have also led to improvements in understanding a patient’s prognosis and the likelihood of the disease responding to a particular treatment. Historically, new molecular markers have been studied in breast cancer as a single gene product. DNA microarray analyses now permit the rapid assessment of the expression pattern of the entire genome of a tumor. By comparing the expression pattern with the clinical outcome, investigators have been able to identify gene sets that predict the development of distant metastases, and this ability may eventually lead to a more selective use of available therapeutics.79 In addition, we have recently identified an expression profile of a set of genes that correlated with the probability of achieving a pCR after paclitaxel/FAC chemotherapy.80 Such insights eventually may allow for therapies to be selected for each individual patient based on the genetic characteristics of the patient’s disease; this would be an improvement on offering uniform treatments to entire classes of breast cancer patients.
8 Breast Cancer Facts and Figures 2009-2010. Atlanta, Ga.: American Cancer Society, 2009. pp 1-25
13 Turpin E, Bieche I, Bertheau P, et al. Increased incidence of ERBB2 overexpression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593-7597.
14 Kleer CG, Zhang Y, Pan Q, et al. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110-R115.
16 Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br J Cancer. 2003;88:718-725.
17 Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61:5231-5241.
20 Yang WT, Le-Petross HT, Macapinlac H, et al. Inflammatory breast cancer. PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109:417-426.
26 Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460-469.
27 Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy. Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778-785.
28 van Nes JG, Putter H, Julien JP, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up. Clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009;115:101-113.
31 Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. The M. D. Anderson Cancer Center experience. J Clin Oncol. 2004;22:2303-2312.
32 Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. A prognostic index for clinical decision making. Cancer. 2005;103:689-695.
33 Partridge SC, Gibbs JE, Lu Y, et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193-1199.
36 Group EBCTC. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival. An overview of the randomised trials. Lancet. 2005;366:2087-2106.
39 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949-955.
40 Overgaard M, Jensen MB, Overgaard J, et al. Randomized trail evaluating postoperative radiotherapy in high risk postmenopausal breast cancer patients given adjuvant tamoxifen. Results from the DBCG 82c trial. Lancet. 1999;353:1641-1648.
41 Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer—risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147-155.
42 Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy. Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268-2275.
44 Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy. 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116-126.
45 Harris JR, Halpin-Murphy P, McNeese M, et al. Consensus statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:989-990.
46 Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy. Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539-1569.
47 Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy. Implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62:1035-1039.
48 Truong PT, Lesperance M, Culhaci A, et al. Patient subsets with T1-T2, node-negative breast cancer at high locoregional recurrence risk after mastectomy. Int J Radiat Oncol Biol Phys. 2005;62:175-182.
49 Overgaard M, Christensen JJ, Johansen H, et al. Evaluation of radiotherapy in high-risk breast cancer patients. Report from the Danish Breast Cancer Cooperative Group (DBCG 82) Trial. Int J Radiat Oncol Biol Phys. 1990;19:1121-1124.
50 Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247-253.
51 Darby S. (presenter): 2006 Update from the Early Breast Cancer Trialists’ Collaborative Group Overview of Radiation Therapy for Early Breast Cancer. 2007 ASCO Annual Meeting, Chicago, Illinois, June, 2007. Available at www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Speaker?&spk=Darby%2C+Sarah+%5Bfau%5D
52 Woodward WA, Strom EA, Tucker SL, et al. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy radiation. Implications for patients with early stage disease and predictors for recurrence after radiation. Int J Radiat Oncol Biol Phys. 2003;57:336-344.
53 Macdonald SM, Abi-Raad RF, Alm El-Din MA, et al. Chest wall radiotherapy. Middle ground for treatment of patients with one to three positive lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2009;75:1297-1303.
54 Katz A, Strom EA, Buchholz TA, et al. Loco-regional recurrence patterns following mastectomy and doxorubicin-based chemotherapy. Implications for postoperative irradiation. J Clin Oncol. 2000;18:2817-2827.
55 Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735-742.
56 Recht A, Gray R, Davidson NE, et al. Locoregional failure ten years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation. Experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689-1700.
57 Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy. Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247-4254.
58 Wallgren A, Bonetti M, Gelber RD, et al. Risk factors for locoregional recurrence among breast cancer patients. Results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21:1205-1213.
59 Buchholz TA, Katz A, Strom EA, et al. Pathologic tumor size and lymph node status predict for different rates of locoregional recurrence after mastectomy for breast cancer patients treated with neoadjuvant versus adjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;53:880-888.
60 Huang EH, Tucker SL, Strom EA, et al. Radiation treatment improves local-regional control and cause-specific survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22:4691-4699.
61 McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004-1009.
62 Garg A, Strom EA, McNeese MD, et al. T3 disease at presentation or pathologic involvement of four or more lymph nodes predict for local-regional recurrence in stage II breast cancer treated with neoadjuvant chemotherapy and mastectomy without radiation. Int J Radiat Oncol Biol Phys. 2004;59:138-145.
63 Nagar H, Mittendorf E, Strom EA, et al. Local-regional recurrence with and without radiation therapy after neoadjuvant chemotherapy and mastectomy for clinically-staged T3N0 breast cancer. Int J Radiat Oncol Biol Phys. 2010. (in press)
64 Yu TK, Mittendorf EA, Nagar H, et al. Local-regional recurrence with and without radiation after neoadjuvant chemotherapy and mastectomy for T1-2/N0-1 breast cancer patients. Int J Radiat Oncol Biol Phys. 2008;72(1 Suppl):S88-S89.
65 Mamounas E. Sentinel node biopsy after neoadjuvant chemotherapy. The pros. Presentation at Preoperative Therapy in Invasive Breast Cancer. Reviewing the State of the Science and Exploring New Research Directions. Bethesda, Maryland: National Cancer Institute; March 26, 2007. Available at http://ctep.cancer.gov/bcmeeting/mamounas.pdf
66 Buchholz TA, Lehman CD, Harris JR, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer. A National Cancer Institute conference. J Clin Oncol. 2008;26:791-797.
67 Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis. Analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379-387.
69 Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction. An evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356-362.
70 Anderson PR, Hanlon AL, Fowble BL, et al. Low complication rates are achievable after postmastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:1080-1087.
71 Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78-82.
72 Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:76-82.
73 Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617-1628.
75 Chang S, Parker SL, Pham T, et al. Inflammatory breast carcinoma incidence and survival. The Surveillance, Epidemiology, and End Results program of the National Cancer Institute, 1975-1992. Cancer. 1998;82:2366-2372.
76 Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer. The M. D. Anderson Cancer Center experience. Clin Breast Cancer. 2004;4:415-419.
77 Bristol IJ, Woodward WA, Strom EA, et al. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:474-484.
1 Lee-Feldstein A, Anton-Culver H, Feldstein PJ. Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. JAMA. 1994;271:1163-1168.
2 Fracchia AA, Evans JF, Eisenberg BC. Stage III carcinoma of the breast. A detailed analysis. Ann Surg. 1980;192:705.
3 Haagensen CD, Cooley E. Radical mastectomy for mammary carcinoma. Ann Surg. 1969;170:884.
4 Haagensen CD, Stout AP. Carcinoma of the breast. Criteria of inoperability. Ann Surg. 1943;118:859-870.
5 Hortobagyi GN, Singletary ES, Buchholz TA. Locally advanced breast cancer. In: Singletary ES, Hortobagyi GN, Robb GL, editors. Advanced therapy of breast disease. ed 2. Hamilton, Ontario, Canada: BC Decker; 2004:498-508.
6 Breast Cancer Facts and Figures 2005-2006. Atlanta, Georgia: American Cancer Society, 2006. pp 1-25
7 Cancer Facts and Figures 2006. Atlanta, Georgia: American Cancer Society, 2006. pp 1-58
8 Breast Cancer Facts and Figures 2009-2010. Atlanta, Georgia: American Cancer Society, 2009. pp 1-25
9 Elledge RM, Clark GH, Chamness GC, et al. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705-712.
10 Li CL, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601-607.
11 Guerin M, Gabillot M, Mathieu M, et al. Structure and expression c-erB-2 and EGFR receptor genes in inflammatory and non-inflammatory breast cancer. Prognostic significance. Int J Cancer. 1989;43:201-208.
12 Pro B, Cristofanilli M, Buzdar AU, et al. The evaluation of p53, HER-2/neu, and serial MDR protein expression as possible markers of chemoresistance and their use as prognostic markers in inflammatory breast cancer. Proc Am Soc Clin Oncol. 1998;17:553a.
13 Turpin E, Bieche I, Bertheau P, et al. Increased incidence of ERBB2 overexpression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593-7597.
14 Kleer CG, Zhang Y, Pan Q, et al. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110-R115.
15 van Golen KL, Davies S, Wu ZF, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511-2519.
16 Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br J Cancer. 2003;88:718-725.
17 Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61:5231-5241.
18 Shirakawa K, Tsuda H, Heike Y, et al. Absence of endothelial cells, central necrosis, and fibrosis are associated with aggressive inflammatory breast cancer. Cancer Res. 2001;61:445-451.
19 McCarthy NJ, Yang X, Linnoila IR, et al. Microvessel density, expression of estrogen receptor alpha, MIB-1, p53, and c-erbB-2 in inflammatory breast cancer. Clin Cancer Res. 2002;8:3857-3862.
20 Yang WT, Le-Petross HT, Macapinlac H, et al. Inflammatory breast cancer. PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109:417-426.
21 Boccardo F, Bruzzi P, Cionini L, et al. Appropriateness of the use of clinical and radiologic examinations and laboratory tests in the follow-up of surgically-treated breast cancer patients. Results of the Working Group on the Clinical Aspects of Follow-up. Ann Oncol. 1995;6(Suppl 2):57-59.
22 Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer. Findings from National Surgical Adjuvant Breast and Bowell Project B-18. J Clin Oncol. 1997;15:2483-2493.
23 Fisher ER, Wang J, Bryant J, et al. Pathobiology of preoperative chemotherapy. Findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681-695.
24 van der Hage JA, Cornelis JH, van de Velde CJ, et al. Preoperative chemotherapy in primary operable breast cancer. Results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224-4237.
25 Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients withoperable breast cancer. Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. Cancer. 2001;30:96-102.
26 Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460-469.
27 Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy. Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778-785.
28 van Nes JG, Putter H, Julien JP, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up. Clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009;115:101-113.
29 Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm. A unicentre randomized trial with a 124-month median follow-up. Ann Oncol. 1999;10:47-52.
30 Rouizer R, Extra JM, Carton M, et al. Primary chemotherapy for operable breast cancer. Incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828-3835.
31 Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. The M. D. Anderson Cancer Center experience. J Clin Oncol. 2004;22:2303-2312.
32 Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. A prognostic index for clinical decision making. Cancer. 2005;103:689-695.
33 Partridge SC, Gibbs JE, Lu Y, et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193-1199.
34 Cuzick J, Stewart H, Peto R, et al. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:15-29.
35 Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447-453.
36 Group EBCTC. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival. An overview of the randomised trials. Lancet. 2005;366:2087-2106.
37 Van de Steene J, Soete G, Storme G. Adjuvant radiotherapy for breast cancer significantly improves overall survival. The missing link. Radiother Oncol. 2000;55:263-272.
38 Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000;18:1220-1229.
39 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949-955.
40 Overgaard M, Jensen MB, Overgaard J, et al. Randomized trail evaluating postoperative radiotherapy in high risk postmenopausal breast cancer patients given adjuvant tamoxifen. Results from the DBCG 82c trial. Lancet. 1999;353:1641-1648.
41 Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer–risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147-155.
42 Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy. Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268-2275.
43 Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956-962.
44 Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy. 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116-126.
45 Harris JR, Halpin-Murphy P, McNeese M, et al. Consensus statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:989-990.
46 Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy. Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539-1569.
47 Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy. Implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62:1035-1039.
48 Truong PT, Lesperance M, Culhaci A, et al. Patient subsets with T1-T2, node-negative breast cancer at high locoregional recurrence risk after mastectomy. Int J Radiat Oncol Biol Phys. 2005;62:175-182.
49 Overgaard M, Christensen JJ, Johansen H, et al. Evaluation of radiotherapy in high-risk breast cancer patients. Report from the Danish Breast Cancer Cooperative Group (DBCG 82) Trial. Int J Radiat Oncol Biol Phys. 1990;19:1121-1124.
50 Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247-253.
51 Darby S. (presenter): 2006 Update from the Early Breast Cancer Trialists’ Collaborative Group Overview of Radiation Therapy for Early Breast Cancer. The 2007 ASCO Annual Meeting, Chicago, Illinois, June, 2007. Available at www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Speaker?&spk=Darby%2C+Sarah+%5Bfau%5D
52 Woodward WA, Strom EA, Tucker SL, et al. Locoregional recurrence after doxorubicin-based chemotherapy and postmastectomy radiation. Implications for patients with early stage disease and predictors for recurrence after radiation. Int J Radiat Oncol Biol Phys. 2003;57:336-344.
53 Macdonald SM, Abi-Raad RF, Alm El-Din MA, et al. Chest wall radiotherapy. Middle ground for treatment of patients with one to three positive lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2009;75:1297-1303.
54 Katz A, Strom EA, Buchholz TA, et al. Loco-regional recurrence patterns following mastectomy and doxorubicin-based chemotherapy. Implications for postoperative irradiation. J Clin Oncol. 2000;18:2817-2827.
55 Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735-742.
56 Recht A, Gray R, Davidson NE, et al. Locoregional failure ten years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation. Experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689-1700.
57 Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy. Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247-4254.
58 Wallgren A, Bonetti M, Gelber RD, et al. Risk factors for locoregional recurrence among breast cancer patients. Results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21:1205-1213.
59 Buchholz TA, Katz A, Strom EA, et al. Pathologic tumor size and lymph node status predict for different rates of locoregional recurrence after mastectomy for breast cancer patients treated with neoadjuvant versus adjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;53:880-888.
60 Huang EH, Tucker SL, Strom EA, et al. Radiation treatment improves local-regional control and cause-specific survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22:4691-4699.
61 McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004-1009.
62 Garg A, Strom EA, McNeese MD, et al. T3 disease at presentation or pathologic involvement of four or more lymph nodes predict for local-regional recurrence in stage II breast cancer treated with neoadjuvant chemotherapy and mastectomy without radiation. Int J Radiat Oncol Biol Phys. 2004;59:138-145.
63 Nagar H, Mittendorf E, Strom EA, et al. Local-regional recurrence with and without radiation therapy after neoadjuvant chemotherapy and mastectomy for clinically-staged T3N0 breast cancer. Int J Radiat Oncol Biol Phys. 2010. (in press)
64 Yu TK, Mittendorf EA, Nagar H, et al. Local-regional recurrence with and without radiation after neoadjuvant chemotherapy and mastectomy for T1-2/N0-1 breast cancer patients. Int J Radiat Oncol Biol Phys. 2008;72(1 Suppl):S88-S89.
65 Mamounas E. Sentinel node biopsy after neoadjuvant chemotherapy. The pros. Presentation at Preoperative Therapy in Invasive Breast Cancer. Reviewing the State of the Science and Exploring New Research Directions. Bethesda, Maryland: National Cancer Institute; March 26, 2007. Available at ctep.cancer.gov/bcmeeting/mamounas.pdf
66 Buchholz TA, Lehman CD, Harris JR, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer. A National Cancer Institute conference. J Clin Oncol. 2008;26:791-797.
67 Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis. Analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379-387.
68 Liao Z, Strom EA, Buzdar AU, et al. Locoregional irradiation for inflammatory breast cancer. Effectiveness of dose escalation in decreasing recurrence. Int J Radiat Oncol Biol Phys. 2000;47:1191-1200.
69 Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction. An evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356-362.
70 Anderson PR, Hanlon AL, Fowble BL, et al. Low complication rates are achievable after postmastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:1080-1087.
71 Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78-82.
72 Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:76-82.
73 Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617-1628.
74 Levine PH, Steinhorn SC, Ries LG, et al. Inflammatory breast cancer. The experience of the Surveillance, Epidemiology, and End Results (SEER) program. J Natl Cancer Inst. 1985;74:291-297.
75 Chang S, Parker SL, Pham T, et al. Inflammatory breast carcinoma incidence and survival. The Surveillance, Epidemiology, and End Results program of the National Cancer Institute, 1975-1992. Cancer. 1998;82:2366-2372.
76 Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer. The M. D. Anderson Cancer Center experience. Clin Breast Cancer. 2004;4:415-419.
77 Bristol IJ, Woodward WA, Strom EA, et al. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:474-484.
78 Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44-52.
79 van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009.
80 Ayers M, Symmans WF, Stec J, et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J Clin Oncol. 2004;22:2284-2293.