CHAPTER 53 Breast augmentation
Physical evaluation
Anatomy
These structures receive their blood supply via the lateral thoracic and internal thoracic arteries by means of the intercostal perforators. These vessels, in turn, converge to form a dense subdermal vascular plexus, which supplies the skin. The nipple and areola are innervated predominately by the 4th intercostal nerve, as well as the 3rd and 5th, to some extent, by way of the anterolateral and anteromedial cutaneous branches.
Technical steps
Placement
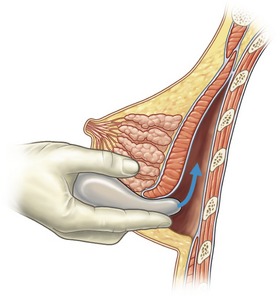
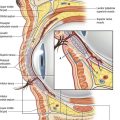
The breast pocket may be created in either the subglandular or subpectoral space (Fig. 53.1). The subglandular technique is usually reserved for patients who have substantial breast tissue or a mild degree of ptosis, since greater projection may be obtained. Also, women who are avid bodybuilders may prefer subglandular implants for the reason that placing them submuscularly, in some instances, may produce breast animation and distortion when the pectoral muscles are flexed. Increased risks of capsular contracture, rippling and implant palpability are typically issues discouraging the routine use of the subglandular plane.
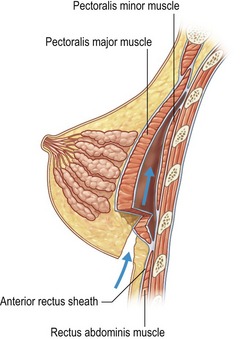
In patients with a paucity of breast tissue and little to no ptosis, the subpectoral technique, in our opinion, produces optimum results. The pectoralis major drapes the superomedial aspect of the prosthesis, softening the transition, and thus creating a more anatomically shaped breast mound (Fig. 53.2). This method also achieves a natural feel, which is especially desirable when using saline implants. The submuscular plane additionally tends to be less vascular, and is associated with fewer sensory alterations of the nipple areolar complex. Also, rates of fibrous capsular contracture are demonstrably lowered with submuscular placement versus subglandular. In addition to improved aesthetic outcomes, there are moreover prospective functional advantages in regard to breast-feeding as well as cancer screening.
Surgical approach
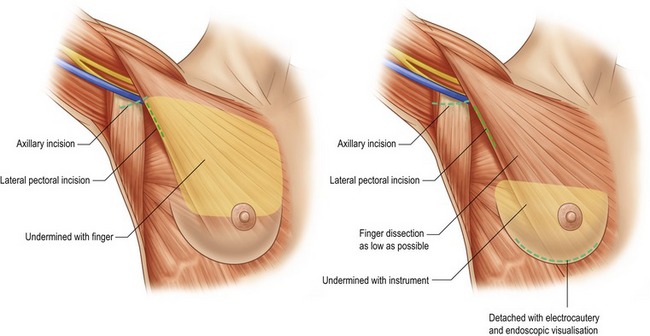
Although numerous methods have been described in the literature, there are three preferred approaches for breast augmentation: inframammary, periareolar and transaxillary (Fig. 53.3). Dissections may be performed in either plane, with or without the aid of an endoscope.
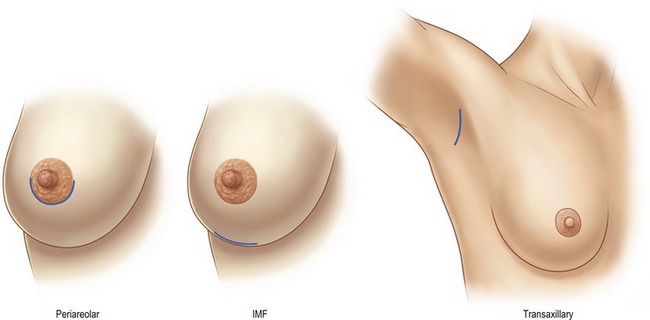
Fig. 53.3 Incision sites: Examples of periareolar, inframammary crease, and transaxillary incision sites are shown. Note how all heal well with proper placement and patient selection given individual breast anatomy. (This figure incorporates images from Figs 53.5, 53.7 and 53.10.)
The inframammary fold incision (Fig. 53.4) provides excellent results in terms of inconspicuous scars, accessibility for both implant types, and fold modifications (Fig. 53.5). This incision allows for optimal view of the pectoral muscle and breast parenchyma, which permits the surgeon to perform an accurate dissection in either, the submuscular or subglandular plane. The downsides of this approach are the potential increased risk of iatrogenic rupture during wound reapproximation, and implant exposure during the postoperative period given the weight of the implant on the healing incision. Additionally, the access incision must be carefully designed to avoid scar migration to the chest wall or inferior pole of the breast.
In patients not requiring fold adjustment, the incision is made at the inframammary fold and is approximately 2.5–3 cm in length, extended laterally from below the nipple, and carried deep until the lateral aspect of the pectoralis major muscle is reached. With the author’s preferred technique, if undertaking a subpectoral placement the inferior muscular attachments are freed by means of a fine mosquito clamp and cautery dissection. The pectoralis major can then be elevated from the chest wall using blunt finger dissection or an Agris–Dingman dissector, which is then replaced by a lighted retractor to allow assessment for hemostasis and the transection of any residual muscular bands via electrocautery. The only medial pectoralis muscle fibers transected are those that appear anomalous and medially displaced. Otherwise, purely inferior muscle fibers are released. In cases of ptosis or tuberous breast deformity, the dual plane technique as described by Tebbetts, may be utilized to aid in redraping the breast tissue over the implant.
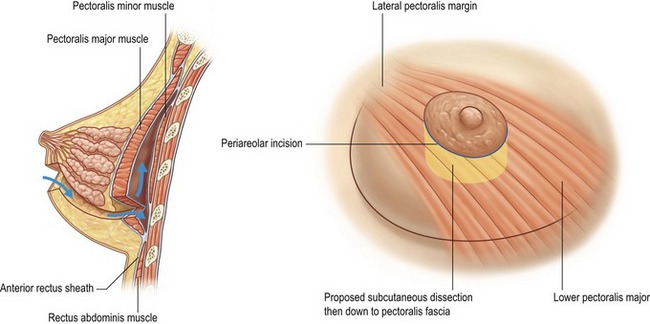
The periareolar incision (Fig. 53.6) requires a somewhat higher degree of technical skill, but provides a central point of access for pocket development as well as radial release. This incision is especially suitable for those who have an ill defined fold, constricted breast tissue, or require nipple-areolar resizing. It also allows for conservative excision of redundant lower pole skin. In order to achieve discrete scars, the patient must have a sufficiently sized nipple-areolar complex to accommodate the selected implant, a well-defined areolar edge, and differentiation of pigment (Figs 53.7, 53.8). The approach is generally limited to smaller silicone implants, but this is less of an issue with saline prostheses. Given the proximity of the incision to the lactiferous ducts, it may however, be a less favorable choice for patients who desire to breastfeed. Also, there may be an increased likelihood of nipple dysesthesias due to proximity of the dissection.
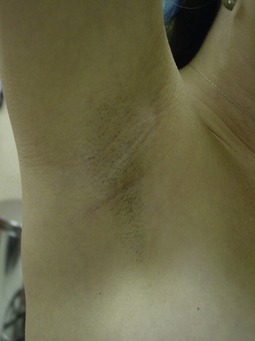
The transaxillary endoscopic technique (Fig. 53.9) is ideal for patients with minimal glandular tissue, little or no ptosis and an ill-defined inframammary fold. The incision is best suited for saline implants that can be filled once they have been placed, but silicone implants may be placed with extension of the incision up to 4 cm. The main advantage of this technique is that there are no scars created on the breast (Fig. 53.10). However, as the breast pocket is further away from the incision, it is more difficult to precisely alter the inframammary fold and correct asymmetries. Inadequate muscle release with this technique may lead to persistently high riding implants. This approach is not appropriate for patients with tubular breast deformities, and although possible, is impractical for subglandular placement. Also, revision surgeries may require an alternate counterincision if substantial modifications are to be made.
To achieve this, we utilize an angled retractor, which accommodates a 10 mm, 30-degree endoscope, along with a standard breast endoscopic instrumentation set. Snowden Pencer (Cardinal Health, Dublin, OH) has provided us with the most effective instrumentation for this procedure. For optimal results, the dissection should be performed bilaterally prior to implant placement. Once the pockets have been irrigated and the implants inserted, the patient is elevated to a sitting position to assess cosmesis as well as symmetry, and allow the surgeon to make any final adjustments prior to closing. The wound is then sutured in a layered fashion using 3-0 and 4-0 Monocryl, with running 5-0 Nylon for skin.
Postoperative care
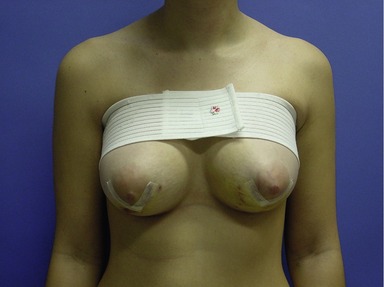
Once the surgical wounds have been reapproximated, Steri-Strips (3M, St Paul, MN) are placed on the incisions, and a 6-inch elastic wrap is placed around the superior chest and the breasts. This bandage is positioned fairly taut in order to prevent displacement of the implants, as well as to minimize edema and ecchymosis. Antibiotics with Gram-positive coverage are prescribed and begun in the immediate postoperative period. The patient is also placed on a narcotic analgesic for pain management and diazepam for its anxiolytic and muscle relaxing effects. At the first postoperative visit, the dressings are removed and replaced with a surgical bra or fitted bandeau, depending upon the specifics of the surgical technique (Fig. 53.11). For smooth-shelled implants, daily massage is recommended postoperatively at three weeks to facilitate in keeping the breast capsules soft and involve the patient in her own care. Patients are advised to refrain from exercise or heavy lifting (over 10 lb) for a minimum of two weeks after surgery.
Complications
Although rare, there is an estimated 0.5% incidence of hematoma following breast augmentation surgery (Baker, 1998). This postoperative complication is typically heralded by the onset of unilateral pain with progressive enlargement and ecchymosis. Risks are minimized by careful hemostasis intraoperatively as well as limiting hypertension and excessive activity in the postoperative period. Infection is also a potential complication, typically caused by flora indigenous to breast tissue. Intraoperatively, this is addressed with antibiotic irrigation and appropriate in travenous antibiotic prior to the incision being made. Nipple and areola sensation may be affected by breast surgery, resulting in hyperesthesia or dysesthesia. The vast majority of these changes are temporary, and tend to resolve over time. Capsular contracture may arise, and is commonly believed to be associated with residual blood or subclinical bacterial contamination, although the predisposing factors are still frequently debated. Again meticulous hemostasis, triple antibiotic pocket irrigation and the use of antimicrobial prophylaxis are certainly helpful, and a daily postoperative massage regimen may also be advantageous. If subglandular placement is planned, the use of a textured implant may be of benefit as has been previously suggested by the plastic surgery literature. However, downsides to textured implants include their thicker shell and potential palpability in patients with thin tissues.
Pearls & pitfalls
Pearls
• To assure symmetry in breast augmentation, arm boards should be attached to the operative table in identical positions and angles. We position them at 90-degree angles to the table.
• Surgical draping for breast augmentation should allow easy access both above and below the arm boards to facilitate pocket modification.
• When replacing saline with silicone implants, silicone requires a greater volume to appear comparable in size to the saline implant, given that most styles of silicone implants in the United States are underfilled.
• Marking the patient in a standing position and upright positioning during the operation aids in precise incision design and implant positioning.
• When employing an endoscopic technique, applying the evacuation tubing to the retractor as opposed to the cautery wand reduces potential clogging of the suction.
Pitfalls
• Breast augmentation is a surgery that requires clear communication with the patient regarding risks, benefits and trade-offs of the various approaches and implants so that informed choices can be made.
• Consider an alternate to the transaxillary approach in patients with a long chest and/or low breast position.
• Avoid selection of prostheses with a significantly greater base diameter than the natural breast, as this often produces unnatural looking results and leads to palpability of the implant edges.
• Upon initial dissection, when releasing pectoral attachments from the inferior chest wall, be certain to do so above the surface of a rib to avoid entry into the pleural space.
• Avoid underfilling saline implants as this increases the risk of rippling and potentially early failure of the shell.
Summary of steps
1. After informed consent has been obtained, the patient is marked in an upright position using a surgical pen. These markings, at minimum, should reflect the midline below the sternal notch, as well as an outline of the inframammary folds and proposed breast pockets with incisions.
2. Intravenous access is established and antibiotic prophylaxis is administered prior to commencing with the operation.
3. The patient is placed in the supine position and anesthetized under general endotracheal anesthesia, or by means of a laryngeal mask airway. The author prefers this to local anesthesia with sedation, as it allows for optimal muscular relaxation and subsequently a more precise pocket dissection.
4. The arms are secured on arm boards at 90 degrees, and at the level of the shoulders. Joint surfaces are inspected and padded.
5. The proposed access incisions and inframammary fold are injected with an approximate total of 50 mL 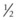 % lidocaine with epinephrine in a 1 : 200,000 ratio.
% lidocaine with epinephrine in a 1 : 200,000 ratio.
6. The patient is then widely prepped and draped in a sterile fashion, with a chest-breast or split sheet, allowing access both above and below the arm boards.
7. An incision is made, and one of the three approaches previously discussed is used.
8. Once the dissection is carried out to the pectoral fascia, a breast pocket is created in either the submuscular or subglandular plane, and hemostasis is attained. The author prefers a partial submuscular or dual plane technique in the vast majority of cases.
9. In moderate to severe cases of asymmetry using saline implants, and in most cases of asymmetry using silicone implants, an implant sizer is placed to determine size (and then overfilled with saline for intraoperative tissue expansion and tamponade).
10. Attention is then directed to the contralateral breast, and the operation is repeated in a similar manner.
11. After the appropriate implant has been selected, the sizers are removed.
12. The breast pockets are then copiously irrigated with an antibiotic solution, and again assessed for hemostasis. Triple antibiotic solution with bacitracin, cefazolin and gentamicin is preferred.
13. The prostheses are dispensed to the sterile field, bathed in the antibiotic solution, and prepared as required.
14. Once the implants have been placed, the patient is maneuvered into a sitting position to assess contour and symmetry, allowing the surgeon to make any final modifications. Assessment of the shoulders and clavicles is necessary to ensure proper positioning and therefore symmetry.
15. When completed, the patient is returned to the supine position and the surgical wounds are closed in a layered fashion.
16. Steri Strips are applied to the incisions, and a 6-inch elastic wrap is placed circumferentially, prior to emergence and transfer to the Recovery Room.
CASE STUDY: PATIENT NO. 1
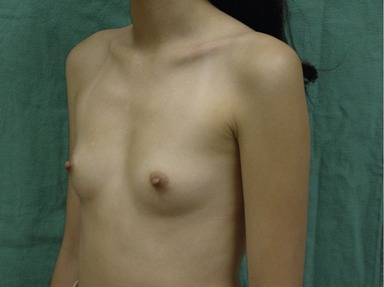
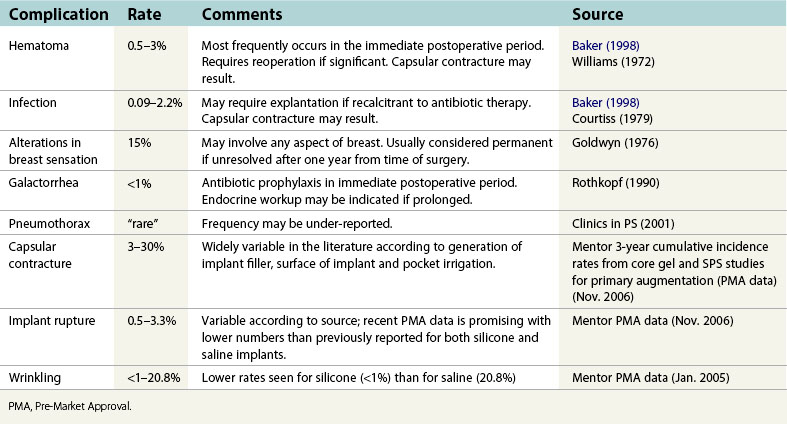
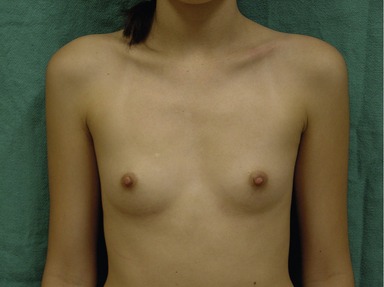
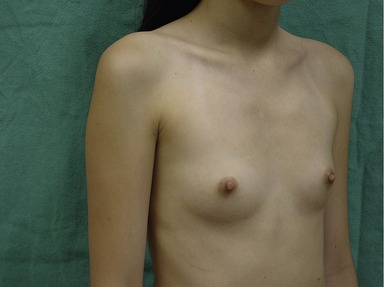
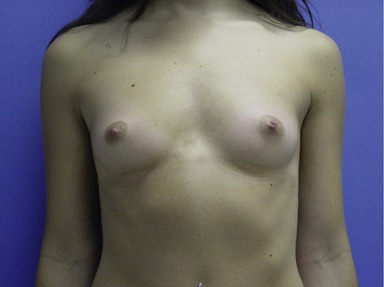
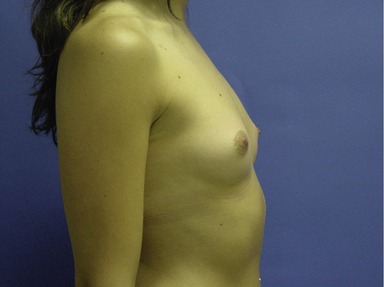
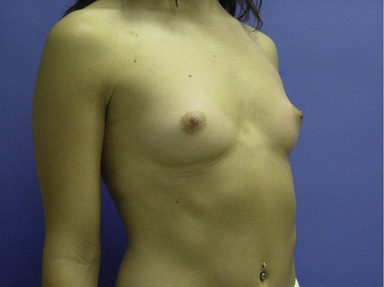
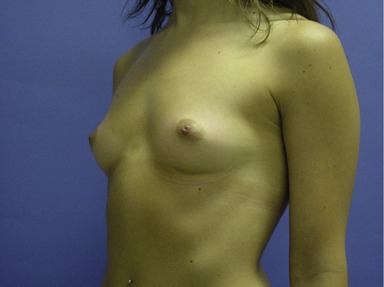
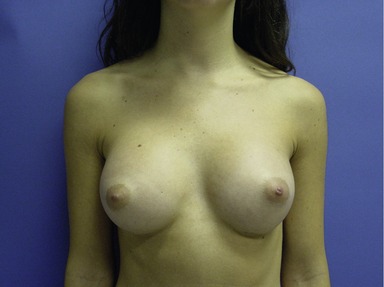
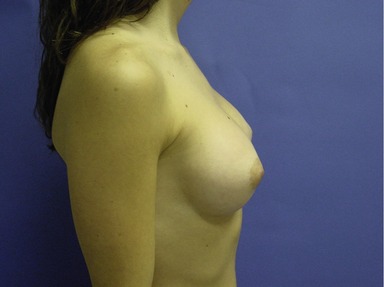
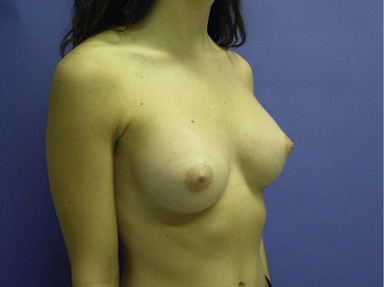
Figs 53.12, 13, 14 (Preoperative.) Patient No. 1: This is a 24-year-old woman with hypomastia, inframammary fold asymmetry, and a mild pectus excavatum who desires a modest breast augmentation before the FDA silicone gel implant reapproval in the U.S. With a height of 5 ft 5 in, weight of 110 lb, and base diameter of 10.5 cm, 250 mL smooth round moderate profile saline-filled implants were chosen. Implants have been placed through an inframammary crease incision in a submuscular pocket to produce a naturally enlarged look with more symmetric folds.

Figs 53.15, 16, 17 (Postoperative.) Patient No. 1: This is a 24-year-old woman with hypomastia, inframammary fold asymmetry, and a mild pectus excavatum who desires a modest breast augmentation before the FDA silicone gel implant reapproval in the U.S. With a height of 5 ft 5 in, weight of 110 lb, and base diameter of 10.5 cm, 250 mL smooth round moderate profile saline-filled implants were chosen. Implants have been placed through an inframammary crease incision in a submuscular pocket to produce a naturally enlarged look with more symmetric folds.
CASE STUDY: PATIENT NO. 2

Figs 53.18, 19, 20, 21, 22 (Preoperative.) Patient No. 2: This is a 23-year-old woman with vertically short breasts, high bases, and the right IMF slightly higher than the left. She desires an enlargement to a “C-cup”, has a height of 5 ft 3 in, weight of 109 lb, and has a base diameter of 12 cm. Postoperative photos show breast augmentation results with 286 mL midrange profile silicone-gel filled implants placed through the inframammary crease in a submuscular pocket. Note how the lower pole has expanded and given her a more aesthetic breast shape and symmetric fold height.

Figs 53.23, 24, 25, 26, 27 (Postoperative.) Patient No. 2: This is a 23-year-old woman with vertically short breasts, high bases, and the right IMF slightly higher than the left. She desires an enlargement to a “C-cup”, has a height of 5 ft 3 in, weight of 109 lb, and has a base diameter of 12 cm. Postoperative photos show breast augmentation results with 286 mL midrange profile silicone-gel filled implants placed through the inframammary crease in a submuscular pocket. Note how the lower pole has expanded and given her a more aesthetic breast shape and symmetric fold height.
Acknowledgment
The author would like to thank Walter Lampeter and Sarah Bruce for their assistance with this chapter.
Adams WP, Jr., Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: Six year prospective clinical study. Plast Reconstr Surg Adv Breast Augment. 2006;118((7S) Suppl):46S–52S.
Baker JL, Jr. Chapter 63. In: Spear SL, ed. Augmentation mammaplasty, surgery of the breast principles and art. Philadelphia: Lippincott-Raven; 1998:845–854.
Bostwick J, III. Plastic and reconstructive breast surgery, 2nd edn. St Louis: MO: Quality Medical Publishing; 2000.
Burkhardt BR, Demas CP. The effect of siltex texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg. 1994;93(1):123–130.
FDA approves silicone gel-filled breast implants after in-depth evaluation news release. http://www.fda.gov/bbs/topics/NEWS/2006/NEW01512.html, 16 November 2006.
Hidalgo DA. Breast augmentation: Choosing the optimal incision, implant, and pocket plane. Plast Reconstr Surg. 2000;105(6):2202–2216.
Muzaffar AR, Rohrich RJ. The silicone gel-filled breast implant controversy: An update. Plast Reconstr Surg. 2004;109(2):742–748.
Netter FH. Atlas of human anatomy. Basle: Ciba-Geigy Corporation; 1989.
Takayanagi S, Chisato N, Sugimoto Y. Augmentation mammaplasty: Where should the implant be placed? Aesthet Plast Surg. 2004;28:83–88.
Tebbetts JB. Dual plane breast augmentation: Optimizing implant–soft tissue relationships in a wide range of breast types. Plast Reconstr Surg. 2001;107(5):1255–1272.

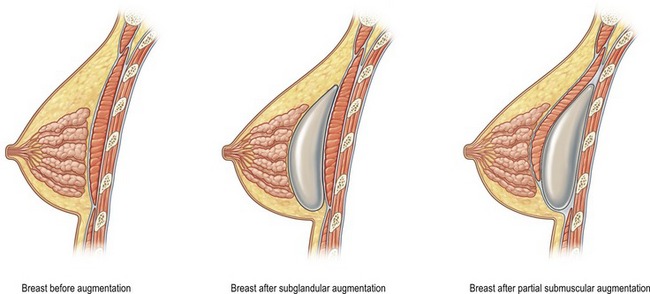
 of the implant is covered by breast gland and fascia, and the top
of the implant is covered by breast gland and fascia, and the top  by pectoralis major muscle.
by pectoralis major muscle.