Chapter 53 Branch Vein Occlusion
Introduction
Branch retinal vein occlusion (BRVO) is a common cause of retinal vascular disease.1 The Beaver Dam Study estimated the 15-year cumulative incidence of retinal vein occlusions (RVO) at 2.3% in the population, with a majority of these (78%) being BRVO.2 BRVO affects males and females equally and occurs most frequently between the ages of 60 and 70. The pathologic interruption of venous flow in these eyes almost always occurs at a retinal arteriovenous intersection, where a retinal artery crosses over a retinal vein.3–7 Systemic vascular diseases such as hypertension and arteriosclerosis are risk factors for BRVO, probably because they lead to thickening of the retinal artery.3,7 Other risk factors for BRVO include diabetes, smoking, hyperlipidemia, glaucoma, and ocular inflammatory disease.8 Antiphospholipid antibodies, elevated plasma homocysteine levels, and low serum folate levels have also been associated with increased risk of vein occlusion.9–11 A decreased risk is present in those with higher serum levels of high-density lipoprotein and light to moderate alcohol consumption.8 Other studies have suggested an increased risk of BRVO in eyes with shorter axial lengths.12–15 This section discusses the pathophysiology, clinical features, evaluation, and treatment of patients with BRVO.
Pathogenesis
Because BRVO mostly occurs at arteriovenous crossings,4,8,16 underlying arterial disease may play a causative role. In 99% of 106 eyes with BRVO, the artery was located anterior to the vein at the obstructed site.7,8 Histopathologically, the retinal artery and vein share a common adventitial sheath, and in some cases, a common medium.17 The lumen of the vein may be compressed up to 33% at the crossing site.7,18 The vitreous may also play a role in compression of susceptible arteriovenous crossing sites, as evidenced by studies demonstrating that eyes with decreased axial length and higher likelihood of vitreomacular attachment at the arteriovenous crossing are at increased risk of BRVO.13,19
Some have postulated that turbulent blood flow at the crossing site causes focal swelling of the endothelium and deeper vein wall tissue, leading to venous obstruction.17,19,20 Other reports have demonstrated actual venous thrombus formation at the point of occlusion.3,18 The resulting venous obstruction leads to elevation of venous pressure that may overload the collateral drainage capacity21 and lead to macular edema and ischemia by mechanisms that are still under investigation. Unrelieved venous pressure can also result in rupture of the vein wall with intraretinal hemorrhage.17 Vision loss from RVOs is typically due to macular ischemia, macular edema, or complications from neovascular disease.
Clinical features
Signs
Patients typically present with a wedge-shaped distribution of intraretinal hemorrhage that is less marked if the occlusion is perfused (or nonischemic), and more extensive if the occlusion is nonperfused (or ischemic) and associated with retinal capillary nonperfusion. The Branch Vein Occlusion Study Group defined ischemic BRVO as those with greater than a total of five disc diameters of nonperfusion on fluorescein angiography (FA).1 The location of the venous blockage determines the distribution of the intraretinal hemorrhage; if the venous obstruction is at the optic nerve head, two quadrants of the fundus may be involved, whereas if the occlusion is peripheral to the disc, one quadrant or less may be involved with the intraretinal hemorrhage. If the venous blockage is peripheral to tributary veins draining the macula, there may be no macular involvement and no decrease in visual acuity. The most common location for BRVOs is in the superotemporal quadrant.5,6,22 This favored location may be attributed to a larger number of arteriovenous crossings in the superotemporal quadrant.
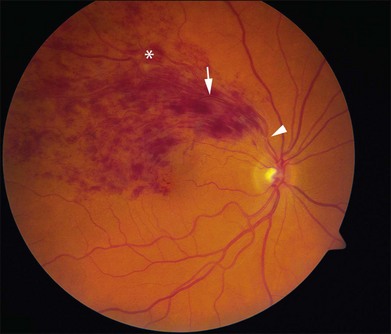
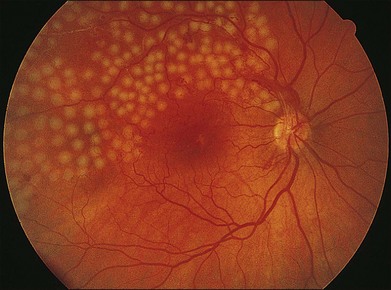
Figure 53.1 demonstrates the typical acute appearance of a BRVO involving the superotemporal quadrant of the right eye. A narrowed branch retinal vein passing under a retinal artery can sometimes be identified proximal to the hemorrhage. Rarely, a patient may present initially with very little intraretinal hemorrhage, which then becomes more extensive in the succeeding weeks to months. In these instances, it is presumed that an incomplete block at the arteriovenous crossing has progressed to more complete occlusion.
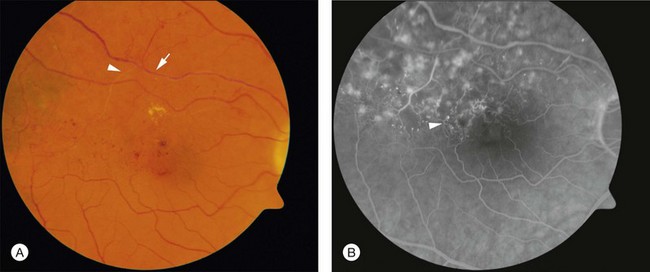
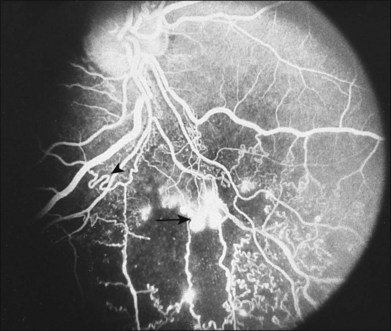
Over time the intraretinal hemorrhage may completely resorb. Without the characteristic segmental distribution of intraretinal hemorrhage, the ophthalmoscopic diagnosis may be more difficult, but the segmental distribution of retinal vascular abnormalities that occurred during the acute phase will persist and be apparent on FA. In many cases, macular edema may also be detected by optical coherence tomography (OCT). Consequently, in the chronic phase of the disease, after intraretinal hemorrhage absorption, the diagnosis may depend on detecting a segmental distribution of retinal vascular abnormalities that may include capillary nonperfusion, dilation of capillaries, microaneurysms, telangectatic vessels, and collateral vessel formation (Fig. 53.2).
Nonocular signs such as systemic hypertension have been associated with BRVOs.2,8,23 Thus, systemic blood pressure may be elevated. In bilateral cases or cases involving young patients, systemic manifestations of infectious disease, inflammatory or autoimmune conditions, neoplasm, or hypercoagulable states may be present.
Complications
There are three common vision-limiting complications of BRVO: (1) macular edema; (2) macular ischemia; and (3) sequelae of neovascularization. It is important to appreciate the variability of these complications before considering the benefits of treatment.24–26
Retinal and iris neovascularization, vitreous hemorrhage, traction retinal detachment, and neovascular glaucoma are complications that manifest late in the course of the disease due to ischemia. With the exception of macular ischemia, these complications can largely be treated or prevented. Thus, it is important that patients with BRVO be closely followed. From the Branch Vein Occlusion Study, 31–41% of patients with ischemic BRVO (defined as >5 disc diameters of nonperfusion on FA) developed neovascularization or vitreous hemorrhage, compared with 11% of patients with nonischemic BRVO.1
Clinical evaluation
Clinical examination
Fluorescein angiography
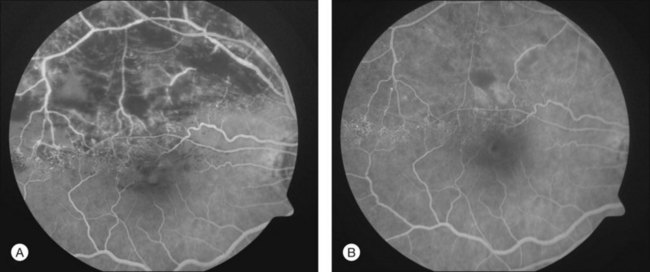
To help verify the diagnosis and evaluate for complications, FA should be obtained to delineate the retinal vascular characteristics that may have prognostic significance: macular leakage and edema, macular ischemia, and large segments of capillary nonperfusion that may portend eventual neovascularization. FA is the only technique that will accurately define the capillary abnormalities in BRVO; it is therefore particularly important that high-quality angiography be obtained (Fig. 53.3).
When FA demonstrates macular leakage and edema with cystoid involvement of the fovea, but no capillary nonperfusion, it is presumed that the macular edema is the cause of vision loss. Under these circumstances, about one-third of patients will spontaneously regain some vision. However, patients who have had decreased vision for over 1 year as a result of macular edema are much less likely to regain vision spontaneously. When macular edema is present ophthalmoscopically within the first 6 months after a BRVO and there is little or no leakage on FA, macular ischemia may be the cause of the macular edema. In such circumstances, the edema almost always spontaneously resorbs in the first year after the occlusion, often with return of vision.27
Wide-field angiography
Wide-field angiography is not a commonly used imaging modality for patients with BRVO, but a recent study supports its utility. Ultrawide-field FA using the Optos C200MA revealed a correlation between nonperfusion in the peripheral retina with macular edema and retinal neovascularization.28 Future studies to determine if laser photocoagulation of the nonperfused peripheral retina decreases macular edema and neovascularization may alter the therapeutic paradigm. In patients with recalcitrant macular edema or retinal neovascularization, wide-field angiography may reveal peripheral areas of nonperfusion helping to guide targeted laser photocoagulation.
Optical coherence tomography
OCT has arguably become the most important imaging modality in the treatment of patients with BRVO and macular edema. OCT offers a noninvasive and rapid method of quantitatively measuring macular edema. OCT is frequently used to monitor the response to treatment of macular edema and has been used in place of FA in some treatment trials for BRVO. Unlike FA, intraretinal hemorrhages have a minimal effect on the interpretation of OCT, making this imaging modality helpful, even in the acute setting with foveal hemorrhage. The characteristic findings of BRVO on OCT are cystoid edema, intraretinal hyperreflectivity from hemorrhages, shadowing from edema and hemorrhages, and occasionally subretinal fluid29,30 (Fig. 53.4A). In chronic cases, photoreceptor inner-segment–outer-segment junction abnormalities from long-standing macular ischemia and macular edema may also be seen (Fig. 53.4B).
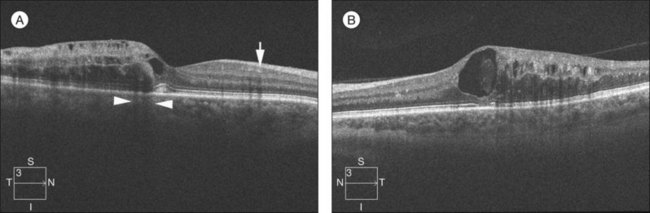
Fig. 53.4 Spectral-domain optical coherence tomography of an eye with a branch retinal vein occlusion (BRVO). (A) Raster scan of the BRVO in Figure 53.1 reveals cystoid macular edema, intraretinal fluid, and shadowing (between the arrowheads). Intraretinal heme (arrow) appears hyperreflective and produces a shadow on optical coherence tomography. (B) Raster scan of a chronic BRVO with inner-segment–outer-segment abnormalities and a large cyst.
Diagnostic workup
Young patient
BRVO typically occurs in patients beyond their sixth decade of life. When they present in younger patients, an underlying predisposing condition may be suspected. In this younger patient population, a detailed history should be taken, including the use of oral contraception in females, or the use of other medications that can promote a hypercoagulable state or thromboembolism. Workup should be performed in consultation with an internist. Systemic blood pressure should be checked and the patient should be screened for diabetes. In suspected cases, infectious causes such as Lyme disease, syphilis, or human immunodeficiency virus should be screened. In young patients with other systemic findings suggestive of inflammatory disease or coagulopathy, workup should include a complete blood count, prothrombin time/partial thromboplastin time/international normalized ratio, lipid panel, serum homocysteine, anticardiolipin antibodies, antinuclear antibodies with lupus anticoagulant, protein C/S, and activated protein C resistance (factor V Leiden).10,11,31
Bilateral or numerous BRVO patients
In bilateral cases or in cases with a history of multiple BRVOs, searching for an infectious or inflammatory disorder or hypercoagulopathy may be warranted. There are numerous case reports of patients with bilateral vein occlusions and systemic inflammatory disorders or hypercoagulopathies; however the vast majority can be attributed to systemic hypertension.32–37 The workup should proceed in the manner described for young patients.
Treatment options
Laser treatment
Branch Vein Occlusion Study for macular edema
The collaborative Branch Vein Occlusion Study (BVOS),38 a multicenter randomized clinical trial supported by the National Eye Institute, reported that argon laser photocoagulation may reduce visual loss from macular edema for those eyes that meet study eligibility criteria and are treated according to that protocol. Important eligibility criteria included fluorescein-proven perfused macular edema involving the foveal center, absorption of intraretinal hemorrhage from the foveal center, recent BRVO (usually 3–18 months’ duration), no diabetic retinopathy, and vision reduced to 20/40 or worse after best refraction.
In the BVOS,38 argon laser photocoagulation was applied in a grid pattern throughout the leaking area demonstrated by FA (Fig. 53.5). Coagulation extended no closer to the fovea than the edge of the capillary-free zone and no further into the periphery than the major vascular arcade. Recommended treatment parameters included a duration of 0.1 second, a 100-µm diameter spot size, and a power setting sufficient to produce a “medium” white burn. FA was repeated 2–4 months after the treatment, and additional photocoagulation was applied to residual areas of leakage if reduced visual acuity persisted. Improvement in visual acuity was assessed in several ways.38 When improvement was defined as reading two or more Snellen lines (beyond baseline) at two consecutive visits, treated eyes showed visual improvement more often than untreated eyes. After 3 years of follow-up, 63% of treated eyes gained two or more lines of vision, compared to 36% of untreated eyes. The average gain in visual acuity for treated eyes was one more Snellen line than in untreated eyes.
Before laser photocoagulation is performed, it is important to obtain high-quality FAs of the macula; the FA must demonstrate that the macular edema involves the center of the fovea and that there is not a large amount of capillary nonperfusion adjacent to the capillary-free zone that could explain the visual loss. In addition, it is important to follow patients for a length of time sufficient to ascertain that macular edema is not resolving spontaneously. During this period of follow-up, it should be demonstrated that there is clearing of intraretinal hemorrhage and that there is no hemorrhage in the center of the fovea that could account for a spontaneously reversible cause of visual loss. In the application of the grid photocoagulation, laser absorption occurs at the level of the pigment epithelium; photocoagulation is not applied to close the leaking and dilated capillary vasculature directly and immediately. Although it is not understood how the laser treatment may act in lessening edema, it is interesting to note that preliminary experimental studies in the normal primate have shown a decrease in capillary diameter when this form of therapy is used and when laser absorption occurs at the level of the pigment epithelium.39 One explanation for the effect of grid pattern photocoagulation is that it results in a thinning of the retina (in particular the outer retina), reducing oxygen consumption and increasing choroidal delivery of oxygen to the inner retina, producing a consequent autoregulatory constriction of the retinal vasculature in the leaking area and thereby decreasing the edema.
For the grid treatment used in the BVOS, the argon blue-green wavelength was employed.38 This is the only wavelength that has been proven effective and it is unknown whether argon green and krypton red photocoagulation are equally effective. In other diseases, when laser treatment is applied inside the capillary-free zone, it is recognized that krypton red and argon green laser photocoagulation are absorbed less than blue-green by the xanthophyll pigment of the inner retina that is present in increasing concentrations close to the foveal center. However, because the grid treatment never comes closer to the fovea than the capillary-free zone, the BVOS did not encounter any problems with the argon blue-green laser in this region; consequently, this laser continues to be recommended.
Branch Vein Occlusion Study for neovascularization
A separate group of patients in the BVOS were randomized to receive scatter panretinal photocoagulation to prevent neovascular complications. The BVOS demonstrated that prophylactic scatter laser photocoagulation can lessen subsequent neovascularization and, if neovascularization already exists, that peripheral scatter laser photocoagulation can lessen subsequent vitreous hemorrhage.1 Only eyes with the type of BRVO that shows large areas (>5 disc diameters) of retinal capillary nonperfusion are at risk for developing neovascularization. About 40% of these eyes develop neovascularization, and of this 40%, about 60% will experience periodic vitreous hemorrhage. Retinal or disc neovascularization, or both, may develop at any time within the first 3 years after an occlusion but are most likely to appear within the first 6–12 months after the occlusion. If peripheral scatter laser photocoagulation is applied in eyes with large areas of nonperfusion, the incidence of neovascularization can be reduced from about 40% to 20%. However, if one were to treat prophylactically, many eyes (60%) that would never develop neovascularization would receive peripheral scatter laser photocoagulation. For this reason, it is recommended that laser photocoagulation be applied only after neovascularization is observed.
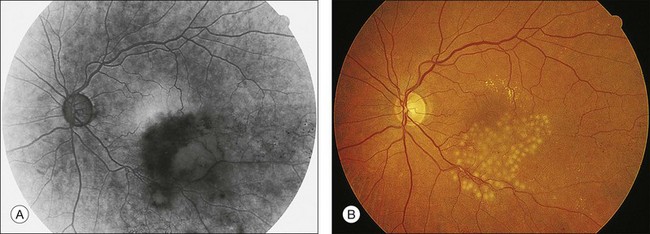
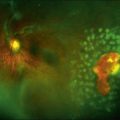
Iris neovascularization is a rare complication of BRVO; it appears, however, that diabetes (with or without retinopathy) may increase this risk. Retinal neovascularization is particularly difficult to recognize in BRVO because the collaterals that develop frequently may mimic neovascularization. Arising presumably from pre-existing capillaries, these collaterals occur as vein-to-vein channels around the blockage site, across the temporal raphe, and in other locations to bypass the blocked retinal segment. These collaterals frequently become quite tortuous, mimicking the appearance of neovascularization if they are evaluated by ophthalmoscopy alone. When it is unclear whether an abnormal vascular pattern represents collateral formation or true neovascularization, the FA (Fig. 53.6) can be helpful because leakage from neovascularization is more prominent than from collateral vessels.
The BVOS data strongly suggest that photocoagulation after the development of neovascularization is as effective in preventing vitreous hemorrhage as is photocoagulation before the development of neovascularization.1 When neovascularization is unequivocally confirmed by FA, peripheral scatter laser photocoagulation can reduce the likelihood of vitreous hemorrhage from about 60% to 30%. As demonstrated in Figure 53.7, the scatter laser photocoagulation can be applied with argon blue-green laser to achieve “medium” white burns (200–500 µm in diameter) spaced one burn width apart and covering the entire area of capillary nonperfusion, as defined by FA, but extending no closer than two disc diameters from the center of the fovea and extending peripherally at least to the equator. Retrobulbar anesthesia is used as needed for discomfort associated with the scatter photocoagulation.
Steroid treatment
Macular edema results from increased vascular permeability mediated at least in part by an increase in VEGF.40,41 Corticosteroids have been shown to inhibit the expression of VEGF and therefore reduce macular edema in animal models.42,43 The anti-inflammatory effects of corticosteroids may further potentiate its anti-VEGF effects and help attenuate macular edema. Intraocular corticosteroids, however, have significant side-effects, including cataract formation and glaucoma. Several trials have evaluated the use of corticosteroids in the treatment of macular edema in BRVO.
SCORE (triamcinolone) study
In the Standard care vs Corticosteroid for Retinal vein occlusion (SCORE) BRVO study, the effectiveness and safety of intravitreal triamcinolone acetate (IVTA) for the treatment of macular edema from BRVO were evaluated.44 In this multicenter, randomized controlled study, 411 patients were randomized to receive macular grid laser, 1 mg IVTA, or 4 mg IVTA. Retreatment was allowed every 4 months for each group unless the treatment was successful, futile, or contraindicated. There was no significant difference in vision or the reduction of macular edema measured by OCT at the end of 12 months between each group. Respectively, 29%, 26%, and 27% of eyes in the laser, 1 mg IVTA, and 4 mg IVTA groups gained a visual acuity score of ≥15 ETDRS letters. Subgroup analysis of pseudophakic eyes also failed to demonstrate a significant difference in vision. Median (interquartile range) center point thicknesses measured by OCT were 228 (163–364) µm, 354 (183–486) µm, and 274 (180–481) µm in the laser, 1 mg, and 4 mg IVTA groups, respectively. Three-year results from 128 patients suggested that the laser group maintained a significantly greater average increase in vision (12.9 letters) compared with the two IVTA groups (4.4 letters, 1 mg and 8.0 letters, 4 mg). Significant side-effects from IVTA included cataract formation and elevation of intraocular pressure requiring treatment. Both side-effects were dose-dependent.
GENEVA (dexamethasone implant) study
The Global Evaluation of implantable dexamethasone in retinal Vein occlusion with macular edema (GENEVA) study evaluated a sustained-release, biodegradable, dexamethasone intravitreal implant (Ozurdex, Allergan, Irvine, CA) for the treatment of macular edema in central retinal vein occlusion (CRVO) and BRVO patients.45 Ozurdex is a biodegradable copolymer of poly (d,l-lactide-co-glycolide) acid (PLGA) containing micronized dexamethasone. It is injected intravitreally through a pars plana route using a 23-gauge custom injector, and it gradually releases the total dose of dexamethasone over several months via Krebs cycle breakdown of the PLGA into lactic and glycolic acid, and finally into water and carbon dioxide. In this multicenter, randomized controlled study, an increase in best-corrected visual acuity (BCVA) of ≥15 ETDRS letters was achieved in 30% of the Ozurdex 0.7 mg group (n = 291), 26% of the 0.35 mg group (n = 260), and 13% of the sham group (n = 279) 60 days after injection (peak response) in patients with BRVO (P < 0.001 for each group versus sham). A statistically significant difference between both Ozurdex groups and sham was seen up to 90 days after injection. At 90 days after injection, there was a significant improvement (P < 0.001) in central retinal thickness measured by OCT in both Ozurdex groups, compared with the sham group. The mean ± sd decrease in central retinal thickness at 90 days was 208 ± 201 µm, 177 ± 197 µm, and 85 ± 173 µm in the 0.7 mg, 0.35 mg, and sham groups, respectively. The OCT results are from pooled data including both BRVO and CRVO patients. The only complications that were significantly greater in the Ozurdex groups compared with sham were elevated intraocular pressure and anterior-chamber cell. Most eyes with elevated intraocular pressures were successfully managed with topical therapy, but five eyes required a procedure to lower the pressure adequately. In the 6 months of this study, there was no difference in the rate of cataract formation, and no endophthalmitis cases were reported. A long-term study of repeated treatments is currently underway and will help determine the safety and optimal interval for retreatment.
Anti-VEGF treatment
Macular edema results from increased vascular permeability as a response to retinal nonperfusion. In patients with BRVO, retinal ischemia leads to the secretion of VEGF, which leads to increased vascular permeability, vasodilation, migration of endothelial cells, and neovascularization.40,41,46 Increased vascular permeability and perhaps vasodilation lead to retinal edema. Thus, inhibition of VEGF is an attractive treatment for macular edema from BRVO. There are several anti-VEGF agents currently being investigated for use in treatment of RVOs. We will discuss the use of ranibizumab (Lucentis), bevacizumab (Avastin), pegaptanib (Macugen), and aflibercept (Eylea). Bevacizumab is a full-length, humanized monoclonal antibody that binds all VEGF-A isoforms and is FDA-approved for colorectal cancer, but is used off-label in the eye. Ranibizumab is an affinity-purified, humanized monoclonal antibody fragment (Fab) that binds all VEGF-A isoforms. Pegaptanib is an aptamer targeted against only the VEGF 165 isoform, and no other isoforms. Aflibercept is a fusion protein composed of key binding domains from VEGF receptors 1 and 2 fused to the Fc portion of human immunoglobulin G. Aflibercept binds with high affinity to all VEGF-A isoforms and placental growth factor. At this time, only ranibizumab is FDA-approved for the treatment of RVO.
BRAVO (ranibizumab) study
The Branch Retinal Vein Occlusion (BRAVO) study was a prospective, multicenter, randomized controlled study to evaluate the efficacy and safety of ranibizumab in the treatment of macular edema from BRVO.47 Patients were randomized into three groups: (1) sham injection (n = 132); (2) 0.3 mg ranibizumab (n = 134); and (3) 0.5 mg ranibizumab (n = 131). In the first 6 months, injections were given monthly. A 28-day screening period excluded patients with spontaneous and rapid improvement in vision of >10 ETDRS letters. At month 3, a patient was eligible for rescue laser if a gain of <5 ETDRS letters, or improvement of <50 µm in central subfield thickness was observed compared with the visit 3 months prior. If rescue laser was not applied at month 3, the same criteria were used to determine eligibility for rescue laser at each subsequent monthly visit. At 6 months, both ranibizumab groups gained +16.6 and +18.3 ETDRS letters (0.3 mg and 0.5 mg groups, respectively) compared with a gain of +7.3 letters in the control group (P < 0.0001 for each group versus sham). The percentage of patients who improved greater than 15 ETDRS letters was 55.2% and 61.1% (0.3 mg and 0.5 mg groups, respectively) compared with 28.8% in the control (P < 0.0001 for each group versus sham). Concurrent with the improvement in visual acuity, the mean decrease in OCT central retinal thickness was –337.3 µm and –345.2 µm (0.3 mg and 0.5 mg groups, respectively) compared with –157.7 µm in the control (P < 0.0001 for each group versus sham). During the first 6 months, 54.5% of the control group required rescue laser therapy compared with 18.7% in the 0.3 mg and 19.8% in the 0.5 mg ranibizumab groups.
Other anti-VEGF inhibitors
Bevacizumab
There are numerous case series and small prospective studies evaluating the efficacy of intravitreal bevacizumab in treating macular edema from BRVO.48–55 All of these studies show that bevacizumab is effective at improving visual acuity and decreasing macular edema, as measured by OCT. One of the larger retrospective studies compared two different doses of bevacizumab, 1.25 and 2.5 mg.50 Forty-five patients with an average 35.2 weeks of follow-up completed the study. There were no functional or anatomical differences between the two dosages, and both required similar numbers of injections. At 6 months the 1.25 mg group improved by an average +5.1 lines compared with +4.8 lines in the 2.5 mg group. The central macular thickness decreased by –184 µm in the 1.25 mg group and –145 µm in the 2.5 mg group. A second large retrospective review with 1 year of follow-up showed similar results with a mean improvement of +13 letters at 6 months and +15 letters at 1 year with a decrease in central retinal thickness of –161 µm at 6 months and –205 µm at 1 year.53
Pegaptanib
A phase II study of pegaptanib for the treatment of BRVO showed significant improvements in vision and central macular thickness.56 However, due to limited enrollment with the increasing use of bevacizumab and ranibizumab, larger trials with pegaptanib have not been conducted. For the most part, with the availability of bevacizumab and ranibizumab, pegaptanib is not used as a primary treatment for macular edema in BRVO patients.
Aflibercept
Currently, there are no trials evaluating intravitreal aflibercept specifically for macular edema from BRVO. However, two multicenter, randomized controlled phase III trials (COPERNICUS and GALILEO) are evaluating its use in patients with macular edema secondary to CRVO. In the COPERNICUS study, patients with macular edema from CRVO were randomized to receive 2 mg aflibercept (n = 114) or sham (n = 74). Only patients with vision between 20/40 and 20/320 with central retinal thickness of ≥250 µm were included. Patients were retreated every 4 weeks with aflibercept. Preliminary results of the COPERNICUS study57 showed that, after 24 weeks, 56.1% of the treatment group gained ≥15 ETDRS letters compared with 12.3% of the sham group (P < 0.0001). There was an average of +17.3 ETDRS letters gained in the treatment group compared with –4.0 letters lost in the sham (P < 0.001). There was a reduction of –457.8 µm in central retinal thickness in the treatment group compared with –144.8 µm in the sham group (P < 0.001). A parallel phase III clinical trial (GALILEO) is currently underway with preliminary results that are similar to the COPERNICUS study.
Experimental treatments
Surgical management
Vitrectomy with or without sheathotomy
The majority of the venous lesions in BRVO occur downstream from the arteriovenous crossing site. In a retrospective review of color photographs and FAs of patients with BRVO, Kumar and associates19 identified venous narrowing at the crossing site, and in the majority of cases, evidence of downstream hemodynamic changes on angiogram, including venous-phase leakage, abnormal flow, and presumed thrombi. The authors also suggested that removal of the compressive factor by sectioning the adventitial sheath (sheathotomy) may be an effective treatment for BRVO.
In the first report of sheathotomy for BRVO, Osterloh and Charles58 reported significant visual improvement in the one case presented (20/200 to 20/25+ over 8 months). In the second report, Opremcak and Bruce59 reported equal or improved visual acuity in 12 of 15 patients (80%). Ten of those patients (67%) had improved postoperative visual acuities, with an average gain of four lines of vision. Three patients had a decline in visual acuity, with an average of two lines of vision lost. All patients had marked resolution of the intraretinal hemorrhage and edema. Visual symptoms from BRVO ranged from 1 to 12 months, with an average of 3.3 months. In 2 patients, intraoperative retinal vascular bleeding was controlled with intraocular diathermy. No patient in the series developed worsening edema, ischemia, retinal neovascularization, or secondary vitreous hemorrhage. Mester and Dillinger reported 43 cases of BRVO treated with sheathotomy with similar results. In 16 of the cases, removal of the internal limiting membrane in the area of the arteriovenous crossing was also performed.60 In contrast, Cahill and colleagues reported 27 cases of BRVO treated with vitrectomy and sheathotomy without a statistically significant improvement in postoperative median visual acuity.61
Other authors have experienced difficulty in separating the artery from the vein at the crossing site. Han and colleagues reported 20 cases of vitrectomy and attempted sheathotomy. While the visual outcome results were similar to those reported by Opremcak and Bruce, in 19 of the 20 cases, the authors were unable to separate the artery from the vein.62 No randomized, controlled study evaluating the benefit of sheathotomy has been published.63
There is evidence that vitreomacular attachment itself may contribute to the development of macular edema in BRVO.64 Saika and coworkers reported reduction in macular edema and restoration of normal foveal contour in 10 of 19 eyes after vitrectomy, posterior hyaloid separation, and intraocular gas tamponade.65 Possible explanations for the clinical improvements in these studies include removal of vitreous traction, increased oxygenation of the macula, and tamponade of the macula by intraocular gas.
1 Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Arch Ophthalmol. 1986;104:34–41.
2 Klein R, Moss SE, Meuer SM, et al. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–518.
3 Bowers DK, Finkelstein D, Wolff SM, et al. Branch retinal vein occlusion. A clinicopathologic case report. Retina. 1987;7:252–259.
4 Weinberg D, Dodwell DG, Fern SA. Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am J Ophthalmol. 1990;109:298–302.
5 Feist RM, Ticho BH, Shapiro MJ, et al. Branch retinal vein occlusion and quadratic variation in arteriovenous crossings. Am J Ophthalmol. 1992;113:664–668.
6 Zhao J, Sastry SM, Sperduto RD, et al. Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Ophthalmology. 1993;100:423–428.
7 Frangieh GT, Green WR, Barraquer-Somers E, et al. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982;100:1132–1140.
8 Eye Disease Case-control Study Group. Risk factors for branch retinal vein occlusion. Am J Ophthalmol. 1993;116:286–296.
9 Lahey JM, Kearney JJ, Tunc M. Hypercoagulable states and central retinal vein occlusion. Curr Opin Pulm Med. 2003;9:385–392.
10 Lahey JM, Tunc M, Kearney J, et al. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology. 2002;109:126–131.
11 Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol. 2003;136:1136–1150.
12 Ariturk N, Oge Y, Erkan D, et al. Relation between retinal vein occlusions and axial length. Br J Ophthalmol. 1996;80:633–636.
13 Majji AB, Janarthanan M, Naduvilath TJ. Significance of refractive status in branch retinal vein occlusion. A case-control study. Retina. 1997;17:200–204.
14 Simons BD, Brucker AJ. Branch retinal vein occlusion. Axial length and other risk factors. Retina. 1997;17:191–195.
15 Timmerman EA, de Lavalette VW, van den Brom HJ. Axial length as a risk factor to branch retinal vein occlusion. Retina. 1997;17:196–199.
16 Duker JS, Brown GC. Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol. 1989;107:998–1000.
17 Seitz R. The retinal vessels. Saint Louis: CV Mosby; 1964. pp. 20–74
18 Bandello F, Tavola A, Pierro L, et al. Axial length and refraction in retinal vein occlusions. Ophthalmologica. 1998;212:133–135.
19 Kumar B, Yu DY, Morgan WH, et al. The distribution of angioarchitectural changes within the vicinity of the arteriovenous crossing in branch retinal vein occlusion. Ophthalmology. 1998;105:424–427.
20 Clemett RS. Retinal branch vein occlusion. Changes at the site of obstruction. Br J Ophthalmol. 1974;58:548–554.
21 Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999;106:2054–2062.
22 Klein R, Klein BE, Moss SE, et al. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. discussion 141–3
23 Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–1247.
24 Gutman FA, Zegarra H. Macular edema secondary to occlusion of the retinal veins. Surv Ophthalmol. 1984;28(Suppl):462–470.
25 Hayreh SS, Rojas P, Podhajsky P, et al. Ocular neovascularization with retinal vascular occlusion – III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488–506.
26 Joffe L, Goldberg RE, Magargal LE, et al. Macular branch vein occlusion. Ophthalmology. 1980;87:91–98.
27 Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol. 1992;110:1427–1434.
28 Prasad PS, Oliver SC, Coffee RE, et al. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117:780–784.
29 Spaide RF, Lee JK, Klancnik JK, Jr., et al. Optical coherence tomography of branch retinal vein occlusion. Retina. 2003;23:343–347.
30 Lerche RC, Schaudig U, Scholz F, et al. Structural changes of the retina in retinal vein occlusion – imaging and quantification with optical coherence tomography. Ophthalmic Surg Lasers. 2001;32:272–280.
31 Turello M, Pasca S, Daminato R, et al. Retinal vein occlusion: evaluation of “classic” and “emerging” risk factors and treatment. J Thromb Thrombolysis. 2010;29:459–464.
32 Chai SM, Mathur R, Ong SG. Retinal vasculopathy in Fanconi anemia. Ophthalmic Surg Lasers Imaging. 2009;40:498–500.
33 Sungur G, Hazirolan D, Hekimoglu E, et al. Late-onset Behcet’s disease: demographic, clinical, and ocular features. Graefes Arch Clin Exp Ophthalmol. 2010;248:1325–1330.
34 De Salvo G, Li Calzi C, Anastasi M, et al. Branch retinal vein occlusion followed by central retinal artery occlusion in Churg–Strauss syndrome: unusual ocular manifestations in allergic granulomatous angiitis. Eur J Ophthalmol. 2009;19:314–317.
35 Mandel ER, Schwartz PL, Rosen DA. Bilateral retinal branch vein occlusion. Ann Ophthalmol. 1982;14(387):390–391.
36 Tseng MY, Chen YC, Lin YY, et al. Simultaneous bilateral central retinal vein occlusion as the initial presentation of acute myeloid leukemia. Am J Med Sci. 2010;339:387–389.
37 Yau JW, Lee P, Wong TY, et al. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904–910.
38 Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98:271–282.
39 Wilson DJ, Finkelstein D, Quigley HA, et al. Macular grid photocoagulation. An experimental study on the primate retina. Arch Ophthalmol. 1988;106:100–105.
40 Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487.
41 Noma H, Minamoto A, Funatsu H, et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2006;244:309–315.
42 Zhang X, Bao S, Lai D, et al. Intravitreal triamcinolone acetonide inhibits breakdown of the blood–retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57:1026–1033.
43 McAllister IL, Vijayasekaran S, Chen SD, et al. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009;147:838–846. 846.e1–2
44 Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115–1128.
45 Haller JA, Bandello F, Belfort R, Jr., et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. e3
46 Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262–271.
47 Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–1112. e1
48 Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007;27:419–425.
49 Kriechbaum K, Michels S, Prager F, et al. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol. 2008;92:518–522.
50 Wu L, Arevalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008;28:212–219.
51 Gunduz K, Bakri SJ. Intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Eye (Lond). 2008;22:1168–1171.
52 Jaissle GB, Leitritz M, Gelisken F, et al. One-year results after intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2009;247:27–33.
53 Gregori NZ, Rattan GH, Rosenfeld PJ, et al. Safety and efficacy of intravitreal bevacizumab (avastin) for the management of branch and hemiretinal vein occlusion. Retina. 2009;29:913–925.
54 Figueroa MS, Contreras I, Noval S, et al. Results of bevacizumab as the primary treatment for retinal vein occlusions. Br J Ophthalmol. 2010;94:1052–1056.
55 Pai SA, Shetty R, Vijayan PB, et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol. 2007;143:601–606.
56 Wroblewski JJ, Wells JA, 3rd., Gonzales CR. Pegaptanib sodium for macular edema secondary to branch retinal vein occlusion. Am J Ophthalmol. 2010;149:147–154.
57 VEGF Trap-Eye In CRVO. Primary endpoint results of the phase 3 COPERNICUS study. ARVO 2011; May 3, 2011; 2011.
58 Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol. 1988;106:1469–1471.
59 Opremcak EM, Bruce RA. Surgical decompression of branch retinal vein occlusion via arteriovenous crossing sheathotomy: a prospective review of 15 cases. Retina. 1999;19:1–5.
60 Mester U, Dillinger P. Vitrectomy with arteriovenous decompression and internal limiting membrane dissection in branch retinal vein occlusion. Retina. 2002;22:740–746.
61 Cahill MT, Kaiser PK, Sears JE, et al. The effect of arteriovenous sheathotomy on cystoid macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2003;87:1329–1332.
62 Han DP, Bennett SR, Williams DF, et al. Arteriovenous crossing dissection without separation of the retina vessels for treatment of branch retinal vein occlusion. Retina. 2003;23:145–151.
63 Cahill MT, Fekrat S. Arteriovenous sheathotomy for branch retinal vein occlusion. Ophthalmol Clin North Am. 2002;15:417–423.
64 Takahashi MK, Hikichi T, Akiba J, et al. Role of the vitreous and macular edema in branch retinal vein occlusion. Ophthalmic Surg Lasers. 1997;28:294–299.
65 Saika S, Tanaka T, Miyamoto T, et al. Surgical posterior vitreous detachment combined with gas/air tamponade for treating macular edema associated with branch retinal vein occlusion: retinal tomography and visual outcome. Graefes Arch Clin Exp Ophthalmol. 2001;239:729–732.