CHAPTER 203 Brainstem Glioma
History and Definition
Brainstem gliomas (BSGs) are a histologically heterogeneous group of tumors that are primarily defined by their location. They present unique challenges to the operative neurosurgeon, are some of the most difficult-to-treat pediatric brain tumors, and require a multidisciplinary approach. However, progress has been made since the beginning of the 20th century, when diffuse pontine glioma (DPG), one of the most commonly encountered tumors in this group, was viewed with a sense of hopelessness.1 Contemporary imaging with magnetic resonance imaging (MRI) has allowed better anatomic localization and classification of these tumors. Although DPG still remains a fatal tumor, multimodality treatment of the other brainstem tumors provides long-term survival in the majority of these patients.
By definition, BSGs are tumors that arise within the anatomic structures that make up the brainstem. Advances in neuroimaging have allowed us to better define these tumors, and a number of different classification schemes that have also incorporated some of their known biologic features have been suggested.2–4 In addition to DPG, definition plus classification into tectal, focal pontine, and midbrain tumors, dorsal exophytic tumors, and tumors of the cervicomedullary junction (CMJ) is practical and most useful.
Epidemiology
BSGs account for 10% to 20% of primary pediatric brain tumors and about 25% of tumors arising in the posterior fossa, with an incidence of 150 to 300 new cases per year in the United States. The mean age at diagnosis in several large series is 6.5 to 9 years (ranging from several months to the late teens), without a predilection for gender or race and without outstanding genetic inheritance.5 DPGs are the most commonly seen BSGs in the pediatric population and represent approximately 60% to 75% of these tumors.
Signs and Symptoms
Diffuse Pontine Glioma
Most children with DPG have evidence of cerebellar dysfunction (87%), multiple lower cranial nerve palsies (77%), and motor paresis and sensory changes secondary to long-tract involvement (53%).5 The presence of this classic triad almost always makes the diagnosis on a clinical basis alone.
The extent and duration of symptoms are usually less than expected given the size of the tumor on imaging studies and the involved brainstem structures. The duration of symptoms is relatively short, and 94% of patients will have had symptoms for less than 6 months at time of initial evaluation. The duration of symptoms is a predictor of outcome, with a better outcome if symptoms were present for 6 months or longer and a significantly worse outcome for tumors with symptoms lasting 1 month or less.5
Tectal Glioma
Tectal gliomas are considered a subgroup of focal BSGs and are most often initially manifested as hydrocephalus secondary to aqueductal stenosis.6–8 Despite involvement of the quadrigeminal plate, the classic Parinaud syndrome is not as commonly seen with these tumors as with pineal tumors causing compression on this structure. Most patients have had a long-standing history of headaches and on occasion “clumsiness” or ataxia, and imaging studies are usually obtained in the course of investigation of the headaches, thus establishing the diagnosis. Tectal lesions may in rare cases be accompanied by oculomotor palsy.6 Many patients will have macrocephaly on examination, in addition to the other neurological findings. Rapidly progressive symptoms should raise concern about the biologic behavior and pathology of these tumors. Atypical or more aggressive tectal gliomas tend to occur in younger patients.9
Focal Tumors
Focal tumors can arise anywhere in the brainstem and cause symptoms that reflect the involved anatomic structures. Midbrain lesions are commonly manifested as hydrocephalus and focal neurological deficits, depending on the location of the tumor. Focal pontine lesions are characterized by isolated facial palsies, long-tract signs, or hearing loss. Lesions in the medulla oblongata involve the lower cranial nerves and can cause respiratory difficulties (including apnea), recurrent upper respiratory tract infections and pneumonia, and swallowing difficulties with aspiration of food and saliva. In infants, failure to thrive may be the initial symptom. The gag reflex may be affected, and tongue asymmetry might be present.10
Exophytic Tumors
These tumors differ from the other subgroups of BSGs in that they are the only BSG originating from the subependymal glia in the floor of the fourth ventricle and growing posteriorly, thereby filling the fourth ventricle.11 Only about 10% of the tumor mass growth occurs in the brainstem, which is spared for a long time and thus results in very gradually progressive symptoms. Careful history taking commonly reveals that the initial symptoms have been present for longer than 1 year.12 Obstruction of cerebrospinal fluid (CSF) pathways in the fourth ventricle is not uncommon and leads to intractable vomiting and failure to thrive in infants and headaches, vomiting, and ataxia in older children. Papilledema and torticollis are frequently present on examination and indicate increased intracranial pressure and chronic tonsillar herniation.12
Diagnosis
Computed Tomography
CT can show the rare presence of calcifications in brainstem tumors better than MRI can. If present, this usually implies an atypical BSG rather than a DPG.15
Single-photon emission CT using thallium 201 shows accumulation of the radioactive tracer in the tumor, which is 90% specific for a BSG but is not correlated with tumor grade, enhancement patterns of other contrast media, or necrosis.16
Magnetic Resonance Imaging
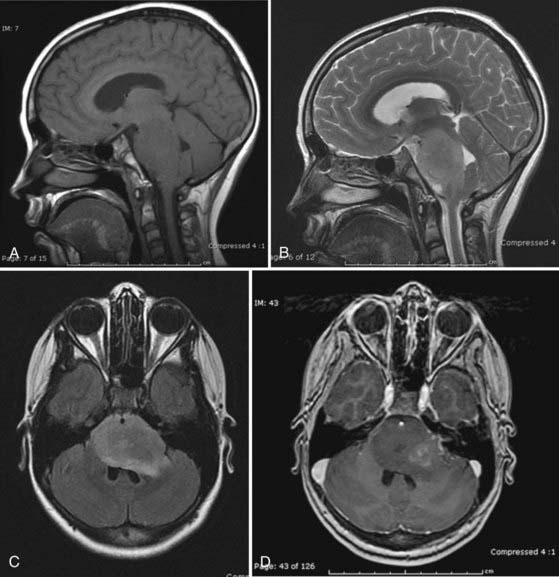
MRI is the preferred method for imaging brainstem tumors. DPGs are generally hypointense on T1-weighted sequences with diffuse enlargement of the pons and indistinct margins. T1-weighted images tend to underestimate the true extent of the tumor, which is best appreciated on T2-weighted images and is hyperintense on these sequences. The tumors characteristically engulf the basilar artery ventrally. Contrast enhancement is variable and may be nonhomogeneous inside or around the tumor or may be absent altogether in about a third of patients,15 so it is of limited prognostic value (Fig. 203-1).17 Dissemination along the CSF pathways is rare, although it can be seen at diagnosis in about 15% of cases, and therefore it is imperative that the entire CNS axis be imaged at time of initial diagnosis.
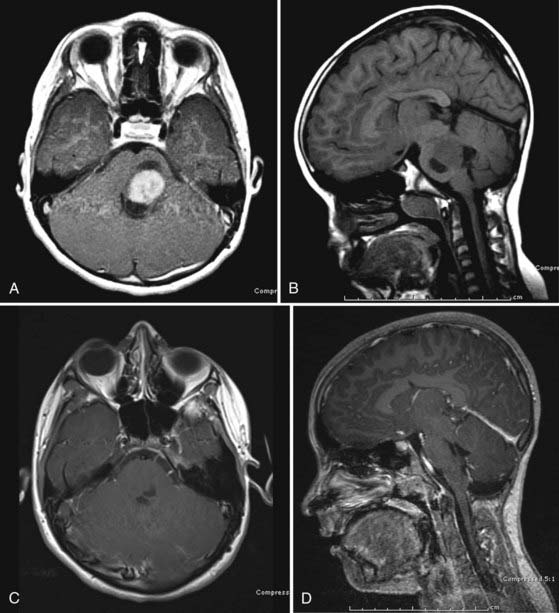
Focal lesions appear to be better circumscribed and are generally isointense on T1-weighted sequences and brightly enhancing. They occupy less than 50% of a brainstem region, thus leading to the designation “focal.” Focal tumors are more common in the midbrain and medulla and least common in the pons; they are usually well delineated without a large amount of edema or evidence of focal infiltration (Fig. 203-2).2,17
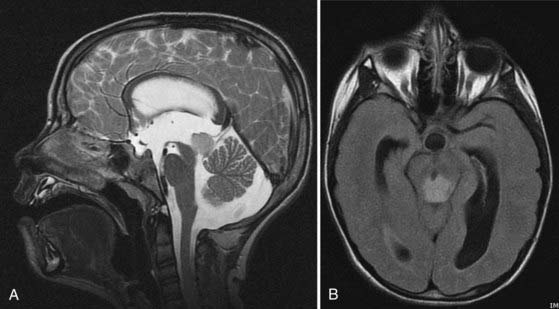
Tectal tumors are infiltrating lesions in the tectal plate that are poorly delineated and rarely enhance. They are usually hypointense on T1-weighted images and bright on T2-weighted sequences (Fig. 203-3). Size larger than 2 cm and enhancement are features associated with a worse outcome and suggest an atypical tectal tumor.18
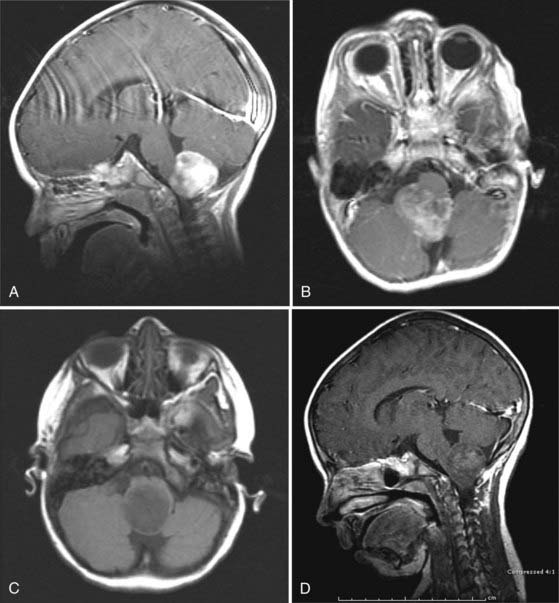
CMJ tumors are similar in appearance to infiltrating gliomas, with evidence of growth in the medulla and cervical spinal cord. The epicenter is usually at the foramen magnum area, and there is frequently a large exophytic component that is either obstructing the outlet of the fourth ventricle or located in the cerebellopontine angle and causing compression and distortion of the lower medulla (Fig. 203-4).
Magnetic resonance spectroscopy in patients with BSG has shown lower N-acetylaspartate levels than in normal controls, which may be helpful in distinguishing them from the benign diffuse pontine enlargements seen in patients with neurofibromatosis type 1 (NF1) or acute demyelinating encephalomyelitis.19,20 Lesions associated with NF1 tend to be multifocal, frequently also extend into the cerebellar peduncles, and may show contrast enhancement.21
Fluorodeoxyglucose positron emission tomography has been used to differentiate between low-grade and high-grade lesions and also to evaluate response to treatment. Diffusion tensor imaging has recently been introduced in an attempt to identify involvement of white matter tracts by the tumor and potentially aid in the surgical management of these tumors.22
Differential Diagnosis
Most diffuse lesions in the pediatric population are fibrillary infiltrating gliomas, but the histologic grade can vary considerably between low grade (37%), anaplastic (56%), and glioblastoma (5%).5 The true incidences are not known because of the low biopsy and resection rate of these tumors. Other pathologies include pilocytic astrocytoma, infantile ganglioglioma, ganglioglioma and gangliocytoma, primitive neuroectodermal tumor, and atypical teratoid-rhabdoid tumor.
Pathobiology
Patients with NF1 may have intrinsic lesions in the brainstem that at least radiographically resemble diffuse BSGs but appear to follow a more benign course. Focal lesions in this context may be indolent.23 In a series of 21 patients with brainstem masses and NF1, only 9 had radiographic and 3 had clinical disease progression with a follow-up period of 3.75 years.24–26
Fibrillary Astrocytoma
Most BSGs are diffuse fibrillary astrocytomas. However, grading is difficult because of the issue of sampling and the relative rarity of specimens available for pathologic examination. They are predominantly composed of fibrillary neoplastic astrocytes with nuclear atypia. Mitotic activity (Ki-67/MIB-1 labeling index) is usually less than 4%, and necrosis and microvascular proliferation can be present. Molecular characterization commonly shows TP53 mutations (>60%), although they are not a predictor of outcome, loss of heterozygosity of 22q (in about a third), and less often a number of other chromosomal abnormalities.27
The cytogenetic abnormalities of diffuse BSGs resemble those of adult malignant gliomas, including TP53 mutations and mutated epidermal growth factor receptor genes. In a study of six patients, 50% had multiple abnormalities and progressed rapidly.28 ERBB1 was found to be overexpressed in some DPGs, and the grade of amplification correlated with the grade of the tumor.29
Pilocytic Astrocytoma
Cytogenetic analysis of 132 tumors showed a normal karyotype in the majority of cases. Focal brainstem lesions are most often pilocytic astrocytomas and pathologically do not show any difference from pilocytic astrocytomas elsewhere in the central nervous system. However, pilocytic astrocytomas arising in patients with NF1 are genetically distinct from the sporadic tumors. Differences include loss of normal NF1 expression, Ras activation, and hyperactivation of the mTOR (mammalian target of rapamycin) pathway. In a large series of pediatric pilocytic astrocytomas in NF1 patients, the tumors were found to be less aggressive than non-NF1 lesions.30 They differ from diffuse fibrillary astrocytomas in their altered and increased activation of immune response genes and higher content of proliferating microglia.27
Ganglioglioma and Gangliocytoma
Chromosomal abnormalities were found in about a third of 30 patients who were studied, and gain of chromosome 7 was the most common alteration.27,31 Although the power of correlation is questionable, it was noted that in 3 patients with adverse outcomes, chromosomal abnormalities were present. Anaplastic changes and high MIB-1 and TP53 labeling indices may indicate more aggressive behavior.27
Atypical Teratoid/Rhabdoid Tumor
First described in 1985, this tumor entity occurs commonly in the infratentorial compartment and brainstem in children younger than 6 years. Previously, these tumors were diagnosed as either primitive neuroectodermal tumors, medulloblastomas, or malignant gliomas. Mutation or loss of the INI1 locus on 22q11.2 is the typical chromosomal abnormality of atypical teratoid/rhabdoid tumor. Age older than 3 years at the time of diagnosis is associated with longer survival, possibly related to the ability to use more aggressive therapies.27
Treatment
DPG is a universally fatal tumor, and patients usually succumb to their illness within 6 to 24 months after diagnosis and treatment and about 3 to 6 months after local progression.32 Median survival is 10 months, and just 13% of patients survive 3 years.5 Systemic metastasis has been reported in rare cases, but leptomeningeal disease occurs in 30% of patients after relapse.33
Biopsy
Diagnostic biopsy plays only a limited role in the management of BSGs. The prognosis of patients with DPG is not related to its histologic grade,34 and contemporary management is mostly independent of the histology of the tumor, thus making biopsy not indicated in cases in which the imaging features are characteristic of DPG. Atypical features are an indication for biopsy, although overall prognosis and outcome have not been significantly affected.
Biopsy can at times be indicated for some of the focal or exophytic lesions in the brainstem. Techniques include open biopsy or stereotactic biopsy (either frameless or fixed). In one series of patients with BSG who underwent stereotactic biopsy, there was a 6% to 11% morbidity rate.35 Transient and permanent morbidity rates of 5.6% and 1.4%, respectively, were reported in another series of CT-guided stereotactic biopy.36
Surgery
The exceptions are tectal tumors in which hydrocephalus is the initial and, frequently, the sole symptom and the only treatment required is for the hydrocephalus. There is no need for biopsy of tectal tumors, and the treatment recommended for the hydrocephalus is endoscopic third ventriculostomy.37,38
Focal
Surgical risk is related to the anatomy and type of the tumor—surgery for cystic and pilocytic tumors is generally associated with less morbidity than that for solid or infiltrating fibrillary astrocytomas, and although some patients will undoubtedly benefit from this procedure, others may experience significant morbidity.39
Surgery on tumors in the medulla carries a significant risk for the need for tracheostomy and feeding gastrostomy. Fortunately, the considerable recovery potential in children makes these interventions temporary.40 Surgery in the region of the pons and midbrain carries a risk for permanent eye movement problems and injury to the long tracts.
Exophytic
The approach to exophytic tumors, which frequently fill a large portion of the fourth ventricle, is generally through a midline posterior fossa craniotomy. There is usually no indication to split the vermis, and elevation of the cerebellar tonsils via a telovelar approach will allow access to the tumor.41–44
Adjuvant Therapy
Radiation Therapy
The administration of temozolomide after RT was found to be efficacious for high-grade gliomas but did not alter the poor prognosis associated with newly diagnosed DPG in children.45,46
Many studies have examined the effects of dose escalation and hyperfractionation but have not shown evidence of dose-dependent improvement of the 2-year survival rate, which remained at 3% to 21%, and of median survival, which also stayed at 9 to 13 months.47–51 Transient clinical stabilization occurs in about 70% of patients with therapy. Prados and colleagues reported reduction of tumor size in 30%, cessation of tumor growth in 40%, and progression in 30%.51 Currently, there is no role for stereotactic radiosurgery in the management of DPG.
Stereotactic RT, either fractionated or single dose, can be very effective for the treatment of recurrent or residual low-grade focal or infiltrative tumors that are unresectable or have failed chemotherapy.52 One series reported 89% tumor control after 4 years with the use of three-dimensional conformal RT in small fractions.17
Chemotherapy
Chemotherapy has been used in the treatment of DPG for the past 4 decades without significant success. Response rates of up to 15% to 20% have been observed, but no survival benefit has been found in a number of phase II trials evaluating various regimens of cyclophosphamide, carboplatin, cisplatin, etoposide, and thiotepa, some in combination with blood-brain barrier–disrupting agents.53–57 Metronomic therapy involves the administration of continuous low-dose chemotherapy to block mechanisms stimulating the growth of new blood vessels needed to feed the tumor and has been investigated for the treatment of central nervous system malignancies in children.58 It has shown promising results in the management of gliomas, although it has not had a significant effect on DPG. Adjuvant therapy for the remainder of the BSGs follows the same principles as for gliomas in other locations in the central nervous system.
Combination Therapy
A Pediatric Oncology Group study did not find a significant difference between hyperfractionated irradiation with 78 and 54 Gy in conventional one-per-day fractions combined with cisplatin.59 Combining hyperfractionation with interferon beta therapy had a more benign side effect profile but failed to change survival.60 Combinations with radiosensitizers have also been studied, but thus far no combination has demonstrated more benefit than RT has alone. An aggressive therapy scheme was found to be toxic in a series of 11 patients but yielded long-term objective good outcomes in more than 50% of patients with histologically confirmed DPG and dorsal exophytic BSGs.61
Prognosis and Outcomes
Two radiographic features provide the most useful prognostic information: epicenter of the tumor and diffuseness. In a large series of patients with BSGs, the 5-year survival rate was 75% and 65% for patients with lesions in the midbrain and medulla, respectively, versus an 18% survival rate for patients with diffuse pontine lesions.62 Focal tumors had a 5-year survival rate of 70%, whereas patients with diffuse tumors had just a 22% rate.62 Other studies have reported even lower 3-year survival rates of 10%.5
Preoperative symptoms such as upper respiratory tract infections, pneumonia, and voice alterations were associated with postoperative ventilator dependency. In the same series of 24 patients, preoperative swallowing difficulties correlated closely with the need for a feeding gastrostomy postoperatively.39
Abbott R, Shiminski-Maher T, Epstein FJ. Intrinsic tumors of the medulla: predicting outcome after surgery. Pediatr Neurosurg. 1996;25:41.
Abbott R, Shiminski-Maher T, Wisoff JH, et al. Intrinsic tumors of the medulla: surgical complications. Pediatr Neurosurg. 1991;17:239.
Albright AL. Diffuse brainstem tumors: when is a biopsy necessary? Pediatr Neurosurg. 1996;24:252.
Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16:73.
Donahue B, Allen J, Siffert J, et al. Patterns of recurrence in brain stem gliomas: evidence for craniospinal dissemination. Int J Radiat Oncol Biol Phys. 1998;40:677.
Epstein F, Wisoff J. Intra-axial tumors of the cervicomedullary junction. J Neurosurg. 1987;67:483.
Fischbein NJ, Prados MD, Wara W, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9.
Jallo GI, Shiminski-Maher T, Velazquez L, et al. Recovery of lower cranial nerve function after surgery for medullary brainstem tumors. Neurosurgery. 2005;56:74.
Kaplan AM, Albright AL, Zimmerman RA, et al. Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg. 1996;24:185.
Packer RJ. Brain stem gliomas: therapeutic options at time of recurrence. Pediatr Neurosurg. 1996;24:211.
Pollack IF, Hoffman HJ, Humphreys RP, et al. The long-term outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg. 1993;78:859.
Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9:145.
Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology. 1996;46:1652.
Poussaint TY, Kowal JR, Barnes PD, et al. Tectal tumors of childhood: clinical and imaging follow-up. AJNR Am J Neuroradiol. 1998;19:977.
Robertson PL, Allen JC, Abbott IR, et al. Cervicomedullary tumors in children: a distinct subset of brainstem gliomas. Neurology. 1994;44:1798.
Robertson PL, Muraszko KM, Brunberg JA, et al. Pediatric midbrain tumors: a benign subgroup of brainstem gliomas. Pediatr Neurosurg. 1995;22:65.
Rubin G, Michowitz S, Horev G, et al. Pediatric brain stem gliomas: an update. Childs Nerv Syst. 1998;14:167.
Ullrich NJ, Raja AI, Irons MB, et al. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007;61:762.
Vandertop WP, Hoffman HJ, Drake JM, et al. Focal midbrain tumors in children. Neurosurgery. 1992;31:186.
1 Baily P, Buchanan DN, Bucy P. Intracranial Tumors of Infancy and Childhood. Chicago: Chicago University Press; 1939. 188
2 Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16:73.
3 Rubin G, Michowitz S, Horev G, et al. Pediatric brain stem gliomas: an update. Childs Nerv Syst. 1998;14:167.
4 Epstein F, Constantini S. Practical decisions in the treatment of pediatric brain stem tumors. Pediatr Neurosurg. 1996;24:24.
5 Kaplan AM, Albright AL, Zimmerman RA, et al. Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg. 1996;24:185.
6 Vandertop WP, Hoffman HJ, Drake JM, et al. Focal midbrain tumors in children. Neurosurgery. 1992;31:186.
7 Robertson PL, Muraszko KM, Brunberg JA, et al. Pediatric midbrain tumors: a benign subgroup of brainstem gliomas. Pediatr Neurosurg. 1995;22:65.
8 May PL, Blaser SI, Hoffman HJ, et al. Benign intrinsic tectal “tumors” in children. J Neurosurg. 1991;74:867.
9 Molloy PT, Yachnis AT, Rorke LB, et al. Central nervous system medulloepithelioma: a series of eight cases including two arising in the pons. J Neurosurg. 1996;84:430.
10 Abbott R, Shiminski-Maher T, Wisoff JH, et al. Intrinsic tumors of the medulla: surgical complications. Pediatr Neurosurg. 1991;17:239.
11 Hoffman HJ, Becker L, Craven MA. A clinically and pathologically distinct group of benign brain stem gliomas. Neurosurgery. 1980;7:243.
12 Pollack IF, Hoffman HJ, Humphreys RP, et al. The long-term outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg. 1993;78:859.
13 Robertson PL, Allen JC, Abbott IR, et al. Cervicomedullary tumors in children: a distinct subset of brainstem gliomas. Neurology. 1994;44:1798.
14 Epstein F, Wisoff J. Intra-axial tumors of the cervicomedullary junction. J Neurosurg. 1987;67:483.
15 Zimmerman RA. Neuroimaging of primary brainstem gliomas: diagnosis and course. Pediatr Neurosurg. 1996;25:45.
16 Nadvi SS, Ebrahim FS, Corr P. The value of 201thallium-SPECT imaging in childhood brainstem gliomas. Pediatr Radiol. 1998;28:575.
17 Farmer JP, Montes JL, Freeman CR, et al. Brainstem gliomas. A 10-year institutional review. Pediatr Neurosurg. 2001;34:206.
18 Poussaint TY, Kowal JR, Barnes PD, et al. Tectal tumors of childhood: clinical and imaging follow-up. AJNR Am J Neuroradiol. 1998;19:977.
19 Warren KE, Frank JA, Black JL, et al. Proton magnetic resonance spectroscopic imaging in children with recurrent primary brain tumors. J Clin Oncol. 2000;18:1020.
20 Broniscer A, Gajjar A, Bhargava R, et al. Brain stem involvement in children with neurofibromatosis type 1: role of magnetic resonance imaging and spectroscopy in the distinction from diffuse pontine glioma. Neurosurgery. 1997;40:331.
21 Ullrich NJ, Raja AI, Irons MB, et al. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007;61:762.
22 Helton KJ, Weeks JK, Phillips NS, et al. Diffusion tensor imaging of brainstem tumors: axonal degeneration of motor and sensory tracts. J Neurosurg Pediatr. 2008;1:270.
23 Raffel C, McComb JG, Bodner S, et al. Benign brain stem lesions in pediatric patients with neurofibromatosis: case reports. Neurosurgery. 1989;25:959.
24 Ullrich NJ, Raja AI, Irons MB, et al. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007;61:762.
25 Klimo PJr, Goumnerova LC. Endoscopic third ventriculostomy for brainstem tumors. J Neurosurg. 2006;105(suppl 4):271.
26 Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology. 1996;46:1652.
27 Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007.
28 Chadduck WM, Boop FA, Sawyer JR. Cytogenetic studies of pediatric brain and spinal cord tumors. Pediatr Neurosurg. 1991;17:57.
29 Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620.
30 Rosser T, Packer RJ. Intracranial neoplasms in children with neurofibromatosis 1. J Child Neurol. 2002;17:630.
31 Squire JA, Arab S, Marrano P, et al. Molecular cytogenetic analysis of glial tumors using spectral karyotyping and comparative genomic hybridization. Mol Diagn. 2001;6:93.
32 Packer RJ. Brain stem gliomas: therapeutic options at time of recurrence. Pediatr Neurosurg. 1996;24:211.
33 Donahue B, Allen J, Siffert J, et al. Patterns of recurrence in brain stem gliomas: evidence for craniospinal dissemination. Int J Radiat Oncol Biol Phys. 1998;40:677.
34 Albright AL, Packer RJ, Zimmerman R, et al. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery. 1993;33:1026.
35 Albright AL. Diffuse brainstem tumors: when is a biopsy necessary? Pediatr Neurosurg. 1996;24:252.
36 Rajshekhar V, Chandy MJ. Computerized tomography–guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg. 1995;82:976.
37 Ternier J, Wray A, Puget S, et al. Tectal plate lesions in children. J Neurosurg. 2006;104(suppl 6):369.
38 Wellons JC3rd, Tubbs RS, Banks JT, et al. Long-term control of hydrocephalus via endoscopic third ventriculostomy in children with tectal plate gliomas. Neurosurgery. 2002;51:63.
39 Abbott R, Shiminski-Maher T, Epstein FJ. Intrinsic tumors of the medulla: predicting outcome after surgery. Pediatr Neurosurg. 1996;25:41.
40 Jallo GI, Shiminski-Maher T, Velazquez L, et al. Recovery of lower cranial nerve function after surgery for medullary brainstem tumors. Neurosurgery. 2005;56:74.
41 Mussi AC, Rhoton ALJr. Telovelar approach to the fourth ventricle: microsurgical anatomy. J Neurosurg. 2000;92:812.
42 Dietze DDJr, Mickle JP. Cerebellar mutism after posterior fossa surgery. Pediatr Neurosurg. 1990;16:25.
43 Rekate HL, Grubb RL, Aram DM, et al. Muteness of cerebellar origin. Arch Neurol. 1985;42:697.
44 Wisoff JH, Epstein FJ. Pseudobulbar palsy after posterior fossa operation in children. Neurosurgery. 1984;15:707.
45 Verschuur AC, Grill J, Lelouch-Tubiana A, et al. Temozolomide in paediatric high-grade glioma: a key for combination therapy? Br J Cancer. 2004;91:425.
46 Broniscer A, Iacono L, Chintagumpala M, et al. Role of temozolomide after radiotherapy for newly diagnosed diffuse brainstem glioma in children: results of a multiinstitutional study (SJHG-98). Cancer. 2005;103:133.
47 Freeman CR, Krischer J, Sanford RA, et al. Hyperfractionated radiotherapy in brain stem tumors: results of a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1988;15:311.
48 Freeman CR, Krischer J, Sanford RA, et al. Hyperfractionated radiation therapy in brain stem tumors. Results of treatment at the 7020 cGy dose level of Pediatric Oncology Group study #8495. Cancer. 1991;68:474.
49 Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group phase I/II trial. Cancer. 1993;72:1414.
50 Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group phase I/II trial. Cancer. 1994;74:1827.
51 Prados MD, Wara WM, Edwards MS, et al. The treatment of brain stem and thalamic gliomas with 78 Gy of hyperfractionated radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:85.
52 Hadjipanayis CG, Kondziolka D, Gardner P, et al. Stereotactic radiosurgery for pilocytic astrocytomas when multimodal therapy is necessary. J Neurosurg. 2002;97:56.
53 Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227.
54 Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9:145.
55 Estlin EJ, Lashford L, Ablett S, et al. Phase I study of temozolomide in paediatric patients with advanced cancer. United Kingdom Children’s Cancer Study Group. Br J Cancer. 1998;78:652.
56 Blaney SM, Phillips PC, Packer RJ, et al. Phase II evaluation of topotecan for pediatric central nervous system tumors. Cancer. 1996;78:527.
57 Heideman RL, Douglass EC, Langston JA, et al. A phase II study of every other day high-dose ifosfamide in pediatric brain tumors: a Pediatric Oncology Group study. J Neurooncol. 1995;25:77.
58 Choi LM, Rood B, Kamani N, et al. Feasibility of metronomic maintenance chemotherapy following high-dose chemotherapy for malignant central nervous system tumors. Pediatr Blood Cancer. 2008;50:970.
59 Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:959.
60 Packer RJ, Prados M, Phillips P, et al. Treatment of children with newly diagnosed brain stem gliomas with intravenous recombinant beta-interferon and hyperfractionated radiation therapy: a Children’s Cancer Group phase I/II study. Cancer. 1996;77:2150.
61 Benesch M, Lackner H, Moser A, et al. Outcome and long-term side effects after synchronous radiochemotherapy for childhood brain stem gliomas. Pediatr Neurosurg. 2001;35:173.
62 Fischbein NJ, Prados MD, Wara W, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9.