CHAPTER 111 Brain Tumors during Pregnancy
In the past, the literature on brain tumors during pregnancy gave contradictory accounts of the relationship between pregnancy and the development or progression of such tumors. All types of nervous system tumors have been reported during pregnancy, but no single type is predominant.1 Many authors describe a relapsing pattern in tumors relative to pregnancy, menstruation, and menopausal status,2–7 and it has been suggested that steroid hormones may play a role in the development of brain tumors, in particular, meningiomas.7,8 However, literature reviews and population-based studies indicate that the incidence of brain tumors in pregnant women is lower than that in age-matched nonpregnant women.1,9–11
Neuroimaging in Pregnancy
As a general rule, imaging studies other than ultrasound should be avoided in pregnant women unless there is a clear indication. However, the well-being and safety of the mother should not be jeopardized because of concerns over radiation. Computed tomography (CT) of the head is relatively benign in pregnant women because the radiation exposure to the fetus is less than 0.005 mGy.12 It has been estimated that the radiation emanating from a CT scan to a fetus that is 30 cm or more from the scanner results in a dose 2 orders of magnitude below the maximal permissible dose of 0.5 mSv.13,14 Hence, the radiation resulting from CT of the mother’s head presents little risk to the fetus.14,15 Magnetic resonance imaging (MRI), which does not use ionizing radiation, is considered relatively harmless to the fetus.16,17 However, MRI is recommended only after 4 months of gestation.18 From a practical standpoint, we therefore tend to avoid follow-up MRI during the first trimester, whereas initial diagnostic MRI studies, when indicated by a new onset of symptoms suggesting an intracranial neoplasm, are regularly performed. Obtaining a timely diagnosis that can appropriately guide the medical management of the rest of the pregnancy clearly outweighs the small risks associated with the procedures.
Unfortunately, the accuracy of MRI and CT relies on intravenous contrast agents, which have as of yet unknown effects on fetal development. Neither iodine- nor gadolinium-based intravenous contrast agents have been shown to be entirely safe in pregnancy.19 Although specific teratogenic effects have yet to be identified or reported in humans, these agents, as a general rule, should be used only when absolutely necessary and only after careful evaluation of the risk-to-benefit ratio.20
Neuroanesthesia in Pregnancy
Anesthesia for Pregnant Women Requiring Craniotomy
The multiple drugs required during craniotomy can increase the risk for developmental abnormalities if administered during the first trimester. In later trimesters, drug-induced abnormalities are less likely. Surgery during pregnancy often increases the risk for a first- or second-trimester spontaneous abortion, but it does not seem to increase the incidence of congenital abnormalities,21 nor does surgery appear to induce premature labor.22 Because of the physiologic changes that occur during pregnancy, issues such as surgical positioning and the dosage of drugs administered during anesthesia must be handled carefully. Pregnancy increases plasma volume and total blood volume with a dilutional anemia. Cardiac output is increased by 50%, and typically, anesthetic requirements are decreased by 30%.23 Because lung-closing capacity is increased, a supine patient is at an increased risk for atelectasis. Gastric motility is decreased, and the patient is at an increased risk for vomiting and aspiration on induction of anesthesia.24
Maintenance of normal uterine blood flow is important to avoid uterine hypoperfusion and fetal hypoxia.25 Monitoring the fetal heart rate by Doppler ultrasonography after the 16th week of gestation provides a fair indication of the adequacy of oxygen delivery to the fetus.25 Moreover, lowered uterine perfusion may lead to premature contractions.22 Thus, judicious use of vasoconstrictors such as phenylephrine or hyperventilation is recommended. A reduction in PaCO2 from 32 mm Hg, the normal level for pregnancy, to 25 mm Hg decreases uterine artery blood flow by 25%, but the effect may be caused by mechanical ventilation rather than simple changes in PaCO2.26
Precautions must be taken in positioning the patient to avoid mechanical compression of the vena cava by the uterus.27 The supine position, which increases both intrathoracic and intra-abdominal pressure, may decrease uterine blood flow, a condition called supine hypotension syndrome. In this case, a folded sheet can be placed under the right hip of the patient to minimize vena cava compression. Both the park bench and sitting positions have little harmful effect on uterine blood flow, although the latter allows better respiratory function.28 The prone position should be avoided. Therefore, even with a posterior fossa approach, the park bench position should be preferred over the prone position.
Controlled hypotension is necessary in certain instances. Several case reports of hypotension, produced by either isoflurane or nitroprusside, indicate that a mean arterial blood pressure of 40 to 50 mm Hg for up to 40 minutes does not result in injury to the fetus.29–31 In any event, this measure should be judiciously limited to the most challenging surgical situations that dictate it; otherwise, normotensive anesthetic support is encouraged.
Delivery in Patients with Cranial Lesions
The time of delivery is of prime significance to infant viability. In the past, delivery was postponed until after 36 to 38 weeks of pregnancy to decrease the likelihood of respiratory distress syndrome. Improvements in the use of surfactant have demonstrated that safe delivery at 32 weeks of pregnancy may be a reasonable option.32
The anesthetic plan for delivery is greatly affected by the intracranial pathology of the patient. If delivery and craniotomy are to be accomplished concurrently, the anesthetic management plan should be that required for a craniotomy. Rapid initiation of general anesthesia with intravenous agents, endotracheal intubation, and mild hyperventilation do not seem to have an adverse effect on the fetus. If delivery is to be accomplished before neurosurgical treatment, the presence of an intracranial lesion causing a mass effect requires a different strategy. Patients with such lesions who are ready to deliver are best anesthetized with general endotracheal anesthesia. The effect of active labor on the central nervous system of such patients is little understood, but it is unlikely to produce a positive effect. A cesarean section under general anesthesia is preferable because it is quick and safe and has minimal disadvantages to the fetus. Respiratory depression in the newborn, which is a potential problem associated with such delivery, is easily treated. Regional anesthesia should be avoided in patients with a demonstrated significant intracranial mass effect and possible shift because of the risk of loss of cerebrospinal fluid and the consequent potential risk of herniation through the foramen magnum. Regional anesthesia, specifically epidural anesthesia, is sufficient for lesions that do not exert a mass effect. In such instances, instrumented delivery should be encouraged. In general, we tend to discourage, unless rigorously dictated by a deteriorating clinical/neurological condition, craniotomies at the time of or shortly after the delivery because of the transient coagulopathy that is known to frequently develop during the immediate postpartum period.32a
Pituitary Brain Tumors
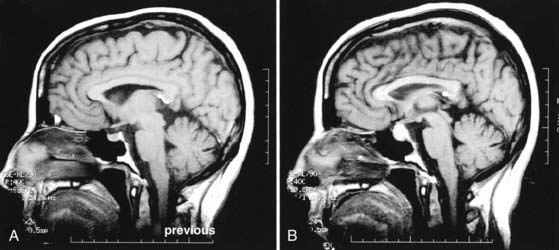
Conversely, pregnancy may affect the evolution of a pituitary adenoma (Fig. 111-1). Since the late 18th century, it has been noted that the pituitary gland enlarges during pregnancy as a physiologic consequence of gestation.33–36 This is due to an increase in the size and number of lactotroph cells.35,37,38 The increased levels of estrogens during pregnancy have been associated with hypertrophic and hyperplastic changes in lactotroph cells.37,38 This hypothesis is also supported by the observation that exogenous administration of estrogens induces enlargement of the pituitary gland in rats.39 MRI studies conducted in pregnant women have shown that the volume of the pituitary gland may increase by 45%40 at a growth rate of 0.08 mm/wk.41 Theoretically, the stimulatory effect of peripheral hormone surges during gestation could result in enlargement of a pituitary adenoma as well. Indeed, it has been reported that pituitary adenomas may grow more rapidly in pregnant women.42 As a result, tumors are at risk for hemorrhage because of enhanced pituitary vascularity and edema, in addition to estrogen-mediated pituitary hyperplasia.35 Studies show that tumor enlargement during pregnancy occurs more frequently with macroadenomas than with microadenomas—5% to 20% of cases versus 1% of cases, respectively—and that the effect is more pronounced in the second and third trimesters.42,43 A recent review of the literature also revealed that pregnancy exacerbated acromegaly in 4 of 24 patients (17%), with therapeutic abortion being necessary in 1 patient.44 Oral contraceptives have not been implicated in the development of prolactinomas.45,46
Although pregnancy may cause enlargement of an existing adenoma (directly or indirectly through enlargement of the gland), there is no evidence that it increases the incidence of adenomas per se. One study showed that among 69 women who died during pregnancy, after abortion, or during the postpartum period, 12% had noninvasive microadenomas, an incidence rate similar to that found in studies conducted on a general sampling of adult autopsies.36,47
In the clinical setting, endocrinopathy and local compression are symptomatic of pituitary tumors. Functional adenomas secrete excessive amounts of pituitary hormones and can frequently be diagnosed when they are small—less than 5 mm in diameter. Nonfunctional adenomas are larger at the time of diagnosis and can be discerned by their direct compression of surrounding structures. Compression of surrounding structures by pituitary tumors may lead to three major consequences: pituitary insufficiency, caused by compression of the pituitary gland; compromise of the hypothalamic-hypophysial axis, caused by compression of the pituitary stalk; or visual problems such as bitemporal hemianopia, caused by compression of the optic pathways. In addition, such tumors may cause oculomotor problems and sometimes stroke by compressing the cavernous sinuses and the neurovascular structures contained in them. Headaches are commonly associated with these tumors as well because the surrounding dura mater is stretched. Patients harboring microadenomas have been shown to have a significantly smaller risk for the development of visual loss than do patients affected by adenomas greater than 1.2 cm.48
Pituitary apoplexy is a rare event that can occur in a patient with a pituitary adenoma. It involves a sudden hemorrhage or ischemic or hemorrhagic infarction of the pituitary adenoma, which causes a rapid increase in intrasellar pressure. Common symptoms are sudden or violent headaches frequently accompanied by vomiting and rapid deterioration of vision or ocular motility. Emergency treatment with a decompressive surgical procedure is needed to avoid progression of the syndrome and potential death.49
Diagnosis
Imaging studies for evaluation of patients with pituitary tumors have improved significantly. Skull radiographs and polytomography, which identify indirect signs of pituitary tumors in the form of bony erosion of the sella turcica and clinoids, have been replaced by high-resolution CT and MRI. CT and MRI can usually outline even the smallest tumors and their effects on surrounding neurovascular structures.50–52 MRI is the preferred diagnostic procedure for pituitary adenomas because it allows visualization of the details of vascular structures, thereby virtually eliminating the need for an angiogram, and it offers the advantage of multiplanar views, which are essential for a complete evaluation. CT, however, provides detailed definition of the sella and surrounding bony structures. This information is of particular importance in the preoperative evaluation of the sphenoidal bones when transsphenoidal resection is being planned.
Preliminary hormonal evaluations to determine the functioning of the anterior and posterior pituitary involve the following measurements: urine volume; serum electrolytes and osmolarity; serum prolactin; early-morning cortisol level; serum gonadotropins; and thyroxine, triiodothyronine, and thyroid-stimulating hormone levels. If the clinical examination and laboratory testing reveal a specific endocrinopathy, more targeted tests should be conducted. For instance, urinary free cortisol, dexamethasone suppression tests, and adrenocorticotropic hormone levels help diagnose Cushing’s syndrome. Serum levels of antidiuretic hormone help diagnose diabetes insipidus. Growth hormone (GH), insulin-like growth factor-I levels, and glucose suppression tests help diagnose acromegaly53; however, such a diagnosis may be difficult to make during pregnancy because of placental secretion of GH. Thus, to diagnose acromegaly during pregnancy, one should measure GH levels by radioimmunoassay with monoclonal antibodies that recognize specific epitopes of pituitary and placental GH. Sometimes, loss of physiologic pulsatile secretion of pituitary GH helps determine the diagnosis.43,44 Insulin-like growth factor-I levels are less useful because they are elevated in normal as well as in acromegalic pregnancies.44,54
Treatment
Bromocriptine is highly effective in the treatment of hyperprolactinemic patients. Bromocriptine can significantly slow or even arrest the growth of a prolactin-secreting tumor and often results in restoration of normal endocrine function and a concurrent reduction in the size of the adenoma.55 As already indicated, patients with prolactinomas and the classic amenorrhea-galactorrhea syndrome who receive bromocriptine therapy may resume regular ovulatory cycles and eventually become pregnant. Hyperprolactinemia also occurs in 30% to 40% of acromegalic patients,56 and bromocriptine has been shown to be effective in restoring normal ovulatory cycles in these patients as well.57–60 Several reports have shown that bromocriptine is safe when administered through the first weeks of gestation in hyperprolactinemic patients.56,61–63 After 9 years’ follow-up of children of mothers treated with bromocriptine during the first weeks of pregnancy, teratogenicity rates were no higher than those expected in an untreated population.64 The only reported fetal malformation secondary to bromocriptine use is talipes, and it was the sole complication in 114 pregnancies in the study.65 In other studies, continuation of bromocriptine treatment throughout pregnancy resulted in uncomplicated deliveries of normal infants.58,59 Because bromocriptine crosses the placenta, it is recommended that women discontinue taking the drug while they are trying to conceive to minimize any possible effects on the developing fetus.44,66 Also, hypertension, stroke, and seizures have been reported in the puerperium after suppression of lactation with bromocriptine.67 In addition to bromocriptine, cabergoline has recently gained popularity in the treatment of prolactinomas in the general population; however, at this time, data on its side effect profile in pregnant patients are limited.
There are multiple reported cases of pregnancy after successful treatment of acromegaly with the somatostatin analogue octreotide.44,68,69 In all cases, treatment was discontinued within the first month of gestation, and in no instance did exposure to the drug cause adverse events in either the fetus or the mother.44,68,69 Of the 14 reports of pregnant patients treated with octreotide, no malformations were reported in their children.70 Nevertheless, until more safety data are available, use of this drug should be discontinued during pregnancy.44
The metabolic and cardiovascular complications of acromegaly require special consideration in the management of pregnant women affected by GH-secreting adenomas. In particular, GH antagonizes the action of insulin and results in carbohydrate intolerance or diabetes mellitus.71 Because pregnancy itself is an insulin-resistant state, pregnant patients harboring GH-secreting adenomas are at greater risk for hyperglycemia.44
Medical therapies available for the treatment of hyperfunctional pituitary adenomas are less effective for Cushing’s disease.72–74 Ketoconazole has been associated with intrauterine growth retardation in two cases.75,76 Aminoglutethimide and mitotane are contraindicated in pregnancy. In these cases, operative intervention appears to be the safer option.
To summarize, most pregnant women with pituitary adenomas can be safely observed with frequent ophthalmic evaluations and MRI. The safety of continuous bromocriptine or octreotide therapy has not been fully assessed, and women should be advised to discontinue such treatment after pregnancy is confirmed.43,44 This approach has been shown to be safe and carries only a small risk of tumor enlargement in cases of microprolactinomas and nonsecreting or GH-secreting adenomas. However, there is a greater than 15% risk of symptomatic enlargement of a macroprolactinoma during pregnancy, thus mandating close surveillance. Periodic assessment of visual fields every 3 months in women with microadenomas and every 6 weeks in those with macroadenomas has been recommended.44 Deterioration in the results of neuro-ophthalmic examination indicates the need for imaging studies and, potentially, therapeutic intervention. Nonetheless, only a small percentage of pregnant women with pituitary adenomas require further surgical treatment before delivery. Finally, the notion that breastfeeding induces the growth of prolactin-secreting adenomas should lead to particular vigilance when managing women with larger tumors during the puerperium.
Glial Tumors
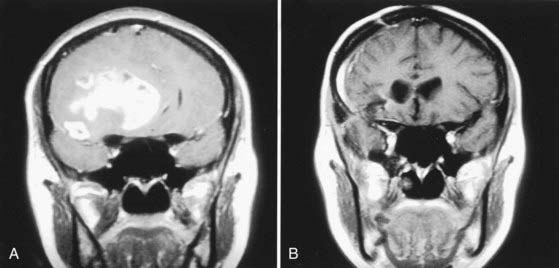
Glial tumors are the most common primary brain tumors in adults and represent about 35% of all intracranial tumors.77 It has been suggested that the hormonal changes associated with pregnancy may play a role in causing glioma (Fig. 111-2)7 or have a direct effect on tumor growth, but no experimental evidence has been provided to support this assertion.1 However, the tendency to retain extracellular and intracellular fluid during pregnancy is considered a predisposing factor for the development of more extensive perineoplastic brain edema and, hence, more severe symptoms.78,79
Diagnosis
CT and MRI are the preferred imaging tests when the presence of a neoplastic lesion is suspected. These diagnostic tests provide precise information on the configuration of the lesion, its relative vascularity, the presence of cystic components or concomitant obstructive hydrocephalus, and the degree of compression of surrounding structures. These imaging studies can also provide information on the histologic type and grade of the malignancy. Angiography, which uses higher doses of radiation,51 is rarely needed. Electroencephalography is sometimes helpful for the optimal management of seizures.
Treatment
Synthetic corticosteroids, such as dexamethasone and methylprednisolone, are used to ameliorate perineoplastic brain edema. Corticosteroids control the progression of symptoms and aid in postponing surgical intervention. As with other agents for which teratogenicity has not been determined, however, unless the benefits of treatment clearly outweigh the potential hazards to the mother and fetus, the use of corticosteroids is discouraged during early pregnancy. Furthermore, the possible development of hypoadrenalism in the newborn mandates careful observation after birth in such instances.80
Antiepileptic drugs are used for seizure control and prophylaxis. An alternative approach should be taken in pregnant women, however, because of the association of anticonvulsants with teratogenicity. To date, no conclusive information is available on which of the four major antiepileptic drugs (phenytoin, carbamazepine, valproate, and phenobarbital) is the most teratogenic.81 There is evidence that such drugs represent the major cause of teratogenesis associated with epilepsy.82–85 Infants of women taking antiepileptic drugs have higher rates of major malformations than do those of women with untreated epilepsy.86,87 Carbamazepine and valproate have been specifically associated with an increased incidence of neural tube defects.88–91 Although Koch and colleagues consider phenytoin and phenobarbital “weakly teratogenic,”92 both drugs have been associated with serious malformations of the heart and orofacial and urogenital structures in infants.93,94 Thus, because no anticonvulsant is completely free of significant teratogenic effects, the use of such drugs, even in patients with brain tumors, should be limited to those with generalized motor seizures or multiple seizures that are jeopardizing the health of the mother and fetus. If a single focal seizure is reported, initiation of anticonvulsant treatment should be deferred if possible. Similarly, prophylactic anticonvulsant treatment should be avoided in women undergoing craniotomy who have no previous history of seizures. A single-agent regimen is preferable if anticonvulsant treatment is necessary.95 Phenobarbital and carbamazepine are generally preferred over the other drugs, but good comparative data are lacking.81,95 Trimethadione and valproic acid should be avoided because of their well-established teratogenic effects.96,97
Because low folate levels have been associated with spontaneous abortion and fetal malformations in animal models and because some antiepileptic drugs (phenytoin, carbamazepine, barbiturates) may decrease folate absorption,87 dietary folic acid supplementation has been proposed for the prevention of neural tube defects in the fetuses of pregnant women with epilepsy, but the results are inconclusive.81,95 A daily dose of 0.4 mg of folic acid has been recommended by the Centers for Disease Control and Prevention as dietary supplementation for all women of reproductive age for the primary prevention of neural tube defects.98 Higher doses, up to 5 mg/day, are recommended for secondary prevention.99 Likewise, vitamin K supplementation has been recommended during the last month of gestation for pregnant women with epilepsy who receive enzyme-inducing antiepileptic drugs (carbamazepine, primidone, phenytoin, phenobarbital, topiramate) to prevent hemorrhagic disease of the newborn.81
Conventional radiation therapy is important in the treatment of glial tumors as an adjuvant measure after surgery. Stereotactic radiosurgery, which uses precisely defined converging radiation beams such as with the Gamma Knife and linear accelerator modalities (i.e., CyberKnife), might be considered an alternative to surgery for the treatment of small, deep-seated lesions because it has minimal scatter to even surrounding brain tissue.100–102 However, the precise role of these modalities in the treatment of malignant glial tumors is still being debated. As already mentioned, it is generally accepted that a fetus should not absorb more than 0.5 mSv at any time during pregnancy.13,14,103 Acute radiation exposure of 1 Gy or more during the 8th to 15th weeks of pregnancy presents a serious risk for abortion, mental retardation, and congenital defects in surviving embryos.104 However, because there is a relatively long distance between the mother’s brain and her abdomen and the ionizing radiation does not scatter through her body, the fetus can be adequately protected from dangerous radiation levels by appropriate lead shielding.
Chemotherapeutic agents should be avoided during the first trimester of pregnancy.105 In particular, antimetabolites such as aminopterin and methotrexate have been associated with fetal abnormalities when administered at this time.106 Animal studies have indicated a teratogenic effect of BCNU (carmustine, the most widely used agent for malignant gliomas) when administered early in pregnancy,107 but there is no evidence of an increased risk for teratogenicity with the administration of cytotoxic drugs in later trimesters.105,108,109 The possibility of reducing the systemic effects of chemotherapy by interstitial implantation of biocompatible polymers impregnated with chemotherapeutic agents has been demonstrated.110 One of the important clinical applications of this method could be the adjuvant treatment of pregnant women with malignant brain tumors.
Meningiomas
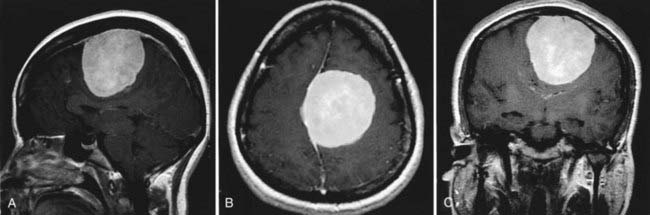
It is often reported that meningiomas develop and progress during pregnancy (Fig. 111-3).79,111,112 Meningiomas account for 18% of all primary intracranial tumors and occur twice as frequently in females as in males.113 It has been observed that pregnancy-related meningiomas tend to have a relapsing course in relation to pregnancy and menstruation; they increase in size during pregnancy,4,112 regress after delivery,1,6 and recur during a subsequent pregnancy.5 An association between the development of meningioma and breast cancer has also been reported.114–118 These observations have led to the hypothesis, supported by laboratory findings, that female hormones may play a role in the development of meningiomas.119–126 However, population-based epidemiologic studies,7,10,11 as well as literature reviews of pregnancy-related meningiomas,1,127,128 do not support the notion of an increased risk for the development of meningiomas during pregnancy.
Other Tumors
Hemangioblastomas have been frequently reported to develop during pregnancy,129–131 and they characteristically occur in the posterior fossa of young adults. These benign tumors may progress rapidly during pregnancy, and symptoms may disappear after delivery.131 Other primary brain tumors that occur during adulthood can develop in pregnant women. Acoustic neuromas,132 ependymomas,127 medulloblastomas,133 and choroid plexus papillomas134 have reportedly been found in pregnant women. Treatment options, particularly whether to postpone the operation, vary according to the progression of the lesion and the patient’s condition.
The incidence of primary brain lymphoma has increased with the rise in the occurrence of acquired immunodeficiency syndrome.135 Because lymphomas are usually sensitive to chemotherapy and, to a lesser extent, radiation therapy, surgical intervention, in general, focuses solely on tissue diagnosis by stereotactic biopsy.135
Metastatic tumors, which are more common in older patients, can also be found in women of childbearing age. Their treatment is generally palliative and varies according to the nature of the primary tumor and the extent of the systemic and central nervous system dissemination.136 Definitive tissue diagnosis is essential. Evidence suggests that surgical resection followed by radiation therapy produces the greatest chance of survival in patients with surgically accessible, solitary brain metastases.137 Radiosurgical approaches have also provided good results in controlling intracranial metastatic disease (many references available).
Although melanoma, breast cancer, and lung cancer are statistically the tumors that metastasize to the brain most frequently, choriocarcinoma also has a propensity to occur during pregnancy, and brain metastases are the most frequent neural complication.138–140 This tumor has a well-documented tendency to proliferate, infiltrate vascular structures, and cause hemorrhagic events.141,142 Irradiation and chemotherapy are the primary treatment methods for this tumor143; surgical treatment should be used only in specific cases.
Pseudotumor Cerebri
Diagnosis involves a process of exclusion. Imaging studies do not always show the typical small ventricles, but these studies are still important in eliminating the possibility of other structural diseases. Patients with pseudotumor cerebri should have periodic complete ophthalmic evaluations. Medical treatment with small doses of corticosteroids and acetazolamide or other diuretics frequently helps control symptoms. Serial lumbar punctures or surgical insertion of a lumboperitoneal shunt should be reserved for patients with documented progression of visual impairment who are refractory to medical therapy. In these cases, surgical nerve sheath decompression has been proposed as an alternative to shunt placement. Pseudotumor cerebri does not represent a contraindication to vaginal delivery.144
Buescher MA, McClamrock HD, Adashi EY. Cushing syndrome in pregnancy. Obstet Gynecol. 1992;79:130-137.
Carroll RS, Glowacka D, Dashner K, et al. Progesterone receptor expression in meningiomas. Cancer Res. 1993;53:1312-1316.
Carroll RS, Zhang J, Dashner K, et al. Progesterone and glucocorticoid receptor activation in meningiomas. Neurosurgery. 1995;37:92-97.
Dineen R, Banks A, Lenthall R. Imaging of acute neurological conditions in pregnancy and the puerperium. Clin Radiol. 2005;60:1156-1170.
Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol. 1989;16:337-346.
Elster AD, Sanders TG, Vines FS, et al. Size and shape of the pituitary gland during pregnancy and post partum: measurement with MR imaging. Radiology. 1991;181:531-535.
Gianopoulos JG. Establishing the criteria for anesthesia and other precautions for surgery during pregnancy. Surg Clin North Am. 1995;75:33-45.
Gonzalez JG, Elizondo G, Saldivar D, et al. Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med. 1988;85:217-220.
Jick SS, Terris BZ. Anticonvulsants and congenital malformations. Pharmacotherapy. 1997;17:561-564.
Lambe M, Coogan P, Baron J. Reproductive factors and the risk of brain tumors: a population-based study in Sweden. Int J Cancer. 1997;72:389-393.
Lindhout D, Meinardi H, Meijer JW, et al. Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology. 1992;42(4 suppl 5):94-110.
Molitch ME. Pituitary diseases in pregnancy. Semin Perinatol. 1998;22:457-470.
Roelvink NC, Kamphorst W, van Alphen HA, et al. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987;44:209-215.
Scheithauer BW, Sano T, Kovacs KT, et al. The pituitary gland in pregnancy: a clinicopathologic and immunohistochemical study of 69 cases. Mayo Clin Proc. 1990;65:461-474.
Schlehofer B, Blettner M, Wahrendorf J. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84:1346-1349.
Smith B. Obstetrics. In: Martin JT, Warner MA, editors. Positioning in Anesthesia and Surgery. Philadelphia: WB Saunders; 1997:267-279.
von Schoultz E, Bixo M, Backstrom T, et al. Sex steroids in human brain tumors and breast cancer. Cancer. 1990;65:949-952.
Waters CH, Belai Y, Gott PS, et al. Outcomes of pregnancy associated with antiepileptic drugs. Arch Neurol. 1994;51:250-253.
Zahn C. Neurological care of pregnant women with epilepsy. Epilepsia. 1998;39(suppl 8):S26-S31.
1 Roelvink NC, Kamphorst W, van Alphen HA, et al. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987;44:209-215.
2 Hagedoorn A. The chiasmal syndrome and retrobulbar neuritis in pregnancy. Am J Ophthalmol. 1937;20:690-699.
3 Rand CW, Andler M. Tumors of the brain complicating pregnancy. Arch Neurol Psychiatry. 1950;63:1. 41, illust
4 Weyand RD, MacCarty CS, Wilson RB. The effect of pregnancy on intracranial meningiomas occurring about the optic chiasm. Surg Clin North Am. 1951;31:1225-1233.
5 Bickerstaff ER, Small JM, Guest IA. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21:89-91.
6 Michelsen JJ, New PF. Brain tumour and pregnancy. J Neurol Neurosurg Psychiatry. 1969;32:305-307.
7 Schlehofer B, Blettner M, Wahrendorf J. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84:1346-1349.
8 Cantor KP, Lynch CF, Johnson D. Reproductive factors and risk of brain, colon, and other malignancies in Iowa (United States). Cancer Causes Control. 1993;4:505-511.
9 Haas JF. Pregnancy in association with a newly diagnosed cancer: a population-based epidemiologic assessment. Int J Cancer. 1984;34:229-235.
10 Haas JF, Janisch W, Staneczek W. Newly diagnosed primary intracranial neoplasms in pregnant women: a population-based assessment. J Neurol Neurosurg Psychiatry. 1986;49:874-880.
11 Lambe M, Coogan P, Baron J. Reproductive factors and the risk of brain tumors: a population-based study in Sweden. Int J Cancer. 1997;72:389-393.
12 Sharp C, Shrimpton JA, Bury RF. Diagnostic Medical Exposures: Advice on Exposure to Ionising Radiation during Pregnancy. Didcot, UK: National Radiological Protection Board; 1998.
13 Hall EJ, editor. Radiobiology for the Radiologist. Philadelphia: JB Lippincott, 1994.
14 Felmlee JP, Gray JE, Leetzow ML, et al. Estimated fetal radiation dose from multislice CT studies. AJR Am J Roentgenol. 1990;154:185-190.
15 Mole RH. Irradiation of the embryo and fetus. Br J Radiol. 1987;60:17-31.
16 Weinreb JC, Brown CE, Lowe TW, et al. Pelvic masses in pregnant patients: MR and US imaging. Radiology. 1986;159:717-724.
17 Weinreb JC, Wolbarsht LB, Cohen JM, et al. Prevalence of lumbosacral intervertebral disk abnormalities on MR images in pregnant and asymptomatic nonpregnant women. Radiology. 1989;170:125-128.
18 Yamashita Y, Namimoto T, Abe Y, et al. MR imaging of the fetus by a HASTE sequence. AJR Am J Roentgenol. 1997;168:513-519.
19 Dineen R, Banks A, Lenthall R. Imaging of acute neurological conditions in pregnancy and the puerperium. Clin Radiol. 2005;60:1156-1170.
20 Weinreb JC. Which study when? Is gadolinium-enhanced MR imaging safer than iodine-enhanced CT? Radiology. 2008;249:3-8.
21 Duncan PG, Pope WD, Cohen MM, et al. Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64:790-794.
22 Gianopoulos JG. Establishing the criteria for anesthesia and other precautions for surgery during pregnancy. Surg Clin North Am. 1995;75:33-45.
23 Palahniuk RJ, Shnider SM, Eger EI2nd. Pregnancy decreases the requirement for inhaled anesthetic agents. Anesthesiology. 1974;41:82-83.
24 Pedersen H, Finster M. Anesthetic risk in the pregnant surgical patient. Anesthesiology. 1979;51:439-451.
25 Barron WM. The pregnant surgical patient: medical evaluation and management. Ann Intern Med. 1984;101:683-691.
26 Levinson G, Shnider SM, DeLorimier AA, et al. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974;40:340-347.
27 Smith B. Obstetrics. In: Martin JT, Warner MA, editors. Positioning in Anesthesia and Surgery. Philadelphia: WB Saunders; 1997:267-279.
28 Giannini A, Bricchi M. Posterior fossa surgery in the sitting position in a pregnant patient with cerebellopontine angle meningioma. Br J Anaesth. 1999;82:941-944.
29 Donchin Y, Amirav B, Sahar A, et al. Sodium nitroprusside for aneurysm surgery in pregnancy. Report of a case. Br J Anaesth. 1978;50:849-851.
30 Lam AM, Gelb AW. Cardiovascular effects of isoflurane-induced hypotension for cerebral aneurysm surgery. Anesth Analg. 1983;62:742-748.
31 Newman B, Lam AM. Induced hypotension for clipping of a cerebral aneurysm during pregnancy: a case report and brief review. Anesth Analg. 1986;65:675-678.
32 Davis JM, Veness-Meehan K, Notter RH, et al. Changes in pulmonary mechanics after the administration of surfactant to infants with respiratory distress syndrome. N Engl J Med. 1988;319:476-479.
32a Andra J. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;26:326.
33 Comte L. Contribution a l’etude de l’hypophyse humaine [dissertation]. Lausanne, Switzerland: 1898.
34 Erdheim J, Stumme E. Uber der schwanger schafts-veranderung der Hypophuse. Ziegler’s Beitr Pathol Anat. 46(1), 1909.
35 Goluboff LG, Ezrin C. Effect of pregnancy on the somatotroph and the prolactin cell of the human adenohypophysis. J Clin Endocrinol Metab. 1969;29:1533-1538.
36 Scheithauer BW, Sano T, Kovacs KT, et al. The pituitary gland in pregnancy: a clinicopathologic and immunohistochemical study of 69 cases. Mayo Clin Proc. 1990;65:461-474.
37 Friesen HG, Fournier P, Desjardins P. Pituitary prolactin in pregnancy and normal and abnormal lactation. Clin Obstet Gynecol. 1973;16:25-45.
38 Ylikorkala O, Kivinen S, Reinila M. Serial prolactin and thyrotropin responses to thyrotropin-releasing hormone throughout normal human pregnancy. J Clin Endocrinol Metab. 1979;48:288-292.
39 Rudin M, Briner U, Doepfner W. Quantitative magnetic resonance imaging of estradiol-induced pituitary hyperplasia in rats. Magn Reson Med. 1988;7:285-291.
40 Gonzalez JG, Elizondo G, Saldivar D, et al. Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med. 1988;85:217-220.
41 Elster AD, Sanders TG, Vines FS, et al. Size and shape of the pituitary gland during pregnancy and post partum: measurement with MR imaging. Radiology. 1991;181:531-535.
42 Gemzell C, Wang CF. Outcome of pregnancy in women with pituitary adenoma. Fertil Steril. 1979;31:363-372.
43 Molitch ME. Pituitary diseases in pregnancy. Semin Perinatol. 1998;22:457-470.
44 Herman-Bonert V, Seliverstov M, Melmed S. Pregnancy in acromegaly: successful therapeutic outcome. J Clin Endocrinol Metab. 1998;83:727-731.
45 Wingrave SJ, Kay CR, Vessey MP. Oral contraceptives and pituitary adenomas. Br Med J. 1980;280:685-686.
46 Maheux R, Jenicek M, Cleroux R, et al. Oral contraceptives and prolactinomas: a case-control study. Am J Obstet Gynecol. 1982;143:134-138.
47 Bergland RM, Ray BS, Torack RM. Anatomical variations in the pituitary gland and adjacent structures in 225 human autopsy cases. J Neurosurg. 1968;28:93-99.
48 Kupersmith MJ, Rosenberg C, Kleinberg D. Visual loss in pregnant women with pituitary adenomas. Ann Intern Med. 1994;121:473-477.
49 Cardoso ER, Peterson EW. Pituitary apoplexy: a review. Neurosurgery. 1984;14:363-373.
50 Kulkarni MV, Lee KF, McArdle CB, et al. 1.5-T MR imaging of pituitary microadenomas: technical considerations and CT correlation. AJNR Am J Neuroradiol. 1988;9:5-11.
51 Young SC, Grossman RI, Goldberg HI, et al. MR of vascular encasement in parasellar masses: comparison with angiography and CT. AJNR Am J Neuroradiol. 1988;9:35-38.
52 Creasy JL. CT and MRI compete in diagnosis of CNS disease. Diagn Imaging (San Franc). 1990;12:96-101.
53 Barrow DL, Tindall SC, Tindall GT. Management of pituitary adenomas. J Med Assoc Ga. 1983;72:837-844.
54 Wilson DM, Bennett A, Adamson GD, et al. Somatomedins in pregnancy: a cross-sectional study of insulin-like growth factors I and II and somatomedin peptide content in normal human pregnancies. J Clin Endocrinol Metab. 1982;55:858-861.
55 Thorner MO, Martin WH, Rogol AD, et al. Rapid regression of pituitary prolactinomas during bromocriptine treatment. J Clin Endocrinol Metab. 1980;51:438-445.
56 Molitch ME. Pregnancy and the hyperprolactinemic woman. N Engl J Med. 1985;312:1364-1370.
57 Aono T, Shioji T, Kohno M, et al. Pregnancy following 2-bromo-alpha-ergocryptine (CB-154)-induced ovulation in an acromegalic patient with galactorrhea and amenorrhea. Fertil Steril. 1976;27:341-344.
58 Espersen T, Ditzel J. Pregnancy and delivery under bromocriptine therapy. Lancet. 1977;2:985-986.
59 Bigazzi M, Ronga R, Lancranjan I, et al. A pregnancy in an acromegalic woman during bromocriptine treatment: effects on growth hormone and prolactin in the maternal, fetal, and amniotic compartments. J Clin Endocrinol Metab. 1979;48:9-12.
60 Luboshitzky R, Dickstein G, Barzilai D. Bromocriptine-induced pregnancy in an acromegalic patient. JAMA. 1980;244:584-586.
61 Turkalj I, Braun P, Krupp P. Surveillance of bromocriptine in pregnancy. JAMA. 1982;247:1589-1591.
62 Hammond CB, Haney AF, Land MR, et al. The outcome of pregnancy in patients with treated and untreated prolactin-secreting pituitary tumors. Am J Obstet Gynecol. 1983;147:148-157.
63 de Wit W, Coelingh Bennink HJ, Gerards LJ. Prophylactic bromocriptine treatment during pregnancy in women with macroprolactinomas: report of 13 pregnancies. Br J Obstet Gynaecol. 1984;91:1059-1069.
64 Raymond JP, Goldstein E, Konopka P, et al. Follow-up of children born of bromocriptine-treated mothers. Horm Res. 1985;22:239-246.
65 Krupp P, Monka C. Bromocriptine in pregnancy: safety aspects. Klin Wochenschr. 1987;65:823-827.
66 Evans W, Thorner M. Bromocriptine. In: Wilkins RN, Rengachary S, editors. Neurosurgery. New York: McGraw-Hill; 1985:873-878.
67 Katz M, Kroll D, Pak I, et al. Puerperal hypertension, stroke, and seizures after suppression of lactation with bromocriptine. Obstet Gynecol. 1985;66:822-824.
68 Landolt AM, Schmid J, Wimpfheimer C, et al. Successful pregnancy in a previously infertile woman treated with SMS-201-995 for acromegaly. N Engl J Med. 1989;320:671-672.
69 Montini M, Pagani G, Gianola D, et al. Acromegaly and primary amenorrhea: ovulation and pregnancy induced by SMS 201-995 and bromocriptine. J Endocrinol Invest. 1990;13:193.
70 Okada Y, Morimoto I, Ejima K, et al. A case of active acromegalic woman with a marked increase in serum insulin-like growth factor-1 levels after delivery. Endocr J. 1997;44:117-120.
71 Berelowitz M, Fischette C, Cefalu W, et al. Comparative efficacy of a once-daily controlled-release formulation of glipizide and immediate-release glipizide in patients with NIDDM. Diabetes Care. 1994;17:1460-1464.
72 Aron DC, Schnall AM, Sheeler LR. Cushing’s syndrome and pregnancy. Am J Obstet Gynecol. 1990;162:244-252.
73 Buescher MA, McClamrock HD, Adashi EY. Cushing syndrome in pregnancy. Obstet Gynecol. 1992;79:130-137.
74 Guilhaume B, Sanson ML, Billaud L, et al. Cushing’s syndrome and pregnancy: aetiologies and prognosis in twenty-two patients. Eur J Med. 1992;1:83-89.
75 Amado JA, Pesquera C, Gonzalez EM, et al. Successful treatment with ketoconazole of Cushing’s syndrome in pregnancy. Postgrad Med J. 1990;66:221-223.
76 Berwaerts J, Verhelst J, Mahler C, et al. Cushing’s syndrome in pregnancy treated by ketoconazole: case report and review of the literature. Gynecol Endocrinol. 1999;13:175-182.
77 Burger P, Scheithauer B, Vogel S. Brain tumors. In: Burger P, Scheithauer B, Vogel S, editors. Surgical Pathology of the Nervous System and Its Coverings. New York: Churchill Livingstone; 1991:193-437.
78 O’Connell JE. Neurosurgical problems in pregnancy. Proc R Soc Med. 1962;55:577-582.
79 Kempers RD, Miller RH. Management of pregnancy associated with brain tumors. Am J Obstet Gynecol. 1963;87:858-864.
80 Ohrlander S, Gennser G, Nilsson KO, et al. ACTH test to neonates after administration of corticosteroids during gestation. Obstet Gynecol. 1977;49:691-694.
81 Zahn C. Neurological care of pregnant women with epilepsy. Epilepsia. 1998;39(suppl 8):S26-S31.
82 Battino D, Binelli S, Caccamo ML, et al. Malformations in offspring of 305 epileptic women: a prospective study. Acta Neurol Scand. 1992;85:204-207.
83 Waters CH, Belai Y, Gott PS, et al. Outcomes of pregnancy associated with antiepileptic drugs. Arch Neurol. 1994;51:250-253.
84 King PB, Lie RT, Irgens LM. Spina bifida and cleft lip among newborns of Norwegian women with epilepsy: changes related to the use of anticonvulsants. Am J Public Health. 1996;86:1454-1456.
85 Jick SS, Terris BZ. Anticonvulsants and congenital malformations. Pharmacotherapy. 1997;17:561-564.
86 Speidel BD, Meadow SR. Maternal epilepsy and abnormalities of the fetus and newborn. Lancet. 1972;2:839-843.
87 Nakane Y, Okuma T, Takahashi R, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia. 1980;21:663-680.
88 Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2:937.
89 Lindhout D, Schmidt D. In-utero exposure to valproate and neural tube defects. Lancet. 1986;1:1392-1393.
90 Rosa FW. Spina bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674-677.
91 Kallen AJ. Maternal carbamazepine and infant spina bifida. Reprod Toxicol. 1994;8:203-205.
92 Koch S, Losche G, Jager-Roman E, et al. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42(4 suppl 5):83-88.
93 Kaneko S, Otani K, Kondo T, et al. Malformation in infants of mothers with epilepsy receiving antiepileptic drugs. Neurology. 1992;42(4 suppl 5):68-74.
94 Lindhout D, Meinardi H, Meijer JW, et al. Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology. 1992;42(4 suppl 5):94-110.
95 Delgado-Escueta AV, Janz D. Consensus guidelines: preconception counseling, management, and care of the pregnant woman with epilepsy. Neurology. 1992;42(4 suppl 5):149-160.
96 Dalessio DJ. Current concepts. Seizure disorders and pregnancy. N Engl J Med. 1985;312:559-563.
97 Tein I, MacGregor DL. Possible valproate teratogenicity. Arch Neurol. 1985;42:291-293.
98 Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14):1-7.
99 Daly LE, Kirke PN, Molloy A, et al. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698-1702.
100 Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154-160.
101 Lunsford LD, Flickinger J, Coffey RJ. Stereotactic gamma knife radiosurgery. Initial North American experience in 207 patients. Arch Neurol. 1990;47:169-175.
102 Lunsford LD, Flickinger J, Lindner G, et al. Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery. 1989;24:151-159.
103 Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16:347-368.
104 Otake M, Schull WJ. In utero exposure to A-bomb radiation and mental retardation; a reassessment. Br J Radiol. 1984;57:409-414.
105 Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol. 1989;16:337-346.
106 Warkany J. Aminopterin and methotrexate: folic acid deficiency. Teratology. 1978;17:353-357.
107 Druckrey H, Ivankovic S, Preussmann R. Teratogenic and carcinogenic effects in the offspring after single injection of ethylnitrosourea to pregnant rats. Nature. 1966;210:1378-1379.
108 Nordlund JJ, DeVita VT, Cabbone PP. Severe vinblastine-induced leukopenia during late pregnancy with delivery of a normal infant. Ann Intern Med. 1968;69:581-582.
109 Lowenthal RM, Marsden KA, Newman NM, et al. Normal infant after treatment of acute myeloid leukaemia in pregnancy with daunorubicin. Aust N Z J Med. 1978;8:431-432.
110 Lawson CH, Sampath P, Bohan E, et al. Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience. J Neurooncology. 2007;83:61-70.
111 Toakley G. Brain tumours in pregnancy. Aust N Z J Surg. 1965;35:149-154.
112 Donaldson JO. Neurology of Pregnancy. Philadelphia: WB Saunders; 1989.
113 Faith D, Preston-Martin S. Epidemiology. In: Bigner DD, McLendon RE, Bruner JM, editors. Russell and Rubinstein’s Pathology of Tumors of the Nervous System. Seven Oaks, Kent, England: Edward Arnold; 1998:5-45.
114 Schoenberg BS, Christine BW, Whisnant JP. Nervous system neoplasms and primary malignancies of other sites. The unique association between meningiomas and breast cancer. Neurology. 1975;25:705-712.
115 Magdelenat H, Pertuiset BF, Poisson M, et al. Steroid receptor status difference in recurrent intracranial meningioma and breast cancer in the same patient. J Neurooncol. 1986;4:155-157.
116 Helseth A, Mork SJ, Glattre E. Neoplasms of the central nervous system in Norway. V. Meningioma and cancer of other sites. An analysis of the occurrence of multiple primary neoplasms in meningioma patients in Norway from 1955 through 1986. APMIS. 1989;97:738-744.
117 Rubinstein AB, Schein M, Reichenthal E. The association of carcinoma of the breast with meningioma. Surg Gynecol Obstet. 1989;169:334-336.
118 von Schoultz E, Bixo M, Backstrom T, et al. Sex steroids in human brain tumors and breast cancer. Cancer. 1990;65:949-952.
119 Donnell MS, Meyer GA, Donegan WL. Estrogen-receptor protein in intracranial meningiomas. J Neurosurg. 1979;50:499-502.
120 Blankenstein MA, Blaauw G, Lamberts SW, et al. Presence of progesterone receptors and absence of oestrogen receptors in human intracranial meningioma cytosols. Eur J Cancer Clin Oncol. 1983;19:365-370.
121 Cahill DW, Bashirelahi N, Solomon LW, et al. Estrogen and progesterone receptors in meningiomas. J Neurosurg. 1984;60:985-993.
122 Martuza RL, Miller DC, MacLaughlin DT. Estrogen and progestin binding by cytosolic and nuclear fractions of human meningiomas. J Neurosurg. 1985;62:750-756.
123 Blaauw G, Blankenstein MA, Lamberts SW. Sex steroid receptors in human meningiomas. Acta Neurochir (Wien). 1986;79:42-47.
124 Grunberg SM. The role of progesterone receptors in meningioma. Cancer Treat Res. 1991;58:127-137.
125 Carroll RS, Zhang J, Dashner K, et al. Progesterone and glucocorticoid receptor activation in meningiomas. Neurosurgery. 1995;37:92-97.
126 Carroll RS, Glowacka D, Dashner K, et al. Progesterone receptor expression in meningiomas. Cancer Res. 1993;53:1312-1316.
127 Simon RH. Brain tumors in pregnancy. Semin Neurol. 1988;8:214-221.
128 Roelvink NC, Kamphorst W, August H, et al. Literature statistics do not support a growth stimulating role for female sex steroid hormones in haemangiomas and meningiomas. J Neurooncol. 1991;11:243-253.
129 Scarcella G, Allen MBJr, Andy OJ. Vascular lesions of the posterior fossa during pregnancy. Am J Obstet Gynecol. 1961;82:836-840.
130 Ferrante L, Celli P, Fraioli B, et al. Haemangioblastomas of the posterior cranial fossa. Acta Neurochir (Wien). 1984;71:283-294.
131 Kasarskis EJ, Tibbs PA, Lee C. Cerebellar hemangioblastoma symptomatic during pregnancy. Neurosurgery. 1988;22:770-772.
132 Allen J, Eldridge R, Koerber T. Acoustic neuroma in the last months of pregnancy. Am J Obstet Gynecol. 1974;119:516-520.
133 Smolik EA, Nash FP, Clawson JW. Neurological and neurosurgical complications associated with pregnancy and the puerperium. South Med J. 1957;50:561-572.
134 Barnes JE, Abbott KH. Cerebral complications incurred during pregnancy and the puerperium. Am J Obstet Gynecol. 1961;82:192-207.
135 O’Neill BP, Illig JJ. Primary central nervous system lymphoma. Mayo Clin Proc. 1989;64:1005-1020.
136 Galicich JH, Sundaresan N, Arbit E, et al. Surgical treatment of single brain metastasis: factors associated with survival. Cancer. 1980;45:381-386.
137 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
138 Adeloye A, Osuntokun BO, Hendrickse JP, et al. The neurology of metastatic chorion carcinoma of the uterus. J Neurol Sci. 1972;16:315-329.
139 Stilp TJ, Bucy PC, Brewer JI. Cure of metastatic choriocarcinoma of the brain. JAMA. 1972;221:276-279.
140 Weed JCJr, Hunter VJ. Diagnosis and management of brain metastasis from gestational trophoblastic disease. Oncology (Williston Park). 1991;5:48-50.
141 Aguilar MJ, Rabinovitch R. Metastatic chorionepithelioma simulating multiple strokes. Neurology. 1964;14:933-937.
142 Nakagawa Y, Tashiro K, Isu T, et al. Occlusion of cerebral artery due to metastasis of chorioepithelioma. Case report. J Neurosurg. 1979;51:247-250.
143 Hammond CB, Borchert LG, Tyrey L, et al. Treatment of metastatic trophoblastic disease: good and poor prognosis. Am J Obstet Gynecol. 1973;115:451-457.
144 Kassam SH, Hadi HA, Fadel HE, et al. Benign intracranial hypertension in pregnancy: current diagnostic and therapeutic approach. Obstet Gynecol Surv. 1983;38:314-321.