Brain tumors
CORRIE J. STAYNER, PT, MS, RACHEL M. LOPEZ, PT, MPT, NCS and KARLA M. TUZZOLINO, PT, NCS
After reading this chapter the student or therapist will be able to:
1. Identify the categories of primary brain tumors.
2. Recognize and interpret signs and symptoms of primary brain tumors specific to tumor location.
3. Recognize current diagnostic tests used to detect brain tumors.
4. Identity the types of medical and surgical management for brain tumors and how that management will affect functional movement.
5. Describe the side effects associated with the treatment of brain tumors and recognize their impact on therapeutic intervention.
6. Discuss the multiple considerations necessary to plan and execute an intervention program for the client with a brain tumor.
7. Recognize the emotional and psychosocial impact of the disease process on the client, the client’s support system, and the interdisciplinary team.
An overview of brain tumors
The rehabilitation clinician serves many different populations, including clients with brain tumors. Despite the prognosis for limited survival associated with primary brain tumors, these individuals have shown progress in the rehabilitation setting similar to that noted in clients with diagnoses of stroke or traumatic brain injury.1–3 Advances in medical and surgical treatment for clients with cancer have resulted in improved survival rates and longer life expectancy. However, individuals are often faced with progressive impairments resulting from the disease process.2 These impairments may be physical or cognitive, or both, and require an interdisciplinary team approach to best facilitate the individual’s participation in a meaningful lifestyle. In addition, clinicians must recognize the psychological and emotional needs of the individual given this diagnosis and be sensitive and flexible in accommodating the patient’s feelings. Improved quality of life, especially the opportunity to return home, remains the ultimate goal of the rehabilitation process.
Incidence and etiology
The incidence of adult brain tumors is on the rise in the United States, with an estimated 62,930 new cases of primary benign or malignant brain and CNS tumors for 2010. The statistics for children include 4030 new cases for the same 12-month period, of which 2880 will occur in children younger than 15 years of age.4,5
The exact cause of the increase in incidence of brain tumors is not known. Studies suggest that the increase is the result of more tumors being diagnosed with improved tumor imaging, rather than an actual increase in the occurrence of malignant brain tumors.6,7
In the United States, brain tumors typically occur in two distinct categories of patients: (1) children aged 0 to 15 years and (2) adults in the fifth to seventh decades of life. In adults, white Americans have a higher incidence than black Americans, and in both pediatric and adult populations males are more frequently affected than females.8,9 In children, a primary brain tumor is now the most common cause of solid tumor cancer death in the 0- to 15-year-old age group and the second overall cancer after leukemia.5
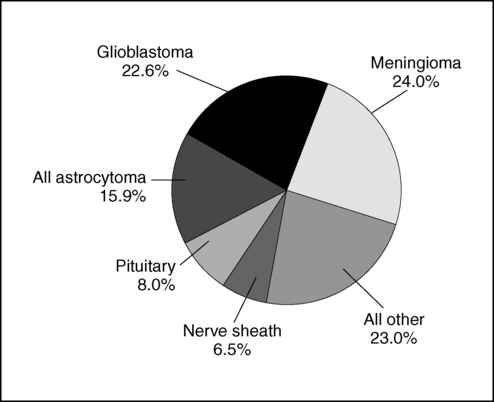
The frequently occurring meningioma, typically benign, accounts for 33.8% of all primary brain tumors. Glioblastoma multiforme, a malignant tumor, accounts for 17.1% of adult primary tumors (Figure 25-1).5 The largest percentage of childhood tumors (17%) are located in the frontal, temporal, parietal, and occipital lobes of the brain, followed by 16% in the cerebellum and 11% in the brain stem.5
The etiology of brain tumors remains unclear. Theories suggest that heredity is a contributing factor, but studies show familial incidence can be explained by a common toxic or infectious exposure.10,11 Research indicates an association, but not a causal relationship, linking brain tumors to certain chemicals and materials (petrochemicals, organic solvents, rubber). These materials are frequently found in specific occupations, such as farming and manufacturing. Electromagnetic field exposure is associated with an increased incidence of brain tumor.12 Ionizing radiation, used therapeutically in high doses to treat tumors, was found to have a causal relationship to the development of a second brain tumor.13
Classification of tumors
The World Health Organization (WHO) first published a universal classification system for CNS tumors in 1979. This system classifies tumors according to their microscopic characteristics and has been accepted as the universal method for the classification of brain tumors.14,15
Primary brain tumors
Gliomas are primary tumors that arise from supportive tissues of the brain and are frequently located in the cerebral hemispheres. These tumors may also occur in the brain stem, optic nerve, and spinal cord. In children, the cerebellum is a primary location for gliomas.13,16 Gliomas have four primary categories and are classified by their predominant cellular components: astrocytomas and oligodendrogliomas originate from glial cells, ependymomas from ependymal cells, and medulloblastomas from primitive cells.17
Astrocytomas are derived from astrocytes, which are star-shaped glial cells, and are the most common primary brain tumor in adults and children.18 Astrocytomas vary in morphology and biological behavior, from those that are diffuse and infiltrate surrounding brain structures, to those that are circumscribed with a decreased likelihood of progression. Astrocytomas are typically found in the cerebrum, originating in the frontal lobe in adults, and in the cerebellum in children. In adults the primary age at onset is typically in the third to fifth decades of life.7,14
Astrocytomas are further classified into four grades: pilocytic, well-differentiated, relatively benign low-grade tumors most common in childhood and young adults (grade I); diffuse, well differentiated, low-grade tumors (grade II); anaplastic, high-grade tumors (grade III); and glioblastoma multiforme, high-grade (grade IV). The higher the grade, the poorer the prognosis.7
Low-grade tumors (grade II) grow slowly and are typically subtotally resected through surgery when accessible, whereas grade I tumors occur primarily in children and are typically cured with complete surgical resection. As a result of incomplete resections, recurrence is common.7 As these tumors recur, their form and structure often change to that of an anaplastic astrocytoma or glioblastoma.17 Anaplastic, midgrade (grade III) tumors grow rapidly, typically carry malignant cell traits, and routinely progress toward glioblastoma multiforme tumors.14
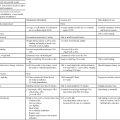
Astrocytomas are typically treated with surgery, radiation therapy, and chemotherapy, depending on the grade, location of the tumor, age of the patient, and Karnofsky performance scale score (Table 25-1).7,16,19 Pilocytic astrocytomas carry a 5-year survival rate of 94%; however, patients with grade III astrocytomas have a 5-year survival rate of only 27%.5
Adapted from Karnofsky DA, Burchenal JH: The clinical evaluation of chemotherapeutic agents in cancer. In Macleod C, editor: Evaluation of chemotherapeutic agents, New York, 1949, Columbia University Press.
Glioblastoma multiforme is the distinct name given to the highly malignant grade IV astrocytoma. These tumors grow rapidly, invade nearby tissue, and contain highly malignant cells. Glioblastomas are predominantly located in the deep white matter of the cerebral hemispheres but may be found in the brain stem, cerebellum, or spinal cord. Fifty percent of these tumors are bilateral or occupy more than one lobe of a hemisphere.7,14,16 Glioblastomas account for 17% of all primary brain tumors. They are most common in older adults and uncommon in children, with males having a 1.6:1 incidence rate over females.5 The medical prognosis is poor for persons with glioblastoma: less than 33% survive more than 1 year and less than 5% survive 5 years.5 The most important prognostic variables are age, tumor histology, and postoperative score on the Karnofsky performance status scale. These tumors are treated by surgical resection, radiation therapy, stereotactic radiosurgery, and chemotherapy.7
Oligodendrogliomas are slow-growing but progressive tumors that typically develop over a period of several years, with 50% involving multiple lobes. Fifty percent of these tumors occur in the frontal lobe, 42% in the temporal lobe, and 32% in the parietal lobe. Many clients have seizures as the only clinical manifestation of the tumor.14,20,21 Oligodendrogliomas typically appear in the fourth to sixth decades of life, and the ratio of affected males to females is 2:1.22 The prognosis with oligodendrogliomas varies considerably and is dependent on age at diagnosis and tumor grade. Positive prognostic indicators have been age at onset of less than 40 years and a tumor grade of I or II. These patients have a 5-year survival rate of 79% and 10-year survival rate of 64%.5 The 5-year survival rate decreases to 47% with anaplastic oligodendroglioma.5 Treatment is dependent on symptoms and ranges from observation and seizure control with anticonvulsant drugs to surgical resection followed by radiation and chemotherapy.7,17,20 Negative prognostic indicators include age at onset over 40 years, hemiparesis, and cognitive changes.23
Ependymomas and ependymoblastomas are tumors arising from ependymal cells, cells that line the ventricles of the brain and central canal of the spinal cord.16,17 These cells have glial and epithelial characteristics. The tumors grow into the ventricle or adjacent brain tissue. The most common site is the fourth ventricle (70% originate here); they occur less frequently in lateral and third ventricles.22 For supratentorial tumors, the age at onset is evenly distributed across the life span, whereas tumors originating in the fourth ventricle more frequently occur in childhood.22 Ependymomas are primarily treated with surgical resection followed by radiation therapy, but chemotherapy is also used.7,16,17,22 These tumors frequently recur, and prognosis is dependent on the success of resection, with a 5-year survival rate approaching 82%.5
Medulloblastomas are malignant embryonal tumors thought to arise from primitive neuroectodermal cells, specifically pluripotential stem cells that have been prevented from maturing to their normal growth-arrested state. The exact cell of origin, however, is still unknown.22 These tumors are typically located in the posterior fossa, originating laterally in the cerebellar hemispheres in young adults and in the vermis in children.14 Medulloblastomas typically grow into the fourth ventricle, blocking cerebrospinal fluid (CSF) flow, causing hydrocephalus and increased intracranial pressure (ICP).7 These tumors primarily occur in children, accounting for 20% of childhood (0 to 19 years) brain tumors, with the most common age of onset being 4 to 8 years old; they are more prevalent in males than females.22 An overall 5-year survival rate of 61% has been noted among adults and children; the most common treatment is surgery followed by radiation and chemotherapy.5,14,22
Meningiomas are slow-growing tumors that primarily originate from cells located in the dura mater or arachnoid membrane and account for 33% of reported brain tumors.5,18,22 Frequently these tumors are found incidentally during imaging studies or at autopsy.24 Approximately 25% of patients are symptomatic when diagnosed.18,24,25 Meningiomas are classified by their cytoarchitecture and genetic origin into four categories: (1) meningothelial or syncytial, (2) fibroblastic, (3) angioblastic variants, and (4) malignant.22 The incidence increases with age, and they occur in females at a 2:1 ratio over males.3,5,26,27 Resectable tumors are primarily treated by surgery, and recurring tumors are treated with surgery, radiation therapy, or stereotactic radiosurgery.17 Patients with nonmalignant meningiomas have a 5-year survival rate of 70% versus a 5-year survival rate of 55% with malignant meningiomas.5,28
Pituitary adenomas are benign epithelial tumors originating from the adenohypophysis of the pituitary gland and frequently encroach on the optic chiasm.14,17,22 These tumors are characterized by hypersecretion or hyposecretion of hormones.7,16 Age at onset spans all ages, but pituitary adenomas are rare before puberty.7 The female-to-male ratio of incidence is 3:1.5 These tumors are primarily treated by surgical resection and drug therapy.7,16,17 Prognosis is related to size and tumor cell type, with a 5-year survival rate of 70%.5
Schwannomas are encapsulated tumors composed of neoplastic Schwann cells that can arise on any cranial or spinal nerve.16,17 The eighth cranial nerve is the cranial nerve usually involved, and a schwannoma here is called an acoustic neuroma.14 Acoustic neuromas produce otological, focal or generalized neurological impairments, depending on the location of the tumor. These tumors are typically located in the internal auditory canal but may extend into the cerebellopontine angle.7,22 These tumors are frequently treated by surgical resection, but stereotactic radiosurgery is increasing in popularity as an alternative method of treatment.16,29,30 The prognosis for patients with these tumors is good, yet complications can result from treatment, including facial paralysis, deafness, and equilibrium impairments. Resulting activity limitations after surgery vary depending on the size and location of the tumor. Currently these tumors rarely result in death, and with the increasing use of noninvasive procedures the eighth cranial nerve is more frequently being preserved.30
Primary CNS lymphoma represents only 1% of intracranial tumors, although the incidence has significantly increased in the last two decades. This increase may be a result of its frequency in individuals with acquired immunodeficiency syndrome (AIDS) and other immunosuppressed states. Surprisingly, there is an increased frequency in immunocompetent persons; however, no evidence-based explanation has been found. These lymphomas have a slightly higher incidence in men and peak in the fifth through seventh decades of life, or in the third and fourth decades in individuals with AIDS. The tumor cells are similar in histology to systemic non-Hodgkin lymphoma cells, but it is uncertain how this tumor arises, as the CNS lacks lymphatic tissue.22,31 The tumor may be solitary or multifocal, forming a poorly defined mass that may be difficult to distinguish from an astrocytoma.22 Although CSF cytology may be diagnostic, stereotactic brain biopsy is often needed for definitive diagnosis.31 Primary brain lymphomas may arise in the cerebrum, cerebellum, or brain stem; however, 60% occur in the cerebral hemisphere. More frequently, presenting symptoms are behavioral and personality changes, confusion, dizziness, and focal cerebral signs rather than headache and other signs of increased ICP.22 Surgical resection is typically ineffective because of the deep location of these tumors. Cranial irradiation and corticosteroids frequently yield a partial or complete response; however, the tumor recurs in 90% of these individuals.22 A more recent favorable treatment includes the administration of intravenous methotrexate and leucovorin over 2- to 3-week intervals with corticosteroids to control neurologic symptoms.22 CNS lymphoma carries a poor prognosis, with only 27% of patients surviving longer than 5 years.5
Secondary brain tumors: metastatic brain tumors
Metastatic brain tumors originate from malignancies outside of the CNS and spread to the brain, typically through the arterial circulation.7,22 Approximately 25% of individuals with systemic cancer develop metastatic brain tumors, approximately 80% in cerebral hemispheres and 20% in the posterior fossa.22,31,32 One third of brain metastases originate in the lung, followed by the breast, skin, gastrointestinal tract, and kidneys in order of frequency. The frontal lobe is the most common site for metastatic disease from these systemic sources. Common clinical manifestations of metastatic brain tumors are similar to those of gliomas, including seizures, headache, focal weakness, mental and behavioral limitations, ataxia, aphasia, and signs of increased ICP.22
Treatment for these tumors is tailored to the individual and dependent on the management of the systemic disease, the accessibility of the lesion, and the number of lesions.32,33 Current treatment regimens use combinations of corticosteroids, brain irradiation, surgical intervention, and chemotherapy.22 The prognosis varies, with positive prognostic indicators including the Karnofsky performance scale score of 70 or greater, age 60 years or younger, remission or resolution of the primary cancer, and metastases located in the brain only.32,33 The average survival with treatment is approximately 6 months but varies widely and is affected by the extent of other systemic metastases. With some radiosensitive tumors, survival increases to 15% to 30% for 1 year and 5% to 10% for 2 years.22
Signs and symptoms
The clinical manifestation of a brain tumor can range from a decreased speed in comprehension or a minor personality change to progressive hemiparesis or seizure, depending on the type and site of the tumor. Patients with brain tumors typically have headaches, seizures, nonspecific cognitive or personality changes, or focal neurological signs.22,34 The presenting sign in some may be a general sign, a specific neurological symptom, or a combination of both.
General signs and symptoms
1. The headache that interrupts sleep or is worse on waking and improves throughout the day
2. The headache that is elicited by postural changes, coughing, or exercise
3. The headache of recent onset that is more severe or of a different type than usual
4. The new onset of headache in a previously asymptomatic person
5. The headache associated with nausea and vomiting, papilledema, or focal neurological signs1,7
The mechanism of the headache is not clearly understood but may be related to local swelling, distortion of blood vessels, direct invasion of the meninges, and increased ICP. When the tumor has grown to a volume large enough to cause compression and displacement of the brain, the onset and severity of the headache seem to correlate with changes in ICP.35 With increased ICP, a bifrontal or bioccipital headache is present regardless of the tumor location.22,36
Seizure activity is the presenting symptom in one third of cases and is present in 50% to 70% of cases at some stage of the disease.13,34 Approximately 10% to 20% of adults with new-onset seizure activity have brain tumors. Seizures are usually focal but may become generalized and cause loss of consciousness.37 Frontal lobe gliomas produce seizures in 59% of all cases. The percentages of patients exhibiting seizures from gliomas in other lobes are as follows: parietal, 42%; temporal, 35%; and occipital, 33%.34
Altered mental status is the initial symptom in 15% to 20% of individuals with brain tumors and is frequently present at the time of diagnosis. Mental status changes can range from subtle changes in concentration, memory, affect, personality, initiative, and abstract reasoning to severe cognitive problems and confusion.34 Subtle changes may be incorrectly attributed to worry, anxiety, or depression.22 Changes in mentation are common with frontal lobe tumors and in the presence of elevated ICP. Increased ICP causes drowsiness and decreased level of consciousness, which can progress to stupor or coma if treatment is not initiated.34
The incidence of papilledema, swelling of the optic nerve, is less frequent today because brain tumors are being diagnosed earlier with the use of sensitive imaging techniques. Papilledema is associated with symptoms of transient visual loss, especially with positional changes, and reflects evidence of increased intracranial hemorrhage transmitted through the optic nerve sheath. It is more common in children and with slow-growing tumors and posterior fossa tumors.34 Other, less common symptoms are vomiting and frank positional vertigo, usually accompanying tumors found in the posterior fossa.22
Specific signs and symptoms
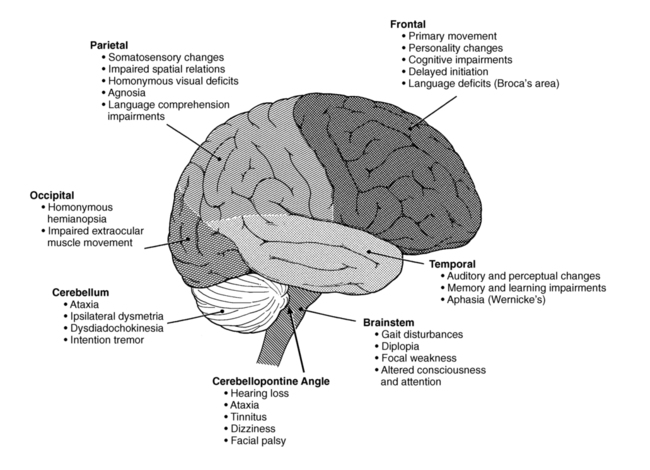
Certain clinical features are related to functional areas of the brain and thus have a specific localizing value in medically diagnosing a brain tumor.34 Therefore it is essential that clinicians be familiar with the lobes of the brain and their distinct functions to effectively manage the impairments resulting from the tumor (Figure 25-2). These symptoms may vary among individuals and result in activity limitations that range from mild to severe.35
The frontal lobe is responsible for motor functioning, initiation of action, and interpretation of emotion, including motor speech, motor praxis, attention, cognition, emotions, intelligence, judgment, motivation, and memory.38,39 Therefore frontal lobe tumors may result in movement disorders such as hemiparesis, seizures, aphasia, and gait difficulties. Initially the tumor may be clinically silent. As the tumor grows, however, there may be personality changes, including disinhibition, irritability, impaired judgment, and lack of initiation.34 Bifrontal disease usually associated with infiltrative gliomas and primary CNS lymphomas may cause bilateral hemiplegia; spastic bulbar palsy; severe cognitive impairment; emotional lability; dementia; and prominent primitive grasp, suck, and snout reflexes.35
The parietal lobe processes complex sensory and perceptual information related to somesthetic sensation, spatial relations, body schema, and praxis. General symptoms of a parietal lobe tumor include contralateral sensory loss and hemiparesis, homonymous visual deficits or neglect, agnosias, apraxias, and visual-spatial disorders. If the dominant parietal lobe is involved, aphasia and seizures may be present. With nondominant parietal lobe involvement, contralateral neglect and decreased awareness of impairments can commonly be found.26,34,36
The occipital lobe is the primary processing area of visual information. Therefore lesions of the occipital lobe often result in dysfunction of eye movement and homonymous hemianopsia. If the parieto-occipital junction is involved, visual agnosia and agraphia are often present. Although less common, visual seizures may be present, characterized by lights, colors, and formed geometric patterns.33,34,36 Bilateral occipital tumors may cause cortical blindness.35
The temporal lobe is responsible for auditory and limbic processing. Anterior temporal lobe lesions may be clinically silent until they have become quite large, resulting in seizures. If the lateral hemispheres are involved, auditory and perceptual changes may occur. When the medial aspects of the lobe are involved, changes in cognitive integration, long-term memory, learning, and emotions may be seen. When the dominant temporal lobe is involved, aphasia may be present. Anomia, agraphia, acalculia, and Wernicke aphasia, characterized by fluent, nonsensical speech, are specific to left temporal lobe lesions.33,34,36 In comparison with bifrontal tumors, bitemporal tumor involvement is rare and causes memory deficits and possible dementia.35
The cerebellum is responsible for coordination and equilibrium.26(Refer to Chapters 21 and 22A.) The most common symptoms of cerebellar tumors in adults include headache, nausea and vomiting in 40% of cases, and ataxia in 25% of cases. Lesions of the midline cause truncal and gait ataxia, and lesions of the hemispheres cause unilateral appendicular ataxia, most commonly seen in the upper extremities. Lesions of either hemisphere may cause ipsilateral dysmetria, dysdiadochokinesia, and intention tremor. If the tumor involves the cerebellopontine angle, hearing loss, headache, ataxia, dizziness, tinnitus, and facial palsy may occur. If the tumor invades the meninges at the foramen magnum or increased ICP causes cerebellar tonsil herniation, nuchal rigidity and head tilt away from the lesion may be seen. Abnormal posturing of the head is observed in children but not adults.35 Because the cerebellum is located in an extremely confined space, even minimal increases in pressure can cause death from cerebellar tonsil herniation.7,34,35,40
The brain stem, which communicates information to and from the cerebral cortex via fiber tracts, controls basic life functions. The reticular formation specifically controls consciousness and attention. Even small changes in tumors invading or compressing the brain stem can lead to death or devastating signs and symptoms. Symptoms of a brain stem tumor have an insidious onset and may include gait disturbances, diplopia, focal weakness, headache, vomiting, facial numbness and weakness, and personality changes.7 If the dorsal midbrain is involved, Parinaud syndrome, characterized by loss of upward gaze, pupillary areflexia to light, and loss of convergence, may be seen. If the reticular system of the pons and medulla is involved, symptoms of apnea, hypoventilation or hyperventilation, orthostatic hypotension, or syncope may occur.7,40
The pituitary gland is an endocrine gland that secretes hormones that regulate many bodily processes. Pituitary tumors are typically large and affect pituitary function by compressing its structure or hypersecreting hormones. An enlarging tumor causes a loss of pituitary function and decreases hormone secretion, resulting in pituitary disorders specific to the type of hormone involved (e.g., Cushing disease, hypothyroidism, Addison disease, diabetes). As the tumor enlarges it may invade or compress nearby structures. Lateral extension involving the third and fourth cranial nerves causes diplopia; fifth cranial nerve involvement causes ipsilateral facial numbness; and internal carotid artery occlusion causes cerebral infarction. Upward extension is more common and may compress the optic chiasm, hypothalamus, or third ventricle. Downward extension may compress the sphenoid sinus, typically without clinical signs.7
Medical diagnosis of disease or pathology
Advances in research and imaging technology have greatly improved brain tumor medical diagnosis. When a physician suspects a brain tumor, many specialized tests may be used to gather clinical, radiological, pathological, and laboratory information to confirm the diagnosis.31,34
Clinical diagnosis
A clinical diagnosis consists of information the physician gathers during a comprehensive evaluation. First, a thorough medical history, including the specific nature of signs and symptoms, must be obtained. A neurological examination is then performed to test reflexes and assess visual, cognitive, sensory, and motor function.40 If the presence of a brain tumor is suspected after the neurological examination, the next diagnostic step, tumor imaging, is warranted.22
Radiological diagnosis
The modern era of CNS imaging began with the introduction of computed tomography (CT) in 1973 and with magnetic resonance imaging (MRI) in 1979.7 The availability of sensitive imaging allows for earlier tumor detection and has revolutionized the diagnosis and management of brain tumors.34,41 Tumor imaging has continued to develop and can be classified into three categories: static, dynamic, and computer integration imaging. Each type of scan shows different features and function of the brain; therefore several scans may be needed for an accurate diagnosis.16
Static imaging.
Static neurological imaging includes CT and MRI, which are noninvasive techniques that provide accurate anatomical and functional analysis of intracranial structures.34 CT uses ionizing radiation, thin bands of x-rays, to produce images of slices of brain tissue.31 It was the first brain imaging technique to allow determination of tumor size. Contrast enhancement helps to identify isodense tumor from surrounding parenchyma, hypodense lesions in edematous areas, and optimal sites for tumor biopsy.7,34 After surgical intervention, CT can be used to confirm the proper tissue biopsy site and determine the success of tumor resection. Although MRI has become the preferred method, CT scanning offers lower cost, a shorter scanning time, and a more sensitive method to detect calcification and bony involvement.22
Magnetic resonance imaging.
MRI is the initial diagnostic imaging procedure of choice. MRI uses magnetic fields rather than ionizing radiation and is superior to CT scanning in detecting and localizing brain tumors, as well as evaluating edema, hydrocephalus, or hemorrhage.22,34,35 CT scans can miss structural lesions, especially posterior fossa tumors and low-grade gliomas.37 MRI is a more sensitive imaging modality than CT for identifying lesions and margin abnormalities by providing greater anatomical detail with thin slices and multiplanar images. With MRI, different signal intensities differentiate between normal brain and tumor. Contrast enhancement with gadolinium sharpens the definition of a lesion.7,22,36 Under certain conditions, MRI enhanced with gadolinium can distinguish between tumor and edema. However, not all high-grade astrocytomas enhance with gadolinium, and MRI signals may imitate imaging abnormalities seen in low-grade astrocytomas or nonmalignant conditions. MRI also cannot accurately predict tumor type or grade of malignancy, for which surgical biopsy is necessary.7,34
Dynamic imaging.
Dynamic functional imaging includes positron emission tomography (PET), single-photon emission CT (SPECT), magnetic resonance spectroscopy (MRS), and functional MRI. PET is a noninvasive technique using a cyclotron and specific isotopes to obtain dynamic information about the metabolism and physiology of the brain tumor and the surrounding brain tissue. PET scans using radioactive markers to measure glucose metabolism can be useful in determining the grade of primary brain tumors and in differentiating tumor regrowth from radiation necrosis.9,36,42 PET can also be helpful in studying the metabolic effects of chemotherapy, radiation therapy, and steroids on the tumor.34 However, PET is expensive and less reliable in patients treated heavily with chemotherapy and radiation therapy.7,22
Single-photon emission computed tomography.
SPECT is a functional imaging technique evolved from PET and uses isotopes without cyclotron to assess cerebral blood flow and determine tumor location.7,22,34 SPECT is used to identify high- and low-grade tumors and to differentiate between tumor recurrence and radiation necrosis.7,34 SPECT is used preoperatively with static imaging to localize the highest metabolic area within tumor for biopsy. Although SPECT is a less sensitive method of obtaining physiological information on brain tumors, it is more readily available and less expensive.7
Magnetic resonance spectroscopy.
MRS is a noninvasive technique used in conjunction with static MRI to measure the metabolism of brain tumors.7 MRS has been proved to differentiate successfully normal brain from malignant tumor and recurrent tumor from radiation necrosis. It also has been used to document early treatment response and provide information regarding histological grade of astrocytomas.43,44 In the future, MRS targeting may enhance the diagnostic yield of brain biopsy and possibly be a noninvasive alternative to surgical biopsy.43,45 Magnetic resonance angiography (MRA) generates images of blood vessels without dye or ionizing radiation to evaluate the blood flow and position of vessels leading to the brain tumor.31
Functional magnetic resonance imaging.
Functional MRI uses a conventional MRI scanner fitted with echo planar technology to map cerebral blood flow at the capillary level. Its intended purpose is to provide information regarding the diffusion of contrast into tumor, resulting in better resolution of tumor and edema.7 Functional MRI can also be used to identify the motor, sensory, and language areas of the brain or the functional eloquent cortex.16,46
Modern computer technology allows for the two- and three-dimensional reconstruction of identical planes in cranial space by combining tumor images from different modalities, including CT, MRI, PET, and SPECT. Computed integration imaging involves the simultaneous display of images from different techniques in a single imaging system that is transposed to a reference stereotactic frame. This development has resulted in significant advances in stereotactic biopsy, interstitial radiotherapy, and laser-guided stereotactic resection.7 By improving targeting and visualization of tissues, stereotaxis provides a safer, more accurate method of tissue acquisition and biopsy. A correct tissue diagnosis can be made in 95% of cases with this technique.47 (Refer to Chapter 37 for additional discussion on all types of imaging and visual examples.)
Biopsy
Surgical biopsy is performed to obtain tumor tissue as part of tumor resection or as a separate diagnostic procedure.13 Stereotactic biopsy is a computer-directed needle biopsy. When guided by advanced imaging tools, stereotactic biopsy yields the lowest surgical morbidity and highest degree of diagnostic information. This technique is frequently used with deep-seated tumors in functionally important or inaccessible areas of the brain in order to preserve function.48
Laboratory diagnosis
Laboratory testing is often used to further assess focal deficits during the diagnosis and management of brain tumors. Perimetry is the measurement of visual fields used when evaluating tumors near the optic chiasm. Electroencephalography (EEG) is used to monitor brain activity and detect seizures but has limited value during screening because EEG findings are often normal in clients with brain tumors.34 Lumbar puncture is used to analyze CSF, which is useful in the diagnosis and detection of dissemination of certain brain tumors. However, lumbar puncture is risky in patients with increased ICP and should be avoided in those cases.22,34,36 Audiometry and vestibular testing are useful for diagnosing tumors in the cerebellopontine angle. Endocrine testing is used to examine endocrine abnormalities with tumors in the pituitary gland and hypothalamus.34
Medical and surgical management
After diagnosis of a brain tumor has been confirmed, specific treatment must be selected. The ultimate goals of tumor management are to improve quality of life and extend survival, by preserving or improving body function and structures.49 These goals are accomplished by removing or decreasing the size of the tumor. Treatment techniques are determined by histological type, location, grade, and size of tumor; age at onset; and medical history of the patient.7,17,49 Four types of treatment are discussed: (1) traditional surgery, (2) chemotherapy, (3) radiation therapy, and (4) stereotactic radiosurgery.
Traditional surgery
The primary goal of traditional surgery is maximal tumor resection with the least amount of damage to neural or supporting structures.7 Gross total resection is associated with longer survival rates and decreased neurological impairment.37 Benign tumors, if accessible, are resected completely, whereas malignant tumors are typically partially resected secondary to location or size of the tumor.7,49 The purposes of surgery in the management of brain tumors include the following:
1. Biopsy to establish a diagnosis
2. Partial resection to decrease the tumor mass to be treated by other methods
Biopsies are performed through open, needle, and stereotactic needle techniques. Open biopsies involve exposure of the tumor followed by removal of a sample through surgical excision. Needle biopsies involve insertion of a needle into the tumor through a hole in the skull and the excision of the tissue sample drawn through the needle. Stereotactic needle biopsies use computers and MRI or CT scanning equipment to assist in directing the needle into the tumor. This type of biopsy is useful for deep-seated or multiple brain lesions.7,16
Partial and complete resections are accomplished through craniotomy. Craniotomy involves removal of a portion of the skull and separation of the dura mater to expose the tumor. Stereotactic craniotomy uses technology to create computed three-dimensional pictures of the brain to guide the neurosurgeon during the procedure. CT scanning and MRI scanners are used to provide an evaluation of the tumor resection during the procedure.7,50 Awake craniotomy allows for intraoperative brain mapping that helps to identify and protect functional cortex and in recent years has become an alternative surgical treatment for most supratentorial tumors.46
Preoperative management.
Before surgery, clients are evaluated for general surgical risks and the possibility of tumors in additional locations. Unless medically contraindicated, steroids are administered before surgery if brain edema is present or if extensive manipulation will be occurring during surgery. Anticonvulsant medications are also administered preoperatively to prevent seizures during or after surgery.7,49
Intraoperative management.
During surgery, precautions are taken to prevent an increase in edema or ICP. Mannitol, a vasodiuretic to decrease ICP, is used to shrink the surrounding brain tissue, thus providing easier access to the tumor. Steroid use is continued and antibiotics are administered to prevent infection. Hyperventilation, with a CO2 level of 25 mEq/L, is also used to reduce ICP.7,49
Postoperative management.
Patients are observed in an intensive care unit for at least 24 hours for possible intracranial bleeding or seizures. Blood pressure is monitored continuously. After surgery, patients are at risk for developing deep vein thrombosis or pulmonary embolism secondary to decreased muscle activity, but because these patients are at risk for intracranial bleeding, anticoagulants cannot be given.51 Therefore compression stockings are used prophylactically in an attempt to prevent deep vein thrombosis. Steroids are tapered after surgery over 5 to 10 days. Anticonvulsant medications are continued after surgery, with the length of time dependent on the presence of seizure activity before and after surgery.7,49 The primary limitations of traditional surgery include the following:
Chemotherapy
Chemotherapy is another treatment frequently used to manage brain tumors. It can be used independently or as an adjuvant to surgery or radiation. Chemotherapeutic drugs are not effective on all types of tumors. Some tumors are known to be resistant to certain drugs, and therefore other treatments are more successful in treating these tumors. Drugs can be given in combination to target all cell types present within the tumor. Because different drugs have different modes of action and side effects, combined drug therapy often proves to be one of the most effective treatments.52 Chemotherapy can be administered in a number of different ways. Most agents are given intravenously through a peripheral intravenous line or through a catheter such as a peripherally inserted central catheter (PICC) or Groshong catheter. Other drugs are placed directly into the tumor bed or are given intramuscularly, orally, or by means of an implanted device.
Chemotherapy drugs impede cellular replication of the tumor cells, interfering with their ability to copy deoxyribonucleic acid (DNA) and reproduce. Once the replicating capability of the tumor cell has been disrupted, the cell dies. In this way the tumor is prevented from growing and is destroyed at the cellular level.52
Methotrexate is a highly toxic drug and is usually paired with an antidote drug, leucovorin, to reverse the side effects on normal cells.53 Typically methotrexate is used to treat cancer outside of the CNS; however, it is the major chemotherapy drug used to treat CNS lymphoma.22 Methotrexate has been found to produce a high degree of neurotoxicity when used in combination with radiation therapy.54
Neurotoxic to surrounding tissue, methotrexate and ara-C are drugs able to be introduced directly into the CSF through an intraventricular Ommaya reservoir.35 The reservoir, implanted under the scalp, is filled by use of a syringe, and the medication is then circulated through the ventricles to the brain.52 The drugs are typically given in a clinic setting by a registered nurse certified in chemotherapy administration. A patient’s chemotherapy schedule varies depending on the drug given. An on-off cycle is used to allow the patient to recover from the toxic effects of the drug.
One of the challenges in delivering cytotoxic drugs to the brain is the blood-brain barrier (BBB). The BBB is the brain’s natural protective barrier against transmission of foreign substances from the blood into the brain.52 One class of drugs that does penetrate the BBB is the nitrosoureas. These include BCNU (carmustine) and CCNU (lomustine), which are lipid soluble and cell cycle specific. These drugs are given in high doses and typically used to treat glioblastoma multiforme and anaplastic astrocytoma; however, often these high-grade tumors invade and destroy the BBB.
BCNU can also be administered in the form of wafers placed by the neurosurgeon directly into the brain tumor. An initial study for recurrent malignant gliomas found that patients’ tumors responded to the treatment.55 This report was followed by an up-front study for glioblastoma multiforme.56 U.S. Food and Drug Administration (FDA) approval for these wafers (Gliadel) followed.
Temozolomide is an orally available chemotherapeutic agent introduced in the 1990s for the treatment of malignant gliomas.57 Initial results in treating recurrent anaplastic astrocytoma58 and glioblastoma59 were so successful that the drug was approved for the treatment of recurrent brain tumors by the FDA. For recurrent tumors the drug is administered orally 5 days per month. Temozolomide was then tested for the up-front treatment of glioblastoma. This occurred in a multicenter study in which the drug was given daily as part of the initial treatment with radiation therapy, followed by five doses per month for maintenance treatment.60 Survival increased substantially with this regimen, and as a result the FDA approved the use of temozolomide as part of first-line treatment of glioblastoma. Temozolomide is also used to treat anaplastic gliomas.
Another major breakthrough in chemotherapy of brain tumors was the finding that the antiangiogenesis monoclonal antibody Avastin (bevacizumab) improved the progression-free survival and the tumor images on MRIs of patients with glioblastoma.61–63 The drug targets vascular endothelial growth factor (VEGF) and is administered intravenously. It has recently been approved by the FDA for the treatment of recurrent glioblastoma multiforme. It is usually administered with another chemotherapy agent, for example, irinotecan.
Radiation therapy
Radiotherapy consists of the delivery of high-powered photons, with energies in a much greater range than that of standard x-rays, as an external beam directly at the tumor site. The external beam is transmitted to the tumor through a linear accelerator or a cobalt machine that uses cobalt isotopes as the radiation source. External beam radiation is the most widely used form of radiation treatment.7
Conventional radiation therapy as described previously is fractionated into small doses delivered over a period of time.16 Often, if a large fraction is to be delivered, the dose is divided and given more than once per day; this is called hyperfractionation. Hyperfractionated radiation therapy is believed to increase the efficacy and decrease the long-term side effects of radiation. More studies need to be completed to know its exact benefits.
Conformal radiation is the use of high-dose external beam radiation, produced by a linear accelerator, to precisely match or “conform” to the tumor shape. One such method of conformal radiation delivery is the Peacock system. This method attempts to deliver a uniform amount of radiation to the tumor and minimize irradiation of healthy brain tissue.16
Radiosurgery involves relatively high-dose hypofractionated radiation beams directed at small tumor areas through the use of computer imaging.16 This type of treatment includes the Gamma Knife, linear accelerators, and the cyberknife, which are discussed later.
Radiation therapy has considerable limitations and disadvantages. There is an accepted maximum lifetime dosage of radiation that the brain and body can tolerate. As doses come close to this limit, the risk of radiation necrosis increases. Because the brains of young children are particularly vulnerable to radiation, other therapies, such as chemotherapy, are used until the developing brain is more tolerant of radiation. Metastatic lesions have invaded multiple organs or body systems, and a more systemic treatment such as chemotherapy is most effective for this type of brain cancer.16
Stereotactic radiosurgery
Stereotactic radiosurgery is defined as delivery of a high dose of ionizing radiation, in a single fraction, to a small, precisely defined volume of tissue.7,33,49,64 The high-energy accelerators involved with stereotactic radiosurgery improve the physical effect of radiation by allowing energy to travel more precisely in a straight line and penetrate deeper before dissipating.64 The goal of stereotactic radiosurgery is to arrest tumor growth.65 This technique has been shown to be most beneficial for treating centrally located lesions less than 3 cm in size and for patients with increased surgical risk factors.33,64 Advantages of stereotactic radiosurgery are as follows:
1. Is a noninvasive procedure using local anesthesia and sedation to place the stereotactic frame
2. Avoids risks of general anesthesia and immediate postoperative risks such as bleeding, CSF leak, and infection
3. Lowers treatment cost and shortens hospital stays7,30,64,66
Stereotactic radiosurgery is used to treat benign and malignant tumors, vascular malformations, and functional disorders.49,64 The primary modes of administration for stereotactic radiosurgery include the Gamma Knife, linear accelerators, and the cyberknife.7,17,49
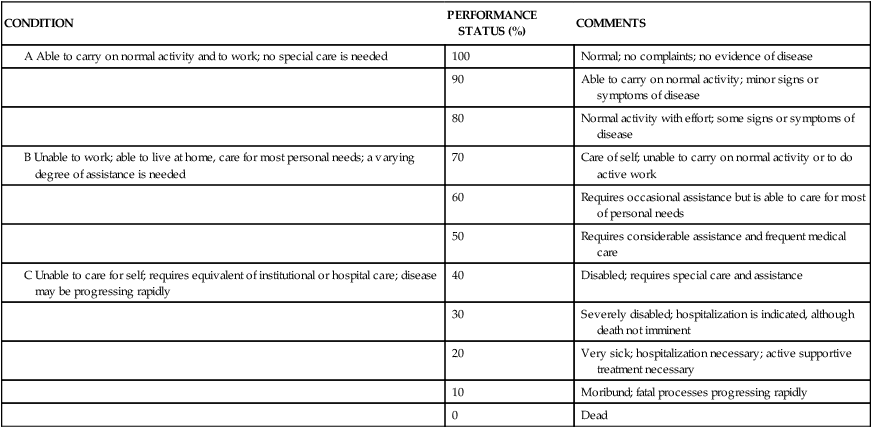
The Gamma Knife was first introduced in Sweden in 1968 and is now used worldwide at 65 sites (Figure 25-3). The Gamma Knife uses 201 discrete sources of cobalt 60, which are focused precisely to one point in three-dimensional space within the cranium.33,49,64,67 The Gamma Knife is typically used for deeply embedded small tumors that require precise delivery of radiation.33
 The Leksell Gamma Knife.
The Leksell Gamma Knife.MRI, CT scanning, or angiography is used to identify the exact location of the lesion to be treated after the stereotactic frame is placed on the client’s head. The stereotactic frame is then fixed to the machine and attached to a collimator helmet containing 201 holes for the radiation to pass through. The patient is then locked into position. The prescribed dose is given over 20 minutes to 2 hours. After treatment the frame is removed; the client is observed and is frequently discharged after 24 hours. Return to previous activity typically occurs within a few days.30,33,49,67
With the Gamma Knife, the full dose of radiation is received only at the point where the 201 beams intersect, thereby giving only a minimal dose to uninvolved tissue when targeted accurately. Side effects are rare, but headache and nausea may occur.7 The primary limitations of the Gamma Knife are the limited brain volume that can be treated with one dose and the cost of the Gamma Knife machine.7,49
Linear accelerators used for conventional radiation can be modified for stereotactic radiosurgery. The brain lesion to be targeted is stereotactically placed in the center of the arc of rotation of the machine. A single, highly focused beam of radiation is delivered over multiple sweeps around the brain lesion. Linear accelerators can be used to treat larger tumors with precise shape while maintaining uniform dose. Because linear accelerators are used for conventional radiation, a quality check for beam accuracy is imperative before the machine is used for stereotactic radiosurgery.7,49
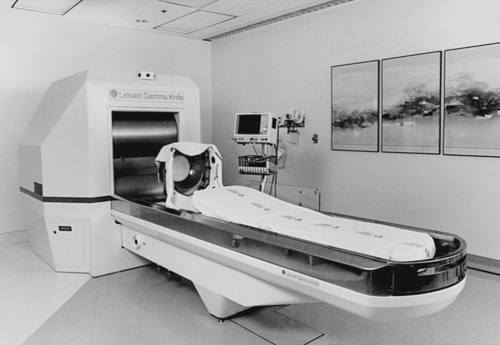
The cyberknife uses a compact linear accelerator mounted on a robotic arm, with the robotic arm moving around the linear accelerator to multiple precalculated positions (Figure 25-4). At each position the accelerator fires a beam of radiation at the tumor or lesion. A high cumulative dose of radiation is achieved at the tumor or lesion because of the convergence of the beams. This dose is typically strong enough to destroy the abnormal cells while minimizing the damaging effects of radiation to healthy surrounding tissue. The cyberknife differs from other stereotactic radiosurgery because a linear accelerator is combined with an image guidance system. The robotic arm allows the cyberknife to target difficult-to-reach areas of the body, as well as adjust quickly for changes in target location during treatment.
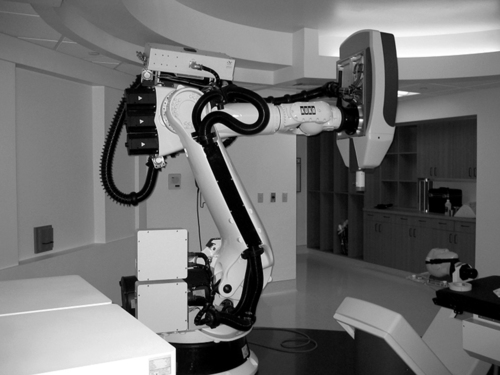
 The cyberknife.
The cyberknife.Several research studies have reported on the use of stereotactic radiosurgery, including the Gamma Knife and linear accelerators, and compared this modality with microsurgery; however, studies involving the cyberknife are limited. In patients with brain metastases, the Gamma Knife is typically indicated for small lesions that are centrally located. Surgical resection is indicated for superficial lesions greater than 3 cm in diameter, when a significant mass effect of the tumor exists, or if edema is present in the cranium.33 The Gamma Knife has been shown to achieve tumor control rates as high as those for surgery and whole-body radiation therapy combined and to halt or reverse neurological progression in 78% of patients treated.68,69
Microsurgical resection has shown a 90% cure rate for acoustic neuromas less than 3 cm in size. Stereotactic radiosurgery avoids the risk of an open procedure, but the tumor is controlled rather than removed. Thus far, a 92% tumor control rate has been noted, but the patients in this study have not had a 10-year follow-up.29,30
Research exists for both low- and high-grade gliomas, but large, controlled studies are few. With low-grade tumors, small studies have shown increased survival after stereotactic radiosurgery, but these studies are uncontrolled and limited by the small number of participants.66 For high-grade tumors, recent studies have found median survival rates ranging from 9.5 to 17 months with use of stereotactic radiosurgery.39,70–72 For recurrent malignant gliomas, survival after fractionated and nonfractionated stereotactic radiosurgery has been shown to be 8 to 11 months.51,73–75 The addition of radiosurgery to surgery and radiation therapy produced only modest improvement when compared with surgery and radiation therapy alone.66
The preferred treatment for meningiomas is surgical resection, if complete resection is possible. When surgery is not an option and the tumor is less than 3 cm in size or 5 mm away from the optic nerve, stereotactic radiosurgery is indicated.27,76,77 Four-year survival rates of 91% in benign meningiomas and 21.5% in malignant meningiomas have been demonstrated after use of the Gamma Knife.27,76 In a survey taken 5 to 10 years after radiosurgery, 96% of patients believed radiosurgery had provided a satisfactory outcome.76
Rehabilitation
Overview
Rehabilitation is a key component in the management of the client with a brain tumor. With advances in technology and treatment intervention, survival rates of people with cancer have improved. Consequently, people are living longer with physical impairments resulting from the disease or its treatment, necessitating interdisciplinary therapeutic intervention.2 Rehabilitating the body function and structural impairments complicates the medical and psychological issues typically associated with cancer diagnosis.19 By preventing complications, maximizing function, and providing support, rehabilitation specialists ultimately improve the client’s quality of life.19 Research has shown that the functional outcomes and discharge to home for individuals with brain tumors are comparable to those of individuals with stroke or traumatic brain injury.78 The most effective rehabilitation plan is flexible, to allow for increasing impairment, and sensitive, to accommodate the highly emotional impact that accompanies the diagnosis of a primary brain tumor. The tumor’s invasion is marked by complaints of pain and growing activity limitations. These functional consequences of the disease process are the target of the rehabilitation team. In addition to the side effects of therapeutic intervention, functional progress may be affected by cerebral edema, hydrocephalus, tumor regrowth, infection, and radiation necrosis. Compared with clients with other diagnoses, clients with brain tumors have a higher rate of unplanned transfers back to acute care, primarily because of infection.79
The management of a client with a brain tumor is different from that of other CNS disorders, despite a similar clinical presentation. To establish an appropriate plan of care, the clinician must understand the nature of the specific tumor, consider the client’s fluctuating neurological status, and prepare for the likelihood of progressive decline. The preferred approach is holistic, addressing quality of life issues such as physical, psychosocial, and emotional needs, incorporated into the systems model of motor control. Factors defining quality of life are unique to each individual, and therefore clinicians should identify and use these factors to construct a meaningful treatment program.80 Individuals with advanced cancer who participated in exercise therapy have reported increased physical functioning, improved quality of life, and decreased fatigue.78
Evaluation
Although the neurological examination yields important information regarding strength, reflexes, sensation, vision, and cognition, it is important not to rely solely on its findings to determine an appropriate intervention. Because multiple systems interact to produce normal movement, it is difficult to examine isolated systems and apply the findings accurately to movement patterns. Therefore clinicians are encouraged to examine all systems through functional tasks to understand how the impaired neurological, musculoskeletal, and cognitive systems are affecting the client’s movement. During the evaluation process the clinician notes systems that are functioning normally, identifies abnormal components of movement, and determines appropriate interventions to optimize motor recovery.38 The progressive nature of the disease necessitates ongoing evaluation followed by accommodating intervention.
Goal setting
The functional impairments and objective neurological findings provide the clinician with valuable information to assess prognosis, establish goals, and determine a treatment plan. Despite the progressive nature of the disease, treatment goals should maximize the potential for function, introduce effective, task-oriented movement strategies, and offer multiple movement options.38
Because the rehabilitation potential for clients with brain tumors varies greatly, it is imperative that the client, family members, rehabilitation team, and third-party payers understand and agree with the purpose of the client’s rehabilitation program. Pathways can be extremely instrumental in clarifying rehabilitation goals and identifying the caregiver’s role on discharge (Table 25-2). If a client has a poor prognosis, the rehabilitation team can successfully train family members and order equipment within 1 week if the family understands the goals and the need to be present during treatment sessions. The pathway serves as a guideline assisting the rehabilitation team in achieving the client’s goals in an effective and efficient manner.
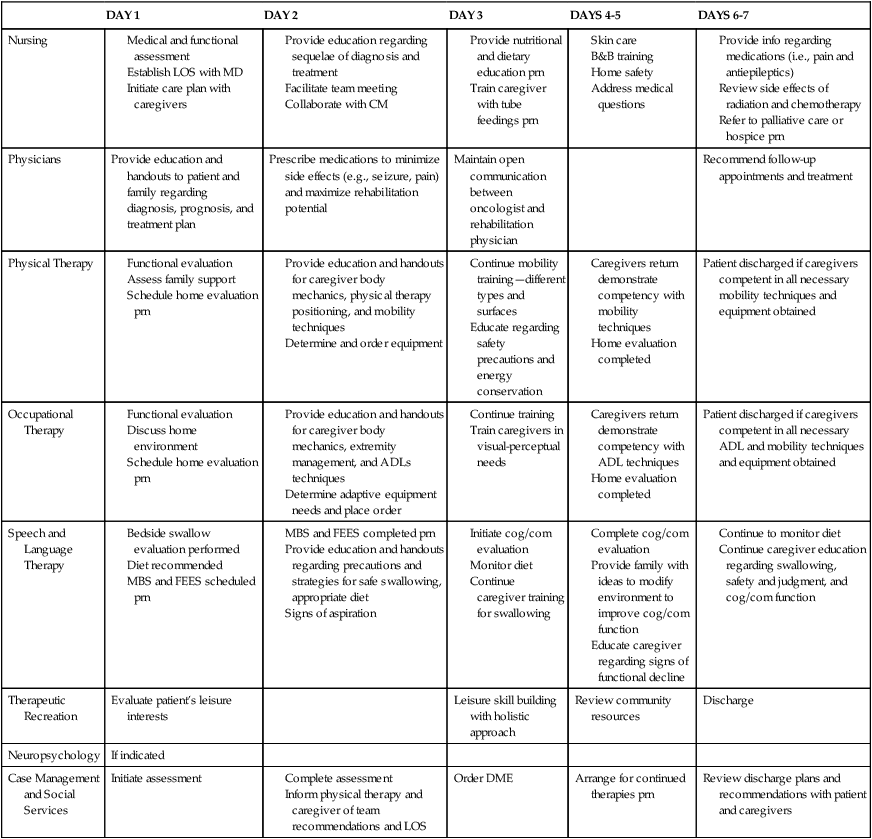
Adapted with permission from Barrow Rehabilitation.
Functional assessment
Historically persons with primary malignant brain tumors have not been considered rehabilitation candidates because of the progressive nature of their disease. Physicians, health care providers, and third-party payers have questioned the efficacy of rehabilitation in this population because of poor prognoses and limited survival rates. However, advances in medical diagnosis and intervention are resulting in longer survival of people with multiple limitations that require rehabilitation. Functional assessment scales provide objective evidence that rehabilitation is effective and worthwhile for these clients.3,78,81
The functional assessment is a critical component in the development of the treatment intervention. It provides a method of analyzing deficits, compiling a problem list, developing a treatment plan, and measuring functional outcomes. The Functional Independence Measure (FIM) is a functional assessment tool used to measure degree of disability, regardless of underlying pathology, and burden of care to demonstrate functional outcomes of rehabilitation and assist clinicians with discharge planning.51
Functional outcome scales such as the FIM provide a means of documenting the client’s response to therapy intervention for clinicians, physicians, and third-party payers in the rehabilitation setting. Research using FIM data demonstrates efficacy for inpatient rehabilitation of brain tumor clients similar to that noted in those with traumatic brain injury or stroke when matched by age, sex, and functional status on admission.1,3,29,81
Physicians use specific functional evaluation scales to measure the success of treatment. The Karnofsky performance scale, which rates patients’ functional performance, is the tool most widely used in clinical research and treatment decisions (see Table 25-1).19 The client receives a score from 0 to 100 based on independence or level of assistance required for normal activity. The scale is used in research to evaluate an individual’s physical response to treatment.19,51,82,83
Side effects and considerations
Through advances in chemotherapy and radiation therapy, the ability to reduce tumor mass has greatly improved. Unfortunately, despite the often favorable long-term results of these treatments, the immediate effects create physical and psychological challenges for the client and clinician. Clients who are being treated aggressively during the rehabilitation phase will probably experience a decline in neurological or hematological status. These declines often limit the individual’s tolerance for treatment intervention and increase client and caregiver feelings of depression and hopelessness. Clinicians have the opportunity to provide more than physical restorative services and should offer psychosocial support when possible to enhance successful rehabilitation.84
Not everyone undergoing chemotherapy or radiation treatment will experience physical side effects; however, the possibilities include hair loss, fatigue, nausea, skin burns or irritation, difficulty eating or digesting food, anorexia, and dry, sore mouth.53,85 The side effects are caused by the toxic effects the drugs have on healthy, rapidly dividing cells, including bone marrow cells, cells lining the mucosa, and hair cells.52,85
The toxic effect chemotherapy has on bone marrow impairs the client’s ability to produce red and white blood cells and platelets.5,52 The client may develop anemia, infection, or hemorrhage as a result of depressed hematological values.
The lining of the mouth, esophagus, and intestines may become inflamed and irritated and interfere with the ability to eat or digest food. The client may experience nausea, vomiting, diarrhea, or constipation, any of which will impair mobility and energy for daily activities.17
Hair loss is a common side effect of brain radiation and chemotherapy. This requires an especially difficult adjustment for most people because it causes a drastic change in appearance.16,52
Intervention
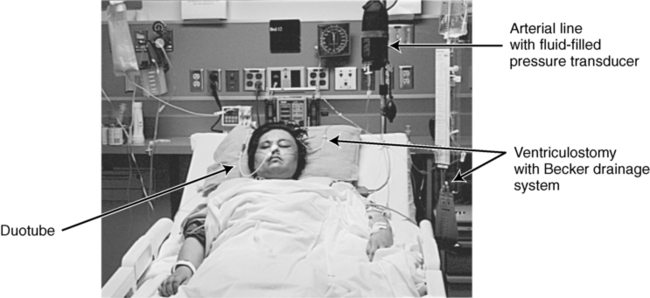
In the intensive care unit, communication with nursing staff regarding the client’s present medical status and an understanding of ICP, hemodynamic values, and monitoring devices are crucial to determining tolerance for therapy intervention (Figure 25-5). For a ventriculostomy, a catheter is placed in the third ventricle to drain CSF and to monitor ICP. Mobilizing a patient with a ventriculostomy is possible, but nursing staff must close the drain before any positional change and should inform the clinician of appropriate treatment measures. A client’s dependence on these monitoring devices does not prevent therapeutic intervention, but the critical status of these individuals must be considered. The monitoring equipment provides constant feedback that assists the clinician in assessing the client’s tolerance to activity and his or her ability to proceed with treatment.
The restoration of previous functional movement patterns is desired. The literature reports increasing evidence that the CNS has dynamic properties, including neural regeneration and collateral sprouting, which supports the concept of plasticity. Plasticity allows intact neural centers to recognize and assume functions of areas of the brain impaired or destroyed by the lesion or its medical management.86 The treatment focus may need to turn to compensatory strategies if the potential for motor recovery and learning is lacking. Once compensatory patterns are established, it is not clearly known whether recovery of normal movement will be achieved.86 Compensatory techniques may be beneficial in increasing safety and efficiency with mobility and activities of daily living, or in providing more independence for the client.51 Increasing independence can assist in improving quality of life for the client and may permit return to work or participation in previous recreational activity.51 For example, an avid golfer with right-sided hemiparesis and impaired standing balance can modify his clubs and return to the game at the wheelchair level.
The rehabilitation program should prepare the client and caregivers for an efficient transition from the structured care setting to the home. Using motor learning principles (refer to Chapter 4) to teach functional mobility will best produce transfer of learning from a constant environment to an unpredictable home environment. Repetitious practice of specific parts of a skill in fixed surroundings, with physical and verbal guidance throughout the movement and frequent feedback during and after completion of the task, are beneficial in teaching acquisition of a specific movement or activity.87–89 Practicing the whole activity in a variable context, with irregular feedback and decreased physical and verbal guidance, expedites learning.89–91
Discharge planning
Client and caregiver training and education constitute an integral part of discharge planning. Before discharge, the client and caregiver should be instructed in functional mobility and activities of daily living, informed of equipment needs and vendor resources, and provided with community resources for support and education. During individual training, the clinician is able to provide feedback to the caregiver and client to facilitate an easier transition to home. Documentation of caregiver education and training should be included in the progress and discharge notes. A sample form for interdisciplinary documentation of education is provided in Figure 25-6.
Information about local community and national resources specific to brain tumors also should be provided before discharge. These resources can be found on the Internet, in the local phone book, or through communication with previous patients or other health care professionals familiar with these organizations. Support groups provide the caregiver and the client with an opportunity to share experiences and information, prevent isolation, foster hope, allow the client and caregiver to discover coping skills, and offer emotional support.92 A study conducted to describe experiences and needs of clients with brain tumors found that “attendance and participation in a support group empowers people to seek the most out of life following a brain tumor diagnosis.”92 (Refer to Chapter 6 for additional information regarding support groups.) National organizations can provide educational information and support to clients (Box 25-1). These organizations can help the client find local resources unfamiliar to the clinician.
Hospice care
A time may come when traditional tumor treatment is ineffective and local control is no longer expected. Patient and family must make a decision regarding the living environment and type of care desired. One option available is hospice care. In the United States, the hospice movement in health care has evolved to include specific standards, licensure requirements, and certification. Providing physical, emotional, and psychosocial support to patients and their families in their final days is the intent of hospice care.93 Hospice recognizes the impact terminal illness has on a patient’s family system, and the demands, both physical and emotional, it places on the caregiver.94 The use of hospice implies a holistic approach that allows families the opportunity to be directly involved in the patient’s care and encourages the expression of grief, love, support, and acceptance.
Psychosocial care
With many clients living extended lives with brain tumors, it is important to measure the efficacy of treatment not only in terms of functional outcome, but also in terms of its effect on quality of life. Quality of life is the individual’s subjective sense of well-being as a whole and has been studied closely in the treatment of clients with brain tumor.83 Health-related quality of life (HRQOL) refers to an individual’s overall quality of life—physical, emotional, spiritual, and intellectual functioning. HRQOL has become an important secondary end point for treatment and clinical studies of individuals with gliomas and metastatic brain tumors. Recently, several self-reporting instruments to measure HRQOL specific for individuals with brain tumors have been developed.95 Some additional tools used clinically include the Functional Living Index, the Karnofsky performance scale, the Index of Independence in Activity of Daily Living, the State-Trait Anxiety Inventory, and the Self-Rating Depression Scale.96 The World Health Organization (WHO) has developed two additional scales to measure quality of life used in all types of medical as well as movement diagnoses that might lead to a decrease in perceived quality of life.97,98 For further discussion and general information refer to the WHO website.99
The development of a strong supportive relationship with client and caregivers is key to successful rehabilitation. This process begins with respecting the client’s unique experience and involves continually evaluating and addressing his or her changing psychosocial needs.86 Many individuals with brain tumors experience higher levels of anxiety and depression as well as feelings of a loss of independence, loss of self, and loss of relationships.100 The clinician must feel invested, demonstrate good communication skills, and exhibit self-confidence in discussing sensitive issues for a caring relationship to develop. By active listening, the clinician can identify the client’s true concerns and feelings and assist the client and family in coping with cancer. The clinician’s consistent interaction with the client can foster a supportive and safe environment in which emotional and spiritual feelings can be shared. Once a trusting relationship has been established, the clinician’s empathy can help decrease common feelings of isolation and helplessness and support the client through the different stages of the disease.86
Hope is a key psychosocial need of the individual with cancer. It is an important coping strategy that can help clients with brain tumors face an uncertain and often fearful future. Hope gives the client something to look forward to each day. Clinicians can create a hopeful environment by encouraging clients to share their expectations, identify realistic short-term goals, and acknowledge hopes, even if they are unrealistic. It is important to recognize that hope must be balanced with reality and honest disclosure regarding diagnosis and prognosis.86,101
Psychological and social problems are not identified in 80% of physically ill persons, possibly owing to clinicians’ personal behaviors or beliefs. Clinicians may find it easier to focus on the physical aspect of care to avoid becoming emotional or experiencing the client’s distress. Persons with cancer often experience feelings of powerlessness and isolation, which may be increased by distancing behaviors demonstrated by clinicians. Before offering support to clients, clinicians need to examine their own thoughts, feelings, and past experiences with death and dying. This awareness may prevent the clinician from internalizing the client’s grief, from protecting the client and family members from the pain of grieving, and from allowing personal values to adversely influence their psychosocial support.101 By recognizing that psychosocial care involves holistic healing, clinicians will be able to develop the best environment for interventions to improve multiple aspects of the client’s quality of life.101