Chapter 105
Brachiocephalic Artery
Surgical Treatment
Sapan S. Desai, Hazim J. Safi
Based on a chapter in the seventh edition by David L. Lau and Hazim J. Safi
The innominate, left common carotid, and left subclavian arteries constitute the branches of the transverse aortic arch. Known as the brachiocephalic arteries, together these vessels supply blood to the upper extremities, head, and neck. Atherosclerosis of these vessels can lead to flow-limiting stenosis or distal embolization, which can result in transient ischemic attacks, stroke, upper extremity ischemia, and vertebrobasilar insufficiency. In addition to atherosclerosis, aneurysmal degeneration, dissection, vasculitides (including Takayasu disease and giant cell arteritis), infection, and fibrosis, such as from radiation therapy, can develop in the brachiocephalic arteries. In this chapter, we discuss the open surgical management of brachiocephalic arterial occlusive and/or aneurysmal disease.
Anatomy
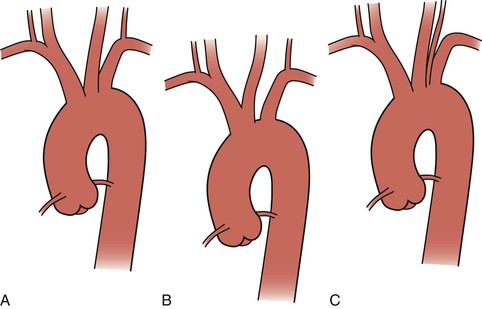
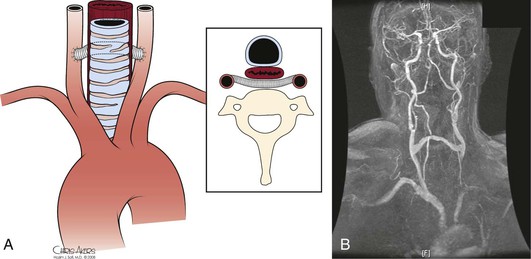
The thoracic aorta develops during the fourth week of gestation, eventually leading to development of the common carotid arteries from the third aortic arch and the brachiocephalic arteries from the third and fourth aortic arches (see Chapter 2). Anatomically, the ascending aorta and transverse arch can be divided into three segments (Fig. 105-1): (1) the aortic root houses the aortic valve, three sinuses, and the two main coronary arteries; (2) the tubular portion of the ascending aorta lies between the supracoronary ascending aorta and the innominate artery; and (3) the transverse arch is located between the innominate artery and the left subclavian artery. The brachiocephalic arteries arise from the dorsal aspect of the transverse arch.
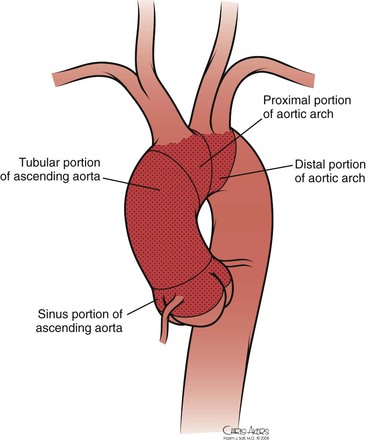
Figure 105-1 Normal anatomy of the ascending and arch aorta.
The most common configuration of the arch is three separate trunks, with the innominate artery giving rise to the right subclavian and right common carotid arteries, the left common carotid in close proximity, and the left subclavian artery originating posterior to and to the left of the left common carotid artery. However, a variety of congenital alterations to the aortic arch exist. The true bovine arch is a single, large, common brachiocephalic trunk that comes off the apex of the arch and then separates into the right and left subclavian arteries and a bicarotid trunk. The bicarotid trunk bifurcates into the right and left common carotid arteries. A true bovine arch is much rarer than the commonly named “bovine arch,” in which the left common carotid artery comes off as a branch off the brachiocephalic trunk instead of the aortic arch. Up to 20% of patients may have the so-called bovine arch variation.1 Typically in an aortic origin of the vertebral artery (6%), the left vertebral artery arises between the left common carotid and left subclavian arteries (Fig. 105-2). An aberrant right subclavian artery (0.5%-1%) occurs when the right subclavian artery arises distal to the left subclavian artery (Fig. 105-3).2
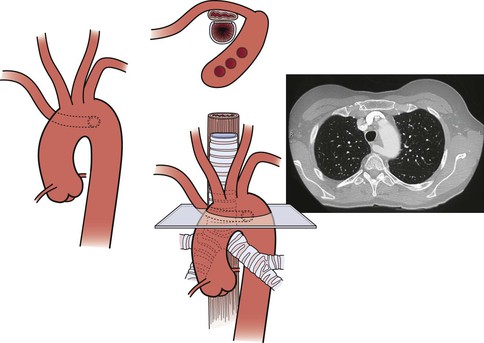
Figure 105-3 Aberrant right subclavian artery with a retroesophageal course. Variations of aberrant subclavian artery include aberrant left subclavian artery in association with a right-sided aortic arch and the association of such aberrant arteries with a diverticulum of Kommerell, which in turn may be aneurysmal (see Chapter 140).
Clinical Findings
The clinical manifestations of lesions involving the brachiocephalic arteries depend on the etiology of the disease, the presence of single-vessel or multivessel disease, and anatomic location. Atherosclerosis is the most common disease affecting the brachiocephalic arteries. Severe disease is defined as stenosis greater than 75% of the vessel’s diameter. Deep ulcerated plaque or thrombus within the arterial lumen is also considered a severe lesion. Atherosclerotic disease can be unifocal, multifocal, single vessel, or multivessel. In the series of 282 transthoracic and transcervical brachiocephalic revascularizations reported by Berguer et al,3,4 there was a 40% incidence of multivessel disease (Fig. 105-4).
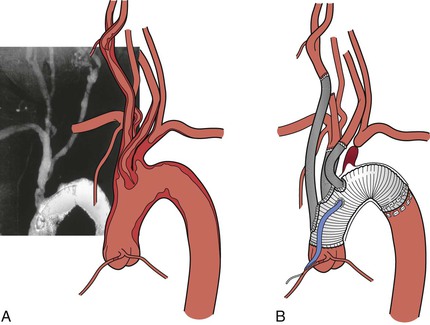
Figure 105-4 A, Severe multivessel atherosclerotic disease with involvement of the ascending and arch aorta. B, Replacement of the ascending and arch aorta with individual brachiocephalic bypasses.
Single-vessel atherosclerotic occlusive disease typically causes symptoms as a result of hemispheric or upper extremity emboli. When the innominate artery is involved, patients may suffer from stroke, transient ischemic attack, and/or upper extremity ischemia. Isolated common carotid disease commonly manifests as stroke or transient ischemic attack. Single-artery occlusion involving the origin of the subclavian artery can cause subclavian-vertebral steal and lead to vertebrobasilar insufficiency, myocardial ischemia (in the case of prior LIMA coronary artery bypass grafting), and hemiparesis or aphasia.5 Reversal of flow in the ipsilateral vertebral artery to provide blood supply to the arm also may result in so-called subclavian steal syndrome, resulting in vertebrobasilar insufficiency. This syndrome is often accompanied by vertigo, nausea, vomiting, imbalance, and diplopia (Fig. 105-5) (see Chapter 97). In patients who have previously undergone coronary artery bypass with the internal mammary artery, angina may recur because of flow reversal in the bypass, which can lead to myocardial ischemia (subclavian-coronary steal).6 Occlusion of the innominate artery can manifest as subclavian-carotid steal, with reversal of flow in the ipsilateral carotid artery causing anterior cerebral symptoms, such as aphasia and hemiparesis.7 Multiple-vessel disease usually results in vertebrobasilar insufficiency, secondary to a low-flow state.8–10
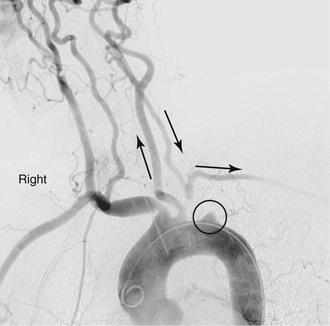
Figure 105-5 Subclavian steal in a patient with an occluded proximal left subclavian artery. Late filling of subclavian artery via retrograde flow in left vertebral artery.
Overall, symptomatic disease from stenosis of the brachiocephalic artery is uncommon because of the numerous collateral vessels present and the relatively low incidence of atherosclerotic disease affecting the upper extremity. Pain and fatigue from repeated use of the upper extremity can be a sign of subclavian or brachiocephalic artery compromise. In more severe cases, rest pain may be present, eventually leading to digital gangrene. Some patients may also present with findings consistent with Raynaud’s syndrome.11 In addition, stenotic and/or ulcerative lesions can be a source of microembolic phenomena to either the brain or hand.
Infectious processes (including syphilis and tuberculosis) can lead to aneurysmal degeneration of the brachiocephalic arteries (Fig. 105-6) and, in particular, the subclavian artery.12 These are rare causes of proximal (intrathoracic) aneurysm. Blunt traumatic injuries can also lead to proximal aneurysm formation, usually involving the origin of the innominate artery.13 Penetrating trauma may affect the brachiocephalic vessels in any location (Fig. 105-7) (see Chapters 156 and 157). Distal (extrathoracic) subclavian artery aneurysms are commonly related to a cervical rib, causing arterial thoracic outlet syndrome (Fig. 105-8) (see Chapter 124). The pathogenesis of the aneurysm is thought to be related to repetitive trauma to the artery at the level of the thoracic outlet.14,15 The clinical consequences of aneurysmal degeneration are usually related to the distal embolisms causing arm claudication, pain at rest, and digital ulcerations. Compressive symptoms, involving the recurrent laryngeal nerve (hoarseness), brachial plexus (upper extremity weakness, pain) and rupture can also occur.16 The more proximal aneurysms affecting the ascending aorta and arch are commonly dealt with by cardiothoracic surgeons and involve cardiopulmonary bypass.
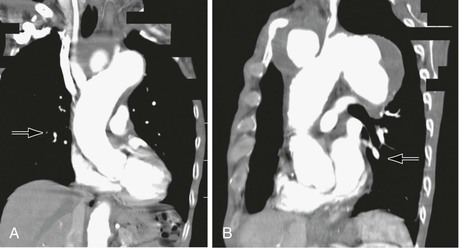
Figure 105-6 A, CT scan of a syphilitic aneurysm. B, CT scan of a syphilitic aneurysm with left carotid artery occlusion.
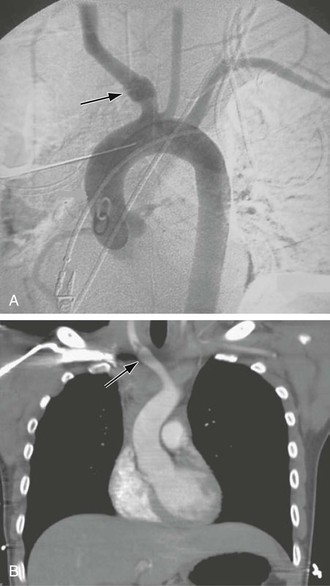
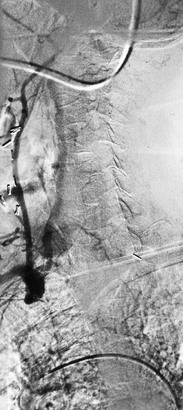
Figure 105-7 A, Aortogram demonstrating blunt traumatic injury (arrow) to the innominate artery. B, CT scan demonstrating an intimal flap in the innominate artery after blunt traumatic injury (arrow).
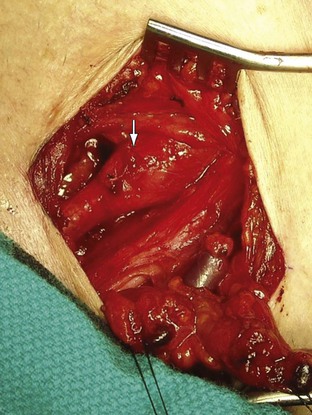
Figure 105-8 Distal right subclavian artery aneurysm in a patient with arterial thoracic outlet syndrome exposed via an infraclavicular incision. The patient’s head is at the top, left corner, and feet to the bottom, right corner.
Takayasu’s disease frequently involves all the proximal portion of the aortic arch branches (Fig. 105-9) (see Chapter 80). The disease is characterized by an acute inflammatory phase and a “burned-out” sclerotic phase. The inflammatory process leads to fibrosis and thickening of the arterial wall, whereas sclerosis leads to stenosis. Symptoms are typically related to vertebrobasilar insufficiency once the disease has progressed to multivessel occlusion. Chronic disease can also manifest as aneurysmal degeneration with embolic potential.17
Diagnostic Evaluation
In addition to a comprehensive physical examination, careful assessment of the upper extremity pulses, bilateral upper extremity blood pressure measurements, and auscultation of the carotid and subclavian arteries for bruit are effective tools for the diagnosis of brachiocephalic vascular disease. Duplex ultrasound examination of the aortic arch may be of some utility in identifying flow reversal in the vertebral arteries and excluding significant disease in the extracranial carotid arteries (see Chapter 98).18,19
Noninvasive imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT) scan, provide excellent imaging of the aorta and great vessels (see Chapter 93). Body habitus, claustrophobia, and metallic implants may limit the use of these imaging techniques. CT or MRI of the brain should also be performed before any planned revascularization to identify lesions susceptible to bleeding or areas of prior stroke, as reperfusion injury may develop in recent areas of stroke as a result of a transient ischemic state during surgery.20
The “gold standard” imaging modality is aortography. However, disadvantages of this invasive study include iatrogenic arterial injury, risk of stroke, and nephrotoxicity from iodinated contrast material. Because the incidence of concomitant coronary atherosclerosis approaches 40%, cardiac evaluation should also be performed, especially if transthoracic revascularization is planned.21 We routinely obtain a transthoracic echocardiogram and 12-lead electrocardiogram. Patients with a low ejection fraction (<50%) or ischemic electrocardiographic changes are referred for additional evaluation with a stress test or coronary angiography.22
Transesophageal echocardiography (TEE) is another important diagnostic tool because of its ability to accurately identify atherosclerotic disease in the proximal ascending and descending portions of the thoracic aorta.23 Disadvantages of TEE are that accurate evaluation requires an experienced sonographer and that the patient usually needs conscious sedation during the procedure. If transthoracic revascularization is planned, the proximal ascending aorta should be devoid of any atheromatous plaque or dissection. If accurate preoperative or intraoperative TEE is not available, a hand-held ultrasound probe (epiaortic ultrasound) can be used intraoperatively, once the aorta is exposed, to rule out atheromatous plaque in the ascending aorta.24
Revascularization
Indications
General indications for brachiocephalic revascularization include stroke or transient ischemic attacks due to atheroembolism; low-flow states, leading to vertebrobasilar insufficiency or, less commonly, cortical symptoms; and steal syndrome from proximal innominate or subclavian artery occlusion, leading to vertebrobasilar insufficiency, myocardial ischemia after internal mammary artery bypass or anterior cerebral TIA, or stroke. Upper extremity pain with exertion and/or digital ischemia is a common indication for intervention.25,26 A significant number of patients with proximal subclavian stenosis may demonstrate reversal of flow in the vertebral artery without displaying symptoms of vertebrobasilar insufficiency. Such patients can be safely observed until symptoms develop.27 There are few natural history data to guide decision making for patients with asymptomatic brachiocephalic disease. Some authorities suggest that asymptomatic patients with severe (>75%) stenosis of the innominate or common carotid artery should undergo revascularization if they have reasonable surgical risk,27a but this is controversial and data are inadequate for resolution. There is agreement, however, that revascularization of severe (>75%) asymptomatic stenosis of the subclavian artery should be performed if coronary artery bypass with the ipsilateral internal mammary artery is planned.27b
Options for Revascularization
Anatomic (transthoracic) revascularization is preferred for patients with multivessel disease and low surgical risk. Proximal innominate and subclavian aneurysms and traumatic injuries are also best treated with a bypass from the ascending aorta to the involved brachiocephalic arteries (Fig. 105-10).
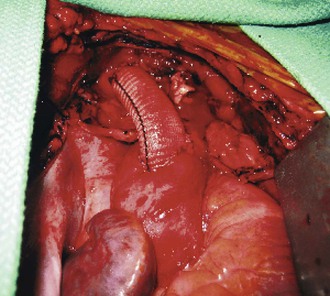
Figure 105-10 Completed aorto-innominate bypass in a patient (see also Fig. 105-7) with blunt traumatic injury to the proximal innominate artery.
Extraanatomic cervical revascularization is ideal for single-vessel subclavian disease or in patients for whom median sternotomy presents prohibitive risk. This approach is often combined with endovascular treatment of thoracic aortic aneurysms. Transposition of the left subclavian artery or carotid-subclavian bypass can extend the proximal “landing zone” to the left carotid artery. A right-to-left carotid bypass can further extend the proximal landing zone to the level of the innominate artery (see Chapters 136 and 137).
Anatomic Revascularization
The ascending aorta and transverse aortic arch are approached through a median sternotomy. The procedure is initiated with a skin incision from just below the sternal notch to the xiphoid process. Electrocautery is used to divide the soft tissues down to the sternum. A sternal saw is then used to divide the sternum from superior to inferior, and a sternal retractor to gently free the mediastinal structures from the undersurface of the sternum. Incision of the pericardium exposes the heart, aorta, innominate vein, and brachiocephalic arteries. Anatomic revascularization of the brachiocephalic arteries can be accomplished by either endarterectomy or bypass.
Endarterectomy
Endarterectomy is an effective strategy for focal lesions involving the midsection of the proximal innominate or common carotid artery. When the atherosclerotic lesion is located at the orifice of the brachiocephalic trunk, the transverse arch is usually involved in this process. However, in this setting, endarterectomy can be fraught with the dangers of embolization, incomplete endarterectomy, and aortic dissection. Also, endarterectomy is unsuitable for patients with disease involving a bovine arch variant (left common carotid artery arising from the innominate artery) because clamping of the innominate artery would cause ischemia in both cerebral hemispheres.
Bypass Grafts
Bypasses can be constructed from the ascending aorta to the brachiocephalic arteries if the ascending aorta is free of atherosclerotic disease (Fig. 105-11). The presence of atheroma can be confirmed by intraoperative TEE or epiaortic ultrasound.23 The patient undergoes heparinization with 1 mg/kg (body weight) (90 units/kg) of intravenous heparin. A partial-occlusion clamp is applied after heparinization (see Fig. 105-11) and a 12- to 14-mm woven Dacron tube graft impregnated with collagen or gelatin is sewn to the ascending aorta in end-to-side fashion with 4-0 polypropylene suture. The partial-occlusion clamp is released with the patient in the Trendelenburg position, and a clamp is placed distally on the graft. The innominate artery is then transected distal to the diseased portion and the proximal stump is oversewn with 4-0 polypropylene suture. The distal anastomosis is completed in an end-to-end fashion with 4-0 or 5-0 polypropylene suture.
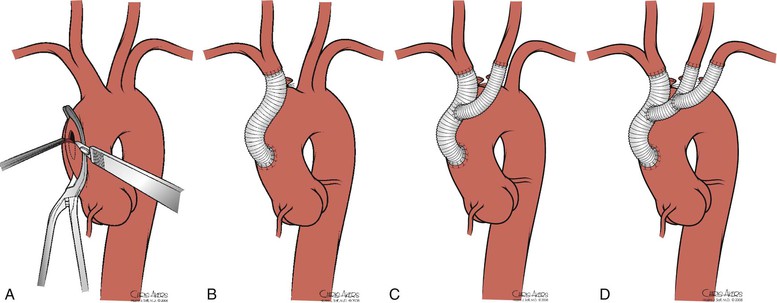
Figure 105-11 A, Partial-occlusion clamp placed on the ascending aorta with creation of a punch arteriotomy. B, Creation of an aorto-innominate bypass. C, Sidearm graft to the left carotid artery. D, Sidearm graft to the left subclavian artery.
When the left common carotid artery is involved, we avoid the use of a bifurcation graft because the volume of this graft creates difficulty with closing the sternotomy. We use a separate sidearm graft hand sewn to the innominate bypass graft in an end-to-side fashion with 4-0 polypropylene suture. This graft is then sewn end to end to the left common carotid artery with 5-0 polypropylene suture.
If the left subclavian artery also has a proximal lesion, a separate bypass is constructed in a similar fashion. There is a misperception that the left subclavian artery cannot be exposed with a median sternotomy. Actually, exposure of the proximal left subclavian artery is achieved by downward pressure retraction on the ascending aorta and the transverse arch. This maneuver allows transection of the left subclavian artery and end-to-end anastomosis. After completion of all anastomoses, the anticoagulated state is reversed with protamine sulfate. We insert two large (32 or 36 Fr) chest tubes in the mediastinum for drainage.
Ascending Aorta and Arch Replacement
Patients with severe atheromatous plaque involving the ascending and transverse arch aorta have a significant risk of stroke from atheroembolic debris with any manipulation of the vessel (i.e., clamping). In patients who need anatomic reconstruction, we replace the ascending and arch aorta and reconstruct the brachiocephalic arteries with a prefabricated branched graft (Fig. 105-12). Our center has low mortality and neurologic morbidity with use of cardiopulmonary bypass, profound hypothermia, and cardiocirculatory arrest. Cerebral function is monitored with transcranial Doppler ultrasonography or infrared spectroscopy, and 10-lead electroencephalography is used to monitor neurologic activity.28–31
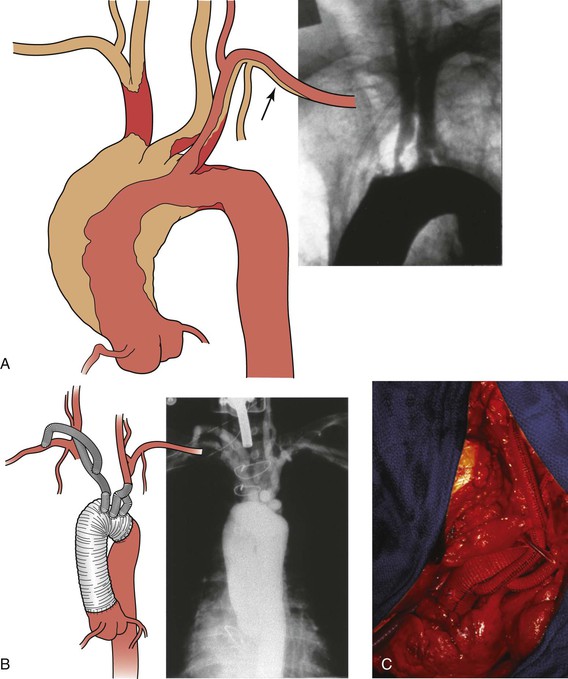
Figure 105-12 A, Illustration and preoperative aortogram of patient with multivessel ascending aorta, arch, and brachiocephalic (arrow)atherosclerosis. B, Illustration and postoperative aortogram after replacement of the ascending aorta and aortic arch with individual bypasses to the brachiocephalic arteries. C, Completed replacement of the ascending aorta and aortic arch with individual bypasses to the brachiocephalic arteries.
Following median sternotomy, cardiopulmonary bypass is achieved after heparinization and the patient is cooled until the electroencephalogram is isoelectric, a point that typically coincides with a nasopharyngeal temperature between 15°C-20°C. Once the pupils are fixed and dilated, bypass is discontinued and circulatory arrest is established. Retrograde cardioplegia is initiated through the superior vena cava cannula, and sufficient flow given to achieve reversal flow in the middle cerebral artery.
The arch is then repaired, starting with resection of the two lateral walls along with the lesser curvature. A Dacron graft is used to replace this section of the arch. The graft travels approximately 15 cm into the descending thoracic aorta, and the collar of the graft is sewn into the thoracic aorta, just beyond the left subclavian artery. The brachiocephalic, carotid, and subclavian vessels are sewn back onto the graft via side holes. After hemostasis, cerebral perfusion is restarted and the patient rewarmed. The tubular portion of the ascending aorta is then excised, and the graft is sewn into the supracoronary ascending aorta. A composite valve graft prosthesis is used if there is aortic root dilatation. The ascending aortic clamp is then released and coronary circulation resumed. The patient is weaned from cardiopulmonary bypass once he or she resumes normal body temperature and maintains satisfactory blood pressure and heart rate.
Operative Results
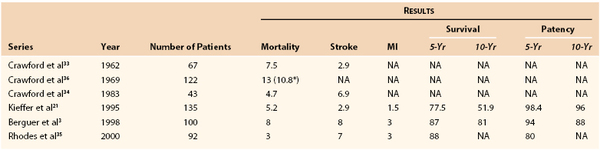
DeBakey et al32 performed the first aortoinnominate bypass in 1957. Crawford et al33 subsequently examined the initial results of direct brachiocephalic revascularization (combining endarterectomy and bypass) in 1962 and reported a 30-day mortality of 7.5%. In 1983, the same group reported their own series of brachiocephalic revascularization, with stroke and 30-day mortality rates of 6.9% and 4.7%, respectively, after thoracic bypass.34 Since that time, there have been several reports of excellent initial and long-term results after transthoracic revascularization of the brachiocephalic arteries. Kieffer et al21 reported 2.9% stroke, 1.5% myocardial infarction, and 5.2% mortality rates, with 5- and 10-year patency rates of 98.4% and 96%, respectively. Rhodes et al35 reported 7% stroke, 3% myocardial infarction, and 3% mortality rates, with a 5-year patency rate of 80%.35 Berguer et al3 reported 8% stroke, 3% myocardial infarction, and 8% mortality rates, with 5- and 10-year patency rates of 94% and 88%, respectively (Table 105-1).21,33–36
Postoperative Management
In the immediate postoperative period, patients are observed in a monitored unit for the first 24 hours. Mediastinal drains are removed once the drainage is less than 200 mL/day. Patients are discharged with strict poststernotomy precautions. Although there are no universally accepted guidelines for poststernotomy precautions, our institutional policy includes not lifting a weight of more than 5 pounds, avoiding any upper extremity or chest weight-bearing exercises, and limiting arm abduction to less than 90 degrees. After the initial postoperative visit, we monitor patients with duplex ultrasound of the extracranial carotid system and the graft itself every 6 months for the first year and yearly thereafter.
Extraanatomic Revascularization
Extraanatomic reconstructions are suitable for patients with single-vessel disease or very high-risk patients in whom median sternotomy presents prohibitive risk. A bypass can be constructed between the carotid and subclavian, bilateral carotid, bilateral subclavian, or bilateral axillary arteries. The most common finding is isolated subclavian artery stenosis,4 and there are several options for revascularization.
Carotid-Subclavian Transposition.
Direct subclavian-carotid transposition carries the advantage of avoiding prosthetic material. However, it requires more extensive dissection of the proximal subclavian artery and individual isolation of the vertebral and internal mammary arteries (Fig. 105-13). The procedure is initiated via a short transverse supraclavicular incision between the two heads of the sternocleidomastoid muscle. Platysmal flaps are created, with care taken to avoid trauma to the external jugular vein. The omohyoid is divided and the common carotid artery is circumferentially dissected and mobilized medially. If a left-sided exposure is being performed, the thoracic duct is identified and ligated; accessory lymphatic channels on either side are also meticulously identified and ligated to avoid a major lymph leak. The internal jugular vein and vagus nerve are retracted laterally, and exposure of the subclavian artery and its proximal branches proceeds after division of the vertebral vein. Once the vertebral and internal mammary arteries and thyrocervical trunk are controlled, the patient is heparinized and the proximal subclavian artery is transected proximal to the origin of the vertebral artery. Absolute control must be maintained until the proximal stump can be oversewn, because transected stump lost in the thoracic cavity can be disastrous.
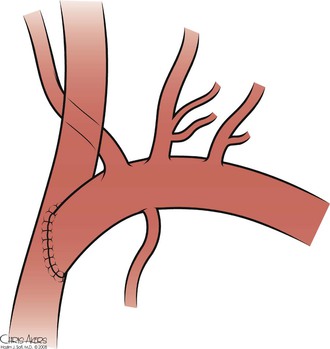
Figure 105-13 Subclavian-carotid transposition.
The common carotid artery is then clamped, and an arteriotomy created. An end-to-side anastomosis is created with 5-0 or 6-0 polypropylene suture. Because of the tight spaces and acute angles involved, a parachute technique can be used to facilitate creation of the anastomosis. Additional length can be created by further mobilizing the common carotid artery and swinging it more inferolaterally, toward the subclavian artery. Flow is then reestablished (a drain may be placed if there is sufficient leakage of lymph). The incision is closed after reapproximation of the platysma.
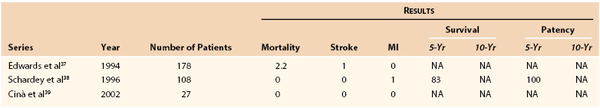
Transposition is contraindicated in patients with an early origin of the vertebral artery and in patients with patent internal mammary coronary artery bypass grafts (Table 105-2).37–40 Care should be taken to preserve the vertebral artery whenever possible during transposition. Sometimes, however, the vertebral artery must be ligated if it cannot be mobilized adequately to avoid tethering the proximal subclavian artery. Our preference is to perform the bypass. This issue is especially important in patients who have greater than 60% bilateral vertebral artery stenosis or one-sided occlusion, hypoplasia of the contralateral vertebral artery, or aberrant termination of the vertebral artery into the posteroinferior cerebellar artery. In rare cases, if absolute vertebral artery preservation is required, the proximal vertebral artery may be transposed with the common carotid artery to maintain patency.41,42
Carotid-Subclavian Bypass.
Carotid-subclavian bypass is a straightforward procedure with excellent results (Fig. 105-14, Table 105-3).34,36,43–50 It does not require extensive dissection of the proximal subclavian artery. However, a conduit (usually prosthetic) must be used. A bypass is typically indicated when the vertebral artery originates very proximally from the subclavian artery or when the patient has previously had a coronary artery bypass graft with the internal mammary artery. The procedure begins similarly to the carotid-subclavian transposition. A transverse supraclavicular incision extending lateral to the clavicular head of the sternocleidomastoid muscle provides exposure for both the carotid and subclavian arteries. The platysma is divided and the sternocleidomastoid is retracted medially. The jugular vein is dissected free and retracted medially to expose the common carotid artery, which is encircled and mobilized sufficiently to obtain proximal and distal control. Exposure of the subclavian artery is completed by dividing the anterior scalene muscle at its attachment on the first rib. Care must be taken to identify and protect the phrenic nerve. Meticulous attention should be given to careful ligation of the thoracic duct and all its tributaries and to avoid trauma to the surrounding brachial plexus. The thyrocervical trunk can be divided for further mobilization of the subclavian artery. Once sufficient length is obtained for control of the artery and space for an arteriotomy, the patient undergoes heparinization.
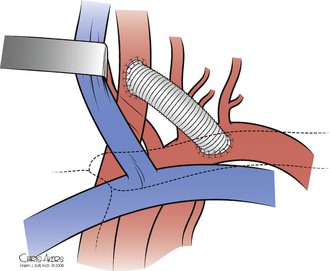
Figure 105-14 Carotid-subclavian bypass.
The target portion of the subclavian artery for the anastomosis is the retroscalene portion, and the type of graft that is used is typically prosthetic conduit. The subclavian anastomosis is created first in an end-to-side fashion with 5-0 or 6-0 polypropylene suture. The graft is passed under the jugular vein, flushed, and cut to the appropriate length. Carotid clamps are then applied, and a clamp is placed on the graft near the subclavian anastomosis. The carotid anastomosis is completed in an end-to-side fashion with 5-0 or 6-0 polypropylene suture (see Fig. 105-14). If the procedure is being performed for proximal common carotid artery disease involving ulcerated plaque, we transect the carotid artery distal to the plaque and perform an end-to-end anastomosis with the graft. Our preference is to use 8-mm woven Dacron, although the use of autologous vein and polytetrafluoroethylene has also been described. Prosthetic conduit appears to have superior patency.45,51,52
Ziomek et al52 initially examined their experience in the choice of conduit for carotid-subclavian bypass in 1986. Their series consisted of 36 revascularizations, 18 of which were bypasses with a prosthetic graft, 13 were bypasses with autogenous vein, and 5 were transpositions. The prosthetic graft patency rate at 5 years was 94.1%, whereas the vein graft patency rate at 5 years was 58.3% (P<.01). On the basis of this initial experience, the group subsequently adjusted their practice by decreasing their use of autogenous vein as conduit. In 1995, they reported their series, which consisted of 60 revascularizations, 25 with polytetrafluoroethylene (5-year patency rate, 95.2% ± 4.6%), 15 with Dacron (5-year patency rate, 83.9% ± 10.5%), 11 with autogenous vein (5-year patency, 64.8 ± 16.5%), and 9 transpositions (5-year patency rate, 100%).45 Prosthetic grafts again demonstrated an advantage in patency over autogenous vein, but because the 5-year patency rate for all revascularizations was 87.5%, it was not statistically significant. Given the relatively large-diameter, high-flow nature of the reconstruction, coupled with the short graft length, a consensus appears to have developed that prosthetic conduits are preferred for carotid subclavian bypass.
Axilloaxillary and Subclavian-Subclavian Bypass.
A third option to revascularize the subclavian artery is axilloaxillary bypass (Fig. 105-15). The axillary artery is exposed bilaterally by transverse infraclavicular incisions along the lateral third of the clavicle. The pectoralis major is split along its fibers, and the axillary artery is identified just inferior to the clavicle, posterior to the deep pectoral fascia. A subcutaneous tunnel is created between the two arteries, and an end-to-side anastomosis with prosthetic graft is performed bilaterally. Although good results have been reported (Table 105-4),53–55 the potential risks of graft infection, skin erosion, and need for possible future sternotomy have limited widespread use of this technique. A subclavian-subclavian bypass can be used, and most surgeons prefer it over an axilloaxillary bypass, if required by the patient’s anatomy.
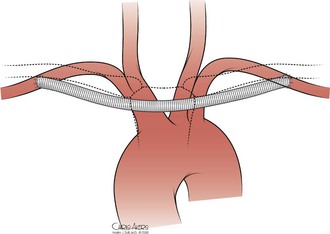
Figure 105-15 Axilloaxillary bypass.
Carotid-Carotid Bypass.
Occasionally, no ipsilateral source artery is suitable for bypass. In these situations, the contralateral carotid artery can provide inflow for either carotid or subclavian revascularization (Fig. 105-16). Bilateral exposure of the carotid artery is achieved through a longitudinal incision overlying the anterior border of the sternocleidomastoid muscle on each side. The carotid sheath is opened and the common carotid artery is dissected free bilaterally. The pharynx is identified medially, and blunt dissection is used to create a tunnel posterior to the pharynx and anterior to the prevertebral fascia. This retropharyngeal tunnel, as described by Berguer et al,56 eliminates the risk of skin erosion, enables transposition of the carotid artery, and places any graft safely away from possible future tracheostomy. Once the tunnel is created, heparinization is achieved, and the inflow carotid artery is clamped proximally and distally. An arteriotomy is created, and a prosthetic graft is sewn end to side with 5-0 or 6-0 polypropylene suture. The graft is passed through the tunnel, the carotid clamps are released, and the graft is clamped. The target carotid artery is then clamped, and an end-to-side anastomosis with 5-0 or 6-0 polypropylene suture is performed (see Fig. 105-16). If the proximal plaque on the target carotid artery is ulcerated, we transect the artery distal to the plaque. The proximal stump is oversewn, and an end-to-end anastomosis to the graft is performed with 5-0 or 6-0 polypropylene suture (Table 105-5).56,57
Carotid-Contralateral Subclavian Bypass.
If the target vessel is the contralateral subclavian artery, exposure of the subclavian artery is performed as described earlier for subclavian transposition, and the graft is sewn end to end with 5-0 or 6-0 polypropylene suture. If transposition is contraindicated, the graft can be sutured end to side at a more distal location on the subclavian artery.
Operative Results.
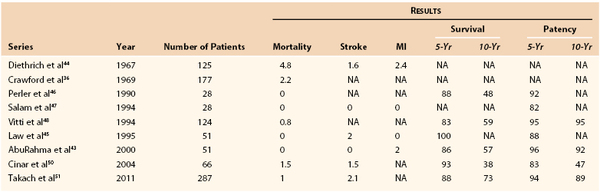
The short-term outcome of cervical brachiocephalic revascularization is excellent. The review by Diethrich et al44 of carotid-subclavian bypass in 1967 demonstrated 1.6% stroke, 2.4% myocardial infarction, and 4.8% mortality rates and greatly promoted the use of extraanatomic bypass for isolated subclavian (or common carotid) artery disease. Crawford et al36 updated this series in 1969 and then reviewed their series in 1983, which demonstrated 1% stroke and 2.2% mortality rates. More recently, Vitti et al48 reported a mortality rate of 0.8% with 5- and 10-year patency rates of 95%. Schardey et al38 reported in 1996 that subclavian transposition carries no additional risk for stroke or death and has superior 5-year patency rates, approaching 100%.
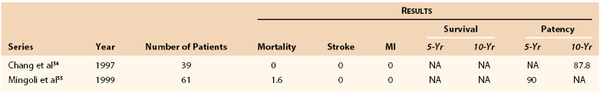
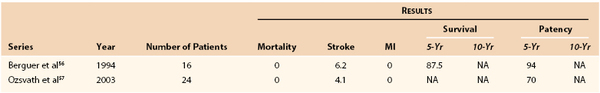
Chang et al54 in 1997 and Mingoli et al55 in 1999 demonstrated that axilloaxillary bypass could be performed safely, with their combined stroke, myocardial infarction, and mortality rates being 0% and 1.6%, respectively. Chang et al54 also reported a 10-year patency rate of 88%, whereas Mingoli et al55 reported a 5-year patency rate of 90% (see Table 105-4). The efficacy of carotid-carotid bypass was demonstrated by Berguer et al56 in 1994, and their series had no deaths and a 6.2% incidence of stroke. The 5-year patency rate was 94%. Others have reported similar results.
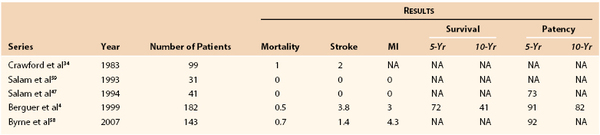
Berguer et al4 reviewed a large series of varied cervical reconstructions in 1999. Stroke, myocardial infarction, and mortality rates were 3.8%, 3%, and 0.5%, respectively. Five- and 10-year survival rates were 72% and 41%, with 5- and 10-year patency rates of 91% and 82%. In 1997, Byrne et al58 reported a series of 143 patients, with stroke, myocardial infarction, and mortality rates of 1.4%, 4.3%, and 0.7%, respectively. The 5-year patency rate was 92% (Table 105-6).4,34,47,59
Postoperative Management.
The physiologic stress after extraanatomic revascularization is less than that after direct revascularization. The primary postoperative concern is the presence of neurologic deficits in patients in whom the carotid artery was clamped. All patients are observed in the operating room for gross motor function before transfer to a recovery room for 1 hour of observation. If patients do not exhibit any neurologic changes, we move them to a telemetry floor for 24 hours of observation before discharge. After the initial postoperative visit, graft patency is monitored by duplex ultrasound examination every 6 months for the first year, followed by yearly scans.
Acknowledgments
We wish to thank Dr. Anahita Dua and Troy Brown for editing, and Chris Akers for providing illustrations.
Selected Key References
Berguer R, Kieffer E. Surgery of the arteries to the head. Springer-Verlag: New York, NY; 1992.
Textbook of techniques for revascularization of the arteries to the head..
Berguer R, Morasch MD, Kline RA. Transthoracic repair of innominate and common carotid artery disease: immediate and long-term outcome for 100 consecutive surgical reconstructions. J Vasc Surg. 1998;27:34–41.
Presents a large series of transthoracic innominate revascularizations..
Crawford ES, Stowe CL, Powers RW Jr. Occlusion of the innominate, common carotid, and subclavian arteries: long-term results of surgical treatment. Surgery. 1983;94:781–791.
Provides long-term results of brachiocephalic arterial reconstruction..
DeBakey ME, Morris GC Jr, Jordan GL Jr, Cooley DA. Segmental thrombo-obliterative disease of branches of aortic arch; successful surgical treatment. JAMA. 1958;166:998–1003.
The first description of treatment of the atherosclerotic brachiocephalic arteries..
Kieffer E, Sabatier J, Koskas F, Bahnini A. Atherosclerotic innominate artery occlusive disease: early and long-term results of surgical reconstruction. J Vasc Surg. 1995;21:326–337.
A large series of innominate artery revascularizations..
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Desai S, et al: Vascular surgery principles. Clinical review of vascular surgery. Chapter 1, pages 6–7.
2. Bergman RA. Compendium of human anatomic variation: text, atlas, and world literature. Urban & Schwarzenberg: Baltimore; 1988.
3. Berguer R, et al. Transthoracic repair of innominate and common carotid artery disease: immediate and long-term outcome for 100 consecutive surgical reconstructions. J Vasc Surg. 1998;27:34–41.
4. Berguer R, et al. Cervical reconstruction of the supra-aortic trunks: a 16-year experience. J Vasc Surg. 1999;29:239–246.
5. Fisher CM. A new vascular syndrome: “the subclavian steal.”. N Engl J Med. 1961;265:912–913.
6. Marshall WG Jr. The coronary-subclavian steal syndrome: report of a case and recommendations for prevention and management. Ann Thorac Surg. 1988;46:93–96.
7. Mozersky DJ, et al. Hemodynamics of innominate artery occlusion. Ann Surg. 1973;178:123–127.
8. Berguer R, et al. Vertebrobasilar arterial disease. Quality Medical: St. Louis; 1992.
9. Savitz SI, et al. Vertebrobasilar disease. N Engl J Med. 2005;352:2618–2626.
10. Toursarkissian B. Surgical treatment of patients with symptomatic vertebrobasilar insufficiency. Ann Vasc Surg. 1998;12:28–33.
11. Wylie EJ, et al. Surgery of the aortic arch branches and vertebral arteries. Surg Clin North Am. 1979;59:669–680.
12. Daniel RA Jr. Syphilitic aneurysm of the subclavian artery. Ann Surg. 1951;134:251–258.
13. Johnston RH Jr, et al. Innominate artery trauma: a thirty-year experience. J Vasc Surg. 1993;17:134–139.
14. Mathes SJ, et al. Subclavian artery aneurysm; sequela of thoracic outlet syndrome. Surgery. 1974;76:506–510.
15. Pairolero PC, et al. Subclavian-axillary artery aneurysms. Surgery. 1981;90:757–763.
16. Bower TC, et al. Brachiocephalic aneurysm: the case for early recognition and repair. Ann Vasc Surg. 1991;5:125–132.
17. Hall S, et al. Takayasu arteritis: a study of 32 North American patients. Medicine (Baltimore). 1985;64:89–99.
18. Weinberger J, et al. A new noninvasive technique for imaging atherosclerotic plaque in the aortic arch of stroke patients by transcutaneous real-time B-mode ultrasonography: an initial report. Stroke. 1998;29:673–676.
19. Yip PK, et al. Subclavian steal phenomenon: a correlation between duplex sonographic and angiographic findings. Neuroradiology. 1992;34:279–282.
20. Estol CJ, et al. Intracerebral hemorrhage after carotid revascularization procedures. Semin Cerebrovasc Dis Stroke. 2005;5:194–201.
21. Kieffer E, et al. Atherosclerotic innominate artery occlusive disease: early and long-term results of surgical reconstruction. J Vasc Surg. 1995;21:326–337.
22. Fleisher LA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007;50:e159–e241.
23. Vaduganathan P, et al. Pathologic correlates of aortic plaques, thrombi and mobile “aortic debris” imaged in vivo with transesophageal echocardiography. J Am Coll Cardiol. 1997;30:357–363.
24. Sylivris S, et al. The intraoperative assessment of ascending aortic atheroma: epiaortic imaging is superior to both transesophageal echocardiography and direct palpation. J Cardiothorac Vasc Anesth. 1997;11:704–707.
25. Cherry KJ. Direct reconstruction of the innominate artery. Cardiovasc Surg. 2002;10:383–388.
26. Takach TJ, et al. Concomitant subclavian and coronary artery disease. Ann Thorac Surg. 2001;71:187–189.
27. Nicholls SC, et al. Clinical significance of retrograde flow in the vertebral artery. Ann Vasc Surg. 1991;5:331–336.
27a. Cherry KJ. Direct reconstruction of the innominate artery. Cardiovasc Surg. 2002;10:383–388.
27b. Takach TJ, et al. Concomitant subclavian and coronary artery disease. Ann Thorac Surg. 2001;71:187–189.
28. Estrera AL, et al. Replacement of the ascending and transverse aortic arch: determinants of long-term survival. Ann Thorac Surg. 2002;74:1058–1064.
29. Estrera AL, et al. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation. 2008;118(Suppl):S160–S166.
30. Estrera AL, et al. Repair of the transverse arch using retrograde cerebral perfusion during repair of acute type A aortic dissection. Oper Tech Thorac Cardiovasc Surg Compar Atlas. 2005;10:3–22.
31. Estrera AL, et al. Ascending and transverse aortic arch repair: the impact of glomerular filtration rate on mortality. Ann Surg. 2008;247:524–529.
32. DeBakey ME, et al. Segmental thrombo-obliterative disease of branches of aortic arch; successful surgical treatment. JAMA. 1958;166:998–1003.
33. Crawford ES, et al. Thrombo-obliterative disease of the great vessels arising from the aortic arch. Thorac Cardiovasc Surg. 1962;43:38–53.
34. Crawford ES, et al. Occlusion of the innominate, common carotid, and subclavian arteries: long-term results of surgical treatment. Surgery. 1983;94:781–791.
35. Rhodes JM, et al. Aortic-origin reconstruction of the great vessels: risk factors of early and late complications. J Vasc Surg. 2000;31:260–269.
36. Crawford ES, et al. Surgical treatment of occlusion of the innominate, common carotid, and subclavian arteries: a 10-year experience. Surgery. 1969;65:17–31.
37. Edwards WH Jr, et al. Subclavian revascularization: a quarter century experience. Ann Surg. 1994;219:673–677.
38. Schardey HM, et al. Subclavian carotid transposition: an analysis of a clinical series and a review of the literature. Eur J Vasc Endovasc Surg. 1996;12:431–436.
39. Cinà CS, et al. Subclavian carotid transposition and bypass grafting: consecutive cohort study and systematic review. J Vasc Surg. 2002;35:422–429.
40. Morasch MD. Technique for subclavian to carotid transposition, tips, and tricks. J Vasc Surg. 2009;49:251–254.
41. Edwards WH, et al. The surgical reconstruction of the proximal subclavian and vertebral artery. J Vasc Surg. 1985;2:634–642.
42. Imparato AM. Vertebral arterial reconstruction: a nineteen-year experience. J Vasc Surg. 1985;2:626–634.
43. AbuRahma AF, et al. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32:411–419.
44. Diethrich EB, et al. Occlusive disease of the common carotid and subclavian arteries treated by carotid-subclavian bypass: analysis of 125 cases. Am J Surg. 1967;114:800–808.
45. Law MM, et al. Carotid-subclavian bypass for brachiocephalic occlusive disease choice of conduit and long-term follow-up. Stroke. 1995;26:1565–1571.
46. Perler BA, et al. Carotid-subclavian bypass: a decade of experience. J Vasc Surg. 1990;12:716–722.
47. Salam TA, et al. Subclavian artery revascularization: a decade of experience with extrathoracic bypass procedures. J Surg Res. 1994;56:387–392.
48. Vitti MJ, et al. Carotid-subclavian bypass: a twenty-two-year experience. J Vasc Surg. 1994;20:411–417.
49. Morasch MD. Technique for subclavian to carotid transposition, tips, and tricks. J Vasc Surg. 2009;49:251–254.
50. Cinar B, et al. Carotid-subclavian bypass in occlusive disease of subclavian artery: more important today than before. Tohoku J Exp Med. 2004;204:53–62.
52. Ziomek S, et al. The superiority of synthetic arterial grafts over autologous veins in carotid-subclavian bypass. J Vasc Surg. 1986;3:140–145.
53. Mingoli A, et al. Comparative results of carotid-subclavian bypass and axillo-axillary bypass in patients with symptomatic subclavian disease. Eur J Vasc Surg. 1992;6:26–30.
54. Chang JB, et al. Long-term results with axillo-axillary bypass grafts for symptomatic subclavian artery insufficiency. J Vasc Surg. 1997;25:173–178.
55. Mingoli A, et al. Long-term results and outcomes of crossover axilloaxillary bypass grafting: a 24-year experience. J Vasc Surg. 1999;29:894–901.
56. Berguer R, et al. Revascularization by the retropharyngeal route for extensive disease of the extracranial arteries. J Vasc Surg. 1994;19:217–224.
57. Ozsvath KJ, et al. Carotid-carotid crossover bypass: is it a durable procedure? J Vasc Surg. 2003;37:582–585.
58. Byrne J, et al. Long-term outcome for extra-anatomic arch reconstruction: an analysis of 143 procedures. Eur J Vasc Endovasc Surg. 2007;34:444–450.
59. Salam TA, et al. Extrathoracic bypass procedures for proximal common carotid artery lesions. Am J Surg. 1993;166:163–166.