Chapter 123 Bone Graft Harvesting
Bone Graft Specifications
Autograft
Autografts are commonly used in spine surgery, and they remain the gold standard for fusion. An ideal autograft should include strong cortical bone for structural support and cancellous bone for more rapid incorporation and fusion.1 Revascularization of cancellous bone is completed within several weeks, whereas the same process takes several months, or longer, for cortical bone.2,3 Autografts commonly are used in conjunction with spinal instrumentation, but can also be used for dorsal onlay fusions without instrumentation. A significant advantage of autologous bone is that there is no risk of disease transmission.
Allograft
Allografts are commercially prepared and typically are obtained from cadaver bone. They are characterized by delayed vascularization and incorporation, which is believed to be due to antigenic recognition by the host. Allografts are appropriate in a variety of clinical situations.4,5 They are most commonly used for ventral cervical interbody fusions, where single-level allografts generally lead to solid arthrodesis, similar to the fusion rate with autograft.6 However, they incorporate relatively slowly,7 and, if used for multilevel fusions, are associated with a pseudarthrosis rate of 63% to 70%.7,8 Fibular allografts are preferred for cervical corpectomy, because the harvesting of a fibular autograft is associated with significant morbidity, including pedal edema, ankle pain, and the risk for peroneal nerve injury.5
Bone Graft Substitutes
Nonbiologic materials such as hydroxyapatite,9,10 ceramic, and polymethylmethacrylate11 also have been used, either in conjunction with, or in lieu of, of bone graft materials. They have the advantage of being able to be manufactured in a variety of sizes, shapes, and quantities. Polymethylmethacrylate is an inert substance that is rarely used, except in cases where life expectancy is severely limited, as in providing structural support in a patient with metastatic disease to the spine and a very short survival.
Bone Graft Types
Local Bone
Bone that is removed during surgical decompression commonly is used in spinal fusions. It consists of both cancellous and cortical components. It can be morselized manually with a rongeur or can be placed in a bone mill to provide a more consistent substrate for grafting.12 The quality of local bone generally is perceived as being not as good as iliac bone grafts because of its cortical content.
Iliac Crest
The most commonly used donor site is the iliac crest. The iliac crest is a readily available source of cancellous bone, which provides rapid incorporation. Its disadvantages include donor site pain, which is a common complaint13; limited volume, which can be a concern for procedures requiring a copious amount of bone; and limited utility for procedures requiring a large piece of structural bone for reconstruction.
Ventral Iliac Crest Grafts
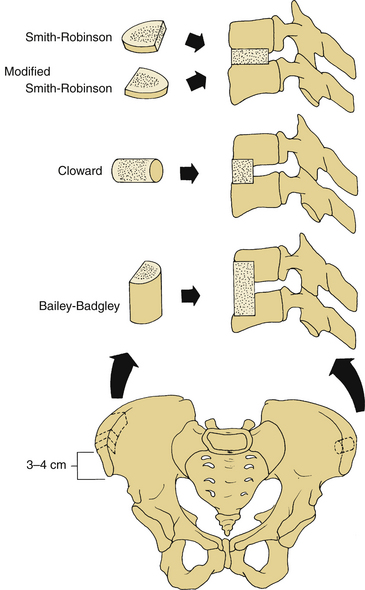
Ventral iliac crest grafts commonly are used to provide bone for various types of ventral cervical fusions (Fig. 123-1). The incision should be just caudal to the crest, to minimize discomfort that would be caused if the incision lay directly over the graft site, and should be placed approximately 3 to 4 cm lateral to the anterior superior iliac spine (ASIS) to minimize the risk of inadvertent injury to the lateral femoral cutaneous nerve. This nerve lies lateral to the ASIS in 90% of patients; in 10% it lies medial to the ASIS. When harvesting a tricortical graft for anterior cervical discectomy and fusion, an incision 6 to 8 cm long and a subperiosteal dissection of both the inner and outer wall of the ilium are performed. The iliac crest also can serve as a source of structural graft for cervical corpectomy and can provide an 8- to 10-cm length of tricortical strut graft.14–17 Dissection of the iliacus muscle from the inner wall of the crest should be minimized to reduce the risk of hematoma formation and to reduce the risk of postoperative pain. The fascia should be closed meticulously to prevent a herniation of the pelvic contents.18,19
Dorsal Iliac Crest Grafts
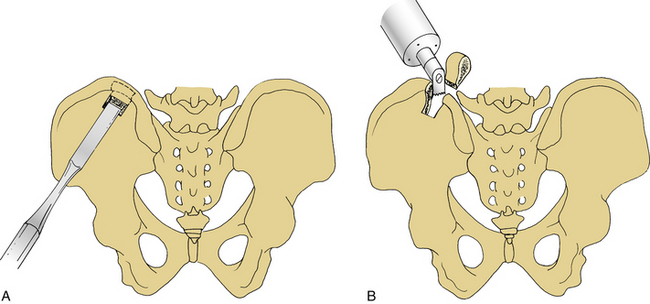
The dorsal iliac crest is predominantly used to obtain large quantities of dorsal onlay graft material for dorsolateral lumbar fusions. More bone is available from the dorsal iliac crest than from the ventral crest.20 Bone graft may be obtained through the midline lumbar skin incision used to perform the concomitant decompression or through a separate lateral skin incision over the iliac crest. The midline skin incision usually is used to obtain dorsal iliac graft. The optimal site for the underlying fascial incision is 6 to 8 cm lateral to the midline. If the fascial incision is placed lateral to this point, injury to the cluneal nerves may occur, which can result in numbness or pain over the buttocks. This is more likely if a large graft is required. Laterally, the sacroiliac ligaments and joints must be avoided. Care should be taken to minimize the depth of the osteotomy to avoid the sciatic notch where the superior gluteal artery and nerve could be injured. Injury to these structures is unlikely if the dissection is performed subperiosteally. Cancellous bone can be harvested with a gouge, which is helpful in removing strips of cancellous bone (Figs. 123-2 and 123-3).
Fibula
The fibula has the advantages of providing strength and length, and being relatively easily harvested. Its disadvantages include a slow rate of incorporation and harvest site complications. A fibular strut graft should be harvested from the middle third of the fibular shaft through a long skin incision on the lateral side of the leg, extending through the lateral intermuscular septum, preserving the periosteum (Fig. 123-4). During fibula graft harvesting, the peroneal nerve must be protected. Distally, the fibula should be harvested no more than 10 cm proximal to the ankle joint to minimize the risk of injury to the ankle syndesmosis, which is important for the stability of the ankle joint. The peroneal muscles should also be preserved. The middle third of the fibula should be osteotomized using an oscillating saw rather than an osteotome, which could cause fracture of the graft (Fig. 123-5). After fibula harvest, a few days of compressive leg wrapping with elevation of the leg will minimize swelling and discomfort.
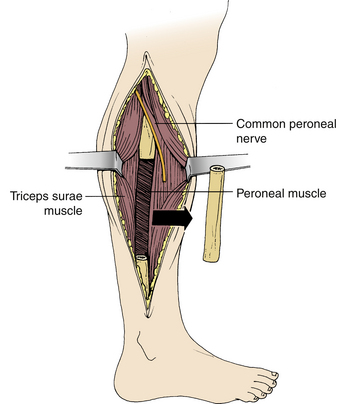
FIGURE 123-5 Fibula graft harvesting. The midportion of the fibula is removed with an oscillating saw.
Care must be exercised when harvesting a long segment of fibula to avoid proximal extension of the graft to the region of the neck of the fibula, where the common peroneal nerve is in jeopardy of injury. Injury to this nerve may result in pain and weakness in the foot and ankle.21
Tibia
The subcutaneous ventromedial aspect of the tibia historically has been a site used occasionally for bone grafting. Currently, it is rarely used, because other, more suitable, autogenous or allograft alternatives exist. The potential morbidity associated with tibial grafts is significant, with tibial fracture being its main disadvantage. The tibia must be protected for several months to prevent fracture.22
Rib
Rib can be easily harvested, especially during thoracic spine operations. It is, however, a weak, poorly vascularized graft. Biomechanically it is inferior to the fibula. If taken with its artery, it is suitable for use as a vascularized strut graft.23–25 It is used almost exclusively with thoracic or thoracolumbar fusions.
Complications of Graft Harvesting
Graft harvesting complications are common, and pain from a bone graft harvest site sometimes is more severe than the pain from the actual surgical procedure.18,26–33 Although the complications usually are minor, a review of 1244 cases from multiple series demonstrated that their occurrence is about 20%, whereas only a 0.2% complication rate was reported at the neck incision site.34
Chronic Pain
Graft donor site pain is nearly universal in the early postoperative period13 but may be persistent in up to one third of patients35,36 and may continue throughout the first 3 months postoperatively in up to 15% of patients.29 One study reported that donor site pain was present for more than 10 years following surgery in more than one third of patients.36 The reason for this chronic pain is not well understood, but it often is associated with the patient’s overall pain syndrome. In addition, there are specific reasons for chronic donor site pain, including sacroiliac joint disruption, hernia through the graft site, fracture at the graft donor site (predominantly on the ventral ilium), and heterotopic bone formation.37
Nerve Injury and Pain
Incisions for bone grafts may injure nerves or may cause entrapment of nerves due to scar formation (see Fig. 123-2A).
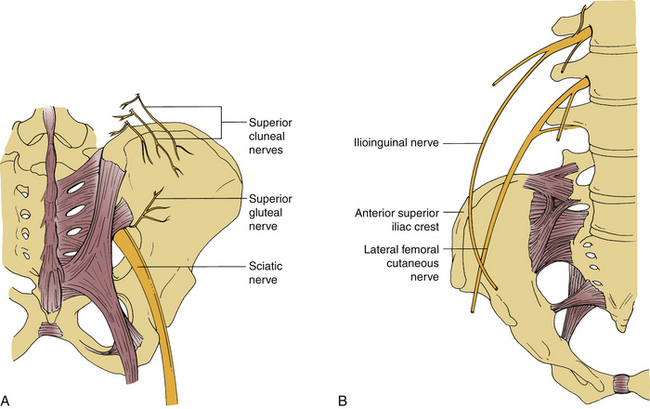
FIGURE 123-2 Possible nerve injury sites during dorsal (A) and ventral (B) iliac crest bone graft harvesting.
The lateral femoral cutaneous nerve can be injured during ventral iliac crest harvest procedures, especially if the incision is very close to the ASIS.38 Injury to this nerve can result in meralgia paresthetica and has been reported in 1% to 14% of cases.32,37,38 It is characterized by numbness or dysesthesia on the ventrolateral thigh.30,32 The nerve usually passes beneath the inguinal ligament, approximately 1 cm medial to the ASIS. However, in about 10% of cases it may pass above the inguinal ligament and just lateral to the ASIS.39
To avoid this complication, the incision must be kept at least 2 cm dorsal to the ASIS.38
The superior cluneal nerves are the most commonly injured cutaneous nerves following dorsal iliac crest bone grafting.40 They arise from the superficial fascia, 6 to 8 cm lateral to the posterior superior iliac spine (PSIS). Injury to these nerves during dorsal iliac crest graft harvesting may cause analgesia over the buttock or painful neuromas. A hockey-stick or longitudinal incision may be helpful for avoiding this problem. Exposing the iliac crest under the deep fascia also may avoid injuring these nerves. Another technique recommended to avoid their injury is to make a separate vertical skin incision medial to the cluneal nerves.41
Other cutaneous nerves, such as the ilioinguinal, iliohypogastric, genitofemoral, superior gluteal, and femoral nerves may, rarely, be injured.37
Vascular and Other Visceral Organ Injuries
The superior gluteal artery can be injured during dorsal iliac crest graft harvesting.42,43 This can cause severe hemorrhage. It lies between the gluteus medius and minimus muscles, and is avoided by careful subperisoteal dissection. If the artery is transected, it may retract into the pelvis, and controlling the bleeding may be difficult. Dissection of the gluteal muscles from the pelvis distally may be necessary.43 In addition to vascular structures, the ureter and other visceral organs also may be injured.44
Hematoma Formation
Hematoma formation has been reported in 9% of iliac crest graft cases.35 The bleeding typically comes from the adjacent muscles and bone surfaces and can, therefore, be minimized by the use of bone wax prior to closure. For larger bone graft defects, use of a drain may be helpful.
Pelvic Fracture or Instability
Pelvic fracture or instability is rare, but may occur during either ventral or dorsal iliac graft harvesting procedures. Bone graft harvest performed too close to the ASIS can result in avulsion of the ASIS. Staying 2 cm behind the ASIS during bone resection will reduce the likelihood of this occurrence and also will reduce the risk of injury to the lateral femoral cutaneous nerve.45 In addition, aggressive dorsal iliac crest grafting can result in injury to the sacroiliac joint or can produce a stress fracture to the ilium. Either of these can result in instability and can cause severe pain with ambulation.
Local Infection
Infections from the bone graft site are uncommon and have been reported in fewer than 1% of cases.29 The risk of dorsal iliac graft site infection can be minimized by taking the bone graft through a separate facial incision, rather than from the laminectomy/fusion incision. A separate deep facial incision reduces the risk of cross-contamination between the laminectomy/fusion site and the bone graft site.
Where and When to Use a Bone Graft
Ventral Cervical Operations
Discectomy
Following cervical discectomy, the depth of the disc space is measured, and the graft is sized accordingly. The cartilaginous surfaces of the end plates are prepared, but the underlying cortical bone is preserved to minimize the risk of graft subsidence. A rasp or high-speed bur is used to create two parallel surfaces to optimize contact between the end plates and graft surface. A tricortical iliac crest graft of the appropriate size is harvested.
To facilitate insertion of the graft, distraction of the vertebral bodies is performed, either by cervical traction or by distracting across pins placed in the adjacent vertebral bodies (Caspar Cervical Distractor; Aesculap, Inc., Center Valley, PA).50 Care must be exercised in gently tapping the bone into the distracted disc space to minimize risk of neural injury and to reduce the likelihood of cracking the graft. If ventral plating is not used, the graft should be recessed approximately 1 to 2 mm to lock the graft in the interspace.
Iliac crest grafts can be used in a three- or two-cortex construct. Three well-known graft configurations are used for ventral cervical spine surgery (see Fig. 123-1):
1. The Smith-Robinson–type graft is a tricortical horseshoe-type graft. Smith and Robinson have described the harvesting of the graft from the ventral iliac crest at a depth of approximately 1 cm and a width of 5 to 6 mm.51 This graft is inserted into the intervertebral space, so that its cancellous portion is directed dorsally and the cortical surface ventrally. To prevent collapse, extrusion, or nonunion, Bloom and Raney have modified the graft position, and the cortical portion is inserted directly dorsally.52
2. The Cloward-type graft is a dowel graft. After harvesting the graft from iliac crest with a cylindrical bur, it is impacted in the intended graft site with a 1-mm, narrower bur. A special instrument set is used for graft harvesting, graft bed preparation, and graft impaction. If a Cloward type of graft is used in multilevel fusions, avascular necrosis of the vertebra may be encountered as a complication53 if two adjacent levels are fused. The use of allograft bone rather than autograft is more common currently.4 For multilevel fusions, a Smith-Robinson type of graft may be inserted at one disc level with a dowel graft at the adjacent space. Other alternatives for multilevel fusions include multilevel tricortical grafts (i.e., the Smith-Robinson technique) or cervical corpectomy.
3. The Bailey-Badgley–type graft is an iliac crest strut graft in which a trough is prepared in the ventral aspect of the vertebral body at a limited depth (0.5 cm).19 The trough is cut to the full vertical height of the vertebra, and the discs are cleaned with a rongeur to a depth of approximately 1.2 cm.19,34 A cortical cancellous iliac graft is then placed into the trough.
The strongest constructs are provided by the Smith-Robinson type of graft.54 Tricortical grafts are stronger than bicortical grafts,55 although graft breakage is a relatively uncommon complication. The optimal autologous graft has a substantial cancellous surface to facilitate bone incorporation.
Corpectomy
Reconstruction following cervical corpectomy typically involves use of either an iliac crest autograft or a fibular allograft. Iliac crest graft can be used for shorter segmental defects, but will not withstand axial stress following reconstruction for multilevel corpectomies.5 In addition, the curved nature of the iliac crest sometimes makes it difficult to insert the graft when reconstructing a multilevel defect. The use of iliac crest autograft to reconstruct a multilevel corpectomy also leaves a significant defect in the iliac crest. A fibular allograft is used, therefore, for more than a two-level corpectomy and usually is the preferred choice for even a single-level corpectomy. We prefer to pack the medullary canal of the fibula with autogenous bone from the corpectomy to increase the healing potential of the fibular allograft.
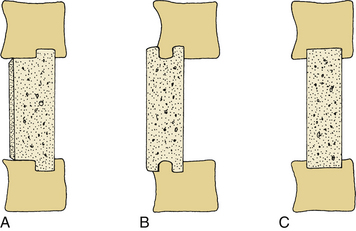
Several methods are available for inserting a fibular graft into a corpectomy site (Fig. 123-6). In general, it is preferable to preserve the cortical end plates to minimize the risk of graft subsidence into the vertebral bodies. In the “keystone method” (see Fig. 123-6C) a defect is made in the midportion of both the rostral and caudal vertebral end plates.5,56 In the “dovetail method” (see Fig. 123-6B) the bone is keyed into the ventral cortex of the vertebral body,57 and the graft is inserted first into the rostral end, and then gently tapped into the caudal vertebra. In this instance, manual or skeletal traction may be helpful.58 The iliac crest graft also can be tailored in a T-shaped form in which the cortical portion of the graft is faced dorsally (see Fig. 123-6A).
Ventral Thoracic or Thoracolumbar Operations
In the upper thoracic spine, a rib often is used for ventral bone grafting, since removal of a rib is commonly performed as part of the exposure. The iliac crest also can be used because the patient typically is in the lateral decubitus position and the iliac crest is readily accessible. Because the lower thoracic and lumbar vertebral bodies are relatively large, a bigger graft often is necessary. For autogenous graft, a tricortical iliac crest can be used, whereas for allograft, femur or tibia can be utilized.
Dorsal Cervical and Thoracolumbar Operations
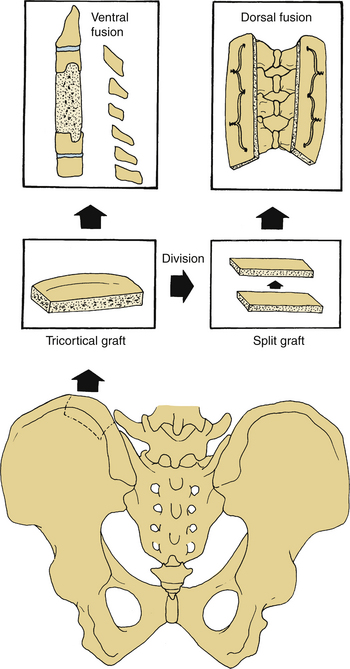
If wiring of the dorsal cervical spine is performed, a rectangular piece of iliac crest can be used following decortication of the dorsal elements. Either a unicortical, rectangular piece of bone or a bicortical piece that can then be split longitudinally can be harvested from the dorsal iliac crest and placed along the cervical spine to help maintain stability. Wires are passed through the facets or spinous processes and then through holes in the graft to help fasten the graft onto the laminar facet surfaces (see Fig. 123-3). With the introduction of cervical instrumentation, wire fixation is no longer commonly used. It has been replaced by lateral mass screws and rods, with bone grafting consisting of cancellous bone from either the dorsal iliac crest or allograft chips.
Hamby W.B., Glaser H.T. Replacement of spinal intervertebral discs with locally polymerizing methylmethacrylate. Experimental study of effects upon tissue and report of a small clinical series. J Neurosurg. 1959;16:311-313.
Kostuik J.P., Frymoyer J.W. Failures after spinal fusion: causes and surgical treatment results. In: Frymoyer J.W., et al, editors. The adult spine: principles and practice. New York: Raven; 1991:2027-2068.
Kurtz L.T., Garfin S.R., Booth R.E.Jr. Harvesting autologous iliac bone grafts—a review of complications and techniques. Spine (Phila Pa 1976). 1989;14:1324-1331.
Stevenson S., Emery S.E., Goldberg V.M. Factors affecting bone graft incorporation—review. Clin Orthop Relat Res. 1996;324:66-74.
Zdeblick T.A., Ducker T.B. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976). 1991;16:726-729.
1. Stevenson S., Emery S.E., Goldberg V.M. Factors affecting bone graft incorporation: review. Clin Orthop Relat Res. 1996;324:66-74.
2. Deleu J., Trueta J. Vascularization of bone grafts in the anterior chamber of the eye. J Bone Joint Surg [Br]. 1965;47:319.
3. Kingma M.J., Hampe J.F. The behaviour of blood vessels after experimental transplantation of bone. J Bone Joint Surg [Br]. 1964;46:141-150.
4. Cloward R.B. Gas-sterilized cadaver bone grafts for spinal fusion operations. A simplified bone bank. Spine. 1980;5:4-10.
5. Rengachary S.S., Redford J.B. Partial median corpectomy for cervical spondylotic myelopathy. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery, update II. New York: McGraw Hill; 1991:356-359.
6. Rish B.L., McFadden J.T., Penix J.O. Anterior cervical fusion using homologous bone grafts: a comparative study. Surg Neurol. 1976;5:119-121.
7. Zdeblick T.A., Ducker T.B. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976). 1991;16:726-729.
8. Ferynhough J.C., White J.I., LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine (Phila Pa 1976). 1992;16:S561-S564.
9. Cook S.D., Whitecloud T.S., Reynolds M.C., et al. Hydroxylapatite graft materials for cervical spine fusions. In: Kehr P., Weidner A., editors. Cervical spine I, Strasbourg 1985. New York: Springer-Verlag; 1987:257-262.
10. Senter H.J., Kortyna R., Kemp W.R. Anterior cervical fusion with hydroxylapatite fusion. Neurosurgery. 1989;25:39-43.
11. Hamby W.B., Glaser H.T. Replacement of spinal intervertebral discs with locally polymerizing methylmethacrylate. Experimental study of effects upon tissue and report of a small clinical series. J Neurosurg. 1959;16:311-313.
12. Keene J.S., McKinley N.E. Iliac crest versus spinous process grafts in posttraumatic spinal fusions. Spine (Phila Pa 1976). 1992;17:790-794.
13. Summers B.N., Eissenstein S.M. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg [Am]. 1989;71:677-680.
14. Wolfinbarger L.Jr., Zhang Y., Adam B.L., et al. A comprehensive study of physical parameters, biomechanical properties, and statistical correlations of iliac crest bone wedges used in spinal fusion surgery. I. Physical parameters and their correlations. Spine (Phila Pa 1976). 1994;19:277-283.
15. Wolfinbarger L.Jr., Zhang Y., Adam B.L., et al. A comprehensive study of physical parameters, biomechanical properties, and statistical correlations of iliac crest bone wedges used in spinal fusion surgery. II. Mechanical properties and correlation with physical parameters. Spine (Phila Pa 1976). 1994;19:284-295.
16. Zhang Y., Wolfinbarger L.Jr. A comprehensive study of physical parameters, biomechanical properties, and statistical correlations of iliac crest bone wedges used in spinal fusion surgery. III. Multivariable regression analysis and practical formulas for strength prediction. Spine (Phila Pa 1976). 1994;19:296-303.
17. Zhang Y., Homsi D., Gates K., et al. A comprehensive study of physical parameters, biomechanical properties, and statistical correlations of iliac crest bone wedges used in spinal fusion surgery. IV. Effect of gamma irradiation on mechanical and material properties. Spine (Phila Pa 1976). 1994;19:304-308.
18. Albee F.H. Bone graft surgery—the classic. Clin Orthop Relat Res. 1996;324:5-12.
19. Bailey R.W., Badgley C.E. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg [Am]. 1960;42:565-594.
20. Brantigan J.W., Cunningham B.W., Warden K., McAfee P.C., Steffee A.D. Compression strength of donor bone for posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1993;18:1213-1221.
21. Gore D.R., Gardner G.M., Sepic S.B., et al. Function following partial fibulectomy. Clin Orthop Relat Res. 1987;220:206.
22. Crenshaw A.H. Campbell’s operative orthopaedics, vol 1. St Louis: Mosby Year Book; 1992.
23. Hendel P.M., Hattner R.S., Rodrigo J., Buncke H.J. The functional vascular anatomy of rib. Plast Reconstruct Surg. 1982;70:578-587.
24. McElvein R.B., Nasca R.J., Dunham W.K., Zorn G.L.Jr. Transthoracic exposure for anterior spinal surgery. Ann Thorac Surg. 1988;45:278-283.
25. Nakamura H., Yamano Y., Seki M., Konishi S. Use of folded vascularized rib graft in anterior fusion after treatment of thoracic and upper lumbar lesions. Technical note. J Neurosurg. 2001;94(Suppl 2):323-327.
26. Arrington E.D., Smith W.J., Chambers H.G., et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300-309.
27. Banwart J.C., Asher M.A., Hassanein R.S. Iliac crest bone graft harvest donor site morbidity—a statistical evaluation. Spine (Phila Pa 1976). 1995;20:1055-1060.
28. Ebraheim N., Elgafy H., Xu R. Bone graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg. 2001;9:210-218.
29. Kurtz L.T., Garfin S.R., Booth R.E.Jr. Harvesting autologous iliac bone grafts: a review of complications and techniques. Spine (Phila Pa 1976). 1989;14:1324-1331.
30. Massey E.W. Meralgia paresthetica secondary to trauma of bone graft. J Trauma. 1980;20:342-343.
31. Robertson P.A., Wray A.J. Natural history of posterior iliac crest bone graft donation for spinal surgery. A prospective analysis of morbidity. Spine (Phila Pa 1976). 2001;26:1473-1476.
32. Weikel A.M., Habal M.B. Meralgia paresthetica: a complication of iliac bone procurement. Plast Reconstr Surg. 1977;60:572-574.
33. Younger E.M., Chapman M.W. Morbidity at bone graft donor site. J Orthop Trauma. 1989;3:192-195.
34. Whitecloud T.S. Cervical spondylosis: the anterior approach. In: Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven; 1991:1165-1185.
35. DePalma A.F., Rothman R.H., Lewinnek G.E., et al. Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet. 1972;134:755-758.
36. Frymoyer J.W., Howe J., Kuhlmann D. The long-term effects of spinal fusion on the sacroiliac joints and ilium. Clin Orthop Relat Res. 1978;134:196-201.
37. Kostuik J.P., Frymoyer J.W. Failures after spinal fusion: causes and surgical treatment results. In: Frymoyer J.W., et al, editors. The adult spine: principles and practice. New York: Raven; 1991:2027-2068.
38. Mirovsky Y., Neuwirth M. Injuries to the lateral femoral cutaneous nerve during spine surgery. Spine. 2000;25:1266-1269.
39. Ghent W.R. Further studies on meralgia paresthetica. Can Med Assoc J. 1961;85:871-875.
40. Fernyhough J.C., Schimandle J.J., Weigel M.C., et al. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine (Phila Pa 1976). 1992;17:1474-1480.
41. Colterjohn N.R., Bednar D.A. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J Bone Joint Surg [Am]. 1997;79:756-759.
42. Lim E.V., Lavadia W.T., Roberts J.M. Superior gluteal artery injury during iliac bone grafting for spinal fusion. A case report and literature review. Spine (Phila Pa 1976). 1996;21:2376-2378.
43. Shin A.Y., Moran M.E., Wenger D.R. Superior gluteal artery injury secondary to posterior iliac crest bone graft harvesting. A surgical technique to control hemorrhage. Spine (Phila Pa 1976). 1996;21:1371-1374.
44. Escalas F., DeWald R.L. Combined traumatic arteriovenous fistula and ureteral injury: a complication of iliac bone-grafting. J Bone Joint Surg [Am]. 1977;59:270-271.
45. Kuhn D.A., Moreland M.S. Complications following iliac crest bone grafting. Clin Orthop Relat Res. 1986;209:224-226.
46. Bosworth D.M. Repair of herniae through iliac-crest defects. J Bone Joint Surg [Am]. 1955;37:1069-1073.
47. Moeller K.H., Lawrence D.P. Hernia through an iliac bone graft defect. Mil Med. 1996;161:176-178.
48. Wolfe S.A., Kawamoto H.K. Taking the iliac-bone graft: a new technique. J Bone Joint Surg [Am]. 1978;60:411.
49. Hochshuler S.H., Guyer R.D., Stith W.J., et al. Proplast reconstruction on iliac crest defects. Spine (Phila Pa 1976). 1988;13:378-379.
50. Caspar W. Anterior stabilization with the trapezial osteosynthetic plate technique in cervical spine injuries. In: Kehr P., Weidner A., editors. Cervical spine I. Wien, New York: Springer Verlag; 1987:198-203.
51. Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by the anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
52. Bloom M.H., Raney F.L. Anterior intervertebral fusion of the cervical spine. A technical note. J Bone Joint Surg [Am]. 1982;63:842.
53. Mosdal C. Cervical osteochondrosis and disc herniation: eighteen years’ use of interbody fusion by Cloward’s technique in 755 cases. Acta Neurochir (Wien). 1984;70:207-255.
54. White A.A., Hirsch C. An experimental study of the immediate load bearing capacity of some commonly used iliac bone grafts. Acta Orthop Scand. 1971;42:482-490.
55. Wittenberg R.H., Moeller J., Shea M., et al. Compressive strength of autologous and allogenous bone grafts for thoracolumbar and cervical spine fusion. Spine (Phila Pa 1976). 1990;15:1073-1078.
56. Simmons E.H., Bhalla S.K. Anterior cervical discectomy and fusion: a clinical and biomechanical study with eight year follow-up. J Bone Joint Surg [Br]. 1969;51:225-237.
57. Gore D.R. Technique of cervical interbody fusion. Clin Orthop Relat Res. 1984;188:191-195.
58. Saunders R.L. Ventral exposures of the subaxial cervical spine. In: Benzel E.C., editor. Surgical exposure of the spine: an extensile approach. American Association of Neurological Surgeons; Rolling Meadows, IL, 1995:55-68.