Bone Density and Imaging of Osteoporosis
An NIH conference1 described osteoporosis as a “skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength primarily reflects the integration of bone density and bone quality.” Bone density corresponds to the amount of mineral within an area or a volume, and bone quality depends on architecture, bone turnover, damage accumulation, and mineralization. Compromised bone strength (bone fragility) increases the risk of fractures resulting from moderate trauma, defined as a fall from standing height or less, which has led to the concept of “fragility fracture.” Therefore, osteoporosis is a risk factor for fracture.
The concept of osteoporosis has progressively evolved from criteria based on histology, to fracture occurrence, to assessment of bone mass, and currently to the individual assessment of fracture risk, including history of fracture, clinical risk factors for fracture, and bone mass evaluation. Indeed, a fracture-based diagnosis unacceptably delays intervention in a condition for which prevention of fracture is essential. Changes encountered in osteoporosis can be assessed by noninvasive techniques measuring bone mineral density (BMD), such as dual x-ray absorptiometry (DXA). BMD accounts for 75% to 85% of the variance in ultimate strength of bone tissue2 and is well correlated with the load-bearing capacity of the skeleton in vitro.3 Prospective studies demonstrate that the risk of fragility fractures increases as BMD declines, with a 1.5- to 3-fold increased risk of fracture for each standard deviation (SD) fall in BMD.4 The main advantage of a density-based diagnosis is the possibility of an early intervention, before fractures. This approach was carried out by the World Health Organization (WHO) in its definition of osteoporosis in 1994.5 In women, osteoporosis can be diagnosed if the BMD or the bone mineral content (BMC) at the spine, femoral neck, or forearm is 2.5 SD or more below the mean value of a reference population of young, healthy premenopausal women, with the following categories:
1. Normal: BMD higher than 1 SD below the young adult mean
2. Osteopenia, or low bone mass: BMD between 1 and 2.5 SD below the young adult mean
3. Osteoporosis: BMD lower than 2.5 SD below the young adult mean
4. Established (or severe) osteoporosis: BMD lower than 2.5 SD below the young adult mean and the presence of one or more fragility fractures
Thus, osteoporosis is defined in practice by a surrogate marker (i.e., BMD), not a health outcome (fracture), even if other factors can influence the likelihood of fractures. The relationship between BMD and fractures is very similar to that between hypertension and stroke and stronger than that between serum cholesterol and coronary heart disease (Fig. 13-1). The gradient of the risk of fracture with decreasing BMD is as steep as that between diastolic blood pressure and stroke.
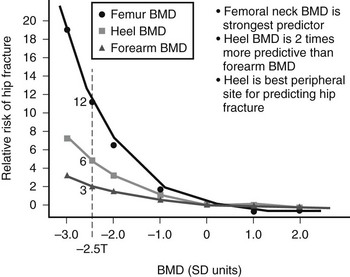
FIGURE 13-1 Hip fracture incidence as a function of BMD measured at the hip, spine, and forearm in postmenopausal women. (Data from Cummings SR, Black DM, Nevitt MC, et al: Bone density at various sites for prediction of hip fractures, Lancet 341:72–75, 1993.)
It is estimated that with this 2.5 SD threshold, 30% of white women older than 50 years have osteoporosis,6 a fraction that is similar to the lifetime risk of fracture at the hip, spine, and forearm for a 50-year-old woman.7 By this definition, about 0.6% of young adult women have osteoporosis, and 16% have low bone mass. The prevalence of osteoporosis increases with age because of the decline in bone mass. Not all women who have osteoporosis by the WHO criteria will sustain fractures, and conversely, many women who do not have osteoporosis by this definition may deserve treatment because of other risk factors that will increase their risk of fracture. Thus, this operational WHO definition of osteoporosis provided the concept of osteoporosis as a risk factor for fracture, which is important for assessing the number of affected individuals, but patient diagnosis must include other risk factors for fracture. This is the role of the individual fracture risk assessment of fracture.
Recently, a WHO Scientific Group issued a technical report including hierarchical levels of evidence for the ability of risk factors to identify individuals at risk for fracture.8 Those risk factors were validated by meta-analyses of population-based cohorts from Asia, Australia, Europe, and North America. Those risk factors were BMD at the femoral neck, low body mass index, a prior fragility fracture, glucocorticoid exposure, a parental history of hip fracture, smoking, excessive intake of alcohol, and rheumatoid arthritis. The combination of those risk factors allowed for building fracture probability models, providing individual probabilities of fracture that will be the basis for therapeutic decision making, thus shifting the former therapeutic threshold paradigm, which was often based only on the BMD T score.
Epidemiology
In women, osteoporosis is rare before menopause. With aging, more and more women are affected by osteoporosis; by the age of 80 years, 27% have low bone mass and 70% have osteoporosis at the hip, spine, or forearm.7 Sixty percent of osteoporotic women will experience one or more fragility fractures. In the United States, it is estimated that 16.8 million (54%) of postmenopausal white women have low bone mass, and 9.4 million (30%) have osteoporosis.7 These rates of prevalence are lower in nonwhite women and in men. NHANES III (Third National Health and Nutritional Examination Survey) data indicate that 10,103,000 Americans (8,021,000 women and 2,082,000 men) have osteoporosis and that 18,557,000 (15,434,000 women and 3,123,000 men) have low bone mass and thus an increased risk of osteoporosis in comparison to those who do not have low bone mass.8 The prevalence of vertebral fractures can vary according to the definition of fracture used. Thus 10% to 25% of women older than 50 years are estimated to have vertebral fractures.9,10 In 1990, 1.66 million hip fractures were estimated worldwide in those older than 35 years.11
Osteoporosis results in high rates of morbidity. In 1986 in the United States, osteoporosis was responsible for an estimated 321,909 hospitalizations in white women 45 years and older—167,421 for hip fractures, 35,106 for fragility vertebral fractures, 120,636 for wrist fractures, and 20,369 for humerus fractures.12 Osteoporosis-related fractures also significantly contribute to the economic burden of health care. In 1995, direct costs for osteoporosis fractures were estimated to be $13.8 billion,13 whereas it was 5 to 6 billion annually 10 years earlier.12 Being elderly, patients with hip fractures often have other medical conditions, thus increasing their risk of complications such as pressure sores or infections. Hip fractures often result in admission to nursing homes.14 The number of hip fractures and their associated costs are expected to sharply increase and could triple by the year 2040. Indeed, the number of elderly people is quickly growing, and fracture incidence rates increase with age.15 This phenomenon is observed in developed countries, but the projected rapid expansion of the elderly populations of Latin America and Asia could lead to an increase in hip fractures from 1.66 million worldwide in 1990 to 6.26 million in 2050, with only 25% occurring in North America and Europe.11 Osteoporosis is also associated with mortality. For each standard deviation decrease in bone mineral density, the mortality risk is increased 1.5-fold.
The lifetime risk of fractures can be estimated, given the probability of fracture and the odds of reaching a given age. Most data have been generated in white populations of Western countries. The lifetime risk of sustaining an osteoporotic fracture has been estimated in the United States to be close to 40% for women and 13% for men. For vertebral fractures, the estimated risk is 15.6% for women and 5% for men. The risk of hip fracture is 17.5% for women and 6% for men and for wrist fracture, 16% for women and 2.5% for men.7
Site-Specific Fractures
Hip fracture is the most serious complication of osteoporosis. It results in high morbidity and sometimes mortality. The most striking characteristic of hip fractures is that they are associated with a 12% to 20% reduction in expected survival.16 Many of the deaths after hip fractures are related to other diseases.17 Few deaths are actually due to or hastened by the fracture, but the risk of dying of another disease is sometimes increased by the hip fracture. About 5% of women sustaining a hip fracture die during the following year as a consequence of fracture. In women living in nursing homes, 36% of those having a hip fracture will die within a year. Thirty percent to 50% of these patients are unable to regain the level of function they had before the hip fracture. The patient’s physical condition before the fracture may be the best predictor of postfracture functional outcome.
The incidence of hip fracture increases exponentially with age in both genders. In women younger than 35 years, the incidence is 2 per 100,000 person years, whereas it is 3032 per 100,000 person years in women older than 85 years.18 In men the rates are 4 and 1909 per 100,000 person years, respectively. Most hip fractures occur in elderly people: 52% after age 80 years and 90% after age 50 years.18 The decline in BMD and the increase in frequency of falls in elderly people are responsible for this high incidence of hip fracture. Only 1% of falls lead to a hip fracture, but 90% of hip fractures are related to a fall from standing height or less.19 Hip fractures tend to be the consequence of falls on hard surfaces, the fall not being stopped by protective reflexes of upper limbs.20 Fifty percent of the falls leading to hip fractures result from slipping or tripping, 20% from loss of consciousness, and 20% to 30% of loss of balance and other factors.
Vertebral Fracture
The epidemiology of vertebral fractures is less well characterized than that for hip fractures because symptoms after vertebral fracture are unidentified in up to 70% of cases, and standardized radiographic diagnostic criteria for vertebral fractures are lacking. Less than a third of patients with vertebral deformities seek medical assistance, and 2% to 8% need hospital admission.23 In women, 90% of vertebral deformities are a consequence of mild to moderate trauma, whereas this proportion is only 50% in men.24
The incidence of clinical vertebral fractures increases gradually with age in both genders. The age-adjusted prevalence is thought to be between 8% and 25% in women older than 50 years. The difference in prevalence figures is due to the definition used.24 Early definitions of vertebral fracture were based on the type of deformity and thus had poor precision. New techniques using vertebral morphometry give more reliable results.25 Vertebral deformities are mostly noted in women, with a ratio of 2 : 1, but this ratio becomes narrower in elderly people older than 80 years. It has been suggested in a large epidemiologic study involving 15,570 European men and women that the incidence of vertebral deformities might be equal in both genders. In the same cohort, vertebral fracture incidence in women over 50 was estimated at 10.7/1000 person years in women and 5.7/1000 per person years in men.26 Changes in the gross appearance of the vertebral body encompass a wide range of morphologic characteristics, from increased endplate concavity to complete destruction of the vertebral anatomy in vertebral crush fractures.27 It must be kept in mind that vertebral deformity is not synonymous with vertebral fracture; for example, Scheuermann’s disease is frequently discussed as a differential diagnosis.28
New vertebral fractures, even those not recognized clinically, are associated with substantial increases in back pain and functional limitation due to back pain, at least in elderly women.29 Ten percent to 20% of elderly women with worsening of their functional status because of back pain have had a vertebral fracture. Common complications of vertebral deformities are chronic back pain, back disability, height loss, limitations in activity, emotional difficulties stemming from physical appearance, and more medical consumption. Kyphosis from vertebral fractures adds to the overall back pain. For instance, after adjustment for age, a 15% increase in kyphosis increases the odds ratio of severe upper back pain 2.1-fold. However, no association has been found between the number of fractures and impairment in quality of life. The occurrence of vertebral fracture is predictive of new fracture, with RR = 5 to 11. Vertebral fractures are associated with increased mortality at 5 years, as for hip fracture. The increase in mortality is progressive over this period, as opposed to hip fracture. The risk of death appears to be much higher for clinically apparent vertebral fracture, and the presence of multiple vertebral fractures further increases this risk.30,31
Wrist Fracture
Wrist fractures, also called distal forearm fractures or Colles’ fractures, are very common. The incidence of wrist fracture is hard to calculate because only a minority of patients is hospitalized. A Norwegian study showed that it was the most common fracture responsible for admission to a local university hospital.32 Incidence varies among different ethnic groups. Wrist fracture risk is lower in black people than white people.33 Increasing incidence was observed between 1950 and 1982,34 but this trend seemed to have leveled off during the second part of the 1980s.
The pattern of incidence of wrist fracture is different from that of hip or vertebral fractures. The age-adjusted female-to-male ratio is 4 : 1, with a linear increase in incidence in women from ages 40 to 60 years, followed by a plateau.35 In men, the incidence is almost constant between the ages of 20 and 80 years, for unknown reasons. Wrist fractures are not associated with an increase in mortality and are usually thought to be free of long-term poor outcome.36 Other data, however, suggest that half of the patients do not report good functional status at 6 months because of complications such as algodystrophy, neuropathies, and posttraumatic osteoarthritis.37
Wrist fractures must be considered as an important predictor of subsequent vertebral and hip fractures. Indeed, it has been shown that men and women with distal forearm fractures have on average a twofold increase in the risk of sustaining a hip fracture.38 Previous wrist fractures are also significant predictors of overall osteoporotic fractures, with a doubled risk. In younger women, the predictive value of a previous wrist fracture for subsequent osteoporotic fractures seems even more important, with a relative risk of 2.67 (1.02 to 6.94) for women aged 40 to 49 years. This predictive value is of the same magnitude as that provided by a 1-SD decrease in BMD measured by bone densitometry.39 Thus, the occurrence of a wrist fracture in an early postmenopausal woman is suggestive of osteoporosis and should trigger appropriate investigation such as BMD measurement.
Variability In Fracture Incidence
The incidence of hip fracture is markedly lower in black and Asian people and varies among different countries and populations. Rates are higher in Scandinavia than in Western Europe and Oceania.21 A north-south gradient in age-standardized risk is found in Europe and the United States,22 with a higher rate in the north. The age-adjusted increase in incidence that has been observed in several countries over the past 50 years appears to have leveled off in some of these countries. The incidence increases with socioeconomic difficulties, decreased winter sunlight, and water fluoridation. Fractures are more frequent in winter months. This seasonal trend could be due to altered neuromuscular coordination and vitamin D deficiency in winter months, because most fractures occur indoors and thus cannot be explained only by slippery winter conditions.22 About 80% of hip fractures occur in women, because the age-adjusted incidence in men is two times lower than in women, and more elderly women are alive than are elderly men.
Costs
In the United States, direct medical expenditures for osteoporotic fractures were estimated at $20 billion in 2000,40 two-thirds of which are accounted for by hip fracture alone. An important determinant of rising cost is increased age. The estimated cost for care in the United States during the first year after a hip fracture is $21,000 per person and is much higher in women older than 80 years. If the cost of the subsequent nursing home is attributed to the hip fracture, the cost of care for those who stay in nursing homes after 1 year is $93,378 per person. The average cost for vertebral fracture during the first year is $1200 per patient, and it is $800 for Colles’ fracture. Costs are expected to double in 2050, due to the increasing number of fractures resulting from the aging population.
Osteoporosis: A Neglected Disease
It has been shown in recent years that even after marketing of several effective therapies for osteoporosis, most patients who have sustained an osteoporotic fracture (forearm fracture, vertebral fracture, and even hip fracture) do not receive adequate secondary prevention for future fractures, whereas the risk of repeated fractures is markedly increased, including that of second hip fracture.41
Pathogenesis
Clinical Risk Factors
Clinical risk factors can shed light on the pathophysiology of fragility fractures. Bone mineral density is a key risk factor for fracture,42 but numerous studies have evaluated other risk factors for fractures.16,43–50 The use of such factors (Table 13-1) to identify high-risk subjects is frequently advocated. Different types of fractures have different risk-factor profiles.44
Table 13-1
Risk Factors for Hip Fracture With and Without Adjustment for Calcaneal Bone Density and History of Prior Fracture
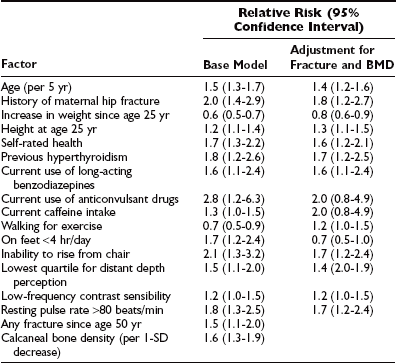
Data from Study of Osteoporotic Fractures.
Estrogen Deficiency
Estrogen deficiency has been associated with osteoporosis since first suggested by Fuller Albright in the 1940s. It is the main cause of bone loss in women after menopause. Premature menopause—natural or surgically induced—extends the period during which a woman is exposed to a hypogonadal state, thus increasing the total duration of bone loss occurring after menopause.51 Similarly, late menarche and primary or secondary amenorrhea also increase the risk for osteoporosis.52 Luteal deficiency in premenopausal women has been suggested to be associated with bone loss,53 but the evidence is still controversial. Estrogen deficiency is also associated with bone loss during the 3 to 4 years preceding the actual menopause—the perimenopause—with a rate similar to that observed in the early postmenopausal years.54 It is likely that women with the lowest concentrations of residual estrogen have faster bone loss and fracture more often, although this might be confounded by the effect of body weight or other sex steroids.55–57 Also, women receiving aromatase inhibitors as adjuvant therapy for breast cancer lose bone more rapidly and sustain an increased rate of fragility fracture.58
Other Factors
Many factors increase the risk of fracture. For instance, the Study of Osteoporotic Fractures49 identified 16 independent risk factors for hip fracture in addition to low BMD (see Table 13-1). Numerous studies have described risk factors such as female gender, white or Asian race, age, previous fractures, thinness, cigarette smoking, family history of hip fracture, use of sedative hypnotics, and impairment in visual and neuromuscular function. Lack of physical activity may also be an important cause of bone loss,59,60 and it has been hypothesized that some of the age-related bone loss and the burden of skeletal fragility result from a decline in physical activity, in particular, in Western societies. Black people have less osteoporosis and fewer fractures than white and Asian people do. Excessive alcohol consumption increases the risk of fractures, whereas moderate intake might exert a protective effect. Undernutrition in the elderly may contribute to bone loss, the risk of falling, or the response to injury.61 It is widely believed that inadequate calcium intake throughout life is a significant risk factor.62
In a cohort of 7575 French women aged 75 years or older, the risk of subsequent hip fracture was significantly increased (even after adjustment for femoral hip BMD) in women with a slow gait speed, in women who had difficulty performing a tandem (heel-to-toe) walk (i.e., neuromuscular impairment), and in women with visual acuity less than 2/10.63 The incidence of hip fracture among women classified as being at high risk because of both a high fall risk status and a low BMD was found to be 29 per 1000 women years. It was 11 per 1000 in women at risk because of either a high fall risk status or low BMD, and the risk was 5.4 per 1000 women years in those at low risk according to both criteria. Even with this combined approach, about a third of women with a hip fracture had not been identified as being at high risk, thus indicating that other factors are important in the pathogenesis of hip fracture. Although these clinical risk factors can be easily assessed with a rapid questionnaire and physical examination, they are probably of limited value in younger women. A personal history of hip fracture is still predictive, but its prevalence is much lower in women younger than 65 years. Fall-related factors have not been tested but are likely to be poor discriminants in younger and healthier women.
Candidate risk factors have been examined by a WHO working group in individual data meta-analyses of 12 prospective population-based cohorts. Data were collected from 60,000 men and women participating in the Rotterdam study in the Netherlands; the European Vertebral Osteoporosis Study and the European Prospective Osteoporosis Study; the Canadian Multicenter Osteoporosis Study; the Dubbo Osteoporosis Epidemiology Study in Australia; the EPIDOS and OFELY cohorts in France; and cohorts from Rochester, Minnesota; Sheffield, England; Kuopio, Finland; Hiroshima, Japan; and Gothenburg, Sweden. Several risk factors have been selected on the basis of the possibility to modify them and ease of use in clinical practice: BMD at the femoral neck, low body mass index, a prior fragility fracture, glucocorticoid exposure, a parental history of hip fracture, smoking, excessive intake of alcohol, and rheumatoid arthritis.64–68 Those factors are modeled to provide individual probabilities of fracture that can be easily calculated using the computer-driven WHO fracture risk assessment tool called FRAX (available at Internet websites such as http://www.shef.ac.uk/FRAX/).69
Peak Bone Mass
Blacks achieve higher peak bone mass than whites do, who have greater peak bone mass than Asians, particularly Japanese. Twin studies have shown a strong correspondence of peak bone mass in monozygotic twins, who have closer concordance in BMD than dizygotic twins.70 Which genes are the most important is still unknown. It has been reported by Morrison et al. that polymorphism of a noncoding sequence of the vitamin D receptor gene was associated with peak bone mass variance in monozygotic twins, and that these polymorphisms accounted for most of the genetic effect.71 This association has not been confirmed by most subsequent studies72–74 but has initiated searches for other candidate genes, including the estrogen receptor, interleukin 6 (IL-6), transforming growth factor β (TGF-β), and type I collagen. For example, the collagen Iα1 Sp1 polymorphism is moderately associated with BMD and fracture, as shown in a meta-analysis of 13 studies involving 3642 patients.75 More recently, some variants of LRP5 have been found associated with BMD and fracture.76–78 Even if many advances have been made in understanding the role of genetic factors in the last 10 years, a great deal of additional research is required to identify the genes relevant to the pathogenesis of osteoporotic fracture. Indeed, most studies so far have been underpowered. In the future, it is likely that a combination of large-scale studies and technologic improvements such as genomewide associations will help identify candidate genes in the regulation of bone strength.77–79
Dietary calcium intake may be important for attaining optimal peak bone mass. Three placebo-controlled trials in children or adolescents, including one performed in twins, have shown a modest but significant increase in BMD in those receiving calcium supplementation.80–82 Children with very low calcium intake are more likely to benefit from an increase in calcium, preferably from a dietary source.
Other factors may be important, such as those influencing the progression of puberty. Exercise during growth may contribute to the level of peak bone mass. It has been shown that intensive exercise before puberty may enhance bone acquisition that might persist in adulthood,83 but the role of exercise in the normal physiologic range is unknown. In girls, areal BMD gains stop around ages 16 to 20 and in boys around ages 20 to 22. Bone width, however, will continue to increase throughout life, even after linear growth cessation, to maintain bending strength despite areal BMD decline.84 Diet, physical activity, and toxic substances exposure, including maternal cigarette smoking during pregnancy (programming), may also influence the level of peak bone mass.85,86
Bone Loss With Aging
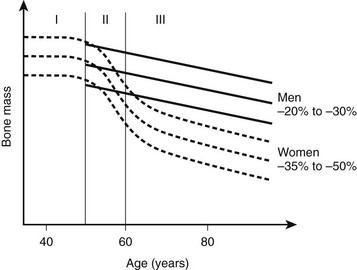
Changes in bone mass as a function of age are presented in Fig. 13-2. Throughout life, women lose 35% to 50% of their bone mass, depending on the skeletal site. Although osteoporosis is a systemic disease affecting the whole skeleton (with the exception of the bones of the skull and face), the pattern of bone loss differs slightly and depends on the type of bone. It has been generally believed that bone loss starts around menopause. In fact, a recent study showed that trabecular bone loss—assessed at the distal forearm with high-resolution peripheral quantitative computed tomography (HR-pQCT)—starts in young adults of both sexes, accounting for a third of the total trabecular bone loss over the entire life.87 Menopause induces accelerated bone loss within 5 to 8 years, followed by a linear rate of bone loss that may accelerate after the age of 75 years.88 Because of a much slower rate of bone remodeling, cortical bone loss may start later, but the total amount of cortical bone mass that is lost in women in their 80s is probably similar to the amount of cancellous bone lost. The proportion of overall bone loss related to menopause and to the estrogen-independent aging process is debated, but clearly bone loss in the elderly is still under the influence of estrogen deficiency and can be prevented by hormone replacement therapy (HRT).89
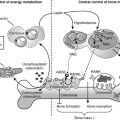
Two major mechanisms underlie bone loss in women. First, an age-related decrease in osteoblast activity occurs and leads to an imbalance between the amount of bone resorbed and the amount of bone formed within a remodeling unit (BMU). Second, menopause induces a marked increase in the number of remodeling units activated within the cancellous and cortical bone envelopes per unit of time, which will amplify the small deficits observed at each BMU. This overall increase in bone turnover peaks within the first 2 to 3 years after menopause but persists throughout life, even in women in their 80s, as shown by the sustained increase in bone markers of resorption and formation in elderly postmenopausal women. The mechanism by which estrogen deficiency results in increased bone resorption and bone loss is not yet completely understood, but evidence indicates that estrogens act indirectly through cytokines and growth factors such as IL-1α, IL-1 receptor antagonist, tumor necrosis factor α (TNF-α), IL-7, and TGF-β. Estrogen also stimulates production of osteoprotegerin—a decoy receptor that is a potent inhibitor of osteoclast activity—in osteoblastic cells and is also an inhibitor of receptor activator of nuclear factor κB (RANKL) and RANKL-stimulated osteoclastogenesis, thus explaining a large part of the antiresorptive action of estrogen in bone.90–92 Estrogen results in an increase in IL-7 in target organs such as bone, thymus, and spleen, at least in part through decreases in TGF-β and increased IGF-1 production, leading to a first wave of T-cell activation.93 Those activated T cells release IFN-γ, increasing antigen presentation by dendritic cells and macrophages as a result of up-regulation of MHCII expression through the transcription factor CIIA.94 Estrogen deficiency also amplifies osteoclastogenesis and T cell activation by down-regulating antioxidant pathways, resulting in an upswing in ROS, which in turn stimulates antigen presentation and TNF production by mature osteoclasts. As a result, T-cell activation is again amplified and promotes release of the osteoclastogenic factors RANKL and TNF. In addition, TNF stimulates osteoblastic RANKL and M-CSF production, in part by up-regulating IL-1, thus leading to osteoclastic formation.95,96 TNF and IL-7 also directly blunt bone formation through their effects on osteoblasts.
Bone turnover in osteoporosis, however, varies widely with patients with high, normal, and low bone turnover.97 In osteoporosis, trabecular plates are perforated and transform into rods, leading to thinning of trabeculae and loss of connectivity, with increased risk of fracture as a result. Cortices also thin because of reduced periosteal apposition failing to compensate for bone loss at the endosteal surfaces. Modifications in collagen matrix (e.g., variations in the proportions of various crosslinks and isomerization of C-telopeptide) and the degree of mineralization may also influence bone strength.
Serum intact parathyroid hormone (PTH) levels increase with advanced age in both genders. This hyperparathyroidism is secondary to the calcium and/or vitamin D deficiency commonly found in the elderly, especially in those institutionalized, and may contribute to bone loss in both women and men. A classification of osteoporotic fractures based on clinical features and underlying mechanisms has been used. Type I osteoporosis includes mainly wrist and vertebral fractures, occurring mostly in women younger than 70 years, and is predominantly due to loss of cancellous bone because of estrogen deficiency. Type II osteoporosis includes mainly hip fractures that occur in both elderly men and women as a result of cancellous and cortical bone loss driven primarily by secondary hyperparathyroidism. It has been proposed that this model should be unitary, with bone loss caused by estrogen deficiency in both phases and in both genders.43 In this model, the accelerated phase of bone loss after menopause involves a disproportionate loss of cancellous bone, mainly due to estrogen deficiency. Compared to normal postmenopausal women, osteoporotic women seem to have impaired responsiveness to postmenopausal low levels of estrogen. The subsequent phase of slow bone loss involves proportionate losses of cancellous and cortical bone and is associated with secondary hyperparathyroidism. In aging men, low testosterone and estrogen levels contribute to bone loss. In the elderly, decreased bone formation also contributes to bone loss, probably due to stem cells which tend to differentiate toward the adipocyte lineage rather than the osteoblastic lineage.
Bone Quality
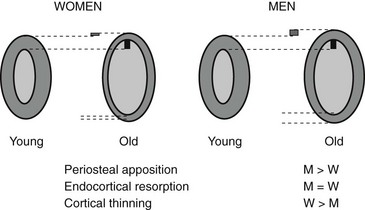
Bone strength is determined by its material composition and structure.98 Bone must be stiff to resist deformation, thereby making loading possible, and it must also be flexible to absorb energy by deforming. Bones shorten and widen when compressed, and lengthen and narrow in tension. When bone is brittle (i.e., too stiff and unable to deform a little), the energy imposed during loading leads to structural failure, initially by the development of microcracks and then by complete fracture. When bone is too flexible and deforms beyond its peak strain, it breaks. Long bones are mainly made of cortical bone, favoring rigidity over flexibility, whereas mainly trabecular vertebrae can absorb more energy by deforming more before breaking. Differences in bone dimensions explain the better tolerance to load in men compared with women and in some races compared with others.99 Men and women generally have similar vertebral trabecular volumetric density and similar vertebral heights. The larger vertebral cross-sectional area in men contributes to sex-based differences in bone strength. Black people tend to have wider but shorter vertebral bodies and higher trabecular volumetric density than do white people, owing to thicker trabeculae. The geometry of the hip (hip axis length) influences the risk of hip fractures. Femoral neck length is a predictor of future fracture, and individuals with particularly long femoral necks are more likely to have hip fractures. This feature has been noted in white women from the United States100 and France.101 The increase in height over the second part of the 20th century could partly explain the increase in the incidence of hip fracture during this period.102 Bone modeling (construction) produces a change in size and shape of bone when new bone is deposited without previous bone resorption. During bone remodeling (reconstruction), resorption by osteoclasts precedes bone formation by osteoblasts. Bone modeling and remodeling modify the external size and contours of bone and its internal architecture by the deposition or removal of bone from the surface of bone. They result in cortical and trabecular thickening during growth and thinning during aging. Bone loss occurring with menopause and aging is associated with disturbances in bone microarchitecture.103 Osteoclastic resorption leads to focal perforations in cancellous bone plates, which results in loss of connection of the horizontal plates, along with detachment of vertical bars throughout the bone marrow cavity. Thus the probability of crush fracture is increased in bones rich in cancellous bone, such as vertebrae. The thickness of cancellous bone plates is about 100 to 150 µm, and osteoclasts dig resorption defects of 50 to 100 µm during normal remodeling. Perforations in cancellous plates can be a consequence of increased osteoclast activity and lead to impairment in bone mechanical properties and therefore to an increased risk of fracture. With continued remodeling, trabeculae perforate and some disappear completely. Active endocortical and intracortical remodeling “trabecularizes” cortical bone (i.e., creates cortical bone with more surface area).104 Older, more densely mineralized interstitial bone distant from surface remodeling has a reduced number of osteocytes and accumulates microdamage.105 Both cortical thinning and cortical porosity reduce the resistance of bone to the propagation of cracks, leading to complete fracture in case of a fall. The loss of bone strength due to cortical thinning and porosity is partially offset by deposition of new bone on the external surface (periosteal apposition), so cortical thickness is better maintained than would occur without periosteal apposition (Fig. 13-3).106,107 However, details of changes in periosteal apposition with aging—along with the effects of site, sex, and race—are difficult to evaluate prospectively, given the small magnitude of periosteal apposition throughout adult life. The notion that periosteal apposition is greater in men than in women remains controversial.108 More recent studies suggest that sex-based differences may occur at some but not all sites.99,109 Sex-based differences may also vary across races.
Role of Falls
Skeletal determinants of bone strength do not reflect all the factors related to fracture risk. For any given bone density, the risk of fracture is greater in the elderly. The increased frequency of falling, the type of fall that occurs among the elderly, and the loss of protective soft tissue may all explain the larger contribution of age and the less important role of bone mass in the elderly. Among postmenopausal women in the United States, the frequency of at least one annual fall rises from about one in five at 60 to 64 years of age to one in three at 80 to 84 years of age.110 Propensity for falling has been assessed in several studies48 with parameters such as gait speed, inability to rise from a chair without using one’s arms, and of course, visual impairment. These parameters are associated with a risk of falling. The increase in falling is nevertheless not sufficient to account for the increasing incidence of fractures, because only 5% to 6% of falls result in a fracture (1% of hip fractures and 4% to 5% for other fractures). Fracture risk is also related to the seriousness of the trauma on the femur and the direction of the fall. Indeed, the risk of hip fracture is 13 times higher when the impact is delivered directly over the hip.110 A great amount of force can be dissipated by the thickness of soft tissue over the femur, and patients with low fat mass may be at higher risk of hip fracture.
Clinical Features
Wrist fractures or distal forearm fractures are mainly of two types: a Colles’ fracture is a consequence of dorsal angulation, and the less frequent type, a Smith fracture, results from volar angulation. These fractures usually have a favorable outcome, but some patients suffer from algodystrophy, osteoarthritis, or neuropathies.36
Two types of hip fracture are cervical and trochanteric. The femoral trochanter is composed of more cancellous bone than the femoral neck is. It seems that the predictive value of BMD and ultrasonic parameters may be higher for trochanteric fracture than for cervical fracture.111 Hip fractures are associated with more morbidity and mortality, and the prefracture functional state is restored in less than half of patients (see earlier in Epidemiology).
Vertebral fracture requires separate consideration. Vertebral fracture results in back pain, which often appears after some strain on the back, such as lifting a suitcase or working in the garden. The pain is commonly severe and often confines the patient to bed. This pain localizes to the back and rarely radiates to the legs; cord compression is exceptional, and in this latter case, one must consider other diagnoses such as metastases or myeloma. Pain from the fracture usually eases over a period of 6 to 8 weeks and disappears. Nevertheless, it has been estimated that about two-thirds of vertebral fractures are not diagnosed at the time they occur because they are considered by patients or physicians as nonspecific back pain. Loss of height is another main feature of vertebral fracture, but often patients do not report it spontaneously, so it needs to be sought by asking about the individual’s height in early adulthood and measuring the current height. Therefore, it is worthwhile to record the patient’s height at each clinical visit. Height loss of 3 cm or more should alert the physician to the possibility of a new vertebral fracture. Detection of asymptomatic vertebral fractures is clinically relevant because they are associated with a threefold to fivefold increased risk of new vertebral fractures, independent of the level of BMD. In addition, the incidence of new vertebral fracture in the year following a vertebral fracture has been estimated to be 20%.112 Kyphosis is generally a consequence of vertebral crush fractures in the thoracic spine and sometimes results in decreased lung capacity. Vertebral fractures in the lumbar spine result in decreased abdominal volume, which causes protrusion of the abdomen and impingement of the costal margin on the iliac crest. This iliocostal contact provokes pain and a grating sensation.
Diseases and Treatments Contributing To Osteoporosis
A variety of disorders are associated with an increased risk of osteoporosis (Table 13-2). In most of these cases, osteoporosis appears to be multifactorial and cannot be attributed to only a specific disease (“secondary osteoporosis”). We will discuss only the most common disorders associated with osteoporosis.
Table 13-2
Glucocorticoid-Induced Osteoporosis
Consistent evidence indicates that glucocorticoids impair bone formation113 by directly inhibiting osteoblastic activity. Osteoblastic synthesis of type I collagen, osteocalcin, and alkaline phosphatase is decreased; the production of insulin-like growth factor 1 (IGF-1) and IGF-2 is also inhibited by glucocorticoids. Measurement of serum osteocalcin provides a good index of this osteoblast inhibition by glucocorticoids. The effect of corticosteroids on bone resorption is less clear. High doses have been associated (in some but not all studies) with an increased rate of bone resorption resulting from decreased intestinal calcium absorption, increased urine calcium excretion, decreased osteoprotegerin levels, and hypogonadism. Glucocorticoids contribute to adrenal and gonadal deficiency because of inhibition of pituitary hormone secretion, with a dose-dependent reduction in free testosterone in men. Consequently, exposure to supraphysiologic doses of glucocorticoids leads to substantial and rapid bone loss in most individuals, especially in the first year of therapy and with doses above 7.5 mg of prednisone. Thus fractures are very common, especially vertebral fractures, which occur in 30% to 50% of corticosteroid-treated patients; the risk of vertebral and hip fractures is generally more than doubled.114 Patients undergoing organ transplantation may have a higher fracture risk than other steroid-treated patients, perhaps because of the preexisting condition and the osteopenic effect of other immunosuppressive drugs. One of the main problems with corticosteroid treatment is the minimal effective dose to avoid side effects. Some evidence suggests that corticosteroid-induced inhibition of bone formation occurs even with low doses of inhaled corticosteroids, with a marked decrease in serum osteocalcin.115 With oral corticosteroids, the increase in fracture risk starts as low as 2.5 mg/day.116 Data from cross-sectional and prospective studies suggest that the bone mass of asthmatic patients treated with inhaled corticosteroids is lower than that of controls, in a dose-dependent fashion.117 The increased fracture risk rapidly offsets on cessation of therapy.118 BMD values are not good predictors of fracture risk in patients on corticosteroids, since the risks start to rise with T score around 0.0.119
Endocrine Diseases
Patients with hyperthyroidism are subject to high-turnover osteoporosis with or without mild hypercalcemia, and they have an increased risk of fracture.49 The decrease in BMD in thyrotoxic patients is reversible after treatment of the hyperthyroidism.120 The deleterious effects of supraphysiologic doses of thyroid hormone on bone may also occur (but to a lesser extent) in patients who receive suppressive doses of thyroxin for the treatment of thyroid carcinomas and nontoxic goiter. Conversely, hypothyroidism does not seem to significantly affect BMD. The potential role of calcitonin deficiency in bone loss after thyroidectomy is unclear.
Studies by DXA in mild primary hyperparathyroidism have shown that bone density is reduced in regions of cortical bone but is normal in areas of cancellous bone. This decrease in BMD is likely to increase the risk of fracture. Skeletal recovery after surgical treatment of parathyroid adenoma is very significant, with an increased BMD of about 6% at the femoral neck and 8% at the spine 1 year after surgery.121This improvement is sustained 10 years after surgery. These patients were the more severely affected patients whose surgery was dictated by the guidelines of the National Institutes of Health.
Other Conditions
Rheumatologic conditions such as rheumatoid arthritis and ankylosing spondylitis are associated with osteoporosis and an increased rate of fracture. Pregnancy is very uncommonly associated with bone loss in the last trimester. At intakes higher than 40 g of ethanol per day, alcoholism increases the risk of osteoporotic fractures, particularly in men. In addition to the direct effect of alcohol on osteoblasts, the role of hypogonadism and liver disease may be of importance. Caffeine consumption has also been associated with reduced bone mass and hip fracture risk in some studies.49,59 Medroxyprogesterone acetate is a progestational agent that suppresses gonadotropin, thus causing anovulation and hypoestrogenemia. Its prolonged use is associated with decreased spinal bone density in about 10%. Other drugs, such as some anticonvulsants, methotrexate, heparin, and cyclosporine, may increase the risk of osteoporosis. More recently, aromatase inhibitors58 and the thiazolidinediones used in the treatment of type 2 diabetes have been found to increase fracture risk.122
Osteoporosis In Young Adults
A few young adults have osteoporotic fractures corresponding to either mild osteogenesis imperfecta or idiopathic osteoporosis. Osteogenesis imperfecta is an inherited syndrome characterized by fragile bones and recurrent fractures that can lead to skeletal deformities.123 Inheritance and phenotypic expression are very heterogeneous. Clinical features of osteogenesis imperfecta also include short stature, blue sclerae, dentinogenesis imperfecta, hearing loss, scoliosis, and joint laxity. Osteogenesis imperfecta generally results from mutations of type I collagen.
Idiopathic juvenile osteoporosis is a very rare condition123 of children and adolescents before puberty. This disease does not seem to be of familial origin. Vertebral fractures usually occur over a 2- to 4-year period. In severe cases, patients may have deformities of the extremities and kyphoscoliosis.
Idiopathic adult osteoporosis is more often recognized because bone densitometry is more widely available. Although the condition may resemble mild osteogenesis imperfecta, these patients do not have dentinogenesis imperfecta, blue sclerae, or hearing loss. They do, however, have joint laxity and mild scoliosis, and a familial history is sometimes found.123
Osteoporosis In Men
Fractures are more prevalent in men than in women from childhood to middle life, probably because of a higher incidence of trauma. After 40 years of age, fractures are less common in men than in women, but the incidence of fracture as a result of mild trauma increases with aging. The incidence of hip fracture in men rises exponentially with age, as in women. The sex ratio (female to male) is about 2 : 1 in northern Europe, but it may vary in other areas and reflects the lower life expectancy of men. Mortality related to hip fracture is significantly higher in men than in women. Although vertebral deformities are common in men, many of them are unrelated to osteoporosis. Vertebral fractures in men are associated with height loss, kyphosis, and increased disability, as in women. The incidence of osteoporotic vertebral fractures seems to be half that in women.23 Vertebral fracture in men is associated with lower BMD than in controls.124 The incidence of limb fracture begins to rise at a later age in men than in women.
As in women, osteoporosis in men can result from an inadequate peak bone mass and/or accelerated bone loss. As discussed later, gonadal status may be critical for the achievement of peak bone mass in the male. Age-related bone loss is less pronounced in men than in women, around 15% to 20% from 30 to 80 years of age. The mechanisms underlying bone loss with aging in men are unknown, but some evidence indicates decreased osteoblastic activity in males with idiopathic osteoporosis.125 In elderly men, secondary hyperparathyroidism may contribute to bone loss.
The same risk factors for fragility fractures have been described in men and women and include smoking and excessive alcohol intake; these factors are often combined in a man with osteoporosis. About 50% of men with osteoporosis are considered to have secondary osteoporosis. The most common causes are chronic glucocorticoid therapy, hypogonadism, alcoholism, gastrectomy, and other gastrointestinal disorders. Male hypogonadism has a major influence on the occurrence of osteoporosis. Peak bone mass may be impaired by disorders of puberty, and men with abnormal or delayed puberty have reduced bone mass.126 Estrogen status is believed to be critical for the acquisition of peak bone mass in men since the observation that aromatase deficiency in a man, resulting in estrogen deficiency, led to the absence of epiphyseal closure and to osteopenia, abnormalities that can be successfully treated with estrogen.127 Androgens are also essential for the maintenance of bone mass in adult men insofar as hypogonadism in men is associated with low bone mass. Osteoporosis is encountered in many forms of hypogonadism, such as hyperprolactinemia, castration, anorexia, hemochromatosis, and Klinefelter’s syndrome. Prolonged abuse of alcohol has detrimental effects on the skeleton, with reduced bone mass resulting mainly from impaired osteoblast activity, nutritional deficiencies, and hypogonadism. Gastrointestinal disorders, particularly gastrectomy, are also associated with osteoporosis in men. Some but not all studies have linked nephrolithiasis or hypercalciuria with reduced bone mass, but their impact on the mechanisms of bone loss remains unclear.
The absolute risk of various osteoporotic fractures is similar in men and women with the same BMD level, but fewer men than women have a low BMD, and men are generally older if they reach such low levels.128 This difference in age and biomechanical factors such as bone size may also modulate the influence of BMD on fracture risk. There is currently no consensus on T scores that may be used as thresholds for therapeutic decision making in men. The −2.5 cutoff T score (if the reference values are obtained from a male population) is often used, given the similar relationship between BMD and fracture in men and women. The FRAX calculator69 can also be used in men to assess the individual probability of fracture, but the T score to enter in the software must be calculated using a reference curve obtained with women data (NHANES III database).
Diagnosis
Investigation of patients with osteoporosis is intended to fulfill the following purposes:
• To establish the diagnosis and eliminate the possibility of conditions mimicking osteoporosis
• To identify factors contributing to osteoporosis
• To determine the prognosis of the disease, with quantification of bone mass, identification of previous fractures, identification of factors that influence the risk of fractures independently of bone mass, and assessment of the rate of bone loss with biochemical markers
• To select the most appropriate treatment
• To obtain baseline measurements that can be useful for monitoring treatment efficacy
Differential Diagnosis and Causes of Osteoporosis
Evaluation of a patient suspected of having osteoporosis includes an adequate history and physical examination, and it must assess the potential causes of secondary osteoporosis and diseases mimicking osteoporosis. Biological abnormalities are commonly found in osteoporotic women, but few major medical problems are identified with screening,129 and routine laboratory testing may not be cost effective.130 However, a biochemical profile is necessary in patients with vertebral fractures, including assays of serum calcium, phosphate, creatinine, 25-hydroxycholecalciferol [25(OH)D], and alkaline phosphatase; serum protein electrophoresis; test for proteinuria; urine calcium; and in some cases, urine protein immunoelectrophoresis and blood cell count. Thyroid-stimulating hormone measurement and, in men, total and bioavailable testosterone and prolactin assays may be useful in some cases. In the elderly, 25(OH)D and PTH assays are appropriate when vitamin D deficiency is suspected. Assessment is guided by the clinical findings because some patients with apparent primary osteoporosis turn out to have mild hyperparathyroidism, osteomalacia, systemic mastocytosis, or late appearance of atypical osteogenesis imperfecta.
Radiologic Evaluation
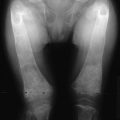
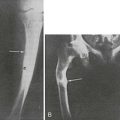
The estimation of spine BMD on conventional radiographs is insensitive and inaccurate because the subjective assessment is dependent on radiographic exposure, patient size, and film-processing techniques. BMD has to decline by as much as a third before it can be detected on plain radiographs. The radiographic manifestation of osteopenia of the axial skeleton includes increased radiolucency of the vertebrae, sometimes vertical striation, framed appearance of the vertebrae (“empty box” or “picture framing”) and increased concavity of the vertebral endplates resulting from protrusion of the intervertebral disk into the weakened vertebral body (Fig. 13-4).131 Those signs of osteopenia, however, do not reflect the bone mineral status accurately, so they have been replaced by bone densitometry.
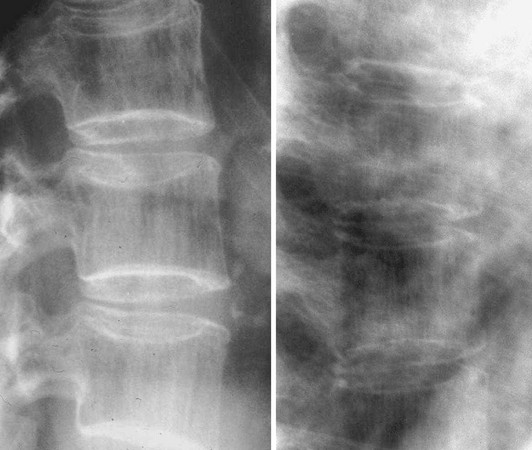
FIGURE 13-4 Reinforcement of the vertical primary trabeculae and loss of the horizontal trabeculae in postmenopausal osteoporosis led to vertical striations on a radiograph of the lumbar and thoracic spine, combined with an overall radiolucency and “empty box” appearance.
Radiographs are important tools to confirm fractures and their potential etiology. Plain radiographs with anteroposterior and lateral views are required for both the thoracic and lumbar spine. Vertebral fractures include wedge deformities, endplate (“biconcave”) deformities, and compression (or crush) fractures (Fig. 13-5). Some vertebral deformities unrelated to osteoporosis include Scheuermann’s disease, malignancies, and osteomalacia. The diagnosis of mild vertebral fracture can be difficult because of the overlap with the normal range of vertebral body shape. Algorithms to define vertebral fractures that were developed for epidemiologic studies and clinical trials have led to a consensus proposal for the radiologic diagnosis of vertebral fractures.132
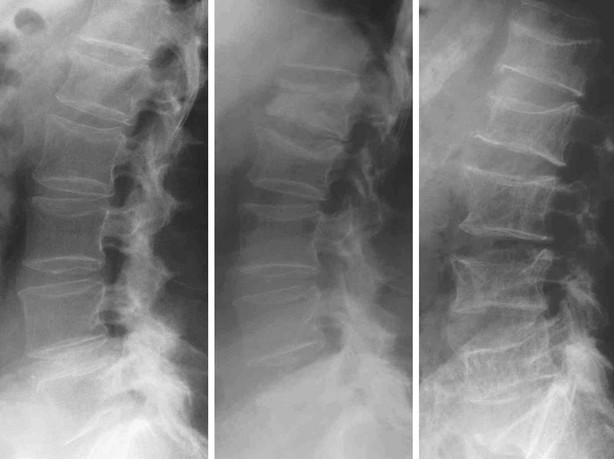
FIGURE 13-5 Progressive evolution of spinal osteoporosis in a postmenopausal woman with vertebral fracture cascade, at baseline, 10, and 20 years.
Vertebral fracture evaluation, however, may be challenging because of a considerable variability in fracture identification if clinicians or radiologists interpret radiographs without specific training. Several qualitative and quantitative methods have been developed to standardize visual vertebral fracture evaluation, but the most widely used currently is Genant’s semiquantitative assessment.133 Vertebral fractures are distinguished from other nonfracture deformities, and the severity of the vertebral fracture is assessed by visual determination of the magnitude of vertebral height reduction and morphologic change. The type of deformity (wedge, biconcave, compression) is not linked to the extent of vertebral height reduction—that is, the grading of the fracture. Vertebrae from T4 to L4 are graded on visual inspection as normal (grade 0), mildly deformed (grade 1, 20% to 25% in anterior, middle, and/or posterior height), moderately deformed (grade 2, 25% to 40% in anterior, middle, and/or posterior height), and severely deformed (grade 3, >40% in anterior, middle, and/or posterior height) (Fig. 13-6). Semiquantitative interpretation has excellent intraobserver and interobserver precision, provided careful training and standardization have been applied.133,134
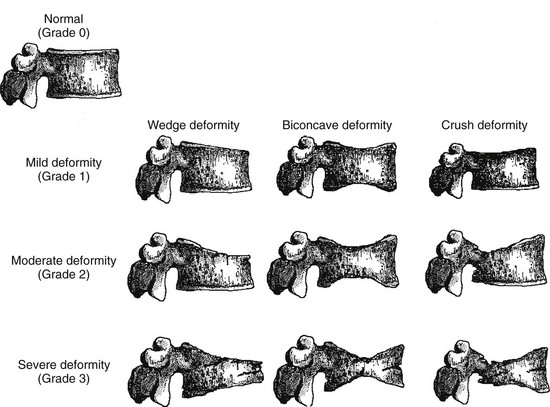
FIGURE 13-6 Schematic representations of wedge, biconcave, and crush vertebral deformities with grading normal to severe deformity. The figure illustrates the semiquantitative visual grading system of Genant. (Drawn by C.Y. Wu.)
The axial skeleton is not the only site where changes due to osteoporosis can be observed. Widening of the medullary canal and thinning of the cortices is commonly described in older individuals as a result of longstanding endosteal bone resorption in the appendicular skeleton (Fig. 13-7). Conventional radiographs can also be used to assess the bone structure, with image procedures relying on texture or fractal analyses.135
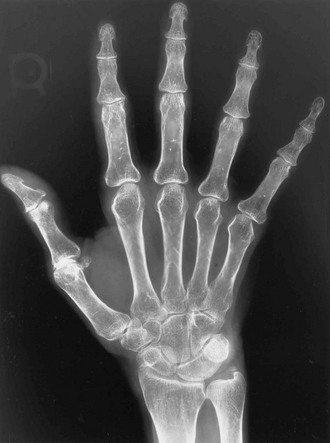
FIGURE 13-7 PA radiograph of the hand in an elderly osteoporotic woman showing severe diffuse cortical thinning, principally by endosteal resorption.
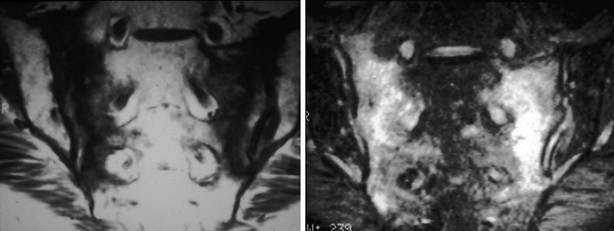
A variety of diseases and medications are associated with bone loss, but some differences can be seen in the appearance of those secondary osteoporoses compared with postmenopausal or senile osteoporosis. The characteristic radiographic appearance of Cushing’s or corticosteroid-induced osteoporosis, in the extreme, consists of a marginal condensation of the fractured vertebral bodies, resulting from exuberant callus formation (Fig. 13-8). Primary hyperparathyroidism may affect all bone surfaces and result in subperiosteal, intracortical, endosteal, subchondral, and trabecular bone resorption. Those signs, however, are seldom encountered because most patients with primary hyperparathyroidism have mild forms of the disease. In osteomalacia, radiographic abnormalities include osteopenia, non-sharp delineation of cortical bone, deformities, and stress and true fractures. Hyperparathyroidism secondary to renal insufficiency associates vertebral osteosclerosis, amyloid deposits, avascular necrosis, and vascular calcifications (Fig. 13-9A,B). The typical radiographic appearance of steroid-induced osteoporosis consists of a marginal condensation of the fractured vertebral bodies secondary to exuberant callus formation. In multiple myeloma, the radiographic appearance of spine radiographs can imitate osteoporosis, and other diagnostic procedures like skull radiographs (Fig. 13-10) or protein electrophoresis will make the diagnosis. Additional imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and bone scintigraphy may be useful in the differential diagnosis of the various conditions associated with osteoporosis or vertebral fractures (e.g., trauma [Fig. 13-11], multiple myeloma [Fig. 13-12], storage diseases, leukemia, sickle cell anemia, and thalassemia). Bone scintigraphy or MRI are the ideal techniques to diagnose sacrum stress fracture (Fig. 13-13) that may be encountered in elderly patients with osteoporosis or osteomalacia.
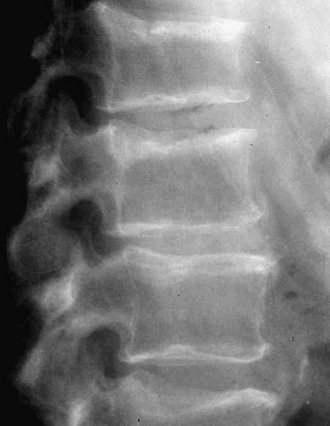
FIGURE 13-8 Lateral radiograph of the spine showing diffuse osteoporosis, dense thickened endplate, or marginal sclerosis, all characteristic of Cushing’s- or steroid-induced secondary osteoporosis.
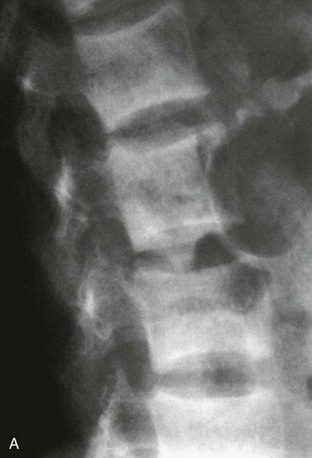
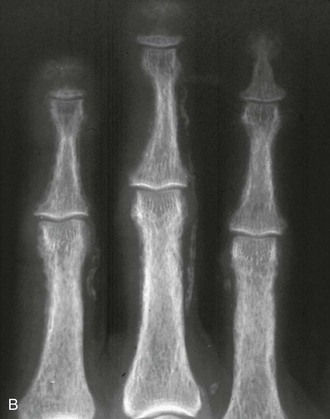
FIGURE 13-9 A, Lateral radiograph of the lumbar spine showing typical changes of renal osteodystrophy with rugger jersey spine. B, PA radiograph of the fingers shows tuft and subperiosteal resorption and vascular calcification.
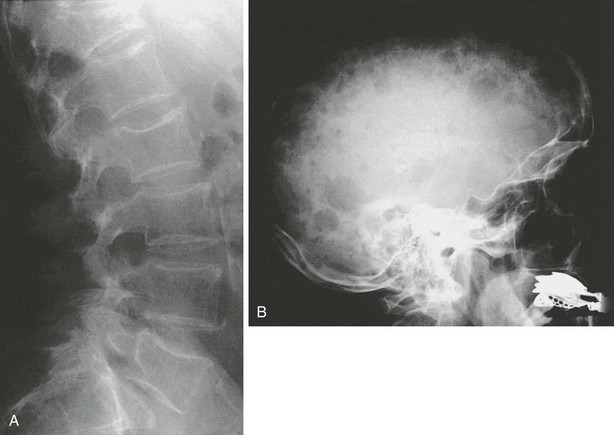
FIGURE 13-10 Changes in the spine in multiple myeloma (A), here seen with increased radiolucency and vertebral deformities, may easily be confused with osteoporotic changes. However, additional typical lesions at other sites (besides clinical features and relatively typical laboratory values) may reveal the nature of the underlying disease. In this example, multiple osteolytic lesions in the skull (B) support the diagnosis of multiple myeloma.
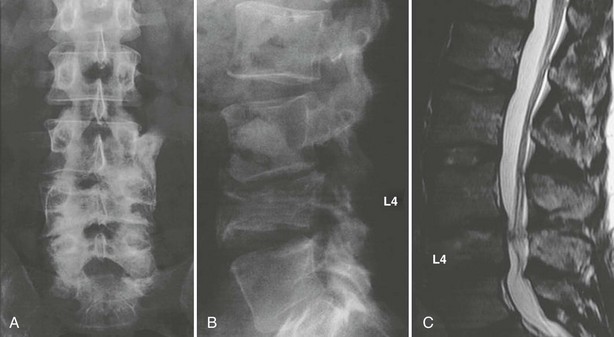
FIGURE 13-11 Conventional radiographs of the lumbar spine in the anteroposterior (A) and lateral (B) views reveal an old traumatic fracture of the fourth lumbar vertebra, resulting in instability of the lumbar spine and modest spinal stenosis, as shown on the corresponding MRI (C).
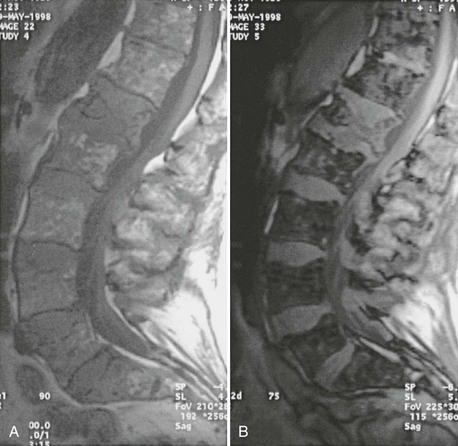
FIGURE 13-12 MRI of the spine in patients with multiple myeloma may give information on the infiltration of spinal bone marrow. In this patient, a diffuse infiltration pattern may be seen on T1-weighted spin echo (A) and gradient echo (B) images. In addition, the patient has a pathologic fracture of the first lumbar vertebra.
Bone Mass Measurements
Measurement of bone mass is a critical step in the investigation of an osteoporotic patient to (1) confirm the reduction in bone mass and (2) assess the magnitude of bone loss and therefore the risk of further fracture. Some of the terms may be confusing, so they need to be defined.136 What is measured with the various densitometric techniques is an apparent density, including the bone itself and the bone marrow. The term bone mineral density is related to the mass of bone tissue. The true bone mineral density—the mass of bone per unit volume, excluding the marrow and non-bone tissue—is not determined. DXA provides an areal apparent bone density because of a two-dimensional image, so the density is expressed as the bone density per unit area, not per volume, in g/cm2. Even if QCT provides a volumetric bone density, usually expressed in mg/cm3, it is also an apparent density, because it includes the marrow spaces of vertebrae.
Dual X-Ray Absorptiometry
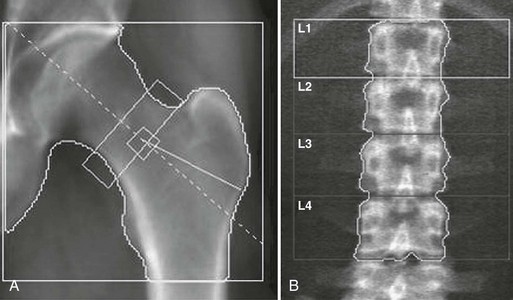
The physical principle of DXA is to measure the attenuation of x-rays with two different energies through the body, thus accounting for variable soft-tissue thickness and composition, at sites like the axial skeleton, the hip, and the whole body. The dual x-ray can be generated by either K-edge filters or kVp switching. The preferred anatomic sites for BMD measurement are the lumbar spine (L1 to L4) and the proximal femur (Fig. 13-14), although the whole body and peripheral sites can also be scanned (calcaneus, distal forearm). Measurement of attenuation of the x-ray photons of two distinct energies also allows accurate assessment of body fat content.137 The areal density that is measured by DXA is an integral of both cortical and trabecular BMC normalized to the size of the projected area. Scan times have been shortened from about 5 to 10 minutes for pencil-beam scanners to 10 to 30 seconds for the fan-beam systems, which perform a single sweep across the patient instead of a two-dimensional raster scan. Fan-beam systems also allow for easier identification of vertebral structures and artifacts because of better image resolution, but at the expense of greater x-ray exposure.
Diagnostic Use
Several sites can be measured with DXA. The spine measurement is limited to the lumbar spine, because the thoracic spine evaluation would be compounded by air in the lungs and ribs and sternum overlying the scan field. The scan usually includes L1 to L4 (sometimes L2 to L4). The spine should be centered in the scan field and properly aligned. One should remain alert to artifacts such as those induced by patient movement. The presence of vertebral fracture, deformities, osteoarthritis, aortic calcification, or curvature such as scoliosis modifies BMD,138 so in individuals older than 65 or 70 years, those commonly affected by osteoarthritis, the spine measurement is often of limited clinical utility. On the DXA report, large differences in vertebral height, area, BMC, and/or BMD suggest fracture or degenerative changes. Affected vertebral bodies should be excluded from the analysis; the entire spine scan is often discarded because of these kinds of artifacts. The hip DXA scan includes the proximal end of the femur and a portion of the pelvis. Only the proximal femur bone is assessed. Small changes in femur rotation can induce significant changes in hip BMD,139 so special positioning devices are typically used to position the measured limb at 15 to 30 degrees inward rotation. This way, the femoral neck is visible because it is aligned to the table and perpendicular to the x-ray beam. The right and left hips being similar within an individual, one can choose either side and repeat measurements on the same side. If degenerative changes, previous fracture, localized bone disease (hip osteonecrosis, Paget’s disease of bone, etc,) affect only one hip, the scan will be performed on the opposite side. On the report image, the lesser trochanter should be only slightly visible, indicating an appropriate rotation. Machines evaluate different regions of the hip, including the femoral neck, the trochanteric region, Ward’s triangle, and the total region. The diagnosis relies on the femoral neck and total regions values. The forearm can also be measured, typically on the nondominant side. The radial shaft is a good indicator of cortical bone density. Total body scans mainly reflect the cortical bone and are very precise. They are generally performed to assess body composition parameters such as lean and fat mass.
Several prospective studies have shown that BMD measured by DXA at various skeletal sites (spine, hip, forearm, calcaneus, total body) predicts the risk of fragility fractures, with a relative risk of 1.5 to 3.0 for each 1-SD decrease in BMD.4 The prediction of fracture is higher when BMD is measured at the site of fracture—for example, measuring hip BMD for hip fracture. Although BMD is commonly measured at the spine and hip, measuring multiple sites has little advantage.
Observed changes on follow-up should not be due to precision errors. The bone content and area should be checked for consistency. The precision error—expressed as a coefficient of variation (CV)—is usually around 1% at the spine and 1.5% to 2% at the hip. This precision error should be evaluated in a group of subjects representative of the clinic population setting. The precision error can be estimated by measuring a group of 14 individuals, each at least three times, with interim repositioning.140 The least significant change—the minimal BMD variation considered statistically significant within an individual—is calculated as 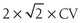 . Thus, the least significant change in BMD is generally around 3% at the spine and 5% to 6% at the femoral neck. The precision error can also be expressed as an absolute BMD value difference, which is more accurate for extreme BMD values.
. Thus, the least significant change in BMD is generally around 3% at the spine and 5% to 6% at the femoral neck. The precision error can also be expressed as an absolute BMD value difference, which is more accurate for extreme BMD values.
Other Techniques
Quantitative Computed Tomography
QCT measures the volumetric BMD of the lumbar spine and proximal femur, allows differentiation between cortical and trabecular BMD, and provides a sensitive measure of compartmental changes; however, it has several drawbacks, including relatively high radiation exposure and a higher precision error than that of DXA.136,141 QCT was originally implemented to determine bone mineral density in single slices through the midsections of the lumbar vertebral bodies (Fig. 13-15) or the distal radius.136,141,142 With the advent of whole body multislice/multidetector (spiral) CT, a technique in which the examination table is continually advanced during data acquisition and one that allows more rapid scanning of large sections of the body, three-dimensional volumetric QCT (vQCT) techniques were developed. These advances improved the precision for measuring spine or hip from approximately 2% to 3% for single-slice to approximately 1% to 2% for volumetric measurements and enhanced the sensitivity and accuracy of the original approach by covering larger volumes.143–145 As a volumetric technique, vQCT can determine BMD or bone mineral content of the entire bone or of specified subregions and, similar to single-slice QCT, can provide a separate analysis of the trabecular or cortical components. Geometric measurements, such as cross-sectional area or hip axis length, and derivation of mechanical properties, such as cross-sectional moment of inertia, are possible (Fig. 13-16). For further central skeletal analyses, the vQCT data can be used for high-resolution imaging of bone structure146,147 and for finite element analysis (FEA).148,149 This technique computes distributions of mechanical stress and strain throughout the bone under simulated loading conditions in an iterative process. Finite element modeling may improve the assessment of bone strength and the prediction of fracture healing, because it integrates the geometric and densitometric information that has been acquired from the QCT scan.
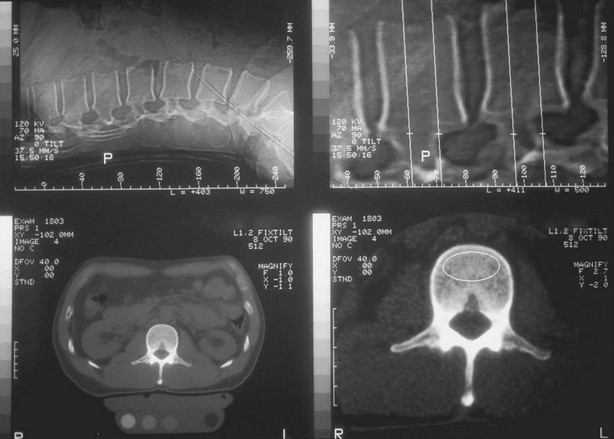
FIGURE 13-15 Traditional quantitative computed tomography (QCT) with single slice per vertebra is illustrated, along with the scout localization, the simultaneously scanned phantom, and the ellipse region of interest in the vertebral centrum.
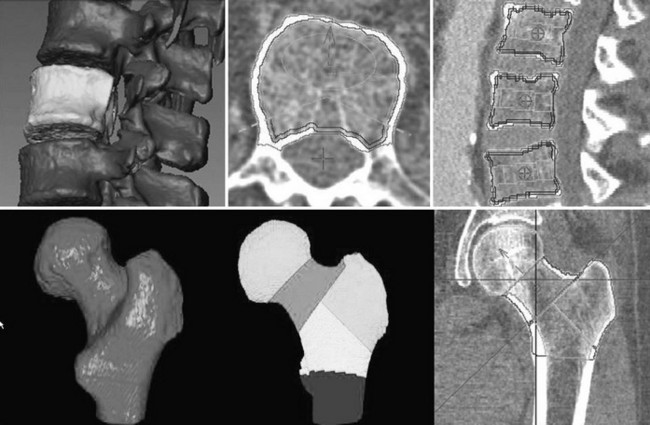
FIGURE 13-16 Volumetric quantitative computed tomography of the spine (top) and hip (bottom) may be used to analyze bone mineral density (BMD) in various bone compartments and to accurately measure BMD and geometry. Top left, Segmented vertebral body selected for analysis with removed processes. Top center and right, Integral and trabecular and peeled trabecular volumes of interest along with the traditional elliptical and Pacman volumes of interest (VOIs). Bottom left, Segmented proximal femur. Bottom center and right, Analysis VOIs in the hip.
High-Resolution Peripheral Quantitative Computed Tomography
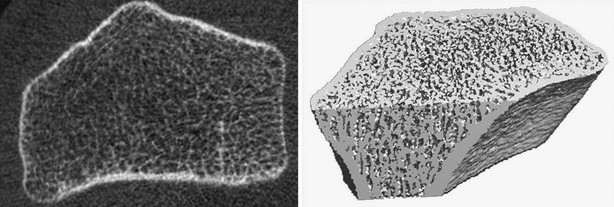
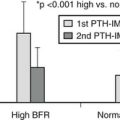
HR-pQCT allows for three-dimensional evaluation of BMD and quantification of architecture. These devices were introduced more than 10 years ago, but their performance has been improved more recently, with machines evaluating trabecular architecture and volumetric BMD at the distal radius and tibia with a nominal isotropic voxel size of 82 µm (Fig. 13-17). Precision of HR-pQCT measurements was 0.7% to 1.5% for total, trabecular, and cortical densities and 2.5% to 4.4% for trabecular architecture.150 In the OFELY cohort, postmenopausal women had lower density, trabecular number, and cortical thickness than premenopausal women at both radius and tibia. Osteoporotic women had lower density, cortical thickness, and increased trabecular separation than osteopenic women at both sites. Furthermore, although spine and hip BMD were similar, fractured osteopenic women had lower trabecular density and more heterogeneous trabecular distribution at the radius compared with unfractured osteopenic women.150 At the distal radius, women with fractures had lower volumetric total (D tot), trabecular (D trab) BMDs, trabecular bone volume (BV/TV), cortical thickness (Cort Th), trabecular number (TbN), trabecular thickness (TbTh), and higher trabecular separation (TbSp) and distribution of trabecular separation (TbSpSd) than controls without fractures. In a logistic model, each SD decrease of volumetric total and trabecular densities was associated with a significantly increased risk of fracture at both sites (ORs ranged from 2.00 to 2.47). After adjusting for aBMD measured by DXA at the ultradistal radius, differences between cases and controls remained significant for D trab, and there was a similar trend for TbN, TbSp, and TbSpSd, with adjusted ORs ranging from 1.32 to 1.50. In postmenopausal women, vertebral and nonvertebral fractures are associated with low volumetric BMD and architectural alterations of trabecular and cortical bone that can be assessed noninvasively and that are partially independent of aBMD assessed by DXA.151 Assessment of bone mechanical properties by FEA may improve identification of those at high risk for fracture. The three-dimensional data obtained with HR-pQCT may help to achieve this goal. Thus, the proportion of load carried by cortical versus trabecular bone seemed to be associated with wrist fracture independently of BMD and microarchitecture in the same set of women from the OFELY cohort. These results suggest that bone mechanical properties assessed by microFE may provide information about skeletal fragility and fracture risk not assessed by BMD or architecture measurements alone and are therefore likely to enhance the prediction of wrist fracture risk.152
Quantitative Ultrasonography
Quantitative ultrasonography may be a surrogate technique for DXA and may provide additional information on the material properties of bone. Ultrasound velocity (speed of sound) and ultrasound attenuation through bone (broadband attenuation) are recorded at various peripheral bones such as the calcaneus, patella, phalanges, and tibia.153 In general, ultrasound devices have a low precision error, but their accuracy is unknown. Both speed of sound and broadband attenuation decrease with age, with a magnitude that varies considerably from device to device. Prospective studies have shown that ultrasound measurement of the os calcis is a valid predictor of hip fracture risk in elderly women.153,154 Quantitative ultrasonography is a technique for the broad diagnosis of osteoporosis, with the availability of cheap and portable devices. Until each device is adequately validated and precise diagnostic and therapeutic thresholds are defined, quantitative ultrasound should be still considered a research tool.
Who Should Be Screened With Dxa?
Even if BMD is an important predictor of fracture risk, it is too insensitive to be used solely as an indication for treatment, so targeted BMD testing of postmenopausal women who have clinical risk factors for fracture or low BMD is generally recommended, rather than universal testing.155 The U.S. Preventive Services Task Force has concluded that the benefits of BMD testing are clear for all women 65 and older.156 The use of other risk factors such as smoking, family history, race, decreased physical activity, alcohol or caffeine use, or low calcium intake could not be used to identify women at high risk. Most North American guidelines consider that BMD testing is appropriate in all women aged 65 and older. In younger women, BMD testing should be limited to those with BMD-independent risk factors. In other regions of the world, specifically in Europe, a case finding strategy is often adopted, so that BMD testing is proposed in women who have risk factors for low bone mass; for example, low BMI, smoking, excessive alcohol consumption, history of steroid use, personal or familial history of fragility fracture, diseases associated with accelerated bone loss (hyperparathyroidism, uncontrolled hyperthyroidism, osteogenesis imperfecta, malabsorption), or premature menopause, regardless of age.
Vertebral Fracture Assessment
With most recent DXA systems, it is possible to scan the entire thoracic and lumbar spine to evaluate vertebral height.157 The patient is scanned in supine position if the machine has a rotating arm, or in the lateral position. Vertebral bodies can be assessed qualitatively to screen for vertebral deformities, typically using the semiquantitative scoring method also applied to radiographs.133,134 Those deformities can be quantified with a software that measures the height of vertebral bodies. The computer—or better, the technologist—places three points on the posterior, mid-, and anterior margins of both endplates of each vertebra from T4 to L4 to calculate the vertebral heights. Significant changes in vertebral height are indicative of vertebral fracture. This technique is attractive because evaluation of vertebral fracture prevalence can be performed without a conventional radiograph at the same time as the BMD measurement, thus saving time, unnecessary irradiation, and cost. This is an important advantage because vertebral fractures are often overlooked. The interpretation must be cautious, however, because the image quality is variable across individuals, so the observation of deformities is not synonymous for vertebral fracture and should lead to radiographs for confirmation. In addition, the upper thoracic spine may be difficult to visualize, especially among older individuals, with false-negative readings as a result. The specificity and the negative predictive value remain high despite these limitations, because most vertebral fractures occur at lumbar and lower thoracic levels where the image quality is still acceptable.158 VFA is now widely used as a screening tool for vertebral fracture.
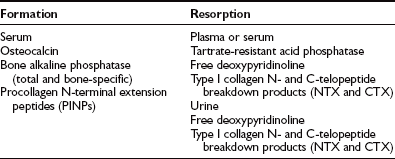
Biochemical Markers
Bone markers are usually classified as markers of bone formation and markers of bone resorption (Table 13-3), but in diseases in which both processes are coupled and disclose similar variation, markers reflect the overall rate of bone turnover. Markers cannot distinguish between bone turnover changes originating in the cortical or in the trabecular envelopes.
Bone Formation Markers
Markers of bone formation are serum total and bone measurements of alkaline phosphatase, osteocalcin, and procollagen I extension peptides. Serum alkaline phosphatase is the most commonly used marker of bone formation but lacks sensitivity and specificity. New assays have been developed to improve specificity in order to separate bone and liver isoenzymes—in particular, immunoassays using monoclonal antibodies with a cross-reactivity of only 15% to 20%.159 Thus, bone alkaline phosphatase is a sensitive marker of increased turnover in postmenopausal women, and since it is an enzyme activity and not a protein that is cleared by the kidney, it is also a marker of choice in the evaluation of bone turnover in chronic kidney failure.
Serum osteocalcin, also called bone Gla protein, is a small (49 amino acids) noncollagenous protein that is specific for bone tissue and dentin and produced only by osteoblasts and odontoblasts. Serum osteocalcin levels correlate with skeletal growth during puberty, as well as with increase in bone formation rate in conditions characterized by increased bone turnover, such as primary and secondary hyperparathyroidism, hyperthyroidism, or acromegaly. Conversely, osteocalcin is decreased in hypothyroidism, hypoparathyroidism, and glucocorticoid-treated patients, conditions associated with a decreased rate of bone formation. When resorption and formation are coupled, serum osteocalcin is a good marker of bone turnover. The most robust and sensitive assays measure both the intact molecule and the N-midfragment (which is the largest product of degradation of osteocalcin).160 Although osteocalcin was discovered 30 years ago, its function remains elusive.161 It might be involved in the regulation of energy metabolism, its action depending on its degree of gamma-carboxylation.162
Propeptides of type I procollagen (N-terminal and C-terminal extension peptides of type I collagen [PINP and PICP, respectively]) are cleaved during the extracellular processing of collagen. They also reflect bone formation, because type I collagen is the most abundant organic component of bone matrix. PICP exhibits smaller variations in postmenopausal osteoporosis than other markers, so it is probably less useful. However, it seems valuable in the monitoring of growth hormone treatment.163 After the menopause, PINP augments to a similar extent that osteocalcin and bone alkaline phosphatase does. It is the most sensitive marker to monitor PTH treatment.164
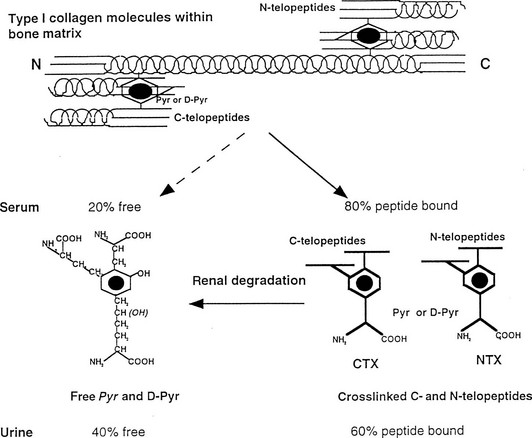
Bone Resorption Markers
Pyridinoline (Pyr) and deoxypyridinoline (D-Pyr) are nonreducible pyridinium cross-links in the mature form of collagen (Fig. 13-18). This posttranslational covalent cross-linking creates interchain bonds stabilizing the molecule of collagen. Concentration of Pyr and D-Pyr in biological fluids is derived predominantly from bone. Pyr and D-Pyr are released from bone matrix during osteoclastic bone resorption. They are excreted in urine in a free form (around 40%) and in a peptide-bound form (60%). Enzyme-linked immunosorbent assays have been developed against the N-telopeptide to helix (NTX) and against breakdown products of type I collagen C-telopeptide (CTX) in urine and serum. These cross-links are markedly increased at the time of menopause and return to premenopausal levels with estrogen and bisphosphonate therapy.165
Clinical Use of Bone Markers
Bone Markers for Assessment of Bone Loss
A sharp increase in bone markers occurs after menopause. This increase is sustained long after the start of menopause, even in elderly women, and markers are negatively correlated with bone mass assessed by DXA,166 which suggests that a high bone turnover rate is associated with increased bone loss. A long-term study suggested that the rate of bone loss measured over 12 years by densitometry is increased in women classified as rapid losers at baseline and significantly higher than that of slow losers.167 Thus a combination of markers and BMD measurement could be useful in assessment of the risk of osteoporosis.168
Bone Markers for Assessment of Fracture Risk
It has been reported that women classified as fast losers have a vertebral and wrist fracture risk double that of those classified as normal or slow losers.169 Large prospective studies have shown that increased bone resorption (i.e., above the premenopausal values) is associated with an increased risk in vertebral and peripheral fractures, independently of BMD.170,171 Women with both a low BMD and increased bone resorption had a fourfold to fivefold increase in the risk of hip fracture. Measurements of bone resorption markers may be combined with other risk factors for fracture to calculate individual probability of fracture, but markers do not enter into the calculation of individual probabilities of fracture in the FRAX calculator, because data were not available in most cohorts used in model building.
Bone Markers for Monitoring Treatment of Osteoporosis
Antiresorptive therapies such as calcitonin, estrogen, and bisphosphonates induce a significant decrease in markers, which return to the premenopausal range within 3 to 6 months for resorption markers and within 6 to 9 months for formation markers. The significant decrease in bone turnover seen after treatment of osteoporotic women with a bisphosphonate significantly correlates with an increase in BMD at the lumbar spine after 2 years, with a low rate of false-positive and false-negative results.172 There is also an association between the decrease in bone turnover markers in response to antiresorptive agents such as bisphosphonates or raloxifene and the degree of reduction in fracture risk.173,174 Given the precision of bone mass measurement with DXA and the expected change induced by antiresorptive treatment, it is usually necessary to wait for 2 years to determine whether the treatment is effective in an individual patient. Determination of markers after a few months of treatment is likely to provide useful information on efficacy and may improve compliance.175 Therapies stimulating bone formation, such as teriparatide, may be monitored using bone formation markers (e.g., PINP).
Treatment
Lifestyle
Patients who have sustained vertebral fractures need specific guidelines in their daily activities to prevent additional fractures. They should avoid weightlifting and learn how to bend to avoid excessive strain on their spine. All lifestyle factors that might be deleterious to bone metabolism should be corrected. Thus patients should not smoke, they should consume moderate amounts of alcohol, and conditions predisposing to osteoporosis should be treated. Although no controlled trial has shown that cessation of smoking increases BMD or reduces the risk of osteoporotic fractures, sufficient evidence indicates that smoking is a risk factor for vertebral and hip fractures.48 Drugs predisposing to osteoporosis should be avoided as much as possible.
Nutrition
No universal consensus has been reached on the daily calcium requirement by age, but according the Dietary Reference Intake (DRI) published in 1997,176 1300 mg/day is the recommended dose for adolescents, 1000 to 1200 mg/day for adults up to 70 years, and 1200 mg/day after the age of 70 years. Although most studies have shown a beneficial effect of calcium supplementation, as discussed later, the long-term effect of high dietary calcium intake on bone health is unknown. Conversely, there seems to be a threshold of calcium intake—around 400 mg/day—under which increasing calcium intake appears to be beneficial and necessary, both in children and in women older than 60 years.
Several nutritional factors influence the calcium requirement, such as sodium, protein, caffeine, fiber, and vitamin D status. The effect of fiber and caffeine is relatively small, whereas sodium and protein intake may be more relevant because they increase urinary excretion of calcium. A sodium-rich diet has been associated with increased incidence of kidney stones. The effects of phosphorus and fat intake do not substantially influence bone metabolism. Vitamin D promotes calcium absorption and thereby influences calcium requirements. Vitamin D status commonly deteriorates in older people, with serum levels of 25(OH)D lower than those in young adults because of low vitamin D intake and decreased sunlight exposure (see Chapters 3 and 15). NOF recommends an intake of 800 to 1000 international units (IU) of vitamin D per day for adults aged 50 and older. This intake will bring the average adult’s serum 25(OH)D concentration to the desired level of 30 ng/mL (75 nmol/L) or higher.176–179 The low levels of 25(OH)D commonly seen in the elderly, especially those institutionalized, contribute to secondary hyperparathyroidism, which may play a role in bone loss in the elderly.
Exercise
Immobilization induces bone loss, as documented after prolonged bedrest, space flight, paralysis from spinal cord injury, and casting of limbs. The beneficial effect of exercise on bone mass and bone strength in normal and osteoporotic individuals, however, is still unclear. Several controlled trials have looked at the effect of various exercise programs on BMD, including walking, weight training, aerobics, and high-impact and low-impact exercise. Most of them show a small (1% to 2%) increase in BMD in comparison to either baseline or the control group at some but not all skeletal sites; the increase is not sustained once the exercise program is stopped. Both clinical trials and observational studies suggest that load-bearing exercise is more effective in preserving or increasing bone mass than other types of exercise are. The dominant arm of tennis players or the limbs of gymnasts usually have higher bone mass than other sites do.180 Skeletal sites must be directly strained to be affected by exercise. In addition, it is likely that fitness might indirectly preserve individuals from fractures by improving mobility and reducing the risk of falls. No randomized controlled trial has been conducted to assess the effect of exercise on fracture risk. Because of the many nonskeletal benefits of exercise, it seems appropriate to recommend regular and moderate exercise in postmenopausal women, but it cannot replace pharmacologic prevention. After vertebral fracture, a supervised exercise program to maintain strength and flexibility of the thoracic and lumbar spine is recommended in the elderly.
It is critical to develop specific interventions aimed at preventing falls and their consequences in the elderly. So far, few studies have shown that specific strategies prevent falls. For example, one trial has shown that older men and women trained to practice tai chi regularly (better balance training) were less prone to fall than untrained counterparts.181
Experimental studies have shown that the soft tissues covering the hip may have an impact on energy absorption of the fall. Some clinical trials have shown that hip protectors substantially decrease the risk of hip fracture in elderly people living in institution (up to 80%), but those results were not found in all trials, and poor compliance remains an issue in all studies.182,183
Pharmacologic Intervention
The goal of therapy is to prevent fragility fractures, and drugs for osteoporosis should demonstrate their ability to significantly decrease the incidence of fractures in adequately powered, prospective, randomized placebo-controlled studies. Conducting such placebo-controlled trials has been debated, but they remain the cornerstone of sound drug evaluation.184 The diagnosis of nonvertebral fractures is easy, whereas the diagnosis of existing and new vertebral fractures requires adequate morphometric evaluation of spinal radiographs to exclude vertebral deformities unrelated to osteoporosis. Changes in BMD are commonly monitored and are usually seen as a surrogate marker of treatment efficacy. Actually, most drugs that inhibit bone turnover induce a small increase in BMD (i.e., 2% to 10% according to the skeletal site of measurement) that does not account for their marked reduction in fracture rate (i.e., 30% to 50% for vertebral fractures). In addition, some drugs—such as fluoride—may induce a marked increase in BMD without decreasing the fracture rate. Bone turnover markers are another surrogate for treatment efficacy of antiresorptive drugs. Their diminution in response to antiresorptive therapies is associated with the decreased fracture rate. High bone turnover is associated with an increased risk of fractures that is independent of the level of BMD,170 and antiresorptive therapy such as raloxifene and bisphosphonates induces a dose-dependent decrease in bone markers that is sustained throughout treatment. Anabolic therapies such as PTH induce a substantial increase in markers of bone formation (e.g., PINP).
Anticatabolic Agents
Estrogen: Several controlled trials have shown that estrogen stops bone loss in early and late postmenopausal women by inhibiting bone resorption and that it results in a small increment in BMD (5% to 10% over a period of 1 to 3 years). Estrogen reduces bone turnover and increases bone density in postmenopausal women of all ages, as well as improving calcium homeostasis. Results from observational studies185 and randomized placebo-controlled trials186–188 show that estrogen decreased the risk of hip fracture by about 30% and the risk of spine fracture by 30% to 50%. So, HRT has been a standard treatment for prevention and treatment of postmenopausal osteoporosis for years. The reduction in fracture risk exceeds that expected by BMD alone. Calcium supplements enhance the effect of estrogen on BMD.189 When HRT is stopped, bone loss resumes at the same rate as after menopause. In the Study of Osteoporotic Fractures,190 the relative risk for nonspinal fracture was 0.66 in postmenopausal women currently taking HRT as compared with those not taking HRT, but it was not decreased in previous users regardless of the duration of treatment. This positive effect was not affected by age and was more significant for women who started HRT soon after menopause (within 5 years).
The long-term risks of HRT, however, outweigh the benefits, as has been shown in a large randomized placebo-controlled trial, the Women’s Health Initiative (WHI) randomized trial.191 Indeed, among more than 16,000 women aged 50 to 79 followed for an average 5.2 years, treated using conjugated equine estrogen and medroxyprogesterone acetate, although hip fracture and colon cancer risks were reduced,191 increased risks of coronary heart disease (CHD) (nonfatal myocardial infarction or death due to CHD),192 stroke,193 breast cancer,194 and dementia195 were observed. In addition, in this trial, estrogen plus progestin did not have clinically meaningful effect on health-related quality of life, such as sleep disturbance or vasomotor symptoms.196 Those results in primary prevention are consistent with those obtained in secondary CHD prevention in a well-conducted randomized placebo-controlled trial.197 More recent subgroup analyses from the WHI trial have suggested that women who initiated hormone therapy closer to menopause (<10 years) tended to have reduced CHD risk compared with the increase in CHD risk among women more distant from menopause, but this trend did not meet strict criteria for statistical significance. A similar non-significant trend was observed for total mortality, but the risk of stroke was elevated regardless of years since menopause. These data should be considered in regard to the short-term treatment of menopausal symptoms.198 The fall in HRT prescription observed after the release of the WHI results has been associated with a decline in new breast cancer incidence in several countries.199 Thus, estrogen replacement therapy is no longer recommended as a first-line therapy for prevention and treatment of osteoporosis. It should be used only to relieve menopausal symptoms for as short a time and at the lowest dose possible.
Bisphosphonates: Bisphosphonates are stable analogs of pyrophosphate characterized by two P-C-P bonds. By substituting for hydrogens on the carbon atom, a variety of bisphosphonates have been synthesized, the potency of which depends on the length and structure of the side chain. Bisphosphonates have a strong affinity for bone apatite, both in vitro and in vivo, which is the basis for their clinical use. Bisphosphonates are potent inhibitors of bone resorption and produce their effect by reducing the recruitment and activity of osteoclasts and increasing their apoptosis. This activity varies greatly from compound to compound and ranges from 1 to 10,000. The mechanism of action on osteoclasts includes inhibition of the proton vacuolar ATPase of some phosphatases and alteration of the cytoskeleton and the ruffled border. Aminobisphosphonates also inhibit several steps of the mevalonate pathway, thereby modifying the isoprenylation of guanosine triphosphatate–binding proteins.200
Etidronate, given in an intermittent regimen, has led to an increase of about 3.5% in spine BMD after 2 years, with a reduction in the vertebral fracture rate,201 but this reduction in vertebral fracture rate was no longer significant after 3 years of treatment. Etidronate does not appear to be effective in preventing bone loss at the hip and in reducing nonvertebral fractures.202 Inhibition of mineralization can occur when etidronate is given in high dose.
Alendronate at 5 mg/day is effective in preventing bone loss in postmenopausal women to nearly the same extent as HRT.203 In women with vertebral fractures, alendronate given for 3 years resulted in an 8.8% increase in BMD at the lumbar spine and hip204 (Fig. 13-19). This treatment reduced the incidence of new vertebral, wrist, and hip fractures by half and prevented height loss. Alendronate also reduces fracture risk in postmenopausal women at the highest risk of fracture (i.e., those older than 75 years or those with very low BMD).205 In women without preexisting vertebral fracture but with a hip T score lower than 2.5, alendronate is able to decrease clinical fractures of all types.206 Alendronate is approved in most countries for the treatment of osteoporosis at the 10-mg/day dose and for the prevention of osteoporosis at the 5-mg/day dose in the United States. Alendronate is safe but can induce mild upper gastrointestinal tract disturbances and heartburn; on rare occasions, these side effects are related to the esophagitis sometimes caused by inappropriate administration of the drug. The antifracture efficacy of another oral nitrogen-containing bisphosphonate, risedronate, has been established in randomized placebo-controlled trials. This compound has been able to halve the rate of vertebral fracture in postmenopausal women in two studies.207,208 A trial in elderly women has shown a reduction of about a third of the incidence of hip fracture in women with hip BMD in the osteoporosis range, whereas women selected using clinical risk factors for fracture—and who generally were not osteoporotic—did not benefit from the treatment.209 Although these drugs were developed with daily dosages, they are now prescribed to be taken only on a weekly basis. Compliance has been slightly improved by weekly regimens, using 70 mg for alendronate and 35 mg for risedronate.
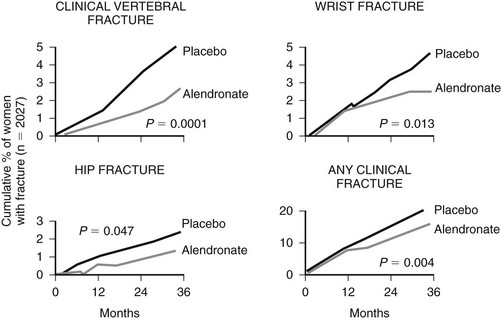
FIGURE 13-19 Effects of alendronate (aln) on the risk of fragility fracture. (Data from Black DM, Cummings SR, Karpf DB, et al: Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures, Lancet 348:1535–1541, 1996.)
Ibandronate, given either daily (2.5 mg) or intermittently (20 mg every other day for the first 24 days, followed by 9 weeks without the treatment) has reduced the incidence in vertebral fracture of about 50% in a randomized trial involving postmenopausal osteoporotic women.210 It has been marketed as a monthly 150-mg tablet and a 3-mg, every-3-months IV injection.
Intravenous zoledronate (e.g., 4 mg once a year or 1 mg every 3 months) has been shown to decrease bone turnover and increase BMD with the same magnitude as alendronate in a randomized phase II trial.211 Two phase III trials have been published on the antifracture efficacy of annual IV zoledronic acid 5 mg. In the HORIZON Prevention Fracture Trial,212 the rate of vertebral fracture was reduced by 65% at 3 years, hip fracture by 40%, and nonvertebral fracture by 25%. In the Recurrent Fracture Trial,213 men and women with a recent hip fracture who received annual 5 mg zoledronic acid had a 35% reduction in clinical fractures and a 28% reduction in all-cause mortality. Other bisphosphonates have been studied in the treatment of osteoporosis. Pamidronate can increase BMD, but no valuable data on its antifracture efficacy have been presented. Clodronate may have an antifracture efficacy, but no adequate data have been published so far.214
Bisphosphonates can also be used in secondary osteoporosis, such as corticosteroid-induced osteoporosis. Etidronate prevents bone loss at the spine in men and women receiving corticosteroids, but not entirely at the femoral neck.215 At a dose of 5 or 10 mg/day, alendronate is an effective therapy for the prevention and treatment of glucocorticoid-induced osteoporosis, preventing bone loss at all skeletal sites in men and women and reducing the rate of new vertebral fractures in postmenopausal women.216 Risedronate (5 mg daily) also increases BMD in patients receiving long-term treatments with corticosteroids and may have favorable effects on fracture rate.217 Annual IV zoledronic acid has also shown favorable effects in prevention of steroid-induced osteoporosis.
Selective Estrogen Receptor Modulators: Selective estrogen receptor modulators (SERMs), also called estrogen analogs, act as estrogen agonists or antagonists, depending on the target tissue. Tamoxifen, which has long been used for the adjuvant treatment of breast cancer, is an estrogen antagonist in breast tissue but a partial agonist on bone, cholesterol metabolism, and endometrium. Tamoxifen does not entirely prevent bone loss in postmenopausal women and increases the risk of endometrial cancer, which precludes its use in healthy postmenopausal women. Raloxifene is a benzothiophene that competitively inhibits the action of estrogen in the breast and the endometrium and also acts as an estrogen agonist of bone and lipid metabolism. In a large 2-year randomized placebo-controlled trial of raloxifene in postmenopausal women,218 raloxifene increased lumbar spine (2.4%), total hip (2.4%), and total body (2.0%) BMD and significantly reduced markers of bone turnover. Serum cholesterol concentrations and its low-density lipoprotein (LDL) fraction were reduced without stimulation of the endometrium. Results of the MORE trial (Multiple Outcomes of Raloxifene Evaluation),219 performed in 7705 osteoporotic women randomized to receive either raloxifene or placebo, showed that the incidence of estrogen receptor–positive breast cancer is reduced by two-thirds in postmenopausal women. In this study, raloxifene also lowered the incidence of vertebral fractures in osteoporotic women with (−30%) or without (−50%) existing fracture but had no significant effect on nonvertebral fractures.220,221 In a post hoc analysis of women with prevalent vertebral fractures that were at highest risk of subsequent fractures, raloxifene has decreased the risk of both vertebral and nonvertebral fractures.222 In another subgroup post hoc analysis of this trial,223 women at higher cardiovascular risk were also protected by raloxifene compared to those on placebo; but in the RUTH trial,224 in which the primary endpoint was cardiovascular events, raloxifene did not reduce cardiovascular events or mortality in a high-cardiovascular-risk population of postmenopausal women. In addition, two trials with incidence of breast cancer as the primary endpoint have confirmed the reduction in receptor-positive breast cancer incidence that was suggested in the MORE trial.224,225
In a 3-year randomized trial,226 arzoxifene was compared to placebo and raloxifene among 6847 postmenopausal osteoporotic women. A vertebral fracture risk reduction of 42% was obtained with arzoxifene compared to placebo, which was similar to that observed with raloxifene. It is only in a subgroup analysis of patients at high risk that nonvertebral fracture risk was diminished. The incidence of breast cancer was low and did not differ between groups. The adverse events profile was comparable to that of raloxifene.
In a 5-year randomized placebo-controlled trial,227 lasofoxifene 0.5 mg/day use was associated with a 42% reduction in vertebral fracture risk and a 22% reduction in nonvertebral fracture risk. The risk of estrogen receptor–positive breast cancer decreased by 67%.
Denosumab: Denosumab is a human monoclonal anti-RANKL antibody. It is a potent antiresorptive agent which is administered by a 60-mg subcutaneous injection every 6 months. In a large randomized placebo-controlled trial involving postmenopausal women with osteoporosis,228 denosumab has been shown to reduce the risk of vertebral fracture by 68% at 3 years. The risk of hip fracture was diminished by 40% and nonvertebral fractures by 20%. The compound was well tolerated. In a trial among 1189 postmenopausal osteoporotic women randomized to receive either weekly alendronate or denosumab 60 mg every 6 months, the increase in BMD and the decrease in markers of bone resorption was larger in the denosumab group than in the alendronate group.229 In a trial conducted in 252 women at high risk of rapid loss because of an antiaromatase inhibitor therapy prescribed for nonmetastatic breast cancer over 24 months, twice-yearly administration of denosumab led to significant increases in BMD over 24 months at trabecular and cortical bone, with overall adverse events rates similar to those of placebo.230 In another trial, postmenopausal women with low BMD were randomized to denosumab every 3 months (Q3M; 6, 14, or 30 mg) or every 6 months (Q6M; 14, 60, 100, or 210 mg); placebo; or open-label oral alendronate weekly.231 After 24 months, patients receiving denosumab either continued treatment at 60 mg Q6M for an additional 24 months, discontinued therapy, or discontinued treatment for 12 months then reinitiated denosumab (60 mg Q6M) for 12 months. The placebo cohort was maintained. Alendronate-treated patients discontinued alendronate and were followed. The main finding was that the effects on bone turnover were fully reversible with discontinuation of denosumab, and its effects were restored with subsequent retreatment.
Calcitonin: Calcitonin, a peptide produced by thyroid C cells, reduces bone resorption by inhibiting osteoclast activity. It is traditionally given by subcutaneous or intramuscular injection, with poor tolerance (nausea, facial flushes, diarrhea). The development of intranasal salmon calcitonin has made this therapy more acceptable, with fewer side effects. The minimum intranasal dose to have an effect on BMD is 200 IU/day. Calcitonin is less effective in preventing cortical bone loss than cancellous bone loss in postmenopausal women.232 A small controlled study in osteoporotic women suggested a reduction in new vertebral fractures.233 In the PROOF (Prevent Recurrence of Osteoporotic Fractures) study,234 which is a 5-year double-blind, randomized, placebo-controlled study of 1255 postmenopausal women with osteoporosis, 200 IU/day of intranasal salmon calcitonin significantly reduced the rate of vertebral (but not peripheral) fractures by about 30% in comparison to placebo, but doses of 100 and 400 IU had no effect, and no consistent effect on BMD and bone turnover markers was seen in this trial with a high dropout rate. Thus the role of nasal calcitonin in the management of postmenopausal osteoporosis is not yet clearly documented.235 Studies examining the effect of oral calcitonin are ongoing.
Anabolic Agents
Parathyroid Hormone: When administered intermittently, PTH stimulates bone formation. It can be administered as the intact hormone (1-84) or as the 1-34 fragment (teriparatide). Teriparatide increases osteoblast birth rate and prevents osteoblasts apoptosis, thereby increasing the number of osteoblasts and the rate of new bone formation. When given once daily subcutaneously, teriparatide increases BMD and improves trabecular architecture, cortical geometry, and strength. In a randomized placebo-controlled trial involving 1637 postmenopausal women with severe osteoporosis, the risk of vertebral fracture was reduced by 65% in those receiving 20 µg/day compared to those on placebo, and their reduction in peripheral fracture risk was of 53% after a median duration of treatment of 21 months (see Fig. 13-16).236 Treatment was well tolerated, with a transient rise in serum calcium in fewer than 10% of patients.
PTH is prescribed mostly in patients with severe osteoporosis. Those women, however, have often received other therapies for osteoporosis (e.g., raloxifene or bisphosphonates) before PTH may be started because they continue to fracture. In an observational prospective study, Ettinger et al.237 have treated osteoporotic women who had been either on raloxifene or alendronate for 18 to 36 months, with teriparatide 20 µg/day for 18 months. They found that teriparatide stimulated bone turnover in patients pretreated with raloxifene and alendronate, but the increase in markers of bone formation was blunted during the first months among those on prior alendronate. Moreover, among those on prior raloxifene, the teriparatide-induced increase in BMD was comparable to that reported in treatment-naïve patients, whereas prior treatment with alendronate delayed increase in BMD, particularly during the first 6 months. One may think that activated bone resorption is a prerequisite for an effect of PTH on bone formation. Thus, in old female sheep treated with the bisphosphonate tiludronate, PTH, or PTH and tiludronate, it has been shown that in the combined-therapy PTH and tiludronate group, the anabolic effect of PTH—as assessed using biochemical markers of bone formation and bone histomorphometry—was abolished.238 Comparable findings have been made in clinical trials in humans. In postmenopausal women treated with both alendronate and PTH(1-84), the increase in volumetric density was comparable to that observed in those receiving alendronate alone and significantly lower than that of women taking PTH alone, suggesting that concurrent use of alendronate and PTH may reduce the anabolic effects of PTH.239 Similar findings have been made in men taking alendronate, PTH(1-34), or PTH plus alendronate.240
Such results suggest that PTH and bisphosphonates should not be prescribed simultaneously because the bisphosphonate reduces the anabolic effect of PTH. In contrast, in patients who have taken raloxifene before, the effect of PTH does not appear to be modified, and in those who have taken a bisphosphonate before, the anabolic effect of PTH is still obtained but delayed. This initial blunting of anabolic effect seems to depend on the potency of the antiresorptive agent preceding the PTH treatment, (1) since the increase in markers of bone formation is greater in patients who have taken risedronate previously compared to those who have taken alendronate241 and (2) because there is no blunting after raloxifene.237,242
The antifracture efficacy of intact PTH(1-84) has also been tested. In a trial among 2532 postmenopausal women with mildly severe osteoporosis, PTH could reduce the risk of vertebral fracture by 40%, but not that of nonvertebral fracture. Study results interpretation was hampered by a high dropout rate. The incidence of hypercalcemia among patients on PTH was 23%.243 This form of PTH is marketed in some European countries but not in the United States.
Other Medications
Calcium: Calcium partially decreases the rate of bone loss, especially in women in late postmenopause. It is generally prescribed as an adjunct to other drugs such as bisphosphonates, and in most clinical trials, both the active and placebo groups receive calcium supplements.
Calcium is likely to be partially effective in preventing bone loss, particularly in older women and those with low calcium intake. Two studies244,245 have shown a slight reduction in the incidence of fractures in patients receiving calcium supplements. In one study,246 women older than 60 years were supplemented with 1200 mg/d when their calcium intake was below 1000 mg/d, and this regimen resulted in prevention of bone loss from the forearm over a 4-year period and decreased the rate of vertebral fractures by 59% in women who had vertebral fractures at baseline but not in those without existing vertebral fractures. Calcium supplementation on the order of 500 to 1500 mg/day is safe. Mild gastrointestinal disturbances such as constipation are commonly reported. The risk of kidney stone disease related to hypercalciuria appears to be minimally increased. Bioavailability is greater during meals and varies with different calcium salts, but this factor is probably of little clinical significance.
Vitamin D: In a French study of 3270 institutionalized women with a mean age of 84 years who were treated with calcium (1200 mg/d) and vitamin D (800 IU/day) for 1.5 years, the risk of hip fracture and other nonvertebral fractures was decreased 43% and 32%, respectively, when compared with the placebo group.178 Treatment increased serum 25(OH)D, decreased serum PTH, and increased femoral neck BMD. However, in a Dutch study of 2578 women of similar age treated with 400 IU of vitamin D or placebo for 3.5 years but without supplemental calcium because of higher dietary calcium intake, the rate of hip fracture was the same in the two groups.247 These women were healthier and more ambulatory, and the hip fracture rate was much lower than in the French study. In a more recent smaller study, 389 men and women older than 63 years were treated with vitamin D (700 IU/day) and calcium (500 mg/day); the study noted a trend toward a decrease in nonvertebral fractures.248 Another study showed a decrease in nonvertebral fractures when vitamin D was given annually by intramuscular injection.249 These studies show the utility of adequate calcium (500 to 1500 mg/day) and vitamin D (700 IU or equivalent) in calcium-deficient and vitamin D–deficient elderly women and probably men. A reduction of about 25% has also been found in men and women aged 65 and older living in the community, after receiving 100,000 IU of vitamin D every 4 months for 5 years.250 In elderly women recovering from a first hip fracture, a supplement in vitamin D has been able to increase BMD and reduce the incidence of falls, these effects being more marked with the addition of calcium.251
Strontium Ranelate: Strontium ranelate is a compound containing two atoms of non-radioactive strontium; it improves bone mass by inhibiting bone resorption while preserving bone formation. Strontium ranelate prevents bone loss induced by estrogen deficiency and immobilization in animal models and also improves bone strength in those models. In phase II trials, this compound has been able to prevent bone loss at the dose of 1 g/day.252,253 In a randomized placebo-controlled trial conducted in 1649 postmenopausal women with osteoporosis (mean age 69) treated for 3 years, strontium ranelate use has been associated with a 41% reduction in vertebral fracture risk, this diminution being apparent as early as the first year.254 In another randomized trial, the primary endpoint being incidence of nonvertebral fracture, all peripheral fractures were significantly but slightly reduced (−16%). The reduction in the incidence of hip fracture did not reach statistical significance in the intent-to-treat analysis but only in a per-protocol analysis (−41%, P = 0.025).255 In those trials, strontium ranelate has been well tolerated. An analysis based on preplanned pooling of data from these two international phase III, randomized, placebo-controlled, double-blind studies included 1488 women between 80 and 100 years of age followed for 3 years. In this study, treatment with strontium ranelate reduced the risk of vertebral (−59%) and nonvertebral (−41%) fractures in women 80 years and older with osteoporosis.256 Strontium ranelate is not approved by the U.S. Food and Drug Administration.
Others: Ipriflavone is a synthetic compound that belongs to the family of isoflavones. In a large randomized placebo-controlled trial, it failed to demonstrate prevention of bone loss.257 In addition, a sizeable proportion of women on ipriflavone had lymphopenia.
Tibolone is a synthetic analog of anabolic steroids with estrogen-like, androgen-like, and progestin-like properties. It is marketed in some countries and generally used as HRT in postmenopausal women. It prevents postmenopausal bone loss and has positive effects on hot flashes. In the randomized LIFT study,258 4538 women who were between the ages of 60 and 85 years and had a BMD T score of −2.5 or less at the hip or spine or a T score of −2.0 or less and radiologic evidence of a vertebral fracture were assigned to receive once-daily tibolone (at a dose of 1.25 mg) or placebo. Tibolone reduced the risk of vertebral fracture (−45%), nonvertebral fracture (−26%), and invasive breast cancer (−68%) and possibly colon cancer, but increased the risk of stroke.
Growth hormone has been used for its alleged bone and muscle anabolic properties. However, growth hormone has produced conflicting results in the prevention of bone loss in postmenopausal osteoporosis.259
Thiazide diuretics reduce tubular reabsorption of calcium and slow cortical bone loss in normal postmenopausal women, so they should not be used as a monotherapy in the treatment of postmenopausal osteoporosis.260
Who Should Be Treated?
Therapeutic recommendations for women rely on results of clinical trials whose main endpoint was the reduction in fracture risk.261 There was no clinical trial in which the main endpoint was reduction in fracture risk in men, although bisphosphonates clearly increase BMD, reduce levels of biochemical markers, and are likely to reduce fracture risk to the same extent as in women. Similarly, teriparatide has shown beneficial effects in men with surrogates, but without the fracture outcome. The decision-making process should be based on an analysis of the patient’s individual risk of fracture and the efficacy and tolerance of the drugs to be prescribed. This decision should rely on age, the existence of clinical risk factors, the magnitude of bone loss as assessed by the level of BMD, and the presence or not of previous fragility fractures.
Most recent therapeutic recommendations rely at least partly on the use of the FRAX algorithm. Each country can adapt the available models to its public health priorities. For example, in the United Kingdom, a cost-effectiveness analysis using the probabilities of fracture calculated with the FRAX algorithm at various ages and the cost of medications in the United Kingdom found that treatment is cost effective at all ages when the 10-year probability of a major fracture exceeds 7%.262 The intervention threshold at the age of 50 years corresponds to a 10-year probability of a major osteoporotic fracture of 7.5% and rises progressively with age up to 30% at the age of 80 years, so that intervention is cost effective at all ages. The use of these thresholds in a case-finding strategy would identify 23% to 46% as eligible for treatment, depending on age. The same threshold can be used in men.
In a United States–specific cost-effectiveness analysis,263 osteoporosis treatment was considered cost effective when the 10-year hip fracture probability reached approximately 3%. Although the RR at which treatment became cost effective varied markedly between genders and by race/ethnicity, the absolute 10-year hip fracture probability at which intervention became cost effective was similar across race/ethnicity groups and tended to be slightly higher for men than for women. The new WHO fracture prediction algorithm has been combined with an updated economic analysis to evaluate existing NOF guidance for osteoporosis prevention and treatment.264 The WHO fracture prediction algorithm was calibrated to the U.S. population using national age-, sex- and race-specific death rates and age- and sex-specific hip fracture incidence rates. Thus, it is cost effective to treat patients with a fragility fracture and those with osteoporosis by WHO criteria, as well as older individuals at average risk and osteopenic patients with additional risk factors. However, the estimated 10-year fracture probability was lower in men and nonwhite women compared to postmenopausal white women. This analysis generally endorsed existing clinical practice recommendations and concluded that specific treatment decisions must be individualized. So, in the latest NOF recommendations, postmenopausal women and men aged 50 and older presenting with the following should be considered for treatment:
• A hip or vertebral (clinical or morphometric) fracture
• T score ≤−2.5 at the femoral neck or spine after appropriate evaluation to exclude secondary causes
• Low bone mass (T score between −1.0 and −2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture ≥3% or a 10-year probability of a major osteoporosis-related fracture ≥20% based on the U.S.-adapted WHO algorithm.
Treatment Monitoring
Monitoring of Treatment With Densitometry
Whether the long-term antifracture efficacy of antiosteoporotic drugs will depend on the extent to which treatment can increase or maintain BMD is controversial. In one meta-analysis, 16% of vertebral fracture risk reduction after treatment with alendronate was attributed to an increase in BMD at the lumbar spine.265 For patients treated with risedronate or raloxifene, changes in BMD predict even more poorly the degree of reduction in vertebral (raloxifene) or nonvertebral (risedronate) fractures. In women taking alendronate and losing BMD (0 to −4%) at the spine during the first or second year of treatment, the effect of alendronate on vertebral fracture risk reduction is similar to that observed in those women who gained BMD.266 For bone-forming agents, increases in BMD account for approximately one-third of the vertebral fracture risk reduction with teriparatide.267 Further data are needed on the role of BMD in monitoring patients treated with bone-forming agents, but it appears to be of greater value than its use with inhibitors of bone resorption.
Monitoring of Treatment With Biochemical Markers of Bone Turnover
Antiresorptive therapies such as calcitonin, estrogen, SERMs, and bisphosphonates induce a significant decrease in bone markers that return to the premenopausal range within 3 to 6 months for the resorption markers and within 6 to 9 months for markers of formation. A significant association has been reported between the short-term decrease and the absolute level of markers of bone turnover with the use of antiresorptive agents (raloxifene and bisphosphonates) on the one hand, and the magnitude of the reduction of the risk of vertebral and nonvertebral fractures on the other hand.173,174,268 In addition, a large prospective study suggests that the use of markers of bone turnover in the monitoring of bisphosphonate therapy is associated with a greater persistence with therapy than in those not monitored.175 Thus, measurement of markers of bone turnover after a few months of treatment may provide useful information on efficacy and improve persistence. Changes in markers of bone turnover with strontium ranelate are of small magnitude and are unlikely to be clinically useful for the monitoring of treatment.
References
1. NIH Consensus development panel on osteoporosis prevention, diagnosis and therapy. Osteoporosis prevention diagnosis and therapy. JAMA. 2001;285:785–795.
2. Hayes, WC. Biomechanics of fractures. In: Riggs BL, Melton LJ, III., eds. Osteoporosis diagnosis and management. ed 2. Philadelphia: Lippincott-Raven; 1995:93–114.
3. Mosekilde, L, Bentzen, SM, Ortoft, G, et al. The predictive value of quantitative computed tomography for vertebral body compressive strength and ash density. Bone. 1989;10:465–470.
4. Cummings, SR, Black, DM, Nevitt, MC, et al. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–75.
5. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser. 1994.
6. Kanis, JA, Melton, LJ, III., Christiansen, C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141.
7. Melton, LJ, III. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175–177.
8. Kanis JA on behalf of the World health Organization scientific Group: Assessment of osteoporosis at the primary health-care level. Technical report. World Health organization collaborating centre for metabolic bone diseases, University of Sheffield, UK, 2007.
9. National Osteoporosis Foundation. 1996 and 2015: Osteoporosis prevalence figures: state-by-state report. Washington, DC: National Osteoporosis Foundation; 1997.
10. O’Neill, TW, Felsenberg, D, Varlow, J, et al. The prevalence of vertebral deformity in European men and women: the European vertebral osteoporosis study. J Bone Miner Res. 1996;11:1010–1018.
11. Spector, TD, McCloskey, EV, Doyle, DV, et al. Prevalence of vertebral fractures in women and the relationship with bone density and symptoms: the Chingford study. J Bone Miner Res. 1993;7:817–822.
12. Cooper, C, Campion, G, Melton, LJ, III. Hip fractures in the elderly: a worldwide projection. Osteoporos Int. 1992;2:285–289.
13. Phillips, S, Fox, N, Jacobs, J, et al. The direct medical costs for osteoporosis for American women aged 45 and older, 1986. Bone. 1988;9:271–279.
14. Ray, NF, Chan, JK, Thaemer, M, et al. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1994. J Bone Miner Res. 1997;12:24–35.
15. Jensen, JS, Bagger, J. Long-term social prognosis after hip fractures. Acta Orthop Scand. 1982;53:97–101.
16. Schneider, EL, Guralnik, JM. The aging of America: impact on health care costs. JAMA. 1990;263:2335–2350.
17. Cummings, SR, Kelsey, JL, Nevitt, MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178–208.
18. Browner, WS, Pressman, AR, Nevitt, MC, et al. Mortality following fractures in older women: the Study of Osteoporotic Fractures. Arch Intern Med. 1996;156:1521–1525.
19. Cooper, C, Melton, LJ, III. Epidemiology of osteoporosis. Trends Endocrinol Metab. 1992;314:224–229.
20. Gallagher, JC, Melton, LJ, Riggs, BL, et al. Epidemiology of fractures of the proximal femur in Rochester, Minnesota. Clin Orthop. 1980;150:163–171.
21. Nevitt, MC, Cummings, SR, Study of Osteoporotic Fractures Research Group. type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. Am J Geriatr Soc. 1993;41:1226–1234.
22. Melton, LJ, III. Differing patterns of osteoporosis across the world. In: Chesnut CH, III., eds. New dimensions in osteoporosis in the 1990s. Hong Kong: Excerpta Medica; 1991:13–18.
23. Melton, LJ, III. Epidemiology of age-related fractures. In: Avioli LV, ed. The osteoporotic syndrome: detection, prevention and treatment. New York: Wiley-Liss; 1993:17–18.
24. Cooper, C, Atkinson, EJ, O’Fallon, WM, et al. The incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227.
25. Melton, LJ, III., Lane, AW, Cooper, C, et al. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–119.
26. EPOS Group. Incidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study. J Bone Miner Res. 2002;17:716–724.
27. McCloskey, EV, Spector, TD, Eyres, KS, et al. The assessment of vertebral deformity. A method for use in population studies and clinical trials. Osteoporos Int. 1993;3:138–147.
28. Hedlund, LR, Gallagher, JC. Vertebral morphometry in diagnosis of spinal fractures. Bone Miner. 1988;5:59–67.
29. Kleerekoper, M, Nelson, DA. Vertebral fracture or vertebral deformity? Calcif Tissue. 1992;50:5–6.
30. Kado, DM, Browner, WS, Palermo, L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220.
31. Center, JR, Nguyen, TV, Pocock, NA, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882.
32. Nevitt, MC, Ettinger, B, Black, DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793–800.
33. Sahlin, Y. Occurrence of fractures in a defined population: a 1-year study. Injury. 1990;21:158–160.
34. Baron, JA, Barrett, J, Malenka, D, et al. Racial differences in fracture risk. Epidemiology. 1994;5:42–47.
35. Bengnér, U, Johnell, O. Increasing incidence of forearm fractures. Acta Orthop Scand. 1985;56:158–160.
36. Owen, RA, Melton, LJ, III., Johnson, KA, et al. Incidence of Colles fracture in a North American community. Am J Public Health. 1982;72:605–607.
37. Cooper, C, Atkinson, EJ, Jacobsen, SJ, et al. Population-based study of survival following osteoporotic fractures. Am J Epidemiol. 1993;137:1001–1005.
38. Kaukonen, JP, Karaharju, EO, Porras, M, et al. Functional recovery after fractures of the distal forearm. Ann Chir Gynaecol. 1988;77:27–31.
39. Klotzbuecher, CM, Ross, PD, Landsmann, PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739.
40. Woolf, AD, Akesson, K. Preventing fractures in elderly people. BMJ. 2003;327:89–95.
41. Chapurlat, RD, Bauer, DC, Nevitt, M, et al. Incidence and risk factors for a second hip fracture: the Study of Osteoporotic Fractures. Osteoporos Int. 2003;14:130–136.
42. Cummings, SR, Black, DM, Nevitt, MC, et al. Bone densitometry at various sites for prediction of hip fractures. Lancet. 1993;341:72–75.
43. Riggs, BL, Khosla, S, Melton, LJ, III. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773.
44. Kelsey, JL, Browner, WS, Seeley, DG, et al. Risk factors for fractures of the distal forearm and proximal humerus. Am J Epidemiol. 1992;135:477–489.
45. Cooper, C, Barker, DJP, Wickham, C. Physical activity, muscle strength, and calcium intake in fracture of the proximal femur in Britain. BMJ. 1988;297:1443–1446.
46. Farmer, ME, Harris, T, Madans, JH, et al. Anthropometric indicators and hip fracture: The NHANES I epidemiologic follow-up study. J Am Geriatr Soc. 1989;37:9–16.
47. Paganini-Hill, A, Chao, A, Ross, RK, et al. Exercise and other factors in the prevention of hip fracture: The Leisure World study. Epidemiology. 1991;2:16–25.
48. Grisso, JA, Kelsey, JL, Strom, BL, et al. Risk factors for falls as a cause for hip fractures in women. N Engl J Med. 1991;324:1326–1331.
49. Cummings, SR, Nevitt, MC, Browner, WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–773.
50. Johnell, O, Gullberg, B, Kanis, JA, et al. Risk factors for hip fracture in European women—the MEDOS study. J Bone Miner Res. 1995;10:1802–1815.
51. Ohta, H, et al. Which is more osteoporosis-inducing, menopause or oophorectomy? Bone Miner. 1992;19:273–285.
52. Davies, MC, Hall, ML, Jacobs, HS. Bone mineral loss in young women with amenorrhea. BMJ. 1990;301:790–793.
53. Prior, JC, Vigna, YM, Schechter, MT, et al. Spinal bone loss and ovulatory disturbances. N Engl J Med. 1990;323:1211–1227.
54. Chapurlat, RD, Garnero, P, Sornay-Rendu, E, et al. Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int. 2000;11:493–498.
55. Cummings, SR, Browner, WS, Bauer, DC, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–738.
56. Chapurlat, RD, Garnero, P, Bréart, G, et al. Serum estradiol and sex hormone-binding globulin and the risk of hip fracture in elderly women: the EPIDOS study. J Bone Miner Res. 2000;15:1835–1841.
57. Lee, JS, LaCroix, AZ, Lu, S, et al. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab. 2008;93:1796–1803.
58. Eastell, R, Adams, JE, Coleman, RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–1057.
59. Bauer, DC, Browner, WS, Cauley, JA, et al. Factors associated with appendicular bone mass in older women. Ann Intern Med. 1993;118:657–665.
60. Mosekilde, L. Osteoporosis and exercise. Bone. 1995;17:193–195.
61. Tinetti, ME, Speechley, M, Ginter, SF. Risk factors for falls among persons living in the community. N Engl J Med. 1988;319:1701–1707.
62. Nordin, BEC, Heaney, RP. Calcium supplementation of the diet: justified by the present evidence. BMJ. 1990;300:1056–1060.
63. Dargent-Molina, P, Favier, F, Grandjean, H, et al. Fall-related factors and risk for hip fracture: the EPIDOS prospective study. Lancet. 1996;348:145–149.
64. Kanis, JA, Johansson, H, Oden, O, et al. A family history of fracture and fracture risk. Bone. 2004;35:1029–1037.
65. Kanis, JA, Johnell, O, De Laet, C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382.
66. Kanis, JA, Oden, O, Johnell, O, et al. The burden of osteoporotic fracture: a method for setting intervention thresholds. Osteoporos Int. 2001;12:417–424.
67. Higgins, JP, Thompson, SG, Deeks, JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
68. Kanis, JA, Johnell, O, Oden, O, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995.
69. http://www.shef.ac.uk/FRAX
70. Smith, DM, Nance, WE, Kang, KW, et al. Genetic factors in determining bone mass. J Clin Invest. 1973;52:2800–2808.
71. Morrison, NA, Qi, JC, Tokita, A, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–287.
72. Morrison, NA, Qi, JC, Tokita, A, et al. Prediction of bone density from vitamin D alleles. Nature. 1997;387:106.
73. Peacock, M. Vitamin D receptor gene alleles and osteoporosis: a contrasting view. J Bone Miner Res. 1995;10:1294–1297.
74. Garnero, P, Borel, O, Sornay-Rendu, E, et al. Vitamin D receptor gene polymorphisms are not related to bone turnover, rate of bone loss, and bone mass in post-menopausal women: The OFELY study. J Bone Miner Res. 1996;11:827–834.
75. Efstathiadou, Z, Tsatsoulis, A, Ioannidis, JP. Association of collagen I alpha 1 Sp1 polymorphism with the risk of prevalent fractures: a meta-analysis. J Bone Miner Res. 2001;16:1586–1592.
76. Tran, BN, Nguyen, ND, Eisman, JA, et al. Association between LRP5 polymorphism and bone mineral density: a Bayesian meta-analysis. BMC Med Genet. 2008;9:55.
77. van Meurs, JB, Trikalinos, TA, Ralston, SH, et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–1290.
78. Richards, JB, Rivadeneira, F, Inouye, M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–1512.
79. Langdahl, BL, Uitterlinden, AG, Ralston, SH, et al. Large-scale analysis of association between polymorphisms in the transforming growth factor beta 1 gene (TGFB1) and osteoporosis: the GENOMOS study. Bone. 2008;42:969–981.
80. Bonjour, J-P, Carrie, A-L, Ferrari, S, et al. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest. 1997;99:1287–1294.
81. Cadogan, J, Eastell, R, Jones, N, et al. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ. 1997;315:1255–1260.
82. Johnston, CC, Miller, JZ, Slemenda, CW, et al. Calcium supplementation and increases in bone mineral density in children. N Engl J Med. 1992;327:82–87.
83. Bass, S, Pearce, G, Bradney, M, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–507.
84. Ahlborg, HG, Johnell, O, Turner, CH, et al. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334.
85. Lanham, SA, Roberts, C, Cooper, C, et al. Intrauterine programming of bone. Part 1: alteration of the osteogenic environment. Osteoporos Int. 2008;19:147–156.
86. Lanham, SA, Roberts, C, Perry, MJ, et al. Intrauterine programming of bone. Part 2: alteration of skeletal structure. Osteoporos Int. 2008;19:157–167.
87. Riggs, BL, Melton, LJ, Robb, RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214.
88. Riggs, BL, Wahner, HW, Dunn, WL, et al. Differential changes in bone mineral density of the appendicular and axial skeleton with ageing: relationship to spinal osteoporosis. J Clin Invest. 1981;67:328–335.
89. Lindsay, R, Hart, DM, Forrest, C, et al. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980;2:1151–1153.
90. Hofbauer, LC, Khosla, S, Dunstan, CR, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370.
91. Shevde, NK, Bendixen, AC, Dienger, KM, et al. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A. 2000;97:7829–7834.
92. Eghbali-Faterouchi, G, Khosla, S. Role of RANK ligand in mediating bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230.
93. Weitzmann, MN, Roggia, G, Toraldo, L, et al. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110:1643–1650.
94. Cenci, S, Toraldo, G, Weitzmann, MN, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and life-span through IFN-gamma-induced class II transactivator. Proc Nat Acad Sci U S A. 2003;100:10405–10410.
95. Lam, J, Takeshita, S, Barker, JE, et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANL ligand. J Clin Invest. 2000;106:1229–1237.
96. Wei, S, Kitaura, H, Zhou, P, et al. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290.
97. Chavassieux, P, Seeman, E, Delmas, PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–164.
98. Seeman, E, Delmas, PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261.
99. Duan, Y, Wang, XF, Evans, A, et al. Structural and biomechanical basis of racial and sex differences in vertebral fragility in Chinese and Caucasians. Bone. 2005;36:987–998.
100. Faulkner, KG, Cummings, SR, Black, D, et al. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–1217.
101. Duboeuf, F, Hans, D, Schott, AM, et al. Different morphometric and densitometric parameters predict cervical and trochanteric hip fracture: the EPIDOS Study. J Bone Miner Res. 1997;12:1895–1902.
102. Reid, IR, Chin, K, Evans, MC, et al. Relation between increase in length of hip axis in older women between 1950s and 1990s and increase in age specific rates of hip fracture. BMJ. 1994;309:508–509.
103. Parfitt, AM, Matthews, CHE, Villanueva, AR, et al. Relationship between surface, volume and thickness of iliac trabecular bone on aging and in osteoporosis: implications for the micro-anatomic and cellular mechanism of bone loss. J Clin Invest. 1983;72:1396–1409.
104. Foldes, J, Parfitt, AM, Shih, M-S, et al. Structural and geometric changes in iliac bone: relationship to normal aging and osteoporosis. J Bone Miner Res. 1991;6:759–766.
105. Qiu, S, Rao, DS, Fyhrie, DP, et al. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone. 2005;37:10–15.
106. Seeman, E. Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med. 2003;349:320–323.
107. Ahlborg, HG, Johnell, O, Turner, CH, et al. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334.
108. Riggs, BL, Melton, LJ, III., Robb, RA, et al. A population-based study of age and sex differences in bone volumetric density, size, geometry and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954.
109. Wang, XF, Duan, Y, Beck, T, et al. Varying contributions of growth and ageing to racial and sex differences in femoral neck structure and strength in old age. Bone. 2005;36:978–986.
110. Berry, SD, Kiel, DP. Falls as risk factors for fracture. In: Marcus R, Feldman D, Nelson DA, et al, eds. Osteoporosis. ed 3. San Diego: Academic press; 2008:911–922.
111. Hans, D, Dargent-Molina, P, Schott, AM, et al. Ultrasonographic heel measurements to predict hip fracture in elderly women: The EPIDOS prospective study. Lancet. 1996;348:511–514.
112. Lindsay, R, Silverman, SL, Cooper, C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;17(285):320–323.
113. Dempster, DW. Bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Miner Res. 1989;4:137–141.
114. Van Staa, TP, Leufkens, HGM, Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787.
115. Teelucksingh, S, Padfield, PL, Tibi, L, et al. Inhaled corticosteroids, bone formation, and osteocalcin. Lancet. 1991;338:60–61.
116. van Staa, TP, Gueusens, P, Pols, HA, et al. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–198.
117. Israel, E, Banerjee, TR, Fitzmaurice, GM, et al. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med. 2001;345:941–947.
118. van Staa, TP, Leufkens, HG, Abenhaim, M, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000.
119. van Staa, TP, Laan, RF, Barton, IP, et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229.
120. Toh, SH, Claunch, BC, Brown, PH. Effect of hyperthyroidism and its treatment on bone mineral content. Arch Intern Med. 1985;145:883–886.
121. Silverberg, SJ, Shane, E, Jacobs, TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255.
122. McDonough, AK, Rosenthal, RS, Cao, X, et al. The effect of thiazolidinediones on BMD and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008;4:507–513.
123. Shapiro, JR. Osteogenesis imperfecta and other defects of bone development as occasional causes of adult osteoporosis. In: Marcus R, Feldman D, Nelson DA, et al, eds. Osteoporosis. San Diego, CA: Academic; 2008:1247–1281.
124. Mann, T, Oviatt, SK, Wilson, D, et al. Vertebral deformity in men. J Bone Miner Res. 1992;7:1259–1265.
125. Marie, PJ, De Vernejoul, MC, Connes, C, et al. Decreased DNA synthesis by cultures of osteoblastic cells in eugonadal osteoporotic men with defective bone formation. J Clin Invest. 1991;88:1167–1172.
126. Finkelstein, JS, Klibanski, A, Neer, RM, et al. Osteoporosis in men with idiopathic hypogonadotrophic hypogonadism. Ann Intern Med. 1987;106:354–361.
127. Bilezikian, JP, Morishima, A, Bell, J, et al. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med. 1998;339:599–603.
128. Melton, LJ, III., Orwoll, ES, Wasnich, RD. Does bone density predict fractures comparably in men and women? Osteoporos Int. 2001;12:707–709.
129. Tannenbaum, C, Clark, J, Schwartzmann, K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431–4437.
130. Jamal, SA, Leiter, RE, Bayoumi, AM, et al. Clinical utility of laboratory testing in women with osteoporosis. Osteoporos Int. 2005;16:534–540.
131. Twomey, LT, Taylor, JR. Age changes in lumbar vertebrae and intervertebral discs. Clin Orthop Relat Res. 1987;224:97–104.
132. National Osteoporosis Foundation Working Group on Vertebral Fractures. assessing vertebral fractures. J Bone Miner Res. 1995;10:518–523.
133. Genant, HK, Wu, CY, van Kuijk, C, et al. Vertebral fracture assessment using a semi-quantitative technique. J Bone Miner Res. 1993;8:1137–1148.
134. Wu, CY, Li, J, Jergas, M, et al. Comparison of semi-quantitative and quantitative techniques for the assessment of prevalent and incident fractures. Osteoporos Int. 1995;5:354–370.
135. Benhamou, CL, Lespessailles, E, Jacquet, G, et al. Fractal organization of trabecular bone images on calcaneus radiographs. J Bone Miner Res. 1994;9:1909–1918.
136. Genant, HK, Engelke, K, Fuerst, T, et al. Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res. 1996;11:707–730.
137. Faulkner, KG, Miller, PD. Clinical use of bone densitometry. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, eds. Osteoporosis. ed 3. San Diego, CA: Academic Press, San Diego; 2007:1493–1518.
138. Reid, IR, Evans, MC, Ames, R, et al. The influence of osteophytes and aortic calcification on spinal mineral density. The influence of osteophytes and aortic calcification on spinal mineral density in postmenopausal women. J Clin Endocrinol Metab. 1991;72:1372–1374.
139. Goh, JCH, Low, SL, Bose, K. Effect of femoral rotation on bone mineral density measurements with dual x-ray absorptiometry. Calcif Tissue Int. 1995;57:340–343.
140. Gluer, CC, Blake, G, Lu, Y, et al. Accurate assessment of precision errors: how to measure the reproducibility errors of bone densitometry techniques. Osteoporos Int. 1995;5:262–270.
141. Genant, H, Cann, C, Ettinger, B, et al. Quantitative computed tomography of vertebral spongiosa: a sensitive method for detecting early bone loss after oophorectomy. Ann Intern Med. 1982;97:699–705.
142. Rüegsegger, P, Koller, B, Müller, R. A microtomographic system for the non-destructive evaluation of bone architecture. Calcif Tis Int. 1996;58:24–29.
143. Kang, Y, Engelke, K, Fuchs, S, et al. Anatomic coordinate system of the femoral neck for highly reproducible BMD measurements using 3D QCT. Comput Med Imaging Graph. 2005;29:533–541.
144. Lang, TF, Li, J, Harris, ST, et al. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–137.
145. Bousson, V, LeBras, A, Roqueplan, F, et al. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int. 2006;17:855–864.
146. Gordon, C, Lang, T, Augat, P, et al. Image-based assessment of spinal trabecular bone structure from high-resolution CT images. Osteoporos Int. 1998;8:317–325.
147. Ito, M, Ikeda, K, Nishiguchi, M, et al. Multi-detector row CT imaging of vertebral microstructure for evaluation of fracture risk. J Bone Miner Res. 2005;20:1828–1836.
148. Kevak, JH, Rossi, SA, Jones, KA, et al. Prediction of femoral fracture load using automated finite element modelling. J Biomech. 1998;31:125–133.
149. Crawford, RP, Cann, CE, Keaveny, TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33:44–50.
150. Boutroy, S, Bouxsein, ML, Munoz, F, et al. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515.
151. Sornay-Rendu, E, Boutroy, S, Munoz, F, et al. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–433.
152. Boutroy, S, Van Rietbergen, B, Sornay-Rendu, E, et al. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–399.
153. Hans, D, Gluer, CC, Njeh, CF. Ultrasonic evaluation of osteoporosis. In: Meunier PJ, ed. Osteoporosis, Diagnosis and management. London: Martin Dunitz; 1998:59–78.
154. Bauer, DC, Gluer, CC, Cauley, JA, et al. Bone ultrasound predicts fractures strongly and independently of densitometry in older women: a prospective study. Arch Intern Med. 1997;157:629–634.
155. Kanis, JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936.
156. Nelson, HD, Helfand, M, Woolf, SH, et al. Screening for postmenopausal osteoporosis: a review of the evidence for the US preventive Services Task Force. Ann Int Med. 2002;137:529–541.
157. Duboeuf, F, Bauer, DC, Chapurlat, RD, et al. Assessment of vertebral fracture using densitometric morphometry. J Clin Densitom. 2005;8:362–368.
158. Chapurlat, RD, Duboeuf, F, Marion-Audibert, HO, et al. Effectiveness of instant vertebral assessment to detect prevalent vertebral fracture. Osteoporos Int. 2006;17:1189–1195.
159. Garnero, P, Delmas, PD. Assessment of the serum levels of bone alkaline phosphatase with a new immunometric assay in patients with metabolic bone disease. J Clin Endocrinol Metab. 1993;77:1046–1053.
160. Garnero, P, Grimaux, M, Demiaux, B, et al. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992;7:1389–1398.
161. Ducy, P, Desbois, C, Boyce, B, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1986;382:448–452.
162. Lee, NK, Sowa, H, Hinoi, E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469.
163. Szulc, P, Seeman, E, Delmas, PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–294.
164. Chen, P, Satterwhite, JH, Licata, AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–970.
165. Uebelhart, D, Schlemmer, A, Johansen, J, et al. Effect of menopause and hormone replacement therapy on the urinary excretion of pyridinium crosslinks. J Clin Endocrinol Metab. 1991;72:367–373.
166. Garnero, P, Sornay-Rendu, E, Chapuy, MC, et al. Increased bone turnover in late post-menopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–349.
167. Hansen, MA, Kirsten, O, Riis, BJ, et al. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 years study. BMJ. 1991;303:961–964.
168. Garnero, P, Dargent-Molina, P, Hans, D, et al. Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int. 1998;8:563–569.
169. Riis, SBL, Hansen, AM, Jensen, K, et al. Low bone mass and fast rate of bone loss at menopause—equal risk factors for future fracture. A 15 year follow-up study. Bone. 1996;19:9–12.
170. Garnero, P, Hausher, M, Chapuy, C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J Bone Miner Res. 1996;11:1531–1538.
171. Chapurlat, RD, Garnero, P, Bréart, PJ, et al. serum type I collagen breakdown products (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS study. Bone. 2000;27:283–286.
172. Garnero, P, Shih, WJ, Gineyts, E, et al. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–1700.
173. Bjarnason, NH, Sarkar, S, Duong, T, et al. Six and twelve months changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001;12:922–930.
174. Eastell, R, Barton, I, Hannon, RA, et al. Relationship of early changes in bone resorption in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056.
175. Delmas, PD, Vrijens, B, Eastell, R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis: the IMPACT study. J Clin Endocrinol Metab. 2007;92:1296–1304.
176. Institute of Medicine. Dietary references intakes for calcium, phosphorus, magnesium vitamin D and fluoride. Washington DC: National Academy Press; 1997.
177. Ooms, ME, Roos, JC, Bezemer, PD, et al. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab. 1995;80:1052–1058.
178. Chapuy, MC, Arlot, ME, Duboeuf, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;237:1637–1642.
179. Dawson-Hughes, B, Dallal, GE, Krall, EA, et al. Effects of vitamin D supplementation on overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115:505–512.
180. Kannus, P, Haapasalo, H, Sankelo, M, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31.
181. Wolf, SL, Barnhart, HX, Kutner, NJ, et al. Reducing frailty and falls in older persons: an investigation of tai chi and computerized balance training. J Am Geriatr Soc. 1996;44:489–497.
182. Kannus, P, Parkkari, J, Niemi, S, et al. Prevention of hip fracture in elderly people with use of hip protector. N Engl J Med. 2000;343:1506–1513.
183. van Schoor, NM, Smit, JH, Twisk, JWR, et al. Prevention of hip fractures by external hip protectors. a randomized controlled trial. JAMA. 2003;289:1957–1962.
184. Kanis, JA, Oden, A, Johnell, O, et al. Uncertain future of trials in osteoporosis. Osteoporos Int. 2002;13:443–449.
185. Grady, D, Rubin, SM, Pettiti, DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1037.
186. Cauley, JA, Robbins, J, Chen, Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density. The Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738.
187. Torgerson, DJ, Bell-Syer, SEM. Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomized trials. BMC Musculoskelet Disord. 2001;2:2–7.
188. Torgerson, DJ, Bell-Syer, SEM. Hormone replacement therapy and prevention of nonvertebral fractures. A met-analysis of randomized trials. JAMA. 2001;285:2891–2897.
189. Nieves, JW, Komar, L, Cosman, F, et al. Calcium potentiates the effect of estrogen and calcitonin on bone mass: review and analysis. Am J Clin Nutr. 1998;67:18–24.
190. Cauley, JA, Seeley, DJ, Ensrud, K, et al. Estrogen replacement therapy and fractures in older women: study of Osteoporotic Fractures Research Group. Ann Intern Med. 1995;122:9–16.
191. Writing group for the Women’s Health Initiative investigators. risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333.
192. Manson, JE, Hsia, J, Johnson, KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534.
193. Wassertheil-Smoller, S, Hendrix, SL, Limacher, M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. The Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684.
194. Chlebowski, RT, Hendrix, SL, Langer, RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. The Women’s Health Initiative randomized trial. JAMA. 2003;289:3243–3253.
195. Schumaker, SA, Legault, C, Rapp, SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory study: a randomized controlled trial. JAMA. 2003;289:2651–2662.
196. Hays, J, Ockene, JK, Brunner, RL, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–1854.
197. Hulley, S, Grady, D, Bush, T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613.
198. Rossouw, JE, Prentice, RL, Manson, JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477.
199. Kumle, M. Declining breast cancer incidence and decreased HRT use. Lancet. 2008;372:608–610.
200. Chapurlat, RD, Delmas, PD. Drug insight: Bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211–219.
201. Watts, NB, Harris, ST, Genant, HK, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323:73–79.
202. Cranney, A, Guyatt, G, Krolicki, N, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2001;12:140–151.
203. Hosking, D, Chilvers, CED, Christiansen, C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med. 1998;338:485–492.
204. Black, DM, Cummings, SR, Karpf, DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541.
205. Ensrud, KE, Black, DM, Palermo, L, et al. Treatment with alendronate prevents fractures in women at highest risk. Arch Intern Med. 1997;157:2617–2624.
206. Cummings, SR, Black, DM, Thompson, DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA. 1998;280:2077–2082.
207. Harris, ST, Watts, N, Genant, HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. JAMA. 1999;282:1344–1352.
208. Reginster, J-Y, Minne, HW, Sorensen, OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11:83–91.
209. McClung, MR, Geusens, P, Miller, PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–340.
210. Chestnut, CHIII, Skag, A, Christiansen, C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
211. Reid, IR, Brown, JP, Burckardt, P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661.
212. Black, DM, Delmas, PD, Eastell, R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2006;356:1809–1822.
213. Lyles, KW, Colon-Emeric, CS, Magaziner, JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809.
214. McCloskey, E, Selby, P, de Takats, D, et al. Effects of clodronate on vertebral fracture risk in osteoporosis: a 1-year interim analysis. Bone. 2001;28:310–315.
215. Adachi, JD, Bensen, WG, Brown, J, et al. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med. 1997;337:382–387.
216. Saag, KG, Emkey, R, Schnitzer, TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292–299.
217. Wallach, S, Cohen, S, Reid, DM, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277–285.
218. Delmas, PD, Bjarnason, NH, Mitlak, BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647.
219. Cummings, SR, Eckert, S, Krueger, KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple outcomes on raloxifene evaluation. JAMA. 1999;281:2189–2197.
220. Ettinger, B, Black, DM, Mitlack, BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. JAMA. 1999;282:637–645.
221. Delmas, PD, Ensrud, KE, Adachi, JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
222. Delmas, PD, Genant, HK, Crans, GG, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and non vertebral: results from the MORE trial. Bone. 2003;33:522–532.
223. Barrett-Connor, E, Grady, D, Sashegyi, A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women. Four-year results from the MORE randomized trial. JAMA. 2002;287:847–857.
224. Barrett-Connor, E, Mosca, L, Collins, P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137.
225. Vogel, VG, Costantino, JP, Wickerham, DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741.
226. Silverman, SL, Christiansen, C, Genant, HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results of a 3-year randomized placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923–1934.
227. Cummings, SR, Eastell, R, Ensrud, K, et al. The effects of lasofoxifene on fractures and breast cancer: 3-year results from the PEARL Trial. J Bone Miner Res. 2008;23(Suppl 1):S81.
228. Cummings, SR, McClung, MR, Christiansen, C, et al. A phase III study of the effects of denosumab on vertebral, non vertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. J Bone Miner Res. 2008;23(Suppl 1):S80.
229. Brown, JP, Prince, RL, Deal, C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–161.
230. Ellis, GK, Bone, HG, Chlebowski, R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882.
231. Miller, PD, Bolognese, MA, Liewecki, EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
232. Overgaard, K, Riis, BJ, Christiansen, C, et al. Effect of salcatonin given intranasally on early postmenopausal bone loss. BMJ. 1989;299:477–479.
233. Overgaard, K, Hansen, MA, Jensen, SB, et al. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ. 1992;305:556–561.
234. Chestnut, CH, 3rd., Silverman, S, Andriano, K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence of Osteoporotic Fractures study. PROOF Study Group. Am J Med. 2000;109:267–276.
235. Cummings, SR, Chapurlat, RD. What PROOF proves about calcitonin and clinical trials. Am J Med. 2000;109:330–331.
236. Neer, RM, Arnaud, CD, Zanchetta, JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:434–441.
237. Ettinger, B, San Martin, J, Crans, G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–751.
238. Delmas, PD, Vergnaud, P, Arlot, ME, et al. The anabolic effect of human PTH (1–34) on bone formation is blunted when bone resorption in inhibited by the bisphosphonate tiludronate—Is activated resorption a prerequisite for the in vivo effect of PTH on formation in a remodeling system? Bone. 1995;16:603–610.
239. Black, DM, Greenspan, SL, Ensrud, KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215.
240. Finkelstein, JS, Hayes, A, Hunzelman, JL, et al. The effects of parathyroid hormone, alendronate, or both, in men with osteoporosis. N Engl J Med. 2003;349:1216–1226.
241. Miller, PD, Delmas, PD, Lindsay, R, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93:3785–3793.
242. Obermayer-Pietsch, BM, Marin, F, McCloskey, EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23:1591–1600.
243. Geenspan, SL, Bone, HG, Ettinger, MP, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146(5):326–339.
244. Dawson-Hughes, B, Dallal, GE, Krall, EA, et al. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med. 1990;323:878–883.
245. Reid, IR, Ames, RW, Evans, MC, et al. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women: a randomized controlled trial. Am J Med. 1995;98:331–335.
246. Recker, RR, Hinders, S, Davies, KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996;11:1961–1966.
247. Lips, P, Graafmans, WC, Ooms, ME, et al. Vitamin D supplementation and fracture incidence in elderly persons: a randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400–406.
248. Dawson-Hughes, B, Harris, SS, Krall, EA, et al. N Engl J Med. 1997;337:670–676.
249. Heikinheimo, RJ, Inkovaraa, JA, Harju, EJ, et al. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int. 1992;51:105–110.
250. Trivedi, DP, Doll, R, Khaw, KY. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomized double blind controlled trial. BMJ. 2003;326:469–475.
251. Harwood, RH, Sahota, O, Gaynor, K, et al. A randomized, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: the Nottingham neck of femur (NONOF) study. Age Ageing. 2004;33:45–51.
252. Meunier, PJ, Slosman, DO, Delmas, PD, et al. Strontium ranelate: dose-dependent effects in established postmenopausal osteoporosis—a two-year randomized placebo controlled trial. J Clin Endocrinol Metab. 2002;87:2060–2066.
253. Reginster, JY, Deroisy, R, Dougados, M, et al. Prevention of early postmenopausal bone loss by strontium ranelate: the randomized, two-year, double-masked, dose ranging, placebo-controlled PREVOS trial. Osteoporos Int. 2002;13:925–931.
254. Meunier, PJ, Roux, C, Seeman, E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–468.
255. Reginster, JY, Seeman, E, De Vernejoul, MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90(5):2816–2822.
256. Seeman, E, Vellas, B, Benhamou, C, et al. Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res. 2006;21:1113–1120.
257. Alexandersen, P, Toussaint, A, Christiansen, C, et al. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. JAMA. 2001;285:1482–1488.
258. Cummings, SR, Ettinger, B, Delmas, PD, et al. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008;359:697–708.
259. Holloway, L, Kohlmeier, L, Kent, K, et al. Skeletal effects of cyclic recombinant human growth hormone and salmon calcitonin on osteopenic postmenopausal women. J Clin Endocrinol Metab. 1997;82:1111–1117.
260. Reid, IR, Ames, RW, Orr-Walker, BJ, et al. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med. 2000;109:362–370.
261. Delmas, PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–2026.
262. Kanis, JA, McCloskey, EV, Johansson, H, et al. Case finding for the management of osteoporosis with FRAX–assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–1408.
263. Tosteson, ANA, Melton, LJ, III., Dawson-Hugues, B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437–447.
264. Dawson-Hughes, B, Tosteson, ANA, Melton, LJ, III., et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
265. Cummings, SR, Karpf, DB, Harris, F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–289.
266. Chapurlat, RD, Palermo, L, Ramsay, P, et al. Risk of fracture among women who lose bone density during treatment with alendronate. The Fracture Intervention Trial Osteoporos Int. 2005;16:842–848.
267. Chen, P, Satterwhite, JH, Licata, AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–970.
268. Bauer, DC, Black, DM, Garnero, P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.