Bloodstream Infections
1. Identify and describe some of the medical consequences that occur when the bloodstream is infected by microorganisms.
2. Name the most common causes of bacterial bloodstream infection, and explain the route of transmission and source of infection.
3. Define the following bloodstream infections: bacteremia, fungemia, and septicemia.
4. List the most common fungi associated with bloodstream infections and the population of patients most often affected by this type of infection.
5. Explain what causes mortality in most cases of parasitic blood-borne infections.
6. Differentiate between intravascular and extravascular bloodstream infections.
7. Define continuous bacteremia, and provide an example.
8. Describe the development of infective endocarditis, including the contributing factors and the microorganisms that are the primary cause for the condition.
9. Define mycotic aneurysms and suppurative thrombophlebitis, and describe the causes for these conditions.
10. Explain the pathogenic features of S. epidermidis that make it uniquely suited for causing catheter-related infections.
11. Explain the importance of collection parameters associated with blood cultures for suspected cases of bloodstream infections, including collection time, the number of cultures, and the volume of blood required.
12. List and briefly describe some of the blood culture systems available to the microbiologist, including the self-contained systems, the lysis centrifugation systems, and instrument-based systems.
13. List some of the most common causes of bloodstream infection associated with the blood cultures from HIV-infected patients.
14. Define the acronym AACEK, and describe the type of blood-borne infections these organisms are most often associated with.
15. Outline the guidelines used to determine if agents isolated from blood cultures are true pathogens or probable contaminants.
General Considerations
Etiology
Bacteria
The organisms most commonly isolated from blood are gram-positive cocci, including coagulase-negative staphylococci, Staphylococcus aureus, and Enterococcus spp., and other organisms likely to be inhabitants of the hospital environment that colonize the skin, oropharynx, and gastrointestinal tract of patients. Some of the most common, clinically significant bacteria isolated from blood cultures are listed in Box 68-1. In general, the number of fungi and coagulase-negative staphylococci has increased, whereas the number of clinically significant anaerobic isolates has decreased since the early 2000s.
Parasites
Parasites in the bloodstream are usually detected by direct visualization. Those parasites for which traditional diagnosis is dependent on observation of the organism in peripheral blood smears include Plasmodium, Trypanosoma, and Babesia. Patients with malaria or filariasis may display a periodicity in their episodes of fever that allows the physician to time the collection of blood for microscopic examination intended for optimal detection. Rapid serological methods and molecular methods are currently used to detect malaria, babesiosis, and trypanosomiasis. These tests are described in Chapter 49.
Types of Bloodstream Infections
Intravascular Infections
Infective Endocarditis.
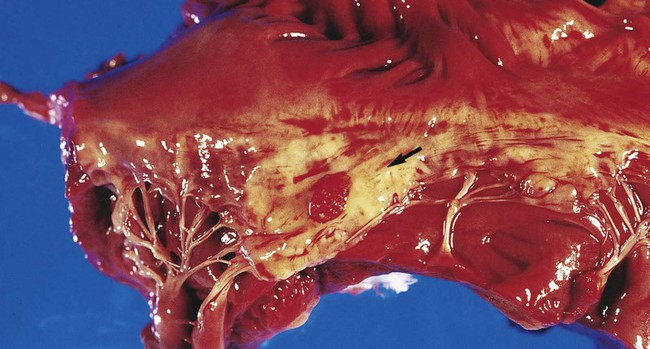
The development of infective endocarditis (infection of the endocardium most commonly caused by bacteria) is believed to involve several independent events. Cardiac abnormalities, such as congenital valvular diseases that lead to turbulence in blood flow or direct trauma from IV catheters, can damage cardiac endothelium. This damage to the endothelial surface results in the deposition of platelets and fibrin. If bacteria transiently gain access to the bloodstream (this can occur after an innocuous procedure such as brushing the teeth) after alteration of the capillary endothelial cells, the organisms may stick to and then colonize the damaged cardiac endothelial cell surface. After colonization, the surface will rapidly be covered with a protective layer of fibrin and platelets. This protective environment is favorable to further bacterial multiplication. This web of platelets, fibrin, inflammatory cells, and entrapped organisms is called a vegetation (Figure 68-1). The resulting vegetations ultimately seed bacteria into the blood at a slow but constant rate.
The primary causes of infective endocarditis are the viridans streptococci, comprising several species (Box 68-2). These organisms are normal inhabitants of the oral cavity, often gaining entrance to the bloodstream as a result of gingivitis, periodontitis, or dental manipulation. Heart valves, especially those previously damaged, present convenient surfaces for attachment of these bacteria. Streptococcus sanguis and Streptococcus mutans are frequently isolated in streptococcal endocarditis. Gram-negative bacilli, known as the AACEK group, Aggregatibacter aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae, can also be associated with endocarditis.
Mycotic Aneurysm and Suppurative Thrombophlebitis.
Intravenous Catheter–Associated Bacteremia.
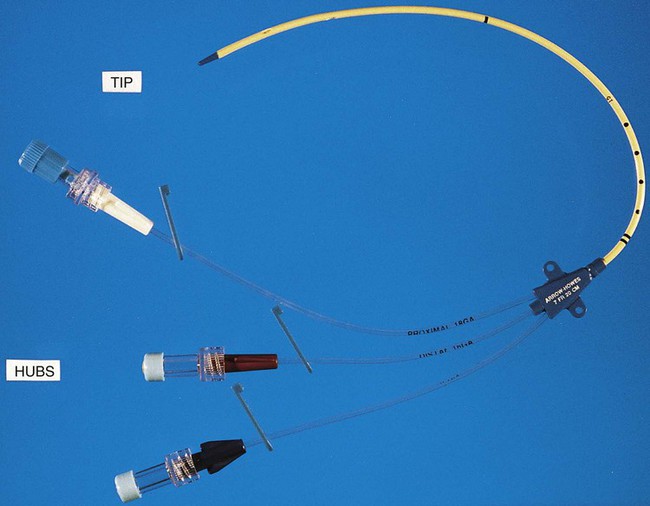
IV catheters are an integral part of the care for many hospitalized patients. More than 3 million central venous catheters are used annually in the United States. For example, central venous catheters are used to administer fluids, blood products, medications, antibiotics, and nutrition, and for hemodynamic monitoring. A short-term, triple-lumen (channel opening within a tube) central venous catheter is shown in Figure 68-2. Unfortunately, a major consequence of these medical devices is colonization of the catheter by either bacteria or fungi, which can lead to catheter infection and serious bloodstream infection. This consequence is a major nosocomial source of illness and even death.
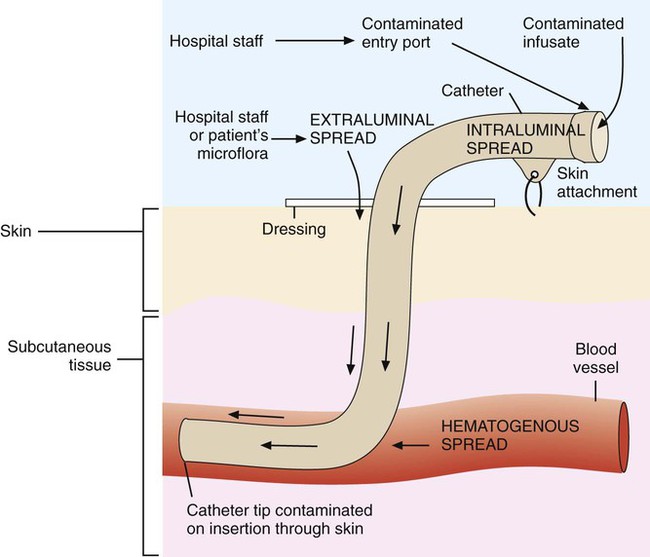
IV catheter–associated bacteremia (or fungemia) is believed to occur primarily by two routes (Figure 68-3). The first route involves the movement of organisms from the catheter entry site through the patient’s skin and down the external surface of the catheter to the catheter tip within the bloodstream. After arriving at the tip, the organisms multiply and may cause a bacteremia. The second way that IV catheter–associated bacteremia may occur is by migration of organisms along the inside of the catheter (the lumen) to the catheter tip. The catheter’s hub, where tubing connects into the IV catheter, is considered the site at which organisms gain access to the patient’s bloodstream through the catheter lumen. The most common etiologic agents for IV catheter–associated bloodstream infections, regardless of the route of infection, are organisms found on the skin (Box 68-3). Certain strains of S. epidermidis appear to be uniquely suited for causing catheter-related infections because of their ability to produce a biofilm or “slime” that consists of complex sugars (polysaccharides) believed to help the organism adhere to the catheter’s surface. The initial attachment of S. epidermidis to the catheter’s polystyrene surface is related to a cell surface protein. Once attached, the organism proliferates, subsequently forming a biofilm. Uncommon routes of IV catheter–tip infection include contaminated fluids or blood-borne seeding from another infection site.
Extravascular Infections
The most common portals of entry for bacteremia are the genitourinary tract (25%), respiratory tract (20%), abscesses (10%), surgical wound infections (5%), biliary tract (5%), miscellaneous sites (10%), and uncertain sites (25%). For the most part, the probability of bacteremia occurring from an extravascular site depends on the site of infection, its severity, and the organism. For example, any organism producing meningitis is likely to produce bacteremia at the same time. Of importance, certain organisms causing extravascular infections commonly invade the bloodstream; some of these organisms are listed in Table 68-1. In addition to these organisms, a large number of other bacteria and fungi that cause extravascular infections are also capable of invading the bloodstream. Whether these organisms invade the bloodstream depends on the host’s ability to control the infection and the organism’s pathogenic potential. Some of the organisms associated with potential bloodstream infections from a localized site include members of the family Enterobacteriaceae, Streptococcus pneumoniae, Staphylococcus aureus, Neisseria gonorrhoeae, anaerobic cocci, Bacteroides, Clostridium, beta-hemolytic streptococci, and Pseudomonas. These are only some of the organisms frequently isolated from blood. Almost every known bacterial species and many fungal species have been implicated in extravascular bloodstream infections.
TABLE 68-1
Organisms Commonly Associated with Bloodstream Invasion from Extravascular Sites of Infection
| Organism | Extravascular Site of Infection |
| Anaerobic organisms | Wound, soft tissue |
| Brucella spp. | Reticuloendothelial system |
| Candida albicans | Genitourinary tract |
| Chlamydia pneumoniae | Respiratory |
| Clostridium spp. | Wound, soft tissue |
| Coagulase negative staphylococci | Wound, soft tissue |
| Enterobacteriaceae (E.coli, Klebsiella spp., Enterobacter spp., Proteus spp., Enterococcus spp.) | Genitourinary tract infections, central nervous system |
| Haemophilus influenzae | Meninges (CNS), epiglotitis, periorbital region, respiratory |
| Legionella spp. | Respiratory |
| Listeria monocytogenes | Meninges (CNS) |
| Neisseria meningitidis | Meninges (CNS) |
| Pseudomonas aeruginosa | Wound, soft tissue, central nervous system |
| Salmonella enterica typhi | Small intestine, regional lymph nodes of the intestine, reticuloendothelial system |
| Streptococcus penumoniae | Meninges (CNS), respiratory |
| Streptococcus pyogenes | Wound, soft tissue |
| Staphylococcus aureus | Wound, soft tissue, meninges (CNS) |
Clinical Manifestations
Shock is the gravest complication of septicemia. In septic shock, the presence of bacterial products and the host’s response act to shut down major host physiologic systems. Clinical manifestations include a drop in blood pressure, increase in heart rate, functional impairment in vital organs (brain, kidney, liver, and lungs), acid-base alterations, and bleeding problems. Gram-negative bacteria contain a substance in their cell walls, called endotoxin, which has a strong effect on several physiologic functions. This substance, a lipopolysaccharide (LPS) comprising part of the cell wall structure (see Chapter 2), may be released during the normal growth cycles of bacteria or after the destruction of bacteria by host defenses. Endotoxin (or the core of the LPS, lipid A) has been shown to mediate numerous systemic reactions, including a febrile response, and the activation of complement and certain blood-clotting factors. Although gram-positive bacteria do not contain the lipid A endotoxin, many produce exotoxins, and the effects of their presence in the bloodstream may be equally devastating to the patient.
Detection of Bacteremia
Specimen Collection
Preparation of the Site
Antisepsis.
Once a vein is selected, the skin site is defatted (fat removal) with 70% isopropyl alcohol and an antiseptic is applied to kill surface and subsurface bacteria. Regardless of the antiseptic used, it is critical to follow the manufacturer’s recommendation for the length of time the antiseptic is allowed to remain on the skin. Available data indicate that iodine tincture (iodine in alcohol) and chlorhexidine are equivalent for skin preparation before drawing blood cultures. The steps necessary for drawing blood for culture are given in Procedure 68-1, which can be found on the Evolve site.
Specimen Volume
Children.
It is not safe to take large samples of blood from children, particularly infants. The optimal volume of blood required for successful identification of organisms from infants and children has not been clearly delineated. Similar to adults, this patient population has low level (small numbers of organisms) bacteremia. In light of low-level bacteremia in infants and children and based on the premise that it is safe to obtain as much as 4% to 4.5% of a patient’s known total blood volume for culture and the relationship between blood volume and patient weight, Baron and colleagues have determined recommendations for blood volumes for cultures from infants and children (Table 68-2). For infants and small children, only 1 to 5 mL of blood should be drawn for bacterial culture. Blood culture bottles are available designed specifically for the pediatric patient. Because blood specimens from septic children may yield fewer than 5 CFU/mL of the organism, quantities less than 1 mL may not be adequate to detect pathogens. Nevertheless, smaller volumes should still be cultured because high levels of bacteremia (more than 1000 CFU/mL of blood) are detected in some infants.
TABLE 68-2
Suggested Blood Volumes for Cultures from Infants and Children
| Weight of Patient | Total Blood Volume (mL) | Recommended Volume of Blood for Culture (mL) | % of Total Blood Volume | |||
| kg | lb | Culture No. 1 | Culture No. 2 | Total Volume for Culture (mL) | ||
| ≤1 | ≤2.2 | 50-99 | 2 | 2 | 4 | |
| 1.1-2 | 2.2-4.4 | 100-200 | 2 | 2 | 4 | 4 |
| 2.1-12.7 | 4.5-27 | >200 | 4 | 2 | 6 | 3 |
| 12.8-36.3 | 28-80 | >800 | 10 | 10 | 20 | 2.5 |
| >36.3 | >80 | >2200 | 20-30 | 20-30 | 40-60 | 1.8-2.7 |
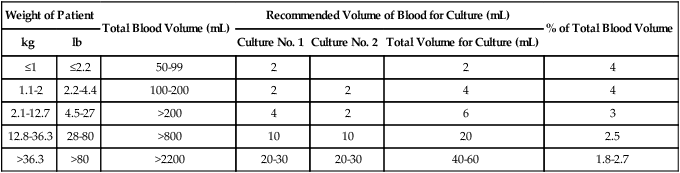
Note: Volumes and recommendations may vary based on automated system and manufacturer’s guidelines.
From Baron EJ, Weinstein MP, Dunne WM, et al: Blood cultures IV. In Baron EJ, coordinating editor, Cumitech 1C, Washington, DC, 2005, American Society for Microbiology, reprinted with permission.
Culture Techniques
Self-Contained Subculture System
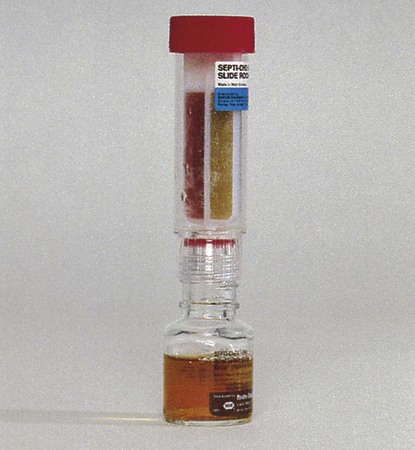
A modification of the biphasic blood culture medium is the BD Septi-Chek system (Becton Dickinson Microbiology Systems, Sparks, Maryland) (Figure 68-4) consisting of a conventional blood culture broth bottle with an attached chamber containing a slide coated with agar or several types of agars. Special media for isolation of fungi and mycobacteria are also available. To subculture, the entire broth contents are allowed to contact the agar surface by inverting the bottle, a simple procedure that does not require opening the bottle or using needles. The large volume of broth subcultured and the enclosed method provide faster detection for many organisms than is possible with conventional systems. The Septi-Chek system appears to enhance the recovery of Streptococcus pneumoniae, but such biphasic systems do not efficiently recover anaerobic isolates.
Lysis Centrifugation
The Isolator (Alere, Waltham, MA) is a lysis centrifugation system commercially available. The Isolator consists of a stoppered tube containing saponin to lyse blood cells and SPS as an anticoagulant (Figure 68-5). After centrifugation, the supernatant is discarded, the sediment containing the pathogen is vigorously vortexed, and the entire sediment is plated to solid agar. Benefits of this system include rapid and improved recovery of filamentous fungi, the presence of actual colonies for direct identification and susceptibility testing after initial incubation, the ability to quantify the colony-forming units present in the blood, rapid detection of polymicrobial bacteremia, dispensing with the need for a separate antibiotic-removal step, the ability to choose special media for initial culture setup based on clinical impression (e.g., direct plating onto media supportive of Legionella spp. or Mycobacterium spp.), and potential enhanced recovery of intracellular microorganisms caused by lysis of host cells. Possible limitations of the system seem to be a relatively high rate of plate contamination and a decreased ability to detect certain bacteria, such as Streptococcus pneumoniae, Listeria monocytogenes, Haemophilus influenzae, and anaerobic bacteria, compared with conventional systems. If a mixed infection is suspected, an additional blood culture collection tube should be inoculated simultaneously.
Instrument-Based Systems
BACTEC Systems.
Subsequent modifications further automated the incubation and measuring device, and detection was accomplished by nonradioactive means. The BACTEC blood culture systems are fully automated with the incubator, shaker, and detector all in one instrument. These fully automated blood culture systems use fluorescence to measure CO2 released by organisms; a gas-permeable fluorescent sensor is on the bottom of each vial (Figure 68-6). As CO2 diffuses into the sensor and dissolves in water present in the sensor matrix, hydrogen (H+) ions are generated. These H+ ions cause a decrease in pH, which, in turn, increases the fluorescent output of the sensor. There is continuous monitoring of each bottle and detection is external to the bottle. Of importance, the noninvasion of the blood culture bottle eliminates the potential for cross-contamination of cultures.
BacT/ALERT Microbial Detection System.
Other laboratories use the BacT/Alert System (bioMérieux, Durham, North Carolina), which measures CO2-derived pH changes with a colorimetric sensor in the bottom of each bottle (see Figure 68-6). The sensor is separated from the broth medium by a membrane permeable to CO2. As organisms grow, they release CO2, which diffuses across the membrane and is dissolved in water present in the matrix of the sensor. As CO2 is dissolved, free hydrogen ions are generated. These free hydrogen ions cause a color change in the sensor (blue to light green to yellow as the pH decreases); a sensor in the instrument reads this color change.
Versa TREK System.
The Versa TREK system (Thermo Scientific, TREK Diagnostics, Cleveland, Ohio) utilizes a unique agitation system during blood culture inoculation. The aerobic media bottles each contain a small magnetic stir bar enhancing oxygenation during incubation. Like the other systems, this is also a continuously monitoring instrument. Table 68-3 summarizes characteristics of some blood culture instruments that are available at the time of printing of the text.
TABLE 68-3
Summary Characteristics of the More Commonly Used Continuous-Monitoring Blood Culture Systems
| System | Bottles Available* | Inoculum Volume (mL)** | Blood: Broth | Detection |
| BacT/ALERT | SA Aerobic | 5-10 | 1 : 4 | Colorimetric detection of CO2 |
| SN Anaerobic | 5-10 | 1 : 4 | ||
| FA, FN (aerobic and anaerobic bottles) | 5-10 | 1 : 4 | ||
| PF Pediatric | 1-4 | 1 : 5 | ||
| MB (mycobacteria whole blood) | 0.50 | ∼1 : 5 | ||
| MP (mycobacteria processed specimen or body fluid other than blood) | 0.50 | |||
| BACTEC | Standard aerobic/F | 8-10 | 1 : 4 | Fluorescent detection of CO2 |
| Standard anaerobic/F | 5-7 | 1 : 4 | ||
| Plus aerobic/F | 8-10 | 1 : 2.5 | ||
| Plus anaerobic/F | 5-7 | 1 : 2.5 | ||
| Peds Plus/F | 0.5-5 | 1 : 8 | ||
| Lytic/10 anaerobic/F | 8-10 | 1 : 4 | ||
| Myco/F Lytic medium (for fungi and mycobacteria) | 1-5 | 1 : 8 | ||
| VersaTREK | REDOX (aerobic) | 10 | 1 : 9 | Detection of O2 consumption and/or CO2, H2, and/or N2 production |
| REDOX (anaerobic) | 10 | 1 : 9 | ||
| EZ Draw REDOX 1 aerobic | 5 | 1 : 9 | ||
| EZ Draw REDOX 2 anaerobic | 5 | 1 : 9 |
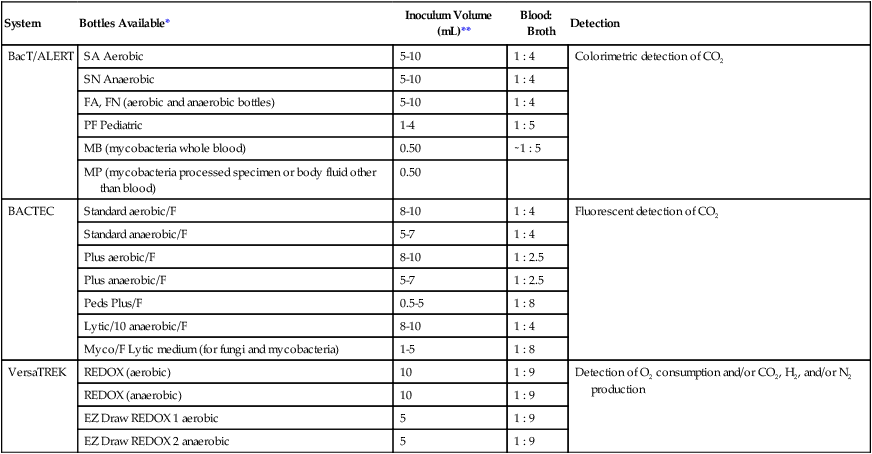
NOTE: Due to the modular design of automated blood culture systems, various models and arrangements of modular units provide a customized specimen capacity to suit the laboratories needs.
*No venting required on any bottles listed.
**Minimum sample volumes. Increased volume will enhance the recovery of the organisms.
Interpretation of Blood Culture Results
Note: Bacillus anthracis must be ruled out before dismissing Bacillus species as a probable contaminant.
• Growth of multiple organisms from one of several cultures (polymicrobial bacteremia is uncommon)
• The clinical presentation or course is not consistent with sepsis (physician-based, not laboratory-based criteria)
• The organism causing the infection at a primary site of infection is not the same as that isolated from the blood culture
• Growth of the same organism in repeated cultures obtained either at different times or from different anatomic sites
• Growth of certain organisms in cultures obtained from patients suspected of endocarditis, such as enterococci, or gram-negative rods in patients with clinical gram-negative sepsis
• Growth of certain organisms such as members of Enterobacteriaceae, Streptococcus pneumoniae, gram-negative anaerobes, and Streptococcus pyogenes
• Isolation of commensal microbial flora from blood cultures obtained from patients suspected to be bacteremic (e.g., immunosuppressed patients or those having prosthetic devices)
Special Considerations for Other Relevant Organisms Isolated From Blood
The organisms discussed in this section require somewhat different conditions for their successful recovery from blood culture samples. Most of these organisms are infrequently isolated from blood. Therefore, it is important for the physician to notify the laboratory of remarkable patient history, such as travel abroad. In light of recent events and concerns about bioterrorism, it is also important the laboratory be aware of organisms isolated from blood cultures that are considered potential agents for bioterrorist attacks. These bacteria include Bacillus anthracis, Francisella tularensis, Brucella spp., and Yersinia pestis. Finally, in addition to the organisms discussed later that require special different conditions for isolation from blood, a number of organisms are unable to grow on artificial media and are best diagnosed by alternative methods such as serology or molecular amplification assays; these organisms are listed in Box 68-4.
Campylobacter and Helicobacter
Several species of Campylobacter and Helicobacter are occasionally isolated from blood cultures, usually growing within the 5-day incubation protocol. However, these organisms are small, thin, curved, gram-negative rods, which may only be visualized using an AO stain following detection by continuous monitoring instruments. Because of the fastidious nature of these organisms, appropriate media and atmospheric conditions for subculture from blood culture bottles must be employed (see Chapter 34).
Spirochetes
Borrelia
Leptospira
Leptospirosis can be diagnosed by isolating the causative spirochete from blood during the first 4 to 7 days of illness. Leptospires will grow 1 to 3 cm below the surface, usually within 2 weeks. The organisms remain viable in blood with SPS for 11 days, allowing for transport of specimens from distant locations. Direct dark-field examination of peripheral blood is not recommended because many artifacts are present that resemble spirochetes. If blood must be shipped to a reference laboratory for culture, blood may be collected into heparin, oxalate, or citrate tubes and maintained at ambient temperature. One to two drops of blood are inoculated into semi-solid oleic acid-albumin medium at the patient’s bedside. Various commercial mediums are available, such as Fletcher’s medium (BD Diagnostics, Sparks, MD). Multiple cultures are recommended to improve recovery of the organisms. Due to the organism’s failure to grow in conventional blood culture systems, molecular assays may improve detection of the organism, as well as the use of serological markers for rapid diagnosis. (Further information about Borrelia and Leptospira is provided in Chapter 46.)
Bartonella
Based on phenotypic and genotypic characteristics, bacteria of the genus Rochalimaea were reclassified into the genus Bartonella. Bartonella previously contained only a single species, B. bacilliformis, the agent of verruga peruana and a septicemic, hemolytic disease known as Oroya fever (see Chapter 33). New species such as Bartonella henselae and B. elizabethae, as well as Bartonella quintana, have been reported to cause bacteremia and endocarditis in both immunocompetent and immunocompromised patients. B. henselae has also been linked to cat-scratch disease, a common infectious disease in the United States. Cat-scratch disease is characterized by a persistent necrotizing inflammation of the lymph nodes. For the most part, the most reliable method for diagnosis of Bartonella bacteremia is serology.